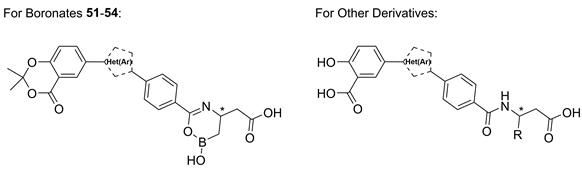
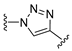
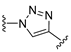
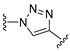
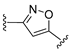
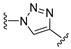
3.1. Chemical Synthesis
All solvents and reagents were purchased from commercial sources and used without further purification. Standard vacuum line techniques were applied. Reactions were monitored via thin-layer silica gel chromatography (TLC) using polyester sheets POLYGRAM SIL G/UV254 coated with 0.2 mm silica gel (Macherey-Nagel, Düren, Germany). Plates were visualized using UV light (254 nm or 365 nm) or staining with KMnO4, curcumin, CAM (ceric ammonium molybdate) or DNPH (dinitrophenylhydrazine). Products were purified by flash column chromatography (normal-phase silica gel chromatography or boric acid-impregnated normal-phase silica gel chromatography) using SiO2 60 (0.040–0.063 mm, 230–400 mesh ASTM) from Merck (Darmstadt, Germany). NMR spectra were recorded with Avance III HD 400 MHz Bruker BioSpin and Avance III HD 500 MHz Bruker BioSpin (1H-NMR: 400 MHz and 500 MHz, 13C-NMR: 101 MHz and 126 MHz) (Bruker, Billerica, MA, USA) using the deuterated solvent stated. Chemical shifts (δ) are quoted in parts per million (ppm) and referenced to the residual solvent peak. Multiplicities are denoted as follows: s—singlet, d—doublet, t—triplet, q—quartet and quin—quintet. Coupling constants J are given in Hz and rounded to the nearest 0.1 Hz. Infrared spectra were recorded from 4000 to 650 cm−1 on a Perkin Elmer Spectrum BX-59343 FT-IR instrument (Perkin Elmer, Shelton, CT, USA). A Smiths Detection DuraSamp IR II Diamond ATR sensor (Smiths Detection, Danbury, CT, USA) was used for detection. The absorption bands are reported in wavenumbers [cm−1]. High-resolution mass spectra (HR-MS) were recorded using a Jeol Mstation 700 or JMS GCmate II Jeol instrument (Jeol, Tokyo, Japan) for electron impact ionization (EI). Thermo Finnigan LTQ (Thermo Finnigan, Somerset, NJ, USA) was used for electrospray ionization (ESI). Melting points were measured with a Büchi Schmelzpunktapparatur B-540 (Büchi, Flawil, Switzerland). HPLC analytical measurements at 210 nm and 254 nm for purities determination was performed with a Zorbax Eclipse Plus C18 column (Waters, Milford, MA, USA) with a diameter of 4.6 mm and a length of 150 mm and a particle size of 3.5 µm.
General Procedure A—N-Boc deprotection with HCl [
33]
. The appropriate
N-Boc-protected amine (1.0 equivalent) was dissolved in 4.0 M HCl dioxane solution (3.2 equivalents) under N
2 atmosphere and stirred at room temperature for 1 h. The solvent was removed in vacuo and then co-evaporated with diethyl ether (4×). The obtained amine hydrochlorides could be used for the subsequent steps without further purification.
General Procedure B—Amide coupling with CDI. To a stirred solution of the appropriate carboxylic acid (1.0 equivalent) in DMF with a concentration of 0.20 M, CDI (1.1 equivalents) was added. The reaction mixture was stirred at room temperature for 15 min. Afterwards, the appropriate amine (1.0 equivalent) was added, and unless stated otherwise, the solution was stirred at room temperature for 3 d. The solution was diluted with EtOAc, and the organic phase was washed with brine (3×). The organic phase was dried using a phase separation paper, and the solvent was removed in vacuo. If necessary, the crude product was purified by FCC using the indicated eluent.
General Procedure C—PIFA-mediated isoxazole formation [
43]
. To a stirred solution of the appropriate alkyne (1.0 equivalent) in MeOH/H
2O (5:1
v/
v) with a concentration of 0.10 M, the appropriate oxime (1.5 equivalents) was added. Then, 0.5 equivalents of PIFA were added every 2 h. After the addition of 1.5 equivalents PIFA, the reaction mixture was stirred at room temperature for another 16 h. The solvents were removed in vacuo, and the crude product was resuspended in EtOAc. Water was added, the resulting two phases were separated, and the aqueous phase was extracted with EtOAc (3×). The combined organic phases were dried using a phase separation paper and the solvent was removed in vacuo. The crude product was purified by FCC using the indicated eluent.
General Procedure D—Copper-catalyzed azide-alkyne cycloaddition. To a stirred solution of the appropriate azide (1.0 equivalent) in t-BuOH/DMSO (21:1 v/v) with a concentration of 0.13 M, we added a solution of the appropriate alkyne (1.2 equivalents) in t-BuOH with a concentration of 0.33 M. A 0.027 M aq. CuSO4 solution (0.2 equivalents) and a 0.13 M aq. sodium ascorbate solution (1.0 equivalent) were added, and the reaction mixture was stirred at room temperature for 18 h. The reaction mixture was diluted with EtOAc, and the organic phase was washed sequentially with 1 M HCl (1×), aq. sat. NaHCO3 (1×) and brine (1×). The organic phase was dried using a phase separation paper, and the solvent was removed in vacuo. The crude product was purified by the indicated method.
General Procedure E—Alkaline deprotection of acetonides and esters. To a stirred solution of the appropriate protected starting material (1.0 equivalent) in THF with a concentration of 0.15 M, an aq. KOH solution was added with the stated concentration and equivalents. The reaction mixture was stirred at the stated temperature for the stated time and then acidified to pH 1 with 2 M HCl. EtOAc was added to the suspension, the resulting two phases were separated, and the aqueous phase was extracted with EtOAc (3×). The combined organic phases were dried using a phase separation paper, and the solvent was removed in vacuo. The crude product was purified by the indicated method.
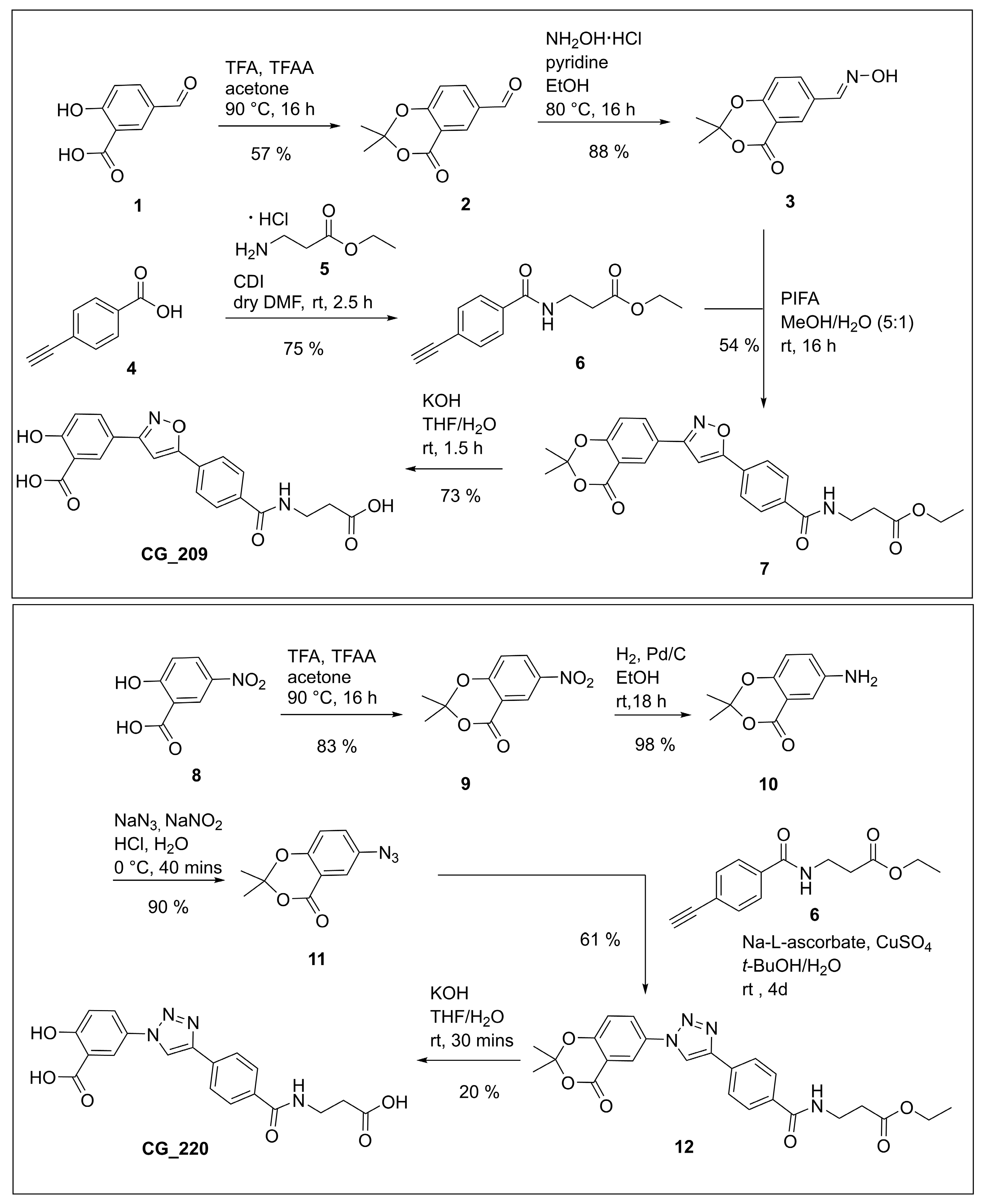
Ethyl 3-(4-ethynylbenzamido)propanoate (6). Prepared according to
General Procedure B from ß-alanine ethyl ester hydrochloride (1.00 g, 6.51 mmol) and 4-ethynylbenzoic acid (1.00 g, 6.51 mmol). The reaction mixture was stirred at room temperature for 2.5 h. The amide
6 (1.20 g, 4.89 mmol, 75%) was obtained as a brownish-yellow solid. Analytical data are in alignment with literature [
22].
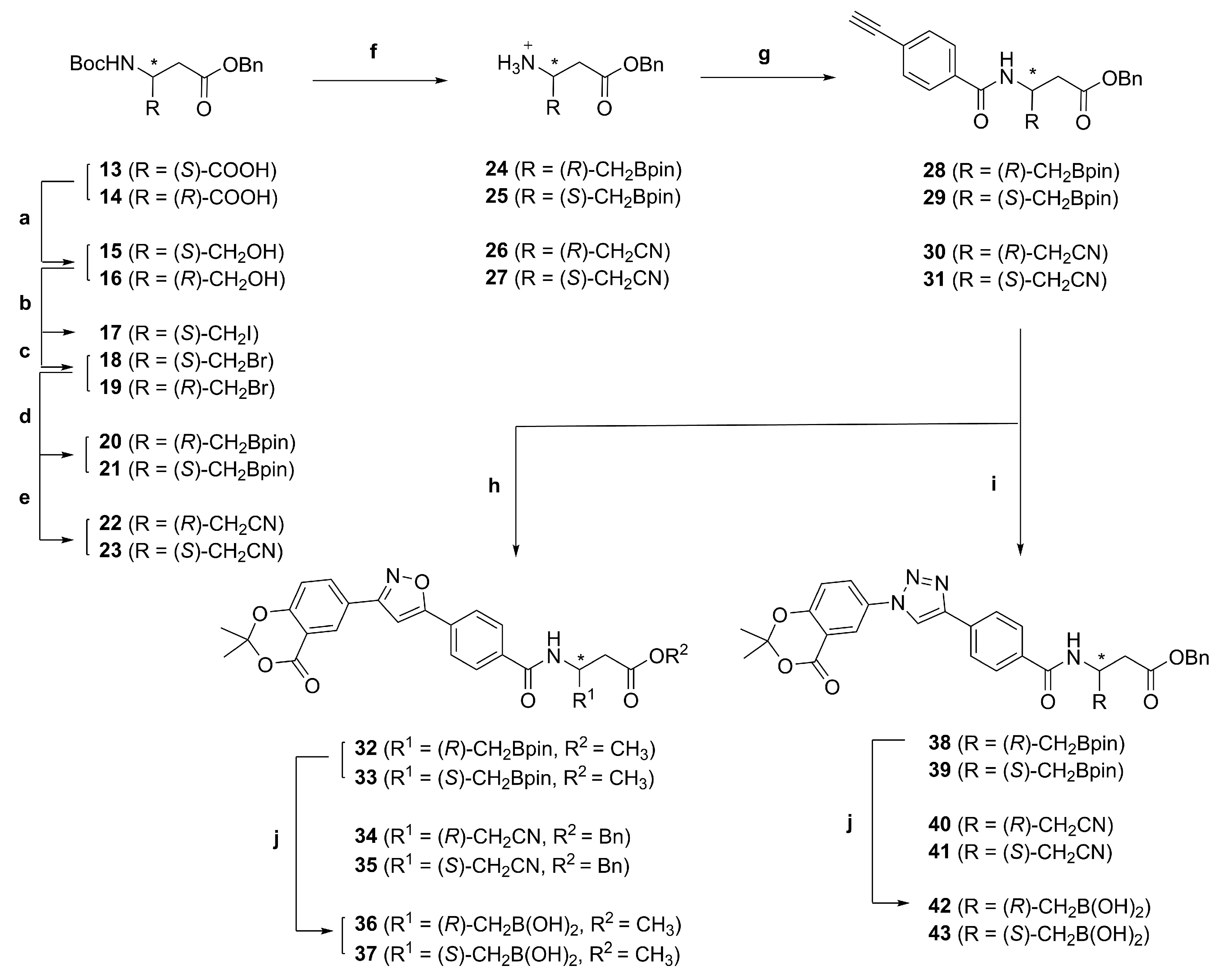
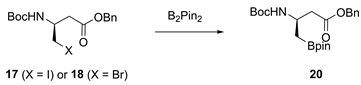
Benzyl (R)-3-((tert-butoxycarbonyl)amino)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)butanoate (20). Pd2(dba)3 (31.9 mg, 0.0348 mmol), di-tert-butyl(methyl)phosphonium tetrafluoroborate (51.8 mg, 0.209 mmol), bis(pinacolato)diboron (2.12 g, 8.35 mmol), K2CO3 (1.92 g, 13.9 mmol) and alkyl bromide 18 (2.59 g, 6.96 mmol) were added to a 100 mL round-bottomed flask and evacuated and backfilled three times with N2. 19 mL of a degassed t-BuOH/H2O (12:1 v/v) solution were added. The reaction mixture was stirred at 65 °C for 6 h, then diluted with EtOAc (200 mL). The organic phase was washed with brine (2 × 200 mL), dried using a phase separation paper, concentrated in vacuo and purified by FCC with boric acid-impregnated silica gel (hexanes/EtOAc 89:11) to give boronic acid pinacol ester 20 (1.75 g, 4.17 mmol, 60%) as a yellow oil. 1H-NMR (400 MHz, (CDCl3): δ (ppm) = 7.37–7.29 (m, 5H, 2′-H, 3′-H, 4′-H, 5′-H and 6′-H), 5.12–5.09 (m, 2H, 1′-CH2), 4.24–4.16 (m, 1H, 3-H), 2.69–2.52 (m, 2H, 2-H), 1.42 (s, 9H, C(CH3)3), 1.24–1.20 (m, 12H, (C(CH3)2)2), 1.15 (d, J = 6.7 Hz, 2H, 4-H). 13C-NMR (101 MHz, (CDCl3): δ (ppm) = 171.59 (C-1), 155.13 (NHCOO), 136.05 (C-1′), 128.67–128.32 (C-2′, C-3′, C-4′, C-5′ and C-6′), 83.59 ((C(CH3)2)2), 79.22 (C(CH3)3), 66.41 (1′-CH2), 44.96 (C-3) 41.17 (C-2), 28.56 (C(CH3)3), 24.97–24.86 ((C(CH3)2)2), 17.78 (C-4). IR (ATR): ṽ (cm−1) = 3348, 2977, 1709, 1514, 1366, 1168, 1140, 1019, 965, 846, 697. HR-MS (ESI): m/z = [M + Na]+ calcd for C22H34BNO6Na+: 442.2377; found: 442.2372. Specific rotation: [α +3 (c 0.1 in DMSO).
Benzyl (S)-3-((tert-butoxycarbonyl)amino)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)butanoate (21). Pd2(dba)3 (49.2 mg, 0.0537 mmol), di-tert-butyl(methyl)phosphonium tetrafluoroborate (80.0 mg, 0.322 mmol), bis(pinacolato)diboron (3.27 g, 12.9 mmol), K2CO3 (2.97 g, 21.5 mmol) and alkyl bromide 19 (4.00 g, 10.7 mmol) were added to a 100 mL round-bottomed flask and evacuated and backfilled three times with N2. 32 mL of a degassed t-BuOH/H2O (12:1 v/v) solution were added. The reaction mixture was stirred at 65 °C for 6 h, then diluted with EtOAc (500 mL). The organic phase was washed with brine (2 × 400 mL), dried using a phase separation paper, concentrated in vacuo and purified by FCC with boric acid-impregnated silica gel (hexanes/EtOAc 89:11) to give boronic acid pinacol ester 21 (2.30 g, 5.48 mmol, 51%) as a yellow oil. 1H-NMR (400 MHz, (CDCl3): δ (ppm) = 7.38–7.29 (m, 5H, 2′-H, 3′-H, 4′-H, 5′-H and 6′-H), 5.13–5.08 (m, 2H, 1′-CH2), 4.27–4.08 (m, 1H, 3-H), 2.75–2.52 (m, 2H, 2-H), 1.45–1.40 (m, 9H, C(CH3)3), 1.25–1.20 (m, 12H, (C(CH3)2)2)), 1.15 (d, J = 6.7 Hz, 2H, 4-H). 13C-NMR (101 MHz, (CDCl3): δ (ppm) = 171.65 (C-1), 155.13 (NHCOO), 136.04 (C-1′), 128.68–128.34 (C-2′, C-3′, C-4′, C-5′ and C-6′), 83.59 ((C(CH3)2)2), 79.16 (C(CH3)3), 66.42 (1′-CH2), 44.87 (C-3), 41.13 (C-2), 28.56 (C(CH3)3), 24.96–24.86 ((C(CH3)2)2)), 17.96 (C-4). IR (ATR): ṽ (cm−1) = 3371, 2977, 1711, 1498, 1366, 1164, 1140, 1019, 967, 846, 696. HR-MS (ESI): m/z = [M + Na]+ calcd for C22H34BNO6Na+: 442.2377; found: 442.2376. Specific rotation: [α −5 (c 0.1 in DMSO).
(R)-4-(Benzyloxy)-4-oxo-1-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)butan-2-aminium chloride (24). Prepared according to General Procedure A from N-Boc-protected amine 20 (3.20 g, 7.63 mmol). The amine hydrochloride 24 (2.45 g, 7.65 mmol, quant.) was obtained as a viscous yellow oil. 1H-NMR (500 MHz, (CD3)2SO): δ (ppm) = 8.04 (s, 3H, NH3+), 7.40–7.32 (m, 5H, 2′-H, 3′-H, 4′-H, 5′-H and 6′-H), 5.14 (d, J = 6.9 Hz, 2H, 1′-CH2), 3.63–3.53 (m, 1H, 2-H), 2.79–2.67 (m, 2H, 3-H), 1.27–1.22 (m, 1H, 1-H), 1.19 (d, J = 2.5 Hz, 12H, (C(CH3)2)2), 1.16–1.13 (m, 1H, 1-H). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 169.87 (C-4), 135.74 (C-1′), 128.45–128.05 (C-2′, C-3′, C-4′, C-5′ and C-6′), 83.51 ((C(CH3)2)2), 66.06 (1′-CH2), 44.96 (C-2), 38.12 (C-3), 24.46 ((C(CH3)2)2), 16.03 (C-1). IR (ATR): ṽ (cm−1) = 3263, 1978, 2883, 2828, 2109, 1727, 1382, 1322, 1204, 1163, 1140, 967, 844, 696. HR-MS (ESI): m/z = [M]+ calcd for C17H27BNO4+: 320.2028; found: 320.2027. Specific rotation: [α −6 (c 0.1 in DMSO).
(S)-4-(Benzyloxy)-4-oxo-1-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)butan-2-aminium chloride (25). Prepared according to General Procedure A from N-Boc-protected amine 21 (2.80 g, 6.68 mmol). The amine hydrochloride 25 (2.14 g, 6.68 mmol, quant.) was obtained as a viscous yellow oil. 1H-NMR (500 MHz, (CD3)2SO): δ (ppm) = 8.04 (s, 3H, NH3+), 7.40–7.33 (m, 5H, 2′-H, 3′-H, 4′-H, 5′-H and 6′-H), 5.14 (d, J = 6.9 Hz, 2H, 1′-CH2), 3.64–3.51 (m, 1H, 2-H), 2.78–2.67 (m, 2H, 3-H), 1.27–1.22 (m, 1H, 1-H), 1.19 (d, J = 2.5 Hz, 12H, C(CH3)2)2), 1.16–1.10 (m, 1H, 1-H). 13C-NMR (101 MHz, (CD3)2SO): δ (ppm) = 169.86 (C-4), 135.74 (C-1′), 128.44–128.04 (C-2′, C-3′, C-4′, C-5′ and C-6′), 83.50 ((C(CH3)2)2), 66.05 (1′-CH2), 44.96 (C-2), 38.12 (C-3), 24.46 ((C(CH3)2)2), 16.02 (C-1). IR (ATR): ṽ (cm−1) = 2977, 2883, 2833, 2034, 1730, 1381, 1324, 1211, 1165, 1139, 966, 845, 696. HR-MS (ESI): m/z = [M]+ calcd for C17H27BNO4+: 320.2028; found: 320.2038. Specific rotation: [α +7 (c 0.1 in DMSO).
(R)-4-(Benzyloxy)-1-cyano-4-oxobutan-2-aminium chloride (26). Prepared according to General Procedure A from N-Boc-protected amine 22 (2.50 g, 7.85 mmol). The amine hydrochloride 26 (1.72 g, 7.84 mmol, quant.) was obtained as a purple-gray solid. m.p.: 107 °C. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 8.59 (s, 3H, NH3+), 7.44–7.32 (m, 5H, 2′-H, 3′-H, 4′-H, 5′-H and 6′-H), 5.15 (s, 2H, 1′-CH2), 3.84 (p, J = 6.3 Hz, 1H, 2-H), 3.06 (dd, J = 6.0, 1.4 Hz, 2H, 1-H), 2.96–2.81 (m, 2H, 3-H). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 168.99 (C-4), 135.58 (C-1′), 128.47–128.16 (C-2′, C-3′, C-4′, C-5′ and C-6′), 116.74 (CN), 66.36 (1′-CH2), 43.86 (C-2), 36.09 (C-3), 20.80 (C-1). IR (ATR): ṽ (cm−1) = 3033, 2923, 2854, 2641, 2500, 2252, 1716, 1541, 1454, 1401, 1217, 1188, 1157, 1117, 972, 754, 696. HR-MS (ESI): m/z = [M]+ calcd for C12H15N2O2+: 219.1128; found: 219.1137. Specific rotation: [α −8 (c 0.1 in DMSO).
(S)-4-(Benzyloxy)-1-cyano-4-oxobutan-2-aminium chloride (27). Prepared according to General Procedure A from N-Boc-protected amine 23 (1.30 g, 4.08 mmol). The amine hydrochloride 27 (895 g, 4.08 mmol, quant.) was obtained as a beige solid. m.p.: 109 °C. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 8.59 (s, 3H, NH3+), 7.43–7.33 (m, 5H, 2′-H, 3′-H, 4′-H, 5′-H and 6′-H), 5.15 (s, 2H, 1′-CH2), 3.84 (p, J = 6.3 Hz, 1H, 2-H), 3.06 (dd, J = 6.0, 1.4 Hz, 2H, 1-H), 2.95–2.80 (m, 2H, 3-H). 13C-NMR (101 MHz, (CD3)2SO): δ (ppm) = 168.98 (C-4), 135.57 (C-1′), 128.46–128.15 (C-2′, C-3′, C-4′, C-5′ and C-6′), 116.74 (CN), 66.36 (1′-CH2), 43.86 (C-2), 36.09 (C-3), 20.80 (C-1). IR (ATR): ṽ (cm−1) = 3033, 2922, 2854, 2641, 2541, 2252, 1716, 1541, 1454, 1401, 1217, 1188, 1157, 1117, 972, 754, 696. HR-MS (ESI): m/z = [M]+ calcd for C12H15N2O2+: 219.1128; found: 219.1124. Specific rotation: [α +8 (c 0.1 in DMSO).
Benzyl (R)-3-(4-ethynylbenzamido)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)butanoate (28). Prepared according to General Procedure B from amine hydrochloride 24 (2.45 g, 7.68 mmol) and 4-ethynylbenzoic acid (1.18 g, 7.68 mmol). The crude product was purified by FCC with boric acid-impregnated silica gel (hexanes/EtOAc 75:25) to give amide 28 (2.73 mg, 6.10 mmol, 80%) as a yellow oil. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 8.38 (d, J = 8.2 Hz, 1H, CONH), 7.79–7.75 (m, 2H, 2′-H and 6′-H), 7.57–7.53 (m, 2H, 3′-H and 5′-H), 7.34–7.25 (m, 5H, 2″-H, 3″-H, 4″-H, 5″-H and 6″-H), 5.06–5.04 (m, 2H, 1″-CH2), 4.50–4.43 (m, 1H, 3-H), 4.36 (s, 1H, C≡CH), 2.69–2.55 (m, 2H, 2-H), 1.14 (s, 12H, C(CH3)2)2), 1.10–1.05 (m, 2H, 4-H). 13C-NMR (101 MHz, (CD3)2SO): δ (ppm) = 170.65 (C-1), 164.56 (CONH), 136.12 (C-1″), 134.80 (C-1′), 131.49 (C-3′ and C-5′), 128.30–127.80 (C-2″, C-3″, C-4″, C-5″ and C-6″), 127.48 (C-2′ and C-6′), 124.19 (C-4′), 82.88 ((C(CH3)2)2), 82.72 (C≡CH and C≡CH), 65.46 (1″-CH2), 43.97 (C-3), 41.04 (C-2), 24.48 ((C(CH3)2)2), 17.81 (C-4). IR (ATR): ṽ (cm−1) = 3263, 2978, 2109, 1729, 1641, 1537, 1372, 1323, 1140, 967, 848, 696. HR-MS (ESI): m/z = [M + H]+ calcd for C26H31BNO5+: 448.2290; found: 448.2288. Specific rotation: [α +6 (c 0.1 in DMSO).
Benzyl (S)-3-(4-ethynylbenzamido)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)butanoate (29). Prepared according to General Procedure B from amine hydrochloride 25 (2.14 g, 6.68 mmol) and 4-ethynylbenzoic acid (1.03 g, 6.68 mmol). The crude product was purified by FCC with boric acid-impregnated silica gel (hexanes/EtOAc 75:25) to give amide 29 (1.92 g, 4.29 mmol, 64%) as a yellow oil. 1H-NMR (500 MHz, (CD3)2SO): δ (ppm) = 8.38 (d, J = 8.2 Hz, 1H, CONH), 7.78–7.75 (m, 2H, 2′-H and 6′-H), 7.57–7.53 (m, 2H, 3′-H and 5′-H), 7.33–7.26 (m, 5H, 2″-H, 3″-H, 4″-H, 5″-H and 6″-H), 5.07–5.04 (m, 2H, 1″-CH2), 4.50–4.43 (m, 1H, 3-H), 4.36 (s, 1H, C≡CH), 2.69–2.55 (m, 2H, 2-H), 1.14 (s, 12H, C(CH3)2)2), 1.10–1.03 (m, 2H, 4-H). 13C-NMR (101 MHz, (CD3)2SO): δ (ppm) = 170.65 (C-1), 164.56 (CONH), 136.12 (C-1′), 134.80 (C-1′), 131.49 (C-3′ and C-5′), 128.30–127.80 (C-2′′, C-3″, C-4″, C-5″ and C-6″), 127.48 (C-2′ and C-6′), 124.20 (C-4′), 82.88 ((C(CH3)2)2, C≡CH and C≡CH), 65.46 (1″-CH2), 43.98 (C-3), 41.04 (C-2), 24.48 ((C(CH3)2)2), 17.80 (C-4). IR (ATR): ṽ (cm−1) = 3284, 2978, 2107, 1731, 1639, 1535, 1372, 1323, 1139, 966, 846, 696. HR-MS (ESI): m/z = [M + H]+ calcd for C26H31BNO5+: 448.2290; found: 448.2295. Specific rotation: [α −5 (c 0.1 in DMSO).
Benzyl (R)-4-cyano-3-(4-ethynylbenzamido)butanoate (30). Prepared according to General Procedure B from amine hydrochloride 26 (1.72 g, 7.84 mmol) and 4-ethynylbenzoic acid (1.21 g, 7.84 mmol). The crude product was purified by FCC (hexanes/EtOAc 80:20) to give amide 30 (1.58 g, 4.56 mmol, 58%) as a white solid. 1H-NMR (500 MHz, CDCl3): δ (ppm) = 7.66 (m, 2H, 2′-H and 6′-H), 7.53 (m, 2H, 3′-H and 5′-H), 7.36 (m, 5H, 2″-H, 3″-H, 4″-H, 5″-H and 6″-H), 7.00 (CONH), 5.18 (s, 2H, 1″-CH2), 4.71 (m, 1H, 3-H), 3.22 (s, 1H, C≡CH), 2.90 (m, 4H, 2-H and 4-H). 13C-NMR (126 MHz, CDCl3): δ (ppm) = 170.69 (C-1), 166.31 (CONH), 135.06 (C-1″), 133.32 (C-1′), 132.54 (C-3′ and C-5′), 128.91–128.65 (C-2″, C-3″, C-4″, C-5″ and C-6″), 127.14 (C-2′ and C-6′), 126.16 (C-4′), 116.88 (CN), 82.73 (C≡CH), 80.05 (C≡CH), 67.51 (1″-CH2), 43.67 (C-3), 36.92 (C-2), 22.68 (C-4). IR (ATR): ṽ (cm−1) = 3366, 3337, 3295, 3263, 2938, 2248, 1731, 1717, 1650, 1607, 1585, 1525, 1426, 1385, 1290, 1272, 1165, 1084, 1001, 967, 900, 853, 766, 751, 699. HR-MS (ESI): m/z = [M + Na]+ calcd for C21H18O3N2Na+: 369.1215; found: 369.1208. Specific rotation: [α −9 (c 0.1 in CHCl3).
Benzyl (S)-4-cyano-3-(4-ethynylbenzamido)butanoate (31). Prepared according to General Procedure B from amine hydrochloride 27 (895 mg, 4.08 mmol) and 4-ethynylbenzoic acid (628 mg, 4.08 mmol). The crude product was purified by FCC (hexanes/EtOAc 70:30) to give amide 31 (998 mg, 2.88 mmol, 71%) as a beige solid. m.p.: 103 °C. 1H-NMR (500 MHz, CDCl3): δ (ppm) = 7.66 (m, 2H, 2′-H and 6′-H), 7.53 (m, 2H, 3′-H and 5′-H), 7.36 (m, 5H, 2′′-H, 3″-H, 4″-H, 5″-H and 6″-H), 7.00 (CONH), 5.18 (s, 2H, 1″-CH2), 4.71 (m, 1H, 3-H), 3.22 (s, 1H, C≡CH), 2.90 (m, 4H, 2-H and 4-H). 13C-NMR (126 MHz, CDCl3): δ (ppm) = 170.69 (C-1), 166.33 (CONH), 135.07 (C-1″), 133.32 (C-1′), 132.54 (C-3′ and C-5′), 128.91–128.64 (C-2″, C-3″, C-4″, C-5″ and C-6″), 127.15 (C-2′ and C-6′), 126.16 (C-4′), 116.89 (CN), 82.73 (C≡CH), 80.05 (C≡CH), 67.51 (1″-CH2), 43.68 (C-3), 36.93 (C-2), 22.68 (C-4). IR (ATR): ṽ (cm−1) = 3366, 3294, 3263, 2962, 2938, 2248, 1729, 1717, 1649, 1607, 1525, 1495, 1454, 1384, 1322, 1273, 1166, 1082, 1012, 967, 900, 853, 766, 750, 730, 699. HR-MS (ESI): m/z = [M + Na]+ calcd for C21H18O3N2Na+: 369.1215; found: 369.1210. Specific rotation: [α +10 (c 0.1 in CHCl3).
Methyl (R)-3-(4-(3-(2,2-dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)isoxazol-5-yl)benzamido)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)butanoate (32). Prepared according to General Procedure C from alkyne 28 (350 mg, 0.782 mmol) and aldoxime 3 (260 mg, 1.17 mmol). The crude product was purified by FCC with boric acid-impregnated silica gel (hexanes/EtOAc 60:40) to give isoxazole 32 (141 mg, 0.239 mmol, 31%) as a pale yellow oil. 1H-NMR (500 MHz, (CD3)2SO): δ (ppm) = 8.44 (d, J = 8.2 Hz, 1H, CONH), 8.39 (d, J = 2.2 Hz, 1H, 5′′′-H), 8.24 (dd, J = 8.6, 2.2 Hz, 1H, 7′′′-H), 8.04–8.00 (m, 2H, 3′-H and 5′-H), 7.99–7.95 (m, 2H, 2′-H and 6′-H), 7.87 (s, 1H, 4″-H), 7.34 (d, J = 8.6 Hz, 1H, 8′′′-H), 4.50–4.41 (m, 1H, 3-H), 3.57 (s, 3H, OCH3), 2.66–2.54 (m, 2H, 2-H), 1.75 (s, 6H, 2′′′-(CH3)2), 1.16–1.15 (m, 12H, (C(CH3)2)2), 1.13–1.08 (m, 2H, 4-H). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 171.22 (C-1), 169.26 (C-5″), 164.50 (CONH), 161.38 (C-3″), 159.75 (C-4′′′), 156.78 (C-8a′′′), 136.25 (C-1′), 134.79 (C-7′′′), 128.74 (C-4′), 128.18 (C-2′ and C-6′), 127.33 (C-5′′′), 125.41 (C-3′ and C-5′), 123.34 (C-6′′′), 118.54 (C-8′′′), 113.56 (C-4a′′′), 107.01 (C-2′′′), 99.67 (C-4″), 82.90 (C(CH3)2)2), 51.32 (OCH3), 43.96 (C-3), 40.95 (C-2), 25.32 (2′′′-(CH3)2), 24.95–24.48 (C(CH3)2)2), 17.76 (C-4). IR (ATR): ṽ (cm−1) = 3324, 2979, 1732, 1626, 1498, 1378, 1286, 1200, 1141, 1046, 846, 826, 673. HR-MS (ESI): m/z = [M + H]+ calcd for C31H36BN2O9+: 591.2514; found: 591.2500. Specific rotation: [α +3 (c 0.1 in DMSO).
Methyl (S)-3-(4-(3-(2,2-dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)isoxazol-5-yl)benzamido)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)butanoate (33). Prepared according to General Procedure C from alkyne 29 (650 mg, 1.45 mmol) and aldoxime 3 (482 mg, 2.18 mmol). The crude product was purified by FCC with boric acid-impregnated silica gel (hexanes/EtOAc 60:40) to give isoxazole 33 (187 mg, 0.316 mmol, 22%) as a pale yellow oil. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 8.44 (d, J = 8.2 Hz, 1H, CONH), 8.39 (d, J = 2.2 Hz, 1H, 5′′′-H), 8.24 (dd, J = 8.6, 2.2 Hz, 1H, 7′′′-H), 8.04–8.00 (m, 2H, 3′-H and 5′-H), 7.98–7.95 (m, 2H, 2′-H and 6′-H), 7.87 (s, 1H, 4″-H), 7.34 (d, J = 8.6 Hz, 1H, 8′′′-H), 4.50–4.41 (m, 1H, 3-H), 3.57 (s, 3H, OCH3), 2.65–2.54 (m, 2H, 2-H), 1.75 (s, 6H, 2′′′-(CH3)2), 1.16–1.15 (m, 12H, (C(CH3)2)2), 1.13–1.09 (m, 2H, 4-H). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 171.22 (C-1), 169.26 (C-5″), 164.51 (CONH), 161.38 (C-3″), 159.75 (C-4′′′), 156.78 (C-8a′′′), 136.26 (C-1′), 134.79 (C-7′′′), 128.74 (C-4′), 128.18 (C-2′ and C-6′), 127.34 (C-5′′′), 125.41 (C-3′ and C-5′), 123.33 (C-6′′′), 118.54 (C-8′′′), 113.56 (C-4a′′′), 107.01 (C-2′′′), 99.68 (C-4″), 82.90 (C(CH3)2)2), 51.33 (OCH3), 43.96 (C-3), 40.95 (C-2), 25.32 (2′′′-(CH3)2), 24.95–24.48 (C(CH3)2)2), 17.73 (C-4). IR (ATR): ṽ (cm−1) = 3388, 2980, 1735, 1624, 1498, 1379, 1288, 1200, 1139, 1052, 847, 828, 673. HR-MS (ESI): m/z = [M + H]+ calcd for C31H36BN2O9+: 591.2514; found: 591.2510. Specific rotation: [α −3 (c 0.1 in DMSO).
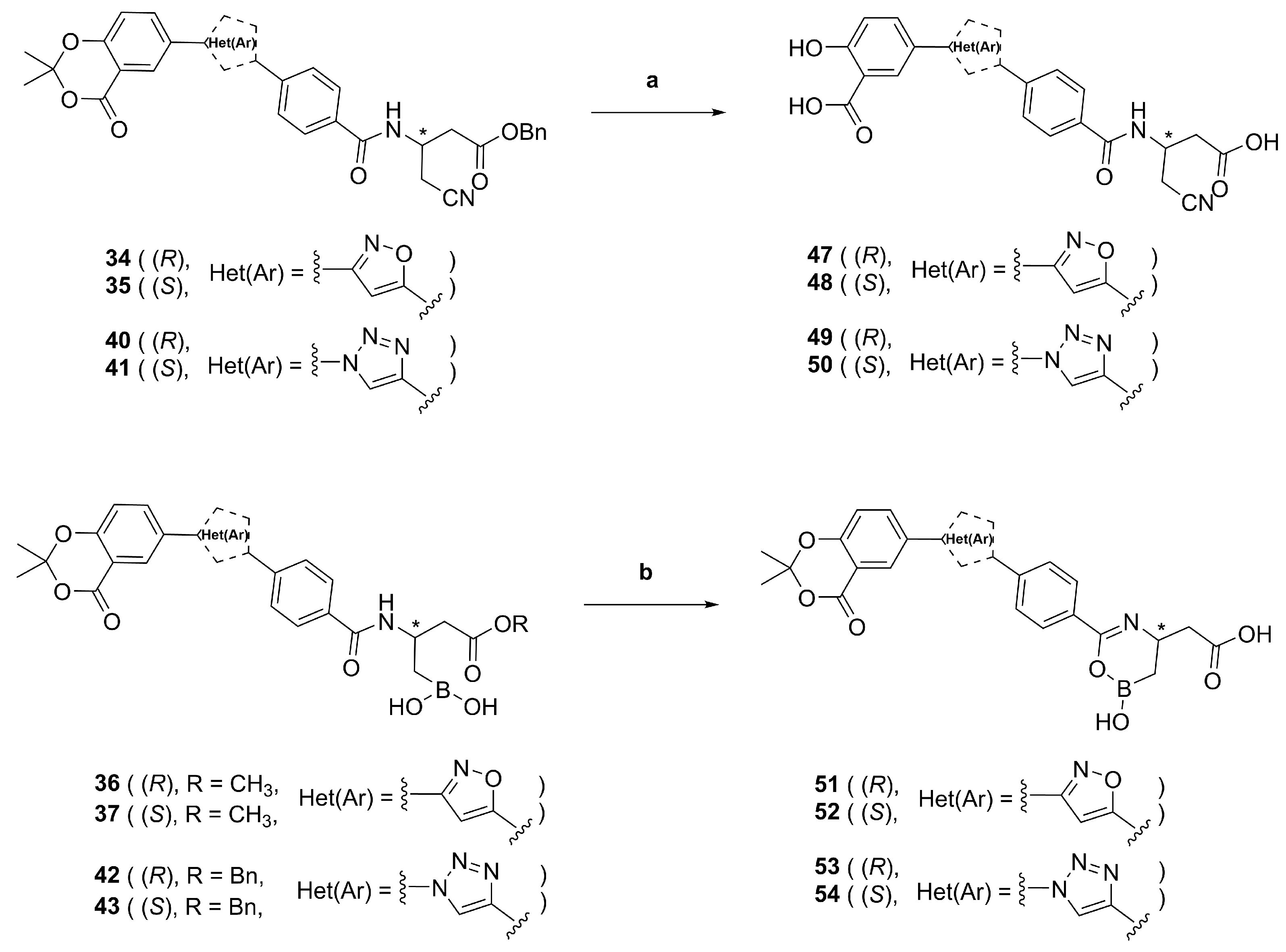
Benzyl (R)-4-cyano-3-(4-(3-(2,2-dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)isoxazol-5-yl)benzamido)butanoate (34). Prepared according to General Procedure C from alkyne 30 (50.0 mg, 0.144 mmol) and aldoxime 3 (47.9 mg, 0.217 mmol). The crude product was purified by FCC (hexanes/EtOAc 60:40) to give isoxazole 34 (11.5 mg, 0.0203 mmol, 14%) as a white solid. m.p.: 161 °C. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 8.88 (d, J = 7.9 Hz, 1H, CONH), 8.40 (d, J = 2.3 Hz, 1H, 5″-H), 8.25 (dd, J = 8.6, 2.3 Hz, 1H, 7′′′-H), 8.02 (m, 4H, 2′-H, 3′-H, 5′-H and 6′-H), 7.90 (s, 1H, 4″-H), 7.35 (d, J = 3.7 Hz, 1H, 8′′′-H), 7.32 (m, 5H, 2′′′′-H, 3′′′′-H, 4′′′′-H, 5′′′′-H and 6′′′′-H), 5.11 (s, 2H,1′′′′-CH2), 4.63 (d, J = 6.9 Hz, 1H, 3-H), 2.93 (d, J = 11.9 Hz, 2H, 4-H), 2.83 (d, J = 9.5 Hz, 2H, 2-H), 1.75 (s, 6H, C(CH3)2). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 169.84 (C-1), 169.15 (C-5″), 165.29 (CONH), 161.43 (C-3″), 159.76 (C-4′′′), 156.79 (C-8a′′′), 135.92 (C-1′′′′), 135.30 (C-1′), 134.81 (C-7′′′), 129.17 (C-4′), 128.35 (C-2′ and C-6′), 128.00 -127.88, (C-2′′′′, C-3′′′′, C-4′′′′, C-5′′′′ and C-6′′′′), 127.36 (C-5′′′), 125.54 (C-3′ and C-5′), 123.31 (C-4a′′′), 118.55 (C-8′′′), 118.28 (CN), 107.02 (C-2′′′), 99.89 (C-4″), 65.82 (1′′′′-CH2), 43.67 (C-3), 37.71 (C-2), 25.32 (C(CH3)2), 22.43 (C-4). IR (ATR): ṽ (cm−1) = 3354, 1735, 1642, 1620, 1598, 1534, 1500, 1417, 1389, 1312, 1291, 1194, 1144, 1059, 981, 925, 855, 816, 774, 745, 697, 672. HR-MS (ESI): m/z = [M − H]− calcd for C32H26O7N3−: 564.1771; found: 564.1770. Specific rotation: [α −25 (c 0.1 in CHCl3).
Benzyl (S)-4-cyano-3-(4-(3-(2,2-dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)isoxazol-5-yl)benzamido)butanoate (35). Prepared according to General Procedure C from alkyne 31 (620 mg, 1.79 mmol) and aldoxime 3 (594 mg, 2.68 mmol). The crude product was purified by FCC (hexanes/EtOAc 60:40) to give isoxazole 35 (239 mg, 0.423 mmol, 24%) as a white solid. m.p.: 162 °C. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 8.88 (d, J = 7.9 Hz, 1H, CONH), 8.40 (d, J = 2.3 Hz, 1H, 5″-H), 8.25 (dd, J = 8.6, 2.3 Hz, 1H, 7′′′-H), 8.02 (m, 4H, 2′-H, 3′-H, 5′-H and 6′-H), 7.90 (s, 1H, 4″-H), 7.35 (d, J = 3.7 Hz, 1H, 8′′′-H), 7.32 (m, 5H, 2′′′′-H, 3′′′′-H, 4′′′′-H, 5′′′′-H and 6′′′′-H), 5.11 (s, 2H, 1′′′′-CH2), 4.63 (d, J = 6.9 Hz, 1H, 3-H), 2.93 (d, J = 11.9 Hz, 2H, 4-H), 2.83 (d, J = 9.5 Hz, 2H, 2-H), 1.75 (s, 6H, C(CH3)2). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 169.84 (C-1), 169.15 (C-5″), 165.29 (CONH), 161.43 (C-3″), 159.75 (C-4′′′), 156.79 (C-8a′′′), 135.92 (C-1′′′′), 135.30 (C-1′), 134.81 (C-7′′′), 129.17 (C-4′), 128.35 (C-2′ and C-6′), 128.00 -127.88 (C-2′′′′, C-3′′′′, C-4′′′′, C-5′′′′ and C-6′′′′), 127.36 (C-5′′′), 125.54 (C-3′ and C-5′), 123.31 (C-4a′′′), 118.54 (C-8′′′), 118.28 (CN), 107.01 (C-2′′′), 99.88 (C-4″), 65.82 (C-7′′′′), 43.67 (C-3), 37.71 (C-2), 25.32 (C(CH3)2), 22.43 (C-4). IR (ATR): ṽ (cm−1) = 3354, 1732, 1642, 1620, 1598, 1533, 1499, 1441, 1389, 1312, 1290, 1193, 1144, 1059, 962, 924, 855, 816, 774, 745, 697, 672. HR-MS (ESI): m/z = [M + Na]+ calcd for C32H27O7N3Na+: 588.1747; found: 588.1737. Specific rotation: [α +13 (c 0.1 in CHCl3).
(R)-(2-(4-(3-(2,2-Dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)isoxazol-5-yl)benzamido)-4-methoxy-4-oxobutyl)boronic acid (36). To a stirred solution of boronic acid pinacol ester 32 (50 mg, 0.0847 mmol) in 3.5 mL THF/water (4:1 v/v), sodium periodate (90.6 mg, 0.423 mmol) was added, and the reaction mixture was stirred vigorously for 45 min at room temperature. 1M HCl (0.102 mmol, 0.102 mL) was then added to the resulting suspension, and the mixture stirred for another 16 h. The suspension was diluted with EtOAc (50 mL) and water (50 mL), the phases were separated, and the aqueous phase was extracted with EtOAc (2 × 50 mL). The combined organic phases were dried with Na2SO4, concentrated in vacuo and purified by FCC with boric acid-impregnated silica gel (DCM/MeOH 97:3 -> 95:5) to give boronic acid 36 (31.2 mg, 0.0614 mmol, 73%) as a white solid. m.p.: 152 °C. 1H-NMR (500 MHz, (CD3)2SO): δ (ppm) = 8.39 (d, J = 2.2 Hz, 1H, 5′′′-H), 8.37 (d, J = 8.1 Hz, 1H, CONH), 8.25 (dd, J = 8.6, 2.2 Hz, 1H, 7′′′-H), 8.03–8.00 (m, 2H, 3′-H and 5′-H), 7.98–7.96 (m, 2H, 2′-H and 6′-H), 7.87 (s, 1H, 4″-H), 7.66 (s, 2H, B(OH)2), 7.34 (d, J = 8.6 Hz, 1H, 8′′′-H), 4.50–4.43 (m, 1H, 2-H), 3.56 (s, 3H, OCH3), 2.62–2.55 (m, 2H, 3-H), 1.75 (s, 6H, 2′′′-(CH3)2), 1.07–1.01 (m, 2H, 1-H). 13C-NMR (126 MHz, (CD3)2SO): 171.47 (C-4), 169.28 (C-5″), 164.65 (CONH), 161.40 (C-3″), 159.76 (C-4′′′), 156.78 (C-8a′′′), 136.32 (C-1′), 134.81 (C-7′′′), 128.73 (C-4′), 128.20 (C-2′ and C-6′), 127.34 (C-5′′′), 125.43 (C-3′ and C-5′), 123.34 (C-6′′′), 118.54 (C-8′′′), 113.56 (C-4a′′′), 107.01 (C-2′′′), 99.68 (C-4″), 51.21 (OCH3), 44.57 (C-2), 40.81 (C-3), 25.32 (2′′′-(CH3)2), 22.16 (C-1). IR (ATR): ṽ (cm−1) = 3323, 2952, 1731, 1623, 1498, 1286, 1200, 927, 764. HR-MS (ESI): m/z = [M − H]− calcd for C25H24BN2O9−: 507.1575; found: 507.1579. Specific rotation: [α −11 (c 0.1 in DMSO).
(S)-(2-(4-(3-(2,2-Dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)isoxazol-5-yl)benzamido)-4-methoxy-4-oxobutyl)boronic acid (37). To a stirred solution of boronic acid pinacol ester 33 (170 mg, 0.288 mmol) in 12 mL THF/water (4:1 v/v), sodium periodate (308 mg, 1.44 mmol) was added, and the reaction mixture was stirred vigorously for 45 min at room temperature. 1M HCl (0.346 mmol, 0.346 mL) was then added to the resulting suspension, and the mixture stirred for another 16 h. The suspension was diluted with EtOAc (100 mL) and water (100 mL), the phases were separated, and the aqueous phase was extracted with EtOAc (2 × 100 mL). The combined organic phases were dried with Na2SO4, concentrated in vacuo and purified by FCC with boric acid-impregnated silica gel (DCM/MeOH 97:3 -> 95:5) to give boronic acid 37 (93.1 mg, 0.183 mmol, 64%) as a white solid. m.p.: 157 °C. 1H-NMR (500 MHz, (CD3)2SO): δ (ppm) = 8.39 (d, J = 2.2 Hz, 1H, 5′′′-H), 8.37 (d, J = 8.2 Hz, 1H), 8.25 (dd, J = 8.6, 2.2 Hz, 1H, 7′′′-H), 8.03–8.00 (m, 2H, 3′-H and 5′-H), 7.99–7.96 (m, 2H, 2′-H and 6′-H), 7.87 (s, 1H, 4″-H), 7.66 (s, 2H, B(OH)2), 7.34 (d, J = 8.6 Hz, 1H, 8′′′-H), 4.51–4.41 (m, 1H, 2-H), 3.56 (s, 3H, OCH3), 2.64–2.55 (m, 2H, 3-H), 1.75 (s, 6H, 2′′′-(CH3)2), 1.08–0.99 (m, 2H, 1-H). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 171.47 (C-4), 169.28 (C-5″), 164.65 (CONH), 161.40 (C-3″), 159.76 (C-4′′′), 156.78 (C-8a′′′), 136.32 (C-1′), 134.81 (C-7′′′), 128.73 (C-4′), 128.20 (C-2′ and C-6′), 127.34 (C-5′′′), 125.43 (C-3′ and C-5′), 123.34 (C-6′′′), 118.54 (C-8′′′), 113.56 (C-4a′′′), 107.01 (C-2′′′), 99.68 (C-4″), 51.22 (OCH3), 44.56 (C-2), 40.82 (C-3), 25.32 (2′′′-(CH3)2), 22.09 (C-1). IR (ATR): ṽ (cm−1) = 3364, 2997, 1730, 1622, 1498, 1287, 1200, 927, 764. HR-MS (ESI): m/z = [M − H]− calcd for C25H24BN2O9−: 507.1575; found: 507.1568. Specific rotation: [α +10 (c 0.1 in DMSO).
Benzyl (R)-3-(4-(1-(2,2-dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)-1H-1,2,3-triazol-4-yl)benzamido)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)butanoate (38). Prepared according to General Procedure D from azide 11 (300 mg, 1.37 mmol) and alkyne 28 (735 mg, 1.64 mmol). The crude product was purified by FCC with boric acid-impregnated silica gel (hexanes/EtOAc 50:50) to give triazole 38 (153 mg, 0.230 mmol, 17%) as a pale yellow solid. m.p.: 140 °C. 1H-NMR (500 MHz, (CD3)2SO): δ (ppm) = 9.54 (s, 1H, 5″-H), 8.42 (d, J = 2.7 Hz, 1H, 5′′′-H), 8.37 (d, J = 8.2 Hz, 1H, CONH), 8.32 (dd, J = 8.9, 2.7 Hz, 1H, 7′′′-H), 8.05–8.00 (m, 2H, 3′-H and 5′-H), 7.95–7.89 (m, 2H, 2′-H and 6′-H), 7.44 (d, J = 8.9 Hz, 1H, 8′′′-H), 7.37–7.27 (m, 5H, 2′′′′-H, 3′′′′-H, 4′′′′-H, 5′′′′-H and 6′′′′-H), 5.09–5.07 (m, 2H, 1′′′′-CH2), 4.55–4.48 (m, 1H, 3-H), 2.73–2.67 (m, 1H, 2-H), 2.65–2.58 (m, 1H, 2-H), 1.78 (s, 6H, 2′′′-(CH3)2), 1.17 (s, 12H, (C(CH3)2)2), 1.16–1.06 (m, 2H, 4-H). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 170.71 (C-1), 164.88 (CONH), 159.48 (C-4′′′), 155.21 (C-8a′′′), 146.69 (C-4″), 136.16 (C-1′′′′), 134.27 (C-1′), 132.54 (C-4′), 131.70 (C-6′′′), 128.66 (C-7′′′), 128.31 (C-2′′′′, C-3′′′′, C-4′′′′, C-5′′′′ and C-6′′′′), 128.03 (C-2′ and C-6′), 127.90–127.81 (C-2′′′′, C-3′′′′, C-4′′′′, C-5′′′′ and C-6′′′′), 124.90 (C-3′ and C-5′), 120.66 (C-5″), 120.18 (C-5′′′), 119.26 (C-8′′′), 113.77 (C-4a′′′), 107.23 (C-2′′′), 82.90 ((C(CH3)2)2), 65.47 (1′′′′-CH2), 43.97 (C-3), 41.10 (C-2), 25.27 (2′′′-(CH3)2), 24.66–24.51 ((C(CH3)2)2), 17.85 (C-4). IR (ATR): ṽ (cm−1) = 3316, 2977, 1736, 1628, 1509, 1378, 1295, 1139, 1047, 853, 697. HR-MS (ESI): m/z = [M + H]+ calcd for C36H40BN4O8+: 667.2939; found: 667.2961. Specific rotation: [α +4 (c 0.1 in DMSO).
Benzyl (S)-3-(4-(1-(2,2-dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)-1H-1,2,3-triazol-4-yl)benzamido)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)butanoate (39). Prepared according to General Procedure D from azide 11 (300 mg, 1.37 mmol) and alkyne 29 (735 mg, 1.64 mmol). The crude product was purified by FCC with boric acid-impregnated silica gel (hexanes/EtOAc 50:50) to give triazole 39 (76.8 mg, 0.115 mmol, 8%) as a pale yellow solid. m.p.: 129 °C. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 9.53 (s, 1H, 5′′-H), 8.41 (d, J = 2.7 Hz, 1H, 5′′′-H), 8.36 (d, J = 8.2 Hz, 1H, CONH), 8.31 (dd, J = 8.9, 2.7 Hz, 1H, 7′′′-H), 8.05–7.98 (m, 2H, 3′-H and 5′-H), 7.95–7.87 (m, 2H, 2′-H and 6′-H), 7.43 (d, J = 8.9 Hz, 1H, 8′′′-H), 7.35–7.26 (m, 5H, 2′′′′-H, 3′′′′-H, 4′′′′-H, 5′′′′-H and 6′′′′-H), 5.08–5.06 (m, 2H, 1′′′′-CH2), 4.55–4.44 (m, 1H, 3-H), 2.72–2.65 (m, 1H, 2-H), 2.64–2.57 (m, 1H, 2-H), 1.77 (s, 6H, 2′′′-(CH3)2), 1.16 (s, 12H, (C(CH3)2)2), 1.16–1.04 (m, 1H, 4-H). 13C-NMR (101 MHz, (CD3)2SO): δ (ppm) = 170.71 (C-1), 164.87 (CONH), 159.48 (C-4′′′), 155.21 (C-8a′′′), 146.69 (C-4″), 136.15 (C-1′′′′), 134.27 (C-1′), 132.53 (C-4′), 131.70 (C-6′′′), 128.65 (C-7′′′), 128.31 (C-2′′′′, C-3′′′′, C-4′′′′, C-5′′′′ and C-6′′′′), 128.02 (C-2′ and C-6′), 127.89–127.80 (C-2′′′′, C-3′′′′, C-4′′′′, C-5′′′′ and C-6′′′′), 124.90 (C-3′ and C-5′), 120.66 (C-5″), 120.18 (C-5′′′), 119.25 (C-8′′′), 113.77 (C-4a′′′), 107.23 (C-2′′′), 82.89 ((C(CH3)2)2), 65.47 (1′′′′-CH2), 43.97 (C-3), 41.09 (C-2), 25.27 (2′′′-(CH3)2), 24.66–24.50 ((C(CH3)2)2), 17.87 (C-4). IR (ATR): ṽ (cm−1) = 3316, 2977, 1734, 1627, 1509, 1377, 1295, 1139, 1046, 847, 697. HR-MS (ESI): m/z = [M + H]+ calcd for C36H40BN4O8+: 667.2939; found: 667.2949. Specific rotation: [α −3 (c 0.1 in DMSO).
Benzyl (R)-4-cyano-3-(4-(1-(2,2-dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)-1H-1,2,3-triazol-4-yl)benzamido)butanoate (40). Prepared according to General Procedure D from azide 11 (150 mg, 0.684 mmol) and alkyne 30 (284 mg, 0.821 mmol). The crude product was resuspended in EtOAc (20 mL), filtered and the solid residue collected to give triazole 40 (157 mg, 0.278 mmol, 41%) as a pale yellow solid. m.p.: 196 °C. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 9.55 (s, 1H, 5′′-H), 8.79 (d, J = 7.9 Hz, 1H, CONH), 8.41 (d, J = 2.7 Hz, 1H, 5′′′-H), 8.31 (dd, J = 8.9, 2.8 Hz, 1H, 7′′′-H), 8.08–8.04 (m, 2H, 3′-H and 5′-H), 7.98–7.94 (m, 2H, 2′-H and 6′-H), 7.44 (d, J = 8.9 Hz, 1H, 8′′′-H), 7.36–7.28 (m, 5H, 2′′′′-H, 3′′′′-H, 4′′′′-H, 5′′′′-H and 6′′′′-H), 5.11 (s, 2H, 1′′′′-CH2), 4.68–4.57 (m, 1H, 3-H), 2.99–2.75 (m, 4H, 2-H and 4-H), 1.77 (s, 6H, (CH3)2). 13C-NMR (101 MHz, (CD3)2SO): δ (ppm) = 169.88 (C-1), 165.63 (CONH), 159.48 (C-4′′′), 155.23 (C-8a′′′), 146.59 (C-4″), 135.94 (C-1′′′′), 133.35 (C-1′), 133.04 (C-4′), 131.69 (C-6′′′), 128.69 (C-7′′′), 128.35 (C-2′′′′, C-3′′′′, C-4′′′′, C-5′′′′ and C-6′′′′), 128.17 (C-2′ and C-6′), 127.99–127.86 (C-2′′′′, C-3′′′′, C-4′′′′, C-5′′′′ and C-6′′′′), 125.04 (C-3′ and C-5′), 120.81 (C-5″), 120.22 (C-5′′′), 119.26 (C-8′′′), 118.32 (CN), 113.77 (C-4a′′′), 107.23 (C-2′′′), 65.81 (1′′′′-CH2), 43.61 (C-3), 37.77 (C-2), 25.27 ((CH3)2), 22.45 (C-4). IR (ATR): ṽ (cm−1) = 3330, 1735, 1721, 1645, 1524, 1506, 1296, 1040. HR-MS (ESI): m/z = [M + H]+ calcd for C31H28N5O6+: 566.2040; found: 566.2034. Specific rotation: [α −9 (c 0.1 in DMSO).
Benzyl (S)-4-cyano-3-(4-(1-(2,2-dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)-1H-1,2,3-triazol-4-yl)benzamido)butanoate (41). Prepared according to General Procedure D from azide 11 (200 mg, 0.912 mmol) and alkyne 31 (379 mg, 1.09 mmol). The crude product was resuspended in EtOAc (20 mL), filtered and the solid residue collected to give triazole 41 (275 mg, 0.487 mmol, 53%) as a yellow solid. m.p.: 198 °C. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 9.55 (s, 1H, 5′′-H), 8.80 (d, J = 7.9 Hz, 1H, CONH), 8.41 (d, J = 2.7 Hz, 1H, 5′′′-H), 8.31 (dd, J = 8.9, 2.7 Hz, 1H, 7′′′-H), 8.10–8.03 (m, 2H, 3′-H and 5′-H), 8.00–7.93 (m, 2H, 2′-H and 6′-H), 7.44 (d, J = 9.0 Hz, 1H, 8′′′-H), 7.37–7.28 (m, 5H, 2′′′′-H, 3′′′′-H, 4′′′′-H, 5′′′′-H and 6′′′′-H), 5.11 (s, 2H, 1′′′′-CH2), 4.67–4.57 (m, 1H, 3-H), 2.99–2.76 (m, 4H, 2-H and 4-H), 1.77 (s, 6H, (CH3)2). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 169.89 (C-1), 165.62 (CONH), 159.48 (C-4′′′), 155.23 (C-8a′′′), 146.59 (C-4″), 135.94 (C-1′′′′), 133.35 (C-1′), 133.04 (C-4′), 131.69 (C-6′′′), 128.69 (C-7′′′), 128.35 (C-2′′′′, C-3′′′′, C-4′′′′, C-5′′′′ and C-6′′′′), 128.17 (C-2′ and C-6′), 127.99–127.87 (C-2′′′′, C-3′′′′, C-4′′′′, C-5′′′′ and C-6′′′′), 125.05 (C-3′ and C-5′), 120.82 (C-5″), 120.22 (C-5′′′), 119.26 (C-8′′′), 118.33 (CN), 113.77 (C-4a′′′), 107.24 (C-2′′′), 65.81 (1′′′′-CH2), 43.61 (C-3), 37.77 (C-2), 25.27 ((CH3)2), 22.45 (C-4). IR (ATR): ṽ (cm−1) = 3325, 1734, 1720, 1636, 1523, 1506, 1295, 1039. HR-MS (ESI): m/z = [M + Na]+ calcd for C31H27N5O6Na+: 588.1859; found: 588.1864. Specific rotation: [α +7 (c 0.1 in DMSO).
(R)-(4-(Benzyloxy)-2-(4-(1-(2,2-dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)-1H-1,2,3-triazol-4-yl)benzamido)-4-oxobutyl)boronic acid (42). Method A: Prepared according to General Procedure D from azide 11 (300 mg, 1.37 mmol) and alkyne 28 (735 mg, 1.64 mmol). The crude product was purified by FCC with boric acid-impregnated silica gel (DCM/MeOH 95:5) to give triazole 42 (193 mg, 0.331 mmol, 24%) as a yellow solid. Method B: To a stirred solution of boronic acid pinacol ester 38 (70 mg, 0.105 mmol) in 5 mL THF/water (4:1 v/v), sodium periodate (112 mg, 0.525 mmol) was added, and the reaction mixture was stirred vigorously for 45 min at room temperature. 1 M HCl (0.126 mmol, 0.126 mL) was added to the resulting suspension, and the mixture stirred for another 16 h. The suspension was then filtered and the filtrate concentrated in vacuo. The crude product was purified by FCC with boric acid-impregnated silica gel (DCM/MeOH 95:5) to give triazole 42 (41.9 mg, 0.0717 mmol, 68%) as a yellow solid. m.p.: 138 °C. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 9.53 (s, 1H, 5″-H), 8.41 (d, J = 2.7 Hz, 1H, 5′′′-H), 8.31 (dd, J = 8.8, 2.5 Hz, 2H, CONH and 7′′′-H), 8.05–8.00 (m, 2H, 3′-H and 5′-H), 7.94–7.88 (m, 2H, 2′-H and 6′-H), 7.67 (s, 2H, B(OH)2), 7.44 (d, J = 8.9 Hz, 1H, 8′′′-H), 7.35–7.26 (m, 5H, 2′′′′-H, 3′′′′-H, 4′′′′-H, 5′′′′-H and 6′′′′-H), 5.05 (s, 2H, 1′′′′-CH2), 4.47–4.55 (m, 1H, 2-H), 2.69–2.62 (m, 2H, 3-H), 1.77 (s, 6H, (CH3)2), 1.08–1.01 (m, 2H, 1-H). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 170.97 (C-4), 165.07 (CONH), 159.49 (C-4′′′), 155.22 (C-8a′′′), 146.69 (C-4″), 136.21 (C-1′′′′), 134.28 (C-1′), 132.55 (C-4′), 131.71 (C-6′′′), 128.69 (C-7′′′), 128.30–128.04 (C-2′′′′, C-3′′′′, C-4′′′′, C-5′′′′ and C-6′′′′), 127.85 (C-2′ and C-6′), 127.81 (C-2′′′′, C-3′′′′, C-4′′′′, C-5′′′′ and C-6′′′′), 124.92 (C-3′ and C-5′), 120.68 (C-5″), 120.20 (C-5′′′), 119.26 (C-8′′′), 113.77 (C-4a′′′), 107.24 (C-2′′′), 65.40 (1′′′′-CH2), 44.53 (C-2), 41.01 (C-3), 25.27 ((CH3)2), 22.49 (C-1). IR (ATR): ṽ (cm−1) = 3307, 1735, 1624, 1509, 1295, 1202, 1047, 932, 697. HR-MS (ESI): m/z = [M + Na]+ calcd for C30H29BN4O8Na+: 607.1976; found: 607.1970. Specific rotation: [α −15 (c 0.1 in DMSO).
(S)-(4-(Benzyloxy)-2-(4-(1-(2,2-dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)-1H-1,2,3-triazol-4-yl)benzamido)-4-oxobutyl)boronic acid (43). Prepared according to General Procedure D from azide 11 (300 mg, 1.37 mmol) and alkyne 29 (735 mg, 1.64 mmol). The crude product was purified by FCC with boric acid-impregnated silica gel (DCM/MeOH 95:5) to give triazole 43 (263 mg, 0.450 mmol, 33%) as a yellow solid. m.p.: 141 °C. 1H-NMR (500 MHz, (CD3)2SO): δ (ppm) = 9.53 (s, 1H, 5″-H), 8.41 (d, J = 2.7 Hz, 1H, 5″′-H), 8.31 (dd, J = 9.0, 2.5 Hz, 2H, CONH and 7′′′-H), 8.05–7.99 (m, 2H, 3′-H and 5′-H), 7.93–7.90 (m, 2H, 2′-H and 6′-H), 7.67 (s, 2H, B(OH)2), 7.44 (d, J = 8.9 Hz, 1H, 8′′′-H), 7.35–7.26 (m, 5H, 2′′′′-H, 3′′′′-H, 4′′′′-H, 5′′′′-H and 6′′′′-H), 5.05 (s, 2H, 1′′′′-CH2), 4.47–4.55 (m, 1H, 2-H), 2.67–2.63 (m, 2H, 3-H), 1.77 (s, 6H, (CH3)2), 1.10–1.00 (m, 2H, 1-H). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 170.98 (C-4), 165.09 (CONH), 159.50 (C-4′′′), 155.23 (C-8a′′′), 146.70 (C-4″), 136.21 (C-1′′′′), 134.28 (C-1′), 132.57 (C-4′), 131.72 (C-6′′′), 128.70 (C-7′′′), 128.31–128.05 (C-2′′′′, C-3′′′′, C-4′′′′, C-5′′′′ and C-6′′′′), 127.87 (C-2′ and C-6′), 127.82 (C-2′′′′, C-3′′′′, C-4′′′′, C-5′′′′ and C-6′′′′), 124.93 (C-3′ and C-5′), 120.69 (C-5″), 120.22 (C-5′′′), 119.27 (C-8′′′), 113.78 (C-4a′′′), 107.25 (C-2′′′), 65.41 (1′′′′-CH2), 44.54 (C-2), 41.02 (C-3), 25.28 ((CH3)2), 22.63 (C-1). IR (ATR): ṽ (cm−1) = 3307, 1733, 1617, 1506, 1296, 1199, 1036, 929, 697. HR-MS (ESI): m/z = [M + Na]+ calcd for C30H29BN4O8Na+: 607.1976; found: 607.1970. Specific rotation: [[α +14 (c 0.1 in DMSO).
(S)-4-(Benzyloxy)-1-bromo-4-oxobutan-2-aminium (44). Prepared according to General Procedure A from N-Boc-protected amine 18 (520 mg, 1.40 mmol). The aminium 44 (380 mg, 1.39 mmol, quant.) was obtained as a white solid. m.p.: 109 °C. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 8.49 (s, 3H, NH3+), 7.45–7.29 (m, 5H, 2′-H, 3′-H, 4′-H, 5′-H and 6′-H), 5.15 (s, 2H, 1′-CH2), 3.88–3.72 (m, 3H, 1-H and 2-H), 2.87 (dd, J = 6.4, 1.4 Hz, 2H, 3-H). 13C-NMR (101 MHz, (CD3)2SO): δ (ppm) = 169.19 (C-4), 135.62 (C-1′), 128.47–128.13 (C-2′, C-3′, C-4′, C-5′ and C-6′), 66.30 (1′-CH2), 47.67 (C-2), 35.66 (C-3), 33.90 (C-1). IR (ATR): ṽ (cm−1) = 3203, 2866, 2812, 2590, 1720, 1708, 1501, 1395, 1348, 1227, 1154, 1138, 1082, 944, 739, 697. HR-MS (ESI): m/z = [M]+ calcd for C11H15BrNO2+: 272.0281; found: 272.0279. Specific rotation: [α −4 (c 0.1 in DMSO).
Benzyl (S)-2-(2-(4-ethynylphenyl)-4,5-dihydrooxazol-4-yl)acetate (45). Prepared according to General Procedure B from aminium 44 (459 mg, 1.68 mmol) and 4-ethynylbenzoic acid (284 mg, 1.85 mmol). The reaction mixture was stirred at room temperature for 16 h. The crude product was purified by FCC (hexanes/EtOAc 75:25) to give oxazoline 45 (269 mg, 0.842 mmol, 50%) as a yellow solid. m.p.: 82 °C. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 7.86–7.82 (m, 2H, 2″-H and 6″-H), 7.60–7.55 (m, 2H, 3″-H and 5″-H), 7.39–7.28 (m, 5H, 2′′′-H, 3′′′-H, 4′′′-H, 5′′′-H and 6′′′-H), 5.13 (d, J = 2.0 Hz, 2H, 1′′′-CH2), 4.63–4.55 (m, 2H, 4′-H and 5′-H), 4.41 (s, 1H, C≡CH), 4.20–4.11 (m, 1H, 5′-H), 2.78–2.73 (m, 2H, 2-H). 13C-NMR (101 MHz, (CD3)2SO): δ (ppm) = 170.65 (C-1), 162.29 (C-2′), 136.08 (C-1′′′), 131.94 (C-3″ and C-5″), 128.40–127.97 (C-2′′′, C-3′′′, C-4′′′, C-5′′′ and C-6′′′), 127.84 (C-2″ and C-6″), 127.41 (C-1″), 124.73 (C-4″), 83.24 (C≡CH or C≡CH), 82.80 (C≡CH or C≡CH), 72.12 (C-5′), 65.53 (1′′′-CH2), 62.91 (C-4′), 39.50 (C-2). IR (ATR): ṽ (cm−1) = 3212, 2812, 2590, 1719, 1710, 1645, 1500, 1348, 1227, 1167, 1082, 954, 739, 697, 679. HR-MS (ESI): m/z = [M + H]+ calcd for C20H18NO3+: 320.1287; found: 320.1280. Specific rotation: [α −42 (c 0.1 in DMSO).
(R)-5-(5-(4-((1-Carboxy-3-cyanopropan-2-yl)carbamoyl)phenyl)isoxazol-3-yl)-2-hydroxybenzoic acid (47). Prepared according to General Procedure E from isoxazole 34 (12.0 mg, 0.0212 mmol) and 0.70 M aq. KOH solution (5.95 mg, 0.106 mmol). The reaction mixture was stirred at room temperature for 2.5 h. The crude product was purified by FCC (hexanes/EtOAc 30:70 + 1% AcOH) to give isoxazole 47 (4.60 mg, 0.0106 mmol, 50%) as a white solid. m.p.: 228 °C. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 12.41 (s, 1H, 1-COOH or 1′′′-COOH), 8.85 (d, J = 7.8 Hz, 1H, CONH), 8.33 (d, J = 2.3 Hz, 1H, 6-H), 8.07 (s, 1H, 4-H), 8.03 (m, 4H, 2″-H, 3″-H, 5″-H and 6″-H), 7.77 (s, 1H, 4′-H), 7.11 (d, J = 8.6 Hz, 1H, 3-H), 4.54 (d, J = 7.2 Hz, 1H, 2′′′-H), 2.89 (d, J = 5.0 Hz, 2H, 3′′′-H), 2.67 (d, J = 7.9 Hz, 2H, 1′′′-H). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 171.53 (1′′′-COOH), 171.21 (1-COOH), 168.73 (C-5′), 165.27 (CONH), 163.49 (C-2), 161.95 (C-3′), 135.23 (C-4″), 132.99 (C-4), 129.32 (C-1″), 128.65 (C-6), 128.28 (C-3″ and C-5″), 125.51 (C-2″ and C-6″), 119.09 (C-5), 118.38 (CN), 118.09 (C-3), 114.62 (C-1), 99.62 (C-4′), 43.61 (C-2′′′), 37.66 (C-1″), 22.34 (C-3″). IR (ATR): ṽ (cm−1) = 2922, 1653, 1598, 1540, 1497, 1423, 1291, 1207, 1074, 948, 798, 768, 686. HR-MS (ESI): m/z = [M − H]− calcd for C22H16O7N3−: 434.0988; found: 434.0986. Specific rotation: [α −28 (c 0.1 in DMSO). Purity (HPLC): 210 nm: >95%; 254 nm: >95%.
(S)-5-(5-(4-((1-Carboxy-3-cyanopropan-2-yl)carbamoyl)phenyl)isoxazol-3-yl)-2-hydroxybenzoic acid (48). Prepared according to General Procedure E from isoxazole 35 (228 mg, 0.403 mmol) and 0.70 M aq. KOH solution (113 mg, 2.02 mmol). The reaction mixture was stirred at room temperature for 2.5 h. The crude product was purified by FCC (hexanes/EtOAc 30:70 + 1% AcOH) to give isoxazole 48 (95.6 mg, 0.220 mmol, 55%) as a white solid. m.p.: 231 °C. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 12.41 (s, 1H, 1-COOH or 1′′′-COOH), 8.85 (d, J = 7.8 Hz, 1H, CONH), 8.33 (d, J = 2.3 Hz, 1H, 6-H), 8.07 (s, 1H, 4-H), 8.03 (m, 4H, 2″-H, 3″-H, 5″-H and 6″-H), 7.77 (s, 1H, 4′-H), 7.11 (d, J = 8.6 Hz, 1H, 3-H), 4.54 (d, J = 7.2 Hz, 1H, 2′′′-H), 2.89 (d, J = 5.0 Hz, 2H, 3′′′-H), 2.67 (d, J = 7.9 Hz, 2H, 1′′′-H). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 171.53 (1′′′-COOH), 171.31 (1-COOH), 168.81 (C-5′), 165.26 (CONH), 162.53 (C-2), 161.82 (C-3′), 135.26 (C-4″), 133.35 (C-4), 129.28 (C-1″), 128.65 (C-6), 128.29 (C-3″ and C-5″), 125.52 (C-2″ and C-6″), 119.47 (C-5), 118.38 (CN), 118.22 (C-3), 113.88 (C-1), 99.64 (C-4′), 43.61 (C-2′′′), 37.66 (C-1″), 22.34 (C-3″). IR (ATR): ṽ (cm−1) = 2922, 1664, 1597, 1533, 1496, 1421, 1287, 1197, 797, 768, 686. HR-MS (ESI): m/z = [M − H]− calcd for C22H16O7N3−: 434.0988; found: 434.0986. Specific rotation: [α +28 (c 0.1 in DMSO). Purity (HPLC): 210 nm: >95%; 254 nm: >95%.
(R)-5-(4-(4-((1-Carboxy-3-cyanopropan-2-yl)carbamoyl)phenyl)-1H-1,2,3-triazol-1-yl)-2-hydroxybenzoic acid (49). Prepared according to General Procedure E from triazole 40 (60.0 mg, 0.106 mmol) and 0.70 M aq. KOH solution (29.8 mg, 0.530 mmol). The reaction mixture was stirred at room temperature for 30 min. The crude product was resuspended in EtOAc (10 mL), filtered and the solid residue collected to give cyanomethyl 49 (18.0 mg, 0.0413 mmol, 39%) as a white solid. m.p.: 290 °C. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 12.43 (s, 1H, 1-COOH or 1′′′-COOH), 9.42 (s, 1H, 5′-H), 8.74 (d, J = 7.7 Hz, 1H, CONH), 8.30 (d, J = 2.8 Hz, 1H, 6-H), 8.12–8.06 (m, 1H, 4-H), 8.08–8.04 (m, 2H, 2″-H and 6″-H), 8.01–7.94 (m, 2H, 3″-H and 5″-H), 7.22 (d, J = 9.0 Hz, 1H, 3-H), 4.58–4.48 (m, 1H, 2′′′-H), 2.98–2.80 (m, 2H, 3′′′-H), 2.77–2.60 (m, 2H, 1′′′-H). 13C-NMR (101 MHz, (CD3)2SO): δ (ppm) = 171.58 (1′′′-COOH), 170.84 (1-COOH), 165.60 (CONH), 160.93 (C-2), 146.40 (C-4′), 133.30 (C-4″), 133.19 (C-1″), 128.48 (C-5), 128.13 (C-3″ and C-5″), 127.37 (C-4), 125.01 (C-2″ and C-6″), 121.67 (C-6), 120.62 (C-5′), 118.68 (C-3), 118.42 (CN), 114.03 (C-1), 43.55 (C-2′′′), 37.71 (C-3′′′), 22.36 (C-1′′′). IR (ATR): ṽ (cm−1) = 3364, 3116, 1728, 1673, 1521, 1291, 1184, 1042, 829, 772, 691. HR-MS (ESI): m/z = [M + H]+ calcd for C21H18N5O6+: 436.1257; found: 436.1250. Specific rotation: [α −18 (c 0.1 in DMSO). Purity (HPLC): 210 nm: >95%; 254 nm: >95%.
(S)-5-(4-(4-((1-Carboxy-3-cyanopropan-2-yl)carbamoyl)phenyl)-1H-1,2,3-triazol-1-yl)-2-hydroxybenzoic acid (50). Prepared according to General Procedure E from triazole 41 (120 mg, 0.212 mmol) and 0.70 M aq. KOH solution (59.5 mg, 1.06 mmol). The reaction mixture was stirred at room temperature for 30 min. The crude product was resuspended in EtOAc (10 mL), filtered and the solid residue collected to give cyanomethyl 50 (53.7 mg, 0.123 mmol, 58%) as a white solid. m.p.: 292 °C. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 12.44 (s, 1H, 1-COOH or 1′-COOH), 9.42 (s, 1H, 5′-H), 8.74 (d, J = 7.7 Hz, 1H, CONH), 8.30 (d, J = 2.8 Hz, 1H, 6-H), 8.11–8.08 (m, 1H, 4-H), 8.10–8.03 (m, 2H, 2″-H and 6″-H), 8.01–7.94 (m, 2H, 3″-H and 5″-H), 7.22 (d, J = 8.9 Hz, 1H, 3-H), 4.58–4.48 (m, 1H, 2′′′′-H), 2.97–2.81 (m, 2H, 3′′′-H), 2.77–2.61 (m, 2H, 1′′′-H). 13C-NMR (101 MHz, (CD3)2SO): δ (ppm) = 171.58 (1′′′-COOH), 170.85 (1-COOH), 165.60 (CONH), 160.91 (C-2), 146.40 (C-4′), 133.31 (C-4″), 133.19 (C-1″), 128.50 (C-5), 128.13 (C-3″ and C-5″), 127.40 (C-4), 125.01 (C-2″ and C-6″), 121.67 (C-6), 120.62 (C-5′), 118.69 (C-3), 118.42 (CN), 113.99 (C-1), 43.55 (C-2′′′), 37.71 (C-3′′′), 22.36 (C-1′′′). IR (ATR): ṽ (cm−1) = 3364, 3116, 1716, 1675, 1546, 1292, 1194, 1045, 828, 768, 691. HR-MS (ESI): m/z = [M − H]− calcd for C21H16N5O6−: 434.1101; found: 434.1102. Specific rotation: [α +12 (c 0.1 in DMSO). Purity (HPLC): 210 nm: >95%; 254 nm: >95%.
(R)-2-(6-(4-(3-(2,2-Dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)isoxazol-5-yl)phenyl)-2-hydroxy-3,4-dihydro-2H-1,5,2-oxazaborinin-4-yl)acetic acid (51). Prepared according to General Procedure E from isoxazole 36 (30.0 mg, 0.0590 mmol) and 0.40 M aq. KOH solution (4.97 mg, 0.0885 mmol). The reaction mixture was stirred at 0 °C for 30 min. The crude product was resuspended in DCM (10 mL), filtered and the solid residue collected to give cyclic boronic acid 51 (15.1 mg, 0.0317 mmol, 54%) as an off-white solid. m.p.: 233 °C. 1H-NMR at 90 °C (400 MHz, (CD3)2SO): δ (ppm) = 8.41–8.37 (m, 1H, 5′′′′-H), 8.25–8.19 (m, 1H, 7′′′′-H), 8.09–7.97 (m, 4H, 2″-H, 3″-H, 5″-H and 6″-H), 7.77–7.68 (m, 1H, 4′′′-H), 7.29 (d, J = 8.6 Hz, 1H, 8′′′′-H), 4.27–4.10 (m, 1H, 4′-H), 3.78–3.53 (m, 2H, 2-H or 3′-H), 2.69–2.54 (m, 2H, 2-H or 3′-H), 1.76 (s, 6H, (CH3)2). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 172.68 (C-1), 169.16 (C-5′′′), 166.79 (C-6′), 161.45 (C-3′′′), 159.75 (C-4′′′′), 156.81 (C-8a′′′′), 134.79 (C-1″ and C-7′′′′), 128.75 (C-4″), 128.37 (C-2″ and C-6″ or C-3″ and C-5″), 127.36 (C-5′′′′), 125.87–125.51 (C-2″ and C-6″ or C-3″ and C-5″), 123.23 (C-6′′′′), 118.56 (C-8′′′′), 113.56 (C-4a′′′′), 107.02 (C-2′′′′), 99.66 (C-4′′′), 64.03 (C-2 or C-3′), 48.18 (C-4′), 25.33 ((CH3)2). IR (ATR): ṽ (cm−1) = 3122, 2995, 1736, 1619, 1287, 1199, 1016, 927, 763, 674. HR-MS (ESI): m/z = [M + H]+ calcd for C24H22BN2O8+: 477.1469; found: 477.1427. Specific rotation: [α −6 (c 0.1 in DMSO). Purity (HPLC): 210 nm: 94%; 254 nm: >95%.
(S)-2-(6-(4-(3-(2,2-Dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)isoxazol-5-yl)phenyl)-2-hydroxy-3,4-dihydro-2H-1,5,2-oxazaborinin-4-yl)acetic acid (52). Prepared according to General Procedure E from isoxazole 37 (20.0 mg, 0.0393 mmol) and 0.40 M aq. KOH solution (3.31 mg, 0.0590 mmol). The reaction mixture was stirred at 0 °C for 30 min. The crude product was resuspended in DCM (10 mL), filtered and the solid residue collected to give cyclic boronic acid 52 (10.4 mg, 0.0218 mmol, 56%) as an off-white solid. m.p.: 188 °C. 1H-NMR at 90 °C (400 MHz, (CD3)2SO): δ (ppm) = 8.41–8.37 (m, 1H, 5′′′′-H), 8.22 (dd, J = 8.6, 2.2 Hz, 1H, 7′′′′-H), 8.08–7.95 (m, 4H, 2″-H, 3″-H, 5″-H and 6″-H), 7.75–7.69 (m, 1H, 4′′′-H), 7.29 (d, J = 8.7 Hz, 1H, 8′′′′-H), 4.41–4.00 (m, 1H, 4′-H), 3.70–3.41 (m, 2H, 2-H or 3′-H), 2.63–2.56 (m, 1H, 2-H or 3′-H), 2.41–2.31 (m, 1H, 2-H or 3′-H), 1.76 (s, 6H, (CH3)2). 13C-NMR (101 MHz, (CD3)2SO): δ (ppm) = 161.07 (C-3″), 159.11 (C-4′′′′), 156.42 (C-8a′′′′), 134.33 (C-7′′′′ and C-1″), 127.92 (C-2″ and C-6″ or C-3″ and C-5″), 126.91 (C-5′′′′), 125.28 (C-2″ and C-6″ or C-3″ and C-5″), 123.05 (C-6′′′′), 117.93 (C-8′′′′), 113.27 (C-4a′′′′), 106.50 (C-2′′′′), 25.01 ((CH3)2). IR (ATR): ṽ (cm−1) = 3118, 2918, 1738, 1619, 1287, 1199, 1018, 926, 762, 674. HR-MS (ESI): m/z = [M − H]− calcd for C24H20BN2O8−: 475.1313; found: 475.1294. Specific rotation: [α +4 (c 0.1 in DMSO). Purity (HPLC): 210 nm: >95%; 254 nm: >95%.
(R)-2-(6-(4-(1-(2,2-Dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)-1H-1,2,3-triazol-4-yl)phenyl)-2-hydroxy-3,4-dihydro-2H-1,5,2-oxazaborinin-4-yl)acetic acid (53). Prepared according to General Procedure E from triazole 42 (180 mg, 0.308 mmol) and 0.40 M aq. KOH solution (25.9 mg, 0.462 mmol). The reaction mixture was stirred at 0 °C for 1 h. The crude product was purified by FCC with boric acid-impregnated silica gel (DCM/MeOH 90:10 + 1% AcOH) to give cyclic boronic acid 53 (56.5 mg, 0.119 mmol, 39%) as a white solid. m.p.: 292 °C (decomposition). 1H-NMR (400 MHz, CD3OD): δ (ppm) = 9.08 (s, 1H, 5″-H), 8.42 (d, J = 2.7 Hz, 1H, 5′′′′-H), 8.22 (dd, J = 8.9, 2.7 Hz, 1H, 7′′′′-H), 8.07–8.02 (m, 2H, 3″-H and 5″-H), 7.97–7.92 (m, 2H, 2″-H and 6″-H), 7.31 (d, J = 8.9 Hz, 1H, 8′′′′-H), 4.38–4.29 (m, 1H, 4′-H), 2.74–2.66 (m, 1H, 2-H), 2.56–2.47 (m, 1H, 2-H), 1.80 (s, 6H, (CH3)2), 0.92–0.83 (m, 1H, 3′-H), 0.77–0.68 (m, 1H, 3′-H). 13C-NMR (101 MHz, CD3OD): δ (ppm) = 174.68 (C-1), 168.94 (C-6′), 161.47 (C-4′′′′), 157.46 (C-8a′′′′), 148.62 (C-4′′′), 136.12 (C-1′ and C-4″), 133.45 (C-6′′′′), 129.95 (C-7′′′′), 129.18 (C-2″ and C-6″), 126.84 (C-3″ and C-5″), 121.99 (C-5′′′′), 121.34 (C-5′′′), 120.35 (C-8′′′′), 115.55 (C-4a′′′′), 108.66 (C-2′′′′), 47.39 (C-4′), 40.50 (C-2), 25.86 ((CH3)2). IR (ATR): ṽ (cm−1) = 3343, 1733, 1618, 1509, 1379, 1299, 1044, 828, 767. HR-MS (ESI): m/z = [M + H]+ calcd for C23H22BN4O7+: 477.1582; found: 477.1564. Specific rotation: [α −12 (c 0.1 in DMSO). Purity (HPLC): 210 nm: >95%; 254 nm: >95%.
(S)-2-(6-(4-(1-(2,2-Dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)-1H-1,2,3-triazol-4-yl)phenyl)-2-hydroxy-3,4-dihydro-2H-1,5,2-oxazaborinin-4-yl)acetic acid (54). Prepared according to General Procedure E from triazole 43 (205 mg, 0.351 mmol) and 0.40 M aq. KOH solution (29.5 mg, 0.526 mmol). The reaction mixture was stirred at 0 °C for 1 h. The crude product was purified by FCC with boric acid-impregnated silica gel (DCM/MeOH 90:10 + 1% AcOH) to give cyclic boronic acid 54 (53.0 mg, 0.111 mmol, 32%) as a pale yellow solid. m.p.: 304 °C (decomposition). 1H-NMR (500 MHz, CD3OD): δ (ppm) = 9.10 (s, 1H, 5′′′-H), 8.42 (d, J = 2.7 Hz, 1H, 5′′′′-H), 8.23 (dd, J = 8.9, 2.7 Hz, 1H, 7′′′′-H), 8.10–8.05 (m, 2H, 3″-H and 5″-H), 8.00–7.95 (m, 2H, 2″-H and 6″-H), 7.31 (d, J = 9.0 Hz, 1H, 8′′′′-H), 4.36–4.26 (m, 1H, 4′-H), 2.76–2.67 (m, 1H, 2-H), 2.64–2.50 (m, 1H, 2-H), 1.80 (s, 6H, (CH3)2), 0.92–0.86 (m, 1H, 3′-H), 0.82–0.73 (m, 1H, 3′-H). 13C-NMR (126 MHz, CD3OD): δ (ppm) = 175.06 (C-1), 169.18 (C-6′), 161.46 (C-4′′′′), 157.48 (C-8a′′′′), 148.51 (C-4′′′), 135.11 (C-1″ and C-4″), 133.43 (C-6′′′′), 129.95 (C-7′′′′), 129.33 (C-2″ and C-6″), 126.91 (C-3″ and C-5″), 121.99 (C-5′′′′), 121.46 (C-5′′′), 120.36 (C-8′′′′), 115.56 (C-4a′′′′), 108.66 (C-2′′′′), 45.77 (C-4′), 40.53 (C-2), 25.86 ((CH3)2). IR (ATR): ṽ (cm−1) = 3343, 1733, 1616, 1508, 1378, 1298, 1041, 826, 766. HR-MS (ESI): m/z = [M + H]+ calcd for C23H22BN4O7+: 477.1582; found: 477.1583. Specific rotation: [α +8 (c 0.1 in DMSO). Purity (HPLC): 210 nm: >95%; 254 nm: >95%.
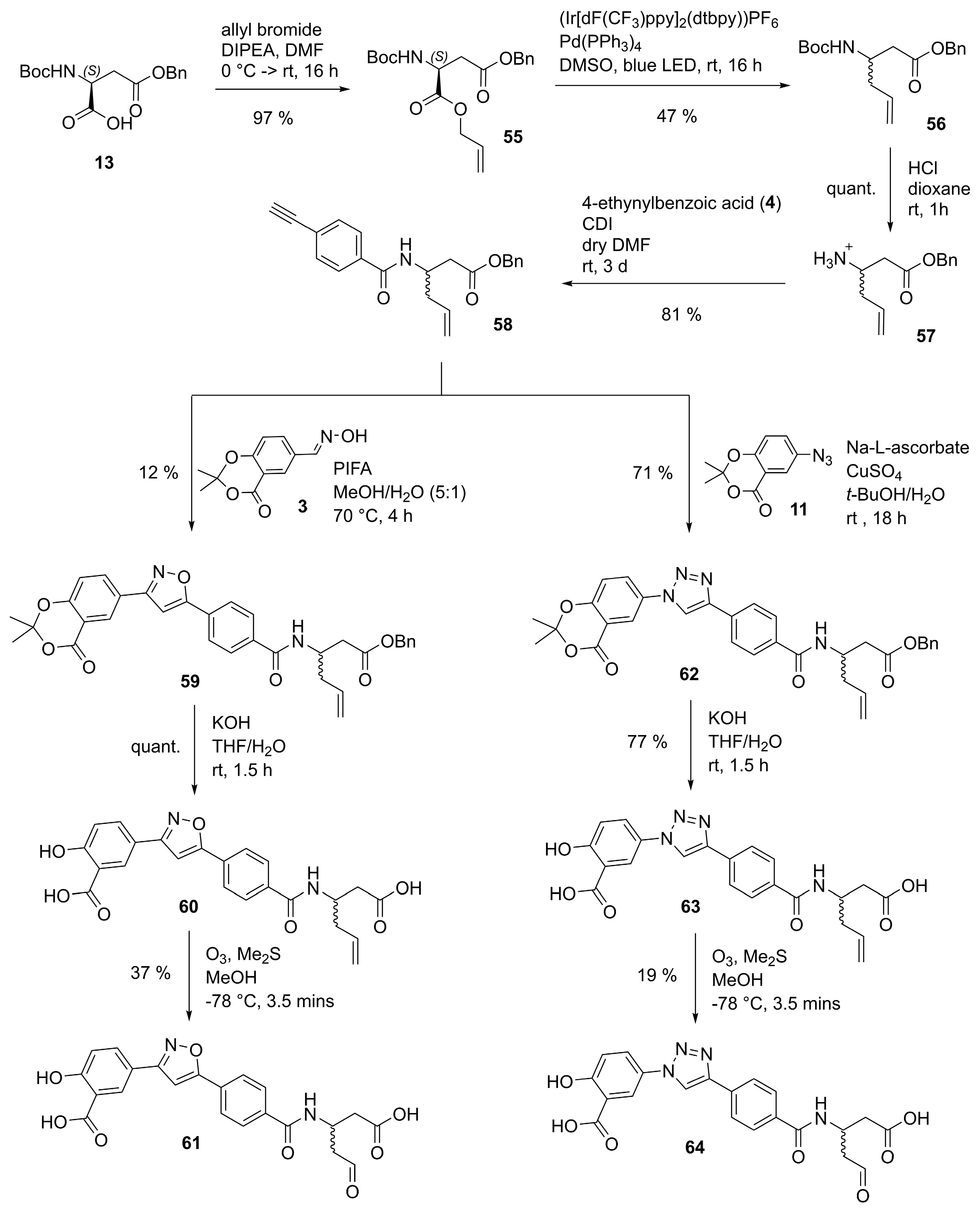
1-(Benzyloxy)-1-oxohex-5-en-3-aminium (57). Prepared according to General Procedure A from N-Boc-protected homoallyl amine 56 (1.37 g, 4.29 mmol). The aminium 57 (945 mg, 4.31 mmol, quant.) was obtained as a viscous yellow oil. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 8.22 (s, 3H, NH3+), 7.40–7.32 (m, 5H, 2′-H, 3′-H, 4′-H, 5′-H and 6′-H), 5.83–5.71 (m, 1H, 5-H), 5.17–5.10 (m, 4H, 6-H and 1′-CH2), 3.58–3.48 (m, 1H, 3-H), 2.81–2.64 (m, 2H, 2-H), 2.47–2.30 (m, 2H, 4-H). 13C-NMR (101 MHz, (CD3)2SO): δ (ppm) = 169.74 (C-1), 135.71 (C-1′), 132.41 (C-5), 128.45 -128.18 (2′-H, 3′-H, 4′-H, 5′-H and 6′-H), 119.51 (C-6), 66.10 (1′-CH2), 46.86 (C-3), 36.42 (C-4), 36.09 (C-2). IR (ATR): ṽ (cm−1) = 2977, 2880, 2834, 1998, 1728, 1601, 1497, 1395, 1216, 1190, 1128, 923, 738, 696. HR-MS (ESI): m/z = [M]+ calcd for C13H18NO2+: 220.1332; found: 220.1353.
Benzyl 3-(4-ethynylbenzamido)hex-5-enoate (58). Prepared according to General Procedure B from aminium 57 (490 mg, 2.23 mmol) and 4-ethynylbenzoic acid (344 mg, 2.23 mmol). The crude product was purified by FCC (hexanes/EtOAc 80:20) to give amide 58 (629 mg, 1.81 mmol, 81%) as a pale yellow solid. m.p.: 104 °C. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 8.42 (d, J = 8.4 Hz, 1H, CONH), 7.81–7.77 (m, 2H, 2′-H and 6′-H), 7.57–7.54 (m, 2H, 3′-H and 5′-H), 7.33–7.27 (m, 5H, 2″-H, 3″-H, 4″-H, 5″-H and 6″-H), 5.77 (ddt, J = 17.2, 10.2, 7.0 Hz, 1H, 5-H), 5.10–4.99 (m, 4H, 6-H and 1″-CH2), 4.45–4.38 (m, 1H, 3-H), 4.37 (s, 1H, C≡CH), 2.66–2.61 (m, 2H, 2-H), 2.33–2.28 (m, 2H, 4-H). 13C-NMR (101 MHz, (CD3)2SO): δ (ppm) = 170.67 (C-1), 164.98 (CONH), 136.08 (C-1″), 134.92 (C-5), 134.62 (C-1′), 131.55 (C-3′ and C-5′), 128.31–127.86 (C-2″, C-3″, C-4″, C-5″ and C-6″), 127.50 (C-2′ and C-6′), 124.32 (C-4′), 117.42 (C-6), 82.90 (C≡CH and C≡CH), 65.54 (1″-CH2), 46.34 (C-3), 38.63 (C-2), 38.46 (C-4). IR (ATR): ṽ (cm−1) = 3317, 3271, 1737, 1716, 1638, 1543, 1536, 1495, 1301, 1258, 1164, 1116, 854, 757, 698, 675. HR-MS (ESI): m/z = [M + H]+ calcd for C22H22NO3+: 348.1600; found: 348.1607.
Benzyl 3-(4-(3-(2,2-dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)isoxazol-5-yl)benzamido)hex-5-enoate (59). To a stirred solution of alkyne 58 (844 mg, 2.43 mmol) in 24 mL MeOH/H2O (5:1 v/v), aldoxime 3 (806 mg, 3.64 mmol) and PIFA (403 mg, 1.82 mmol) were added. The reaction mixture was stirred at 70 °C for 2 h. Another equivalent of PIFA (403 mg, 1.82 mmol) was added, and the reaction mixture was stirred at 70 °C for another 2 h. The solvents were removed in vacuo, and the crude product was redissolved in EtOAc (150 mL). Water (150 mL) was added, the resulting two phases were separated, and the aqueous phase was extracted with EtOAc (3 × 150 mL). The combined organic phases were dried using a phase separation paper, and the solvent was removed in vacuo. The crude product was purified by FCC (hexanes/EtOAc 70:30) to give isoxazole 59 (163.2 mg, 0.290 mmol, 12%) as a white solid. m.p.: 173 °C. 1H-NMR (500 MHz, CDCl3): δ (ppm) = 8.37 (d, J = 2.2 Hz, 1H, 5′′′-H), 8.19 (dd, J = 8.6, 2.2 Hz, 1H, 7′′′-H), 7.89–7.86 (m, 2H, 3′-H and 5′-H), 7.84–7.81 (m, 2H, 2′-H and 6′-H), 7.39–7.31 (m, 5H, 2′′′′-H, 3′′′′-H, 4′′′′-H, 5′′′′-H and 6′′′′-H), 7.11 (d, J = 8.6 Hz, 1H, 8′′′-H), 6.97 (d, J = 8.7 Hz, 1H, CONH), 6.94 (s, 1H, 4″-H), 5.81 (ddt, J = 19.5, 9.6, 7.1 Hz, 1H, 5-H), 5.20–5.09 (m, 4H, 6-H and 1′′′′-CH2), 4.60–4.52 (m, 1H, 3-H), 2.74 (d, J = 5.0 Hz, 2H, 2-H), 2.54–2.38 (m, 2H, 4-H), 1.79 (s, 6H, (CH3)2). 13C-NMR (126 MHz, CDCl3): δ (ppm) = 171.98 (C-1), 169.88 (C-5″), 165.75 (CONH), 161.72 (C-3″), 160.74 (C-4′′′), 157.46 (C-8a′′′), 136.06 (C-1′), 135.57 (C-1′′′′), 134.69 (C-7′′′), 133.95 (C-5), 129.88 (C-4′), 128.85–128.57 (C-2′′′′, C-3′′′′, C-4′′′′, C-5′′′′ and C-6′′′′), 128.35 (C-5′′′), 127.89 (C-2′ and C-6′), 126.12 (C-3′ and C-5′), 123.97 (C-6′′′), 118.77 (C-6), 118.39 (C-8′′′), 113.90 (C-4a′′′), 107.10 (C-2′′′), 98.46 (C-4″), 66.91 (1′′′′-CH2), 46.21 (C-3), 38.59 (C-4), 37.59 (C-2), 26.04 ((CH3)2). IR (ATR): ṽ (cm−1) = 3300, 2922, 2851, 1748, 1720, 1634, 1627, 1534, 1429, 1284, 1200, 1050, 922, 914, 822, 763, 688. HR-MS (ESI): m/z = [M + H]+ calcd for C33H31N2O7+: 567.2131; found: 567.2117.
5-(5-(4-((1-Carboxypent-4-en-2-yl)carbamoyl)phenyl)isoxazol-3-yl)-2-hydroxybenzoic acid (60). Prepared according to General Procedure E from isoxazole 59 (165 mg, 0.291 mmol) and 0.70 M aq. KOH solution (81.4 mg, 1.45 mmol). The reaction mixture was stirred at room temperature for 1.5 h. The crude product was resuspended in EtOAc (20 mL), filtered and the solid residue collected to give vinyl 60 (127 mg, 0.291 mmol, quant.) as a white solid. m.p.: 225 °C. 1H-NMR (500 MHz, (CD3)2SO): δ (ppm) = 12.19 (s, 1H, 1-COOH or 1′′′-COOH), 8.45 (d, J = 8.4 Hz, 1H, CONH), 8.34 (d, J = 2.3 Hz, 1H, 6-H), 8.06 (dd, J = 8.7, 2.3 Hz, 1H, 4-H), 8.05–7.99 (m, 2H, 2′′-H and 6′′-H), 8.01–7.95 (m, 2H, 3′′-H and 5′′-H), 7.76 (s, 1H, 4′-H), 7.14 (d, J = 8.6 Hz, 1H, 3-H), 5.81 (ddt, J = 17.1, 10.2, 7.0 Hz, 1H, 4′′′-H), 5.11–5.01 (m, 2H, 5′′′-H), 4.43–4.34 (m, 1H, 2′′′-H), 2.57–2.51 (m, 2H, 1′′′-H), 2.37–2.30 (m, 2H, 3′′′-H). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 172.42 (1′′′-COOH), 171.32 (1-COOH), 168.91 (C-5′), 164.84 (CONH), 162.53 (C-2), 161.80 (C-3′), 136.02 (C-4″), 135.10 (C-4′′′), 133.35 (C-4), 128.94 (C-1″), 128.65 (C-6), 128.16 (C-3″ and C-5″), 125.42 (C-2″ and C-6″), 119.50 (C-5), 118.22 (C-3), 117.31 (C-5′′′), 113.88 (C-1), 99.48 (C-4′), 46.26 (C-2′′′), 38.71 (C-1′′′), 38.45 (C-3′′′). IR (ATR): ṽ (cm−1) = 3315, 2916, 1729, 1688, 1632, 1589, 1531, 1499, 1421, 1290, 1212, 926, 796, 769, 695. HR-MS (ESI): m/z = [M − H]− calcd for C23H19N2O7−: 435.1192; found: 435.1192.
5-(5-(4-((1-Carboxy-4-oxobutan-2-yl)carbamoyl)phenyl)isoxazol-3-yl)-2-hydroxybenzoic acid (61). A solution of vinyl 60 (85 mg, 0.195 mmol) in 7 mL MeOH was flushed with N2 and cooled to -78 °C. O3 (flowrate: 25–30, power: 35%) was then bubbled into the solution for 3.5 min. Me2S (21.7 µL, 0.292 mmol) was added and the solution stirred at room temperature for 1 h. The solvent was removed in vacuo and the crude product purified by PTLC (DCM/MeOH 88:12 + 1% AcOH) to give formylmethyl 61 (31.4 mg, 0.0716 mmol, 37%) as a beige solid. m.p.: 275 °C. 1H-NMR (500 MHz, (CD3)2SO): δ (ppm) = 9.73 (s, 1H, CONH), 9.68 (s, 1H, 4′′′-H), 8.23 (d, J = 2.4 Hz, 1H, 6-H), 8.04–8.00 (m, 2H, 2′′-H and 6″-H), 7.94–7.90 (m, 2H, 3″-H and 5″-H), 7.71 (dd, J = 8.5, 2.4 Hz, 1H, 4-H), 7.61 (s, 1H, 4′-H), 6.74 (d, J = 8.4 Hz, 1H, 3-H), 4.63–4.53 (m, 1H, 2′′′-H), 2.76–2.55 (m, 2H, 3′′′-H), 2.32–2.22 (m, 2H, 1′′′-H). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 202.04 (C-4′′′), 174.07 (1′′′-COOH), 170.65 (1-COOH), 168.14 (C-5′), 166.02 (C-2), 164.19 (CONH), 162.94 (C-3′), 135.74 (C-4″), 129.76 (C-4), 129.28 (C-1″), 128.61 (C-6), 127.76 (C-3″ and C-5″), 125.50 (C-2″ and C-6″), 120.44 (C-5), 116.97 (C-3), 115.53 (C-1), 99.33 (C-4′), 48.90 (C-3′′′), 43.15 (C-2′′′), 41.28 (C-1′′′). IR (ATR): ṽ (cm−1) = 3300, 2925, 1715, 1635, 1560, 1497, 1392, 1257, 1077, 948, 834, 768, 700. HR-MS (ESI): m/z = [M − H]− calcd for C22H17N2O8−: 437.0985; found: 437.0988. Purity (HPLC): 210 nm: 90%; 254 nm: 91%.
Benzyl 3-(4-(1-(2,2-dimethyl-4-oxo-4H-benzo[d][1,3]dioxin-6-yl)-1H-1,2,3-triazol-4-yl)benzamido)hex-5-enoate (62). Prepared according to General Procedure D from azide 11 (300 mg, 1.37 mmol) and alkyne 58 (571 mg, 1.64 mmol). The crude product was resuspended in EtOAc (20 mL), filtered and the solid residue collected to give triazole 62 (550 mg, 0.971 mmol, 71%) as a white solid. m.p.: 173 °C. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 9.53 (s, 1H, 5′′-H), 8.42–8.38 (m, 2H, CONH and 5′′′-H), 8.31 (dd, J = 8.9, 2.7 Hz, 1H, 7′′′-H), 8.06–8.00 (m, 2H, 3′-H and 5′-H), 7.96–7.90 (m, 2H, 2′-H and 6′-H), 7.43 (d, J = 8.9 Hz, 1H, 8′′′-H), 7.35–7.27 (m, 5H, 2′′′′-H, 3′′′′-H, 4′′′′-H, 5′′′′-H and 6′′′′-H), 5.87–5.74 (m, 1H, 5-H), 5.13–5.01 (m, 4H, 6-H and 1′′′′-CH2), 4.50–4.40 (m, 1H, 3-H), 2.69–2.64 (m, 2H, 2-H), 2.34 (t, J = 6.9 Hz, 2H, 4-H), 1.77 (s, 6H, (CH3)2). 13C-NMR (101 MHz, (CD3)2SO): δ (ppm) = 170.73 (C-1), 165.27 (CONH), 159.48 (C-4′′′), 155.22 (C-8a′′′), 146.67 (C-4″), 136.11 (C-1′′′′), 134.99 (C-5), 134.08 (C-1′), 132.66 (C-4′), 131.70 (C-6′′′), 128.67 (C-7′′′), 128.33 (C-2′′′′, C-3′′′′, C-4′′′′, C-5′′′′ and C-6′′′′), 128.04 (C-2′ and C-6′), 127.92–127.87 (C-2′′′′, C-3′′′′, C-4′′′′, C-5′′′′ and C-6′′′′), 124.95 (C-3′ and C-5′), 120.69 (C-5″), 120.20 (C-5′′′), 119.25 (C-8′′′), 117.41 (C-6), 113.77 (C-4a′′′), 107.23 (C-2′′′), 65.55 (1′′′′-CH2), 46.33 (C-3), 38.71 (C-2 or C-4), 38.53 (C-2 or C-4), 25.27 ((CH3)2). IR (ATR): ṽ (cm−1) = 3313, 1735, 1630, 1507, 1295, 1170, 1047, 932, 851, 697. HR-MS (ESI): m/z = [M + H]+ calcd for C32H31N4O6+: 567.2244; found: 567.2259.
5-(4-(4-((1-Carboxypent-4-en-2-yl)carbamoyl)phenyl)-1H-1,2,3-triazol-1-yl)-2-hydroxybenzoic acid (63). Prepared according to General Procedure E from triazole 62 (422 mg, 0.745 mmol) and 0.70 M aq. KOH solution (208 mg, 3.71 mmol). The reaction mixture was stirred at room temperature for 1.5 h. The crude product was resuspended in EtOAc (50 mL), filtered and the solid residue collected to give vinyl 63 (249 mg, 0.570 mmol, 77%) as a white solid. m.p.: 223 °C. 1H-NMR (400 MHz, (CD3)2SO): δ (ppm) = 12.12 (s, 1H, 1-COOH or 1′′′-COOH), 9.40 (s, 1H, 5′-H), 8.34 (d, J = 8.4 Hz, 1H, CONH), 8.30 (d, J = 2.8 Hz, 1H, 6-H), 8.09 (dd, J = 8.9, 2.8 Hz, 1H, 4-H), 8.06–7.99 (m, 2H, 2′′-H and 6′′-H), 7.98–7.90 (m, 2H, 3′′-H and 5′′-H), 7.22 (d, J = 8.9 Hz, 1H, 3-H), 5.87–5.75 (m, 1H, 4′′′-H), 5.13–5.00 (m, 2H, 5′′′-H), 4.44–4.34 (m, 1H, 2′′′-H), 2.52–2.48 (m, 2-H, 1′′′-H), 2.33 (t, J = 6.9 Hz, 2H, 3′′′-H). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 172.47 (1′′′-COOH), 170.87 (1-COOH), 165.19 (CONH), 160.89 (C-2), 146.49 (C-4′), 135.17 (C-4′′′), 134.06 (C-4″), 132.80 (C-1″), 128.52 (C-5), 128.00 (C-3″ and C-5″), 127.40 (C-4), 124.93 (C-2″ and C-6″), 121.65 (C-6), 120.50 (C-5′), 118.70 (C-3), 117.26 (C-5′′′), 113.96 (C-1), 46.18 (C-2′′′), 38.77 (C-1′′′), 38.49 (C-3′′′). IR (ATR): ṽ (cm−1) = 3119, 1723, 1669, 1593, 1546, 1492, 1288, 1209, 1182, 1046, 828, 689. HR-MS (ESI): m/z = [M + H]+ calcd for C22H21N4O6+: 437.1461; found: 437.1471.
5-(4-(4-((1-Carboxy-4-oxobutan-2-yl)carbamoyl)phenyl)-1H-1,2,3-triazol-1-yl)-2-hydroxybenzoic acid (64). A solution of vinyl 63 (100 mg, 0.229 mmol) in 8 mL MeOH was flushed with N2 and cooled to −78 °C. O3 (flowrate: 25–30, power: 35%) was then bubbled into the solution for 3.5 min. Me2S (25.5 µL, 0.344 mmol) was added and the solution stirred at room temperature for 1 h. The solvent was removed in vacuo and the crude product purified by PTLC (DCM/MeOH 85:15 + 1% AcOH) to give aldehyde 64 (18.9 mg, 0.0431 mmol, 19%) as a beige solid. m.p.: 151 °C (decomposition). 1H-NMR (500 MHz, (CD3)2SO): δ (ppm) = 9.69 (s, 1H, 4′′′-H), 9.65 (s, 1H, CONH), 9.27 (s, 1H, 5′-H), 8.13 (d, J = 2.9 Hz, 1H, 6-H), 8.06–8.01 (m, 2H, 2′′-H and 6″-H), 7.89–7.85 (m, 2H, 3″-H and 5″-H), 7.69 (dd, J = 8.7, 2.9 Hz, 1H, 4-H), 6.82 (d, J = 8.7 Hz, 1H, 3-H), 4.61–4.55 (m, 1H, 2′′′-H), 2.76–2.55 (m, 2H, 3′′′-H), 2.30–2.20 (m, 2H, 1′′′-H). 13C-NMR (126 MHz, (CD3)2SO): δ (ppm) = 202.17 (C-4′′′), 174.34 (1′′′-COOH), 170.08 (1-COOH), 164.53 (CONH), 164.03 (C-2), 146.09 (C-4′), 133.81 (C-4″), 133.18 (C-1″), 127.57 (C-3″ and C-5″), 125.87 (C-5), 125.00 (C-2″ and C-6″), 123.76 (C-4), 121.70 (C-6), 120.71 (C-1), 120.23 (C-5′), 117.03 (C-3), 49.01 (C-3′′′), 43.08 (C-2′′′), 41.44 (C-1′′′). IR (ATR): ṽ (cm−1) = 3300, 2918, 1716, 1636, 1576, 1487, 1374, 1252, 1043, 829, 769, 706. HR-MS (ESI): m/z = [M-H]− calcd for C21H17N4O7−: 437.1097; found: 437.1102. Purity (HPLC): 210 nm: 94%; 254 nm: 92%.
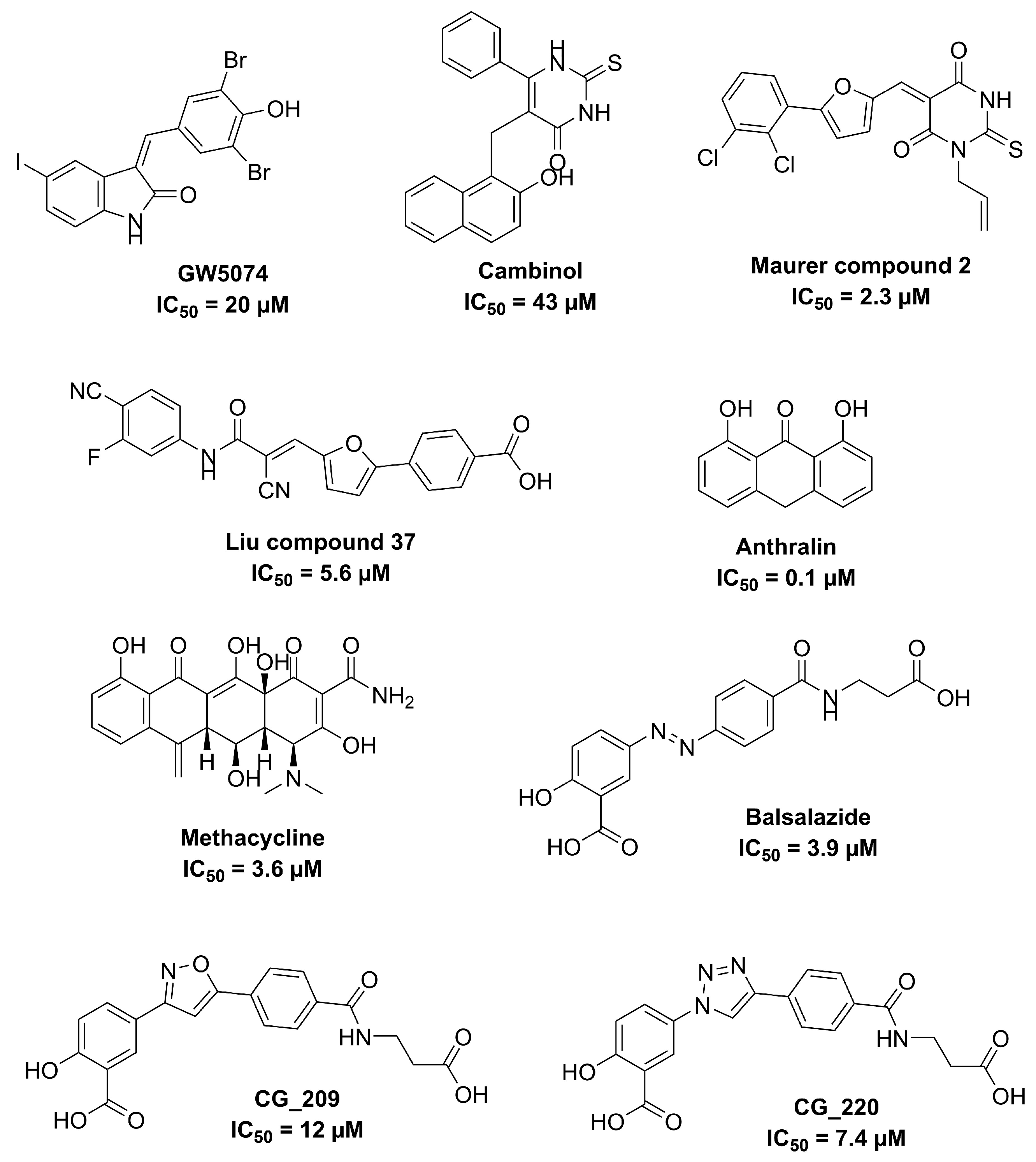


 2 Entry 10b differs from entry 10a in the purification method.
2 Entry 10b differs from entry 10a in the purification method.