Effect of Support and Polymer Modifier on the Catalytic Performance of Supported Palladium Catalysts in Hydrogenation
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Pd Catalysts Deposited on Polymer-Modified Supports
2.2. Catalytic Properties of Pd Catalysts, Deposited on Polymer-Modified Supports, in the Hydrogenation Process
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Pd Catalysts
3.3. Characterization of the Catalysts by Physicochemical Methods
3.4. Methodology of Hydrogenation
4. Conclusions
- −
- Polymer-containing catalysts showed improved activity and selectivity compared to unmodified ones, confirming that polymer modification has a positive effect on the performance of palladium-based catalysts. This improvement is likely associated with the stabilizing effect of the polymer, which promotes the formation of smaller and more uniformly distributed palladium nanoparticles, thereby enhancing catalytic efficiency.
- −
- Catalysts supported on MgO exhibited higher activity in the hydrogenation of 2-propen-1-ol and phenylacetylene compared to those based on SBA-15, which correlates with the formation of smaller and more uniformly dispersed Pd nanoparticles (2–3 nm on MgO vs. 8–10 nm on SBA-15), as confirmed by TEM analysis. In contrast, for the hydrogenation of 2-hexyn-1-ol, the nature of the inorganic support had an insignificant effect on catalytic activity.
- −
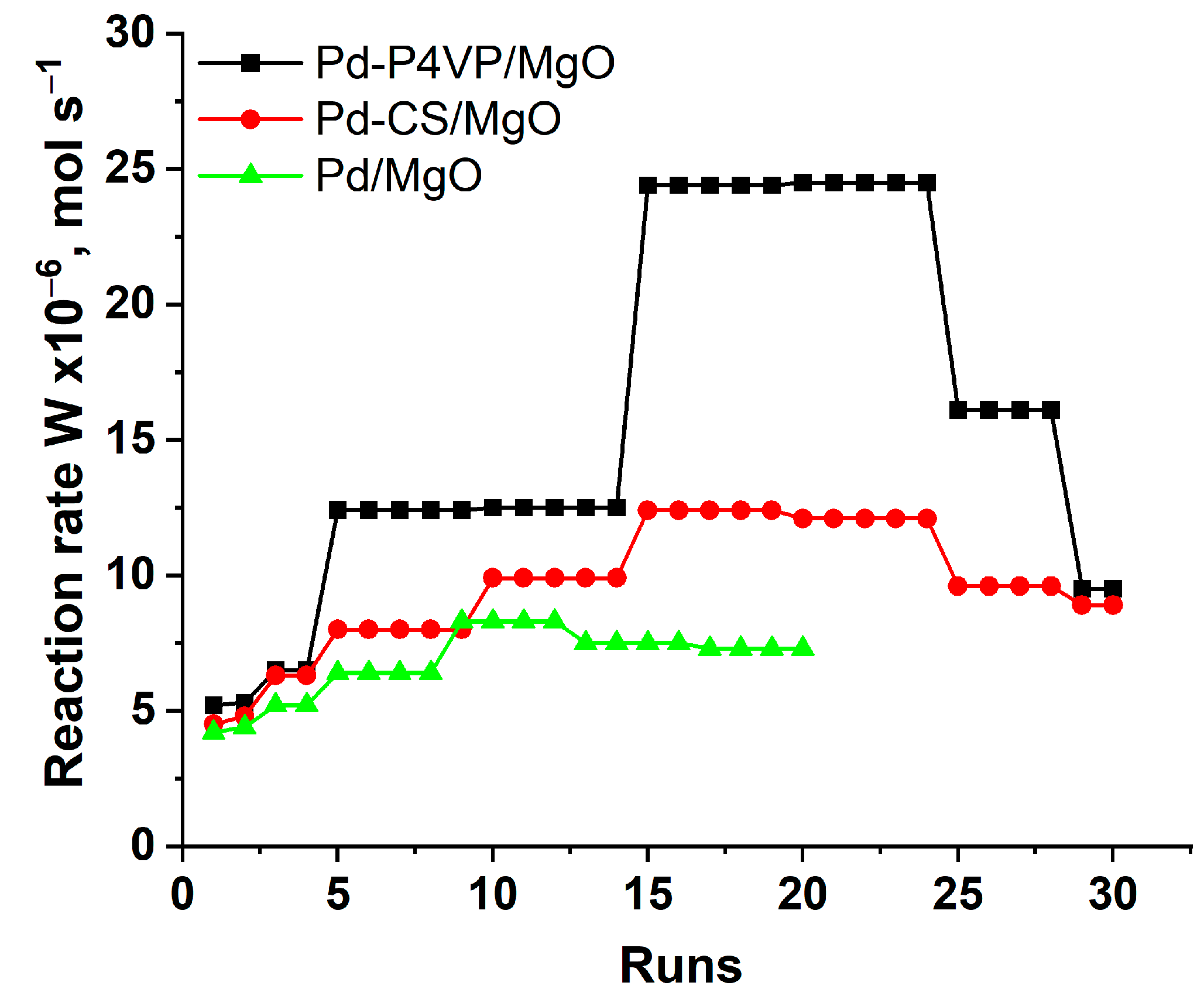
- P4VP-modified catalysts demonstrated higher activity than their chitosan-containing counterparts across all studied hydrogenation reactions. This difference became particularly pronounced during long-term stability tests conducted for Pd–P4VP/MgO and Pd–CS/MgO in the hydrogenation of 2-propen-1-ol. In both cases, the reaction rate increased progressively with each subsequent substrate addition, likely due to swelling of the polymer layer in ethanol. However, the rate enhancement was more significant for the Pd–P4VP/MgO system, which can be attributed to the superior swelling capacity of poly(4-vinylpyridine) compared to chitosan. The chitosan-modified catalyst (1%Pd–CS/MgO) also outperformed the unmodified 1%Pd/MgO system, confirming the beneficial effect of polymer modification. In contrast, the unmodified catalyst showed only a slight increase in activity during repeated cycles, which can be explained by the gradual increase in the fraction of metallic palladium (Pd0) in the presence of alkaline magnesium oxide after each successive cycle.
- −
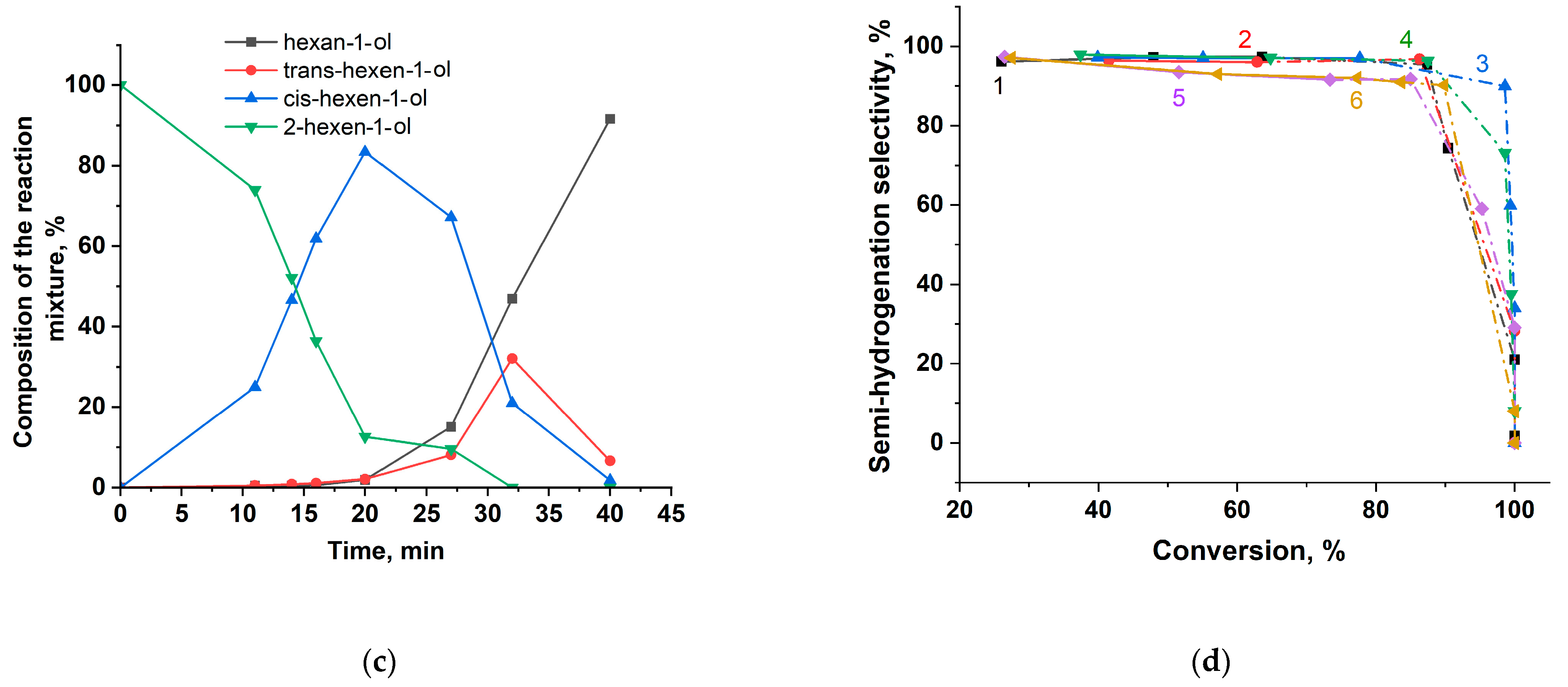
- Polymer modification noticeably increased the selectivity of catalysts toward the target products in all three hydrogenation reactions, which can be explained by the influence of the polymer on the electronic state of palladium. For example, the selectivity to propanol in the hydrogenation of 2-propen-1-ol was higher for polymer-modified catalysts (77–83%) compared to unmodified ones (71–80%). Similarly, in phenylacetylene hydrogenation, the selectivity to styrene for unmodified catalysts was 89–91%, while polymer-modified catalysts achieved 93–95%. In the case of 2-hexyn-1-ol hydrogenation, unmodified catalysts showed selectivity to cis-2-hexen-1-ol of 90–91%, compared to 96–97% for polymer-modified systems. For polymer-modified catalysts, selectivity trends were found to be substrate-dependent. In the hydrogenation of 2-propen-1-ol, MgO-supported catalysts exhibited higher selectivity toward propanol than their SBA-15-supported counterparts. In the case of 2-hexyn-1-ol and phenylacetylene hydrogenation, neither the type of support nor the nature of the polymer modifier significantly affected selectivity: all catalysts exhibited consistently high selectivity to cis-2-hexen-1-ol (96–97%) and styrene (93–95%), respectively.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friend, C.M.; Xu, B. Heterogeneous Catalysis: A Central Science for a Sustainable Future. Acc. Chem. Res. 2017, 50, 517–521. [Google Scholar] [CrossRef]
- Pouramiri, B.; Hadadianpour, E.; Saghebi, S. Palladium supported on alumina as a highly active heterogeneous catalyst for direct arylation of 6-substituted imidazo[2,1-b]thiadiazole via C single bond H bond activation. Tetrahedron Lett. 2024, 150, 155286. [Google Scholar] [CrossRef]
- Lee, S.; Shin, S.-J.; Baek, H.; Choi, Y.; Hyun, K.; Seo, M.; Kim, K.; Koh, D.-Y.; Kim, H.; Choi, M. Dynamic metal-polymer interaction for the design of chemoselective and long-lived hydrogenation catalysts. Sci. Adv. 2020, 6, eabb7369. [Google Scholar] [CrossRef]
- Erigoni, A.; Diaz, U. Porous Silica-Based Organic-Inorganic Hybrid Catalysts: A Review. Catalysts 2021, 11, 79. [Google Scholar] [CrossRef]
- Prajapati, P.; Shrivastava, S.; Sharma, V.; Srivastava, P.; Shankhwar, V.; Sharma, A.; Srivastava, S.K.; Agarwal, D.D. Karanja seed shell ash: A sustainable green heterogeneous catalyst for biodiesel production. Results Eng. 2023, 18, 101063. [Google Scholar] [CrossRef]
- Simon, A.; Mathai, S. Recent advances in heterogeneous magnetic Pd-NHC catalysts in organic transformations. J. Organomet. Chem. 2023, 996, 122768. [Google Scholar] [CrossRef]
- Gross, E.; Toste, F.D.; Somorjai, G.A. Polymer-Encapsulated Metallic Nanoparticles as a Bridge Between Homogeneous and Heterogeneous Catalysis. Catal. Lett. 2015, 145, 126–138. [Google Scholar] [CrossRef]
- Van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–997. [Google Scholar] [CrossRef]
- Gao, M.; Lyalin, A.; Taketsugu, T. Role of the Support Effects on the Catalytic Activity of Gold Clusters: A Density Functional Theory Study. Catalysts 2011, 1, 18–39. [Google Scholar] [CrossRef]
- Dong, Z.; Pan, H.; Chen, J.; Fan, L.; Guo, J.; Wang, W. Palladium supported on urea-containing porous organic polymers as heterogeneous catalysts for C–C cross coupling reactions and reduction of nitroarenes. J. Saudi Chem. Soc. 2021, 25, 101317. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, B.; Zhou, L.; Zhang, Z.-M.; Zhang, J. Polymer-supported chiral palladium-based complexes as efficient heterogeneous catalysts for asymmetric reductive Heck reaction. Green Synth. Catal. 2024, 5, 102–107. [Google Scholar] [CrossRef]
- Bukharbayeva, F.; Zharmagambetova, A.; Talgatov, E.; Auyezkhanova, A.; Akhmetova, S.; Jumekeyeva, A.; Naizabayev, A.; Kenzheyeva, A.; Danilov, D. The Synthesis of Green Palladium Catalysts Stabilized by Chitosan for Hydrogenation. Molecules 2024, 29, 4584. [Google Scholar] [CrossRef] [PubMed]
- Zharmagambetova, A.; Auyezkhanova, A.; Talgatov, E.; Jumekeyeva, A.; Buharbayeva, F.; Akhmetova, S.; Myltykbayeva, Z.; Nieto, J.M.L. Synthesis of polymer protected Pd–Ag/ZnO catalysts for phenylacetylene hydrogenation. J. Nanoparticle Res. 2022, 24, 236. [Google Scholar] [CrossRef]
- Ródenas, M.; El Haskouri, J.; Ros-Lis, J.V.; Marcos, M.D.; Amorós, P.; Úbeda, M.Á.; Pérez-Pla, F. Highly Active Hydrogenation Catalysts Based on Pd Nanoparticles Dispersed along Hierarchical Porous Silica Covered with Polydopamine as Interfacial Glue. Catalysts 2020, 10, 449. [Google Scholar] [CrossRef]
- Wissing, M.; Niehues, M.; Ravoo, B.J.; Studer, A. Synthesis and Immobilization of Metal Nanoparticles Using Photoactive Polymer-Decorated Zeolite L Crystals and Their Application in Catalysis. Adv. Synth. Catal. 2020, 362, 2245–2253. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, X.; Liu, Q.; Xu, M.; Yang, S.; Zeng, M. Chitosan supported Pd0 nanoparticles encaged in Al or Al-Fe pillared montmorillonite and their catalytic activities in Sonogashira coupling reactions. Appl. Clay Sci. 2020, 195, 105721. [Google Scholar] [CrossRef]
- Nagarjuna, R.; Sharma, S.; Rajesh, N.; Ganesan, R. Effective Adsorption of Precious Metal Palladium over Polyethyleneimine-Functionalized Alumina Nanopowder and Its Reusability as a Catalyst for Energy and Environmental Applications. ACS Omega 2017, 2, 4494–4504. [Google Scholar] [CrossRef]
- Rakap, M.; Kalu, E.E.; Özkar, S. Polymer-immobilized palladium supported on TiO2 (Pd–PVB–TiO2) as highly active and reusable catalyst for hydrogen generation from the hydrolysis of unstirred ammonia–borane solution. Int. J. Hydrogen Energy 2011, 36, 1448–1455. [Google Scholar] [CrossRef]
- Szubiakiewicz, E.; Modelska, M.; Brzezinska, M.; Binczarski, M.J.; Severino, C.J.; Stanishevsky, A.; Witonska, I. Influence of modification of supported palladium systems by polymers: PVP, AMPS and AcrAMPS on their catalytic properties in the reaction of transformation of biomass into fuel bio-components. Fuel 2020, 271, 117584. [Google Scholar] [CrossRef]
- Antonetti, C.; Toniolo, L.; Cavinato, G.; Forte, C.; Ghignoli, C.; Ishak, R.; Cavani, F.; Raspolli Galletti, A.M. A hybrid polyketone–SiO2 support for palladium catalysts and their applications in cinnamaldehyde hydrogenation and in 1-phenylethanol oxidation. Appl. Catal. A Gen. 2015, 496, 40–50. [Google Scholar] [CrossRef]
- Auyezkhanova, A.S.; Zharmagambetova, A.K.; Talgatov, E.T.; Jumekeyeva, A.I.; Akhmetova, S.N.; Myltykbayeva, Z.K.; Kalkan, I.; Koca, A.; Naizabayev, A.A.; Zamanbekova, A.T. Chitosan-Modified SBA-15 as a Support for Transition Metal Catalysts in Cyclohexane Oxidation and Photocatalytic Hydrogen Evolution. Catalysts 2025, 15, 650. [Google Scholar] [CrossRef]
- Talgatov, E.; Auyezkhanova, A.; Zharmagambetova, A.; Tastanova, L.; Bukharbayeva, F.; Jumekeyeva, A.; Aubakirov, T. The Effect of Polymer Matrix on the Catalytic Properties of Supported Palladium Catalysts in the Hydrogenation of Alkynols. Catalysts 2023, 13, 741. [Google Scholar] [CrossRef]
- Lu, Q.; Wei, X.; Zhang, Q.; Zhang, X.; Chen, L.; Liu, J.; Chen, Y.; Ma, L. Efficient selective hydrogenation of terminal alkynes over Pd–Ni nanoclusters encapsulated inside S-1 zeolite. Micropor. Mesopor. Mat. 2024, 365, 112883. [Google Scholar] [CrossRef]
- Lv, J.; Wang, D.; Guo, X.; Ding, W.; Yang, W. Selective hydrogenation of phenylacetylene over high-energy facets exposed nanotubular alumina supported palladium catalysts. Catal. Commun. 2023, 181, 106715. [Google Scholar] [CrossRef]
- Bonrath, W.; Medlock, J.; Schutz, J.; Wustenberg, B.; Netscher, T. Hydrogenation in the Vitamins and Fine Chemicals Industry—An Overview. In Hydrogenation; Karamé, I., Ed.; IntechOpen: London, UK, 2012; pp. 17–47. [Google Scholar] [CrossRef]
- Pietrantonio, K.; Coccia, F.; Tonucci, L.; d’Alessandro, N.; Bressan, M. Hydrogenation of allyl alcohols catalyzed by aqueous palladium and platinum nanoparticles. RSC Adv. 2015, 5, 68493–68499. [Google Scholar] [CrossRef]
- Behnami, A.; Croué, J.-P.; Aghayani, E.; Pourakbar, M. A catalytic ozonation process using MgO/persulfate for degradation of cyanide in industrial wastewater: Mechanistic interpretation, kinetics and by-products. RSC Adv. 2021, 11, 36965–36977. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Velavan, R.; Batoo, K.M.; Raslan, E.H. Microstructure, optical and photocatalytic properties of MgO nanoparticles. Results Phys. 2020, 16, 103013. [Google Scholar] [CrossRef]
- Martínez, I.; Santillán, R.; Fuentes Camargo, I.; Rodríguez, J.L.; Andraca Adame, J.A.; Martínez Gutiérrez, H. Synthesis and Characterization of Poly(2-vinylpyridine) and Poly(4-vinylpyridine) with Metal Oxide (TiO2, ZnO) Films for the Photocatalytic Degradation of Methyl Orange and Benzoic Acid. Polymers 2022, 14, 4666. [Google Scholar] [CrossRef]
- Wu, L.; Pang, T.; Wu, L.; Guan, Y.; Yin, L. In situ synthesis of the Fe3O4@poly(4-vinylpyridine)-block-polystyrene magnetic polymer nanocomposites via dispersion RAFT polymerization. Nanocomposites 2022, 8, 227–237. [Google Scholar] [CrossRef]
- Elías, V.R.; Ferrero, G.O.; Oliveira, R.G.; Eimer, G.A. Improved stability in SBA-15 mesoporous materials as catalysts for photo-degradation processes. Micropor. Mesopor. Mat. 2016, 236, 218–227. [Google Scholar] [CrossRef]
- Harry Krishna Charan, P.; Ranga Rao, G. Investigation of chromium oxide clusters grafted on SBA-15 using Cr-polycation sol. J. Porous Mater. 2012, 20, 81–94. [Google Scholar] [CrossRef]
- Conesa, J.M.; Morales, M.V.; Rodriguez-Ramos, I.; Rocha, M. CuPd Bimetallic Nanoparticles Supported on Magnesium Oxide as an Active and Stable Catalyst for the Reduction of 4-Nitrophenol to 4-Aminophenol. Int. J. Green Technol. 2018, 3, 51–62. [Google Scholar] [CrossRef]
- Morad, M.; Nowicka, E.; Douthwaite, M.; Iqbal, S.; Miedziak, P.; Edwards, J.K.; Brett, G.L.; He, Q.; Knight, D.W.; Morgan, D.J.; et al. Multifunctional supported bimetallic catalysts for a cascade reaction with hydrogen auto transfer: Synthesis of 4-phenylbutan-2-ones from 4-methoxybenzyl alcohols. Catal. Sci. Technol. 2017, 7, 1928–1936. [Google Scholar] [CrossRef]
- Major, G.H.; Fairley, N.; Sherwood, P.M.A.; Linford, M.R.; Terry, J.; Fernandez, V.; Artyushkova, K. Practical guide for curve fitting in x-ray photoelectron spectroscopy. J. Vac. Sci. Technol. 2020, 38, 061203. [Google Scholar] [CrossRef]
- Han, S.; Liu, Y.; Li, J.; Li, R.; Yuan, F.; Zhu, Y. Improvement Effect of Ni to Pd-Ni/SBA-15 Catalyst for Selective Hydrogenation of Cinnamaldehyde to Hydrocinnamaldehyde. Catalysts 2018, 8, 200. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, P.; Tan, W.; Jiang, F.; Tang, R. Palladium supported on amino functionalized magnetic MCM-41 for catalytic hydrogenation of aqueous bromate. RSC Adv. 2014, 4, 38743–38749. [Google Scholar] [CrossRef]
- Yang, J.; Yang, L.; Zhang, L.; Yu, T.; Zhai, D.; Wang, H.; Zhou, W.; Li, Y.; Ren, G.; Sun, L.; et al. Hydrogenation Reactions with Synergistic Catalysis of Pd single atoms and nanoparticles under Near-Ambient Conditions. Chem. Eur. J. 2022, 29, e202203108. [Google Scholar] [CrossRef]
- Talgatov, E.; Naizabayev, A.; Kenzheyeva, A.; Myltykbayeva, Z.; Koca, A.; Bukharbayeva, F.; Akhmetova, S.; Yersaiyn, R.; Auyezkhanova, A. Investigation of the Performances of TiO2 and Pd@TiO2 in Photocatalytic Hydrogen Evolution and Hydrogenation of Acetylenic Compounds for Application in Photocatalytic Transfer Hydrogenation. Catalysts 2024, 14, 665. [Google Scholar] [CrossRef]
- Zharmagambetova, A.K.; Talgatov, E.T.; Auyezkhanova, A.S.; Tumabayev, N.Z.; Bukharbayeva, F.U. Effect of polyvinylpyrrolidone on the catalytic properties of Pd/γ-Fe2O3 in phenylacetylene hydrogenation. Reac. Kinet. Mech. Cat. 2020, 131, 153–166. [Google Scholar] [CrossRef]
- Montsch, T.; Heuchel, M.; Traa, Y.; Klemm, E.; Stubenrauch, C. Selective hydrogenation of 3-Hexyn-1-ol with Pd nanoparticles synthesized via microemulsions. Appl. Catal. A Gen. 2017, 539, 19–28. [Google Scholar] [CrossRef]
- Liguori, F.; Barbaro, P.; Giordano, C.; Sawa, H. Partial hydrogenation reactions over Pd-containing hybrid inorganic/polymeric catalytic membranes. Appl. Catal. A Gen. 2013, 459, 81–88. [Google Scholar] [CrossRef]
- Shakirov, I.I.; Boronoev, M.P.; Sinikova, N.A.; Karakhanov, E.A.; Maksimov, A.L. Selective Hydrogenation of Phenylacetylene on a Pd-Containing Catalyst Based on a Polymer Layered Substrate. Russ. J. Appl. Chem. 2020, 93, 258–267. [Google Scholar] [CrossRef]
- Zhao, L.; Qin, X.; Zhang, X.; Cai, X.; Huang, F.; Jia, Z.; Diao, J.; Xiao, D.; Jiang, Z.; Lu, R.; et al. A Magnetically Separable Pd Single-Atom Catalyst for Efficient Selective Hydrogenation of Phenylacetylene. Adv. Mater. 2022, 34, 2110455. [Google Scholar] [CrossRef]
- Zharmagambetova, A.K.; Talgatov, E.T.; Auyezkhanova, A.S.; Tumabayev, N.Z.; Bukharbayeva, F.U. Behavior of Pd-supported catalysts in phenylacetylene hydrogenation: Effect of combined use of polyvinylpyrrolidone and NaOH for magnetic support modification. Polym. Adv. Technol. 2021, 32, 2735–2743. [Google Scholar] [CrossRef]
- Yang, L.; Jin, Y.; Fang, X.; Cheng, Z.; Zhou, Z. Magnetically Recyclable Core–Shell Structured Pd-Based Catalysts for Semihydrogenation of Phenylacetylene. Ind. Eng. Chem. Res. 2017, 56, 14182–14191. [Google Scholar] [CrossRef]
- Zharmagambetova, A.K.; Talgatov, E.T.; Auyezkhanova, A.S.; Bukharbayeva, F.U.; Jumekeyeva, A.I. Polysaccharide-Stabilized PdAg Nanocatalysts for Hydrogenation of 2-Hexyn-1-ol. Catalysts 2023, 13, 1403. [Google Scholar] [CrossRef]
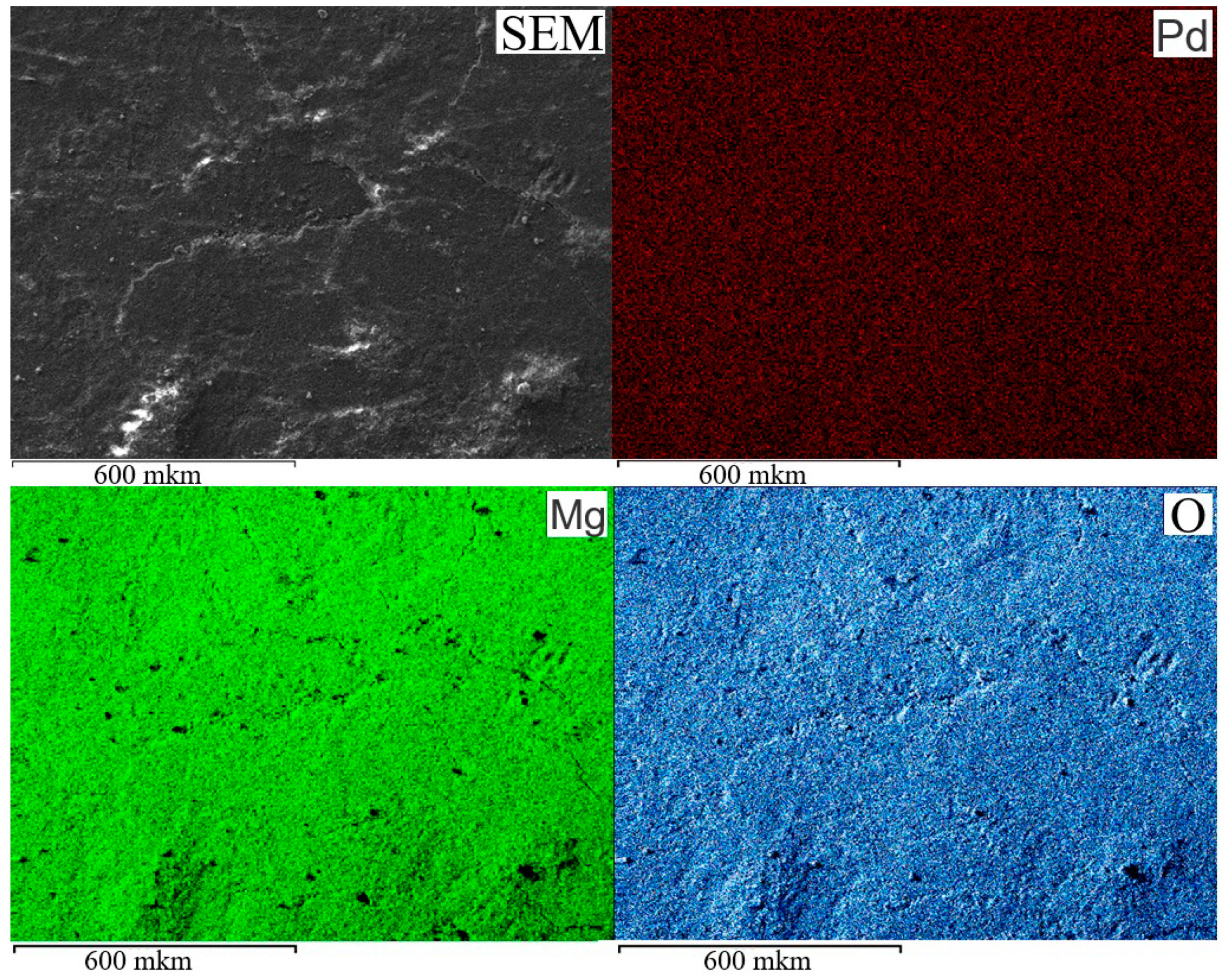


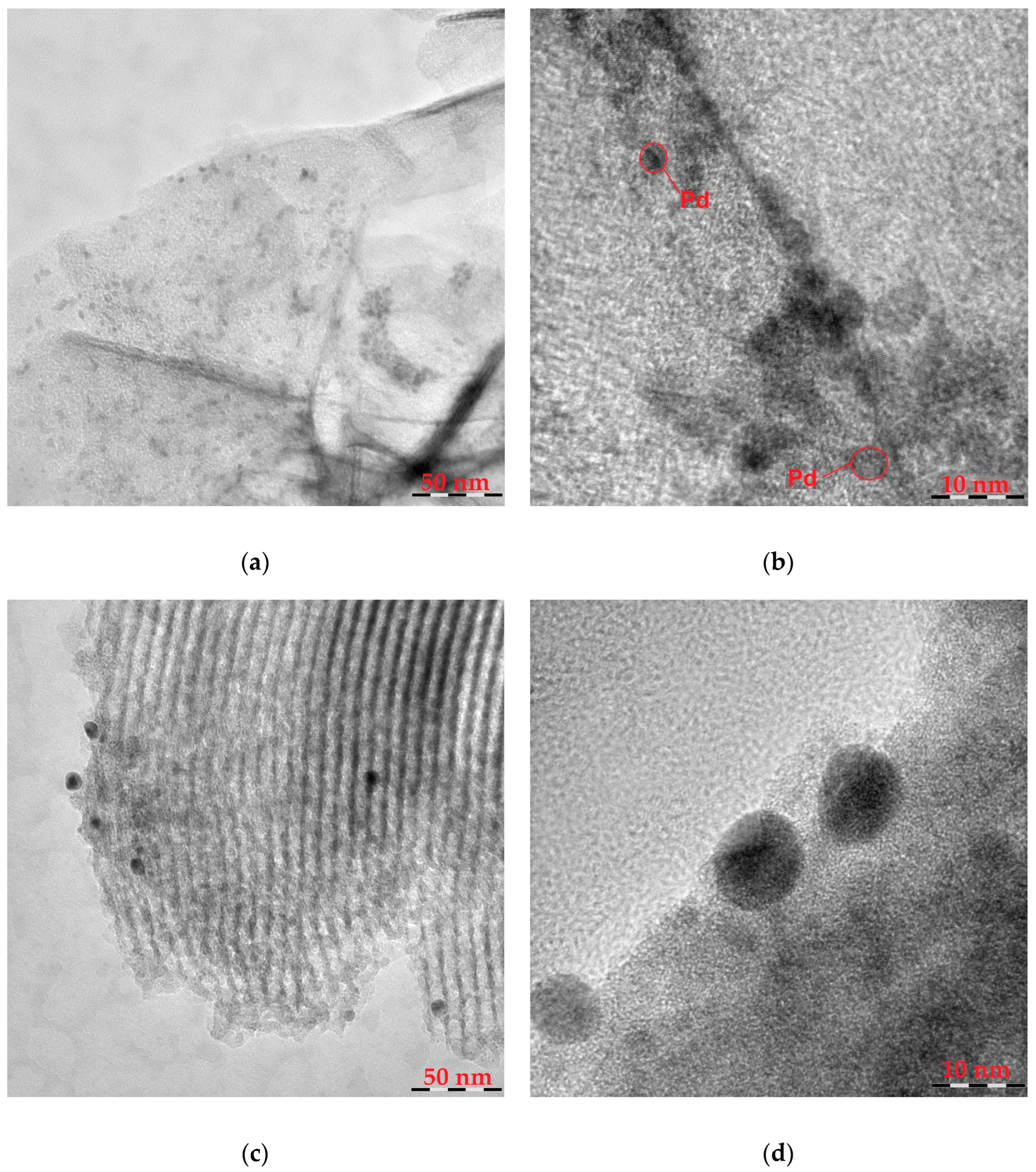
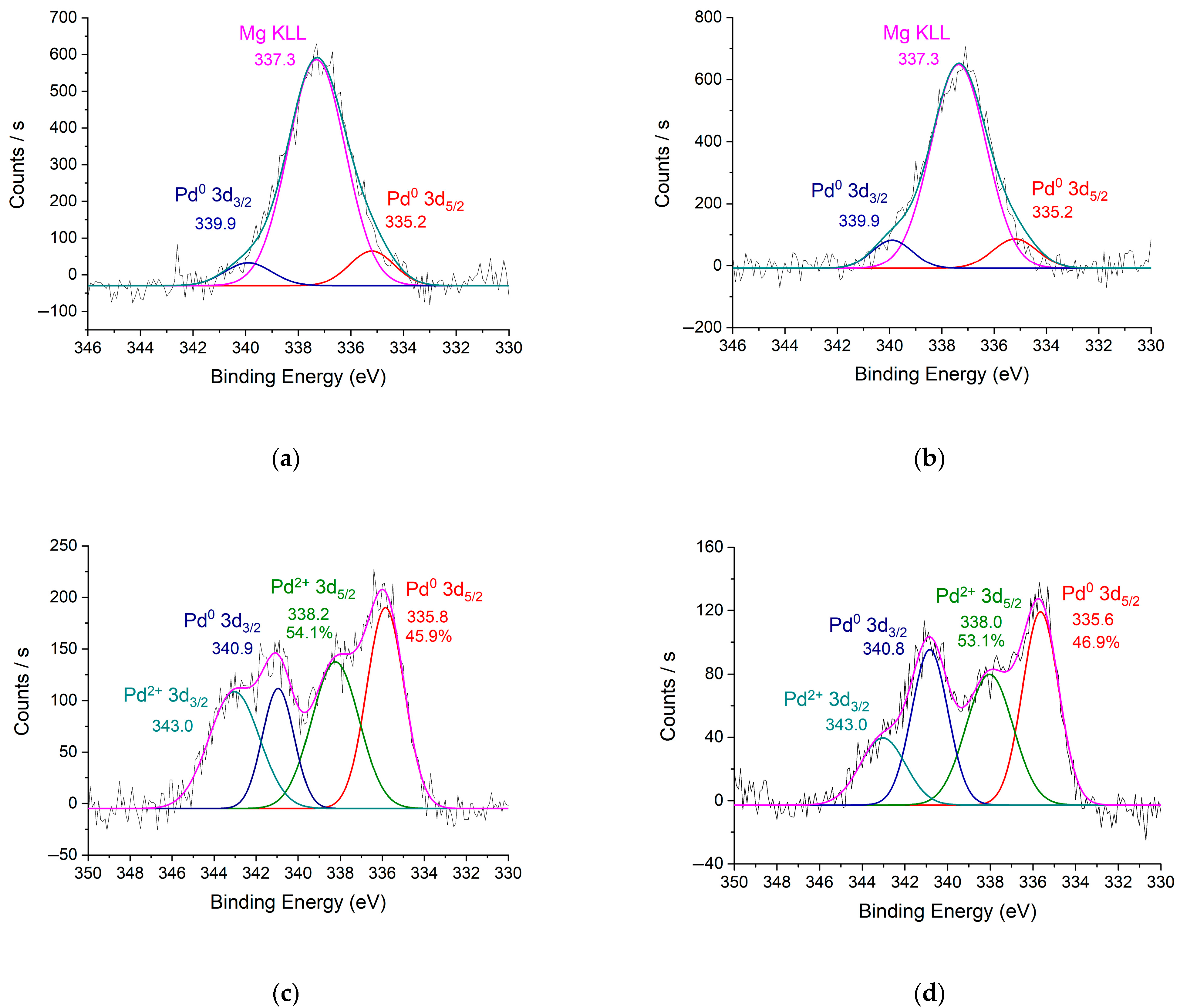
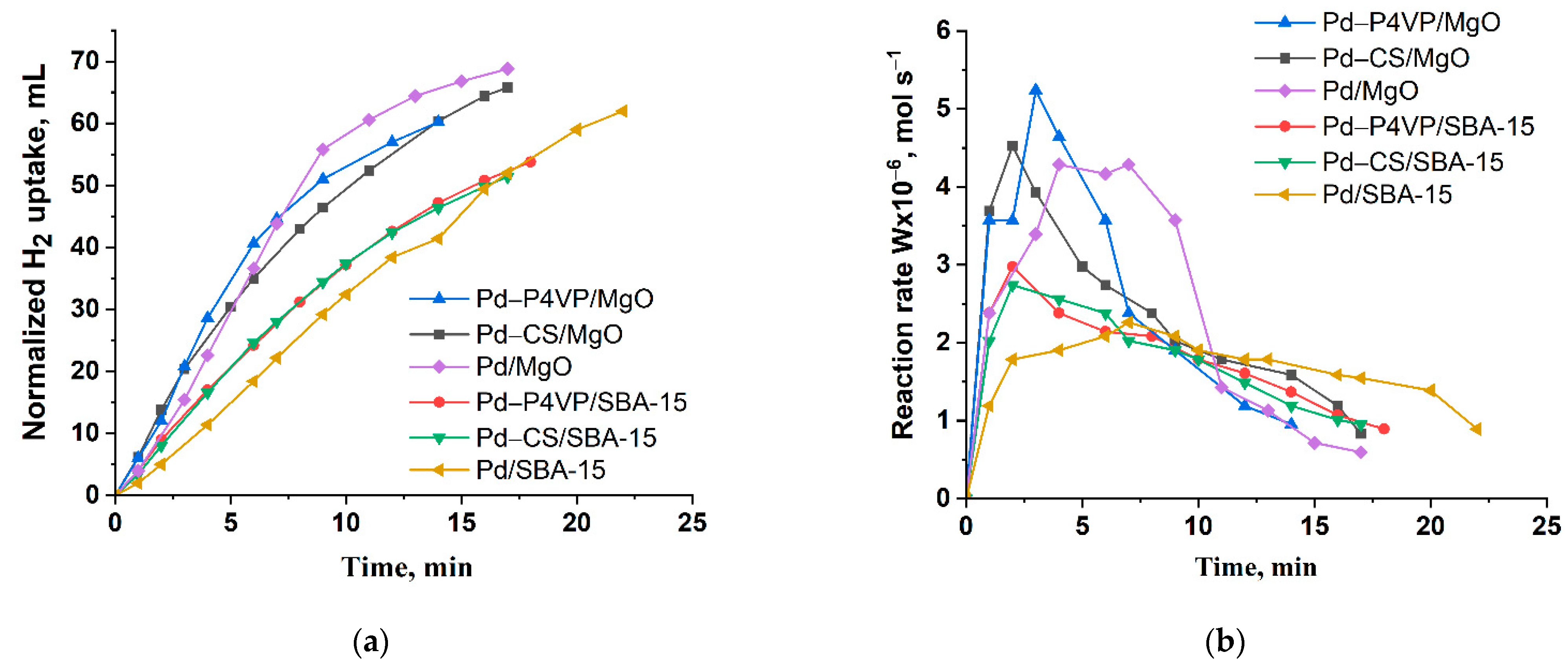


| Catalyst | m(Pd) Before Adsorption, Mg | m(Pd) After Adsorption, Mg | Ads. Degree, % | Pd Content, % | |
|---|---|---|---|---|---|
| PEC | EDX | ||||
| 1%Pd–P4VP/MgO | 10.1 | 0.1 | 99 | 1.0 | 1.0 |
| 1%Pd–P4VP/MgO (after 30 cycles) | - | - | - | 1.0 | |
| 1%Pd–CS/MgO | 0.1 | 99 | 1.0 | 1.0 | |
| 1%Pd–P4VP/SBA-15 | 0.4 | 96 | 1.0 | n.a.* | |
| 1%Pd–CS/SBA-15 | 0.5 | 95 | 1.0 | n.a.* | |
| 1%Pd/MgO | 0.5 | 95 | 1.0 | 1.0 | |
| 1%Pd/SBA-15 | 0.5 | 95 | 1.0 | 1.0 | |
| Catalyst | Wmax·10−6, mol s−1 | Selectivity, % | Conversion, % | |
|---|---|---|---|---|
| Propanal | Propanol | |||
| 1%Pd–P4VP/MgO | 5.2 | 16.6 | 83.4 | 100 |
| 1%Pd–CS/MgO | 4.5 | 17.8 | 82.2 | 100 |
| 1%Pd/MgO | 4.2 | 19.2 | 80.2 | 100 |
| 1%Pd–P4VP/SBA-15 | 3.0 | 22.6 | 77.4 | 100 |
| 1%Pd–CS/SBA-15 | 2.7 | 22.0 | 78.0 | 100 |
| 1%Pd/SBA-15 | 2.2 | 14.0 | 71.0 | 100 |
| Catalyst | Wmax·10−6, mol s−1 | Selectivity to Styrene, % | Conversion, % | |
|---|---|---|---|---|
| C≡C | C=C | |||
| 1%Pd–P4VP/MgO | 3.0 | 3.6 | 94 | 77 |
| 1%Pd–CS/MgO | 2.5 | 2.7 | 93 | 93 |
| 1%Pd/MgO | 1.9 | 2.1 | 91 | 88 |
| 1%Pd–P4VP/SBA-15 | 2.4 | 2.7 | 95 | 88 |
| 1%Pd–CS/SBA-15 | 1.3 | 1.8 | 94 | 87 |
| 1%Pd/SBA-15 | 1.1 | 1.2 | 89 | 87 |
| Catalyst | W·10−6, mol s−1 | Selectivity to cis-2-hexen-1-ol, % | Conversion, % | |
|---|---|---|---|---|
| C≡C | C=C | |||
| 1%Pd–P4VP/MgO | 3.7 | 6.4 | 97 | 81 |
| 1%Pd–CS/MgO | 2.7 | 3.7 | 97 | 78 |
| 1%Pd/MgO | 2.1 | 2.3 | 91 | 80 |
| 1%Pd–P4VP/SBA-15 | 3.7 | 4.6 | 97 | 86 |
| 1%Pd–CS/SBA-15 | 2.5 | 2.7 | 96 | 88 |
| 1%Pd/SBA-15 | 1.9 | 2.1 | 90 | 89 |
| Catalyst | Substrate | T, °C | Pressure, MPa | TOF, s−1 | Selectivity, % | Ref. |
|---|---|---|---|---|---|---|
| 1%Pd–P4VP/MgO | 2-hexyn-1-ol | 40 | 0.1 | 0.7 | 97 | This study |
| Pd@TiO2CA | 2-hexyn-1-ol | 40 | 0.1 | 1.2 | 96 | [40] |
| Pd/FDU-12(4.5)_CAB_0.02 | 3-hexyn-1-ol | 35 | 0.3 | - | 95 | [41] |
| Pd@NKZPD | 3-hexyn-1-ol | r.t.* | 1.0 | 0.4 | 55 | [42] |
| 1%Pd–P4VP/MgO | phenylacetylene | 40 | 0.1 | 0.6 | 94 | This study |
| HHTPTA-Pd | phenylacetylene | 40 | 0.1 | - | 97 | [43] |
| Pd/Ni@G | phenylacetylene | 30 | 0.2 | 2.0 | 93 | [44] |
| Pd-PVP/MNPs | phenylacetylene | 40 | 0.1 | 0.9 | 94 | [45] |
| Fe3O4@ZIF-8/Pd | phenylacetylene | 40 | 0.1 | 0.5 | 93 | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auyezkhanova, A.S.; Talgatov, E.T.; Akhmetova, S.N.; Jumekeyeva, A.I.; Naizabayev, A.A.; Zamanbekova, A.T.; Malgazhdarova, M.K. Effect of Support and Polymer Modifier on the Catalytic Performance of Supported Palladium Catalysts in Hydrogenation. Molecules 2025, 30, 3820. https://doi.org/10.3390/molecules30183820
Auyezkhanova AS, Talgatov ET, Akhmetova SN, Jumekeyeva AI, Naizabayev AA, Zamanbekova AT, Malgazhdarova MK. Effect of Support and Polymer Modifier on the Catalytic Performance of Supported Palladium Catalysts in Hydrogenation. Molecules. 2025; 30(18):3820. https://doi.org/10.3390/molecules30183820
Chicago/Turabian StyleAuyezkhanova, Assemgul S., Eldar T. Talgatov, Sandugash N. Akhmetova, Aigul I. Jumekeyeva, Akzhol A. Naizabayev, Aigul T. Zamanbekova, and Makpal K. Malgazhdarova. 2025. "Effect of Support and Polymer Modifier on the Catalytic Performance of Supported Palladium Catalysts in Hydrogenation" Molecules 30, no. 18: 3820. https://doi.org/10.3390/molecules30183820
APA StyleAuyezkhanova, A. S., Talgatov, E. T., Akhmetova, S. N., Jumekeyeva, A. I., Naizabayev, A. A., Zamanbekova, A. T., & Malgazhdarova, M. K. (2025). Effect of Support and Polymer Modifier on the Catalytic Performance of Supported Palladium Catalysts in Hydrogenation. Molecules, 30(18), 3820. https://doi.org/10.3390/molecules30183820






