The Efficacy of Melatonergic Receptor Agonists Used in Clinical Practice in Insomnia Treatment: Melatonin, Tasimelteon, Ramelteon, Agomelatine, and Selected Herbs
Abstract
1. Introduction
2. Methodology
3. Melatonergic Receptor Neurophysiology
3.1. Characterization of MT1 (Initiation) and MT2 (Diurnal Phase) Receptors
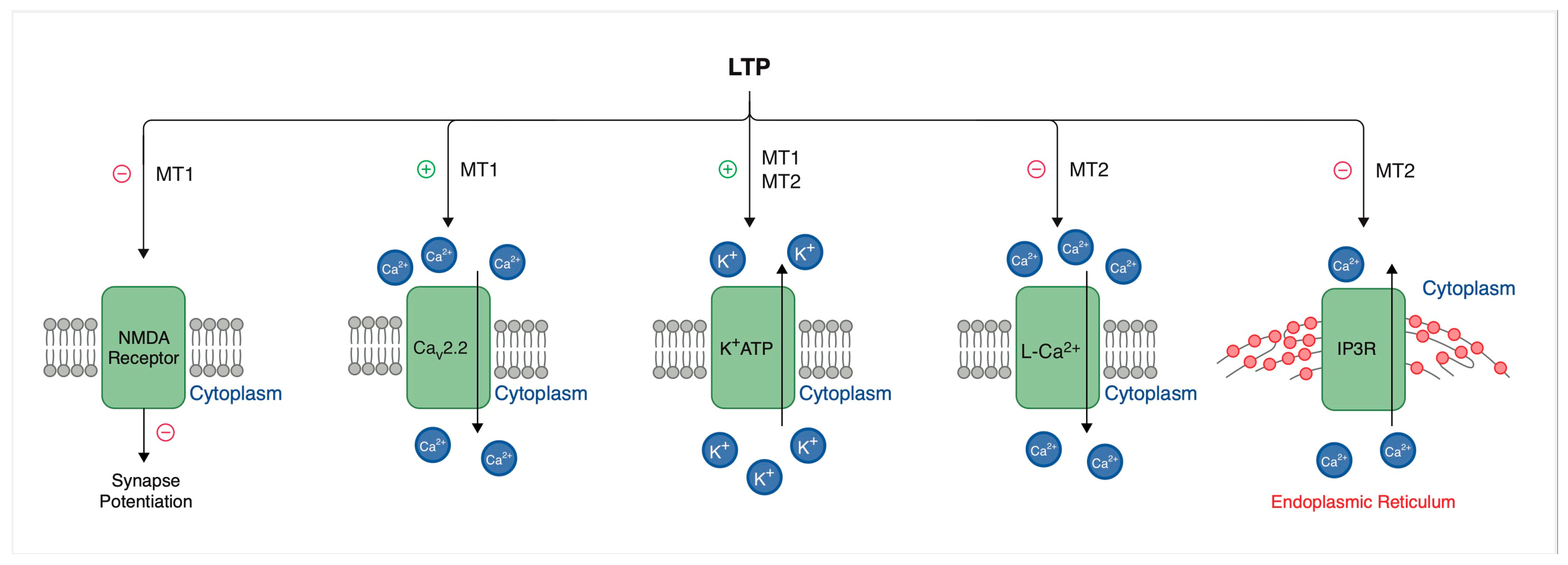
3.2. Long-Term Potentiation Variability
3.3. Intracellular Pathways, Modulation of Neuronal Activity of Suprachiasmatic Nucleus and Other CNS Areas (Hypothalamus, Cortex, Hippocampus, N. accumbens)—Effect of Modulation on a Diurnal Cycle
3.3.1. MT1 Receptor Signaling Pathway
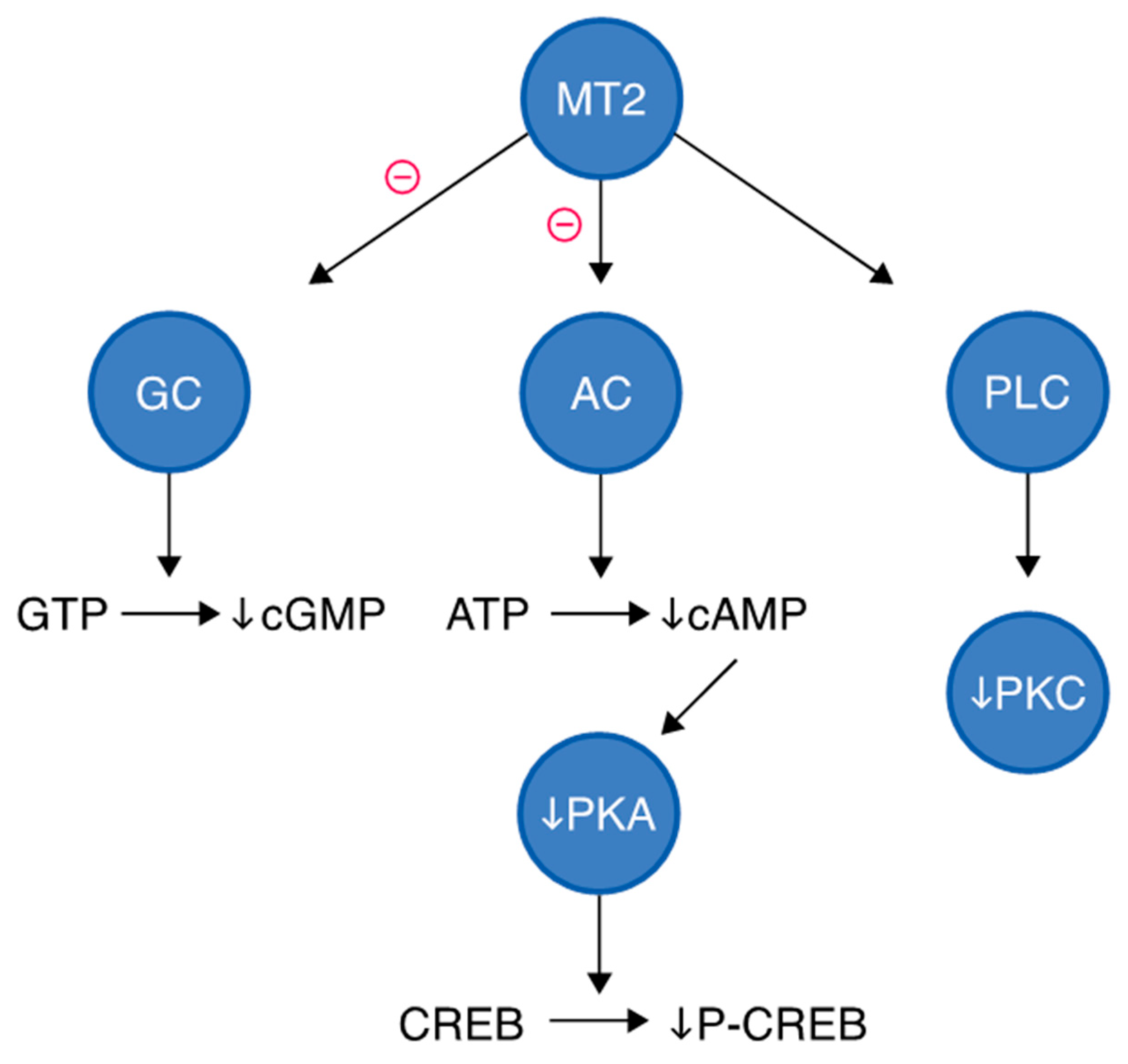
3.3.2. MT2 Receptor Signaling Pathway
3.3.3. Modulation of Neuronal Activity of SCN
3.4. Interaction with Serotonergic, Dopaminergic and GABAergic, Orexinergic Systems
3.4.1. Serotonergic System
3.4.2. GABAergic System
3.4.3. Dopaminergic System
3.4.4. Orexinergic System
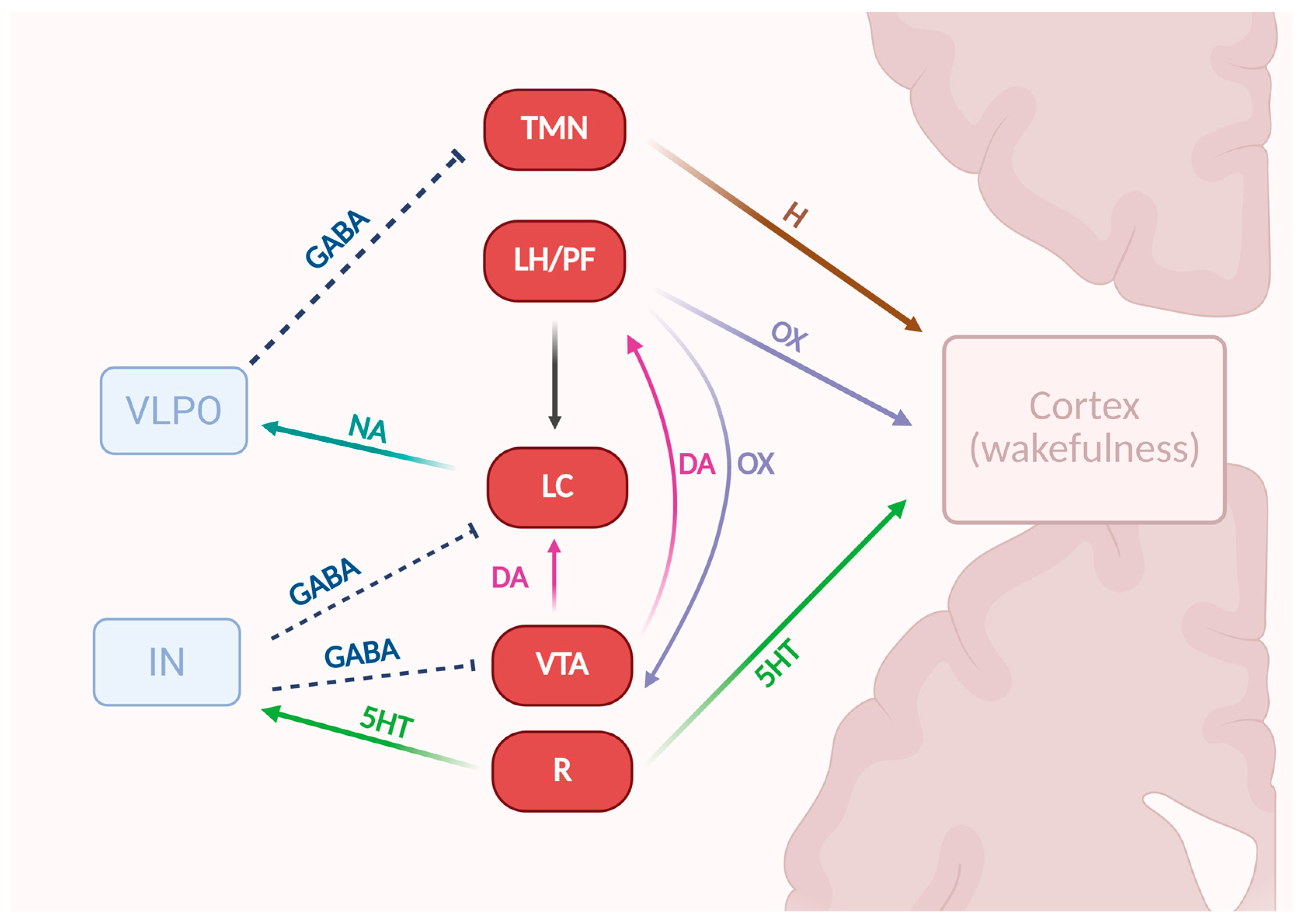
3.4.5. Arousal-Controlling Neuronal Network
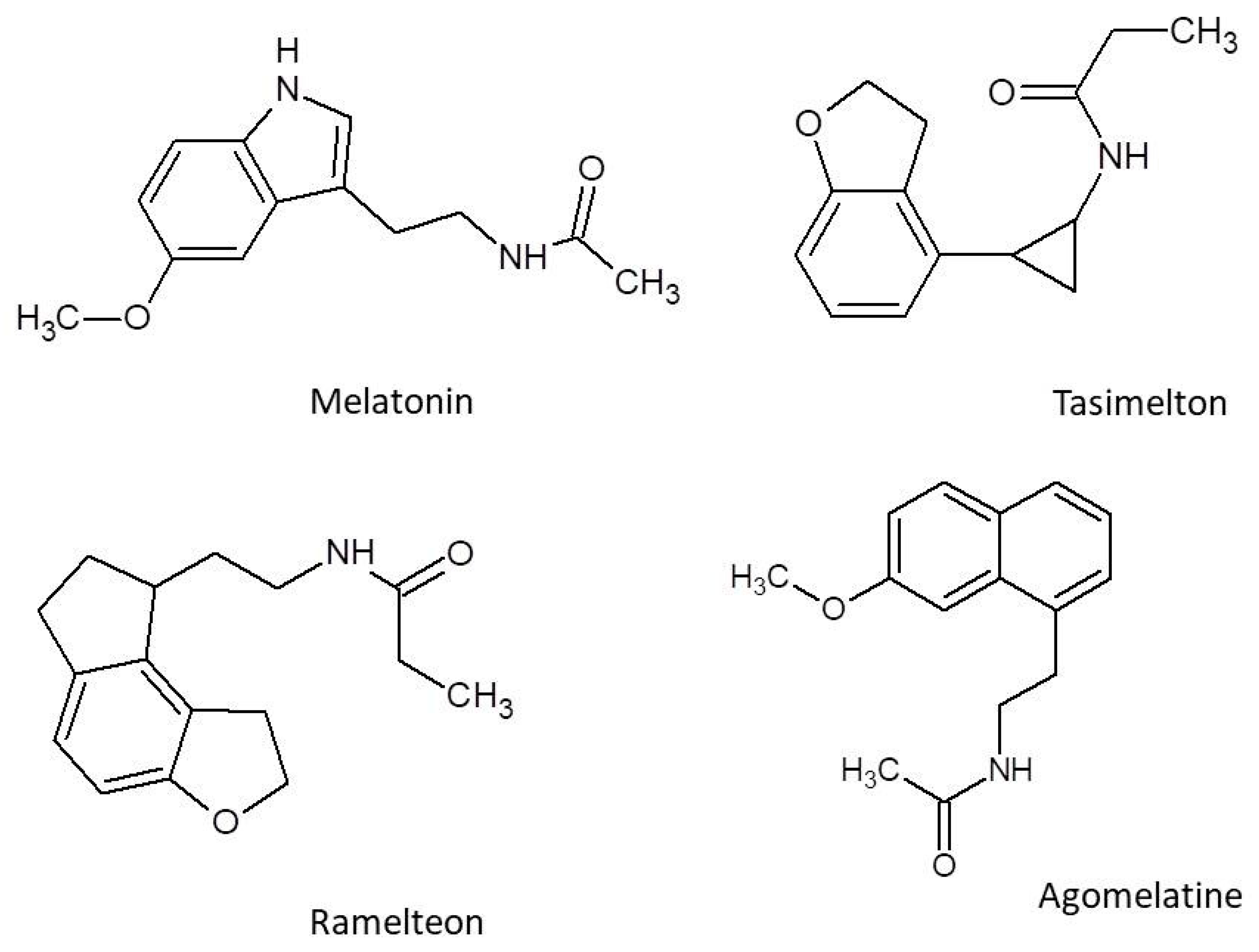
4. Characteristic of Melatonergic Receptor Agonists
4.1. Melatonin
4.1.1. Characteristics of Melatonin
4.1.2. Pharmacokinetics of Melatonin
4.1.3. Efficacy of Melatonin in Sleep-Related Clinical Trials
4.2. Tasimelteon
4.2.1. Characteristics of Tasimelteon
4.2.2. Pharmacokinetics of Tasimelteon
4.2.3. Clinical Efficacy
Non-24-Hour Sleep–Wake Disorder
Jet Lag and Insomnia After Sleep-Time Shift
Safety and Tolerance of Tasimelteon
4.3. Ramelteon
4.3.1. Characteristics of Ramelteon
4.3.2. Pharmacokinetics of Ramelteon
4.3.3. Safety and Tolerance of Ramelteon
4.4. Agomelatine
4.4.1. Characteristics of Agomelatine
4.4.2. Pharmacokinetics of Agomelatine
4.4.3. Safety and Tolerance of Agomelatine
5. Herbal Medicines
5.1. Matricaria chamomilla L. (Chamomile); Asteraceae
5.2. Melissa officinalis L. (Lemon Balm)
5.3. Nigella sativa L. (Black Cumin)
5.4. Valerian (Valeriana Officinalis)
5.5. Passiflora incarnata L. (Passionflower)
5.6. Lavandula Angustifolia Mill. (Lavender)
5.7. Melatonin-Containing Plants (MCP)
5.8. Comparison of Efficacy and Cost: Melatonergic Drugs vs. Herbal Preparations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICSD-3-TR | The International Classification of Sleep Disorders, Third Edition |
| ICD-11 | 11th Revision of the International Classification of Diseases |
| DSM-5 | 5th edition of the Diagnostic and Statistical Manual of Mental Disorders |
| SCN | Suprachiasmatic nucleus |
| LTP | Long-term potentiation |
| cAMP | Cyclic adenosine monophosphate |
| AC | Adenylate cyclase |
| PKA | Protein kinase A |
| P-CREB | Phosphorylated CREB |
| PKC | Protein kinase C |
| GC | Guanylyl cyclase |
| PLC | Phospholipase C |
| cGMP | Cyclic GMP |
| DAG | Diacylglycerol |
| IP3 | Inositol trisphosphate |
| PVN | Paraventricular nucleus |
| VTA | Ventral tegmental area |
| OX1R/OX2R | Orexin-1-receptor/Orexin-2-receptor |
| GIRK | G protein–regulated inward rectifier |
| NMDA | N-methyl-D-aspartate |
| DMH | Hypothalamus |
| NREM | Non-rapid eye movement |
| REM | Rapid eye movement |
| VLPO | Ventrolateral preoptic nucleus |
| TMN | Tuberomammillary nucleus |
| LC | Locus coeruleus |
| LH/PF | Lateral hypothalamus/Perifornical area |
| GPCR | G protein-coupled receptor |
| NQO2 | Quinone reductase 2 |
| TST | Total sleep time |
| sTST | Subjective total sleep time |
| SL | Sleep latency |
| sSL | Subjective sleep latency |
| ASD | Autism spectrum disorder |
| LPS | Latency to persistent sleep |
| SE | Sleep efficiency |
| oSOL | Objective sleep onset latency |
| EMA | European Medicines Agency |
| MDD | Major depressive disorder |
| LSEQ | Leeds Sleep Evaluation Questionnaire |
| CGI | Clinical Global Impression |
| OR | Odds ratio |
| PSQI | Pittsburgh Sleep Quality Index |
| TCIM | Integrative medicines |
| WHO | World Health Organization |
| GAD | Generalized anxiety disorder |
| HRSD | Hamilton Rating Scale for Depression |
| GABA | Gamma-aminobutyric acid |
| NO | Nitric oxide |
References
- Khara, N.; Apte, A.; Vyas, Y.; Prajapati, D.; Kshatriya, R.; Patel, S. The Intricacies of Insomnia: A Comprehensive Exploration. J. Sleep Med. 2024, 21, 65–72. [Google Scholar] [CrossRef]
- American Academy of Sleep. Medicine International Classification of Sleep Disorders. Available online: https://aasm.org/clinical-resources/international-classification-sleep-disorders/ (accessed on 25 March 2025).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5-TR, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2022. [Google Scholar]
- World Health Organization. ICD-11 for Mortality and Morbidity Statistics. Available online: https://icd.who.int/browse/2025-01/mms/en#1038292737 (accessed on 25 March 2025).
- American Academy of Sleep. Medicine Insomnia Sleep Disorder. Available online: https://sleepeducation.org/sleep-disorders/insomnia/ (accessed on 25 March 2025).
- Sateia, M.J. Insomnia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Knutson, K.L.; Ryden, A.M.; Mander, B.A.; Van Cauter, E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch. Intern. Med. 2006, 166, 1768–1774. [Google Scholar] [CrossRef]
- Kasasbeh, E.; Chi, D.S.; Krishnaswamy, G. Inflammatory aspects of sleep apnea and their cardiovascular consequences. South. Med. J. 2006, 99, 58–68. [Google Scholar] [CrossRef]
- Schwartz, D.J.; Kohler, W.C.; Karatinos, G. Symptoms of depression in individuals with obstructive sleep apnea may be amenable to treatment with continuous positive airway pressure. Chest 2005, 128, 1304–1309. [Google Scholar] [CrossRef]
- Hillman, D.R.; Murphy, A.S.; Antic, R.; Pezzullo, L. The economic cost of sleep disorders. Sleep 2006, 29, 299–305. [Google Scholar] [CrossRef]
- Morin, C.M.; Jarrin, D.C. Epidemiology of Insomnia: Prevalence, Course, Risk Factors, and Public Health Burden. Sleep Med. Clin. 2022, 17, 173–191. [Google Scholar] [CrossRef]
- Wickwire, E.M.; Juday, T.R.; Kelkar, M.; Heo, J.; Margiotta, C.; Frech, F.H. Economic Burden of Comorbid Insomnia in 5 Common Medical Disease Subgroups. J. Clin. Sleep Med. 2023, 19, 1293–1302. [Google Scholar] [CrossRef]
- Morphy, H.; Dunn, K.M.; Lewis, M.; Boardman, H.F.; Croft, P.R. Epidemiology of insomnia: A longitudinal study in a UK population. Sleep 2007, 30, 274–280. [Google Scholar] [CrossRef]
- Naha, S.; Sivaraman, M.; Sahota, P. Insomnia: A Current Review. Mo. Med. 2024, 121, 44–51. [Google Scholar] [PubMed]
- Ogeil, R.P.; Chakraborty, S.P.; Young, A.C.; Lubman, D.I. Clinician and Patient Barriers to the Recognition of Insomnia in Family Practice: A Narrative Summary of Reported Literature Analysed Using the Theoretical Domains Framework. BMC Fam. Pract. 2020, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Yeom, J.W.; Cho, C.-H. Herbal and Natural Supplements for Improving Sleep: A Literature Review. Psychiatry Investig. 2024, 21, 810–821. [Google Scholar] [CrossRef]
- Wachełko, O.; Szpot, P.; Tusiewicz, K.; Nowak, K.; Chłopaś-Konowałek, A.; Zawadzki, M. An Ultra-Sensitive UHPLC-QqQ-MS/MS Method for Determination of 54 Benzodiazepines (Pharmaceutical Drugs, NPS and Metabolites) and z-Drugs in Biological Samples. Talanta 2023, 251, 123816. [Google Scholar] [CrossRef]
- Schifano, F.; Chiappini, S.; Corkery, J.M.; Guirguis, A. An Insight into Z-Drug Abuse and Dependence: An Examination of Reports to the European Medicines Agency Database of Suspected Adverse Drug Reactions. Int. J. Neuropsychopharmacol. 2019, 22, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bell, J.S.; Visvanathan, R.; Hilmer, S.N.; Emery, T.; Robson, L.; Hughes, J.M.; Tan, E.C.K. The Association between Benzodiazepine Use and Sleep Quality in Residential Aged Care Facilities: A Cross-Sectional Study. BMC Geriatr. 2016, 16, 196. [Google Scholar] [CrossRef]
- Reid Finlayson, A.J.; Macoubrie, J.; Huff, C.; Foster, D.E.; Martin, P.R. Experiences with Benzodiazepine Use, Tapering, and Discontinuation: An Internet Survey. Ther. Adv. Psychopharmacol. 2022, 12, 20451253221082386. [Google Scholar] [CrossRef] [PubMed]
- Kripke, D.F. Hypnotic drug risks of mortality, infection, depression, and cancer: But lack of benefit. F1000Research 2016, 5, 918. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.J.; Page, A.T. Herbal medicine for insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 2015, 24, 1–12. [Google Scholar] [CrossRef]
- Shi, Y.; Dong, J.W.; Zhao, J.H.; Tang, L.N.; Zhang, J.J. Herbal insomnia medications that target GABAergic systems: A review of the psychopharmacological evidence. Curr. Neuropharmacol. 2014, 12, 289–302. [Google Scholar] [CrossRef]
- Rios, P.; Cardoso, R.; Morra, D.; Nincic, V.; Goodarzi, Z.; Farah, B.; Harricharan, S.; Morin, S.M.; Leech, J.; Straus, S.E.; et al. Comparative effectiveness and safety of pharmacological and non-pharmacological interventions for insomnia: An overview of reviews. Syst. Rev. 2019, 8, 281. [Google Scholar] [CrossRef]
- Zee, P.C.; Attarian, H.; Videnovic, A. Circadian Rhythm Abnormalities. Contin. Lifelong Learn. Neurol. 2013, 19, 132–147. [Google Scholar] [CrossRef]
- Reiter, R.J. Melatonin: The Chemical Expression of Darkness. Mol. Cell. Endocrinol. 1991, 79, C153–C158. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol. 2015, 56, 361–383. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.M.; Zisapel, N.; Cardinali, D.P. Physiological Effects of Melatonin: Role of Melatonin Receptors and Signal Transduction Pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef]
- Witt-Enderby, P.A.; Bennett, J.; Jarzynka, M.J.; Firestine, S.; Melan, M.A. Melatonin Receptors and Their Regulation: Biochemical and Structural Mechanisms. Life Sci. 2003, 72, 2183–2198. [Google Scholar] [CrossRef]
- Arshad, D.; Joyia, U.M.; Fatima, S.; Khalid, N.; Rishi, A.I.; Abdul Rahim, N.U.; Bukhari, S.F.; Shairwani, G.K.; Salmaan, A. The Adverse Impact of Excessive Smartphone Screen-Time on Sleep Quality among Young Adults: A Prospective Cohort. Sleep Sci. 2021, 14, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Crooke, A.; Guzman-Aranguez, A.; Mediero, A.; Alarma-Estrany, P.; Carracedo, G.; Pelaez, T.; Peral, A.; Pintor, J. Effect of Melatonin and Analogues on Corneal Wound Healing: Involvement of Mt2 Melatonin Receptor. Curr. Eye Res. 2015, 40, 56–65. [Google Scholar] [CrossRef]
- Gobbi, G.; Comai, S. Differential Function of Melatonin MT1 and MT2 Receptors in REM and NREM Sleep. Front. Endocrinol. 2019, 10, 87. [Google Scholar] [CrossRef]
- Legros, C.; Devavry, S.; Caignard, S.; Tessier, C.; Delagrange, P.; Ouvry, C.; Boutin, J.A.; Nosjean, O. Melatonin MT1 and MT2 receptors display different molecular pharmacologies only in the G-protein coupled state. Br. J. Pharmacol. 2014, 171, 186–201. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, Q.; Guo, Q.; Teng, M.; Gong, Q.; Li, X.; Du, Y.; Tao, Y. Structural basis of the ligand binding and signaling mechanism of melatonin receptors. Nat. Commun. 2022, 13, 454. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Huang, X.P.; Grandner, J.M.; Johansson, L.C.; Stauch, B.; McCorvy, J.D.; Liu, Y.; Roth, B.; Katritch, V. Structure-based discovery of potent and selective melatonin receptor agonists. eLife 2020, 9, e53779. [Google Scholar] [CrossRef]
- de Lima Menezes, G.; Sales Bezerra, K.; Oliveira, J.I.N.; Araújo, J.F.; Galvão, D.S.; da Silva, R.A.; Saivish, M.V.; Fulco, U.L. Quantum mechanics insights into melatonin and analogs binding to melatonin MT1 and MT2 receptors. Sci. Rep. 2024, 14, 10922. [Google Scholar] [CrossRef]
- Cecon, E.; Liu, L.; Jockers, R. Melatonin Receptor Structures Shed New Light on Melatonin Research. J. Pineal Res. 2019, 67, e12606. [Google Scholar] [CrossRef]
- Ikram, M.; Park, H.Y.; Ali, T.; Kim, M.O. Melatonin as a Potential Regulator of Oxidative Stress, and Neuroinflammation: Mechanisms and Implications for the Management of Brain Injury-Induced Neurodegeneration. J. Inflamm. Res. 2021, 14, 6251–6264. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Suthana, N.A.; Chaudhury, D.; Weaver, D.R.; Colwell, C.S. Melatonin Inhibits Hippocampal Long-Term Potentiation. Eur. J. Neurosci. 2005, 22, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Jilg, A.; Bechstein, P.; Saade, A.; Dick, M.; Li, T.X.; Tosini, G.; Rami, A.; Zemmar, A.; Stehle, J.H. Melatonin modulates daytime-dependent synaptic plasticity and learning efficiency. J. Pineal Res. 2019, 66, e12553. [Google Scholar] [CrossRef]
- Chang, H.-M.; Lin, H.-C.; Cheng, H.-L.; Liao, C.-K.; Tseng, T.-J.; Renn, T.-Y.; Lan, C.-T.; Chen, L.-Y. Melatonin Successfully Rescues the Hippocampal Molecular Machinery and Enhances Anti-oxidative Activity Following Early-Life Sleep Deprivation Injury. Antioxidants 2021, 10, 774. [Google Scholar] [CrossRef]
- Gumuslu, E.; Mutlu, O.; Sunnetci, D.; Ulak, G.; Celikyurt, I.K.; Cine, N.; Akar, F.; Savlı, H.; Erden, F. The Antidepressant Agomelatine Improves Memory Deterioration and Upregulates CREB and BDNF Gene Expression Levels in Unpredictable Chronic Mild Stress (UCMS)-Exposed Mice. Drug Target Insights 2014, 8, 11–21. [Google Scholar] [CrossRef]
- Lopez-Canul, M.; Palazzo, E.; Dominguez-Lopez, S.; Luongo, L.; Lacoste, B.; Comai, S.; Angeloni, D.; Fraschini, F.; Boccella, S.; Spadoni, G.; et al. Selective Melatonin MT2 Receptor Ligands Relieve Neuropathic Pain through Modulation of Brainstem Descending Antinociceptive Pathways. Pain 2015, 156, 305–317. [Google Scholar] [CrossRef]
- Emet, M.; Ozcan, H.; Ozel, L.; Yayla, M.; Halici, Z.; Hacimuftuoglu, A. A Review of Melatonin, Its Receptors and Drugs. Eurasian J. Med. 2016, 48, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kornhauser, J.M.; Zee, P.C.; Mayo, K.E.; Takahashi, J.S.; Turek, F.W. Effects of Aging on Light-Induced Phase-Shifting of Circadian Behavioral Rhythms, Fos Expression and CREB Phosphorylation in the Hamster Suprachiasmatic Nucleus. Neuroscience 1996, 70, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.R.; Capodice, C.E. Postmortem Stability of Melatonin Receptor Binding and Clock-Relevant MRNAs in Mouse Suprachiasmatic Nucleus. J. Biol. Rhythms 2001, 16, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 Melatonin Receptors in Mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Dubocovich, M.L. Melatonin Receptors: Role on Sleep and Circadian Rhythm Regulation. Sleep Med. 2007, 8, 34–42. [Google Scholar] [CrossRef]
- Ma, M.A.; Morrison, E.H. Neuroanatomy, Nucleus Suprachiasmatic. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hattar, S.; Liao, H.W.; Takao, M.; Berson, D.M.; Yau, K.W. Melanopsin-Containing Retinal Ganglion Cells: Architecture, Projections, and Intrinsic Photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef]
- Blume, C.; Garbazza, C.; Spitschan, M. Effects of Light on Human Circadian Rhythms, Sleep and Mood. Somnologie 2019, 23, 147–156. [Google Scholar] [CrossRef]
- Foulkes, N.S.; Borjigin, J.; Snyder, S.H.; Sassone-Corsi, P. Rhythmic Transcription: The Molecular Basis of Circadian Melatonin Synthesis. Trends Neurosci. 1997, 20, 487–492. [Google Scholar] [CrossRef]
- Bell, A.; Hewins, B.; Bishop, C.; Fortin, A.; Wang, J.; Creamer, J.L.; Collen, J.; Werner, J.K. Traumatic Brain Injury, Sleep, and Melatonin—Intrinsic Changes with Therapeutic Potential. Clocks Sleep 2023, 5, 177–203. [Google Scholar] [CrossRef]
- Kozaki, T.; Arata, T.; Kubokawa, A. Salivary Melatonin Concentrations in a Sitting and a Standing Position. J. Horm. 2013, 2013, 236325. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.; Mahmoud, A.; Awad, M.; et al. Role of Melatonin in Plant Tolerance to Soil Stressors: Salinity, PH and Heavy Metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.K.; Johnson, P.L.; Hay-Schmidt, A.; Mikkelsen, J.D.; Shekhar, A.; Lowry, C.A. Serotonergic Systems Associated with Arousal and Vigilance Behaviors Following Administration of Anxiogenic Drugs. Neuroscience 2005, 133, 983–997. [Google Scholar] [CrossRef]
- Graham, S.J.; Langley, R.W.; Verduzco, A.M.B.; Bradshaw, C.M.; Szabadi, E. Effects of Ketanserin and Haloperidol on Prepulse Inhibition of the Acoustic Startle (Eyeblink) Response and the N1/P2 Auditory Evoked Response in Man. J. Psychopharmacol. 2002, 16, 15–22. [Google Scholar] [CrossRef]
- Future Treatments for Depression, Anxiety, Sleep Disorders, Psychosis, and ADHD—Neurotransmitter.Net. Available online: https://www.neurotransmitter.net/newdrugs.html (accessed on 5 April 2025).
- Millan, M.J.; Gobert, A.; Lejeune, F.; Dekeyne, A.; Newman-Tancredi, A.; Pasteau, V.; Rivet, J.M.; Cussac, D. The Novel Melatonin Agonist Agomelatine (S20098) Is an Antagonist at 5-Hydroxytryptamine2C Receptors, Blockade of Which Enhances the Activity of Frontocortical Dopaminergic and Adrenergic Pathways. J. Pharmacol. Exp. Ther. 2003, 306, 954–964. [Google Scholar] [CrossRef]
- Al Hasan, M.S.; Bhuia, M.S.; Chowdhury, R.; Husain, Z.; Saifuzzaman, M.; Mia, E.; Akbor, M.S.; Yana, N.T.; Islam, M.A.; Ansari, S.A.; et al. Tangeretin Enhances Sedative Activity of Diazepam in Swiss Mice through GABAA Receptor Interaction: In Vivo and In Silico Approaches. Neuroscience 2025, 572, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Edwards, Z.; Preuss, C.V. GABA Receptor Positive Allosteric Modulators. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Li, J.; Wei, Y.; Xiang, J.; Zhang, D. Role of the Ventral Tegmental Area in General Anesthesia. Eur. J. Pharmacol. 2025, 986, 177145. [Google Scholar] [CrossRef]
- Oishi, Y.; Suzuki, Y.; Takahashi, K.; Yonezawa, T.; Kanda, T.; Takata, Y.; Cherasse, Y.; Lazarus, M. Activation of Ventral Tegmental Area Dopamine Neurons Produces Wakefulness through Dopamine D2-like Receptors in Mice. Brain Struct. Funct. 2017, 222, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Bubser, M.; Fadel, J.R.; Jackson, L.L.; Meador-Woodruff, J.H.; Jing, D.; Deutch, A.Y. Dopaminergic Regulation of Orexin Neurons. Eur. J. Neurosci. 2005, 21, 2993–3001. [Google Scholar] [CrossRef] [PubMed]
- Mignot, E.; Taheri, S.; Nishino, S. Sleeping with the Hypothalamus: Emerging Therapeutic Targets for Sleep Disorders. Nat. Neurosci. 2002, 5, 1071–1075. [Google Scholar] [CrossRef]
- Scammel, T.E.; Winrow, C.J. Orexin Receptors: Pharmacology and Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 243–266. [Google Scholar] [CrossRef]
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors That Regulate Feeding Behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef]
- Chou, T.C.; Scammell, T.E.; Gooley, J.J.; Gaus, S.E.; Saper, C.B.; Lu, J. Critical Role of Dorsomedial Hypothalamic Nucleus in a Wide Range of Behavioral Circadian Rhythms. J. Neurosci. 2003, 23, 10691–10702. [Google Scholar] [CrossRef]
- Kilduff, T.S.; Peyron, C. The Hypocretin/Orexin Ligand-Receptor System: Implications for Sleep and Sleep Disorders. Trends Neurosci. 2000, 23, 359–365. [Google Scholar] [CrossRef]
- Sutcliffe, J.G.; de Lecea, L. The Hypocretins: Setting the Arousal Threshold. Nat. Rev. Neurosci. 2002, 3, 339–348. [Google Scholar] [CrossRef]
- Pace-Schott, E.F.; Hobson, J.A. The Neurobiology of Sleep: Genetics, Cellular Physiology and Subcortical Networks. Nat. Rev. Neurosci. 2002, 3, 591–605. [Google Scholar] [CrossRef]
- Samuels, E.R.; Szabadi, E. Functional Neuroanatomy of the Noradrenergic Locus Coeruleus: Its Roles in the Regulation of Arousal and Autonomic Function Part II: Physiological and Pharmacological Manipulations and Pathological Alterations of Locus Coeruleus Activity in Humans. Curr. Neuropharmacol. 2008, 6, 254–285. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.H.; Freeman, C.; Langley, R.W.; Szabadi, E.; Bradshaw, C.M. Does Modafinil Activate the Locus Coeruleus in Man? Comparison of Modafinil and Clonidine on Arousal and Autonomic Functions in Human Volunteers. Psychopharmacology 2005, 181, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.E.; Lu, J.; Guo, T.; Saper, C.B.; Franks, N.P.; Maze, M. The A2-Adrenoceptor Agonist Dexmedetomidine Converges on an Endogenous Sleep-Promoting Pathway to Exert Its Sedative Effects. Anesthesiology 2003, 98, 428–436. [Google Scholar] [CrossRef]
- Szabadi, E. Drugs for Sleep Disorders: Mechanisms and Therapeutic Prospects. Br. J. Clin. Pharmacol. 2006, 61, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Epstein, F.H.; Brzezinski, A. Review Articles Mechanisms of Disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar]
- Dawson, D.; Encel, N. Melatonin and Sleep in Humans. J. Pineal Res. 1993, 15, 1–12. [Google Scholar] [CrossRef]
- Boutin, J.A.; Ferry, G. Is There Sufficient Evidence that the Melatonin Binding Site MT3 Is Quinone Reductase 2? J. Pharmacol. Exp. Ther. 2019, 368, 59–65. [Google Scholar] [CrossRef]
- Ng, K.Y.; Leong, M.K.; Liang, H.; Paxinos, G. Melatonin Receptors: Distribution in Mammalian Brain and Their Respective Putative Functions. Brain Struct. Funct. 2017, 222, 2921–2939. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; de Jonge, L.; Tadio, K.; Sharifi-Rad, J.; Posmyk, M.M.; Martorell, M.; Martins, N.; et al. Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans. Cells 2019, 8, 681. [Google Scholar] [CrossRef]
- Letelier, M.E.; Jara-Sandoval, J.; Molina-Berríos, A.; Faúndez, M.; Aracena-Parks, P.; Aguilera, F. Melatonin Protects the Cytochrome P450 System through a Novel Antioxidant Mechanism. Chem. Biol. Interact. 2010, 185, 208–214. [Google Scholar] [CrossRef]
- Ma, X.; Idle, J.R.; Krausz, K.W.; Gonzalez, F.J. Metabolism of Melatonin by Human Cytochromes P450. Drug Metab. Dispos. 2005, 33, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, C.; Błońska, A.; Chojnacki, J. The Effects of Melatonin on Elevated Liver Enzymes during Statin Treatment. BioMed Res. Int. 2017, 2017, 3204504. [Google Scholar] [CrossRef]
- Zhdanova, I.V.; Wurtman, R.J.; Balcioglu, A.; Kartashov, A.I.; Lynch, H.J. Endogenous Melatonin Levels and the Fate of Exogenous Melatonin: Age Effects. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1998, 53, B293–B298. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, A.; Ritschel, W.A. Pharmacokinetics of Melatonin in Human Sexual Maturation. J. Clin. Endocrinol. Metab. 1996, 81, 1882–1886. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gunn, P.J.; Middleton, B.; Davies, S.K.; Revell, V.L.; Skene, D.J. Sex Differences in the Circadian Profiles of Melatonin and Cortisol in Plasma and Urine Matrices under Constant Routine Conditions. Chronobiol. Int. 2016, 33, 39–50. [Google Scholar] [CrossRef]
- Harpsøe, N.G.; Andersen, L.P.H.; Gögenur, I.; Rosenberg, J. Clinical Pharmacokinetics of Melatonin: A Systematic Review. Eur. J. Clin. Pharmacol. 2015, 71, 901–909. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Werner, M.U.; Rosenkilde, M.M.; Harpsøe, N.G.; Fuglsang, H.; Rosenberg, J.; Gögenur, I. Pharmacokinetics of Oral and Intravenous Melatonin in Healthy Volunteers. BMC Pharmacol. Toxicol. 2016, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Pepping, J. Melatonin. Am. J. Health-Syst. Pharm. 1999, 56, 2520–2527. [Google Scholar] [CrossRef]
- Zetner, D.; Andersen, L.P.K.; Alder, R.; Jessen, M.L.; Tolstrup, A.; Rosenberg, J. Pharmacokinetics and Safety of Intravenous, Intravesical, Rectal, Transdermal, and Vaginal Melatonin in Healthy Female Volunteers: A Cross-Over Study. Pharmacology 2021, 106, 169–176. [Google Scholar] [CrossRef]
- Demuro, R.L.; Nafziger, A.N.; Blask, D.E.; Menhinick, A.M.; Bertino, J.S. The Absolute Bioavailability of Oral Melatonin. J. Clin. Pharmacol. 2000, 40, 781–784. [Google Scholar] [CrossRef]
- Gooneratne, N.S.; Edwards, A.Y.Z.; Zhou, C.; Cuellar, N.; Grandner, M.A.; Barrett, J.S. Melatonin Pharmacokinetics Following Two Different Oral Surge-Sustained Release Doses in Older Adults. J. Pineal Res. 2012, 52, 437–445. [Google Scholar] [CrossRef]
- Lemoine, P.; Wade, A.G.; Katz, A.; Nir, T.; Zisapel, N. Efficacy and Safety of Prolonged-Release Melatonin for Insomnia in Middle-Aged and Elderly Patients with Hypertension: A Combined Analysis of Controlled Clinical Trials. Integr. Blood Press. Control 2012, 5, 9–17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luthringer, R.; Muzet, M.; Zisapel, N.; Staner, L. The effect of prolonged-release melatonin on sleep measures and psychomotor performance in elderly patients with insomnia. Int. Clin. Psychopharmacol. 2009, 24, 239–249. [Google Scholar] [CrossRef]
- Wade, A.G.; Ford, I.; Crawford, G.; McConnachie, A.; Nir, T.; Laudon, M.; Zisapel, N. Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: A randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety. BMC Med. 2010, 8, 51. [Google Scholar] [CrossRef]
- Appleton, R.E.; Jones, A.P.; Gamble, C.; Williamson, P.R.; Wiggs, L.; Montgomery, P.; Sutcliffe, A.; Barker, C.; Gringras, P. The use of MElatonin in children with neurodevelopmental disorders and impaired sleep: A randomised, double-blind, placebo-controlled, parallel study (MENDS). Health Technol. Assess. 2012, 16, 1–239. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.G.; Nagtegaal, E.E.; van der Heijden, J.; Coenen, A.M.; Kerkhof, G.A. Melatonin for chronic sleep onset insomnia in children: A randomized placebo-controlled trial. J. Child Neurol. 2001, 16, 86–92. [Google Scholar] [CrossRef]
- Garfinkel, D.; Zisapel, N.; Wainstein, J.; Laudon, M. Facilitation of Benzodiazepine Discontinuation by Melatonin A New Clinical Approach. Arch. Intern. Med. 1999, 159, 2456–2460. [Google Scholar] [CrossRef]
- Yue, J.L.; Chang, X.W.; Zheng, J.W.; Shi, L.; Xiang, Y.J.; Que, J.Y.; Yuan, K.; Deng, J.H.; Teng, T.; Li, Y.Y.; et al. Efficacy and Tolerability of Pharmacological Treatments for Insomnia in Adults: A Systematic Review and Network Meta-Analysis. Sleep Med. Rev. 2023, 68, 101746. [Google Scholar] [CrossRef]
- Wang, L.; Pan, Y.; Ye, C.; Guo, L.; Luo, S.; Dai, S.; Chen, N.; Wang, E. A Network Meta-Analysis of the Long- and Short-Term Efficacy of Sleep Medicines in Adults and Older Adults. Neurosci. Biobehav. Rev. 2021, 131, 489–496. [Google Scholar] [CrossRef]
- Sugumaran, R.; Sai Krishna, K.S.; Saibaba, J.; Narayan, S.K.; Sandhiya, S.; Rajeswari, M. Melatonin on Sleep in Parkinson’s Disease: A Randomized Double Blind Placebo Controlled Trial. Sleep Med. 2024, 124, 502–509. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, M.; Park, S.; Jang, W.; Park, J.; Oh, E.; Cho, J.W.; Kim, J.S.; Youn, J. Prolonged-Release Melatonin in Parkinson’s Disease Patients with a Poor Sleep Quality: A Randomized Trial. Park. Relat. Disord. 2020, 75, 50–54. [Google Scholar] [CrossRef]
- Xiong, M.; Li, F.; Liu, Z.; Xie, X.; Shen, H.; Li, W.; Wei, L.; He, R. Efficacy of Melatonin for Insomnia in Children with Autism Spectrum Disorder: A Meta-Analysis. Neuropediatrics 2022, 54, 167–173. [Google Scholar]
- Hayashi, M.; Mishima, K.; Fukumizu, M.; Takahashi, H.; Ishikawa, Y.; Hamada, I.; Sugioka, H.; Yotsuya, O.; Yamashita, Y. Melatonin Treatment and Adequate Sleep Hygiene Interventions in Children with Autism Spectrum Disorder: A Randomized Controlled Trial. J. Autism Dev. Disord. 2022, 52, 2784–2793. [Google Scholar] [CrossRef] [PubMed]
- de Seabra, M.L.V.; Bignotto, M.; Pinto, L.R., Jr.; Tufik, S. Randomized, Double-blind Clinical Trial, Controlled with Placebo, of the Toxicology of Chronic Melatonin Treatment. J. Pineal Res. 2000, 29, 193–200. [Google Scholar] [CrossRef]
- Buscemi, N.; Vandermeer, B.; Hooton, N.; Pandya, R.; Tjosvold, L.; Hartling, L.; Baker, G.; Klassen, T.P.; Vohra, S. The Efficacy and Safety of Exogenous Melatonin for Primary Sleep Disorders: A Meta-Analysis. J. Gen. Intern. Med. 2005, 20, 1151–1158. [Google Scholar] [CrossRef]
- Boutin, J.A.; Kennaway, D.J.; Jockers, R. Melatonin: Facts, Extrapolations and Clinical Trials. Biomolecules 2023, 13, 943. [Google Scholar] [CrossRef]
- Bishop-Freeman, S.C.; Young, K.A.; Labay, L.M.; Beuhler, M.C.; Hudson, J.S. Melatonin Supplementation in Undetermined Pediatric Deaths. J. Anal. Toxicol. 2022, 46, 808–816. [Google Scholar] [CrossRef]
- Kim, H.K.; Yang, K.I. Melatonin and Melatonergic Drugs in Sleep Disorders. Transl. Clin. Pharmacol. 2022, 30, 163–171. [Google Scholar] [CrossRef]
- Nishimon, S.; Nishino, N.; Nishino, S. Advances in the Pharmacological Management of Non-24-h Sleep-Wake Disorder. Expert Opin. Pharmacother. 2021, 22, 1039–1049. [Google Scholar] [CrossRef]
- European Medicines Agency. Hetlioz—EPAR Product Information. 2022. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/hetlioz (accessed on 29 August 2025).
- Zlotos, D.P.; Jockers, R.; Cecon, E.; Rivara, S.; Witt-Enderby, P.A. MT1 and MT2 Melatonin Receptors: Ligands, Models, Oligomers, and Therapeutic Potential. J. Med. Chem. 2014, 57, 3161–3185. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, B.W.; Torres, R.; Dressman, M.A.; Kramer, W.G.; Baroldi, P. Clinical Assessment of Drug-Drug Interactions of Tasimelteon, a Novel Dual Melatonin Receptor Agonist. J. Clin. Pharmacol. 2015, 55, 1004–1011. [Google Scholar] [CrossRef]
- Torres, R.; Kramer, W.G.; Baroldi, P. Pharmacokinetics of the Dual Melatonin Receptor Agonist Tasimelteon in Subjects with Hepatic or Renal Impairment. J. Clin. Pharmacol. 2015, 55, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Atkin, T.; Comai, S.; Gobbi, G. Drugs for Insomnia beyond Benzodiazepines: Pharmacology, Clinical Applications, and Discovery. Pharmacol. Rev. 2018, 70, 197–245. [Google Scholar] [CrossRef] [PubMed]
- Bonacci, J.M.; Venci, J.V.; Gandhi, M.A. Tasimelteon (HetliozTM): A New Melatonin Receptor Agonist for the Treatment of Non-24-Hour Sleep-Wake Disorder. J. Pharm. Pract. 2015, 28, 473–478. [Google Scholar] [CrossRef]
- Lockley, S.W.; Dressman, M.A.; Licamele, L.; Xiao, C.; Fisher, D.M.; Flynn-Evans, E.E.; Hull, J.T.; Torres, R.; Lavedan, C.; Polymeropoulos, M.H. Tasimelteon for Non-24-Hour Sleep-Wake Disorder in Totally Blind People (SET and RESET): Two Multicentre, Randomised, Double-Masked, Placebo-Controlled Phase 3 Trials. Lancet 2015, 386, 1754–1764. [Google Scholar] [CrossRef]
- Connolly, P.J.; Quigg, M.; Davis, E.M. Improvement in Non-24-h Sleep-Wake Rhythm Disorder in a Sighted Individual Treated with a Melatonin Receptor Agonist. Sleep Med. 2024, 116, 41–42. [Google Scholar] [CrossRef]
- Polymeropoulos, C.M.; Mohrman, M.A.; Keefe, M.S.; Brzezynski, J.L.; Wang, J.; Prokosch, L.S.; Polymeropoulos, V.M.; Xiao, C.; Birznieks, G.; Polymeropoulos, M.H. Efficacy of Tasimelteon (HETLIOZ®) in the Treatment of Jet Lag Disorder Evaluated in an 8-h Phase Advance Model; A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Front. Neurol. 2020, 11, 611. [Google Scholar] [CrossRef]
- Rajaratnam, S.M.; Polymeropoulos, M.H.; Fisher, D.M.; Roth, T.; Scott, C.; Birznieks, G.; Klerman, E.B. Melatonin Agonist Tasimelteon (VEC-162) for Transient Insomnia after Sleep-Time Shift: Two Randomised Controlled Multicentre Trials. Lancet 2009, 373, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Torres, R.; Dressman, M.A.; Kramer, W.G.; Baroldi, P. Absolute Bioavailability of Tasimelteon. Am. J. Ther. 2015, 22, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Torres, R.; Fisher, M.; Birznieks, G.; Polymeropoulos, C.; Kay, G.G.; Xiao, C.; Polymeropoulos, M.H. Simulated Driving Performance in Healthy Adults after Night-Time Administration of 20 Mg Tasimelteon. J. Sleep Res. 2022, 31, e13430. [Google Scholar] [CrossRef]
- Zuo, T.; Sun, S.; Yang, J.; Wu, H.; Peng, W. Assessment of Adverse Events of Tasimelteon: A Real-World Pharmacovigilance Study Based on FAERS. Expert Opin. Drug Saf. 2025, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gerdin, M.J.; Masana, M.I.; Rivera-Bermúdez, M.A.; Hudson, R.L.; Earnest, D.J.; Gillette, M.U.; Dubocovich, M.L. Melatonin Desensitizes Endogenous MT 2 Melatonin Receptors in the Rat Suprachiasmatic Nucleus: Relevance for Defining the Periods of Sensitivity of the Mammalian Circadian Clock to Melatonin. FASEB J. 2004, 18, 1646–1656. [Google Scholar] [CrossRef]
- Spadoni, G.; Bedini, A.; Lucarini, S.; Mor, M.; Rivara, S. Pharmacokinetic and Pharmacodynamic Evaluation of Ramelteon: An Insomnia Therapy. Expert Opin. Drug Metab. Toxicol. 2015, 11, 1145–1156. [Google Scholar] [CrossRef]
- Levinsohn, E.A.; Radhakrishnan, V.; Euting, H.; Kaplan, G.B. Pharmacological Management of Sleep–Wake Disturbances in Delirium. J. Clin. Pharmacol. 2025, 65, 285–302. [Google Scholar] [CrossRef]
- Kato, K.; Hirai, K.; Nishiyama, K.; Uchikawa, O.; Fukatsu, K.; Ohkawa, S.; Kawamata, Y.; Hinuma, S.; Miyamoto, M. Neurochemical Properties of Ramelteon (TAK-375), a Selective MT1/MT2 Receptor Agonist. Neuropharmacology 2005, 48, 301–310. [Google Scholar] [CrossRef]
- Shimura, A.; Kanno, T.; Inoue, T. Ultra-Low-Dose Early Night Ramelteon Administration for the Treatment of Delayed Sleep-Wake Phase Disorder: Case Reports with a Pharmacological Review. J. Clin. Sleep Med. 2022, 18, 2861–2865. [Google Scholar] [CrossRef]
- Miyamoto, M. Pharmacology of Ramelteon, a Selective MT1/MT2 Receptor Agonist: A Novel Therapeutic Drug for Sleep Disorders. CNS Neurosci. Ther. 2009, 15, 32–51. [Google Scholar] [CrossRef]
- Srujitha, M.; Daniel, A.; Ashley, M.C.; Marion, K.S.; Jeannie, K.L. Use of Melatonin and/on Ramelteon for the Treatment of Insomnia in Older Adults: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 5138. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Brzezinski, A.; Pandi-Perumal, S.R.; Spence, D.W.; Cardinali, D.P.; Brown, G.M. Melatonin Agonists in Primary Insomnia and Depression-Associated Insomnia: Are They Superior to Sedative-Hypnotics? Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 913–923. [Google Scholar] [CrossRef]
- Hardeland, R.; Poeggeler, B. Melatonin and Synthetic Melatonergic Agonists: Actions and Metabolism in the Central Nervous System. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 189–216. [Google Scholar] [CrossRef]
- Kuriyama, A.; Honda, M.; Hayashino, Y. Ramelteon for the Treatment of Insomnia in Adults: A Systematic Review and Meta-Analysis. Sleep Med. 2014, 15, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Maruani, J.; Reynaud, E.; Chambe, J.; Palagini, L.; Bourgin, P.; Geoffroy, P.A. Efficacy of Melatonin and Ramelteon for the Acute and Long-Term Management of Insomnia Disorder in Adults: A Systematic Review and Meta-Analysis. J. Sleep Res. 2023, 32, e13939. [Google Scholar] [CrossRef] [PubMed]
- de Bodinat, C.; Guardiola-Lemaitre, B.; Mocaër, E.; Renard, P.; Muñoz, C.; Millan, M.J. Agomelatine, the First Melatonergic Antidepressant: Discovery, Characterization and Development. Nat. Rev. Drug Discov. 2010, 9, 628–642. [Google Scholar] [CrossRef]
- Gahr, M. Agomelatine in the Treatment of Major Depressive Disorder: An Assessment of Benefits and Risks. Curr. Neuropharmacol. 2014, 12, 387–398. [Google Scholar] [CrossRef]
- Green, B. Focus on Agomelatine. Curr. Med. Res. Opin. 2011, 27, 745–749. [Google Scholar] [CrossRef]
- Lemoine, P.; Guilleminault, C.; Alvarez, E. Improvement in Subjective Sleep in Major Depressive Disorder with a Novel Antidepressant, Agomelatine: Randomized, Double-Blind Comparison with Venlafaxine. J. Clin. Psychiatry 2007, 68, 1723–1732. [Google Scholar] [CrossRef]
- Quera-Salva, M.-A.; Hajak, G.; Philip, P.; Montplaisir, J.; Keufer-Le Gall, S.; Laredo, J.; Guilleminault, C. Comparison of Agomelatine and Escitalopram on Nighttime Sleep and Daytime Condition and Efficacy in Major Depressive Disorder Patients. Int. Clin. Psychopharmacol. 2011, 26, 252–262. [Google Scholar] [CrossRef]
- Hsing, S.C.; Jin, Y.T.; Tzeng, N.S.; Chung, C.H.; Chen, T.Y.; Chang, H.A.; Kao, Y.C.; Chien, W.C. Is Agomelatine Associated with Less Sedative-Hypnotic Usage in Patients with Major Depressive Disorder? A Nationwide, Population-Based Study. Neuropsychiatr. Dis. Treat. 2020, 16, 1727. [Google Scholar] [CrossRef]
- Grosshans, M.; Mutschler, J.; Luderer, M.; Mann, K.; Kiefer, F. Agomelatine Is Effective in Reducing Insomnia in Abstinent Alcohol-Dependent Patients. Clin. Neuropharmacol. 2014, 37, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Gorwood, P.; Benichou, J.; Moore, N.; Álvarez Martínez, E.; Mertens, J.; Aguglia, E.; Figueira, M.L.; Falkai, P.; Olivier, V.; Wattez, M.; et al. The Safety of Agomelatine in Standard Medical Practice in Depressed Patients: A 26-Week International Multicentre Cohort Study. Hum. Psychopharmacol. 2021, 36, 1–11. [Google Scholar] [CrossRef]
- Longo, L.P.; Johnson, B. Addiction: Part I. Benzodiazepines—Side effects, abuse risk and alternatives. Am. Fam. Physician 2000, 61, 2121–2128. [Google Scholar]
- Stewart, S.A. The effects of benzodiazepines on cognition. J. Clin. Psychiatry 2005, 66, 9–13. [Google Scholar] [PubMed]
- Yeung, W.-F.; Chung, K.-F.; Poon, M.M.K.; Ho, F.Y.-Y.; Zhang, S.-P.; Zhang, Z.-J.; Ziea, E.T.-C.; Wong, V.T. Chinese herbal medicine for insomnia: A systematic review of randomized controlled trials. Sleep Med. Rev. 2012, 16, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.G.; Goode, M.; Heinrich, M. Herbal medicines and botanicals for managing insomnia, stress, anxiety, and depression: A critical review of the emerging evidence focusing on the Middle East and Africa. PharmaNutrition 2024, 29, 100399. [Google Scholar] [CrossRef]
- Mihyaoui, A.E.; Esteves Da Silva, J.C.G.; Charfi, S. Chamomile (Matricaria chamomilla L.): A review of ethnomedicinal use, phytochemistry and pharmacological uses. Life 2022, 12, 479. [Google Scholar] [CrossRef]
- Amsterdam, J.D.; Li, Y.; Soeller, I.; Rockwell, K.; Mao, J.J.; Shults, J. A randomized, double-blind, placebo-controlled trial of Oral Matricaria Recutita (Chamomile) extract therapy of generalized anxiety disorder. J. Clin. Psychopharmacol. 2009, 29, 378–382. [Google Scholar] [CrossRef]
- Miraj, S.; Alesaeidi, S. A systematic review study of therapeutic effects of Matricaria Recuitta Chamomile (Chamomile). Electron. Physician 2016, 8, 3024–3031. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H. Anesthetic agents of plant origin: A review of phytochemicals with anesthetic activity. Molecules 2017, 22, 1369. [Google Scholar] [CrossRef]
- Adib-Hajbaghery, M.; Mousavi, S.N. The effects of chamomile extract o sleep quality among elderly people: A clinical trial. Complement. Ther. Med. 2017, 35, 109–114. [Google Scholar]
- Amsterdam, J.D.; Li, Q.S.; Xie, S.X. Putative antidepressant effect of Chamomile (Matricaria chamomilla L.) oral extract in subjects with comorbid generalized anxiety disorder and depression. J. Alter. Complement Med. 2020, 26, 813–819. [Google Scholar] [CrossRef]
- Chang, S.-M.; Chen, C.-H. Effects of an intervention with drinking chamomile tea on sleep quality and depression in sleep disturbed postnatal women: A randomized controlled trial, Randomized Controlled Trial. J. Adv. Nurs. 2016, 72, 306–315. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with bright future. Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [CrossRef]
- Pierro, F.; Sisti, D.; Rocchi, M.; Belli, A.; Bertuccioli, A.; Cazzaniga, M.; Palazzi, C.M.; Tanda, M.L.; Zerbinati, N. Effects of Melissa officinalis Phytosome on Sleep Quality: Results of a Prospective, Double-Blind, Placebo-Controlled, and Cross-Over Study. Nutrients 2024, 16, 4199. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, M.; Firoozabadi, A.; Salehi, A.; Ghorbanifar, Z.; Zarshenas, M.M.; Sadeghniiat-Haghighi, K.; Rezaeizadeh, H. Effects of Herbal combination (Melissa officinalis L. and Nepeta menthoides Boiss. & Buhse) on insomnia severity, anxiety and depression in insomniacs: Randomized placebo controlled trial. Integr. Med. Res. 2018, 7, 328–332. [Google Scholar] [CrossRef] [PubMed]
- McCarter, S.J.; Hagen, P.T.; St Louis, E.K.; Rieck, T.M.; Haider, C.R.; Holmes, D.R.; Morgenthaler, T.I. Physiological markers of sleep quality: A scoping review. Sleep Med. Rev. 2022, 64, 101657. [Google Scholar] [CrossRef]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L. A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef]
- Kara, M.; Sahin, S.; Rabbani, F. An in vitro analysis of an innovative standardized phospholipid carrier-based Melissa officinalis L. Extract as a potential neuromodulator for emotional distress and related conditions. Front. Mol. Biosci. 2024, 11, 1359177. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M. Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double blind, randomised, placebo controlled trial. J. Neurol. Neurosurg. Psychiatry 2003, 74, 863. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Little, W.; Scholey, A.B. Attenuation of laboratory-induced stress in humans after acute administration of Melissa officinalis (Lemon Balm). Psychosom. Med. 2004, 66, 607–613. [Google Scholar] [CrossRef]
- Scholey, A.; Gibbs, A.; Neale, C. Anti-stress effects of lemon balm-containing foods. Nutrients 2014, 6, 4805–4821. [Google Scholar] [CrossRef] [PubMed]
- Petrisor, G.; Motelica, L.; Craciun, L.N.; Oprea, O.C.; Ficai, D.; Ficai, A. Melissa officinalis: Composition, Pharmacological Effects and Derived Release Systems-A Review. Int. J. Mol. Sci. 2022, 23, 3591. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Rahman, M.A.; Sohag, A.M. Black Cumin (Nigella sativa L.): A comprehensive review on phytochemistry, health benefits, molecular pharmacology, and safety. Nutrients 2021, 13, 1784. [Google Scholar] [CrossRef]
- Beheshti, F.; Khazaei, M.; Hosseini, M. Neuropharmacological effects of Nigella sativa. Avicenna J. Phytomed. 2016, 6, 104–116. [Google Scholar]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. A review on possible therapeutic effect of Nigella sativa and Thymoquinone in neurodegenerative diseases. CNS Neurol. Disord. Drug Targets 2018, 17, 412–420. [Google Scholar] [CrossRef]
- Mohan, M.E.; Thomas, J.V.; Mohan, M.C. A proprietary Black Cumin oil extract (Nigella sativa) (Blaqmax®) modulates stress-sleep-immunity axis safely: Randomized double-blind placebo controlled study. Front. Nutr. 2023, 10, 1152680. [Google Scholar] [CrossRef]
- Sayeed, M.S.B.; Shams, T.; Hossain, S.F. Nigella sativa L. seeds modulate mood, anxiety and cognition in healthy adolescent males. J. Ethnopharmacol. 2014, 152, 156–162. [Google Scholar] [CrossRef]
- Asadi, M.; Molavi, F.; Qorbani, M.; Tanha, F.D. Comparative Efficacy of Zolpidem and Nigella Sativa in Treatment of Sleep Disorder and Vasomotor Symptoms in Menopausal Women of Women’s General Hospital. J. Family Reprod. Health 2020, 14, 186–191. [Google Scholar] [CrossRef]
- Morris, C.A.; Avorn, J. Internet marketing of herbal products. JAMA 2003, 290, 1505–1509. [Google Scholar] [CrossRef]
- Yao, M.; Ritchie, H.E.; Brown-Woodman, P.D. A developmental toxicity-screening test of valerian. J. Ethnopharmacol. 2007, 113, 204–209. [Google Scholar] [CrossRef]
- Trauner, G.; Khom, S.; Baburin, I.; Benedek, B.; Hering, S.; Kopp, B. Modulation of GABAA receptors by valerian extracts is related to the content of valerenic acid. Planta Med. 2008, 74, 19–24. [Google Scholar] [CrossRef]
- Schroeck, J.L.; Ford, J.; Conway, E.L.; Kurtzhalts, K.E.; Gee, M.E.; Vollmer, K.A.; Mergenhagen, K.A. Review of Safety and Efficacy of Sleep Medicines in Older Adults. Clin. Ther. 2016, 38, 2340–2372. [Google Scholar] [CrossRef]
- Ziegler, G.; Ploch, M.; Miettinen-Baumann, A.; Collet, W. Efficacy and tolerability of valerian extract LI 156 compared with oxazepam in the treatment of non-organic insomnia—A randomized, double-blind, comparative clinical study. Eur. J. Med. Res. 2002, 7, 480–486. [Google Scholar]
- Tammadon, M.R.; Nobahar, M.; Hydarinia-Naieni, Z.; Ebrahimian, A.; Ghorbani, R.; Vafaei, A.A. The Effects of Valerian on Sleep Quality, Depression, and State Anxiety in Hemodialysis Patients: A Randomized, Double-blind, Crossover Clinical Trial. Oman. Med. J. 2021, 36, e255. [Google Scholar] [CrossRef]
- Dominguez, R.A.; Bravo-Valverde, R.L.; Kaplowitz, B.R.; Cott, J.M. Valerian as a hypnotic for Hispanic patients. Cultur. Divers. Ethnic. Minor. Psychol. 2000, 6, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.L.; Atherton, P.J.; Bauer, B.A.; Moore, D.F., Jr.; Mattar, B.I.; Lavasseur, B.I.; Rowland, K.M., Jr.; Zon, R.T.; Lelindqwister, N.A.; Nagargoje, G.G.; et al. The use of Valeriana officinalis (Valerian) in improving sleep in patients who are undergoing treatment for cancer: A phase III randomized, placebo-controlled, double-blind study (NCCTG Trial, N01C5). J. Support. Oncol. 2011, 9, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.P.; Bent, S.; Tice, J.A.; Blackwell, T.; Cummings, S.R. An internet-based randomized, placebo-controlled trial of kava and valerian for anxiety and insomnia. Medicine 2005, 84, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Oxman, A.D.; Flottorp, S.; Håvelsrud, K.; Fretheim, A.; Odgaard-Jensen, J.; Austvoll-Dahlgren, A.; Carling, C.; Pallesen, S.; Bjorvatn, B. A televised, web-based randomised trial of an herbal remedy (valerian) for insomnia. PLoS ONE 2007, 2, e1040. [Google Scholar] [CrossRef] [PubMed]
- Taibi, D.M.; Bourguignon, C.; Taylor, A.G. A feasibility study of valerian extract for sleep disturbance in person with arthritis. Biol. Res. Nurs. 2009, 10, 409–417. [Google Scholar] [CrossRef]
- Dhawan, K.; Dhawan, S.; Sharma, A. Passiflora: A review update. J. Ethnopharmacol. 2004, 94, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, K.; Kumar, S.; Sharma, A. Anti-anxiety studies on extracts of Passiflora incarnata Linneaus. J. Ethnopharmacol. 2001, 78, 165–170. [Google Scholar] [CrossRef]
- Movafegh, A.; Alizadeh, R.; Hajimohamadi, F.; Esfehani, F.; Nejatfar, M. Preoperative oral Passiflora incarnata reduces anxiety in ambulatory surgery patients: A double-blind, placebo-controlled study. Anesth. Analg. 2008, 106, 1728–1732. [Google Scholar] [CrossRef]
- Cronin, J.R. Passionflower: Reigniting Male Libido and Other Potential Uses. Altern. Complement. Ther. 2004, 9, 89–92. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Naghavi, H.R.; Vazirian, M.; Shayeganpour, A.; Rashidi, H.; Khani, M. Passionflower in the treatment of generalized anxiety: A pilot double-blind randomized controlled trial with oxazepam. J. Clin. Pharm. Ther. 2001, 26, 363–367. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Akhondzadeh, S. Passiflora incarnata in the teartment of attention-deficit hyperactivity disorder in children and adolescents. Therapy 2005, 2, 609–614. [Google Scholar] [CrossRef]
- Peng, A.; Ji, S.; Lai, W.; Hu, D.; Wang, M.; Zhao, X.; Chen, L. The bidirectional relationship between sleep disturbance and anxiety: Sleep disturbance is a stronger predictor of anxiety. Sleep Med. 2024, 121, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ngan, A.; Conduit, R. A double-blind, placebo-controlled investigation of the effects of Passiflora incarnata (passionflower) herbal tea on subjective sleep quality. Phytother. Res. 2011, 25, 1153–1159. [Google Scholar] [CrossRef]
- Kim, G.-H.; Lim, K.; Yang, H.S.; Lee, J.-K.; Kim, Y.; Park, S.-K.; Kim, S.-H.; Park, S.; Kim, T.-H.; Moon, J.-S.; et al. Improvement in neurogenesis and memory function by administration of Passiflora incarnata L. extract applied to sleep disorder in rodent models. J. Chem. Neuroanat. 2019, 98, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Christoffoli, M.T.; Bachesk, A.B.; Farah, G.J.; Ferreira, G.Z. Assessment of Passiflora incarnata L. for conscious sedation of patients during the extraction of mandibular third molars: A randomized, split-mouth, double-blind, crossover study. Quintessence Int. 2021, 52, 868–878. [Google Scholar] [CrossRef]
- La Tempa, A.; Ferraiuolo, G.; Pranzetti, B.; Pruccoli, J.; Parmeggiani, A. Passiflora incarnata L. Herba in the Treatment of Anxiety Symptoms and Insomnia in Children and Adolescents with Feeding and Eating Disorders. Adolescents 2025, 5, 24. [Google Scholar] [CrossRef]
- Bradley, B.F.; Starkey, N.J.; Brown, S.L.; Lea, R.W. Anxiolytic effects of Lavandula angustifolia odour on the Mongolian gerbil elevated plus maze. J. Ethnopharmacol. 2007, 111, 517–525. [Google Scholar] [CrossRef]
- Coelho, L.S.; Correa-Netto, N.F.; Masukawa, M.Y.; Lima, A.C.; Maluf, S.; Linardi, A.; Santos-Junior, J.G. Inhaled Lavandula angustifolia essential oil inhibits consolidation of contextual- but not tone-fear conditioning in rats. J. Ethnopharmacol. 2018, 215, 34–41. [Google Scholar] [CrossRef]
- Bikmoradi, A.; Seifi, Z.; Poorolajal, J.; Araghchian, M.; Safiaryan, R.; Oshvandi, K. Effect of inhalation aromatherapy with lavender essential oil on stress and vital signs in patients undergoing coronary artery bypass surgery: A single-blinded randomized clinical trial. Complement. Ther. Med. 2015, 23, 331–338. [Google Scholar] [CrossRef]
- Karadag, E.; Samancioglu, S.; Ozden, D.; Bakir, E. Effects of aromatherapy on sleep quality and anxiety of patients. Nurs. Crit. Care 2017, 22, 105–112. [Google Scholar] [CrossRef]
- Lillehei, A.S.; Halcón, L.L.; Savik, K.; Reis, R. Effect of inhaled lavender and sleep hygiene on self-reported sleep issues: A randomized controlled trial. J. Altern. Complement. Med. 2015, 21, 430–438. [Google Scholar] [CrossRef]
- Najafi, Z.; Tagharrobi, Z.; Shahriyari-Kale-Masihi, M. Effect of aromatherapy with Lavender on sleep quality among patients undergoing hemodialysis. Feyz Med. Sci. J. 2014, 18, 145–150. [Google Scholar]
- Luo, J.; Jiang, W. A critical review on clinical evidence of the efficacy of lavender in sleep disorders. Phytother. Res. 2022, 36, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H. The effects of the inhalation method using essential oils on blood pressure and stress responses of clients with essential hypertension. J. Korean Acad. Nurs. 2006, 36, 1123–1134. [Google Scholar] [CrossRef]
- Lee, E.; Kim, K.S. The effects of aroma hand massage on anxiety and sleep in cancer patients during hospitalization. Perspect. Nurs. Sci. 2011, 8, 42–53. [Google Scholar]
- Kim, O.J.; Kim, K.H.; Park, K.S. The effect of aroma inhalation on stress, anxiety and sleep pattern in patients with hemodialysis. Clin. Nurs. Res. 2007, 13, 99–111. [Google Scholar]
- Lee, Y.M.; Ahn, H.Y. Effect of the aromatherapy on anxiety and discomfort in patients having colonoscopy. J. Korean Acad. Fundam. Nurs. 2010, 17, 539–547. [Google Scholar]
- Cho, M.; Min, E.; Hur, M.; Lee, M. Effects of aromatherapy on the anxiety, vital signs, and sleep quality of percutaneous coronary intervention patients in intensive care units. Evid.-Based Complement. Alternat. Med. 2013, 2013, 381381. [Google Scholar] [CrossRef] [PubMed]
- Lytle, J.; Mwatha, C.; Davis, K. Effect of lavender aromatherapy on vital signs and perceived quality of sleep in the intermediate care unit: A pilot study. Am. J. Crit. Care 2014, 23, 24–29. [Google Scholar] [CrossRef]
- Hirokawa, K.; Nishimoto, T.; Taniguchi, T. Effects of lavender aroma on sleep quality in healthy Japanese students. Percept. Mot. Skills 2012, 114, 111–122. [Google Scholar] [CrossRef]
- Chien, L.; Cheng, S.; Liu, C. The effect of lavender aromatherapy on autonomic nervous system in midlife women with insomnia. Evid.-Based Complement. Altern. Med. 2012, 2012, 740813. [Google Scholar] [CrossRef]
- Lewith, G.T.; Godfrey, A.D.; Phillip, P. A single-blinded, randomized pilot study evaluating the aroma of Lavandula augustifolia as a treatment for mild insomnia. J. Altern. Complement. Med. 2005, 11, 631–637. [Google Scholar] [CrossRef]
- Fismer, K.; Pilkington, K. Lavender and sleep: A systematic review of the evidence. Eur. J. Integr. Med. 2012, 4, e436–e447. [Google Scholar] [CrossRef]
- Lari, Z.N.; Hajimonfarednejad, M.; Riasatian, M.; Abolhassanzadeh, Z.; Iraji, A.; Vojoud, M.; Heydari, M.; Shams, M. Efficacy of inhaled Lavandula angustifolia Mill. Essential oil on sleep quality, quality of life and metabolic control in patients with diabetes mellitus type II and insomnia. J. Ethnopharmacol. 2020, 251, 112560. [Google Scholar] [CrossRef]
- Moeini, M.; Khadibi, M.; Bekhradi, R.; Mahmoudian, S.A.; Nazari, F. Effect of aromatherapy on the quality of sleep in ischemic heart disease patients hospitalized in intensive care units of heart hospitals of the Isfahan University of Medical Sciences. Iran. J. Nurs. Midwifery Res. 2010, 15, 234–239. [Google Scholar] [PubMed]
- Kukula-Koch, W.; Szwajgier, D.; Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Głowniak, K.; Meissner, H.O. Is Phytomelatonin Complex Better Than Synthetic Melatonin? The Assessment of the Antiradical and Anti-Inflammatory Properties. Molecules 2021, 26, 6087. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, M.K.; Yu, J.; Zhou, J. Phytomelatonin: Recent advances and future prospects. J. Pineal Res. 2018, 65, e12526. [Google Scholar] [CrossRef]
- Oladi, E.; Mohamadi, M.; Shamspur, T.; Mostafavi, A. Expression of Concern to Spectrofluorimetric Determination of Melatonin in Kernels of Four Different Pistacia Varieties after Ultrasound-Assisted Solid-Liquid Extraction. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 326–329. [Google Scholar] [CrossRef]
- Lautie, E.; Russo, O.; Ducrot, P.; Boutin, J.A. Unraveling Plant Natural Chemical Diversity for Drug Discovery Purposes. Front. Pharmacol. 2020, 11, 397. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Zhou, Z.; Cruz, M.H.C.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting plants to survive and thrive. Molecules 2015, 20, 7396–7437. [Google Scholar] [CrossRef]
- Maronde, E.; Stehle, J. The mammalian pineal gland: Known facts, unknown facets. Trends Endocrinol. Metabol. 2007, 18, 142–149. [Google Scholar] [CrossRef]
- Hardeland, R.; Madrid, J.A.; Tan, D.X.; Reiter, R.J. Melatonin, the circadian multioscillator system and health: The need for detailed analysis of peripheral melatonin signal. J. Pineal Res. 2012, 52, 139–166. [Google Scholar] [CrossRef]
- Tan, D.; Xu, B.; Zhou, X.; Reiter, R. Pineal calcification, melatonin production, aging, associated health consequences and rejuvenation of the pineal gland. Molecules 2018, 23, 301. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2016, 6, 1230. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C.; Rengel, Z.; Song, Z.B.; Chen, Q. Phytomelatonin receptor PMTR 1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef]
- Wade, A.G.; Crawford, G.; Ford, I.; McConnachie, A.; Nir, T.; Laudon, M.; Zisapel, N. Prolonged release melatonin in the treatment of primary insomnia: Evaluation of the age cut-off for short- and long-term response. Curr. Med. Res. Opin. 2011, 27, 87–98. [Google Scholar] [CrossRef]
- Erman, M.; Seiden, D.; Zammit, G.; Sainati, S.; Zhang, J. An efficacy, safety, and dose-response study of Ramelteon in patients with chronic primary insomnia. Sleep Med. 2006, 7, 17–24. [Google Scholar] [CrossRef]
- Shinomiya, K.; Inoue, T.; Utsu, Y.; Tokunaga, S.; Masuoka, T.; Ohmori, A.; Kamei, C. Hypnotic activities of chamomile and passiflora extracts in sleep-disturbed rats. Biol. Pharm. Bull. 2005, 28, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Mathews, I.M.; Eastwood, J.; Lamport, D.J.; Le Cozannet, R.; Fanca-Berthon, P.; Williams, C.M. Clinical Efficacy and Tolerability of Lemon Balm (Melissa officinalis L.) in Psychological Well-Being: A Review. Nutrients 2024, 16, 3545. [Google Scholar] [CrossRef]

| Population | TST/sTST (min) | SL/sSL (min) | Dose (mg) | References |
|---|---|---|---|---|
| 40 patients ≥ 55 years with primary insomnia (20 PRM, 20 placebo) | PRM: 391.7 Placebo: 389.5 | PRM: 13.7 Placebo: 22.6 (−9 min) | 2 mg prolonged-release melatonin | [95] |
| Adults with primary insomnia, age 65–80 (PRM n = 137, Placebo n = 144) | After 3 weeks: +7.0 min After 6 months: +7.5 min | After 3 weeks: −15.6 min After 6 months: −14.5 min | 2 mg prolonged-release melatonin | [96] |
| 110 children (3–15 yrs) with chronić sleep problems | +22.4 min vs. placebo (sleep diaries, p = 0.04); +13.3 min vs. placebo (actigraphy, NS) | −37.5 min vs. placebo (sleep diaries, p < 0.0001); −45.3 min vs. placebo (actigraphy, p = 0.0003) | 0.5–12 mg | [97] |
| 40 children (6–12 yrs) with chronic sleep onset insomnia | +41 min | −63 min (diary), −75 min (actigraphy) | 5 mg | [98] |
| Population | TST (min) | SL/sSL (min) | oTST/sTST (min) | Dose (mg) | References |
|---|---|---|---|---|---|
| Adults ≥ 50 years with insomnia | +21 (objective) | −13.8 | Not reported | 4–8 mg | [131] |
| Adults with insomnia | +7.26 (objective) | −9.36/−4.3 | +3.23 min (sTST), not statistically significant | 4–32 mg | [134] |
| Adults with insomnia without comorbidities | +17.9 (oTST), +11.7 (sTST) | −14 (oSOL)/−8.74 (sSOL) | +2.02/+14.5 (long-term treatment) | 4–16 mg (commonly 8) | [135] |
| Population | TST/sTST (min) | SL/sSL (min) | Dose (mg) | Refrerences |
|---|---|---|---|---|
| Adults with MDD (n = 332) | ↑ subjective sleep quality (LSEQ) | ↑ getting to sleep (p = 0.001) | 25–50 | [139] |
| Adults with MDD (n = 138) | Preserved sleep cycles (vs. ↓ in escitalopram) | ↓ sleep latency (p < 0.05) | 25–50 | [140] |
| Adults with depression (n = 7961) | Not reported | No increase in hypnotic use (p = 0.520) | 25–50 | [141] |
| Patients with alcohol dependence and insomnia (n = 9) | ↓ PSQI score from 13.1 to 7.8 | Improved sleep onset latency | 25–50 | [142] |
| Plant Name (Family) | Key Metabolites | Therapeutic Uses & Effects | Research Findings & Clinical Trials | Reference |
|---|---|---|---|---|
| Matricaria chamomilla L. (Chamomile); Asteraceae | Terpenoids, Phenolic compounds, Essential oils | Anxiolytic, anti-depressant, sleep quality improvement, potential in GAD and depression. | Clinical trials have shown effectiveness in treating anxiety and improving sleep quality. | [148,149,150,151,152,153,154,155] |
| Melissa officinalis L. (Lemon balm); Lamiaceae | Rosmarinic acid, Volatile metabolites: geranial, neral, citronellal, geraniol | Cognitive improvement, mood enhancement, sleep quality improvement. | Clinical trials have shown significant improvement in sleep quality and mood enhancement. | [16,156,157,158,159,160,161,162,163,164] |
| Nigella sativa L. (Black cumin); Ranunculaceae | Thymoquinone | Anti-anxiety properties, memory enhancement, stress management. | Animal trials show an increased sleep quality and reduced anxiety; human trials proved stress and sleep management | [165,166,167,168,169,170] |
| Valeriana officinalis (Valerian); Caprifoliaceae | essential oils, ketones, phenols, iridoid esters, valeric acid, aminobutyric acid, arginine, tyrosine, glutamine, as well as noncyclic, monocyclic, and bicyclic hydrocarbons | It has sedative and hypnotic properties, relieving anxiety and sleep disorders. It inhibits the uptake and stimulates the release of GABA, a partial agonist of the 5-hydroxytryptamine 2A receptor, which increases melatonin release. | Clinical trials have shown that Valerian was found to significantly improve sleep quality, anxiety, and depression symptoms in patients. | [171,172,173,174,175,176,177,178,179,180] |
| Passiflora incarnata (passionflower), Passifloraceae | alkaloids, phenolic compounds, flavonoids, and cyanogenic glycosides. The primary phytochemicals found in the passionflower are flavonoids (apigenin, luteolin, quercetin, and kaempferol) and flavonoid glycosides (vitexin, isovitexin, orientin, and isoorientin) | A sedative, anxiolytic, and hypnotic agent used to treat sleep disorders. It modulates the neurotransmitter system, primarily gamma-aminobutyric acid (GABA), serotonin, and the adrenergic system. | Clinical trials have shown that Passiflora incarnata (passionflower) regulates mood, anxiety and stress response | [181,182,183,184,185,186,187,188,189,190,191,192] |
| Lavandula angustifolia (Lavender) | Lavender oil contains linalyl acetate, linalool, α-aminobutyric acid | Used in aromatherapy, essential oils treat sleep disorders. Lavender’s scent is an anti-anxiety, anticonvulsant, analgesic, sedative, and sleep-inducing agent. | Clinical trials have shown that L. angustifolia relieves stress, reduces anxiety, and improves sleep quality in patients | [193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211] |
| Melatonin-containing plants | Melatonin | Activity of the plants resembles that of melatonin, and additionally, a marked antiradical and anti-inflammatory action supported by the complex of metabolites. | Melatonin was identified in several plant species, including Coffea, Piper, Lycium, Brassica, Medicago, Chlorella and Oryza species | [81,169,212,213,214,215,216,217,218,219,220,221,222] |
| Criterion | Melatonergic Drugs | Herbal Preparations |
|---|---|---|
| Examples | Melatonin, Ramelteon, Tasimelteon, Agomelatine | M. chamomilla, M. officinalis, N. sativa |
| Mechanism of Action | MT1/MT2 agonism, 5-HT2C antagonism (agomelatine) | GABA modulation, serotonin activity, antioxidant effects |
| Clinical Efficacy | High (multiple RCTs and meta-analyses) | Limited (mostly pilot trials) |
| Safety | High, low abuse potential | High, but risk of interactions and allergies |
| Use in Severe Insomnia | Confirmed by multiple trials | Not confirmed |
| Cost | Moderate to high | Low |
| Availability | Prescription only (except melatonin OTC in some regions) | OTC, dietary supplements |
| Additional Benefits | Antidepressant effects (agomelatine) | Anxiolytic, adaptogenic potential |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żełabowski, K.; Pichowicz, W.; Skowron, I.; Szwach, J.; Biedka, K.; Wesołowski, M.; Błaszczyk, K.; Ziobro, O.; Petrov, W.; Kukula-Koch, W.; et al. The Efficacy of Melatonergic Receptor Agonists Used in Clinical Practice in Insomnia Treatment: Melatonin, Tasimelteon, Ramelteon, Agomelatine, and Selected Herbs. Molecules 2025, 30, 3814. https://doi.org/10.3390/molecules30183814
Żełabowski K, Pichowicz W, Skowron I, Szwach J, Biedka K, Wesołowski M, Błaszczyk K, Ziobro O, Petrov W, Kukula-Koch W, et al. The Efficacy of Melatonergic Receptor Agonists Used in Clinical Practice in Insomnia Treatment: Melatonin, Tasimelteon, Ramelteon, Agomelatine, and Selected Herbs. Molecules. 2025; 30(18):3814. https://doi.org/10.3390/molecules30183814
Chicago/Turabian StyleŻełabowski, Kacper, Wojciech Pichowicz, Izabela Skowron, Jagoda Szwach, Kamil Biedka, Michał Wesołowski, Katarzyna Błaszczyk, Oliwia Ziobro, Wiktor Petrov, Wirginia Kukula-Koch, and et al. 2025. "The Efficacy of Melatonergic Receptor Agonists Used in Clinical Practice in Insomnia Treatment: Melatonin, Tasimelteon, Ramelteon, Agomelatine, and Selected Herbs" Molecules 30, no. 18: 3814. https://doi.org/10.3390/molecules30183814
APA StyleŻełabowski, K., Pichowicz, W., Skowron, I., Szwach, J., Biedka, K., Wesołowski, M., Błaszczyk, K., Ziobro, O., Petrov, W., Kukula-Koch, W., & Chłopaś-Konowałek, A. (2025). The Efficacy of Melatonergic Receptor Agonists Used in Clinical Practice in Insomnia Treatment: Melatonin, Tasimelteon, Ramelteon, Agomelatine, and Selected Herbs. Molecules, 30(18), 3814. https://doi.org/10.3390/molecules30183814








