Oxidative Stress Protection and Anti-Inflammatory Activity of Polyphenolic Fraction from Urtica dioica: In Vitro Study Using Human Skin Cells
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of Fraction Isolated from Urtica dioica Flower and Leaves
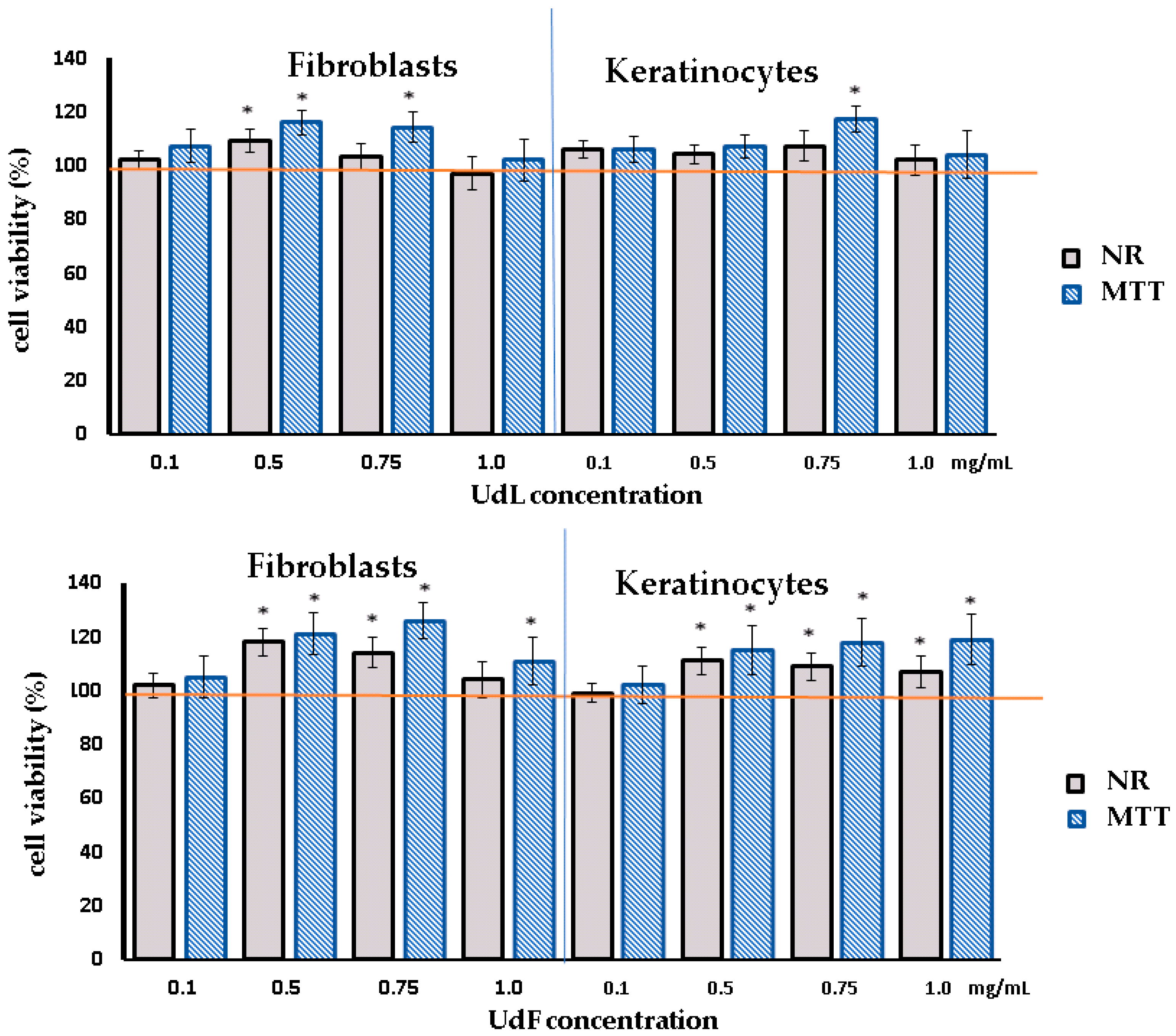
2.2. Cytotoxicity Test
2.3. Antioxidant Tests
2.3.1. Chemical Test
2.3.2. Intracellular ROS Scavenging Activity
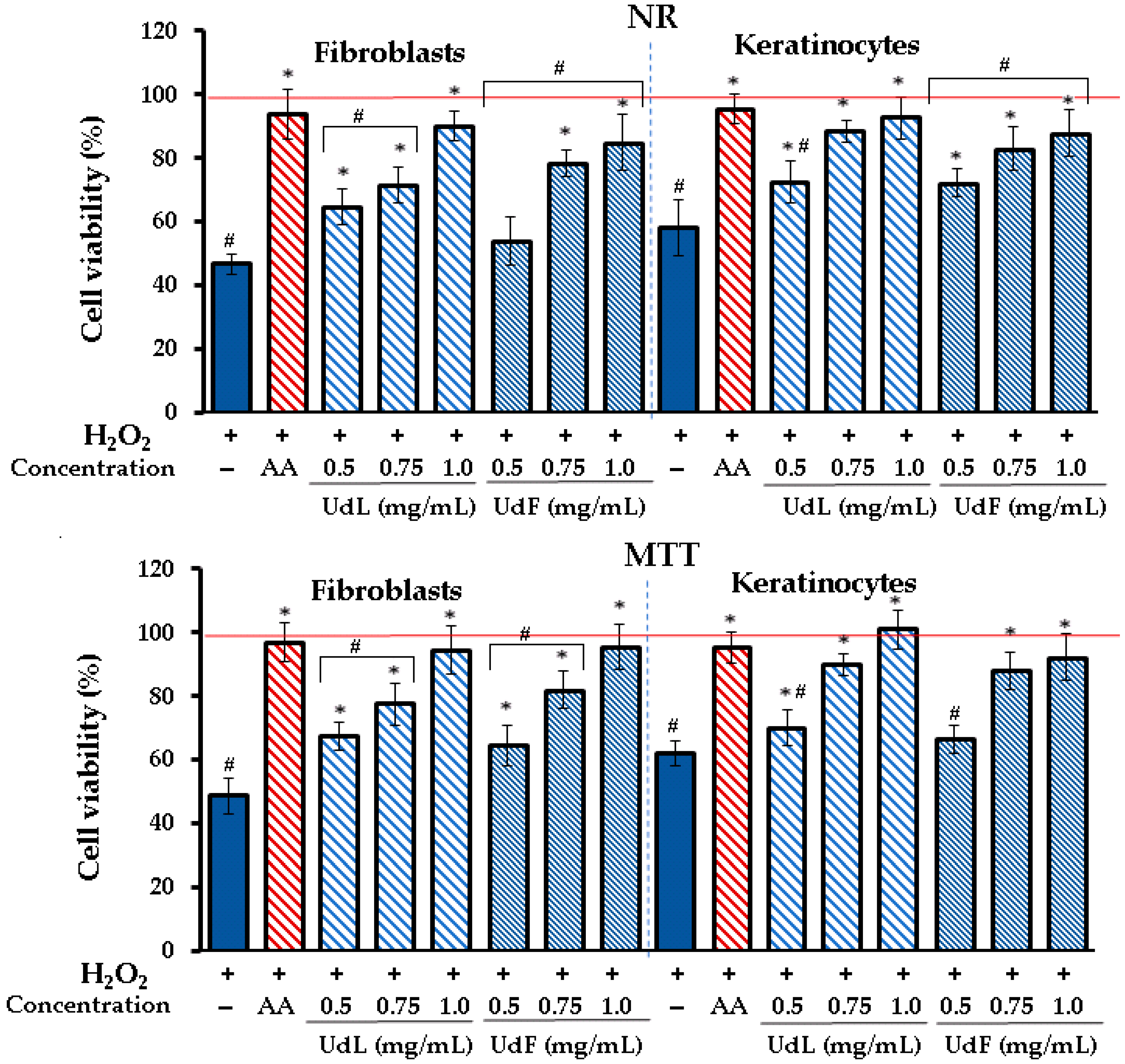
2.3.3. Prevention Against H2O2-Induced Cytotoxicity
2.3.4. Impact on Antioxidant Enzymes
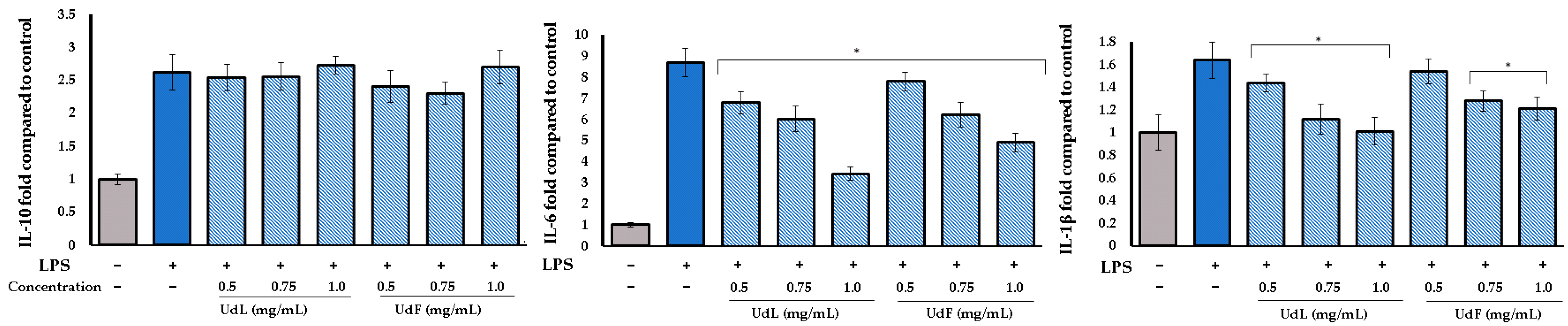
2.4. Anti-Inflammatory Activity
3. Discussion
4. Materials and Methods
4.1. Extract Preparation and Phytochemical Characterization
4.2. Antioxidant Tests
4.2.1. Free Radical Scavenging Activity
4.2.2. Total Polyphenols Content (TPC)
4.2.3. Ferric Ion Reducing Antioxidant Power Assay
4.2.4. Cupric Ion Reducing Antioxidant Capacity Assay
4.3. Biological Assays
4.3.1. Cell Cultures
4.3.2. Cell Viability Assays
4.3.3. Intracellular ROS Scavenging Activity
4.3.4. SOD, CAT, and MDA Levels.
4.3.5. Anti-Inflammatory Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nemzer, B.V.; Al-Taher, F.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Health-Improving Effects of Polyphenols on the Human Intestinal Microbiota: A Review. Int. J. Mol. Sci. 2025, 26, 1335. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, K.; Boda, A.K.; Dogra, S.; Bose, I.; Yadav, P.N.; Aidhen, I.S. Discovery of an Isocoumarin Analogue That Modulates Neuronal Functions via Neurotrophin Receptor TrkB. Bioorganic Med. Chem. Lett. 2019, 29, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Saadati, F.; Modarresi Chahardehi, A.; Jamshidi, N.; Jamshidi, N.; Ghasemi, D. Coumarin: A Natural Solution for Alleviating Inflammatory Disorders. Curr. Res. Pharmacol. Drug Discov. 2024, 7, 100202. [Google Scholar] [CrossRef]
- Lee, Y.I.; Choi, S.; Roh, W.S.; Lee, J.H.; Kim, T.-G. Cellular Senescence and Inflammaging in the Skin Microenvironment. Int. J. Mol. Sci. 2021, 22, 3849. [Google Scholar] [CrossRef] [PubMed]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef]
- Toplicean, I.-M.; Ianuș, R.-D.; Datcu, A.-D. An Overview on Nettle Studies, Compounds, Processing and the Relation with Circular Bioeconomy. Plants 2024, 13, 3529. [Google Scholar] [CrossRef]
- Subba, S.; Pradhan, K. A Comprehensive Review on Common Plants with Remarkable Medicinal Properties: Urtica dioica. J. Med. Plants Stud. 2022, 10, 87–91. [Google Scholar] [CrossRef]
- Repajić, M.; Cegledi, E.; Zorić, Z.; Pedisić, S.; Elez Garofulić, I.; Radman, S.; Palčić, I.; Dragović-Uzelac, V. Bioactive Compounds in Wild Nettle (Urtica dioica L.) Leaves and Stalks: Polyphenols and Pigments upon Seasonal and Habitat Variations. Foods 2021, 10, 190. [Google Scholar] [CrossRef]
- Flórez, M.; Cazón, P.; Vázquez, M. Antioxidant Extracts of Nettle (Urtica dioica) Leaves: Evaluation of Extraction Techniques and Solvents. Molecules 2022, 27, 6015. [Google Scholar] [CrossRef]
- Bhusal, K.K.; Magar, S.K.; Thapa, R.; Lamsal, A.; Bhandari, S.; Maharjan, R.; Shrestha, S.; Shrestha, J. Nutritional and Pharmacological Importance of Stinging Nettle (Urtica dioica L.): A Review. Heliyon 2022, 8, e09717. [Google Scholar] [CrossRef]
- Đurović, S.; Kojić, I.; Radić, D.; Smyatskaya, Y.A.; Bazarnova, J.G.; Filip, S.; Tosti, T. Chemical Constituents of Stinging Nettle (Urtica dioica L.): A Comprehensive Review on Phenolic and Polyphenolic Compounds and Their Bioactivity. Int. J. Mol. Sci. 2024, 25, 3430. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M.; Savo, V. Perceived Health Properties of Wild and Cultivated Food Plants in Local and Popular Traditions of Italy: A Review. J. Ethnopharmacol. 2013, 146, 659–680. [Google Scholar] [CrossRef] [PubMed]
- Leporatti, M.L.; Corradi, L. Ethnopharmacobotanical Remarks on the Province of Chieti Town (Abruzzo, Central Italy). J. Ethnopharmacol. 2001, 74, 17–40. [Google Scholar] [CrossRef]
- Jarić, S.; Popović, Z.; Mačukanović-Jocić, M.; Djurdjević, L.; Mijatović, M.; Karadžić, B.; Mitrović, M.; Pavlović, P. An Ethnobotanical Study on the Usage of Wild Medicinal Herbs from Kopaonik Mountain (Central Serbia). J. Ethnopharmacol. 2007, 111, 160–175. [Google Scholar] [CrossRef]
- Roschek, B.; Fink, R.C.; McMichael, M.; Alberte, R.S. Nettle Extract (Urtica dioica) Affects Key Receptors and Enzymes Associated with Allergic Rhinitis. Phytother. Res. 2009, 23, 920–926. [Google Scholar] [CrossRef]
- Johnson, T.A.; Sohn, J.; Inman, W.D.; Bjeldanes, L.F.; Rayburn, K. Lipophilic Stinging Nettle Extracts Possess Potent Anti-Inflammatory Activity, Are Not Cytotoxic and May Be Superior to Traditional Tinctures for Treating Inflammatory Disorders. Phytomedicine 2013, 20, 143–147. [Google Scholar] [CrossRef]
- Gülçin, İ.; Küfrevioǧlu, Ö.İ.; Oktay, M.; Büyükokuroǧlu, M.E. Antioxidant, Antimicrobial, Antiulcer and Analgesic Activities of Nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef]
- Devkota, H.P.; Paudel, K.R.; Khanal, S.; Baral, A.; Panth, N.; Adhikari-Devkota, A.; Jha, N.K.; Das, N.; Singh, S.K.; Chellappan, D.K.; et al. Stinging Nettle (Urtica dioica L.): Nutritional Composition, Bioactive Compounds, and Food Functional Properties. Molecules 2022, 27, 5219. [Google Scholar] [CrossRef] [PubMed]
- Abi Sleiman, M.; Younes, M.; Hajj, R.; Salameh, T.; Abi Rached, S.; Abi Younes, R.; Daoud, L.; Doumiati, J.L.; Frem, F.; Ishak, R.; et al. Urtica dioica: Anticancer Properties and Other Systemic Health Benefits from In Vitro to Clinical Trials. Int. J. Mol. Sci. 2024, 25, 7501. [Google Scholar] [CrossRef]
- Hodroj, M.H.; Al Bast, N.A.H.; Taleb, R.I.; Borjac, J.; Rizk, S. Nettle Tea Inhibits Growth of Acute Myeloid Leukemia Cells In Vitro by Promoting Apoptosis. Nutrients 2020, 12, 2629. [Google Scholar] [CrossRef]
- Levy, A.; Sivanesan, D.; Murugan, R.; Jornadal, J.; Quinonez, Y.; Jaffe, M.; Rathinavelu, A. Urtica dioica Induces Cytotoxicity in Human Prostate Carcinoma LNCaP Cells: Involvement of Oxidative Stress, Mitochondrial Depolarization and Apoptosis. Trop. J. Pharm. Res. 2014, 13, 711–717. [Google Scholar] [CrossRef]
- Zouari Bouassida, K.; Bardaa, S.; Khimiri, M.; Rebaii, T.; Tounsi, S.; Jlaiel, L.; Trigui, M. Exploring the Urtica dioica Leaves Hemostatic and Wound-Healing Potential. BioMed Res. Int. 2017, 2017, 1047523. [Google Scholar] [CrossRef] [PubMed]
- Skalska-Kamińska, A.; Wójciak, W.; Żuk, M.; Paduch, R.; Wójciak, M. Protective Effect of Urtica dioica Extract against Oxidative Stress in Human Skin Fibroblasts. Life 2023, 13, 2182. [Google Scholar] [CrossRef]
- Ciupei, D.; Colişar, A.; Leopold, L.; Stănilă, A.; Diaconeasa, Z.M. Polyphenols: From Classification to Therapeutic Potential and Bioavailability. Foods 2024, 13, 4131. [Google Scholar] [CrossRef]
- Dini, I.; Grumetto, L. Recent Advances in Natural Polyphenol Research. Molecules 2022, 27, 8777. [Google Scholar] [CrossRef] [PubMed]
- Repajić, M.; Cegledi, E.; Kruk, V.; Pedisić, S.; Çınar, F.; Bursać Kovačević, D.; Žutić, I.; Dragović-Uzelac, V. Accelerated Solvent Extraction as a Green Tool for the Recovery of Polyphenols and Pigments from Wild Nettle Leaves. Processes 2020, 8, 803. [Google Scholar] [CrossRef]
- Koraqi, H.; Qazimi, B.; Khalid, W.; Stanoeva, J.P.; Sehrish, A.; Siddique, F.; Çesko, C.; Ali Khan, K.; Rahim, M.A.; Hussain, I.; et al. Optimized Conditions for Extraction, Quantification and Detection of Bioactive Compound from Nettle (Urtica dioica L.) Using the Deep Eutectic Solvents, Ultra-Sonication and Liquid Chromatography-Mass Spectrometry (LC-DAD-ESI-MS/MS). Int. J. Food Prop. 2023, 26, 2171–2185. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MSnIdentification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and Antioxidant Assays of Polyphenols: A Review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Vajic, U.-J.; Grujic-Milanovic, J.; Miloradovic, Z.; Jovovic, D.; Ivanov, M.; Karanovic, D.; Savikin, K.; Bugarski, B.; Mihailovic-Stanojevic, N. Urtica dioica L. Leaf Extract Modulates Blood Pressure and Oxidative Stress in Spontaneously Hypertensive Rats. Phytomedicine 2018, 46, 39–45. [Google Scholar] [CrossRef]
- Wójciak, M.; Paduch, R.; Drozdowski, P.; Wójciak, W.; Żuk, M.; Płachno, B.J.; Sowa, I. Antioxidant and Anti-Inflammatory Effects of Nettle Polyphenolic Extract: Impact on Human Colon Cells and Cytotoxicity Against Colorectal Adenocarcinoma. Molecules 2024, 29, 5000. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ahmad, N.; Alkhars, S.; Alkhars, A.; Alyousif, M.; Bukhamseen, A.; Abuthayn, S.; Aqeel, M.; Aljamea, A. Green Accelerated Solvent Extraction (ASE) with Solvent and Temperature Effect and Green UHPLC-DAD Analysis of Phenolics in Pepper Fruit (Capsicum annum L.). J. Food Compos. Anal. 2021, 97, 103766. [Google Scholar] [CrossRef]
- Wójciak, M.; Mazurek, B.; Wójciak, W.; Kostrzewa, D.; Żuk, M.; Chmiel, M.; Kubrak, T.; Sowa, I. Optimizing the Extraction of the Polyphenolic Fraction from Defatted Strawberry Seeds for Tiliroside Isolation Using Accelerated Solvent Extraction Combined with a Box–Behnken Design. Molecules 2024, 29, 3051. [Google Scholar] [CrossRef] [PubMed]
- Senevirathna, N.; Hassanpour, M.; O’Hara, I.; Karim, A. Sustainable Extraction of Fresh Banana Inflorescence by ASE: Optimization and Characterization of Anthocyanin Rich Extracts by LC-UV-MS/MS. Foods 2025, 14, 1299. [Google Scholar] [CrossRef]
- Vajic, U.-J.; Zivkovic, J.; Ivanov, M.; Jovovic, D.; Savikin, K.; Bugarski, B.; Mihailovic-Stanojevic, N. Optimization of the Extraction of Antioxidants from Stinging Nettle Leaf Using Response Surface Methodology. Maced. J. Chem. Chem. Eng. 2022, 41, 119–128. [Google Scholar] [CrossRef]
- Vajic, U.-J.; Mihailovic-Stanojevic, N.; Karanovic, D.; Zivotic, M.; Ivanov, M.; Jovovic, D.; Grujic-Milanovic, J.; Miloradovic, Z. Urtica dioica L. Leaf Extract Dose-Dependently Modulates Oxidative Stress in the Kidney and Exerts Anti-Fibrotic and Anti-Inflammatory Properties by the Molecular Mechanisms Independent of NRF-2 Signalization Mirroring the Effects of Losartan in SHR. Int. J. Mol. Sci. 2024, 25, 13272. [Google Scholar] [CrossRef]
- Pająk, J.; Nowicka, D.; Szepietowski, J.C. Inflammaging and Immunosenescence as Part of Skin Aging—A Narrative Review. Int. J. Mol. Sci. 2023, 24, 7784. [Google Scholar] [CrossRef]
- Kruk, J.; Duchnik, E. Oxidative Stress and Skin Diseases: Possible Role of Physical Activity. Asian Pac. J. Cancer Prev. 2014, 15, 561–568. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Masłowski, M.; Aleksieiev, A.; Miedzianowska, J.; Efenberger-Szmechtyk, M.; Strzelec, K. Antioxidant and Anti–Aging Activity of Freeze–Dried Alcohol–Water Extracts from Common Nettle (Urtica dioica L.) and Peppermint (Mentha piperita L.) in Elastomer Vulcanizates. Polymers 2022, 14, 1460. [Google Scholar] [CrossRef]
- Goya, L.; Sánchez-Medina, A.; Redondo-Puente, M.; Dupak, R.; Bravo, L.; Sarriá, B. Main Colonic Metabolites from Coffee Chlorogenic Acid May Counteract Tumor Necrosis Factor-α-Induced Inflammation and Oxidative Stress in 3T3-L1 Cells. Molecules 2023, 29, 88. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-K.; Kang, I.-J.; Kim, B.; Sim, H.J.; Kim, D.-W.; Ahn, J.H.; Lee, J.-C.; Ryoo, S.; Shin, M.C.; Cho, J.H.; et al. Experimental Pretreatment with Chlorogenic Acid Prevents Transient Ischemia-Induced Cognitive Decline and Neuronal Damage in the Hippocampus through Anti-Oxidative and Anti-Inflammatory Effects. Molecules 2020, 25, 3578. [Google Scholar] [CrossRef]
- Mei, Y.; Sun, H.; Du, G.; Wang, X.; Lyu, D. Exogenous Chlorogenic Acid Alleviates Oxidative Stress in Apple Leaves by Enhancing Antioxidant Capacity. Sci. Hortic. 2020, 274, 109676. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Chlorogenic Acid (CGA) Isomers Alleviate Interleukin 8 (IL-8) Production in Caco-2 Cells by Decreasing Phosphorylation of P38 and Increasing Cell Integrity. Int. J. Mol. Sci. 2018, 19, 3873. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Yang, B.; Du, J.; Chen, L.; Li, Y.; Guo, F. Structures, Sources, Identification/Quantification Methods, Health Benefits, Bioaccessibility, and Products of Isorhamnetin Glycosides as Phytonutrients. Nutrients 2023, 15, 1947. [Google Scholar] [CrossRef]
- Boubaker, J.; Sghaier, M.B.; Skandrani, I.; Ghedira, K.; Chekir-Ghedira, L. Isorhamnetin 3-O-Robinobioside from Nitraria Retusa Leaves Enhance Antioxidant and Antigenotoxic Activity in Human Chronic Myelogenous Leukemia Cell Line K562. BMC Complement. Altern. Med. 2012, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Cao, Y.; Bao, B.; Zhang, L.; Ding, A. Antioxidant Capacity of Typha Angustifolia Extracts and Two Active Flavonoids. Pharm. Biol. 2017, 55, 1283–1288. [Google Scholar] [CrossRef]
- Bouhlel, I.; Skandrani, I.; Nefatti, A.; Valenti, K.; Ghedira, K.; Mariotte, A.M.; Hininger-Favier, I.; Laporte, F.; Dijoux-Franca, M.G.; Chekir-Ghedira, L. Antigenotoxic and Antioxidant Activities of Isorhamnetin 3-O Neohesperidoside from Acacia salicina. Drug Chem. Toxicol. 2009, 32, 258–267. [Google Scholar] [CrossRef]
- Lv, H.; Liu, L.; Zou, W.; Yang, Y.; Li, Y.; Yang, S.; Liang, A.; Yang, L. Isorhamnetin Ameliorates Non-Esterified Fatty Acid-Induced Apoptosis, Lipid Accumulation, and Oxidative Stress in Bovine Endometrial Epithelial Cells via Inhibiting the MAPK Signaling Pathway. Antioxidants 2025, 14, 156. [Google Scholar] [CrossRef]
- Chen, T.-L.; Zhu, G.-L.; Wang, J.-A.; Zhang, G.-D.; Liu, H.-F.; Chen, J.-R.; Wang, Y.; He, X.-L. Protective Effects of Isorhamnetin on Apoptosis and Inflammation in TNF-α-Induced HUVECs Injury. Int. J. Clin. Exp. Pathol. 2015, 8, 2311–2320. [Google Scholar]
- Ghasemi, S.; Moradzadeh, M.; Mousavi, S.H.; Sadeghnia, H.R. Cytotoxic Effects of Urtica dioica Radix on Human Colon (HT29) and Gastric (MKN45) Cancer Cells Mediated through Oxidative and Apoptotic Mechanisms. Cell. Mol. Biol. 2016, 62, 90–96. [Google Scholar] [PubMed]
- D’Abrosca, B.; Ciaramella, V.; Graziani, V.; Papaccio, F.; Della Corte, C.M.; Potenza, N.; Fiorentino, A.; Ciardiello, F.; Morgillo, F. Urtica dioica L. Inhibits Proliferation and Enhances Cisplatin Cytotoxicity in NSCLC Cells via Endoplasmic Reticulum-Stress Mediated Apoptosis. Sci. Rep. 2019, 9, 4986. [Google Scholar] [CrossRef] [PubMed]
- Obertreis, B.; Giller, K.; Teucher, T.; Behnke, B.; Schmitz, H. [Anti-inflammatory effect of Urtica dioica folia extract in comparison to caffeic malic acid]. Arzneimittelforschung 1996, 46, 52–56. [Google Scholar]
- Nematgorgani, S.; Agah, S.; Shidfar, F.; Janani, L.; Faghihi, A.; Hosseini, S. The Effect of Urtica dioica Leaf Extract Intake on Serum TNF-α, Stool Calprotectin and Erythrocyte Sedimentation Rate in Patients with Inflammatory Bowel Disease: A Double-Blind, Placebo-Controlled, Randomized, Clinical Trial. Mediterr. J. Nutr. Metab. 2020, 13, 75–87. [Google Scholar] [CrossRef]
- Abd-Nikfarjam, B.; Abbasi, M.; Memarzadeh, M.; Farzam, S.-A.; Jamshidian, A.; Dolati-Somarin, A. Therapeutic Efficacy of Urtica dioica and Evening Primrose in Patients with Rheumatoid Arthritis: A Randomized Double-Blind, Placebo-Controlled Clinical Trial. J. Herb. Med. 2022, 32, 100556. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Pavlíková, N. Caffeic Acid and Diseases—Mechanisms of Action. Int. J. Mol. Sci. 2022, 24, 588. [Google Scholar] [CrossRef]
- Chen, D.; Pan, D.; Tang, S.; Tan, Z.; Zhang, Y.; Fu, Y.; Lü, G.; Huang, Q. Administration of Chlorogenic Acid Alleviates Spinal Cord Injury via TLR4/NF-κB and P38 Signaling Pathway Anti-inflammatory Activity. Mol. Med. Rep. 2017, 17, 1340–1346. [Google Scholar] [CrossRef]
- Bagdas, D.; Gul, Z.; Meade, J.A.; Cam, B.; Cinkilic, N.; Gurun, M.S. Pharmacologic Overview of Chlorogenic Acid and Its Metabolites in Chronic Pain and Inflammation. Curr. Neuropharmacol. 2020, 18, 216–228. [Google Scholar] [CrossRef]
- Zielińska, D.; Zieliński, H.; Laparra-Llopis, J.M.; Szawara-Nowak, D.; Honke, J.; Giménez-Bastida, J.A. Caffeic Acid Modulates Processes Associated with Intestinal Inflammation. Nutrients 2021, 13, 554. [Google Scholar] [CrossRef]
- Da Cunha, F.M.; Duma, D.; Assreuy, J.; Buzzi, F.C.; Niero, R.; Campos, M.M.; Calixto, J.B. Caffeic Acid Derivatives: In Vitro and In Vivo Anti-Inflammatory Properties. Free Radic. Res. 2004, 38, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Afnan; Saleem, A.; Akhtar, M.F.; Sharif, A.; Akhtar, B.; Siddique, R.; Ashraf, G.M.; Alghamdi, B.S.; Alharthy, S.A. Anticancer, Cardio-Protective and Anti-Inflammatory Potential of Natural-Sources-Derived Phenolic Acids. Molecules 2022, 27, 7286. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Lee, H.J.; Kim, S.; Kwon, Y.; Chun, W. Isorhamnetin-3-O-Glucuronide Suppresses JNK and P38 Activation and Increases Heme-Oxygenase-1 in Lipopolysaccharide-Challenged RAW264.7 Cells. Drug Dev. Res. 2016, 77, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.A.; Xu, X.; Wang, Y.; Shie, P.-H.; Qiu, L. A New Anti-Inflammatory Flavonoid Glycoside from Tetraena aegyptia. Nat. Prod. Res. 2021, 35, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; Esmat, A. Antioxidant and Anti-Inflammatory Activities of the Major Phenolics from Zygophyllum simplex L. J. Ethnopharmacol. 2017, 205, 51–56. [Google Scholar] [CrossRef]
- Fu, Y.; Jia, Y.; Sun, Y.; Liu, X.; Yi, J.; Cai, S. Dietary Flavonoids Alleviate Inflammation and Vascular Endothelial Barrier Dysfunction Induced by Advanced Glycation End Products In Vitro. Nutrients 2022, 14, 1026. [Google Scholar] [CrossRef]
- Osman, S.; El Kashak, W.; Wink, M.; El Raey, M. New Isorhamnetin Derivatives from Salsola Imbricata Forssk. Leaves with Distinct Anti-Inflammatory Activity. Pharmacogn. Mag. 2016, 12, S47–S51. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; Martínez-Vitela, C.; Serna-Saldívar, S.O. Topical Anti-Inflammatory Effects of Isorhamnetin Glycosides Isolated from Opuntia ficus-indica. BioMed Res. Int. 2015, 2015, 847320. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Z.; Lu, H.; Xu, Z.; Tong, R.; Shi, J.; Jia, G. Recent Advances of Natural Polyphenols Activators for Keap1-Nrf2 Signaling Pathway. Chem. Biodivers. 2019, 16, e1900400. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Zagórska-Dziok, M.; Bujak, T.; Wójciak, M.; Sowa, I. Evaluation of Cosmetic and Dermatological Properties of Kombucha-Fermented Berry Leaf Extracts Considered to Be By-Products. Molecules 2022, 27, 2345. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Ziemlewska, A.; Zagórska-Dziok, M.; Mokrzyńska, A.; Wójciak, M.; Sowa, I. Apiaceae Bioferments Obtained by Fermentation with Kombucha as an Important Source of Active Substances for Skin Care. Molecules 2025, 30, 983. [Google Scholar] [CrossRef] [PubMed]
- Bort, A.; Alvarado-Vazquez, P.A.; Moracho-Vilrriales, C.; Virga, K.G.; Gumina, G.; Romero-Sandoval, A.; Asbill, S. Effects of JWH015 in Cytokine Secretion in Primary Human Keratinocytes and Fibroblasts and Its Suitability for Topical/Transdermal Delivery. Mol. Pain 2017, 13, 1744806916688220. [Google Scholar] [CrossRef] [PubMed]
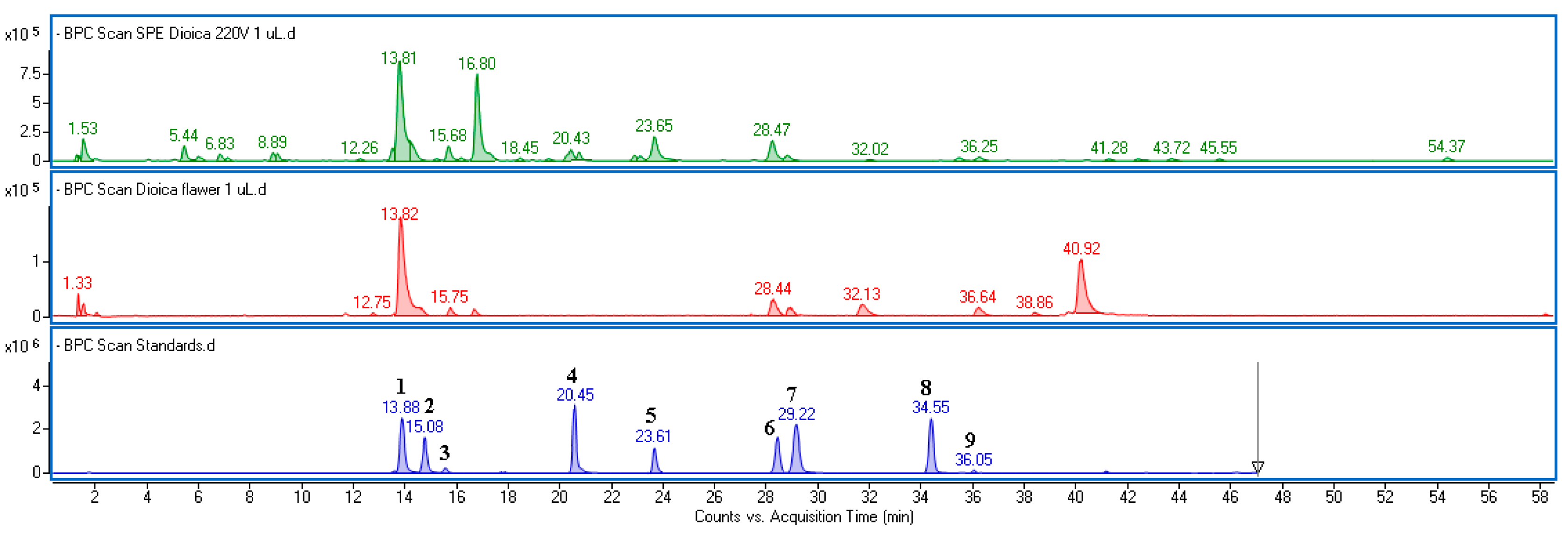



| RT (min) | Mass Data (m/z–H) | Fragment (m/z–H) | Formula | Δ ppm | Component | UdL | UdF |
|---|---|---|---|---|---|---|---|
| 5.60 | 315.07261 | (152) | C13H16O9 | 1.44 | Dihydroxybenzoic acid hexoside | 0.21 ± 0.01 | nd |
| 6.22 | 371.06188 | (209,135,179) | C15H16O11 | −0.28 | Caffeoylglucaric acid | 2.21 ± 0.08 | nd |
| 7.31 | 315.07289 | (152) | C13H16O9 | 2.32 | Dihydroxybenzoic acid hexoside | 0.18 ± 0.01 | nd |
| 13.82 | 353.08887 | (191,135,179) | C16H18O9 | 3.01 | 5-O-caffeoylquinic acid * | 35.12 ± 1.11 | 17.51 ± 0.98 |
| 15.08 | 179.03551 | (135) | C9H8O4 | 2.93 | Caffeic acid * | 4.09 ± 0.20 | 0.32 ± 0.01 |
| 15.68 | 353.08863 | (191,135,179) | C16H18O9 | 2.33 | 4-O-caffeoylquinic acid * | 0.37 ± 0.03 | 0.11 ± 0.01 |
| 16.80 | 295.04614 | (133,135,179) | C13H12O8 | 0.67 | Caffeoylmalic acid | 27.18 ± 1.53 | 2.49 ± 0.11 |
| 20.43 | 163.04071 | C9H8O3 | 3.92 | p-coumaric acid * | 4.21 ± 0.22 | nd | |
| 23.65 | 193.05011 | C10H10O4 | −2.67 | Ferulic acid * | 1.52 ± 0.05 | nd | |
| 28.47 | 609.14562 | (300,463) | C27H30O16 | −0.80 | Quercetin-3-O-rutinoside * | 1.51 ± 0.04 | 1.94 ± 0.95 |
| 29.22 | 463.08785 | (300) | C21H20O12 | -0.75 | Quercetin 3-O-glucoside * | 0.21 ± 0.01 | 1.11 ± 0.18 |
| 32.13 | 505.10076 | (463,300) | C23H22O13 | 3.94 | Quercetin acetylglucoside | 0.18 ± 0.00 | 0.96 ± 0.04 |
| 34.55 | 593.14999 | (285) | C27H30O15 | −2.03 | Kaempferol 3-O-rutinoside * | 0.09 ± 0.00 | 0.24 ± 0.01 |
| 36.05 | 623.16183 | C28H32O16 | 0.11 | Isorhamnetin 3-O-rutinoside * | 0.01 ± 0.00 | 0.10 ± 0.00 | |
| 36.64 | 477.10394 | (315) | C22H22O12 | 0.19 | Isorhamnetin-3-O-glucoside | 0.05 ± 0.01 | 1.94 ± 0.17 |
| 38.91 | 489.10519 | (284) | C23H22O12 | 2.73 | Kaempferol acetylglucoside | nd | 0.29 ± 0.01 |
| 40.92 | 519.11690 | (315) | C24H24O13 | 4.78 | Isorhamnetin acetylglucoside | nd | 4.93 ± 0.25 |
| DPPH * | ABTS | FRAP | CUPRAC | TPC | |
|---|---|---|---|---|---|
| UdL | 78.56 ± 0.85 b | 8.37 ± 0.28 b | 15.53 ± 0.82 b | 59.67 ± 1.99 b | 37.58 ± 1.92 b |
| UdF | 124.77 ± 1.38 c | 20.98 ± 0.12 c | 10.70 ± 0.87 a | 38.22 ± 1.28 a | 24.08 ± 1.02 a |
| AA | 25.55 ± 0.20 a | 4.24 ± 0.23 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik-Borowska, K.; Wójciak, W.; Żuk, M.; Luchowski, P.; Skalska-Kamińska, A.; Pacuła, W.; Sowa, I.; Wójciak, M. Oxidative Stress Protection and Anti-Inflammatory Activity of Polyphenolic Fraction from Urtica dioica: In Vitro Study Using Human Skin Cells. Molecules 2025, 30, 2515. https://doi.org/10.3390/molecules30122515
Wójcik-Borowska K, Wójciak W, Żuk M, Luchowski P, Skalska-Kamińska A, Pacuła W, Sowa I, Wójciak M. Oxidative Stress Protection and Anti-Inflammatory Activity of Polyphenolic Fraction from Urtica dioica: In Vitro Study Using Human Skin Cells. Molecules. 2025; 30(12):2515. https://doi.org/10.3390/molecules30122515
Chicago/Turabian StyleWójcik-Borowska, Katarzyna, Weronika Wójciak, Magdalena Żuk, Piotr Luchowski, Agnieszka Skalska-Kamińska, Wiktoria Pacuła, Ireneusz Sowa, and Magdalena Wójciak. 2025. "Oxidative Stress Protection and Anti-Inflammatory Activity of Polyphenolic Fraction from Urtica dioica: In Vitro Study Using Human Skin Cells" Molecules 30, no. 12: 2515. https://doi.org/10.3390/molecules30122515
APA StyleWójcik-Borowska, K., Wójciak, W., Żuk, M., Luchowski, P., Skalska-Kamińska, A., Pacuła, W., Sowa, I., & Wójciak, M. (2025). Oxidative Stress Protection and Anti-Inflammatory Activity of Polyphenolic Fraction from Urtica dioica: In Vitro Study Using Human Skin Cells. Molecules, 30(12), 2515. https://doi.org/10.3390/molecules30122515






