s-Triazine-Based Ligands Possessing Identical Heteroatom-Bridged Substituents—Unexpected Triazine-O Bond Cleavage
Abstract
1. Introduction
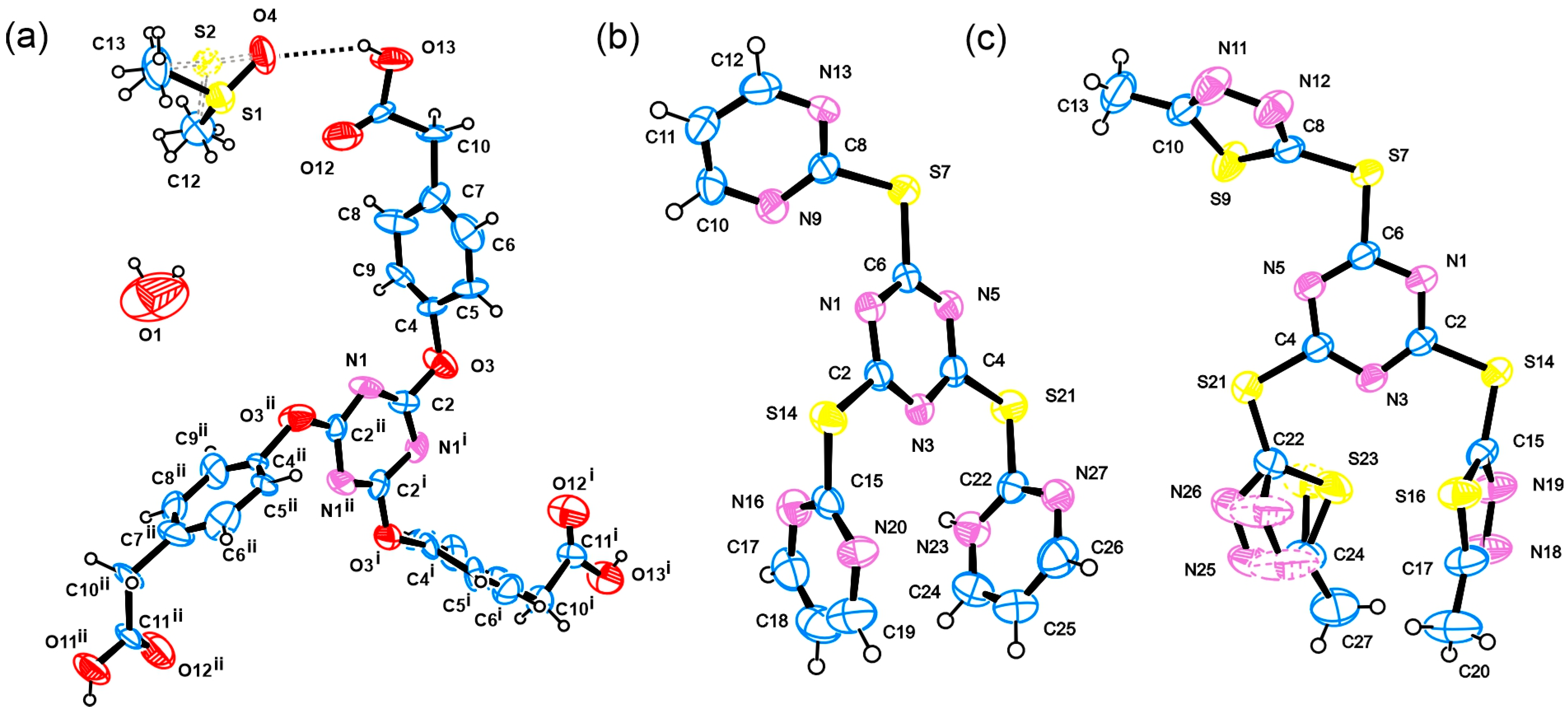
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis of Ligands 3
3.3. Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MOF(s) | Metal–organic framework(s) |
| H3TCPT | 2,4,6-tris-(4-carboxyphenoxy)-1,3,5-triazine |
| H3TATAB | 4,4′,4″-((1,3,5-triazine-2,4,6-triyl)tris(azanediyl))tribenzoic acid |
| H3TATMB | 3,3′,3″-((1,3,5-triazine-2,4,6-triyl)tris(azanediyl))tribenzoic acid |
| NMR | Nuclear magnetic resonance |
| XRD | X-Ray diffraction |
| HESI HRMS | Heated electrospray ionization high resolution mass spectrometry |
| DMSO-d6 | Deuterated dimethyl sulfoxide |
| CDCl3 | Deuterochloroform |
| TMS | Tetramethyl silane |
| HSQC | Heteronuclear single quantum coherence |
| HMBC | Heteronuclear multiple bond correlation |
| ROESY | Rotating-frame nuclear overhauser effect spectroscopy |
| THF | Tetrahydrofuran |
| DCM | Dichloromethane |
| COST | Cooperation in Science and Technology |
References
- Pilgrim, B.S.; Champness, N.R. Metal-organic frameworks and metal-organic cages—A perspective. ChemPlusChem 2020, 85, 1842–1856. [Google Scholar] [CrossRef]
- Seidi, F.; Jouyandeh, M.; Taghizadeh, M.; Taghizadeh, A.; Vahabi, H.; Habibzadeh, S.; Formela, K.; Saeb, M.R. Metal-organic framework (MOF)/epoxy coatings: A review. Materials 2020, 13, 2881. [Google Scholar] [CrossRef]
- Ejsmont, A.; Andreo, J.; Lanza, A.; Galarda, A.; Macreadie, L.; Wuttke, S.; Canossa, S.; Ploetz, E.; Goscianska, J. Applications of reticular diversity in metal–organic frameworks: An ever-evolving state of the art. Coord. Chem. Rev. 2021, 430, 213655. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Lou, Z. Recent advancements in MOF/biomass and bio-MOF multifunctional materials: A review. Sustainability 2022, 14, 5768. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Liu, X.; Li, K.; Xu, Q. Recent advances in MOF-bio-interface: A review. Nanotechnology 2023, 34, 202002. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Soni, S.; Bajpai, P.K.; Arora, C. A review on metal-organic framework: Synthesis, properties and application. Charact. Appl. Nanomater. 2020, 3, 87–106. [Google Scholar] [CrossRef]
- Bull, O.S.; Bull, I.; Amadi, G.K.; Odu, C.O.; Okpa, E. A review on metal- organic frameworks (MOFS), synthesis, activation, characterisation, and application. Orient. J. Chem. 2022, 38, 490–516. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, S.; Yan, X.; Lv, Y. Recent advances in metal-organic frameworks: Synthesis, application and toxicity. Sci. Total Environ. 2023, 902, 165944. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, W.; Yu, S.; Xia, S.-L.; Liu, Y.-N.; Yang, G.-J. Application of MOF-based nanotherapeutics in light-mediated cancer diagnosis and therapy. J. Nanobiotechnol. 2022, 20, 421. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, A.; Khiar, N.; Carrillo-Carrión, C. Recent progress of metal–organic frameworks as sensors in (bio)analytical fields: Towards real-world applications. Anal. Bioanal. Chem. 2023, 415, 2005–2023. [Google Scholar] [CrossRef]
- Sadiq, S.; Khan, S.; Khan, I.; Khan, A.; Humayun, M.; Wu, P.; Usman, M.; Khan, A.; Alanazi, A.F.; Bououdina, M. A critical review on metal-organic frameworks (MOFs) based nanomaterials for biomedical applications: Designing, recent trends, challenges, and prospects. Heliyon 2024, 10, e25521. [Google Scholar] [CrossRef]
- Damian-Buda, A.-I.; Alipanah, N.; Bider, F.; Sisman, O.; Neščáková, Z.; Boccaccini, A.R. Metal-organic framework (MOF)-bioactive glass (BG) systems for biomedical applications—A review. Mater. Today Bio 2025, 30, 101413. [Google Scholar] [CrossRef]
- Patil, P.D.; Gargate, N.; Tiwari, M.S.; Nadar, S.S. Two-dimensional metal-organic frameworks (2D–MOFs) as a carrier for enzyme immobilization: A review on design and bio-applications. Int. J. Biol. Macromol. 2025, 291, 138984. [Google Scholar] [CrossRef] [PubMed]
- Moharramnejad, M.; Karim, A.; Gharanli, S.; Malekshah, R.E.; Amini, A.h.; Sharifi, M.S.; Basmenj, Z.S.; Salariyeh, Z.; Mohammadkhani, M.; Shahi, M.; et al. A comprehensive review of MOFs based on electrochemical biosensors as smart platforms in cancer biomarkers detection. Microchem. J. 2025, 208, 112498. [Google Scholar] [CrossRef]
- Li, D.; Yadav, A.; Zhou, H.; Roy, K.; Thanasekaran, P.; Lee, C. Advances and applications of metal-organic frameworks (MOFs) in emerging technologies: A comprehensive review. Glob. Chall. 2024, 8, 2300244. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, T.; Yuan, G.; Li, Q.; Pang, H. MOF and MOF-derived composites for flexible energy storage devices. Compos. Commun. 2024, 52, 102144. [Google Scholar] [CrossRef]
- Han, Z.; Yang, Y.; Rushlow, J.; Huo, J.; Liu, Z.; Hsu, Y.-C.; Yin, R.; Wang, M.; Liang, R.; Wang, K.-Y.; et al. Development of the design and synthesis of metal–organic frameworks (MOFs)—from large scale attempts, functional oriented modifications, to artificial intelligence (AI) predictions. Chem. Soc. Rev. 2025, 54, 367–395. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhu, G. A review on metal organic frameworks (MOFs) modified membrane for remediation of water pollution. Environ. Eng. Res. 2021, 26, 190435. [Google Scholar] [CrossRef]
- Jeong, C.; Ansari, M.Z.; Anwer, A.H.; Kim, S.-H.; Nasar, A.; Shoeb, M.; Mashkoor, F. A review on metal-organic frameworks for the removal of hazardous environmental contaminants. Sep. Pur. Technol. 2023, 305, 122416. [Google Scholar] [CrossRef]
- Motshekga, S.C.; Oyewo, O.A.; Makgato, S.S. Recent and prospects of synthesis and application of metal-organic frameworks (MOFs) in water treatment: A review. J. Inorg. Organomet. Polym. Mater. 2024, 34, 3907–3930. [Google Scholar] [CrossRef]
- Shafti, D.M.; Dahlan, I.; Din, A.T.M. A review of the effectiveness of metal–organic frameworks in removing dye effluents. Water Pract. Technol. 2024, 19, 4699–4733. [Google Scholar] [CrossRef]
- Chang, J.; Bian, Y.; Wang, Y. MOFs-coupled fiber membranes: A versatile platform for water purification. Sep. Purif. Technol. 2025, 357, 130059. [Google Scholar] [CrossRef]
- He, L.; Wang, Z.; Wang, H.; Wu, Y.-N. Are MOFs ready for environmental applications: Assessing stability against natural stressors? Coord. Chem. Rev. 2025, 526, 216361. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Mustafa, F.S.; Hamarawf, R.F.; Omer, K.M. Adsorptive removal of toxic heavy metals from aquatic environment by metal organic framework (MOF): A review. J. Water Process Eng. 2025, 70, 106867. [Google Scholar] [CrossRef]
- Letwaba, J.; Uyor, U.O.; Mavhungu, M.L.; Achuka, N.O.; Popoola, P.A. A review on MOFs synthesis and effect of their structural characteristics for hydrogen adsorption. RSC Adv. 2024, 14, 14233–14253. [Google Scholar] [CrossRef] [PubMed]
- Alamro, A.; Balbaied, T. Boron nitride nanostructures (BNNS) within metal–organic frameworks (MOFs): Electrochemical platform for hydrogen sensing and storage. Analytica 2024, 5, 599–618. [Google Scholar] [CrossRef]
- Sutton, A.L.; Mardel, J.I.; Hill, M.R. Metal-organic frameworks (MOFs) as hydrogen storage materials at near-ambient temperature. Chem. Eur. J. 2024, 30, e202400717. [Google Scholar] [CrossRef] [PubMed]
- Gangu, K.K.; Jonnalagadda, S.B. A review on metal-organic frameworks as congenial heterogeneous catalysts for potential organic transformations. Front. Chem. 2021, 9, 747615. [Google Scholar] [CrossRef]
- Khan, M.S.; Li, Y.; Li, D.-S.; Qiu, J.; Xu, X.; Yang, H.Y. A review of metal–organic framework (MOF) materials as an effective photocatalyst for degradation of organic pollutants. Nanoscale Adv. 2023, 5, 6318–6348. [Google Scholar] [CrossRef]
- Bautista, K.A.; Mata, E.J.D.C.; Mercado, C.D.B.; Placio, R.J.C.; Alano, V.H.O.; Soriano, A.N.; Rubi, R.V.C. A systematic review of metal–organic framework (MOF)-based nanocomposites and their application in photocatalytic degradation of pharmaceutical compounds. Eng. Proc. 2024, 67, 33. [Google Scholar]
- Amjad, A.A.; Murtaza, M.; Shah, S.S.A.; Ahmad, I.; Alawadhi, H.; Shah, W.A.; Waseem, A. Atomically precise MOF-based electrocatalysts by design: Hydrogen evolution applications. Fuel 2025, 385, 134021. [Google Scholar] [CrossRef]
- Virender, V.; Pandey, V.; Singh, G.; Sharma, P.K.; Bhatia, P.; Solovev, A.A.; Mohan, B. Hybrid metal-organic frameworks (MOFs) for various catalysis applications. Top. Curr. Chem. 2025, 383, 3. [Google Scholar] [CrossRef]
- Yusuf, V.F.; Malek, N.I.; Kailasa, S.K. Review on metal–organic framework classification, synthetic approaches, and influencing factors: Applications in energy, drug delivery, and wastewater treatment. ACS Omega 2022, 7, 44507–44531. [Google Scholar] [CrossRef]
- Alluhaibi, M.S.; Shariq, M.; Alkhayri, F.; Karmouch, R.; Hussain, S.; Ali, S.K.; Azeez, N.A.; Farid, A.; Khan, M. Unlocking the power of MOF-inspired nanomaterials: Enhancing solar cell efficiency through advanced structures and properties. Synth. Met. 2025, 311, 117823. [Google Scholar] [CrossRef]
- Abazari, R.; Sanati, S.; Fan, W.K.; Tahir, M.; Nayak, S.; Parida, K.; El-Shahat, M.; Abdelhameed, R.M.; Nesterov, D.S.; Kirillov, A.M.; et al. Design and engineering of MOF/LDH hybrid nanocomposites and LDHs derived from MOF templates for electrochemical energy conversion/storage and environmental remediation: Mechanism and future perspectives. Coord. Chem. Rev. 2025, 523, 216256. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, R.; Draksharapu, A. Correlating structure-activity-stability relationship of high-valent 3d-metal-based MOFs and MOF-derived materials for electrochemical energy conversion and storage. Coord. Chem. Rev. 2025, 523, 216239. [Google Scholar] [CrossRef]
- Li, W.; Li, C.; Guo, J.; Jiang, T.; Kang, W.; Pang, H. Advanced alkali metal batteries based on MOFs and their composites. ChemSusChem 2025, 18, e202402289. [Google Scholar] [CrossRef]
- Mooibroek, T.J.; Gamez, P. The s-triazine ring, a remarkable unit to generate supramolecular interactions. Inorg. Chim. Acta 2007, 360, 381–404. [Google Scholar] [CrossRef]
- Yu, M.-H.; Liu, X.-T.; Space, B.; Chang, Z.; Bu, X.-H. Metal-organic materials with triazine-based ligands: From structures to properties and applications. Coord. Chem. Rev. 2021, 427, 213518. [Google Scholar] [CrossRef]
- Mondal, S.; Alam, N.; Sarma, D. Triazine core anchored lanthanide driven soft gels: Photo switching emission, robust anticounterfeiting, and smart sensor probe for nitroexplosive/nitrofuran antibiotics. ACS Appl. Eng. Mater. 2024, 2, 1467–1482. [Google Scholar] [CrossRef]
- Park, H.J.; Suh, M.P. Enhanced isosteric heat, selectivity, and uptake capacity of CO2 adsorption in a metal-organic framework by impregnated metal ions. Chem. Sci. 2013, 4, 685–690. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.-Z.; Zhang, D.-S.; Zhu, B.; Li, J.-R. A hydrothermal stable Zn(II)-based metal-organic framework: Structural modulation and gas adsorption. Dalton Trans. 2015, 44, 15697–15702. [Google Scholar] [CrossRef]
- Pal, S.; Bhunia, A.; Jana, P.P.; Dey, S.; Mçllmer, J.; Janiak, C.; Nayek, H.P. Microporous La–metal–organic framework (MOF) with large surface area. Chem. Eur. J. 2015, 21, 2789–2792. [Google Scholar] [CrossRef]
- Wang, J.; Sun, W.; Chang, S.; Liu, H.; Zhang, G.; Wang, Y.; Liu, Z. A terbium metal–organic framework with stable luminescent emission in wide pH range that acts as a quantitative detection material for nitroaromatics. RSC Adv. 2015, 5, 48574–48579. [Google Scholar] [CrossRef]
- Etemadi-Davan, E.; Iranpoor, N. Efficient Ni-catalyzed conversion of phenols protected with 2,4,6-trichloro-1,3,5-triazine (TCT) to olefins. Chem. Commun. 2017, 53, 12794–12797. [Google Scholar] [CrossRef]
- Xiao, Y.; You, Z.X.; Xing, Y.H.; Bai, F.Y.; Shi, Z. Three-pole wheel paddle luminescent metal organic frameworks (Lmofs) based on the oxygen substituted triazine tricarboxylic acid ligand: Recognition and detection of small drug molecules and aromatic amine molecules. Dalton Trans. 2022, 51, 9336–9347. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.F.; Xu, F.; Zhang, N.; Hou, C.Y.; Sun, L.X.; Xing, Y.H.; Bai, F.Y. High-symmetry Co/Ni triazine polycarboxylate diverse frameworks constructed by MX(COO)Y building blocks: Characterization and catalytic performance evaluation of p-nitrophenol. Inorg. Chem. 2022, 61, 19951–19960. [Google Scholar] [CrossRef]
- Fang, Q.-R.; Yuan, D.-Q.; Sculley, J.; Li, J.-R.; Han, Z.-B.; Zhou, H.-C. Functional mesoporous metal-organic frameworks for the capture of heavy metal ions and size-selective catalysis. Inorg. Chem. 2010, 49, 11637–11642. [Google Scholar] [CrossRef] [PubMed]
- Aliev, S.B.; Samsonenko, D.G.; Rakhmanova, M.I.; Dybtsev, D.N.; Fedin, V.P. Syntheses and structural characterization of lithium carboxylate frameworks and guest-dependent photoluminescence study. Cryst. Grwght Des. 2014, 14, 4355–4363. [Google Scholar] [CrossRef]
- Xia, T.; Song, T.; Zhang, G.; Cui, Y.; Yang, Y.; Wang, Z.; Qian, G. A terbium metal–organic framework for highly selective and sensitive luminescence sensing of Hg2+ ions in aqueous solution. Chem. Eur. J. 2016, 22, 18429–18434. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Song, T.; Cui, Y.; Yang, Y.; Qian, G. Dye encapsulated terbium-based metal-organic framework for ratiometric temperature sensing. Dalton Trans. 2016, 45, 18689–18695. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Zhang, S.-R.; Wang, W.; Xu, Y.-H.; Che, G.-B. A 3D metal–organic framework with dual-aerial-octahedral trinucleate building units: Synthesis, structure and fluorescent sensing property. New J. Chem. 2018, 42, 14648–14654. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, W.; Yao, M.; Wu, Z.; Jiao, Y.; Qu, H. Novel triazine-based metal-organic frameworks: Synthesis and mulifunctional application of flame retardant, smoke suppression and toxic attenuation on EP. Mater. Des. 2023, 226, 111664. [Google Scholar] [CrossRef]
- Sun, C.-Y.; To, W.-P.; Wang, X.-L.; Chan, K.-T.; Su, Z.-M.; Che, C.-M. Metal–organic framework composites with luminescent gold(III) complexes. Strongly emissiveand long-lived excited states in open air and photocatalysis. Chem. Sci. 2015, 6, 7105–7111. [Google Scholar] [CrossRef]
- Ghosh, S.; De Adhikari, A.; Nath, J.; Nayak, G.C.; Nayek, H.P. Lanthanide (III) metal-organic frameworks: Syntheses, structures and supercapacitor application. ChemistrySelect 2019, 4, 10624–10631. [Google Scholar] [CrossRef]
- Yousafa, A.; Xua, N.; Arifa, A.M.; Zhoua, J.; Suna, C.-Y.; Wanga, X.-L.; Su, Z.-M. A triazine-based metal-organic framework with solvatochromic behaviour and selectively sensitive photoluminescent detection of nitrobenzene and Cu2+ ions. Dyes Pigm. 2019, 163, 159–167. [Google Scholar] [CrossRef]
- Yin, Z.; Zhou, Y.-L.; Zeng, M.-H.; Kurmoo, M. The concept of mixed organic ligands in metal–organic frameworks: Design, tuning and functions. Dalton Trans. 2015, 44, 5258–5275. [Google Scholar] [CrossRef]
- Pullen, S.; Clever, G.H. Mixed-ligand metal–organic frameworks and heteroleptic coordination cages as multifunctional scaffolds—A comparison. Acc. Chem. Res. 2018, 51, 3052–3064. [Google Scholar] [CrossRef] [PubMed]
- Viciano-Chumillas, M.; Liu, X.; Leyva-Pérez, A.; Armentano, D.; Ferrando-Soria, J.; Pardo, E. Mixed component metal-organic frameworks: Heterogeneity and complexity at the service of application performances. Coord. Chem. Rev. 2022, 451, 214273. [Google Scholar] [CrossRef]
- Mylonas-Margaritis, I.; Mayans, J.; Efthymiou, C.G.; McArdle, P.; Papatriantafyllopoulou, C. Mixed-ligand metal-organic frameworks: Synthesis and characterization of new MOFs containing pyridine-2,6-dimethanolate and benzene-1,4-dicarboxylate ligands. Eur. J. Inorg. Chem. 2022, 2022, e202200140. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, S.; Fu, S.; Wang, X.; Liua, G.; Yang, H. Pyridine-induced caused structural reconfiguration forming ultrathin 2D metal–organic frameworks for the oxygen evolution reaction. J. Mater. Chem. A 2024, 12, 8885–8892. [Google Scholar] [CrossRef]
- Angelova, M.; Lazarova, H.; Kurteva, V.; Nikolova, R.; Rusew, R.; Shivachev, B. A novel zinc-based MOF featuring 2,4,6-tris-(4-carboxyphenoxy)-1,3,5-triazine: Structure, adsorption and photocatalytic activity. Crystals 2025, 15, 348. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, X.-H.; Wang, X.-D. 2,4,6-Tris(pyrimidin-2-ylsulfanyl)-1,3,5-triazine. Acta Cryst. 2005, E61, o1133–o1134. [Google Scholar] [CrossRef]
- Cornella, J.; Zarate, C.; Martin, R. Metal-catalyzed activation of ethers via C–O bond cleavage: A new strategy for molecular diversity. Chem. Soc. Rev. 2014, 43, 8081–8097. [Google Scholar] [CrossRef]
- Jian, Y.; Meng, Y.; Li, H. Selectivity control of C-O bond cleavage for catalytic biomass valorization. Front. Energy Res. 2022, 9, 827680. [Google Scholar] [CrossRef]
- Lang, M.; Li, H. Heterogeneous metal-based catalysts for cyclohexane synthesis from hydrodeoxygenation of lignin-derived phenolics. Fuel 2023, 344, 128084. [Google Scholar] [CrossRef]
- Li, P.; Zhang, M.; Zhang, L. Recent advances in C–O bond cleavage of aryl, vinyl, and benzylic ethers. Top. Curr. Chem. 2024, 382, 38. [Google Scholar] [CrossRef]
- De Smet, G.; Bai, X.; Maes, B.U.W. Selective C(aryl)–O bond cleavage in biorenewable phenolics. Chem. Soc. Rev. 2024, 53, 5489–5551. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Desper, J.; Urbina, J.F. Is conformational flexibility in a supramolecular reagent advantageous for high-yielding co-crystallization reactions? CrystEngComm 2005, 7, 193–201. [Google Scholar] [CrossRef]
- Bruker. APEX6, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2025. [Google Scholar]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]



| Series 1 | Series 2 | Series 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Substituent XR | Yield, % | Substituent OAr | Yield, % | Substituent XAr | Yield, % | |||
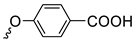 | 3a | 87 1 | 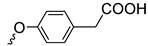 | 3j | 99 | 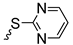 | 3o | 84 |
 | 3b | 89 |  | 3k | 96 | 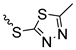 | 3p | 87 |
 | 3c | 91 | 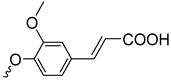 | 3l | 91 |  | 3q | 88 |
 | 3d | 93 | ||||||
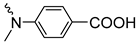 | 3e | 65 | 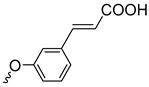 | 3m | 96 |  | 3r | 67 |
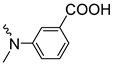 | 3f | 66 | ||||||
 | 3g | 48 | 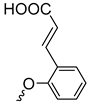 | 3n | 88 | Additional | ||
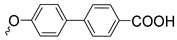 | 3s | 89 | ||||||
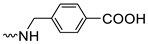 | 3h | 55 | 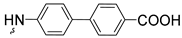 | 3t | 69 | |||
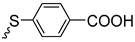 | 3i | 90 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurteva, V.B.; Rusew, R.I.; Petkova, Z.S.; Angelova, M.; Shivachev, B.L. s-Triazine-Based Ligands Possessing Identical Heteroatom-Bridged Substituents—Unexpected Triazine-O Bond Cleavage. Molecules 2025, 30, 3811. https://doi.org/10.3390/molecules30183811
Kurteva VB, Rusew RI, Petkova ZS, Angelova M, Shivachev BL. s-Triazine-Based Ligands Possessing Identical Heteroatom-Bridged Substituents—Unexpected Triazine-O Bond Cleavage. Molecules. 2025; 30(18):3811. https://doi.org/10.3390/molecules30183811
Chicago/Turabian StyleKurteva, Vanya B., Rusi I. Rusew, Zhanina S. Petkova, Magdalena Angelova, and Boris L. Shivachev. 2025. "s-Triazine-Based Ligands Possessing Identical Heteroatom-Bridged Substituents—Unexpected Triazine-O Bond Cleavage" Molecules 30, no. 18: 3811. https://doi.org/10.3390/molecules30183811
APA StyleKurteva, V. B., Rusew, R. I., Petkova, Z. S., Angelova, M., & Shivachev, B. L. (2025). s-Triazine-Based Ligands Possessing Identical Heteroatom-Bridged Substituents—Unexpected Triazine-O Bond Cleavage. Molecules, 30(18), 3811. https://doi.org/10.3390/molecules30183811








