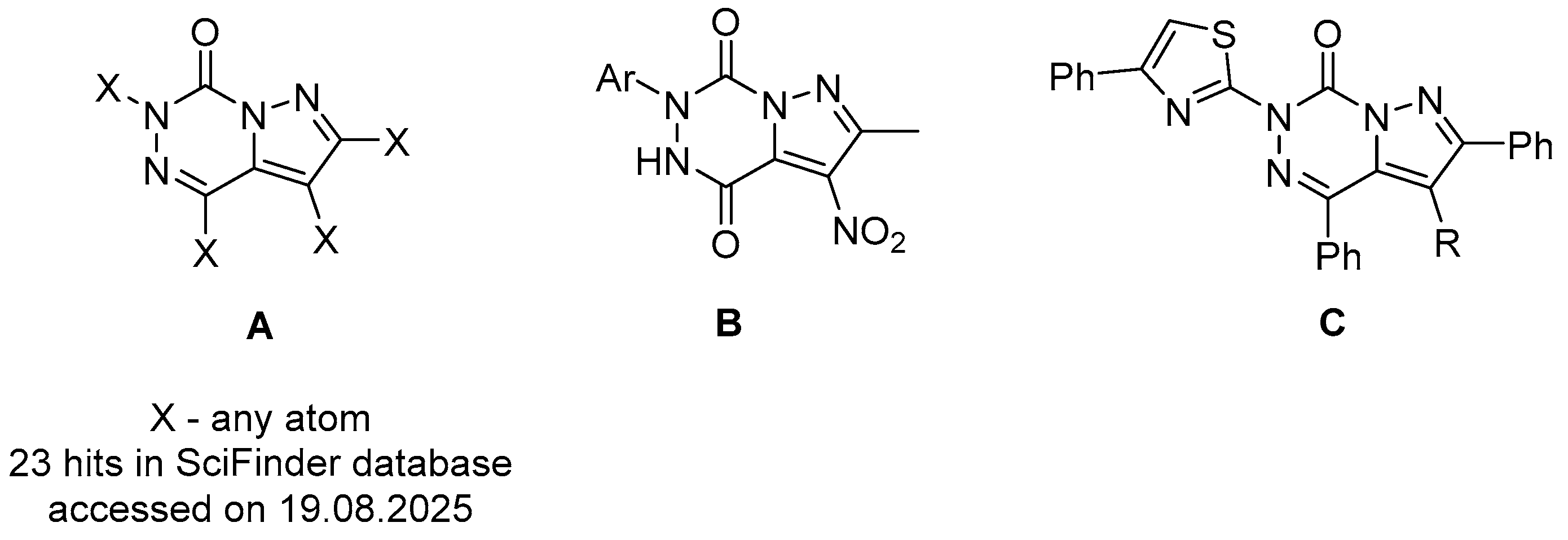
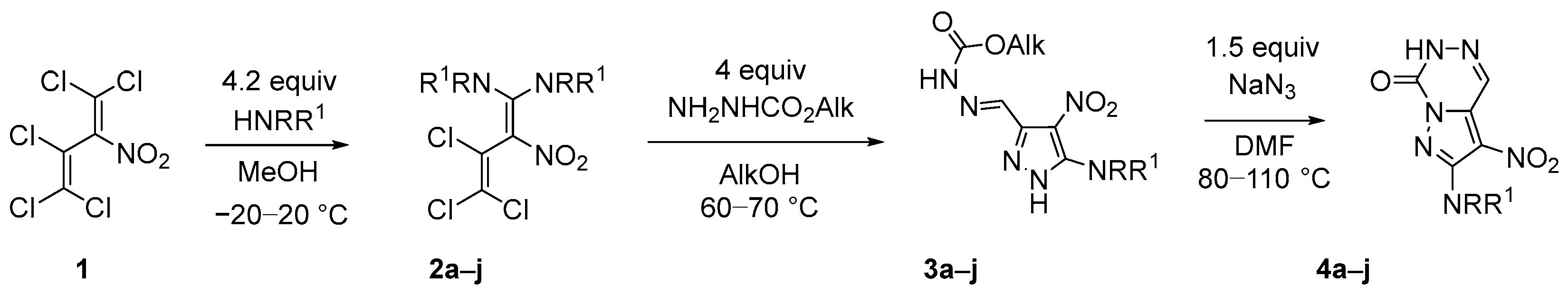
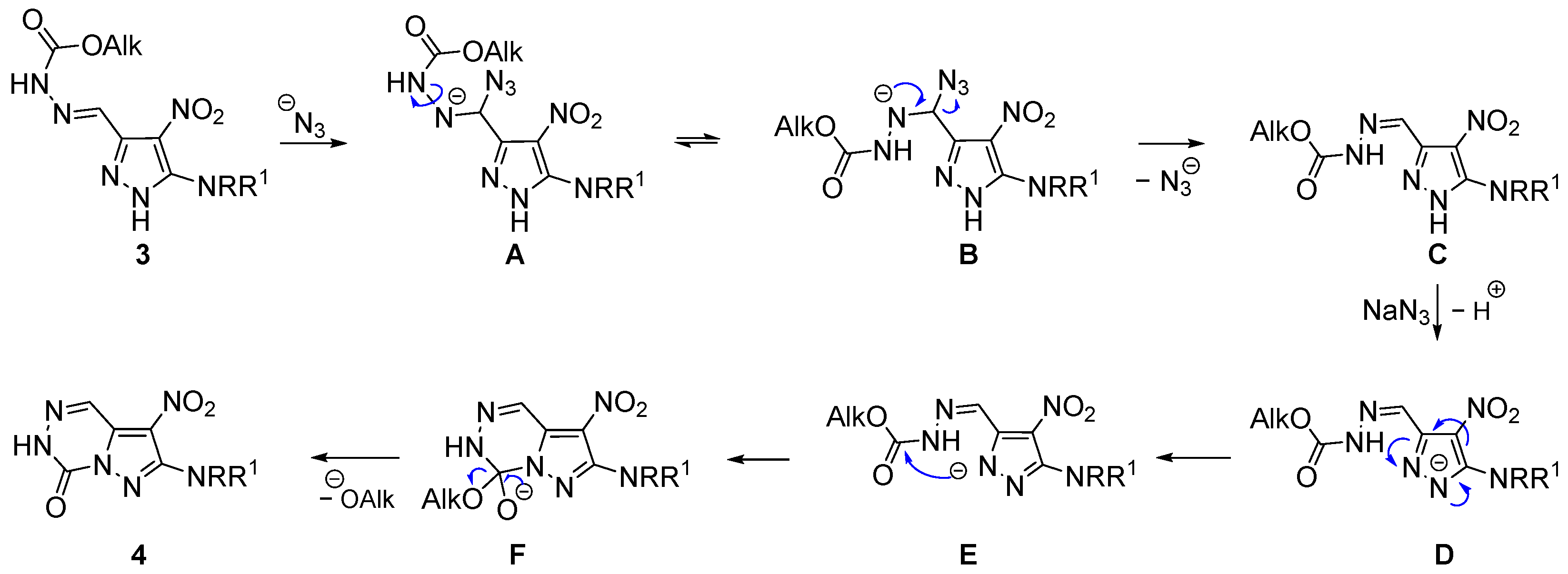
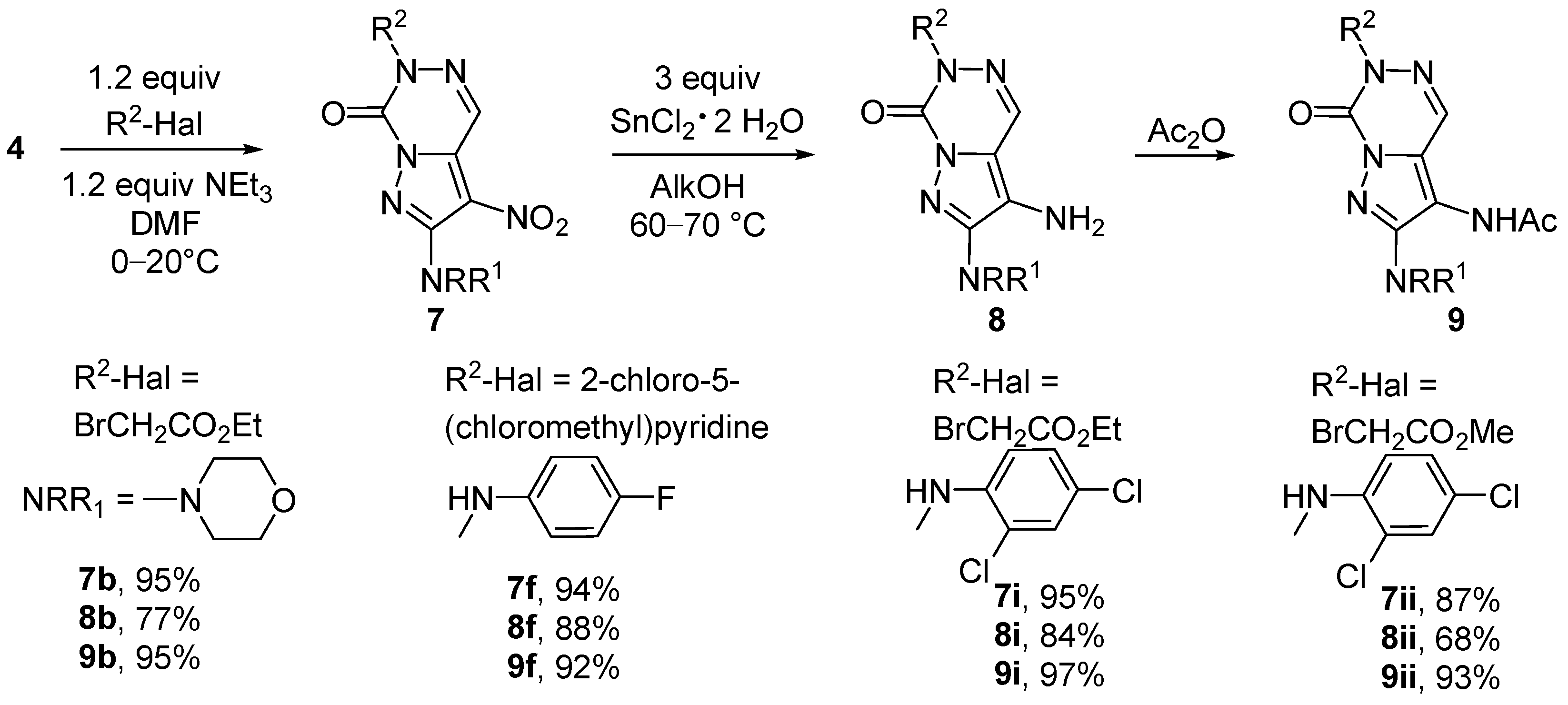
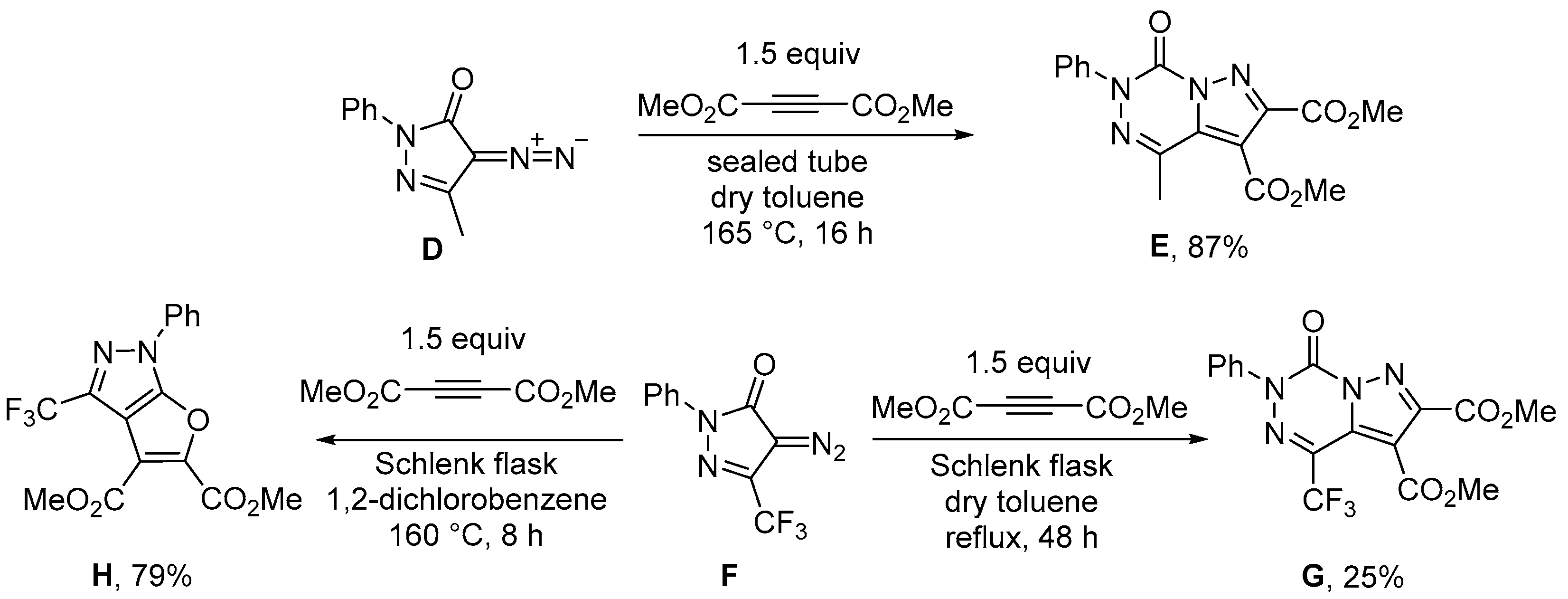3.1.2. Synthesis Protocols
2-Nitro-pentachloro-1,3-butadiene (
1). The product was prepared from 2
H–pentachloro-1,3-butadiene at a 53% yield (bp 69–71 °C/1 mbar), according to the literature [
14].
1,1-Bis(methylamino)-3,4,4-trichloro-2-nitrobuta-1,3-diene (2a). A 33% methylamine (3.92 g, 42 mmol) solution (soln.) in ethanol (EtOH) was added dropwise to a soln. of nitrodiene 1 (2.71 g, 10 mmol) in 30 mL methanol (MeOH) at −20 °C within 15 min. The resulting mixture was kept at the same temperature for an additional 1 h and 2 h at room temperature (rt) with stirring. Subsequently, the supernatant liquid was concentrated in vacuo to a volume of 10 mL and cooled to 10 °C. The resulting precipitate was filtered off, washed with water (2 × 10 mL), and then dried in vacuo to give 2a as a colorless solid; yield: 1.44 g (5.50 mmol, 55%) mp 130–131 °C. IR (KBr): ṽmax = 3218, 1630, 1573, 1409, 1318, 1138, 1039, 1012, 950, 840, 793, 698, 5940 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 3.11 (d, 1JC-H = 139.8 Hz, 3H), 3.09 (d, 1JC-H = 139.6 Hz, 3H) ppm, NH groups could not be detected. 1H-NMR (DMSO-d6, 400 MHz): δ = 8.33 (br. s, 2H, NH), 2.83 (d, J = 5.5 Hz, 6H) ppm. 13C-NMR (DMSO-d6, 101 MHz): δ = 158.9 (NCN), 127.2 and 113.2 (CCl2 = CCl), 121.4 (C-NO2), 30.5 (2 CH3) ppm. In DMSO-d6 compound 2a slowly hydrolyzes to 1,1-bis(methylamino)-4,4-dichloro-2-nitrobut-1-en-3-one: 1H-NMR (400 MHz): δ = 9.46 (q, J = 5.5 Hz, 1H, NH), 8.91 (q, J = 5.5 Hz, 1H, NH), 7.53 (d, 1JC-H = 185.1 Hz, 1H, CHCl2), 2.72 (d, J = 5.5 Hz, 6H) ppm. 13C-NMR (101 MHz): δ = 174.6 (C=O), 160.3 (NCN), 104.4 (C-NO2), 69.5 (CHCl2), 29.9 (CH3), 29.2 (CH3) ppm. MS: m/z (%) = 259 (6) [M+] 224 (12) [M—Cl]+, 177 (12) [M—Cl—HNO2]+, 114 (100) [M—C2Cl3—CH4]+. HRMS-ESI: m/z was calc. for C6H8Cl3N3O2Na [M + Na]+ 281.9574 and found 281.9569.
1,1-Bis(morpholino)-3,4,4-trichloro-2-nitrobuta-1,3-diene (
2b). A soln. of morpholine (3.66 g, 42 mmol) in 10 mL MeOH was added dropwise to a soln. of nitrodiene
1 (2.71 g, 10 mmol) in 30 mL MeOH at −20 °C within 15 min. The resulting mixture was kept at the same temperature for an additional 1 h and 2 h at rt with stirring. Subsequently, the resulting precipitate was filtered off, washed with water (2 × 10 mL) and MeOH (2 × 10 mL), and then dried in vacuo to give
2b as a yellowish solid; yield: 3.20 g (8.60 mmol, 86%); mp 251–252 °C. IR (KBr):
ṽmax = 2909, 2862, 1536, 1497, 1383, 1290, 1108, 1030, 876, 800, 716, 575 cm
−1.
1H-NMR (CDCl
3, 400 MHz):
δ = 4.09–3.64 (m, 6H), 3.64–3.49 (m, 4H), 3.47–3.33 (m, 5H), 3.32–3.21 (m, 1H) ppm.
1H-NMR (DMSO-
d6, 400 MHz):
δ = 3.88–3.13 (m, 16H) ppm. All spectral data and the mp are in accordance with the literature [
15].
1,1-Bis(2-(4-chlorophenoxy)ethylamino)-3,4,4-trichloro-2-nitrobuta-1,3-diene (2c). A soln. of 2-(4-chlorophenoxy)ethan-1-amine (7.21 g, 42 mmol) in 20 mL MeOH was added dropwise to a soln. of nitrodiene 1 (2.71 g, 10 mmol) in 30 mL MeOH at −20 °C within 15 min. The resulting mixture was kept at the same temperature for an additional 1 h and 2 h at rt with stirring. Subsequently, the supernatant liquid was concentrated in vacuo to a volume of 20 mL, cooled to 10 °C, and diluted with 100 mL cold water. The resulting precipitate was filtered off, washed with water (3 × 10 mL) and diethyl ether (2 × 10 mL), and then dried in vacuo to give 2c as a colorless solid, yield: 4.77 g (8.80 mmol, 88%) mp 118–120 °C. IR (ATR): ṽmax = 002993, 2930, 2870, 1632, 1489, 1338, 1234, 1048, 819, 716, 611, 505 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 8.43 (br.s, 2H, 2NH), 7.24 (d, J = 8.9 Hz, 4CH), 6.82 (d, J = 8.9 Hδz, 4CH), 4.14 (t, J = 4.8 Hz, 4H, 2OCH2) 3.72 (q, J = 5.4 Hz, 4H, 2NCH2) ppm. 13C-NMR (CDCl3, 101 MHz): δ = 159.2 (NCN), 156.2 (Cq-O), 129.7 (4CH), 127.1 (2CAr-Cl), 125.5 and 125.1 (CCl2 = CCl), 115.9 (4CH), 107.4 (C-NO2), 67.9 (2OCH2), 43.9 (2NCH2) ppm. MS: m/z (%) = 541 (1) [M+], 422 (12) [M—Cl—HCl—NO2]+, 352 (4) [M—ArOCH2—NO2]+, 128 (100) [p-Cl-C6H4-OH]+. HRMS (ESI): m/z was calc. for C20H18Cl5N3O4Na [M + Na]+ 563.9603 and found 563.9608.
1,1-Bis(2,6-difluorobenzylamino)-3,4,4-trichloro-2-nitrobuta-1,3-diene (2d). The product was prepared according to diene 2c from nitrodiene 1 (2.71 g, 10 mmol) and (2,6-difluorophenyl)methanamine (6.01 g, 42 mmol): colorless solid, yield 4.56 g (9.40 mmol, 94%) mp 130–131 °C. IR (ATR): ṽmax = 3340, 1593, 1538, 1469, 1336, 1136, 1013, 783, 608, 497, 467, 411 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 7.52 (br.s, 2H, 2NH), 7.35 (tt, J = 6.8 (C-F), 8.1 (C-H) Hz, 2CH), 6.95 (t, J = 8.1 Hz, 4CH), 4.59 (d, J = 5.6 Hz, 4H, 2NCH2) ppm. 13C-NMR (CDCl3, 101 MHz): δ = 161.2 (d, 1JC-F = 249.8 Hz, 2Cq-F), 161.1 (d, 1JC-F = 249.9 Hz, 2Cq-F), 158.0 (NCN), 131.0 (t, 3JC-F = 10.5 Hz, 2CH), 127.2 and 123.8 (CCl2=CCl), 111.9 (t, 2JC-F = 17.4 Hz, 2Cq), 111.8 (dd, 2JC-F = 19.3 Hz, 4JC-F = 6.2 Hz, 4CH), 108.7 (C-NO2), 37.0 (2NCH2) ppm. MS: m/z (%) = 483 (1) [M+], 448 (1) [M—Cl]+, 413 (3) [M—2 Cl]+, 127 (100) [CH2C6H4F2+].
1,1-Bis(phenylamino)-3,4,4-trichloro-2-nitrobuta-1,3-diene (
2e). The product was prepared according to diene
2c from nitrodiene
1 (2.71 g, 10 mmol) and aniline (3.91 g, 42 mmol): pale solid, yield 3.39 g (8.80 mmol, 88%), mp 165–166 °C. IR (ATR):
ṽmax = 3032, 1630, 1580, 1496, 1380, 1327, 1125, 856, 804, 689, 588 cm
−1.
1H-NMR (DMSO-
d6, 400 MHz):
δ = 10.25 (br.s, 2H, 2NH), 7.27–7.07 (m, 10CH) ppm.
13C-NMR (DMSO-
d6, 101 MHz):
δ = 152.2 (NCN), 138.5 (2Cq-NH), 129.2 (4CH), 126.2 and 122.5 (CCl
2 = CCl), 115.2 (2CH), 121.9 (4CH), 109.8 (C-NO
2) ppm. All spectral data are in accordance with the literature [
16].
1,1-Bis(4-fluorophenylamino)-3,4,4-trichloro-2-nitrobuta-1,3-diene (
2f) was prepared according to the literature [
9]. All spectral data are in accordance with the literature [
9].
1,1-Bis(4-chlorophenylamino)-3,4,4-trichloro-2-nitrobuta-1,3-diene (
2g) was prepared according to the literature [
9]. All spectral data are in accordance with the literature [
9].
1,1-Bis(2-chlorophenylamino)-3,4,4-trichloro-2-nitrobuta-1,3-diene (
2h) was prepared according to the literature [
9]. All spectral data are in accordance with the literature [
17].
1,1-Bis(2,4-dichlorophenylamino)-3,4,4-trichloro-2-nitrobuta-1,3-diene (2i). The product was prepared according to diene 2b from nitrodiene 1 (2.71 g, 10 mmol) and 2,4-dichloroaniline (6.81 g, 42 mmol): light brown solid, yield 4.56 g (8.60 mmol, 86%), mp 156–157 °C. IR (KBr): ṽmax = 3087, 1614, 1571, 1476, 1282, 1127, 1101, 1058, 868, 792, 690, 600 cm−1. 1H-NMR (DMSO-d6, 400 MHz): δ = 10.31 (br.s, 2H, 2NH), 7.62 (d, J = 2.1 Hz, 2CH), 7.36 (dd, J = 8.6, 2.1 Hz, 2CH), 7.27 (d, J = 8.6 Hz, 2CH) ppm. 13C-NMR (DMSO-d6, 101 MHz): δ = 154.8 (NCN), 133.7 (2Cq-NH), 132.1 (2Cq-Cl), 131.1 (2Cq-Cl), 129.5 (2CH), 129.2 (2CH), 128.1 (2CH), 125.1 and 125.0 (CCl2 = CCl), 108.9 (C-NO2) ppm. MS: m/z (%) = 521 (5) [M+], 484 (12) [M—Cl]+, 475 (12) [M—NO2]+, 161 (100) [2,4-dichloroaniline—H]+.
1,1-Bis(2-methyl-3-trifluoromethylphenylamino)-3,4,4-trichloro-2-nitrobuta-1,3-diene (2j). A soln. of 2-methyl-3-trifluoromethylaniline (7.36 g, 42 mmol) in 20 mL MeOH was added dropwise to a soln. of nitrodiene 1 (2.71 g, 10 mmol) in 30 mL MeOH at −20 °C within 15 min. The resulting mixture was kept at the same temperature for an additional 1 h and 1 d at rt with stirring. Subsequently, the supernatant liquid was concentrated in vacuo to a volume of 20 mL, cooled to 10 °C, and diluted with 100 mL hydrochloric acid (5% in water). The resulting precipitate was filtered off, washed with water (3 × 10 mL) and PE (2 × 10 mL), and then dried in vacuo to give 2j as a colorless solid; yield: 4.66 g (8.50 mmol, 85%) mp 155–156 °C. IR (ATR): ṽmax = 3234, 1605, 1581, 1518, 1313, 1233, 1171, 1111, 1063, 1014, 803, 614, 522, 477 cm−1. 1H-NMR (CDCl3, 400 MHz): δ = 11.86 (br.s, 1H, NH), 7.40 (d, J = 7.6 Hz, 2CH), 7.14 (d, J = 7.8 Hz, 2CH), 7.09 (dd, J = 7.8, 7.6 Hz, 2CH), 6.19 (br.s, 1H, NH), 2.33 (s, 6H, 2CH3) ppm. 13C-NMR (CDCl3, 101 MHz): δ = 154.7 (NCN), 136.0 (2Cq), 133.1 (2Cq), 130.6 (q, 2JC-F = 30.3 Hz, 2Cq), 129.7 (2CH), 128.9 and 122.7 (CCl2 = CCl), 126.5 (2CH), 125.5 (q, 3JC-F = 5.3 Hz, 2CH), 123.5 (q, 1JC-F = 274.0 Hz, 2Cq), 109.9 (C-NO2), 14.1 (q, 4JC-F = 2.4 Hz, 2CH3) ppm. MS: m/z (%) = 548 (3) [M+], 512 (5) [M—Cl]+, 477 (40) [M—2 Cl]+, 417 (10) [M—C2Cl3]+, 109 (100).
Methyl 2-((5-(methylamino)-4-nitro-1H-pyrazol-3-yl)methylene)hydrazine-1-carboxylate (3a). To a suspension of 261 mg (1.00 mmol) diene 2a in 20 mL MeOH, 361 mg (4.00 mmol) methyl hydrazinecarboxylate was added, and the resulting mixture was refluxed for 8 h. Subsequently, the supernatant liquid was concentrated in vacuo to a volume of 10 mL and cooled to rt. The precipitate was filtered off, washed with MeOH (3 mL), 5% hydrochloric acid (3 × 5 mL), water (5 mL), and MeOH again (3 mL), and finally dried under reduced pressure; yield: 162 mg (0.67 mmol, 67%) pyrazole 2a as a single isomer, yellow solid, mp 243–245 °C. IR (ATR): ṽmax = 3401, 3195, 1744, 1656, 1579, 1538, 1507, 1489, 1425, 1354, 1309, 1237, 1207, 1163, 1125, 1092, 1043, 1004, 969, 891, 854, 752, 710, 648, 578, 544, 457, 413 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.33 (br. s, 1H, NH), 11.92 (br. s, 1H, NH), 7.94 (q, J = 5.1 Hz, 1H, NH-Me), 7.86 (s, 1H, HC=N), 3.73 (s, 3H, OMe), 2.94 (d, J = 5.1 Hz, 3H, NH-Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 153.8 (C=O), 148.1 (Cq), 140.6 (Cq), 127.9 (HC=N), 114.5 (CNO2), 52.8 (OCH3), 30.1 (NCH3) ppm. MS: m/z (%) = 242 (25) [M+], 196 (10) [M—NO2]+, 102 (100) [M—C3H5N2O2]+.
Methyl 2-((5-(morpholino)-4-nitro-1H-pyrazol-3-yl)methylene)hydrazine-1-carboxylate (3b). The product was prepared according to pyrazole 3a from diene 2b (373 mg, 1.00 mmol) and methyl hydrazinecarboxylate (361 mg, 4.00 mmol). Reaction time was 12 h. The product was a yellow solid, yield 254 mg (0.85 mmol, 85%), mp 234–236 °C. IR (ATR): ṽmax = 3236, 2854, 1726 (CO), 1547 (NO2), 1457, 1350 (NO2), 1249, 1114, 1060, 919, 770, 609 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.84 (br. s, 1H, NH), 11.65 (br. s, 1H, NH), 8.46 (d, 1JC-H = 176.8 Hz, 1H, CH=N), 3.72 (br. s, 7H, OMe + 2OCH2), 3.16 (br. s, 4H, 2NCH2) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 153.8 (C=O), 153.6 (Cq), 137.8 (Cq), 132.6 (HC=N), 122.9 (CNO2), 65.9 (2OCH2), 52.5 (OCH3), 49.9 (2NCH2) ppm. MS: m/z (%) = 298 (10) [M+], 384 (22), 267 (100) [M—OMe]+.
Methyl 2-((5-((2-(4-chlorophenoxy)ethyl)amino)-4-nitro-1H-pyrazol-3-yl)methylene)-hydrazine-1-carboxylate (3c). The product was prepared according to pyrazole 3a from diene 2c (542 mg, 1.00 mmol) and methyl hydrazinecarboxylate (361 mg, 4.00 mmol). Reaction time was 8 h. Yellow solid, yield 249 mg (0.65 mmol, 65%) mp 236–238 °C. IR (ATR): ṽmax = 3398, 3293, 2951, 1745, 1646, 1550, 1491, 1244, 1052, 819, 764, 669, 502 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.63 (br. s, 0.4H, NH) and 13.01 (br. s, 0.6H, NH), 11.62 (br. s, 0.4H, NH) and 11.31 (br. s, 0.6H, NH), 8.44 (d, 1JC-H = 173.5 Hz, 1H, CH=N), 7.81 (br. s, 1H, NH), 7.31 (d, J = 8.8 Hz, 2CH), 6.97 (d, J = 8.8 Hz, 2CH), 6.69 (br. s, 1H, NH), 4.16 (t, J = 5.7 Hz, 2H, OCH2), 3.77 (br. s, 5H, OMe + NHCH2) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 157.7 (Cq-O), 154.0 (C=O), 148.1 (Cq), 135.9 (Cq), 129.7 (HC=N), 129.5 (2CH), 124.6 (C-Cl), 116.4 (2CH), 114.6 (CNO2), 66.6 (OCH2), 52.3 (OCH3), 42.4 (HNCH2) ppm. MS: m/z (%) = 382 (25) [M+], 347 (21) [M—Cl]+, 255 (45) [M—C6H4OCl]+, 241 (80) [M—C7H4OCl]+, 128 (60) [M—C6H4OCl—C2H3O]+.
Methyl 2-((5-(2,6-difluorobenzylamino)-4-nitro-1H-pyrazol-3-yl)methylene)hydrazine-1-carboxylate (3d). The product was prepared according to pyrazole 3a from diene 2d (485 mg, 1.00 mmol) and methyl hydrazinecarboxylate (361 mg, 4.00 mmol). Reaction time was 12 h. The product was purified by column chromatography (CC) using a mixture of PE/EA 2:1 as eluent. Orange solid, yield 301 mg (0.85 mmol, 85%), mp 238–241 °C. IR (ATR): ṽmax = 3421, 3369, 3222, 3131, 3064, 1704, 1592, 1469, 1383, 1337, 1260, 1165, 1041, 763, 663, 495 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.65 (br. s, 0.65H, NH) and 13.16 (br. s, 0.35H, NH), 11.60 (br. s, 0.65H, NH) and 11.32 (br. s, 0.35H, NH), 8.43 (d, 1JC-H = 175.4 Hz, 1H, CH=N), 7.86 (br. s, 0.35H, NH) and 6.68 (br. s, 0,65H, NH), 7.40 (br. s, 1CH), 7.09 (br. s, 2CH), 4.65 (br. s, 0.70H, NCH2) and 4.58 (br. s, 1.30H, NCH2), 3.71 (s, 3H, OMe) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 161.2 (d, 1JC-F = 246.4 Hz, 2Cq-F), 153.7 (Cq), 151.4 (Cq), 147.8 (Cq), 135.7 (CH), 130.2 (HC=N), 118.7 (Cq), 114.8 (C-NO2), 111.9 (2CH), 52.5 (OCH3), 34.6 (NCH2) ppm. MS: m/z (%) = 354 (2) [M+], 308 (5) [M—NO2]+, 267 (100).
Methyl 2-((5-(phenylamino)-4-nitro-1H-pyrazol-3-yl)methylene)hydrazine-1-carboxylate (3e). The product was prepared according to pyrazole 3a from diene 2e (385 mg, 1.00 mmol) and methyl hydrazinecarboxylate (361 mg, 4.00 mmol). Reaction time was 7 h. Red solid, yield 225 mg (0.74 mmol, 74%), mp 233–235 °C. IR (ATR): ṽmax = 3381, 3247, 3180, 3055, 1731, 1596, 1539, 1366, 1306, 1285, 1232, 1157, 1054, 958, 916, 740, 648, 559, 498 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.95 (br. s, 1H, NH), 11.70 (br. s, 1H, NH), 8.63 (br. s, 1H, NH), 8.51 (d, 1JC-H = 177.2 Hz, 1H, CH=N), 7.70 (d, J = 7.4 Hz, 2CH), 7.31 (t, J = 7.4 Hz, 2CH), 6.96 (t, J = 7.3 Hz, 1CH), 3.73 (s, 1JC-H = 147.4 Hz, 3H, OMe) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 153.8 (C=O), 147.6 (Cq), 140.3 (Cq-NH), 135.7 (Cq), 132.2 (CH=N), 129.0 (2CH), 121.6 (1CH), 119.4 (C-NO2), 118.2 (2CH), 52.6 (OCH3) ppm. MS: m/z (%) = 304 (70) [M+], 273 (15) [M—CH3O]+, 272 (100) [M—CH3O—H]+.
Ethyl 2-((5-(phenylamino)-4-nitro-1H-pyrazol-3-yl)methylene)hydrazine-1-carboxylate (3ee). The product was prepared in EtOH according to pyrazole 3a from diene 2e (385 mg, 1.00 mmol) and ethyl hydrazinecarboxylate (417 mg, 4.00 mmol). Reaction time was 7 h. Red solid, yield 191 mg (0.60 mmol, 60%) mp 249–251 °C. IR (KBr): ṽmax = 3199, 1713 (CO), 1603, 1554 (NO2), 1362 (NO2), 1254, 1168, 1058, 916, 741, 563 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.94 (br. s, 1H, NH), 11.69 (br. s, 1H, NH), 8.64 (br. s, 1H, NH), 8.51 (s, 1H, CH=N), 7.69 (d, J = 7.4 Hz, 2CH), 7.31 (t, J = 7.4 Hz, 2CH), 6.97 (t, J = 7.4 Hz, 1CH), 4.19 (q, J = 7.0 Hz, 2H, OCH2), 1.25 (t, J = 7.0 Hz, 3H, CH3) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 153.2 (C=O), 147.6 (Cq), 140.3 (Cq-NH), 135.9 (Cq), 132.2 (CH=N), 129.0 (2CH), 121.6 (1CH), 119.3 (C-NO2), 118.2 (2CH), 61.2 (OCH2), 14.6 (CH3) ppm. MS: m/z (%) = 318 (50) [M+], 272 (100) [M—NO2]+. HRMS-ESI: m/z calcd. for C13H14N6O4Na [M + Na]+: 341.0969; found: 341.0965.
Methyl 2-((5-(4-fluorophenylamino)-4-nitro-1H-pyrazol-3-yl)methylene)hydrazine-1-carboxylate (3f). The product was prepared according to pyrazole 3a from diene 2f (421 mg, 1.00 mmol) and methyl hydrazinecarboxylate (361 mg, 4.00 mmol). Reaction time was 5 h. Orange solid, yield 168 mg (0.52 mmol, 52%) mp 225–227 °C. IR (KBr): ṽmax = 3378, 3230, 1720 (CO), 1613, 1554 (NO2), 1510, 1364 (NO2), 1255, 1165, 1055, 827, 790, 506 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.93 (br. s, 1H, NH), 11.70 (br. s, 1H, NH), 8.66 (br. s, 1H, NH), 8.51 (s, 1H, CH=N), 7.74 (dd, J = 8.4, 5.4 Hz, 2CH), 7.14 (t, J = 8.4 Hz, 2CH), 3.73 (s, 3H, OMe) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 157.3 (d, 1JC-F = 237.2 Hz, Cq-F), 153.8 (C=O), 147.7 (Cq), 136.9 (Cq-NH), 135.8 (Cq), 132.2 (CH=N), 120.0 (d, 3JC-F = 8.1 Hz, 2CH), 119.6 (C-NO2), 115.4 (d, 2JC-F = 22.5 Hz, 2CH), 52.6 (OCH3) ppm. MS: m/z (%) = 322 (90) [M+], 290 (100) [M—OMe—H]+, 247 [M—NHCO2Me]+.
Ethyl 2-((5-(4-fluorophenylamino)-4-nitro-1H-pyrazol-3-yl)methylene)hydrazine-1-carboxylate (3ff). The product was prepared in EtOH according to pyrazole 3a from diene 2f (421 mg, 1.00 mmol) and ethyl hydrazinecarboxylate (417 mg, 4.00 mmol). Reaction time was 4 h. Orange solid, yield 185 mg (0.55 mmol, 55%) mp 251–253 °C. IR (KBr): ṽmax = 3381, 3196, 1715 (CO), 1612, 1587, 1557 (NO2), 1510, 1362 (NO2), 1258, 1169, 1061, 826, 791, 561, 509 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.92 (br. s, 1H, NH), 11.71 (br. s, 1H, NH), 8.68 (br. s, 1H, NH), 8.50 (s, 1H, CH=N), 7.74 (br. s, 2CH), 7.15 (t, J = 8.1 Hz, 2CH), 4.18 (q, J = 6.9 Hz, 2H, OCH2), 1.25 (t, J = 6.9 Hz, 3H, CH3) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 157.3 (d, 1JC-F = 238.2 Hz, Cq-F), 153.3 (C=O), 147.7 (Cq), 136.9 (Cq-NH), 135.8 (Cq), 132.1 (CH=N), 119.9 (d, 3JC-F = 7.7 Hz, 2CH), 119.3 (C-NO2), 115.4 (d, 2JC-F = 22.7 Hz, 2CH), 61.3 (OCH2), 14.7 (CH3) ppm. MS: m/z (%) = 322 (90) [M+], 290 (100) [M—OMe—H]+, 247 [M—NHCO2Me]+.
Methyl 2-((5-(4-chlorophenylamino)-4-nitro-1H-pyrazol-3-yl)methylene)hydrazine-1-carboxylate (3g). The product was prepared according to pyrazole 3a from diene 2g (454 mg, 1.00 mmol) and methyl hydrazinecarboxylate (361 mg, 4.00 mmol). Reaction time was 8 h. Orange solid, yield 305 mg (0.90 mmol, 90%) mp 248–251 °C. IR (ATR): ṽmax = 3365, 3279, 3211, 1729, 1599, 1545,1359, 1248, 1164, 1052, 815, 763, 606, 506 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.93 (br. s, 1H, NH), 11.70 (br. s, 1H, NH), 8.76 (br. s, 1H, NH), 8.50 (s, 1H, CH=N), 7.75 (d, J = 8.6 Hz, 2CH), 7.34 (d, J = 8.6 Hz, 2CH), 3.73 (s, 3H, CH3) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 153.7 (C=O), 147.2 (Cq), 139.4 (Cq-NH), 135.8 (Cq), 132.1 (CH=N), 128.7 (2CH), 125.0 (C-Cl), 119.8 (2CH), 119.4 (C-NO2), 52.6 (OCH3) ppm. MS: m/z (%) = 338 (75) [M+], 308 (50) [M—CH3O]+, 305 (100) [M—Cl]+, 250 (55) [M—C2H4N2O2]+.
Ethyl 2-((5-(4-chlorophenylamino)-4-nitro-1H-pyrazol-3-yl)methylene)hydrazine-1-carboxylate (3gg). The product was prepared in EtOH according to pyrazole 3a from diene 2g (454 mg, 1.00 mmol) and ethyl hydrazinecarboxylate (417 mg, 4.00 mmol). Reaction time was 4 h. Red solid, yield 282 mg (0.80 mmol, 80%) mp 251–253 °C. IR (KBr): ṽmax = 3375, 3193, 1711 (CO), 1604, 1564 (NO2), 1493, 1363 (NO2), 1255, 1169, 1058, 916, 822, 764, 569, 506 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.98 (br. s, 1H, NH), 11.71 (br. s, 1H, NH), 8.76 (br. s, 1H, NH), 8.50 (s, 1H, CH=N), 7.76 (d, J = 8.5 Hz, 2CH), 7.35 (d, J = 8.5 Hz, 2CH), 4.19 (q, J = 7.1 Hz, 2H, OCH2), 1.25 (t, J = 7.1 Hz, 3H, CH3) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 153.2 (C=O), 147.2 (Cq), 139.4 (Cq-NH), 135.9 (Cq), 132.0 (CH=N), 128.7 (2CH), 125.0 (C-Cl), 119.8 (2CH), 119.4 (C-NO2), 61.3 (OCH2), 14.6 (CH3) ppm. MS: m/z (%) = 352 (18) [M+], 306 (28) [M—NO2]+, 267 (100).
Methyl 2-((5-(2-chlorophenylamino)-4-nitro-1H-pyrazol-3-yl)methylene)hydrazine-1-carboxylate (
3h) was prepared according to the literature [
17]. All spectral data are in accordance with the literature [
17].
Methyl 2-((5-(2,4-dichlorophenylamino)-4-nitro-1H-pyrazol-3-yl)methylene)hydrazine-1-carboxylate (3i). The product was prepared according to pyrazole 3a from diene 2i (522 mg, 1.00 mmol) and methyl hydrazinecarboxylate (361 mg, 4.00 mmol). Reaction time was 5 h. Orange solid, yield 347 mg (0.93 mmol, 93%) mp 237–238 °C. IR (KBr): ṽmax = 3278, 1718 (CO), 1601, 1556 (NO2), 1453, 1375 (NO2), 1256, 1168, 1058, 850, 765, 583 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 14.19 (br. s, 1H, NH), 11.76 (br. s, 1H, NH), 9.03 (br. s, 1H, NH), 8.50 (d, J = 178.5 Hz, 1H, CH=N), 8.39 (d, J = 9.0 Hz, 1CH), 7.69 (d, J = 2.5 Hz, 1CH), 7.49 (dd, J = 9.0, 2.5 Hz, 1CH), 3.74 (s, 3H, OMe) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 153.7 (C=O), 146.4 (Cq), 136.1 (Cq), 135.5 (Cq), 131.8 (CH=N), 128.7 (1CH), 128.4 (1CH), 125.1 (C-Cl), 121.1 (C-Cl), 119.7 (C-NO2), 119.0 (1CH), 52.6 (OCH3) ppm. MS: m/z (%) = 372 (12) [M+], 337 (30) [M—Cl]+, 302 [M—2Cl]+.
Methyl 2-((5-(2-methyl-3-(trifluoromethyl)phenylamino)-4-nitro-1H-pyrazol-3-yl)methylene)hydrazine-1-carboxylate (3j). The product was prepared according to pyrazole 3a from diene 2j (549 mg, 1.00 mmol) and methyl hydrazinecarboxylate (361 mg, 4.00 mmol). Reaction time was 5 h. Red solid, yield 301 mg (0.78 mmol, 78%) mp 233–234 °C. IR (ATR): ṽmax = 3375, 3284, 3165, 1737, 1569, 1537, 1366, 1311, 1238, 113, 1020, 788, 623, 578 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 14.01 (br. s, 1H, NH), 11.73 (br. s, 1H, NH), 8.59 (br. s, 1H, NH), 8.51 (s, 1H, CH=N), 8.32 (d, J = 7.9 Hz, 1CH), 7.44 (t, J = 7.9 Hz, 1CH), 7.38 (d, J = 7.9 Hz, 1CH), 3.73 (s, 3H, OMe), 2.38 (s, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 153.7 (C=O), 147.8 (Cq), 140.1 (Cq), 136.0 (Cq), 132.0 (CH=N), 128.4 (q, 2JC-F = 29.7 Hz, Cq), 127.0 (CH), 125.1 (Cq), 123.2 (q, 1JC-F = 274.3 Hz, CF3), 123.2 (CH), 119.6 (C-NO2), 119.4 (CH), 52.6 (OMe), 13.3 (2CH3) ppm. MS: m/z (%) = 400 (1) [M+], 386 (100) [M—CH3 + H]+, 354 [M—NO2]+, 323 (20) [M—NO2—CH3O]+, 267 (85) [M—C4H7N2O2—F + H]+.
2-(Methylamino)-3-nitropyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (4a). To a soln. of 242 mg (1.00 mmol) pyrazole 3a in 10 mL DMF, 98 mg (1.50 mmol) sodium azide was added at rt, and the resulting mixture was stirred at 85–90 °C for 10 h. Subsequently, after cooling to rt and the addition of 60 mL cold water, 1 mL conc. hydrochloric acid was added dropwise. After 5 min of stirring, the precipitate was filtered off, washed with 5 mL water and cold MeOH (2 × 3 mL), and finally dried under reduced pressure to give 198 mg (0.94 mmol, 94%) of the desired triazinone 4a as a yellow solid, mp 307–309 °C. IR (ATR): ṽmax = 3411, 3235, 1765, 1623, 1580, 1470, 1413, 1370, 1318, 1295, 1175, 1141, 1037, 899, 765, 728, 638, 614, 528, 457, 415 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.48 (br. s, 1H, NH), 8.58 (d, 1JC-H = 201.5 Hz, 1H, HC=N), 7.29 (q, J = 4.6 Hz, 1H, NH), 2.94 (d, J = 4.6 Hz, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 153.3 (C=O), 142.9 (Cq-NH), 134.3 (Cq-N), 128.1 (CH=N), 114.9 (CNO2), 29.5 (Me) ppm. MS: m/z (%) = 210 (100) [M+], 164 (15) [M—NO2]+, 135 (15) [M—NO2—NHCH3]+, 96 (25) [M—C3H4N3O2]+. HRMS-ESI: m/z calcd. for C6H6N6O3Na [M + Na]+: 233.0394; found: 233.0393.
2-(Morpholino)-3-nitropyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (4b). The product was prepared according to triazinone 4a from pyrazole 3b (298 mg, 1.00 mmol) and sodium azide (98 mg, 1.50 mmol) at 95–100 °C. Reaction time was 4 h. Yellow solid, yield 347 mg (0.90 mmol, 90%) mp 251–253 °C. IR (ATR): ṽmax = 3178, 2968, 1748 (CO), 1601, 1514 (NO2), 1423, 1358 (NO2), 1323, 1194, 1105, 955, 849, 735, 643 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.57 (br. s, 1H, NH), 8.66 (d, 1JC-H = 204.5 Hz, 1H, HC=N), 3.92–3.56 (m, 4H, 2OCH2), 3.43–3.27 (m, 4H, 2NCH2) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 154.7 (C=O), 142.6 (Cq-N), 136.0 (Cq-N), 128.9 (CH=N), 118.7 (CNO2), 65.7 (2OCH2), 49.6 (2NCH2) ppm. MS: m/z (%) = 266 (30) [M+], 249 (100) [M—OH]+, 231 (37) [M—OH—NO2]+.
2-(2-(4-Chlorophenoxy)ethylamino)-3-nitropyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (4c). The product was prepared according to triazinone 4a from pyrazole 3c (383 mg, 1.00 mmol) and sodium azide (98 mg, 1.50 mmol) at 95–100 °C. Reaction time was 12 h. Yellow solid, yield 235 mg (0.67 mmol, 67%) mp 198 °C. IR (ATR): ṽmax = 3395, 3260, 1757, 1703, 1613, 1578, 1491, 1464, 1297, 1242, 1169, 1092, 1057, 892, 820, 765, 722, 666, 504 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.53 (br. s, 1H, NH), 8.60 (s, 1H, HC=N), 7.41 (t, J = 6.0 Hz, 1H, NH), 7.31 (d, J = 8.2 Hz, 2CH), 7.02 (d, J = 8.2 Hz, 2CH), 4.22 (t, J = 6.2 Hz, 2H, OCH2), 3.77 (q, J = 6.0 Hz, 2H, NHCH2) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 157.4 (Cq-O), 152.7 (C=O), 142.8 (NCN), 134.3 (Cq), 129.4 (2CH), 128.3 (HC=N), 124.6 (C-Cl), 116.5 (2CH), 115.1 (CNO2), 65.7 (OCH2), 41.7 (NCH2) ppm. MS: m/z (%) = 350 (15) [M+], 305 (10) [M—NO2 + H]+, 223 (100) [M—C6H4ClO]+.
2-(2,6-Difluorobenzylamino)-3-nitropyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (4d). The product was prepared according to triazinone 4a from pyrazole 3d (354 mg, 1.00 mmol) and sodium azide (98 mg, 1.50 mmol) at 80–85 °C. Reaction time was 12 h. Yellow solid, yield 277 mg (0.86 mmol, 86%) mp 236–238 °C. IR (ATR): ṽmax = 3415, 31, 25, 2929, 1728, 1621, 1580, 1467, 1326, 1174, 1022, 873, 808785, 768, 730, 640, 532, 489, 439 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.52 (br. s, 1H, NH), 8.58 (d, 1JC-H = 202.8 Hz, 1H, HC=N), 7.50 (t, J = 5.6 Hz, 1NH), 7.39 (tt, J = 6.8, 8.2 Hz, 1CH), 7.09 (t, J = 8.2 Hz, 2CH), 4.68 (d, 1JC-H = 142.2 Hz, d J = 5.6 Hz, 2H, CH2) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 161.5 (d, 1JC-F = 248.8 Hz, Cq-F), 161.4 (d, 1JC-F = 248.0 Hz, Cq-F), 152.2 (NCN), 142.8 (C=O), 134.4 (Cq), 130.2 (t, 3JC-F = 10.5 Hz, CH), 128.0 (HC=N), 115.0 (C-NO2), 114.0 (t, 2JC-F = 18.5 Hz, Cq), 111.7 (dd, 2JC-F = 19.3 Hz, 4JC-F = 6.5 Hz, 2CH), 34.8 (t, 3JC-F = 4.0 Hz, NCH2) ppm. MS: m/z (%) = 292 (50) [M—CH2NH—H]+ 164 (60) [M—C7H5F2—NH—NO2]+, 149 (25) [M—C7H5F2—NO2]+, 127 (100) [C7H5F2]+.
2-(Phenylamino)-3-nitropyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (4e). The product was prepared according to triazinone 4a either from pyrazole 3e (304 mg, 1.00 mmol) or from pyrazole 3ee (318 mg, 1.00 mmol) and sodium azide (98 mg, 1.50 mmol) at 105–110 °C. Reaction time was 4 h. Orange solid, yield 253 mg (0.93 mmol, 93%) from 3e and 240 mg (0.88 mmol, 88%) from 3ee, mp 275–277 °C. IR (KBr): ṽmax = 3345, 1721 (CO), 1615, 1501 (NO2), 1376 (NO2), 1321, 1293, 1173, 1019, 903, 840, 764, 693, 642 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.64 (br. s, 1H, NH), 9.04 (br. s, 1H, NH), 8.70 (d, 1JC-H = 204.7 Hz, 1H, HC=N), 7.84 (d, J = 7.9 Hz, 2CH), 7.39 (t, J = 7.9 Hz, 2CH), 7.08 (t, J = 7.9 Hz, 1CH) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 148.9 (Cq), 142.7 (Cq), 139.3 (Cq), 133.9 (Cq), 129.0 (2CH), 128.4 (HC=N), 123.0 (CH), 119.4 (2CH), 115.8 (CNO2) ppm. MS: m/z (%) = 272 (100) [M+], 226 (5) [M—NO2]+, 149 (10) [M—C6H5—NO2]+.
2-(4-Fluorophenylamino)-3-nitropyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (4f). The product was prepared according to triazinone 4a either from pyrazole 3f (322 mg, 1.00 mmol) or from pyrazole 3ff (336 mg, 1.00 mmol) and sodium azide (98 mg, 1.50 mmol) at 105–110 °C. Reaction time was 4 h. Orange solid, yield 276 mg (0.95 mmol, 95%) from 3e and 261 mg (0.90 mmol, 90%) from 3ee, mp 290 °C. IR (KBr): ṽmax = 3368, 3151, 1727 (CO), 1616, 1585, 1509, 1371 (NO2), 1315, 1171, 907, 834, 765, 644, 509 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.64 (br. s, 1H, NH), 9.01 (br. s, 1H, NH), 8.68 (d, 1JC-H = 204.7 Hz, 1H, HC=N), 7.84 (dd, J = 8.1, 5.2 Hz, 2CH), 7.22 (t, J = 8.1 Hz, 2CH) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 158.2 (d, 1JC-F = 239.3 Hz, Cq-F), 149.1 (Cq), 142.7 (Cq), 135.8 (d, 4JC-F = 2.2 Hz, Cq-NH), 133.9 (Cq), 128.4 (CH=N), 121.5 (d, 3JC-F = 7.7 Hz, 2CH), 115.6 (C-NO2), 115.5 (d, 2JC-F = 22.3 Hz, 2CH) ppm. MS: m/z (%) = 290 (100) [M+], 256 (2), 244 (1) [M—NO2]+.
2-(4-Chlorophenylamino)-3-nitropyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (4g). The product was prepared according to triazinone 4a either from pyrazole 3g (339 mg, 1.00 mmol) or from pyrazole 3gg (353 mg, 1.00 mmol) and sodium azide (98 mg, 1.50 mmol) at 105–108 °C. Reaction time was 12 h. Orange solid, yield 261 mg (0.85 mmol, 85%) from 3g and 255 mg (0.83 mmol, 83%) from 3gg, mp 275–278 °C. IR (KBr): ṽmax = 3348, 3177, 1746 (CO), 1613, 1572, 1371 (NO2), 1320, 1172, 906, 822, 766, 639, 507 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.66 (br. s, 1H, NH), 9.20 (br. s, 1H, NH), 8.71 (s, 1H, HC=N), 7.88 (d, J = 8.8 Hz, 2CH), 7.43 (d, J = 8.8 Hz, 2CH) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 148.7 (Cq), 142.6 (Cq), 138.5 (Cq-NH), 133.9 (Cq), 128.9 (2CH), 128.4 (CH=N), 126.6 (C-Cl), 121.1 (2CH), 115.9 (C-NO2) ppm. MS: m/z (%) = 305 (100) [M—H]+, 271 (4) [M—Cl]+, 225 (4) [M—Cl—NO2]+.
2-(2-Chlorophenylamino)-3-nitropyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (
4h) was prepared according to literature [
17]. All spectral data are in accordance with the literature [
17].
2-(2,4-Dichlorophenylamino)-3-nitropyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (4i). The product was prepared according to triazinone 4a from pyrazole 3i (373 mg, 1.00 mmol) and sodium azide (98 mg, 1.50 mmol) at 106–108 °C. Reaction time was 6 h. Orange solid, yield 290 mg (0.85 mmol, 85%), mp 274 °C. IR (KBr): ṽmax = 3340, 3148, 1726 (CO), 1620, 1594, 1552 (NO2), 1476, 1374 (NO2), 1319, 1173, 907, 854, 764, 638, 611 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.76 (br. s, 1H, NH), 9.18 (br. s, 1H, NH), 8.73 (s, 1H, HC=N), 8.38 (d, J = 9.0 Hz, 1CH), 7.74 (d, J = 2.3 Hz, 1CH), 7.55 (dd, J = 9.0, 2.3 Hz, 1CH) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 148.0 (Cq), 142.4 (Cq), 134.4 (Cq-NH), 133.7 (Cq), 129.0 (CH), 128.6 (CH), 128.3 (CH=N), 127.1 (C-Cl), 122.7 (C-Cl), 120.4 (CH), 115.9 (C-NO2) ppm. MS: m/z (%) = 340 (85) [M+], 305 (100) [M—Cl]+, 289 (5) [M—Cl—O]+.
2-(2-Methyl-3-trifluoromethylphenylamino)-3-nitropyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (4j). The product was prepared according to triazinone 4a from pyrazole 3j (386 mg, 1.00 mmol) and sodium azide (98 mg, 1.50 mmol) at 105–107 °C. Reaction time was 12 h. Orange solid, yield 149 mg (0.42 mmol, 42%) mp 218 °C. IR (KBr): ṽmax = 3376, 3254, 1739, 1701, 1611, 1579, 1504, 1313, 1278, 1165, 1133, 1110, 1021, 898, 799, 723, 643, 580, 531 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 13.63 (br. s, 1H, NH), 8.94 (br. s, 1H, NH), 8.69 (s, 1H, CH=N), 8.10 (d, J = 8.0 Hz, 1CH), 7.56 (d, J = 8.0 Hz, 1CH), 7.50 (dd, J = 8.0, 8.0 Hz, 1CH), 2.39 (s, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 150.0 (Cq), 142.6 (Cq), 139.2 (Cq), 134.1 (Cq), 129.4 (q, 3JC-F = 1.7 Hz, Cq), 128.6 (q, 2JC-F = 29.1 Hz, Cq), 128.3 (CH), 127.4 (CH), 127.1 (CH=N), 124.6 (q, 1JC-F = 273.9 Hz, CF3), 122.2 (q, 3JC-F = 5.8 Hz, CH), 115.7 (C-NO2), 13.6 (q, 4JC-F = 2.4 Hz, CH3) ppm. MS: m/z (%) = 354 (100) [M+], 307 (25) [M—NO2]+, 194 (25) [M—ArCH3CF3]+.
3-Amino-2-(methylamino)pyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (5a). To a suspension of 210 mg (1.00 mmol) of triazinone 4a in a mixture of MeOH (10 mL) and conc. hydrochloric acid (1 mL), 677 mg (3.00 mmol) tin(II) chloride dihydrate was added at rt, and the resulting mixture was stirred at 60–65 °C for 15 h. Subsequently, after cooling to rt and the addition of 60 mL cold water and 5 mL saturated sodium bicarbonate soln. under stirring, the resulting mixture was extracted with EA (6 × 50 mL), washed with water (1 × 100 mL), and dried with sodium sulfate (Na2SO4). After purification with CC (EA/PE 3:1), evaporation of the solvents, and drying under reduced pressure, the amine 5a was obtained as a brownish solid, yield 87 mg (0.48 mmol, 48%), mp 316–318 °C. IR (ATR): ṽmax = 3379, 3321, 3197, 1672, 1566, 14, 16, 12, 32, 1048, 717, 591 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 11.47 (br. s, 1H, NH), 8.04 (d, 1JC-H = 189.8 Hz, 1H, HC=N), 5.76 (q, J = 5.0 Hz, 1H, NH), 4.80 (br. s, 2H, NH2), 2.83 (d, J = 5.0 Hz, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 152.7 (C=O), 144.5 (Cq-NH), 130.1 (CH=N), 118.8 (Cq), 115.5 (Cq), 29.7 (Me) ppm. MS: m/z (%) = 180 (100) [M+], 163 (15) [M—NH2—H]+, 95 (20) [M—C7H3N3]+.
3-Amino-2-morpholinopyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (5b). To a suspension of 266 mg (1.00 mmol) of triazinone 4b in a mixture of EtOH (15 mL) and conc. hydrochloric acid (1.5 mL), 677 mg (3.00 mmol) tin(II) chloride dihydrate was added at rt, and the resulting mixture was stirred at rt for 1 d and at 40–45 °C for 8 h. Subsequently, after cooling to rt, the precipitate was filtered off, washed with 5 mL water and cold MeOH (3 mL), and finally dried under reduced pressure to give 227 mg (0.96 mmol, 96%) of the desired triazinone 5b as a brownish solid, mp 314–318 °C. IR (ATR): ṽmax = 3391, 3221, 2844, 1694, 1498, 1358, 1231, 1109, 1050, 953, 851, 743, 717, 673, 588, 566, 517, 440 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 11.83 (br. s, 1H, NH), 8.23 (d, 1JC-H = 191.1 Hz, 1H, HC=N), 4.91 (br. s, 2H, NH2), 3.79–3.68 (m, 4H, 2OCH2), 3.23–3.12 (m, 4H, 2NCH2) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 153.0 (C=O), 144.3 (Cq-NH), 130.5 (CH=N), 121.2 (Cq), 118.7 (Cq), 66.0 (OCH2), 48.3 (NCH2) ppm. MS: m/z (%) = 236 (100) [M+], 151 (50) [M—NC4H8O + H]+. HRMS-ESI: calcd. for C9H12N6O2Na [M + Na]+: 259.0914; found: 259.0914.
3-Amino-2-((2-(4-chlorophenoxy)ethyl)amino)pyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (5c). To a suspension of 351 mg (1.00 mmol) of triazinone 4c in a mixture of MeOH (20 mL) and conc. hydrochloric acid (2 mL), 677 mg (3.00 mmol) tin(II) chloride dihydrate was added at rt, and the resulting mixture was stirred at 50–55 °C for 12 h. Subsequently, after cooling to rt, the precipitate was filtered off, washed with 7 mL water and cold MeOH (5 mL), and finally dried under reduced pressure to give 237 mg (0.74 mmol, 74%) of the triazinone 5c as a colorless solid, mp 221–223 °C. IR (ATR): ṽmax = 3387, 3270, 1689, 1551, 1491, 1395, 1357, 1245, 1058, 823, 666 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 11.55 (br. s, 1H, NH), 8.06 (d, 1JC-H = 190.0 Hz, 1H, HC=N), 7.32 (d, J = 9.0 Hz, 2CH), 7.02 (d, J = 9.0 Hz, 2CH), 6.08 (t, J = 5.6 Hz, 1H, NH), 4.90 (br. s, 2H, NH2), 4.16 (t, J = 5.5 Hz, 2H, OCH2), 3.62 (q, J = 5.5 Hz, 2H, NHCH2) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 157.5 (Cq-O), 151.4 (C=O), 144.4 (NCN), 130.1 (HC=N), 129.4 (2CH), 124.5 (C-Cl), 118.9 (Cq), 116.5 (2CH), 115.6 (Cq), 66.6 (OCH2), 42.3 (NCH2). MS: m/z (%) = 320 (25) [M+], 193 (80) [M—C6H4OCl]+, 179 (100) [M—CO—C6H4Cl]+.
3-Amino-2-((2,6-difluorobenzyl)amino)pyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (5d). To a suspension of 322 mg (1.00 mmol) triazinone 4d in a mixture of MeOH (20 mL) and conc. hydrochloric acid (2 mL), 677 mg (3.00 mmol) tin(II) chloride dihydrate was added at rt, and the resulting mixture was stirred at 60–65 °C for 18 h. Subsequently, after cooling to rt, 120 mL cold water was added, and the resulting mixture was extracted with EA (6 × 50 mL), washed with water (1 × 100 mL), and dried with Na2SO4. After purification with CC (EA/PE 2:1), evaporation of solvents, and drying under reduced pressure, the amine 5d was obtained as a brownish solid, yield 246 mg (0.84 mmol, 84%), mp 217–219 °C. IR (ATR): ṽmax = 3340, 2863, 1680, 1556, 1465, 1390, 1345, 1216, 1046, 900, 796, 723, 694, 663, 585, 491, 433 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 12.13 (br. s, 1H, NH), 8.25 (d, 1JC-H = 195.8 Hz, 1H, HC=N), 7.44 (tt, J = 6.7, 7.9 Hz, 1CH), 7.14 (t, J = 7.9 Hz, 2CH), 6.37 (br. s, 1NH), 5.60 (br. s, 2H, NH2), 4.48 (d, 1JC-H = 141.9 Hz, 2H, CH2) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 161.4 (d, 1JC-F = 248.3 Hz, Cq-F), 161.3 (d, 1JC-F = 247.9 Hz, Cq-F), 152.2 (Cq), 143.9 (Cq), 130.5 (t, 3JC-F = 10.5 Hz, 1CH), 129.2 (HC=N), 125.0 (Cq), 114.3 (t, 2JC-F = 19.0 Hz, Cq), 111.8 (dd, 2JC-F = 19.1 Hz, 4JC-F = 6.3 Hz, 2CH), 105.5 (Cq), 34.6 (t, 3JC-F = 3.8 Hz, NCH2) ppm. MS: m/z (%) = 292 (55) [M+], 179 (5) [M—C6H3F2]+, 164 (70) [M—C6H3F2CH2]+, 127 (100) [C6H3F2CH2]+.
3-Amino-2-(phenylamino)pyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (5e). The product was prepared according to 5a from 4e (272 mg, 1.00 mmol) and tin(II) chloride dihydrate (677 mg, 3.00 mmol). Reaction time was 9 h at 70–75 °C in EtOH. Yellowish solid, yield 216 mg (0.89 mmol, 89%), mp 280–282 °C. IR (ATR): ṽmax = 3400, 3325, 3220, 1690, 1598, 1566, 1495, 1387, 1351, 1306, 1206, 1066, 901, 121, 734, 682, 597, 541, 461 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 11.78 (br. s, 1H, NH), 8.46 (br. s, 1H, NH), 8.19 (d, 1JC-H = 190.8 Hz, 1H, HC=N), 7.65 (dd, J = 8.6, 1.0 Hz, 2CH), 7.30 (dd, J = 8.6, 7.4 Hz, 2CH), 6.88 (tt, J = 7.4, 1.0 Hz, 1CH), 5.12 (br. s, 2H, NH2) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 146.7 (Cq), 144.4 (Cq), 141.7 (Cq), 130.3 (HC=N), 129.0 (2CH), 120.2 (1CH), 118.7 (Cq), 117.0 (Cq), 116.4 (2CH) ppm. MS: m/z (%) = 242 (100) [M+], 150 (25) [M—C6H6N]+, 93 (60) [C6H6N]+.
3-Amino-2-(4-fluorophenylamino)pyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (5f). The product was prepared according to 5a from 4f (290 mg, 1.00 mmol) and tin(II) chloride dihydrate (677 mg, 3.00 mmol). Reaction time was 10 h at 65–70 °C. Light brown solid, yield 247 mg (0.95 mmol, 95%), mp 295–297 °C. IR (KBr): ṽmax = 3407, 3243, 1690 (CO), 1615, 1579 (NO2), 1510, 1394 (NO2), 1209, 1069, 819, 712, 500 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 11.97 (br. s, 1H, NH), 8.72 (br. s, 1H, NH), 8.23 (s, 1H, HC=N), 7.66 (dd, J = 8.8, 4.8 Hz, 2CH), 7.17 (t, J = 8.8 Hz, 2CH), 4.33 (br. s, 2H, NH2)m ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 156.3 (d, 1JC-F = 236.3 Hz, Cq-F), 147.3 (Cq), 144.2 (Cq), 138.1 (d, 4JC-F = 2.1 Hz, Cq-NH), 130.0 (CH=N), 120.6 (Cq), 117.8 (d, 3JC-F = 7.5 Hz, 2CH), 115.6 (d, 2JC-F = 22.1 Hz, 2CH), 113.6 (Cq) ppm. MS: m/z (%) = 260 (100) [M+], 244 (10) [M—NH2]+, 231 (10).
3-Amino-2-(4-chlorophenylamino)pyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (5g). The product was prepared according to 5a from 4g (307 mg, 1.00 mmol) and tin(II) chloride dihydrate (677 mg, 3.00 mmol). Reaction time was 12 h at 65–70 °C. Brown solid, yield 249 mg (0.90 mmol, 90%) mp 327–330 °C. IR (KBr): ṽmax = 3406, 3241, 1696 (CO), 1602, 1565 (NO2), 1492, 1390 (NO2), 1205, 1067, 815, 713, 459 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 11.84 (br. s, 1H, NH), 8.65 (br. s, 1H, NH), 8.20 (s, 1H, HC=N), 7.67 (d, J = 8.9 Hz, 2CH), 7.34 (d, J = 8.9 Hz, 2CH), 5.14 (br. s, 2H, NH2) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 144.6 (Cq), 144.3 (Cq), 140.7 (Cq), 130.4 (CH=N), 128.9 (2CH), 123.4 (C-Cl), 118.8 (Cq), 117.9 (2CH), 117.1 (Cq) ppm. MS: m/z (%) = 276 (44) [M+], 241 (20) [M—Cl]+, 225 (12) [M—Cl—NH2]+, 58 (100).
3-Amino-2-(2-chlorophenylamino)pyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (5h). The product was prepared according to 5a from 4h (307 mg, 1.00 mmol) and tin(II) chloride dihydrate (677 mg, 3.00 mmol). Reaction time was 12 h at 65–70 °C. Light brown solid, yield 235 mg (0.85 mmol, 85%), mp 277–279 °C. IR (KBr): ṽmax = 3387, 3278, 1709 (CO), 1601, 1561 (NO2), 1475, 1386 (NO2), 1212, 1051, 898, 737, 603 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 11.94 (br. s, 1H, NH), 8.27 (s, 1H, HC=N), 8.05 (dd, J = 8.2, 1.2 Hz, 1CH), 7.62 (br. s, 1H, NH), 7.45 (dd, J = 7.8, 1.2 Hz, 1CH), 7.31 (ddd, J = 8.2, 7.4, 1.2 Hz, 1CH), 6.95 (ddd, J = 7.8, 7.4, 1.2 Hz, 1CH), 5.24 (br. s, 2H, NH2) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 146.4 (Cq), 144.3 (Cq), 138.1 (Cq), 130.5 (CH=N), 129.6 (CH), 127.9 (CH), 121.9 (CH), 121.1 (Cq), 120.2 (Cq), 118.8 (CH), 118.1 (Cq) ppm. MS: m/z (%) = 276 (100) [M]+, 241 (70) [M—Cl]+, 225 (15) [M—Cl—NH2]+, 212 (35).
3-Amino-2-(2,4-dichlorophenylamino)pyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (5i). The product was prepared according to 5a from 4i (341 mg, 1.00 mmol) and tin(II) chloride dihydrate (677 mg, 3.00 mmol). Reaction time was 8 h at 75–80 °C in EtOH. Brown solid, yield 236 mg (0.76 mmol, 76%), mp 198–200 °C. IR (ATR): ṽmax = 3387, 3346, 3312, 2899, 1697, 1599, 1553, 1469, 1378, 1306, 1205, 1050, 807, 707, 581, 544, 473, 430 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 11.96 (br. s, 1H, NH), 8.27 (s, 1H, HC=N), 8.05 (d, J = 8.8 Hz, 1CH), 7.74 (br. s, 1H, NH), 7.60 (d, J = 2.3 Hz, 1CH), 7.39 (dd, J = 8.8, 2.3 Hz, 1CH), 5.25 (br. s, 2H, NH2) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 145.8 (Cq), 144.2 (Cq), 137.4 (Cq), 130.5 (CH=N), 128.9 (CH), 128.0 (CH), 124.3 (Cq), 121.7 (Cq), 120.2 (Cq), 119.5 (CH), 118.3 (Cq) ppm. MS: m/z (%) = 314 (100) [M+], 275 (18) [M—Cl]+, 243 (20) [M—2 Cl]+.
3-Amino-2-(2,4-dichlorophenylamino)pyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (5j). The product was prepared according to 5a from 4j (354 mg, 1.00 mmol) and tin(II) chloride dihydrate (677 mg, 3.00 mmol). Reaction time was 8 h at 60–65 °C. Yellowish solid, yield 285 mg (0.88 mmol, 88%) mp 223–225 °C. IR (ATR): ṽmax = 3220, 1693, 1557, 1505, 1378, 1316, 1280, 1217, 1168, 1110, 1020, 894, 793, 717, 567, 537 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 11.87 (br. s, 1H, NH), 8.24 (d, 1JC-H = 190.8 Hz, 1H, HC=N), 7.87 (d, J = 7.7 Hz, 1CH), 7.60 (br. s, 1H, NH), 7.39–7.27 (m, 2CH), 5.12 (br. s, 2H, NH2), 2.37 (s, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 146.9 (Cq), 144.3 (Cq), 141.9 (Cq), 130.5 (CH), 128.5 (q, 2JC-F = 28.6 Hz, Cq), 126.7 (CH=N), 125.6 (q, 3JC-F = 1.7 Hz, Cq), 124.9 (q, 1JC-F = 273.9 Hz, CF3), 122.9 (CH), 119.9 (Cq), 118.7 (q, 3JC-F = 5.8 Hz, CH), 118.3 (Cq), 13.8 (q, 4JC-F = 2.4 Hz, CH3) ppm. MS: m/z (%) = 324 (100) [M+], 308 (60) [M—NH2]+, 268 (20) [M—3 F]+.
N-(3-Acetamido-7-oxo-6,7-dihydropyrazolo[1,5-d][1,2,4]triazin-2-yl)-N-methylacetamide (6a). A soln. of 180 mg (1.00 mmol) amine 5a in 3 mL acetic anhydride (Ac2O) was stirred at rt for 2 d. The formed precipitate was filtered off, washed with cold MeOH (1 mL) and water (2 × 3 mL), and finally dried under reduced pressure to give 248 mg (0.94 mmol, 94%) of the acetamide 6a as a colorless solid, mp 244–246 °C. IR (ATR): ṽmax = 3087, 1747, 1646, 1578, 1489, 13, 25, 1268, 1236, 1147, 1005, 818, 728, 666, 616, 566, 530, 465 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 12.81 (br. s, 1H, NH), 10.05 (br. s, 1H, NH), 8.40 (d, 1JC-H = 199.2 Hz, 1H, HC=N), 3.10 (s, 3H, N-Me), 2.10 (s, 3H, Me), 1.80 (s, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 169.8 (C=O), 168.7 (C=O), 149.2 (C=O), 143.5 (Cq), 132.2 (CH=N), 129.0 (Cq), 111.8 (Cq), 34.8 (N-Me), 22.8 (C-Me), 21.8 (C-Me) ppm. MS: m/z (%) = 264 (15) [M+], 178 (20) [M—2 C2H3O]+, 149 (85) [M—C2H3O—C7H6F2N]+, 96 (100) [C3H2N3O]+.
N-(2-Morpholino-7-oxo-6,7-dihydropyrazolo [1,5-d][1,2,4]triazin-3-yl)acetamide (6b). The product was prepared according to 6a from 5b (236 mg, 1.00 mmol) and 3 mL Ac2O. Reaction time was 6 h at 40–45 °C. Colorless solid, yield 245 mg (0.88 mmol, 88%), mp 318–321 °C. IR (ATR): ṽmax = 3277, 3090, 2994, 1716, 1665, 14, 89, 1370, 1264, 119, 971, 810, 725, 677, 611, 584, 519 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 12.52 (br. s, 1H, NH), 9.62 (br. s, 1H, NH), 8.06 (d, 1JC-H = 195.7 Hz, 1H, HC=N), 3.78–3.67 (m, 4H, 2OCH2), 3.30–3.21 (m, 4H, 2NCH2), 2.06 (d, 1JC-H = 123.6 Hz, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 169.2 (C=O), 156.0 (C=O), 143.7 (NCN), 131.6 (Cq), 130.4 (CH=N), 104.5 (Cq), 65.8 (2OCH2), 47.8 (2NCH2), 22.9 (Me) ppm. MS: m/z (%) = 278 (75) [M+], 235 (100) [M—C2H3O]+.
N-(2-((2-(4-Chlorophenoxy)ethyl)amino)-7-oxo-6,7-dihydropyrazolo[1,5-d][1,2,4]triazin-3-yl)acetamide (6c). The product was prepared according to 6a from 5c (321 mg, 1.00 mmol) and 3 mL Ac2O. Reaction time was 24 h at 40–45 °C. Colorless solid, yield 308 mg (0.85 mmol, 85%), mp 250–252 °C. IR (ATR): ṽmax = 3266, 29, 32, 1713, 1657, 1631, 1577, 1517, 1492, 1376, 1281, 1247, 1028, 902, 824, 725, 665, 613, 587, 425 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 12.28 (br. s, 1H, NH), 9.68 (br. s, 1H, NH), 8.15 (d, 1JC-H = 195.5 Hz, 1H, HC=N), 7.32 (d, J = 9.0 Hz, 2CH), 7.03 (d, J = 9.0 Hz, 2CH), 6.15 (t, J = 5.6 Hz, 1H, NH), 4.17 (t, J = 5.6 Hz, 2H, OCH2), 3.63 (q, J = 5.5 Hz, 2H, NHCH2), 2.07 (s, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 168.6 (C=O), 157.5 (Cq-O), 153.5 (C=O), 143.9 (NCN), 130.5 (HC=N), 129.5 (2CH), 127.6 (C-Cl), 124.5 (Cq), 116.5 (2CH), 103.5 (Cq), 66.5 (OCH2), 42.2 (NCH2), 23.0 (Me) ppm. MS: m/z (%) = 362 (25) [M+], 235 (30) [M—C6H4ClO]+, 221 (90) [M—COC6H4Cl]+, 179 (100) [M—C2H3O—COC6H4Cl]+.
N-(2-((2,6-Difluorobenzyl)amino)-7-oxo-6,7-dihydropyrazolo[1,5-d][1,2,4]triazin-3-yl)acetamide (6d). The product was prepared according to 6a from 5d (292 mg, 1.00 mmol) and 3 mL Ac2O. Reaction time was 2 h at 0 °C and 24 h at rt. After purification by CC (EA/PE 1:1), the complete separation of 6d and 6dd proved impossible due to similar physical properties. The isolated acetamide 6d contained 20% of 6dd. Brownish solid, yield 281 mg (0.84 mmol, 84%) mp 294–297 °C. IR (ATR): ṽmax = 3277, 1719, 1655, 1636, 1578, 1521, 1472, 1350, 1068, 1043, 900, 786, 663, 613, 547, 471, 422 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 12.29 (br. s, 1H, NH), 9.50 (br. s, 1H, NH), 8.17 (s, 1H, HC=N), 7.44 (br. s, 1CH), 7.14 (t, J = 7.9 Hz, 2CH), 6.17 (br. s, 1H, NH), 4.49 (br. s, 2H, CH2), 2.04 (s, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 168.5 (C=O), 161.4 (d, 1JC-F = 248.1 Hz, Cq-F), 161.3 (d, 1JC-F = 247.6 Hz, Cq-F), 152.9 (Cq), 143.9 (Cq), 130.7 (HC=N), 130.4 (t, 3JC-F = 10.2 Hz, 1CH), 127.5 (Cq), 114.4 (t, 2JC-F = 19.6 Hz, Cq), 111.8 (dd, 2JC-F = 18.5 Hz, 4JC-F = 6.2 Hz, 2CH), 103.5 (Cq), 34.6 (t, 3JC-F = 4.4 Hz, NCH2), 23.0 (Me) ppm. MS: m/z (%) = 334 (40) [M+], 291 (25) [M—C2H3O]+, 164 (30) [M—C2H3O—C7H5F2]+.
N-(3-Acetamido-7-oxo-6,7-dihydropyrazolo[1,5-d][1,2,4]triazin-2-yl)-N-(2,6-difluorobenzyl)acetamide (6dd). The product was prepared according to 6a from 5d (292 mg, 1.00 mmol) and 3 mL Ac2O. Reaction time was 24 h at 40–45 °C. Colorless solid, yield 331 mg (0.88 mmol, 88%), mp 292–295 °C. IR (ATR): ṽmax = 3294, 1725, 1700, 1646, 1543, 1469, 1343, 1250, 1145, 991, 769, 670, 610, 471 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 12.79 (br. s, 1H, NH), 10.00 (br. s, 1H, NH), 8.34 (d, 1JC-H = 198.4 Hz, 1H, HC=N), 7.33 (tt, J = 6.9, 8.0 Hz, 1CH), 6.96 (t, J = 7.9 Hz, 2CH), 4.92 (br. s, 2H, CH2), 2.03 (s, 3H, Me), 1.80 (br. s, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 169.5 (C=O), 168.2 (C=O), 161.4 (d, 1JC-F = 249.1 Hz, Cq-F), 161.3 (d, 1JC-F = 248.9 Hz, Cq-F), 146.7 (Cq), 143.4 (Cq), 132.6 (HC=N), 130.7 (t, 3JC-F = 10.5 Hz, 1CH), 128.4 (Cq), 112.8 (Cq), 111.9 (t, 2JC-F = 19.6 Hz, Cq), 111.6 (dd, 2JC-F = 19.0 Hz, 4JC-F = 5.9 Hz, 2CH), 37.2 (NCH2), 22.7 (Me), 22.4 (Me) ppm. MS: m/z (%) = 376 (25) [M+], 333 (50) [M—C2H3O]+, 127 (100) [C7H5F2]+. HRMS-ESI: calcd. for C16H14F2N6O3Na [M + Na]+: 399.0988; found: 399.0993.
N-(7-Oxo-2-(phenylamino)-6,7-dihydropyrazolo[1,5-d][1,2,4]triazin-3-yl)acetamide (6e). The product was prepared according to 6a from 5e (242 mg, 1.00 mmol) and 3 mL Ac2O. Reaction time was 24 h at 40–45 °C. Colorless solid, yield 256 mg (0.90 mmol, 90%), mp 297–299 °C. IR (ATR): ṽmax = 3288, 3106, 2946, 1713, 1658, 1605, 1571, 1503, 1372, 1354, 1281, 1210, 1123, 1023, 896, 822, 744, 686, 622, 493, 435 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 12.49 (br. s, 1H, NH), 9.68 (br. s, 1H, NH), 8.49 (br. s, 1H, NH), 8.20 (s, 1H, HC=N), 7.65 (d, J = 8.4, 2CH), 7.32 (dd, J = 8.4, 7.4 Hz, 2CH), 6.92 (t, J = 7.3, 1CH), 2.13 (d, 1JC-H = 128.6 Hz, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 169.1 (C=O), 149.6 (Cq), 143.8 (Cq), 141.2 (Cq), 130.6 (HC=N), 129.1 (2CH), 127.8 (Cq), 120.7 (1CH), 116.9 (2CH), 104.6 (Cq), 23.2 (Me) ppm. MS: m/z (%) = 284 (100) [M]+, 241 (25) [M—C2H3O]+. HRMS-ESI: m/z calcd. for C13H12N6O2Na [M + Na]+: 307.0914; found: 307.0917.
N-(7-Oxo-2-(4-fluorophenylamino)-6,7-dihydropyrazolo[1,5-d][1,2,4]triazin-3-yl)acetamide (6f). The product was prepared according to 6a from 5f (260 mg, 1.00 mmol) and 3 mL Ac2O. Reaction time was 8 h at 40–45 °C. Colorless solid, yield 284 mg (0.94 mmol, 94%), mp 312–314 °C. IR (ATR): ṽmax = 3292, 3111, 2949, 1714, 1580, 1504, 1373, 1279, 128, 896, 828, 723, 644, 585, 446 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 12.49 (br. s, 1H, NH), 9.65 (br. s, 1H, NH), 8.52 (br. s, 1H, NH), 8.19 (d, 1JC-H = 198.0 Hz 1H, HC=N), 7.67 (dd, J = 8.6, 4.8 Hz, 2CH), 7.17 (t, J = 8.7 Hz, 2CH), 2.13 (d, 1JC-H = 128.2 Hz, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 169.2 (C=O), 156.7 (d, 1JC-F = 236.6 Hz, Cq-F), 149.8 (Cq), 143.8 (Cq), 137.8 (d, 4JC-F = 2.0 Hz, Cq-NH), 130.5 (CH=N), 128.0 (Cq), 118.4 (d, 3JC-F = 8.3 Hz, 2CH), 115.6 (d, 2JC-F = 22.2 Hz, 2CH), 104.4 (Cq), 23.2 (Me) ppm. MS: m/z (%) = 302 (100) [M+], 259 (60) [C2H3O]+, 94 (60) [C6H4F]+.
N-(7-Oxo-2-(4-chlorophenylamino)-6,7-dihydropyrazolo[1,5-d][1,2,4]triazin-3-yl)acetamide (6g). The product was prepared according to 6a from 5g (277 mg, 1.00 mmol) and 3 mL Ac2O. Reaction time was 24 h at 40–45 °C. Colorless solid, yield 312 mg (0.98 mmol, 98%), mp 328–331 °C. IR (ATR): ṽmax = 3288, 3113, 2952, 1718, 1661, 1610, 1562, 1497, 1354, 1275, 1209, 1125, 896, 824, 724, 639, 574, 535, 502, 429 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 12.52 (br. s, 1H, NH), 9.67 (br. s, 1H, NH), 8.66 (br. s, 1H, NH), 8.20 (d, 1JC-H = 196.9 Hz, 1H, HC=N), 7.68 (d, J = 9.0 Hz, 2CH), 7.37 (d, J = 9.0 Hz, 2CH), 2.13 (s, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ 169.2 (C=O), 149.4 (Cq), 143.7 (Cq), 140.2 (Cq), 130.5 (CH=N), 128.9 (2CH), 128.1 (Cq), 124.1 (C-Cl), 118.4 (2CH), 104.7 (Cq), 23.2 (Me) ppm. MS: m/z (%) = 318 (100) [M+], 275 (25) [M—C2H3O]+, 240 (20) [M—C2H3O—Cl]+.
N-(7-Oxo-2-(2-chlorophenylamino)-6,7-dihydropyrazolo[1,5-d][1,2,4]triazin-3-yl)acetamide (6h). The product was prepared according to 6a from 5h (277 mg, 1.00 mmol) and 3 mL Ac2O. Reaction time was 4 h at rt. Brownish solid, yield 284 mg (0.89 mmol, 89%) mp 309–311 °C. IR (ATR): ṽmax = 3218, 1712, 16662, 1597, 1557, 1516, 1361, 1313, 1207, 1137, 1035, 905, 746, 723, 636, 532, 438 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 12.60 (br. s, 1H, NH), 10.33 (br. s, 1H, NH), 8.35 (br. s, 1H, NH), 8.27 (s, 1H, HC=N), 8.23 (d, J = 8.1 Hz, 1CH), 7.46 (d, J = 8.0 Hz, 1CH), 7.33 (dd, J = 8.1, 7.6 Hz, 1CH), 6.96 (dd, J = 8.0, 7.6 Hz, 1CH), 2.16 (s, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 169.8 (C=O), 148.5 (Cq), 143.6 (Cq), 137.7 (Cq), 129.8 (CH=N), 129.5 (CH), 128.0 (CH), 127.3 (Cq), 121.9 (CH), 120.7 (C-Cl), 118.1 (CH), 106.3 (Cq), 22.9 (Me) ppm. MS: m/z (%) = 318 (80) [M+], 283 (25) [M—Cl]+, 240 (100) [M—C2H3O—Cl]+, 127 (65) [M—C6H4NH—Cl]+.
N-(7-Oxo-2-(2,4-dichlorophenylamino)-6,7-dihydropyrazolo[1,5-d][1,2,4]triazin-3-yl)acetamide (6i). The product was prepared according to 6a from 5i (311 mg, 1.00 mmol) and 3 mL Ac2O. Reaction time was 3 h at rt. Yellowish solid, yield 314 mg (0.89 mmol, 89%) mp >340 °C. IR (ATR): ṽmax = 3424, 1734 (CO), 1671 (CO), 1596 (NO2), 1551, 1524, 1369 (NO2), 1248, 1218, 1094, 1052, 820, 566 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 12.63 (br. s, 1H, NH), 10.36 (br. s, 1H, NH), 8.52 (br. s, 1H, NH), 8.28 (s, 1H, HC=N), 8.24 (d, J = 8.8 Hz, 1CH), 7.61 (d, J = 2.3 Hz, 1CH), 7.42 (dd, J = 8.8, 2.3 Hz, 1CH), 2.16 (s, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 170.0 (C=O), 148.1 (Cq), 143.6 (Cq), 136.9 (Cq), 129.8 (CH=N), 128.9 (CH), 128.1 (CH), 127.3 (Cq), 124.4 (C-Cl), 121.4 (C-Cl), 118.9 (CH), 106.5 (Cq), 22.9 (Me) ppm. MS: m/z (%) = 352 (100) [M+], 317 (10) [M—Cl]+, 309 (25) [M—Ac]+, 274 (27) [M—Cl—Ac]+.
N-(2-((2-Methyl-3-(trifluoromethyl)phenyl)amino)-7-oxo-6,7-dihydropyrazolo[1,5-d][1,2,4]triazin-3-yl)acetamide (6j). The product was prepared according to 6a from 5j (324 mg, 1.00 mmol) and 3 mL Ac2O. Reaction time was 6 h at 40–45 °C. Colorless solid, yield 352 mg (0.96 mmol, 96%) mp 313–315 °C. IR (ATR): ṽmax = 3262, 3113, 2947, 1711, 1663, 1573, 1503, 1325, 1281, 1109, 1022, 9001, 791, 715, 637, 611, 540, 468, 426 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 12.55 (br. s, 1H, NH), 10.10 (br. s, 1H, NH), 8.24 (s, 1H, HC=N), 8.15 (br. s, 1H, NH), 8.07 (d, J = 7.7 Hz, 1CH), 7.42–7.29 (m, 2CH), 2.35 (s, 3H, Me), 2.13 (s, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 169.7 (C=O), 149.6 (Cq), 143.7 (Cq), 141.3 (Cq), 130.1 (CH), 128.5 (q, 2JC-F = 28.5 Hz, Cq), 127.8 (Cq), 126.8 (CH), 125.3 (Cq), 124.8 (q, 1JC-F = 274.3 Hz, CF3), 122.6 (CH), 118.9 (q, 3JC-F = 5.8 Hz, CH), 106.0 (Cq), 23.0 (Me), 13.7 (q, 4JC-F = 2.4 Hz, CH3) ppm. MS: m/z (%) = 366 (100) [M+], 323 (15) [M—C2H3O]+, 308 (50) [M—C2H3ONH]+.
Ethyl 2-(2-morpholino-3-nitro-7-oxopyrazolo[1,5-d][1,2,4]triazin-6(7H)-yl)acetate (7b). To a soln. of 266 mg (1.00 mmol) of triazinone 4b and 200 mg (1.20 mmol) of ethyl 2-bromoacetate in 7 mL DMF, 121 mg (1.20 mmol) triethylamine at 10 °C was added, and the resulting mixture was kept off with stirring at rt for 8 h. After the addition of 70 mL cold water and 1 mL conc. hydrochloric acid, the precipitate was filtered off, washed with water (2 × 5 mL) and cold MeOH (3 mL), and was then dried in vacuo to give 7b as a yellow solid, yield 335 mg (0.95 mmol, 95%) mp 130–131 °C. IR (KBr): ṽmax = 2983, 2856, 1762 (CO), 1733 (CO), 1597 (NO2), 1515, 1442, 1388, 1312, 1204, 1116, 962, 820, 678 cm−1. 1H-NMR (400 MHz, CDCl3): δ = 8.71 (d, 1JC-H = 204.4 Hz, 1H, HC=N), 4.96 (d, 1JC-H = 144.4 Hz, 2H, NCH2), 4.27 (q, J = 7.1 Hz, 2H, OCH2), 3.91–3.84 (m, 4H, 2OCH2), 3.55–3.49 (m, 4H, 2NCH2), 1.30 (t, J = 7.1 Hz, 3H, Me) ppm. 13C-NMR (101 MHz, CDCl3): δ = 166.5 (C=O), 155.5 (C=O), 142.4 (Cq), 135.1 (Cq), 128.4 (CH=N), 119.5 (CNO2), 66.2 (2OCH2), 62.3 (OCH2), 53.4 (NCH2), 49.7 (2NCH2), 14.1 (Me) ppm. MS: m/z (%) = 352 (20) [M+] 334 (70) [M—OH]+, 306 (12) [M—NO2]+, 289 (58) [M—OH—NO2]+, 279 (12) [M—CO2Et]+.
6-((6-Chloropyridin-3-yl)methyl)-2-((4-fluorophenyl)amino)-3-nitropyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (7f). The product was prepared according to 7b from 4f (290 mg, 1.00 mmol), 194 mg (1.20 mmol) 2-chloro-5-(chloromethyl)pyridine, 121 mg (1.20 mmol) triethylamine, and 7 mL DMF. Reaction time was 8 h at 75–80 °C. Orange solid, yield 391 mg (0.94 mmol, 94%) mp 205–206 °C. IR (KBr): ṽmax = 3365, 3071, 1736 (CO), 1614, 1587 (NO2), 1511, 1377 (NO2), 1312, 1024, 843, 667, 518 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 9.14 (br. s, 1H, NH), 8.78 (s, 1H, HC=N), 8.48 (d, J = 2.0 Hz, CH=N), 7.96–7.71 (m, 3CH), 7.53 (d, J = 8.1 Hz, 1CH), 7.23 (t, J = 8.1 Hz, 2CH), 5.41 (br. s, 2H, NCH2) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 158.2 (d, 1JC-F = 239.7 Hz, Cq-F), 150.0 (C-Cl), 149.9 (CH=N), 149.3 (Cq), 142.7 (Cq), 140.0 (CH), 135.7 (d, 4JC-F = 2.7 Hz, Cq-NH), 133.7 (Cq), 131.1 (Cq), 128.3 (CH=N), 124.4 (CH), 121.6 (d, 3JC-F = 7.9 Hz, 2CH), 115.7 (C-NO2), 115.6 (d, 2JC-F = 22.4 Hz, 2CH), 52.5 (NCH2) ppm. MS: m/z (%) = 415 (70) [M+], 167 (65), 126 (100) [C6H5ClN]+. HRMS-ESI: m/z calcd. for C17H11ClFN7O3Na [M + Na]+: 438.0488; found: 438.0492.
Ethyl 2-(2-((2,4-dichlorophenyl)amino)-3-nitro-7-oxopyrazolo[1,5-d][1,2,4]triazin-6(7H)-yl)acetate (7i). The product was prepared according to 7b from 4i (341 mg, 1.00 mmol), 200 mg (1.20 mmol) of ethyl 2-bromoacetate, 121 mg (1.20 mmol) of triethylamine, and 7 mL DMF. Reaction time was 10 h at rt. Yellow solid, yield 406 mg (0.95 mmol, 95%) mp 175–177 °C. IR (KBr): ṽmax = 3623, 3333, 1730 (CO), 1610, 1595 (NO2), 1556, 1476, 1379 (NO2), 1241, 1018, 842, 766, 617 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 9.21 (br. s, 1H, NH), 8.87 (d, 1JC-H = 202.6 Hz, 1H, HC=N), 8.35 (d, J = 8.8 Hz, 1CH), 7.78 (d, J = 2.1 Hz, 1CH), 7.59 (dd, J = 8.8, 2.1 Hz, 1CH), 5.07 (s, 2H, NCH2), 4.21 (q, J = 7.1 Hz, 2H, OCH2), 1.23 (t, J = 7.1 Hz, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 167.2 (C=O), 148.7 (Cq), 142.3 (Cq), 134.5 (Cq), 133.4 (Cq), 129.2 (CH), 128.6 (CH), 128.2 (CH), 127.5 (C-Cl), 123.3 (C-Cl), 121.0 (CH), 116.6 (C-NO2), 61.8 (OCH2), 54.0 (NCH2), 14.2 (Me) ppm. MS: m/z (%) = 426 (33) [M+], 390 (380) [M—HCl]+, 354 (8) [M—2HCl]+.
Methyl 2-(2-((2,4-dichlorophenyl)amino)-3-nitro-7-oxopyrazolo[1,5-d][1,2,4]triazin-6(7H)-yl)acetate (7ii). The product was prepared according to 7b from 4i (341 mg, 1.00 mmol), 184 mg (1.20 mmol) methyl 2-bromoacetate, 121 mg (1.20 mmol) triethylamine, and 7 mL DMF. Reaction time was 8 h at rt. Yellow solid, yield 359 mg (0.87 mmol, 87%) mp 201–202 °C. IR (ATR): ṽmax = 3315, 1737, 1598, 1558, 1502, 1482, 1375, 1321, 1220, 1134, 1032, 983, 925, 829, 765, 666, 626, 556, 429 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 9.22 (br. s, 1H, NH), 8.88 (d, 1JC-H = 205.0 Hz, 1H, HC=N), 8.36 (d, J = 8.9 Hz, 1CH), 7.79 (d, J = 2.3 Hz, 1CH), 7.60 (dd, J = 8.9, 2.3 Hz, 1CH), 5.09 (d, 1JC-H = 145.5 Hz, 2H, NCH2), 3.74 (d, 1JC-H = 148.2 Hz, 3H, OMe) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 167.7 (C=O), 148.7 (Cq), 142.3 (Cq), 134.5 (Cq), 133.4 (Cq), 129.2 (CH), 128.6 (CH), 128.3 (CH), 127.5 (C-Cl), 123.4 (C-Cl), 121.1 (CH), 116.7 (C-NO2), 53.8 (NCH2), 52.8 (OMe) ppm. MS: m/z (%) = 412 (100) [M+], 378 (70) [M—Cl]+, 224 (10) [M—C6H4NHF—NO2—Cl]+.
Ethyl 2-(3-amino-2-morpholino-7-oxopyrazolo[1,5-d][1,2,4]triazin-6(7H)-yl)acetate (8b). The product was prepared according to 5a from 7b (352 mg, 1.00 mmol) and tin(II) chloride dihydrate (677 mg, 3.00 mmol) in EtOH. Reaction time was 8 h at 40–45 °C. Brownish solid, yield 248 mg (0.77 mmol, 77%) mp 211–213 °C. IR (ATR): ṽmax = 3369, 2970, 1748, 1703, 15009, 1452, 1373, 1205, 1112, 1013, 953, 925, 902, 854, 755, 720, 573, 471, 441 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 8.30 (d, 1JC-H = 191.5 Hz, 1H, HC=N), 5.09 (br. s, 2H, NH2), 4.74 (d, 1JC-H = 143.0 Hz, 2H, NCH2), 4.15 (q, J = 7.1 Hz, 2H, OCH2), 3.77–3.72 (m, 4H, 2OCH2), 3.21–3.16 (m, 4H, 2NCH2), 1.20 (t, J = 7.1 Hz, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 168.5 (C=O), 153.5 (C=O), 144.1 (Cq), 130.7 (CH=N), 120.6 (Cq), 119.8 (Cq), 66.0 (2OCH2), 61.3 (OCH2), 52.1 (NCH2), 48.2 (2NCH2), 14.2 (Me) ppm. MS: m/z (%) = 322 (98) [M+], 306 (20) [M -NH2]+, 191 (100) [M—C2H5-– NC4H8O—NH2]+.
3-Amino-6-((6-chloropyridin-3-yl)methyl)-2-((4-fluorophenyl)amino)pyrazolo[1,5-d][1,2,4]triazin-7(6H)-one (8f). The product was prepared according to 5a from 7f (416 mg, 1.00 mmol) and tin(II) chloride dihydrate (677 mg, 3.00 mmol). Reaction time was 1 d at 65–70 °C. Brown solid, yield 339 mg (0.88 mmol, 88%) mp 206–208 °C. IR (KBr): ṽmax = 3338, 1698 (CO), 1619, 1581 (NO2), 1508, 1385 (NO2), 1213, 1096, 826, 716, 586 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 8.73 (br. s, 1H, NH), 8.41 (d, J = 1.9 Hz, 1H, HC=N), 8.29 (s, 1H, CH=N), 7.83 (dd, J = 8.2, 1.9 Hz, 1CH), 7.65 (dd, J = 8.4, 4.7 Hz, 2CH), 7.48 (d, J = 8.1 Hz, 1CH), 7.14 (t, J = 8.4 Hz, 2CH), 5.18 (br. s, 2H, NCH2), 4.07 (br. s, 2H, NH2) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 156.3 (d, 1JC-F = 237.2 Hz, Cq-F), 149.6 (C-Cl), 149.5 (CH=N), 147.3 (Cq), 144.1 (Cq), 139.6 (CH), 138.1 (d, 4JC-F = 1.9 Hz, Cq-NH), 132.8 (Cq), 130.7 (CH=N), 124.4 (CH), 118.8 (Cq), 117.7 (d, 3JC-F = 7.5 Hz, 2CH), 116.6 (Cq), 115.3 (d, 2JC-F = 22.4 Hz, 2CH), 50.5 (NCH2) ppm. MS: m/z (%) = 385 (7) [M+], 315 (5) [M—Cl—F—NH2]+, 243 (5) [M—NH2—C6H5ClN]+, 126 (40) [C6H5ClN]+.
Ethyl 2-(3-amino-2-((2,4-dichlorophenyl)amino)-7-oxopyrazolo[1,5-d][1,2,4]triazin-6(7H)-yl)acetate (8i). The product was prepared according to 5a from 7i (427 mg, 1.00 mmol) and tin(II) chloride dihydrate (677 mg, 3.00 mmol) in EtOH. Reaction time was 16 h at 65–70 °C. Beige solid, yield 334 mg (0.84 mmol, 84%), mp 275–278 °C. IR (ATR): ṽmax = 3385, 3251, 1732, 1675, 1544, 1474, 1381, 1216, 1102, 1014, 813, 710, 643, 573, 479, 426 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 8.33 (d, 1JC-H = 192.1 Hz, 1H, HC=N), 8.01 (d, J = 9.0 Hz, 1CH), 7.83 (br. s, 1H, NH), 7.61 (d, J = 2.3 Hz, 1CH), 7.39 (dd, J = 9.0, 2.3 Hz, 1CH), 5.41 (br. s, 2H, NH2), 4.77 (d, 1JC-H = 143.0 Hz, 2H, NCH2), 4.16 (q, J = 7.1 Hz, 2H, OCH2), 1.21 (t, J = 7.1 Hz, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 168.4 (C=O), 146.4 (Cq), 144.0 (Cq), 137.3 (Cq), 130.6 (CH), 129.0 (CH), 128.0 (CH), 124.7 (C-Cl), 122.1 (C-Cl), 120.0 (CH), 119.5 (Cq), 119.4 (Cq), 61.3 (OCH2), 52.2 (NCH2), 14.2 (Me) ppm. MS: m/z (%) = 396 (100) [M+], 360 (25) [M—Cl]+, 324 (35) [M—2 Cl]+. HRMS-ESI: m/z calcd. for C15H14Cl2N6O3Na [M + Na]+: 419.0397; found: 319.0404.
Methyl 2-(3-amino-2-((2,4-dichlorophenyl)amino)-7-oxopyrazolo[1,5-d][1,2,4]triazin-6(7H)-yl)acetate (8ii). The product was prepared according to 5a from 7ii (414 mg, 1.00 mmol) and tin(II) chloride dihydrate (677 mg, 3.00 mmol) in MeOH. Reaction time was 18 h at 65–70 °C. Light brown solid, yield 261 mg (0.68 mmol, 68%) mp 273–275 °C. IR (KBr): ṽmax = 3391, 3254, 1742 (CO), 1677 (CO), 1548 (NO2), 1475, 1392 (NO2), 1228, 1040, 813, 714, 552 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 8.34 (s, 1H, HC=N), 8.00 (d, J = 8.9 Hz, 1CH), 7.85 (br. s, 1H, NH), 7.61 (d, J = 2.4 Hz, 1CH), 7.39 (dd, J = 8.9, 2.4 Hz, 1CH), 5.44 (br. s, 2H, NH2), 4.79 (s, 2H, NCH2), 3.69 (s, 3H, OCH3) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 169.0 (C=O), 146.5 (Cq), 144.0 (Cq), 137.3 (Cq), 130.7 (CH), 129.0 (CH), 128.0 (CH), 124.8 (C-Cl), 122.2 (C-Cl), 122.1 (Cq), 120.0 (CH), 119.5 (Cq), 52.5 (OCH3), 52.1 (NCH2) ppm. MS: m/z (%) = 382 (90) [M]+, 347 (40) [M—Cl]+, 323 (45) [M—CO2Me]+.
Ethyl 2-(3-acetamido-2-morpholino-7-oxopyrazolo[1,5-d][1,2,4]triazin-6(7H)-yl)acetate (9b). The product was prepared according to 6a from 8b (322 mg, 1.00 mmol) and 3 mL Ac2O. Reaction time was 96 h at rt. Colorless solid, yield 346 mg (0.95 mmol, 95%) mp 204–205 °C. IR (ATR): ṽmax = 3297, 2954, 2863, 1747, 1698, 1488, 1353, 1213, 1109, 1019, 960, 928, 765, 740, 6001, 546, 523 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 9.77 (br. s, 1H, NH), 8.17 (d, 1JC-H = 197.3 Hz, 1H, HC=N), 4.86 (d, 1JC-H = 143.6 Hz, 2H, NCH2), 4.17 (q, J = 7.1 Hz, 2H, OCH2), 3.79–3.67 (m, 4H, 2OCH2), 3.32–3.23 (m, 4H, 2NCH2), 2.08 (d, 1JC-H = 128.2 Hz, 3H, CH3), 1.21 (t, J = 7.1 Hz, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 169.3 (C=O), 168.1 (C=O), 156.4 (C=O), 143.5 (Cq), 131.1 (Cq), 130.4 (CH=N), 105.3 (Cq), 65.8 (2OCH2), 61.5 (OCH2), 52.6 (NCH2), 47.8 (2NCH2), 22.9 (Me), 14.2 (Me) ppm. MS: m/z (%) = 364 (99) [M+], 321 (65) [M—C2H3O]+, 190 (100) [M—C2H5O—NC4H8O—C2H3O]+.
N-(6-((6-Chloropyridin-3-yl)methyl)-2-((4-fluorophenyl)amino)-7-oxo-6,7-dihydropyrazolo[1,5-d][1,2,4]triazin-3-yl)acetamide (9f). The product was prepared according to 6a from 8f (386 mg, 1.00 mmol) and 3 mL Ac2O. Reaction time was 8 h at 40–45 °C. Colorless solid, yield 394 mg (0.92 mmol, 92%) mp 274–276 °C. IR (ATR): ṽmax = 3271, 1718, 1657, 1618, 1582, 1505, 1360, 1211, 116, 826, 745, 722, 664, 511 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 9.71 (br. s, 1H, NH), 8.55 (br. s, 1H, NH), 8.45 (d, J = 2.3 Hz, 1H, HC=N), 8.29 (s, 1H, CH=N), 7.85 (dd, J = 8.3, 2.3 Hz, 1CH), 7.66 (dd, J = 9.0, 4.8 Hz, 2CH), 7.50 (d, J = 8.3 Hz, 1CH), 7.17 (t, J = 9.0 Hz, 2CH), 5.27 (d, 1JC-H = 142.6 Hz, 2H, NCH2), 2.13 (d, 1JC-H = 128.3 Hz, 3H, CH3) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 169.0 (C=O), 156.8 (d, 1JC-F = 236.5 Hz, Cq-F), 149.85 (Cq), 149.75 (Cq), 149.7 (CH=N), 143.6 (Cq), 139.7 (CH), 137.7 (d, 4JC-F = 2.2 Hz, Cq-NH), 132.2 (Cq), 130.9 (CH=N), 127.5 (Cq), 124.4 (CH), 118.4 (d, 3JC-F = 7.5 Hz, 2CH), 115.6 (d, 2JC-F = 22.2 Hz, 2CH), 105.0 (Cq), 51.0 (NCH2), 23.2 (Me) ppm. MS: m/z (%) = 427 (100) [M]+, 385 (15) [M -C2H3O]+, 126 (75) [C5NH3CH2Cl]+. HRMS-ESI: m/z calcd. for C19H15ClFN7O2Na [M + Na]+: 450.0852; found: 450.0853.
Ethyl 2-(3-acetamido-2-((2,4-dichlorophenyl)amino)-7-oxopyrazolo[1,5-d][1,2,4]triazin-6(7H)-yl)acetate (9i). The product was prepared according to 6a from 8i (397 mg, 1.00 mmol) and 3 mL Ac2O. Reaction time was 1 d at rt. Beige solid, yield 426 mg (0.97 mmol, 97%) mp 280–282 °C. IR (ATR): ṽmax = 3251, 3009, 1730, 1656, 1593, 1548, 1355, 1213, 1144, 1016, 817, 730, 621, 601, 538, 434 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 10.39 (br. s, 1H, NH), 8.54 (br. s, 1H, NH), 8.37 (s, 1H, HC=N), 8.18 (d, J = 9.0 Hz, 1CH), 7.62 (br. s, 1CH), 7.43 (d, J = 9.0 Hz, 1CH), 4.88 (d, 1JC-H = 145.0 Hz, 2H, NCH2), 4.18 (q, J = 7.3 Hz, 2H, OCH2), 2.17 (s, 3H, Me), 1.22 (t, J = 7.3 Hz, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 169.9 (C=O), 168.1 (C=O), 148.7 (Cq), 143.3 (Cq), 136.8 (Cq), 130.0 (CH), 128.9 (CH), 128.1 (CH), 126.9 (Cq), 124.8 (C-Cl), 121.8 (C-Cl), 119.2 (CH), 107.3 (Cq), 61.5 (OCH2), 52.7 (NCH2), 22.9 (Me), 14.2 (Me) ppm. MS: (%) = 439 (100) [M+], 360 (75) [M—C2H3O—Cl]+.
Methyl 2-(3-acetamido-2-((2,4-dichlorophenyl)amino)-7-oxopyrazolo[1,5-d][1,2,4]triazin-6(7H)-yl)acetate (9ii). The product was prepared according to 6a from 8ii (383 mg, 1.00 mmol) and 3 mL Ac2O. Reaction time was 6 h at rt. Colorless solid, yield 395 mg (0.93 mmol, 93%) mp 289–292 °C. IR (KBr): ṽmax = 3251, 1733 (CO), 1657, 1596 (NO2), 1551, 1354 (NO2), 1225, 1144, 1041, 820, 729, 661 cm−1. 1H-NMR (400 MHz, DMSO-d6): δ = 10.40 (br. s, 1H, NH), 8.54 (br. s, 1H, NH), 8.37 (s, 1H, HC=N), 8.18 (d, J = 9.0 Hz, 1CH), 7.62 (d, J = 2.5 Hz, 1CH), 7.43 (dd, J = 9.0, 2.5 Hz, 1CH), 4.91 (s, 2H, NCH2), 3.72 (s, 3H, OCH3), 2.17 (s, 3H, Me) ppm. 13C-NMR (101 MHz, DMSO-d6): δ = 169.9 (C=O), 168.6 (C=O), 148.7 (Cq), 143.3 (Cq), 136.8 (Cq), 130.1 (CH), 128.9 (CH), 128.1 (CH), 127.0 (Cq), 124.8 (C-Cl), 121.8 (C-Cl), 119.2 (CH), 107.3 (Cq), 52.7 (NCH2), 52.6 (OCH3), 22.9 (Me) ppm. MS: m/z (%) = 424 (100) [M+], 388 (18) [M—HCl]+, 381 (15) [M—Ac]+, 365 (8) [M—CO2Me]+, 346 (80) [M—HCl—Ac]+.