Abstract
We have developed a highly sensitive colorimetric probe based on starch–iodine (I2) crystallization for the precise discrimination of ethanol–water clusters (E-Wc) within binary ethanol–water solutions (E-Ws). This probe enables the identification of specific E-Wc species and their corresponding transition points. Notably, two distinct transition points were identified at ethanol volume fractions of 40–45% and 75–77%. The former corresponds to the structural transition from (H2O)m(EtOH) to (H2O)m(EtOH)n, characterized by a significant loss of blue coloration, while the latter signifies the transition from (H2O)m(EtOH)n to (H2O)(EtOH)n, as evidenced by alterations in the absorption intensity of the starch–I2 complex. Mechanistic studies demonstrate that the observed starch–I2 crystallization is governed by supramolecular E-Wc rather than individual ethanol or water molecules in the binary solution. By leveraging starch–I2 crystallization as a colorimetric bridge, we establish a direct correlation between E-Wc transitions and the iodine chromogenic effect. This approach enables the visual detection of transitions in colorless supramolecular assemblies, offering new insights into supramolecular science. Furthermore, as a simple, rapid, and visually interpretable detection method, this colorimetric probe holds promising applications in fields such as the food industry and supramolecular science.
1. Introduction
Supramolecular assemblies and clusters formed through non-covalent interactions (e.g., hydrogen bonds, π-π stacking, hydrophobic effects) demonstrate remarkable potential in materials science, biosensing, and drug delivery. However, colorless supramolecular assemblies/clusters are particularly challenging to study because their transparent nature makes it difficult to directly observe state transitions in solution. This absence of visible optical signals complicates the investigation of their dynamic behavior and functional mechanisms.
Research indicates that the hydrogen bonding (HB) configurations in ethanol–water mixtures exhibit significant differences compared to those observed in pure water or pure ethanol, owing to the formation of distinct E-Wc species that occurs in such binary systems [1]. Concurrently with the dynamic processes of HB formation and dissociation, the structural complexity of E-Wc undergoes substantial variations [2]. Consequently, elucidating the patterns of E-Wc in such binary mixtures is essential for advancing the understanding of ethanol–water systems across diverse application domains. To achieve this objective, a comprehensive suite of experimental techniques has been employed, including mass spectrometry [3,4], Raman spectroscopy [5,6], nuclear magnetic resonance (NMR) spectroscopy [7], neutron diffraction [8,9,10], X-ray diffraction (XRD) [4,11], and infrared spectroscopy [7,12,13], to investigate the molecular interactions between ethanol and water. Furthermore, quantum chemical calculations have also been increasingly adopted as complementary methods to these experimental approaches [14,15,16,17], providing valuable insights into the alterations of HB interactions between ethanol and water molecules.
Compared to conventional experimental approaches that often entail inherent complexity, time-consuming procedures, and significant costs, optical probes offer notable advantages characterized by operational simplicity, rapid response times, high analytical precision, and exceptional sensitivity to molecular environmental changes. These distinctive features make optical probes particularly suitable for tracking hydrogen bonding configuration changes through their responsive behavior to molecular environmental variations. However, the currently reported fluorescent probes can only detect the water content in ethanol or bioethanol by affecting their absorption and emission characteristics through sensitivity to solvent polarity or hydrogen bonding interactions with water molecules [18,19]. To date, no research has documented the application of fluorescent probes for elucidating hydrogen bonding pattern variations in E-Wc systems. Furthermore, the potential utilization of straightforward colorimetric probes in this specific research domain remains entirely unexplored. Given these research gaps, the development of a direct, user-friendly, and visually interpretable colorimetric probe—despite the numerous technical challenges that persist—holds considerable scientific significance and practical value for both fundamental research and potential applications.
Starch functions as the principal carbohydrate constituent in cereal grains and represents a vital nutritional resource for humans. Chemically, it consists of two distinct glucose polymers: amylose, the minor constituent, which exhibits a predominantly linear molecular architecture with molecular weights spanning from 105 to 106, and amylopectin, the predominant component, characterized by its highly branched chain structure with molecular weights ranging between 107 and 108 [20]. The linear amylose molecules typically adopt a helical conformation stabilized by intramolecular hydrogen bonds (HBs) [21]. Upon interaction with iodine molecules (I2), starch forms a distinctive helical complex through non-covalent interactions. This starch–I2 complex is formed via the accommodation of I2 within the hydrophobic cavities of the starch helix, resulting in a thermodynamically stable configuration with notable optical properties. A particularly remarkable characteristic of this complex is its intense colorimetric response, typically manifesting as a deep blue hue. This phenomenon arises from the non-covalent interaction between starch and I2, which facilitates charge transfer to generate triiodide ions (I3−), thereby producing the characteristic blue coloration [22]. The distinct colorimetric behavior of the starch–I2 complex has established it as a widely utilized optical probe for analyzing the structural features of starch in aqueous solutions. Previous studies have demonstrated that in middle-concentration ethanol–water systems (48–68 vol%), amylose can form single, left-handed helical inclusion complexes with ethanol, referred to as V-type crystals [23]. More recent investigations have revealed that in 70 vol% E-W systems, starch gelatinization can be entirely suppressed following annealing treatment, with V-type complex formation between starch and ethanol initiating at temperatures exceeding 110 °C [24]. Thus, the incorporation of ethanol into aqueous solutions not only inhibits starch granule swelling but also promotes the formation of V-type single helical complexes, thereby stabilizing starch chains [25]. Consequently, annealing starch in E-W systems has been employed to improve its thermal, shear, and acid resistance, broadening its applicability in the food industry [26].
In general, molecular-level interactions between starch and ethanol primarily involve HBs, hydrophobic interactions, and van der Waals forces [26]. However, in fact, the underlying mechanisms are considerably more complex, as ethanol molecules in E-W systems are never isolated but instead form various E-Wc through complexation with water. The intrinsic mechanism behind it has not been truly solved; in particular, the mechanism between starch and E-Wc and their effects on starch crystallinity have never been investigated. This study aims to address this knowledge gap by employing the starch–I2 complex as an optical probe to elucidate the transition behavior of E-Wc. The starch–I2 complex, renowned for its sensitivity and distinct colorimetric response, serves as an effective tool for monitoring subtle structural changes within E-Ws. Unlike conventional methods that often rely on indirect measurements or complex theoretical models, our approach leverages the unique optical properties of the starch–I2 complex to directly track alterations in the local environment of E-Ws. The transitions of E-Wc in different E-Ws are visually demonstrated through the color changes of the starch–I2 complex following thermal-annealing treatments in various E-Ws media. The crystalline structures of starch formed within E-Ws were further characterized employing advanced analytical techniques, including XRD, scanning electron microscopy (SEM), and Fourier-transform infrared spectroscopy (FT-IR), thereby enabling a comprehensive elucidation of the underlying responsive mechanisms. This integrated approach effectively bridges the gap between macroscopic observations and atomic-scale insights. Notably, the starch–I2 complex facilitates the correlation of optical property changes with specific structural rearrangements in E-Ws, thereby providing a more refined understanding of their behavior. This methodology establishes a novel pathway for demonstrating transitions in colorless supramolecular assemblies through the use of readily accessible optical probes. Furthermore, it offers a unique capability to visualize and quantify dynamic changes in E-Ws in real time, presenting a new paradigm for investigating their behavior.
2. Results
2.1. The Colorimetry Responses of the I2/I− System to Starch Crystals Formed in Various Ethanol–Water Solutions (E-Ws)
Figure 1 clearly illustrates that the starch solution, without undergoing thermal annealing treatment, was incubated with E-Ws at different concentrations. Following the addition of the I2/I− solution, the mixture was further incubated and subjected to centrifugation to remove large particles. The resulting supernatant remained colorless visually. The UV-vis absorption peaks observed at 290 and 360 nm are attributed to the anionic iodine species (I3−) [27].
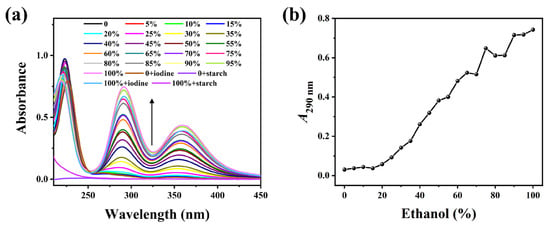
Figure 1.
(a) Absorption spectra of the mixture of soluble starch and I2/I− in different ethanol–water solutions (0–100 vol%) without heat treatment. (The arrow indicates the trend of spectral intensity change.) (b) Relationship curves of A290 with ethanol concentrations.
Following hydrothermal annealing treatment with varying volumes of E-Ws (Approach I), the introduction of I2/I− generates a blue solution exhibiting a broad absorption peak centered at 590 nm (Figure 2a), which is attributed to the formation of the starch–I2 complex [26]. As the ethanol concentration increases, the solution color progressively diminishes, transitioning sequentially from blue to purplish-red and ultimately to colorless. Specifically, within the ethanol concentration range of 40–45 vol%, the color evolves from light pink to complete decolorization. Further elevation of ethanol concentration produces negligible additional changes in solution color (Figure 2b). Concurrently, absorption peaks at 290 and 360 nm are observed in the starch-I3− aqueous mixture, indicative of free I3− species. Notably, the intensities of these peaks exhibit a positive correlation with increasing ethanol concentration, suggesting a reduced formation of the starch–I2 complex.
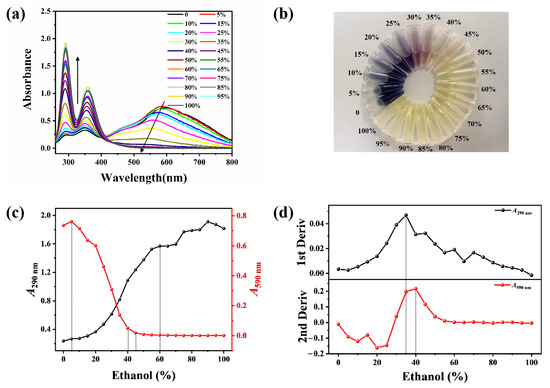
Figure 2.
(a) UV-vis absorption spectra of the mixture of starch and I2/I− in different ethanol–water solutions (0–100 vol%), being prepared based on Approach I. (The arrows indicate the trend of spectral intensity change.) (b) Corresponding photos of (a) being measured under daylight. (c) Relationship curves between A290 and A590 with the ethanol concentrations. (d) Corresponding first derivative curve of A290 with ethanol concentrations (upper) and second derivative curve of A590 with ethanol concentrations (bottom). (The dotted lines indicate the inflection point of the trend change).
The absorbance values at 290 nm (A290) and 590 nm (A590) as functions of ethanol concentration are presented in Figure 2c. With increasing ethanol concentration, A290 exhibits a continuous upward trend, whereas A590 demonstrates a corresponding decline. Notably, within the ethanol concentration range of 5–40 vol%, both A290 and A590 undergo significant changes: A290 increases sharply while A590 decreases markedly. When the ethanol concentration surpasses 45 vol%, A290 continues to rise rapidly, while A590 remains relatively stable with minimal variation. At ethanol concentrations exceeding 60 vol%, A290 displays irregular fluctuation patterns, whereas A590 maintains remarkable stability (Figure 2c).
The second derivative curve of A590 values as a function of ethanol concentration exhibits a distinct transition region between 35 and 40 vol%, which corresponds to the inflection point observed at 35 vol% in the first derivative curve of A290. Notably, when the ethanol concentration surpasses 60 vol%, the curve becomes linear and loses sensitivity to the structural change occurring at 75 vol% (Figure 2d). Consequently, thermal annealing treatments of starch were performed using E-Ws with concentrations ranging from 60–79 vol% (in 2 vol% increments), as outlined in Approach II.
As shown in Figure 3, distinct absorption peaks were observed within the wavelength range of 500–800 nm in the PS and I2/I− mixture, indicating the formation of an amylose–I2 complex (Figure 3a,c). Typically, absorbance values serve as an indicator of the complexation affinity between starch and I2. In this interaction, starch chains adopt a single helical conformation, enabling electron transfer between iodine molecules and starch upon entry into the helical cavity, which consequently generates coloration (with the amylose–I2 complex exhibiting a blue hue and the amylopectin-I2 complex displaying a purple-red coloration) [28]. Thus, a stronger starch–I2 interaction corresponds to higher absorbance values in the 500–800 nm range and a more intense blue coloration.
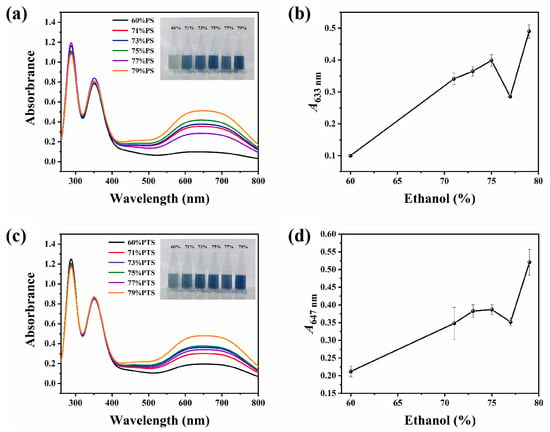
Figure 3.
(a) UV-vis absorption spectra and the corresponding photos (insert) of the mixture of PS and I2/I− under daylight after thermal annealing treatments in 60–79 vol% E-Ws (approach II). (b) The plot of the relationship between corresponding A633 and ethanol concentrations. (c) UV-vis absorption spectra and the corresponding photos (insert) of the mixture of PTS and I2/I− under daylight after thermal annealing treatments in 60–79 vol% E-Ws (Approach II). (d) The plot of the relationship between corresponding A647 and ethanol concentrations.
As demonstrated in the inset of Figure 3a, with progressive increases in ethanol concentration, the color of the PS-I2 complex transitions from light to dark blue. However, when the ethanol concentration increases from 75 vol% to 77 vol%, an anomalous fluctuation is observed, where both the color intensity and the A633 value deviate from the previously established increasing trend (Figure 3b). A similar phenomenon was also observed for PTS (Figure 3c,d), further confirming this irregularity in the PS system.
2.2. Structure and Morphology Characterizations of Starch Crystal
2.2.1. Long-Range Helical Structures Revealed by X-Ray Diffraction (XRD)
XRD is widely utilized to characterize the helical structures of long-range ordered crystals formed in starch. The XRD patterns of PS and PTS before and after thermal annealing in 75 vol% and 77 vol% E-Ws are compared in Figure 4. Initially, prior to thermal treatment (black curves in Figure 4a), PS exhibited a typical C-type crystalline structure [26], characterized by a distinct peak at 5.83°, two peaks at 14.9° and 23.0°, and an additional pair of peaks at 17.0° and 18.1°. Following thermal annealing, however, the peak at 5.83° nearly disappeared while the other four peaks remained well-defined, indicating a thermal-induced transition from C-type to A-type crystalline structure [29]. The RC was subsequently calculated based on the integrated area of characteristic diffraction peaks. The results revealed an overall decrease in RC from 37.19% to 33.57% and 31.97% for the two starch samples after thermal annealing in E-Ws.
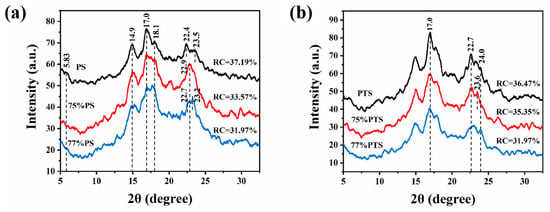
Figure 4.
X-ray diffraction curves of (a) native pea starch (PS), and (b) native potato starch (PTS), and after thermal annealing in 75 vol% (red) and 77 vol% (blue) E-Ws (Approach II), respectively.
Moreover, the XRD analysis unveils several significant structural modifications (Figure 4a). The peak intensity corresponding to starch at 14.9° exhibited a marked reduction following thermal annealing treatment, concomitant with a broadening of the peak width. Simultaneously, the peaks at 17.0° and 18.1° progressively widened, accompanied by a decrease in their relative intensity ratio. Furthermore, the peak morphology near 23.0° became sharper, while its intensity displayed fluctuations post-annealing. These observations provide compelling evidence of the structural alterations in starch crystallinity induced by thermal annealing.
Additionally, based on Equation (1), the relative RC of PS in 77% E-Ws (31.97%) was lower than that in 75% E-W solution (33.57%). This difference can be attributed to variations in starch granule gelatinization during thermal treatment [26], leading to greater fracture of the crystalline domains formed during the annealing process in the 77% E-W system.
As illustrated in Figure 4b, in contrast to the untreated sample, the annealed PTS specimen exhibited a typical B-type crystal structure, which is commonly observed in native PTS, indicating negligible changes in crystallinity. Notably, thermal annealing treatment resulted in a decrease in peak intensity and an increase in peak width at 15.0°, 17.0°, and 22.7°, along with fluctuations in peak intensity near 24.0°. These changes enabled the evaluation of RC for PTS before and after thermal annealing in systems containing either 75 vol% or 77 vol% E-Ws. Based on Equation (1), the RC value of PTS in the 77 vol% E-Ws system (31.97%) was significantly lower than that in the 75 vol% E-Ws system (35.35%), which is in excellent agreement with the previously observed trends for PS.
2.2.2. Granular Morphologies of Starches Revealed by SEM Images
The morphological features and surface characteristics of native PS and thermally annealed PS are presented in Figure 5. Native starch granules exhibit a smooth surface devoid of cavities or fractures, with shapes varying from kidney-shaped and spherical to oval and displaying a broad size distribution. Smaller granules predominantly adopt a spherical morphology, whereas larger granules tend toward an oval configuration [30]. The kidney shape, however, remains a consistent morphological feature across all size ranges (Figure 5a,d).
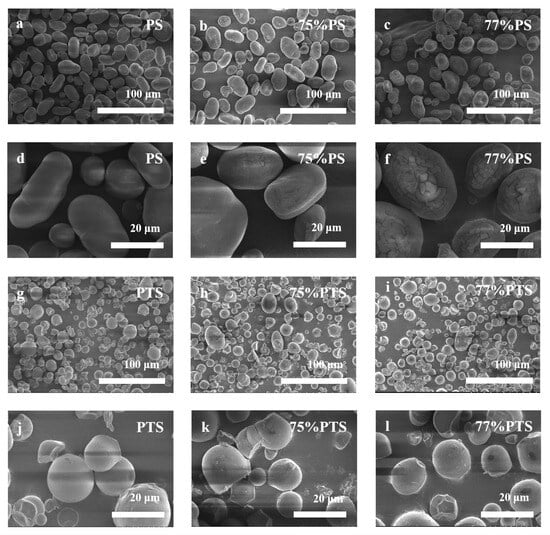
Figure 5.
SEM images of (a,d) native PS and thermal annealing-treated PS by (b,e) 75 vol%, (c,f) 77 vol% E-Ws; (g,j) native PTS and thermal annealing-treated PTS by (h,k) 75 vol%, (i,l) 77 vol% E-Ws (Approach II) at different scales, respectively.
Thermal annealing treatment induces only minor surface disruptions without significantly altering the overall granule morphology. While some granules develop indentations or minor fractures following annealing, the primary structural integrity is preserved. Comparative analysis between PS samples in 75 vol% E-Ws (Figure 5b,e) and those in 77 vol% E-Ws (Figure 5c,f) reveals that the latter exhibit deeper and more pronounced surface cracks. This difference can be attributed to the greater degree of partial swelling and gelatinization experienced by starch granules during annealing in the 77 vol% E-Ws, followed by shrinkage during the drying process [31]. Notably, the 77% PS system undergoes more extensive swelling compared to the 75% PS system, leading to the formation of deeper and larger fractures.
Native PTS granules showed smooth surfaces with truncated, circular shapes (Figure 5g,j). After E-Ws thermal annealing, PTS granules developed surface indentations and small fractures, exhibiting roughened surfaces and occasional irregular, blocky structures. The comparison between 75% PTS (Figure 5h,k) and 77% PTS (Figure 5i,l) demonstrated deeper cracks and more prevalent blocky structures in the 77% PTS samples. Notably, the morphological changes in PTS following thermal annealing closely mirrored those observed in PS under identical treatment conditions.
2.2.3. Fine Structural Differences in Native and Annealed Starch Samples Revealed by FT-IR Spectroscopy
FT-IR spectroscopy is a crucial method for analyzing the composition and structure of samples. It allows for the determination of ligand complexation with starch and helps identify structural differences between samples. Using FT-IR spectroscopy, detailed investigations were carried out on the material composition, conformation, configuration, and crystalline structure of starch samples. The results obtained from the analysis of native and annealed starch samples are presented in Figure 6 for comparison.
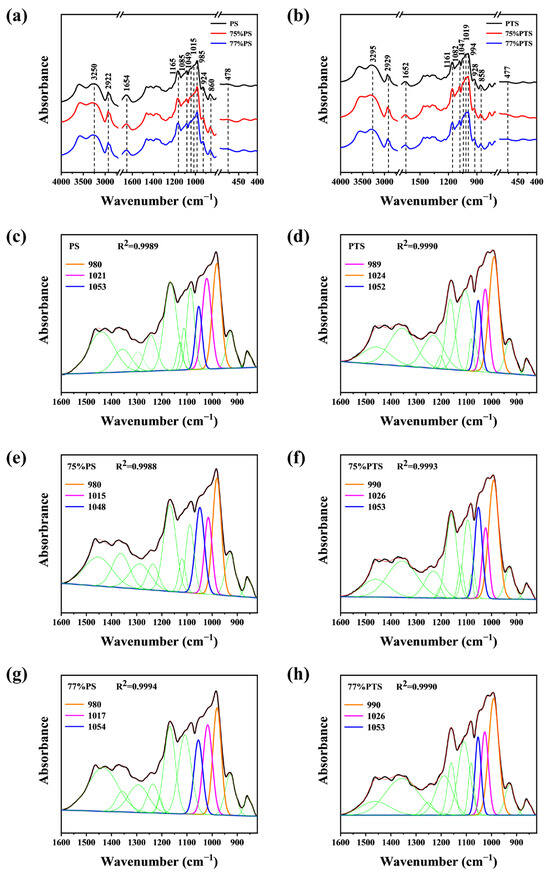
Figure 6.
FT-IR patterns of native and thermal annealing-treated (a) PS, and (b) PTS by E-Ws. Split-peak fitting curve of (c) native PS, (d) 75 vol%, and (e) 77 vol% E-Ws-treated PS, and (f) native PTS, and (g) 75 vol% (h) 77 vol% E-Ws-treated PTS (Approach II). (The black lines in panels (c) to (f) represent the experimental infrared data of the sample, while the green lines correspond to 9 distinct peaks obtained from Gaussian deconvolution within the spectral region of 1600–820 cm−1, excluding the peaks at 1047, 1022, and 995 cm−1).
All samples, including both native starch and those subjected to thermal annealing in the E-Ws, exhibited a broad absorption band at ~3250 cm−1, attributed to abundant O-H groups in starch molecules. A minor absorption peak at 2922 cm−1 was assigned to C-H stretching vibrations [32]. Additionally, the peak near 1654 cm−1 corresponds to the vibrational frequency of the aldehyde group (C=O) in starch. Absorption bands at 1165 cm−1 and 1085 cm−1 were observed in all samples, corresponding to C-O stretching vibrations. Furthermore, the absorption peak at ~985 cm−1 indicates C-OH vibration, associated with intramolecular hydrogen bonds at the C6 position of starch [33,34]. Besides, characteristic bands at 478 cm−1, 860 cm−1, 924 cm−1, and 2922 cm−1 were linked to CH2, C1-O-C4, C1-O-C5, and C-H vibrational modes, respectively [35].
The remarkable similarity evident in the FTIR spectra across all samples indicates that the short-range ordering of the annealed PS samples closely resembles that of native amylose starch. Likewise, thermal annealing of PTS resulted in a short-range molecular arrangement that aligns closely with that of natural PTS, as corroborated by X-ray diffraction analysis [36]. Nevertheless, subtle structural distinctions between these materials can be effectively identified through subsequent spectral split curve-fitting analyses.
The split-peak fitting results for both native and annealed samples in the 1600–820 cm−1 spectral region are presented in Figure 6c–h, respectively. Gaussian deconvolution revealed 12 distinct peaks within this wavenumber range, with peak intensities quantified by their corresponding peak areas. Taking native PS as an example, the absorption bands at 1049 cm−1 and 985 cm−1 are attributed to the crystalline regions, while the band at 1015 cm−1 corresponds to the amorphous regions of starch. Consequently, the short-range ordered helical structures of starch can be effectively characterized by analyzing the area ratios of 1049/1015 and 1015/985, as previously reported [37]. Specifically, higher 1049/1015 ratios and lower 1015/985 ratios indicate a more ordered short-range internal structure [24].
Following peak deconvolution, the calculated area ratios are summarized in Table 1. Comparative analysis with the native PS spectrum demonstrated significant increases in both the 1049/1015 and 1015/985 absorbance ratios for PS starch, suggesting enhanced short-range helical ordering. Furthermore, when compared to the 75%PS sample, the 77%PS exhibited a reduced degree of short-range ordering, which is in good agreement with the XRD findings.

Table 1.
The ratios of absorbance between 1047, 1022, and 995 cm−1 after deconvolution of infrared spectra.
Thermal annealing treatment similarly induced an increase in the short-range helical structure of PTS. Similarly, the short-range helix of 77%PTS was lower than that of 75%PTS, illustrating again the gelatinization difference of starch granules during the thermal process [26], resulting in more fractures of the crystalline granules during annealing of 77%PS.
3. Discussion
3.1. Small Molecule Interference on Amylose Crystallinity
Starch consists of a mixture of amylose and amylopectin molecules. The linear segments of amylose chains and the side chains of amylopectin can form complexes with various small molecules, including iodine, alcohols, fatty acids, aromatic compounds, salicylic acid, and even ibuprofen [38]. Depending on environmental conditions, amylose in aqueous solution may adopt a left-handed helical conformation or exist as random coils. Notably, when amylose is in a single-helical state, its interior exhibits greater hydrophobicity than its surface [39]. Upon thermal treatment of starch, numerous single helices can interact with hydrophobic guest molecules and subsequently reassemble into new V-type crystalline structures. Thus, the preformation of V-type starch serves as a promising strategy for accommodating small guest molecules via hydrophobic interactions and diffusion driven by concentration gradients [40].
Chen et al. employed the characteristic color reaction between starch and iodine to evaluate the complexation affinity between starch and ethanol. Their findings suggest that when ethanol molecules occupy the helical cavity of amylose, the available binding sites for iodine–amylose interaction are reduced, thereby diminishing the intensity of the color reaction [26]. This phenomenon indicates that ethanol–starch complexation alters the crystalline structure of amylose, consequently reducing its iodine-binding capacity. Moreover, through systematic investigation of the amylose-I2/I− system, they observed subtle shifts in the absorption maximum (~600 nm) under varying conditions, including different iodide concentrations and amylose chain lengths modified by amylase treatment [41].
3.2. Macromolecule Interference on the Amylose Crystallinity
In addition to small-molecule compounds, macromolecular substances, including proteins, cellulose, and pectin, can also interact with starch to form complexes. These interactions can substantially modify the physicochemical properties and structural characteristics of the starch system, including viscosity, dynamic modulus, gel enthalpy, and even crystallinity [42,43,44]. The formation of starch–guest complexes is predominantly governed by non-covalent interactions, encompassing van der Waals forces, HBs, and hydrophobic interactions. Among these, hydrophobic interactions play a particularly pivotal role, as the helical cavities formed by amylose chains exhibit inherent hydrophobicity. Notably, the internal hydrophobic environment of the helix enables the binding of non-polar regions of guest molecules through such non-covalent interactions. Concurrently, the polar regions of the guest molecules can establish HBs with the hydroxyl groups located on the outer surface of the helix.
Furthermore, due to the large molecular size of guest compounds, steric hindrance can significantly influence the formation of starch helical structures. For instance, to examine the effects of high-pressure homogenization (HPH) on starch structure, the honokiol (HK) and arctiin (AC), were selected as representative model compounds of lignans [45]. Under high-shear conditions, lignans were observed to form complexes with starch molecules via hydrophobic interactions or HBs, potentially inducing amylose to form double helices. These complexes could further assemble into crystalline structures and ultimately become entangled into aggregates.
Moreover, it was observed that starch subjected to HPH treatment exhibited a reduced double helical structure when combined with AC compared to HK. Besides, upon the addition of the same lignan, HK demonstrated a greater propensity to form V-type crystallinity than AC. These results indicate that the smaller molecular size of HK facilitates its penetration into the hydrophobic cavity of amylose, thereby promoting the formation of a single helix. This, in turn, enables the amylose molecules to arrange more regularly into a double helical structure due to the relatively weak steric hindrance. In contrast, AC encountered greater difficulty in forming an inclusion complex with amylose or inducing the self-assembly of starch molecules, as its larger molecular size resulted in stronger steric hindrance during complexation. Consequently, this led to a reduction in starch crystallinity when AC was employed.
3.3. Interference of Supramolecular Complex with Starch Crystallinity
It is widely established that pure liquid water forms a dynamic three-dimensional (3D) hydrogen-bonded network [46]. Consequently, the introduction of a small fraction of alcohol into water typically disrupts the intermolecular HBs among water molecules, resulting in an endothermic process. Surprisingly, however, experimental observations reveal that the mixing of water and alcohol generates excessive heat evolution [46]. As early as 1945, Frank and Evans proposed an explanation for the observed negative excess entropies and enthalpies in E-W systems, attributing these phenomena to the formation of an “iceberg” structure around the hydrophobic groups of alcohol molecules in solution [47]. This hypothesis has since motivated extensive research into various molecular association mechanisms, including clathrate-like structures, in E-W mixtures. Nevertheless, a substantial body of both experimental and theoretical investigations has demonstrated that ethanol–water mixtures actually form percolating 3D networks. In this structural model, ethanol and water molecules are interconnected through hydrogen bonding to establish a dynamic and percolating network architecture. Notably, this network exhibits a “percolation threshold” within a specific concentration range: when the molar fraction of either component reaches a critical value, its HB network abruptly becomes continuous and assumes dominant control over the structural organization of the system [8,48,49].
In addition, these mesoscopic clathrate structures play a wide range of roles in aqueous solutions and exert a significant influence on the HB network of water molecules [46]. Given the significance of E-W mixtures across various fields, researchers have conducted detailed investigations into the different supramolecular E-Wc present in this binary system. They used fluorescence spectroscopy and 2D correlation analysis at different volumes of E-W mixtures and incubation times after mixing [50]. Through 2D correlation analysis of E-W mixtures with ethanol concentrations ranging from 10 vol% to 100 vol% at each incubation time, it was found that the correlation intensity of the emission peak at 330 nm for (H2O)m(EtOH)n not only correlated with ethanol concentration but also increased gradually with incubation time. These findings suggest that the (H2O)m(EtOH)n cluster presented is the most stable one, as it tends to form over longer incubation periods. Moreover, the correlation results indicate that in the low-concentration range of 10–45 vol%, the dominant cluster is the hydrophilic (H2O)m(EtOH), corresponding to an emission peak at approximately 373 nm. Conversely, in the high-concentration range of 80–99 vol%, the predominant cluster is the hydrophobic (H2O)(EtOH)n, corresponding to an emission peak at approximately 310 nm. However, the most stable cluster of (H2O)m(EtOH)n, corresponding to an emission peak at 330 nm, not only predominates in the concentration range of 50–75 vol% but also exists in all E-W mixtures after longer incubation times.
Additionally, researchers employed Raman spectroscopy, Fourier transform infrared-attenuated total reflection (FTIR-ATR), terahertz time-domain spectroscopy, and pulsed-field gradient nuclear magnetic resonance (PFG-NMR), combined with molecular dynamics simulations and density functional theory, to deduce the possible structures of ethanol–water clusters at different concentrations [51,52,53,54,55]. The results indicate that in the water-rich region at low ethanol concentrations, where the concentration of water exceeds that of ethanol, the interactions between water molecules are stronger. Thus, the primary clusters formed are (H2O)m(EtOH), with ethanol molecules embedded within cage-like clusters (H2O)m composed of numerous water molecules. The hydrogen bond strength between ethanol and water reaches its maximum at a 40 vol% ethanol concentration. As the ethanol concentration increases further, the hydration structure becomes less stable, the hydrogen bond strength between ethanol and water weakens, and ethanol molecules begin to self-associate. They form (H2O)m(EtOH)n clusters with water molecules through hydrogen bonding, which are closed or open planar ring-like clusters with high rigidity, where water molecules bridge ethanol molecules via hydrogen bonds. In the high ethanol concentration region, ethanol molecules preferentially self-associate to form short-chain molecular clusters, while water molecules primarily engage in hydrogen bonding with the hydrophilic hydroxyl groups of these ethanol clusters, forming E-Wc-type structures represented as (H2O)(EtOH)n.
In summary, both small and large molecules can form complexes with starch via HBs, van der Waals forces, and/or hydrophobic interactions. Generally, as the concentration of small molecules increases, a greater number of complexes are formed with starch. However, for macromolecules, steric hindrance effects make their complexation with starch more challenging. Molecules with relatively small steric hindrance can arrange into a regular double-helical structure, whereas those with greater steric hindrance have difficulty forming inclusion complexes with starch or effectively inducing starch self-assembly [45]. In this study, after subjecting starch to thermal annealing treatments under varying E-W ratios, the subsequent addition of iodine induced distinctly different color responses in the treated samples. Notably, when the ethanol concentration increased from 75 vol% to 77 vol%, the blue color of the complex became noticeably lighter, and the absorbance value at 633 nm (A633) did not exhibit a gradual decline with rising ethanol concentrations. Based on these observations, we further investigated the influence of supramolecular complexes on starch crystallinity, as illustrated in Figure 7.
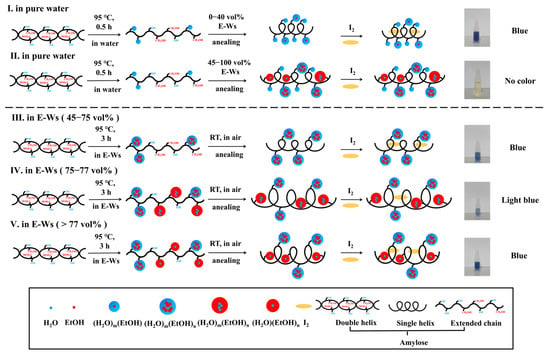
Figure 7.
The mechanism of color changes showing for starch–I2 along with thermal-heating treatments in different vol% of E-Ws.
3.4. Intrinsic Mechanism of Starch–I2 to Show Different Colors
3.4.1. Phase Transition Behavior of 40–45 vol% in E-Ws?
The hydrophilic hydroxyl groups of α [1→4] glucan helices are predominantly located on the exterior surface, while the hydrophobic hydroxymethyl groups and oxygen atoms of the glycosidic bonds reside within the interior, forming a hydrophobic cavity capable of encapsulating hydrophobic ligands. This structural arrangement establishes the amylose helix as a hydrophobic interior with a hydrophilic exterior [56].
Amylose is known to form crystalline inclusion complexes with both polar and non-polar compounds, exhibiting a well-defined V-type amylose helical structure. Early X-ray diffraction studies demonstrated that the helical conformation of this polysaccharide is strongly influenced by the size of the complexing agent. Specifically: (i) linear alcohols and fatty acids induce the formation of 6-fold helices (6 D-glucosyl residues per turn); (ii) Branched-chain alkyl compounds, such as tert-butyl alcohol, promote 7-fold helices (7 D-glucosyl residues per turn); (iii) bulky molecules like 1-naphthol result in 8-fold helices (8 D-glucosyl residues per turn) [57].
Furthermore, iodine molecules readily incorporate into the hydrophobic cavity of the amylose helix via van der Waals interactions, forming a characteristic left-handed single-chain helical complex [58]. This complex typically exhibits an outer diameter of ~13 Å and a pitch of 8 Å (with six 1,4-glucose units per pitch), hosting an internal cavity of ~5 Å, where the iodine alongside iodide is accommodated with I-I distances of ~3.1 Å [41]. The subtle variations in the blue coloration of starch–I2 complexes observed in different E-W systems can be attributed to differences in the E-Wc size and its phase transition behavior within the 40–45 vol% E-W range.
The proposed mechanisms governing the color evolution of starch–I2 complexes in pure water and various E-W mixtures are schematically illustrated in Figure 7. When starch granules are heated in pure water, they undergo hydration-induced swelling, ultimately leading to the disruption of both granular and crystalline structures at elevated temperatures (95 °C). This thermal treatment facilitates the dissociation of the double helical structure into extended single chains. Subsequent interactions between water molecules (or their clusters) and hydroxyl groups via non-covalent forces, combined with annealing and I2/I− incorporation, promote the formation of single helices that bind to I2, resulting in the characteristic blue coloration.
In contrast, the introduction of varying ethanol concentrations in the annealing medium alters this process. Ethanol forms E-Wc within the mixture, which significantly influences the final structural configuration. The resulting single-stranded helices may or may not bind to I2, depending critically on the size and physicochemical properties of the E-Wc formed under different ethanol–water compositions.
- (I)
- When the ethanol concentration is below 40 vol%, the E-W system is primarily composed of (H2O)m(EtOH) clusters, where ethanol molecules are embedded within cage-like (H2O)m clusters formed by numerous water molecules, exhibiting internal hydrophobicity and external hydrophilicity [51,52,53,54,55]. Upon heating at 95 °C in aqueous solution followed by annealing in such E-W systems, non-covalent interactions occur between these clusters and the hydroxyl groups of liberated amylose. These interactions do not hinder the formation of single-helix amylose structures or their encapsulation of I2 within the helical cavity, resulting in a characteristic blue solution, as illustrated in Figure 7 (I).
- (II)
- When the ethanol concentration ranges from 45 to 75 vol%, larger and more structurally complex (H2O)m(EtOH)n clusters predominantly form in the E-W system. Under these conditions, the clusters may interact with either the hydrophobic or hydrophilic regions of the amylose chain and localize either on the exterior or interior of the helix following annealing. However, due to steric constraints imposed by the E-W system, the iodine–helix interaction is likely attenuated, leading to partial or complete decolorization of the blue coloration, as illustrated in Figure 7 (II).
3.4.2. Phase Transition Behavior of 75–77 vol% in E-Ws?
- (III)
- Within the ethanol volume fraction range of 45–75%, the predominant aggregates in E-Ws exist as (H2O)m(EtOH)n (m > n) configurations. Owing to the persistent exposure of hydroxyl groups from water molecules, these aggregates maintain pronounced hydrophilic characteristics. During the thermal treatment of starch in E-Ws, as temperature progressively increases, amylose initially dissolves and leaches from the starch granules. At this stage, supramolecular E-Wc engages in interactions with the extended starch chains, facilitating the formation of a composite structure.
Following room-temperature annealing in E-Ws, the cluster of (H2O)m(EtOH)n remains stably adsorbed onto the exterior surface of the single helix. This spatial arrangement not only permits uninterrupted iodine access but also sustains the characteristic blue coloration of the solution, as illustrated in Figure 7 (III).
- (IV)
- When the ethanol concentration rises to 75–77 vol%, the E-Ws systems contain both hydrophilic and hydrophobic (H2O)m(EtOH)n clusters. These clusters can selectively interact with the hydrophilic hydroxyl groups and hydrophobic hydroxymethyl groups of the α [1→4] glucan helix through specific recognition mechanisms. During the annealing process, a composite structure forms where hydrophilic clusters localize on the outer surface of the helix, while hydrophobic clusters embed within the helical cavity. This structural reorganization of starch helices may lead to an increase in the helical pitch [59]. Simultaneously, the larger molecular dimensions of (H2O)m(EtOH)n, compared to (H2O)(EtOH)n [60], not only augment the overall helix diameter but also attenuate its binding affinity with iodine molecules. Ultimately, the diminished formation of starch–I2 complexes leads to a noticeable weakening of the blue coloration in the solution, as illustrated in Figure 7 (IV).
- (V)
- When the ethanol concentration exceeds 77 vol%, the hydrophobic clusters (H2O)m(EtOH)n (m < n) in solution reorganize into an externally hydrophobic and internally hydrophilic structure, (H2O)(EtOH)n [51]. Compared to the original (H2O)m(EtOH)n clusters, the reduced size of (H2O)(EtOH)n leads to a decrease in helix spacing during amylose chain helix formation. Upon iodine binding, the interaction sites between iodine and the hydrophobic spiral cavities are re-established, restoring the characteristic blue complex, as illustrated in Figure 7 (V).
In summary, within E-W systems at varying concentrations, ethanol and water exist predominantly as E-Wc rather than as individual molecules. The distinct structural configurations, sizes, and surface properties of these E-Wc result in differential binding sites and interaction modes with amylose through non-covalent forces, which are governed by the helical pitch and hydrophobic cavity dimensions of the single-helix structure formed after annealing. Further iodine addition induces color changes in the solution, corresponding to the formation of specific starch–I2 complexes. Consequently, this phenomenon enables the differentiation of E-Wc across different ethanol–water concentration regimes. We propose that this approach not only facilitates the identification of E-Wc in E-Ws but also holds potential for distinguishing various types of emulsifiers or surfactants under appropriately designed experimental conditions.
4. Materials and Methods
4.1. Materials
Soluble starch was purchased from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). Pea starch (PS), was procured from Xiushan Huolang Food Development Co., Ltd. (Chongqing, China). Potato starch (PTS) was acquired from Hebei Gufu Food Co., Ltd. (Langfang, China). Potassium iodide was obtained from Tianjin Bodi Chemical Co., Ltd. (Tianjin, China). Iodine was sourced from Tianjin Xinbote Chemical Co., Ltd. (Tianjin, China). Absolute ethanol was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China).
4.2. Heating in Water and Annealing in E-Ws of Soluble Starch (Approach I)
A total of 100 mg of soluble starch and 1 mL of distilled water were mixed and heated at 95 °C for 30 min. Then, 10 μL of it was mixed with 1 mL of E-Ws (0–100 vol%, with 5 vol% increment) and 40 μL of iodine/iodide solution (I2/I−; 0.005 mol/L I2 and 0.01 mol/L KI) for annealing and complexing with I2. After further incubation at room temperature (RT) for 1 h, the mixture was centrifuged, and the supernatant was collected for UV-vis absorption spectral measurement.
4.3. Thermal Heating and Annealing of PS and PTS in E-Ws (Approach II)
The thermal heating and annealing treatments of starch in E-Ws were conducted according to an established method [26], with minor modifications. In brief, 12 g of PS or PTS were mixed with 28 mL of E-Ws with different ethanol volumes (vol%), and then placed in sealed containers, which were shaken in a 95 °C water bath for 3 h. After annealing at RT for 1 h, the mixture was centrifuged. Finally, the starch slurry was dried in an oven (45 °C) for 24 h to become solid for the measurements of XRD and FT-IR spectrum. The starch of PS and PTS after being treated with thermal heating and annealing by different volumes of E-Ws is abbreviated as X%PS or X%PTS, where the letter X stands for the volume ratio of ethanol to water.
The sample (0.4 g) and distilled water (4.6 mL) were mixed in a centrifuge tube and heated at 100 °C for 30 min. Twenty-five milliliters of distilled water were added to the centrifuge tube and cooled to room temperature. The mixture (50 μL) was taken out, mixed with distilled water (1.5 mL) and iodine/iodide solution (0.2 mL), and then the spectra of the annealed starch–I2 complex were measured with an ultraviolet-visible spectrophotometer at 260–800 nm.
4.4. UV-Vis Absorption Spectrum
The maximum absorption wavelength (λmax) of native and annealed starches was determined following a previously established method [26] after minor adjustments, both for the native and treated starches achieved in approaches I and II. All the spectra were recorded using a Shimadzu UV-3600 spectrophotometer (Kyoto, Japan) over the optical range of 260–800 nm.
4.5. XRD
The samples were conditioned for 48 h in a desiccator containing saturated sodium chloride solution (relative humidity 75%) to stabilize the moisture content at above 20% [61,62]. Subsequently, the crystalline structures of the samples were analyzed using an X-ray diffractometer (D8 Venture, Bruker, Germany) and scanned utilizing Cu-Kα radiation with a scanning speed of 3° per minute between two thetas of 4° and 40°.
The relative crystallinity (RC) of the starch in each sample was determined using Origin software (version 6.5, Materials Data Inc., Livermore, CA, USA), by comparing the integrated area under the specific peaks for crystalline with the area of the total region using the following formula:
where CDA denotes the dispersed area of crystalline, and TDA denotes the total dispersed area.
RC (%) = CDA × 100/TDA,
4.6. SEM
The morphology of the starch samples was examined using a scanning electron microscope (SEM, Hitachi Regulus 8100, Hitachi, Japan). To prepare the samples, they were mounted on an aluminum stub using double-sided adhesive tape and then coated with gold palladium using a sputter coater. Subsequently, the stub was positioned under the scanning electron microscope with an acceleration voltage of 10 kV. To gain a detailed understanding of the surface morphology, representative digital images of the samples were captured at magnifications of 500× and 2000×, enabling a closer examination of the intricate features present on the surface.
4.7. FT-IR Spectroscopy
The FT-IR spectra of native and treated PS and PTS were obtained using an infrared spectrometer (VERTEX 80V, Bruker, Germany). The solid sample was mixed with spectroscopic-grade KBr and then pressed into tablets. Calibration was carried out by using potassium bromide as a blank. Then, the spectrum was acquired using the software provided by the instrument company. Spectra of the PS and PTS were obtained from 4000–400 cm−1 with 64 scans. Origin software was employed for the process of peak splitting and the detailed analysis of the infrared spectrum.
5. Conclusions
To elucidate the phase transition point of E-Wc across varying volume ratios, a highly sensitive colorimetric assay based on starch–I2 crystallization was developed in this study. The results visually demonstrate two distinct transition points, occurring at volume ratios of 40–45 vol% and 75–77 vol% for E-Wc. The first transition is characterized by the disappearance of the characteristic blue coloration, while the second is marked by a significant alteration in the absorption intensity of the starch–I2 complex. Mechanistic investigations reveal that the supramolecular structure of E-Wc, rather than individual ethanol or water molecules within the binary mixture, governs the starch–I2 crystallization behavior. By employing medium-regulated starch–I2 crystallization as an analytical platform, we establish a direct correlation between the phase transition of E-Wc and the iodine chromogenic effect. This approach provides a novel and accessible optical probing strategy for investigating the transitions of colorless supramolecular assemblies. The unique value of the starch–iodine system as a cluster environment probe lies in its sensitivity to disturbances in the hydrogen bond network, which provides a new idea for the development of in situ detection techniques.
Author Contributions
H.-S.L.: writing—original draft, visualization, project administration, investigation, data curation, formal analysis. H.-J.B.: writing—original draft, visualization, project administration, investigation, data curation, formal analysis. Y.-Q.W.: writing—review and editing, supervision, software, project administration. H.-W.L.: supervision, formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Jilin Province Science and Technology Development Plan Project (No. 20230204040YY), NSFC (No. 21875085), and the Innovation & Opening Program of the State Key Laboratory of Supramolecular Structure and Materials, Jilin University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank Yi Li for his contribution to this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| E-Wc | Ethanol–water cluster(s) |
| E-Ws | Ethanol–water solution(s) |
| starch-I2 | starch–iodine (system) |
| HB(s) | hydrogen bonding(s) |
| I2 | iodine molecules |
| I3− | triiodide ions |
| PS | pea starch |
| PTS | potato starch |
| I2/I− | iodine/iodide solution |
| RT | room temperature |
| HPH | high-pressure homogenization |
| HK | honokiol |
| AC | arctin |
| XRD | X-ray diffraction |
| RC | relative crystallinity |
| SEM | scanning electron microscopy |
| FT-IR | Fourier transform infrared |
References
- Hu, N.; Schaefer, D.W. Effect of Impurity Compounds on Ethanol Hydration. J. Mol. Liq. 2010, 155, 29–36. [Google Scholar] [CrossRef]
- Miller, M.A.; Jimenez-Ruiz, M.; Bermejo, F.J.; Birge, N.O. Comparison of the Structural and Orientational Glass-Transition Dynamics in Ethanol. Phys. Rev. B 1998, 57, R13977–R13980. [Google Scholar] [CrossRef][Green Version]
- Wakisaka, A.; Komatsu, S.; Usui, Y. Solute-Solvent and Solvent-Solvent Interactions Evaluated through Clusters Isolated from Solutions: Preferential Solvation in Water-Alcohol Mixtures. J. Mol. Liq. 2001, 90, 175–184. [Google Scholar] [CrossRef]
- Nishi, N.; Takahashi, S.; Matsumoto, M.; Tanaka, A.; Muraya, K.; Takamuku, T.; Yamaguchi, T. Hydrogen-Bonded Cluster Formation and Hydrophobic Solute Association in Aqueous Solutions of Ethanol. J. Phys. Chem. 1995, 99, 462–468. [Google Scholar] [CrossRef]
- Egashira, K.; Nishi, N. Low-Frequency Raman Spectroscopy of Ethanol−Water Binary Solution: Evidence for Self-Association of Solute and Solvent Molecules. J. Phys. Chem. B 1998, 102, 4054–4057. [Google Scholar] [CrossRef]
- Snow, J.B.; Qian, S.-X.; Chang, R.K. Stimulated Raman Scattering from Individual Water and Ethanol Droplets at Morphology-Dependent Resonances. Opt. Lett. 1985, 10, 37. [Google Scholar] [CrossRef]
- Mizuno, K.; Miyashita, Y.; Shindo, Y.; Ogawa, H. NMR and FT-IR Studies of Hydrogen Bonds in Ethanol-Water Mixtures. J. Phys. Chem. 1995, 99, 3225–3228. [Google Scholar] [CrossRef]
- Dixit, S.; Crain, J.; Poon, W.C.K.; Finney, J.L.; Soper, A.K. Molecular Segregation Observed in a Concentrated Alcohol–Water Solution. Nature 2002, 416, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Soper, A.K.; Dougan, L.; Crain, J.; Finney, J.L. Excess Entropy in Alcohol−Water Solutions: A Simple Clustering Explanation. J. Phys. Chem. B 2006, 110, 3472–3476. [Google Scholar] [CrossRef] [PubMed]
- Dougan, L.; Bates, S.P.; Hargreaves, R.; Fox, J.P.; Crain, J.; Finney, J.L.; Réat, V.; Soper, A.K. Methanol-Water Solutions: A Bi-Percolating Liquid Mixture. J. Chem. Phys. 2004, 121, 6456–6462. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Hayashi, H.; Iijima, T. Temperature Dependence of the Concentration Fluctuation, the Kirkwood-Buff Parameters, and the Correlation Length of Tert-Butyl Alcohol and Water Mixtures Studied by Small-Angle x-Ray Scattering. J. Phys. Chem. 1989, 93, 6559–6565. [Google Scholar] [CrossRef]
- Alavi, S.; Takeya, S.; Ohmura, R.; Woo, T.K.; Ripmeester, J.A. Hydrogen-Bonding Alcohol-Water Interactions in Binary Ethanol, 1-Propanol, and 2-Propanol+methane Structure II Clathrate Hydrates. J. Chem. Phys. 2010, 133, 074505. [Google Scholar] [CrossRef] [PubMed]
- Sobczyk, L.; Grabowski, S.J.; Krygowski, T.M. Interrelation between H-Bond and Pi-Electron Delocalization. Chem. Rev. 2005, 105, 3513–3560. [Google Scholar] [CrossRef]
- Alavi, S.; Ohmura, R.; Ripmeester, J.A. A Molecular Dynamics Study of Ethanol–Water Hydrogen Bonding in Binary Structure I Clathrate Hydrate with CO2. J. Chem. Phys. 2011, 134, 054702. [Google Scholar] [CrossRef]
- Perera, A.; Sokolić, F.; Zoranić, L. Microstructure of Neat Alcohols. Phys. Rev. E 2007, 75, 060502. [Google Scholar] [CrossRef]
- Mejía, S.M.; Espinal, J.F.; Restrepo, A.; Mondragón, F. Molecular Interaction of (Ethanol)2−Water Heterotrimers. J. Phys. Chem. A 2007, 111, 8250–8256. [Google Scholar] [CrossRef]
- Masella, M.; Flament, J.P. Relation between Cooperative Effects in Cyclic Water, Methanol/Water, and Methanol Trimers and Hydrogen Bonds in Methanol/Water, Ethanol/Water, and Dimethylether/Water Heterodimers. J. Chem. Phys. 1998, 108, 7141–7151. [Google Scholar] [CrossRef]
- Sun, X.; Cai, L.; He, W.; Cao, X.; Liu, B.; Wang, H. A Novel Ratiometric Fluorescent Probe for Water Content in Ethanol and Temperature Sensing. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 264, 120266. [Google Scholar] [CrossRef]
- Passos, W.E.; Oliveira, I.P.; Michels, F.S.; Trindade, M.A.G.; Falcão, E.A.; Marangoni, B.S.; Oliveira, S.L.; Caires, A.R.L. Quantification of Water in Bioethanol Using Rhodamine B as an Efficient Molecular Optical Probe. Renew. Energy 2021, 165, 42–51. [Google Scholar] [CrossRef]
- Yulianti, R.; Liao, H.-J. V-Amylose Nanocarriers Complexed with Debranched Sweet Potato Starch: Structural Characteristics and Digestibility. Food Biophys. 2023, 18, 389–401. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Li, Y.; Qian, H.; Qi, X.; Zhang, H.; Wang, L. Understanding the Molecular Weight Distribution, in Vitro Digestibility and Rheological Properties of the Deep-Fried Wheat Starch. Food Chem. 2020, 331, 127315. [Google Scholar] [CrossRef] [PubMed]
- Pesek, S.; Silaghi-Dumitrescu, R. The Iodine/Iodide/Starch Supramolecular Complex. Molecules 2024, 29, 641. [Google Scholar] [CrossRef]
- Dries, D.M.; Gomand, S.V.; Delcour, J.A.; Goderis, B. V-Type Crystal Formation in Starch by Aqueous Ethanol Treatment: The Effect of Amylose Degree of Polymerization. Food Hydrocoll. 2016, 61, 649–661. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Jin, Z. Effect of High-Temperatures and Aqueous Ethanol Treatment on the Formation Process and Properties of V-Type Granular Starch (VGS). Carbohydr. Polym. 2021, 258, 117713. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, G.; Gao, Q. Preparation and Properties of Granular Cold-Water-Soluble Porous Starch. Int. J. Biol. Macromol. 2020, 144, 656–662. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Ji, N.; Li, M.; Wang, Y.; Xiong, L.; Sun, Q. The Effect of Ethanol Solution Annealing on the Physicochemical Properties of Pea and Potato Starches. Food Hydrocoll. 2022, 125, 107428. [Google Scholar] [CrossRef]
- Gardner, J.M.; Abrahamsson, M.; Farnum, B.H.; Meyer, G.J. Visible Light Generation of Iodine Atoms and I−I Bonds: Sensitized I− Oxidation and I3− Photodissociation. J. Am. Chem. Soc. 2009, 131, 16206–16214. [Google Scholar] [CrossRef]
- Lu, H.; Xiong, L.; Li, M.; Chen, H.; Xiao, J.; Wang, S.; Qiu, L.; Bian, X.; Sun, C.; Sun, Q. Separation and Characterization of Linear Glucans Debranched from Normal Corn, Potato and Sweet Potato Starches. Food Hydrocoll. 2019, 89, 196–206. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, B.; Li, J.; Shu, Z.; Wang, P.; Zeng, X. Structure and Properties of Octenyl Succinic Anhydride-Modified High-Amylose Japonica Rice Starches. Polymers 2021, 13, 1325. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, H.; Shi, M. Effect of Ethanol–Water Solution on the Crystallization of Short Chain Amylose from Potato Starch. Starch-Stärke 2016, 68, 683–690. [Google Scholar] [CrossRef]
- Lopez-Silva, M.; Bello-Perez, L.A.; Agama-Acevedo, E.; Alvarez-Ramirez, J. Effect of Amylose Content in Morphological, Functional and Emulsification Properties of OSA Modified Corn Starch. Food Hydrocoll. 2019, 97, 105212. [Google Scholar] [CrossRef]
- Ijaz; Uddin, M.; Khan, N.M.; Ali, F.; Khan, Z.U.; Muhammad, N.; Iqbal, J.; Rehman, N.; Jan, A.K.; Ahmad, S. Green Production and Structural Evaluation of Maize Starch–Fatty Acid Complexes Through High Speed Homogenization. J. Polym. Environ. 2020, 28, 3110–3115. [Google Scholar] [CrossRef]
- van Soest, J.J.G.; Tournois, H.; de Wit, D.; Vliegenthart, J.F.G. Short-Range Structure in (Partially) Crystalline Potato Starch Determined with Attenuated Total Reflectance Fourier-Transform IR Spectroscopy. Carbohydr. Res. 1995, 279, 201–214. [Google Scholar] [CrossRef]
- Guo, T.; Hou, H.; Liu, Y.; Chen, L.; Zheng, B. In Vitro Digestibility and Structural Control of Rice Starch-Unsaturated Fatty Acid Complexes by High-Pressure Homogenization. Carbohydr. Polym. 2021, 256, 117607. [Google Scholar] [CrossRef]
- Mutungi, C.; Passauer, L.; Onyango, C.; Jaros, D.; Rohm, H. Debranched Cassava Starch Crystallinity Determination by Raman Spectroscopy: Correlation of Features in Raman Spectra with X-Ray Diffraction and 13C CP/MAS NMR Spectroscopy. Carbohydr. Polym. 2012, 87, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Julian McClements, D.; Wang, L.; Li, C. Formation and Characterization of Starch-Based Spherulite: Effect of Molecular Weight of Potato Amylose Starch. Food Chem. 2022, 371, 131060. [Google Scholar] [CrossRef]
- Chen, J.; Cai, H.; Yang, S.; Zhang, M.; Wang, J.; Chen, Z. The Formation of Starch-Lipid Complexes in Instant Rice Noodles Incorporated with Different Fatty Acids: Effect on the Structure, in Vitro Enzymatic Digestibility and Retrogradation Properties during Storage. Food Res. Int. 2022, 162, 111933. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Chen, L.; McClements, D.J.; Zhao, J.; Zhou, X.; Qiu, C.; Long, J.; Ji, H.; Xu, Z.; Meng, M.; et al. Starch-Guest Complexes Interactions: Molecular Mechanisms, Effects on Starch and Functionality. Crit. Rev. Food Sci. Nutr. 2023, 64, 7550–7562. [Google Scholar] [CrossRef]
- Liu, H.; Yao, Y.; Zhang, Y.; Zheng, B.; Zeng, H. Ultrasonication-Mediated Formation of V-Type Lotus Seed Starch for Subsequent Complexation with Butyric Acid. Int. J. Biol. Macromol. 2023, 236, 124000. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, S.; Tan, C.P.; Feng, Y.; Zhang, B.; Fu, X.; Huang, Q. Solid Encapsulation of Lauric Acid into “Empty” V-Type Starch: Structural Characteristics and Emulsifying Properties. Carbohydr. Polym. 2021, 267, 118181. [Google Scholar] [CrossRef]
- Pesek, S.; Lehene, M.; Brânzanic, A.M.V.; Silaghi-Dumitrescu, R. On the Origin of the Blue Color in The Iodine/Iodide/Starch Supramolecular Complex. Molecules 2022, 27, 8974. [Google Scholar] [CrossRef]
- Li, W.; Sun, S.; Gu, Z.; Cheng, L.; Li, Z.; Li, C.; Hong, Y. Effect of Protein on the Gelatinization Behavior and Digestibility of Corn Flour with Different Amylose Contents. Int. J. Biol. Macromol. 2023, 249, 125971. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, S.; Zhao, R.; Wang, N.; Kan, J.; Zhang, F. Effect of Four Viscous Soluble Dietary Fibers on the Physicochemical, Structural Properties, and in Vitro Digestibility of Rice Starch: A Comparison Study. Food Chem. 2021, 362, 130181. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhang, H.; Xing, J.; Sang, S.; Zhan, X.; Liu, Y.; Jia, L.; Li, J.; Luo, X. Long-Term Retrogradation Properties and In Vitro Digestibility of Waxy Rice Starch Modified with Pectin. Foods 2023, 12, 3981. [Google Scholar] [CrossRef]
- Guo, T.; Zheng, B.; He, H.; Chen, L. Effects of Non-Covalent Binding of Lignans with Rice Starch Driven by High-Pressure Homogenization on the Starch Structure and in Vitro Nutritional Characteristics. Food Funct. 2022, 13, 9243–9253. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-H.; Yen, T.-C.; Chen, C.-C.; Yang, C.-W.; Fang, C.-K.; Hwang, I.-S. Observation of Mesoscopic Clathrate Structures in Ethanol-Water Mixtures. J. Mol. Liq. 2022, 366, 120299. [Google Scholar] [CrossRef]
- Frank, H.S.; Evans, M.W. Free Volume and Entropy in Condensed Systems III. Entropy in Binary Liquid Mixtures; Partial Molal Entropy in Dilute Solutions; Structure and Thermodynamics in Aqueous Electrolytes. J. Chem. Phys. 1945, 13, 507–532. [Google Scholar] [CrossRef]
- Guo, J.-H.; Luo, Y.; Augustsson, A.; Kashtanov, S.; Rubensson, J.-E.; Shuh, D.K.; Ågren, H.; Nordgren, J. Molecular Structure of Alcohol-Water Mixtures. Phys. Rev. Lett. 2003, 91, 157401. [Google Scholar] [CrossRef]
- Gereben, O.; Pusztai, L. Cluster Formation and Percolation in Ethanol-Water Mixtures. Chem. Phys. 2017, 496, 1–8. [Google Scholar] [CrossRef]
- Jia, X.-Q.; Li, Y.; Zhang, C.-X.; Gao, Y.-C.; Wu, Y. Supramolecular Clusters Clarification in Ethanol-Water Mixture by Using Fluorescence Spectroscopy and 2D Correlation Analysis. J. Mol. Struct. 2020, 1219, 128569. [Google Scholar] [CrossRef]
- Wu, B.; Liu, Y.; Han, C. Hydration of Liquid Ethanol Probed by Raman Spectra. Spectrosc. Spectr. Anal. 2011, 31, 2738–2741. [Google Scholar]
- Zeinalipour-Yazdi, C.D.; Loizidou, E.Z. An Experimental FTIR-ATR and Computational Study of H-Bonding in Ethanol/Water Mixtures. Chem. Phys. 2021, 550, 111295. [Google Scholar] [CrossRef]
- Dong, Q.; Yu, C.; Li, L.; Nie, L.; Li, D.; Zang, H. Near-Infrared Spectroscopic Study of Molecular Interaction in Ethanol-Water Mixtures. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 222, 117183. [Google Scholar] [CrossRef]
- Li, R.; D’Agostino, C.; McGregor, J.; Mantle, M.D.; Zeitler, J.A.; Gladden, L.F. Mesoscopic Structuring and Dynamics of Alcohol/Water Solutions Probed by Terahertz Time-Domain Spectroscopy and Pulsed Field Gradient Nuclear Magnetic Resonance. J. Phys. Chem. B 2014, 118, 10156–10166. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.; Jiang, M.; Tian, J.; Chen, M.; Huang, D. Investigation of Liquor Microstructure (Ethanol-Water Clusters): Molecular Dynamics Simulation and Density Functional Theory. J. Mol. Graph. Model. 2024, 133, 108864. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chao, C.; Cai, J.; Niu, B.; Copeland, L.; Wang, S. Starch–Lipid and Starch–Lipid–Protein Complexes: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1056–1079. [Google Scholar] [CrossRef] [PubMed]
- Biliaderis, C.G.; Galloway, G. Crystallization Behavior of Amylose-V Complexes: Structure-Property Relationships. Carbohydr. Res. 1989, 189, 31–48. [Google Scholar] [CrossRef]
- Gessler, K.; Usón, I.; Takaha, T.; Krauss, N.; Smith, S.M.; Okada, S.; Sheldrick, G.M.; Saenger, W. V-Amylose at Atomic Resolution: X-Ray Structure of a Cycloamylose with 26 Glucose Residues (Cyclomaltohexaicosaose). Proc. Natl. Acad. Sci. USA 1999, 96, 4246–4251. [Google Scholar] [CrossRef]
- Peng, M.; Ruan, X.; Fan, H.; Chen, Z. AFM Probing on the Microstructure of the Starch and the Starch-Iodine Complex. Polym. Mater. Sci. Eng. 2009, 25, 102–104. [Google Scholar] [CrossRef]
- Pothoczki, S.; Pethes, I.; Pusztai, L.; Temleitner, L.; Ohara, K.; Bakó, I. Properties of Hydrogen-Bonded Networks in Ethanol–Water Liquid Mixtures as a Function of Temperature: Diffraction Experiments and Computer Simulations. J. Phys. Chem. B 2021, 125, 6272–6279. [Google Scholar] [CrossRef]
- Ji, N.; Ge, S.; Li, M.; Wang, Y.; Xiong, L.; Qiu, L.; Bian, X.; Sun, C.; Sun, Q. Effect of Annealing on the Structural and Physicochemical Properties of Waxy Rice Starch Nanoparticles: Effect of Annealing on the Properties of Starch Nanoparticles. Food Chem. 2019, 286, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, X.; Zhang, B.; Fu, X.; Li, L.; Huang, Q. Structure, Physicochemical and in Vitro Digestion Properties of Ternary Blends Containing Swollen Maize Starch, Maize Oil and Zein Protein. Food Hydrocoll. 2018, 76, 88–95. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).