Abstract
Bismuth-based oxyfluorides (BiOxF3−2x) have recently emerged as promising photocatalysts due to their unique electronic structures and tunable physicochemical properties. This review provides a comprehensive overview of these materials, focusing on their crystal structures, band gap characteristics, and photocatalytic performance. Particular attention is given to BiOF, Bi7O5F11, and β-BiOxF3−2x, highlighting the influence of fluorine’s high electronegativity and internal electric fields on charge separation and light absorption. The potential of Aurivillius-type oxyfluorides is also discussed. Structural modifications, such as the introduction of oxygen vacancies, morphology control, and metal/non-metal doping, are examined for their effects on photocatalytic efficiency. Furthermore, various synthesis techniques and heterojunction engineering strategies involving semiconductors, carbon-based materials, and metal nanoparticles are explored to improve light harvesting and reduce charge recombination. Applications in pollutant degradation and CO2 photoconversion are reviewed, demonstrating the versatility of these materials. Despite their promise, the challenges associated with phase identification and composition control are also emphasized, underlining the need for rigorous structural characterization. Future directions for optimizing the photocatalytic activity of bismuth-based oxyfluorides are outlined, focusing on strategies to enhance their performance.
1. Introduction
Energy shortages and environmental pollution are challenging issues that people are facing all over the world and which demand significant attention. Since its first report by Fujishima and Honda, photocatalysis has been considered a promising technology to solve such issues [1]. It could resolve the problems of solar energy harvesting, conversion, and storage. Solar energy can be converted into electrical and chemical energy through photoinduced redox reactions on photocatalysts. When a semiconductor photocatalyst is irradiated with photon energy equal to or greater than its band gap, the electrons (e−) are excited to the conduction band (CB), leaving holes (h+) in the valence band (VB). Some of these charges recombine, reducing the photocatalytic activity. However, other photogenerated e−/h+ pairs separate efficiently and migrate to the surface, inducing oxidation and reduction photocatalytic reactions, leading to pollutant degradation, water splitting, or CO2 photoconversion. This process can be attained when the potential of the created electrons and holes is appropriate for the reduction and oxidation species adsorbed on the surface of the photocatalyst. Moreover, the oxidation and reduction reactions depend tremendously on the photocatalyst used. Thus, the photocatalyst plays the most important role in determining the process efficiency. It not only determines the thermodynamics of the process through the crystal and electronic structure but it also determines the kinetics of photocatalysis. The surface reaction kinetics consist of light accumulation, charge separation, charge transport or recombination, and charge utilization. Hence, the photocatalytic performance can be enhanced by increasing the light harvesting, reducing the carrier recombination rate in the material bulk or surface, and increasing the specific surface area, thanks to doping, heterojunction construction, using a plasmonic effect, or playing with the microstructure and O-vacancies. Therefore, different classes of materials have been developed for photocatalytic applications. TiO2 has been widely used in a variety of environmental and energy photocatalytic reactions due to its high oxidizing and reductive potential, high stability, low cost, and low toxicity [2,3,4]. Nevertheless, TiO2 cannot satisfy the requirements for industrialization for many reasons. At this stage, photocatalysts have several disadvantages, such as low utilization of sunlight, low quantum efficiency, low recyclability, and low photocatalyst efficiency [5].
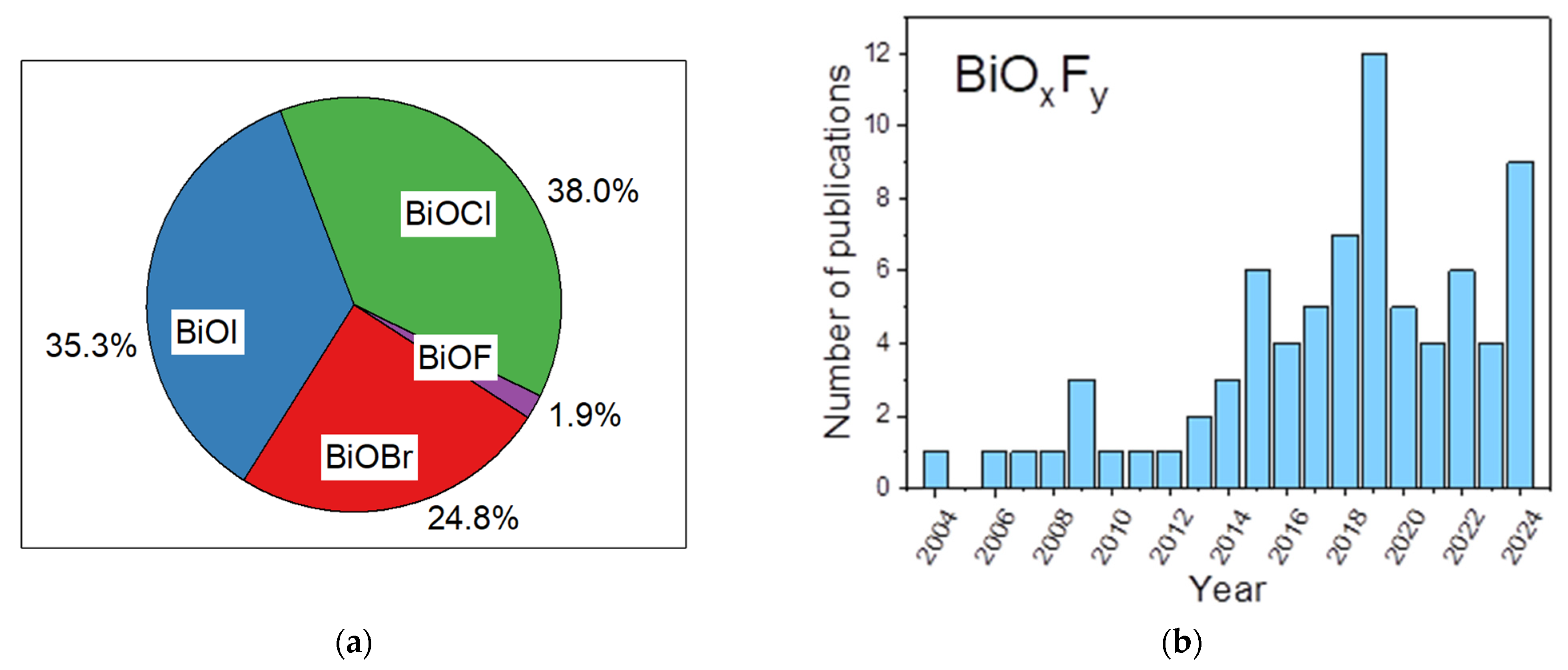
Recently, bismuth-based materials have attracted significant attention in different domains, such as optoelectronics [6,7], gas sensors [8,9], and photocatalysis [10,11]. For example, bismuth-based oxide semiconductors including Bi2O3 [12,13], BiVO4 [14], Bi2MoO6 [15], and Bi2WO6 [16] have relevant photocatalytic activities. Furthermore, pristine bismuth oxyhalides (BiOX, X = F, Cl, Br and I) have been widely studied as important V–VI–VII ternary semiconductors due to their unique layered structure and excellent physicochemical properties [17,18]. They crystallize into a tetragonal matlockite (PbFCl-type) structure and are built of interleaved fluorite-like [Bi2O2] slabs with double halogen slabs. Although much attention has been focused on bismuth oxyhalide photocatalysts, it has principally been centered on BiOCl, BiOBr, and BiOI more than BiOF [19,20,21,22]. Because of its high Eg of 4.2 eV, fluorinated bismuth derivative compounds were initially considered less interesting materials for photocatalytic applications compared to other bismuth-based halides, as extending light absorption into the visible range was the most widely explored strategy [19,20,21,22]. However, charge separation is also one of the crucial steps limiting photocatalytic activity. Recent studies have demonstrated that the formation of internal electric fields is a promising route to limit charge recombination [23,24]. Recently, bismuth oxyfluoride materials have attracted significant attention since fluorine has distinctive chemistry properties from those of chlorine, bromine, and iodine [19,20,21,22]. Due to its high electronegativity, fluorine can trap electrons firmly and creates internal electric fields, thus leading to an uneven electron distribution and promoting charge separation, thereby enhancing the photocatalytic efficiency [25,26,27]. Its wide band gap gives it higher oxidation and reduction potentials than those of other oxyhalides. Moreover, unlike other bismuth oxyhalides, BiOF exhibits a direct band gap, which leads to more efficient light absorption. Although the total number of studies on BiOF photocatalysts remains limited compared to those on other bismuth oxyhalides (1.9%) (Figure 1a), there has been a gradual increase in research activity since 2013, indicating emerging interest from the scientific community (Figure 1b).
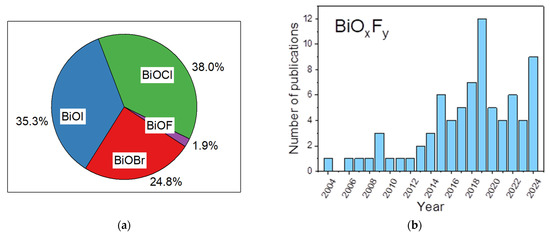
Figure 1.
As per Web of Science data, (a) pie chart of articles dealing with tetragonal matlockite (PbFCl-type) oxyhalides BiOBr, BiOCl, BiOI, and BiOF by percentage; (b) number of publications on “Bismuth oxyfluoride”, “BiOF”, “Bi7O5F11”, “BiO0.5F2”, “BiO0.51F1.98”,“BiO0.67F1.66”, “BiO1.18F0.64”, or “Bi50O59F32” from 2004 to 2024 (Web of Science, 31 December 2024).
To date, several excellent reviews have considered BiOCl, BiOBr, and BiOI materials from different perspectives, such as their activity sites and structural defects. Xiong et al. reported various methods for the controllable synthesis of bismuth-rich BiaObXc, in addition to different strategies for tailoring the photocatalytic behavior [28]. Ye et al. introduced the hybridization, facet effect, and photocatalysis mechanism for a visible-light-driven (VLD) BiOX photocatalyst with remarkably enhanced photocatalytic activity [29]. Jin et al. introduced the methods of morphology control, internal electric field adjustment, and surface modification for the synthesis of a highly efficient BiOxX3−2x photocatalyst [30]. Regarding bismuth oxyfluorides (BiOxF3−2x), the only review available to date is that by Vinoth et al. (2024) [31], which focused exclusively on BiOF. However, bismuth oxyfluorides represent a broad family of compounds with varying O/F compositions (BiOxF3−2x) and crystalline phases, which display markedly different physicochemical properties. In the literature, a lack of rigor often results from the frequent confusion between BiOF and other BiOxF3−2x phases, despite their fundamental differences. As a result, the current body of work remains sometimes inconsistent. A comprehensive and systematic review is therefore required to establish a clearer framework and provide solid foundations for future investigations into this family of photocatalysts. In this paper, we focus on recent advances for the development of significant bismuth-based oxyfluoride materials and their different photocatalytic applications. Firstly, we briefly discuss the unique crystal and electronic structures of known BiOxF3−2x phases and, by extension, of bismuth-based oxyfluorides presenting Aurivillius structures. Then, we present potential applications and various strategies to enhance the photocatalytic activity of these compounds. Finally, we conclude with the existing challenges and prospects for achieving greener and more efficient photocatalytic applications using these photocatalysts.
2. Crystal and Electronic Structures of Bismuth-Based Oxyfluorides
Physicochemical characterization of bismuth oxyfluorides is of particular importance due to the structural complexity and diversity of the compositions reported in the literature. Among these compounds, BiOF is the most frequently described phase. However, for other compositions, it appears that their attribution is sometimes erroneous, due to confusion between all oxyfluoride and fluoride compounds, exhibiting distinct O/F ratios and crystal structures. This section aims to present in a comparative manner the main existing Bismuth oxyfluoride phases and the analytical methods used in the literature to characterize these materials. The objective is to clarify the criteria for reliable structural identification and to highlight recurring pitfalls encountered in the interpretation of experimental data.
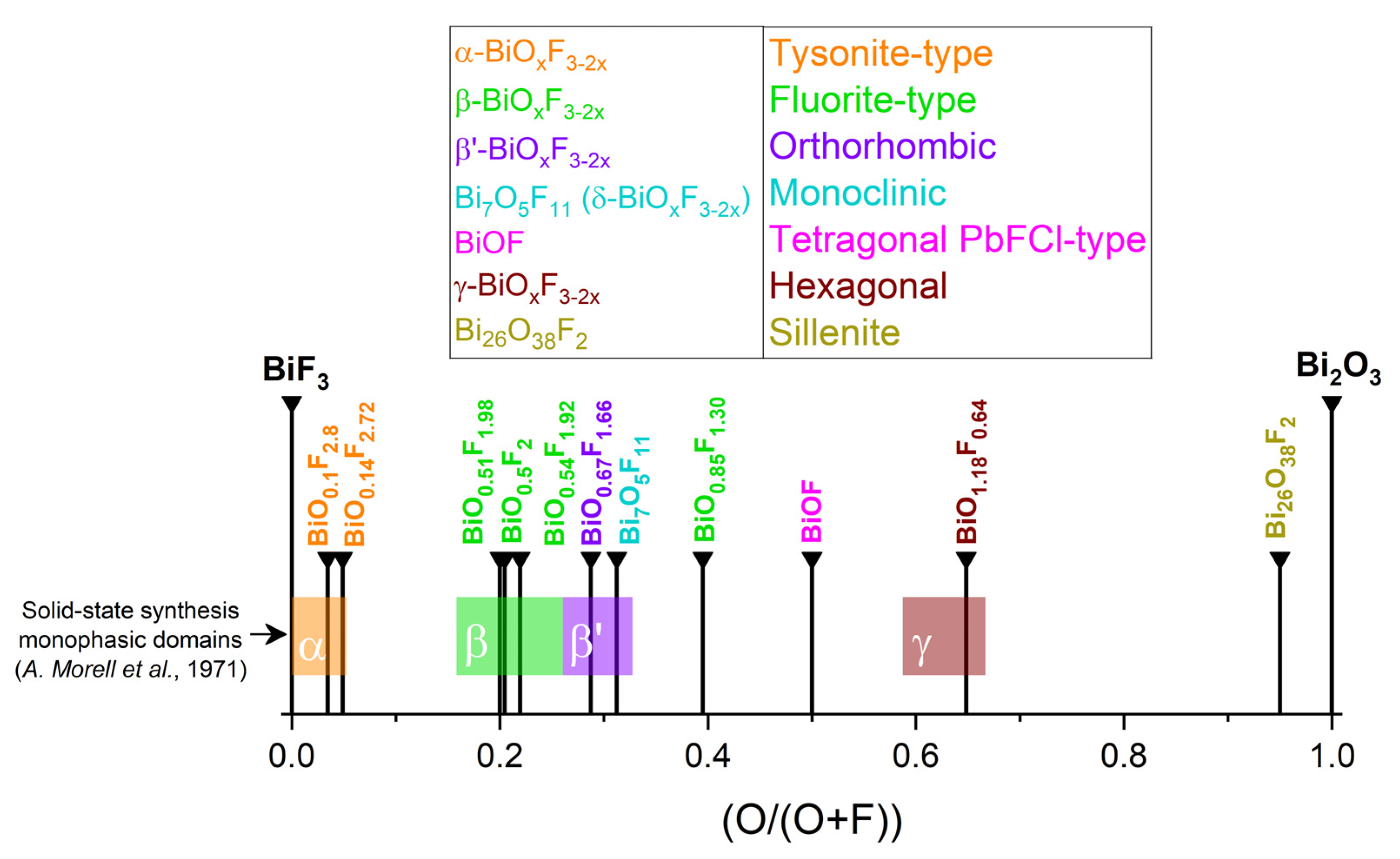
The first studies attempting to describe the different phases of bismuth oxyfluorides were carried out by the Swedish chemist Bengt Aurivillius in the mid-20th century and focused on a description of the Bi2O3–BiF3 system [32,33,34]. Subsequently, the work of Antoinette Morell, through solid-state synthesis of oxyfluoride from BiF3 and Bi2O3 at 670 °C for 15 h under argon, made it possible to demonstrate the existence of a series of phases with the composition BiOxF3−2x (0 ≤ x ≤ 1.5) [35]. Figure 2 summarizes this phase continuum, with particular emphasis on the phase domains described in Antoinette Morell’s solid-state study [35]. The colored horizontal stripes represent the phasic domains of the phases observed by Antoinette Morell et al. [35] (with α: 0 ≤ O/O + F ≤ 0.5; β: 0.16 ≤ O/O + F ≤ 0.26; β’: 0.26 ≤ O/O + F ≤ 0.33; γ: 0.59 ≤ O/O + F ≤ 0.67).
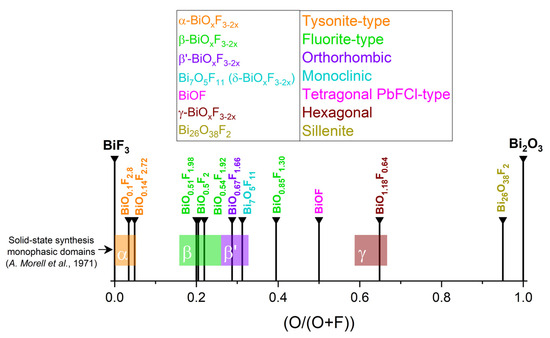
Figure 2.
Known BiOxF3−2x compositions and related phases [35].
2.1. BiOF
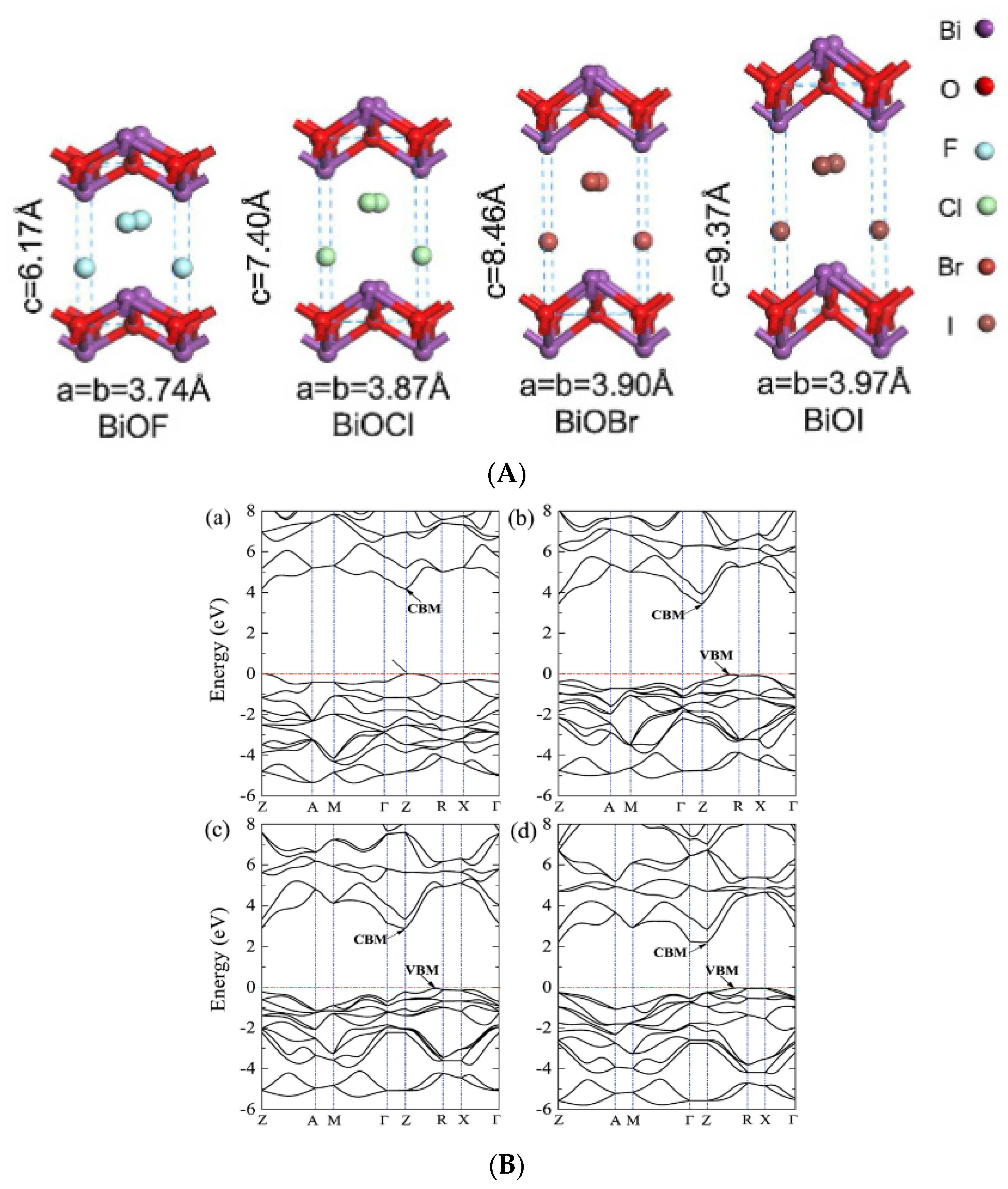
BiOF is the most described bismuth oxyfluoride because of its structural similarity to other well-known BiOX photocatalysts (X = Cl, Br, I) [36]. Figure 3A shows the crystal structure of BiOX [37]. As determined from density functional theory (DFT), relaxed BiOCl, BiOBr, and BiOI show indirect band gaps. BiOF exhibits a direct or slightly indirect band gap, depending on whether Bi 5d states are considered or not [38]. The maximum band gap values of relaxed BiOF, BiOCl, BiOBr, and BiOI are 3.34, 2.92, 2.65, and 1.75 eV, respectively [38]. The width of the valence band increases gradually from relaxed BiOCl to BiOI. However, the width of the conduction band decreases from relaxed BiOF to BiOI. When Bi 5d states are considered, the estimated dipole moments of BiO4×4 polyhedra within the relaxed species are in the order of BiOF > BiOI > BiOBr > BiOCl [38]. Figure 3B shows the electronic band structures of the relaxed bismuth oxyhalide compounds [39].
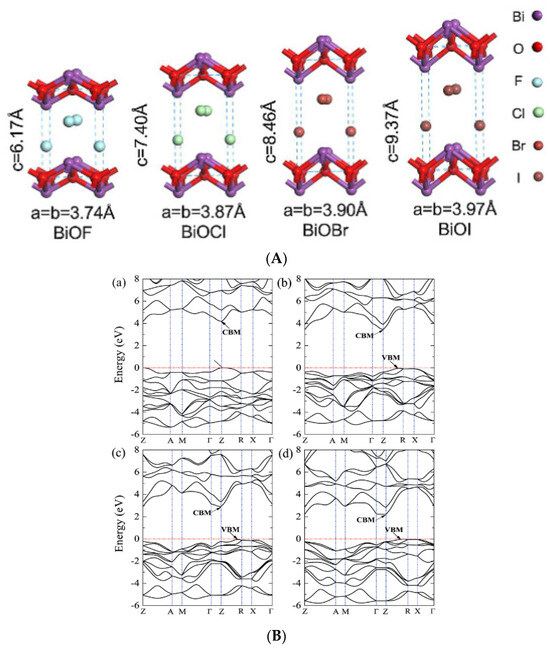
Figure 3.
(A) A schematic diagram of BiOX’s crystal structure [37]; (B) the band structures of (a) BiOF, (b) BiOCl, (c) BiOBr, and (d) BiOI. The horizontal dashed lines represent the Fermi levels [39].
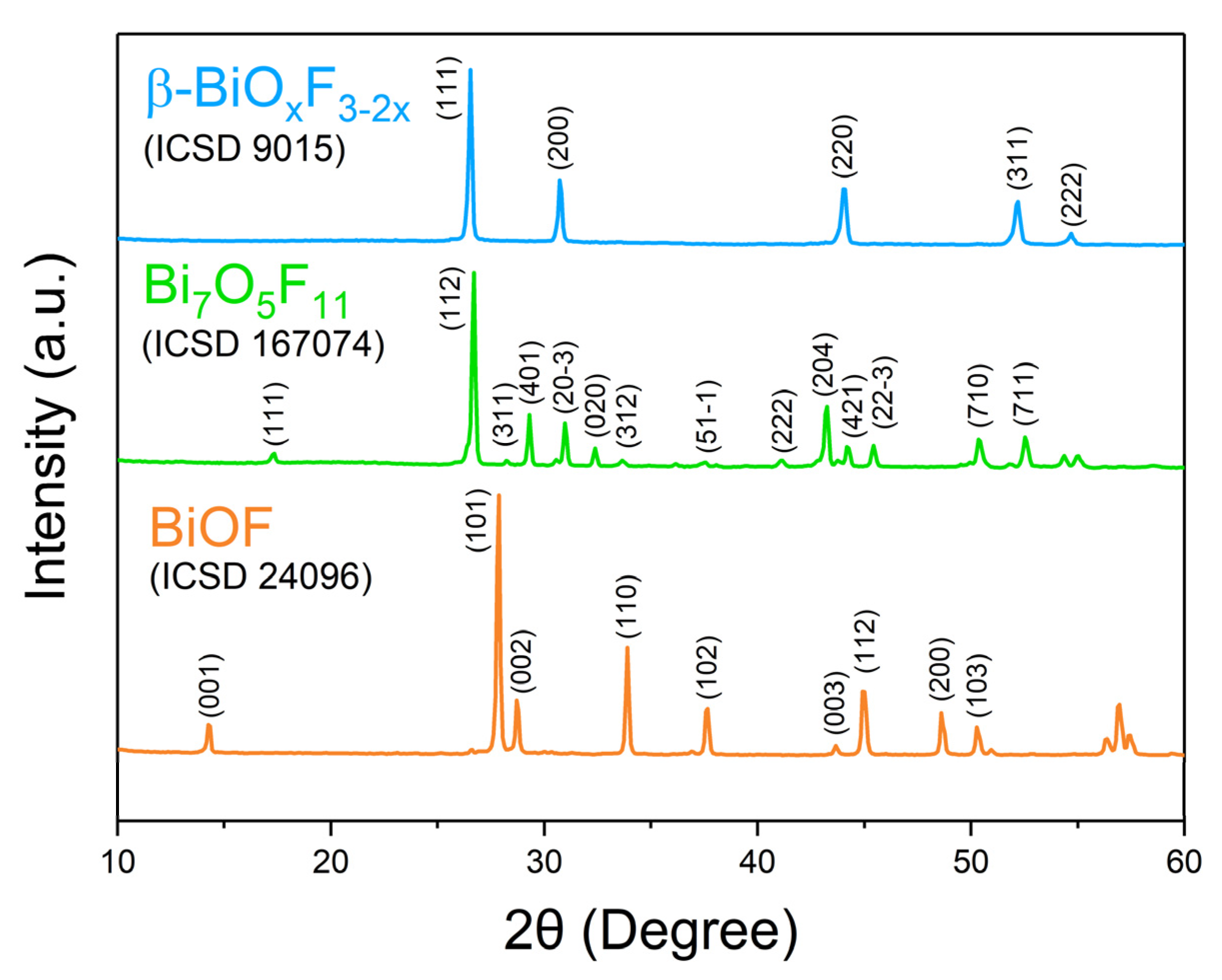
BiOF exhibits a unique layered structure characterized by fluorite-like [Bi2O2]2+ slabs interleaved by double slabs of F− ions. Similar to other bismuth oxyhalides, there is an internal electric field (IEF) between the [Bi2O2]2+ slabs and the anionic halogen layers, which can effectively induce efficient separation of the photoexcited charge carriers [17,18]. The stronger internal electric field, introduced by the higher electronegativity of the fluorine atoms in BiOF, can more efficiently reduce the recombination of photoelectron–hole pairs and enhance the photocatalytic performance of BiOX. Figure 4 shows the X-ray diffraction (XRD) pattern of BiOF. Its diffraction peaks appear at 14.2°, 27.8°, 28.7°, 33.8°, and 37.5° and correspond to the (001), (101), (002), (110), and (102) crystal planes of its tetragonal phase.
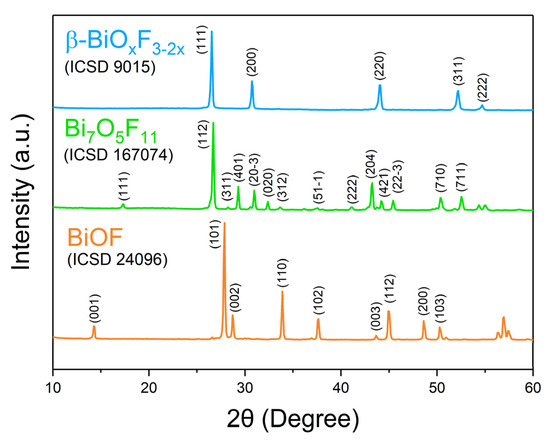
Figure 4.
XRD patterns of BiOF, Bi7O5F11, and β-BiOxF3−2x [40].
BiOF exhibits a phase transition under high pressures from a tetragonal PbFCl-type structure with the space group P4/nmm to an orthorhombic structure with the space group Cmcm at about 19.6 GPa [41]. Under pressure, these structures exhibit different predicted band gaps, 2.74 eV for P4/nmm and 2.47 eV for Cmcm [41]. At the transition pressure, the volume of BiOF experiences an obvious discontinuous change, characterizing its first-order nature [41].
2.2. Bi7O5F11
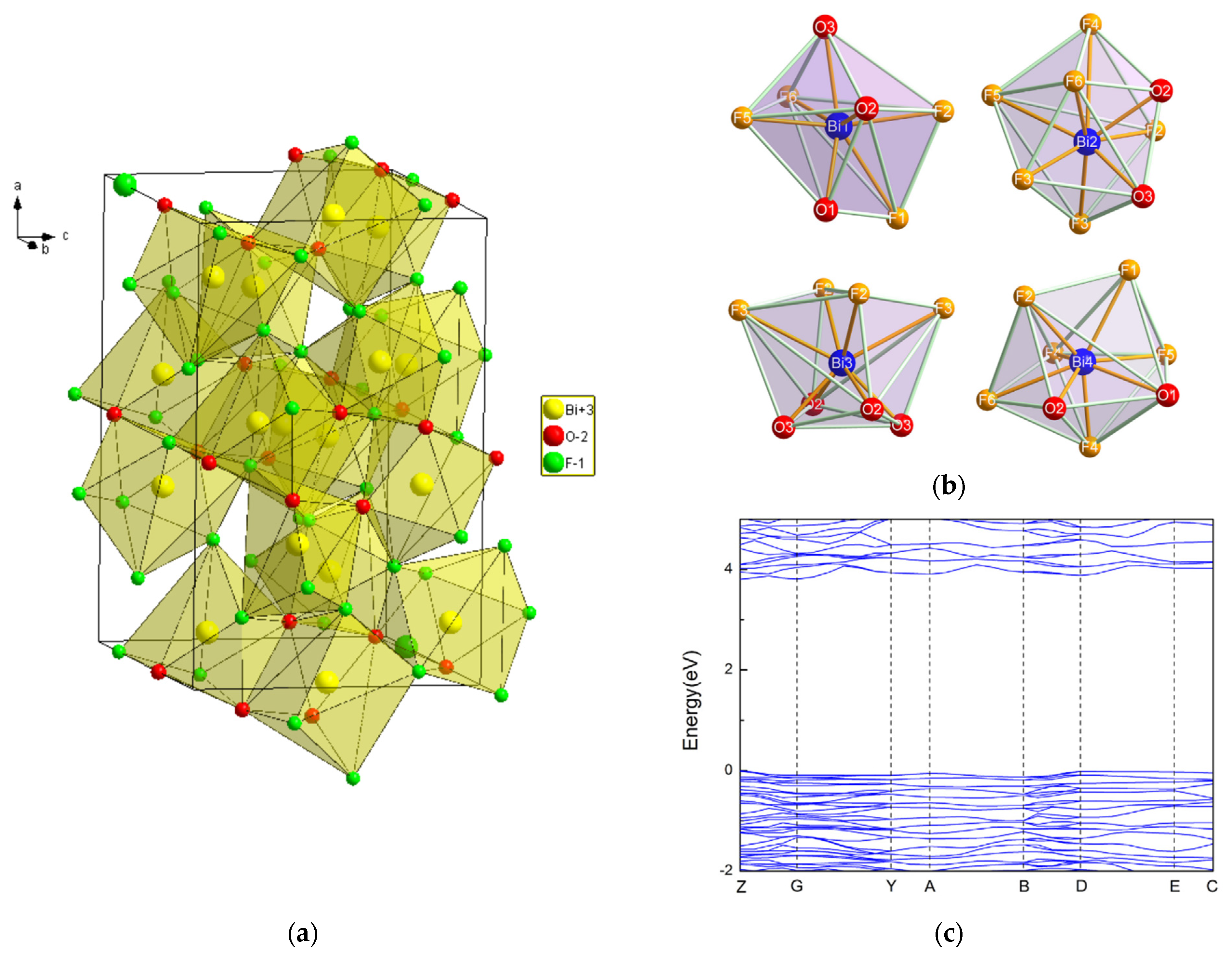
While Aurivillius had already synthesized the Bi7O5F11 monoclinic phase in 1955 and named it δ-BiOxF3−2x [32], this structure was first determined by Laval et al. in 1994 after synthesizing it through the solid-state reaction of Bi2O3 and BiF3 at 290 °C [42]. Bi7O5F11 crystallizes in the monoclinic space group C2. Bi7O5F11 has an intricate three-dimensional framework of interconnecting Bi-centered polyhedra: Figure 5a,b presents the crystal structure and schematics of the fluorine–oxygen polyhedra around the Bi3+ cations in Bi7O5F11 [39]. In Figure 4, peaks in the Bi7O5F11 pattern appear at 17.2°, 26.7°, 28.2°, 29.3°, and 31.0° and correspond to the (111), (112), (311), (401), and (20-3) crystal planes.
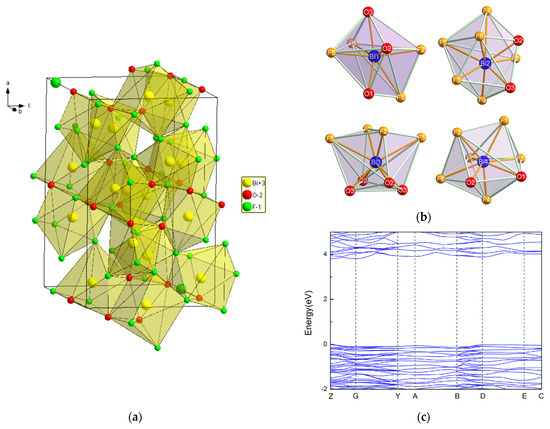
Figure 5.
(a) A perspective view of the hexagonal Bi7O5F11 bismuth oxyfluoride structure; (b) schematic representations of the various polyhedra around the Bi atoms in Bi7O5O11 [43]; (c) the band structure of Bi7O5O11 [43].
Theoretical calculations based on the DFT method were performed by W. Hu et al. [43]. Figure 5c shows the band structure of Bi7F11O5. The tops of the valence bands and the bottoms of the conduction bands are located at the Z point, making Bi7F11 O5 a direct band gap semiconductor with a band gap value of 3.81 eV.
2.3. β-BiOxF3−2x
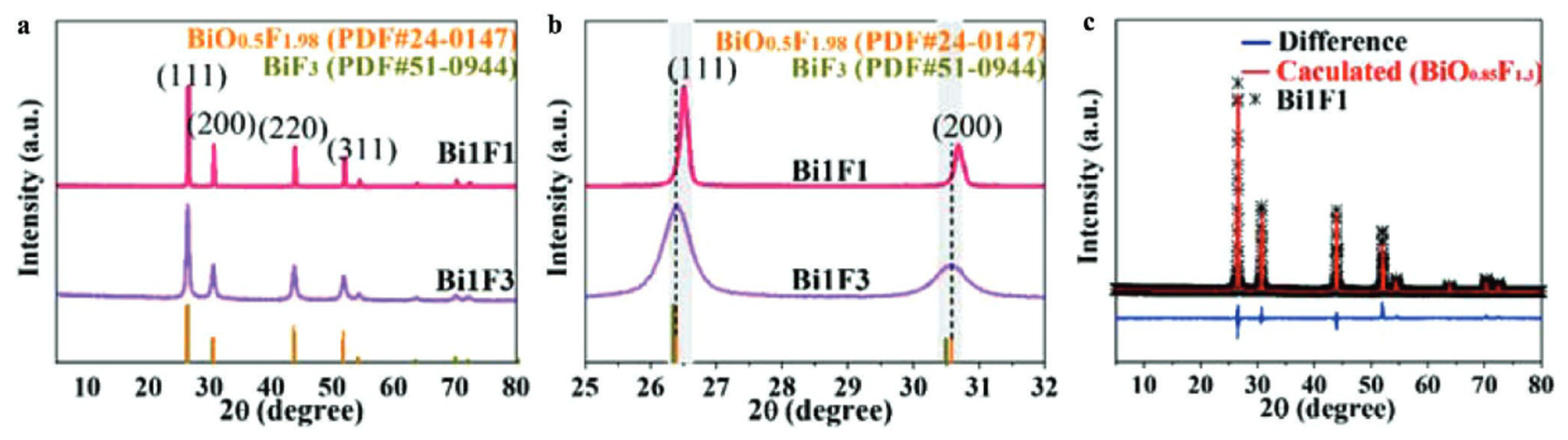
A face-centered cubic phase with a variable composition, named β-BiOxF3−2x by Aurivillius, was first synthesized through the solid-state reaction of Bi2O3 and BiF3 at 670 °C [34]. β-BiOxF3−2x crystallizes in the Fm-3m space group. Figure 3 presents the classically observed β-BiOxF3−2x pattern. The peaks at 26.5°, 30.7°, 44.0°, 52.2°, and 54.7° correspond to the (111), (200), (220), (311), and (222) crystal planes of the cubic β-BiOxF3−2x phase. Its lattice parameters being close to those of α-BiF3 make them easy to confuse using XRD.
Moreover, the modification of the O/F ratio in the β-BiOxF3−2x phase leads to a very slight shift in the diffraction peak position and a variation in the intensity of the (200) to (111) peaks. A. Morell et al. determined that the monophasic domain of β-BiOxF3−x synthesized through the solid-state reaction of Bi2O3 and BiF3 at 670 °C [35] is 0.41 ≤ x ≤ 0.62. Two β-BiOxF3−2x specimens were synthesized through stirring and precipitation in ethylene glycol (EG) for 96 h at room temperature [44]. The composition can be modulated by varying the quantities of Bi3+ and F− precursors (here, Bi(NO3)3.5H2O and NH4F). The crystal molecular formulas, extracted from cell parameters calculated through Rietveld XRD refinement, are BiO0.54F1.92 and BiO0.85F1.30 [44]. Figure 6 shows Zhan et al.’s XRD and Rietveld studies on the influence of the graphene oxide surface on the synthesis and structure of β-BiOxFy [44]. Even if the synthesized oxyfluoride patterns agree well with the standard card for BiO0.51F1.98, the diffraction peaks shift slightly to a higher 2θ angle, indicating that the elemental ratio of O to F in the crystal gas increased, as it is associated with a decrease in the lattice parameters. Despite these studies, most authors have not determined the precise composition of their synthesized compounds and have only identified the compounds through an XRD comparison to the standard BiO0.51F1.98, described by A. Morell et al. [35].
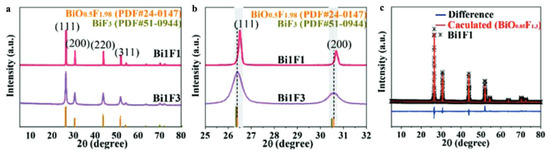
Figure 6.
(a,b) XRD patterns of Bi1F3 (BiO0.54F1.92) and Bi1F1 (BiO0.85F1.3). (c) Rietveld refinement of XRD pattern of Bi1F1 [44].
The experimental band gap of BiO0.51F1.98 synthesized through the hydrothermal method was determined, with a band gap value Eg = 3.35 eV, ECB = 1.73 eV, and EVB = 5.08 eV [45].
2.4. The Less-Described BiOxF3−2x
BiOF, Bi7O5F11, and β-BiOxF3−2x have been detailed most in the literature and have shown the most convincing photocatalytic performance among all bismuth oxyfluorides. However, other bismuth oxyfluoride phases with less-known characteristics are also described in the literature and are presented in this section.
2.4.1. α-BiOxF3−2x
α-BiOxF3−2x was first synthesized by Aurivillius and described as a tysonite-like structure indexing in the P63/mmc space group with the composition BiO0.1F2.8 [32,34]. A. Morell et al. confirmed the hexagonal structure of α-BiOxF3−2x and determined the lattice parameters of the composition BiO0.14F2.72 [35]. This structure does not exist with the composition BiF3 but is stabilized by the occurrence of vacancies in the non-metal framework.
2.4.2. β′-BiOxF3−2x
β′-BiOxF3−2x is an orthorhombic phase whose lattice parameters are derived from β-BiOxF3−2x. The standard composition is BiO0.67F11.66, described by A. Morell et al. [35], with the monophasic domain being 0.62 < x ≤ 0.74. The experimental band gap of BiO0.67F1.66 was determined from BiO0.67F1.66 synthesized through the hydrothermal method with the band gap value Eg = 3.39 eV, ECB = 1.48 eV, and EVB = 4.87 eV [45].
2.4.3. γ-BiOxF3−2x
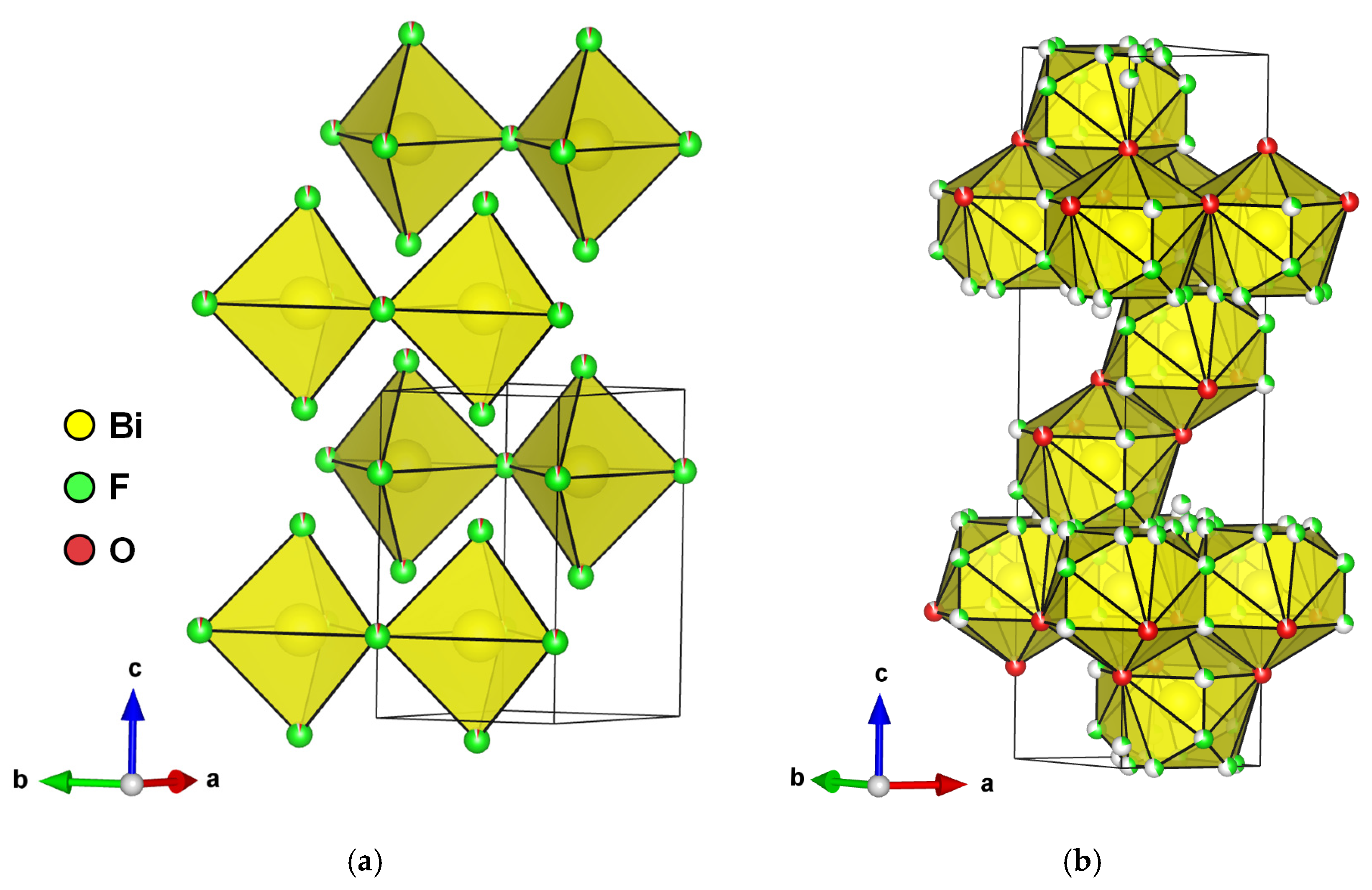
A hexagonal BiOxF3−2x phase, named γ by Aurivillius and described further by A. Morell, was synthesized through the solid-state method with the monophasic domain 1.11 ≤ x ≤ 1.20 (Figure 7a). The composition BiO1.18F0.64 (also referenced as Bi50O59F32) is used as the standard name in the literature.
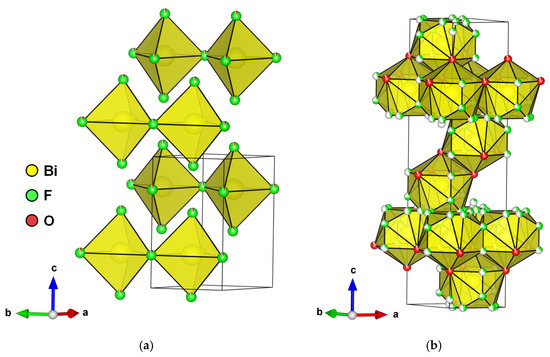
Figure 7.
(a) Perspective view of hexagonal BiO1.18F0.64 bismuth oxyfluoride structure and (b) rhombohedral BiO0.9F2.35 bismuth oxyfluoride.
2.4.4. Other BiOxFy Structures
An anion-excess fluorite defective structure derived from a rhombohedral LnFO type was synthesized through annealing at 500 °C for 12 h in gold sealed tubes and then water-quenched [46]. The structure was solved using XRD on a single crystal in the R-3m space group with the composition BiO0.9F2.35 (Figure 7b). It can be mentioned that this phase was later synthesized through the hydrothermal method by M. Ren et al., and its photocatalytic properties were tested for the degradation of RhB under UV light [47]. Considering the soft synthesis conditions compared to those of Laval et al. [46], it seems unlikely that it was BiO0.9F2.35 but perhaps a confusion with the cubic β phase, which has close diffraction peaks.
Centered cubic-phase Bi26O38F2 with a sillenite structure can be synthesized through precipitation from Bi(NO3)3·5H2O and NaF in NaOH solution [48]. This phase is not considered for its own photocatalytic properties but is known to be used in composite photocatalysts for the formation of heterojunctions, notably with BiOxIy, g-C3N4, or graphene oxide (GO) [49,50,51].
2.5. The Aurivillius Structure
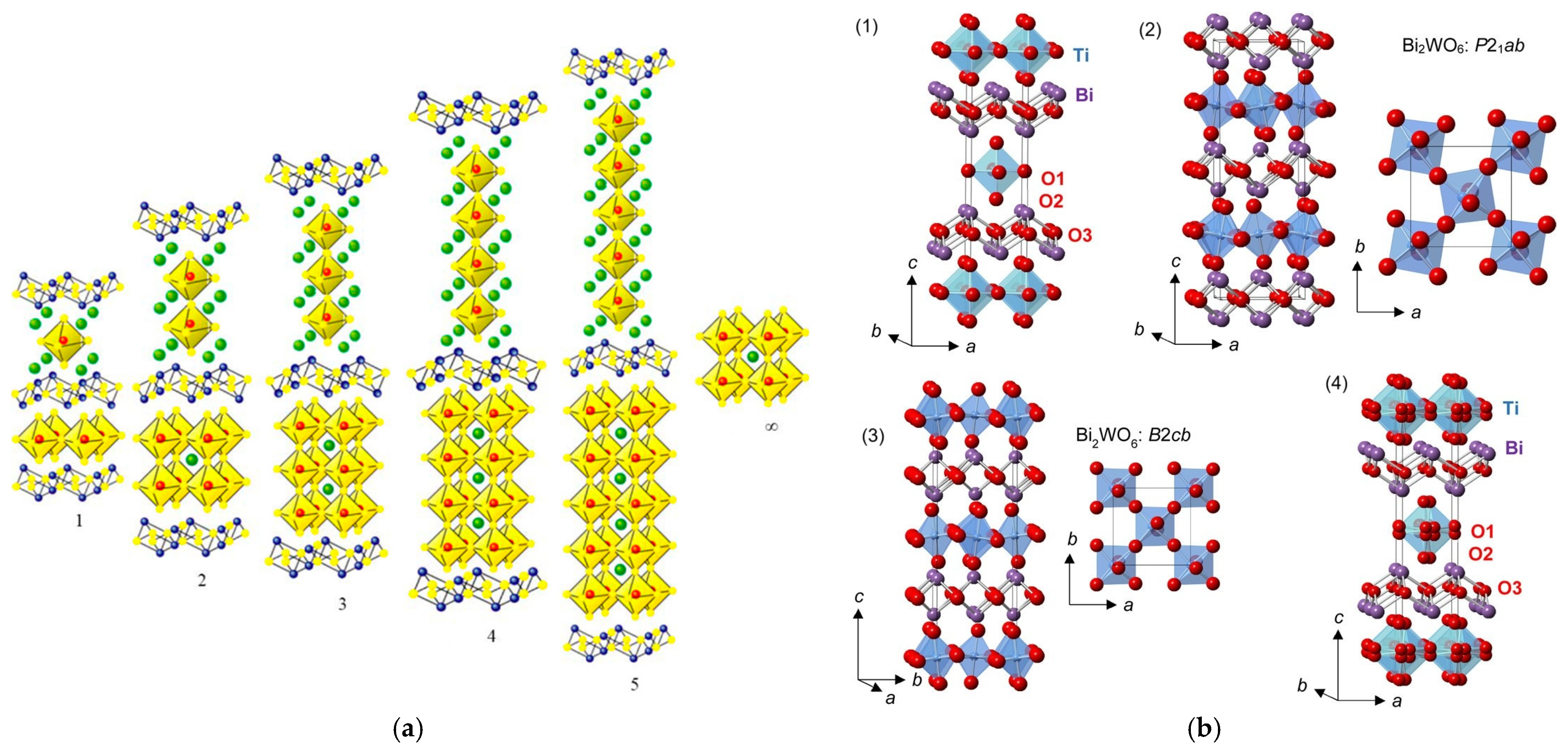
The Aurivillius structure was described by B. Aurivillius in 1949 using the general formula (Bi2O2)(An−1BmO3n+1), where A is a large metal cation in 12 coordination, such as Pb2+, Na+, K+, Ca2+, Sr2+, Ba2+, or Bi3+, and B is a transition metal in an octahedral coordination, such as Ti4+, Nb5+, Ta5+, Fe3+, Mo6+, or Ga3+ (with n ≤ 5) [33]. The structure is made up of fluorite layers [Bi2O2]2+, as shown in Figure 8a, similar to the slabs in the BiOF structure, and n alternating perovskite layers [An−1BmO3n+1]2− along the c axis, as for Bi2MoO6 and Bi4Ti3O12, with n = 1 and 3, respectively. Aurivillius structure oxides generally crystallize in the centrosymmetric tetragonal space group I4/mmm. Substitutions of oxygen by fluorine have allowed for the formation of oxyfluoride Aurivillius structures with distinctive electronic functionalities that induce interesting ferroelectric properties for applications in photocatalysis, as spontaneous polarization allows for an improved surface chemistry and charge separation, induced by an internal electric field.
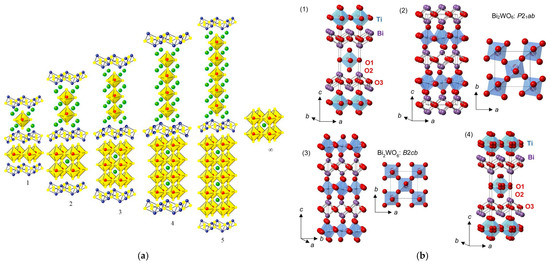
Figure 8.
(a) An Aurivillius structure as a function of the number of pseudo-perovskite layers [52]. (b) An illustration of (1) the ideal ordered I4/mmm structure for an n = 1 Aurivillius phase, (2) a low-temperature P21ab phase for Bi2WO6, (3) an intermediate B2cb phase for Bi2WO6, and (4) a disordered I4/mmm structure of Bi2TiO4F2 (with displacive disorder of the equatorial and apical anion positions to the 16n and 16m sites, respectively) from 100 K NPD Rietveld refinement; TiX6 or WO6 polyhedra, Bi, and O are shown in blue, purple, and red, respectively [53,54].
A centrosymmetric tetragonal structure I4/mmm of the phases Bi2NbO5F, Bi2TaO5F, and Bi2TiO4F2 was proposed in 1952, considering total anionic disorder for the oxygen and fluorine atoms (Figure 8b), and a Bi2VO5F structure was resolved following the same model [55]. Later, the orthorhombic space group Pbca of a lower symmetry was proposed for Bi2NbO5F [56]. Based on calculations of the valence of the anions, the problem of the fluorine atom positions was difficult to elucidate at the level of their localization at the equatorial or apical sites of the Nb(O,F)6 octahedra. Preferential localization of fluorine atoms at the apical sites was ultimately demonstrated.
According to the literature, these three phases are ferroelectric, with transitions measured at Tc = 303, 283, and 284 K for Nb5+, Tb5+, and Ti4+, respectively [56]. The electronic structure of Bi2TiO4F2 has been investigated through DFT calculations by S. Wang et al. and shows that Bi2TiO4F2 exhibits an indirect band gap in the visible part with an energy of 1.46 eV. The band gap energy was also measured through diffuse reflectance spectroscopy and determined to be 3.26 eV [57]. J. Wang at al. investigated the electronic structure of Bi2NbO5F through DFT calculations and demonstrated that Bi2NbO5F is a direct semiconductor with a band gap energy of about 2.50 eV. The band gap energy was also measured experimentally through DRS, with a value of 3.14 eV [58]. Both studies show a larger experimental band gap energy due to underestimation of the band gap in the DFT calculations [59].
3. Challenges in Characterizing BiOxF3−2x
A major contributing factor to the confusion between the different bismuth oxyfluoride phases is the similarity of their XRD patterns, particularly the recurring presence of an intense peak around 27°. Its almost systematic presence complicates the precise identification of the phases and underlines the need for a more detailed analysis, combining XRD with other techniques for reliable attribution of the structures. Moreover, some crystalline phases in BiOxF3−2x bismuth oxyfluorides have variable anionic compositions. Despite this, many studies only report compositions from bibliographic references, without really quantifying the O/F ratio, whereas this variation in the O/F ratio has a direct impact on the physicochemical properties of the material, particularly on its performance in photoelectrochemistry [45]. Rigorous determination of the actual composition is therefore essential to understand and optimize the functional properties of these compounds. Indeed, to avoid misinterpretation in structure–property correlations, complementary characterization techniques should be performed.
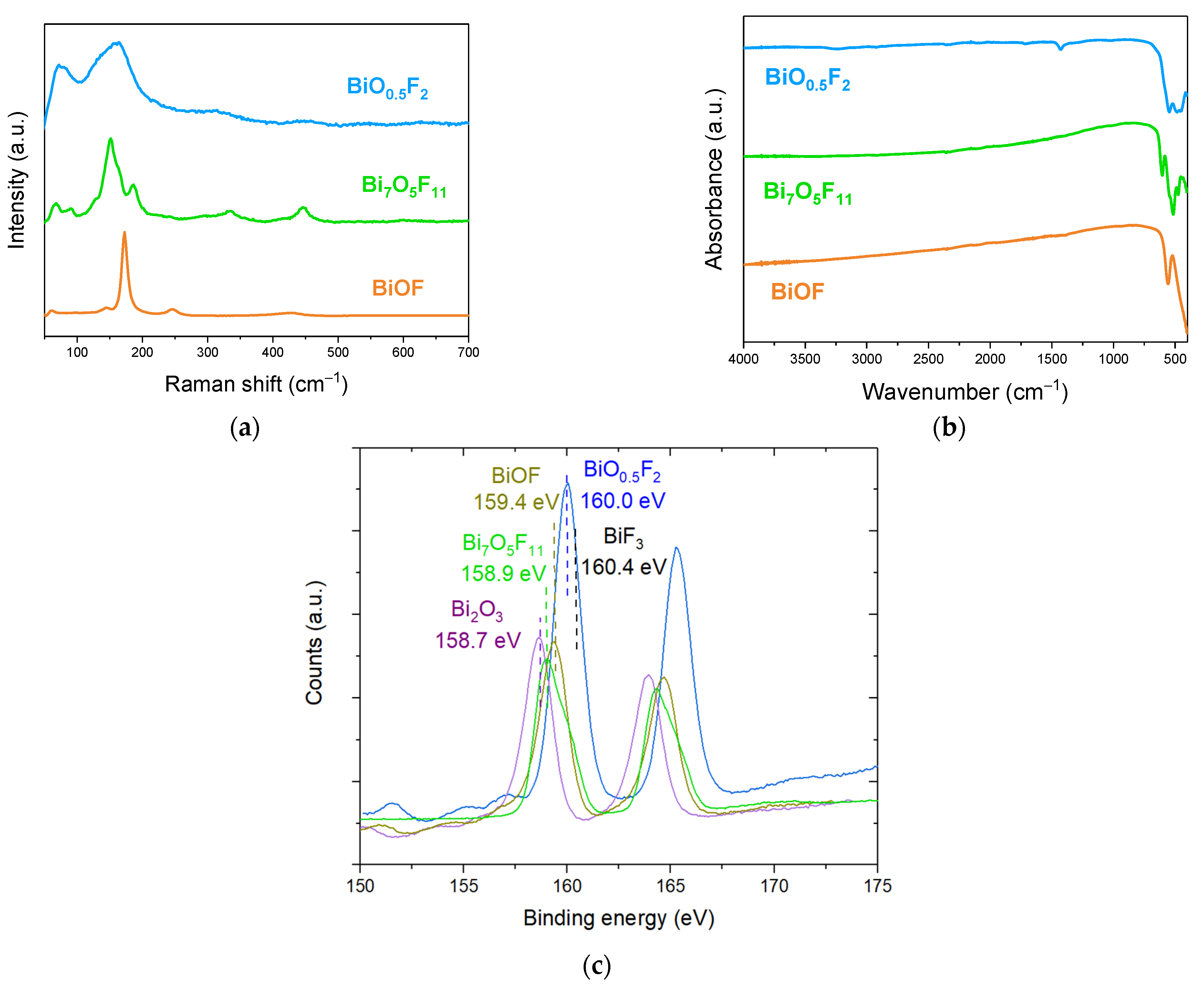
Figure 9a,b present the Raman and FTIR spectra for BiOF, Bi7O5F11, and β-BiOxF3−2x materials. In BiOF’s Raman spectrum, the vibration bands at 169 and 241 cm−1 are attributed to the A1g and Eg Bi-F stretching modes, respectively. In the Bi7O5F11 Raman spectrum, the weak peak at 595 cm−1 and the peak at 444 cm−1 can be assigned to ν(Bi-O) and ν(Bi-F) vibrations, respectively. The peaks below 400 cm−1 are attributable to the bending and stretching vibrations of Bi-O-Bi and Bi-F-Bi [43]. In the Raman spectrum of β-BiOxF3−2x, the band observed at 157 cm−1 can be attributed to Bi-O and Bi-F stretching vibrations, while the band at 313 cm−1 is attributed to the stretching and bending vibrations of Bi-O-Bi and Bi-F-Bi. Finally, the band at 449 cm−1 is attributed to Bi-O−. In the FTIR spectrum of BiOF, the bands observed at 420 and 550 cm−1 correspond to the Bi-O and Bi-F stretching vibrations in BiOF, respectively. In the FTIR spectrum of Bi7O5F11, the two bands at 604 and 526 cm−1 are assigned to the ν(Bi-O) and ν(Bi-F) vibrations of Bi7F11O5. Finally, in the FTIR spectrum of β-BiOxF3−2x the weak bands at 485 and 545 cm−1 correspond, respectively, to the Bi-O and Bi-F stretching vibrations.
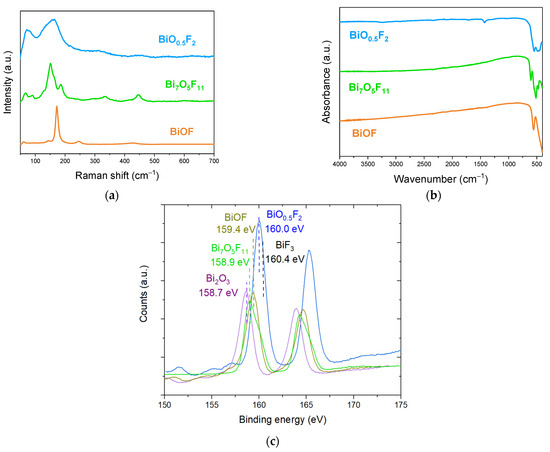
Figure 9.
(a) Raman and (b) FTIR spectra of BiOF, Bi7O5F11, and β-BiO0.5F2; (c) XPS spectra of Bi 4f from Bi2O3, BiOF, Bi7O5F11, and β-BiO0.5F2 and BiF3 compounds [40].
It appears that the characteristic bands of bismuth oxyfluorides in Raman and FTIR spectroscopy are associated with similar bonds, particularly Bi-F and Bi-O, but with shifted wavenumbers and different spectral profiles. Although these analytical techniques do not seem sufficient to clearly identify BiOxF3−2x, they may be relevant techniques to complement XRD characterization and provide further information on the compound.
Fortunately, the position of the Bi 4f peak in XPS is very sensitive to the presence of fluorine. Figure 9c presents the Bi 4f peak spectra for various bismuth oxyfluoride compounds. The Bi 4f peak is composed of a Bi 4f7/2 and Bi 4f5/2 doublet separated by 5.3 eV.
An XPS analysis might be particularly interesting for discriminating compounds presenting a diffraction pattern close to that referenced as β-BiO0.5F2 but that could have a composition ranging from Bi2O3 to BiF3. Indeed, fluorine’s high electronegativity highly influences the position of the Bi3+ 4f7/2 peak, which shifts from 158.7 eV for Bi2O3 to 160.0 eV for BiO0.5F2 and to 160.4 eV for BiF3 [60,61]. Moreover, the exact compound formula can be deduced from the quantitative elemental content analysis allowed by this technique.
Figure 9c also shows the Bi 4f spectra for BiOF, with the position of the Bi3+ 4f7/2 peak at 159.4 eV. Finally, we add the spectrum for Bi7O5F11. Since the latter has been studied very little, the exact position of its 4f7/2 peak is not referenced in the literature, but this position at 158.9 eV seems to be in agreement with the spectrum observed in [62].
Even if an XPS analysis does not give information on the crystalline structures of bismuth-based oxyfluorides, this technique could advantageously give insights into their O-to-F ratios. This could be useful, for instance, for obtaining the exact composition of a material for a structure close to BiO0.5F2.
4. Bismuth-Based Oxyfluorides as Photocatalysts and Strategies to Enhance Their Activity
4.1. Bismuth-Based Oxyfluorure Photocatalysts
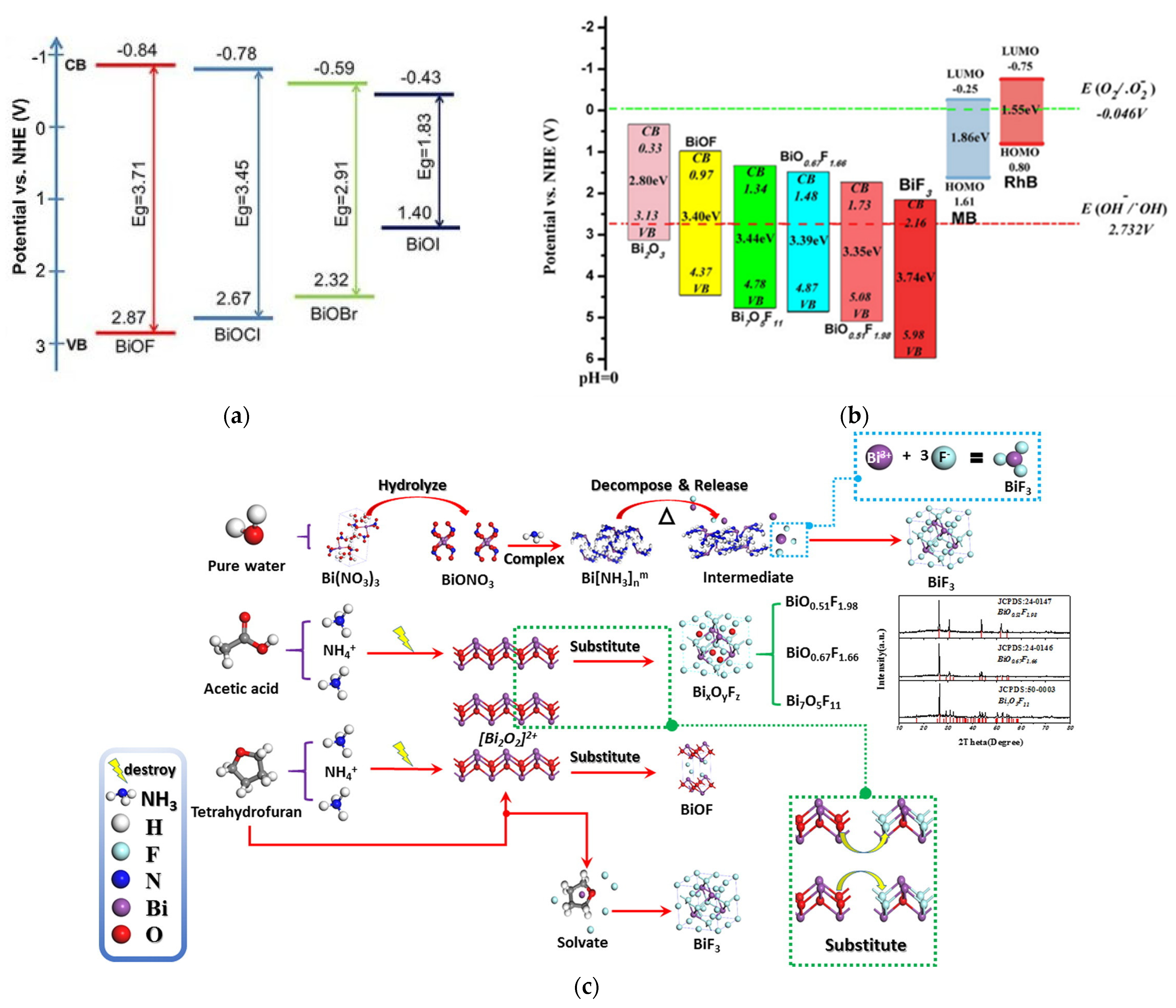
Nowadays, the development of bismuth oxyfluoride as photocatalysts appears to be very promising. BiOF is the most widely studied bismuth oxyfluoride due to its photocatalytic properties. In the BiOX family, as shown in Figure 10a, as the halogen atomic number increases, the valence band position of BiOX gradually becomes more positive, giving BiOF a stronger oxidation potential. Therefore, BiOF is efficient for forming OH radicals from H2O oxidation [63,64]. It has therefore been tested for degrading organic pollutants such as methyl orange, methyl blue, Rhodamine B, Rhodamine 6G, ciprofloxacin, perfluorooctanoic acid, benzyl alcohol, nitrobenzene, crystal violet, 2-hydrobenzoic acid, and tetracycline. In the same way, the conduction band position of BiOX becomes more negative, also making BiOF suitable for activating reduction reactions for CO2 conversion into valuable molecules (CO, CH4…), with redox potentials around −0.106 (pH = 0) for CO2 to CO [65], for industrial CO2 emission abatement [37].
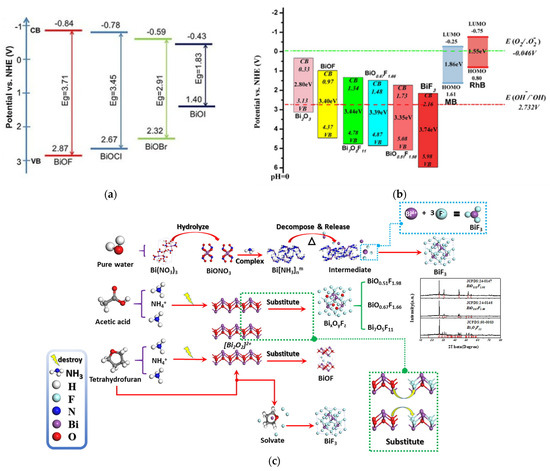
Figure 10.
The redox potentials of photocatalytic reactions with respect to the estimated flat band edge positions of (a) various BiOX [37] and (b) BixOyFz [45] specimens. (c) The plausible conversion mechanism of BixOyFz: in acetic acid/water conditions, acetic acid would hydrolyze to produce H+. H+ and NH4+ as a Lewis acid may partially destroy the [Bi2O2]2+ layer, where the O2− in the [Bi2O2]2+ layer will be substituted with F−, leading to the formation of BixOyFz with different O/F ratios [45].
Besides BiOF, a wide range of BiOxF3−2x materials with different atomic ratios have been synthesized and reported as promising photocatalysts. M. Ren et al. showed that many of these materials are easily accessible through hydrothermal synthesis by modifying synthesis parameters such as the reaction medium’s composition (Figure 10c) [45]. Compared to BiOF, lower-O/F-ratio BiOxF3−2x materials present a broader and more positively positioned valence band, thereby enhancing their oxidative potential, as shown in Figure 10b. Furthermore, the different O/F ratios observed in compounds such as BiO0.51F1.98 (O/F = 0.25), Bi7O5F11 (O/F = 0.45), and BiO0.67F1.66 (O/F = 0.4) could be responsible for the variation in the intensities of their internal electric fields. As the O/F ratio decreases, oxygen defects tend to increase, which correlates with enhanced photocatalytic activity [45].
Concerning Aurivillius compounds, using a mixture of Bi(NO3)3·5 H2O and (NH4)2TiF6 [57] or H2TiF6 [66], nanoparticles and nanospheres of Bi2TiO4F2 have been synthesized through the solvothermal method. Both present good activities for photodegrading pollutants into water under UV–visible light. This strategy even allows porous spheres of Bi2M5+O5F (M = Nb and Ta) with photocatalytic activity to be formed [67]. Nanosheets of Bi2NbO5F were also synthesized through the molten salt method with preferential growth along the (010) plane, which could degrade tetracycline and RhB under UV twice as efficiently as the product synthesized through ceramic synthesis [58]. This synthesis was also adapted to using BiF3 instead of HF. The Bi2NbO5F nanosheets obtained present a photocatalytic activity 15.6 times higher than that of the compound obtained via the solid-state route because of a higher specific surface area [68].
The hydrothermal method can be extended to other Aurivillius compounds with a metal of oxidation number (+II), such as Bi2Co2+O2F4. Its crystal structure has been described in the tetragonal space group I4 with alternating [CoF4]∞ and [BiO2F2]∞ layers via sharing of fluorine atoms at the vertices [69]. The relatively weak interlayer Bi–F bonds offer the possibility of exfoliation through magnetic stirring in isopropanol into nanosheets, exhibiting a higher electrocatalytic activity for water oxidation than that of the initial material thanks to a larger specific surface area [70]. Finally, thin films with an Aurivillius-type structure were investigated. Using a (Bi0.8Ba0.2)FeO2.9 precursor and PVDF for top chemical fluorination at 350 °C, a (Bi0.8Ba0.2)FeO2F film is formed, with promising photocatalytic activity [71].
These findings highlight the value of going beyond the stoichiometric BiOF composition and finely tuning the stoichiometry of bismuth-based oxyfluorides to optimize their photocatalytic performance. Importantly, adjusting the crystal structure, the O/F ratio, or choosing to incorporate other metal cations into the Aurivillius structure provides a powerful strategy for tailoring the electronic structure and reactivity of these materials to meet the specific requirements of targeted photocatalytic applications.
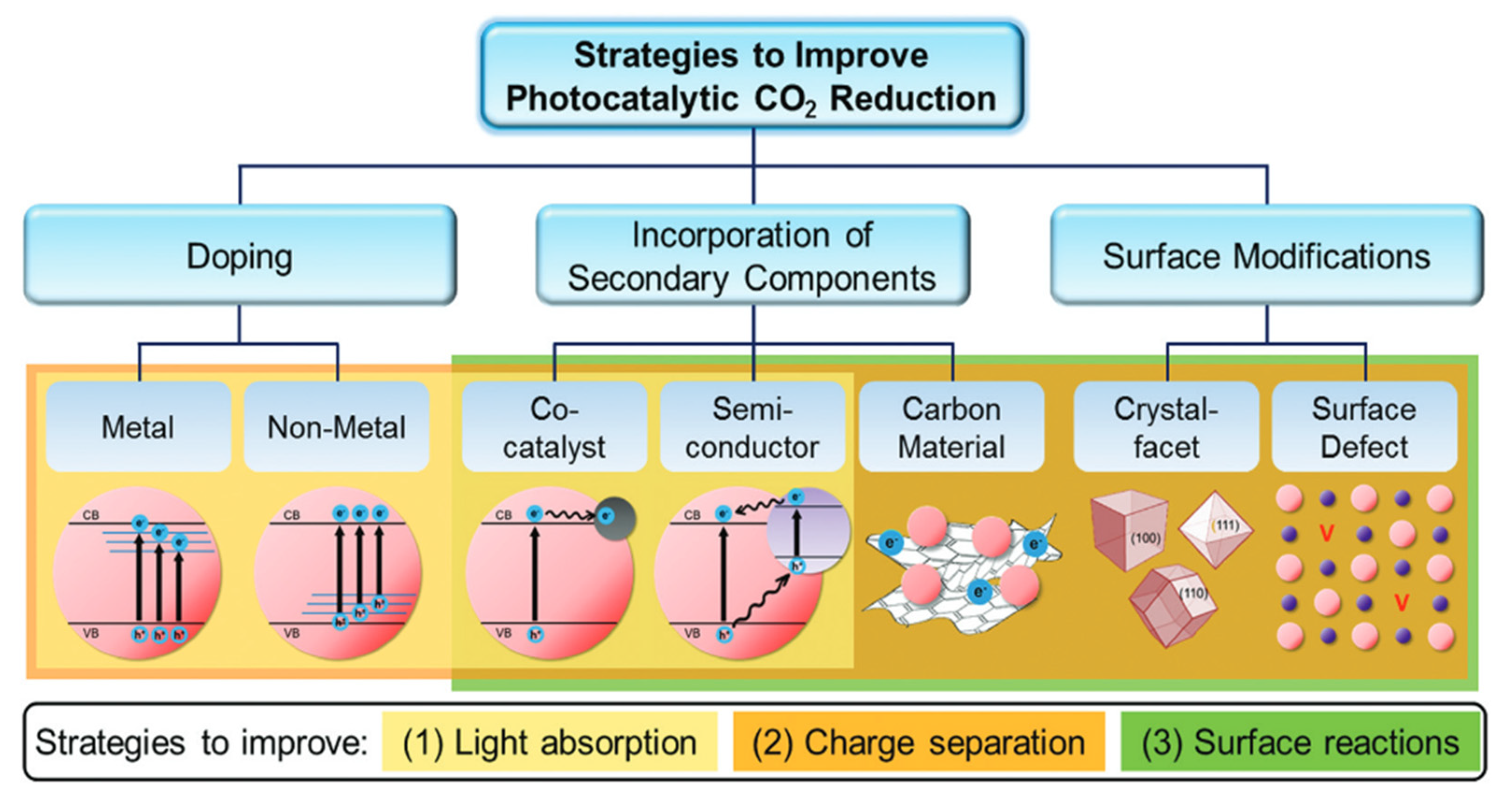
To go further, different approaches to improving the photocatalytic performance of the pure compound have been considered, as illustrated by Figure 11. First, we may play with its morphology and surface to increase the surface reactivity and area. The material may also be doped with metal or non-metal elements to add new levels into the band gap to favor light absorption and carrier separation. Finally, it can be associated with carbon materials, another photocatalyst, or metal in the heterojunction, which favors light harvesting and reduces electron–hole recombination.
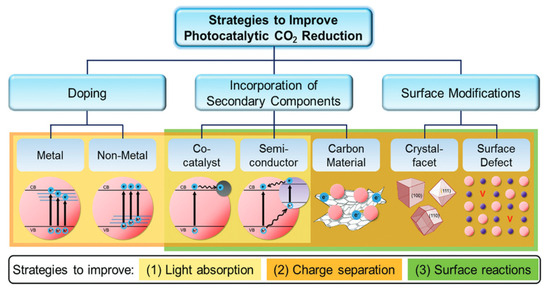
Figure 11.
Strategies for an advanced photocatalytic CO2 reduction performance by improving the light absorption ability, charge separation efficiency, and surface reactions [72].
4.2. Facet Selection and Oxygen Vacancies
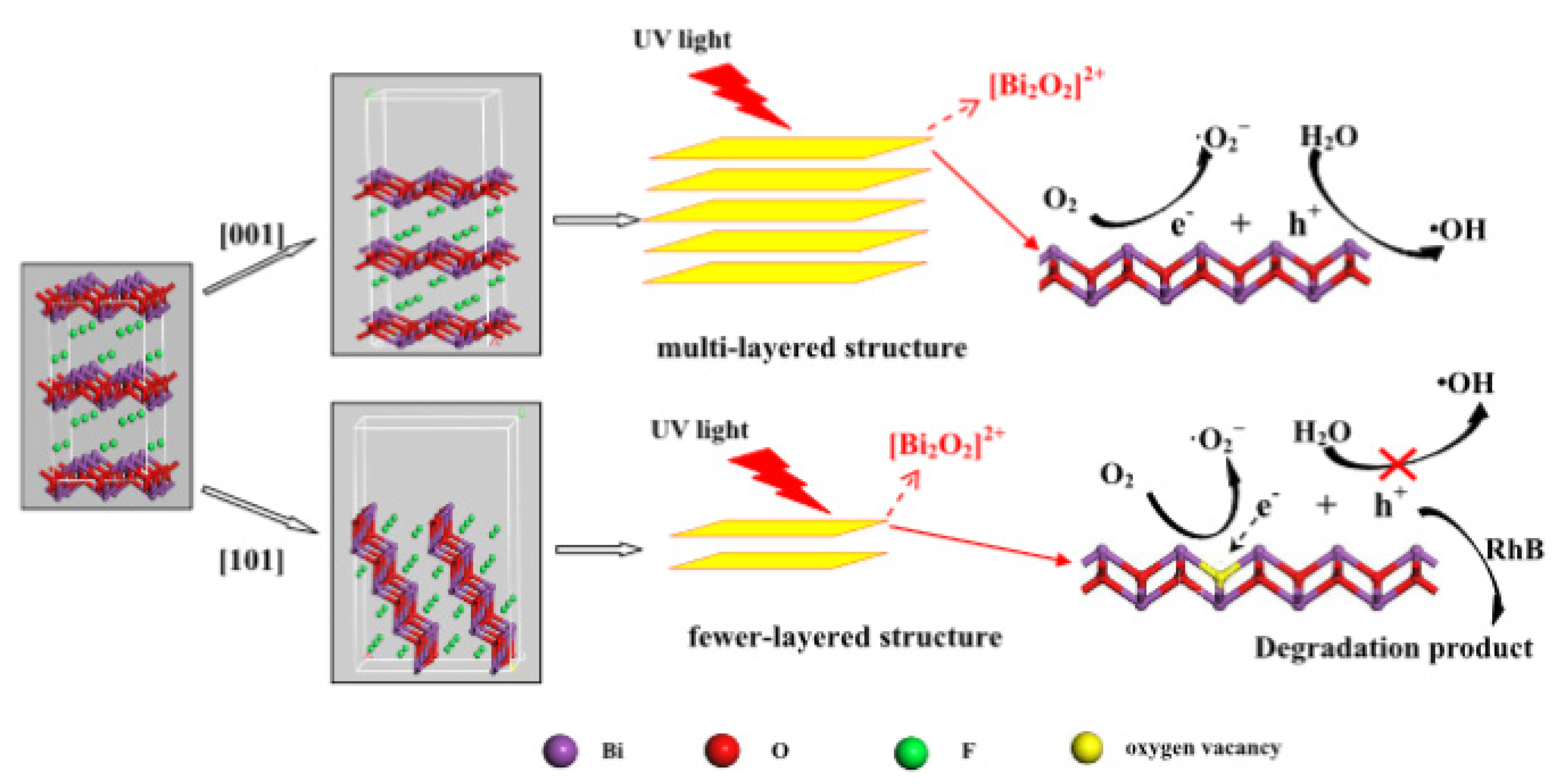
Oxygen vacancy defects have an important effect on the band structure and optical absorption properties of semiconductors. The existence of oxygen vacancies causes the Bi 6p state to fall into the forbidden band, which increases the effective separation of electron–hole pairs, redshifts the edge of the absorption band, and realizes an electronic transition from the F 2p or the O 2p state under visible-light irradiation [41]. Indeed, the presence of O-vacancies induces a decrease in the band gap, enhancing the light absorption, and contributes to carrier separation [73]. It is well known that a BiOF layered structure favors the presence of O-vacancies and accessibility between the [Bi2O2]2+ slabs. Zhang et al. [27] used a simple hydrothermal method to synthesize BiOF, where exposition of the {101} surface, rather than {001} surfaces, is controlled by the pH values. More surface O-vacancies are contained in the {101} nanodisk than common thick BiOF plates, which reduces the band gap and improves the charge transfer and separation, as illustrated in Figure 12. Therefore, the selection of a {101} facet, rather than a {001} facet, drastically improves the photocatalytic properties [27]. With this idea of facet engineering, Zou et al. used a simple hydrothermal method to produce BiOF nanosheets, where 75.4% of the high-energy (002) surface was exposed, showing a higher photocatalytic activity [26].
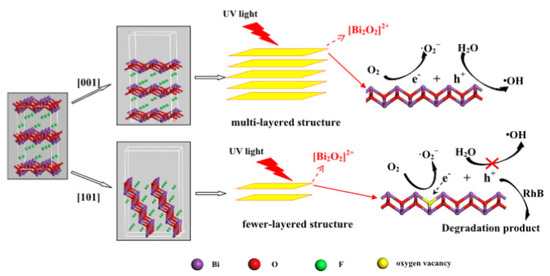
Figure 12.
A schematic of the reaction mechanisms of multi- and few-layered structures [27].
4.3. Metal/Non-Metal Doping
Doping, in the context of materials science, specifically refers to the deliberate incorporation of small quantities of foreign elements into the crystalline lattice of a material, achieved either through substitution or insertion. This distinction is often blurred in the literature concerning BiOF-based materials, where the concept of doping is sometimes confused with that of heterojunctions, defined as the interface formed between two crystalline materials of distinct compositions. In this section, we will present the effect of doping, understood as the incorporation of elements into BiOxF3−2x’s structure. The effect of heterojunction formation is discussed later.
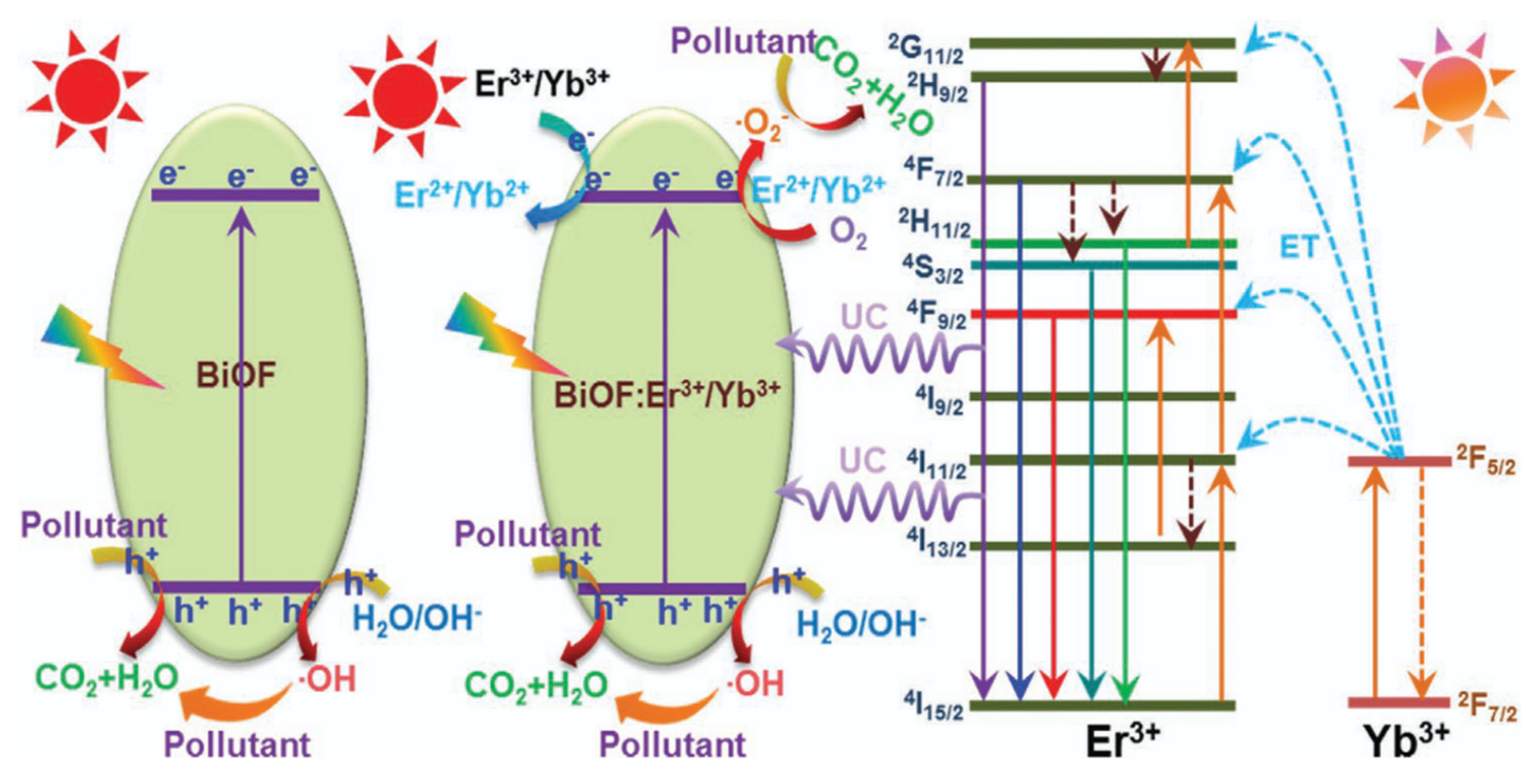
Doping metal/non-metal ions into BiOF can accelerate the separation efficiency of photoelectron–hole pairs and hinder their recombination, in addition to adjusting the band gap. Table 1 below summarizes the work conducted on bismuth oxyfluoride doping and highlights the ubiquity of rare earth ions as an oxide doping element. Doping with these elements is indeed recognized for its effect on the band structure and a shift in the band gap towards the visible region [74]. Wang et al. demonstrated that Sm3+-activated BiOF nanoparticles show excellent photoluminescence properties as a visible-light-driven photocatalyst [75]. Vadivel et al. prepared Y-BiOF Reduced Graphene Oxide (RGO) composites and realized that the effective combination of yttrium ions and RGO sheets reduced the recombination rate of BiOF and improved the absorption of visible light [63]. Er3+/Yb3+-codoped BiOF nanoparticles with an enhanced photocatalytic activity were developed by P. Du et al. through a high-temperature solid-state reaction. The improved photocatalytic activity is linked to various effects: an enlarged absorption edge, the formation of impurity energy levels, and an enhanced upconversion performance, allowing for the degradation of RhB and MB under visible and NIR light, as shown in Figure 13 [76]. R. Saraf et al. synthesized Eu3+doped BiOF and evaluated its performance for RhB degradation under visible light [64]. The structural, electronic, and optical properties of Ag/Pd-doped BiOX (X = F, Cl, Br, I) were calculated by S. Zhou et al. based on DFT calculations. It was highlighted as standing out from other compounds due to its unusually broad absorption peaks, which could be beneficial in photocatalytic systems [77]. M. Elias et al. [78] synthesized BiOF, Ag-doped BiOF, and Ag-doped BiOF–rGO composites and tested their performance for the photodegradation of MB and MO under UV light. Ag-doped BiOF–rGO presents an enhanced photocatalytic performance due to a synergistic effect that promotes the efficient separation of photogenerated electron–hole pairs. It is proposed that the incorporation of Ag+ ions into the BiOF lattice improves the excitation rate, while rGO aids in the separation of e−/h+ pairs by directing them towards the interface, thus increasing the photocatalytic activity of the material [78].
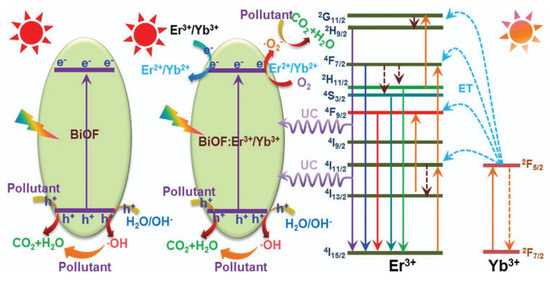
Figure 13.
A schematic diagram of the possible photocatalytic mechanisms of the designed nanoparticles under NIR and visible-light irradiation [76].

Table 1.
Synthesis methods, precursors, and photocatalytic applications of doped-BiOxF3−2x materials.
Table 1.
Synthesis methods, precursors, and photocatalytic applications of doped-BiOxF3−2x materials.
| Materials | Synthesis Method | Precursors | Application | References |
|---|---|---|---|---|
| Ag-doped BiOF–rGO | Solvothermal | GO, Bi(NO3)3·5H2O, AgNO3, NH4F, C2H6O2 | The photodegradation of MB and MO dyes under UV-light and sunlight irradiation | [78] |
| Er3+/Yb3+-codoped BiOF | Solid-state | Bi2O3, NH4F, Yb2O3, Er2O3 | The photodegradation of RhB and MB under visible-light and NIR-light irradiation | [76] |
| Y-BiOF/RGO | Solvothermal | Bi(NO3)3·5H2O, Y(NO3)3·6H2O, PVP mw40.000, C2H6O2 | The degradation of MB under visible-light irradiation | [63] |
| Sm3+-doped BiOF | Solid-state | Bi2O3, NH4F, Sm2O3 | The decomposition of RhB dye under visible-light irradiation | [75] |
| Eu3+doped BiOF | Solid-state | Bi2O3, NH4F, Eu2O3 | The degradation of RhB dye under visible-light irradiation | [64] |
4.4. The Formation of Heterostructures with Other Photocatalysts
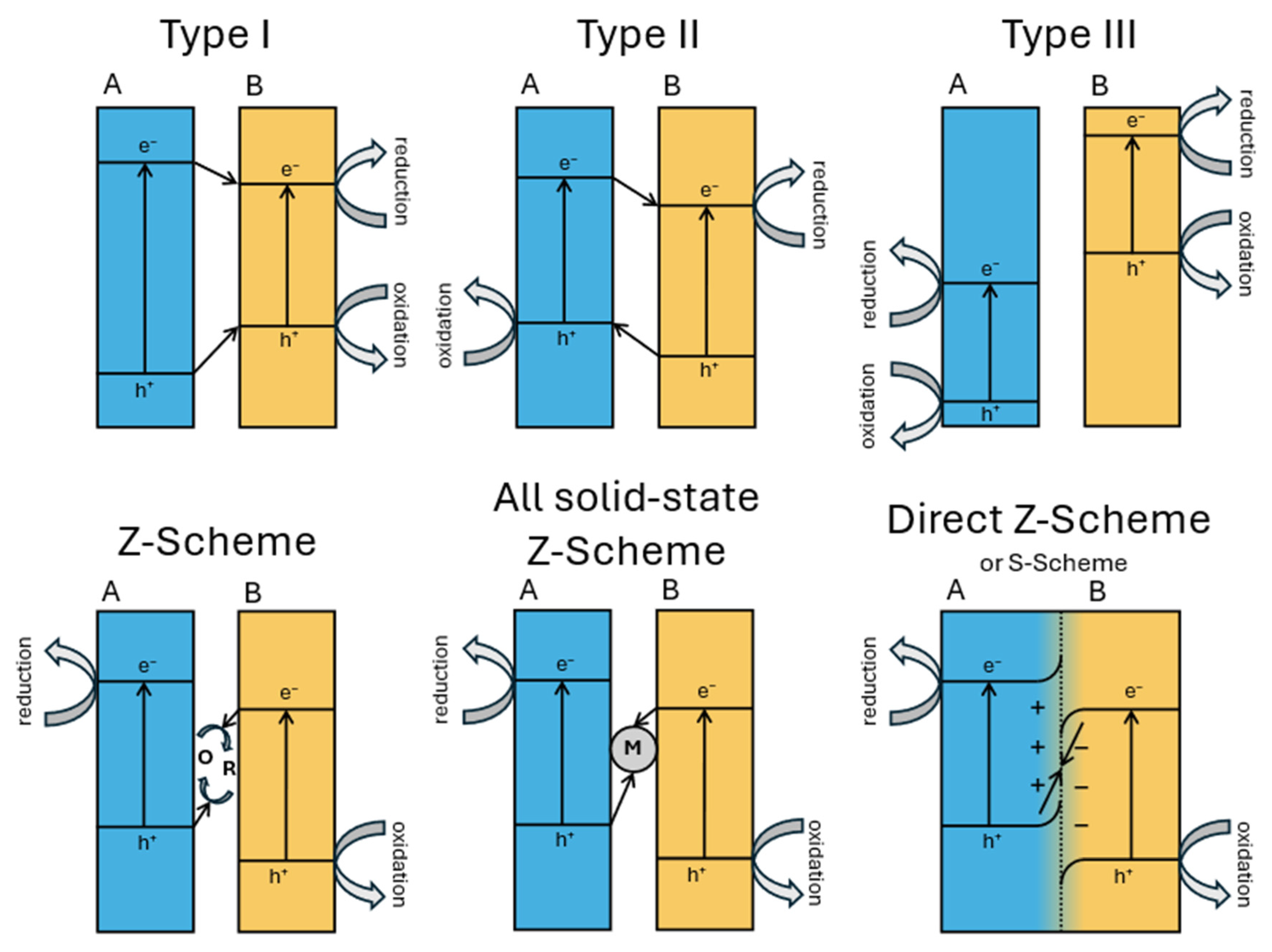
Heterojunction engineering is a central strategy for enhancing the charge separation efficiency in photocatalytic systems. Based on the band structure alignment and interfacial charge transfer behavior, heterojunctions are categorized into three main types (Figure 14): type I, type II, and type III. In type I (straddling gap) heterojunctions, both the conduction band (CB) and the valence band (VB) edges of semiconductor A lie within the band gap of semiconductor B. As a result, both the photogenerated electrons and holes tend to accumulate in semiconductor B, leading to poor spatial separation of the charge carriers and limited redox activity. Type II (staggered gap) heterojunctions exhibit a stepped band alignment, where the CB and VB edges of the two semiconductors are offset such that electrons migrate to the semiconductor with the lower CB and holes to that with the higher VB. This spatial separation improves the charge carrier’s lifetime but significantly reduces the redox potential due to energetic losses during interfacial transfer.
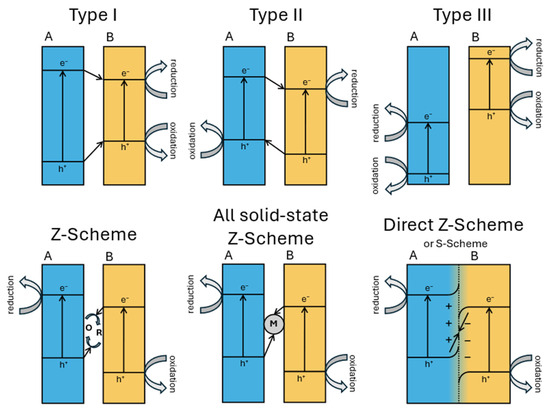
Figure 14.
A schematic representation of band diagrams of different types of heterojunctions.
Type III (broken gap) heterojunctions feature no overlap between the band edges, creating an energy barrier that impedes direct electron–hole recombination but also challenges efficient charge transfer and is thus rarely used in photocatalysis.
Z-scheme heterojunctions involve the recombination of photogenerated electrons from the CB of one semiconductor with holes from the VB of another, leaving behind electrons in a more negative CB and holes in a more positive VB, preserving a high redox potential and enabling stronger photocatalytic reactions. However, traditional Z-schemes have often suffered from complex architectures and recombination losses [79]. To overcome these limitations, the S-scheme (step-scheme) heterojunction was proposed as an evolution of the Z-scheme mechanism. It introduces an internal electric field at the interface due to Fermi level equilibration and band bending, which selectively drives the recombination of low-energy charge carriers (i.e., electrons from the less negative CB and holes from the less positive VB) while retaining high-energy electrons and holes in their respective bands. The S-scheme thus combines the advantages of spatial separation and redox potential retention, without the need for mediators, and has shown promising results in many applications, such as water splitting and pollutant degradation applications [80].
Table 2 shows the different strategies developed to form efficient heterojunctions between bismuth-based oxyfluorides and another visible-light active semiconductor (TiO2, BiVO4, or gC3N4…), other bismuth-based catalysts (which often induce good material compatibility and simple synthesis through one-pot processes), or metallic nanoparticles (to additionally play with their plasmonic effect).
4.4.1. With Semiconductors and Carbon Materials
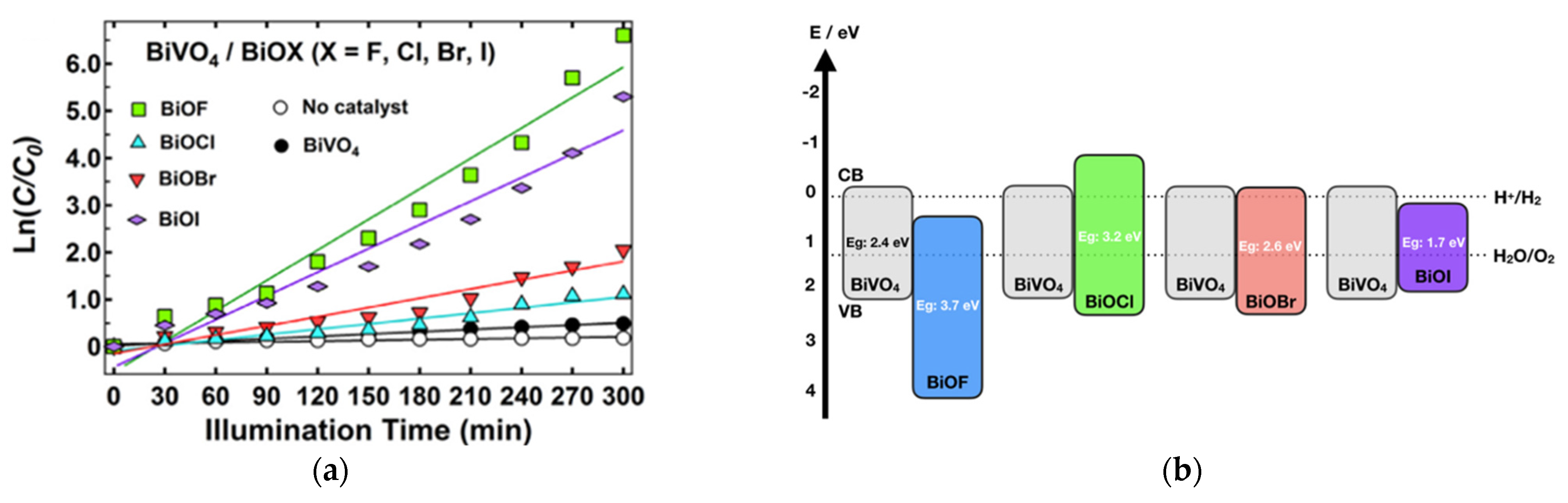
The work of H. Razavi-Khosroshahi et al. nicely illustrates how the respective band positions of both semiconductors might drive the material choice in a heterojunction. Indeed, they propose a hydrothermal synthesis method for preparing various BiVO4/BiOX heterojunctions (with X = F, Cl, Br, I) and find that BiVO4/BiOF shows the best performance for photodegrading methylene blue (Figure 15a) [81]. Compared to other systems, BiVO4/BiOF is the only one that forms a type II heterojunction (Figure 15b), allowing electrons to be driven to BiOF and holes to BiVO4, reducing their recombination. On the opposite side, in other systems, electrons and holes flow to the same material, BiVO4 in BiVO4/BiOCl or BiOI in BiVO4/BiOCl, increasing their recombination. The BiVO4/BiOF association presents as an additional advantage in involving two semiconductors with different band gaps, which increases the range of harvested light. BiVO4/BiO0.67F1.66 cake-like microstructures were successfully prepared through a two-step hydrothermal route by X. Feng et al. [82]. These BiVO4/BiO0.67F1.66 composites demonstrate significantly enhanced photocatalytic activity under visible light for decomposing RhB and phenol in water, compared to that of pure BiVO4, due to their increased surface area and the formation of a unique p–n heterojunction.
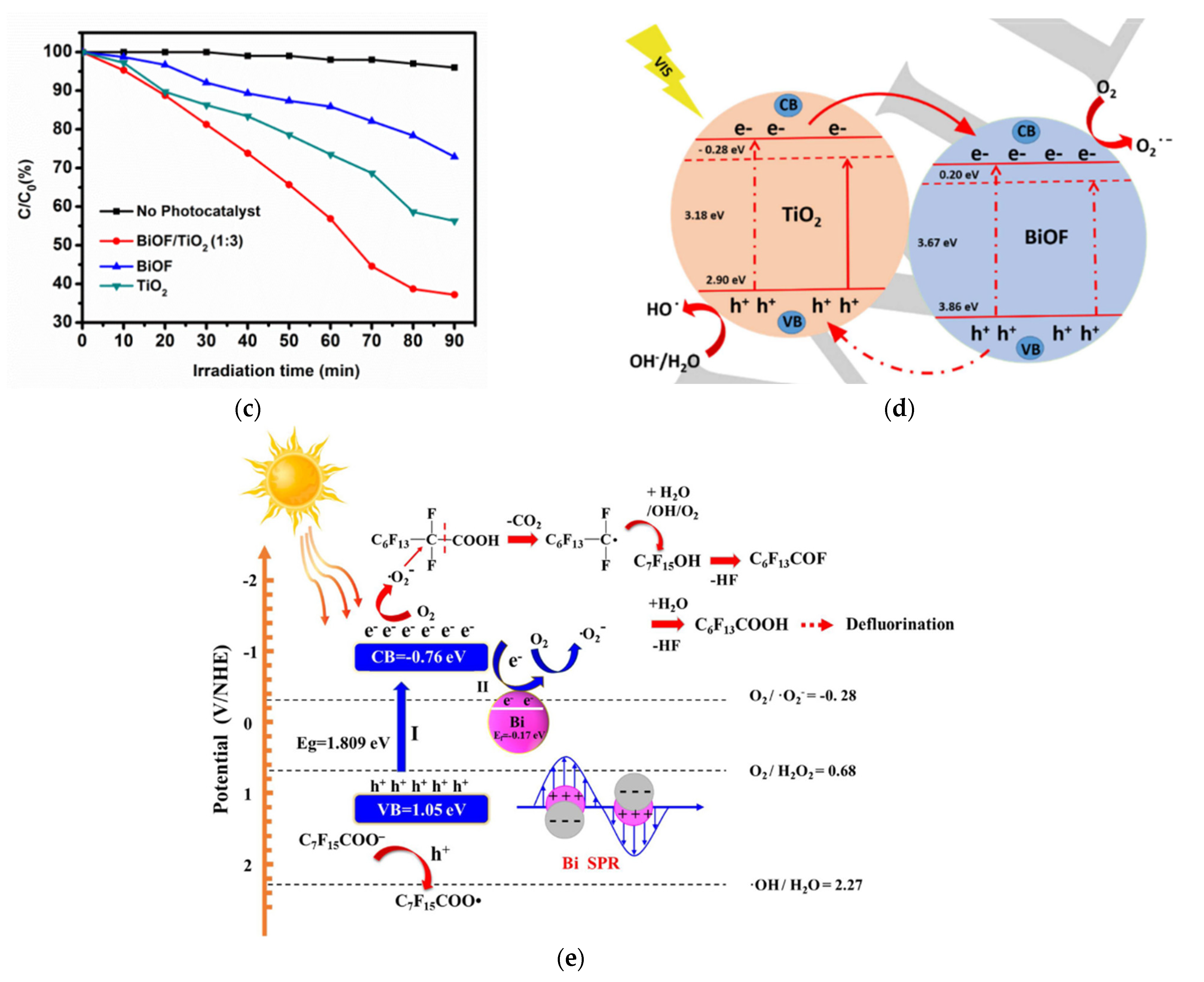
Nasr et al. synthesized a novel BiOF/TiO2 heterostructure and found that the obtained system had a band gap energy of 3.02 eV and could absorb more light and ensure charge separation efficiency (Figure 15c,d) [83]. Qiang et al. designed a series of BiOF/Bi2O3 heterojunction nanosheets and found that they could effectively separate electron–hole pairs and improve the photocatalytic activity [84]. Moreover, the construction of heterojunction structures with a narrower-band-gap semiconductor such as Ag2O strengthens the visible-light response, promotes the separation of the photogenerated charge carriers, and thus enhances the photocatalytic activity [85]. In addition, Hu et al. prepared BiOF/Bi2O3/RGO hybrid composites and found that the involvement of RGO increased the absorption of light, resulting from a smaller band gap energy [86]. Vadivel et al. fabricated a Ag-BiOF/g-C3N4 composite and found that the band gap decreased to 2.72 eV, greatly enhancing the absorption of light [87].
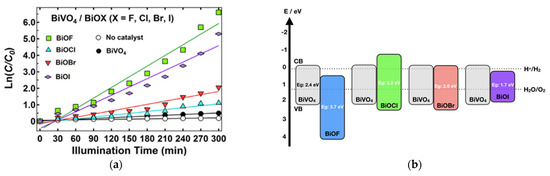
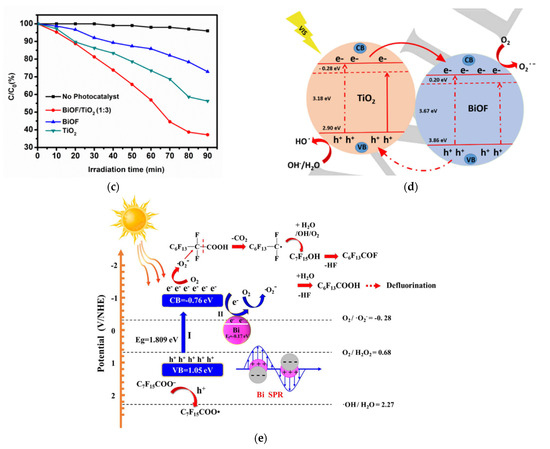
Figure 15.
(a) The kinetics of the photodegradation of methylene blue over the synthesized samples under visible light per area of particles. (b) The band positions of BiVO4, BiOF, BiOCl, BiOBr, and BiOI and the respective possible pn junctions [81]. (c) The photocatalytic degradation of methylene blue without a photocatalyst (black line) and in the presence of the photocatalysts BiOF (blue line), TiO2 (green line), and BiOF/TiO2 (1:3) (red line) under visible-light irradiation [83]. (d) The proposed mechanism for the photogenerated charge separation and migration process in BiOF/TiO2 heterostructures under visible-light irradiation. (e) The proposed photocatalytic degradation mechanism for Bi/BiOI1−xFx (x = 0.2) under visible-light irradiation [88].
Figure 15.
(a) The kinetics of the photodegradation of methylene blue over the synthesized samples under visible light per area of particles. (b) The band positions of BiVO4, BiOF, BiOCl, BiOBr, and BiOI and the respective possible pn junctions [81]. (c) The photocatalytic degradation of methylene blue without a photocatalyst (black line) and in the presence of the photocatalysts BiOF (blue line), TiO2 (green line), and BiOF/TiO2 (1:3) (red line) under visible-light irradiation [83]. (d) The proposed mechanism for the photogenerated charge separation and migration process in BiOF/TiO2 heterostructures under visible-light irradiation. (e) The proposed photocatalytic degradation mechanism for Bi/BiOI1−xFx (x = 0.2) under visible-light irradiation [88].
N. Ghayoumia et al. [89] developed BiOF@ZIF-8 (ZIF-8: (Zn(MeIM) 2; MeIM = imidazolate-2-methyl). This heterogeneous compound has been evaluated for the catalytic conversion of the alcoholic functions of benzylic alcohols into carbonyl groups. BiOF@ZIP-8 is very selective towards benzaldehyde (95%). This new catalyst is thus expected to be useful for green oxidation in organic synthesis and low-cost industrial chemical applications [89].
HF wet etching has also been reported as a technique that can improve the photocatalytic properties of bismuth-oxide-based glass thanks to heterojunction creation. Indeed, this type of etching allowed V.P. Singh and R. Vaish to form a BiO0.51F1.98 layer on SrO-Bi2O3-B2O3 glass ceramic and S. Kumar Sharma et al. to form a BiO0.1F2.8 layer on 2Bi2O3-Bi2O3 (BBO) glass. Both were tested for the photodegradation of methyl blue and Rhodamine 6G and showed an enhanced visible-light-driven photocatalytic activity compared to that of unetched glass ceramics [90,91].
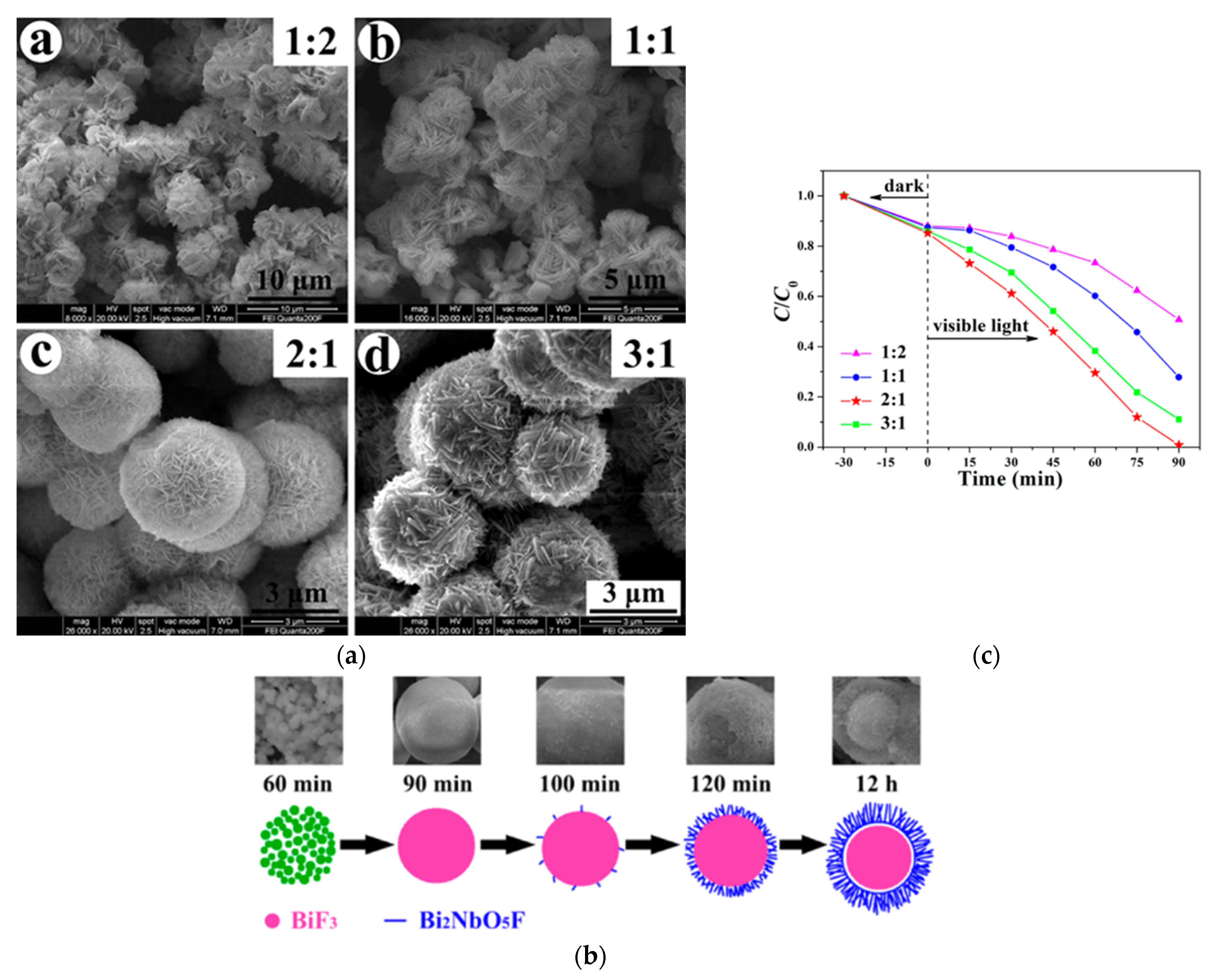
Heterojunctions may also be obtained by forming a shell of a Bi2NbO5F Aurivillius compound around a core of BiF3 nanoparticles through a molten salt method. As illustrated by Figure 16, depending on the time and the EG/H2O ratio, this core–shell structure can be controlled to accelerate the photodegradation of RhB under visible light [92].
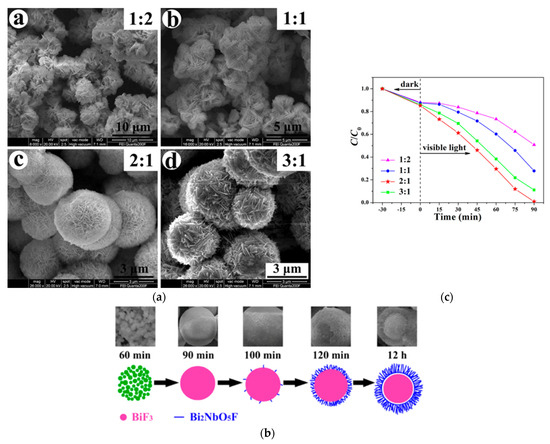
Figure 16.
(a) Morphological evolution as a function of the EG/H2O ratio for BiF3/Bi2NbO5F heterojunction formation; (b) the proposed mechanism of this synthesis; (c) the photocatalytic activity of these structures as a function of the EG/H2O ratio [92].
Thus, the formation of heterostructures with other semiconductors can improve both the light absorption range and the carrier separation efficiency, which enhance the photocatalytic activity further.
4.4.2. Other Bismuth Oxyhalides
The use of different bismuth halides for internal coupling not only can avoid the introduction of impurities but can also change the electronic properties, adjust the band gap energy, and enhance the photocatalytic activity. Wang et al. calculated the band gap of a BiOX/BiOY system using hybrid DFT and found out that the band gap appears between BiOX and BiOY [39]. Zhao et al. systematically studied the lattice constants, band gaps, and optical properties of a BiOX1−xYx solid solution, also using DFT calculations [93]. The results revealed that the visible-light response range of the BiOX1−xYx solid solution was wider and the electron–hole recombination rate was much lower [93].
Yan et al. synthesized BiOBr/BiOF hybrid materials and determined that the band gap was significantly narrowed and the photo-absorption and photocatalytic properties were significantly enhanced [94]. Cheng et al. prepared a BiOCl/BiOF composite photocatalyst and found that good band-matching between the oxygen vacancy states in BiOCl and BiOF resulted in effective separation of the electron–hole pairs [95]. A Bi7F11O5/BiOCl heterojunction was prepared using a simple hydrothermal method by Y. Kan et al. In comparison with pure BiOCl and Bi7O5F11, the composite showed an improved photocatalytic activity due to the separation and transfer of the charges by the heterojunction [96]. Guan et al. prepared BiO0.51F1.98 and calcination polycrystalline BiO0.51F1.98 with self-doped BiOF through solvothermal synthesis. This material showed enhanced photocatalytic activity, which was basically ascribed to the broadened visible-light absorption and lower recombination rate of the charge carriers [97]. Jingzhen Wang et al. synthesized Bi7O5F11/BiOF and demonstrated its very high performance for the photodegradation of perfluorooctanoic acid. The enhanced performance is attributed to the introduction of a built-in electric field into the S-scheme photocatalyst, providing a strong driving force for the reaction [98]. Jing-Ya Fu et al. prepared BiOmFn/BiOxIy/GO with different compositions. Their study noted the excellent photocatalytic activity regarding dye photodegradation of the Bi50O59F32/BiOI/GO composite. Yu-Yun Lin et al. synthesized various quaternary BiOF/BiOI/Bi26O38F2/g-C3N4 photocatalysts by varying the pH and temperature conditions to optimize their composition. The outstanding photocatalytic performance of BiOxFy/BiOpIq/g-C3N4 is mainly due to the formation of heterojunctions, which enhances the photocatalytic efficiency by reducing the recombination rate of the photogenerated electrons and holes. Additional factors contributing to its performance include the composite’s low-energy band structure, high BET surface area, and layered architecture [49,50].
Aurivillius compounds have also been associated with bismuth oxyhalides. Through hydrothermal synthesis, mixing oxides in a sealed gold tube in NH4F 1M medium at a high temperature (≥400 °C) and a high pressure (107 Pa), Bi2NbO5F and Bi2TiO4F2 phases have been obtained with a small amount of BiOF [99]. The hydrothermal method may also be used to grow Bi2TiO4F2 on a BiOBr (001) sheet and then produce a composite exhibiting a better photocatalytic performance for oxygen production due to the larger charge separation-induced polarization at the heterojunction than that in single materials [100].
4.4.3. Metal Nanoparticles
The integration of metallic nanoparticles, particularly those composed of noble metals such as Au, Ag, Pd, Ru, Rh, and Pt, has emerged as a powerful strategy for enhancing the performance of semiconductor-based photocatalysts. This enhancement is largely attributed to localized surface plasmon resonance (LSPR), a phenomenon wherein collective oscillations in the conduction electrons in metallic nanoparticles are excited by incident light, leading to significant amplification of the local electromagnetic field near the nanoparticle surface. This effect induces strong absorption and scattering of light in the visible range. This plasmonic effect also promotes the generation of energetic “hot electrons”. These hot electrons can be injected into the conduction band of the adjacent semiconductor, effectively boosting the charge carrier population and improving the photocatalytic efficiency [101,102]. Furthermore, the presence of metallic nanoparticles can modulate the local Fermi level equilibrium and facilitate the formation of heterojunctions, which enhance the charge separation and reduce electron–hole recombination [103,104]. In some systems, metallic nanoparticles serve a dual function by acting as cocatalysts, providing active sites for redox reactions [105]. Additionally, the incorporation of metallic nanoparticles into Z-scheme photocatalytic architectures allows for spatially separated oxidation and reduction reactions while maintaining strong redox potentials. Beyond purely electronic effects, the plasmonic heating induced by LSPR can also contribute to photothermal catalysis, where localized temperature rises at the nanoparticle–semiconductor interface accelerate the reaction kinetics and facilitate thermally activated surface processes [106]. Overall, the multifunctional roles of metallic nanoparticles in modifying the electronic structure, enhancing light–matter interactions, and facilitating the interfacial charge dynamics represent a promising approach to the design of new photocatalysts. S. Vadivel et al. synthesized both Ag-BiOF/g-C3N4 and BiOF/g-C3N4 through a solvothermal method and showed that the Ag-modified BiOF specimen had a significantly enhanced photocatalytic activity for the degradation of organic pollutants. The increased photocurrent measured confirms the improved separation of the photogenerated charges. Finally, the decreased band gap, to 2.72 eV, greatly enhanced the absorption of light [87].
The plasmon resonance of non-noble metals (Al, Cu, Mg, In, Ni, Ga, Co, Fe, Bi) has also been investigated and could be of interest due to their much lower manufacturing cost potential [107]. Metal Bi exhibits an SPR that is similar to that of noble metals, and recently, Bi nanoparticles have been used to upgrade the photocatalytic properties of many semiconductors, such as BiVO4, TiO2, and Bi2Sn2O7 [108,109,110]. L. Ai et al. prepared a Bi/BiOF/Bi2O2CO3 Z-scheme heterojunction. The enhanced photocatalytic activity for ciprofloxacin degradation can be attributed to use of metal Bi, which extends the light absorption range and whose SPR improves the rate of transfer of the photogenerated electron–hole pairs to the semiconductor’s surface. J. Wang et al. synthesized a Bi/BiOI1−xFx solid solution and demonstrated that compared with pure BiOI and BiOF, the band gap of the metal–oxyhalide compound was significantly reduced, thus promoting visible-light absorbance. Figure 15e shows the photocatalytic mechanism of perfluorooctanoic acid degradation [88]. S. Ibrahim et al. deposited BiO0.5F2 through reactive magnetron sputtering in different Ar/O2/CF4 gas mixtures. By modulating Rf (O2/(O2 + CF4)), it was possible to synthesize one-pot heterojunctions of BiO0.5F2/Bi with a controlled content of both phases. These thin films were tested for the photoconversion of CO2 into CO and compared with a Bi2O3/Bi heterojunction thin film, Bi2O3 powder, and BiOF powder. The BiO0.5F2/Bi heterojunction shows both the best photocatalyst yield and selectivity for CO [111,112].

Table 2.
Synthesis methods, precursors, and photocatalytic applications of BiOxF3−2x-based materials.
Table 2.
Synthesis methods, precursors, and photocatalytic applications of BiOxF3−2x-based materials.
| Materials | Synthesis Method | Precursors | Application | References |
|---|---|---|---|---|
| Ag-BiOF/g-C3N4 | Solvothermal | g-C3N4, Bi(NO3)3·5H2O, AgNO3, C6H12N4, NaF, C2H6O2 | Photodegradation of MB under visible light | [87] |
| Bi/BiOF/Bi2O2CO3 | Hydrothermal | Bi(NO3)3·5H2O, NaF, C2H6O2/H2O (1:1) | Photodegradation of ciprofloxacin under UV-Vis light | [112] |
| Bi/BiOI1−xFx | Hydrothermal | Bi(NO3)3·5H2O, NaF, KI, C2H6O2 | Photodecomposition of perfluorooctanoic acid | [88] |
| BiO0.5F2/Bi | Magnetron sputtering | Bi target, Ar/O2/CF4 atmosphere | Photodegradation of MO | [111] |
| Ag2O/BiOF | Solvothermal | Bi(NO3)3·5H2O, NH4F | Photodegradation of RhB under UV-Vis light | [85] |
| BiOF/Bi2O3/RGO | Precipitation/calcination/reduction | Bi(NO3)3·5H2O, NaF, GO | Photodegradation of RhB under natural sunlight | [86] |
| BiVO4/BiO0.67F1.66 | Two-step hydrothermal synthesis | Bi(NO3)3·5H2O, C2H6O2, NaVO3, NH4F | Photodegradation of RhB and phenol molecules | [82] |
| BiVO4/BiOF | Hydrothermal | Bi(NO3)3·5H2O, C2H6O2, Na3VO4, NH4F | Photodegradation of MB under visible light | [81] |
| BiOF/TiO2 | Solid-state and sintering | Bi2O3, HF, TiO2 | Photodegradation of RhB and MB under visible light | [83] |
| BiOF@ZIF-8 | Hydrothermal | Bi(NO3)3·5H2O, terephthalic acid, NH4F, HF, Zn(NO3)2·6H2O, H-MeIM, DMF, CHCl3, DCM | Oxidation of benzylalcohols | [89] |
| BiOF/Bi2O3 | Hydrothermal | Bi(NO3)3·5H2O, HF/NH3·H2O | Photodegradation of RhB under UV–visible light | [84] |
| BiO0.51F1.98 coated SrO-Bi2O3-B2O3 glass ceramic | Fluorination through controlled etching | SBBO, HF | Photodegradation of MB under visible light | [91] |
| BiO0.1F2.8 coated 2Bi2O3-Bi2O3 (BBO) glass | Wet etching | BBO, HF | Photodegradation of Rh6G dye | [90] |
| BiOBr/BiOF | Solvothermal | CTAB, NaF, HF, Bi(NO3)3·5H2O, C2H6O2 | Photodegradation of MB under visible light | [94] |
| BiOBr/BiOF | Hydrothermal | CTAB, NH4F, Bi(NO3)3·5H2O | Photodegradation of RhB and nitrobenzene under visible light | [113] |
| BiOCl/BiOF | Solvothermal | BiCl3, NH4F, Bi(NO3)3·5H2O, C4H10O2 | Photocatalytic degradation of methyl orange (MO) under UV–Vis.-light irradiation | [95] |
| BiO0.51F1.98/BiOF | Solvothermal | NaF, Bi(NO3)3·5H2O, C2H6O2 | MO degradation under light irradiation | [97] |
| Bi7O5F11/BiOF | Solvothermal | NH4F, Bi(NO3)3·5H2O, C2H6O2, H2O | Photodegradation of PFOA under UV-light irradiation | [98] |
| Bi7F11O5/BiOCl | Hydrothermal | BiOCl, NH4F, C2H6O/H2O | Photodegradation of MO under UV light | [96] |
| BiOmFn/BiOxIy/GO | Hydrothermal | C Graphite, NaNO3, H2SO4., KMnO4, Bi(NO3)3·5H2O, NaOH, KI, KF | Photodegradation of CV and HBA under visible light | [51] |
| BiOF/BiOI/Bi26O38F2/g-C3N4 | Hydrothermal | Melamine, Bi(NO3)3·5H2O, NaOH, HNO3, KF, KI | Photodegradation of CV under visible light and photoconversion of CO2 into CH4 under visible light | [49] |
| Bi2TiO4F2/BiOBr | Solvothermal | Bi(NO3)3·5H2O, (NH4)2TiF6, C2H6O2 | Photocatalytic O2 evolution | [100] |
| BiF3/Bi2NbO5F | Solvothermal | Bi(NO3)3·5H2O, Nb2O5, C2H6O2 | Photodegradation of RhB under visible-light irradiation | [92] |
4.5. The Use of Sensitizers
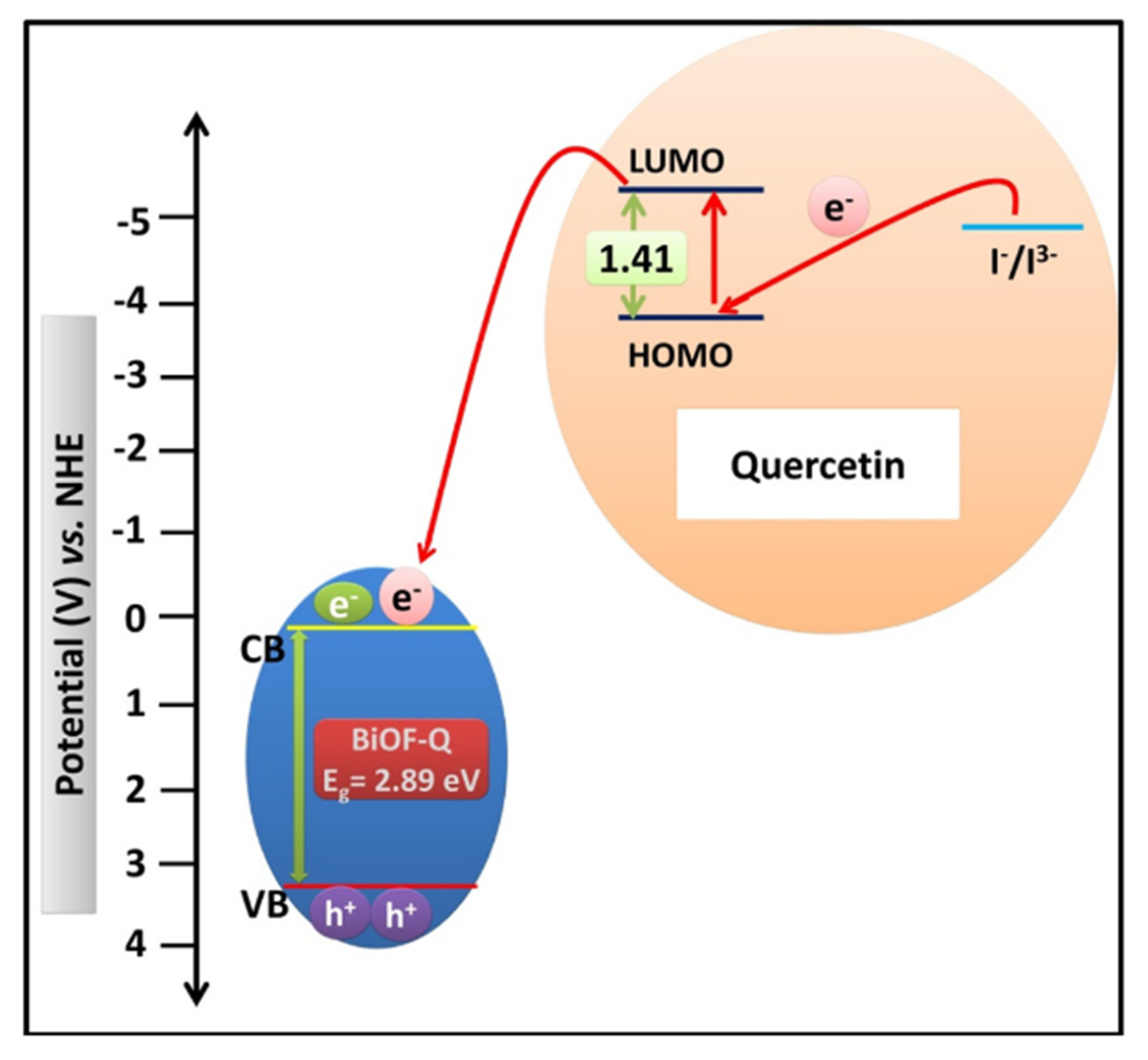
The use of dye sensitizers can increase the absorption of visible light and enrich the photogeneration of electric charge carriers. Yadav et al. synthesized quercetin-sensitized BiOF nanostructures using a non-toxic and economical route. The electron acceptor properties of quercetin promoted the charge injection into BiOF, as shown in Figure 17, resulting in higher light absorption, a narrower BiOF band gap, and a larger specific surface area [114].
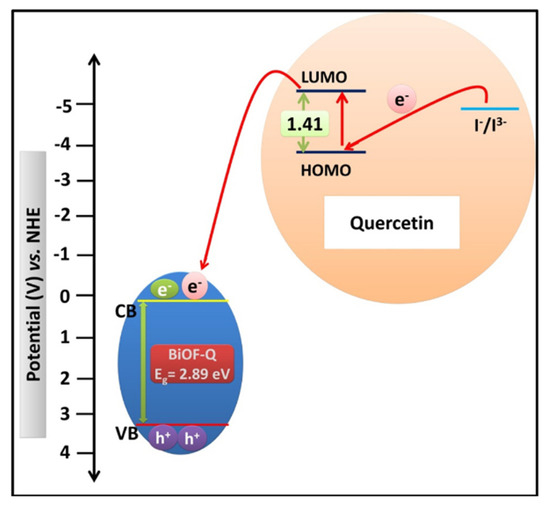
Figure 17.
A schematic illustration of an energy-level diagram displaying the injection of electron from the quercetin molecule into the CB of BiOF-Q and the subsequent regeneration of the quercetin molecule [114].
5. Conclusions
This paper focuses on bismuth-based oxyfluorides as emergent photocatalysts, highlighting their potential to address energy shortages and environmental pollution through photocatalysis. While titanium dioxide (TiO2) has been widely used, it has disadvantages such as low sunlight utilization and quantum efficiency. Recently, bismuth-based materials have garnered significant attention. Bismuth oxyfluoride (BiOF) stands out among these due to its unique layered structure, the high electronegativity of fluorine (which enhances the photocatalytic efficiency by trapping electrons and influencing the electron distribution), and its direct band gap, which facilitates efficient light absorption.
Beyond pure BiOF, BiOxF3−2x compounds with finely varied stoichiometries have started to be investigated, as their O/F ratios influence oxygen defects and photocatalytic activity. Furthermore, Aurivillius bismuth oxyfluorides, which present layered perovskite-like structures, are gaining attention due to their unique electronic functionalities and ferroelectric properties, making them promising for photocatalytic applications. This large variety of compositions allows them to adapt to a targeted redox potential for a specific photocatalytic application.
Moreover, several strategies can be explored for enhancing the photocatalytic activity of these pure materials. First, the facet selection and oxygen vacancies play a critical role, as oxygen vacancies can improve electron–hole separation and enhance light absorption. Metal/non-metal doping is also a common approach to adjusting the band gap and accelerating the charge separation in a photocatalyst. Rare earth ions (e.g., Sm3+, Y3+, Er3+/Yb3+, Eu3+) and noble metals (e.g., Ag, Pd) were demonstrated to be effective dopants for enhancing the photocatalytic activity under visible or NIR light. Moreover, heterostructure formation with other semiconductors (e.g., TiO2, BiVO4, Bi2O3, g-C3N4), other bismuth-based catalysts (e.g., BiOBr, BiOCl), and metal nanoparticles (e.g., Ag, Bi) is a highly effective strategy for reducing electron–hole recombination and extending light absorption. Finally, a few papers have indicated the use of sensitizers, such as quercetin, which can also significantly increase visible-light absorption and charge carrier photogeneration.
Environmental remediation and solar fuel production are then two important applications of photocatalysis that can be addressed by BiOF and its derivatives. In addition, theoretical calculations should be considered in future studies, as they could provide fundamental insights into the photocatalytic mechanisms of bismuth oxyfluorides and guide the rational design of more efficient materials. However, from a practical point of view, it remains difficult to recycle a powder catalyst from liquid and requires the addition of a filtration step. The use of thin films could overcome this problem and lead to reusable and ecofriendly systems. However, up to now, no studies concerning BiOF-based thin films have been published, and very few on BiOxF3−2x materials have been published.
Funding
This work benefited from aid from the French state managed by the Agence Nationale de la Recherche under the France 2030 plan, bearing reference code ANR-22-PESP-0010: Projet ciblé “POWERCO2”, within the PEPR project SPLEEN.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is contained within the article.
Acknowledgments
The authors thank Sujuan Wu at the College of Materials Science and Engineering, Chongqing University, for fruitful discussions with her during the writing of this review. The authors also thank the International Research Center IMOBS3 “Innovative Mobility: Smart and Sustainable Solutions” for its support of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.K.; Kaur, N.; Sandhu, S.; Shahi, J.S.; Singh, V. Influences of a New Templating Agent on the Synthesis of Coral-like TiO2 Nanoparticles and Their Photocatalytic Activity. J. Sci. Adv. Mater. Devices 2017, 2, 347–353. [Google Scholar] [CrossRef]
- Kim, J.H.; Noh, B.H.; Lee, G.-D.; Hong, S.-S. Hydrothermal Synthesis of Titanium Dioxide Using Acidic Peptizing Agents and Their Photocatalytic Activity. Korean J. Chem. Eng. 2005, 22, 370–374. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New Understanding of the Difference of Photocatalytic Activity among Anatase, Rutile and Brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent Advances and Applications of Semiconductor Photocatalytic Technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Shi, H.; Ming, W.; Du, M.-H. Bismuth Chalcohalides and Oxyhalides as Optoelectronic Materials. Phys. Rev. B 2016, 93, 104108. [Google Scholar] [CrossRef]
- Łaczka, M.; Stoch, L.; Górecki, J. Bismuth-Containing Glasses as Materials for Optoelectronics. J. Alloys Compd. 1992, 186, 279–291. [Google Scholar] [CrossRef]
- Sears, W.M. The Gas-Sensing Properties of Sintered Bismuth Iron Molybdate Catalyst. Sens. Actuators 1989, 19, 351–370. [Google Scholar] [CrossRef]
- Poghossian, A.; Abovian, H.; Avakian, P.; Mkrtchian, S.; Haroutunian, V. Bismuth Ferrites: New Materials for Semiconductor Gas Sensors. Sens. Actuators B Chem. 1991, 4, 545–549. [Google Scholar] [CrossRef]
- Ratova, M.; Marcelino, R.B.P.; De Souza, P.P.; Amorim, C.C.; Kelly, P.J. Reactive Magnetron Sputter Deposition of Bismuth Tungstate Coatings for Water Treatment Applications under Natural Sunlight. Catalysts 2017, 7, 283. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Bismuth-Based Photocatalytic Semiconductors: Introduction, Challenges and Possible Approaches. J. Mol. Catal. A Chem. 2016, 423, 533–549. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, C.; Huang, R.; Peng, H.; Tang, X. Investigation of Optical and Photocatalytic Properties of Bismuth Nanospheres Prepared by a Facile Thermolysis Method. J. Phys. Chem. C 2014, 118, 1155–1160. [Google Scholar] [CrossRef]
- Iyyapushpam, S.; Nishanthi, S.T.; Pathinettam Padiyan, D. Synthesis of Room Temperature Bismuth Oxide and Its Photocatalytic Activity. Mater. Lett. 2012, 86, 25–27. [Google Scholar] [CrossRef]
- Jiang, H.; Endo, H.; Natori, H.; Nagai, M.; Kobayashi, K. Fabrication and Photoactivities of Spherical-Shaped BiVO4 Photocatalysts through Solution Combustion Synthesis Method. J. Eur. Ceram. Soc. 2008, 28, 2955–2962. [Google Scholar] [CrossRef]
- Bi, J.; Wu, L.; Li, J.; Li, Z.; Wang, X.; Fu, X. Simple Solvothermal Routes to Synthesize Nanocrystalline Bi2MoO6 Photocatalysts with Different Morphologies. Acta Mater. 2007, 55, 4699–4705. [Google Scholar] [CrossRef]
- Wu, L.; Bi, J.; Li, Z.; Wang, X.; Fu, X. Rapid Preparation of Bi2WO6 Photocatalyst with Nanosheet Morphology via Microwave-Assisted Solvothermal Synthesis. Catal. Today 2008, 131, 15–20. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Dai, Y. Engineering BiOX (X = Cl, Br, I) Nanostructures for Highly Efficient Photocatalytic Applications. Nanoscale 2014, 6, 2009–2026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, L.; Zhou, Z. First-Principles Studies on Facet-Dependent Photocatalytic Properties of Bismuth Oxyhalides (BiOXs). RSC Adv. 2012, 2, 9224–9229. [Google Scholar] [CrossRef]
- Hu, J.; Weng, S.; Zheng, Z.; Pei, Z.; Huang, M.; Liu, P. Solvents Mediated-Synthesis of BiOI Photocatalysts with Tunable Morphologies and Their Visible-Light Driven Photocatalytic Performances in Removing of Arsenic from Water. J. Hazard. Mater. 2014, 264, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Zhang, J.; Miao, M.; Jin, Y. Solvothermal Synthesis of Flower-like BiOBr Microspheres with Highly Visible-Light Photocatalytic Performances. Appl. Catal. B Environ. 2012, 111–112, 334–341. [Google Scholar] [CrossRef]
- Wang, Q.; Hui, J.; Huang, Y.; Ding, Y.; Cai, Y.; Yin, S.; Li, Z.; Su, B. The Preparation of BiOCl Photocatalyst and Its Performance of Photodegradation on Dyes. Mater. Sci. Semicond. Process. 2014, 17, 87–93. [Google Scholar] [CrossRef]
- Li, G.; Jiang, B.; Xiao, S.; Lian, Z.; Zhang, D.; Yu, J.C.; Li, H. An Efficient Dye-Sensitized BiOCl Photocatalyst for Air and Water Purification under Visible Light Irradiation. Environ. Sci. Process. Impacts 2014, 16, 1975–1980. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, P.; Huang, B.; Dai, Y.; Qin, X.; Zhang, X.; Wang, Z.; Liu, Y. Enhancing Charge Separation in Photocatalysts with Internal Polar Electric Fields. ChemPhotoChem 2017, 1, 136–147. [Google Scholar] [CrossRef]
- Guo, Y.; Shi, W.; Zhu, Y. Internal Electric Field Engineering for Steering Photogenerated Charge Separation and Enhancing Photoactivity. EcoMat 2019, 1, e12007. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Q.; Zhong, J.; Li, J.; Huang, S.; Li, X. PVA-Assisted Hydrothermal Preparation of BiOF with Remarkably Enhanced Photocatalytic Performance. Mater. Lett. 2017, 201, 35–38. [Google Scholar] [CrossRef]
- Zou, S.; Teng, F.; Chang, C.; Liu, Z.; Wang, S. Controllable Synthesis of Uniform BiOF Nanosheets and Their Improved Photocatalytic Activity by an Exposed High-Energy (002) Facet and Internal Electric Field. RSC Adv. 2015, 5, 88936–88942. [Google Scholar] [CrossRef]
- Zhang, A.; Teng, F.; Zhai, Y.; Liu, Z.; Hao, W.; Liu, Z.; Yang, Z.; Gu, W. Controllable Synthesis and Efficient Photocatalytic Activity of BiOF Nanodisks Exposed with {101} Facets, Instead of {001} Facets. J. Alloys Compd. 2019, 794, 127–136. [Google Scholar] [CrossRef]
- Xiong, J.; Song, P.; Di, J.; Li, H. Bismuth-Rich Bismuth Oxyhalides: A New Opportunity to Trigger High-Efficiency Photocatalysis. J. Mater. Chem. A 2020, 8, 21434–21454. [Google Scholar] [CrossRef]
- Ye, L.; Su, Y.; Jin, X.; Xie, H.; Zhang, C. Recent Advances in BiOX (X = Cl, Br and I) Photocatalysts: Synthesis, Modification, Facet Effects and Mechanisms. Environ. Sci. Nano 2014, 1, 90–112. [Google Scholar] [CrossRef]
- Jin, X.; Ye, L.; Xie, H.; Chen, G. Bismuth-Rich Bismuth Oxyhalides for Environmental and Energy Photocatalysis. Coord. Chem. Rev. 2017, 349, 84–101. [Google Scholar] [CrossRef]
- Vinoth, S.; Pandikumar, A. Recent Advances in Bismuth Oxyfluoride-Based Photocatalysts for Energy and Environmental Remediation. Mater. Today Chem. 2024, 36, 101924. [Google Scholar] [CrossRef]
- Aurivillius, B.; Lundqvist, T. X-Ray Studies of the System BiF3-Bi2O3. II. A Bismuth Oxide Fluoride of Defective Tysonite Type. Acta Chem. Scand. 1955, 9, 1209–1212. [Google Scholar] [CrossRef]
- Aurivillius, B. The Crystal Structure of Bismuth Oxide Fluoride. Acta Chem. Scand. 1964, 18, 1823–1830. [Google Scholar] [CrossRef]
- Aurivillius, B. X-Ray Studies on the System BiF3–Bi2O3 I. Preliminary Phase Analysis and a Note on the Structure of BiF3. Acta Chem. Scand. (Den.) Divid. Acta Chem. Scand. Ser. A Ser. B 1955, 9, 7. [Google Scholar] [CrossRef]
- Morell, A.; Tanguy, B.; Portier, J. Le Système Bi2O3–BiF3. Bull. Société Chim. Fr. 1971, 7, 2502–2504. [Google Scholar]
- Chen, X.; Chen, P.; Yang, S.; Gao, H. Recent Advances in Bismuth Oxyhalides Photocatalysts and Their Applications. Nanotechnology 2022, 34, 052001. [Google Scholar] [CrossRef]
- Ren, X.; Gao, M.; Zhang, Y.; Zhang, Z.; Cao, X.; Wang, B.; Wang, X. Photocatalytic Reduction of CO2 on BiOX: Effect of Halogen Element Type and Surface Oxygen Vacancy Mediated Mechanism. Appl. Catal. B Environ. 2020, 274, 119063. [Google Scholar] [CrossRef]
- Huang, W.L. Electronic Structures and Optical Properties of BiOX (X = F, Cl, Br, I) via DFT Calculations. J. Comput. Chem. 2009, 30, 1882–1891. [Google Scholar] [CrossRef]
- Wang, G.; Luo, X.; Huang, Y.; Kuang, A.; Yuan, H.; Chen, H. BiOX/BiOY (X, Y = F, Cl, Br, I) Superlattices for Visible Light Photocatalysis Applications. RSC Adv. 2016, 6, 91508–91516. [Google Scholar] [CrossRef]
- Erbland, T.; Ibrahim, S.; Monier, G.; Bonnet, P.; Bousquet, A. On the Identification of BiOF, Bi7O5F11and β-BiO0.5F2 Bismuth Oxyfluorides. 2025. Available online: https://hal.science/hal-05261692v1 (accessed on 3 September 2025).
- Li, S.; Zhang, C.; Min, F.; Dai, X.; Pan, C.; Cheng, W. Electronic Structural Properties of BiOF Crystal and Its Oxygen Vacancy from First-Principles Calculations. Russ. J. Phys. Chem. 2017, 91, 2425–2430. [Google Scholar] [CrossRef]
- Laval, J.P.; Champarnaud-Mesjard, J.-C.; Frit, B.; Britel, A.; Mikou, A. Bi7F11O5: A New Ordered Anion-Excess Fluorite-Related Structure with Columnar Clusters. Eur. J. Solid State Inorg. Chem. 1994, 31, 943–956. [Google Scholar] [CrossRef]
- Hu, W.; Shan, P.; Sun, T.; Liu, H.; Zhang, J.; Liu, X.; Kong, Y.; Xu, J. Preparation, Nonlinear Optical Properties, and Theoretical Analysis of the Non-Centrosymmetric Bismuth Oxyfluoride, Bi7F11O5. J. Alloys Compd. 2016, 658, 788–794. [Google Scholar] [CrossRef]
- Zhan, J.; Lei, Z.; Zhang, Y. Influence of Graphene Oxide Surface on the Synthesis and Structure of BiOxFy at Ambient Conditions. Adv. Mater. Interfaces 2022, 9, 2102042. [Google Scholar] [CrossRef]
- Ren, M.; Teng, F.; Yang, Y.; Zhai, Y.; Gu, W.; Liu, Z.; Liu, Z.; Teng, Y. Influence of O/F Ratio on Oxygen Defect and Photochemical Properties of BixOyFz. Mater. Des. 2017, 131, 402–409. [Google Scholar] [CrossRef]
- Laval, J.P.; Champarnaud-Mesjard, J.C.; Britel, A.; Mikou, A. Bi(F, O)2.45: An Anion-Excess Fluorite Defect Structure Deriving from Rhombohedral LnFO Type. J. Solid State Chem. 1999, 146, 51–59. [Google Scholar] [CrossRef]
- Gu, W.; Zhang, G.; Teng, F.; Teng, Y.; Zhao, Z.; Fan, W. Effect of F/O Atomic Ratio on Photocatalytic Activity of BixOyFz. Chem. Phys. Lett. 2016, 659, 221–224. [Google Scholar] [CrossRef]
- Wang, M.; Huang, Q.-L.; Chen, X.-T.; You, X.-Z. Room Temperature Synthesis of Bismuth Oxyfluoride Nanosheets and Nanorods. Mater. Lett. 2007, 61, 4666–4669. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Hung, K.-Y.; Liu, F.-Y.; Dai, Y.-M.; Lin, J.-H.; Chen, C.-C. Photocatalysts of Quaternary Composite, Bismuth Oxyfluoride/Bismuth Oxyiodide/Graphitic Carbon Nitride: Synthesis, Characterization, and Photocatalytic Activity. Mol. Catal. 2022, 528, 112463. [Google Scholar] [CrossRef]
- Chen, C.-C.; Fu, J.-Y.; Chang, J.-L.; Huang, S.-T.; Yeh, T.-W.; Hung, J.-T.; Huang, P.-H.; Liu, F.-Y.; Chen, L.-W. Bismuth Oxyfluoride/Bismuth Oxyiodide Nanocomposites Enhance Visible-Light-Driven Photocatalytic Activity. J. Colloid Interface Sci. 2018, 532, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.-Y.; Chen, L.-W.; Dai, Y.-M.; Liu, F.-Y.; Huang, S.-T.; Chen, C.-C. BiOmFn/BiOxIy/GO Nanocomposites: Synthesis, Characterization, and Photocatalytic Activity. Mol. Catal. 2018, 455, 214–223. [Google Scholar] [CrossRef]
- Moure, A. Review and Perspectives of Aurivillius Structures as a Lead-Free Piezoelectric System. Appl. Sci. 2018, 8, 62. [Google Scholar] [CrossRef]
- Giddings, A.T.; Scott, E.A.S.; Stennett, M.C.; Apperley, D.C.; Greaves, C.; Hyatt, N.C.; McCabe, E.E. Symmetry and the Role of the Anion Sublattice in Aurivillius Oxyfluoride Bi2TiO4F2. Inorg. Chem. 2021, 60, 14105–14115. [Google Scholar] [CrossRef]
- McDowell, N.A.; Knight, K.S.; Lightfoot, P. Unusual High-Temperature Structural Behaviour in Ferroelectric Bi2WO6. Chem.—Eur. J. 2006, 12, 1493–1499. [Google Scholar] [CrossRef]
- Akopjan, A.V.; Serov, T.V.; Dolgikh, V.A.; Ardaschnikova, E.I.; Lightfoot, P. A New Anion Conductive Bismuth–Vanadium Oxyfluoride. J. Mater. Chem. 2002, 12, 1490–1494. [Google Scholar] [CrossRef]
- Needs, R.L.; Dann, S.E.; Weller, M.T.; Cherryman, J.C.; Harris, R.K. The Structure and Oxide/Fluoride Ordering of the Ferroelectrics Bi2TiO4F2 and Bi2NbO5F. J. Mater. Chem. 2005, 15, 2399–2407. [Google Scholar] [CrossRef]
- Wang, S.; Huang, B.; Wang, Z.; Liu, Y.; Wei, W.; Qin, X.; Zhang, X.; Dai, Y. A New Photocatalyst: Bi2TiO4F2 Nanoflakes Synthesized by a Hydrothermal Method. Dalton Trans. 2011, 40, 12670–12675. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Wang, K.; Gong, J.; Li, Y.; Zhang, G. Controlled Synthesis of Bi2NbO5F Plates with Exposed {010} Facet by Molten Salt Method and Their Photocatalytic Mechanism Insights. J. Alloys Compd. 2019, 776, 586–593. [Google Scholar] [CrossRef]
- Asahi, R.; Taga, Y.; Mannstadt, W.; Freeman, A.J. Electronic and Optical Properties of Anatase TiO2. Phys. Rev. B 2000, 61, 7459–7465. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Liu, J.; Cheng, K.; Sun, W.; Lin, J. Enhanced Visible Light Photocatalysis of Bi2O3 upon Fluorination. J. Phys. Chem. C 2013, 117, 20029–20036. [Google Scholar] [CrossRef]
- Gmitter, A.J.; Halajko, A.; Sideris, P.J.; Greenbaum, S.G.; Amatucci, G.G. Subsurface Diffusion of Oxide Electrolyte Decomposition Products in Metal Fluoride Nanocomposite Electrodes. Electrochim. Acta 2013, 88, 735–744. [Google Scholar] [CrossRef]
- Wei, D.; Huang, Y.; Seo, H.J. Eu3+-Doped Bi7O5F11 Microplates with Simultaneous Luminescence and Improved Photocatalysis. APL Mater. 2020, 8, 081109. [Google Scholar] [CrossRef]
- Vadivel, S.; Paul, B.; Maruthamani, D.; Kumaravel, M.; Vijayaraghavan, T.; Hariganesh, S.; Pothu, R. Synthesis of Yttrium Doped BiOF/RGO Composite for Visible Light: Photocatalytic Applications. Mater. Sci. Energy Technol. 2019, 2, 112–116. [Google Scholar] [CrossRef]
- Saraf, R.; Shivakumara, C.; Behera, S.; Dhananjaya, N.; Nagabhushana, H. Synthesis of Eu3+-Activated BiOF and BiOBr Phosphors: Photoluminescence, Judd–Ofelt Analysis and Photocatalytic Properties. RSC Adv. 2015, 5, 9241–9254. [Google Scholar] [CrossRef]
- Ren, C.; Ni, W.; Li, H. Recent Progress in Electrocatalytic Reduction of CO2. Catalysts 2023, 13, 644. [Google Scholar] [CrossRef]
- Lei, S.; Gao, X.; Cheng, D.; Fei, L.; Lu, W.; Zhou, J.; Xiao, Y.; Cheng, B.; Wang, Y.; Huang, H. A Novel Hierarchically Porous Hollow Structure of Layered Bi2TiO4F2 for Efficient Photocatalysis. Eur. J. Inorg. Chem. 2024, 2017, 1892–1899. [Google Scholar] [CrossRef]
- Lei, S.; Cheng, D.; Gao, X.; Fei, L.; Lu, W.; Zhou, J.; Xiao, Y.; Cheng, B.; Wang, Y.; Huang, H. A New Low-Temperature Solution Route to Aurivillius-Type Layered Oxyfluoride Perovskites Bi2MO5F (M = Nb, Ta) as Photocatalysts. Appl. Catal. B Environ. 2017, 205, 112–120. [Google Scholar] [CrossRef]
- Yin, X.; Li, Y.; Yang, D.; Shen, J.; Han, W.; Zhang, M.; Zhuang, M.; Tang, X.; Gao, R.; Li, X.; et al. Gentle Preparation of Layered Perovskite Oxyfluoride Nanosheets and Exploration of Excellent Catalytic Activity. Surf. Interfaces 2025, 58, 105898. [Google Scholar] [CrossRef]
- Mitoudi Vagourdi, E.; Müllner, S.; Lemmens, P.; Kremer, R.K.; Johnsson, M. Synthesis and Characterization of the Aurivillius Phase CoBi2O2F4. Inorg. Chem. 2018, 57, 9115–9121. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Mitoudi-Vagourdi, E.; Johnsson, M. The Aurivillius Compound CoBi2O2F4—An Efficient Catalyst for Electrolytic Water Oxidation after Liquid Exfoliation. ChemCatChem 2019, 11, 6105–6110. [Google Scholar] [CrossRef]
- Sano, M.; Kamigaito, A.; Wakayama, Y.; Shigematsu, K.; Katayama, T.; Hirose, Y.; Chikamatsu, A. Topochemical Fluorination of Epitaxial Thin Films of Barium-Doped Bismuth Iron Oxyfluoride. Cryst. Growth Des. 2024, 24, 9344–9349. [Google Scholar] [CrossRef]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial Carbon Dioxide Capture and Utilization: State of the Art and Future Challenges. Chem. Soc. Rev. 2024, 49, 8584–8686. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Y.; Yu, Y.; Zhang, B. Oxygen Vacancy Engineering in Photocatalysis. Solar RRL 2020, 4, 2000037. [Google Scholar] [CrossRef]
- Mehtab, A.; Ahmed, J.; Alshehri, S.M.; Mao, Y.; Ahmad, T. Rare Earth Doped Metal Oxide Nanoparticles for Photocatalysis: A Perspective. Nanotechnology 2022, 33, 142001. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ran, W.; Du, P.; Li, W.; Luo, L.; Wang, D. Enhanced Visible Light-Driven Photocatalytic Activities and Photoluminescence Characteristics of BiOF Nanoparticles Determined via Doping Engineering. Inorg. Chem. 2020, 59, 11801–11813. [Google Scholar] [CrossRef]
- Du, P.; Ran, W.; Wang, C.; Luo, L.; Li, W. Facile Realization of Boosted Near-Infrared-Visible Light Driven Photocatalytic Activities of BiOF Nanoparticles through Simultaneously Exploiting Doping and Upconversion Strategy. Adv. Mater. Interfaces 2021, 8, 2100749. [Google Scholar] [CrossRef]
- Zhou, S.; Shi, T.; Chen, Z.; Kilin, D.S.; Shui, L.; Jin, M.; Yi, Z.; Yuan, M.; Li, N.; Yang, X.; et al. First-Principles Study of Optoelectronic Properties of the Noble Metal (Ag and Pd) Doped BiOX (X = F, Cl, Br, and I) Photocatalytic System. Catalysts 2019, 9, 198. [Google Scholar] [CrossRef]
- Elias, M.; Alam, R.; Sarker, S.; Hossain, M.A. Fabrication of Ag-Doped BiOF-Reduced Graphene Oxide Composites for Photocatalytic Elimination of Organic Dyes. Heliyon 2024, 10, e34921. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Garlisi, C.; Guan, Q.; Anwer, S.; Al-Ali, K.; Palmisano, G.; Zheng, L. A Review of Material Aspects in Developing Direct Z-Scheme Photocatalysts. Mater. Today 2021, 47, 75–107. [Google Scholar] [CrossRef]
- Wang, X.; Sayed, M.; Ruzimuradov, O.; Zhang, J.; Fan, Y.; Li, X.; Bai, X.; Low, J. A Review of Step-Scheme Photocatalysts. Appl. Mater. Today 2022, 29, 101609. [Google Scholar] [CrossRef]
- Razavi-Khosroshahi, H.; Mohammadzadeh, S.; Hojamberdiev, M.; Kitano, S.; Yamauchi, M.; Fuji, M. BiVO4/BiOX (X = F, Cl, Br, I) Heterojunctions for Degrading Organic Dye under Visible Light. Adv. Powder Technol. 2019, 30, 1290–1296. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, X.; Chen, L. BiVO4/BiO0.67F1.66 Heterojunction Enhanced Charge Carrier Separation to Boost Photocatalytic Activity. J. Nanopart. Res. 2019, 21, 61. [Google Scholar] [CrossRef]
- Nasr, M.; Huang, W.; Bittencourt, C.; Cui, D.; Sun, Y.; Wang, L.; Caperaa, N.G.; Ning, Y.; Song, P.; Bonnet, P.; et al. Synthesis of BiOF/TiO2 Heterostructures and Their Enhanced Visible-Light Photocatalytic Activity. Eur. J. Inorg. Chem. 2020, 2020, 253–260. [Google Scholar] [CrossRef]
- Qiang, Z.; Zhu, S.; Li, T.; Li, F. Excellent Photo- and Sono- Catalytic BiOF/Bi2O3 Heterojunction Nanoflakes Synthesized via pH-Dependent and Ionic Liquid Assisted Solvothermal Method. Mater. Today Commun. 2020, 23, 100980. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Q.; Zhong, J.; Huang, S.; Li, J.; Song, J.; Burda, C. Enhanced Photocatalytic Performance of Ag2O/BiOF Composite Photocatalysts Originating from Efficient Interfacial Charge Separation. Appl. Surf. Sci. 2017, 416, 666–671. [Google Scholar] [CrossRef]
- Hu, L.; Dong, S.; Li, Q.; Feng, J.; Pi, Y.; Liu, M.; Sun, J.; Sun, J. Facile Synthesis of BiOF/Bi2O3/Reduced Graphene Oxide Photocatalyst with Highly Efficient and Stable Natural Sunlight Photocatalytic Performance. J. Alloys Compd. 2015, 633, 256–264. [Google Scholar] [CrossRef]
- Vadivel, S.; Kamalakannan, V.P.; Kavitha, N.P.; Santhoshini Priya, T.; Balasubramanian, N. Development of Novel Ag Modified BiOF Squares/g-C3N4 Composite for Photocatalytic Applications. Mater. Sci. Semicond. Process. 2016, 41, 59–66. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Cao, C.; Zhang, Y.; Zhang, Y.; Zhu, L. Decomposition of Highly Persistent Perfluorooctanoic Acid by Hollow Bi/BiOI1−xFx: Synergistic Effects of Surface Plasmon Resonance and Modified Band Structures. J. Hazard. Mater. 2021, 402, 123459. [Google Scholar] [CrossRef]
- Ghayoumian, N.; Aliyan, H.; Fazaeli, R. A Novel Nano-Cotton-like Bismuth Oxyfluoride (NC-BiOF) and a Novel Nanosheet Heterogeneous Compound BiOF@ZIF-8 as Catalyst for the Selective and Green Oxidation of Benzylic Alcohols. J. Chin. Chem. Soc. 2019, 66, 363–370. [Google Scholar] [CrossRef]
- Sharma, S.K.; Singh, V.P.; Chauhan, V.S.; Kushwaha, H.S.; Vaish, R. Photocatalytic Active Bismuth Fluoride/Oxyfluoride Surface Crystallized 2Bi2O3-B2O3 Glass–Ceramics. J. Electron. Mater. 2018, 47, 3490–3496. [Google Scholar] [CrossRef]
- Singh, V.P.; Vaish, R. Controlled Crystallization of Photocatalytic Active Bismuth Oxyfluoride/Bismuth Fluoride on SrO-Bi2O3-B2O3transparent Glass Ceramic. J. Eur. Ceram. Soc. 2018, 38, 3635–3642. [Google Scholar] [CrossRef]
- Lei, S.; Wang, C.; Cheng, D.; Gao, X.; Chen, L.; Yan, Y.; Zhou, J.; Xiao, Y.; Cheng, B. Hierarchical BiF3–Bi2NbO5F Core–Shell Structure and Its Application in the Photosensitized Degradation of Rhodamine B under Visible Light Irradiation. J. Phys. Chem. C 2015, 119, 502–511. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Liu, Q.-L.; Dai, W.-W. Structural, Electronic and Optical Properties of BiOX1−xYx (X, Y = F, Cl, Br and I) Solid Solutions from DFT Calculations. Sci. Rep. 2016, 6, 31449. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Feng, H.; Hu, M.; Huang, F.; Jin, T.; Liu, Y.; Li, Q.; Qiang, Y. Influence of CTAB Surfactant on the Phase, Morphology, Light Absorption and Photocatalytic Performance of BiOF Nanomaterials. Mater. Res. Express 2019, 6, 105541. [Google Scholar] [CrossRef]
- Cheng, J.; Frezet, L.; Bonnet, P.; Wang, C. Preparation and Photocatalytic Properties of a Hierarchical BiOCl/BiOF Composite Photocatalyst. Catal. Lett. 2018, 148, 1281–1288. [Google Scholar] [CrossRef]
- Kan, Y.; Yang, Y.; Teng, F.; Yang, L.; Xu, J.; Teng, Y. Synthesis of Bi7F11O5/BiOCl Nanosheets by a Simple Post-Synthesis Method and the Improved Charge Separation by the Heterojunction. Catal. Commun. 2016, 87, 10–13. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, S.; Li, Z.; Ding, X.; Wu, M.; Zhang, M.; Yu, W. Polycrystalline Bismuth Oxyfluoride of BiO0.51F1.98 with Self-Doped BiOF Achieving Distinctly Enhanced Photocatalytic Activity. Mater. Lett. 2020, 262, 127197. [Google Scholar] [CrossRef]
- Wang, J.; Cao, C.-S.; Zhang, Y.; Zhu, L. Insights into the Intrinsic Mechanisms Underlying the Ultra-Highly Efficient Degradation of PFOA over S-Scheme Heterojunction of Bi7O5F11/BiOF. Appl. Catal. B Environ. 2023, 336, 122899. [Google Scholar] [CrossRef]
- Kodama, H.; Izumi, F.; Watanabe, A. New Members of a Family of Layered Bismuth Compounds. J. Solid State Chem. 1981, 36, 349–355. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H.; Zhu, L.; Fu, Z.; Lu, Y. One-Pot Synthesis of Bi2TiO4F2/BiOBr Ferroelectric Heterostructure for Photocatalytic Oxygen Evolution. J. Alloys Compd. 2021, 873, 159847. [Google Scholar] [CrossRef]
- Amirjani, A.; Amlashi, N.B.; Ahmadiani, Z.S. Plasmon-Enhanced Photocatalysis Based on Plasmonic Nanoparticles for Energy and Environmental Solutions: A Review. ACS Appl. Nano Mater. 2023, 6, 9085–9123. [Google Scholar] [CrossRef]
- Hou, W.; Cronin, S.B. A Review of Surface Plasmon Resonance-Enhanced Photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Singh, A.N.; Devnani, H.; Jha, S.; Ingole, P.P. Fermi Level Equilibration of Ag and Au Plasmonic Metal Nanoparticles Supported on Graphene Oxide. Phys. Chem. Chem. Phys. 2018, 20, 26719–26733. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Catalysis with TiO2/Gold Nanocomposites. Effect of Metal Particle Size on the Fermi Level Equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Xu, Z.; Loo, J.S.C. Hybrid Catalysts for Photoelectrochemical Reduction of Carbon Dioxide: A Prospective Review on Semiconductor/Metal Complex Co-Catalyst Systems. J. Mater. Chem. A 2014, 2, 15228–15233. [Google Scholar] [CrossRef]
- Liu, J.; He, H.; Xiao, D.; Yin, S.; Ji, W.; Jiang, S.; Luo, D.; Wang, B.; Liu, Y. Recent Advances of Plasmonic Nanoparticles and Their Applications. Materials 2018, 11, 1833. [Google Scholar] [CrossRef]
- McMahon, J.M.; Schatz, G.C.; Gray, S.K. Plasmonics in the Ultraviolet with the Poor Metals Al, Ga, In, Sn, Tl, Pb, and Bi. Phys. Chem. Chem. Phys. 2013, 15, 5415–5423. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Z.; Fang, J.; Wu, S.; Xu, W. Synthesis and Characterization of Mesoporous Bi/TiO2 Nanoparticles with High Photocatalytic Activity under Visible Light. J. Mater. Res. 2013, 28, 1334–1342. [Google Scholar] [CrossRef]
- Li, N.; Zhao, W.; Zhang, J.; Liu, X.; Gao, Y.; Ge, L. Plasmonic Bi-Modified Bi2Sn2O7 Nanosheets for Efficient Photocatalytic NO Removal. Catalysts 2024, 14, 275. [Google Scholar] [CrossRef]
- Bian, Z.; Lu, B.; Yang, Y.; Zhang, Q. Bi/BiVO4 Tailoring Molecular Oxygen Activation for SPR-Promoted Photocatalytic Contaminant Removal. Surf. Interfaces 2024, 47, 104210. [Google Scholar] [CrossRef]
- Ibrahim, S.; Minasyan, L.; Bonnet, P.; Monier, G.; Tomasella, E.; Sauvage, T.; Richard-Plouet, M.; Le Granvalet, M.; Bonduelle, A.; Pagis, C.; et al. Photocatalytic Properties of Bismuth Oxyfluoride Thin Films Deposited by Reactive Magnetron Sputtering in Ar/O2/CF4 Atmosphere. Preprints 2023. [Google Scholar]
- Ai, L.; Yin, H.; Wang, S.; Wang, J.; Gao, X.; Yin, X.; Dou, K.; Ju, P.; Sun, H. One-Pot Preparation of Bi/BiOF/Bi2O2CO3 Z-Scheme Heterojunction with Enhanced Photocatalysis Activity for Ciprofloxacin Degradation under Simulated Sunlight. Mater. Today Nano 2024, 25, 100446. [Google Scholar] [CrossRef]
- Jiang, T.; Li, J.; Gao, Y.; Li, L.; Lu, T.; Pan, L. BiOBr/BiOF Composites for Efficient Degradation of Rhodamine B and Nitrobenzene under Visible Light Irradiation. J. Colloid Interface Sci. 2017, 490, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Garg, S.; Chandra, A.; Hernadi, K. Quercetin-Sensitized BiOF Nanostructures: An Investigation on Photoinduced Charge Transfer and Regeneration Process for Degradation of Organic Pollutants. J. Photochem. Photobiol. A Chem. 2019, 383, 112014. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).