Continuous Flow-Mode Synthesis of Aromatic Amines in a 3D-Printed Fixed Bed Reactor Loaded with Amino Sugar-Stabilized Re Apparent Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Synthesized Catalytic Materials
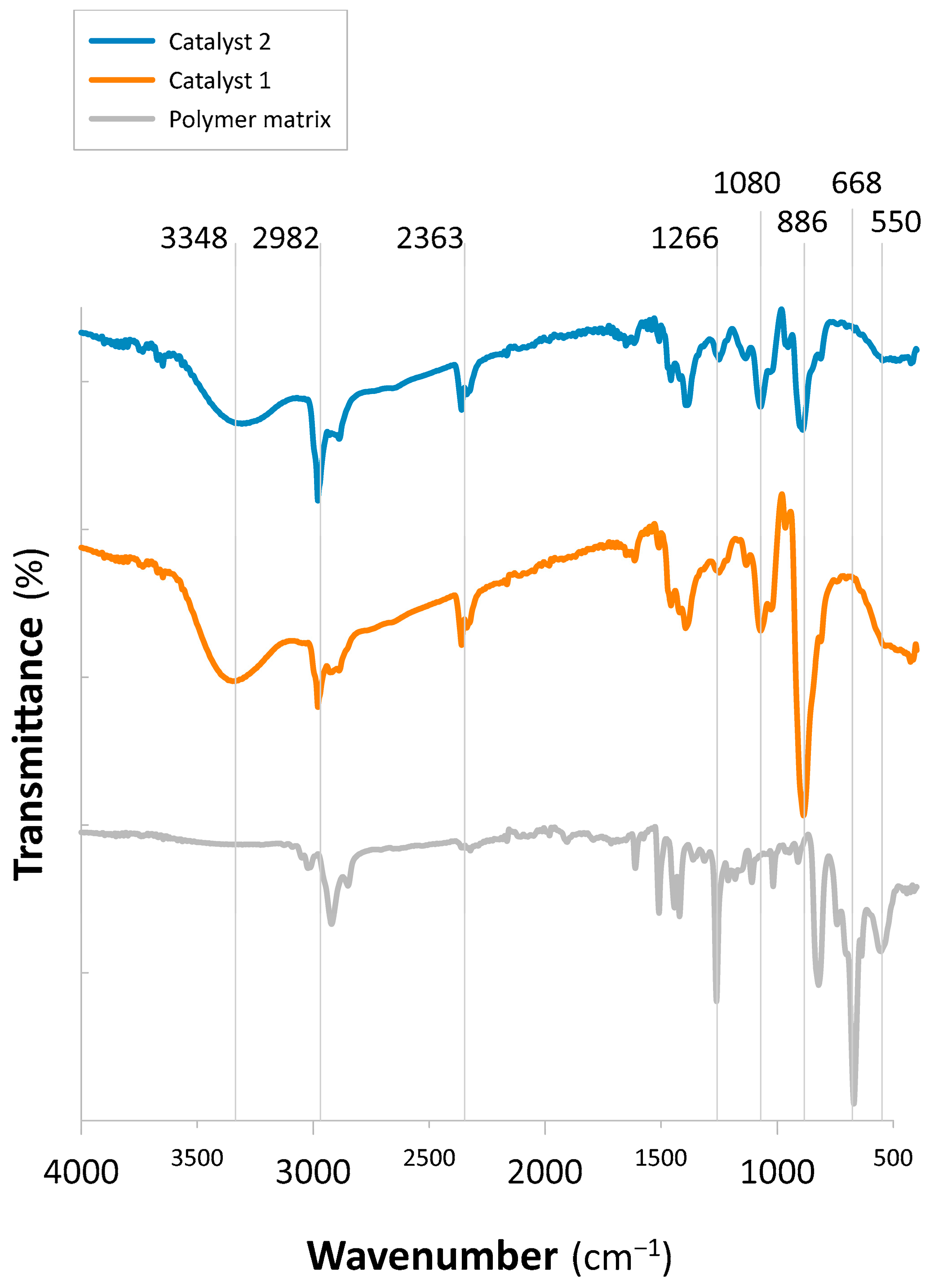
2.1.1. FT-IR Analysis
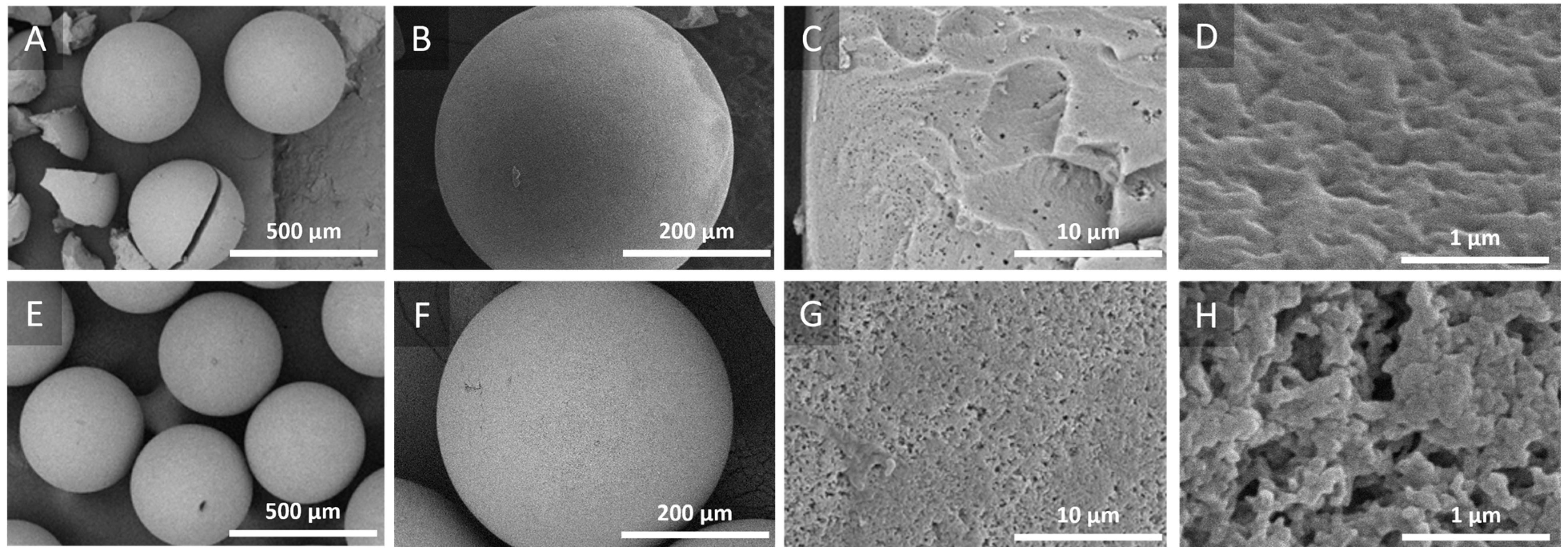
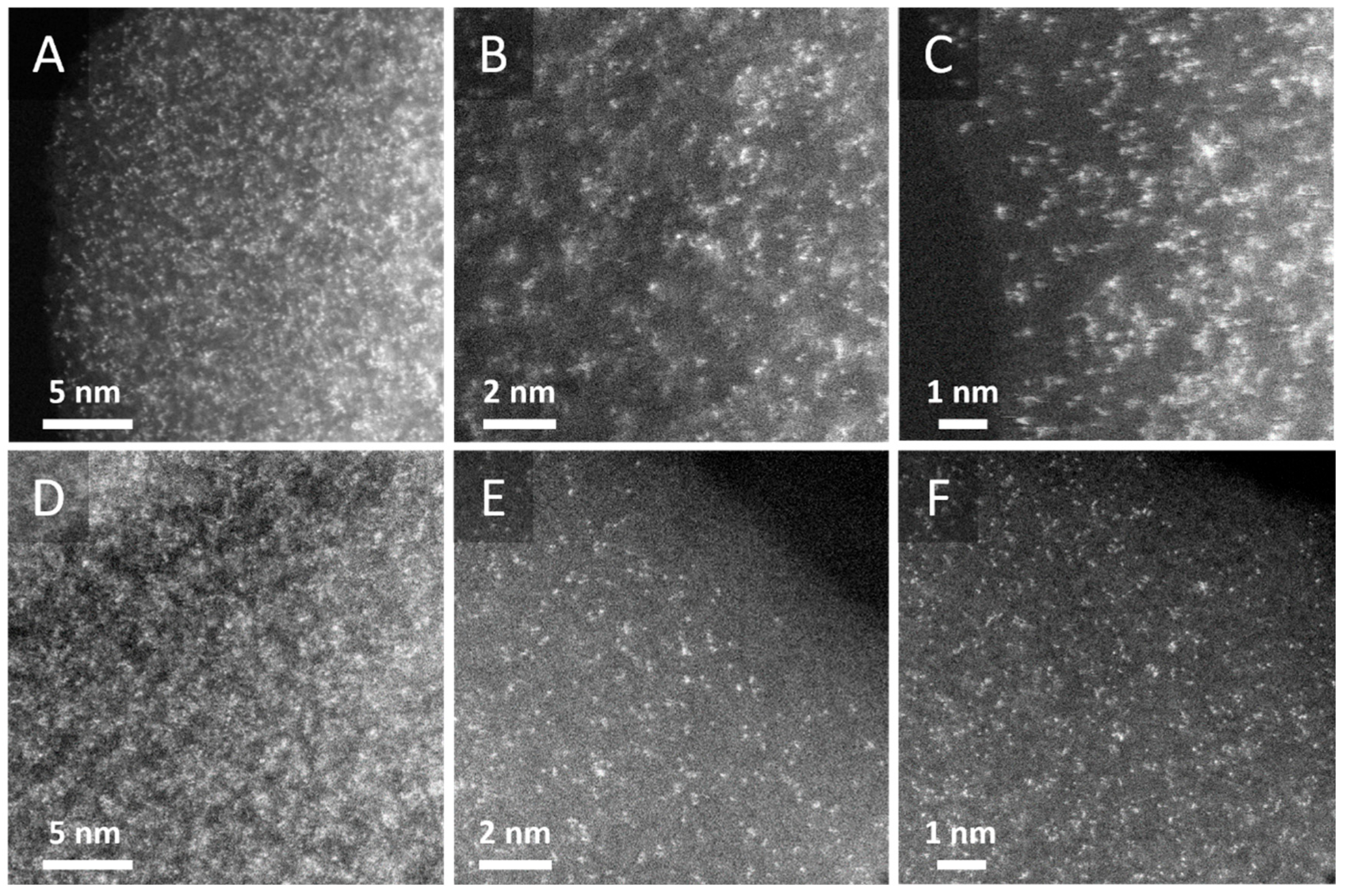
2.1.2. Electron Microscopy
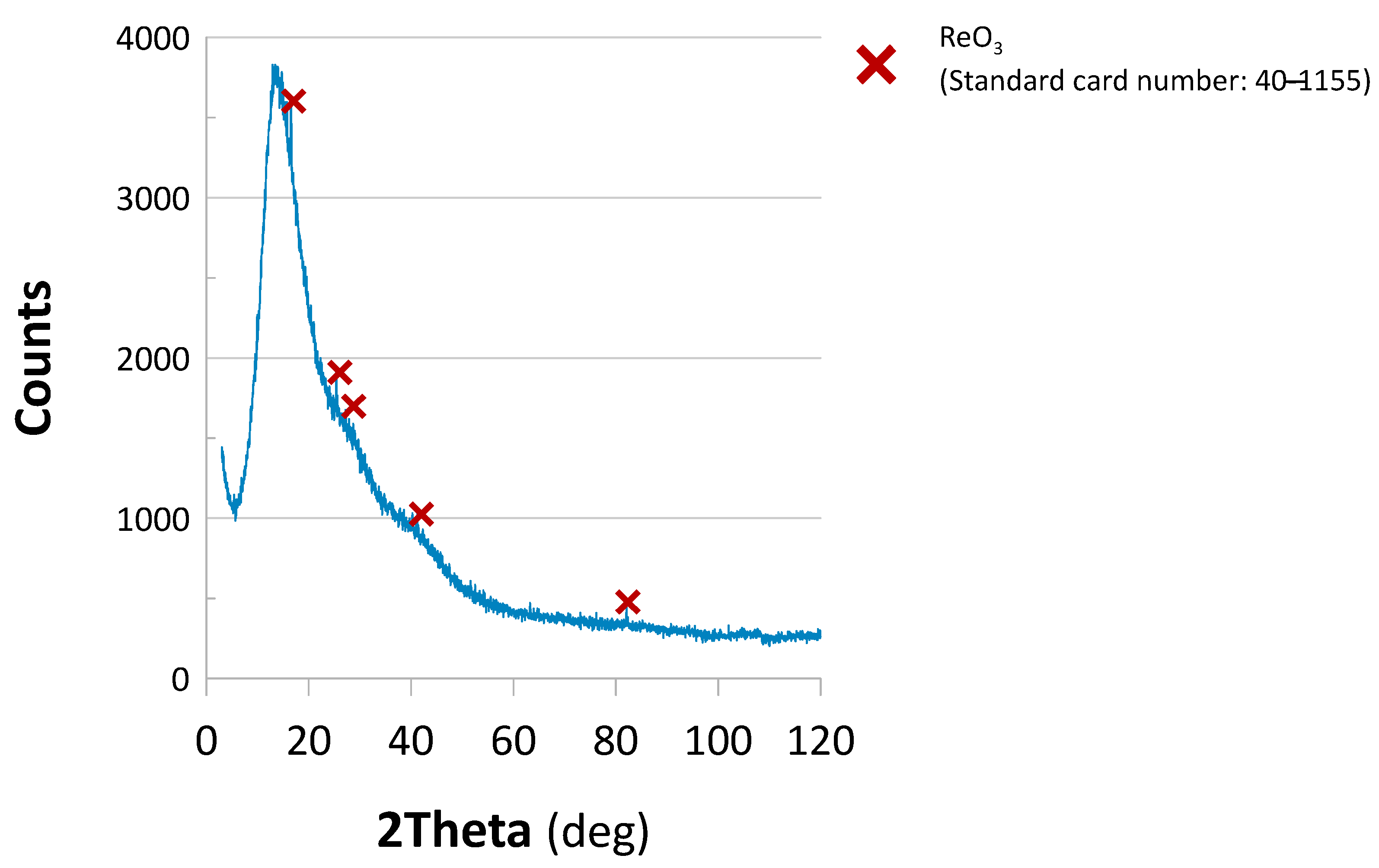
2.1.3. Phase Composition
2.2. Determination of Catalytic Activity in Batch Mode
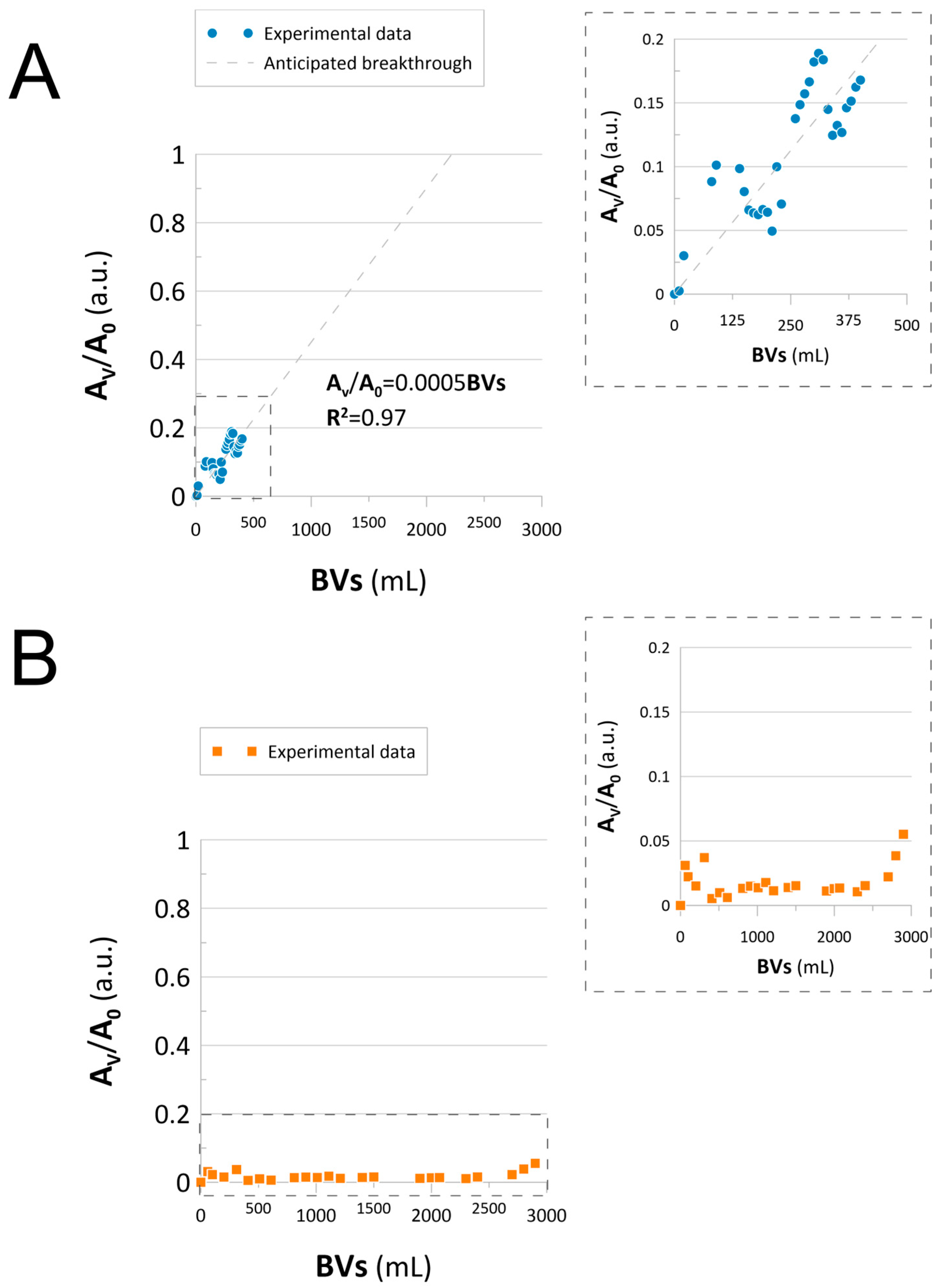
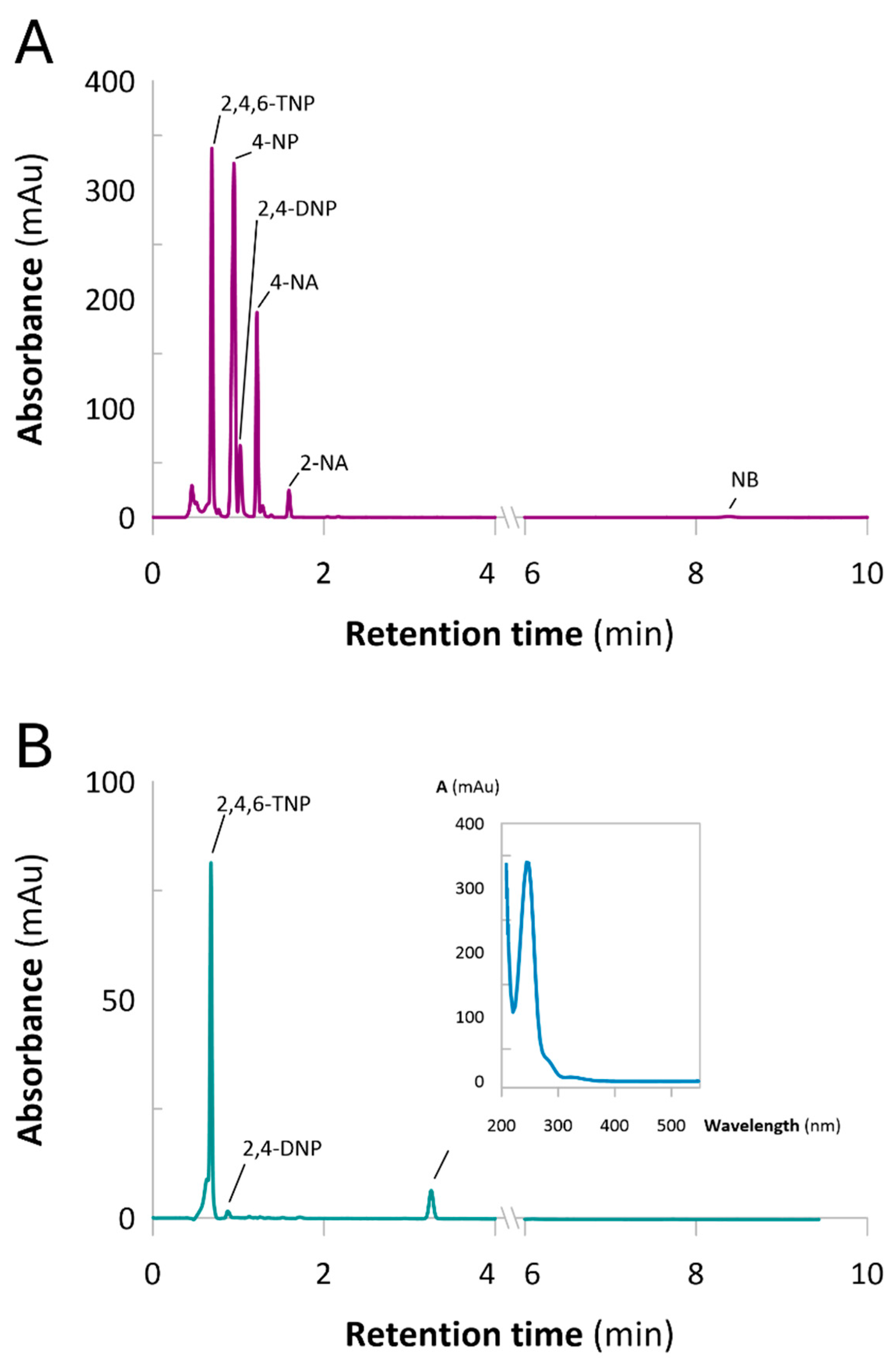
2.3. Continuous Flow-Mode Reduction of NACs
Catalysts Performance and Stability
2.4. Flow-Mode Catalytic Reduction in Multicomponent System
3. Materials and Methods
3.1. Reagents and Materials
3.2. Analytical Methods
3.3. Synthesis of Polymeric Materials Containing Rhenium Apparent Nanoparticles
3.4. Catalytic Reduction of NACs in Batch Mode
3.5. Stereolithographic (SLA) 3D Printing of Reaction Columns
3.6. Catalytic Reduction of NACs in Continuous-Flow Mode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spiekermann, M.L.; Seidensticker, T. Catalytic Processes for the Selective Hydrogenation of Fats and Oils: Reevaluating a Mature Technology for Feedstock Diversification. Catal. Sci. Technol. 2024, 14, 4390–4419. [Google Scholar] [CrossRef]
- Chen, B.; Dingerdissen, U.; Krauter, J.G.E.; Lansink Rotgerink, H.G.J.; Möbus, K.; Ostgard, D.J.; Panster, P.; Riermeier, T.H.; Seebald, S.; Tacke, T.; et al. New Developments in Hydrogenation Catalysis Particularly in Synthesis of Fine and Intermediate Chemicals. Appl. Catal. A Gen. 2005, 280, 17–46. [Google Scholar] [CrossRef]
- Bonrath, W.; Medlock, J.; Müller, M.-A.; Schütz, J. Catalysis for Fine Chemicals; De Gruyter: Berlin, Germany, 2024. [Google Scholar] [CrossRef]
- Yadav, J.K.; Yadav, P.; Awasthi, S.K.; Agarwal, A. Efficient N-Formylation of Primary Aromatic Amines Using Novel Solid Acid Magnetic Nanocatalyst. RSC Adv. 2020, 10, 41229–41236. [Google Scholar] [CrossRef] [PubMed]
- El-Behairy, M.F.; Hassan, R.M.; Sundby, E. Enantioselective Chromatographic Separation and Lipase Catalyzed Asymmetric Resolution of Biologically Important Chiral Amines. Separations 2021, 8, 165. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Bekkum, H. (Eds.) Fine Chemicals Through Heterogeneous Catalysis; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Lipshutz, B.H.; Caravez, J.C.; Iyer, K.S. Nanoparticle-Catalyzed Green Synthetic Chemistry … in Water. Curr. Opin. Green Sustain. Chem. 2022, 38, 100686. [Google Scholar] [CrossRef]
- Das, T.K.; Das, N.C. Advances on Catalytic Reduction of 4-Nitrophenol by Nanostructured Materials as Benchmark Reaction. Int. Nano Lett. 2022, 12, 223–242. [Google Scholar] [CrossRef]
- Wu, B.; Miraghaee, S.; Handa, S.; Gallou, F. Nanoparticles for Catalysis in Aqueous Media. Curr. Opin. Green Sustain. Chem. 2022, 38, 100691. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.V.; Nuzhdin, A.L.; Bukhtiyarova, G.A. Comparative Study of Batch and Continuous Flow Reactors in Selective Hydrogenation of Functional Groups in Organic Compounds: What Is More Effective? Int. J. Mol. Sci. 2023, 24, 14136. [Google Scholar] [CrossRef]
- Devlin, J.F.; Klausen, J.; Schwarzenbach, R.P. Kinetics of Nitroaromatic Reduction on Granular Iron in Recirculating Batch Experiments. Environ. Sci. Technol. 1998, 32, 1941–1947. [Google Scholar] [CrossRef]
- Mhaskar, P.; Garg, A.; Corbett, B. Modeling and Control of Batch Processes; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Chapman, M.R.; Kwan, M.H.T.; King, G.; Jolley, K.E.; Hussain, M.; Hussain, S.; Salama, I.E.; González Nino, C.; Thompson, L.A.; Bayana, M.E.; et al. Simple and Versatile Laboratory Scale CSTR for Multiphasic Continuous-Flow Chemistry and Long Residence Times. Org. Process Res. Dev. 2017, 21, 1294–1301. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Continuous Flow Reactors: A Perspective. Green Chem. 2012, 14, 38–54. [Google Scholar] [CrossRef]
- Morse, P.D.; Beingessner, R.L.; Jamison, T.F. Enhanced Reaction Efficiency in Continuous Flow. Isr. J. Chem. 2017, 57, 218–227. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Continuous Process Technology: A Tool for Sustainable Production. Green Chem. 2013, 16, 55–62. [Google Scholar] [CrossRef]
- What Is Continuous Flow Manufacturing? + Keys to Success | KaiNexus. Available online: https://blog.kainexus.com/continuous-flow-manufacturing (accessed on 28 September 2024).
- Gascon, J.; Corma, A.; Kapteijn, F.; Llabrés I Xamena, F.X. Metal Organic Framework Catalysis: Quo Vadis? ACS Catal. 2014, 4, 361–378. [Google Scholar] [CrossRef]
- Mehrabadi, B.A.T.; Eskandari, S.; Khan, U.; White, R.D.; Regalbuto, J.R. A Review of Preparation Methods for Supported Metal Catalysts. Adv. Catal. 2017, 61, 1–35. [Google Scholar] [CrossRef]
- Mastrorilli, P.; Nobile, C.F. Supported Catalysts from Polymerizable Transition Metal Complexes. Coord. Chem. Rev. 2004, 248, 377–395. [Google Scholar] [CrossRef]
- Colella, M.; Carlucci, C.; Luisi, R. Supported Catalysts for Continuous Flow Synthesis. Top. Curr. Chem. 2018, 376, 1–37. [Google Scholar] [CrossRef]
- Xia, C.; Jin, X.; Parandoust, A.; Sheibani, R.; Khorsandi, Z.; Montazeri, N.; Wu, Y.; Van Le, Q. Chitosan-Supported Metal Nanocatalysts for the Reduction of Nitroaromatics. Int. J. Biol. Macromol. 2023, 239, 124135. [Google Scholar] [CrossRef]
- Dassouki, K.; Dasgupta, S.; Dumas, E.; Steunou, N. Interfacing Metal Organic Frameworks with Polymers or Carbon Based Materials: From Simple to Hierarchical Porous and Nanostructured Composites. Chem. Sci. 2023, 14, 12898–12925. [Google Scholar] [CrossRef]
- Cyganowski, P.; Dzimitrowicz, A.; Marzec, M.M.; Arabasz, S.; Sokołowski, K.; Lesniewicz, A.; Nowak, S.; Pohl, P.; Bernasik, A.; Jermakowicz-Bartkowiak, D. Catalytic Reductions of Nitroaromatic Compounds over Heterogeneous Catalysts with Rhenium Sub-Nanostructures. Sci. Rep. 2023, 13, 12789. [Google Scholar] [CrossRef]
- Kuś, A.; Leśniewicz, A.; Dzimitrowicz, A.; Pohl, P.; Cyganowski, P. Waste-Derived Caffeine for Green Synthesis of Rhenium Nanoparticles with Enhanced Catalytic Activity in the Hydrogenation of 4-Nitrophenol. Int. J. Mol. Sci. 2024, 25, 11319. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Santos, B.; Faustino, R.; Pombeiro, A.; Martins, L. C-Heterogenized Re Nanoparticles as Effective Catalysts for the Reduction of 4-Nitrophenol and Oxidation of 1-Phenylethanol. Catalysts 2022, 12, 285. [Google Scholar] [CrossRef]
- Belousov, V.M.; Palchevskaya, T.A.; Bogutskaya, L.V.; Zyuzya, L.A. Properties of Homogeneous and Heterogeneous Rhenium Catalysts in the Hydrogenation of Nitro Compounds. J. Mol. Catal. 1990, 60, 165–172. [Google Scholar] [CrossRef]
- Khan, M.A.; Cyganowski, P.; Pohl, P.; Jamroz, P.; Tylus, W.; Motyka-Pomagruk, A.; Dzimitrowicz, A. Multiparameter Optimization of Non-Thermal Plasma-Driven Synthesis of Carbohydrate-Stabilized Rhenium Nanoparticles towards Enhancement of Their Catalytical Activity for Reduction of Nitroaromatic Compounds. Colloids Surf. A Physicochem. Eng. Asp. 2024, 695, 134190. [Google Scholar] [CrossRef]
- Malachowski, L.; Holcombe, J.A. Immobilized poly-l-histidine for chelation of metal cations and metal oxyanions. Anal. Chim. Acta 2003, 495, 151–163. [Google Scholar] [CrossRef]
- Dambies, L.; Salinaro, R.; Alexandratos, S.D. Immobilized N-Methyl-D-Glucamine as an Arsenate-Selective Resin. Environ. Sci. Technol. 2004, 38, 6139–6146. [Google Scholar] [CrossRef]
- Yousif, A.M. Synthesis of Chemically Modified Macroreticular Resins for the Preparation of Gold Nanoparticles via Sorption from Aqueous Gold Solution, and the Application of These Nanoparticles in Catalytic Remediation. J. Polym. Res. 2012, 20, 33. [Google Scholar] [CrossRef]
- Pant, P.; Bansal, R.; Gulati, S.; Kumar, S.; Kodwani, R. Porous and Chelated Nanostructured Multifunctional Materials: Recoverable and Reusable Sorbents for Extraction of Metal Ions and Catalysts for Diverse Organic Reactions. J. Nanostruct. Chem. 2016, 6, 145–157. [Google Scholar] [CrossRef]
- Hoshina, H.; Chen, J.; Amada, H.; Seko, N. Chelating Fabrics Prepared by an Organic Solvent-Free Process for Boron Removal from Water. Polymers 2021, 13, 1163. [Google Scholar] [CrossRef]
- Long, D.A. Infrared and Raman Characteristic Group Frequencies. Tables and Charts; John Wiley & Sons, Ltd.: Chichester, UK, 2004. [Google Scholar]
- Larkin, P.J. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Miramontes, O.; Bonafé, F.; Santiago, U.; Larios-Rodriguez, E.; Velázquez-Salazar, J.J.; Mariscal, M.M.; Yacaman, M.J. Ultra-Small Rhenium Clusters Supported on Graphene. Phys. Chem. Chem. Phys. 2015, 17, 7898–7906. [Google Scholar] [CrossRef] [PubMed]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database. J. Chem. Educ. 1993, 70, A25. [Google Scholar] [CrossRef]
- Rouxhet, P.G.; Genet, M.J. XPS Analysis of Bio-Organic Systems. Surf. Interface Anal. 2011, 43, 1453–1470. [Google Scholar] [CrossRef]
- Genet, M.J.; Dupont-Gillain, C.C.; Rouxhet, P.G. XPS Analysis of Biosystems and Biomaterials. In Medical Applictions of Colloids; Matijevic, E., Ed.; Springer Science Business Media, LLC: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Naumkin, A.V.; Kraust-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-Ray Photoelectron Spectroscopy Database; Version 5.0; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2024. [Google Scholar]
- Briggs, D. Surface Analysis of Polymers by XPS and Static SIMS; Cambridge University Press: New York, NY, USA, 2005. [Google Scholar]
- McCormick, N.G.; Feeherry, F.E.; Levinson, H.S. Microbial Transformation of 2,4,6-Trinitrotoluene and Other Nitroaromatic Compounds. Appl. Environ. Microbiol. 1976, 31, 949–958. [Google Scholar] [CrossRef]
- Wu, Q.; Su, W.; Huang, R.; Shen, H.; Qiao, M.; Qin, R.; Zheng, N. Full Selectivity Control over the Catalytic Hydrogenation of Nitroaromatics Into Six Products. Angew. Chem. 2024, 136, e202408731. [Google Scholar] [CrossRef]
- Sassykova, L.R.; Aubakirov, Y.A.; Sendilvelan, S.; Tashmukhambetova, Z.K.; Zhakirova, N.K.; Faizullaeva, M.F.; Batyrbayeva, A.A.; Ryskaliyeva, R.G.; Tyussyupova, B.B.; Abildin, T.S. Studying the Mechanisms of Nitro Compounds Reduction (A-Review). Orient. J. Chem. 2019, 35, 22–38. [Google Scholar] [CrossRef]
- Formlabs. Guide to Stereolithography (SLA) 3D Printing. Formlabs. Available online: https://formlabs.com/blog/ultimate-guide-to-stereolithography-sla-3d-printing/ (accessed on 2 April 2025).

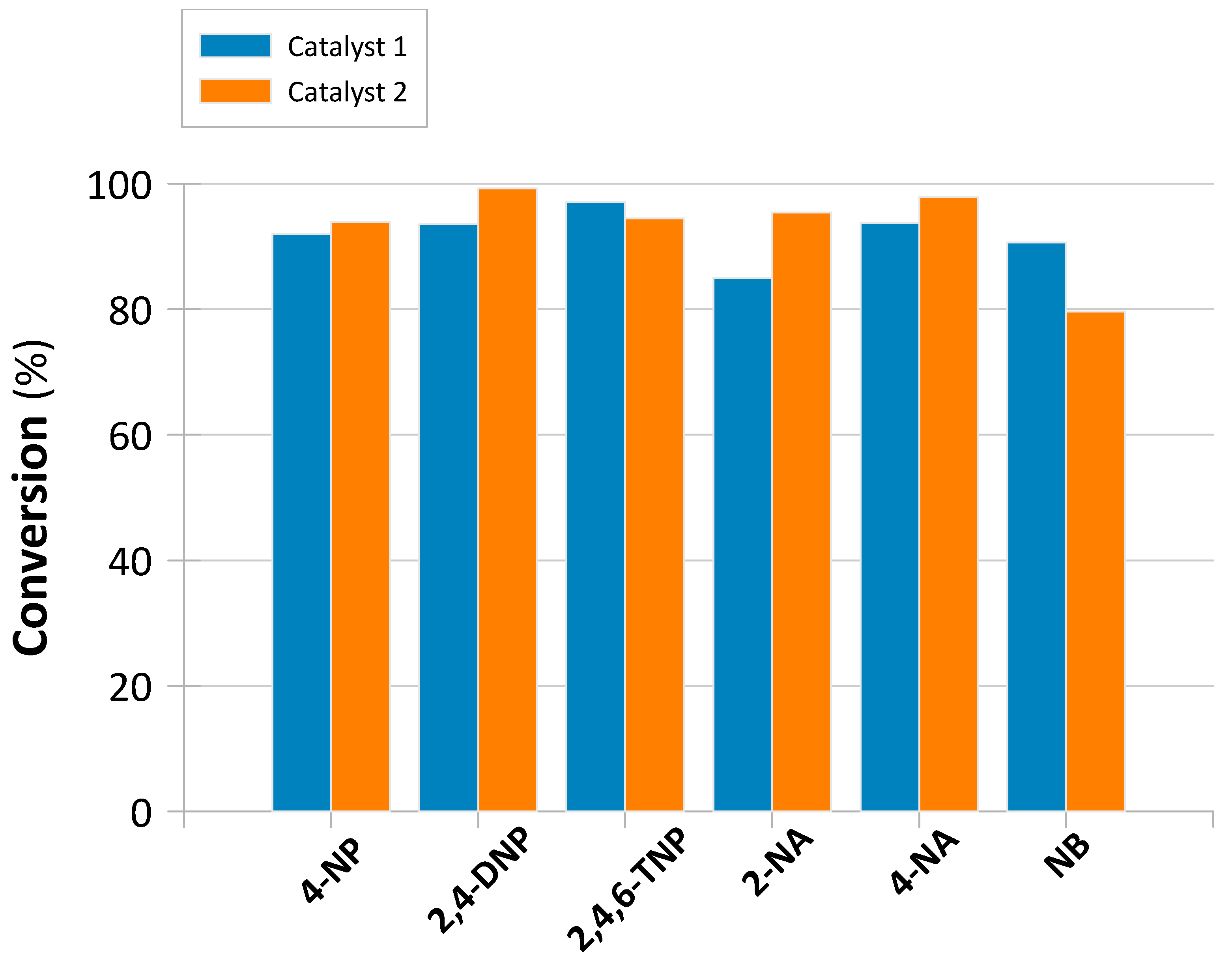

| C | N | O | Si | Cl | Re | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Binding Energy [eV] | 285.0 | 286.7 | 287.9 | 400.6 | 402.3 | 531.5 | 532.7 | 533.8 | 102.1 | 103.4 | 198.3 | 44.2 | 46.3 |
| Compounds/Bonds | C–C, C–H | C–O–C, C–OH, C–N | C=O, O–C–O | C–NH | NH4+ | O–Re, O=C O–Si | O–C, O–Si | OH, H2O ads. | Siloxane | SiO2 | Cl− | Re6+ (ReO3) | Re7+ (Re2O7) |
| Catalyst 1 | 38.1 | 21.3 | 4.1 | 0.9 | 2.2 | 10.3 | 13.2 | 2.9 | 3.1 | 1.8 | 0.0 | 0.4 | 1.9 |
| Catalyst 2 | 43.9 | 19.2 | 2.7 | 2.5 | 1.1 | 6.0 | 15.4 | 1.7 | 3.7 | 2.0 | 0.7 | 0.7 | 0.5 |
| NAC * | Catalyst | k1 a (s−1) | k1IP b (s−1) | R2 | R1IP2 | Conversion (%) | Conversion t (h) | TOF (h−1) |
|---|---|---|---|---|---|---|---|---|
| 4-NP | 1 | 0.841 | 0.992 | - | 91.969 | 0.050 | 1.968 | |
| 2 | 0.621 | 0.040 | 0.976 | 0.981 | 93.892 | 0.125 | 1.234 | |
| 2,4-DNP | 1 | 0.804 | 0.921 | - | 93.587 | 0.063 | 1.602 | |
| 2 | 0.533 | 0.145 | 0.948 | 0.968 | 99.229 | 0.138 | 1.186 | |
| 2,4,6-TNP | 1 | 0.676 | 0.963 | - | 97.066 | 0.092 | 1.133 | |
| 2 | 0.420 | 0.094 | 0.769 | 0.996 | 94.485 | 0.154 | 1.007 | |
| 2-NA | 1 | 0.605 | 0.932 | - | 85.012 | 0.058 | 1.559 | |
| 2 | 0.497 | 0.078 | 0.979 | 0.999 | 95.424 | 0.142 | 1.107 | |
| 4-NA | 1 | 0.263 | 0.998 | - | 93.748 | 0.063 | 1.605 | |
| 2 | 0.317 | 0.085 | 0.936 | 0.980 | 97.863 | 0.125 | 1.287 | |
| NB | 1 | 1.406 | 0.837 | - | 90.652 | 0.029 | 3.326 | |
| 2 | 0.261 | - | 0.927 | - | 79.667 | 0.100 | 1.309 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niyirora, P.; Wolska, J.; Marzec, M.M.; Sokolowski, K.; Leśniewicz, A.; Jamróz, P.; Dzimitrowicz, A.; Bernasik, A.; Cyganowski, P. Continuous Flow-Mode Synthesis of Aromatic Amines in a 3D-Printed Fixed Bed Reactor Loaded with Amino Sugar-Stabilized Re Apparent Nanoparticles. Molecules 2025, 30, 3782. https://doi.org/10.3390/molecules30183782
Niyirora P, Wolska J, Marzec MM, Sokolowski K, Leśniewicz A, Jamróz P, Dzimitrowicz A, Bernasik A, Cyganowski P. Continuous Flow-Mode Synthesis of Aromatic Amines in a 3D-Printed Fixed Bed Reactor Loaded with Amino Sugar-Stabilized Re Apparent Nanoparticles. Molecules. 2025; 30(18):3782. https://doi.org/10.3390/molecules30183782
Chicago/Turabian StyleNiyirora, Patrick, Joanna Wolska, Mateusz M. Marzec, Krystian Sokolowski, Anna Leśniewicz, Piotr Jamróz, Anna Dzimitrowicz, Andrzej Bernasik, and Piotr Cyganowski. 2025. "Continuous Flow-Mode Synthesis of Aromatic Amines in a 3D-Printed Fixed Bed Reactor Loaded with Amino Sugar-Stabilized Re Apparent Nanoparticles" Molecules 30, no. 18: 3782. https://doi.org/10.3390/molecules30183782
APA StyleNiyirora, P., Wolska, J., Marzec, M. M., Sokolowski, K., Leśniewicz, A., Jamróz, P., Dzimitrowicz, A., Bernasik, A., & Cyganowski, P. (2025). Continuous Flow-Mode Synthesis of Aromatic Amines in a 3D-Printed Fixed Bed Reactor Loaded with Amino Sugar-Stabilized Re Apparent Nanoparticles. Molecules, 30(18), 3782. https://doi.org/10.3390/molecules30183782











