Dual-Ionization SPME-GC–HRMS Metabolomic Profiling of Broccoli Volatiles for the Construction of a Broccoli Metabolic Database
Abstract
1. Introduction
2. Results
2.1. Method Development
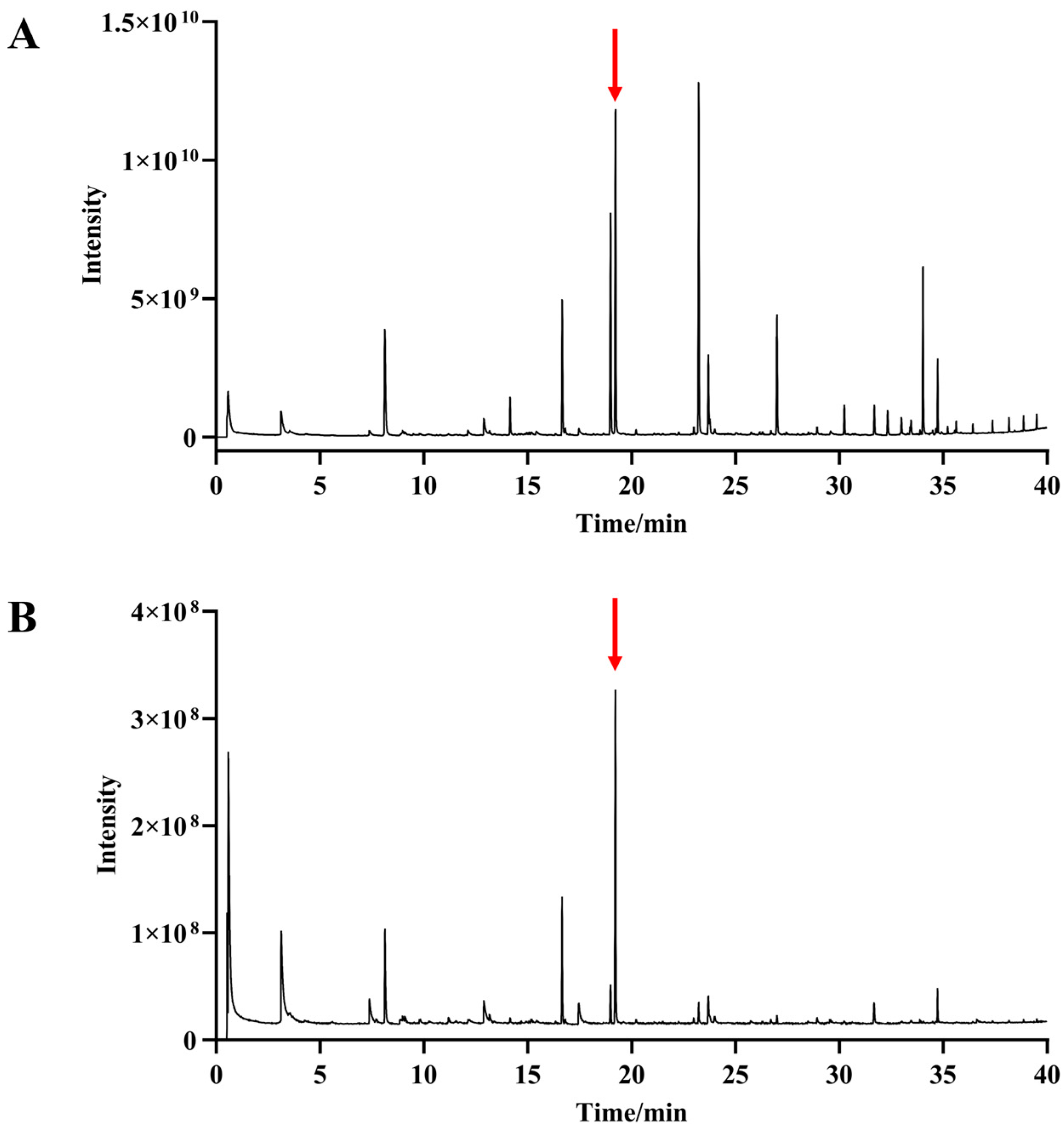
2.2. n-Alkanes (C8–C40) and n-Nonyl Acetate (IS) Analysis
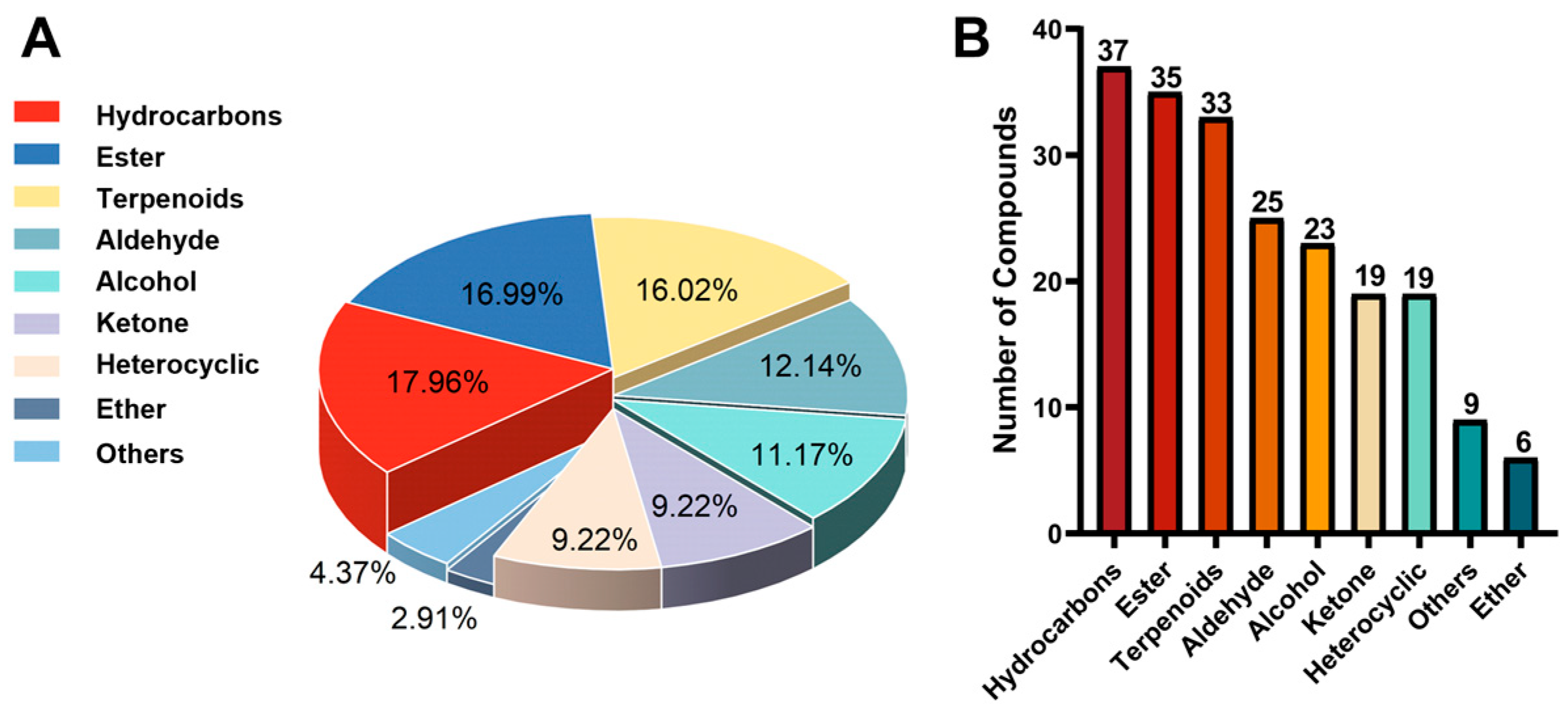
2.3. Database of Volatile Metabolites in Broccoli
2.4. Volatile Metabolites in Different Varieties: Database Validation and Comparison
2.4.1. Database Validation
2.4.2. Esters
2.4.3. Hydrocarbons
2.4.4. Terpenoids
2.4.5. Aldehydes
2.4.6. Alcohols
2.4.7. Ketones
2.4.8. Heterocyclic Compounds
2.4.9. Ethers
2.4.10. Other Compounds
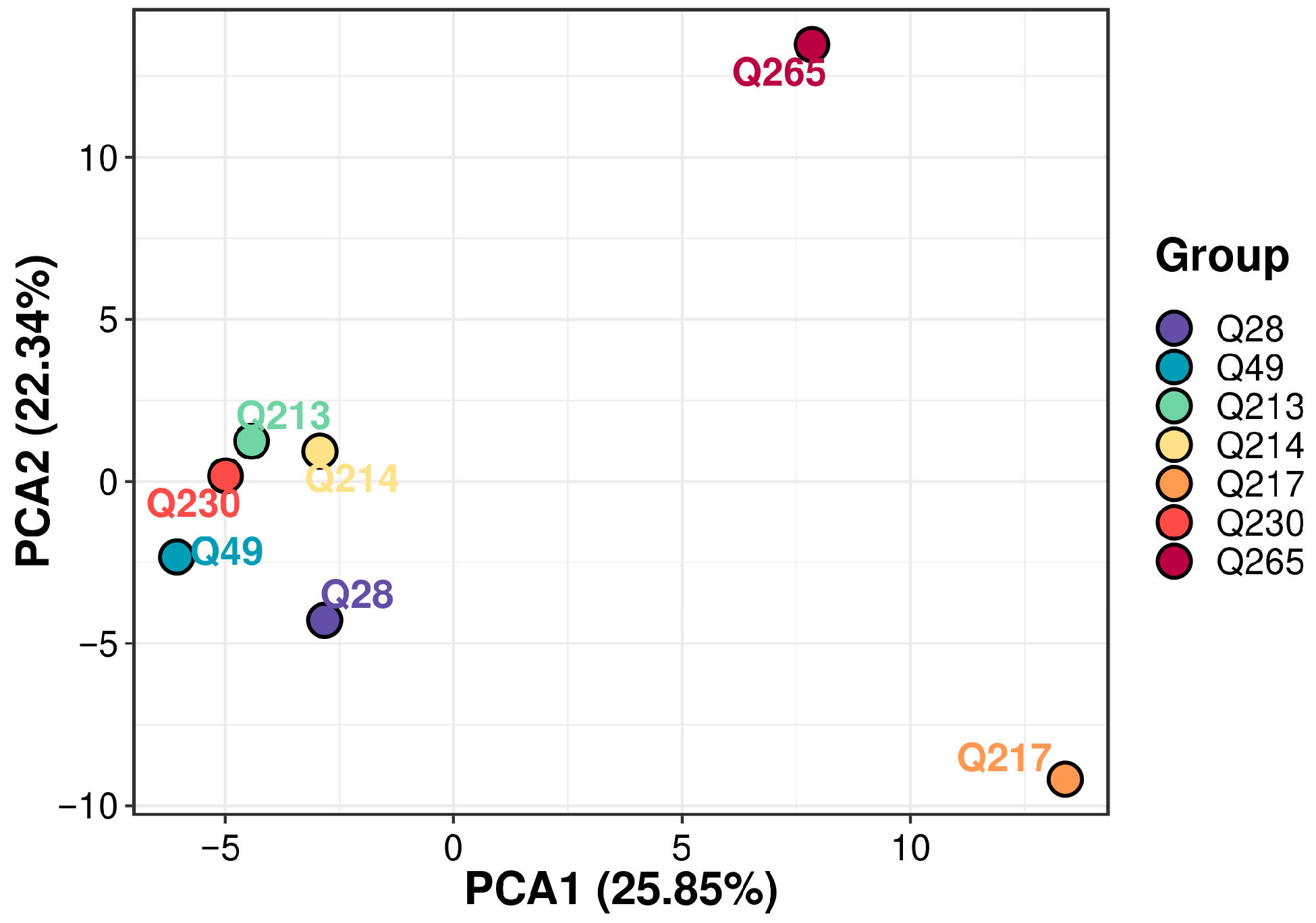
2.4.11. Principal Component Analysis (PCA)
3. Discussion
3.1. Fresh and Freeze-Dried Sample Preparation for Volatile Profiling
3.2. Pooled-Sample Strategy for Comprehensive Volatile Metabolite Database Construction
3.3. Validation and Applicability of the Volatile Metabolite Database
3.4. VOC Profile Differences Among 7 Varieties
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Materials
4.3. SPME Extraction of Volatile Compounds
4.4. GC–Orbitrap MS Analysis of Volatile Metabolites
4.5. Data Processing
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Chemical ionization |
| EI | Electron ionization |
| GC-MS | Gas chromatography–mass spectrometry |
| HRMS | High-resolution mass spectrometry |
| PCA | Principal component analysis |
| SD | Standard deviation |
| SPME | Solid-phase microextraction |
| TIC | Total ion chromatogram |
| VOC | Volatile organic compounds |
References
- Tholl, D.; Hossain, O.; Weinhold, A.; Röse, U.S.; Wei, Q. Trends and applications in plant volatile sampling and analysis. Plant J. 2021, 106, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Zhang, M.; Guo, Z. Discrimination of fresh-cut broccoli freshness by volatiles using electronic nose and gas chromatography-mass spectrometry. Postharvest Biol. Technol. 2019, 148, 168–175. [Google Scholar] [CrossRef]
- Akutsu, M.; Sugie, Y.; Saito, Y. Analysis of 62 synthetic cannabinoids by gas chromatography-mass spectrometry with photoionization. Forensic Toxicol. 2017, 35, 94–103. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, C.; Li, Y.; Zheng, X. Profiling of aroma compounds in vegetables using headspace SPME and GC-MS. Food Anal. Methods 2022, 15, 987–996. [Google Scholar]
- Zhao, D.; Tang, J.; Ding, X. Analysis of volatile components during potherb mustard (Brassica juncea Coss.) pickle fermentation using SPME–GC-MS. LWT-Food Sci. Technol. 2007, 40, 439–447. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Ballesteros-Vivas, D.; Parada-Alfonso, F.; Ibañez, E.; Cifuentes, A. Recent applications of high resolution mass spectrometry for the characterization of plant natural products. TrAC Trends Anal. Chem. 2019, 112, 87–101. [Google Scholar] [CrossRef]
- Rivera-Perez, A.; Romero-González, R.; Garrido Frenich, A. Feasibility of applying untargeted metabolomics with GC-Orbitrap-HRMS and chemometrics for authentication of black pepper (Piper nigrum L.) and identification of geographical and processing markers. J. Agric. Food Chem. 2021, 69, 5547–5558. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Umashankar, S.; Liang, X.; Lee, H.W.; Swarup, S.; Ong, C.N. Characterization of plant volatiles reveals distinct metabolic profiles and pathways among 12 Brassicaceae vegetables. Metabolites 2018, 8, 94. [Google Scholar] [CrossRef]
- Chevilly, S.; Dolz-Edo, L.; Blanca, J.; Yenush, L.; Mulet, J.M. Identification of distinctive primary metabolites influencing broccoli (Brassica oleracea var. italica) taste. Foods 2023, 12, 339. [Google Scholar] [CrossRef]
- Abate, S.; Ahn, Y.G.; Kind, T.; Cataldi, T.R.I.; Fiehn, O. Determination of elemental compositions by gas chromatography/time-of-flight mass spectrometry using chemical and electron ionization. Rapid Commun. Mass Spectrom. 2010, 24, 1172–1180. [Google Scholar] [CrossRef]
- Tsunoi, S.; Yasuhisa, T.; Hisasue, T.; Suzuki, I.; Shibata, I. Differentiating the aromatic positional isomers of methylbuphedrones and methoxybuphedrones via chemical ionization-mass spectrometry. Anal. Sci. Adv. 2024, 5, 2300064. [Google Scholar] [CrossRef]
- Misra, B.B.; Olivier, M. High resolution GC-Orbitrap-MS metabolomics using both electron ionization and chemical ionization for analysis of human plasma. J. Proteome Res. 2020, 19, 2717–2731. [Google Scholar] [CrossRef] [PubMed]
- Capellades, J.; Junza, A.; Samino, S.; Brunner, J.S.; Schabbauer, G.; Vinaixa, M.; Yanes, O. Exploring the use of gas chromatography coupled to chemical ionization mass spectrometry (GC-CI-MS) for stable isotope labeling in metabolomics. Anal. Chem. 2020, 93, 1242–1248. [Google Scholar]
- Wang, L.; Zhang, Y.; Chen, Y.; Liu, S.; Yun, L.; Guo, Y.; Zhang, X.; Wang, F. Investigating the relationship between volatile components and differentially expressed proteins in broccoli heads during storage in high CO2 atmospheres. Postharvest Biol. Technol. 2019, 153, 43–51. [Google Scholar] [CrossRef]
- Sikorska-Zimny, K.; Beneduce, L. The glucosinolates and their bioactive derivatives in Brassica: A review on classification, biosynthesis and content in plant tissues, fate during and after processing, effect on the human organism and interaction with the gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 2544–2571. [Google Scholar] [CrossRef]
- Nagraj, G.S.; Chouksey, A.; Jaiswal, S.; Jaiswal, A.K. Broccoli. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 5–17. [Google Scholar]
- Syed, R.U.; Moni, S.S.; Break, M.K.B.; Khojali, W.M.; Jafar, M.; Alshammari, M.D.; Abdelsalam, K.; Taymour, S.; Alreshidi, K.S.M.; Elhassan Taha, M.M.; et al. Broccoli: A multi-faceted vegetable for health: An in-depth review of its nutritional attributes, antimicrobial abilities, and anti-inflammatory properties. Antibiotics 2023, 12, 1157. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, W.; Xu, Y.; Ji, Y.; Yang, X.; Feng, K. Proteomic analysis validates previous findings on wounding-responsive plant hormone signaling and primary metabolism contributing to the biosynthesis of secondary metabolites based on metabolomic analysis in harvested broccoli (Brassica oleracea L. var. italica). Food Res. Int. 2021, 145, 110388. [Google Scholar] [CrossRef] [PubMed]
- Hassini, I.; Rios, J.J.; Garcia-Ibañez, P.; Baenas, N.; Carvajal, M.; Moreno, D.A. Comparative effect of elicitors on the physiology and secondary metabolites in broccoli plants. J. Plant Physiol. 2019, 239, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Oloyede, O.O.; Lignou, S.; Wagstaff, C.; Methven, L. Taste and flavor perceptions of glucosinolates, isothiocyanates, and related compounds. Mol. Nutr. Food Res. 2018, 62, 1700990. [Google Scholar] [CrossRef]
- Muthusamy, M.; Lee, S.I. Abiotic stress-induced secondary metabolite production in Brassica: Opportunities and challenges. Front. Plant Sci. 2024, 14, 1323085. [Google Scholar] [CrossRef]
- Ilahy, R.; Tlili, I.; Pék, Z.; Montefusco, A.; Siddiqui, M.W.; Homa, F.; Hdider, C.; R’Him, T.; Lajos, H.; Lenucci, M.S. Pre- and post-harvest factors affecting glucosinolate content in broccoli. Front. Nutr. 2020, 7, 147. [Google Scholar] [CrossRef]
- Kamboj, A.; Sharma, S.; Singh, V.P.; Sinha, A.; Yadav, K.S.; Lal, B.; Chaudhary, M.; Devi, L. Phytochemical and therapeutic potential of broccoli (Brassica oleracea): A review. Pharma Innov. J. 2023, 12, 633–638. [Google Scholar]
- Wang, J.; Mao, S.; Liang, M.; Zhang, W.; Chen, F.; Huang, K.; Wu, Q. Preharvest methyl jasmonate treatment increased glucosinolate biosynthesis, sulforaphane accumulation, and antioxidant activity of broccoli. Antioxidants 2022, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Chen, L.F.O.; Shaw, J.F. Senescence-associated genes in harvested broccoli florets. Plant Sci. 2008, 175, 137–144. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov (accessed on 21 January 2025).
- López-Gresa, M.P.; Lisón, P.; Campos, L.; Rodrigo, I.; Rambla, J.L.; Granell, A.; Bellés, J.M. A non-targeted metabolomics approach unravels the VOCs associated with the tomato immune response against Pseudomonas syringae. Front. Plant Sci. 2017, 8, 1188. [Google Scholar] [CrossRef]
- Carraturo, F.; Libralato, G.; Esposito, R.; Galdiero, E.; Aliberti, F.; Amoresano, A.; Guida, M. Metabolomic profiling of food matrices: Preliminary identification of potential markers of microbial contamination. J. Food Sci. 2020, 85, 3467–3477. [Google Scholar] [CrossRef]
- ElNaker, N.A.; Daou, M.; Ochsenkühn, M.A.; Amin, S.A.; Yousef, A.F.; Yousef, L.F. A metabolomics approach to evaluate the effect of lyophilization versus oven drying on the chemical composition of plant extracts. Sci. Rep. 2021, 11, 22679. [Google Scholar] [CrossRef]
- Sánchez-Palomo, E.; Diaz-Maroto, M.C.; Perez-Coello, M.S. Rapid determination of volatile compounds in grapes by HS-SPME coupled with GC–MS. Talanta 2005, 66, 1152–1157. [Google Scholar] [CrossRef]
- Raseetha, S.; Oey, I.; Burritt, D.; Hamid, N. Monitoring colour, volatiles in the headspace and enzyme activity to assess the quality of broccoli florets (Brassica oleracea L. italica cv. Bellstar and Legacy) during postharvest storage. Int. J. Food Sci. Technol. 2014, 49, 280–287. [Google Scholar] [CrossRef]
- Ejigu, B.A.; Valkenborg, D.; Baggerman, G.; Vanaerschot, M.; Witters, E.; Dujardin, J.C.; Burzykowski, T.; Berg, M. Evaluation of normalization methods to pave the way towards large-scale LC-MS-based metabolomics profiling experiments. Omics 2013, 17, 473–485. [Google Scholar] [CrossRef]
- Barnes, S.; Benton, H.P.; Casazza, K.; Cooper, S.J.; Cui, X.; Du, X.; Engler, J.; Kabarowski, J.H.; Li, S.; Pathmasiri, W.; et al. Training in metabolomics research. I. Designing the experiment, collecting and extracting samples and generating metabolomics data. J. Mass Spectrom. 2016, 51, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, M.; Chen, P.; Harnly, J.M.; Sun, J. Mass spectrometry-based nontargeted and targeted analytical approaches in fingerprinting and metabolomics of food and agricultural research. J. Agric. Food Chem. 2022, 70, 11138–11153. [Google Scholar] [CrossRef] [PubMed]
- Majithia, D.; Metrani, R.; Dhowlaghar, N.; Crosby, K.M.; Patil, B.S. Assessment and classification of volatile profiles in melon breeding lines using headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry. Plants 2021, 10, 2166. [Google Scholar] [CrossRef] [PubMed]

| No * | Compound Name | Retention Time | Reference m/z | Avg Peak Area | Formula | CAS No. | Avg Calculated RI | Library RI | Classes |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 2-Hexenal, (E)- | 3.11 | 41.0386 | 551,060,469 | C6H10O | 6728-26-3 | N/C ** | Aldehyde | |
| 5 | Thiophene, 3-ethyl- | 3.55 | 97.0106 | 21,633,341 | C6H8S | 1795-01-3 | N/C | Heterocyclic | |
| 6 | Allylacetone | 3.64 | 43.0178 | 50,094,456 | C6H10O | 109-49-9 | N/C | Ketone | |
| 7 | p-Xylene | 3.78 | 91.0542 | 20,357,362 | C8H10 | 106-42-3 | N/C | Hydrocarbons | |
| 12 | Allyl Isothiocyanate | 4.67 | 99.0137 | 2,235,272 | C4H5NS | 57-06-7 | N/C | Others | |
| 13 | o-Xylene | 4.85 | 91.0542 | 10,171,454 | C8H10 | 95-47-6 | N/C | Hydrocarbons | |
| 14 | Benzocyclobutene | 4.87 | 104.0621 | 25,872,768 | C8H8 | 694-87-1 | N/C | Hydrocarbons | |
| 17 | Caproic acid methyl ester | 6.56 | 74.0362 | 7,124,719 | C7H14O2 | 106-70-7 | 863 | 925 | Ester |
| 18 | 7-Azabicyclo[4.1.0] heptane, 2-methyl- | 7.39 | 96.0808 | 1,110,790 | C7H13N | 55903-15-6 | 896 | 948 | Heterocyclic |
| 20 | n-propylbenzene | 7.52 | 91.0542 | 1,887,762 | C9H12 | 103-65-1 | 901 | 953 | Hydrocarbons |
| 21 | 7-Azabicyclo[4.1.0] heptane, 1-methyl- | 7.71 | 82.0651 | 6,019,497 | C7H13N | 25022-25-7 | 908 | 961 | Heterocyclic |
| 25 | Heptanonitrile | 8.98 | 41.0386 | 113,477,389 | C7H13N | 629-08-3 | 951 | 988 | Others |
| 26 | 2,3-Octanedione | 8.99 | 43.0178 | 81,773,661 | C8H14O2 | 585-25-1 | 951 | 984 | Ketone |
| 27 | 2-Amylfuran | 9.06 | 81.0335 | 47,171,653 | C9H14O | 3777-69-3 | 954 | 993 | Heterocyclic |
| 28 | Cyclopentane, 1-methyl-3-(2-methyl-1-propenyl)- | 9.26 | 81.0699 | 15,627,543 | C10H18 | 75873-01-7 | 960 | 972 | Hydrocarbons |
| 37 | d-Limonene | 10.44 | 68.0621 | 217,256,255 | C10H16 | 5989-27-5 | 1001 | 1018 | Terpenoids |
| 41 | 2,2-Dimethyl-1-aza-spiro[2.4]heptane | 11.2 | 110.0964 | 584,869 | C8H15N | N/A *** | 1023 | 1034 | Heterocyclic |
| 44 | 3-[(E)-3-Methyl-1-butenyl]-1-cyclohexene | 12.03 | 79.0542 | 6,086,431 | C11H18 | 56030-49-0 | 1054 | 1104 | Hydrocarbons |
| 45 | (3E,5E)-3,5-Octadien-2-one | 12.10 | 95.0491 | 187,208,595 | C8H12O | 30086-02-3 | 1056 | 1073 | Ketone |
| 52 | Cyclohexene, 2-ethenyl-1,3,3-trimethyl- | 13.08 | 135.1168 | 8,886,180 | C11H18 | 5293-90-3 | 1089 | 1105 | Hydrocarbons |
| 74 | Naphthalene | 15.76 | 128.0621 | 1,425,023 | C10H8 | 91-20-3 | 1181 | 1182 | Hydrocarbons |
| 82 | Dimethyltetrasulfane | 16.65 | 157.9347 | 831,336,230 | C2H6S4 | 5756-24-1 | 1315 | 1234 | Others |
| 84 | Citral | 16.79 | 69.0699 | 311,692,396 | C10H16O | 5392-40-5 | 1217 | 1276 | Terpenoids |
| 93 | 1,7-Octadiene-3,6-diol, 2,6-dimethyl- | 18.15 | 67.0542 | 2,553,586 | C10H18O2 | 51276-33-6 | 1267 | 1273 | Terpenoids |
| 95 | 1-Methoxyindole | 18.32 | 117.0573 | 50,967,860 | C9H9NO | 54698-11-2 | 1273 | 1218 | Heterocyclic |
| 102 | Methyl caprate | 19.55 | 74.0362 | 122,090,959 | C11H22O2 | 110-42-9 | 1318 | 1325 | Ester |
| 138 | trans-β-lonone | 23.68 | 177.1274 | 1,314,889,389 | C13H20O | 79-77-6 | 1481 | 1486 | Ketone |
| 148 | Benzene, 1,4-bis(1-formylethyl)- | 25.73 | 161.0961 | 13,325,826 | C12H14O2 | N/A *** | 1568 | 1553 | Aldehyde |
| 157 | Tetradecanal | 26.68 | 57.0335 | 282,159,980 | C14H28O | 124-25-4 | 1609 | 1613 | Aldehyde |
| 164 | 7-Methoxy-4-quinolinol | 28.54 | 175.0628 | 2,991,165,529 | C10H9NO2 | 82121-05-9 | 1694 | 1635 | Heterocyclic |
| 166 | 1-Heptadecyne | 28.93 | 81.0699 | 84,475,533 | C17H32 | 26186-00-5 | 1712 | 1709 | Hydrocarbons |
| 169 | Arvelexin | 29.54 | 171.0553 | 846,914 | C11H10N2O | 4837-74-5 | 1740 | 1798 | Heterocyclic |
| 178 | 3-Octadecyne | 31.67 | 67.0542 | 547,120,892 | C18H34 | 61886-64-4 | 1838 | 1828 | Hydrocarbons |
| 180 | 7,10,13-Hexadecatrienoic acid, methyl ester | 33.01 | 79.0542 | 58,497,194 | C17H28O2 | 56554-30-4 | 1896 | 1902 | Ester |
| 181 | Methyl palmitate | 33.45 | 74.0362 | 26,907,298 | C17H34O2 | 112-39-0 | 1924 | 1926 | Ester |
| 197 | Methyl Linolenate | 35.62 | 79.0542 | 86,055,764 | C19H32O2 | 301-00-8 | 2096 | 2098 | Ester |
| 201 | 3,4′-Isopropylidenediphenol | 36.62 | 213.0910 | 156,994,944 | C15H16O2 | 46765-25-7 | 2211 | 2173 | Others |
| No. | Cultivar | Provider |
|---|---|---|
| Q28 | Yacui 60 | Shouguang Syngenta Seed Co., Ltd., Shouguang, China |
| Q49 | Zheqing 161 | Zhejiang Academy of Agricultural Sciences, Hangzhou, China |
| Q213 | W10 | Wenzhou Academy of Agricultural Sciences, Wenzhou, China |
| Q214 | W11 | Wenzhou Academy of Agricultural Sciences, Wenzhou, China |
| Q217 | W14 | Wenzhou Academy of Agricultural Sciences, Wenzhou, China |
| Q230 | Wancui 2 | Shouguang Syngenta Seed Co., Ltd., Shouguang, China |
| Q265 | Zhongqing 518 | Institute of vegetables and flowers, Chinese academy of agricultural Sciences, Beijing, China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; Yan, M.; Lin, S.; Li, J.; Zou, H.; Hu, Z.; Yan, X. Dual-Ionization SPME-GC–HRMS Metabolomic Profiling of Broccoli Volatiles for the Construction of a Broccoli Metabolic Database. Molecules 2025, 30, 3781. https://doi.org/10.3390/molecules30183781
Song C, Yan M, Lin S, Li J, Zou H, Hu Z, Yan X. Dual-Ionization SPME-GC–HRMS Metabolomic Profiling of Broccoli Volatiles for the Construction of a Broccoli Metabolic Database. Molecules. 2025; 30(18):3781. https://doi.org/10.3390/molecules30183781
Chicago/Turabian StyleSong, Chenxue, Meijia Yan, Sue Lin, Junliang Li, Huixi Zou, Zhiwei Hu, and Xiufeng Yan. 2025. "Dual-Ionization SPME-GC–HRMS Metabolomic Profiling of Broccoli Volatiles for the Construction of a Broccoli Metabolic Database" Molecules 30, no. 18: 3781. https://doi.org/10.3390/molecules30183781
APA StyleSong, C., Yan, M., Lin, S., Li, J., Zou, H., Hu, Z., & Yan, X. (2025). Dual-Ionization SPME-GC–HRMS Metabolomic Profiling of Broccoli Volatiles for the Construction of a Broccoli Metabolic Database. Molecules, 30(18), 3781. https://doi.org/10.3390/molecules30183781








