Electrochemical Coagulant Generation via Aluminum-Based Electrocoagulation for Sustainable Greywater Treatment and Reuse: Optimization Through Response Surface Methodology and Kinetic Modelling
Abstract
1. Introduction
2. Results and Discussion
2.1. Greywater Characteristics
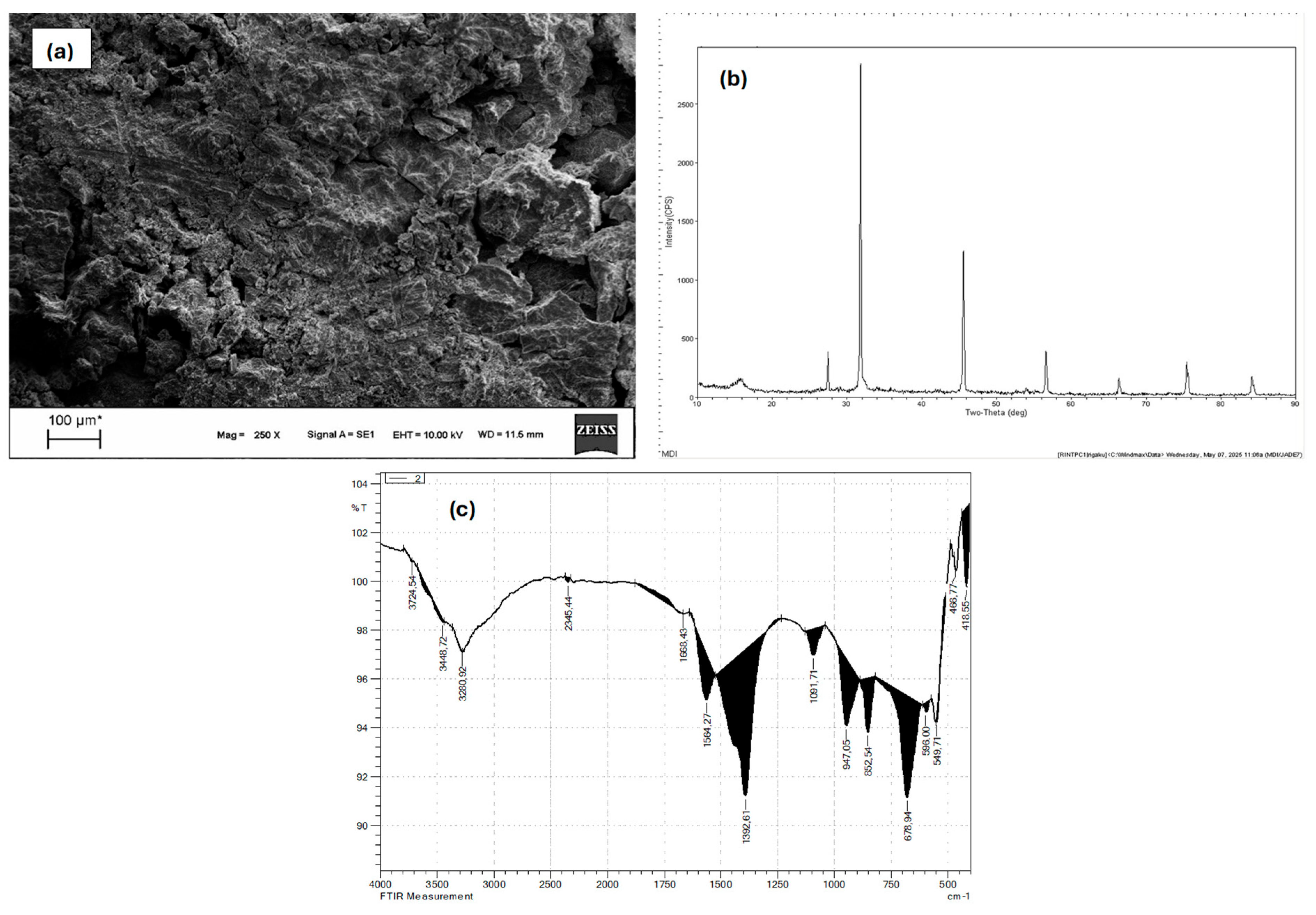
2.2. Electrode Characteristics
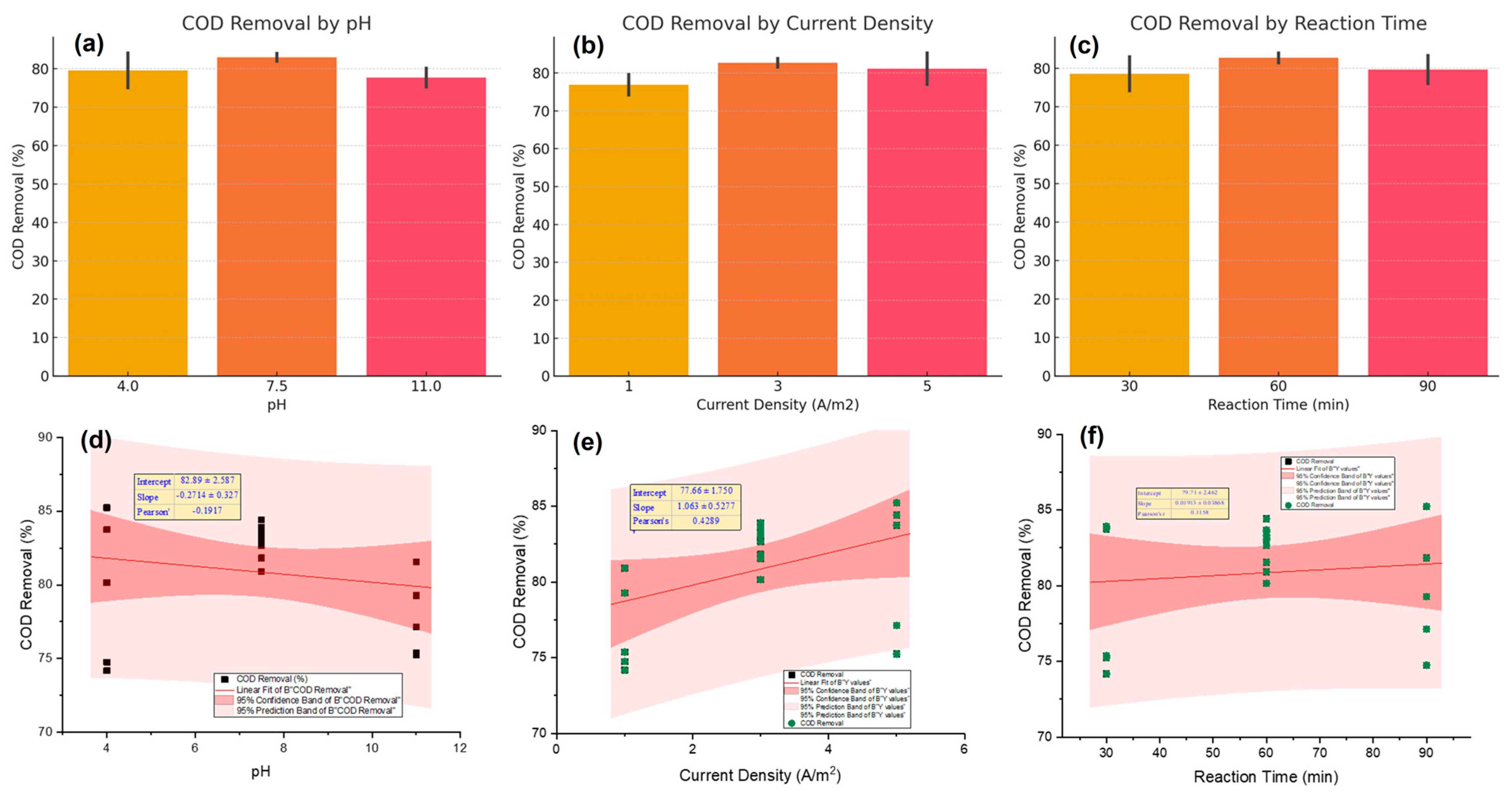
2.3. Effect of Current Density
2.4. Effect of Reaction Time
2.5. Effect of pH
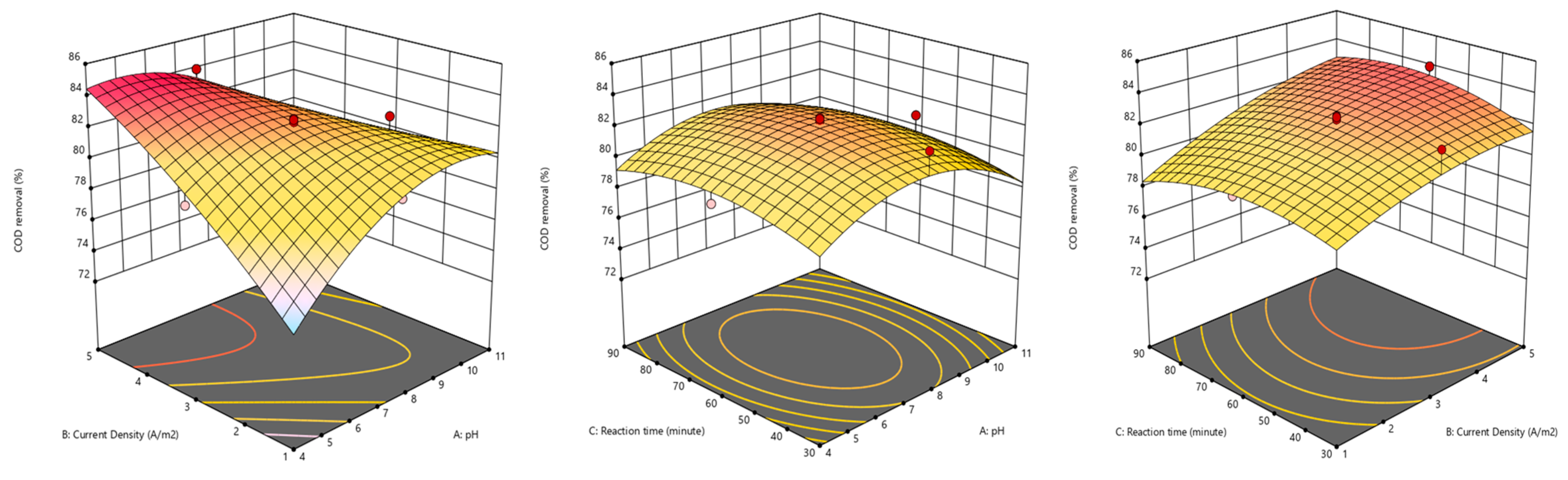
2.6. RSM Optimization
2.7. ANOVA-Based Statistical Evaluation of Experimental Data
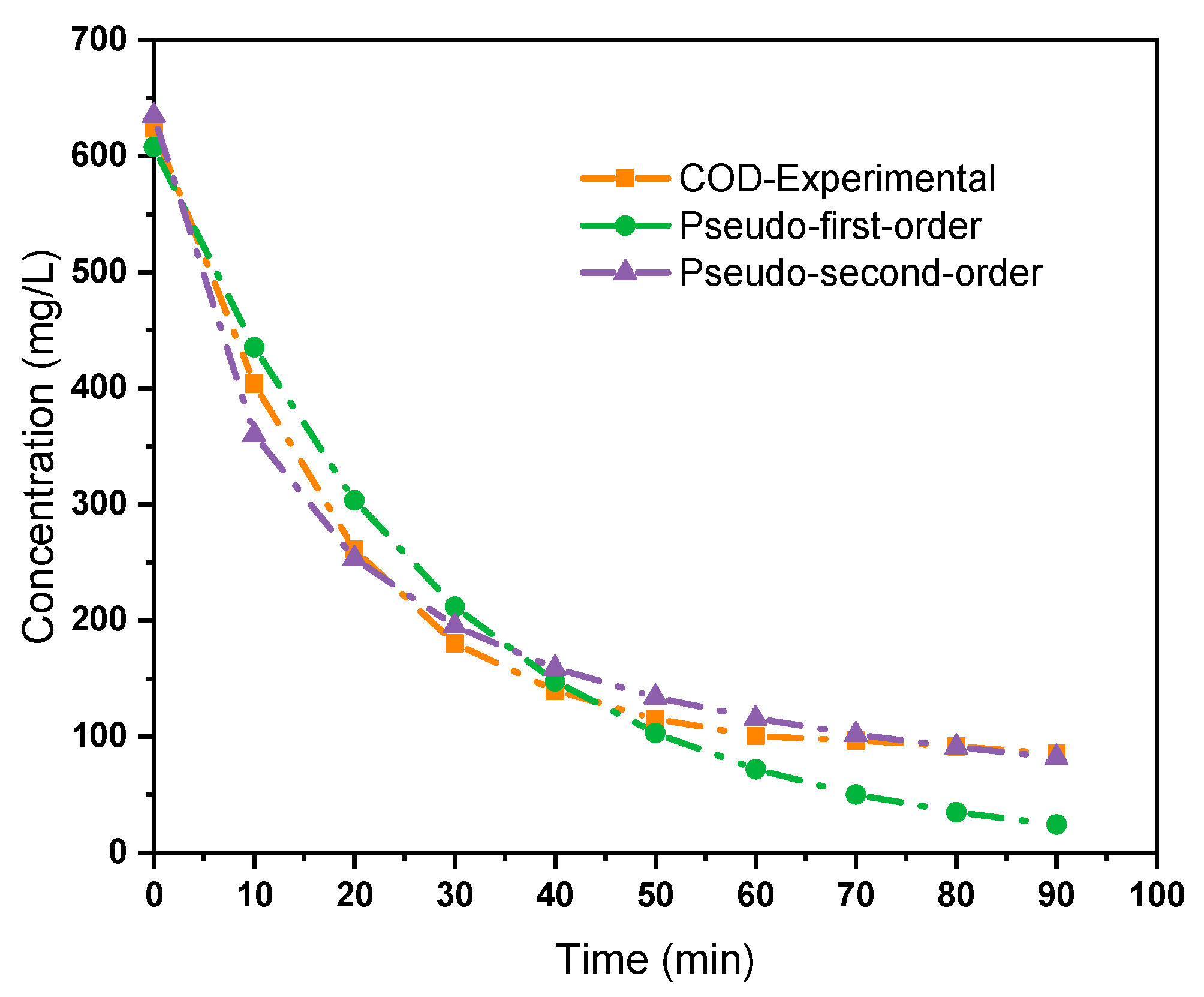
2.8. Kinetic Modelling of COD Removal
2.9. Sludge Characteristics
2.10. Greywater Reuse Potential and Compliance with International Standards
3. Materials and Methods
3.1. Greywater Sampling and Characterization
3.2. Experimental Setup and Procedure
3.3. Response Surface Methodology
3.4. Kinetic Study
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karichappan, S.; Kandasamy, J.; Naidu, S. Optimization of Electrocoagulation Process to Treat Grey Wastewater in Batch Mode Using Response Surface Methodology. Environ. Syst. Res. 2014, 3, 29. [Google Scholar] [CrossRef]
- Bajpai, M.; Katoch, S.S. Reduction of COD from Real Graywater by Electro-Coagulation Using Fe Electrode: Optimization through Box-Behnken Design. Mater. Today Proc. 2021, 43, 303–307. [Google Scholar] [CrossRef]
- Tabash, I.; Elnakar, H.; Khan, M. Optimization of Iron Electrocoagulation Parameters for Enhanced Turbidity and Chemical Oxygen Demand Removal from Laundry Greywater. Sci. Rep. 2024, 14, 16468. [Google Scholar] [CrossRef]
- Ansari, K.; Shrikhande, A.; Malik, M.A.; Alahmadi, A.A.; Alwetaishi, M.; Alzaed, A.N.; Elbeltagi, A. Optimization and Operational Analysis of Domestic Greywater Treatment by Electrocoagulation Filtration Using Response Surface Methodology. Sustainability 2022, 14, 15230. [Google Scholar] [CrossRef]
- Ikumapayi, O.M. Comparative Analysis of Greywater Treatment Systems for Sustainable Water Reuse. In E3S Web of Conferences, Proceedings of the 1st Trunojoyo Madura International Conference (1st TMIC 2023), Surabaya, Indonesia, 29 November 2023; EDP Sciences: Les Ulis, France, 2024; Volume 499, p. 01047. [Google Scholar]
- Oteng-Peprah, M.; Acheampong, M.A.; deVries, N.K. Greywater Characteristics, Treatment Systems, Reuse Strategies and User Perception—A Review. Water Air Soil Pollut. 2018, 229, 255. [Google Scholar] [CrossRef]
- Filali, M.; Bahmani, R.; Bousselmi, L. A Review of Greywater Treatment Systems Using Coagulation Process: Performance and Limitations. Sustainability 2022, 14, 665. [Google Scholar] [CrossRef]
- Veli, S.; Arslan, A.; Bingöl, D. Application of Response Surface Methodology to Electrocoagulation Treatment of Hospital Wastewater. Clean 2016, 44, 1516–1522. [Google Scholar] [CrossRef]
- Awasthi, A.; Gandhi, K.; Rayalu, S. Greywater treatment technologies: A comprehensive review. Int. J. Environ. Sci. Technol. 2024, 21, 1053–1082. [Google Scholar]
- Kariuki, F.W.; Kotut, K.; Ngángá, V.G. The Potential of a Low Cost Technology for the Greywater Treatment. Open Environ. Eng. J. 2011, 4, 32–39. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M.; Al-Sulaiman, A.M. Anaerobic/Aerobic Integration via Uasb/Enhanced Aeration for Greywater Treatment and Unrestricted Reuse. Water Pract. Technol. 2019, 14, 837–850. [Google Scholar] [CrossRef]
- Ebba, M.; Asaithambi, P.; Alemayehu, E. Development of Electrocoagulation Process for Wastewater Treatment: Optimization by Response Surface Methodology. Heliyon 2022, 8, e09383. [Google Scholar] [CrossRef]
- Patil, Y.M.; Munavalli, G.R. Performance Evaluation of an Integrated On-Site Greywater Treatment System in a Tropical Region. Ecol. Eng. 2016, 95, 492–500. [Google Scholar] [CrossRef]
- Devikar, S.; Ansari, K.; Waghmare, C. Solar Based Hybrid Combination of Electrocoagulation and Filtration Process in Domestic Greywater Treatment. Indian J. Sci. Technol. 2021, 14, 2215–2222. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Pareek, V.; Ang, M. A Review of Greywater Characteristics and Treatment Processes. Water Sci. Technol. 2013, 67, 1403–1424. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, Y.; Qi, B. Recent Insights into Greywater Treatment: A Comprehensive Review on Characteristics, Treatment Technologies, and Pollutant Removal Mechanisms. Environ. Sci. Pol. Res. 2022, 29, 54025–54044. [Google Scholar] [CrossRef]
- Ejairu, U.; Aderamo, A.T.; Olisakwe, H.C.; Esiri, A.E.; Adanma, U.M.; Solomon, N.O. Eco-Friendly Wastewater Treatment Technologies (Concept): Conceptualizing Advanced, Sustainable Wastewater Treatment Designs for Industrial and Municipal Applications. CCRET 2024, 2, 83–104. [Google Scholar]
- Khapra, R.; Singh, N. Physical, Chemical, and Biological Evaluation of Domestic Laundry Greywater Discharges to Attract Reclamation Strategies and Reuse Applications in Urban Settings. Environ. Qual. Manag. 2024, 33, 209–221. [Google Scholar] [CrossRef]
- Shaikh, I.N.; Ahammed, M.M. Sand filtration for greywater treatment: Long-term performance evaluation and optimization by response surface methodology. Urban Water J. 2023, 20, 450–464. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Safe Use of Wastewater, Excreta and Greywater in Agriculture and Aquaculture; World Health Organization: Geneva, Switzerland, 2006; Volume 4, ISBN 9789241546850. [Google Scholar]
- USEPA. Guidelines for Water Reuse; EPA/600/R-12/618; United States Environmental Protection Agency (USEPA): Washington, DC, USA, 2012.
- European Union (EU). Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on Minimum Requirements for Water Reuse. In Official Journal of the European Union; (L 177/3); European Union: Brussels, Belgium, 2020. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; FAO Irrigation and Drainage Paper 29, rev. 1; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985; 174p. [Google Scholar]
- Meena, S.K.; Meena, S.O.; Sangal, V.K. Once through Continuous Mode Electrocoagulation for Tannery Wastewater Remediation: Process Optimization and Performance Evaluation. J. Indian Chem. Soc. 2024, 101, 101212. [Google Scholar] [CrossRef]
- Gerek, E.E. Optimized Sizing of Hybrid Renewable Energy Systems for Sustainable Buildings with Seasonal Grey Water Treatment. Sustain. Energy Technol. Assess. 2025, 76, 104287. [Google Scholar] [CrossRef]
- Leong, J.Y.C.; Chong, M.N.; Poh, P.E. Assessment of Greywater Quality and Performance of a Pilot-Scale Decentralised Hybrid Rainwater-Greywater System. J. Clean Prod. 2018, 172, 81–91. [Google Scholar] [CrossRef]
- Vakil, K.A.; Sharma, M.K.; Bhatia, A.; Kazmi, A.A.; Sarkar, S. Characterization of Greywater in an Indian Middle-Class Household and Investigation of Physicochemical Treatment Using Electrocoagulation. Sep. Purif. Technol. 2014, 130, 160–166. [Google Scholar] [CrossRef]
- Govindan, K.; McNamara, P.J.; Lavin, J.; Raja, M.; Samuel, M.; Kuru, W.; Smies, A.; Mayer, B.K. Response Surface Methodology for Evaluating Electrocoagulation Treatment of Bath and Laundry Greywater. J. Water Process Eng. 2025, 71, 107273. [Google Scholar]
- Tibebe, D.; Dejene, F.B.; Shigute, T.; Berhe, T. Characterization of Sludge Generated from Electrocoagulation of Domestic Wastewater: Removal Mechanism of Phosphate and Nitrate. BMC Chem. 2019, 13, 107. [Google Scholar] [CrossRef]
- Al-Marri, J.S.; Abouedwan, A.B.; Ahmad, M.I.; Bensalah, N. Electrocoagulation Using Aluminum Electrodes as a Sustainable and Economic Method for the Removal of Kinetic Hydrate Inhibitor (Polyvinyl Pyrrolidone) from Produced Wastewaters. Front. Water 2023, 5, 1305347. [Google Scholar] [CrossRef]
- Ghernaout, D. An Insight in Electrocoagulation Process through Current Density Distribution (CDD). OA Libr. J. 2020, 7, 1. [Google Scholar]
- Boinpally, S.; Kolla, A.; Kainthola, J.; Kodali, R.; Vemuri, J. A State-of-the-Art Review of the Electrocoagulation Technology for Wastewater Treatment. Water Cycle 2023, 4, 26–36. [Google Scholar]
- Reátegui-Romero, W.; Morales-Quevedo, S.E.; Huanca-Colos, K.W.; Figueroa-Gómez, N.M.; King-Santos, M.E.; Zaldivar-Alvarez, W.F.; Pino, L.V.F.-D.; Yuli-Posadas, R.A.; Bulege-Gutiérrez, W. Effect of Current Density on COD Removal Efficiency for Wastewater Using the Electrocoagulation Process. Des. Water Treat. 2020, 184, 15–29. [Google Scholar] [CrossRef]
- Patel, P.; Gupta, S.; Mondal, P. Electrocoagulation Process for Greywater Treatment: Statistical Modeling, Optimization, Cost Analysis and Sludge Management. Sep. Purif. Technol. 2022, 296, 121327. [Google Scholar] [CrossRef]
- Mahmad, M.K.N.; Rozainy, M.M.R.; Abustan, I.; Baharun, N. Electrocoagulation Process by Using Aluminium and Stainless Steel Electrodes to Treat Total Chromium, Colour and Turbidity. Procedia Chem. 2016, 19, 681–686. [Google Scholar] [CrossRef]
- Khan, S.U.; Khalid, M.; Hashim, K.; Jamadi, M.H.; Mousazadeh, M.; Basheer, F.; Farooqi, I.H. Efficacy of Electrocoagulation Treatment for the Abatement of Heavy Metals: An Overview of Critical Processing Factors, Kinetic Models and Cost Analysis. Sustainability 2023, 15, 1708. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Zghoul, T.M.; Jamrah, A. The Performance of Pharmaceutical Wastewater Treatment System of Electrocoagulation Assisted Adsorption Using Perforated Electrodes to Reduce Passivation. Environ. Sci. Pollut. Res. 2024, 31, 20434–20448. [Google Scholar] [CrossRef] [PubMed]
- Mousazadeh, M.; Esrafili, A.; Farzadkia, M.; Gholami, M. Domestic Greywater Treatment Using Electrocoagulation-Electrooxidation Process: Optimisation and Experimental Approaches. J. Environ. Health Sci. Eng. 2023, 21, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Yazici Karabulut, B.; Atasoy, A.D.; Can, O.T.; Yesilnacar, M.I. Electrocoagulation for Nitrate Removal in Groundwater of Intensive Agricultural Region: A Case Study of Harran Plain, Turkey. Environ. Earth Sci. 2021, 80, 190. [Google Scholar] [CrossRef]
- Barzegar, G.; Wu, J.; Ghanbari, F. Enhanced Treatment of Greywater Using Electrocoagulation/Ozonation: Investigation of Process Parameters. Process Saf. Environ. Prot. 2019, 121, 125–132. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Onukwuli, O.D.; Ighalo, J.O.; Umembamalu, C.J.; Adeniyi, A.G. Comparative Analysis on the Electrochemical Reduction of Colour, COD and Turbidity from Municipal Solid Waste Leachate Using Aluminium, Iron and Hybrid Electrodes. Sustain Water Resour. Manag. 2021, 7, 39. [Google Scholar] [CrossRef]
- Sharma, L.; Prabhakar, S.; Tiwari, V.; Dhar, A.; Halder, A. Optimization of EC Parameters Using Fe and Al Electrodes for Hydrogen Production and Wastewater Treatment. Environ. Adv. 2021, 3, 100029. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Alnaief, M.; Al-Qodah, Z.; Al-Shannag, M.; Elnakar, H.; AlJbour, N.; Alu’dAtt, M.; Alrosan, M.; Ezelden, E. On the Performance of Electrocoagulation Treatment of High-Loaded Gray Water: Kinetic Modeling and Parameters Optimization via Response Surface Methodology. Appl. Water Sci. 2025, 15, 114. [Google Scholar] [CrossRef]
- Pacheco, H.G.J.; Elguera, N.Y.M.; Sarka, H.D.Q.; Ancco, M.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Box-Behnken Response Surface Design for Modeling and Optimization of Electrocoagulation for Treating Real Textile Wastewater. Int. J. Environ. Res. 2022, 16, 43. [Google Scholar] [CrossRef]
- Gasmi, A.; Ibrahimi, S.; Elboughdiri, N.; Tekaya, M.A.; Ghernaout, D.; Hannachi, A.; Mesloub, A.; Ayadi, B.; Kolsi, L. Comparative Study of Chemical Coagulation and Electrocoagulation for the Treatment of Real Textile Wastewater: Optimization and Operating Cost Estimation. ACS Omega 2022, 7, 22456–22476. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
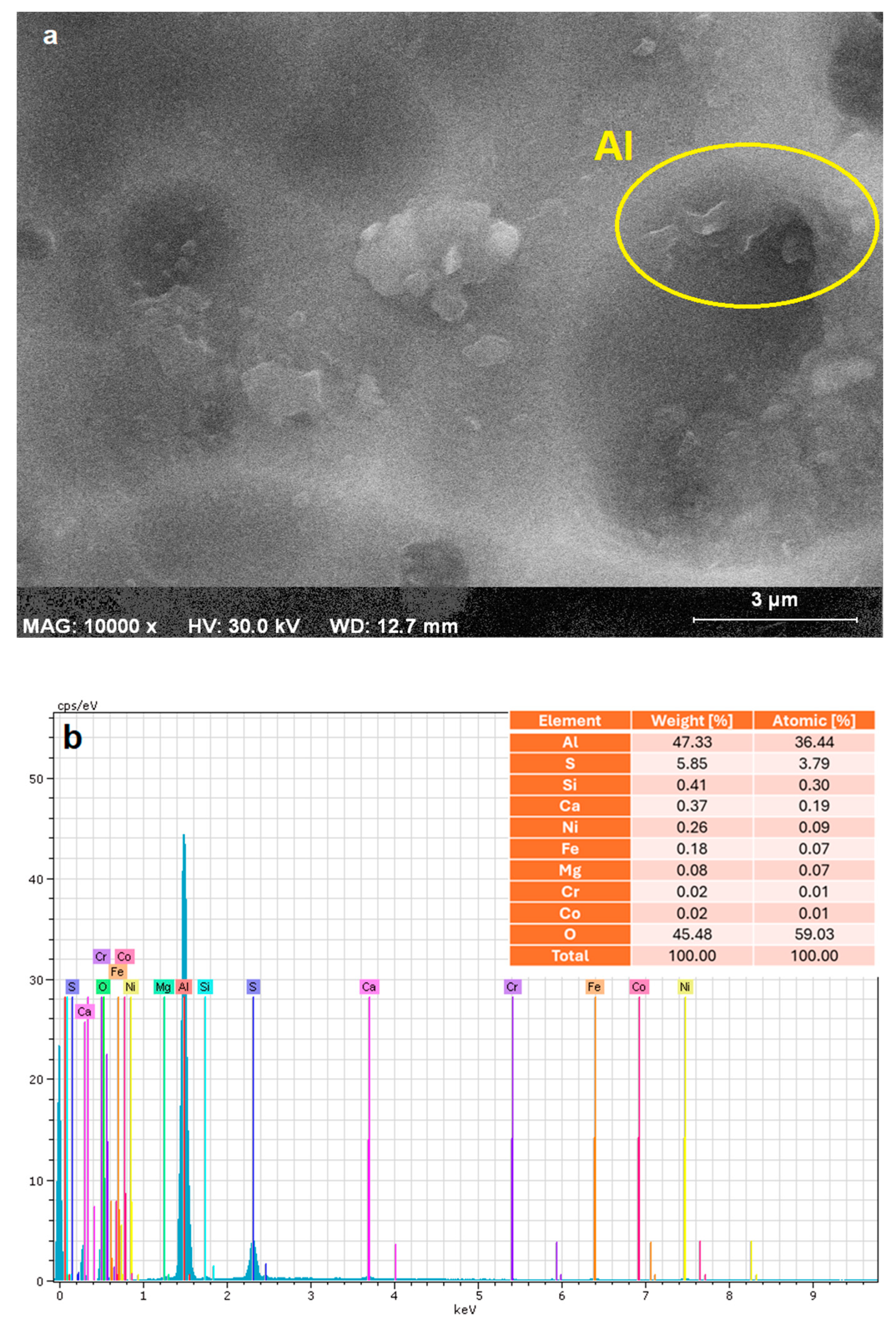


| Parameter | Unit | Raw Greywater | Treated Greywater | WHO [20] | USEPA [21] | EU 2020/741 [22] |
|---|---|---|---|---|---|---|
| pH | – | 8.1 | 7.5 | 6.0–9.0 | 6.5–8.5 | 6.0–9.0 |
| COD | mg/L | 624 | 85.24 | — | ≤50 | ≤125 |
| Turbidity | NTU | 74 | 1.8 | — | ≤2 | ≤5 |
| TSS | mg/L | 286 | 4.5 | ≤30 | ≤5 | ≤35 |
| EC | µg/L | 1350 | 865 | — | — | — |
| Factor 1 | Factor 2 | Factor 3 | Response 1 | |
|---|---|---|---|---|
| Run | A: pH | B: Current Density | C: Reaction Time | COD Removal |
| A/m2 | min | % | ||
| 1 | 11 | 3 | 60 | 80.78 |
| 2 | 7.5 | 3 | 60 | 82.59 |
| 3 | 11 | 5 | 30 | 74.61 |
| 4 | 7.5 | 3 | 90 | 80.34 |
| 5 | 7.5 | 3 | 60 | 82.06 |
| 6 | 7.5 | 1 | 60 | 79.82 |
| 7 | 11 | 5 | 90 | 76.77 |
| 8 | 7.5 | 3 | 60 | 82.37 |
| 9 | 4 | 1 | 90 | 73.85 |
| 10 | 7.5 | 5 | 60 | 83.91 |
| 11 | 7.5 | 3 | 30 | 82.61 |
| 12 | 4 | 3 | 60 | 79.41 |
| 13 | 7.5 | 3 | 60 | 82.11 |
| 14 | 7.5 | 3 | 60 | 82.52 |
| 15 | 11 | 1 | 90 | 78.62 |
| 16 | 4 | 1 | 30 | 73.09 |
| 17 | 11 | 1 | 30 | 79.53 |
| 18 | 4 | 5 | 30 | 82.7 |
| 19 | 7.5 | 3 | 60 | 81.37 |
| 20 | 4 | 5 | 90 | 84.13 |
| ANOVA Results | |||||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | ||
| Model | 194.60 | 9 | 21.62 | 24.38 | <0.0001 | significant | |
| A-pH | 0.8237 | 1 | 0.8237 | 0.9288 | 0.3579 | ||
| B-Current Density | 29.62 | 1 | 29.62 | 33.40 | 0.0002 | ||
| C-Reaction time | 0.1369 | 1 | 0.1369 | 0.1544 | 0.7026 | ||
| AB | 88.84 | 1 | 88.84 | 100.19 | <0.0001 | ||
| AC | 0.1105 | 1 | 0.1105 | 0.1245 | 0.7315 | ||
| BC | 1.75 | 1 | 1.75 | 1.97 | 0.1906 | ||
| A2 | 17.26 | 1 | 17.26 | 19.46 | 0.0013 | ||
| B2 | 1.49 | 1 | 1.49 | 1.68 | 0.2246 | ||
| C2 | 3.48 | 1 | 3.48 | 3.92 | 0.0757 | ||
| Residual | 8.87 | 10 | 0.8868 | ||||
| Lack of Fit | 7.87 | 5 | 1.57 | 7.92 | 0.0002 | significant | |
| Pure Error | 0.9946 | 5 | 0.1989 | ||||
| Cor Total | 203.47 | 19 | |||||
| Model Fit Statistics | |||||||
| Targeted Compounds | Std. Dev. | Mean | C.V. % | R2 | Adjusted R2 | Predicted R2 | Adeq Precision |
| COD | 0.94 | 80.16 | 1.17 | 0.96 | 0.92 | 0.85 | 16.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazıcı Karabulut, B. Electrochemical Coagulant Generation via Aluminum-Based Electrocoagulation for Sustainable Greywater Treatment and Reuse: Optimization Through Response Surface Methodology and Kinetic Modelling. Molecules 2025, 30, 3779. https://doi.org/10.3390/molecules30183779
Yazıcı Karabulut B. Electrochemical Coagulant Generation via Aluminum-Based Electrocoagulation for Sustainable Greywater Treatment and Reuse: Optimization Through Response Surface Methodology and Kinetic Modelling. Molecules. 2025; 30(18):3779. https://doi.org/10.3390/molecules30183779
Chicago/Turabian StyleYazıcı Karabulut, Benan. 2025. "Electrochemical Coagulant Generation via Aluminum-Based Electrocoagulation for Sustainable Greywater Treatment and Reuse: Optimization Through Response Surface Methodology and Kinetic Modelling" Molecules 30, no. 18: 3779. https://doi.org/10.3390/molecules30183779
APA StyleYazıcı Karabulut, B. (2025). Electrochemical Coagulant Generation via Aluminum-Based Electrocoagulation for Sustainable Greywater Treatment and Reuse: Optimization Through Response Surface Methodology and Kinetic Modelling. Molecules, 30(18), 3779. https://doi.org/10.3390/molecules30183779








