Comparative Antioxidant Protection of Cochlear Hair Cells from Ototoxins
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Explant Preparation
2.3. Compound Testing
2.4. Statistical Analysis
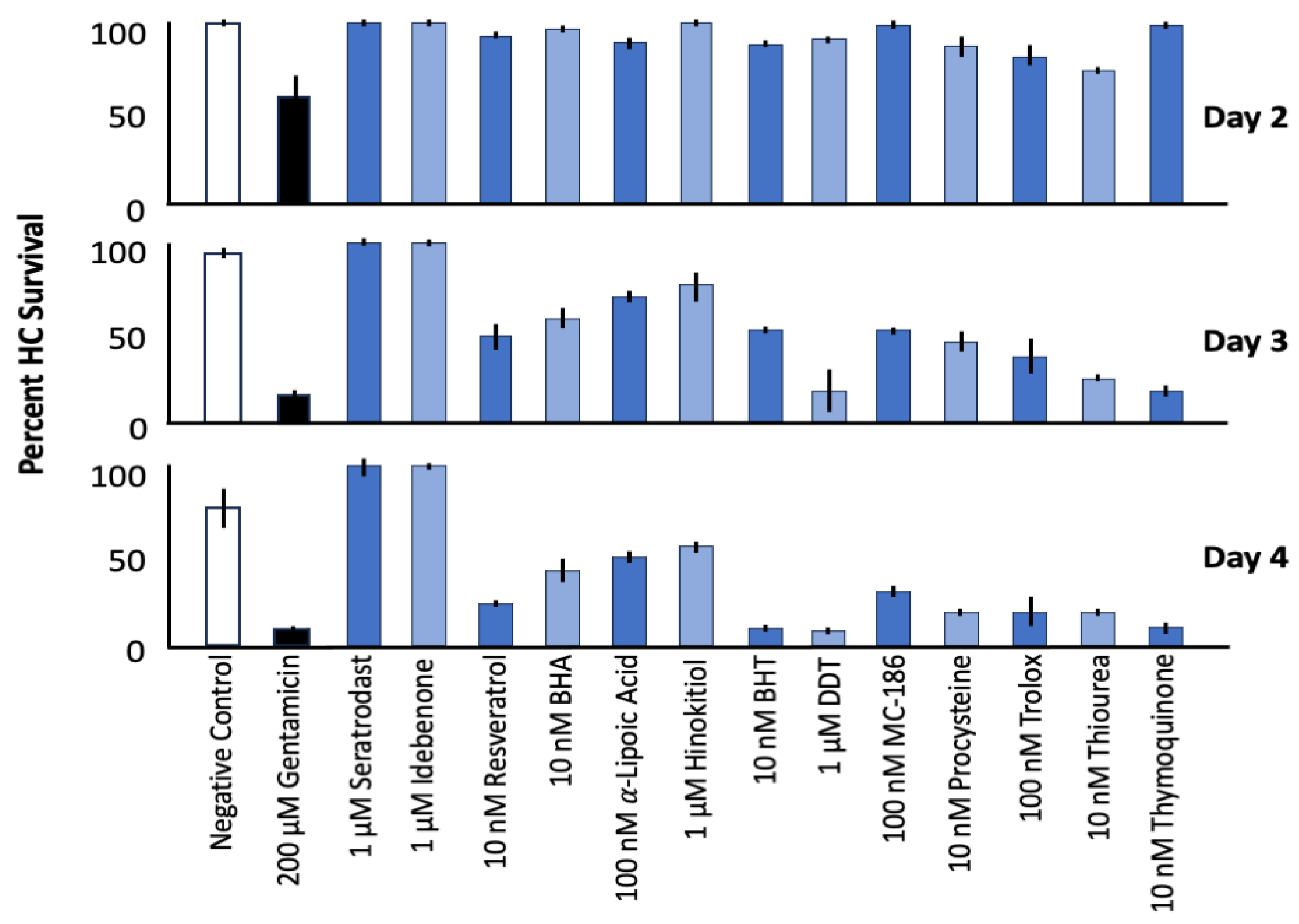
3. Results
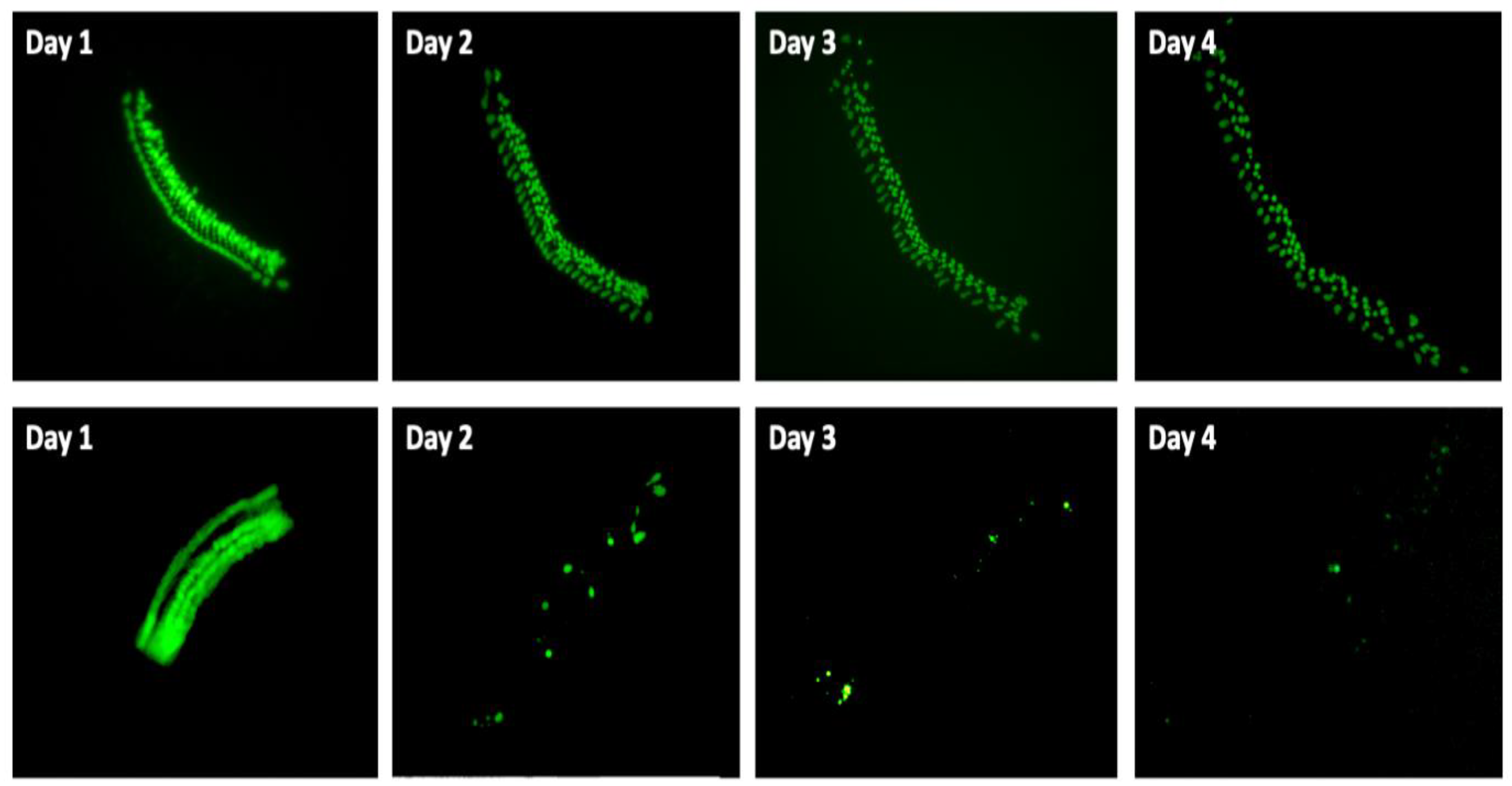
3.1. Control Explants
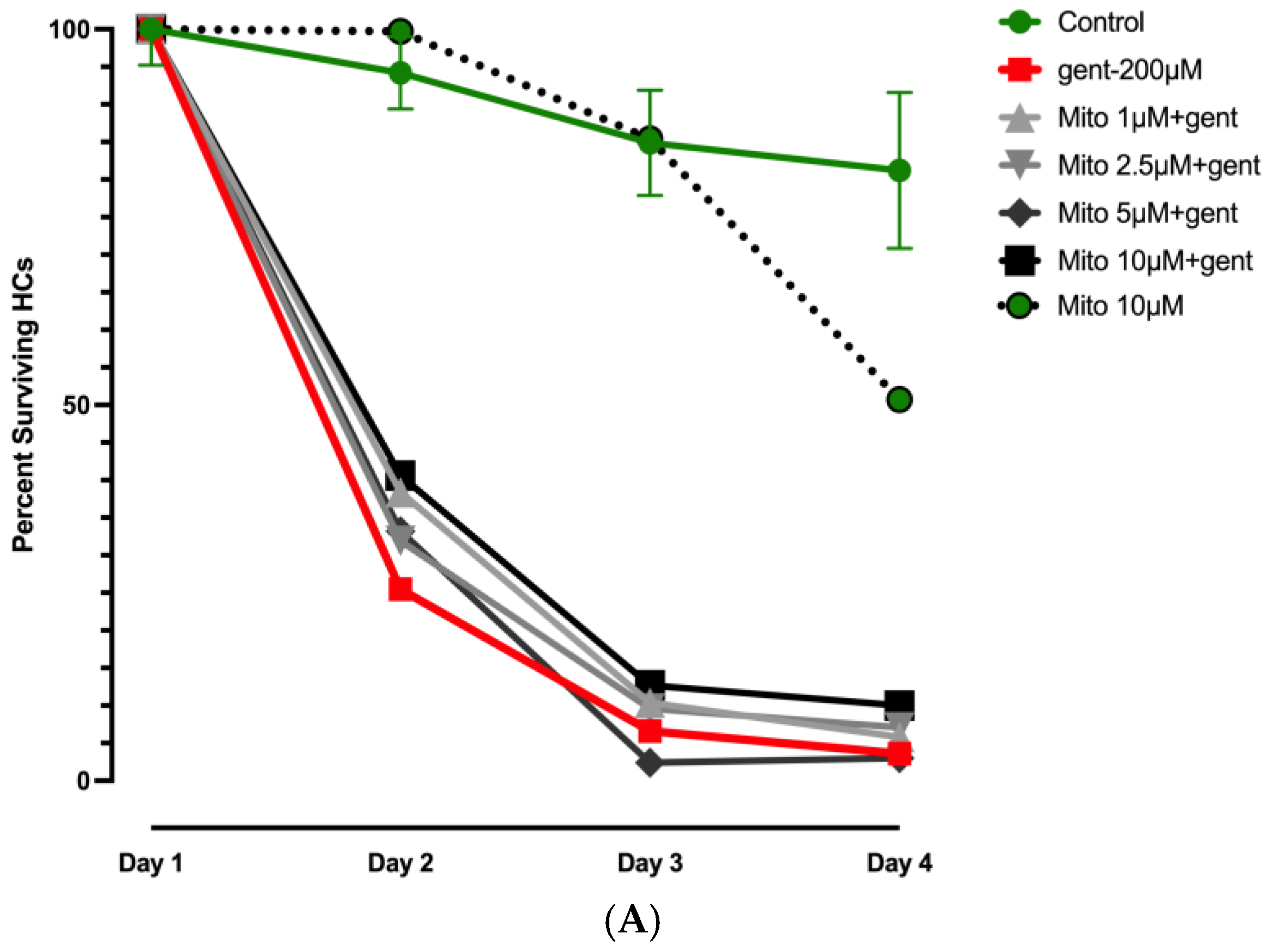
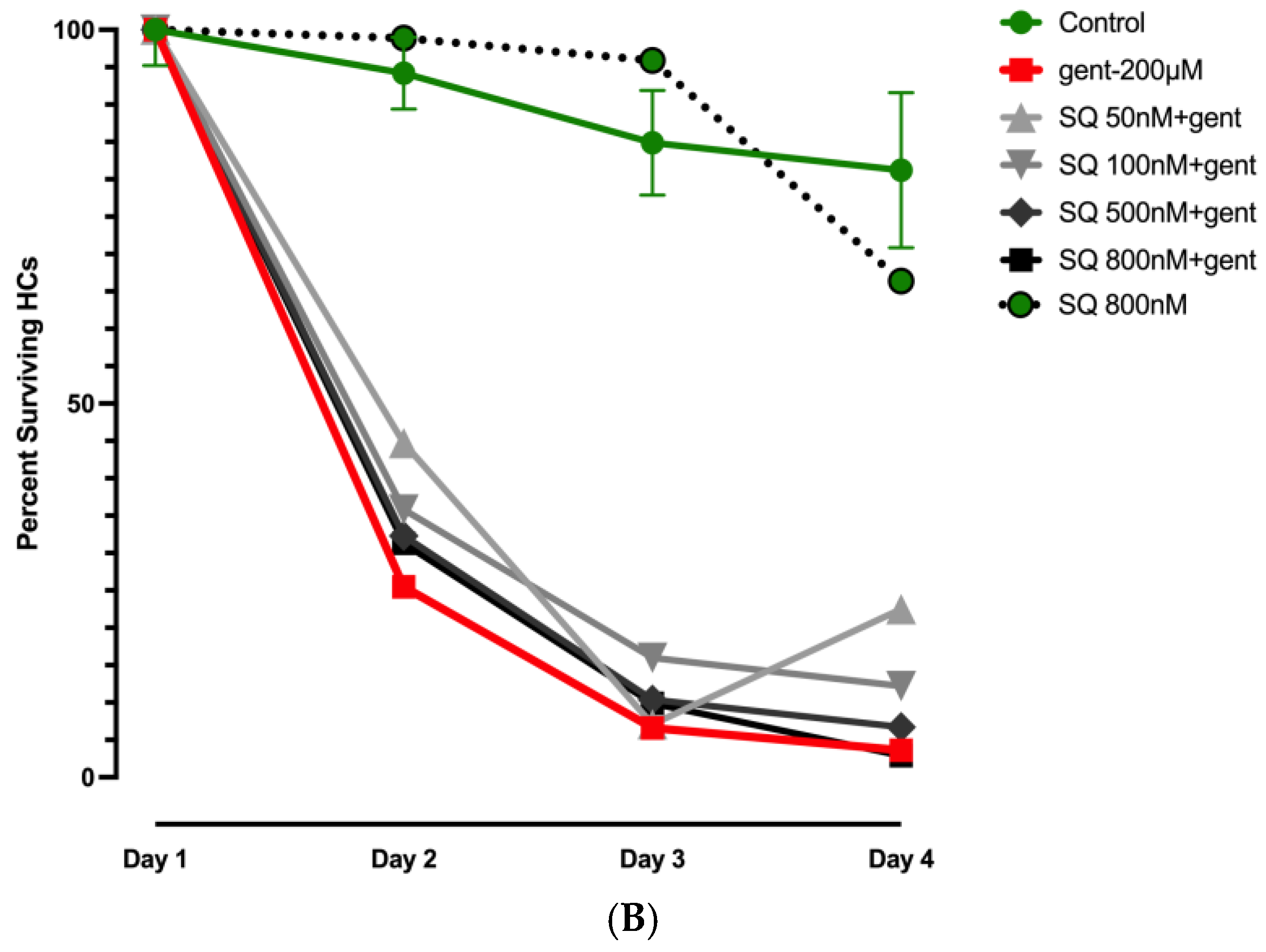
3.2. Effects of Mitochonic Acid or SQ-29548 on Gentamicin-Induced HC Loss
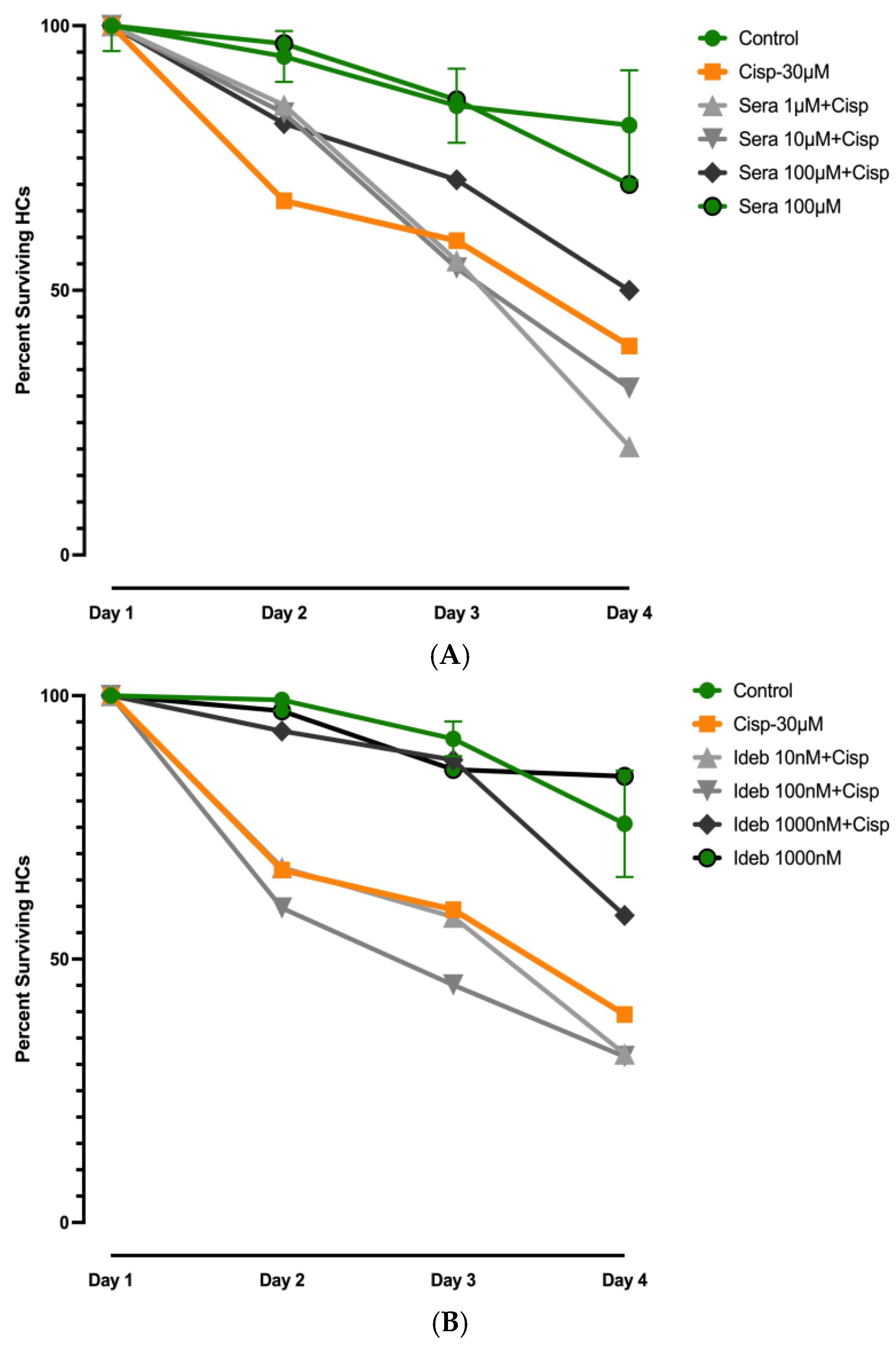
3.3. Effects of Seratrodast or Idebenone on Cisplatin Ototoxicity
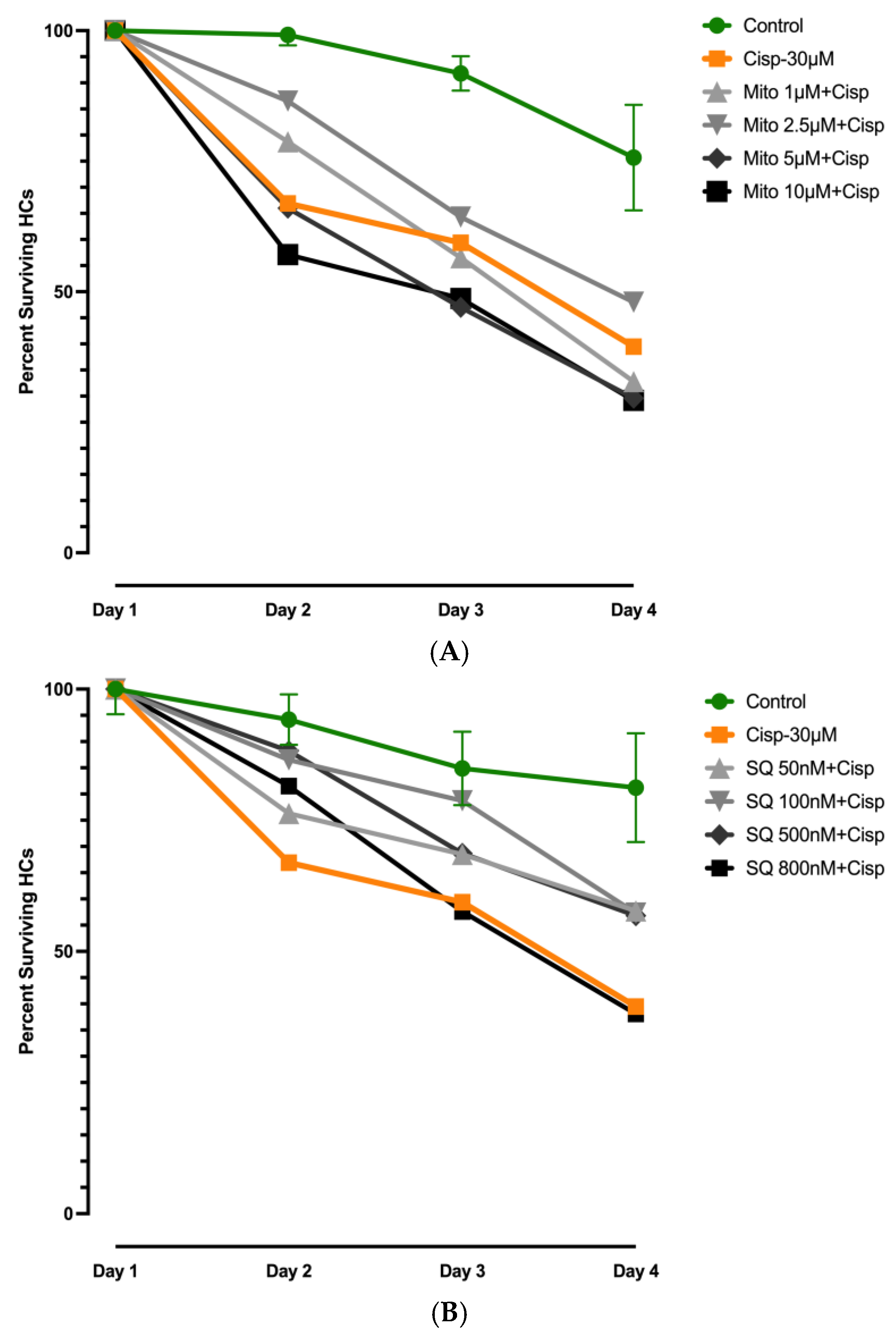
3.4. Effects of Mitochonic Acid or SQ-29548 on Cisplatin-Induced HC Loss
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fausti, S.A.; Henry, J.A.; Schaffer, H.I.; Olson, D.J.; Frey, R.H.; McDonald, W.J. High-frequency audiometric monitoring for early detection of aminoglycoside ototoxicity. J. Infect. Dis. 1992, 165, 1026–1032. [Google Scholar] [CrossRef]
- McKeage, M.J. Comparative adverse effect profiles of platinum drugs. Drug Saf. 1995, 13, 228–244. [Google Scholar] [CrossRef]
- Rybak, L.P.; Mukherjea, D.; Jajoo, S.; Ramkumar, V. Cisplatin ototoxicity and protection: Clinical and experimental studies. Tohoku J. Exp. Med. 2009, 219, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.; Dallos, P. Effect of absence of cochlear outer hair cells on behavioural auditory threshold. Nature 1975, 253, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Kitcher, S.R.; Kirkwood, N.K.; Camci, E.D.; Wu, P.; Gibson, R.M.; Redila, V.A.; Simon, J.A.; Rubel, E.W.; Raible, D.W.; Richardson, G.P.; et al. ORC-13661 protects sensory hair cells from aminoglycoside and cisplatin ototoxicity. JCI Insight. 2019, 4, e126764. [Google Scholar] [PubMed]
- Dinh, C.T.; Goncalves, S.; Bas, E.; Van De Water, T.R.; Zine, A. Molecular regulation of auditory hair cell death and approaches to protect sensory receptor cells and/or stimulate repair following acoustic trauma. Front. Cell Neurosci. 2015, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Esterberg, R.; Hailey, D.W.; Coffin, A.B.; Raible, D.W.; Rubel, E.W. Disruption of intracellular calcium regulation is integral to aminoglycoside-induced hair cell death. J. Neurosci. 2013, 33, 7513–7525. [Google Scholar] [CrossRef]
- Rybak, L.P.; Husain, K.; Whitworth, C.; Somani, S.M. Dose dependent protection by lipoic acid against cisplatin-induced ototoxicity in rats: Antioxidant defense system. Toxicol. Sci. 1999, 47, 195–202. [Google Scholar] [CrossRef][Green Version]
- Sun, Y.; Zou, S.; He, Z.; Chen, X. The role of autophagy and ferroptosis in sensorineural hearing loss. Front. Neurosci. 2022, 16, 1068611. [Google Scholar] [CrossRef]
- Rybak, L.P.; Mukherjea, D.; Ramkumar, V. Mechanisms of cisplatin-Induced ototoxicity and prevention. Semin. Hear. 2019, 40, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.H.; Taylor, R.; Forge, A.; Schacht, J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res. 2001, 155, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Garetz, S.L.; Altschuler, R.A.; Schacht, J. Attenuation of gentamicin ototoxicity by glutathione in the guinea pig in vivo. Hear. Res. 1994, 77, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, K.; Nishimura, B.; Nakamagoe, M.; Hayashi, K.; Nakayama, M.; Hara, A. Ototoxicity: Mechanisms of cochlear impairment and its prevention. Curr. Med. Chem. 2011, 18, 4866–4871. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.H.; Zajic, G.; Epstein, C.J.; Schacht, J. Overexpression of copper/zinc-superoxide dismutase protects from kanamycin-induced hearing loss. Audiol. Neurootol. 2001, 6, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, I.; Vural, A.; Unal, A.; Sipahioglu, M.H.; Yucel, H.E.; Aydemir, S.; Yazici, C.; İlhan Sahin, M.; Oymak, O.; Tokgoz, B. Preventing amikacin related ototoxicity with N-acetylcysteine in patients undergoing peritoneal dialysis. Eur. Arch. Otorhinolaryngol. 2015, 272, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, A.C.; Rosenhall, U.; Olofsson, A.; Hagerman, B. The efficacy of N-acetylcysteine to protect the human cochlea from subclinical hearing loss caused by impulse noise: A controlled trial. Noise Health 2011, 13, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Bagger-Sjöbäck, D.; Strömbäck, K.; Hakizimana, P.; Plue, J.; Larsson, C.; Hultcrantz, M.; Papatziamos, G.; Smeds, H.; Danckwardt-Lillieström, N.; Hellström, S.; et al. A randomised, double blind trial of N-Acetylcysteine for hearing protection during stapes surgery. PLoS ONE 2015, 10, e0115657. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.; Dreisbach, L.; Lockwood, J.; Baldwin, K.; Kopke, R.; Scranton, S.; O′Leary, M. Efficacy of the antioxidant N-acetylcysteine (NAC) in protecting ears exposed to loud music. J. Am. Acad. Audiol. 2006, 17, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Noack, V.; Pak, K.; Jalota, R.; Kurabi, A.; Ryan, A.F. An antioxidant screen identifies candidates for improved protection of cochlear hair cells from gentamicin toxicity. Front. Cell Neurosci. 2017, 11, 242. [Google Scholar] [PubMed]
- Hur, D.; Kurabi, A.; Ryan, A.F. Screening antioxidants for the protection of cochlear sensory cells. Neural Regen. Res. 2018, 13, 62–64. [Google Scholar] [CrossRef]
- Giorgio, V.; Petronilli, V.; Ghelli, A.; Carelli, V.; Rugolo, M.; Lenaz, G.; Bernardi, P. The effects of idebenone on mitochondrial bioenergetics. Biochim. Biophys. Acta 2012, 1817, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Mordente, A.; Martorana, G.E.; Minotti, G.; Giardina, B. Antioxidant properties of 2,3-dimethoxy-5-methyl-6-(10-hydroxydecyl)-1,4-benzoquinone (idebenone). Chem. Res. Toxicol. 1998, 11, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Gueven, N.; Ravishankar, P.; Eri, R.; Rybalka, E. Idebenone: When an antioxidant is not an antioxidant. Redox Biol. 2021, 38, 101812. [Google Scholar] [CrossRef] [PubMed]
- Ojano-Dirain, C.P.; Antonelli, P.J. Prevention of gentamicin-induced apoptosis with the mitochondria-targeted antioxidant mitoquinone. Laryngoscope 2012, 122, 2543–2548. [Google Scholar] [PubMed]
- Sergi, B.; Fetoni, A.R.; Paludetti, G.; Ferraresi, A.; Navarra, P.; Mordente, A.; Troiani, D. Protective properties of idebenone in noise-induced hearing loss in the guinea pig. Neuroreport 2006, 17, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Tschuck, J.; Tonnus, W.; Gavali, S.; Kolak, A.; Mallais, M.; Maremonti, F.; Sato, M.; Rothenaigner, I.; Friedmann Angeli, J.P.; Pratt, D.A.; et al. Seratrodast inhibits ferroptosis by suppressing lipid peroxidation. Cell Death Dis. 2024, 15, 853. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc Biol. 2011, 31, 986–1000. [Google Scholar] [PubMed][Green Version]
- Kurokawa, T.; Matsumoto, T.; Ashida, Y.; Sasada, R.; Iwasa, S. Antagonism of the human thromboxane A2 receptor by an anti-asthmatic agent AA-2414. Biol. Pharm. Bull. 1994, 17, 383–385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gill, N.B.; Dowker-Key, P.D.; Hedrick, M.; Bettaieb, A. Unveiling the Role of Oxidative Stress in Cochlear Hair Cell Death: Prospective Phytochemical Therapeutics against Sensorineural Hearing Loss. Int. J. Mol. Sci. 2024, 25, 4272. [Google Scholar] [CrossRef] [PubMed]
- Matsuhashi, T.; Sato, T.; Kanno, S.I.; Suzuki, T.; Matsuo, A.; Oba, Y.; Kikusato, M.; Ogasawara, E.; Kudo, T.; Suzuki, K.; et al. Mitochonic Acid 5 (MA-5) facilitates ATP synthase oligomerization and cell survival in various mitochondrial diseases. EBioMedicine 2017, 20, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Cai, G.; Xia, W.; Fu, Y. Thromboxane A2 receptor antagonist SQ29548 suppresses the LPS-induced release of inflammatory cytokines in BV2 microglia cells via suppressing MAPK and NF-κB signaling pathways. Mol. Med. Rep. 2017, 16, 2491–2496. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamaguchi, H.; Kikusato, M.; Hashizume, O.; Nagatoishi, S.; Matsuo, A.; Sato, T.; Kudo, T.; Matsuhashi, T.; Murayama, K.; et al. Mitochonic Acid 5 Binds Mitochondria and Ameliorates Renal Tubular and Cardiac Myocyte Damage. J. Am. Soc. Nephrol. 2016, 27, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Yan, A.; Fu, N.; Fu, Y. Thromboxane A2 receptor antagonist SQ29548 attenuates SH-SY5Y neuroblastoma cell impairments induced by oxidative stress. Int. J. Mol. Med. 2018, 42, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Dulon, D.; Pak, K.; Mullen, L.M.; Li, Y.; Erkman, L.; Ryan, A.F. Regulation of Pou4f3 gene expression in hair cells by 5′ DNA in mice. Neuroscience 2011, 197, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Estrada-García, L.; Carrera-Rotllan, J.; Puig-Parellada, P. Effects of oxidative stress and antioxidant treatments on eicosanoid synthesis and lipid peroxidation in long term human umbilical vein endothelial cells culture. Prostaglandins Other Lipid Mediat. 2002, 67, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Hashimoto, T.; Mizuno, M.; Takigawa, H.; Yoshida, M.; Azuma, T.; Kanazawa, K. A lipid peroxidation product 9-oxononanoic acid induces phospholipase A2 activity and thromboxane A2 production in human blood. J. Clin. Biochem. Nutrit. 2013, 52, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Esterberg, R.; Linbo, T.; Pickett, S.B.; Wu, P.; Ou, H.C.; Rubel, E.W.; Raible, D.W. Mitochondrial calcium uptake underlies ROS generation during aminoglycoside-induced hair cell death. J. Clin. Investig. 2016, 126, 3556–3566. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Kim, H.K.; Shim, W.; Anwar, M.A.; Kwon, J.W.; Kwon, H.K.; Kim, H.J.; Jeong, H.; Kim, H.M.; Hwang, D.; et al. Mechanism of cisplatin-induced cytotoxicity is correlated to impaired metabolism due to mitochondrial ros generation. PLoS ONE 2015, 10, e0135083. [Google Scholar] [CrossRef] [PubMed]
- Kleih, M.; Böpple, K.; Dong, M.; Gaißler, A.; Heine, S.; Olayioye, M.A.; Aulitzky, W.E.; Essmann, F. Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis. 2019, 10, 851. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Krishnamurthy, S. Cellular responses to cisplatin-induced DNA damage. J. Nucleic Acids 2010, 2010, 201367. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R. Oxidative DNA damage, antioxidants, and cancer. Bioessays 1999, 21, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front. Cell Neurosci. 2017, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- McFadden, S.L.; Ding, D.; Salvemini, D.; Salvi, R.J. M40403, a superoxide dismutase mimetic, protects cochlear hair cells from gentamicin, but not cisplatin toxicity. Toxicol. Appl. Pharmacol. 2003, 186, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Schrader, A.; Warchol, M.; Sheets, L. Cisplatin exposure acutely disrupts mitochondrial bioenergetics in the zebrafish lateral-line organ. Hear. Res. 2022, 426, 108513. [Google Scholar] [CrossRef] [PubMed]
- Jariyawat, S.; Kigpituck, P.; Suksen, K.; Chuncharunee, A.; Chaovanalikit, A.; Piyachaturawat, P. Protection against cisplatin-induced nephrotoxicity in mice by Curcuma comosa Roxb. ethanol extract. J. Nat. Med. 2009, 63, 430–436. [Google Scholar] [CrossRef]
- Haefeli, R.H.; Erb, M.; Gemperli, A.C.; Robay, D.; Courdier Fruh, I.; Anklin, C.; Dallmann, R.; Gueven, N. NQO1-dependent redox cycling of idebenone: Effects on cellular redox potential and energy levels. PLoS ONE 2011, 6, e17963. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, T.; Miyake, S.; Umino, T.; Inase, N.; Tojo, N.; Yoshizawa, Y. The effect of seratrodast on eosinophil cationic protein and symptoms in asthmatics. J. Asthma 2003, 40, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Jaber, S.M.; Ge, S.X.; Milstein, J.L.; VanRyzin, J.W.; Waddell, J.; Polster, B.M. Idebenone has distinct effects on mitochondrial respiration in cortical astrocytes compared to cortical neurons due to differential NQO1 activity. J. Neurosci. 2020, 40, 4609–4619. [Google Scholar] [CrossRef] [PubMed]
- Suno, M.; Nagaoka, A. Inhibition of brain mitochondrial swelling by idebenone. Arch. Gerontol. Geriatr. 1989, 8, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Murphy, T.H.; Schnaar, R.L.; Coyle, J.T. Antioxidants protect against glutamate-induced cytotoxicity in a neuronal cell line. J. Pharmacol. Exp. Ther. 1989, 250, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ou, Y.; Zhang, C.; Chen, H.; Yue, H.; Yang, Z.; Zhong, X.; Hu, W.; Sun, P. Seratrodast, a thromboxane A2 receptor antagonist, inhibits neuronal ferroptosis by promoting GPX4 expression and suppressing JNK phosphorylation. Brain Res. 2022, 1795, 148073. [Google Scholar] [CrossRef] [PubMed]
| Name | Abbreviation | Structure | Chemical Formula Molecular Weight (g/mol) | Class |
|---|---|---|---|---|
| Seratrodast | Sera | 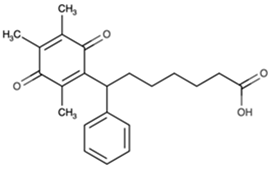 | C22H26O4 (354.45) | Synthetic quinone TXA2 receptor antagonist |
| SQ-29548 | SQ | 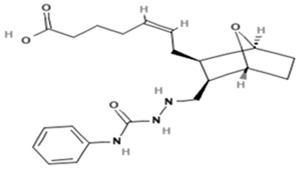 | C21H29N3O4 (387.47) | Synthetic TXA2 receptor antagonist |
| Idebenone | Ideb | 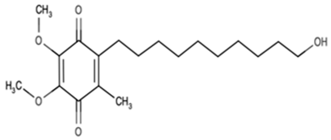 | C19H30O5 (338.44) | Synthetic ubiquinone analog |
| Mitochonic Acid 5 | Mito | 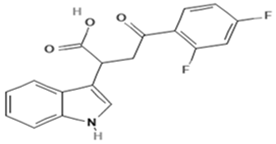 | C19H13F2NO4 (329.30) | Mitochondrial targeted metabolic modulator |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryan, A.F.; Pak, K.; Lee, E.J.; Kurabi, A. Comparative Antioxidant Protection of Cochlear Hair Cells from Ototoxins. Molecules 2025, 30, 3772. https://doi.org/10.3390/molecules30183772
Ryan AF, Pak K, Lee EJ, Kurabi A. Comparative Antioxidant Protection of Cochlear Hair Cells from Ototoxins. Molecules. 2025; 30(18):3772. https://doi.org/10.3390/molecules30183772
Chicago/Turabian StyleRyan, Allen F., Kwang Pak, Eun Jung Lee, and Arwa Kurabi. 2025. "Comparative Antioxidant Protection of Cochlear Hair Cells from Ototoxins" Molecules 30, no. 18: 3772. https://doi.org/10.3390/molecules30183772
APA StyleRyan, A. F., Pak, K., Lee, E. J., & Kurabi, A. (2025). Comparative Antioxidant Protection of Cochlear Hair Cells from Ototoxins. Molecules, 30(18), 3772. https://doi.org/10.3390/molecules30183772





