Indazol-Pyrimidine Hybrids: Design, Synthesis, and Antiproliferative Activity Against Human Cancer Cell Lines
Abstract
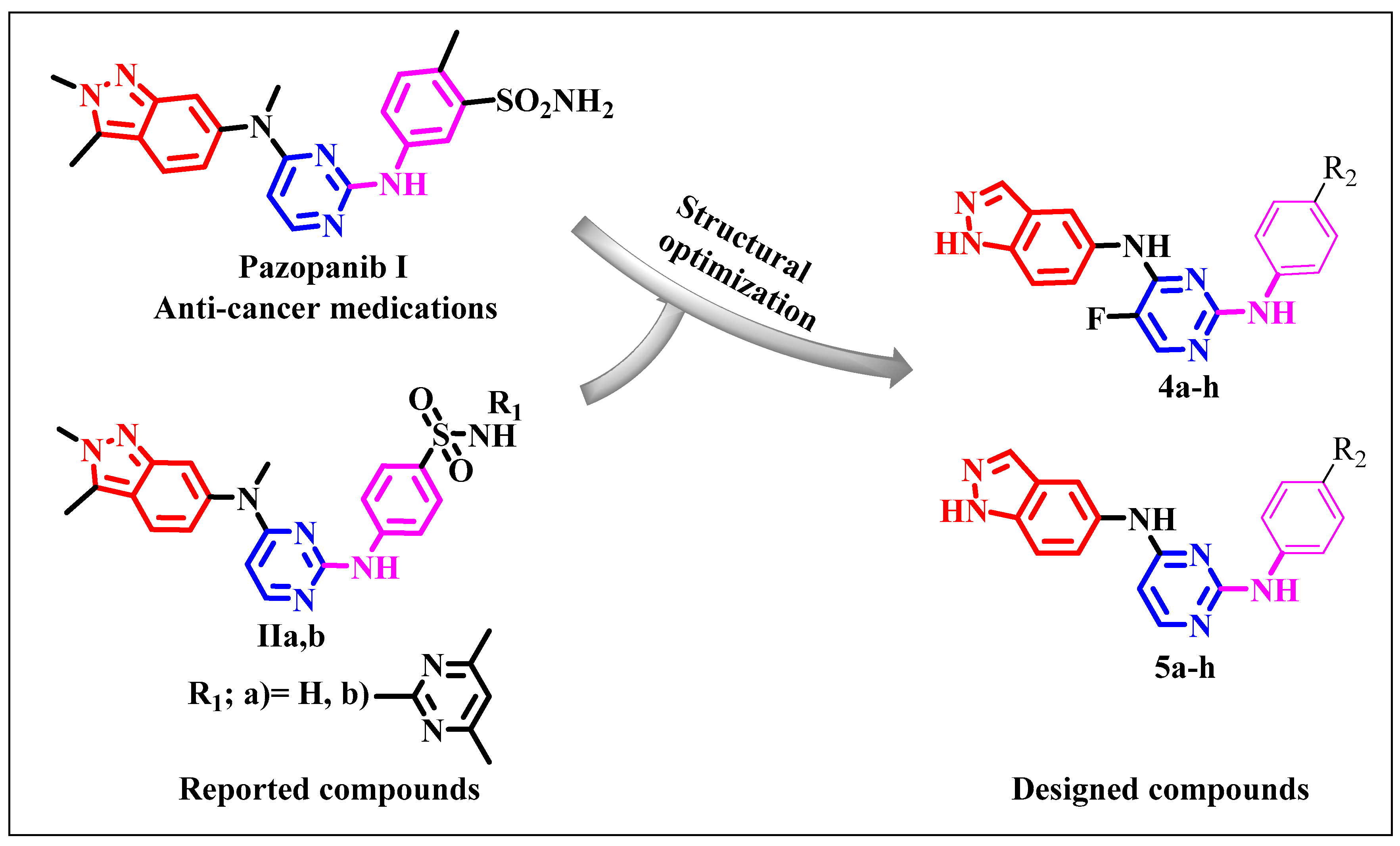
1. Introduction
2. Results and Discussion
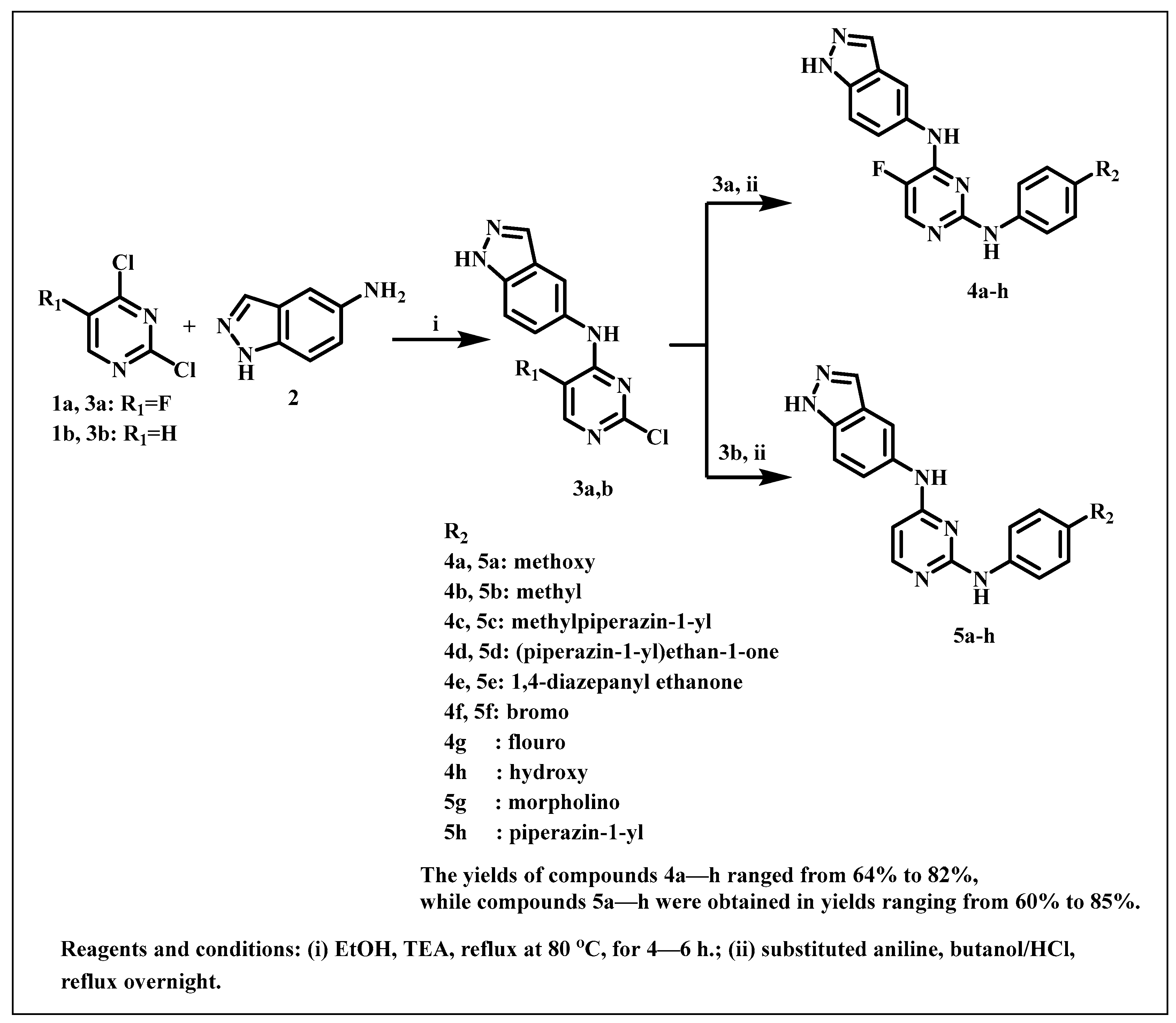
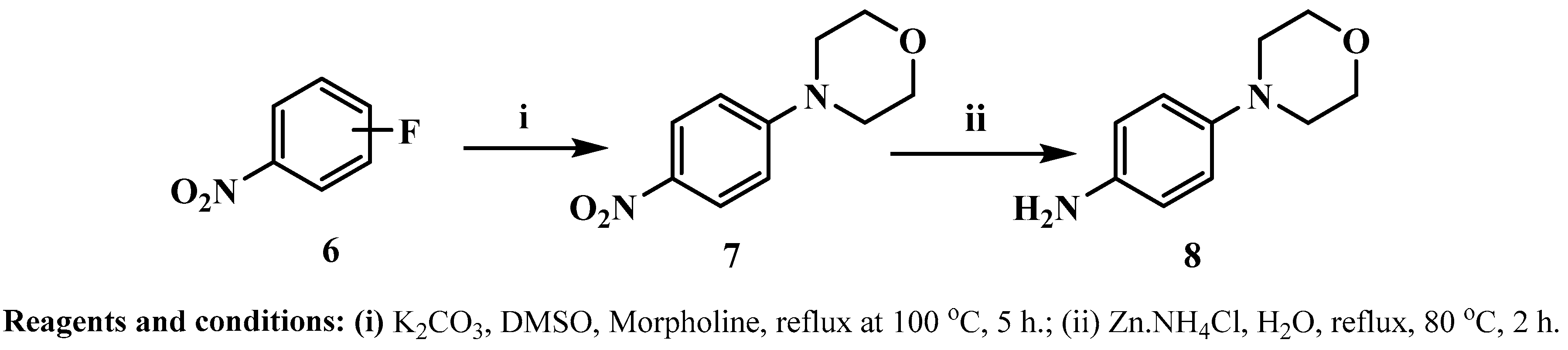
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Molecular Dynamic and System Stability
2.2.2. Binding Interaction Mechanism Based on Binding Free Energy Calculation
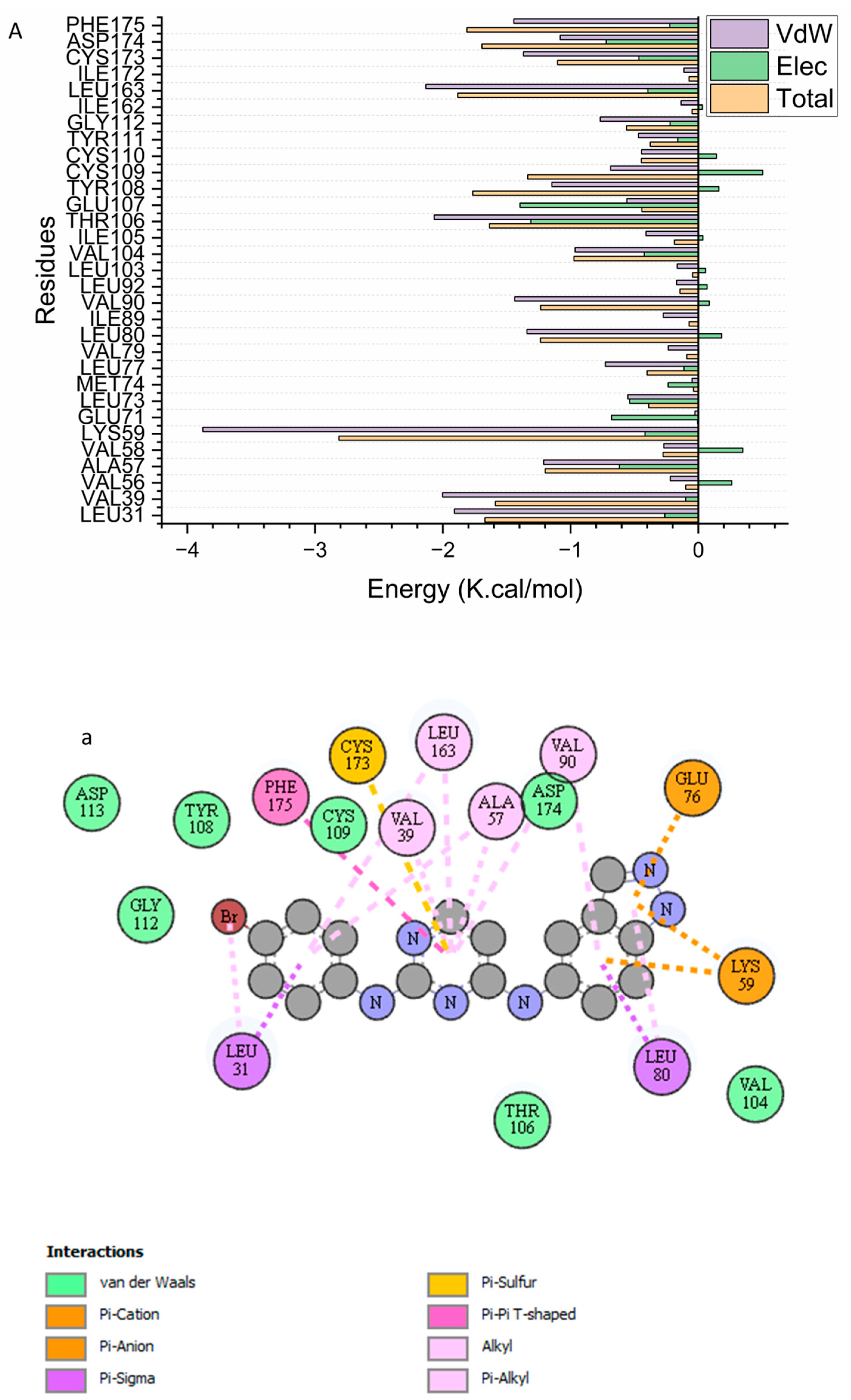
2.2.3. Identification of the Critical Residues Responsible for Ligands Binding
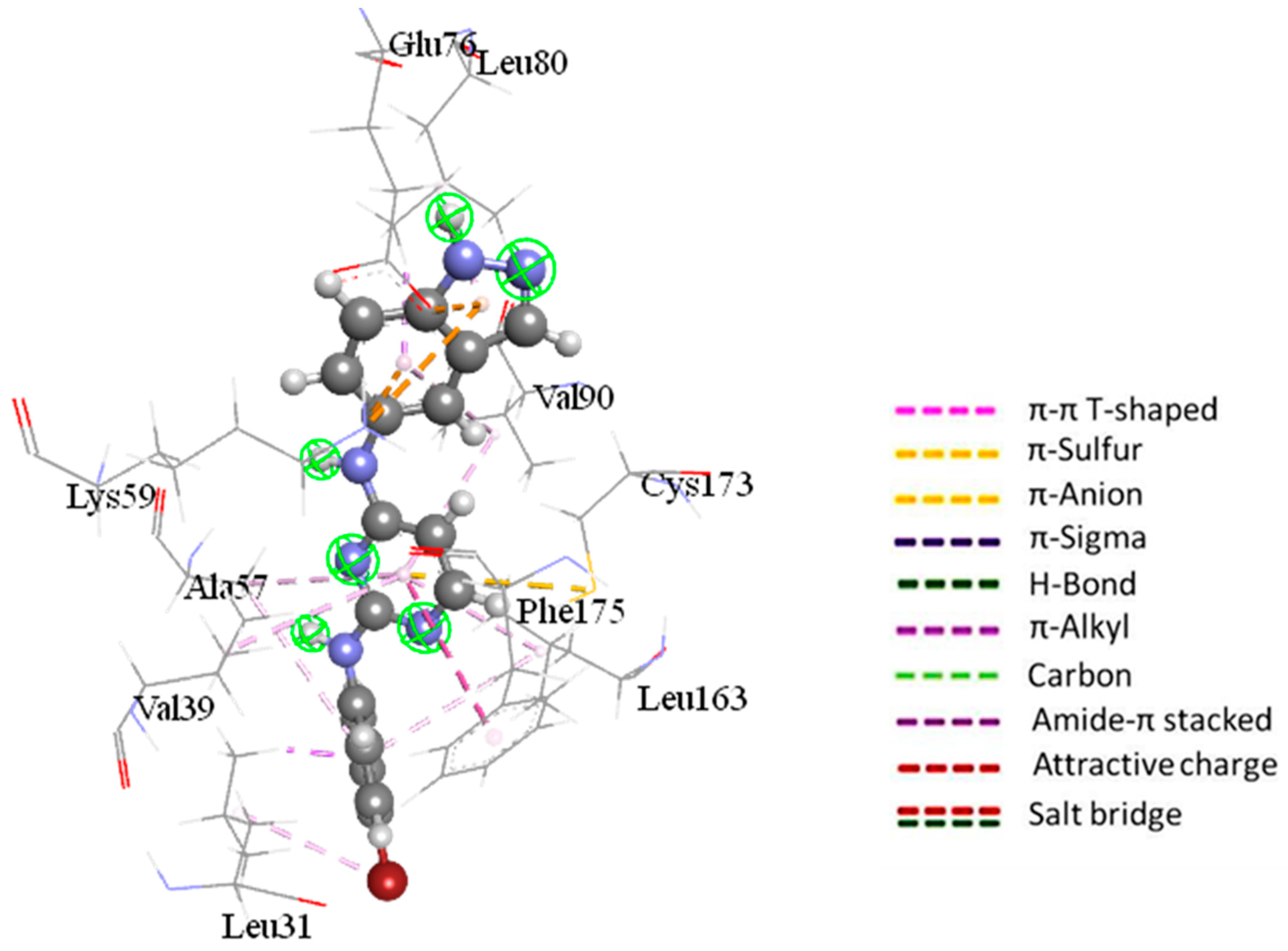
2.2.4. Ligand–Residue Interaction Network Profiles
3. Experimental
3.1. Chemistry
3.1.1. Synthesis of N-(2-chloro-5-substituted pyrimidin-4-yl)-1H-indazol-5-amine (3a,b)
3.1.2. Synthesis of Indazolyl-Pyrimidine Derivatives 4a–h and 5a–h
- 5-fluoro-N4-(1H-indazol-5-yl)-N2-(4-methoxyphenyl)pyrimidine-2,4-diamine 4a
- 5-fluoro-N4-(1H-indazol-5-yl)-N2-(p-tolyl) pyrimidine-2,4-diamine 4b
- 5-fluoro-N4-(1H-indazol-5-yl)-N2-(4-(4-methylpiperazin-1-yl)phenyl)pyrimidine-2,4-diamine 4C
- 1-(4-(4-((4-((1H-indazol-5-yl)amino)-5-fluoropyrimidin-2-yl)amino)phenyl)piperazin-1-yl) ethan-1-one 4d
- 1-(4-(4-((4-((1H-indazol-5-yl)amino)-5-fluoropyrimidin-2-yl)amino)phenyl)-1,4-diazepan-1-yl)ethan-1-one 4e
- N2-(4-bromophenyl)-5-fluoro-N4-(1H-indazol-5-yl) pyrimidine-2,4-diamine 4f
- 5-fluoro-N2-(4-fluorophenyl)-N4-(1H-indazol-5-yl)pyrimidine-2,4-diamine 4g
- 4-((4-((1H-indazol-5-yl)amino)-5-fluoropyrimidin-2-yl)amino)phenol 4h
- N4-(1H-indazol-5-yl)-N2-(4-methoxyphenyl)pyrimidine-2,4-diamine 5a
- N4-(1H-indazol-5-yl)-N2-(p-tolyl)pyrimidine-2,4-diamine 5b
- N4-(1H-indazol-5-yl)-N2-(4-(4-methylpiperazin-1-yl)phenyl)pyrimidine-2,4-diamine 5C
- 1-(4-(4-((4-((1H-indazol-5-yl)amino)pyrimidin-2-yl)amino)phenyl)piperazin-1-yl)ethan-1-one 5d
- 1-(4-(4-((4-((1H-indazol-5-yl)amino)pyrimidin-2-yl)amino)phenyl)-1,4-diazepan-1-yl)ethan-1-one 5e
- N2-(4-bromophenyl)-N4-(1H-indazol-5-yl)pyrimidine-2,4-diamine 5f
- N4-(1H-indazol-5-yl)-N2-(4-morpholinophenyl)pyrimidine-2,4-diamine 5g
- N4-(1H-indazol-5-yl)-N2-(4-(piperazin-1-yl)phenyl)pyrimidine-2,4-diamine 5h
3.2. Biological Assays
MTT Assay
3.3. System Preparation and Molecular Docking
3.4. Molecular Docking
3.5. Molecular Dynamic (MD) Simulations
3.6. Post-MD Analysis
3.7. Thermodynamic Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassanpour, S.H.; Dehghani, M. Review of Cancer from Perspective of Molecular. J. Cancer Res. Pract. 2017, 4, 127–129. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Dave, R.; Friedman, S.; Miller-Sonet, E.; Moore, T.; Peterson, E.; Doran, J.F.; Gianares, B.W.; Schuler, K.W.; Wilson, T. Identifying and Addressing the Needs of Caregivers of Patients with Cancer: Evidence on Interventions and the Role of Patient Advocacy Groups. Futur. Oncol. 2024, 20, 2589–2602. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Burden Growing, Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 30 January 2025).
- Tylińska, B.; Wiatrak, B.; Czyżnikowska, Ż.; Cieśla-Niechwiadowicz, A.; Gębarowska, E.; Janicka-Kłos, A. Novel Pyrimidine Derivatives as Potential Anticancer Agents: Synthesis, Biological Evaluation and Molecular Docking Study. Int. J. Mol. Sci. 2021, 22, 3825. [Google Scholar] [CrossRef]
- Mills, A.D.; Nazer, M.Z.; Haddadin, M.J.; Kurth, M.J. N,N-Bond-Forming Heterocyclization: Synthesis of 3-Alkoxy-2H-Indazoles. J. Org. Chem. 2006, 71, 2687–2689. [Google Scholar] [CrossRef] [PubMed]
- Saluja, S.; Zou, R.; Drach, J.C.; Townsend, L.B. Structure−Activity Relationships among 2-Substituted 5,6-Dichloro-, 4,6-Dichloro-, and 4,5-Dichloro-1-[(2-Hydroxyethoxy)Methyl]- and -1-[(1,3-Dihydroxy-2-Propoxy)Methyl]Benzimidazoles. J. Med. Chem. 1996, 39, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Bursal, E. An in Vitro and in Silico Study on the Synthesis and Characterization of Novel Bis(Sulfonate) Derivatives as Tyrosinase and Pancreatic Lipase Inhibitors. J. Mol. Struct. 2022, 1259, 132734. [Google Scholar] [CrossRef]
- Lolak, N.; Akocak, S.; Türkeş, C.; Taslimi, P.; Işık, M.; Beydemir, Ş.; Gülçin, İ.; Durgun, M. Synthesis, Characterization, Inhibition Effects, and Molecular Docking Studies as Acetylcholinesterase, α-Glycosidase, and Carbonic Anhydrase Inhibitors of Novel Benzenesulfonamides Incorporating 1,3,5-Triazine Structural Motifs. Bioorg. Chem. 2020, 100, 103897. [Google Scholar] [CrossRef]
- Arora, A.; Scholar, E.M. Role of Tyrosine Kinase Inhibitors in Cancer Therapy. J. Pharmacol. Exp. Ther. 2005, 315, 971–979. [Google Scholar] [CrossRef]
- Wan, Y.; He, S.; Li, W.; Tang, Z. Indazole Derivatives: Promising Anti-Tumor Agents. Anti-Cancer Agents Med. Chem. 2018, 18, 1228–1234. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V. Prescribed Drugs Containing Nitrogen Heterocycles: An Overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Pandiyan, S.; Wang, L. In-Silico Design of Novel Potential HDAC Inhibitors from Indazole Derivatives Targeting Breast Cancer through QSAR, Molecular Docking and Pharmacokinetics Studies. Comput. Biol. Chem. 2024, 110, 108035. [Google Scholar] [CrossRef]
- Denya, I.; Malan, S.F.; Joubert, J. Indazole Derivatives and Their Therapeutic Applications: A Patent Review (2013–2017). Expert Opin. Ther. Pat. 2018, 28, 441–453. [Google Scholar] [CrossRef]
- Qin, J.; Cheng, W.; Duan, Y.-T.; Yang, H.; Yao, Y. Indazole as a Privileged Scaffold: The Derivatives and Their Therapeutic Applications. Anti-Cancer Agents Med. Chem. 2021, 21, 839–860. [Google Scholar] [CrossRef]
- Cerecetto, H.; Gerpe, A.; Gonzalez, M.; Aran, V.; de Ocariz, C. Pharmacological Properties of Indazole Derivatives: Recent Developments. Mini Rev. Med. Chem. 2005, 5, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Thangadurai, A.; Minu, M.; Wakode, S.; Agrawal, S.; Narasimhan, B. Indazole: A Medicinally Important Heterocyclic Moiety. Med. Chem. Res. 2012, 21, 1509–1523. [Google Scholar] [CrossRef]
- Edris, N.A.; Kadry, H.H.; Taher, A.T.; Adly, M.E. Pyrimidine-5-Carbonitriles: A Review of Available Synthetic Approaches and Biological Evaluation for Promising Compounds over the Last Decade. Egypt. J. Chem. 2024, 67, 439–462. [Google Scholar] [CrossRef]
- Ma, L.-Y.; Wang, B.; Pang, L.-P.; Zhang, M.; Wang, S.-Q.; Zheng, Y.-C.; Shao, K.-P.; Xue, D.-Q.; Liu, H.-M. Design and Synthesis of Novel 1,2,3-Triazole–Pyrimidine–Urea Hybrids as Potential Anticancer Agents. Bioorg. Med. Chem. Lett. 2015, 25, 1124–1128. [Google Scholar] [CrossRef]
- Xie, F.; Zhao, H.; Zhao, L.; Lou, L.; Hu, Y. Synthesis and Biological Evaluation of Novel 2,4,5-Substituted Pyrimidine Derivatives for Anticancer Activity. Bioorg. Med. Chem. Lett. 2009, 19, 275–278. [Google Scholar] [CrossRef]
- Huang, T.; Wu, X.; Liu, T.; An, L.; Yin, X. Synthesis and Anticancer Activity Evaluation of Novel Oxacalix[2]Arene[2]Pyrimidine Derivatives. Med. Chem. Res. 2019, 28, 580–590. [Google Scholar] [CrossRef]
- Zhao, M.; Ren, H.; Chang, J.; Zhang, D.; Yang, Y.; He, Y.; Qi, C.; Zhang, H. Design and synthesis of novel pyrazolo[1, 5-a]pyrimidine derivatives bearing nitrogen mustard moiety and evaluation of their antitumor activity in vitro and in vivo. Eur. J. Med. Chem. 2016, 119, 183–196. [Google Scholar] [CrossRef]
- Al-Tuwaijri, H.M.; Al-Abdullah, E.S.; El-Rashedy, A.A.; Ansari, S.A.; Almomen, A.; Alshibl, H.M.; Haiba, M.E.; Alkahtani, H.M. New Indazol-Pyrimidine-Based Derivatives as Selective Anticancer Agents: Design, Synthesis, and In Silico Studies. Molecules 2023, 28, 3664. [Google Scholar] [CrossRef] [PubMed]
- Gennigens, C.; Jerusalem, G. Pazopanib (Votrient) in the management of renal cell cancer and soft tissue sarcomas. Rev. Medicale De Liege 2012, 67, 437–442. [Google Scholar]
- Jia, Y.; Zhang, J.; Feng, J.; Xu, F.; Pan, H.; Xu, W. Design, synthesis and biological evaluation of pazopanib derivatives as antitumor agents. Chem. Biol. Drug Des. 2014, 83, 306–316. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Li, C.; Wu, X. An efficient and practical synthesis of antibacterial linezolid. J. Chem. Res. 2009, 2009, 739–740. [Google Scholar] [CrossRef]
- Haiba, M.E.; Al-Abdullah, E.S.; Ahmed, N.S.; Ghabbour, H.A.; Awad, H.M. Efficient and Easy Synthesis of New Benzo[h]Chromene and Benzo[h]Quinoline Derivatives as a New Class of Cytotoxic Agents. J. Mol. Struct. 2019, 1195, 702–711. [Google Scholar] [CrossRef]
- Mirzaei, S.; Eisvand, F.; Hadizadeh, F.; Mosaffa, F.; Ghasemi, A.; Ghodsi, R. Design, Synthesis and Biological Evaluation of Novel 5,6,7-Trimethoxy-N-Aryl-2-Styrylquinolin-4-Amines as Potential Anticancer Agents and Tubulin Polymerization Inhibitors. Bioorg. Chem. 2020, 98, 103711. [Google Scholar] [CrossRef]
- Hasanin, M.; Hashem, A.H.; El-Rashedy, A.A.; Kamel, S. Synthesis of Novel Heterocyclic Compounds Based on Dialdehyde Cellulose: Characterization, Antimicrobial, Antitumor Activity, Molecular Dynamics Simulation and Target Identification. Cellulose 2021, 28, 8355–8374. [Google Scholar] [CrossRef]
- Machaba, K.E.; Mhlongo, N.N.; Soliman, M.E.S. Induced Mutation Proves a Potential Target for TB Therapy: A Molecular Dynamics Study on LprG. Cell Biochem. Biophys. 2018, 76, 345–356. [Google Scholar] [CrossRef]
- Pan, L.; Patterson, J.C.; Deshpande, A.; Cole, G.; Frautschy, S. Molecular Dynamics Study of Zn(Aβ) and Zn(Aβ)2. PLoS ONE 2013, 8, 70681–70688. [Google Scholar] [CrossRef] [PubMed]
- Wijffels, G.; Dalrymple, B.; Kongsuwan, K.; Dixon, N. Conservation of Eubacterial Replicases. IUBMB Life 2005, 57, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Richmond, T.J. Solvent Accessible Surface Area and Excluded Volume in Proteins: Analytical Equations for Overlapping Spheres and Implications for the Hydrophobic Effect. J. Mol. Biol. 1984, 178, 63–89. [Google Scholar] [CrossRef]
- Cournia, Z.; Allen, B.; Sherman, W. Relative Binding Free Energy Calculations in Drug Discovery: Recent Advances and Practical Considerations. J. Chem. Inf. Model. 2017, 57, 2911–2937. [Google Scholar] [CrossRef]
- Nassar, A.E.F.; Kamel, A.M.; Clarimont, C. Improving the Decision-Making Process in the Structural Modification of Drug Candidates: Enhancing Metabolic Stability. Drug Discov. Today 2004, 9, 1020–1028. [Google Scholar] [CrossRef]
- Sheikh, E.; Tran, T.; Vranic, S.; Levy, A.; Bonfil, R.D. Role and Significance of C-KIT Receptor Tyrosine Kinase in Cancer: A Review. Bosn. J. Basic Med. Sci. 2022, 22, 683. [Google Scholar] [CrossRef]
- Alonso-Moreno, C.; Antiñolo, A.; Carrillo-Hermosilla, F.; Otero, A. Guanidines: From Classical Approaches to Efficient Catalytic Syntheses. Chem. Soc. Rev. 2014, 43, 3406–3425. [Google Scholar] [CrossRef]
- Manns-James, L. Bacterial Vaginosis and Preterm Birth. J. Midwifery Women’s Health 2011, 56, 575–583. [Google Scholar] [CrossRef]
- Mol, C.D.; Dougan, D.R.; Schneider, T.R.; Skene, R.J.; Kraus, M.L.; Scheibe, D.N.; Snell, G.P.; Zou, H.; Sang, B.C.; Wilson, K.P. Structural Basis for the Autoinhibition and STI-571 Inhibition of c-Kit Tyrosine Kinase. J. Biol. Chem. 2004, 279, 31655–31663. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Robertson, A.D.; Jensen, J.H. Very Fast Empirical Prediction and Rationalization of Protein pKa Values. Proteins 2005, 61, 704–721. [Google Scholar] [CrossRef]
- BETHANY HALFORD: Reflections on Chem Draw. Chem. Eng. News Arch. 2014, 92, 26–27. [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Bikadi, Z.; Hazai, E. Application of the PM6 Semi-Empirical Method to Modeling Proteins Enhances Docking Accuracy of AutoDock. J. Cheminform. 2009, 1, 15. [Google Scholar] [CrossRef]
- Huey, R.; Molecular, G.M. Using Autodock with Autodocktools: A Tutorial; The Scripps Research Institute Molecular Graphics Laboratory: La Jolla, CA, USA, 2006. [Google Scholar]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J. Comput. Chem. 1639, 19, 16391662. [Google Scholar] [CrossRef]
- Hospital, A.; Goñi, J.R.; Orozco, M.; Gelpí, J.L. Molecular Dynamics Simulations: Advances and Applications. Adv. Appl. Bioinform. Chem. 2015, 8, 37–47. [Google Scholar] [CrossRef]
- Lee, T.S.; Cerutti, D.S.; Mermelstein, D.; Lin, C.; Legrand, S.; Giese, T.J.; Roitberg, A.; Case, D.A.; Walker, R.C.; York, D.M. GPU-Accelerated Molecular Dynamics and Free Energy Methods in Amber18: Performance Enhancements and New Features. J. Chem. Inf. Model. 2018, 58, 2043–2050. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic Atom Type and Bond Type Perception in Molecular Mechanical Calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular Dynamics with Coupling to an External Bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Seifert, E. OriginPro 9.1: Scientific Data Analysis and Graphing Software—Software Review. J. Chem. Inf. Model. 2014, 54, 1552. [Google Scholar] [CrossRef]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W.; et al. Calculating Structures and Free Energies of Complex Molecules: Combining Molecular Mechanics and Continuum Models. Acc. Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef]
- Ylilauri, M.; Pentikäinen, O.T. MMGBSA as a Tool to Understand the Binding Affinities of Filamin-Peptide Interactions. J. Chem. Inf. Model. 2013, 53, 2626–2633. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.M.; Archontis, G. MM-GB(PB)SA Calculations of Protein-Ligand Binding Free Energies. In Molecular Dynamics—Studies of Synthetic and Biological Macromolecules; InTech Open: London, UK, 2012. [Google Scholar]
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the Performance of the MM/PBSA and MM/GBSA Methods. 1. The Accuracy of Binding Free Energy Calculations Based on Molecular Dynamics Simulations. J. Chem. Inf. Model. 2011, 51, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Greenidge, P.A.; Kramer, C.; Mozziconacci, J.C.; Wolf, R.M. MM/GBSA Binding Energy Prediction on the PDBbind Data Set: Successes, Failures, and Directions for Further Improvement. J. Chem. Inf. Model. 2013, 53, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Sitkoff, D.; Sharp, K.A.; Honig, B. Accurate Calculation of Hydration Free Energies Using Macroscopic Solvent Models. J. Phys. Chem. 1994, 98, 1978–1988. [Google Scholar] [CrossRef]
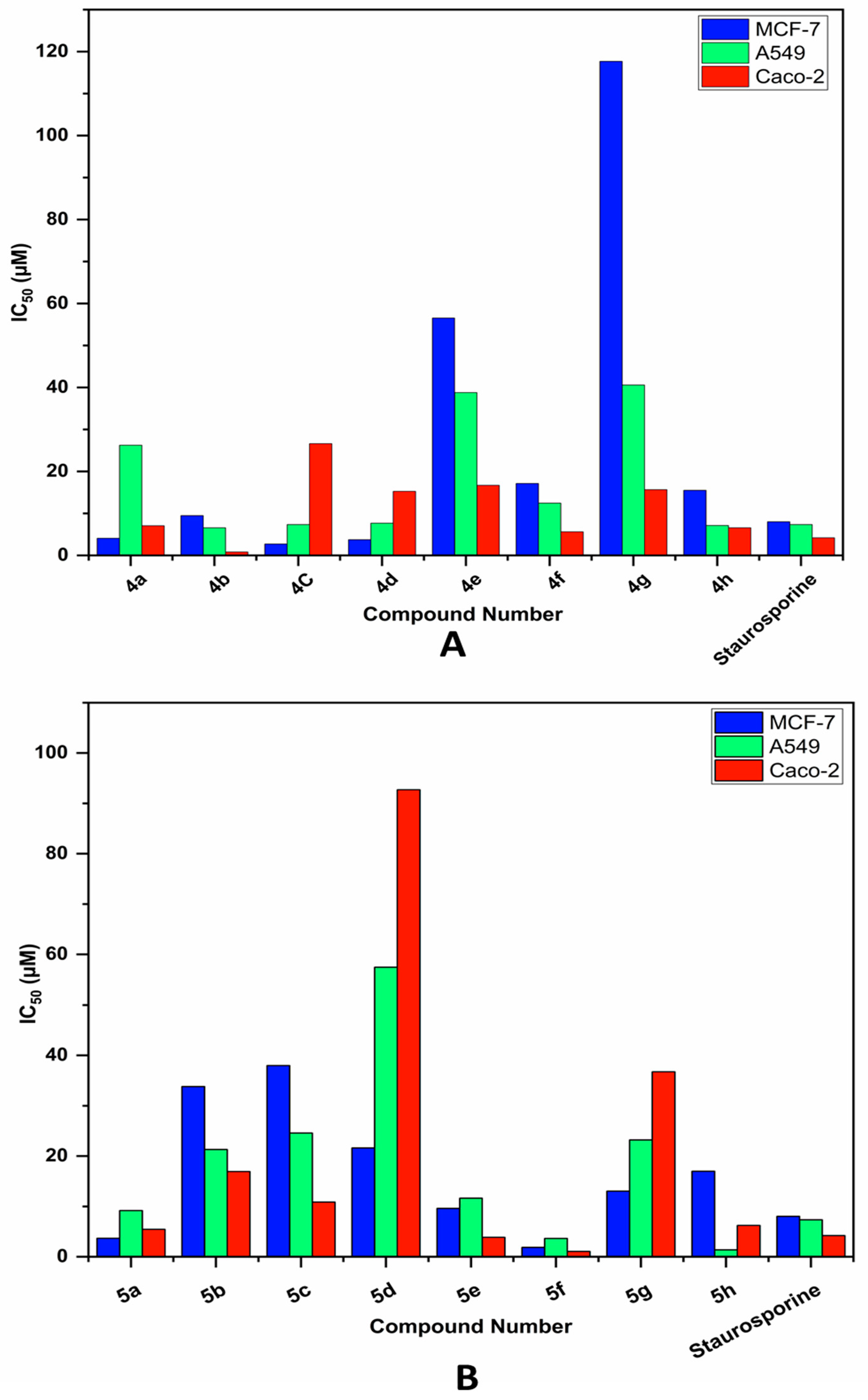

| Compound | Cytotoxicity IC50 (µM) | ||
|---|---|---|---|
| MCF-7 | A549 | Caco-2 | |
| 4a | 4.086 ± 0.16 | 26.24 ± 1.15 | 7.071 ± 0.32 |
| 4b | 9.474 ± 0.38 | 6.576 ± 0.29 | 0.827 ± 0.04 |
| 4C | 2.738 ± 0.11 | 7.354 ± 0.32 | 26.62 ± 1.22 |
| 4d | 3.744 ± 0.15 | 7.696 ± 0.34 | 15.25 ± 0.7 |
| 4e | 56.55 ± 2.24 | 38.79 ± 1.7 | 16.69 ± 0.76 |
| 4f | 17.13 ± 0.68 | 12.46 ± 0.55 | 5.616 ± 0.26 |
| 4g | 117.7 ± 4.66 | 40.59 ± 1.78 | 15.66 ± 0.72 |
| 4h | 15.52 ± 0.61 | 7.136 ± 0.31 | 6.592 ± 0.3 |
| 5a | 3.67 ± 0.15 | 9.18 ± 0.4 | 5.435 ± 0.25 |
| 5b | 33.83 ± 1.34 | 21.3 ± 0.93 | 16.91 ± 0.77 |
| 5C | 37.99 ± 1.5 | 24.58 ± 1.08 | 10.87 ± 0.5 |
| 5d | 21.61 ± 0.86 | 57.52 ± 2.52 | 92.69 ± 4.25 |
| 5e | 9.603 ± 0.38 | 11.65 ± 0.51 | 3.869 ± 0.18 |
| 5f | 1.858 ± 0.05 | 3.628 ± 0.16 | 1.056 ± 0.05 |
| 5g | 13.05 ± 0.52 | 23.21 ± 1.02 | 36.74 ± 1.68 |
| 5h | 16.98 ± 0.67 | 1.378 ± 0.06 | 6.226 ± 0.29 |
| Staurosporine | 8.029 ± 0.12 | 7.354 ± 0.12 | 4.202 ± 0.19 |
| Energy Components (kcal/mol) | |||||
|---|---|---|---|---|---|
| c-KI | |||||
| Complex | ΔEvdW | ΔEelec | ΔGgas | ΔGsolv | ΔGbind |
| Compound 5f–C-kit | −59.79 ± 0.01 | −8.48 ± 0.04 | −68.27 ± 0.03 | 18.89 ± 0.02 | −49.38 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Tuwaijri, H.M.; El-Rashedy, A.A.; Ansari, S.A.; Almomen, A.; Alkahtani, H.M.; Al-Abdullah, E.S.; Haiba, M.E. Indazol-Pyrimidine Hybrids: Design, Synthesis, and Antiproliferative Activity Against Human Cancer Cell Lines. Molecules 2025, 30, 3773. https://doi.org/10.3390/molecules30183773
Al-Tuwaijri HM, El-Rashedy AA, Ansari SA, Almomen A, Alkahtani HM, Al-Abdullah ES, Haiba ME. Indazol-Pyrimidine Hybrids: Design, Synthesis, and Antiproliferative Activity Against Human Cancer Cell Lines. Molecules. 2025; 30(18):3773. https://doi.org/10.3390/molecules30183773
Chicago/Turabian StyleAl-Tuwaijri, Hanaa M., Ahmed A. El-Rashedy, Siddique Akber Ansari, Aliyah Almomen, Hamad M. Alkahtani, Ebtehal S. Al-Abdullah, and Mogedda E. Haiba. 2025. "Indazol-Pyrimidine Hybrids: Design, Synthesis, and Antiproliferative Activity Against Human Cancer Cell Lines" Molecules 30, no. 18: 3773. https://doi.org/10.3390/molecules30183773
APA StyleAl-Tuwaijri, H. M., El-Rashedy, A. A., Ansari, S. A., Almomen, A., Alkahtani, H. M., Al-Abdullah, E. S., & Haiba, M. E. (2025). Indazol-Pyrimidine Hybrids: Design, Synthesis, and Antiproliferative Activity Against Human Cancer Cell Lines. Molecules, 30(18), 3773. https://doi.org/10.3390/molecules30183773





