Analysis of the Reactivity of Z-2-Ar-1-EWG-1-Nitroethene Molecular Segment in the Hetero Diels–Alder Reaction: Experimental and MEDT Quantum Chemical Study
Abstract
1. Introduction
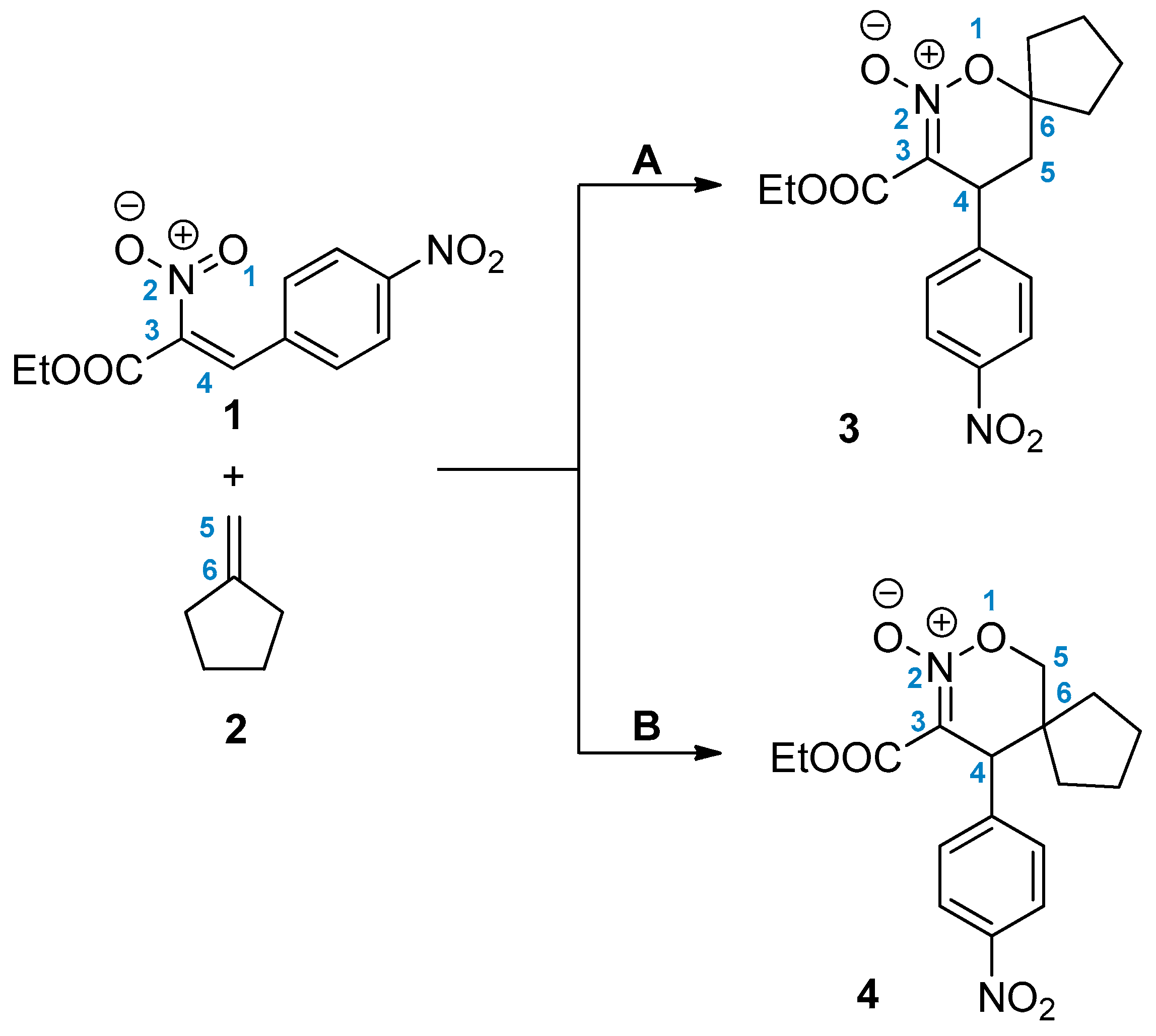
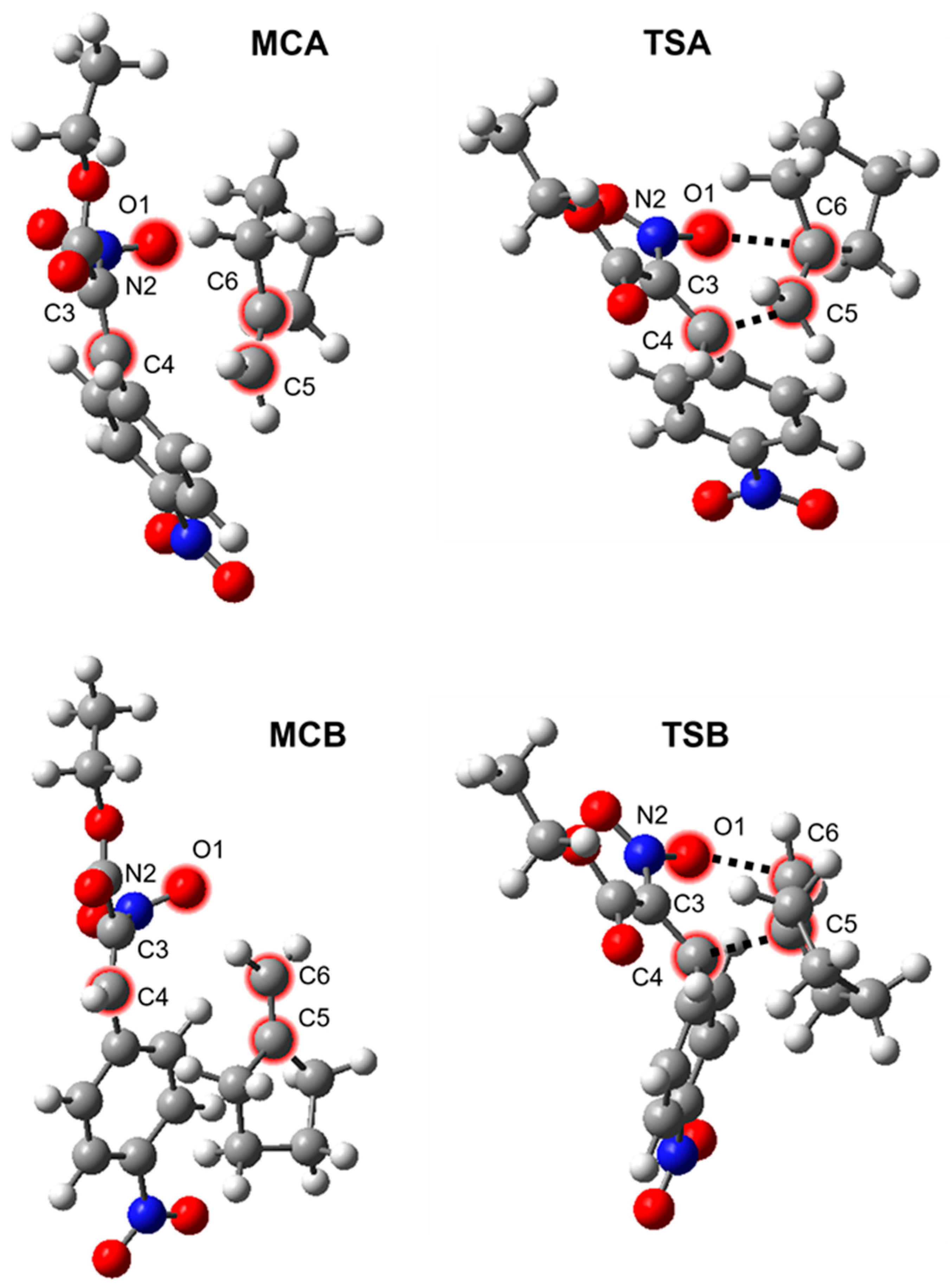
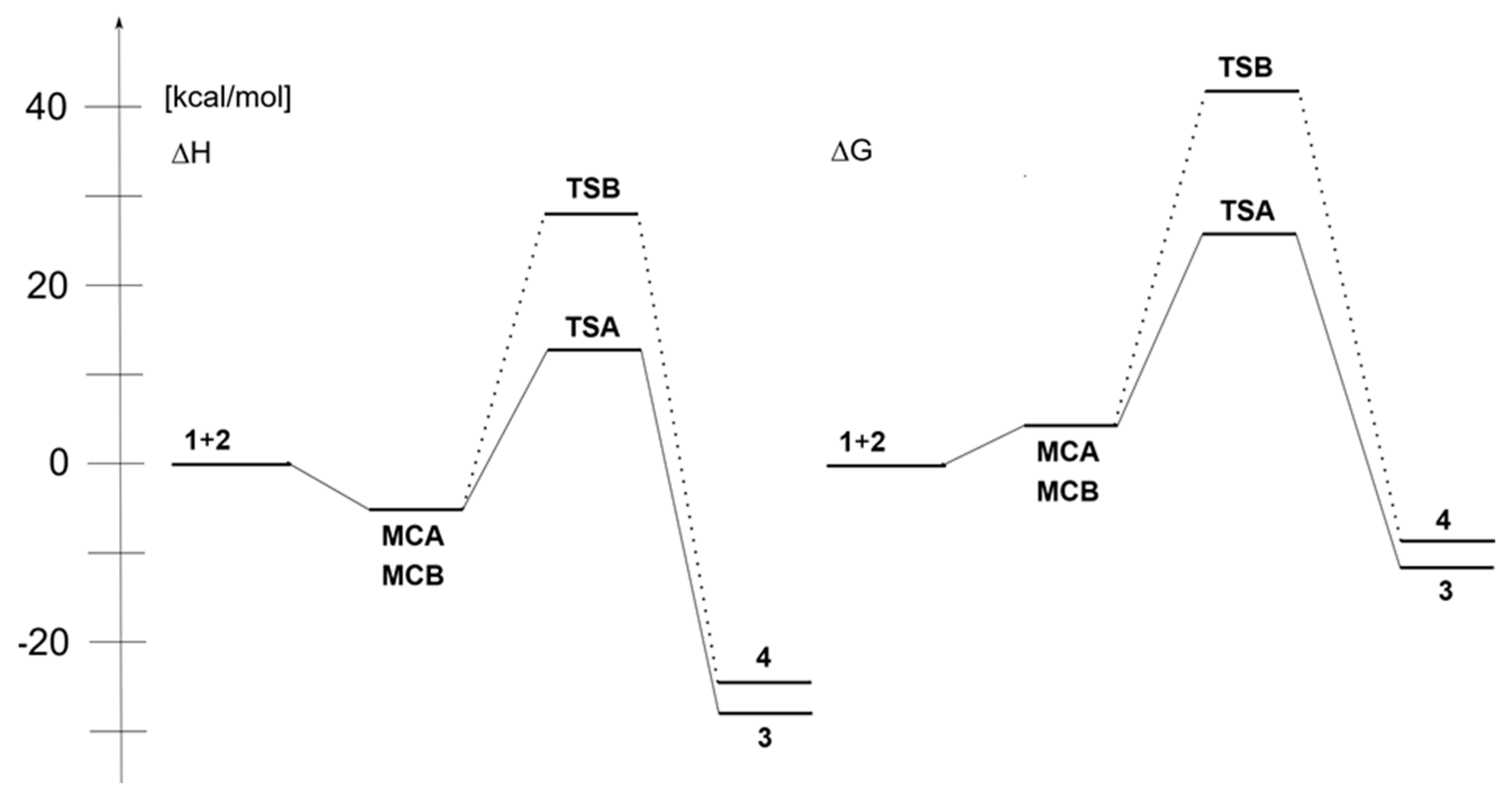
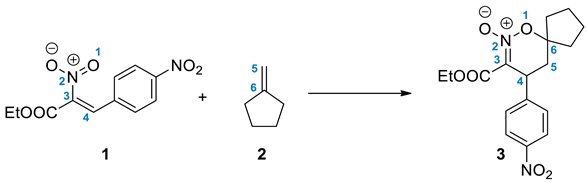
2. Results and Discussion
3. Materials and Methods
3.1. Analytical Techniques
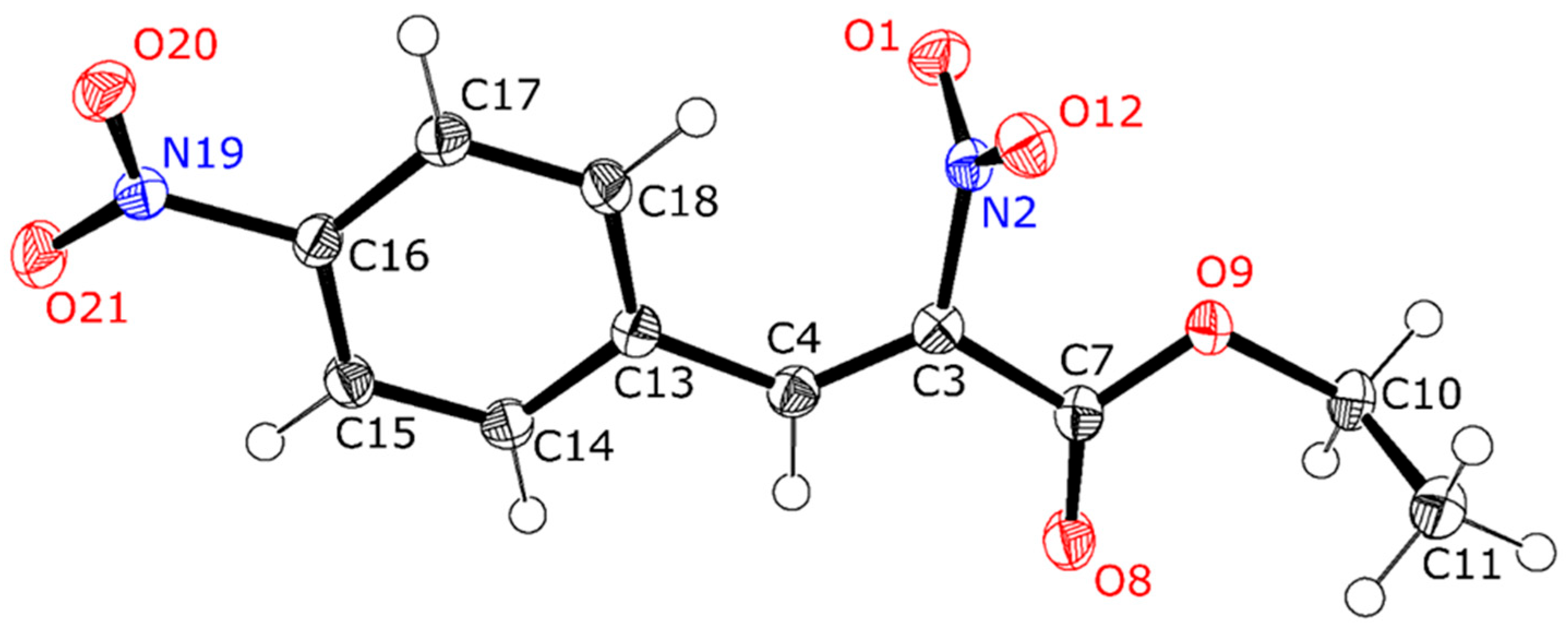
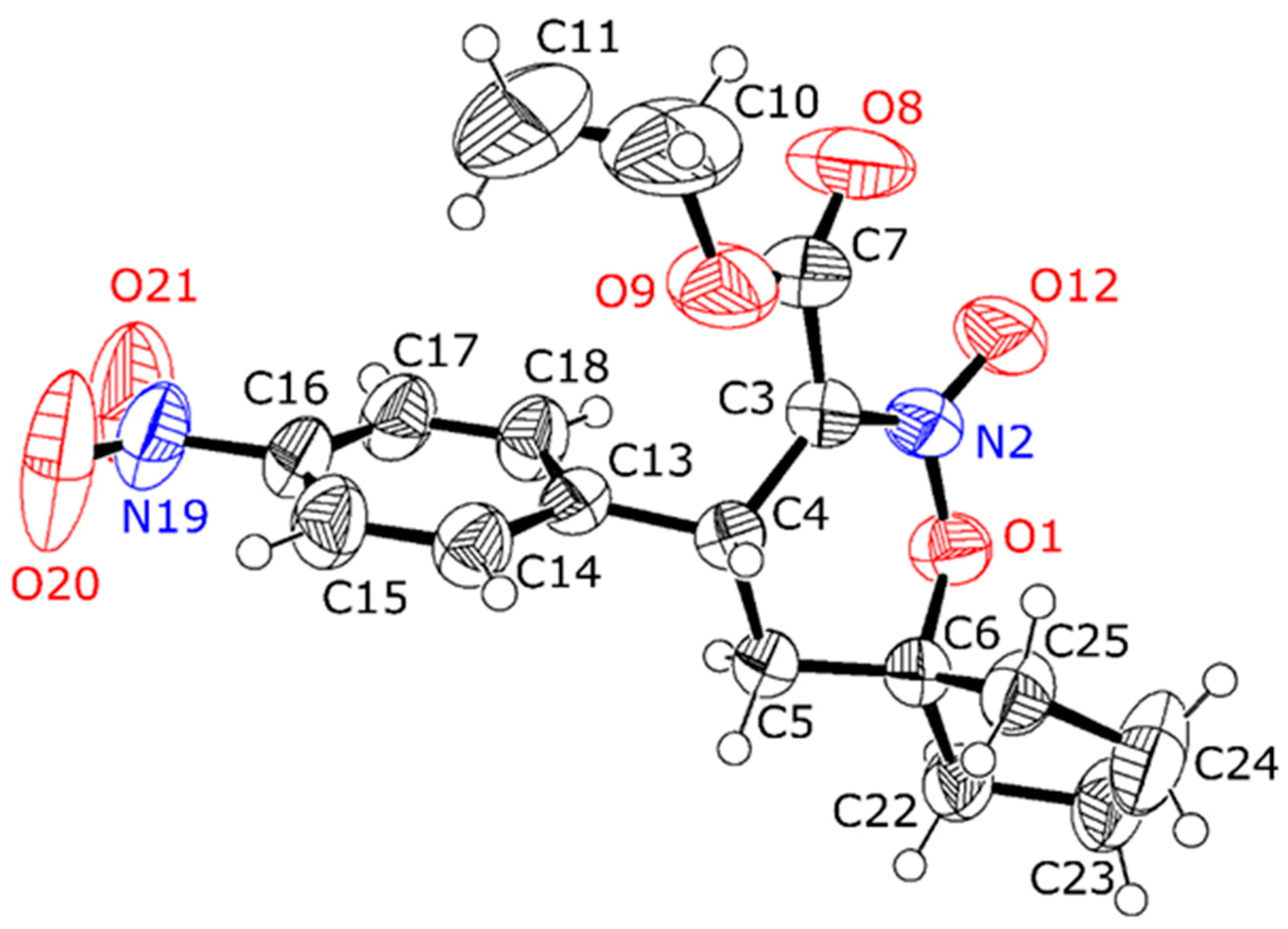
3.2. X-Ray Crystal Structure Determination
3.3. Synthesis of Reaction Components
3.3.1. Stage 1—Preparation of the N-n-propyl-c-(4-nitrophenyl)imine
3.3.2. Stage 2—Preparation of the Ethyl 4,β-Dinitrocinnamate (DNC)
3.4. Preparation of 3-Carboethoxy-4-nitrophenyl-6,6-spirocyclopentyl-1,2-oxazine N-Oxide (3)
3.5. Quantum Chemical Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olender, D.; Zwawiak, J.; Zaprutko, L. Multidirectional Efficacy of Biologically Active Nitro Compounds Included in Medicines. Pharmaceuticals 2018, 11, 54. [Google Scholar] [CrossRef]
- Noriega, S.; Cardoso-Ortiz, J.; López-Luna, A.; Cuevas-Flores, M.D.R.; De La Torre, J.A.F. The Diverse Biological Activity of Recently Synthesized Nitro Compounds. Pharmaceuticals 2022, 15, 717. [Google Scholar] [CrossRef]
- Aitouna, A.O.; Syed, A.; Alfagham, A.T.; Mazoir, N.; de Julián-Ortiz, J.V.; Elgorban, A.M.; El idrissi, M.; Wong, L.S.; Zeroual, A. Investigating the chemical reactivity and molecular docking of 2-diazo-3,3,3-trifluoro-1-nitropropane with phenyl methacrylate using computational methods. Chem. Het. Comp. 2024, 60, 592–599. [Google Scholar] [CrossRef]
- Ustinov, I.I.; Hlytin, N.V. Selective reduction of 8-hydroxy-5,7-dinitroquinoline and synthesis of 2-substituted 5-nitrooxazolo [4,5-h]quinolones. Chem. Het. Comp. 2024, 60, 524–528. [Google Scholar] [CrossRef]
- Shkineva, T.K.; Krasnova, S.A.; Dalinger, I.L. The first example of a cine-substitution in a series of 1,3-dinitropyrazoles. Chem. Het. Comp. 2024, 60, 257–261. [Google Scholar] [CrossRef]
- Sadowski, M.; Kula, K. Nitro-functionalized analogues of 1,3-Butadiene: An overview of characteristic, synthesis, chemical transformations and biological activity. Curr. Chem. Lett. 2024, 13, 15–30. [Google Scholar] [CrossRef]
- Sadowski, M.; Dresler, E.; Zawadzińska, K.; Wróblewska, A.; Jasiński, R. Syn-Propanethial S-Oxide as an Available Natural Building Block for the Preparation of Nitro-Functionalized, Sulfur-Containing Five-Membered Heterocycles: An MEDT Study. Molecules 2024, 29, 4892. [Google Scholar]
- Tafesh, A.M.; Weiguny, J. A Review of the Selective Catalytic Reduction of Aromatic Nitro Compounds into Aromatic Amines, Isocyanates, Carbamates, and Ureas Using CO. Chem. Rev. 1996, 96, 2035–2052. [Google Scholar] [CrossRef]
- Zard, S.Z. Some Aspects of the Chemistry of Nitro Compounds. Helv. Chim. Acta 2012, 95, 1730–1757. [Google Scholar] [CrossRef]
- Lammertsma, K.; Bharatam, P.V. Keto ⇌ Enol, Imine ⇌ Enamine, and Nitro ⇌ aci-Nitro Tautomerism and Their Interrelationship in Substituted Nitroethylenes. Keto, Imine, Nitro, and Vinyl Substituent Effects and the Importance of H-bonding. J. Org. Chem. 2000, 65, 4662–4670. [Google Scholar] [CrossRef]
- Wang, K.; Qian, X.; Cui, J. One step from nitro to oxime: A convenient preparation of unsaturated oximes by the reduction of the corresponding vinylnitro compounds. Tetrahedron 2009, 65, 10377–10382. [Google Scholar] [CrossRef]
- Zagozda, M.; Plenkiewicz, J. Optically active nitrile oxides: Synthesis and 1,3-dipolar cycloaddition reactions. Tetrahedron Asymmetry 2007, 18, 1457–1464. [Google Scholar] [CrossRef]
- Jasiński, R. Synthesis of 1,2-oxazine N-oxides via noncatalyzed hetero-Diels–Alder reactions of nitroalkenes. Chem. Het. Compd. 2024, 60, 121–123. [Google Scholar]
- Zawadzińska, K.; Kula, K. Application of β-Phosphorylated Nitroethenes in [3+2] Cycloaddition Reactions Involving Benzonitrile N-Oxide in the Light of a DFT Computational Study. Organics 2021, 2, 26–37. [Google Scholar] [CrossRef]
- Fryźlewicz, A.; Kącka-Zych, A.; Demchuk, O.M.; Mirosław, B.; Woliński, P.; Jasiński, R. Green synthesis of nitrocyclopropane-type precursors of inhibitors for the maturation of fruits and vegetables via domino reactions of diazoalkanes with 2-nitroprop-1-ene. J. Clean. Prod. 2012, 292, 126079. [Google Scholar]
- Woliński, P.; Kącka-Zych, A.; Demchuk, O.M.; Łapczuk-Krygier, A.; Mirosław, B.; Jasiński, R. Clean and molecularly programmable protocol for preparation of bis-heterobiarylic systems via a domino pseudocyclic reaction as a valuable alternative for TM-catalyzed cross-couplings. J. Clean. Prod. 2020, 275, 122086. [Google Scholar]
- Sadowski, M.; Kula., K. Unexpected Course of Reaction Between (1E,3E)-1,4-Dinitro-1,3-butadiene and N-Methyl Azomethine Ylide—A Comprehensive Experimental and Quantum-Chemical Study. Molecules 2024, 29, 5066. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Gu, M.; Lim, S.S.; Kim, M.J.; Lee, J.H.; Jin, H.G.; Jang, Y.H.; Jung, B. Cu’-Catalysed Enantioselective Alkyl 1,4-Additions to (E)-Nitroalkenes and Cyclic Enones with Phosphino-Oxazoline Ligands. Eur. J. Org. Chem. 2018, 24, 3122–3130. [Google Scholar] [CrossRef]
- Hesse, G.; Jäger, V. Dreikohlenstoff-Tautomerie von Nitropropen. Justus Liebigs Annalen Chemie. 1970, 740, 79–84. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Zimnitskiy, N.S.; Korotaev, V.Y.; Kutyashev, I.B.; Moshkin, V.S.; Sosnovskikh, V.Y. Highly regio- and stereoselective 1,3-dipolar cycloaddition of stabilised azomethine ylides to 3,3,3-trihalogeno-1-nitropropenes: Synthesis of trihalomethylated spiro[indoline-3,2′-pyrrolidin]-2-ones and spiro[indoline-3,3′-pyrrolizin]-2-ones. Tetrahedron 2016, 72, 6825–6836. [Google Scholar]
- Iwata, S.; Ishiguro, Y.; Utsugi, M.; Mitsuhashi, K.; Tanaka, K. 3,3,3-Trifluoro-1-nitropropene as a Novel Trifluoromethyl-Containing Building Block. Bull. Chem. Soc. Japan 1993, 66, 2432–2435. [Google Scholar] [CrossRef]
- Zawadzińska-Wrochniak, K.; Gaurav, G.K.; Demchuk, O.M. Synthesis and physical properties of the (E)-3,3,3-tribromo-1-nitroprop-1-ene. Sci. Radices 2025, 4, 202–208. [Google Scholar] [CrossRef]
- Yoshida, M.; Masaki, E.; Ikehara, H.; Hara, S. Enantioselective synthesis of gabapentin analogues via organocatalytic asymmetric Michael addition of α-branched aldehydes to β-nitroacrylates. Org. Biomol. Chem. 2012, 10, 5289–5297. [Google Scholar] [CrossRef]
- Łapczuk-Krygier, A.; Ponikiewski, Ł. Single crystal X-ray structure of (Z)-1-bromo-1-nitro-2-phenylethene. Curr. Chem. Lett. 2015, 4, 21–26. [Google Scholar] [CrossRef]
- Anisimova, N.A.; Slobodchikova, E.K.; Kuzhaeva, A.A.; Stukan’, E.V.; Bagryanskaya, I.Y.; Berestovitskaya, V.M. 1-Bromo-3,3,3-trifluoro-1-nitropropene: Synthesis and reaction with phenyl azide. Russ. J. Org. Chem. 2016, 52, 1379–1384. [Google Scholar]
- Jasiński, R.; Mirosław, B.; Demchuk, O.M.; Babyuk, D.; Łapczuk-Krygier, A. In the search for experimental and quantumchemical evidence for zwitterionic nature of (2E)-3-[4-(dimethylamino)phenyl]-2-nitroprop-2-enenitrile – An extreme example of donor–π–acceptor push–pull molecule. J. Mol. Str. 2016, 1108, 689–697. [Google Scholar] [CrossRef]
- Boguszewska-Czubara, A.; Kula, K.; Wnorowski, A.; Biernasiuk, A.; Popiołek, Ł.; Miodowski, D.; Demchuk, O.M.; Jasiński, R. Novel functionalized β-nitrostyrenes: Promising candidates for new antibacterial drugs. Saudi Pharm. J. 2019, 27, 593–601. [Google Scholar] [CrossRef]
- Baichurin, R.I.; Baichurina, L.V.; Aboskalova, N.I.; Berestovitskaya, V.M. Synthesis and structure of β-aryl-α-nitroacrylates. Russ. J. Gen. Chem. 2013, 83, 1764–1770. [Google Scholar] [CrossRef]
- Bláha, I.; Lešetický, L. Preparation and Z-E isomerization of substituted nitrostyrenes. Coll. Czech. Chem. Commun. 1986, 51, 1094–1099. [Google Scholar] [CrossRef]
- Kochetkov, K.A.; Babievskii, K.K.; Nalivaiko, E.V.; Garbalinskaya, N.A.; Belikov, V.M. Reaction of acetals with aliphatic nitro compounds. 2. Effect of structure of diethyl acetals of benzaldehydes on C- and O-alkylation of nitroacetic ester. Russ. Chem. Bull. 1981, 30, 466–469. [Google Scholar]
- Sadowski, M.; Utnicka, J.; Wojtowicz, J.; Kula, K. The global and local Reactivity of C,N-diarylnitryle imines in [3+2] cycloaddition processes with trans-B-nitrostyrene according to Molecular Electron Density Theory: A computational study. Curr. Chem. Lett. 2023, 12, 421–430. [Google Scholar] [CrossRef]
- Woliński, P.; Kącka-Zych, A.; Wróblewska, A.; Wielgus, E.; Dolot, R.; Jasiński, R. Fully Selective Synthesis of Spirocyclic-1,2-oxazine N-Oxides via Non-Catalysed Hetero Diels-Alder Reactions with the Participation of Cyanofunctionalysed Conjugated Nitroalkenes. Molecules 2023, 28, 4586. [Google Scholar] [CrossRef]
- Woliński, P.; Kącka-Zych, A.; Mirosław, B.; Wielgus, E.; Olszewska, A.; Jasiński, R. Green, one-pot synthesis of 1, 2-oxazine-type herbicides via non-catalyzed Hetero Diels-Alder reactions comprising (2E)-3-aryl-2-nitroprop-2-enenitriles. J. Clean. Prod. 2022, 356, 131878. [Google Scholar] [CrossRef]
- Fałowska, A.; Grzybowski, S.; Kapuściński, D.; Sambora, D.; Łapczuk, A. Modeling of the General Trends of Reactivity and Regioselectivity in Cyclopentadiene–Nitroalkene Diels–Alder Reactions. Molecules 2025, 30, 2467. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Electrophilicity w and Nucleophilicity N Scales for Cationic and Anionic Species. Sci. Rad. 2025, 4, 1–17. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular-systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Krokidis, X.; Noury, S.; Silvi, B. Characterization of Elementary Chemical Processes by Catastrophe Theory. J. Phys. Chem. A 1997, 101, 7277–7282. [Google Scholar] [CrossRef]
- González, C.; Schlegel, H.B. Improved algorithms for reaction path following: Higher-order implicit algorithms. J. Chem. Phys. 1991, 95, 5853–5860. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Perez, P. Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Pérez, P.; Contreras, R. Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels–Alder reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- Jaramillo, P.; Domingo, L.R.; Chamorro, E.; Pérez, P. A further exploration of a nucleophilicity index based on the gas-phase ionization potentials. J. Mol. Struct. THEOCHEM 2008, 865, 68–72. [Google Scholar] [CrossRef]
- Zou, D.; Wang, W.; Hua, Y.; Jia, T. Nitroarenes and nitroalkenes as potential amino sources for the synthesis of N-heterocycles. Org. Biomol. Chem. 2023, 21, 2254–2271. [Google Scholar] [CrossRef]
- Barrett, A.G.M.; Graboski, G.G. Conjugated nitroalkenes: Versatile intermediates in organic synthesis. Chem. Rev. 1986, 86, 751–762. [Google Scholar] [CrossRef]
- Kras, J.; Mikulska, M.; Allnajar, R.; Kula, K. Unsuccessful synthesis of individual 1,1-dinitroethene. Sci. Rad. 2024, 3, 9–13. [Google Scholar] [CrossRef]
- Wallis, J.D.; Watkin, D.J. Ethyl (Z)-2-nitro-3-(4-nitrophenyl)acrylate. Acta Cryst. (B) 1982, 38, 2057–2059. [Google Scholar] [CrossRef]
- Kula, K.; Łapczuk, A.; Sadowski, M.; Demchuk, O.M.; Kras, J.; Zawadzińska, J.; Gaurav, G.K.; Wróblewska, A.; Jasiński, R. On the Question of the Formation of Nitro-Functionalized 2,4-Pyrazole Analogs on the Basis of Nitrylimine Molecular Systems and 3,3,3-Trichloro-1-Nitroprop-1-Ene. Molecules 2022, 27, 8409. [Google Scholar] [CrossRef] [PubMed]
- Zawadzińska, K.; Gadocha, Z.; Pabian, K.; Wróblewska, A.; Wielgus, E.; Jasiński, R. The First Examples of [3+2] Cycloadditions with the Participation of (E)-3,3,3-Tribromo-1-Nitroprop-1-Ene. Materials 2022, 15, 7584. [Google Scholar] [CrossRef]
- Huo, X.; He, R.; Fu, J.; Zhang, J.; Yang, G.; Zhang, W. Stereoselective and Site-Specific Allylic Alkylation of Amino Acids and Small Peptides via a Pd/Cu Dual Catalysis. J. Am. Chem. Soc. 2017, 139, 9819–9822. [Google Scholar] [CrossRef]
- Kula, K.; Sadowski, M. Regio- and stereoselectivity of [3+2] cycloaddition reactions between (Z)-1-(anthracen-9-yl)-N-methyl nitrone and analogs of trans-β-nitrostyrene on the basis of MEDT computational study. Chem. Het. Comp. 2023, 59, 138–144. [Google Scholar] [CrossRef]
- Tolstikov, G.A.; Shults, E.E.; Malikova, T.S.; Spirikhin, L.V. The Importance of Preliminary Orientation in [4+2]-Cycloadditions of Dienes and Dienophiles with Complex Structures. Mendeleev Commun. 1994, 4, 60–62. [Google Scholar] [CrossRef]
- Baharfar, M.; Hillier, A.C.; Mao, G. Charge-Transfer Complexes: Fundamentals and Advances in Catalysis, Sensing, and Optoelectronic Applications. Adv. Mat. 2024, 36, 2406083. [Google Scholar] [CrossRef]
- Kalita, K.J.; Arikkottira, M.R.; Vijayaraghavan, R.K. Organic charge transfer complex towards functional optical materials. Cryst. Eng. Comm. 2024, 26, 4751–4765. [Google Scholar] [CrossRef]
- Dresler, E.; Wróblewska, A.; Jasiński, R. Understanding the Molecular Mechanism of Thermal and LA-Catalysed Diels–Alder Reactions between Cyclopentadiene and Isopropyl 3-Nitroprop-2-Enate. Molecules 2023, 28, 5289. [Google Scholar] [CrossRef]
- Sadowski, M.; Dresler, E.; Wróblewska., A.; Jasiński, R. A New Insight into the Molecular Mechanism of the Reaction between 2-Methoxyfuran and Ethyl (Z)-3-phenyl-2-nitroprop-2-enoate: An Molecular Electron Density Theory (MEDT) Computational Study. Molecules 2024, 29, 4876. [Google Scholar] [CrossRef]
- Karaś, A.; Łapczuk, A. Computational model of the formation of novel nitronorbornene analogs via Diels–Alder process. React. Kinet. Mech. Catal. 2025, 138, 2671–2689. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT−Integrated space-group and crystal structure determination. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehen-sive constrained, restrained refinement program for the modern computing environment−Olex2 dissected. Acta Crystallogr. 2015, 71, 59–75. [Google Scholar]
- Bakulina, O.; Dar’in, D.; Krasavin, M. Mixed carboxylic–sulfonic anhydride in reaction with imines: A straightforward route to water-soluble β-lactams via a Staudinger-type reaction. Org. Biomol. Chem. 2018, 16, 3989–3998. [Google Scholar] [CrossRef] [PubMed]
- Moodie, R.B.; Schofield, K.; Taylor, P.G.; Baillie, P.J. Electrophilic Aromatic Substitution. Part 25. The Nitration in Aqueous Sulphuric Acid of Some Cinnamic Acids and Other Styrene Derivatives. J. Chem. Soc. Perkin Trans. 2 1981, 5, 842–847. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.J.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 16 Rev A.1; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A molecular electron density theory view of reactivity in organic chemistry. Molecules. 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Noury, S.; Krokidis, X.; Fuster, F.; Silvi, B. Computational tools for the electron localization function topological analysis. Comput. Chem. 1999, 23, 597–604. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView; Version 6.1; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Ahrens, J.; Geveci, B.; Law, C. ParaView: An End-User Tool for Large Data Visualization, Visualization Handbook; Butterworth-Heinemann: Oxford, UK, 2005. [Google Scholar]
- Ayachit, U. The ParaView Guide: A Parallel Visualization Application; Kitware: Clifton Park, NY, USA, 2015. [Google Scholar]
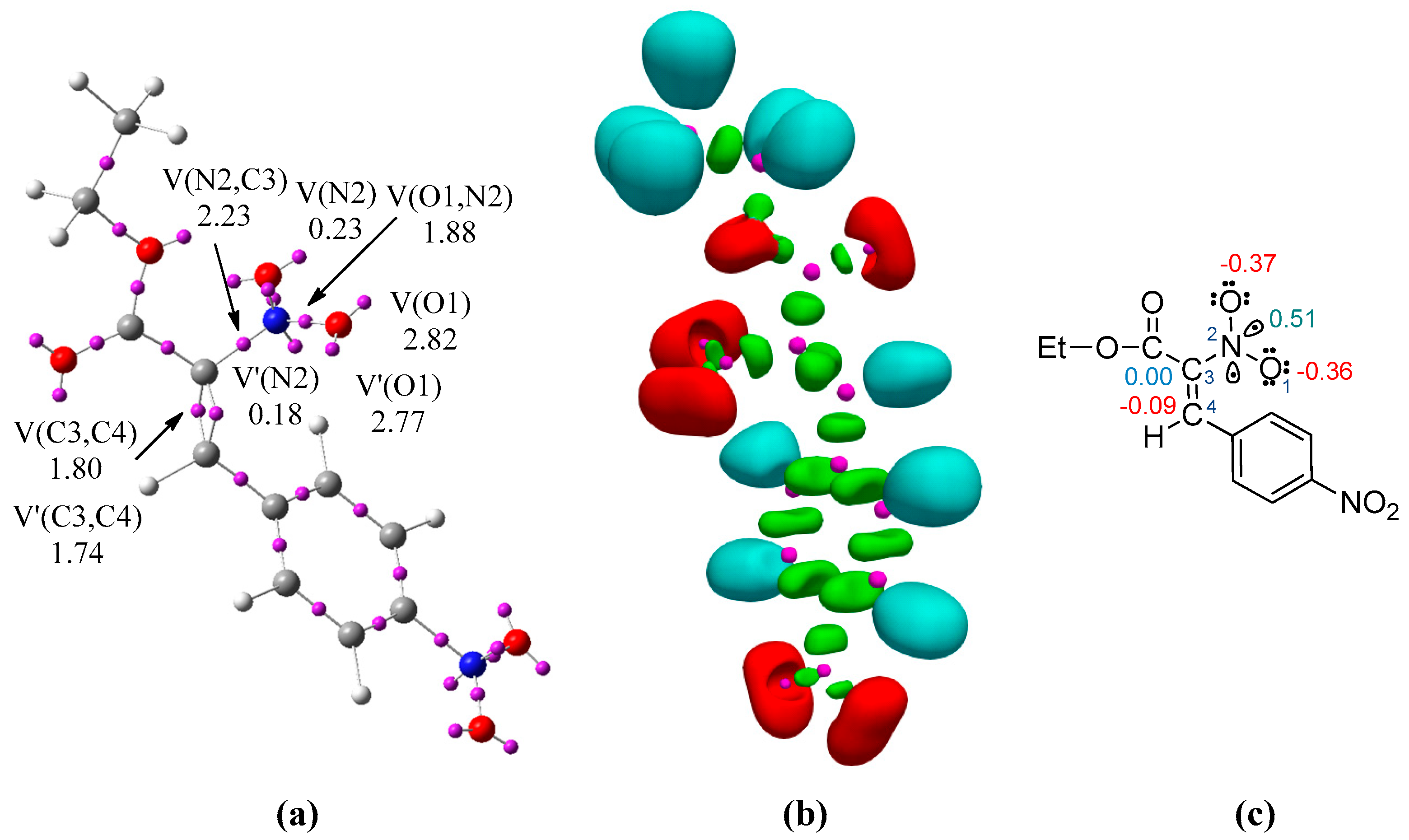


| Global Properties | Local Properties | ||||||
|---|---|---|---|---|---|---|---|
| µ [eV] | η [eV] | ω [eV] | N [eV] | 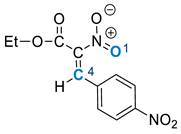 1 | |||
| Pk+ | ωk | ||||||
| 1 | 5.29 | 4.22 | 3.32 | 1.72 | C4 | 0.24 | 0.81 |
| O1 | 0.05 | 0.17 | |||||
| Compound | Compound 1 | Compound 3 |
|---|---|---|
| Crystal data | ||
| CCDC | 2471984 | 2471985 |
| Chemical formula | C11H10N2O6 | C17H20N2O6 |
| Formula weight | 266.21 | 348.35 |
| Crystal system | Triclinic | Triclinic |
| Space group | P1 | P1 |
| Temperature (K) | 99.95(13) | 272.99(14) |
| a [Å] | 7.6679(2) | 7.2551(1) |
| b [Å] | 7.7157(1) | 9.2996(2) |
| c [Å] | 10.9464(2) | 13.9423(2) |
| α [°] | 82.178(2) | 71.538(1) |
| β [°] | 76.778(2) | 85.035(1) |
| γ [°] | 68.692(2) | 77.182(1) |
| V [Å3] | 586.36(2) | 869.90(3) |
| Z | 2 | 2 |
| Z′ | 1 | 1 |
| dcalc [g/cm3] | 1.508 | 1.330 |
| Crystal dimensions [mm] | 0.30 × 0.15 × 0.03 | 0.25 × 0.20 × 0.20 |
| Radiation type | CuKα | CuKα |
| μ [mm−1] | 1.080 | 0.853 |
| Data collection | ||
| Reflections measured | 22714 | 33484 |
| Range/indices (h, k, l) | −9,−9; −9, 9; −13, 13 | −9, 9; −11, 10; −17, 17 |
| θ (max, min) [°] | 80.4, 4.2 | 80.3, 3.3 |
| Total no. of unique data | 2466 | 3697 |
| No. of observed data, I > 2σ(I) | 2239 | 3022 |
| Rint | 0.042 | 0.043 |
| Refinement | ||
| R [F2 > 2σ (F2)] | 0.035 | 0.058 |
| wR(F2) | 0.097 | 0.185 |
| S | 1.08 | 1.11 |
| No. of reflections | 2466 | 3697 |
| No. of parameters | 174 | 228 |
| No. of restraints | 0 | 0 |
| H atom treatment | H atoms treated by constrained refinement | H atoms treated by constrained refinement |
| Δρ (min, max), e/Å3 | −0.28, 0.27 | −0.28, 0.35 |
| Path | Transition | ΔH [kcal/mol] | ΔS [cal/molK] | ΔG [kcal/mol] | Imaginary Frequencies [cm−1] |
|---|---|---|---|---|---|
| 1+2→MCA | −6.5 | −36.0 | 4.2 | ||
| A | 1+2→TSA | 11.5 | −49.7 | 26.4 | −389.3 |
| 1+2→3 | −27.3 | −50.4 | −12.2 | ||
| 1+2→MCB | −6.6 | −36.1 | 4.2 | ||
| B | 1+2→TSB | 27.8 | −49.0 | 42.4 | −414.1 |
| 1+2→4 | −24.9 | −53.0 | −9.1 |
| Path | Structure | Interatomic Distances [A] | GEDT | |||||
|---|---|---|---|---|---|---|---|---|
| O1-N2 | N2-C3 | C3-C4 | C4-C5 | C5-C6 | C6-O1 | [e] | ||
| 1 | 1.214 | 1.475 | 1.330 | |||||
| 2 | 1.329 | |||||||
| A | MCA | 1.212 | 1.475 | 1.330 | 3.629 | 1.330 | 4.282 | 0.00 |
| TSA | 1.238 | 1.418 | 1.404 | 2.029 | 1.372 | 2.991 | 0.41 | |
| 3 | 1.397 | 1.317 | 1.502 | 1.536 | 1.526 | 1.479 | ||
| MCB | 1.213 | 1.474 | 1.330 | 3.700 | 1.326 | 3.649 | 0.00 | |
| B | TSB | 1.258 | 1.386 | 1.423 | 1.973 | 1.381 | 2.366 | 0.40 |
| 4 | 1.396 | 1.311 | 1.510 | 1.551 | 1.512 | 1.433 | ||
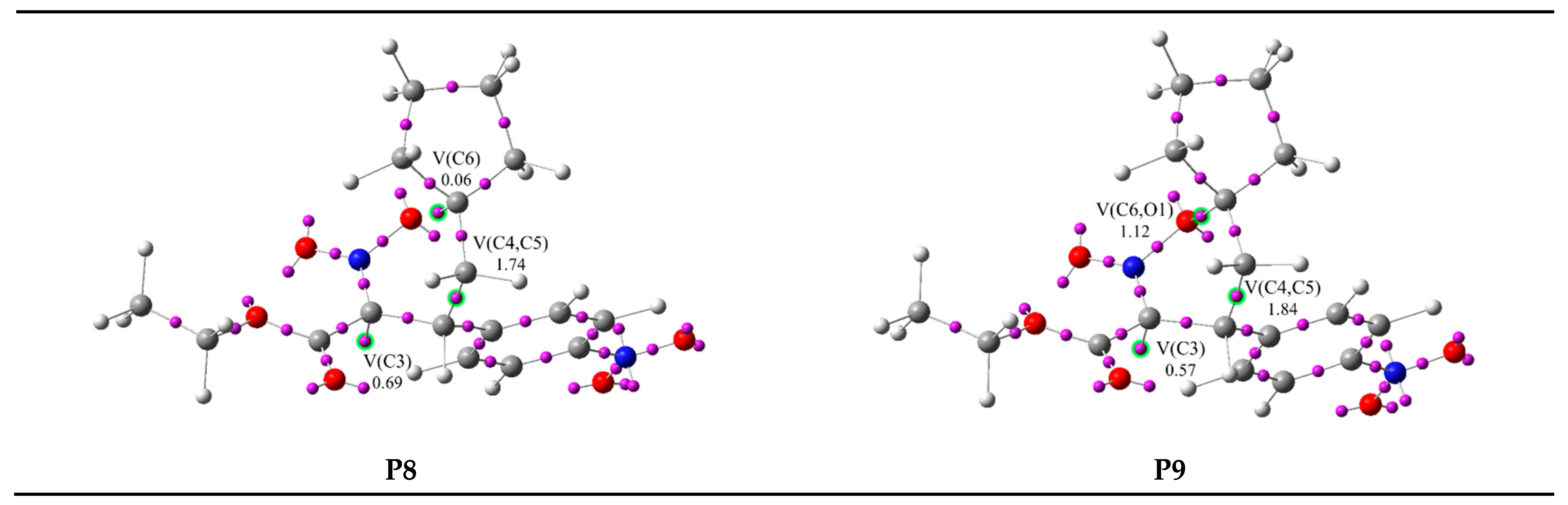
| Points | 1 | 2 | MCA | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phases | I | II | III | IV | V | VI | VII | VIII | IX | X | |||||||||||||
| d(C4-C5) | 2.935 | 2.564 | 2.286 | 2.205 | 2.072 | 2.060 | 2.023 | 2.017 | 1.553 | 1.526 | 1.525 | ||||||||||||
| d(C6-O1) | 3.316 | 3.133 | 3.044 | 3.025 | 2.999 | 2.996 | 2.989 | 2.988 | 2.103 | 1.585 | 1.499 | ||||||||||||
| V(O1,N2) | 1.88 | 1.84 | 1.81 | 1.79 | 1.78 | 1.74 | 1.73 | 1.73 | 1.72 | 1.42 | 1.21 | 1.21 | |||||||||||
| V(O1) | 2.82 | 2.82 | 2.84 | 2.86 | 2.88 | 2.89 | 2.89 | 2.90 | 2.90 | 2.94 | 2.65 | 2.60 | |||||||||||
| V’(O1) | 2.77 | 2.82 | 2.84 | 2.85 | 2.84 | 2.85 | 2.85 | 2.86 | 2.86 | 2.92 | 2.39 | 2.37 | |||||||||||
| V(N2,C3) | 2.23 | 2.29 | 2.32 | 2.36 | 2.56 | 2.75 | 2.76 | 2.77 | 2.78 | 3.26 | 3.57 | 3.60 | |||||||||||
| V(N2) | 0.23 | 0.20 | 0.19 | 0.18 | |||||||||||||||||||
| V’(N2) | 0.18 | ||||||||||||||||||||||
| V(C3,C4) | 1.80 | 1.84 | 3.47 | 3.47 | 3.47 | 2.96 | 2.86 | 2.64 | 2.62 | 2.04 | 2.04 | 2.03 | |||||||||||
| V’(C3,C4) | 1.74 | 1.66 | |||||||||||||||||||||
| V(C5,C6) | 1.78 | 1.76 | 1.75 | 3.29 | 3.26 | 3.22 | 3.21 | 2.84 | 2.82 | 2.15 | 2.02 | 2.01 | |||||||||||
| V’(C5,C6) | 1.77 | 1.71 | 1.66 | ||||||||||||||||||||
| V(C3) | 0.51 | 0.51 | 0.55 | 0.56 | 0.69 | 0.57 | 0.57 | ||||||||||||||||
| V(C4) | 0.11 | 0.16 | |||||||||||||||||||||
| V(C5) | 0.37 | ||||||||||||||||||||||
| V(C4,C5) | 0.53 | 1.74 | 1.84 | 1.84 | |||||||||||||||||||
| V(C6) | 0.06 | ||||||||||||||||||||||
| V(C6,O1) | 1.12 | 1.43 | |||||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woliński, P.; Kącka-Zych, A.; Wielgus, E.; Dolot, R.; Jasiński, R. Analysis of the Reactivity of Z-2-Ar-1-EWG-1-Nitroethene Molecular Segment in the Hetero Diels–Alder Reaction: Experimental and MEDT Quantum Chemical Study. Molecules 2025, 30, 3768. https://doi.org/10.3390/molecules30183768
Woliński P, Kącka-Zych A, Wielgus E, Dolot R, Jasiński R. Analysis of the Reactivity of Z-2-Ar-1-EWG-1-Nitroethene Molecular Segment in the Hetero Diels–Alder Reaction: Experimental and MEDT Quantum Chemical Study. Molecules. 2025; 30(18):3768. https://doi.org/10.3390/molecules30183768
Chicago/Turabian StyleWoliński, Przemysław, Agnieszka Kącka-Zych, Ewelina Wielgus, Rafał Dolot, and Radomir Jasiński. 2025. "Analysis of the Reactivity of Z-2-Ar-1-EWG-1-Nitroethene Molecular Segment in the Hetero Diels–Alder Reaction: Experimental and MEDT Quantum Chemical Study" Molecules 30, no. 18: 3768. https://doi.org/10.3390/molecules30183768
APA StyleWoliński, P., Kącka-Zych, A., Wielgus, E., Dolot, R., & Jasiński, R. (2025). Analysis of the Reactivity of Z-2-Ar-1-EWG-1-Nitroethene Molecular Segment in the Hetero Diels–Alder Reaction: Experimental and MEDT Quantum Chemical Study. Molecules, 30(18), 3768. https://doi.org/10.3390/molecules30183768








