Optimization of Supplemental LED Spectral Quality and Light Dose for Enhancing Biomass and Essential Oil Yield of Ocimum gratissimum L. Under Net House Condition
Abstract
1. Introduction
2. Results and Discussion
2.1. The Effect of Light Spectra on Biomass and Essential Oil Yield of Ocimum gratissimum
2.2. The Effect of Light Spectra on Essential Oil Composition of Ocimum gratissimum
2.3. The Effect of Light Spectra on Antimicrobial Activity of Essential Oils of O. gratissimum
3. Materials and Methods
3.1. Plant Materials, Growth Conditions and Light Treatments
3.2. Essential Oil Extraction and Physical Properties Analysis
3.3. Gas Chromatography/Mass Spectrometry with Flame Ionization Detection Analysis
3.4. Antimicrobial Activity Screening
3.5. Statistical Analysis
4. Conclusions—Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krahmer, J.; Fankhauser, C. Environmental control of hypocotyl elongation. Annu. Rev. Plant Biol. 2024, 75, 489–519. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Effects of light quality on growth and phytonutrient accumulation of herbs under controlled environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef]
- Kang, S.; Kim, J.E.; Zhen, S.; Kim, J. Mild-intensity UV-A radiation applied over a long duration can improve the growth and phenolic contents of sweet basil. Front. Plant Sci. 2022, 13, 858433. [Google Scholar] [CrossRef]
- Lee, J.H.; Oh, M.M.; Son, K.H. Short-term ultraviolet (UV)-A light-emitting diode (LED) radiation improves biomass and bioactive compounds of kale. Front. Plant Sci. 2019, 10, 1042. [Google Scholar] [CrossRef]
- Chen, Y.; Li, T.; Yang, Q.; Zhang, Y.; Zou, J.; Bian, Z.; Wen, X. UVA radiation is beneficial for yield and quality of indoor cultivated lettuce. Front. Plant Sci. 2019, 10, 1563. [Google Scholar] [CrossRef]
- Zhang, Y.; Kaiser, E.; Zhang, Y.; Zou, J.; Bian, Z.; Yang, Q.; Li, T. UVA radiation promotes tomato growth through morphological adaptation leading to increased light interception. Environ. Exp. Bot. 2020, 176, 104073. [Google Scholar] [CrossRef]
- Tarakanov, I.; Yakovleva, O.; Konovalova, I.; Paliutina, G.; Anisimov, A. Light-emitting diodes: On the way to combinatorial lighting technologies for basic research and crop production. Acta Hortic. 2012, 956, 171–178. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Kim, H.H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red-and blue-light-emitting diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef]
- McAusland, L.; Lim, M.T.; Morris, D.E.; Smith-Herman, H.L.; Mohammed, U.; Hayes-Gill, B.R.; Crowe, J.A.; Fisk, I.D.; Murchie, E.H. Growth spectrum complexity dictates aromatic intensity in coriander (Coriandrum sativum L.). Front. Plant Sci. 2020, 11, 462. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and physiological properties of indoor cultivated lettuce in response to additional far-red light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Kim, D.; Son, J.E. Adding far-red to red, blue supplemental light-emitting diode interlighting improved sweet pepper yield but attenuated carotenoid content. Front. Plant Sci. 2022, 13, 938199. [Google Scholar] [CrossRef]
- Ugbogu, O.C.; Emmanuel, O.; Agi, G.O.; Ibe, C.; Ekweogu, C.N.; Ude, V.C.; Uche, M.E.; Nnanna, R.O.; Ugbogu, E.A. A review on the traditional uses, phytochemistry, and pharmacological activities of clove basil (Ocimum gratissimum L.). Heliyon 2021, 7, e08404. [Google Scholar] [CrossRef]
- Yarou, B.B.; Bokonon-Ganta, A.H.; Verheggen, F.J.; Lognay, G.C.; Francis, F. Aphid behavior on Amaranthus hybridus L. (Amaranthaceae) associated with Ocimum spp. (Lamiaceae) as repellent plants. Agronomy 2020, 10, 736. [Google Scholar] [CrossRef]
- Akara, E.U.; Emmanuel, O.; Ude, V.C.; Uche-Ikonne, C.; Eke, G.; Ugbogu, E.A. Ocimum gratissimum leaf extract ameliorates phenylhydrazine-induced anaemia and toxicity in Wistar rats. Drug Metabol. Pers. Ther. 2021, 36, 311–320. [Google Scholar] [CrossRef]
- Udi, O.A.; Oyem, J.C.; Ebeye, O.A.; Chris-Ozoko, L.E.; Igbigbi, P.S.; Olannye, D.U. The effects of aqueous extract of Ocimum gratissimum on the cerebellum of male wistar rats challenged by lead acetate. Clin. Nutr. Open Sci. 2022, 44, 28–41. [Google Scholar] [CrossRef]
- Duru, I.A.; Duru, C.E. Molecular docking of compounds in the essential oil of Ocimum gratissimum leaf against PIM-1 kinase of Escherichia coli. Eur. J. Adv. Chem. Res. 2020, 1, 1–4. [Google Scholar] [CrossRef]
- Singh, P.; Jayaramaiah, R.H.; Agawane, S.B.; Vannuruswamy, G.; Korwar, A.M.; Anand, A.; Dhaygude, V.S.; Shaikh, M.L.; Joshi, R.S.; Boppana, R.; et al. Potential dual role of eugenol in inhibiting advanced glycation end products in diabetes: Proteomic and mechanistic insights. Sci. Rep. 2016, 6, 18798. [Google Scholar] [CrossRef]
- Halayal, R.Y.; Bagewadi, Z.K.; Maliger, R.B.; Al Jadidi, S.; Deshpande, S.H. Network pharmacology based anti-diabetic attributes of bioactive compounds from Ocimum gratissimum L. through computational approach. Saudi J. Biol. Sci. 2023, 30, 103766. [Google Scholar] [CrossRef]
- Vilanova, C.M.; Coelho, K.P.; Luz, T.R.S.A.; Silveira, D.P.B.; Coutinho, D.F.; de Moura, E.G. Effect of different water application rates and nitrogen fertilisation on growth and essential oil of clove basil (Ocimum gratissimum L.). Ind. Crops Prod. 2018, 125, 186–197. [Google Scholar] [CrossRef]
- Fernandes, V.F.; de Almeida, L.B.; Feijó, E.V.D.S.; Silva, D.D.C.; de Oliveira, R.A.; Mielke, M.S.; Costa, L.C.D.B. Light intensity on growth, leaf micromorphology and essential oil production of Ocimum gratissimum. Rev. Bras. Farmacogn. 2013, 23, 419–424. [Google Scholar] [CrossRef]
- Rahman, M.M.; Vasiliev, M.; Alameh, K. LED Illumination spectrum manipulation for increasing the yield of sweet basil (Ocimum basilicum L.). Plants 2021, 10, 344. [Google Scholar] [CrossRef]
- Weeplian, T.; Somsong, P.; Tayjasanant, T.; Kiravittaya, S. Improving phytochemicals and antioxidant activity of clove basil (Ocimum gratissimum L.) with far-red and UV-A light supplementation in late production stages. Acta Hortic. 2025, 1423, 277–284. [Google Scholar] [CrossRef]
- Litvin, A.G.; Currey, C.J.; Wilson, L.A. Effects of supplemental light source on basil, dill, and parsley growth, morphology, aroma, and flavor. J. Am. Soc. Hortic. Sci. 2020, 145, 18–29. [Google Scholar] [CrossRef]
- Semenova, N.A.; Smirnov, A.A.; Ivanitskikh, A.S.; Izmailov, A.Y.; Dorokhov, A.S.; Proshkin, Y.A.; Yanykin, D.V.; Sarimov, R.R.; Gudkov, S.V.; Chilingaryan, N.O. Impact of ultraviolet radiation on the pigment content and essential oil accumulation in sweet basil (Ocimum basilicum L.). Appl. Sci. 2022, 12, 7190. [Google Scholar] [CrossRef]
- Brodersen, C.R.; Vogelmann, T.C. Do changes in light direction affect absorption profiles in leaves? Funct. Plant Biol. 2010, 37, 403–412. [Google Scholar] [CrossRef]
- Kong, J.; Zhao, Y.; Fan, P.; Wang, Y.; Xu, X.; Wang, L.; Li, S.; Duan, W.; Liang, Z.; Dai, Z. Far-red light modulates grapevine growth by increasing leaf photosynthesis efficiency and triggering organ-specific transcriptome remodelling. BMC Plant Biol. 2024, 24, 189. [Google Scholar] [CrossRef] [PubMed]
- Sipos, L.; Balázs, L.; Székely, G.; Jung, A.; Sárosi, S.; Radácsi, P.; Csambalik, L. Optimization of basil (Ocimum basilicum L.) production in LED light environments—A review. Sci. Hortic. 2021, 289, 110486. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Yu-Xin, T.; Qi-Chang, Y. Optimal control of environmental conditions affecting lettuce plant growth in a controlled environment with artificial lighting: A review. S. Afr. J. Bot. 2020, 130, 75–89. [Google Scholar] [CrossRef]
- Kim, S.J.; Hahn, E.J.; Heo, J.W.; Paek, K.Y. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci. Hortic. 2004, 101, 143–151. [Google Scholar] [CrossRef]
- Ahmadi, T.; Shabani, L.; Sabzalian, M.R. LED light sources improved the essential oil components and antioxidant activity of two genotypes of lemon balm (Melissa officinalis L.). Bot. Stud. 2021, 62, 9. [Google Scholar] [CrossRef]
- Verdaguer, D.; Jansen, M.A.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef]
- Brown, C.S.; Schuerger, A.C.; Sager, J.C. Growth and photomorphogenesis of pepper plants under red light-emitting diodes with supplemental blue or far-red lighting. J. Am. Soc. Hortic. Sci. 1995, 120, 808–813. [Google Scholar] [CrossRef]
- Elkins, C.; van Iersel, M.W. Supplemental far-red light-emitting diode light increases growth of foxglove seedlings under sole-source lighting. HortTechnology 2020, 30, 564–569. [Google Scholar] [CrossRef]
- Jin, W.; Urbina, J.L.; Heuvelink, E.; Marcelis, L.F. Adding far-red to red-blue light-emitting diode light promotes yield of lettuce at different planting densities. Front. Plant Sci. 2021, 11, 609977. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.H.; Le, P.T.Q. Chemical profile and antimicrobial activity of Ocimum gratissimum L. essential oil from Dak Lak province, Vietnam. J. Plant Biotechnol. 2024, 51, 50–54. [Google Scholar] [CrossRef]
- Alsahli, A.A. Light effects on growth and essential oil quantity and constituents in some Apiaceae plants. Afr. J. Agric. Res. 2019, 14, 1262–1271. [Google Scholar] [CrossRef]
- Mulas, G.; Gardner, Z.; Craker, L.E. Effect of light quality on growth and essential oil composition in rosemary. Acta Hortic. 2006, 723, 427–432. [Google Scholar] [CrossRef]
- Chen, Y.; Bian, Z.; Marcelis, L.F.; Heuvelink, E.; Yang, Q.; Kaiser, E. Green light is similarly effective in promoting plant biomass as red/blue light–a meta-analysis. J. Exp. Bot. 2024, 75, 259. [Google Scholar] [CrossRef]
- Qian, M.; Kalbina, I.; Rosenqvist, E.; Jansen, M.A.; Strid, Å. Supplementary UV-A and UV-B radiation differentially regulate morphology in Ocimum basilicum. Photochem. Photobiol. Sci. 2023, 22, 2219–2230. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Saleh, M.A. Effect of exposure to light emitted diode (LED) lights on essential oil composition of sweet mint plants. J. Environ. Sci. Health Part A 2019, 54, 435–440. [Google Scholar]
- Sale, A.I.; Uthairatanakij, A.; Laohakunjit, N.; Jitareerat, P.; Kaisangsri, N. Pre-harvest supplemental LED treatments led to improved postharvest quality of sweet basil leaves. J. Photochem. Photobiol. B Biol. 2023, 248, 112788. [Google Scholar]
- Aghakarim, F.; Sarikhani, H.; Azizi, A. Effects of supplemental light quality at the end of day on herb production and some phytochemical properties of lemon balm (Melissa officinalis L.). Int. J. Hortic. Sci. Technol. 2023, 10, 66–88. [Google Scholar]
- Huang, T.; Myanganbayar, A.; Davaasambuu, U. The effects of different color lights on the growth, glandular trichome development and essential oil content of Mentha arvensis L. Proc. Mong. Acad. Sci. 2024, 64, 251. [Google Scholar] [CrossRef]
- Joshi, R.K. Antioxidant Activity Influenced by Seasonal Variation of Essential Oil Constituents of Ocimum gratissimum L. ACS Food Sci. Technol. 2021, 1, 1661–1669. [Google Scholar]
- Freire, C.M.M.; Marques, M.O.M.; Costa, M. Effects of seasonal variation on the central nervous system activity of Ocimum gratissimum L. essential oil. J. Ethnopharmacol. 2006, 105, 161–166. [Google Scholar] [CrossRef]
- Matasyoh, L.G.; Matasyoh, J.C.; Wachira, F.N.; Kinyua, M.G.; Muigai, A.W.T.; Mukiama, T.K. Chemical composition and antimicrobial activity of the essential oil of Ocimum gratissimum L. growing in Eastern Kenya. Afr. J. Biotechnol. 2007, 6, 760–765. [Google Scholar]
- Brada, M.; Khelifa, L.H.; Achour, D.; Wathelet, J.P.; Lognay, G. Essential oil composition of Ocimum basilicum L. and Ocimum gratissimum L. from Algeria. J. Essent. Oil-Bear. Plants 2011, 14, 810–814. [Google Scholar] [CrossRef]
- Joshi, R.K. Chemical composition, in vitro antimicrobial and antioxidant activities of the essential oils of Ocimum gratissimum, O. sanctum and their major constituents. Indian J. Pharm. Sci. 2013, 75, 457. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K. GC—MS analysis of the essential oil of Ocimum gratissimum L. growing desolately in South India. Acta Chromatogr. 2017, 29, 111–119. [Google Scholar] [CrossRef]
- Martins, A.P.; Salgueiro, L.R.; Vila, R.; Tomi, F.; Cañigueral, S.; Casanova, J.; da Cunha, A.P.; Adzet, T. Composition of the essential oils of Ocimum canum, O. gratissimum and O. minimum. Planta Med. 1999, 65, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, A.; Sawadogo, I.; Toé, M.; Hema, M.D.; Bationo, K.R.; Kiendrebeogo, M.; Nébié, C.R. Composition, physico-chemical and antioxidant properties of Ocimum gratissimum L. essential oil from Burkina Faso. J. Appl. Biol. Chem. 2023, 17, 486–499. [Google Scholar] [CrossRef]
- Sellamuthu, R. Eugenol. In Encyclopedia of Toxicology; Academic Press: Cambridge, MA, USA, 2014; pp. 539–541. [Google Scholar]
- Begum, S.N.; Ray, A.S.; Rahaman, C.H. A comprehensive and systematic review on potential anticancer activities of eugenol: From pre-clinical evidence to molecular mechanisms of action. Phytomedicine 2022, 107, 154456. [Google Scholar] [CrossRef]
- Damasceno, R.O.S.; Pinheiro, J.L.S.; Rodrigues, L.H.M.; Gomes, R.C.; Duarte, A.B.S.; Emídio, J.J.; Diniz, L.R.L.; de Sousa, D.P. Anti-Inflammatory and antioxidant activities of eugenol: An update. Pharmaceuticals 2024, 17, 1505. [Google Scholar] [CrossRef]
- Chongtham, A.; Agrawal, N. Neuroprotective potential of eugenol in polyglutamine-mediated neurodegenerative disease using transgenic Drosophila model. Dose-Response 2024, 22, 15593258241291652. [Google Scholar] [CrossRef]
- Ribeiro, T.A.N.; Dos Santos, G.A.; Dos Santos, C.T.; Soares, D.C.F.; Saraiva, M.F.; Leal, D.H.S.; Sachs, D. Eugenol as a promising antibiofilm and anti-quorum sensing agent: A systematic review. Microb. Pathog. 2024, 196, 106937. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Cannabis pharmacology: The usual suspects and a few promising leads. Adv. Pharmacol. 2017, 80, 67–134. [Google Scholar]
- Bülow, N.; König, W.A. The role of germacrene D as a precursor in sesquiterpene biosynthesis: Investigations of acid catalyzed, photochemically and thermally induced rearrangements. Phytochemistry 2020, 55, 141–168. [Google Scholar] [CrossRef]
- Faria, T.D.J.; Ferreira, R.S.; Yassumoto, L.; Souza, J.R.P.D.; Ishikawa, N.K.; Barbosa, A.D.M. Antifungal activity of essential oil isolated from Ocimum gratissimum L. (eugenol chemotype) against phytopathogenic fungi. Braz. Arch. Biol. Technol. 2006, 49, 867–871. [Google Scholar] [CrossRef]
- Lima, E.O.; Gompertz, O.F.; Giesbrecht, A.M.; Paulo, M.Q. Im vitro antifungal activity of essential oils obtained from officinal plants against dermatophytes: Antimyzetische Aktivität ätherischer Öle von Heilpflanzen in vitro gegen Dermatophyten. Mycoses 1993, 36, 333–336. [Google Scholar] [CrossRef]
- Zore, G.B.; Thakre, A.D.; Jadhav, S.; Karuppayil, S.M. Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine 2011, 18, 1181–1190. [Google Scholar] [CrossRef]
- Sikkema, J.A.N.; de Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Chen, H.; Fan, Y.; Shi, Z. Antifungal activity of eugenol against Botrytis cinerea. Trop. Plant Pathol. 2010, 35, 137–143. [Google Scholar] [CrossRef]
- Didehdar, M.; Chegini, Z.; Shariati, A. Eugenol: A novel therapeutic agent for the inhibition of Candida species infection. Front. Pharmacol. 2022, 13, 872127. [Google Scholar] [CrossRef]
- Tabbert, J.M.; Schulz, H.; Krähmer, A. Increased plant quality, greenhouse productivity and energy efficiency with broad-spectrum LED systems: A case study for thyme (Thymus vulgaris L.). Plants 2021, 10, 960. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Q.; Ji, G.; Wang, J.; Ding, Y.; Wang, S. Effects of light intensity and photoperiod on development and photosynthetic characteristics of coriander. Horticulturae 2024, 10, 215. [Google Scholar] [CrossRef]
- SuntrackerTech. DLI Calculator, Version 2.1.5; SunTracker Technologies Ltd.: Victoria, BC, Canada, 2024; Available online: https://dli.suntrackertech.com (accessed on 1 October 2024).
- Ptak, P.; Górecki, K.; Heleniak, J.; Orlikowski, M. Investigations of electrical and optical parameters of some LED luminaires—A study case. Energies 2021, 14, 1612. [Google Scholar] [CrossRef]
- Ministry of Health of Vietnam Vietnamese Pharmacopoeia, V.; Medical Publishing House: Hanoi, Vietnam, 2017; pp. PL274–PL275. (In Vietnamese)
- ISO 279:1998; Essential Oils—Determination of Relative Density at 20 °C—Reference Method. ISO: Geneva, Switzerland, 1998.
- ISO 280:1998; Essential Oils—Determination of Refractive Index. ISO: Geneva, Switzerland, 1998.
- ISO 592:1998; Essential Oils—Determination of Optical Rotation. ISO: Geneva, Switzerland, 1998.
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography, Mass Spectrometry, 4.1th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2017; 804p, ISBN 978-1-932633-21-4. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry Webbook, NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2021; p. 20899. [Google Scholar]
- König, W.A.; Joulain, D.; Hochmuth, D.H. Terpenoids and Related Constituents of Essential Oils; Library of MassFinder: Hamburg, Germany, 2004; Volume 2, Available online: https://www.scirp.org/reference/referencespapers?referenceid=3375220 (accessed on 27 July 2025).
- Hadacek, F.; Greger, H. Testing of antifungal natural products: Methodologies, comparability of results and assay choice. Phytochem. Anal. 2000, 11, 137–147. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
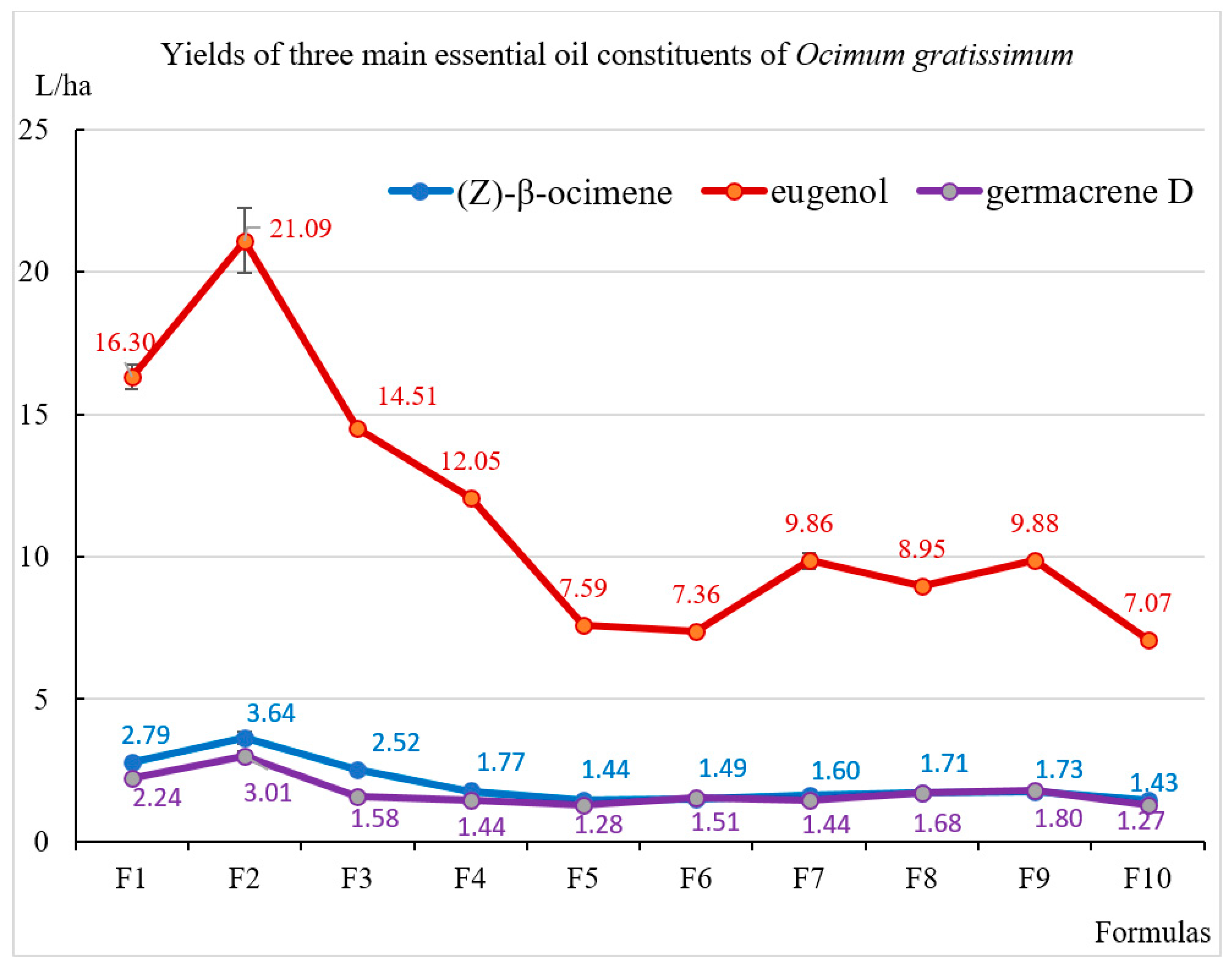
| Formulas | Plant Height (cm/Plant) | Fresh Biomass Yield (Mg/ha) * | Water Content (%) | Dry Biomass Yield (Mg/ha) * | Essential Oil Content (%, Dry v/w) | Essential Oil Yield (L/ha) * |
|---|---|---|---|---|---|---|
| F1 | 104.42 ± 1.51 b | 10.50 ± 0.28 b | 71.31 ± 0.14 i | 3.01 ± 0.08 a | 0.80 ± 0.002 i | 24.05 ± 0.64 b |
| F2 | 117.55 ± 6.07 a | 13.07 ± 0.71 a | 78.63 ± 0.18 c | 2.79 ± 0.15 b | 1.12 ± 0.002 a | 31.39 ± 1.71 a |
| F3 | 110.49 ± 9.62 a | 10.20 ± 0.03 c | 77.93 ± 0.15 d | 2.25 ± 0.01 c | 0.92 ± 0.001 d | 20.79 ± 0.06 c |
| F4 | 101.29 ± 4.24 b | 8.86 ± 0.10 d | 76.30 ± 0.17 e | 2.10 ± 0.02 d | 0.83 ± 0.002 f | 17.34 ± 0.20 d |
| F5 | 104.45 ± 13.31 b | 6.82 ± 0.08 f | 79.87 ± 0.21 a | 1.37 ± 0.02 i | 0.89 ± 0.002 e | 12.22 ± 0.15 g |
| F6 | 98.81 ± 9.25 b | 6.37 ± 0.14 g | 79.82 ± 0.17 a | 1.29 ± 0.03 k | 0.97 ± 0.002 c | 12.52 ± 0.27 g |
| F7 | 101.42 ± 6.75 b | 6.96 ± 0.19 f | 78.90 ± 0.19 b | 1.47 ± 0.04 h | 1.03 ± 0.001 b | 15.14 ± 0.42 f |
| F8 | 101.55 ± 6.12 b | 6.98 ± 0.13 f | 73.20 ± 0.17 h | 1.87 ± 0.04 f | 0.81 ± 0.001 g | 15.17 ± 0.29 f |
| F9 | 102.51 ± 8.04 b | 8.17 ± 0.14 e | 75.39 ± 0.16 f | 2.01 ± 0.03 e | 0.81 ± 0.002 h | 16.20 ± 0.27 e |
| F10 | 97.63 ± 1.28 b | 6.29 ± 0.05 g | 74.60 ± 0.19 g | 1.60 ± 0.01 g | 0.76 ± 0.001 k | 12.08 ± 0.09 g |
| 5%LSD | 12.9709 | 0.4661 | 0.3071 | 0.1049 | 0.0028 | 1.0927 |
| p | 0.129 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Parameters | F1 * | F2 * | F3 * | F4 * | F5 * | F6 * | F7 * | F8 * | F9 * | F10 * |
|---|---|---|---|---|---|---|---|---|---|---|
| Relative density d20 | 0.9951 | 0.9873 | 0.9984 | 0.9910 | 0.9853 | 0.9811 | 0.9900 | 0.9785 | 0.9851 | 0.9788 |
| Refractive index n20 | 1.5155 | 1.5165 | 1.5186 | 1.5190 | 1.5168 | 1.5150 | 1.5170 | 1.5148 | 1.5168 | 1.5138 |
| Optical rotation [α] D20 | [−]21.36 | [−]21.52 | [−]16.53 | [−]18.92 | [−]24.10 | [−]27.78 | [−]21.46 | [−]26.83 | [−]26.65 | [−]24.78 |
| Compounds a | RI b | F1 c | F2 c | F3 c | F4 c | F5 c | F6 c | F7 c | F8 c | F9 c | F10 c |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (3Z)-Hexen-1-ol | 850 | 0.4 | 0.5 | 0.8 | 0.6 | 0.4 | 0.4 | 0.4 | 0.4 | 0.2 | 0.5 |
| α-Thujene | 929 | 0.3 | 0.3 | 0.3 | 0.2 | 0.3 | 0.4 | 0.3 | 0.5 | 0.4 | 0.5 |
| 1-Octen-3-ol | 976 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | 0.1 | 0.3 |
| Sabinene | 978 | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 | 0.4 | 0.4 | 0.5 | 0.5 | 0.5 |
| Myrcene | 991 | 0.3 | 0.3 | 0.3 | 0.2 | 0.4 | 0.4 | 0.3 | 0.4 | 0.4 | 0.5 |
| α-Terpinene | 1021 | 0.1 | Tr | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 |
| p-Cymene | 1029 | Tr | Tr | Tr | Tr | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 |
| (Z)-β-Ocimene | 1038 | 11.6 | 11.6 | 12.1 | 10.2 | 11.8 | 11.9 | 10.6 | 11.3 | 10.7 | 11.8 |
| (E)-β-Ocimene | 1048 | 0.5 | 0.5 | 0.5 | 0.4 | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| γ-Terpinene | 1063 | 0.2 | 0.2 | 0.3 | 0.2 | 0.3 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 |
| cis-Sabinene hydrate | 1072 | 0.2 | 0.2 | Tr | 0.1 | 0.2 | 0.4 | 0.3 | 0.4 | 0.4 | 0.2 |
| Linalool | 1101 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2 | 0.3 | 0.3 | 0.3 |
| allo-Ocimene | 1131 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Menthol | 1179 | Tr | Tr | Tr | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 |
| iso-Menthol | 1179 | Tr | 0.2 | 0.2 | Tr | Tr | Tr | Tr | Tr | Tr | Tr |
| Santalone | 1185 | 0.5 | 0.4 | 0.5 | Tr | Tr | Tr | Tr | Tr | Tr | Tr |
| Terpinen-4-ol | 1186 | Tr | Tr | Tr | 0.5 | 0.6 | 0.5 | 0.7 | 0.8 | 0.7 | 0.9 |
| Methyl chavicol (=Estragole) | 1204 | 1.0 | 0.9 | 1.1 | 1.7 | 1.1 | 1.1 | 0.9 | 0.9 | 0.8 | 0.9 |
| Eugenol | 1369 | 67.8 | 67.2 | 69.8 | 69.5 | 62.1 | 58.8 | 65.1 | 59.0 | 61.0 | 58.5 |
| α-Copaene | 1390 | 0.7 | 0.7 | 0.6 | 0.6 | 0.9 | 1.0 | 0.9 | 1.0 | 1.0 | 1.0 |
| (E)-β-Caryophyllene | 1438 | 4.4 | 4.5 | 3.4 | 4.0 | 5.6 | 6.6 | 5.7 | 6.8 | 6.5 | 6.9 |
| β-Gurjunene (=Calarene) | 1446 | Tr | Tr | Tr | Tr | 0.1 | 0.2 | 0.1 | 0.2 | Tr | 0.2 |
| α-Humulene | 1472 | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 | 0.5 | 0.4 | 0.5 | 0.5 | 0.5 |
| γ-Muurolene | 1491 | Tr | Tr | Tr | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 |
| Germacrene D | 1499 | 9.3 | 9.6 | 7.6 | 8.3 | 10.5 | 12.1 | 9.5 | 11.1 | 11.1 | 10.5 |
| γ-Cadinene | 1531 | Tr | Tr | Tr | Tr | Tr | Tr | Tr | Tr | Tr | 0.2 |
| δ-Cadinene | 1537 | 0.4 | 0.4 | 0.4 | 0.4 | 0.6 | 0.7 | 0.6 | 0.7 | 0.6 | 0.7 |
| Caryophyllene oxide | 1605 | 0.3 | 0.3 | 0.2 | 0.4 | 0.5 | 0.5 | 0.3 | 0.6 | 0.5 | 0.5 |
| epi-α-Cadinol (=τ-Cadinol) | 1659 | Tr | Tr | Tr | 0.2 | 0.3 | Tr | Tr | 0.3 | Tr | Tr |
| epi-α-Muurolol (=τ-Muurolol) | 1660 | Tr | Tr | Tr | Tr | Tr | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 |
| α-Cadinol | 1673 | Tr | Tr | Tr | 0.2 | 0.3 | 0.3 | 0.2 | 0.3 | 0.3 | 0.5 |
| Total | 99.1 | 99.0 | 99.4 | 99.3 | 98.5 | 98.3 | 98.7 | 98.1 | 97.8 | 97.9 | |
| Number of compounds quantified | 21 | 21 | 21 | 25 | 27 | 27 | 27 | 28 | 26 | 28 | |
| Monoterpene hydrocarbons | 13.5 | 13.4 | 14.1 | 11.8 | 14.1 | 14.1 | 12.8 | 14.0 | 13.3 | 14.6 | |
| Oxygenated monoterpenoids | 0.9 | 1.0 | 0.9 | 0.9 | 1.2 | 1.4 | 1.4 | 1.7 | 1.5 | 1.6 | |
| Sesquiterpene hydrocarbons | 15.1 | 15.5 | 12.3 | 13.8 | 18.3 | 21.3 | 17.3 | 20.5 | 19.9 | 20.2 | |
| Oxygenated sesquiterpenoids | 0.3 | 0.3 | 0.2 | 0.8 | 1.1 | 1.0 | 0.7 | 1.4 | 1.0 | 1.3 | |
| Phenylpropanoids | 68.8 | 68.1 | 70.9 | 71.2 | 63.2 | 59.9 | 66.0 | 59.9 | 61.8 | 59.4 | |
| Others | 0.5 | 0.7 | 1.0 | 0.8 | 0.6 | 0.6 | 0.5 | 0.6 | 0.3 | 0.8 |
| Formulas/Reference Compounds | Values (µg/mL) | The Concentration of Essential Oil Inhibiting the Tested Microorganisms (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Gram (+) Bacteria | Gram (−) Bacteria | Yeast | ||||||
| Staphylococcus aureus | Bacillus subtilis | Lactobacillus fermentum | Salmonella enterica | Escherichia coli | Pseudomonas aeruginosa | Candida albicans | ||
| F1 | IC50 | >16,384 | >16,384 | >16,384 | >16,384 | 6051 ± 16 | >16,384 | 3220 ± 8.8 g |
| MIC | >16,384 | >16,384 | >16,384 | >16,384 | 16,384 | >16,384 | 8192 | |
| F2 | IC50 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 2795 ± 15 d |
| MIC | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 8192 | |
| F3 | IC50 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 3006 ± 10.6 g |
| MIC | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 8192 | |
| F4 | IC50 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 3002 ± 12 g |
| MIC | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 8192 | |
| F5 | IC50 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 2916 ± 8.7 e |
| MIC | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 8192 | |
| F6 | IC50 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 3720 ± 10 h |
| MIC | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 16384 | |
| F7 | IC50 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 2566 ± 9.8 c |
| MIC | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 4096 | |
| F8 | IC50 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 2518 ± 9.0 b |
| MIC | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 4096 | |
| F9 | IC50 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 2450 ± 14 a |
| MIC | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 4096 | |
| F10 | IC50 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 2951 ± 21 f |
| MIC | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | >16,384 | 8192 | |
| Ampicillin | IC50 | 0.02 ± 0.005 | 3.62 ± 0.15 | 1.03 ± 0.07 | ||||
| MIC | 0.125 ± 0.0 | 32 ± 0.0 | 32 ± 0.0 | |||||
| Cefotaxime | IC50 | 0.43 ± 0.05 | 0.007 ± 0.002 | 4.34 ± 0.15 | ||||
| MIC | 32 ± 0.0 | 0.5 ± 0.0 | 8 ± 0.0 | |||||
| Nystatin | IC50 | 1.32 ± 0.05 | ||||||
| MIC | 8 ± 0.0 | |||||||
| Formulas | Spectral Distribution | Duration (h/Day) | Lighting Time | Supplemental Light Intensity (µmol·m−2·s−1) | Total Daily Supplemental Light (mol·m−2·d−1) | Total Daily Light (mol·m−2·d−1) |
|---|---|---|---|---|---|---|
| F1 | R:B:UVA ~ 71:20:9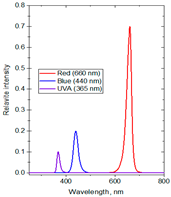 | 8 | 1:00–5:00 a.m. and 19:00–23:00 p.m. | 100 ± 10 | 2.88 | 16.86 |
| F2 | 6 | 2:00–5:00 a.m. and 19:00–22:00 p.m. | 100 ± 10 | 2.16 | 16.14 | |
| F3 | 4 | 3:00–5:00 a.m. and 19:00–21:00 p.m. | 100 ± 10 | 1.44 | 15.42 | |
| F4 | R:B:G ~ 75:21:4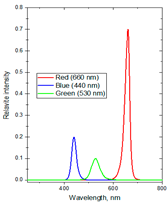 | 8 | 1:00–5:00 a.m. and 19:00–23:00 p.m. | 100 ± 10 | 2.88 | 16.86 |
| F5 | 6 | 2:00–5:00 a.m. and 19:00–22:00 p.m. | 100 ± 10 | 2.16 | 16.14 | |
| F6 | 4 | 3:00–5:00 a.m. and 19:00–21:00 p.m. | 100 ± 10 | 1.44 | 15.42 | |
| F7 | R:B:G:FR ~ 43.5:43.5:8:5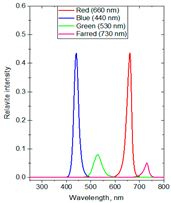 | 6 | 2:00–5:00 a.m. and 19:00–22:00 p.m. | 120 ± 10 | 2.52 | 16.57 |
| F8 | 6 | 2:00–5:00 a.m. and 19:00–22:00 p.m. | 100 ± 10 | 2.16 | 16.14 | |
| F9 | 6 | 2:00–5:00 a.m. and 19:00–22:00 p.m. | 80 ± 10 | 1.73 | 15.71 | |
| F10 | Control | 0 | 0 | 0 | 0 | 13.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, H.T.T.; Vu, T.N.; Dinh, T.T.T.; Do, P.T.; Tien, T.Q.; Tong, Q.C.; Ha, Q.T.; Tran, K.Q.; Setzer, W.N. Optimization of Supplemental LED Spectral Quality and Light Dose for Enhancing Biomass and Essential Oil Yield of Ocimum gratissimum L. Under Net House Condition. Molecules 2025, 30, 3753. https://doi.org/10.3390/molecules30183753
Chu HTT, Vu TN, Dinh TTT, Do PT, Tien TQ, Tong QC, Ha QT, Tran KQ, Setzer WN. Optimization of Supplemental LED Spectral Quality and Light Dose for Enhancing Biomass and Essential Oil Yield of Ocimum gratissimum L. Under Net House Condition. Molecules. 2025; 30(18):3753. https://doi.org/10.3390/molecules30183753
Chicago/Turabian StyleChu, Ha Thi Thu, Thi Nghiem Vu, Thuy Thi Thu Dinh, Phat Tien Do, Tran Quoc Tien, Quang Cong Tong, Quyen Thi Ha, Khanh Quoc Tran, and William N. Setzer. 2025. "Optimization of Supplemental LED Spectral Quality and Light Dose for Enhancing Biomass and Essential Oil Yield of Ocimum gratissimum L. Under Net House Condition" Molecules 30, no. 18: 3753. https://doi.org/10.3390/molecules30183753
APA StyleChu, H. T. T., Vu, T. N., Dinh, T. T. T., Do, P. T., Tien, T. Q., Tong, Q. C., Ha, Q. T., Tran, K. Q., & Setzer, W. N. (2025). Optimization of Supplemental LED Spectral Quality and Light Dose for Enhancing Biomass and Essential Oil Yield of Ocimum gratissimum L. Under Net House Condition. Molecules, 30(18), 3753. https://doi.org/10.3390/molecules30183753









