Phenolic Secondary Metabolites in Aldrovanda vesiculosa L. (Droseraceae)
Abstract
1. Introduction
2. Results
2.1. Phytochemical Characterization Based on UPLC-DAD-(ESI)-MS Analysis
2.2. Comparison of the Metabolite Content in Different Parts of the Plant
3. Discussion
4. Materials and Methods
4.1. Plant Material and Sample Collection
4.2. Extraction of Plant Metabolites
4.3. Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleischmann, A.; Schlauer, J.; Smith, S.A.; Givnish, T.J. Evolution of carnivory in angiosperms. In Carnivorous Plants: Physiology, Ecology, and Evolution; Adamec, L., Ellison, A., Eds.; Oxford University Press: Oxford, UK, 2018; pp. 22–42. [Google Scholar]
- Cross, A. Aldrovanda: The Waterwheel Plant; Redfern Natural History Productions Ltd.: Poole, UK, 2012; ISBN 978-1-908787-04-0. [Google Scholar]
- Adamec, L. Ecological requirements and recent European distribution of the aquatic carnivorous plant Aldrovanda vesiculosa L.—A review. Folia Geobot. 1995, 30, 53–61. [Google Scholar] [CrossRef]
- Adamec, L. Biological flora of Central Europe: Aldrovanda vesiculosa L. Perspect. Plant Ecol. Evol. Syst. 2018, 35, 8–21. [Google Scholar] [CrossRef]
- Cross, A.; Adamec, L. Aldrovanda vesiculosa. The IUCN Red List of Threatened Species 2020: e.T162346A83998419. Available online: https://www.researchgate.net/publication/344197460_Red_List_Assessment_-_Aldrovanda_vesiculosa_Common_Aldrovanda_Waterwheel (accessed on 3 September 2025).
- Adamec, L. The influence of prey capture on photosynthetic rate in two aquatic carnivorous plant species. Aquat. Bot. 2008, 89, 66–70. [Google Scholar] [CrossRef]
- Adamec, L. Rootless aquatic plant Aldrovanda vesiculosa: Physiological polarity, mineral nutrition, and importance of carnivory. Biol. Plant. 2000, 43, 113–119. [Google Scholar] [CrossRef]
- Adamec, L.; Sirová, D.; Vrba, J. Contrasting growth effects of prey capture in two aquatic carnivorous plant species. Fundam. Appl. Limnol. 2010, 176, 153–160. [Google Scholar] [CrossRef]
- Poppinga, S.; Smaij, J.; Westermeier, A.S.; Horstmann, M.; Kruppert, S.; Tollrian, R.; Speck, T. Prey capture analyses in the carnivorous aquatic waterwheel plant (Aldrovanda vesiculosa L., Droseraceae). Sci. Rep. 2019, 9, 18590. [Google Scholar] [CrossRef] [PubMed]
- Adamec, L.; Plačková, L.; Doležal, K. Cytokinins and auxins in organs of aquatic carnivorous plants: What do they reflect? Ann. Bot. 2022, 130, 869–882. [Google Scholar] [CrossRef]
- Horstmann, M.; Heier, L.; Kruppert, S.; Weiss, L.C.; Tollrian, R.; Adamec, L.; Westermeier, A.; Speck, T.; Poppinga, S. Comparative prey spectra analyses on the endangered aquatic carnivorous waterwheel plant (Aldrovanda vesiculosa, Droseraceae) at several naturalized microsites in the Czech Republic and Germany. Integr. Org. Biol. 2019, 1, oby012. [Google Scholar] [CrossRef]
- Iijima, T.; Sibaoka, T. Action potential in the trap-lobes of Aldrovanda vesiculosa. Plant Cell Physiol. 1981, 22, 1595–1601. [Google Scholar] [CrossRef]
- Poppinga, S.; Joyeux, M. Different mechanics of snap-trapping in the two closely related carnivorous plants Dionaea muscipula and Aldrovanda vesiculosa. Phys. Rev. E 2011, 84, 041928. [Google Scholar] [CrossRef]
- Poppinga, S.; Bauer, U.; Speck, T.; Volkov, A.G. Motile traps. In Carnivorous Plants: Physiology, Ecology, and Evolution; Adamec, L., Ellison, A., Eds.; Oxford University Press: Oxford, UK, 2018; pp. 180–193. [Google Scholar]
- Westermeier, A.S.; Sachse, R.; Poppinga, S.; Vögele, P.; Adamec, L.; Speck, T.; Bischoff, M. How the carnivorous waterwheel plant (Aldrovanda vesiculosa) snaps. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180012. [Google Scholar] [CrossRef] [PubMed]
- Westermeier, A.S.; Hiss, N.; Speck, T.; Poppinga, S. Functional–morphological analyses of the delicate snap-traps of the aquatic carnivorous waterwheel plant (Aldrovanda vesiculosa) with 2D and 3D imaging techniques. Ann. Bot. 2020, 126, 1099–1107. [Google Scholar] [CrossRef]
- Atsuzawa, K.; Kanaizumi, D.; Ajisaka, M.; Kamada, T.; Sakamoto, K.; Matsushima, H.; Kaneko, Y. Fine structure of Aldrovanda vesiculosa L: The peculiar lifestyle of an aquatic carnivorous plant elucidated by electron microscopy using cryo-techniques. Microscopy 2020, 69, 214–226. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Świątek, P.; Strzemski, M.; Miranda, V.F.O. Immunocytochemical analysis of the wall ingrowths in the digestive gland transfer cells in Aldrovanda vesiculosa L. (Droseraceae). Cells 2022, 11, 2218. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Wójciak, M.; Świątek, P. Immunocytochemical analysis of bifid trichomes in Aldrovanda vesiculosa L. Traps. Int. J. Mol. Sci. 2023, 24, 3358. [Google Scholar] [CrossRef] [PubMed]
- Wójciak, M.; Feldo, M.; Stolarczyk, P.; Płachno, B.J. Carnivorous plants from Nepenthaceae and Droseraceae as a source of secondary metabolites. Molecules 2023, 28, 2155. [Google Scholar] [CrossRef]
- Schlauer, J.; Fleischmann, A.; Hartmeyer, S.R.H.; Hartmeyer, I.; Rischer, H. Distribution of acetogenic naphthoquinones in Droseraceae and their chemotaxonomic utility. Biology 2024, 13, 97. [Google Scholar] [CrossRef]
- Płachno, B.J.; Strzemski, M.; Dresler, S.; Adamec, L.; Wojas-Krawczyk, K.; Sowa, I.; Danielewicz, A.; Miranda, V.F.O. A chemometry of Aldrovanda vesiculosa L. (waterwheel, Droseraceae) populations. Molecules 2021, 26, 72. [Google Scholar] [CrossRef]
- Wójciak, M.; Feldo, M.; Stolarczyk, P.; Płachno, B.J. Biological potential of carnivorous plants from Nepenthales. Molecules 2023, 28, 3639. [Google Scholar] [CrossRef] [PubMed]
- Makowski, W.; Mrzygłód, K.; Szopa, A.; Kubica, P.; Krychowiak-Maśnicka, M.; Tokarz, K.M.; Tokarz, B.; Ryngwelska, I.; Paluszkiewicz, E.; Królicka, A. Effect of agitation and temporary immersion on growth and synthesis of antibacterial phenolic compounds in genus Drosera. Biomolecules 2024, 14, 1132. [Google Scholar] [CrossRef]
- Zenk, M.H.; Fürbringer, M.; Steglich, W. Occurrence and distribution of 7-methyljuglone and plumbagin in the Droseraceae. Phytochemistry 1969, 8, 2199–2200. [Google Scholar] [CrossRef]
- Culham, A.; Gornall, R.J. The taxonomic significance of naphthoquinones in the Droseraceae. Biochem. Syst. Ecol. 1994, 22, 507–515. [Google Scholar] [CrossRef]
- Adamec, L.; Gastinel, L.; Schlauer, J. Plumbagin content in Aldrovanda vesiculosa shoots. Carniv. Plant Newsl. 2006, 35, 52–55. [Google Scholar] [CrossRef]
- Strzemski, M.; Adamec, L.; Dresler, S.; Mazurek, B.; Dubaj, K.; Stolarczyk, P.; Feldo, M.; Płachno, B.J. Shoots and turions of aquatic plants as a source of fatty acids. Molecules 2024, 29, 2062. [Google Scholar] [CrossRef] [PubMed]
- Adamec, L. How to grow Aldrovanda vesiculosa outdoors. Carniv. Plant Newsl. 1997, 26, 85–88. [Google Scholar] [CrossRef]
- Braunberger, C.; Zehl, M.; Conrad, J.; Fischer, S.; Adhami, H.R.; Beifuss, U.; Krenn, L. LC-NMR, NMR, and LC-MS identification and LC-DAD quantification of flavonoids and ellagic acid derivatives in Drosera peltata. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 932, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Hvattum, E.; Ekeberg, D. Study of the collision-induced radical cleavage of flavonoid glycosides using negative electrospray ionization tandem quadrupole mass spectrometry. J. Mass Spectrom. 2003, 38, 43–49. [Google Scholar] [CrossRef]
- Budzianowski, J. Naphthohydroquinone glucosides of Drosera rotundifolia and D. intermedia from in vitro cultures. Phytochemistry 1996, 42, 1145–1147. [Google Scholar] [CrossRef]
- Tienaho, J.; Reshamwala, D.; Karonen, M.; Silvan, N.; Korpela, L.; Marjomäki, V.; Sarjala, T. Field-grown and in vitro propagated round-leaved sundew (Drosera rotundifolia L.) show differences in metabolic profiles and biological activities. Molecules 2021, 26, 3581. [Google Scholar] [CrossRef]
- Khallouki, F.; Breuer, A.; Merieme, E.; Ulrich, C.M.; Owen, R.W. Characterization and quantitation of the polyphenolic compounds detected in methanol extracts of Pistacia atlantica Desf. fruits from the Guelmim region of Morocco. J. Pharm. Biomed. Anal. 2017, 134, 310–318. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. LC-MS/MS Characterization of phenolic metabolites and their antioxidant activities from Australian native plants. Metabolites 2022, 12, 1016. [Google Scholar] [CrossRef] [PubMed]
- Zehl, M.; Braunberger, C.; Conrad, J.; Crnogorac, M.; Krasteva, S.; Vogler, B.; Beifuss, U.; Krenn, L. Identification and quantification of flavonoids and ellagic acid derivatives in therapeutically important Drosera species by LC-DAD, LC-NMR, NMR, and LC-MS. Anal. Bioanal. Chem. 2011, 400, 2565–2576. [Google Scholar] [CrossRef]
- Dwivedi, S.; Singh, S.; Singh, J. Effect of extraction solvent and plant part on the yield of phenolic compounds, plumbagin and biological activity of Plumbago zeylanica. J. Pharmacogn. Phytochem. 2023, 12, 05–10. [Google Scholar] [CrossRef]
- Adamec, L. Seasonal growth dynamics and overwintering of the aquatic carnivorous plant Aldrovanda vesiculosa at experimental field sites. Folia Geobot. 1999, 34, 287–297. [Google Scholar] [CrossRef]
- Adamec, L. Photosynthetic characteristics of the aquatic carnivorous plant Aldrovanda vesiculosa. Aquat. Bot. 1997, 59, 297–306. [Google Scholar] [CrossRef]
- Šimura, J.; Spíchal, L.; Adamec, L.; Pěnčík, A.; Rolčík, J.; Novák, O.; Strnad, M. Cytokinin, auxin and physiological polarity in the aquatic carnivorous plants Aldrovanda vesiculosa and Utricularia australis. Ann. Bot. 2016, 117, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Schlauer, J.; Hartmeyer, S.R.H.; Hartmeyer, I.; Seppänen-Laakso, T.; Rischer, H. Contrasting dihydronaphthoquinone patterns in closely related Drosera (Sundew) species enable taxonomic distinction and identification. Plants 2021, 10, 1601. [Google Scholar] [CrossRef]
- Kaur, K.; Dolker, D.; Behera, S.; Pati, P.K. Critical factors influencing in vitro propagation and modulation of important secondary metabolites in Withania somnifera (L.) dunal. Plant Cell Tissue Organ Cult. 2022, 149, 41–60. [Google Scholar] [CrossRef]
- Figueiró, A.d.A.; Correa, C.M.; Astarita, L.V.; Santarém, E.R. A manutenção prolongada de culturas in vitro afeta o crescimento e o metabolismo secundärio de hipërico. Cienc. Rural 2010, 40, 2115–2121. [Google Scholar] [CrossRef]
- Kwiecień, I.; Smolin, J.; Beerhues, L.; Ekiert, H. The impact of media composition on production of flavonoids in agitated shoot cultures of the three Hypericum perforatum L. cultivars ‘Elixir,’ ‘Helos,’ and ‘Topas’. Vitr. Cell. Dev. Biol.-Plant 2018, 54, 332–340. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Bednarz, M.; Luczkiewicz, M.; Ekiert, H. Studies on the accumulation of phenolic acids and flavonoids in different in vitro culture systems of Schisandra chinensis (Turcz.) Baill. using a DAD-HPLC method. Phytochem. Lett. 2017, 20, 462–469. [Google Scholar] [CrossRef]
- Idzik, M.; Chanaj-Kaczmarek, J.; Totoń, E.; Hermosaningtyas, A.A.; Gruszka, M.; Gratkowska, M.; Kikowska, M. Do in vitro cultures hold the key? Exploring cultivation systems, hormonal composition and LED elicitation in enhancing shoot biomass and phenolic profiles of Lychnis flos-cuculi. Plant Cell, Tissue Organ Cult. 2025, 162, 5. [Google Scholar] [CrossRef]
- Wu, W.; Wu, H.; Liang, R.; Huang, S.; Meng, L.; Zhang, M.; Xie, F.; Zhu, H. Light regulates the synthesis and accumulation of plant secondary metabolites. Front. Plant Sci. 2025, 16, 1644472. [Google Scholar] [CrossRef]
- Szopa, A.; Ekiert, H.; Szewczyk, A.; Fugas, E. Production of bioactive phenolic acids and furanocoumarins in in vitro cultures of Ruta graveolens L. and Ruta graveolens ssp. divaricata (Tenore) Gams. under different light conditions. Plant Cell Tissue Organ Cult. 2012, 110, 329–336. [Google Scholar] [CrossRef]
- Zielińska, S.; Piątczak, E.; Kozłowska, W.; Bohater, A.; Jezierska-Domaradzka, A.; Kolniak-Ostek, J.; Matkowski, A. LED illumination and plant growth regulators’ effects on growth and phenolic acids accumulation in Moluccella laevis L. in vitro cultures. Acta Physiol. Plant. 2020, 42, 72. [Google Scholar] [CrossRef]
- Kozłowska, W.; Matkowski, A.; Zielińska, S. Light intensity and temperature effect on Salvia yangii (B. T. Drew) metabolic profile in vitro. Front. Plant Sci. 2022, 13, 888509. [Google Scholar] [CrossRef]
- Siatkowska, K.; Chraniuk, M.; Bollin, P.; Banasiuk, R. Light emitting diodes optimisation for secondary metabolites production by Droseraceae plants. J. Photochem. Photobiol. B Biol. 2021, 224, 112308. [Google Scholar] [CrossRef] [PubMed]
- Boonsnongcheep, P.; Sae-foo, W.; Banpakoat, K.; Channarong, S.; Chitsaithan, S.; Uafua, P.; Putha, W.; Kerdsiri, K.; Putalun, W. Artificial color light sources and precursor feeding enhance plumbagin production of the carnivorous plants Drosera burmannii and Drosera indica. J. Photochem. Photobiol. B Biol. 2019, 199, 111628. [Google Scholar] [CrossRef] [PubMed]
- Putalun, W.; Udomsin, O.; Yusakul, G.; Juengwatanatrakul, T.; Sakamoto, S.; Tanaka, H. Enhanced plumbagin production from in vitro cultures of Drosera burmanii using elicitation. Biotechnol. Lett. 2010, 32, 721–724. [Google Scholar] [CrossRef]
- Krolicka, A.; Szpitter, A.; Gilgenast, E.; Romanik, G.; Kaminski, M.; Lojkowska, E. Stimulation of antibacterial naphthoquinones and flavonoids accumulation in carnivorous plants grown in vitro by addition of elicitors. Enzyme Microb. Technol. 2008, 42, 216–221. [Google Scholar] [CrossRef]
- Makowski, W.; Tokarz, K.M.; Tokarz, B.; Banasiuk, R.; Witek, K.; Królicka, A. Elicitation-based method for increasing the production of antioxidant and bactericidal phenolic compounds in Dionaea muscipula J. Ellis tissue. Molecules 2020, 25, 1794. [Google Scholar] [CrossRef]
- Tokunaga, T.; Dohmura, A.; Takada, N.; Ueda, M. Cytotoxic antifeedant from Dionaea muscipula Ellis: A defensive mechanism of carnivorous plants against predators. Bull. Chem. Soc. Jpn. 2004, 77, 537–541. [Google Scholar] [CrossRef]
- Tokunaga, T.; Takada, N.; Ueda, M. Mechanism of antifeedant activity of plumbagin, a compound concerning the chemical defense in carnivorous plant. Tetrahedron Lett. 2004, 45, 7115–7119. [Google Scholar] [CrossRef]
- Dávila-Lara, A.; Rahman-Soad, A.; Reichelt, M.; Mithöfer, A. Carnivorous Nepenthes x ventrata plants use a naphthoquinone as phytoanticipin against herbivory. PLoS ONE 2021, 16, e0258235. [Google Scholar] [CrossRef]
- Adamec, L. Ecophysiological characterization of dormancy states in turions of the aquatic carnivorous plant Aldrovanda vesiculosa. Biol. Plant. 2003, 47, 395–402. [Google Scholar] [CrossRef]
- Płachno, B.J.; Adamec, L.; Kozieradzka-Kiszkurno, M.; Świątek, P.; Kamińska, I. Cytochemical and ultrastructural aspects of aquatic carnivorous plant turions. Protoplasma 2014, 251, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Adamec, L. Ecophysiological characteristics of turions of aquatic plants: A review. Aquat. Bot. 2018, 148, 64–77. [Google Scholar] [CrossRef]
- Grevenstuk, T.; Gonçalves, S.; Domingos, T.; Quintas, C.; Van der Hooft, J.J.; Vervoort, J.; Romano, A. Inhibitory activity of plumbagin produced by Drosera intermedia on food spoilage fungi. J. Sci. Food Agric. 2012, 92, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.V.; Baranwal, G.; Chatterjee, M.; Sachu, A.; Vasudevan, A.K.; Bose, C.; Banerji, A.; Biswas, R. Antimicrobial activity of plumbagin, a naturally occurring naphthoquinone from Plumbago rosea, against Staphylococcus aureus and Candida albicans. Int. J. Med. Microbiol. 2016, 306, 237–248. [Google Scholar] [CrossRef]
- Svobodová, I.; Adamec, L. Preliminary identification of the agent causing the fungal disease of Aldrovanda vesiculosa. Carniv. Plant Newsl. 2020, 49, 56–64. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Parzymies, M.; Pogorzelec, M.; Świstowska, A. Optimization of propagation of the Polish strain of Aldrovanda vesiculosa in tissue culture. Biology 2022, 11, 1389. [Google Scholar] [CrossRef] [PubMed]
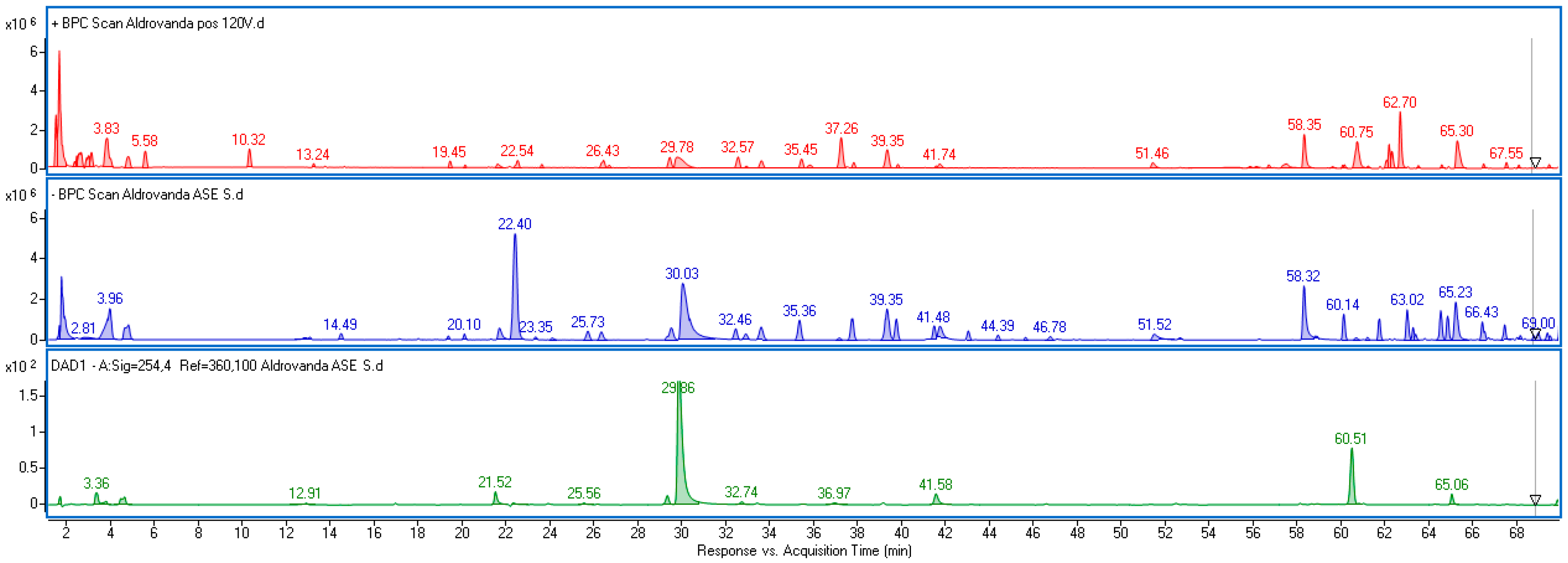
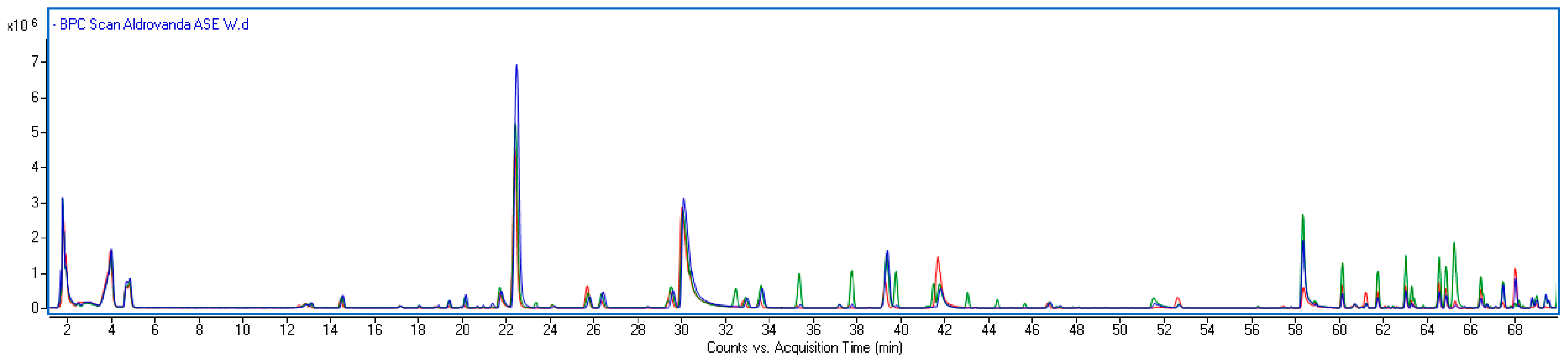

| Rt (min) | [M − H]−/(Fragments) | Error (ppm) | [M + H]+ | Error (ppm) | Formula | UV–Vis (nm) | Identified | Ref |
|---|---|---|---|---|---|---|---|---|
| 1.90 | 331.06793 (169,125) | 2.59 | 333.08196 | 1.01 | C13H16O10 | 218,280 | Galloyl hexoside | [33] |
| 3.96 | 331.06730 (169,125) | 0.69 | 333.08208 | 1.38 | C13H16O10 | 218,280 | Galloyl hexoside | [33] |
| 4.79 | 169.01417 (125) | −0.45 | 171.02889 | 0.53 | C7H6O5 | 215,270 | Gallic acid | str, [34] |
| 8.54 | 331.06709 (169,125) | 0.06 | - | C13H16O10 | 218,280 | Galloyl hexoside | [33] | |
| 12.91 | 483.07725 (331,169) | −1.61 | - | C20H20O14 | 218,280 | Digalloyl hexoside | [33] | |
| 13.06 | 397.1136 (173) | −1.06 | 399.12908 | 1.27 | C18H22O10 | 220,275 | Unknown | |
| 14.49 | 513.16042 (351) | −1.84 | 515.17603 | 0.22 | C23H30O13 | 230,305 | Dihydroplumbagin dihex. | [32] |
| 17.16 | 625.10608 (463,299) | 2.30 | - | C26H26O18 | 255,370 | Ellagic acid dihexoside | [34] | |
| 18.04 | 625.10535 (463,299) | 1.74 | - | C26H26O18 | - | Ellagic acid dihexoside | [34] | |
| 19.39 | 353.12433 (173) | 0.39 | 355.13916 | 1.17 | C17H22O8 | - | Unknown | |
| 20.10 | 609.14637 | 0.43 | 611.16078 | 0.19 | C27H30O16 | 265,365 | Kaempferol dihexoside | [35] |
| 21.32 | 457.07711 (169,125) | −1,15 | - | C22H18O11 | - | Epigallocatechin gallate | str, [34] | |
| 21.70 | 463.05150 (301) | −0.68 | 465.06688 | 1.11 | C20H16O13 | 255,360 | Ellagic acid glucoside | [33] |
| 22.40 | 351.10802 (189) | −1.48 | 353.12420 | 3.14 | C17H20O8 | 230,305 | Dihydroplumbagin hex. | [32] |
| 23.35 | 561.18252 (515) | 0.05 | - | C24H34O15 | - | Unknown | ||
| 24.17 | 477.06643 (315) | −2.16 | - | C21H18O13 | - | Methylellagic acid hex. | [33,34] | |
| 25.73 | 365.08786 (203,175) | 0.15 | - | C17H18O9 | 250,280,380 | Unknown | ||
| 26.42 | 479.08243 (316) | −1.43 | - | C21H20O13 | 255,370 | Myricetin 3-O-glucoside | str, [33,36] | |
| 29.50 | 477.06834 (315) | 1.83 | 479.08346 | 3.02 | C21H18O13 | 250,370 | methylellagic acid hex. | [33,34] |
| 30.03 | 301.00033 | 4.43 | 303.01365 | 0.35 | C14H6O8 | 255,370 | Ellagic acid | str, [33] |
| 32.46 | 635.34872 | −1.32 | - | C27H56O16 | - | Unknown | ||
| 33.01 | 491.08192 (301) | −2.43 | 493.09789 | 0.45 | C22H20O13 | 250,375 | Dimethylellagic acid hex. | [33] |
| 33.61 | 463.08231 (301) | −4.58 | 465.10287 | 0.25 | C21H20O12 | 255,365 | Quercetin 3-O-glucoside | str, [36] |
| 35.36 | 679.37499 | −1.15 | - | C29H60O17 | - | Unknown | ||
| 37.78 | 723.40212 | 0.18 | - | C31H64O18 | - | Unknown | ||
| 39.35 | 447.09514 (284) | 4.14 | 449.10854 | 1.57 | C21H20H11 | 265,365 | Kaempferol 3-O-glucoside | str, [36] |
| 39.80 | 767.42832 | 0.15 | - | C33H68O19 | - | Unknown | ||
| 41.24 | 317.03023 | −0.19 | - | C15H10O8 | 255,370 | Myricetin | str, [36] | |
| 41.76 | 315.01482 | 0.57 | 317.02984 | 2.05 | C15H8O8 | 250,375 | Methylellagic acid | [33] |
| 51.52 | 301.03451 | −2.87 | 303.05043 | 1.66 | C15H10O7 | 255,370 | Quercetin | str, [33,36] |
| 58.32 | 285.04111 | 2.27 | 287.05589 | 3.06 | C15H10O6 | 265,365 | Kaempferol | str, [33,36] |
| 60.71 | - | - | 189.0549 [M + H]+ | 1.49 | C11H8O3 | 270,420 | Plumbagin | str, [32] |
| Components | B | M | A |
|---|---|---|---|
| Gallic acid and derivatives (mg/g) | |||
| Gallic acid | 1.81 ± 0.16 c | 2.17 ± 0.24 b | 2.73 ± 0.31 a |
| Galloyl hexosides (total) 1 | 3.28 ± 0.35 a | 3.13 ± 0.25 a | 3.36 ± 0.38 a |
| Digalloyl hexosides 1 | 0.58 ± 0.03 b | 1.08 ± 0.06 a | 0.98 ± 0.01 a |
| Total: | 5.67 ± 0.26 c | 6.38 ± 0.29 b | 7.07 ± 0.31 a |
| ellagic acid and derivatives (mg/g) | |||
| Ellagic acid glucoside 2 | 0.52 ± 0.02 c | 0.72 ± 0.04 a | 0.60 ± 0.03 b |
| Methylellagic acid glucoside 2 | 0.41 ± 0.02 b | 0.54 ± 0.03 a | 0.42 ± 0.04 b |
| Ellagic acid | 14.25 ± 1.4 b | 14.51 ± 1.56 b | 19.31 ± 2.17 a |
| Dimethylellagic acid hexoside 2 | 0.22 ± 0.02 b | 0.25 ± 0.03 a | 0.26 ± 0.02 a |
| Methylellagic acid 2 | 2.38 ± 0.13 a | 0.83 ± 0.03 b | 0.70 ± 0.04 c |
| Total: | 17.78 ± 0.77 b | 16.86 ± 0.83 b | 21.29 ± 1.31 a |
| Flavonoids (µg/g) | |||
| Kaempferol dihexoside 3 | Det. | 59.88 ± 6.59 b | 77.71 ± 7.60 a |
| Quercetin 3-O-glucoside (Isoquercetin) | 28.74 ± 2.00 b | 35.67 ± 2.84 a | 33.17 ± 2.31 a |
| Kaempferol 3-O-glucoside (Astragalin) | 340.8 ± 7.64 c | 968.2 ± 18.32 b | 1070.4 ± 10.9 a |
| Quercetin | Det. | 28.99 ± 3.15 a | 17.12 ± 1.81 b |
| Kaempferol | 101.5 ± 12.76 c | 516.6 ± 52.8 a | 370.2 ± 35.20 b |
| Total: | 471.04 ± 21.31 b | 1609.34 ± 80.2 a | 1568.6 ± 71.39 a |
| Naphtoquinones (mg/g) | |||
| Plumbagin * | 6.71 ± 0.27 c | 7.80 ± 0.31 b | 8.67 ± 0.26 a |
| Dihydroplumbagin hexoside 4 | 6.36 ± 0.45 c | 9.52 ± 0.52 b | 16.3 ± 1.23 a |
| Total: | 13.07 ± 1.02 c | 17.32 ± 1.23 b | 24.97 ± 2.29 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójciak, M.; Sowa, I.; Strzemski, M.; Parzymies, M.; Pogorzelec, M.; Stolarczyk, P.; Płachno, B.J. Phenolic Secondary Metabolites in Aldrovanda vesiculosa L. (Droseraceae). Molecules 2025, 30, 3746. https://doi.org/10.3390/molecules30183746
Wójciak M, Sowa I, Strzemski M, Parzymies M, Pogorzelec M, Stolarczyk P, Płachno BJ. Phenolic Secondary Metabolites in Aldrovanda vesiculosa L. (Droseraceae). Molecules. 2025; 30(18):3746. https://doi.org/10.3390/molecules30183746
Chicago/Turabian StyleWójciak, Magdalena, Ireneusz Sowa, Maciej Strzemski, Marzena Parzymies, Magdalena Pogorzelec, Piotr Stolarczyk, and Bartosz J. Płachno. 2025. "Phenolic Secondary Metabolites in Aldrovanda vesiculosa L. (Droseraceae)" Molecules 30, no. 18: 3746. https://doi.org/10.3390/molecules30183746
APA StyleWójciak, M., Sowa, I., Strzemski, M., Parzymies, M., Pogorzelec, M., Stolarczyk, P., & Płachno, B. J. (2025). Phenolic Secondary Metabolites in Aldrovanda vesiculosa L. (Droseraceae). Molecules, 30(18), 3746. https://doi.org/10.3390/molecules30183746











