Immobilization of the Proteolytic Fraction P1G10 from Vasconcellea pubescens in Alginate–Chitosan Complex and Enzyme Activity Release
Abstract
1. Introduction
2. Results and Discusion
2.1. Encapsulation of P1G10 with ALG/CS Complex
 , ALG-CS-P1G10 biocatalyst formation yield;
, ALG-CS-P1G10 biocatalyst formation yield;  , P1G10 protein encapsulation yield. Method 1 (CS → ALG → P1G10), method 2 (CS → P1G10 → ALG), method 3 (CS → P1G10 → ALG → CaCl2), method 4 (CS → P1G10 → ALG → CaCl2 → TPP) and method 5 (CS → P1G10 → TPP → ALG → CaCl2). Capital letters indicate statistically significant differences in the formation yield of the ALG-CS-P1G10 biocatalyst (p < 0.05), according to Fisher’s LSD test. Lowercase letters indicate statistically significant differences in the encapsulation yield of the P1G10 protein (p < 0.05), also according to Fisher’s LSD test.
, P1G10 protein encapsulation yield. Method 1 (CS → ALG → P1G10), method 2 (CS → P1G10 → ALG), method 3 (CS → P1G10 → ALG → CaCl2), method 4 (CS → P1G10 → ALG → CaCl2 → TPP) and method 5 (CS → P1G10 → TPP → ALG → CaCl2). Capital letters indicate statistically significant differences in the formation yield of the ALG-CS-P1G10 biocatalyst (p < 0.05), according to Fisher’s LSD test. Lowercase letters indicate statistically significant differences in the encapsulation yield of the P1G10 protein (p < 0.05), also according to Fisher’s LSD test.
 , ALG-CS-P1G10 biocatalyst formation yield;
, ALG-CS-P1G10 biocatalyst formation yield;  , P1G10 protein encapsulation yield. Method 1 (CS → ALG → P1G10), method 2 (CS → P1G10 → ALG), method 3 (CS → P1G10 → ALG → CaCl2), method 4 (CS → P1G10 → ALG → CaCl2 → TPP) and method 5 (CS → P1G10 → TPP → ALG → CaCl2). Capital letters indicate statistically significant differences in the formation yield of the ALG-CS-P1G10 biocatalyst (p < 0.05), according to Fisher’s LSD test. Lowercase letters indicate statistically significant differences in the encapsulation yield of the P1G10 protein (p < 0.05), also according to Fisher’s LSD test.
, P1G10 protein encapsulation yield. Method 1 (CS → ALG → P1G10), method 2 (CS → P1G10 → ALG), method 3 (CS → P1G10 → ALG → CaCl2), method 4 (CS → P1G10 → ALG → CaCl2 → TPP) and method 5 (CS → P1G10 → TPP → ALG → CaCl2). Capital letters indicate statistically significant differences in the formation yield of the ALG-CS-P1G10 biocatalyst (p < 0.05), according to Fisher’s LSD test. Lowercase letters indicate statistically significant differences in the encapsulation yield of the P1G10 protein (p < 0.05), also according to Fisher’s LSD test.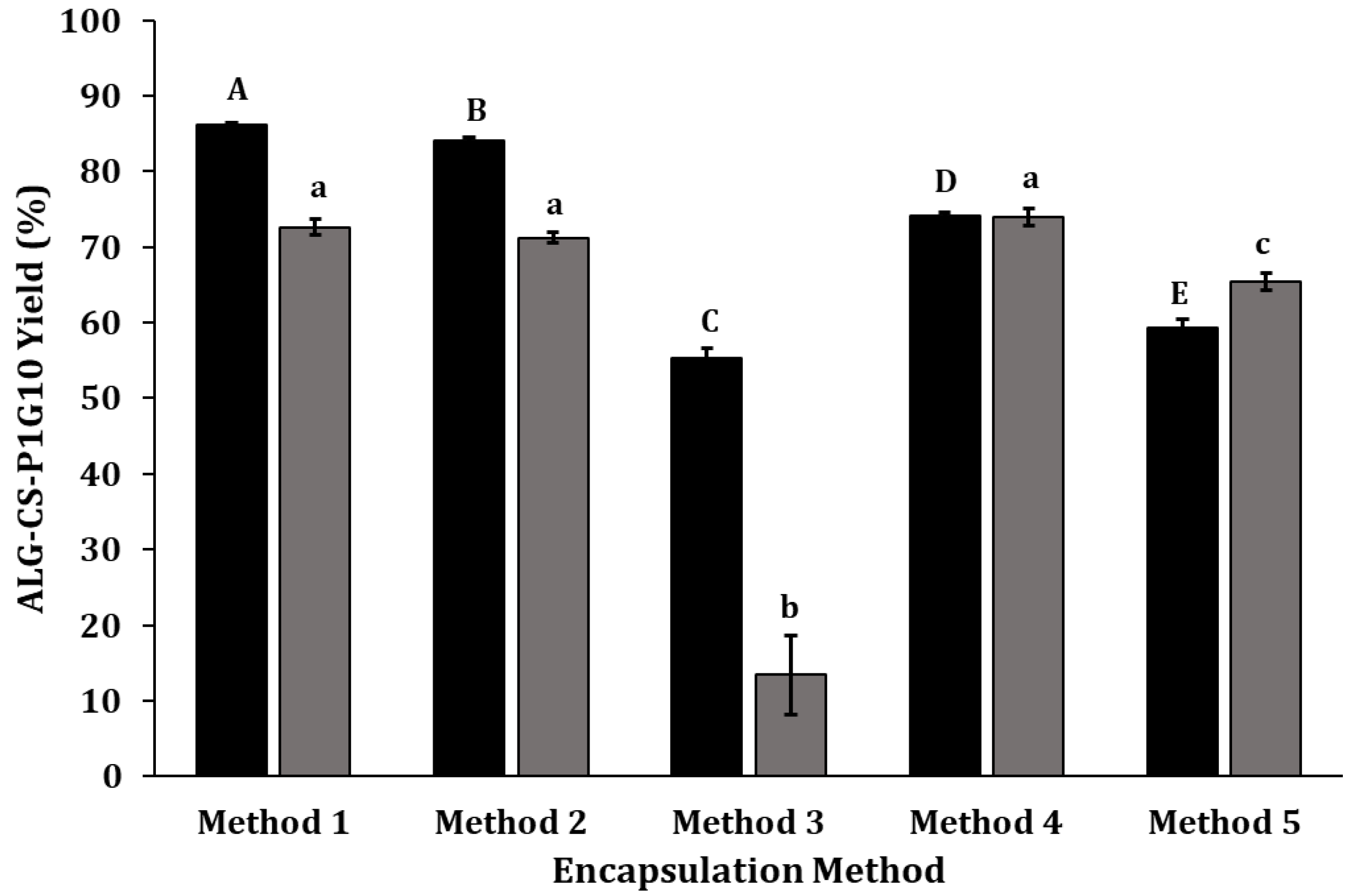
| Component | |||||
|---|---|---|---|---|---|
| Method | CS | P1G10 | ALG | CaCl2 | TPP |
| Amount (mg) | 25.0 | 7.5 | 25.0 | 25.0 | 12.0 |
| 1 | First | Third | Second | - | - |
| 2 | First | Second | Third | - | - |
| 3 | First | Second | Third | Fourth | - |
| 4 | First | Second | Third | - | Fourth |
| 5 | First | Second | Fourth | Fifth | Third |
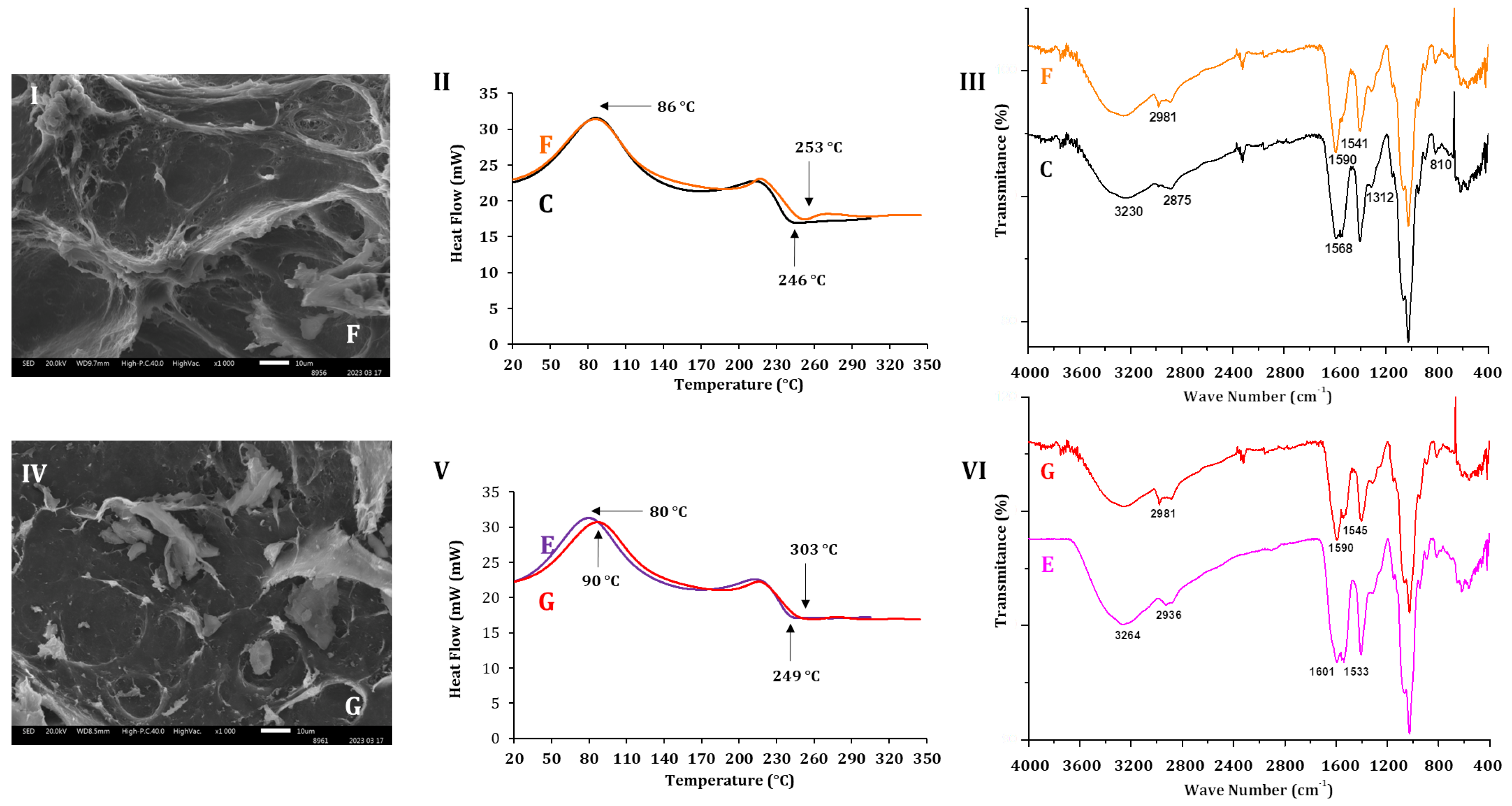
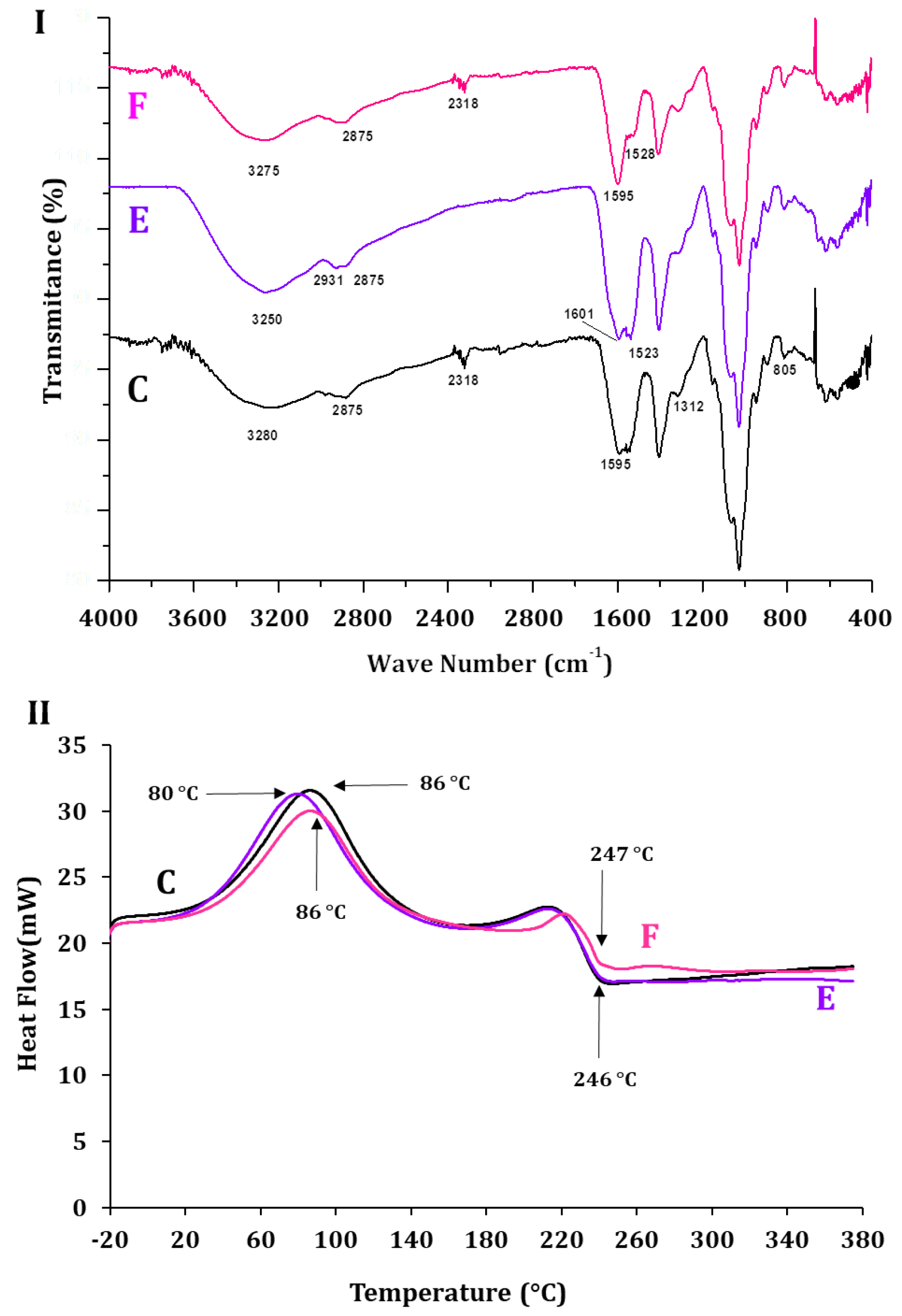
2.2. Morphology and Physico-Chemical Attributes of AlG-CS-P1G10 Biocatalyst
| Sample | Code | Tc (°C) | Tm (°C) |
|---|---|---|---|
| ALG | A | 88 | 258 |
| CS | B | 91 | 308 |
| ALG-CS | C | 86 | 246 |
| P1G10 | D | 76 | 242 |
| ALG-CS-P1G10 | E | 80 | 246 |
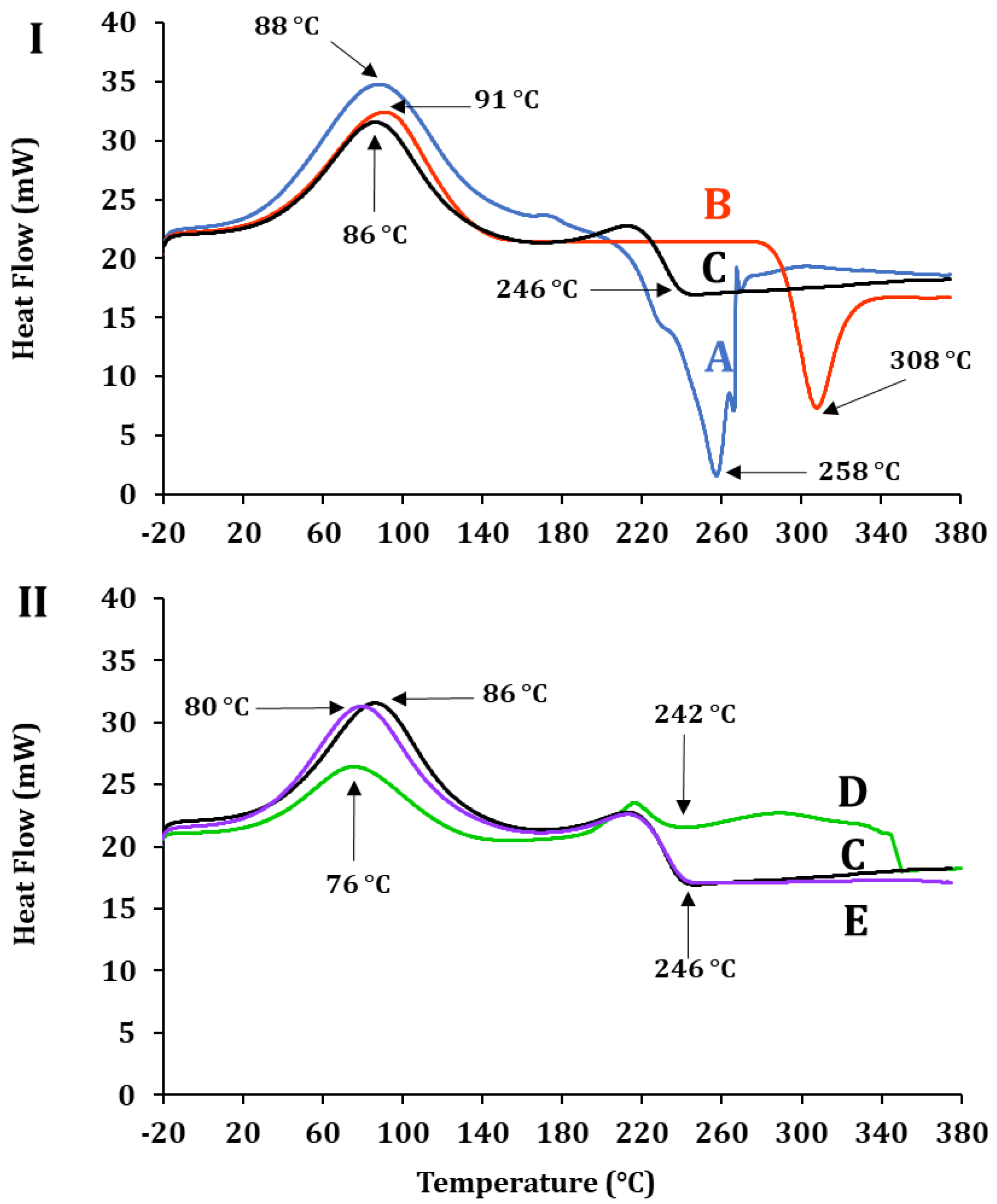
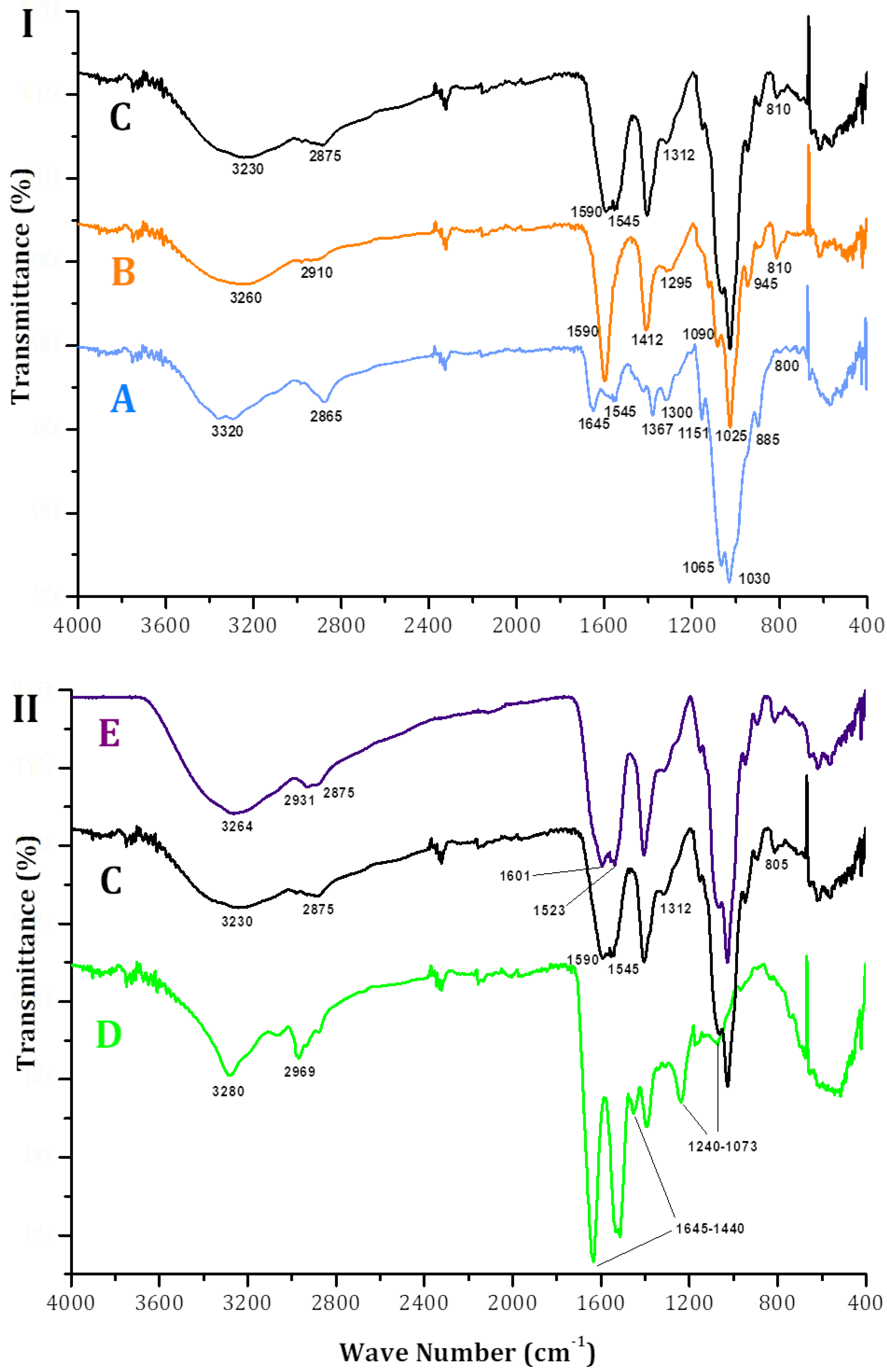
2.3. P1G10 Release: Kinetics, Enzyme Activity and Material Characterization
3. Materials and Methods
3.1. Papaya Latex Collection
3.2. Protein Extraction from Freeze-Dried Papaya Latex
3.3. Encapsulation of the Proteolytic Fraction P1G10
3.4. Protein Quantification
3.5. Determination of Enzyme Activity
3.6. Evaluation of Encapsulating Parameters
3.7. Determination of the Mass Ratio ALG/CS
3.8. Scanning Electron Microscopy (SEM)
3.9. Differential Scanning Calorimetry (DSC)
3.10. Fourier Transform Infrared Spectrometry (FTIR)
3.11. Release Kinetics
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dwivedi, M.; Singh, P.; Pandey, A.K. Botrytis fruit rot management: What have we achieved so far? Food Microbiol. 2024, 122, 104564. [Google Scholar] [CrossRef]
- Olmedo, G.M.; Cerioni, L.; González, M.M.; Cabrerizo, F.M.; Rapisarda, V.A.; Volentini, S.I. Antifungal activity of β-carbolines on Penicillium digitatum and Botrytis cinerea. Food Microbiol. 2017, 62, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xia, X.-M.; Wang, H.-Y.; Li, P.-P.; Wang, K.-Y. Inhibitory effect of lactoferrin against gray mould on tomato plants caused by Botrytis cinerea and possible mechanisms of action. Int. J. Food Microbiol. 2013, 161, 151–157. [Google Scholar] [CrossRef]
- Wroński, M.; Trawiński, J.; Skibiński, R. Antifungal drugs in the aquatic environment: A review on sources, occurrence, toxicity, health effects, removal strategies and future challenges. J. Hazard. Mater. 2023, 465, 133167. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)—Prospects and challenges. Biocontrol Sci. Technol. 2018, 29, 207–228. [Google Scholar] [CrossRef]
- Kfoury, M.; Lounès-Hadj Sahraoui, A.; Bourdon, N.; Laruelle, F.; Fontaine, J.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Solubility, photostability and antifungal activity of phenylpropanoids encapsulated in cyclodextrins. Food Chem. 2016, 196, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-González, A.E.; Palou, E.; López-Malo, A. Antifungal activity of essential oils of clove (Syzygium aromaticum) and/or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innov. Food Sci. Emerg. Technol. 2015, 32, 181–185. [Google Scholar] [CrossRef]
- Soylu, E.M.; Kurt, Ş.; Soylu, S. In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int. J. Food Microbiol. 2010, 143, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.V.; Demarco, D.; Souza, I.C.d.C.; de Freitas, C.D.T. Laticifers, Latex, and Their Role in Plant Defense. Trends Plant Sci. 2019, 24, 553–567. [Google Scholar] [CrossRef]
- Freitas, K.M.; Veloso, E.S.; Ferreira, Ê.; Caliari, M.V.; Salas, C.E.; Lopes, M.T. Effects of P1G10 against UVB-induced damage: Reduction of antioxidant stress, inflammation and cell proliferation. J. Photochem. Photobiol. 2025, 25, 100255. [Google Scholar] [CrossRef]
- Baeza, G.; Correa, D.; Salas, C. Proteolytic enzymes in Carica candamarcensis. J. Sci. Food Agric. 1990, 51, 1–9. [Google Scholar] [CrossRef]
- Lemos, F.O.; Villalba, M.I.C.; Tagliati, C.A.; Cardoso, V.N.; Salas, C.E.; Lopes, M.T. Biodistribution, pharmacokinetics and toxicity of a Vasconcellea cundinamarcensis proteinase fraction with pharmacological activity. Rev. Bras. Farm. 2016, 26, 94–101. [Google Scholar] [CrossRef]
- Teixeira, R.D.; Ribeiro, H.A.; Gomes, M.-T.R.; Lopes, M.T.; Salas, C.E. The proteolytic activities in latex from Carica candamarcensis. Plant Physiol. Biochem. 2008, 46, 956–961. [Google Scholar] [CrossRef]
- Torres-Ossandón, M.J.; Vega-Gálvez, A.; Salas, C.E.; Rubio, J.; Silva-Moreno, E.; Castillo, L. Antifungal activity of proteolytic fraction (P1G10) from (Vasconcellea cundinamarcensis) latex inhibit cell growth and cell wall integrity in Botrytis cinerea. Int. J. Food Microbiol. 2019, 289, 7–16. [Google Scholar] [CrossRef]
- Denadai, Â.M.L.; Santoro, M.M.; Lopes, M.T.P.; Chenna, A.; de Sousa, F.B.; Avelar, G.M.; Gomes, M.R.T.; Guzman, F.; Salas, C.E.; Sinisterra, R.D. A Supramolecular Complex between Proteinases and β-Cyclodextrin that Preserves Enzymatic Activity. BioDrugs 2006, 20, 283–291. [Google Scholar] [CrossRef]
- Rezagholizade-Shirvan, A.; Soltani, M.; Shokri, S.; Radfar, R.; Arab, M.; Shamloo, E. Bioactive compound encapsulation: Characteristics, applications in food systems, and implications for human health. Food Chem. X 2024, 24, 101953. [Google Scholar] [CrossRef]
- Wen, C.; Tang, J.; Cao, L.; Fan, M.; Lin, X.; Liu, G.; Liang, L.; Liu, X.; Zhang, J.; Li, Y.; et al. Strategic Approaches for Co-Encapsulation of Bioactive Compounds: Technological Advances and Mechanistic Insight. Foods 2025, 14, 2024. [Google Scholar] [CrossRef] [PubMed]
- Worrall, E.A.; Hamid, A.; Mody, K.T.; Mitter, N.; Pappu, H.R. Nanotechnology for Plant Disease Management. Agronomy 2018, 8, 285. [Google Scholar] [CrossRef]
- Zabot, G.L.; Rodrigues, F.S.; Ody, L.P.; Tres, M.V.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.S.; Best, I.; Olivera-Montenegro, L. Encapsulation of Bioactive Compounds for Food and Agricultural Applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Khawar, K.M.; Camara, M.C.; Carvalho, L.B.; Fraceto, L.F.; Morsi, R.E.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Ullah, H.; et al. Chitosan-based delivery systems for plants: A brief overview of recent advances and future directions. Int. J. Biol. Macromol. 2020, 154, 683–697. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Ozaltin, K.; Postnikov, P.S.; Trusova, M.E.; Sedlarik, V.; Di Martino, A. Polysaccharides based microspheres for multiple encapsulations and simultaneous release of proteases. Int. J. Biol. Macromol. 2019, 132, 24–31. [Google Scholar] [CrossRef]
- Yi, Y.; Liu, Y.; Chu, Y.; Hou, Z.; Liu, S.; Yang, Q.; Liu, S.; Zuo, P.; Hu, Y. Encapsulation of CBP1 antifungal protein into sodium alginate and chitosan to control the Aspergillus flavus mediated decay of cherry tomatoes. Food Control 2023, 156, 110147. [Google Scholar] [CrossRef]
- Boškov, I.A.; Savić, I.M.; Stanisavljević, N.Đ.G.; Kundaković-Vasović, T.D.; Selgrad, J.S.R.; Gajić, I.M.S. Stabilization of Black Locust Flower Extract via Encapsulation Using Alginate and Alginate–Chitosan Microparticles. Polymers 2024, 16, 688. [Google Scholar] [CrossRef]
- Zhao, B.; Alonso, N.F.; Miras, J.; Vílchez, S.; García-Celma, M.J.; Morral, G.; Esquena, J. Triggered protein release from calcium alginate/chitosan gastro-resistant capsules. Colloids Surfaces A: Physicochem. Eng. Asp. 2024, 693, 133998. [Google Scholar] [CrossRef]
- Upadhyay, U.; Kolla, S.; Maredupaka, S.; Priya, S.; Srinivasulu, K.; Chelluri, L.K. Development of an alginate–chitosan biopolymer composite with dECM bioink additive for organ-on-a-chip articular cartilage. Sci. Rep. 2024, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Khatibi, N.; Naimi-Jamal, M.R.; Balalaie, S.; Shokoohmand, A. Development and evaluation of a pH-sensitive, naturally crosslinked alginate-chitosan hydrogel for drug delivery applications. Front. Biomater. Sci. 2024, 3, 1457540. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, W.; Ma, X.; Li, X.; Zhang, Q.; Ma, W.; Chen, N.; Zhou, X.; Tang, X. Preparation and property evaluation of oral colon targeted protein delivery system with sodium alginate and chitosan. Sci. Rep. 2025, 15, 21598. [Google Scholar] [CrossRef] [PubMed]
- Yeerong, K.; Chantawannakul, P.; Anuchapreeda, S.; Juntrapirom, S.; Kanjanakawinkul, W.; Müllertz, A.; Rades, T.; Chaiyana, W. Chitosan Alginate Nanoparticles of Protein Hydrolysate from Acheta domesticus with Enhanced Stability for Skin Delivery. Pharmaceutics 2024, 16, 724. [Google Scholar] [CrossRef]
- Di Santo, M.C.; Antoni, C.L.D.; Rubio, A.P.D.; Alaimo, A.; Pérez, O.E. Chitosan-tripolyphosphate nanoparticles designed to encapsulate polyphenolic compounds for biomedical and pharmaceutical applications—A review. Biomed. Pharmacother. 2021, 142, 111970. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.J.; Marques, A.M.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Physicochemical properties of alginate-based films: Effect of ionic crosslinking and mannuronic and guluronic acid ratio. Food Hydrocoll. 2018, 81, 442–448. [Google Scholar] [CrossRef]
- Shi, X.; Du, Y.; Sun, L.; Zhang, B.; Dou, A. Polyelectrolyte complex beads composed of water-soluble chitosan/alginate: Characterization and their protein release behavior. J. Appl. Polym. Sci. 2006, 100, 4614–4622. [Google Scholar] [CrossRef]
- Patel, M.A.; AbouGhaly, M.H.; Schryer-Praga, J.V.; Chadwick, K. The effect of ionotropic gelation residence time on alginate cross-linking and properties. Carbohydr. Polym. 2017, 155, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Caetano, L.A.; Almeida, A.J.; Gonçalves, L.M. Effect of Experimental Parameters on Alginate/Chitosan Microparticles for BCG Encapsulation. Mar. Drugs 2016, 14, 90. [Google Scholar] [CrossRef]
- Coppi, G.; Iannuccelli, V.; Leo, E.; Bernabei, M.T.; Cameroni, R. Chitosan-Alginate Microparticles as a Protein Carrier. Drug Dev. Ind. Pharm. 2001, 27, 393–400. [Google Scholar] [CrossRef]
- Thu, T.T.M.; Krasaekoopt, W. Encapsulation of protease from Aspergillus oryzae and lipase from Thermomyces lanuginoseus using alginate and different copolymer types. Agric. Nat. Resour. 2016, 50, 155–161. [Google Scholar] [CrossRef]
- Li, X.; Kong, X.; Shi, S.; Zheng, X.; Guo, G.; Wei, Y.; Qian, Z. Preparation of alginate coated chitosan microparticles for vaccine delivery. BMC Biotechnol. 2008, 8, 89. [Google Scholar] [CrossRef]
- Vandenberg, G.; Drolet, C.; Scott, S.; de la Noüe, J. Factors affecting protein release from alginate–chitosan coacervate microcapsules during production and gastric/intestinal simulation. J. Control. Release 2001, 77, 297–307. [Google Scholar] [CrossRef]
- Dumitriu, S. Inclusion and release of proteins from polysaccharide-based polyion complexes. Adv. Drug Deliv. Rev. 1998, 31, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Holkar, A.; Srivastava, S. Protein–Polyelectrolyte Complexes and Micellar Assemblies. Polymers 2019, 11, 1097. [Google Scholar] [CrossRef]
- Gomes, M.T.R.; Teixeira, R.D.; Ribeiro, H.d.A.L.; Turchetti, A.P.; Junqueira, C.F.; Lopes, M.T.P.; Salas, C.E.; Nagem, R.A.P. Purification, crystallization and preliminary X-ray analysis of CMS1MS2: A cysteine proteinase from Carica candamarcensis latex. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2008, 64, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Sankalia, M.G.; Mashru, R.C.; Sankalia, J.M.; Sutariya, V.B. Reversed chitosan–alginate polyelectrolyte complex for stability improvement of alpha-amylase: Optimization and physicochemical characterization. Eur. J. Pharm. Biopharm. 2007, 65, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Ahn, D.U. Enhanced lutein stability under UV-Light and high temperature by loading it into alginate-chitosan complex. LWT 2022, 164, 113663. [Google Scholar] [CrossRef]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; da Rosa, C.G.; da Silva, M.M. Elaboration of microparticles of carotenoids from natural and synthetic sources for applications in food. Food Chem. 2016, 202, 324–333. [Google Scholar] [CrossRef]
- Jana, S.; Trivedi, M.K.; Tallapragada, R.M.; Branton, A.; Trivedi, D.; Nayak, G.; Mishra, R. Characterization of Physicochemical and Thermal Properties of Chitosan and Sodium Alginate after Biofield Treatment. Pharm. Anal. Acta 2015, 6. [Google Scholar] [CrossRef]
- Wang, L.; Khor, E.; Lim, L.Y. Chitosan-Alginate-CaCl2 System for Membrane Coat Application. J. Pharm. Sci. 2001, 90, 1134–1142. [Google Scholar] [CrossRef]
- Pendekal, M.S.; Tegginamat, P.K. Hybrid drug delivery system for oropharyngeal, cervical and colorectal cancer—In vitro and in vivo evaluation. Saudi Pharm. J. 2012, 21, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Thai, H.; Nguyen, C.T.; Thach, L.T.; Tran, M.T.; Mai, H.D.; Nguyen, T.T.T.; Le, G.D.; Van Can, M.; Tran, L.D.; Bach, G.L.; et al. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci. Rep. 2020, 10, 909. [Google Scholar] [CrossRef]
- Hosseini, S.; Varidi, M. Optimization of Microbial Rennet Encapsulation in Alginate – Chitosan Nanoparticles. Food Chem. 2021, 352, 129325. [Google Scholar] [CrossRef]
- Calvo, T.A.; Santagapita, P. Physicochemical Characterization of Alginate Beads Containing Sugars and Biopolymers. J. Qual. Reliab. Eng. 2016, 2016, 9184039. [Google Scholar] [CrossRef]
- Han, J.; Guenier, A.-S.; Salmieri, S.; Lacroix, M. Alginate and Chitosan Functionalization for Micronutrient Encapsulation. J. Agric. Food Chem. 2008, 56, 2528–2535. [Google Scholar] [CrossRef]
- Gomes, M.T.R.; Teixeira, R.D.; Lopes, M.T.P.; Nagem, R.A.P.; Salas, C.E. X-ray crystal structure of CMS1MS2: A high proteolytic activity cysteine proteinase from Carica candamarcensis. Amino Acids 2012, 43, 2381–2391. [Google Scholar] [CrossRef]
- Solgaard, G.; Thorsen, K.H.; Draget, K.I. Encapsulation of a proteolytically active novel bioproduct; controlling the release of proteinous components. Food Bioprod. Process. 2008, 87, 40–45. [Google Scholar] [CrossRef]
- Lindhoud, S.; de Vries, R.; Schweins, R.; Stuart, M.A.C.; Norde, W. Salt-induced release of lipase from polyelectrolyte complex micelles. Soft Matter 2009, 5, 242–250. [Google Scholar] [CrossRef]
- Mi, F.-L.; Sung, H.-W.; Shyu, S.-S. Drug release from chitosan–alginate complex beads reinforced by a naturally occurring cross-linking agent. Carbohydr. Polym. 2002, 48, 61–72. [Google Scholar] [CrossRef]
- Jahromi, L.P.; Ghazali, M.; Ashrafi, H.; Azadi, A. A comparison of models for the analysis of the kinetics of drug release from PLGA-based nanoparticles. Heliyon 2020, 6, e03451. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.; Al-Osaimi, A.A.; Alghamdi, B.A. Sodium Chloride (NaCl)-Induced Physiological Alteration and Oxidative Stress Generation in Pisum sativum (L.): A Toxicity Assessment. ACS Omega 2022, 7, 20819–20832. [Google Scholar] [CrossRef]
- Singh, J.; Sastry, E.V.D.; Singh, V. Effect of salinity on tomato (Lycopersicon esculentum Mill.) during seed germination stage. Physiol. Mol. Biol. Plants 2011, 18, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Sun, J.; Tan, H.; Yuan, G.; Li, J.; Jia, Y.; Xiong, D.; Chen, G.; Lai, J.; Ling, Z.; et al. Covalently polysaccharide-based alginate/chitosan hydrogel embedded alginate microspheres for BSA encapsulation and soft tissue engineering. Int. J. Biol. Macromol. 2019, 127, 340–348. [Google Scholar] [CrossRef]
- Mello, V.J.; Gomes, M.T.R.; Lemos, F.O.; Delfino, J.L.; Andrade, S.P.; Lopes, M.T.; Salas, C.E. The gastric ulcer protective and healing role of cysteine proteinases from Carica candamarcensis. Phytomedicine 2008, 15, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, H.; Razzak, A.; Kim, E.J.; Choi, S.S. Crosslinker-free Bovine Serum Albumin-loaded Chitosan/alginate Nanocomplex for pH-responsive Bursting Release of Oral-administered Protein. Biotechnol. Bioprocess Eng. 2022, 27, 40–50. [Google Scholar] [CrossRef]
- Ataide, J.A.; Gérios, E.F.; Cefali, L.C.; Fernandes, A.R.; Teixeira, M.D.C.; Ferreira, N.R.; Tambourgi, E.B.; Jozala, A.F.; Chaud, M.V.; Oliveira-Nascimento, L.; et al. Effect of Polysaccharide Sources on the Physicochemical Properties of Bromelain—Chitosan Nanoparticles. Polymers 2019, 11, 1681. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Deng, Q.-Y.; Zhou, C.-R.; Luo, B.-H. Preparation and Characterization of Chitosan Nanoparticles Containing Lysozyme. Pharm. Biol. 2006, 44, 336–342. [Google Scholar] [CrossRef]
- Bernal, C.; Poveda-Jaramillo, J.C.; Mesa, M. Raising the enzymatic performance of lipase and protease in the synthesis of sugar fatty acid esters, by combined ionic exchange -hydrophobic immobilization process on aminopropyl silica support. Chem. Eng. J. 2018, 334, 760–767. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Grumezescu, A.M. Applications of Chitosan-Alginate-Based Nanoparticles—An Up-to-Date Review. Nanomaterials 2022, 12, 186. [Google Scholar] [CrossRef]
- Bahreini, E.; Aghaiypour, K.; Abbasalipourkabir, R.; Mokarram, A.R.; Goodarzi, M.T.; Saidijam, M. Preparation and nanoencapsulation of l-asparaginase II in chitosan-tripolyphosphate nanoparticles and in vitro release study. Nanoscale Res. Lett. 2014, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, J.; Liu, F.; Majeed, H.; Qi, J.; Yokoyama, W.; Zhong, F. Physicochemical and morphological properties of size-controlled chitosan–tripolyphosphate nanoparticles. Colloids Surfaces A: Physicochem. Eng. Asp. 2015, 465, 137–146. [Google Scholar] [CrossRef]
- Lazaridou, M.; Christodoulou, E.; Nerantzaki, M.; Kostoglou, M.; Lambropoulou, D.A.; Katsarou, A.; Pantopoulos, K.; Bikiaris, D.N. Formulation and In-Vitro Characterization of Chitosan-Nanoparticles Loaded with the Iron Chelator Deferoxamine Mesylate (DFO). Pharmaceutics 2020, 12, 238. [Google Scholar] [CrossRef]
- Sáez, M.; Barros, A.; Vizcaíno, A.; López, G.; Alarcón, F.; Martínez, T. Effect of alginate and chitosan encapsulation on the fate of BSA protein delivered orally to gilthead sea bream (Sparus aurata). Anim. Feed Sci. Technol. 2015, 210, 114–124. [Google Scholar] [CrossRef]
 , ALG-CS-P1G10 biocatalyst formation yield;
, ALG-CS-P1G10 biocatalyst formation yield;  , P1G10 protein encapsulation yield. Values are expressed as mean ± standard deviation. Different uppercase and lowercase letters indicate statistically significant differences (p < 0.05) in biocatalyst formation and protein encapsulation yields, respectively, as determined by Fisher’s LSD test.
, P1G10 protein encapsulation yield. Values are expressed as mean ± standard deviation. Different uppercase and lowercase letters indicate statistically significant differences (p < 0.05) in biocatalyst formation and protein encapsulation yields, respectively, as determined by Fisher’s LSD test.
 , ALG-CS-P1G10 biocatalyst formation yield;
, ALG-CS-P1G10 biocatalyst formation yield;  , P1G10 protein encapsulation yield. Values are expressed as mean ± standard deviation. Different uppercase and lowercase letters indicate statistically significant differences (p < 0.05) in biocatalyst formation and protein encapsulation yields, respectively, as determined by Fisher’s LSD test.
, P1G10 protein encapsulation yield. Values are expressed as mean ± standard deviation. Different uppercase and lowercase letters indicate statistically significant differences (p < 0.05) in biocatalyst formation and protein encapsulation yields, respectively, as determined by Fisher’s LSD test.
 ), ALG-CS-P1G10 biocatalyst formation yield; (II) (
), ALG-CS-P1G10 biocatalyst formation yield; (II) ( ), P1G10 protein encapsulation yield and (III) (
), P1G10 protein encapsulation yield and (III) ( ), ALG-CS-P1G10 biocatalyst activity. Values are expressed as mean ± standard deviation. Different uppercase and lowercase letters indicate statistically significant differences (p < 0.05) determined by Fisher’s LSD test Based on the above results and the data from the literature [37], it is apparent that the amount of protein encapsulated influences biocatalyst formation and yield. In contrast, ref. [38] showed that by increasing the BSA protein from 1 to 8 mg/mL, the encapsulation yield decreased from 65 to 30% in ALG-CS microparticles. Similarly, their results show that the encapsulation yield decreases from 90 to about 15% when increasing the ALG/CS mass ratio from 1 to 5. Meanwhile, ref. [39] encapsulated BSA with ALG and CS, obtaining yields higher than 90% that remained high when the BSA/ALG mass ratio increased from 25 to 100. The disparity in yields is attributed to several factors influencing complexation between ALG-CS and the protein, including the molecular structure of the protein, its solubility, the ALG-CS protein mixing ratio, or the concentration and size of polyelectrolytes [40]. The authors of [41] pointed out that the choice of polyelectrolyte must include its charge density, molecular size, and pKa, while for the protein to be encapsulated, one should consider parameters such as pI, charge density, and hydrophobic distribution. The (pI) of P1G10 isoforms rank between 9.4 and 9.6 [42] and thus encompasses net positive charge over a wide pH range; CS has a pKa of 6.2 [22], suggesting a less favorable condition for electrostatic interaction at pH 5.1, as used in these experiments. On the other hand, the interaction between amino groups of CS and the carboxyl groups of ALG reported by [43] would allow trapping of P1G10, as shown by the encapsulation yields obtained at ALG/CS ratios 0.80–1.0. Based on the biocatalyst formation yield and the expressed enzyme activity, 13.2 mg of protein was selected in further encapsulation experiments.
), ALG-CS-P1G10 biocatalyst activity. Values are expressed as mean ± standard deviation. Different uppercase and lowercase letters indicate statistically significant differences (p < 0.05) determined by Fisher’s LSD test Based on the above results and the data from the literature [37], it is apparent that the amount of protein encapsulated influences biocatalyst formation and yield. In contrast, ref. [38] showed that by increasing the BSA protein from 1 to 8 mg/mL, the encapsulation yield decreased from 65 to 30% in ALG-CS microparticles. Similarly, their results show that the encapsulation yield decreases from 90 to about 15% when increasing the ALG/CS mass ratio from 1 to 5. Meanwhile, ref. [39] encapsulated BSA with ALG and CS, obtaining yields higher than 90% that remained high when the BSA/ALG mass ratio increased from 25 to 100. The disparity in yields is attributed to several factors influencing complexation between ALG-CS and the protein, including the molecular structure of the protein, its solubility, the ALG-CS protein mixing ratio, or the concentration and size of polyelectrolytes [40]. The authors of [41] pointed out that the choice of polyelectrolyte must include its charge density, molecular size, and pKa, while for the protein to be encapsulated, one should consider parameters such as pI, charge density, and hydrophobic distribution. The (pI) of P1G10 isoforms rank between 9.4 and 9.6 [42] and thus encompasses net positive charge over a wide pH range; CS has a pKa of 6.2 [22], suggesting a less favorable condition for electrostatic interaction at pH 5.1, as used in these experiments. On the other hand, the interaction between amino groups of CS and the carboxyl groups of ALG reported by [43] would allow trapping of P1G10, as shown by the encapsulation yields obtained at ALG/CS ratios 0.80–1.0. Based on the biocatalyst formation yield and the expressed enzyme activity, 13.2 mg of protein was selected in further encapsulation experiments.
 ), ALG-CS-P1G10 biocatalyst formation yield; (II) (
), ALG-CS-P1G10 biocatalyst formation yield; (II) ( ), P1G10 protein encapsulation yield and (III) (
), P1G10 protein encapsulation yield and (III) ( ), ALG-CS-P1G10 biocatalyst activity. Values are expressed as mean ± standard deviation. Different uppercase and lowercase letters indicate statistically significant differences (p < 0.05) determined by Fisher’s LSD test Based on the above results and the data from the literature [37], it is apparent that the amount of protein encapsulated influences biocatalyst formation and yield. In contrast, ref. [38] showed that by increasing the BSA protein from 1 to 8 mg/mL, the encapsulation yield decreased from 65 to 30% in ALG-CS microparticles. Similarly, their results show that the encapsulation yield decreases from 90 to about 15% when increasing the ALG/CS mass ratio from 1 to 5. Meanwhile, ref. [39] encapsulated BSA with ALG and CS, obtaining yields higher than 90% that remained high when the BSA/ALG mass ratio increased from 25 to 100. The disparity in yields is attributed to several factors influencing complexation between ALG-CS and the protein, including the molecular structure of the protein, its solubility, the ALG-CS protein mixing ratio, or the concentration and size of polyelectrolytes [40]. The authors of [41] pointed out that the choice of polyelectrolyte must include its charge density, molecular size, and pKa, while for the protein to be encapsulated, one should consider parameters such as pI, charge density, and hydrophobic distribution. The (pI) of P1G10 isoforms rank between 9.4 and 9.6 [42] and thus encompasses net positive charge over a wide pH range; CS has a pKa of 6.2 [22], suggesting a less favorable condition for electrostatic interaction at pH 5.1, as used in these experiments. On the other hand, the interaction between amino groups of CS and the carboxyl groups of ALG reported by [43] would allow trapping of P1G10, as shown by the encapsulation yields obtained at ALG/CS ratios 0.80–1.0. Based on the biocatalyst formation yield and the expressed enzyme activity, 13.2 mg of protein was selected in further encapsulation experiments.
), ALG-CS-P1G10 biocatalyst activity. Values are expressed as mean ± standard deviation. Different uppercase and lowercase letters indicate statistically significant differences (p < 0.05) determined by Fisher’s LSD test Based on the above results and the data from the literature [37], it is apparent that the amount of protein encapsulated influences biocatalyst formation and yield. In contrast, ref. [38] showed that by increasing the BSA protein from 1 to 8 mg/mL, the encapsulation yield decreased from 65 to 30% in ALG-CS microparticles. Similarly, their results show that the encapsulation yield decreases from 90 to about 15% when increasing the ALG/CS mass ratio from 1 to 5. Meanwhile, ref. [39] encapsulated BSA with ALG and CS, obtaining yields higher than 90% that remained high when the BSA/ALG mass ratio increased from 25 to 100. The disparity in yields is attributed to several factors influencing complexation between ALG-CS and the protein, including the molecular structure of the protein, its solubility, the ALG-CS protein mixing ratio, or the concentration and size of polyelectrolytes [40]. The authors of [41] pointed out that the choice of polyelectrolyte must include its charge density, molecular size, and pKa, while for the protein to be encapsulated, one should consider parameters such as pI, charge density, and hydrophobic distribution. The (pI) of P1G10 isoforms rank between 9.4 and 9.6 [42] and thus encompasses net positive charge over a wide pH range; CS has a pKa of 6.2 [22], suggesting a less favorable condition for electrostatic interaction at pH 5.1, as used in these experiments. On the other hand, the interaction between amino groups of CS and the carboxyl groups of ALG reported by [43] would allow trapping of P1G10, as shown by the encapsulation yields obtained at ALG/CS ratios 0.80–1.0. Based on the biocatalyst formation yield and the expressed enzyme activity, 13.2 mg of protein was selected in further encapsulation experiments.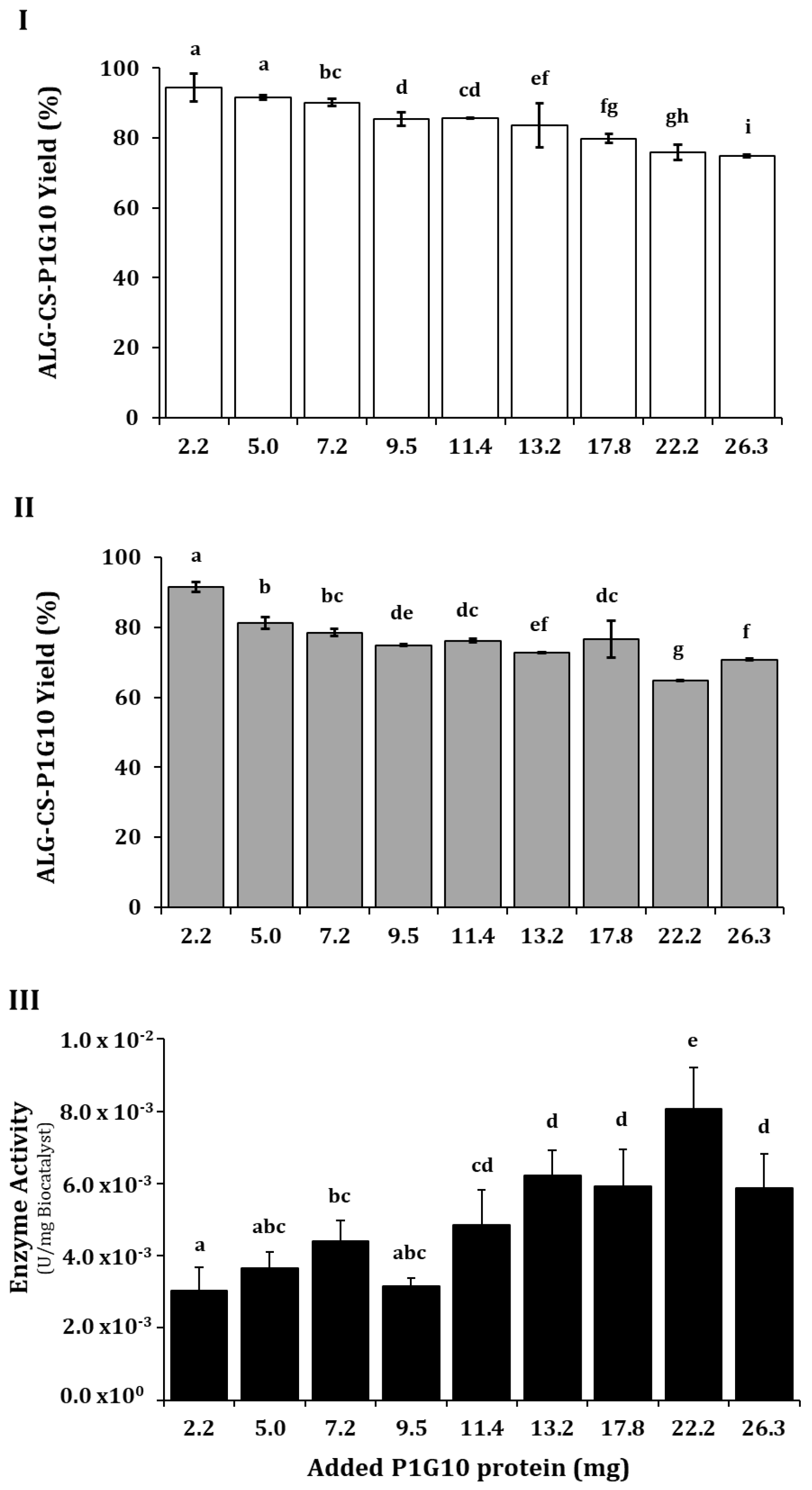

 ), 0.30 M; (◇), 0.40 M. Release kinetics experiments were performed in triplicate. (*) indicates significant differences (p < 0.05) according to LSD test. (II), Enzymatic activity of released P1G10 at three NaCl concentrations: (
), 0.30 M; (◇), 0.40 M. Release kinetics experiments were performed in triplicate. (*) indicates significant differences (p < 0.05) according to LSD test. (II), Enzymatic activity of released P1G10 at three NaCl concentrations: ( ), 0.20 M; (
), 0.20 M; ( ), 0.30 M; (
), 0.30 M; ( ), 0.40 M. No enzymatic activity was detected with NaCl 0.1 M.
), 0.40 M. No enzymatic activity was detected with NaCl 0.1 M.
 ), 0.30 M; (◇), 0.40 M. Release kinetics experiments were performed in triplicate. (*) indicates significant differences (p < 0.05) according to LSD test. (II), Enzymatic activity of released P1G10 at three NaCl concentrations: (
), 0.30 M; (◇), 0.40 M. Release kinetics experiments were performed in triplicate. (*) indicates significant differences (p < 0.05) according to LSD test. (II), Enzymatic activity of released P1G10 at three NaCl concentrations: ( ), 0.20 M; (
), 0.20 M; ( ), 0.30 M; (
), 0.30 M; ( ), 0.40 M. No enzymatic activity was detected with NaCl 0.1 M.
), 0.40 M. No enzymatic activity was detected with NaCl 0.1 M.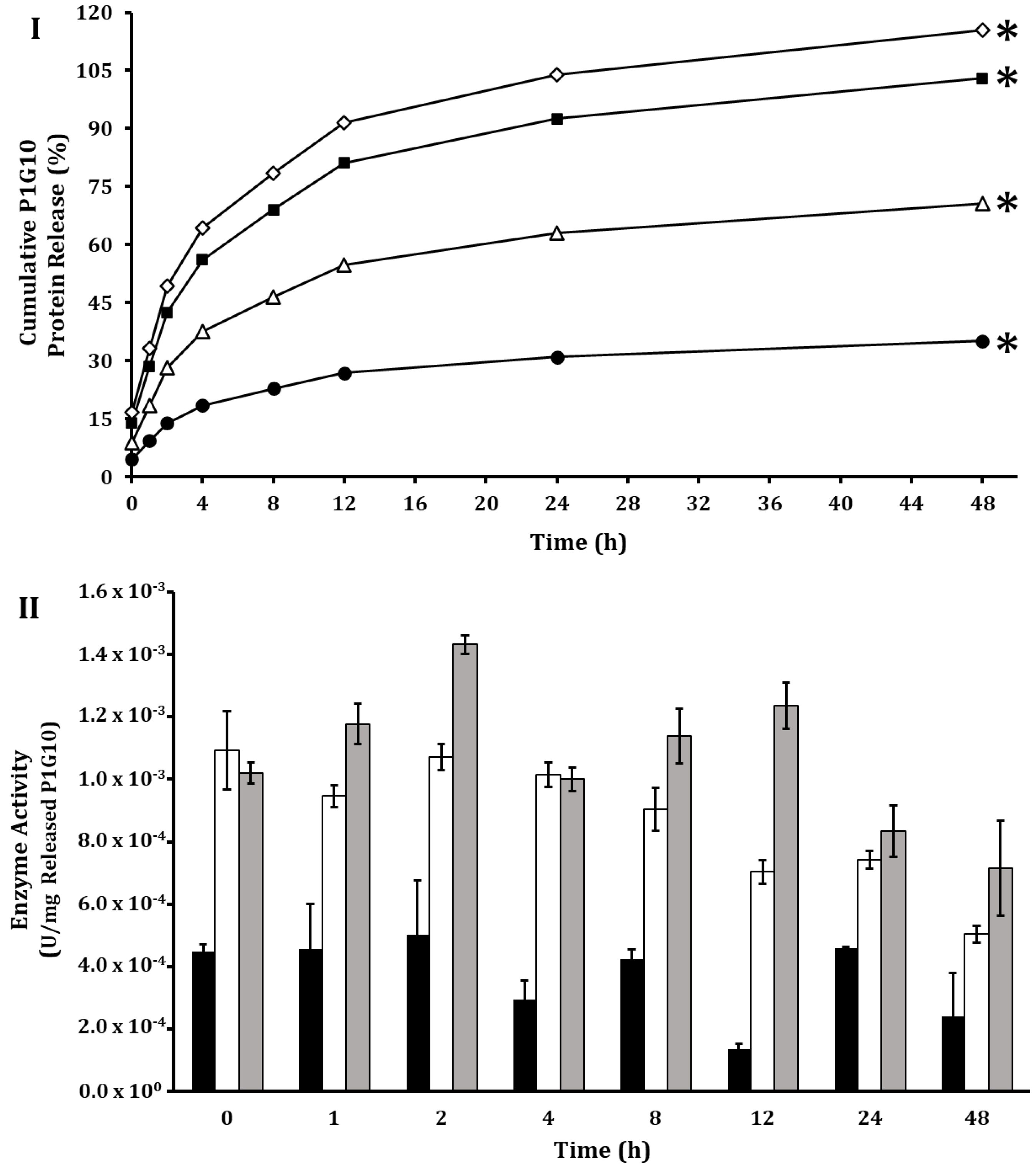
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cisternas-Jamet, J.; Plaza, V.; Salas, C.; Bernal, C.; Castillo, L. Immobilization of the Proteolytic Fraction P1G10 from Vasconcellea pubescens in Alginate–Chitosan Complex and Enzyme Activity Release. Molecules 2025, 30, 3747. https://doi.org/10.3390/molecules30183747
Cisternas-Jamet J, Plaza V, Salas C, Bernal C, Castillo L. Immobilization of the Proteolytic Fraction P1G10 from Vasconcellea pubescens in Alginate–Chitosan Complex and Enzyme Activity Release. Molecules. 2025; 30(18):3747. https://doi.org/10.3390/molecules30183747
Chicago/Turabian StyleCisternas-Jamet, Jonathan, Verónica Plaza, Carlos Salas, Claudia Bernal, and Luis Castillo. 2025. "Immobilization of the Proteolytic Fraction P1G10 from Vasconcellea pubescens in Alginate–Chitosan Complex and Enzyme Activity Release" Molecules 30, no. 18: 3747. https://doi.org/10.3390/molecules30183747
APA StyleCisternas-Jamet, J., Plaza, V., Salas, C., Bernal, C., & Castillo, L. (2025). Immobilization of the Proteolytic Fraction P1G10 from Vasconcellea pubescens in Alginate–Chitosan Complex and Enzyme Activity Release. Molecules, 30(18), 3747. https://doi.org/10.3390/molecules30183747






