Design and Application of Conjugated Oligomers for Imaging and Anticancer and Antibacterial Treatment
Abstract
1. Introduction
2. Conjugated Oligomers
3. Detection Based on Conjugated Oligomers
3.1. Fluorescence Detection
3.2. Photoacoustic Detection
4. Disease Treatment with Conjugated Oligomers
4.1. Antibacterial
4.1.1. Photodynamic Therapy
4.1.2. Photothermal Therapy
4.1.3. Synergistic Therapy
4.2. Anticancer
4.2.1. Photothermal Therapy
4.2.2. Combination Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Okeke, I.N.; de Kraker, M.E.A.; Van Boeckel, T.P.; Kumar, C.K.; Schmitt, H.; Gales, A.C.; Bertagnolio, S.; Sharland, M.; Laxminarayan, R. The scope of the antimicrobial resistance challenge. Lancet 2024, 403, 2426–2438. [Google Scholar] [CrossRef]
- Chen, M.; Gong, N.; Sun, W.; Han, J.; Liu, Y.; Zhang, S.; Zheng, A.; Butt, H.-J.; Liang, X.-J.; Wu, S. Red-Light-Responsive Metallopolymer Nanocarriers with Conjugated and Encapsulated Drugs for Phototherapy Against Multidrug-Resistant Tumors. Small 2022, 18, 2201672. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, H.; Li, Z.; Liao, S.; Yin, B.; Yue, R.; Guan, G.; Chen, B.; Song, G. Enhancing Fractionated Cancer Therapy: A Triple-Anthracene Photosensitizer Unleashes Long-Persistent Photodynamic and Luminous Efficacy. J. Am. Chem. Soc. 2024, 146, 6252–6265. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, Y.; Li, B.; Song, W.; Qi, G.; Tian, J.; Huang, W.; Fan, Q.; Liu, B. Oxygen-independent organic photosensitizer with ultralow-power NIR photoexcitation for tumor-specific photodynamic therapy. Nat. Commun. 2024, 15, 2530. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wang, C.; Zhang, X.; Chen, H.; Wu, M.X.; Lu, M. Photochemical Strategies toward Precision Targeting against Multidrug-Resistant Bacterial Infections. ACS Nano 2024, 18, 14085–14122. [Google Scholar] [CrossRef]
- Wei, K.; Wu, Y.; Zheng, X.; Ouyang, L.; Ma, G.; Ji, C.; Yin, M. A Light-Triggered J-Aggregation-Regulated Therapy Conversion: From Photodynamic/Photothermal Therapy to Long-Lasting Chemodynamic Therapy for Effective Tumor Ablation. Angew. Chem. Int. Ed. 2024, 63, e202404395. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zheng, W.; Wang, X.; Li, Z.; Shen, X.; Chen, Q.; Lu, Y.; Chen, K.; Ai, S.; Zhu, Y.; et al. Enhanced Photodynamic Therapy Synergizing with Inhibition of Tumor Neutrophil Ferroptosis Boosts Anti-PD-1 Therapy of Gastric Cancer. Adv. Sci. 2024, 11, 2307870. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Schanze, K.S. Photophysics of π-conjugated oligomers and polymers that contain transition metal complexes. J. Photochem. Photobiol. C Photochem. Rev. 2002, 3, 1–23. [Google Scholar] [CrossRef]
- Wang, B.; Queenan, B.N.; Wang, S.; Nilsson, K.P.R.; Bazan, G.C. Precisely Defined Conjugated Oligoelectrolytes for Biosensing and Therapeutics. Adv. Mater. 2019, 31, 1806701. [Google Scholar] [CrossRef]
- Zhao, Q.; Qing, G.; Yu, J.; Liu, Y.; Shen, J.; Luo, Y.; Zan, X.; Li, S. De novo design of highly efficient type-I photosensitizer based on π-conjugated oligomer for photodynamic eradication of multidrug-resistant bacterial infections. Chin. Chem. Lett. 2024, 35, 108535. [Google Scholar] [CrossRef]
- Zhao, L.; Chang, M.; He, Z.; Zhao, Y.; Wang, J.; Lu, Y. Photoactive Oligomer with an Acceptor–Donor–Acceptor-Conjugated Structure for Single Near-Infrared Light-Triggered Photothermal/Photodynamic Synergistic Therapy of Tumors. ACS Appl. Polym. Mater. 2023, 5, 1530–1538. [Google Scholar] [CrossRef]
- Qiu, Z.; Hammer, B.A.G.; Müllen, K. Conjugated polymers–Problems and promises. Prog. Polym. Sci. 2020, 100, 101179. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, C.; Serna, J.A.; Biedermann, F.; Levkin, P.A. Binding affinity-based intracellular drug detection enabled by a unimolecular cucurbit [7]uril-dye conjugate. RSC Chem. Biol. 2023, 4, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Liu, B. Conjugated polymers for visible-light-driven photocatalysis. Energy Environ. Sci. 2020, 13, 24–52. [Google Scholar] [CrossRef]
- Zhao, B.; Liang, Z.; Zhang, Y.; Sui, Y.; Shi, Y.; Zhang, X.; Li, M.; Deng, Y.; Geng, Y. Direct Arylation Polycondensation toward Water/Alcohol-Soluble Conjugated Polymers: Influence of Side Chain Functional Groups. ACS Macro Lett. 2021, 10, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, H.; Miyashita, T.; Tamura, K.; Akagi, K. Helically π-Stacked Conjugated Polymers Bearing Photoresponsive and Chiral Moieties in Side Chains: Reversible Photoisomerization-Enforced Switching Between Emission and Quenching of Circularly Polarized Fluorescence. Adv. Funct. Mater. 2010, 20, 1243–1250. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Z.; Shen, X.; Zhu, L.; Wang, D.; Chen, Y.; Zhou, M. A Multiple-Responsive Fluorescence Probe Based on Water-Soluble Fluorescent Conjugated Polymer for Biomolecules Detection. ACS Appl. Polym. Mater. 2023, 5, 2877–2886. [Google Scholar] [CrossRef]
- Yuan, H.; Li, Z.; Zhao, Q.; Jia, S.; Wang, T.; Xu, L.; Yuan, H.; Li, S. Molecular Evolution of Acceptor-Donor-Acceptor-type Conjugated Oligomer Nanoparticles for Efficient Photothermal Antimicrobial Therapy. Adv. Funct. Mater. 2023, 33, 2213209. [Google Scholar] [CrossRef]
- Zhou, C.; Li, Z.; Zhu, Z.; Chia, G.W.N.; Mikhailovsky, A.; Vázquez, R.J.; Chan, S.J.W.; Li, K.; Liu, B.; Bazan, G.C. Conjugated Oligoelectrolytes for Long-Term Tumor Tracking with Incremental NIR-II Emission. Adv. Mater. 2022, 34, 2201989. [Google Scholar] [CrossRef]
- Wei, J.; Liu, Y.; Yu, J.; Chen, L.; Luo, M.; Yang, L.; Li, P.; Li, S.; Zhang, X.-H. Conjugated Polymers: Optical Toolbox for Bioimaging and Cancer Therapy. Small 2021, 17, 2103127. [Google Scholar] [CrossRef]
- Lv, F.; Qiu, T.; Liu, L.; Ying, J.; Wang, S. Recent Advances in Conjugated Polymer Materials for Disease Diagnosis. Small 2016, 12, 696–705. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Q.; Groleau, R.R.; Wu, L.; Mao, Y.; Che, F.; Kotova, O.; Scanlan, E.M.; Lewis, S.E.; Li, P.; et al. Fluorescent Probes for Disease Diagnosis. Chem. Rev. 2024, 124, 7106–7164. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Liu, Y.; Zhang, X.; Hong, G.; Zheng, J.; Cui, J.; Peng, X.; Song, F. Constructing a Local Hydrophobic Cage in Dye-Doped Fluorescent Silica Nanoparticles to Enhance the Photophysical Properties. ACS Cent. Sci. 2020, 6, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ni, W.; Shi, X.; Bian, Y.; Li, H.; Liu, M.; Chen, W.; Zhang, M.; Jiang, S.; Cheng, M.; et al. A Supramolecular Fluorescent Sensor Array Composed of Conjugated Fluorophores and Cucurbit [7]uril for Bacterial Recognition. Anal. Chem. 2024, 96, 14490–14498. [Google Scholar] [CrossRef]
- Zhou, C.; Ho, J.C.S.; Chia, G.W.N.; Moreland, A.S.; Ruan, L.; Liedberg, B.; Kjelleberg, S.; Hinks, J.; Bazan, G.C. Gram-Typing Using Conjugated Oligoelectrolytes. Adv. Funct. Mater. 2020, 30, 2004068. [Google Scholar] [CrossRef]
- Liu, J.; Xiong, Y.; Gao, Y.; Xu, X.; Chen, K.; Shen, Q.; Huang, W.; Fan, Q.; Wang, Q. Molecular Oligomerization and Donor Engineering Strategies for Achieving Superior NIR-II Fluorescence Imaging and Thermotherapy under 1064 nm Laser Irradiation. Small 2023, 19, 2205640. [Google Scholar] [CrossRef]
- Cai, X.; Liu, X.; Liao, L.-D.; Bandla, A.; Ling, J.M.; Liu, Y.-H.; Thakor, N.; Bazan, G.C.; Liu, B. Encapsulated Conjugated Oligomer Nanoparticles for Real-Time Photoacoustic Sentinel Lymph Node Imaging and Targeted Photothermal Therapy. Small 2016, 12, 4873–4880. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, S.; Yuan, Q.; Li, M.; Tang, Y. Cationic Conjugated Oligomers for Efficient and Rapid Antibacterial Photodynamic Therapy via Both Type I and Type II Pathways. Chin. J. Chem. 2024, 42, 61–66. [Google Scholar] [CrossRef]
- He, Y.; Li, N.; Yang, S.; Tan, X.; Tang, L.; Yang, Q. Near-Infrared Molecular Photosensitizer Decorated with Quaternary Ammonium for High-Efficiency Photothermal Treatment of Bacterial Infections. Chemosens. 2023, 11, 164. [Google Scholar] [CrossRef]
- Lian, G.; Zhao, W.; Ma, G.; Zhang, S.; Wu, A.; Wang, L.; Zhang, D.; Liu, W.; Jiang, J. Orthogonally conjugated phthalocyanine-porphyrin oligomer for NIR photothermal-photodynamic antibacterial treatment. Commun. Chem. 2025, 8, 80. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Li, S.; Wan, Y.; Chen, J.-X.; Tian, S.; Huang, Z.; Xiao, Y.-F.; Cui, X.; Xiang, C.; et al. Biodegradable π-Conjugated Oligomer Nanoparticles with High Photothermal Conversion Efficiency for Cancer Theranostics. ACS Nano 2019, 13, 12901–12911. [Google Scholar] [CrossRef]
- Bao, B.; Yuan, Q.; Feng, Q.; Li, L.; Li, M.; Tang, Y. Regulating Signal Pathway Triggers Circular Reactive Oxygen Species Production to Augment Oxidative Stress with Enzyme-Activated Nanoparticles. CCS Chem. 2024, 6, 693–708. [Google Scholar] [CrossRef]
- Zhou, L.; Lv, F.; Liu, L.; Shen, G.; Yan, X.; Bazan, G.C.; Wang, S. Cross-Linking of Thiolated Paclitaxel–Oligo(p-phenylene vinylene) Conjugates Aggregates inside Tumor Cells Leads to “Chemical Locks” That Increase Drug Efficacy. Adv. Mater. 2018, 30, 1704888. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Han, X.; Wang, M.; Li, C.; Jia, T.; Zhao, X. Recent advances in the development of near-infrared organic photothermal agents. Chem. Eng. J. 2021, 417, 128844. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Li, X.; Wang, H.; Yang, Y.; Luo, Y.; Wan, Y.; Lee, C.-S.; Li, S.; Zhang, X.-H. A Stable Open-Shell Conjugated Diradical Polymer with Ultra-High Photothermal Conversion Efficiency for NIR-II Photo-Immunotherapy of Metastatic Tumor. Nano-Micro Lett. 2023, 16, 21. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, Y.; Shi, X.; Wang, K.; Qu, Q.; Liang, Q.; Ma, X.; He, K.; Chi, C.; Tang, J.; et al. NIR-II light in clinical oncology: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2024, 21, 449–467. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Ren, Y.; Bao, B.; Li, M.; Zhang, M.; Wang, J.; Wang, J.; Tang, Y. Conjugated Oligomer-Based NIR-II Chemiluminescence Nanosensor for In Vivo Imaging. Adv. Funct. Mater. 2025, 2424286. [Google Scholar] [CrossRef]
- Park, J.; Choi, S.; Knieling, F.; Clingman, B.; Bohndiek, S.; Wang, L.V.; Kim, C. Clinical translation of photoacoustic imaging. Nat. Rev. Bioeng. 2025, 3, 193–212. [Google Scholar] [CrossRef]
- Menozzi, L.; Yao, J. Deep tissue photoacoustic imaging with light and sound. Npj Imaging 2024, 2, 44. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Ren, Y.; Yuan, Q.; Wang, Y.; Bao, B.; Li, M.; Tang, Y. Chemiluminescent Conjugated Polymer Nanoparticles for Deep-Tissue Inflammation Imaging and Photodynamic Therapy of Cancer. J. Am. Chem. Soc. 2024, 146, 5927–5939. [Google Scholar] [CrossRef]
- Bassler, M.C.; Hiller, J.; Wackenhut, F.; zur Oven-Krockhaus, S.; Frech, P.; Schmidt, F.; Kertzscher, C.; Rammler, T.; Ritz, R.; Braun, K.; et al. Fluorescence lifetime imaging unravels the pathway of glioma cell death upon hypericin-induced photodynamic therapy. RSC Chem. Biol. 2024, 5, 1219–1231. [Google Scholar] [CrossRef]
- McKenzie, L.K.; Flamme, M.; Felder, P.S.; Karges, J.; Bonhomme, F.; Gandioso, A.; Malosse, C.; Gasser, G.; Hollenstein, M. A ruthenium–oligonucleotide bioconjugated photosensitizing aptamer for cancer cell specific photodynamic therapy. RSC Chem. Biol. 2022, 3, 85–95. [Google Scholar] [CrossRef]
- Kolarikova, M.; Hosikova, B.; Dilenko, H.; Barton-Tomankova, K.; Valkova, L.; Bajgar, R.; Malina, L.; Kolarova, H. Photodynamic therapy: Innovative approaches for antibacterial and anticancer treatments. Med. Res. Rev. 2023, 43, 717–774. [Google Scholar] [CrossRef]
- Zhou, T.; Hu, R.; Wang, L.; Qiu, Y.; Zhang, G.; Deng, Q.; Zhang, H.; Yin, P.; Situ, B.; Zhan, C.; et al. An AIE-Active Conjugated Polymer with High ROS-Generation Ability and Biocompatibility for Efficient Photodynamic Therapy of Bacterial Infections. Angew. Chem. Int. Ed. 2020, 59, 9952–9956. [Google Scholar] [CrossRef]
- Puente, B.N.; Kimura, W.; Muralidhar, S.A.; Moon, J.; Amatruda, J.F.; Phelps, K.L.; Grinsfelder, D.; Rothermel, B.A.; Chen, R.; Garcia, J.A.; et al. The Oxygen-Rich Postnatal Environment Induces Cardiomyocyte Cell-Cycle Arrest through DNA Damage Response. Cell 2014, 157, 565–579. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef]
- Chen, D.; Xu, Q.; Wang, W.; Shao, J.; Huang, W.; Dong, X. Type I Photosensitizers Revitalizing Photodynamic Oncotherapy. Small 2021, 17, 2006742. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Feng, G.; Manghnani, P.N.; Cai, X.; Liu, J.; Wu, W.; Xu, S.; Cheng, X.; Teh, C.; Liu, B. A Porphyrin-Based Conjugated Polymer for Highly Efficient In Vitro and In Vivo Photothermal Therapy. Small 2016, 12, 6243–6254. [Google Scholar] [CrossRef] [PubMed]
- Oudjedi, F.; Kirk, A.G. Near-Infrared Nanoparticle-Mediated Photothermal Cancer Therapy: A Comprehensive Review of Advances in Monitoring and Controlling Thermal Effects for Effective Cancer Treatment. Nano Sel. 2025, 6, e202400107. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Xue, Y.; Wu, Y.; Wang, Q.; Xue, L.; Su, Z.; Zhang, C. Transforming Weakness into Strength: Photothermal-Therapy-Induced Inflammation Enhanced Cytopharmaceutical Chemotherapy as a Combination Anticancer Treatment. Adv. Mater. 2019, 31, 1805936. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yong, T.; Wei, Z.; Bie, N.; Zhang, X.; Zhan, G.; Li, J.; Qin, J.; Yu, J.; Zhang, B.; et al. Reversing insufficient photothermal therapy-induced tumor relapse and metastasis by regulating cancer-associated fibroblasts. Nat. Commun. 2022, 13, 2794. [Google Scholar] [CrossRef]
- Shao, W.; Yang, C.; Li, F.; Wu, J.; Wang, N.; Ding, Q.; Gao, J.; Ling, D. Molecular Design of Conjugated Small Molecule Nanoparticles for Synergistically Enhanced PTT/PDT. Nano-Micro Lett. 2020, 12, 147. [Google Scholar] [CrossRef]
- Sultana, N.; Pathak, R.; Samanta, S.; Sen Sarma, N. A comprehensive analysis of photothermal therapy (PTT) and photodynamic therapy (PDT) for the treatment of cancer. Process Biochem. 2025, 148, 17–31. [Google Scholar] [CrossRef]
- He, L.; Chen, T.; You, Y.; Hu, H.; Zheng, W.; Kwong, W.-L.; Zou, T.; Che, C.-M. A Cancer-Targeted Nanosystem for Delivery of Gold(III) Complexes: Enhanced Selectivity and Apoptosis-Inducing Efficacy of a Gold(III) Porphyrin Complex. Angew. Chem. Int. Ed. 2014, 53, 12532–12536. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.-L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef]
- Wu, D.; Duan, X.; Guan, Q.; Liu, J.; Yang, X.; Zhang, F.; Huang, P.; Shen, J.; Shuai, X.; Cao, Z. Mesoporous Polydopamine Carrying Manganese Carbonyl Responds to Tumor Microenvironment for Multimodal Imaging-Guided Cancer Therapy. Adv. Funct. Mater. 2019, 29, 1900095. [Google Scholar] [CrossRef]
- Wang, S.; Yu, G.; Wang, Z.; Jacobson, O.; Tian, R.; Lin, L.-S.; Zhang, F.; Wang, J.; Chen, X. Hierarchical Tumor Microenvironment-Responsive Nanomedicine for Programmed Delivery of Chemotherapeutics. Adv. Mater. 2018, 30, 1803926. [Google Scholar] [CrossRef]
- Wang, T.; Wang, D.; Yu, H.; Wang, M.; Liu, J.; Feng, B.; Zhou, F.; Yin, Q.; Zhang, Z.; Huang, Y.; et al. Intracellularly Acid-Switchable Multifunctional Micelles for Combinational Photo/Chemotherapy of the Drug-Resistant Tumor. ACS Nano 2016, 10, 3496–3508. [Google Scholar] [CrossRef] [PubMed]
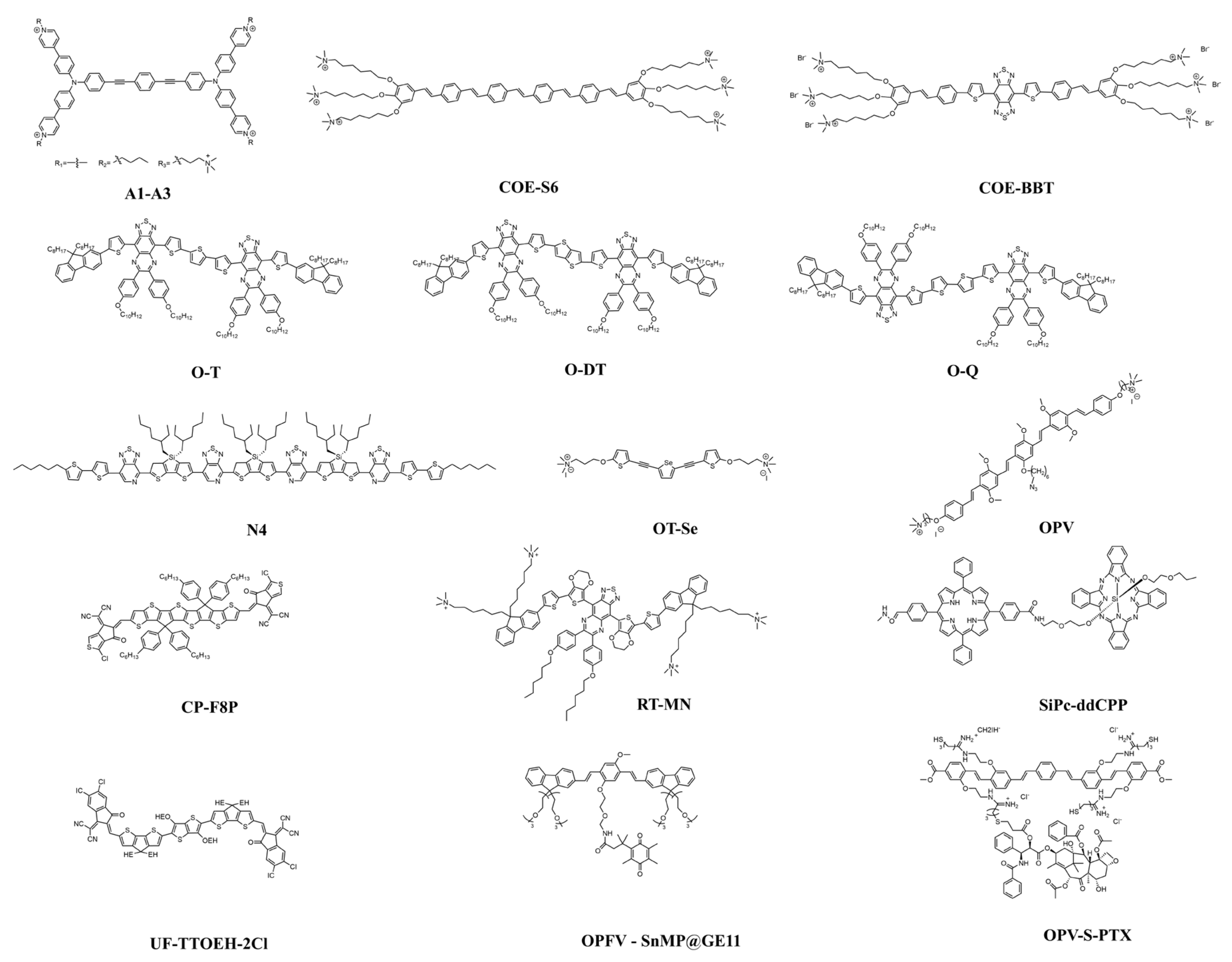
| Material | Laser | Type | In Vivo | In Vitro | Ref. |
|---|---|---|---|---|---|
| OT-Se | White light, 30 mW/cm2 | PDT in hypoxic environments | Trauma surface injection, BALB/c mice | CREC/MRSA | [11] |
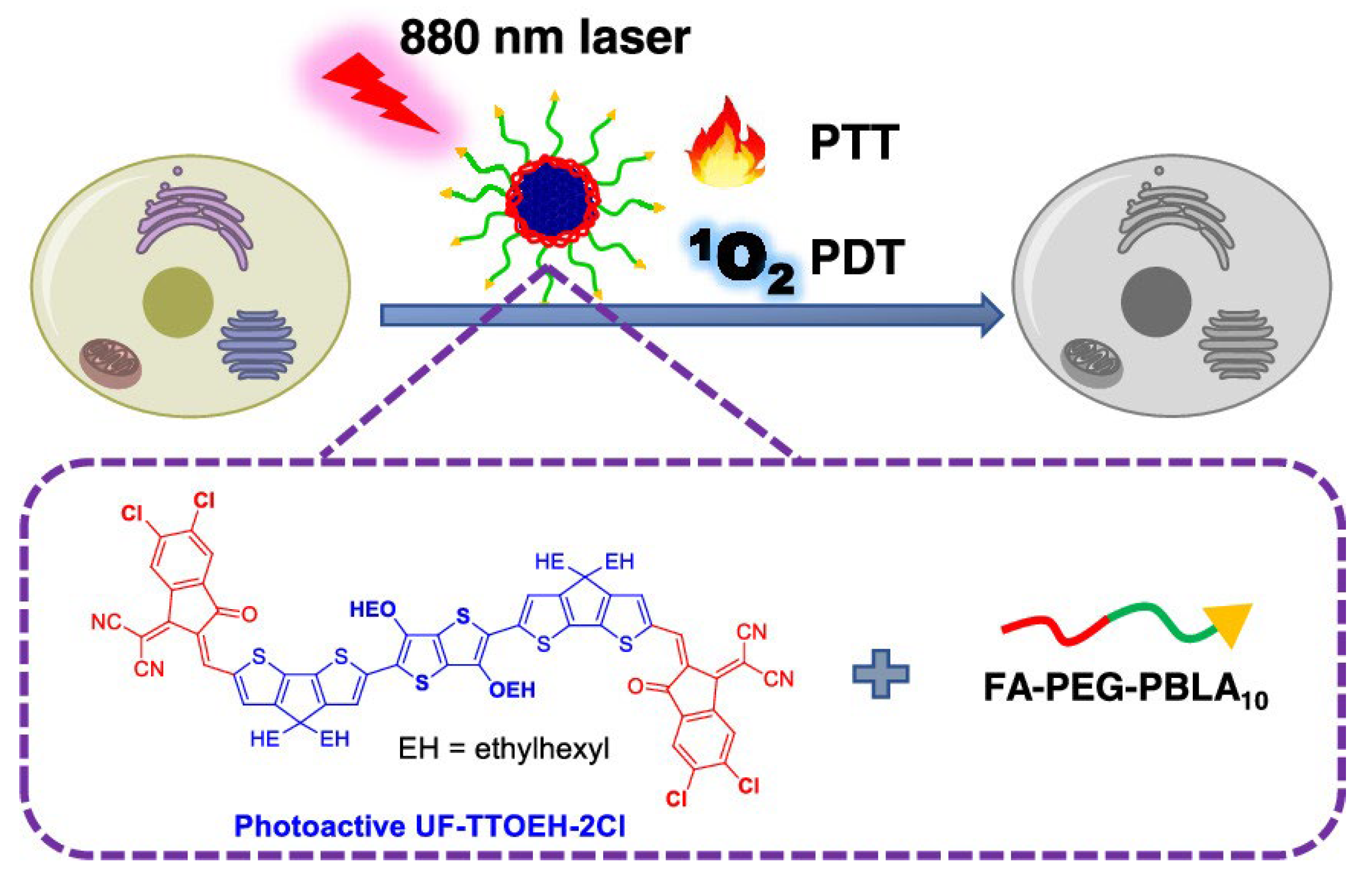
| UF-TTOEH-2Cl | NIR (880 nm), 0.8 W/cm2 | PDT/PTT combination therapy | - | HeLa cells/SiHa cells | [12] |
| CP-F8P NPs | 808 nm, 1 W/cm2 | Kill bacteria by PTT | Trauma surface injection, diabetic mice with wounds | E. coli/S. aureus/C. albicans/MRSA | [19] |
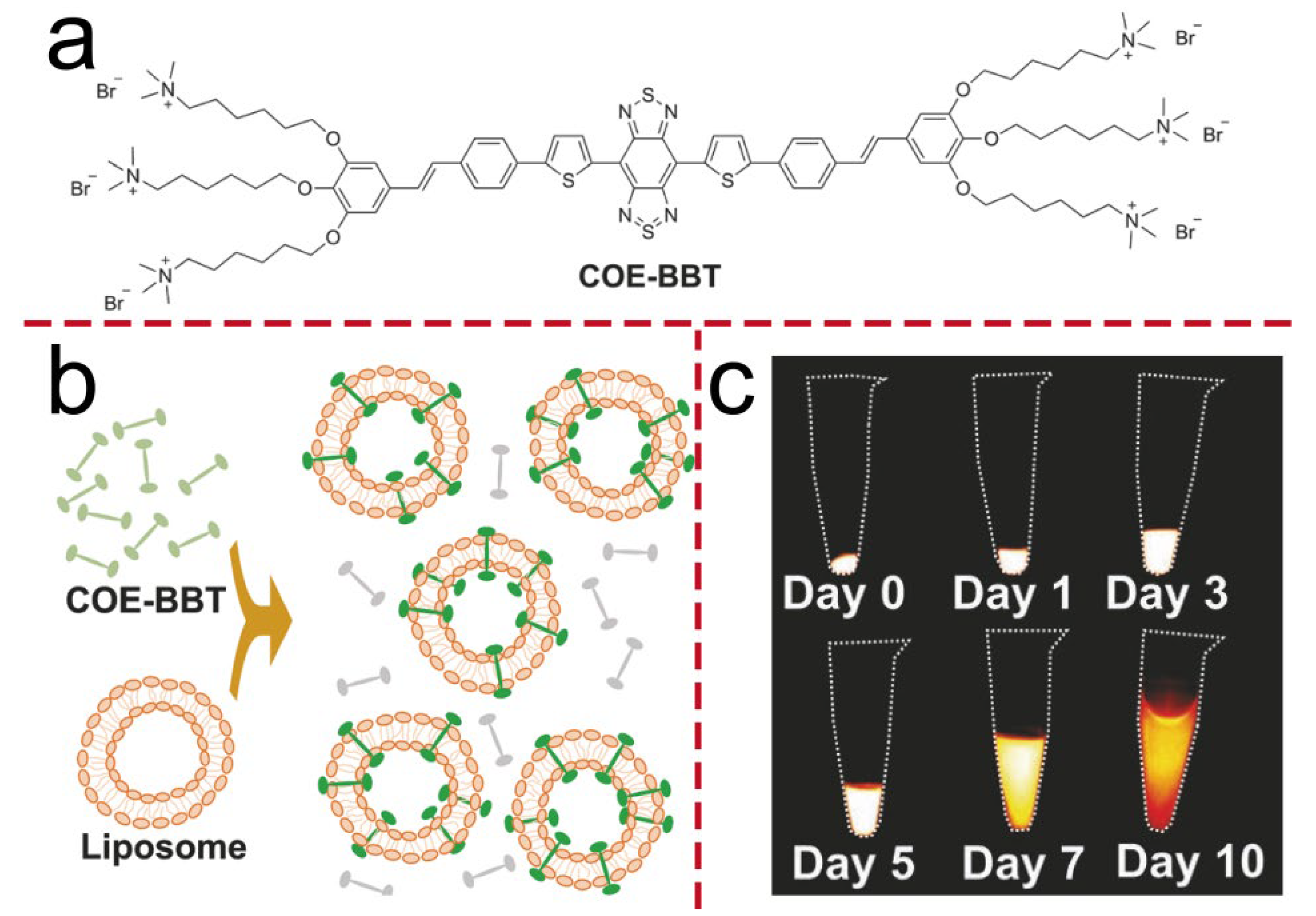
| COE-BBT | NIR-II region (1000–1700 nm) | Fluorescence detection | An orthotopic glioma model, BALB/c mice | A549 cells/C6-Luc glioma cells | [20] |
| Supramolecular Fluorescent Sensor Array | 431 nm | Fluorescence detection | - | 10 distinct bacterial strains | [25] |
| COE-S6 | 405 nm | Fluorescence detection | - | E. faecalis OG1RF/E. coli UTI89 | [26] |
| O-T, O-DT and O-Q | 1064 nm, 1 W/cm2 | NIR-II fluorescence detection | Xenograft, tumor-bearing mice | Hela cells | [27] |
| N4 NPs | 600–900 nm (808 nm, 0.8 W/cm2) | Photoacoustic detection | Injection of the left forepaw pad, Wistar rat | MDA-MB-231 breast cancer cells/NIH-3T3 mice cells | [28] |
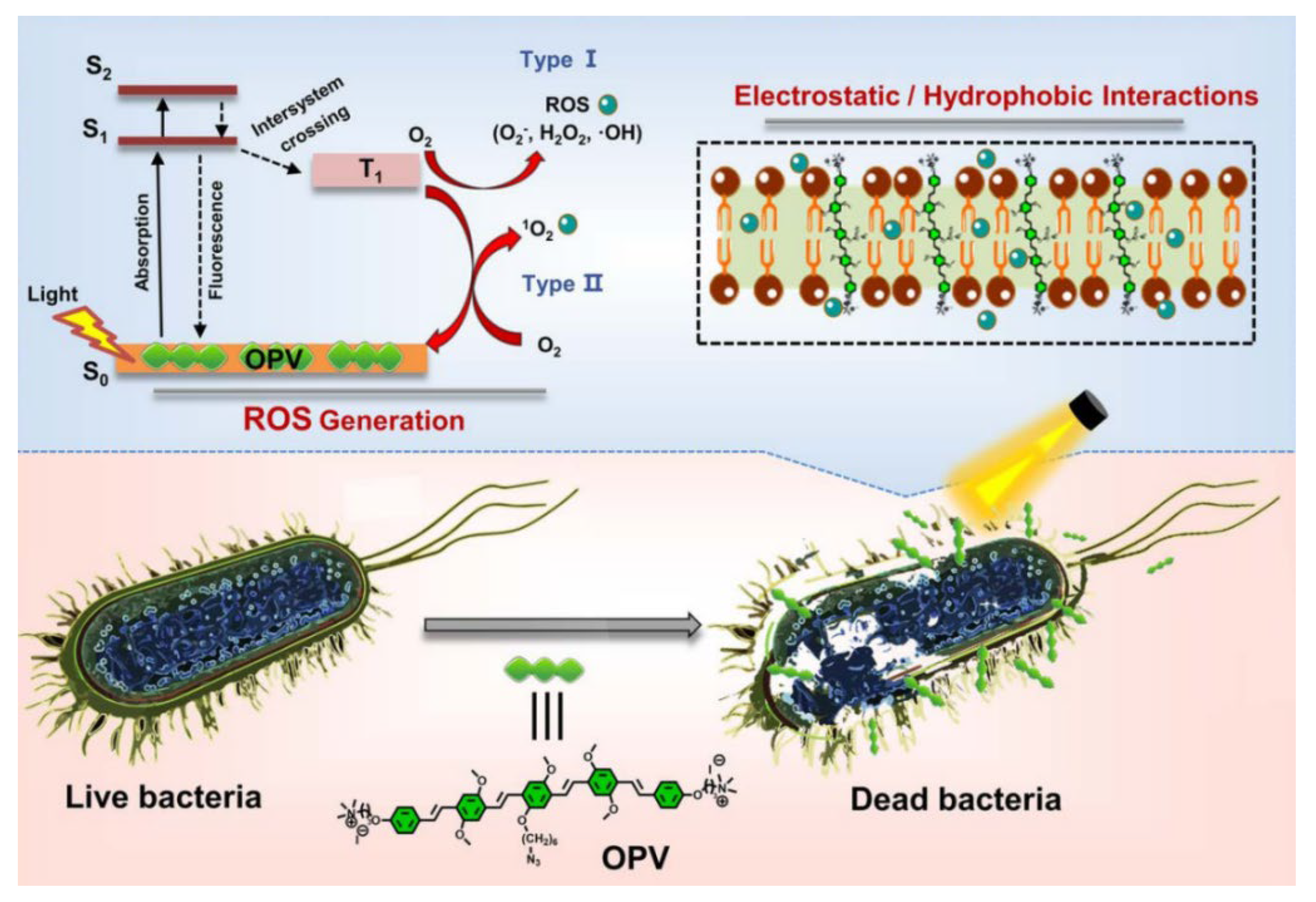
| OPV | 422 nm, 90 mW/cm2 | PDT | - | E. coli/MRSA | [29] |
| RT-MN | 808 nm, 1 W/cm2 | Highly efficient PTT of bacterial infections | Artificial trauma and injection of bacteria, BALB/c mice | E. coli/MRSA | [30] |
| SiPc-ddCPP | NIR (808 nm), 3.5 W/cm2 | PDT/PTT combination therapy | - | S. Aureus/E. coli | [31] |
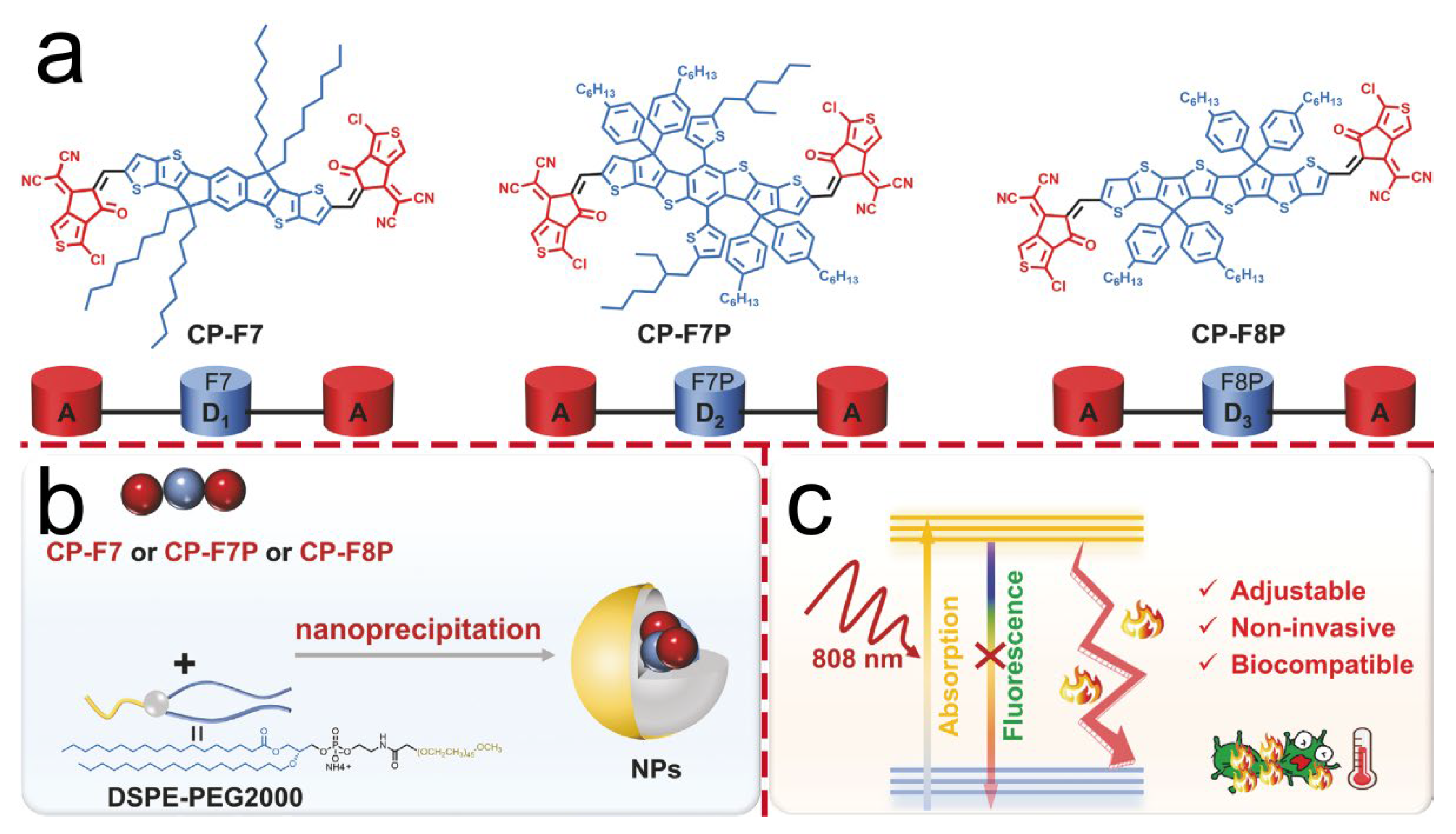
| F8-PEG NPs | NIR (808 nm), 1 W/cm2 | PTT | 4T1 tumor xenograft | A549/4T1/HeLa cells | [32] |
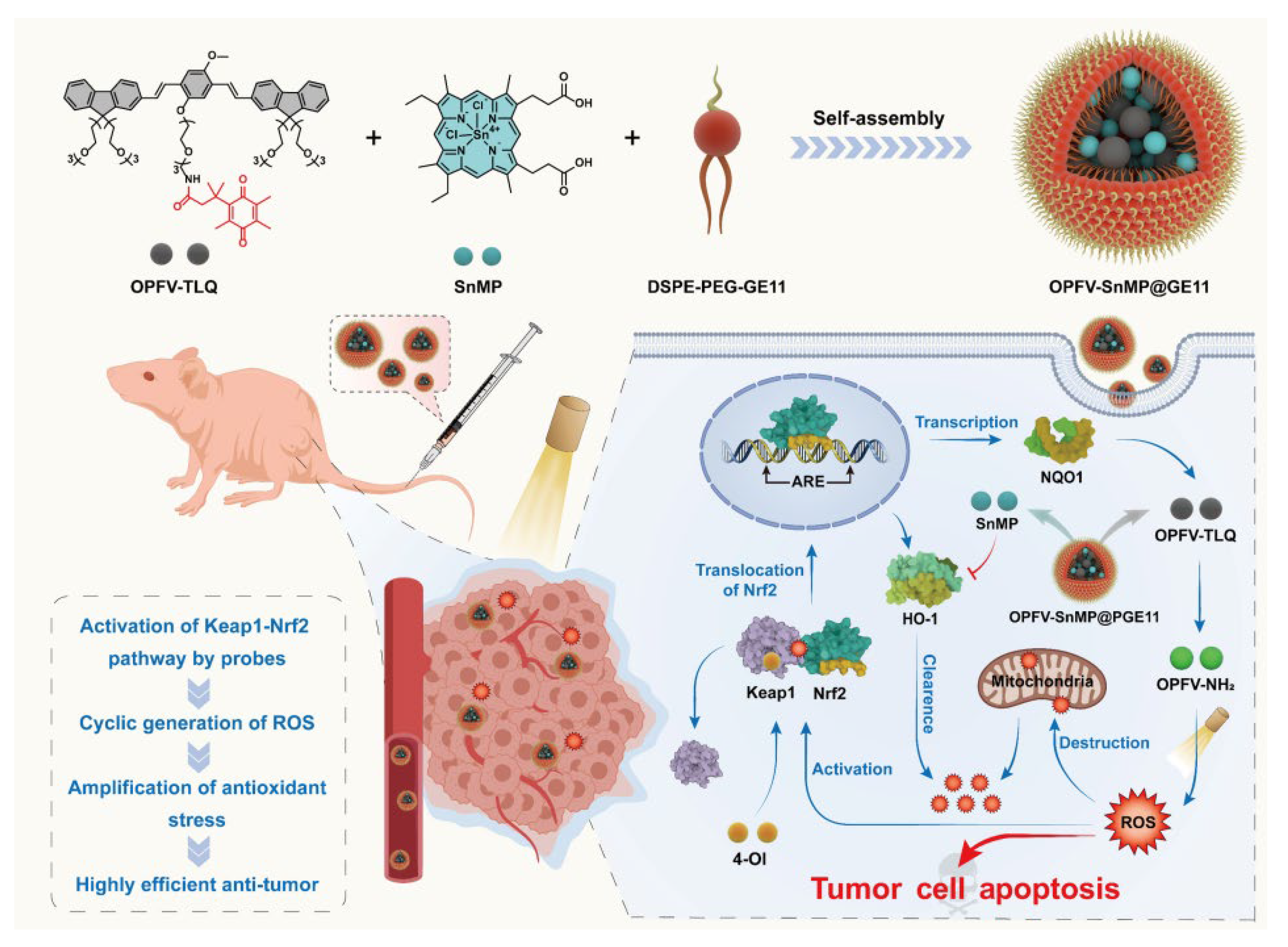
| OPFV-SnMP@GE11 | 418 nm, 25 mW/cm2 | PDT/signaling pathway modulation | Subcutaneous cell injection | A431 cells | [33] |
| OPV-S-PTX | 418 nm | Synergistic PDT-chemotherapy therapy | Xenograft, BALB/c mice | A549/T cells and MCF-7m cells | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Ma, M.; Ding, Y.; Luo, L.; Zuo, Y.; Liu, L. Design and Application of Conjugated Oligomers for Imaging and Anticancer and Antibacterial Treatment. Molecules 2025, 30, 3719. https://doi.org/10.3390/molecules30183719
Wang J, Ma M, Ding Y, Luo L, Zuo Y, Liu L. Design and Application of Conjugated Oligomers for Imaging and Anticancer and Antibacterial Treatment. Molecules. 2025; 30(18):3719. https://doi.org/10.3390/molecules30183719
Chicago/Turabian StyleWang, Jianing, Min Ma, Yuxuan Ding, Lili Luo, Yilin Zuo, and Libing Liu. 2025. "Design and Application of Conjugated Oligomers for Imaging and Anticancer and Antibacterial Treatment" Molecules 30, no. 18: 3719. https://doi.org/10.3390/molecules30183719
APA StyleWang, J., Ma, M., Ding, Y., Luo, L., Zuo, Y., & Liu, L. (2025). Design and Application of Conjugated Oligomers for Imaging and Anticancer and Antibacterial Treatment. Molecules, 30(18), 3719. https://doi.org/10.3390/molecules30183719






