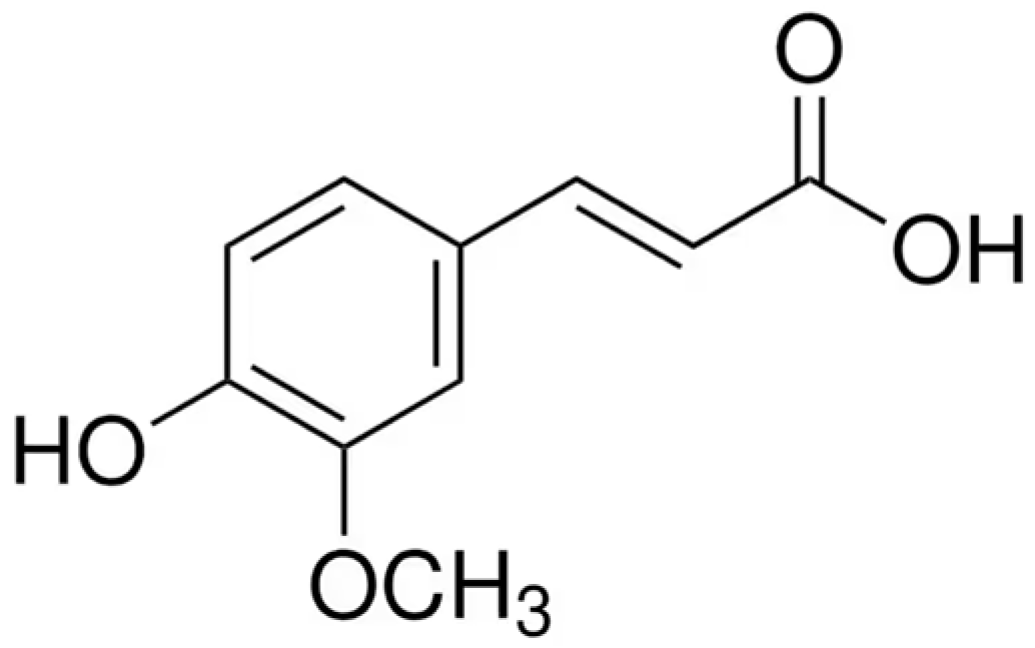
Ferulic Acid: Mechanistic Insights and Multifaceted Applications in Metabolic Syndrome, Food Preservation, and Cosmetics
Abstract
1. Introduction
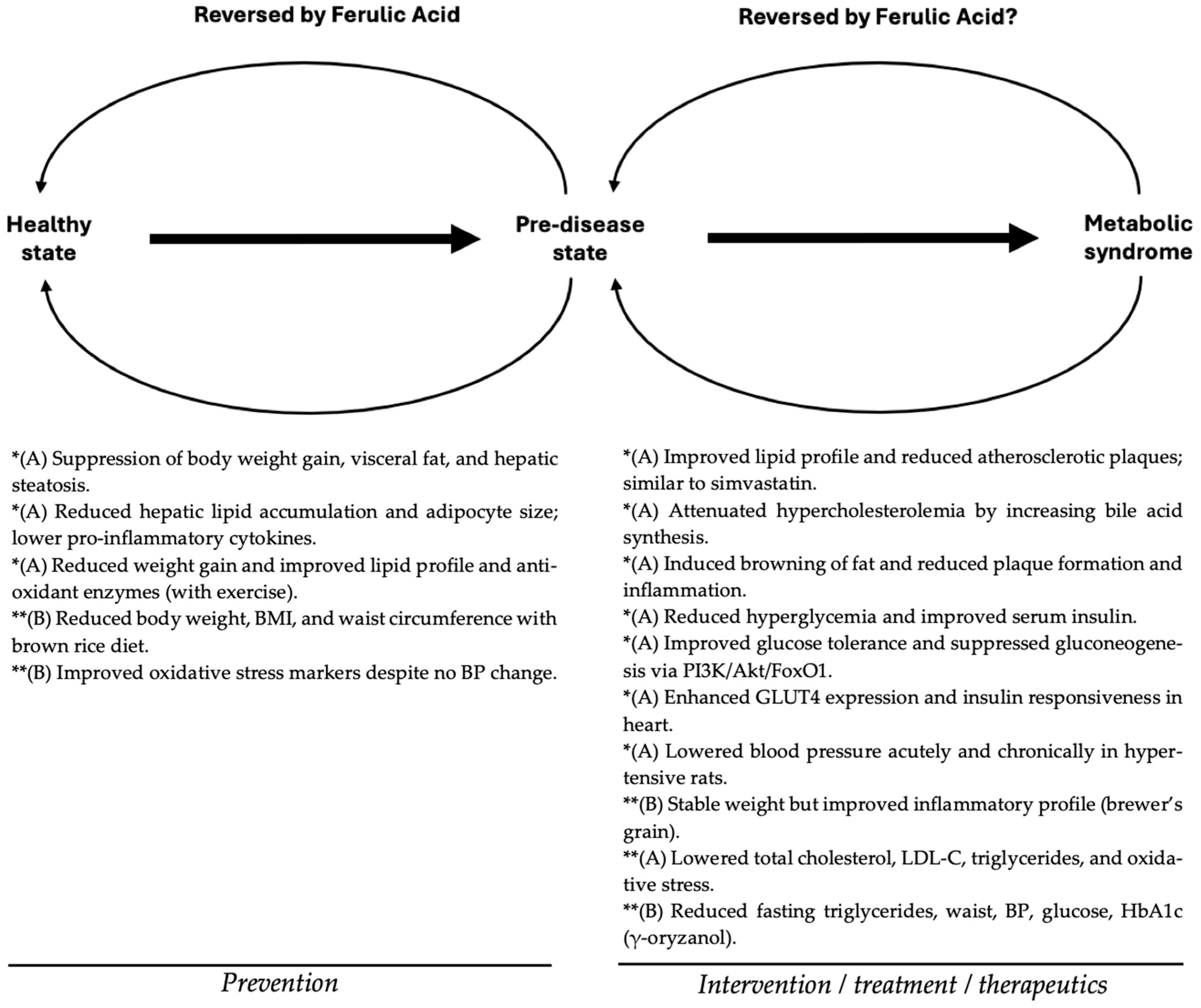
2. Ferulic Acid as a Nutraceutical for Metabolic Syndrome
2.1. Anti-Obesity Effects
2.1.1. Evidence from Animal Studies
2.1.2. Evidence from Human Studies
2.2. Anti-Dyslipidemic and Cardioprotective Effects
2.2.1. Evidence from Animal Studies
2.2.2. Evidence from Human Studies
2.3. Anti-Diabetic and Glycemic Control Effects
2.3.1. Evidence from Animal Studies
2.3.2. Evidence from Human Studies
2.4. Antihypertensive and Vascular Effects
2.4.1. Evidence from Animal Studies
2.4.2. Evidence from Human Studies
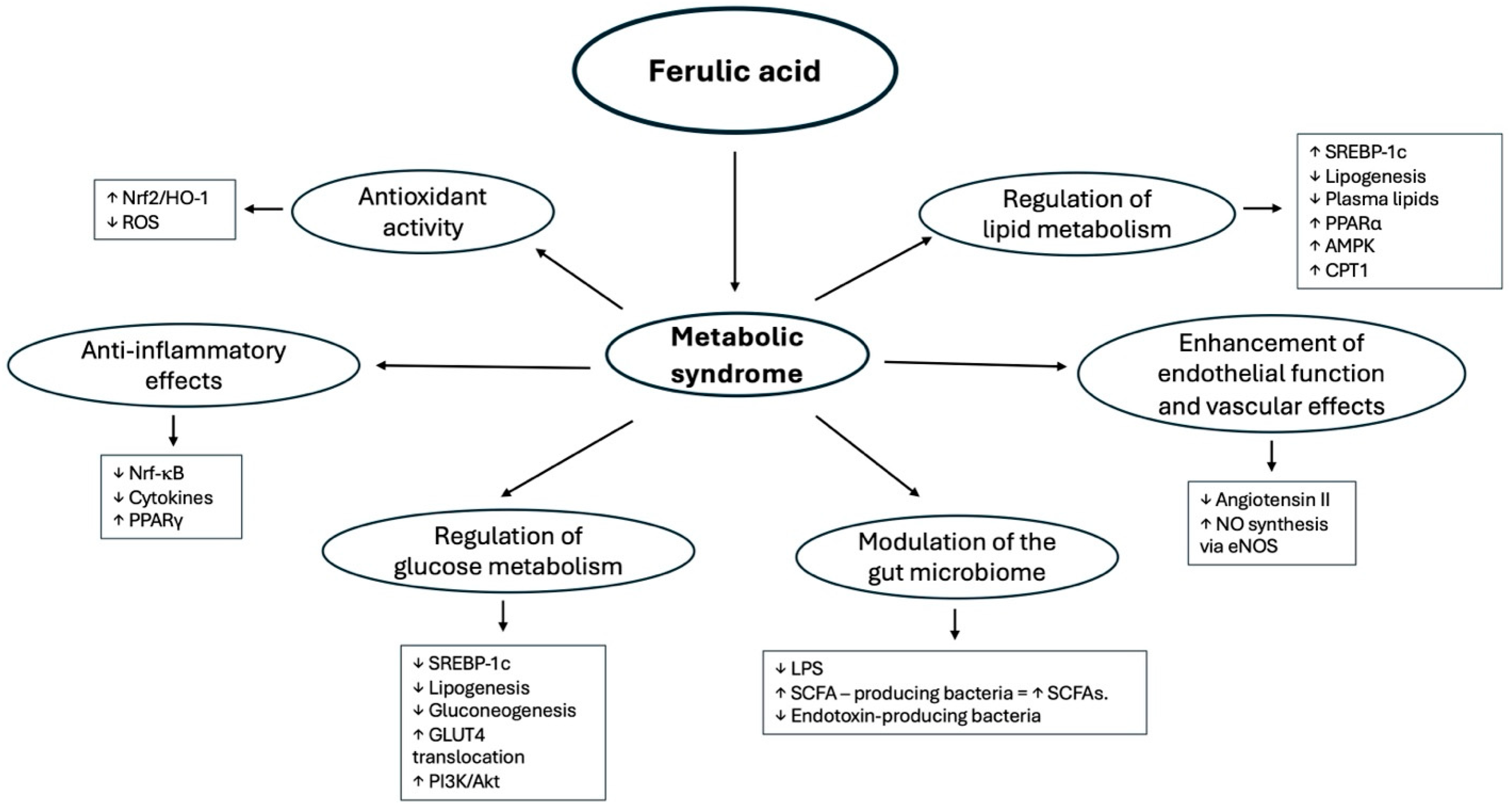
3. Mechanisms of Action of Ferulic Acid in Metabolic Syndrome
3.1. Antioxidant Activity
3.2. Anti-Inflammatory Effects
3.3. Regulation of Glucose Metabolism
3.4. Regulation of Lipid Metabolism
3.5. Enhancement of Endothelial Function and Vascular Effects
3.6. Modulation of the Gut Microbiome
4. Ferulic Acid as a Food Additive: Antimicrobial, Antioxidant, and Prebiotic Properties
4.1. Antimicrobial Activity
4.2. Antioxidant Properties
4.3. Stabilization of Bioactives
4.4. Prebiotic Potential
5. Ferulic Acid in Cosmetic Applications
5.1. Photoprotection
5.2. Skin Repair and Anti-Inflammatory Effects
5.3. Skin Brightening
5.4. Anti-Aging and Anti-Photodamage
5.5. Formulation and Delivery Considerations
5.5.1. Optimal pH and Solvents
5.5.2. Encapsulation Technologies
5.5.3. Derivative Forms
6. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Razzaghi-Asl, N.; Garrido, J.L.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant properties of hydroxycinnamic acids: A review of structure–activity relationships. Curr. Med. Chem. 2013, 20, 4436–4450. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Taméz, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 370438. [Google Scholar] [CrossRef] [PubMed]
- Kroon, P.A.; Faulds, C.B.; Ryden, P.; Robertson, J.A.; Williamson, G. Release of covalently bound ferulic acid from fiber in the human colon. J. Agric. Food Chem. 1997, 45, 661–667. [Google Scholar] [CrossRef]
- Turner, A.L.; Shewry, P.R.; Lovegrove, A.; Spencer, J.P. Release of covalently bound hydroxycinnamate, ferulic acid, from whole-grain. Proc. Nutr. Soc. 2015, 74, OCE1. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; Xu, F.; Chu, C.; Li, X.; Shi, X.; Zheng, W.; Wang, Z.; Jia, Y.; Xiao, W. Use of ferulic acid in the management of diabetes mellitus and its complications. Molecules 2022, 27, 6010. [Google Scholar] [CrossRef]
- Li, Y.; Sair, A.T.; Zhao, W.; Li, T.; Liu, R.H. Ferulic acid mediates metabolic syndrome via regulation of hepatic glucose and lipid metabolism and the insulin/IGF-1 receptor/PI3K/Akt pathway in palmitate-treated HepG2 cells. J. Agric. Food Chem. 2022, 70, 14706–14717. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, W.; Sair, A.T.; Li, T.; Liu, R.H. Ferulic acid restores mitochondrial dynamics and autophagy via AMPK signaling in a palmitate-induced hepatocyte model of metabolic syndrome. Sci. Rep. 2024, 14, 18970. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.; Yadav, A.; Gangwar, P.; Kaur, S.; Kumar, P.; Dhiman, M.; Mantha, A.K. A cross-sectional in vitro study on the synergistic neuroprotective effects of ferulic acid and ginkgolide B against amyloid beta-induced oxidative stress and modulation of multifunctional enzyme APE1/Ref-1 in neuroblastoma SH-SY5Y cells. Cell Biochem. Biophys. 2025. [Google Scholar] [CrossRef]
- Pyrzyńska, K. Ferulic acid—A brief review of its extraction, bioavailability and biological activity. Separations 2024, 11, 204. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef]
- Pangestu, N.P.; Miyagusuku-Cruzado, G.; Giusti, M.M. Copigmentation with chlorogenic and ferulic acids affected color and anthocyanin stability in model beverages colored with Sambucus peruviana, Sambucus nigra, and Daucus carota during storage. Foods 2020, 9, 1476. [Google Scholar] [CrossRef]
- Stompor, M.; Machaczka, M. Recent advances in biological activity, new formulations and prodrugs of ferulic acid. Int. J. Mol. Sci. 2021, 22, 12889. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pan, Y.; Zhou, H.; Wang, L.; Chen, X.; Song, G.; Liu, J.; Li, A. Ferulic acid suppresses obesity and obesity-related metabolic syndromes in high-fat diet–induced obese C57BL/6J mice. Food Agric. Immunol. 2018, 29, 1116–1125. [Google Scholar] [CrossRef]
- De Melo, T.S.; Lima, P.R.; Carvalho, K.M.; Fontenele, T.M.; Solon, F.R.; Tomé, A.R.; De Lemos, T.L.; da Cruz Fonseca, S.G.; Santos, F.A.; Rao, V.S.; et al. Ferulic acid lowers body weight and visceral fat accumulation via modulation of enzymatic, hormonal and inflammatory changes in a mouse model of high-fat diet-induced obesity. Braz. J. Med. Biol. Res. 2017, 50, e5630. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Y.; Li, M.; Huang, Z.; Jiang, J.; Chen, Y.; Zhou, F. Ferulic acid ameliorates atherosclerotic injury by modulating gut microbiota and lipid metabolism. Front. Pharmacol. 2021, 12, 621339. [Google Scholar] [CrossRef]
- Luo, Z.; Li, M.; Yang, J.; Li, J.; Zhang, Y.; Liu, F.; El-Omar, E.; Han, L.; Bian, J.; Gong, L.; et al. Ferulic acid attenuates high-fat diet-induced hypercholesterolemia by activating classic bile acid synthesis pathway. Front. Nutr. 2022, 9, 976638. [Google Scholar] [CrossRef]
- Hong, K.; Wang, J.; Kang, X.; Xue, H.; Gao, Y.; Liang, H.; Huang, W.; Zhan, J.; You, Y. Ferulic acid and protocatechuic acid alleviate atherosclerosis by promoting UCP1 expression to inhibit the NLRP3–IL-1β signaling pathway. Food Funct. 2025, 16, 40–53. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Olofinsan, K.O.; Bharuth, V.; Ijomone, O.M.; Islam, M.S. Ferulic acid improves glucose homeostasis by modulation of key diabetogenic activities and restoration of pancreatic architecture in diabetic rats. Fundam. Clin. Pharmacol. 2023, 37, 324–339. [Google Scholar] [CrossRef] [PubMed]
- Kinyua, A.W.; Ko, C.M.; Doan, K.V.; Yang, D.J.; Huynh, M.K.Q.; Moh, S.H.; Choi, Y.H.; Kim, K.W. 4-Hydroxy-3-methoxycinnamic acid regulates orexigenic peptides and hepatic glucose homeostasis through phosphorylation of FoxO1. Exp. Mol. Med. 2018, 50, e437. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Ghosh, S.; Rashid, K.; Sil, P.C. Deciphering the role of ferulic acid against streptozotocin-induced cellular stress in the cardiac tissue of diabetic rats. Food Chem. Toxicol. 2016, 97, 187–188. [Google Scholar] [CrossRef]
- Suzuki, A.; Kagawa, D.; Fujii, A.; Ochiai, R.; Tokimitsu, I.; Saito, I. Short- and long-term effects of ferulic acid on blood pressure in spontaneously hypertensive rats. Am. J. Hypertens. 2002, 15, 351–357. [Google Scholar] [CrossRef]
- Wang, O.; Zhang, N.; Han, C.; Huang, J. Regular exercise combined with ferulic acid exhibits anti-obesity effect and regulates metabolic profiles in high-fat diet–induced mice. Front. Nutr. 2022, 9, 957321. [Google Scholar] [CrossRef]
- Ye, L.; Hu, P.; Feng, L.P.; Huang, L.L.; Wang, Y.; Yan, X.; Xiong, J.; Xia, H.L. Protective effects of ferulic acid on metabolic syndrome: A comprehensive review. Molecules 2023, 28, 281. [Google Scholar] [CrossRef] [PubMed]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Komindr, S.; Chitchumroonchokchai, C.; Failla, M.L.; Surasiang, R. Ferulic acid supplementation improves lipid profiles, oxidative stress, and inflammatory status in hyperlipidemic subjects: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef] [PubMed]
- Nikooyeh, B.; Zargaraan, A.; Ebrahimof, S.; Kalayi, A.; Zahedirad, M.; Yazdani, H.; Rismanchi, M.; Karami, T.; Khazraei, M.; Jafarpour, A.; et al. Daily consumption of γ-oryzanol-fortified canola oil resulted in better improvement of cardiometabolic biomarkers in adults with type 2 diabetes: Randomized controlled trial. Eur. J. Med. Res. 2023, 28, 1409. [Google Scholar] [CrossRef] [PubMed]
- Costabile, G.; Vitale, M.; Della Pepa, G.; Cipriano, P.; Vetrani, C.; Testa, R.; Mena, P.; Bresciani, L.; Tassotti, M.; Calani, L.; et al. A wheat aleurone-rich diet improves oxidative stress but does not influence glucose metabolism in overweight/obese individuals: Randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 715–726. [Google Scholar] [CrossRef]
- Halter, B.; Ildari, N.; Cline, M.A.; Gilbert, E.R. Ferulic acid, a phytochemical with transient anorexigenic effects in birds. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 259, 111015. [Google Scholar] [CrossRef]
- Tian, B.; Geng, Y.; Wang, P.; Cai, M.; Neng, J.; Hu, J.; Xia, D.; Cao, W.; Yang, K.; Sun, P. Ferulic acid improves intestinal barrier function through altering gut microbiota composition in high-fat diet–induced mice. Eur. J. Nutr. 2022, 61, 3767–3783. [Google Scholar] [CrossRef]
- Gao, J.; Gu, X.; Zhang, M.; Zu, X.; Shen, F.; Hou, X.; Hao, E.; Bai, G. Ferulic acid targets ACSL1 to ameliorate lipid metabolic disorders in db/db mice. J. Funct. Foods 2022, 91, 105009. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, J.; Zhao, L.; Wang, O.; Zhang, L.; Zhou, F. Dietary ferulic acid ameliorates metabolic syndrome–associated hyperuricemia in rats via regulating uric acid synthesis, glycolipid metabolism, and hepatic injury. Front. Nutr. 2022, 9, 946556. [Google Scholar] [CrossRef]
- Wu, X.; Pan, X.; Kang, J.; Huang, Y.; Ren, J.; Pan, J.; Yu, K.; Li, Y. Ferulic acid inhibits ox-LDL-induced ferroptosis and apoptosis in RAW 264.7 cells via the HIF-1 signaling pathway. Front. Pharmacol. 2025, 16, 1524736. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Zhao, D.-S.; Wang, J.; Zhou, H.; Wang, L.; Mao, J.-L.; He, J.-X. The treatment of cardiovascular diseases: A review of ferulic acid and its derivatives. Pharmazie 2021, 76, 55–60. [Google Scholar] [CrossRef]
- Giampieri, F.; Forbes-Hernández, T.Y.; Quiles, J.L.; Battino, M.; La Vignera, S.; Galvano, F. The effect of dietary polyphenols on vascular health and hypertension: Current evidence and mechanisms of action. Nutrients 2022, 14, 545. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Osorio, C.; Schreckinger, E.; Bhargava, P.; Bang, W.Y.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Golden berry and selected tropical (açaí, acerola and maqui) juices. In Handbook of Functional Beverages and Human Health; Shahidi, F., Alasalvar, C., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 251–269. [Google Scholar]
- Jacobo-Velázquez, D.A.; Ortega-Hernández, E.; Cisneros-Zevallos, L. Vegetable-containing juices (carrot, kale and sprout). In Handbook of Functional Beverages and Human Health; Shahidi, F., Alasalvar, C., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 609–626. [Google Scholar]
- Alam, M.A. Anti-hypertensive effect of cereal antioxidant ferulic acid and its mechanism of action. Front. Nutr. 2019, 6, 121. [Google Scholar] [CrossRef]
- Ijabadeniyi, O.A.; Govender, A.; Olagunju, O.F.; Oyedeji, A.B. The antimicrobial activity of two phenolic acids against foodborne Escherichia coli and Listeria monocytogenes and their effectiveness in a meat system. Ital. J. Food Sci. 2021, 33, 39–45. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, T.; Zhao, D.; Gao, S.; Liu, Y.; Yang, X.; Lu, H.; Gao, X. The combined antibacterial mechanism of ferulic acid and ε-polylysine hydrochloride in Shewanella putrefaciens and the effect of their application on refrigerated crayfish. Foods 2025, 14, 1942. [Google Scholar] [CrossRef] [PubMed]
- Abadias, M.; Bobo, G.; Anguera, M.; Ortiz-Solà, J.; Aguiló-Aguayo, I. Fortification of orange and apple juices with ferulic acid: Implications for food safety and quality. Foods 2024, 13, 3288. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, M.L.; Ibarra-Sánchez, L.A.; Takhar, S.R.; Amaya-Llano, S.L.; Miller, M.J. Use of a miniature laboratory fresh cheese model for investigating antimicrobial activities. J. Dairy Sci. 2015, 98, 8515–8524. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Liao, J.; Chen, Y.; Tong, X.; Sun, X.; Yan, J.; Pang, J. Effects of konjac glucomannan/ε-polylysine hydrochloride/ferulic acid composite coating on the freshness and flavor of refrigerated sea bass fillets. Foods 2023, 12, 517. [Google Scholar] [CrossRef]
- Pernin, A.; Bosc, V.; Maillard, M.; Dubois-Brissonnet, F. Ferulic acid and eugenol have different abilities to maintain their inhibitory activity against Listeria monocytogenes in emulsified systems. Front. Microbiol. 2019, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Jaime, A.G.; Castillo-Rangel, F.; Arévalos-Sánchez, M.M.; Rentería-Monterrubio, A.L.; Santellano-Estrada, E.; Tirado-Gallegos, J.M.; Chávez-Martínez, A. Antioxidant and antimicrobial activity of ferulic acid added to dried meat: Shelf-life evaluation. Foods 2025, 14, 708. [Google Scholar] [CrossRef]
- Mikołajczak, N.; Pilarski, W.; Gęsiński, K.; Tańska, M. Effect of ferulic acid and its derivatives on cold-pressed flaxseed oil oxidative stability and bioactive compounds retention during oxidation. Foods 2023, 12, 1088. [Google Scholar] [CrossRef]
- Yang, H.; Feng, K.; Wen, P.; Zong, M.; Lou, W.; Wu, H. Enhancing oxidative stability of encapsulated fish oil by incorporation of ferulic acid into electrospun zein mat. LWT 2017, 84, 82–90. [Google Scholar] [CrossRef]
- Trombino, S.; Serini, S.; Di Nicuolo, F.; Celleno, L.; Andò, S.; Picci, N.; Palozza, P. Antioxidant effect of ferulic acid in isolated membranes and intact cells: Synergistic interactions with α-tocopherol, β-carotene, and ascorbic acid. J. Agric. Food Chem. 2004, 52, 2411–2420. [Google Scholar] [CrossRef]
- Azman, E.M.; Yusof, N.; Chatzifragkou, A.; Charalampopoulos, D. Stability enhancement of anthocyanins from blackcurrant (Ribes nigrum L.) pomace through intermolecular copigmentation. Molecules 2022, 27, 5489. [Google Scholar] [CrossRef]
- Jadhav, R.V.; Bhujbal, S. Effect of copigmentation on thermal stability of Hibiscus sabdariffa anthocyanins. Res. J. Pharm. Technol. 2019, 12, 2949. [Google Scholar] [CrossRef]
- Alves, M.; Gonçalves, M.P.; Rocha, C.M. Effect of ferulic acid on the performance of soy protein isolate-based edible coatings applied to fresh-cut apples. LWT 2017, 80, 409–415. [Google Scholar] [CrossRef]
- LaBouyer, M.; Holtrop, G.; Horgan, G.; Gratz, S.W.; Belenguer, A.; Smith, N.; Walker, A.W.; Duncan, S.H.; Johnstone, A.M.; Louis, P.; et al. Higher total faecal short-chain fatty acid concentrations correlate with increasing proportions of butyrate and decreasing proportions of branched-chain fatty acids across multiple human studies. Gut Microbiome 2022, 3, e2. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Russell, W.R.; Quartieri, A.; Rossi, M.; Parkhill, J.; Walker, A.W.; Flint, H.J. Wheat bran promotes enrichment within the human colonic microbiota of butyrate-producing bacteria that release ferulic acid. Environ. Microbiol. 2016, 18, 2214–2225. [Google Scholar] [CrossRef] [PubMed]
- Martinengo, P.; Kannappan, A.; Shi, C. Polyphenolic antibacterials for food preservation: Review, challenges, and current applications. Foods 2021, 10, 2469. [Google Scholar] [CrossRef] [PubMed]
- Nanditha, B.R.; Prabhasankar, P. Antioxidants in bakery products: A review. Crit. Rev. Food Sci. Nutr. 2008, 49, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Pernin, A.; Guillier, L.; Dubois-Brissonnet, F. Inhibitory activity of phenolic acids against Listeria monocytogenes: Deciphering the mechanisms of action using three different models. Food Microbiol. 2019, 80, 18–24. [Google Scholar] [CrossRef]
- Marimuthu, S.; Sudheer, A.R.; Menon, V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Lin, F.H.; Lin, J.Y.; Gupta, R.D.; Tournas, J.A.; Burch, J.A.; Selim, M.A.; Monteiro-Riviere, N.A.; Grichnik, J.M.; Zielinski, J.; Pinnell, S.R. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J. Investig. Dermatol. 2005, 125, 826–832. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Davies, A.J.; Mazza, G. Copigmentation of simple and acylated anthocyanins with colorless phenolic compounds. J. Agric. Food Chem. 1993, 41, 716–720. [Google Scholar] [CrossRef]
- Gençdağ, E.; Özdemir, E.E.; Demirci, K.; Görgüç, A.; Yılmaz, F.M. Copigmentation and stabilization of anthocyanins using organic molecules and encapsulation techniques. Curr. Plant Biol. 2022, 29, 100238. [Google Scholar] [CrossRef]
- Xue, Z.; Liu, J.; Li, Q.; Yao, Y.; Yang, Y.; Ran, C.; Zhang, Z.; Zhou, Z. Synthesis of lipoic acid ferulate and evaluation of its ability to preserve fish oil from oxidation during accelerated storage. Food Chem. X 2023, 19, 100802. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, P.; Zhao, J. Ferulic acid mediates prebiotic responses of cereal-derived arabinoxylans on host health. Anim. Nutr. 2022, 9, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Wen, T.; Wang, J. Role of the microbiome in mediating health effects of dietary components. J. Agric. Food Chem. 2020, 68, 12820–12835. [Google Scholar] [CrossRef]
- Maruyama, H.; Kawakami, F.; Lwin, T.T.; Imai, M.; Shamsa, F. Biochemical characterization of ferulic acid and caffeic acid which effectively inhibit melanin synthesis via different mechanisms in B16 melanoma cells. Biol. Pharm. Bull. 2018, 41, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.J.; Kim, K.B.; Bae, S.; Choi, B.G.; An, S.; Ahn, K.J.; Kim, S.Y. Pretreatment of ferulic acid protects human dermal fibroblasts against ultraviolet A irradiation. Ann. Dermatol. 2016, 28, 740. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Li, H.; Go, Y.; Chan, X.H.; Huang, Q.; Wu, J. Research advances on the damage mechanism of skin glycation and related inhibitors. Nutrients 2022, 14, 4588. [Google Scholar] [CrossRef] [PubMed]
- Nazaré, A.C.; De Faria, C.M.; Chiari, B.G.; Petrônio, M.S.; Regasini, L.O.; Silva, D.H.; Corrêa, M.A.; Isaac, V.L.; Da Fonseca, L.M.; Ximenes, V.F. Ethyl ferulate, a component with anti-inflammatory properties for emulsion-based creams. Molecules 2014, 19, 8124–8139. [Google Scholar] [CrossRef]
- Choi, J.Y.; Ha, N.G.; Lee, W.J.; Boo, Y.C. Synthetic and natural agents targeting advanced glycation end-products for skin anti-aging: A comprehensive review. Antioxidants 2025, 14, 498. [Google Scholar] [CrossRef]
- Ghaisas, M.M.; Kshirsagar, S.; Sahane, R. Evaluation of wound healing activity of ferulic acid in diabetic rats. Int. Wound J. 2012, 11, 523–532. [Google Scholar] [CrossRef]
- Staniforth, V.; Huang, W.; Kandan, A.; Yang, N. Ferulic acid, a phenolic phytochemical, inhibits UVB-induced matrix metalloproteinases in mouse skin via posttranslational mechanisms. J. Nutr. Biochem. 2012, 23, 443–451. [Google Scholar] [CrossRef]
- Zhou, Z.; Shi, T.; Hou, J.; Li, M. Ferulic acid alleviates atopic dermatitis-like symptoms in mice via its potent anti-inflammatory effect. Immunopharmacol. Immunotoxicol. 2020, 42, 156–164. [Google Scholar] [CrossRef]
- Peres, D.D.A.; Sarruf, F.D.; de Oliveira, C.A.; Velasco, M.V.R.; Baby, A.R. Ferulic acid photoprotective properties in association with UV filters: Multifunctional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B 2018, 185, 46–49. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, X.; Xu, X.G.; Li, Y.H.; Wang, B.; Gao, X.H.; Chen, H.D.; Yatskayer, M.; Oresajo, C. Protective effects of a topical antioxidant complex containing vitamins C and E and ferulic acid against ultraviolet irradiation-induced photodamage in Chinese women. J. Drugs Dermatol. 2013, 12, 464–468. [Google Scholar] [PubMed]
- Addor, F.A.; Gonçalves, J.E.; Szrajbman, M. A double-blind, randomized, comparative study of a facial serum containing 15% L-ascorbic acid, 1% alpha-tocopherol and 0.5% ferulic acid protecting against acute photodamage in Brazilian patients. J. Am. Acad. Dermatol. 2018, 79 (Suppl. 1), AB14. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Lee, Y.I.; Almurayshid, A.; Jung, J.Y.; Lee, J.H. Effect of a topical antioxidant serum containing vitamin C, vitamin E, and ferulic acid after Q-switched 1064-nm Nd: YAG laser for treatment of environment-induced skin pigmentation. J. Cosmet. Dermatol. 2020, 19, 2576–2582. [Google Scholar] [CrossRef]
- Sauce, R.; Pinto, C.A.S.O.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. Ex vivo penetration analysis and anti-inflammatory efficacy of the association of ferulic acid and UV filters. Eur. J. Pharm. Sci. 2021, 156, 105578. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xue, Y.; Zhu, H.; Zhang, J.; Li, M.; Ge, W.; Luo, Z.; Yuan, X.; Zhang, D.; Ma, W. Ferulic acid in the treatment of papulopustular rosacea: A randomized controlled study. J. Cosmet. Dermatol. 2024, 24, e16611. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Hashtroody, B.; Piacentini, M.; Celleno, L. Skin protective effects of an antipollution, antioxidant serum containing Deschampsia antarctica extract, ferulic acid and vitamin C. Clin. Cosmet. Investig. Dermatol. 2019, 12, 393–399. [Google Scholar] [CrossRef]
- Mazurek, K.; Pierzchała, E. Comparison of efficacy of products containing azelaic acid in melasma treatment. J. Cosmet. Dermatol. 2016, 15, 269–282. [Google Scholar] [CrossRef]
- Zduńska-Pęciak, K.; Kołodziejczak, A.; Rotsztejn, H. Two superior antioxidants: Ferulic acid and ascorbic acid in reducing signs of photoaging—A split-face comparative study. Dermatol. Ther. 2022, 35, e15254. [Google Scholar] [CrossRef]
- Zduńska-Pęciak, K.; Dębowska, R.; Kołodziejczak, A.; Rotsztejn, H. Ferulic acid—A novel topical agent in reducing signs of photoaging. Dermatol. Ther. 2022, 35, e15543. [Google Scholar] [CrossRef]
- Pueknang, J.; Saewan, N. Stability and anti-aging of encapsulated ferulic acid in phosphorylated rice starch. Molecules 2022, 27, 3463. [Google Scholar] [CrossRef]
- Mancuso, A.; Cristiano, M.C.; Pandolfo, R.; Greco, M.; Fresta, M.; Paolino, D. Improvement of ferulic acid antioxidant activity by multiple emulsions: In vitro and in vivo evaluation. Nanomaterials 2021, 11, 425. [Google Scholar] [CrossRef] [PubMed]
- Zvezdin, V.; Kasatkina, T.; Kasatkin, I.; Gavrilova, M.; Kazakova, O. Microneedle patch based on dissolving, detachable microneedle technology for improved skin quality of the periorbital region. Part 2: Clinical evaluation. Int. J. Cosmet. Sci. 2020, 42, 429–435. [Google Scholar] [CrossRef]
- Jesus, A.; Mota, S.; Torres, A.; Cruz, M.T.; Sousa, E.; Almeida, I.F.; Cidade, H. Antioxidants in sunscreens: Which and what for? Antioxidants 2023, 12, 138. [Google Scholar] [CrossRef]
- Cavalcanti, G.R.; Duarte, F.Í.C.; Converti, A.; Lima, Á.A.N. Ferulic acid activity in topical formulations: Technological and scientific prospecting. Curr. Pharm. Des. 2021, 27, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Saija, A. In vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int. J. Pharm. 2000, 199, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Jiamphun, S.; Chaiyana, W. Enhancing skin delivery and stability of vanillic and ferulic acids in aqueous enzymatically extracted glutinous rice husk by nanostructured lipid carriers. Pharmaceutics 2023, 15, 1961. [Google Scholar] [CrossRef]
| Disorder | Animal Model (n = Total Number of Animals) | Study Details | Experimental Findings | Reference |
|---|---|---|---|---|
| Obesity | Male C57BL/6J mice (n = 32) | HFD-induced obesity model; diet supplemented with FA (0.5% w/w) for 6 weeks. | FA significantly suppressed HFD-induced weight gain, visceral fat accumulation, adipocyte hypertrophy, and hepatic steatosis; it also improved serum lipid profile and glycemic control (comparable efficacy to the anti-obesity drug sibutramine). | [13] |
| Obesity | HFD-fed mice (n = 30) | Mice on high-fat diet with or without FA supplementation (0.5% of diet). | FA prevented HFD-induced increases in hepatic lipid accumulation and adipocyte size, accompanied by lower levels of pro-inflammatory cytokines (TNF-α, IL-6) in adipose tissue. | [14] |
| Dyslipidemia | ApoE−/− mice (n = 32) | Atherosclerosis-prone mice on high-fat diet ± FA treatment (dose duration not specified). | FA lowered total cholesterol, triglycerides, and LDL-C; improved HDL/total cholesterol ratio; and significantly reduced aortic atherosclerotic plaque area (with enhanced collagen stability). Hepatic steatosis and liver enzymes were improved, comparable to the effects of simvastatin. | [15] |
| Dyslipidemia | Male mice (n = 40) | High-cholesterol diet ± FA (low and high dose) for 4 weeks. | FA supplementation attenuated diet-induced hypercholesterolemia (~13% lower serum cholesterol) by increasing hepatic bile acid synthesis via upregulation of CYP7A1, thereby promoting cholesterol catabolism and fecal excretion. | [16] |
| Dyslipidemia | ApoE−/− mice (n = 24) | High-fat-diet-induced atherosclerosis with or without FA treatment. | FA upregulated brown adipose tissue UCP1 and induced “browning” of fat, increasing energy expenditure. Treated mice had significantly smaller aortic plaques and lower pro-inflammatory cytokine expression in lesions, indicating FA’s anti-atherosclerotic and anti-inflammatory effects. | [17] |
| Diabetes | Fructose/STZ-induced type 2 diabetic rats (n = 32) | Rats rendered diabetic with fructose diet + STZ; treated with FA (150 or 300 mg/kg/day) for 5 weeks. | FA significantly reduced hyperglycemia and improved serum insulin levels in diabetic rats, reflecting enhanced insulin action and glycemic control. | [18] |
| Diabetes | HFD-induced diabetic mice (n = 24) | Obese insulin-resistant mice on HFD with or without FA treatment. | FA improved glucose tolerance and insulin sensitivity, associated with suppression of hepatic gluconeogenic enzymes (PEPCK, G6Pase), and increased hepatic glycogen storage. FA’s activation of insulin signaling (e.g., PI3K/Akt–FoxO1) in liver led to reduced hepatic glucose output and better glycemic control. | [19] |
| Diabetes | STZ-induced diabetic rats (n = 24) | Type 1 diabetic rats treated with FA (dose and duration as per study). | FA increased GLUT4 expression and translocation in insulin-sensitive tissues. In diabetic hearts, FA treatment upregulated myocardial GLUT4 and PI3K/Akt activity, improving cardiac glucose uptake and overall insulin responsiveness. | [20] |
| Hypertension | Spontaneously hypertensive rats (n = 45) | Experiment 1: single oral dose of FA (30–600 mg/kg); experiment 2: diet containing 0.5% FA for 8 weeks. | A single administration of FA acutely lowered blood pressure in SHR (minimum effective dose ~100 mg/kg). Long-term dietary FA intake attenuated the development of hypertension, as FA-fed young SHR showed significantly reduced blood pressure gains over 6–8 weeks compared to controls. | [21] |
| Metabolic syndrome | Male C57BL/6J mice (n = 50) | HFD-induced metabolic syndrome model with interventions: exercise (Ex), FA (100 mg/kg BW oral), or combined (Ex + FA), 13 weeks. | FA plus regular exercise produced greater benefits than either alone: the combo prevented HFD-induced weight gain and adiposity, improved serum lipid profile, enhanced hepatic antioxidant enzyme activities, and improved exercise endurance more than FA or exercise by itself. | [22] |
| Disorder | FA Source | Subjects (n = Total) | Study Details | Experimental Findings | Reference |
|---|---|---|---|---|---|
| Obesity | Brown rice (whole grain, FA-rich) | Overweight adults (n ≈ 50) | 12-week dietary intervention replacing white rice with brown rice (high ferulic acid content). | Modest but significant decreases in body weight, BMI, and waist circumference were observed in the brown rice group compared to the white rice group. | [23] |
| Obesity | Brewer’s spent grain extract (rich in FA) | Prediabetic adults (n ≈ 40) | Randomized cross-over trial of a ferulic-acid-rich brewer’s spent grain supplement vs. placebo. | Body weight remained stable (no gain) in both groups, but the FA-rich supplement led to improved inflammatory profiles (significantly lower levels of inflammatory markers) in participants. | [23] |
| Dyslipidemia | Pure ferulic acid (supplement) | Hyperlipidemic subjects (n = 48) | 6-week double-blind RCT: FA 1000 mg per day vs. placebo. | FA supplementation significantly reduced total cholesterol (~8%↓), LDL-C (~9%↓) and triglycerides (~12%↓), with a slight increase in HDL-C (~4%↑). FA also lowered oxidized LDL and markers of oxidative stress and inflammation (e.g., ~33%↓ in CRP), improving overall cardiovascular risk profile. | [24] |
| Diabetes | γ-Oryzanol–fortified canola oil (ferulate ester-rich) | Adults with type 2 diabetes (n = 92) | 12-week RCT comparing daily use of γ-oryzanol enriched canola oil vs. regular canola or sunflower oil. | Only the ferulic-rich oil group showed significant improvements: fasting triglycerides dropped by ~17.9 mg/dL, and notable reductions in waist circumference, blood pressure, fasting glucose, and HbA1c were achieved. (No significant lipid changes in control oil groups.) These results highlight the efficacy of FA derivatives in improving multiple MetS components. | [25] |
| Hypertension | Wheat-aleurone-rich diet (high in FA) | Overweight individuals at MetS risk (n = 23) | 8-week cross-over diet trial: whole-grain wheat aleurone diet vs. refined wheat diet. | No significant changes in blood pressure were observed over 8 weeks. However, the FA-rich aleurone diet markedly improved oxidative stress indices—urinary 8-iso-prostaglandin F2α (8-isoprostane) excretion decreased by ~33%—indicating enhanced antioxidant status despite no acute BP reduction. | [26] |
| Study Type | Food Matrix or Model System | FA Form or Delivery Method | Main Observed Effect | Reference |
|---|---|---|---|---|
| In vitro (broth culture) and food application (meat model) | Ready-to-eat cold-cut meat inoculated with E. coli O157:H7 and L. monocytogenes | FA combined with caffeic acid (each 150–200 ppm) | Synergistic antibacterial action: ~3.6 log CFU/g reduction of E. coli O157:H7 on meat at 4 °C in 72 h with FA + caffeic (greater than either alone); also significantly inhibited L. monocytogenes, improving pathogen control in cold cuts. | [38] |
| In vitro (culture MIC assay) and food application (seafood preservation) | Shewanella putrefaciens culture; refrigerated crayfish (Procambarus clarkii) tails | FA + ε-polylysine (each at ¼ MIC); applied in storage (with plasma-activated water) | Synergistic inhibition of spoilage bacteria: FA + ε-polylysine completely stopped S. putrefaciens growth in vitro at quarter doses (each alone ineffective). In refrigerated crayfish, the combination slowed spoilage—lower total volatile bases and curtailed fishy off-odor compounds—thereby extending shelf life. | [39] |
| Food application (challenge test in beverage) | Fresh apple and orange juices (inoculated with L. monocytogenes) | FA fortification (1500 mg/L added to juices) | Inhibited L. monocytogenes growth during 4 °C storage: FA-fortified juices had significantly lower Listeria counts after 9 days vs. control, while native juice microbiota were largely unaffected. FA increased juice antioxidant content but caused slight acidification and color change (minor sensory impact). | [40] |
| Food application (dairy product) | High-moisture fresh cheese (e.g., Queso fresco) inoculated with L. monocytogenes | FA incorporated into cheese (alone or with nisin) | Suppressed L. monocytogenes in cheese during refrigeration; FA (± nisin) inhibited Listeria growth and prevented development of resistant subpopulations. Listeria exposed to sublethal FA did not acquire increased tolerance, suggesting low risk of resistance. FA (with nisin) is an effective hurdle to improve cheese safety. | [41] |
| Food application (edible coating) | Refrigerated sea bass fillets (fish) | Edible coating of konjac glucomannan + ε-polylysine + FA | Improved microbial quality and shelf life: fish fillets coated with the FA/ε-PL composite film had significantly lower total viable bacterial counts during 4 °C storage vs. controls. FA in coating inhibited spoilage bacteria like S. putrefaciens, delaying spoilage (lower TVB-N accumulation) and preserving fresh odor longer. | [42] |
| In vitro (food model system) | Oil-in-water emulsion (simulated food matrix) inoculated with L. monocytogenes | Free FA (as antimicrobial additive) compared to eugenol | Maintained anti-Listeria activity in an emulsion: FA continued to effectively inhibit L. monocytogenes in an O/W emulsion, whereas the more lipophilic eugenol lost efficacy due to partitioning into the oil phase. Demonstrates FA’s advantage in emulsified/high-fat foods for pathogen control. | [43] |
| Food application (meat preservation) | Dried beef product (shelf-stable meat snack) | FA added at 0.1% (w/w) in formulation | Strong antioxidant protection: 0.1% FA markedly suppressed lipid oxidation during storage—TBARS remained below sensory rancidity threshold over 6 months (no rancid off-flavors), whereas control showed oxidation by 2 months. FA-treated meat had significantly lower peroxide values and sustained higher antioxidant capacity, effectively extending oxidative shelf life. | [44] |
| In vitro (oil oxidation assay) | Bulk cold-pressed flaxseed oil (rich in ω-3 linolenic acid) | FA added to oil (0.02–0.1%) | Enhanced oxidative stability of oil: FA prolonged the induction time for oxidation in a dose-dependent manner. Even low levels (≤0.1%) delayed peroxide formation and rancidity, extending oil shelf life compared to untreated oil. | [45] |
| Food application (encapsulated oil) | Fish oil encapsulated in electrospun zein fiber mat | FA incorporated into encapsulating fiber matrix | Protection of highly unsaturated lipids: FA in the zein nanofiber walls significantly slowed oxidation of the encapsulated fish oil during storage. Omega-3-rich oil with FA showed reduced peroxidation without negative effects on oil release or sensory properties, indicating improved stability of functional food oils. | [46] |
| In vitro (model membrane and cell assays) | Liposomal membranes and cultured cells (oxidative stress model) | Free FA (alone and combined with vitamins C and E) | Antioxidant efficacy and synergy: FA was the most effective single antioxidant in protecting model membranes from lipid peroxidation, and it synergistically enhanced the protection by α-tocopherol (vitamin E) and ascorbic acid (vitamin C). FA stabilized the radicals of vitamins C/E, regenerating their active forms, thus doubling the overall antioxidant effect compared to vitamins alone. | [47] |
| Food application (beverage color stability) | Model beverages colored with elderberry (Sambucus) and purple carrot anthocyanins | FA added as copigment (in solution) | Improved color intensity and stability: FA addition yielded a hyperchromic effect (deeper initial color) and slowed anthocyanin degradation during 8-week storage. Drinks with FA maintained higher color saturation and anthocyanin levels over time versus controls, demonstrating pigment stabilization by copigmentation. | [11] |
| In vitro (anthocyanin stability test) | Anthocyanin solutions from blackcurrant pomace | FA added as copigment | Extended thermal and pH stability of pigments: copigmentation with FA increased the half-life of blackcurrant anthocyanins at moderate pH (~6) and under heat stress. Ferulic-treated anthocyanins showed significantly better color retention vs. no copigment, indicating enhanced pigment stability at elevated temperature. | [48] |
| In vitro (anthocyanin stability test) | Hibiscus sabdariffa (roselle) anthocyanin extract | FA added as copigment | Higher heat stability of natural colorant: FA incorporation significantly improved the thermal stability of Hibiscus anthocyanins, resulting in less color loss under high-temperature conditions. This suggests FA can help preserve color in heat-processed foods and beverages by protecting anthocyanin pigments. | [49] |
| Food application (edible film on produce) | Fresh-cut apple slices | Soy-protein-isolate-based edible coating with added FA | Improved coating performance and product quality: inclusion of FA in the soy protein coating led to cross-linking that enhanced the film’s tensile strength and water resistance. The stronger, antioxidant-infused coating likely slowed apple browning and moisture loss, thereby preserving the fresh quality of cut apples. | [50] |
| In vivo (animal study) | High-fat diet (HFD)-induced obese mice (12-week study) | Oral FA supplementation (100 mg/kg body weight per day) | Modulation of gut microbiota and metabolites: FA shifted the gut microbiome toward beneficial groups—increased SCFA-producing bacteria (e.g., Faecalibaculum, Olsenella) and reduced endotoxin-producing taxa. Consequently, colonic SCFA levels (especially butyrate) rose and gut barrier function improved (higher tight junction protein, lower LPS), leading to reduced inflammation. Overall metabolic health markers (insulin sensitivity, hepatic fat) were ameliorated via this microbiota modulation. | [28] |
| In vivo (animal study) | ApoE−/− mice (atherosclerosis-prone model) | FA supplementation (diet or gavage, dose as per study) | Altered gut microbiome linked to metabolic benefit: FA treatment reshaped gut microbial composition and fecal metabolites in hyperlipidemic mice, which was associated with downregulation of hepatic SREBP-1 and reduced lipogenesis. The microbiota changes (e.g., enrichment of butyrate producers) contributed to attenuated atherosclerotic injury and improved lipid metabolism in this model. | [15] |
| In vivo (human dietary intervention) | Adult volunteers on a wheat bran/aleurone-rich diet vs. refined wheat diet (8 weeks) | Diet naturally high in bound FA (in cereal fiber) | Prebiotic fermentation and SCFA boost: consuming the FA-rich wheat aleurone diet increased total fecal short-chain fatty acid output and shifted SCFA profile toward higher butyrate proportion, compared to a low-FA refined diet. This indicates enhanced colonic fermentation of ferulate fiber, supporting gut health (butyrogenesis) in humans. | [51] |
| In vitro (fecal culture) and human microbiome analysis | Wheat bran fermentation by human gut microbiota (laboratory batch culture); also observed in vivo in humans consuming bran | Bound FA present in wheat bran fiber (released by gut microbes) | Enrichment of butyrate-producing bacteria: fermentation of wheat bran (rich in ferulate) promoted growth of butyrogenic gut bacteria that liberate FA from fiber. This microbial shift leads to increased butyrate generation in the colon, contributing to gut health and explaining the prebiotic effect of ferulic-containing fiber. | [52] |
| Study Type | Model/Subjects | Application or Formulation | Main Effects | Reference |
|---|---|---|---|---|
| In vitro (enzyme and cell) | Tyrosinase enzyme assay; B16 melanoma cells (mouse) | Ferulic acid (free) added in solution (comparative to caffeic acid) | Competitive inhibition of tyrosinase activity, reducing melanin synthesis. In B16 cells, ferulic acid significantly lowered melanin content without cytotoxicity, indicating a depigmenting effect. | [64] |
| In vitro (cell culture) | Human dermal fibroblasts | Ferulic acid pre-treatment before UVA irradiation | Protected skin cells from UVA-induced damage: fibroblasts showed higher viability and reduced collagenase (MMP) activity after UVA exposure with ferulic acid, compared to untreated cells. | [65] |
| In vitro (biochemical) | Protein glycation assay (BSA + sugar) | Ferulic acid added to protein/sugar reaction mixture | Inhibited formation of advanced glycation end-products (AGEs, e.g., CML) and lowered fructosamine levels. This anti-glycation activity suggests ferulic acid helps prevent collagen cross-linking (anti-aging). | [66] |
| In vitro (formulation efficacy) | Emulsion formulation testing (lab-based) | Cream containing ethyl ferulate (ferulic acid derivative) | Demonstrated significant anti-inflammatory effects in a skin model (reduced inflammation markers) and improved formulation stability versus free ferulic acid. After topical application, skin esterases slowly convert the ester to active ferulic acid. | [67] |
| Ex vivo (human skin) | Human skin explants (laboratory setting) | Cream with ferulic acid + vitamin C + phloretin (antioxidants) | Lowered UVA-induced oxidative damage: treated skin had significantly less lipid peroxidation and collagen damage after UVA exposure compared to untreated controls. | [68] |
| Ex vivo (skin model) | Artificial/excised skin model | Ferulic acid in nanostructured lipid carriers (NLC) vs. free FA | Enhanced delivery and stability of ferulic acid: the NLC formulation increased skin penetration and protected FA from degradation, compared to non-encapsulated ferulic acid. | [43] |
| In vivo (animal) | Diabetic rats (wound-healing model) | Topical ferulic acid treatment on excisional wounds | Accelerated wound closure and repair: ferulic-acid-treated wounds healed faster with enhanced granulation tissue formation and collagen deposition, while excess inflammation was reduced. | [69] |
| In vivo (animal) | Mice (UVB-induced photoaging model) | Topical ferulic acid during chronic UVB exposure | Photoprotection against photoaging: ferulic acid application suppressed UV-induced MMP-2 and MMP-9 upregulation, preventing collagen degradation; treated mice had less collagen damage and wrinkle formation than UV-exposed controls. | [70] |
| In vivo (animal) | Mice (atopic dermatitis model) | Topical ferulic acid on irritated skin | Anti-inflammatory and barrier-protective effects: alleviated dermatitis symptoms (reduced redness, swelling, epidermal thickening) by downregulating pro-inflammatory cytokines (IL-6, TNF-α). Treated mice showed improved skin barrier integrity and less immune cell infiltration. | [71] |
| Clinical (human volunteer study) | Healthy human volunteers (in vivo SPF testing) | Broad-spectrum sunscreen + 1% ferulic acid vs. sunscreen alone | Enhanced photoprotection: adding ferulic acid increased sun protection factor (SPF) by ~37% and UVA-PF by ~26% compared to the same sunscreen without FA. Ferulic acid also reduced UV-induced skin erythema (redness), indicating added anti-inflammatory benefits. | [72] |
| Clinical (controlled trial) | Human volunteers (controlled UV exposure study) | Topical antioxidant serum (15% vitamin C + 1% vitamin E + 0.5% FA) pre-UV | Synergistic photoprotection with vitamins C + E: the ferulic acid serum doubled the photoprotection of skin compared to vitamins C + E alone. Skin pre-treated with the C + E + FA serum showed significantly less UVB-induced erythema and fewer sunburn cells versus untreated skin. | [57] |
| Clinical (controlled trial) | Human volunteers (controlled UV exposure study) | Topical vitamin C + E + ferulic acid serum (same as above) | Confirmed enhanced photoprotection (replicating: pre-treatment with the C + E + FA serum led to significantly reduced UVB-induced erythema and sunburn cell formation compared to no antioxidant treatment, demonstrating ferulic acid’s crucial role in the serum’s efficacy. | [73] |
| Clinical (double-blind RCT) | Human subjects (face; photodamage protection) | Facial serum (15% vitamin C + 1% vitamin E + 0.5% FA) vs. placebo | Protection against acute UV photodamage: after controlled UV exposure, subjects using the C + E + FA serum had significantly less skin redness by day 4 compared to controls, indicating superior photoprotection in a real-world scenario. | [74] |
| Clinical (controlled trial) | Patients after ablative laser resurfacing | Post-procedure use of ferulic acid antioxidant serum vs. no serum | Accelerated wound healing and reduced hyperpigmentation: the ferulic acid serum (with vitamins C/E) sped up post-laser skin recovery—patients had improved texture and significantly less post-inflammatory hyperpigmentation (PIH) than controls—without increasing irritation. | [75] |
| Ex vivo + Clinical (patch test) | Ex vivo human skin; healthy volunteers (patch test) | Sunscreen + ferulic acid vs. sunscreen without FA | Anti-inflammatory benefits in formulations: in ex vivo skin, the FA-enriched sunscreen showed lower UV-induced inflammatory markers than the FA-free version. In human patch tests, skin treated with FA-sunscreen had significantly less chemically induced redness (from an irritant) compared to control, demonstrating ferulic acid’s soothing, anti-inflammatory effect. | [76] |
| Clinical (RCT) | Patients with mild-to-moderate rosacea | Topical ferulic acid added to standard therapy vs. standard alone | Anti-inflammatory and barrier repair in rosacea: after 6 weeks, the ferulic acid group showed greater reduction in inflammatory lesions and redness, plus improved skin barrier function (lower transepidermal water loss, higher hydration) compared to controls. Ferulic acid was well tolerated (minimal irritation) and hastened symptom relief. | [77] |
| Clinical (single-blind trial) | Women in urban high-pollution area (3-month study) | Daily antioxidant serum (Deschampsia antarctica extract + ferulic acid + vitamin C) | Skin-brightening and anti-aging effects: at 4 weeks, the ferulic acid serum significantly reduced dark spots and improved skin brightness; by 3 months, it enhanced skin texture, radiance, and reduced fine lines. These benefits are attributed to the serum’s photoprotective and anti-pollution antioxidant action. | [78] |
| Clinical (split-face study) | Patients with melasma (facial hyperpigmentation) | Ferulic-acid-containing formula on one side of face vs. alternate treatment on other side | Skin lightening in melasma: the side treated with ferulic acid showed a greater decrease in melanin index and pigment intensity than the side treated with the comparative regimen, leading to more pronounced fading of melasma patches (both treatments produced improvement). | [79] |
| Clinical (split-face trial) | Women with photoaged skin | 14% ferulic acid chemical peel on one side vs. 14% ascorbic acid (vitamin C) peel on the other | Anti-aging (collagen remodeling): both peels improved fine wrinkles and firmness, but the ferulic acid peel was as effective as the vitamin C peel in increasing skin elasticity and dermal density. Notably, adding vitamin C to a ferulic acid peel yielded no further benefit, indicating ferulic acid alone robustly stimulated collagen renewal. | [80] |
| Clinical (open-label series) | Individuals with signs of skin aging | Series of 14% ferulic acid chemical peels (multiple sessions) | Anti-aging outcomes: the ferulic acid peel series produced marked improvements in skin hydration and elasticity and a reduction in wrinkle depth. Patients reported high satisfaction with visible results and experienced minimal side effects, underscoring the peels’ safety and efficacy. | [81] |
| Clinical (product comparison) | Human volunteers (skincare efficacy trial) | Topical cream with encapsulated ferulic acid (in phosphorylated starch) vs. cream with free ferulic acid | Improved efficacy via encapsulation: the starch-encapsulated ferulic acid cream provided significantly greater skin-lightening and anti-wrinkle effects than an identical cream with unencapsulated FA. Encapsulation also conferred higher stability of FA under stress conditions, enhancing overall formulation performance. | [82] |
| Clinical (formulation test) | Healthy volunteer skin (UV exposure test sites) | Ferulic acid in multiple emulsion (w/o/w) vs. conventional formulation | Enhanced photoprotection with advanced formulation: the multiple-emulsion formulation protected ferulic acid from oxidation and, when applied to skin, led to higher tissue antioxidant levels and significantly less UV-induced erythema compared to a standard (non-encapsulated) formulation. | [83] |
| Clinical (pilot trial) | Adults with periocular (eye-area) wrinkles | Dissolving microneedle patches with ferulic acid (vs. placebo patches) | Targeted anti-wrinkle efficacy: after several weeks of use, the ferulic acid microneedle patches significantly increased skin elasticity and reduced wrinkle depth in the crow’s-feet area compared to placebo, demonstrating the novel delivery method’s effectiveness. | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobo-Velázquez, D.A. Ferulic Acid: Mechanistic Insights and Multifaceted Applications in Metabolic Syndrome, Food Preservation, and Cosmetics. Molecules 2025, 30, 3716. https://doi.org/10.3390/molecules30183716
Jacobo-Velázquez DA. Ferulic Acid: Mechanistic Insights and Multifaceted Applications in Metabolic Syndrome, Food Preservation, and Cosmetics. Molecules. 2025; 30(18):3716. https://doi.org/10.3390/molecules30183716
Chicago/Turabian StyleJacobo-Velázquez, Daniel A. 2025. "Ferulic Acid: Mechanistic Insights and Multifaceted Applications in Metabolic Syndrome, Food Preservation, and Cosmetics" Molecules 30, no. 18: 3716. https://doi.org/10.3390/molecules30183716
APA StyleJacobo-Velázquez, D. A. (2025). Ferulic Acid: Mechanistic Insights and Multifaceted Applications in Metabolic Syndrome, Food Preservation, and Cosmetics. Molecules, 30(18), 3716. https://doi.org/10.3390/molecules30183716






