Synthesis, Clastogenic and Cytotoxic Potential, and In Vivo Antitumor Activity of a Novel N-Mustard Based on Indole-3-carboxylic Acid Derivative
Abstract
1. Introduction
2. Results
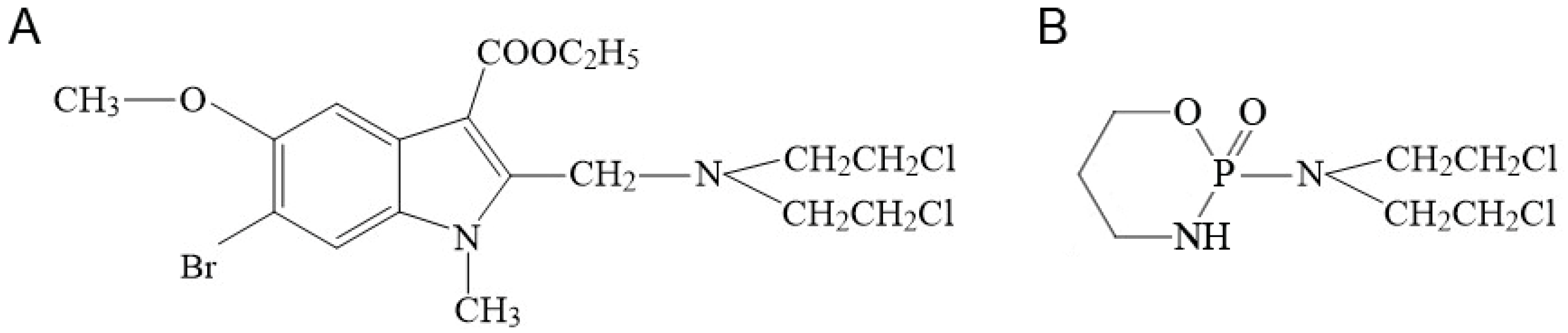
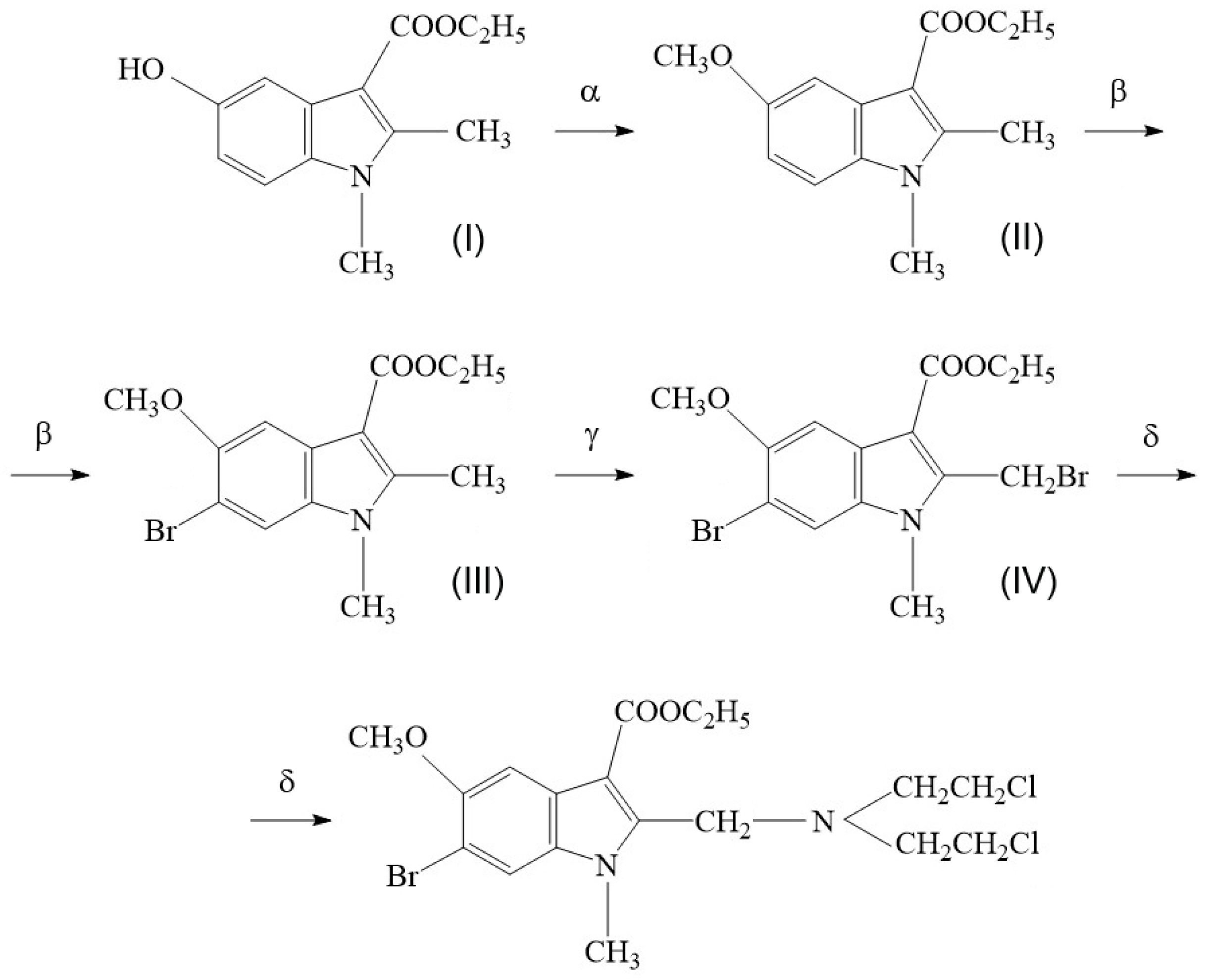
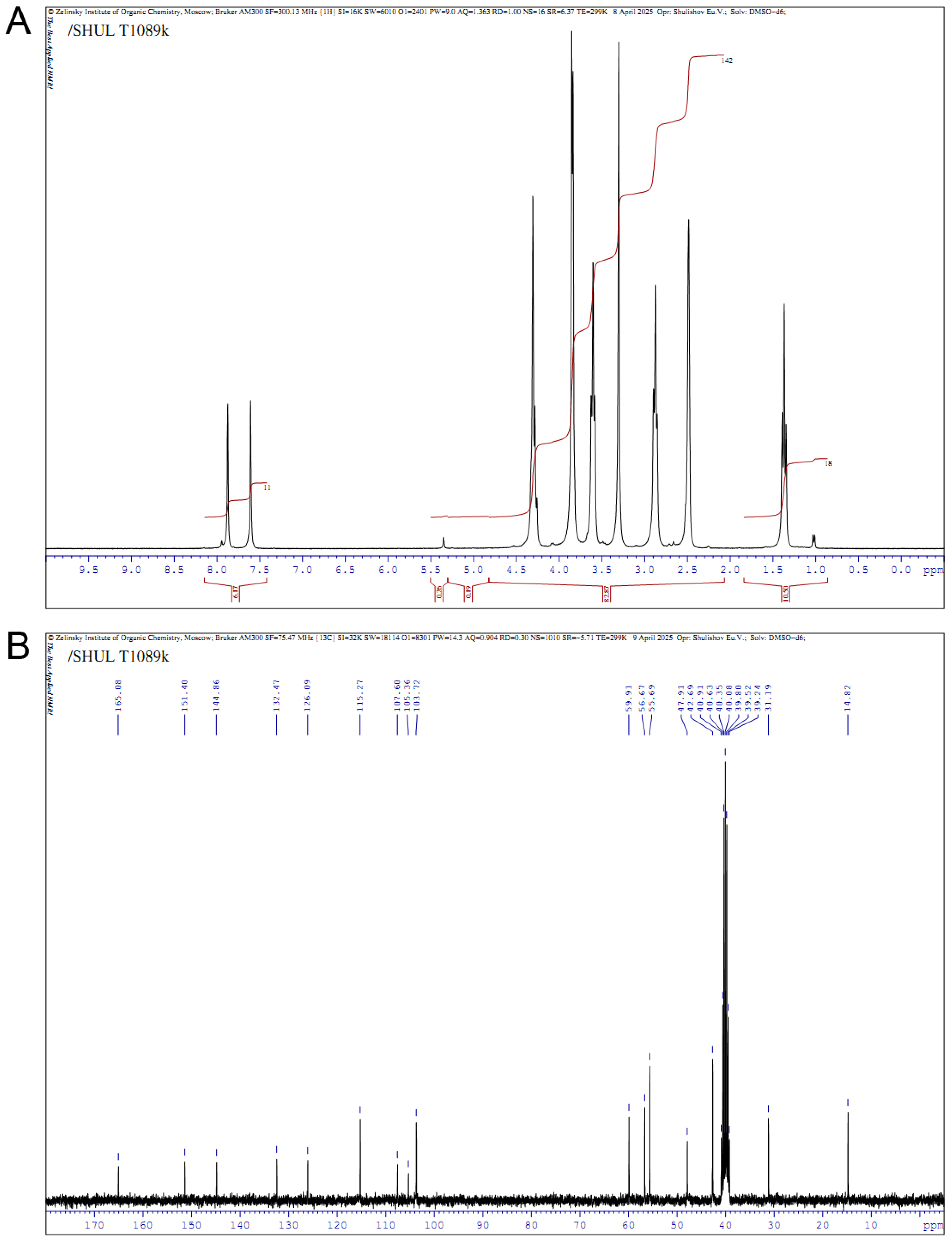
2.1. Synthesis of Compound T1089 and Its Physicochemical Properties
2.2. Toxicological Properties of Compound T1089
- 0.0% (0/5) at 180 mg/kg
- 12.5% (1/8) at 190 mg/kg
- 50.0% (3/6) at 200 mg/kg
- 75.0% (6/8) at 210 mg/kg
- 100.0% (5/5) at 220 mg/kg
- LD10: 188 mg/kg
- LD16: 191 mg/kg
- LD50: 202 ± 6 mg/kg
- LD84: 213 mg/kg
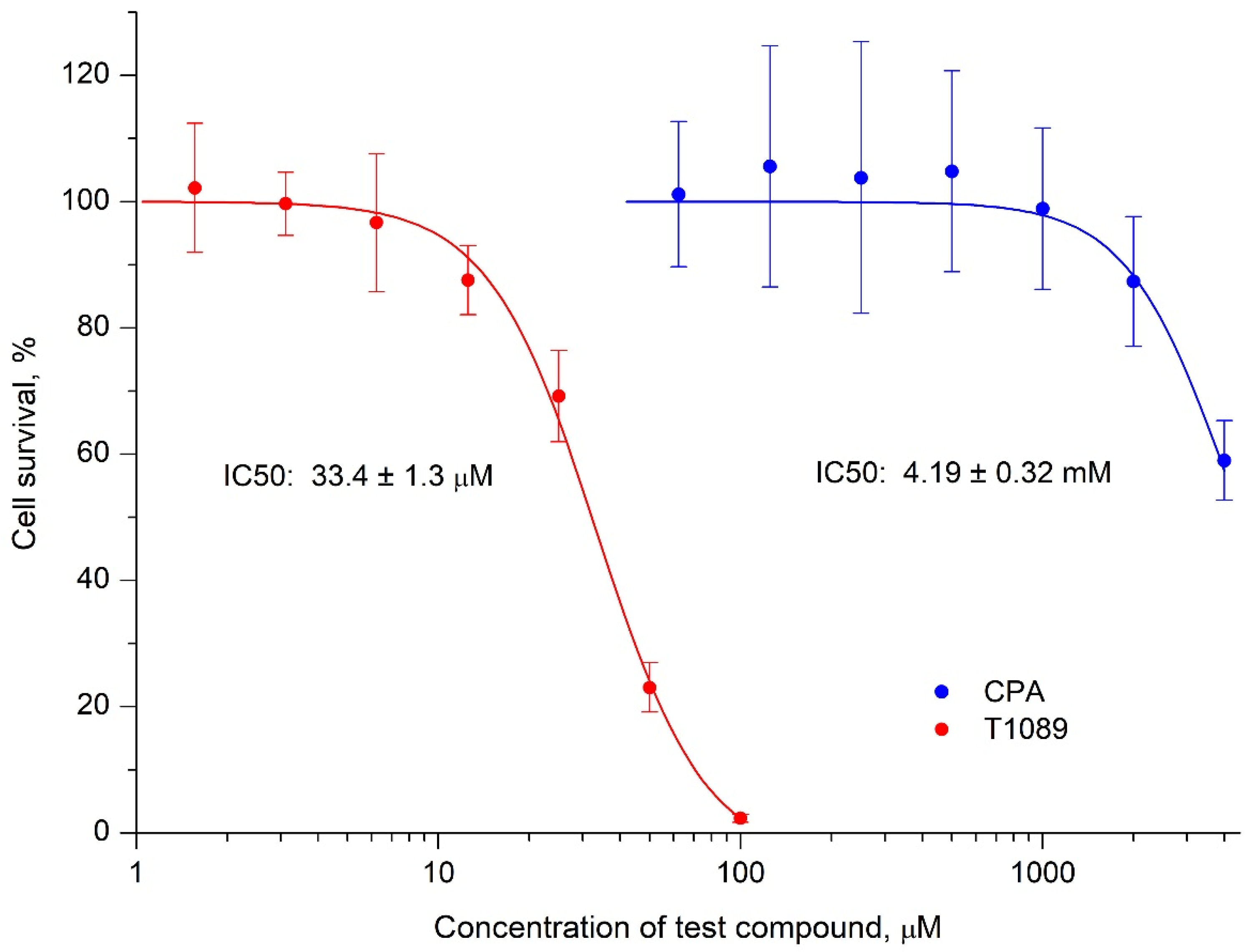
2.3. Cytotoxic Activity of Cyclophosphamide and T1089 In Vitro
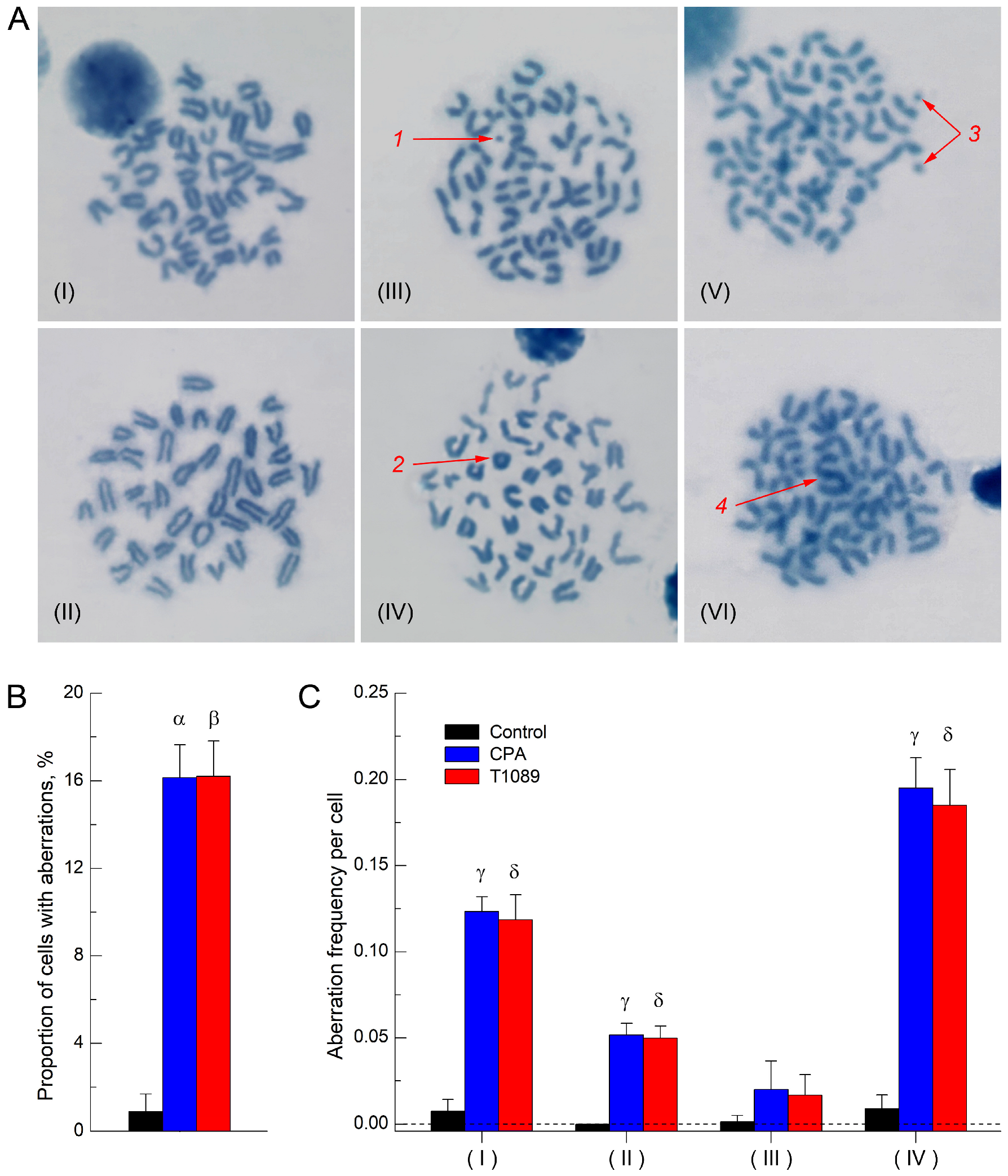
2.4. Clastogenic (Mutagenic) Activity of Cyclophosphamide and T1089 In Vivo
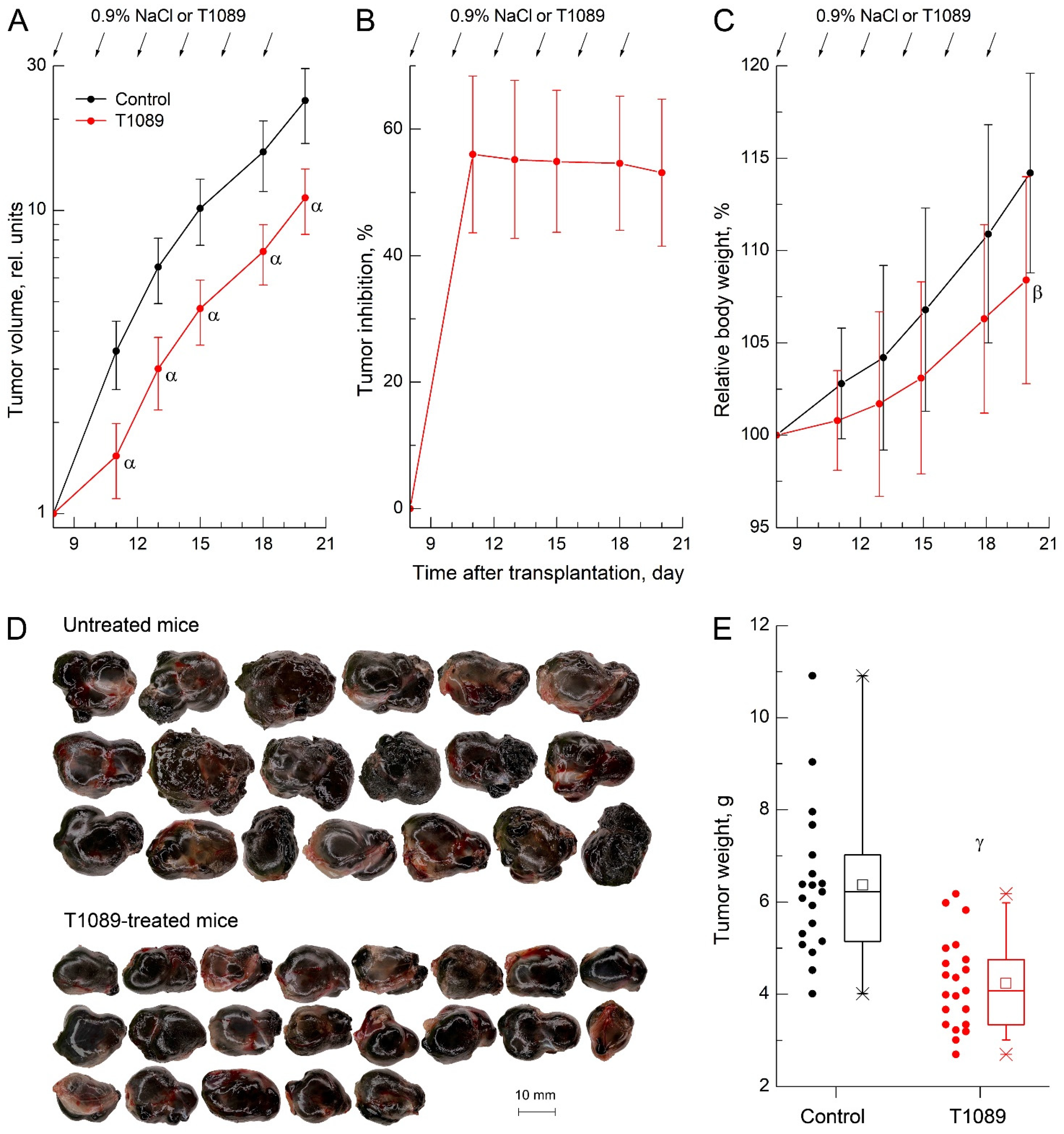
2.5. In Vivo Antitumor Effect of Cyclophosphamide and T1089 on Solid Ehrlich Carcinoma
2.6. In Vivo Antitumor Effect of T1089 on Melanoma B-16
3. Discussion
4. Materials and Methods
4.1. T1089 and Other Compounds and Solutions
4.2. In Vitro Cytotoxic Activity Studies
4.3. Animals
4.4. Toxicological Studies of Compound T1089
4.5. Study of Clastogenic (Mutagenic) Activity of Cyclophosphamide and T1089
4.6. Studies of the Antitumor Activity of Cyclophosphamide and T1089 In Vivo
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.C.; Sharma, A.; Prasad, S.; Singh, K.; Kumar, M.; Sherawat, K.; Tuli, H.S.; Gupta, M. Novel therapeutic agents in clinical trials: Emerging approaches in cancer therapy. Discov. Oncol. 2024, 15, 342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berger, M.F.; Mardis, E.R. The emerging clinical relevance of genomics in cancer medicine. Nat. Rev. Clin. Oncol. 2018, 15, 353–365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, M.; Kumar, V.; Sehrawat, N.; Yadav, M.; Chaudhary, M.; Upadhyay, S.K.; Kumar, S.; Sharma, V.; Kumar, S.; Dilbaghi, N.; et al. Current paradigms in epigenetic anticancer therapeutics and future challenges. Semin. Cancer Biol. 2022, 83, 422–440. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.K.; Poortmans, P.; Chalmers, A.J.; Finn, C.F.; Hall, E.; Huddart, R.A.; Lievens, Y.; Montefiore, D.S.; Coles, C.E. Practice-changing radiation therapy trials for the treatment of cancer: Where are we 150 years after the birth of Marie Curie? Br. J. Cancer 2018, 119, 389–407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Izbicka, E.; Tolcher, A.W. Development of novel alkylating drugs as anticancer agents. Curr. Opin. Investig. Drugs 2004, 5, 587–591. [Google Scholar] [PubMed]
- Hu, Z.; Bousbaa, H. Recent advances in anticancer strategies. Cancers 2025, 17, 173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stewart, M.J. Chemical Warfare Medical Committee Report No. 12; His Majesty’s Stationery Office (HMSO): London, UK, 1918. [Google Scholar]
- Krumbhaar, E.B. Role of the blood and bone marrow in certain forms of gas poisoning. I. Peripheral blood changes and their significance. J. Am. Med. Assoc. 1919, 72, 39–41. [Google Scholar] [CrossRef]
- Adair, F.E.; Bagg, H.J. Experimental and clinical studies on the treatment of cancer by dichlorethylsulphide (mustard gas). Ann. Surg. 1931, 93, 190–199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilman, A.; Philips, F.S. The biological actions and therapeutic applications of the B-chloroethyl amines and sulfides. Science 1946, 103, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Goodman, L.S.; Wintrobe, M.M.; Dameshek, W.; Goodman, M.J.; Gilman, A.; McLennan, M.T. Nitrogen mustard therapy; use of methyl-bis (beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl) amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J. Am. Med. Assoc. 1946, 132, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, C.P. Nitrogen mustards in the treatment of neoplastic disease; official statement. J. Am. Med. Assoc. 1946, 131, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.F.; Fletcher, F. Effect of beta-chloroethylamine hydrochlorides in leukaemia, Hodgkin’s disease, and polycythaemia vera; report on 18 cases. Lancet 1947, 2, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Kumar, S.; Prasad, D.N.; Bhardwaj, T.R. Therapeutic journery of nitrogen mustard as alkylating anticancer agents: Historic to future perspectives. Eur. J. Med. Chem. 2018, 151, 401–433. [Google Scholar] [CrossRef] [PubMed]
- Karati, D.; Mahadik, K.R.; Trivedi, P.; Kumar, D. Alkylating agents, the road less traversed, changing anticancer therapy. Anticancer Agents Med. Chem. 2022, 22, 1478–1495. [Google Scholar] [CrossRef] [PubMed]
- Chiorcea-Paquim, A.-M.; Oliveira-Brett, A.M. Electrochemistry of chemotherapeutic alkylating agents and their interaction with DNA. J. Pharm. Biomed. Anal. 2023, 222, 115036. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Calvo, J.A.; Samson, L.D. Balancing repair and tolerance of DNA damage caused by alkylating agents. Nat. Rev. Cancer 2012, 12, 104–120. [Google Scholar] [CrossRef] [PubMed]
- More, G.S.; Thomas, A.B.; Chitlange, S.S.; Nanda, R.K.; Gajbhiye, R.L. Nitrogen mustards as alkylating agents: A review on chemistry, mechanism of action and current USFDA status of drugs. Anticancer Agents Med. Chem. 2019, 19, 1080–1102. [Google Scholar] [CrossRef] [PubMed]
- Klapacz, J.; Pottenger, L.H.; Engelward, B.P.; Heinen, C.D.; Johnson, G.E.; Clewell, R.A.; Carmichael, P.L.; Adeleye, Y.; Andersen, M.E. Contributions of DNA repair and damage response pathways to the non-linear genotoxic responses of alkylating agents. Mutat. Res. Rev. Mutat. Res. 2016, 767, 77–91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanderson, B.J.; Shield, A.J. Mutagenic damage to mammalian cells by therapeutic alkylating agents. Mutat. Res. 1996, 355, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Montesano, R.; Hall, J.; Wild, C.P. Alkylating agents relating to carcinogenesis in man. Prog. Clin. Biol. Res. 1992, 374, 175–196. [Google Scholar] [PubMed]
- Scaringi, C.; De Sanctis, V.; Minniti, G.; Enrici, R.M. Temozolomide-related hematologic toxicity. Onkologie 2013, 36, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; Jain, D.; Aronow, W.S.; Frishman, W.H. Cardiotoxicity of Cancer Therapies. Cardiol. Rev. 2019, 27, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Aldave, J.E.; Durand-Vásquez, M.D.C.; Chávez-Vásquez, F.S.; Rodríguez-Angulo, A.N.; Gonzáles-Saldaña, S.E.; Alcalde-Loyola, C.C.; Coronado-Arroyo, J.C.; Zavaleta-Gutiérrez, F.E.; Concepción-Urteaga, L.A.; Haro-Varas, J.C.; et al. Ifosfamide-induced nephrotoxicity in oncological patients. Expert Rev. Anticancer Ther. 2024, 24, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Ajithkumar, T.; Parkinson, C.; Shamshad, F.; Murray, P. Ifosfamide encephalopathy. Clin. Oncol. 2007, 19, 108–114. [Google Scholar] [CrossRef] [PubMed]
- De Groot, F.M.; Damen, E.W.; Schere, H.W. Anticancer prodrugs for application in monotherapy: Targeting hypoxia, tumor-associated enzymes, and receptors. Curr. Med. Chem. 2001, 8, 1093–1122. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Ueoka, H. New cytotoxic agents: A review of the literature. Crit. Rev. Oncol. Hematol. 2005, 55, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Diethelm-Varela, B.; Ai, Y.; Liang, D.; Xue, F. Nitrogen mustards as anticancer chemotherapies: Historic perspective, current developments and future trends. Curr. Top. Med. Chem. 2019, 19, 691–712. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.-Y.; Hu, Y.; Hou, X.-F.; Tang, G.-L. Biosynthesis of DNA-alkylating antitumor natural products. Molecules 2022, 27, 6387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Penel, N.; Adenis, A.; Bocci, G. Cyclophosphamide-based metronomic chemotherapy: After 10 years of experience, where do we stand and where are we going? Crit. Rev. Oncol. Hematol. 2012, 82, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Lajous, H.; Lelièvre, B.; Vauléon, E.; Lecomte, P.; Garcion, E. Rethinking alkylating(-like) agents for solid tumor management. Trends Pharmacol. Sci. 2019, 40, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Delahousse, J.; Molina, L.; Paci, A. Cyclophosphamide and analogues; a matter of dose and schedule for dual anticancer activities. Cancer Lett. 2024, 598, 217119. [Google Scholar] [CrossRef] [PubMed]
- Scharovsky, O.G.; Mainetti, L.E.; Rozados, V.R. Metronomic chemotherapy: Changing the paradigm that more is better. Curr. Oncol. 2009, 16, 7–15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhidkov, N.; De Souza, R.; Ghassemi, A.H.; Allen, C.; Piquette-Miller, M. Continuous intraperitoneal carboplatin delivery for the treatment of late-stage ovarian cancer. Mol. Pharm. 2013, 10, 3315–3322. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.; Williams, D.E. Contribution of host immunity to cyclophosphamide therapy of a chemically-induced murine sarcoma. Int. J. Cancer 1973, 11, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Steele, G.; Pierce, G.E. Effects of cyclophosphamide on immunity against chemically-induced syngeneic murine sarcomas. Int. J. Cancer 1974, 13, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Mokyr, M.B.; Dray, S. Some advantages of curing mice bearing a large subcutaneous MOPC-315 tumor with a low dose rather than a high dose of cyclophosphamide. Cancer Res. 1983, 43, 3112–3119. [Google Scholar] [PubMed]
- Kerbel, R.S.; Kamen, B.A. The anti-angiogenic basis of metronomic chemotherapy. Nat. Rev. Cancer 2004, 4, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, F.; Paul, S.; Mancuso, P.; Monestiroli, S.; Gobbi, S.; Shaked, Y.; Kerbel, R.S. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003, 63, 4342–4346. [Google Scholar] [PubMed]
- Hamano, Y.; Sugimoto, H.; Soubasakos, M.A.; Kieran, M.; Olsen, B.R.; Lawler, J.; Sudhakar, A.; Kalluri, R. Thrombospondin-1 associated with tumor microenvironment contributes to low-dose cyclophosphamide-mediated endothelial cell apoptosis and tumor growth suppression. Cancer Res. 2004, 64, 1570–1574. [Google Scholar] [CrossRef] [PubMed]
- Heylmann, D.; Bauer, M.; Becker, H.; van Gool, S.; Bacher, N.; Steinbrink, K.; Kaina, B. Human CD4+CD25+ regulatory T cells are sensitive to low dose cyclophosphamide: Implications for the immune response. PLoS ONE 2013, 8, e83384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, H.; Lai, Y.; Zhang, R.; Daoud, A.; Feng, Q.; Zhou, J.; Shang, J. Low dose cyclophosphamide modulates tumor microenvironment by TGF-β signaling pathway. Int. J. Mol. Sci. 2020, 21, 957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghiringhelli, F.; Menard, C.; Puig, P.E.; Ladoire, S.; Roux, S.; Martin, F.; Solary, E.; Le Cesne, A.; Zitvogel, L.; Chauffert, B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007, 56, 641–648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guerriero, J.L.; Ditsworth, D.; Catanzaro, J.M.; Sabino, G.; Furie, M.B.; Kew, R.R.; Crawford, H.C.; Zong, W.-X. DNA alkylating therapy induces tumor regression through an HMGB1-mediated activation of innate immunity. J. Immunol. 2011, 186, 3517–3526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vacchelli, E.; Ma, Y.; Baracco, E.E.; Sistigu, A.; Enot, D.P.; Pietrocola, F.; Yang, H.; Adjemian, S.; Chaba, K.; Semeraro, M.; et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 2015, 350, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-L.; Zhou, H.-X.; Chen, L.-M.; Wang, S.-S. Metronomic chemotherapy in cancer treatment: New wine in an old bottle. Theranostics 2024, 14, 3548–3564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Filimonova, M.V.; Tsyshkova, N.G.; Narovlyansky, A.N.; Marinchenko, V.P.; Koval, L.S.; Parfenova, T.M.; Izmestyeva, A.V.; Ershov, F.I. Indole-3-Carboxylic Acid Derivatives Having Antiviral Activity. Russian Federation Patent RU 2,552,422, 10 June 2015. (In Russian). [Google Scholar]
- Narovlyansky, A.N.; Filimonova, M.V.; Tsyshkova, N.G.; Pronin, A.; Grebennikova, T.V.; Karamov, E.V.; Larichev, V.F.; Kornilayeva, G.V.; Fedyakina, I.T.; Dolzhikova, I.V.; et al. Indole-3-Carboxylic Acid Derivative Having Antiviral Activity on SARS-CoV-2. Russian Federation Patent RU 2,820,633, 6 June 2024. (In Russian). [Google Scholar]
- Narovlyansky, A.N.; Filimonova, M.V.; Tsyshkova, N.G.; Pronin, A.V.; Grebennikova, T.V.; Karamov, E.V.; Larichev, V.F.; Kornilayeva, G.V.; Fedyakina, I.T.; Dolzhikova, I.V.; et al. In vitro antiviral activity of a new indol-3-carboxylic acid derivative against SARS-CoV-2. Acta Naturae 2023, 15, 83–91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Narovlyansky, A.; Pronin, A.; Poloskov, V.; Sanin, A.; Mezentseva, M.; Fedyakina, I.; Suetina, I.; Zubashev, I.; Ershov, F.; Filimonova, M.; et al. Expression of Toll-like receptor genes and antiviral cytokines in macrophage-like cells in response to indole-3-carboxylic acid derivative. Viruses 2024, 16, 1718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rwandamuriye, F.X.; Wang, T.; Zhang, H.; Elaskalani, O.; Kuster, J.; Ye, X.; Vitali, B.; Schreurs, J.; Morales, M.L.O.; Norret, M.; et al. Local therapy with combination TLR agonists stimulates systemic anti-tumor immunity and sensitizes tumors to immune checkpoint blockade. Oncoimmunology 2024, 13, 2395067. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Everson, R.G.; Hugo, W.; Sun, L.; Antonios, J.; Lee, A.; Ding, L.; Bu, M.; Khattab, S.; Chavez, C.; Billingslea-Yoon, E.; et al. TLR agonists polarize interferon responses in conjunction with dendritic cell vaccination in malignant glioma: A randomized phase II Trial. Nat. Commun. 2024, 15, 3882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Filimonova, M.V.; Surinova, V.I.; Soldatova, O.V.; Shitova, A.A.; Tsyshkova, N.G.; Filimonov, A.S.; Shegay, P.V.; Kaprin, A.D. Indol-3-Carboxic Acid Derivatives with Anti-Tumor Activity. Russian Federation Patent RU 2,782,931, 7 November 2022. (In Russian). [Google Scholar]
- Filimonova, M.V.; Soldatova, O.V.; Surinova, V.I.; Filimonov, A.S.; Shitova, A.A.; Rybachuk, V.A.; Volkova, I.K.; Nikolaev, K.A.; Kosachenko, A.O.; Shegay, P.V.; et al. Method for Obtaining New Original Chloroethylamino-Substituted Derivatives of Aminoalkyl Esters of Indole-3-Carboxylic Acid Possessing Antitumor Activity. Russian Federation Patent RU 2024,127,831, 20 September 2024. (In Russian). [Google Scholar]
- Tsyshkova, N.G.; Trofimov, F.A.; Marinchenko, V.P.; Dubovik, B.V.; Lushnikova, G.A.; Shvedov, V.L. Synthesis 1,2,3,4-tetrahydropyrazino[1,2-a]indole derivatives and their pharmacological investigation. Pharm. Chem. J. 1993, 26, 750–753. [Google Scholar] [CrossRef]
- Berezovskaya, I.V. Classification of substances with respect to acute toxicity for parenteral administration. Pharm. Chem. J. 2003, 37, 139–141. [Google Scholar] [CrossRef]
- Pfizer. Material Safety Data Sheet. Cyclophosphamide Powder for Injection. Version 2.1. Available online: https://cdn.pfizer.com/pfizercom/products/material_safety_data/PZ00021.pdf (accessed on 22 July 2025).
- Organization for Economic Co-Operation and Development, OECD. Test No. 475: Mammalian Bone Marrow Chromosome Aberration Test; OECD Publishing: Paris, France, 1997. [Google Scholar] [CrossRef]
- Thrall, M.A.; Weiser, G.; Allison, R.W.; Campbell, T.W. Veterinary Hematology and Clinical Chemistry, 2nd ed.; Wiley-Blackwell, John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; p. 784. ISBN 978-0-813-81027-0. [Google Scholar]
- Yushkov, B.G.; Korneeva, E.A.; Chereshnev, V.A. The Concept of Norm in Physiology and Pathophysiology. Physiological Constants of Laboratory Animals; Ural Branch of Russian Academy of Sciences: Ekaterinburg, Russia, 2021; p. 864. ISBN 978-5-7691-2542-3. (In Russian) [Google Scholar]
- Savage, J.R. Classification and relationships of induced chromosomal structural changes. J. Med. Genet. 1976, 13, 103–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Natarajan, A.T.; Boei, J.J.W.A. Formation of chromosome aberrations: Insights from FISH. Mutat. Res. 2003, 544, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Filimonova, M.; Shitova, A.; Shevchenko, L.; Soldatova, O.; Surinova, V.; Rybachuk, V.; Kosachenko, A.; Nikolaev, K.; Volkova, I.; Demyashkin, G.; et al. In vitro cytotoxic potential and in vivo antitumor effects of NOS/PDK-inhibitor T1084. Int. J. Mol. Sci. 2024, 25, 9711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bunyatyan, N.D.; Vasiliev, A.N.; Verstakova, O.L.; Zhuravleva, M.V.; Korobov, N.V.; Merkulov, V.A.; Orekhov, S.N.; Sakaeva, I.V.; Uteshev, D.B.; Yavorsky, A.N. Guidelines for Preclinical Drug Research. Part 1; Mironov, A.N., Ed.; Grif & Co.: Moscow, Russia, 2012; p. 944. ISBN 978-5-8125-1466-3. (In Russian) [Google Scholar]
- Dhawan, A.; Bajpayee, M. (Eds.) Genotoxicity Assessment: Methods and Protocols; Humana: Totowa, NJ, USA, 2013; p. 478. ISBN 978-1627035286. [Google Scholar] [CrossRef]
- Filimonova, M.; Shitova, A.; Soldatova, O.; Shevchenko, L.; Saburova, A.; Podosinnikova, T.; Surinova, V.; Shegay, P.; Kaprin, A.; Ivanov, S.; et al. Combination of NOS- and PDK-inhibitory activity: Possible way to enhance antitumor effects. Int. J. Mol. Sci. 2022, 23, 730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filimonova, M.; Soldatova, O.; Shitova, A.; Surinova, V.; Rybachuk, V.; Kosachenko, A.; Nikolaev, K.; Filatova, D.; Prosovskaya, E.; Ivanov, S.; et al. Synthesis, Clastogenic and Cytotoxic Potential, and In Vivo Antitumor Activity of a Novel N-Mustard Based on Indole-3-carboxylic Acid Derivative. Molecules 2025, 30, 3710. https://doi.org/10.3390/molecules30183710
Filimonova M, Soldatova O, Shitova A, Surinova V, Rybachuk V, Kosachenko A, Nikolaev K, Filatova D, Prosovskaya E, Ivanov S, et al. Synthesis, Clastogenic and Cytotoxic Potential, and In Vivo Antitumor Activity of a Novel N-Mustard Based on Indole-3-carboxylic Acid Derivative. Molecules. 2025; 30(18):3710. https://doi.org/10.3390/molecules30183710
Chicago/Turabian StyleFilimonova, Marina, Olga Soldatova, Anna Shitova, Valentina Surinova, Vitaly Rybachuk, Alexander Kosachenko, Kirill Nikolaev, Daria Filatova, Ekaterina Prosovskaya, Sergey Ivanov, and et al. 2025. "Synthesis, Clastogenic and Cytotoxic Potential, and In Vivo Antitumor Activity of a Novel N-Mustard Based on Indole-3-carboxylic Acid Derivative" Molecules 30, no. 18: 3710. https://doi.org/10.3390/molecules30183710
APA StyleFilimonova, M., Soldatova, O., Shitova, A., Surinova, V., Rybachuk, V., Kosachenko, A., Nikolaev, K., Filatova, D., Prosovskaya, E., Ivanov, S., Shegay, P., Kaprin, A., & Filimonov, A. (2025). Synthesis, Clastogenic and Cytotoxic Potential, and In Vivo Antitumor Activity of a Novel N-Mustard Based on Indole-3-carboxylic Acid Derivative. Molecules, 30(18), 3710. https://doi.org/10.3390/molecules30183710






