UV-Induced Photodegradation of 2-Aminothiazole-4-Carboxylic Acid: Identification of New Carbodiimide Molecules
Abstract
1. Introduction
2. Results and Discussion
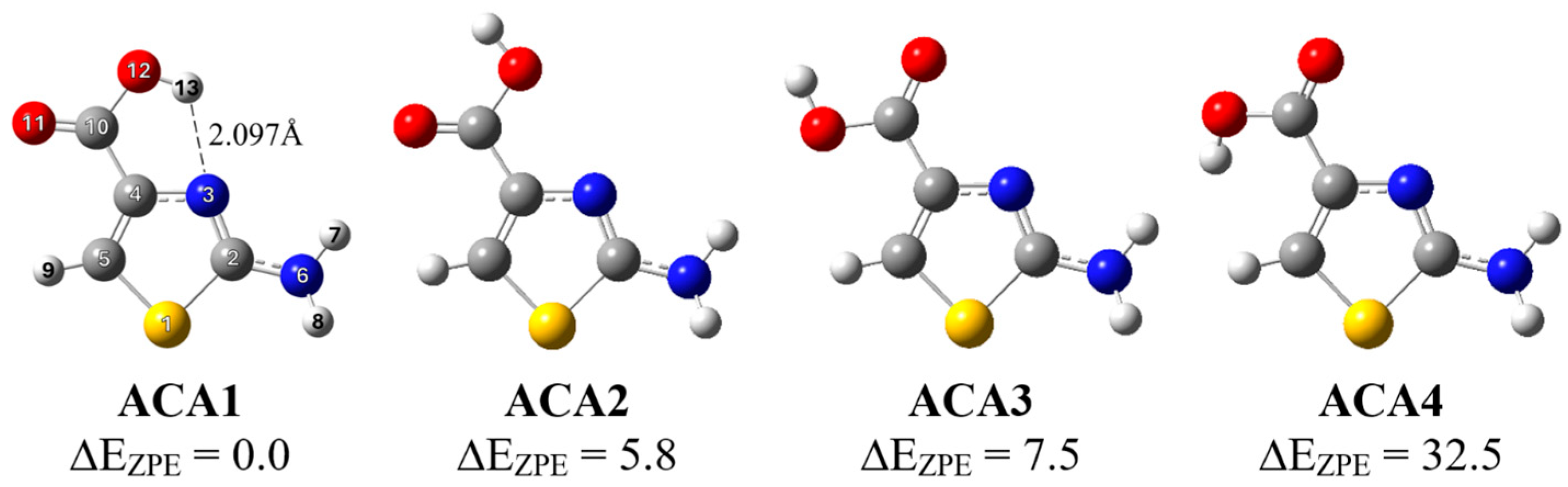
2.1. UV-Induced Photolysis of ACA
2.2. Ring Opening Reactions by Cleavage of the S1-C2 Bond
2.2.1. Formation of Carbodiimides or Cyanamides Containing Sulfur Atom
2.2.2. Formation of Cyanamide or Carbodiimide Complexes
2.3. Ring Opening Reactions by Cleavage of the S1-C5 Bond
2.3.1. Identification of Thiourea Derivatives
2.3.2. Formation of Acetylene Complex
2.3.3. Complexes of Isothiocyanic Acid and Its Isomers
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrou, A.; Fesatidou, M.; Geronikaki, A. Thiazole ring—A biologically active scaffold. Molecules 2021, 26, 3166. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Arshad, M.F.; Ahsan, W.; Alam, M.S. Thiazoles: A valuable insight into the recent advances and biological activities. Int. J. Pharm. Sci. Drug Res. 2009, 1, 136–143. [Google Scholar] [CrossRef]
- Bettendorff, L.; Wins, P. Encyclopedia of Biological Chemistry III, 3rd ed.; Jez, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 1, pp. 302–313. [Google Scholar]
- Hussein, A.H.M.; Khames, A.A.; El-Adasy, A.-B.A.; Atalla, A.A.; Abdel-Rady, M.; Hassan, M.I.A.; Nemr, M.T.M.; Elshaier, Y.A.A.M. Design, synthesis and biological evaluation of new 2-aminothiazole scaffolds as phosphodiesterase type 5 regulators and COX-1/COX-2 inhibitors. RSC Adv. 2020, 10, 29723–29736. [Google Scholar] [CrossRef] [PubMed]
- Mishra, C.B.; Kumari, S.; Tiwari, M. Thiazole: A promising heterocycle for the development of potent CNS active agents. Eur. J. Med. Chem. 2015, 92, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Long, J.; Gao, H.; Tang, Z. 2-aminothiazole: A privileged scaffold for the discovery of anti-cancer agents. Eur. J. Med. Chem. 2021, 210, 112953. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Hashemi, S.M. Development and therapeutic potential of 2-aminothiazole derivatives in anticancer drug discovery. Med. Chem. Res. 2021, 30, 771–806. [Google Scholar] [CrossRef]
- Bardakkaya, M.; Kilic, B.; Sagkan, R.I.; Aksakal, F.; Shakila, S.; Dogruer, D.S. Synthesis and evaluation of multitarget new 2-aminothiazole derivatives as potential anti-Alzheimer’s agents. Arch. Pharm. 2023, 356, e2300054. [Google Scholar] [CrossRef]
- Niu, Z.X.; Wang, Y.T.; Zhang, S.N.; Li, Y.; Chen, X.B.; Wang, S.Q.; Liu, H.M. Application and synthesis of thiazole ring in clinically approved drugs. Eur. J. Med. Chem. 2023, 250, 115172. [Google Scholar] [CrossRef]
- Rostom, S.A.F.; Faidallah, H.M.; Radwan, M.F.; Badr, M.H. Bifunctional ethyl 2-amino-4-methylthiazole-5-carboxylate derivatives: Synthesis and in vitro biological evaluation as antimicrobial and anticancer agents. Eur. J. Med. Chem. 2014, 76, 170–181. [Google Scholar] [CrossRef]
- Pawda, A. Rearrangements in Ground and Excited States; Academic Press: New York, NY, USA, 1980; pp. 501–549. [Google Scholar]
- Venkatasubramanian, R.; Krishnamachari, S.L.N.G. Non-thermal rotational and vibrational excitation of CN produced in the flash photolysis of thiazole. Pramãṇa -J. Phys. 1988, 30, 529–533. [Google Scholar] [CrossRef]
- Lago, A.F.; Januário, R.D.; Simon, M.; Dávalos, J.Z. VUV photodissociation of thiazole molecule investigated by TOF-MS and photoelectron photoion coincidence spectroscopy. J. Mass. Spectrom. 2014, 49, 1163–1170. [Google Scholar] [CrossRef]
- Miyazaki, J.; Takiyama, H.; Nakata, M. Isocyano compounds newly recognized in photochemical reaction of thiazole: Matrix-isolation FT-IR and theoretical studies. RSC Adv. 2017, 7, 4960–4974. [Google Scholar] [CrossRef]
- Pagacz-Kostrzewa, M.; Bumażnik, D.; Coussan, S.; Sałdyka, M. Structure, Spectra and Photochemistry of 2-Amino-4-Methylthiazole: FTIR Matrix Isolation and Theoretical Studies. Molecules 2022, 27, 3897. [Google Scholar] [CrossRef]
- Miyagawa, M.; Akai, N.; Nakata, M. UV-light induced conformational changes of 2-pyridinecarboxylic acid in low-temperature argon matrices. J. Mol. Struct. 2015, 1086, 1–7. [Google Scholar] [CrossRef]
- Pagacz-Kostrzewa, M.; Mucha, K.; Gul, W.; Wierzejewska, M. FTIR spectroscopic evidence for new isomers of 3-aminopyrazine-2-carboxylic acid formed in argon matrices upon UV irradiations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120158. [Google Scholar] [CrossRef]
- Pagacz-Kostrzewa, M.; Szaniawska, W.; Wierzejewska, M. NIR and UV induced transformations of indazole-3-carboxylic acid isolated in low temperature matrices. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 290, 122283. [Google Scholar] [CrossRef] [PubMed]
- Halasa, A.; Lapinski, L.; Reva, I.; Rostkowska, H.; Fausto, R.; Nowak, M.J. Three Conformers of 2-Furoic Acid: Structure Changes Induced with Near-IR Laser Light. J. Phys. Chem. A 2015, 119, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Hałasa, A.; Reva, I.; Łapiński, L.; Nowak, M.J.; Fausto, R. Conformational Changes in Thiazole-2-carboxylic Acid Selectively Induced by Excitation with Narrowband Near-IR and UV Light. J. Phys. Chem. A 2016, 120, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Maier, G.; Endres, J.; Reisenauer, H.P. 2,3-Dihydrothiazol-2-ylidene. Angew. Chem. Int. Ed. Engl. 1997, 36, 1709–1712. [Google Scholar] [CrossRef]
- Bumażnik, D.; Sałdyka, M. Photo-transformations of 2-aminothiazole-4-carboxylic acid in low-temperature matrices. J. Photochem. Photobiol. A Chem. 2024, 455, 115766. [Google Scholar] [CrossRef]
- Schriver, A.; Schriver-Mazzuoli, L.; Vigasin, A.A. Matrix isolation spectra of the carbon dioxide monomer and dimer revisited. Vib. Spectrosc. 2000, 23, 83–94. [Google Scholar] [CrossRef]
- Su, M.-D. A model study on the photochemical isomerization of isothiazoles and thiazoles. Phys. Chem. Chem. Phys. 2014, 32, 17030–17042. [Google Scholar] [CrossRef]
- D’Auria, M. An initio study on the photochemical isomerization of thiazole derivatives. Tetrahedron 2002, 58, 8037–8042. [Google Scholar] [CrossRef]
- Pagacz-Kostrzewa, M.; Sałdyka, M.; Bil, A.; Gul, W.; Wierzejewska, M.; Khomenko, D.M.; Doroschuk, R.O. Phototransformations of 2-(1,2,4-triazol-3-yl)benzoic acid in low temperature matrices. J. Phys. Chem. A 2019, 123, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Pagacz-Kostrzewa, M.; Sałdyka, M.; Gul, W.; Wierzejewska, M.; Khomenko, D.M.; Doroschuk, R.O. Infrared spectra and photochemistry of 2-(tetrazol-5-yl)benzoic acid isolated in nitrogen matrices. J. Photochem. Photobiol. A Chem. 2019, 371, 292–299. [Google Scholar] [CrossRef]
- Duvernay, F.; Chiavassa, T.; Borget, F.; Aycard, J.-P. Carbodiimide Production from Cyanamide by UV Irradiation and Thermal Reaction on Amorphous Water Ice. J. Phys. Chem. A 2005, 109, 603–608. [Google Scholar] [CrossRef] [PubMed]
- King, S.T.; Strope, J.H. Infrared Spectra of the Argon Matrix-Isolated Cyanamide, Cyanamide-d2, and Carbodiimide. J. Chem. Phys. 1971, 54, 1289–1295. [Google Scholar] [CrossRef]
- Kranz, A.; Laureni, J. Matrix Photolysis of 1,2,3-Thiadiazole. On the Possible Involvement of Thiirene. J. Am. Chem. Soc. 1974, 96, 6768–6770. [Google Scholar] [CrossRef]
- Krajewska, M.; Olbert-Majkut, A.; Mielke, Z. Matrix infrared spectra and ab initio calculations of the acetylene complexes with nitric and nitrous acids. Phys. Chem. Chem. Phys. 2002, 4, 4305–4313. [Google Scholar] [CrossRef]
- Wierzejewska, M.; Mielke, Z. Photolysis of isothiocyanic acid HNCS in low-temperature matrices. Infrared detection of HSCN and HSNC isomers. Chem. Phys. Lett. 2001, 349, 227–234. [Google Scholar] [CrossRef]
- Wierzejewska, M.; Olbert-Majkut, A. Matrix Isolation Spectra and ab Initio Calculations of Isothiocyanic Acid Complexes with Carbon Monoxide. J. Phys. Chem. A 2003, 107, 1928–1934. [Google Scholar] [CrossRef]
- Jacox, M.E. Matrix isolation study of the interaction of excited argon atoms with methyl cyanide, vibrational and electronic spectra of ketenimine. Chem. Phys. 1979, 43, 157–172. [Google Scholar] [CrossRef]
- Wierzejewska, M.; Wieczorek, R. Infrared matrix isolation and ab initio studies on isothiocyanic acid HNCS and its complexes with nitrogen and xenon. Chem. Phys. 2003, 287, 169–181. [Google Scholar] [CrossRef]
- Krupa, J.; Wierzejewska, M. Structural and spectroscopic properties of complexes formed between HNCS and SO2 in low temperature matrices. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 183, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Pasinszki, T.; Krebsz, M.; Bazsó, G.; Tarczay, G. First Isolation and Spectroscopic Observation of Thiofulminic acid (HCNS). Chem. Eur. J. 2009, 15, 6100–6102. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron-Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Barone, V. Anharmonic vibrational properties by a fully automated second-order perturbative approach. J. Chem. Phys. 2005, 122, 014108. [Google Scholar] [CrossRef] [PubMed]
- Bloino, J.; Barone, V. A second-order perturbation theory route to vibrational averages and transition properties of molecules: General formulation and application to infrared and vibrational circular dichroism spectroscopies. J. Chem. Phys. 2012, 136, 124108. [Google Scholar] [CrossRef]
- Bloino, J.; Baiardi, A.; Biczysko, M. Aiming at an accurate prediction of vibrational and electronic spectra for medium-to-large molecules: An overview. Int. J. Quantum Chem. 2016, 116, 1543–1574. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The Calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Ligterink, N.F.W.; El-Abd, S.J.; Brogan, C.L.; Hunter, T.R.; Remijan, A.J.; Garrod, R.T.; McGuire, B.M. The Family of Amide Molecules toward NGC 6334I. Astrophys. J. 2020, 901, 1–23. [Google Scholar] [CrossRef]
- Ridgway, S.; Hall, D.; Kleinmann, S.; Weinberger, D.A.; Wojslaw, R.S. Circumstellar acetylene in the infrared spectrum of IRC +10° 216. Nature 1976, 264, 345–346. [Google Scholar] [CrossRef]
- McGuire, B.A.; Loomis, R.A.; Charness, C.M.; Corby, J.F.; Blake, G.A.; Hollis, J.M.F.; Lovas, J.; Jewell, P.R.; Remijan, A.J. Interstellar carbodiimide (HNCNH): A new astronomical detection from the gbt primos survey via maser emission features. Astrophys. J. 2012, 758, L33. [Google Scholar] [CrossRef]
- Salter, C.J.; Ghosh, T.; Catinella, B.; Lebron, M.; Lerner, M.S.; Minchin, R.; Momjian, E. The arecibo arp 220 spectral census. I. Discovery of the pre-biotic molecule methanimine and new cm-wavelength transitions of other molecules. Astron. J. 2008, 136, 389–399. [Google Scholar] [CrossRef]
- Muñoz Caro, G.M.; Carrascosa de Lucas, H.; Martín-Doménech, R. Photochemistry of interstellar ice forming complex organic molecules. Nat. Rev. Chem. 2025, 9, 537–552. [Google Scholar] [CrossRef]
- Tsuge, M.; Watanabe, N. Radical reactions on interstellar icy dust grains: Experimental investigations of elementary processes. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2023, 99, 103–130. [Google Scholar] [CrossRef]
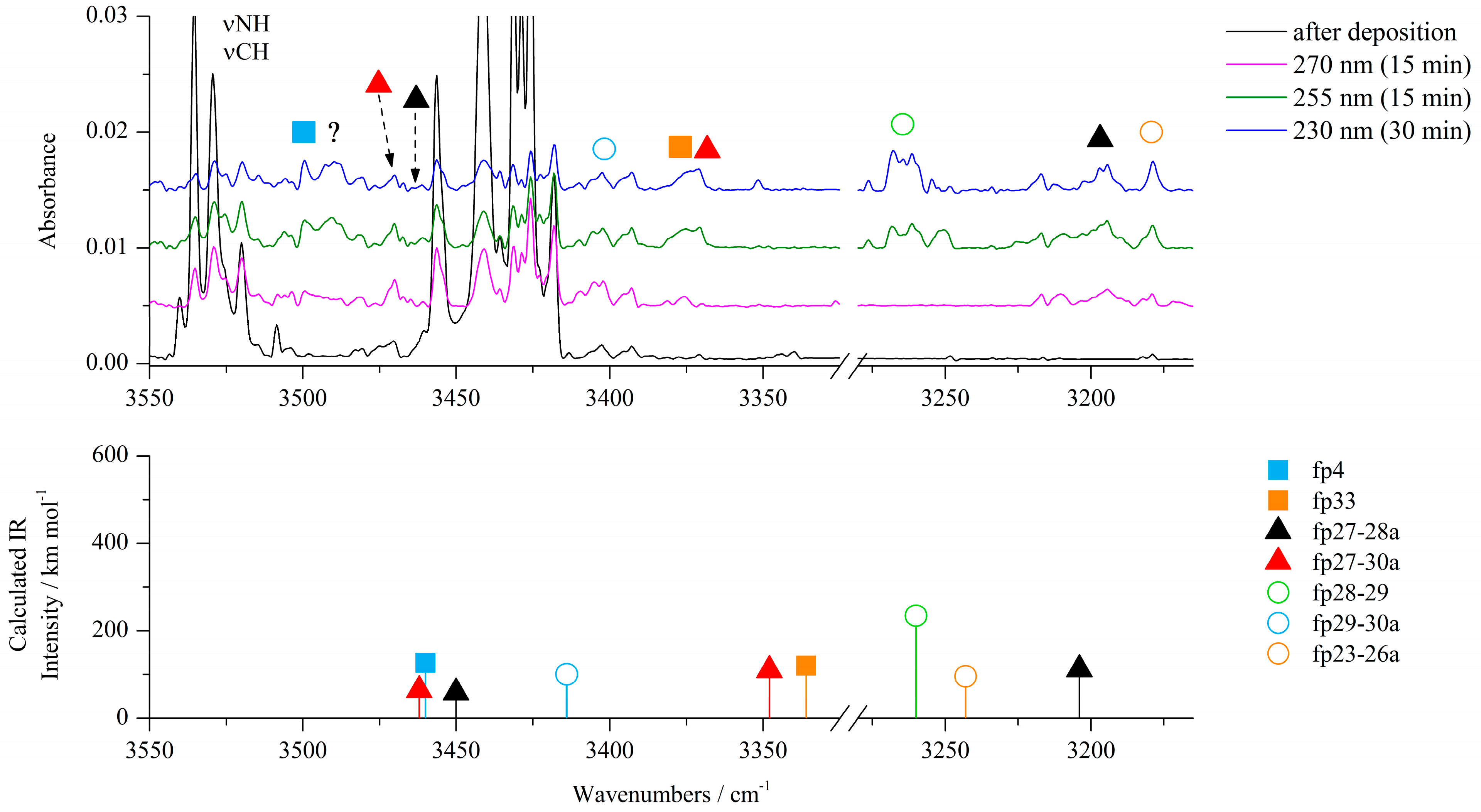



| ACA/Ar | ACA/N2 | ||||||
|---|---|---|---|---|---|---|---|
| fp1 | 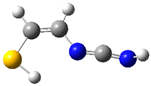 | ||||||
| ν exp | ν harm | ν anharm | ν exp | ν harm | ν anharm | Assignmt b | |
| 2146 | 2228 (1441) a | 2179 | 2145 | 2214 (1430) | 2167 | νasN=C=N | |
| 883 | 906 (431) | 838 | 890 | 906 (403) | 849 | δNH | |
| fp2 | 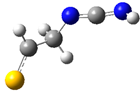 | ||||||
| ν exp | ν harm | ν anharm | ν exp | ν harm | ν anharm | Assignmt | |
| 2146 | 2229 (1148) | 2183 | 2145 | 2218 (1095) | 2172 | νasN=C=N | |
| 917 br | 932 (277) | 869 | - | 936 (275) | 895 | δNH | |
| fp3 |  | ||||||
| ν exp | ν harm | ν anharm | ν exp | ν harm | ν anharm | Assignmt | |
| 2141 | 2224 (1259) | 2173 | 2140 | 2210 (1222) | 2165 | νasN=C=N | |
| 881 | 910 (349) | 846 | 890 | 911 (330) | 865 | δNH | |
| fp4 | 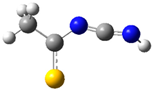 | ||||||
| ν exp | ν harm | ν anharm | ν exp | ν harm | ν anharm | Assignmt | |
| 3499 | 3628 (171) | 3460 | - | 3624 (175) | 3452 | νNH | |
| 2138 | 2217 (1271) | 2181 | 2139 | 2203 (1243) | 2170 | νasN=C=N | |
| 1415 | 1442 (211) | 1418 | 1416 | 1442 (220) | 1414 | νC-N, νsN=C=N | |
| 1233 | 1238 (200) | 1213 | 1232 | 1230 (208) | 1207 | ωCH3, νC=S, | |
| fp33 | 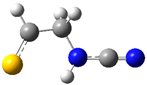 | ||||||
| ν exp | ν harm | ν anharm | ν exp | ν harm | ν anharm | Assignmt | |
| 3387 | 3506 (134) | 3335 | - | 3501 (138) | 3337 | νNH | |
| 2278 | 2334 (237) | 2290 | 2280 | 2328 (258) | 2281 | νC≡N | |
| 1430 | 1480 (100) | 1450 | 1426 | 1476 (99) | 1445 | δNH, δCH2 | |
| H2N–C≡N∙∙∙H–CSC–H | ||||||
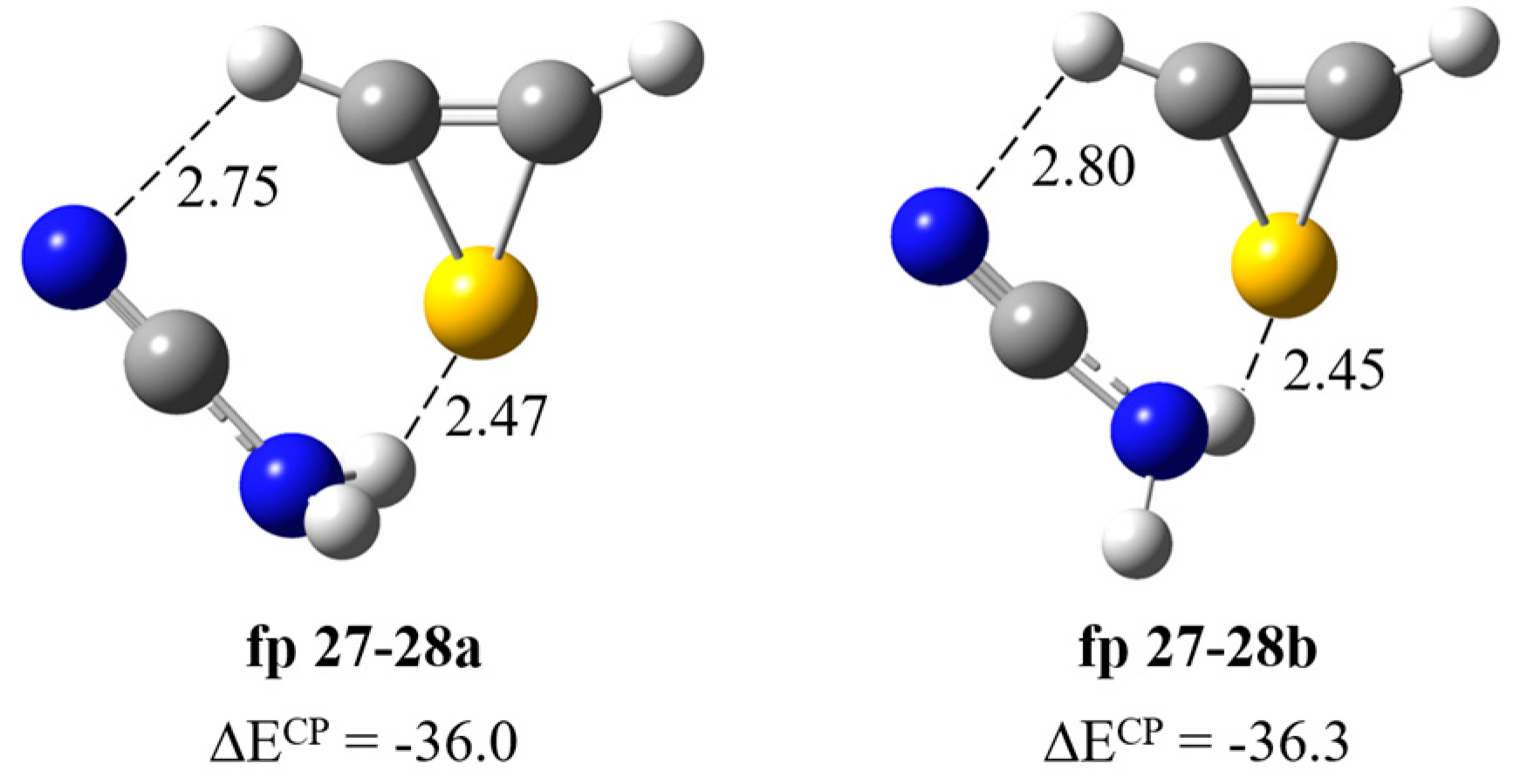
| Experimental | Calculated harmonic | Assignment | ||||
| fp27–28a | fp27–28b | |||||
| ν | Δν | ν | Δν | ν | Δν | |
| 3465 | −21 | 3605 | −33 | 3600 | −38 | νasNH2 |
| 3200 br | −200 | 3348 | −199 | 3336 | −211 | νsNH2 |
| 2260 [2270] | −4 | 2336 | −16 | 2336 | −16 | νC≡N |
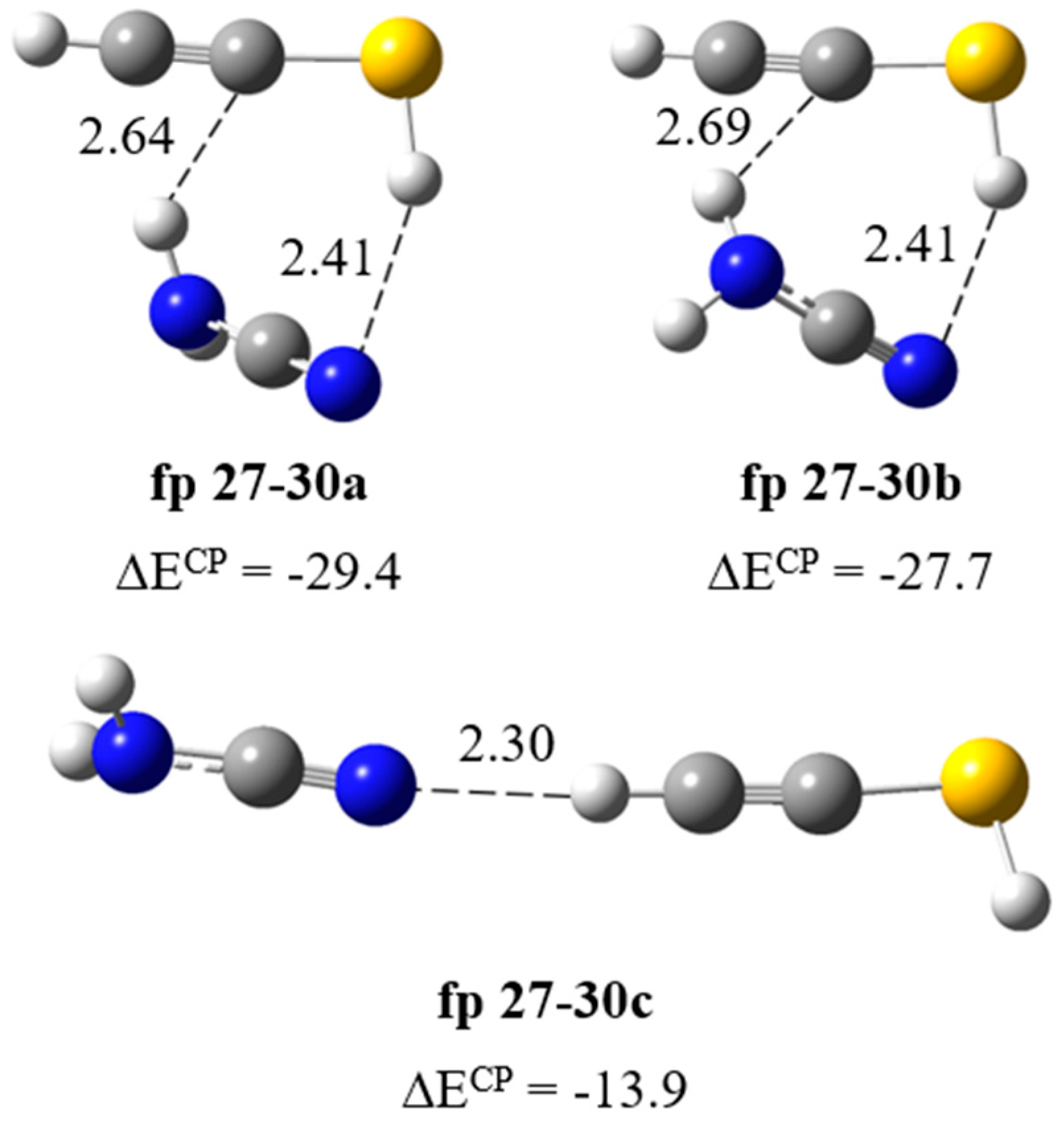
| H2N–C≡N∙∙∙HC≡CSH | ||||||||
| Experimental | Calculated harmonic | Assignment | ||||||
| fp27–30a | fp27–30b | fp27–30c | ||||||
| ν | Δν | ν | Δν | ν | Δν | ν | Δν | |
| 3470 | −16 | 3618 | −19 | 3621 | −16 | 3640 | 3 | νasNH2 |
| 3375 [3370] | −25 | 3498 | −50 | 3503 | −45 | 3548 | 0 | νsNH2 |
| 3310 | −3 | 3461 | −7 | 3462 | −6 | 3387 | −81 | νCH |
| 2260 [2270] | −4 | 2340 | −12 | 2341 | −11 | 2358 | 6 | νC≡N |
| 762 | 34 | 609 | 42 | 572 | 5 | 551 | −16 | ωNH2 |
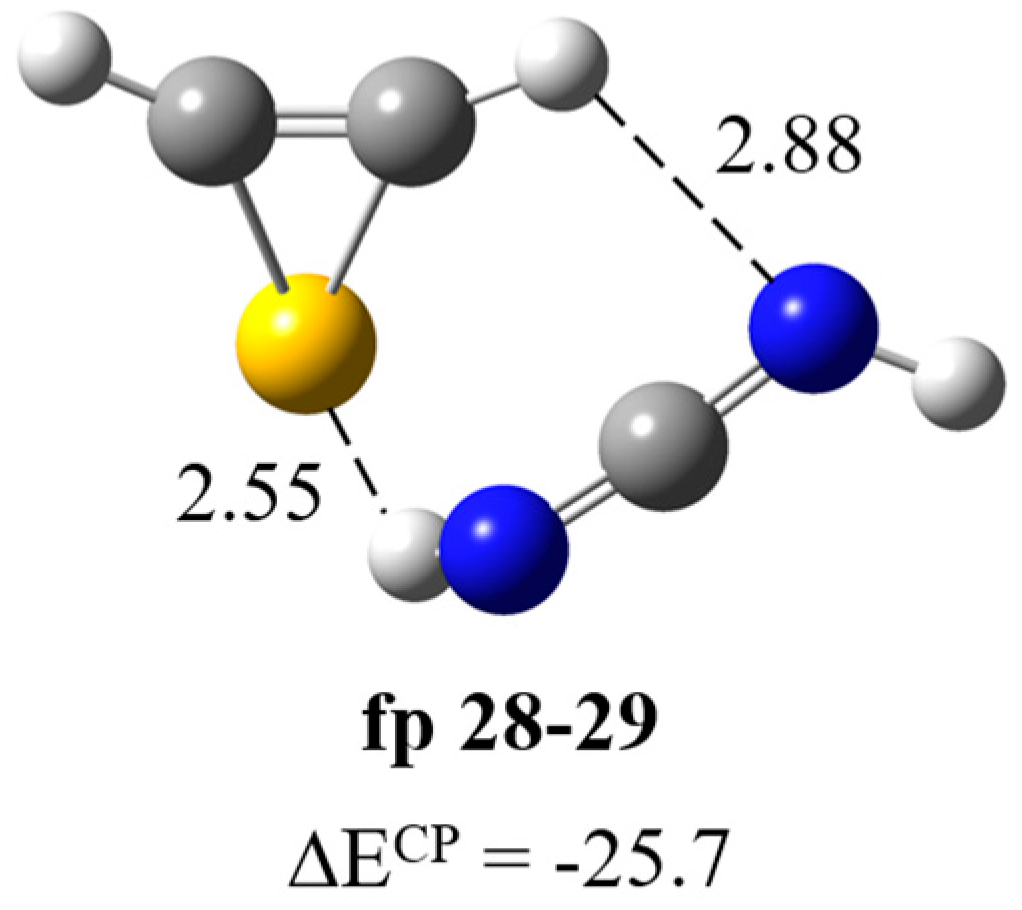
| HN=C=NH∙∙∙H–CSC–H | ||||
| Experimental | Calculated harmonic | Assignment | ||
| fp28–29 | ||||
| ν | Δν | ν | Δν | |
| 3260 [3260] | −165 | 3406 | −200 | νNHbonded |
| 2090 [2090] | −8 | 2212 | −7 | νasNCN |
| 955 | 69 | 974 | 60 | δNHbonded |
| 917 br | 31 | 954 | 40 | δNHfree |
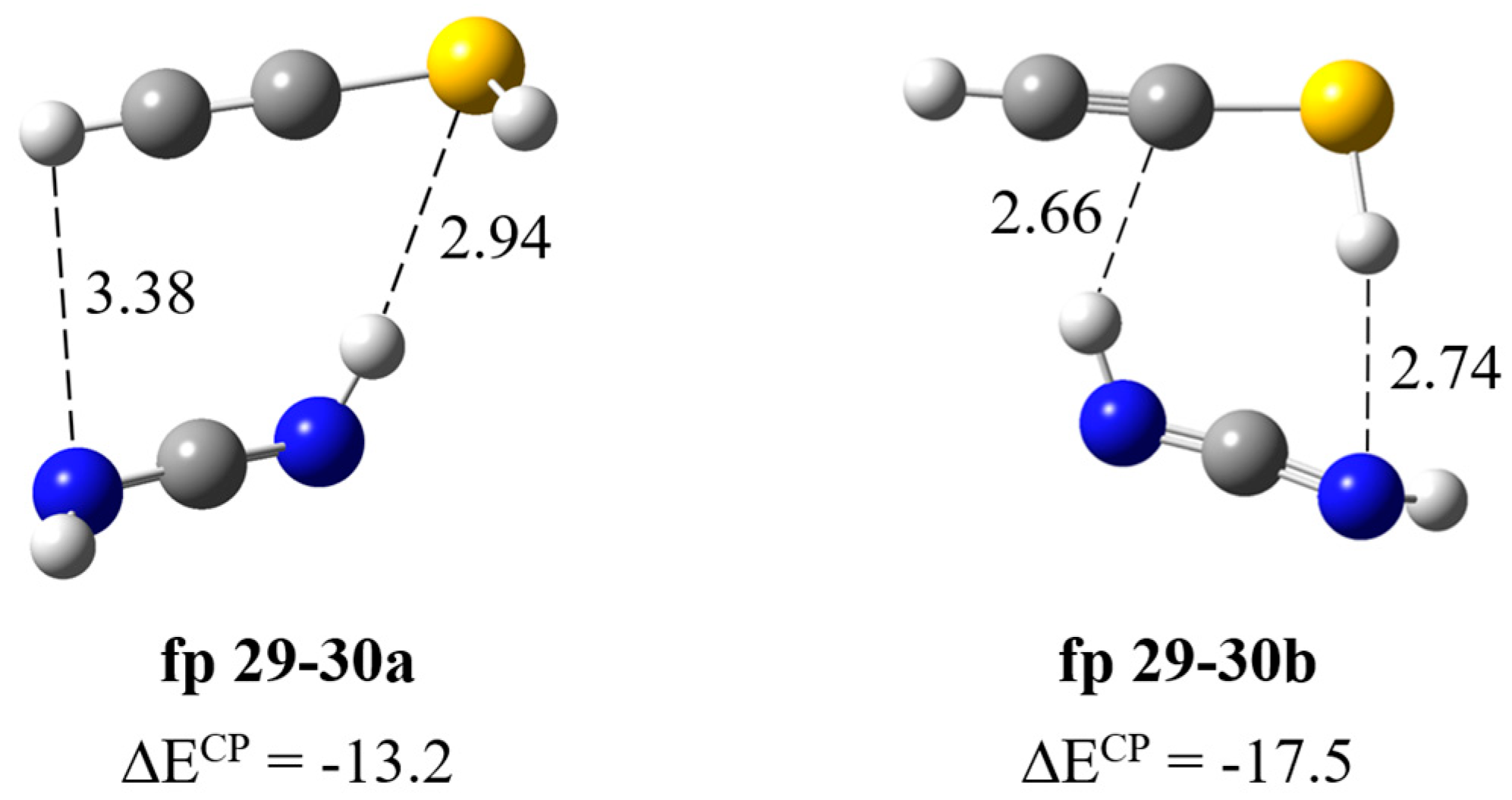
| HN=C=NH∙∙∙HC≡CSH | ||||||
| Experimental | Calculated harmonic | Assignment | ||||
| fp29–30a | fp29–30b | |||||
| ν | Δν | ν | Δν | ν | Δν | |
| 3395 | −30 | 3567 | −33 | 3553 | −42 | νNHbonded |
| 3312 | −1 | 3465 | −3 | 3463 | −5 | νCH |
| 2094 [2097] | −4 | 2216 | −3 | 2216 | −3 | νasNCN |
| 903 [900] | 17 | 936 | 22 | 944 | 30 | δNHbonded |
| 896 [895] | 10 | 925 | 11 | 936 | 22 | δNHfree |
| ACA/Ar | ACA/N2 | ||||||
|---|---|---|---|---|---|---|---|
| fp14 | 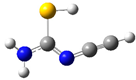 | ||||||
| ν exp | ν harm | ν anharm | ν exp | ν harm | ν anharm | Assignmt b | |
| 2131 | 2191 (165) a | 2160 | 2133 | 2185 (186) | 2152 | νC≡C | |
| 1648 | 1663 (612) | 1624 | 1650 | 1652 (594) | 1606 | δNH2, νC=N | |
| fp15 | 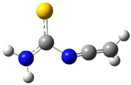 | ||||||
| ν exp | ν harm | ν anharm | ν exp | ν harm | ν anharm | Assignmt | |
| 2061 | 2125 (685) | 2094 | 2052 | 2118 (680) | 2069 | νasN=C=C | |
| 1598 | 1624 (222) | 1584 | - | 1624 (210) | 1557 | δNH2 | |
| 1280 | 1304 (232) | 1287 | 1283 | 1301 (242) | 1270 | νC-N, ρNH2 | |
| 2083 | - | 2084 | - | - | 2069 | δNCN+δCH2 | |
| fp17 | 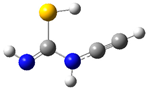 | ||||||
| ν exp | ν harm | ν anharm | ν exp | ν harm | ν anharm | Assignmt | |
| 2162 | 2233 (140) | 2192 | 2163 | 2229 (138) | 2189 | νC≡C | |
| 1641 | 1662 (418) | 1631 | 1638 | 1652 (427) | 1621 | νC=N | |
| 1283 | 1319 (140) | 1271 | 1284 | 1320 (135) | 1270 | νC-N, δNH | |
| 1151 | 1186 (137) | 1154 | - | 1186 (136) | 1151 | δNH, νC-N | |
| fp18 | 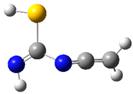 | ||||||
| ν exp | ν harm | ν anharm | ν exp | ν harm | ν anharm | Assignmt | |
| 2070 | 2126 (688) | 2075 | 2060 | 2120 (681) | 2072 | νasN=C=C | |
| 1632 | 1672 (290) | 1632 | 1633 | 1665 (291) | 1623 | νC=N | |
| 1220 | 1274 (230) | 1237 | - | 1273 (225) | 1239 | δNH, νC-N | |
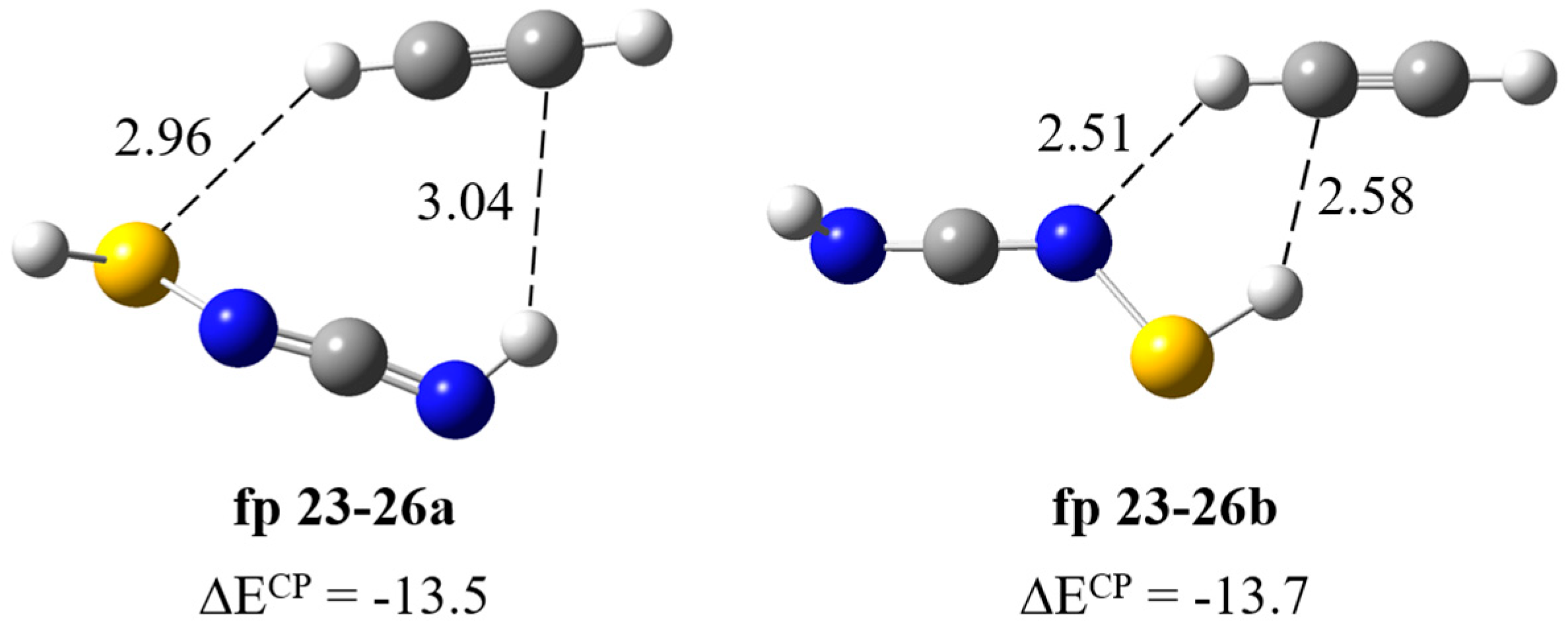
| HS–N=C=NH∙∙∙HC≡CH | ||||||
| Experimental | Calculated harmonic | Assignment | ||||
| fp23–26a | fp23–26b | |||||
| ν | Δν | ν | Δν | ν | Δν | |
| 3178 | −110 | 3389 | −122 | 3389 | −122 | νsCH |
| 2100 [2100] | −61 b | 2208 | −4 | 2209 | −3 | νasNCN |
| 924 [929] | 40 b | 960 | 16 | 942 | −2 | δNH |
| 747 [755] | 11 | 794 | 32 | 787 | 25 | δsCH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bumażnik, D.; Sałdyka, M. UV-Induced Photodegradation of 2-Aminothiazole-4-Carboxylic Acid: Identification of New Carbodiimide Molecules. Molecules 2025, 30, 3713. https://doi.org/10.3390/molecules30183713
Bumażnik D, Sałdyka M. UV-Induced Photodegradation of 2-Aminothiazole-4-Carboxylic Acid: Identification of New Carbodiimide Molecules. Molecules. 2025; 30(18):3713. https://doi.org/10.3390/molecules30183713
Chicago/Turabian StyleBumażnik, Daria, and Magdalena Sałdyka. 2025. "UV-Induced Photodegradation of 2-Aminothiazole-4-Carboxylic Acid: Identification of New Carbodiimide Molecules" Molecules 30, no. 18: 3713. https://doi.org/10.3390/molecules30183713
APA StyleBumażnik, D., & Sałdyka, M. (2025). UV-Induced Photodegradation of 2-Aminothiazole-4-Carboxylic Acid: Identification of New Carbodiimide Molecules. Molecules, 30(18), 3713. https://doi.org/10.3390/molecules30183713








