Structural Characterization and Anti-Tumor Activity of a Polysaccharide from Laetiporus sulphureus in A549 Cells
Abstract
1. Introduction
2. Results and Analysis
2.1. Obtained L. Sulphureus Polysaccharide
2.2. UV-Vis and FT-IR Analysis
2.3. Methylation Analysis
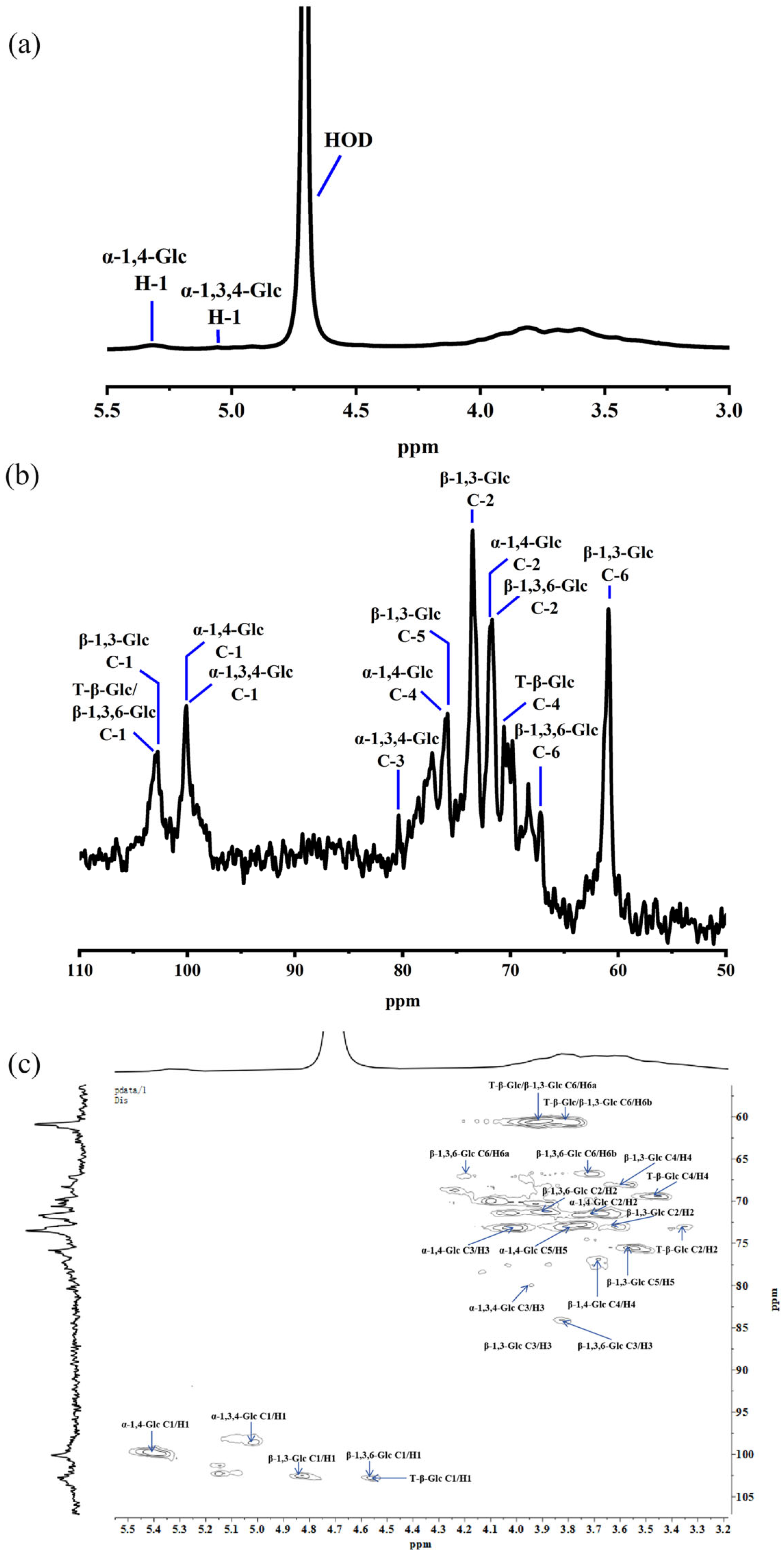
2.4. NMR Analysis
2.5. The Structural Differences Between LSPS1 and LSPS2
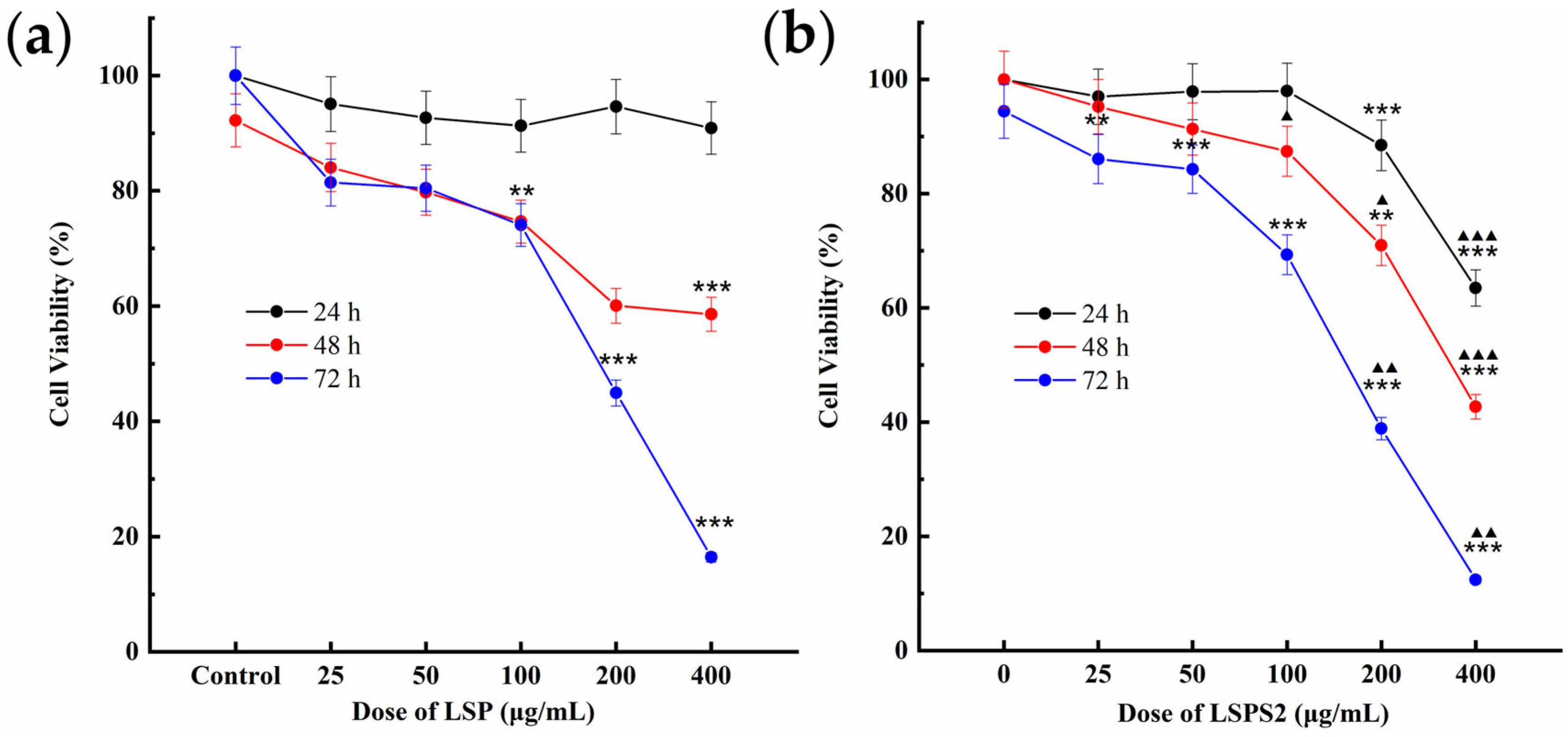
2.6. LSPS2 Decreased A549 Cell Viability
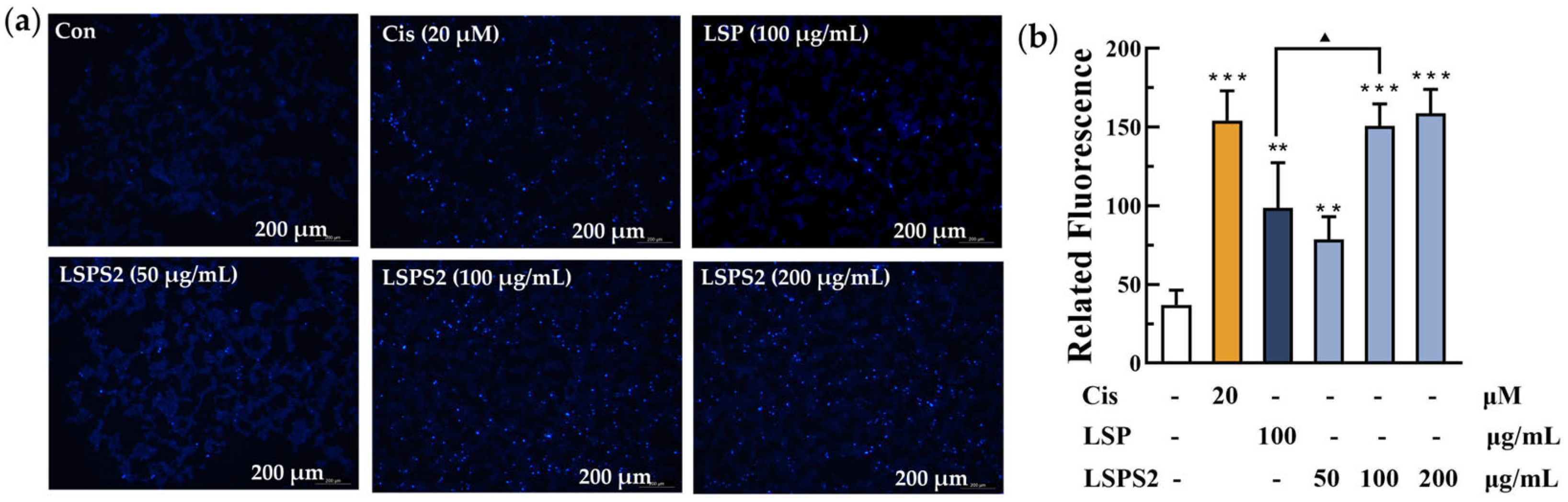
2.7. Influence of LSPS2 Treatment on the Oxidative Stress of A549 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction and Purification of L. sulphureus Polysaccharide
4.3. Analysis of the Composition for Monosaccharide
4.4. The Distribution of Molecular Weight
4.5. UV-Vis Spectroscopy
4.6. Infrared Spectroscopy
4.7. Analysis of Methylation
4.8. NMR Analysis
4.9. Cell Culture
4.10. Cell Viability Assay
4.11. Determination of Cellular MDA, GSH, and SOD
4.12. Detection of Apoptosis and Cell Cycle
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Liu, H.; Li, J.; Wang, Y. Research Progress on Elements of Wild Edible Mushrooms. J. Fungi 2022, 8, 964. [Google Scholar] [CrossRef]
- Sun, Y.; He, H.; Wang, Q.; Yang, X.; Jiang, S.; Wang, D. A Review of Development and Utilization for Edible Fungal Polysaccharides: Extraction, Chemical Characteristics, and Bioactivities. Polymers 2022, 14, 4454. [Google Scholar] [CrossRef]
- Amara, A.A.; El-Baky, N.A. Fungi as a Source of Edible Proteins and Animal Feed. J. Fungi 2023, 9, 73. [Google Scholar] [CrossRef]
- Mustafa, F.; Chopra, H.; Baig, A.A.; Avula, S.K.; Kumari, S.; Mohanta, T.K.; Saravanan, M.; Mishra, A.K.; Sharma, N.; Mohanta, Y.K. Edible Mushrooms as Novel Myco-Therapeutics: Effects on Lipid Level, Obesity and BMI. J. Fungi 2022, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tan, J.; Nima, L.; Sang, Y.; Cai, X.; Xue, H. Polysaccharides from fungi: A review on their extraction, purification, structural features, and biological activities. Food Chem. X 2022, 15, 100414. [Google Scholar] [CrossRef] [PubMed]
- Cerletti, C.; Esposito, S.; Iacoviello, L. Edible Mushrooms and Beta-Glucans: Impact on Human Health. Nutrients 2021, 13, 2195. [Google Scholar] [CrossRef]
- Hassan, K.; Matio Kemkuignou, B.; Stadler, M. Two New Triterpenes from Basidiomata of the Medicinal and Edible Mushroom, Laetiporus sulphureus. Molecules 2021, 26, 7090. [Google Scholar] [CrossRef] [PubMed]
- Adamska, I. The Possibility of Using Sulphur Shelf Fungus (Laetiporus sulphureus) in the Food Industry and in Medicine—A Review. Foods 2023, 12, 1539. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, B.; Shao, J.; Jia, J.; Tian, Y.; Shu, X.; Ren, X.; Guan, Y. Extraction, purification and physicochemical properties of a novel lectin from Laetiporus sulphureus mushroom. LWT 2018, 91, 151–159. [Google Scholar] [CrossRef]
- He, J.-B.; Tao, J.; Miao, X.-S.; Bu, W.; Zhang, S.; Dong, Z.-J.; Li, Z.-H.; Feng, T.; Liu, J.-K. Seven new drimane-type sesquiterpenoids from cultures of fungus Laetiporus sulphureus. Fitoterapia 2015, 102, 1–6. [Google Scholar] [CrossRef]
- Wiater, A.; Waśko, A.; Adamczyk, P.; Gustaw, K.; Pleszczyńska, M.; Wlizło, K.; Skowronek, M.; Tomczyk, M.; Szczodrak, J. Prebiotic Potential of Oligosaccharides Obtained by Acid Hydrolysis of α-(1→3)-Glucan from Laetiporus sulphureus: A Pilot Study. Molecules 2020, 25, 5542. [Google Scholar] [CrossRef]
- Lu, M.-K.; Jen, C.-I.; Chao, C.-H.; Hsu, Y.-C.; Ng, L.-T. SPS, a sulfated galactoglucan of Laetiporus sulphureus, exhibited anti-inflammatory activities. Int. J. Biol. Macromol. 2023, 226, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Jen, C.-I.; Ng, L.-T. Physicochemical Properties of Different Sulfated Polysaccharide Components from Laetiporus sulphureus and Their Anti-Proliferative Effects on MDA-MB-231 Breast Cancer Cells. J. Fungi 2024, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, C.; Zhou, M.; Long, J.; Li, L. Research Progress on the Mechanisms of Polysaccharides against Gastric Cancer. Molecules 2022, 27, 5828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Guan, L.; Wang, K.; Ren, C.; Gao, Y.; Li, J.; Yan, S.; Zhang, X.; Yao, X.; Zhou, Y.; et al. Recent trends in extraction, purification, structural characterization, and biological activities evaluation of Perilla frutescens (L.) Britton polysaccharide. Front. Nutr. 2024, 11, 1359813. [Google Scholar] [CrossRef]
- Cadar, E.; Negreanu-Pirjol, T.; Pascale, C.; Sirbu, R.; Prasacu, I.; Negreanu-Pirjol, B.S.; Tomescu, C.L.; Ionescu, A.M. Natural Bio-Compounds from Ganoderma lucidum and Their Beneficial Biological Actions for Anticancer Application: A Review. Antioxidants 2023, 12, 1907. [Google Scholar] [CrossRef]
- Ji, X.; Yin, M.; Nie, H.; Liu, Y.; Evstigneev, M.P. A Review of Isolation, Chemical Properties, and Bioactivities of Polysaccharides from Bletilla striata. BioMed Res. Int. 2020, 2020, 5391379. [Google Scholar] [CrossRef]
- Li, X.; Ma, L.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumor drug in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 263–296. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Hao, C.; Zeng, P.; Zhang, M.; Liu, Y.; Chang, Y.; Zhang, L. Grifola frondosa polysaccharide: A review of antitumor and other biological activity studies in China. Discov. Med. 2018, 25, 159–176. [Google Scholar]
- Niu, L.; Wu, Y.; Liu, H.; Wang, Q.; Li, M.; Jia, Q. The Structural Characterization of a Novel Water-Soluble Polysaccharide from Edible Mushroom Leucopaxillus giganteus and Its Antitumor Activity on H22 Tumor-Bearing Mice. Chem. Biodivers. 2021, 18, e2001010. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Zhang, G.; Li, M.; Xu, Y.; Peng, L.; Yang, C.; Zhou, Y.; Zhang, J. Antioxidation Effect of Simvastatin in Aorta and Hippocampus: A Rabbit Model Fed High-Cholesterol Diet. Oxidative Med. Cell. Longev. 2016, 2016, 6929306. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Malondialdehyde-Induced Post-Translational Modification of Human Hemoglobin. J. Proteome Res. 2023, 22, 2141–2143. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Date, A.; Chawda, H.; Patel, K. Polysaccharides as potential anticancer agents-A review of their progress. Carbohydr. Polym. 2019, 210, 412–428. [Google Scholar] [CrossRef]
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Huang, H.W.; Tang, J.Y.; Ou-Yang, F.; Wang, H.R.; Guan, P.Y.; Huang, C.Y.; Chen, C.Y.; Hou, M.F.; Sheu, J.H.; Chang, H.W. Sinularin Selectively Kills Breast Cancer Cells Showing G2/M Arrest, Apoptosis, and Oxidative DNA Damage. Molecules 2018, 23, 849. [Google Scholar] [CrossRef]
- Chang, H.W.; Li, R.N.; Wang, H.R.; Liu, J.R.; Tang, J.Y.; Huang, H.W.; Chan, Y.H.; Yen, C.Y. Withaferin A Induces Oxidative Stress-Mediated Apoptosis and DNA Damage in Oral Cancer Cells. Front. Physiol. 2017, 8, 634. [Google Scholar] [CrossRef]
- Bae, T.; Hallis, S.P.; Kwak, M.K. Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer. Exp. Mol. Med. 2024, 56, 501–514. [Google Scholar] [CrossRef]
- Li, M.; Du, X.; Yuan, Z.; Cheng, M.; Dong, P.; Bai, Y. Lentinan triggers oxidative stress-mediated anti-inflammatory responses in lung cancer cells. Mol. Cell. Biochem. 2022, 477, 469–477. [Google Scholar] [CrossRef]
- Liu, L.-X.; Heng, J.-H.; Deng, D.-X.; Zhao, H.; Zheng, Z.-Y.; Liao, L.-D.; Lin, W.; Xu, X.-E.; Li, E.-M.; Xu, L.-Y. Sulconazole Induces PANoptosis by Triggering Oxidative Stress and Inhibiting Glycolysis to Increase Radiosensitivity in Esophageal Cancer. Mol. Cell Proteom. 2023, 22, 100551. [Google Scholar] [CrossRef]
- Zhang, K.; Ping, L.; Du, T.; Wang, Y.; Sun, Y.; Liang, G.; Wang, X.; Xie, X.; Wei, W.; Xiao, X.; et al. A Novel Systematic Oxidative Stress Score Predicts the Prognosis of Patients with Operable Breast Cancer. Oxidative Med. Cell. Longev. 2021, 2021, 9441896. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, P.; Zhao, B.; Xu, J.; Shi, D. Preparation, structural characterization and biological activities of Laetiporus sulphureus polysaccharide and its stabilized selenium nanoparticles. Chem. Biol. Technol. Agric. 2024, 11, 176. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Kong, F.; Shu, L.; Li, Y.; Wang, D.; Li, L. Acidic polysaccharide from Ganoderma tsugae: Structural characterization and antiatherosclerotic related to macrophage polarization. Food Res. Int. 2025, 203, 115913. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yan, Y.; Hou, C.; Shi, M.; Liu, Y. Structural characterization of a galacturonic acid-rich polysaccharide from Ziziphus Jujuba cv. Muzao. Int. J. Biol. Macromol. 2020, 147, 844–852. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Yan, Y.; Shi, M.; Liu, Y. Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. [Google Scholar] [CrossRef]
- Zavadinack, M.; Cantu-Jungles, T.M.; Abreu, H.; Ozturk, O.K.; Cordeiro, L.M.C.; de Freitas, R.A.; Hamaker, B.R.; Iacomini, M. (1 → 3),(1 → 6) and (1 → 3)-β-D-glucan physico-chemical features drive their fermentation profile by the human gut microbiota. Carbohydr. Polym. 2024, 327, 121678. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Yu, H.; Zhou, S.; Zhang, Z.; Wu, D.; Yan, M.; Tang, Q.; Zhang, J. Structural characterization and immuno-enhancing activity of a highly branched water-soluble β-glucan from the spores of Ganoderma lucidum. Carbohydr. Polym. 2017, 167, 337–344. [Google Scholar] [CrossRef]
- Zheng, H.C.; Gao, S.; Liu, Y.Z.; Wang, T.S.; Chen, J.A.; Zhang, J.Z.; Li, C.Z.; Liu, D.C.; Gu, Y.X.; Lei, H.M.; et al. Bioactive glycyrrhizic acid-astragalus polysaccharide hydrogel facilitates gastric ulcer healing via ROS scavenging and anti-apoptotic effects. Carbohydr. Polym. 2025, 362, 13. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Heo, E.; Jeong, M.; Kim, J.H.; Park, J.J.; Chae, J.; Gwon, S.; Do Lee, S.; Kim, H.; Ojulari, O.V.; et al. Acidic polysaccharide from the edible insect Protaetia brevitarsis seulensis activates antiviral immunity to suppress norovirus infection. Carbohydr. Polym. 2025, 347, 122587. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhu, L.; Li, Y.; Jiang, S.; Sun, Q.; Xie, E.; Chen, H.; Zhao, Z.; Qiao, W.; Xu, J.; et al. Structure of a laminarin-type β-(1→3)-glucan from brown algae Sargassum henslowianum and its potential on regulating gut microbiota. Carbohydr. Polym. 2021, 255, 117389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, C.; Lai, P.F.H.; Xie, F.; Xia, Y.; Ai, L. NMR elucidation of a water-soluble β-(1→3, 1→6)-glucan from Russula vinosa Lindblad. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100311. [Google Scholar] [CrossRef]
- Wong, W.Y.; Ismail, S.M.; Phan, C.W.; Tan, Y.S. Size Matters: Influence of Particle Size on Antioxidant, β-Glucan, and Anti-Inflammatory Potential in Pleurotus floridanus (Agaricomycetes). Int. J. Med. Mushrooms 2024, 26, 17–31. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Q.; Farooqi, A.A.; Wang, J.; Yue, Y.; Geng, L.; Wu, N. Opportunities and challenges of fucoidan for tumors therapy. Carbohydr. Polym. 2024, 324, 121555. [Google Scholar] [CrossRef]
- Kumla, J.; Thangrongthong, S.; Kaewnunta, A.; Suwannarach, N. Research advances in fungal polysaccharides: Production, extraction, characterization, properties, and their multifaceted applications. Front. Cell. Infect. Microbiol. 2025, 15, 1604184. [Google Scholar] [CrossRef]
- Rezvani, V.; Pourianfar, H.R.; Mohammadnejad, S.; Madjid Ansari, A.; Farahmand, L. Anticancer potentiality and mode of action of low-carbohydrate proteins and peptides from mushrooms. Appl. Microbiol. Biotechnol. 2020, 104, 6855–6871. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, Y.; Pi, X.; Zhao, S.; Liu, W. In Vitro Hepatoprotective and Human Gut Microbiota Modulation of Polysaccharide-Peptides in Pleurotus citrinopileatus. Front. Cell. Infect. Microbiol. 2022, 12, 892049. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, Y.H. Ultrasound-assisted polysaccharide extraction from Cercis chinensis and properites, antioxidant activity of polysaccharide. Ultrason. Sonochem. 2023, 96, 106422. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, G. Extraction, structure, and activity of polysaccharide from Radix astragali. Biomed. Pharmacother. 2022, 150, 113015. [Google Scholar] [CrossRef]
- Jen, C.I.; Ng, L.T. F2-sulfated polysaccharides of Laetiporus sulphureus suppress triple-negative breast cancer cell proliferation and metastasis through the EGFR-mediated signaling pathway. Int. J. Biol. Macromol. 2025, 306, 141407. [Google Scholar] [CrossRef]
- Jen, C.I.; Lu, M.K.; Lai, M.N.; Ng, L.T. Sulfated polysaccharides of Laetiporus sulphureus fruiting bodies exhibit anti-breast cancer activity through cell cycle arrest, apoptosis induction, and inhibiting cell migration. J. Ethnopharmacol. 2024, 321, 117546. [Google Scholar] [CrossRef]
- Bai, J.H.; Xu, J.; Zhao, J.; Zhang, R. Ganoderma lucidum Polysaccharide Enzymatic Hydrolysate Suppresses the Growth of Human Colon Cancer Cells via Inducing Apoptosis. Cell Transpl. 2020, 29, 963689720931435. [Google Scholar] [CrossRef]
- Lee, I.Y.; Wang, T.C.; Kuo, Y.J.; Shih, W.T.; Yang, P.R.; Hsu, C.M.; Lin, Y.S.; Kuo, R.S.; Wu, C.Y. Astragalus Polysaccharides and Metformin May Have Synergistic Effects on the Apoptosis and Ferroptosis of Lung Adenocarcinoma A549 Cells. Curr. Issues Mol. Biol. 2024, 46, 7782–7794. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiong, F.; Liu, Y.; Liu, F.; Hao, Z.; Chen, H. Total fractionation and characterization of the water-soluble polysaccharides isolated from Enteromorpha intestinalis. Int. J. Biol. Macromol. 2018, 111, 319–325. [Google Scholar] [CrossRef]
- Selvendran, R.R.; Needs, P.W. Avoiding oxidative degradation during sodium hydroxide/methyl iodide-mediated carbohydrate methylation in dimethyl sulfoxide. Carbohydr. Res. 1993, 245, 1–10. [Google Scholar] [CrossRef]


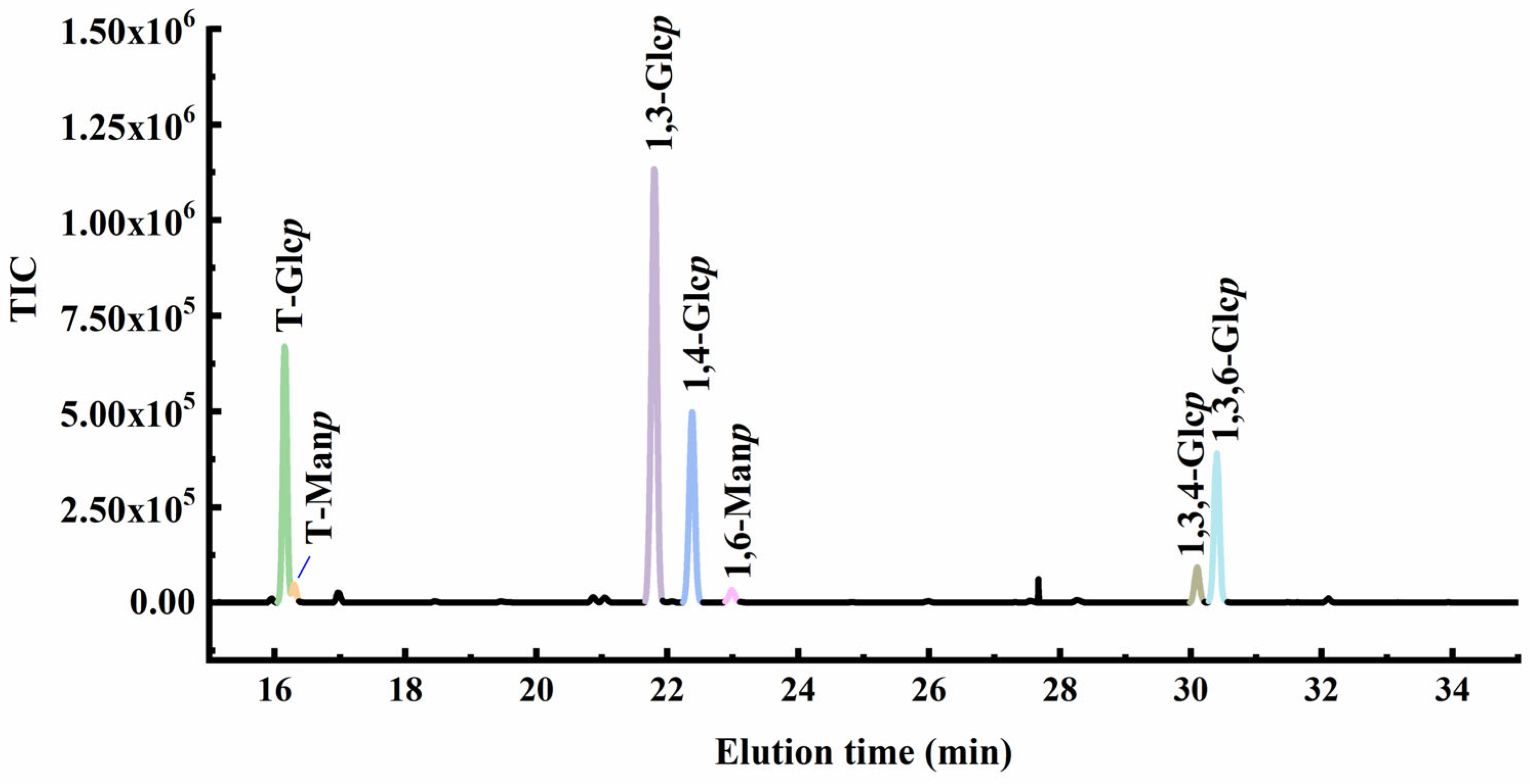

| Time (min) | Partially Methylated Alditol Acetates | Linkages | Molar (%) |
|---|---|---|---|
| 16.155 | 1,5-Di-O-acetyl-1-hydrogen-2,3,4,6-tetra-O-methyl-D-glucitol | T-Glcp | 18.8 |
| 16.295 | 1,5-Di-O-acetyl-1-hydrogen-2,3,4,6-tetra-O-methyl-D-mannitol | T-Manp | 1.4 |
| 21.800 | 1,3,5-Tri-O-acetyl-1-hydrogen-2,4,6-tri-O-methyl-D-glucitol | 1,3-Glcp | 42.4 |
| 22.381 | 1,4,5-Tri-O-acetyl-1-hydrogen-2,3,6-tri-O-methyl-D-glucitol | 1,4-Glcp | 18.9 |
| 22.988 | 1,5,6-Tri-O-acetyl-1-hydrogen-2,3,4-tri-O-methyl-D-mannitol | 1,6-Manp | 1.3 |
| 30.096 | 1,3,4,5-Tetra-O-acetyl-1-hydrogen-2,6-di-O-methyl-D-glucitol | 1,3,4-Glcp | 3.2 |
| 30.397 | 1,3,5,6-Tetra-O-acetyl-1-hydrogen-2,4-di-O-methyl-D-glucitol | 1,3,6-Glcp | 14.1 |
| Residues | Linkage Type | C1 | C2 | C3 | C4 | C5 | C6 | |
|---|---|---|---|---|---|---|---|---|
| H1 | H2 | H3 | H4 | H5 | H6a | H6b | ||
| A | β-D-Glcp-(1→ | 102.93 | 73.02 | - | 69.42 | - | 60.65 | |
| 4.56 | 3.35 | - | 3.46 | - | 3.93 | 3.78 | ||
| B | 3→)-β-D-Glcp-(1→ | 102.48 | 73.02 | 84.04 | 68.07 | 75.49 | 60.65 | |
| 4.83 | 3.61 | 3.83 | 3.55 | 3.54 | 3.93 | 3.78 | ||
| C | 4→)-α-D-Glcp-(1→ | 100.01 | 71.45 | 73.24 | 76.84 | 72.79 | 60.43 | |
| 5.39 | 3.61 | 4.00 | 3.68 | 3.78 | 3.90 | 4.12 | ||
| D | 3,4→)-α-D-Glcp-(1→ | 98.66 | - | 79.99 | - | - | 60.43 | |
| 5.02 | - | 3.94 | - | - | 3.90 | 4.12 | ||
| E | 3,6→)-β-D-Glcp-(1→ | 102.70 | 71.22 | 84.26 | - | - | 66.72 | |
| 4.56 | 3.89 | 3.81 | - | - | 4.19 | 3.72 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Y.; Yang, X.; Zhao, D.; Zhang, P.; Mi, Y.; Xu, J.; Zhao, B.; Shi, D. Structural Characterization and Anti-Tumor Activity of a Polysaccharide from Laetiporus sulphureus in A549 Cells. Molecules 2025, 30, 3706. https://doi.org/10.3390/molecules30183706
Qu Y, Yang X, Zhao D, Zhang P, Mi Y, Xu J, Zhao B, Shi D. Structural Characterization and Anti-Tumor Activity of a Polysaccharide from Laetiporus sulphureus in A549 Cells. Molecules. 2025; 30(18):3706. https://doi.org/10.3390/molecules30183706
Chicago/Turabian StyleQu, Yunhe, Xing Yang, Dongxue Zhao, Pingping Zhang, Yue Mi, Jing Xu, Boya Zhao, and Dongfang Shi. 2025. "Structural Characterization and Anti-Tumor Activity of a Polysaccharide from Laetiporus sulphureus in A549 Cells" Molecules 30, no. 18: 3706. https://doi.org/10.3390/molecules30183706
APA StyleQu, Y., Yang, X., Zhao, D., Zhang, P., Mi, Y., Xu, J., Zhao, B., & Shi, D. (2025). Structural Characterization and Anti-Tumor Activity of a Polysaccharide from Laetiporus sulphureus in A549 Cells. Molecules, 30(18), 3706. https://doi.org/10.3390/molecules30183706





