Abstract
Use of a microwave-labile leaving group, 2,4-dinitrophenyl (2,4-DNP), leads to O-glycosylation in 70–80% yields with high α-selectivity. The 2,4-DNP glycosyl donors, protected with electron-donating benzyl ethers, gave the best results under controlled microwave conditions, which were all conducted in polar aprotic solvents, such as DMF, and were all conducted in the absence of any reagent-activating additives such as a Lewis acid/base or Brønsted–Lowry acid/base. This method represents concerted efforts towards reagent-free neutral glycosylation.
1. Introduction
The chemical synthesis of an acetal, linking sugar units together, is of critical importance for moving the field of glycoscience forward. Chemical glycosylation is normally achieved at the electrophilic anomeric position through the reaction between an activatable glycosyl donor and a nucleophilic glycosyl acceptor employing a promoter, such as a Lewis acid, below ambient temperature [1]. Developing a glycosylation reaction that operates under non-acidic conditions is desirable to allow for the formation of acid-sensitive glycosidic linkages in the presence of acid-sensitive protecting groups.
Many recent reports have developed new glycosylation methods aiming for more reliable, robust, and user-friendly alternatives to the stringent conditions required when working with standard promoters, like Lewis acids. For instance, glycosylation with a hydrogen-bond donor catalyst [2], phenanthroline-catalyzed stereoselective glycosylation [3], chelation-assisted glycosylation [4], and enzymatic transformations all attempt to overcome challenges with stereoselectivity and hydrolysis realized during or after glycosylation has occurred. Among many glycosylation methods, a few reports by the groups of Gervay-Hague [5], Jensen [6], Waldmann [7], and others [4,8], describe employing neutral and mildly basic conditions. Of particular interest to us and relevant to the work described herein, the Jensen group, in particular, described a glycosylation method using dinitro salicylate (DISAL) donors. Under conventional heating conditions, they employed the DISAL donor to obtain a glycosylated product at 40–60 °C within 17 h using LiClO4 as a mild Lewis acid activator. Having also performed this reaction under microwave irradiation, they were able to reduce the reaction time from hours to minutes at elevated reaction temperatures from 60 °C to 100 °C [6,9,10].
Cognizant of Jensen’s approach, we set out to target thermal activation of armed [11] sugar donors with the assistance of microwave irradiation but in the absence of any external additives/promoters. We hypothesized that microwave irradiation [12] has the potential to promote thermally driven glycosylation reactions through localized heating in the absence of reagent additives (only solvent), for short reaction times and improved overall yields. Over the past three decades, microwave-assisted chemistry has attracted a considerable amount of attention in the labs of academic and industrial groups, allowing for the discovery of unprecedented chemical reactivities [13,14,15] and efficiency in glycosylations [16,17]. Along these lines, our longstanding interest has been to develop microwave-labile leaving groups (MWLLGs), which can be used in an orthogonal manner with other common protecting groups. With that idea in mind, we have designed and incorporated ester- and nitrophenol-based microwave-labile leaving groups (donors 1–3, Figure 1) and performed glycosylation reactions under microwave irradiation in the absence of any promoters.
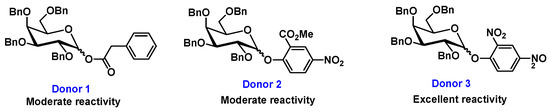
Figure 1.
Ester and nitrophenol-based microwave-labile leaving groups.
2. Results
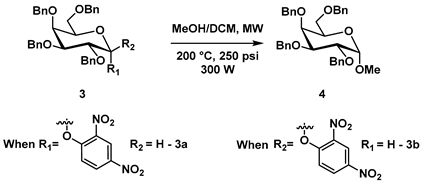
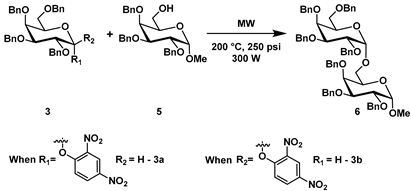
In order to establish that glycosylation reactions would ensue under our ascribed conditions, a series of preliminary solvolysis experiments were conducted (Scheme 1). We investigated D-galactose-derived donors, which are known for their high reactivity. Both galactosyl phenylacetate (donor 1) and 2-methoxycarbonyl-4-nitrophenyl D-galactoside (donor 2), in a MeOH/DCM = 9:1 solvent system using microwave irradiation at 200 °C, 300 W, and 250 psi, gave methyl glycoside (4) in yields above 75% (all synthetic procedures and characterization data are presented in supplementary material). In these solvolysis cases, methanol acted as an acceptor and DCM was simply used to dissolve the donors. After realizing our solvolysis approach, we thought that, for the preparation of a simple disaccharide in our glycosylation system (Scheme 1; glycosylation reactions), the reaction of donor 1 with acceptor (5) at 200 °C, 300 W, and 250 psi in DMF would be sufficient. However, in practice, we observed a yield of 35% for compound 6. The solvent change from MEOH/DCM to DMF is rationalized as follows: DMF, as the polar aprotic solvent with a large dipole moment (µ = 3.86 D at 25 °C), high boiling point (153 °C) and dielectric constant (ε = 37.51 at 25 °C), has been recognized as an optimal solvent for microwave-based reactions [18]. However, when we employed DMF as our solvent in our glycosylation reaction with donor 2 and 6-OH D-galactosyl acceptor 5 (Scheme 1), we only observed a 50% yield of compound 6.
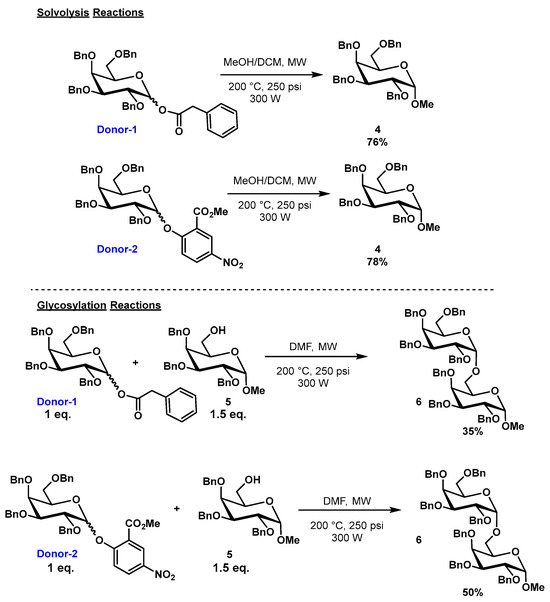
Scheme 1.
Microwave reaction conditions for glycosylation using sugar donors 1 and 2.
While we were pleased to obtain results noted in Scheme 1, the issue of low yield plagued us, and we decided to replace the anomeric moieties noted in Scheme 1 with more electron-deficient aryl dinitro groups by employing 2,4-DNP donor 3. We elected to employ 2,4-DNP as a glycosyl donor, given its leaving group potential in water but also understanding that it is not reactive enough to undergo glycosylation on its own. To our delight, donor 3 reacted under our prescribed solvolysis conditions and gave methyl glycoside 4 in excellent yields of 81–87% (Table 1; entry 1, 2, 3). In every example conducted, 2,4-dinitrophenol was isolated as the sole by-product (recyclable in our process), which was isolated from the aqueous layer using size exclusion chromatography. Prepared 2,4-DNP donor 3 (see SI) was dissolved in a dry methanol/DCM solution and then subjected to microwave irradiation at 200 °C, 300 W (power), and 250 psi (Table 1). When the reaction was conducted in a 1:1 mixture of 3a and 3b, it resulted in 1.3:1 mixture of α and β products (Table 1; entry 3). It is important to note that we observed no reaction with 3a and 3b utilizing traditional refluxing conditions (Table 1; entry 4). The reaction pH was monitored and noted to be neutral, even though the by-product of 2,4-dinitrophenol has a pKa ~4. These initial results confirmed that the 2,4-DNP donor was highly susceptible to microwave irradiation under a high temperature and pressure. It was also rewarding to observe that benzylated donors and acceptors did not decompose at high temperature microwave conditions and the 2,4-DNP donor 3 was stable at room temperature without any decomposition over long periods of time (months). These results demonstrated that we could overcome issues with yields and gave us confidence that an entirely neutral glycosylation method (without additives/promoters) employing thermal microwave energy could indeed be realized.

Table 1.
Solvolysis of 2,4-DNP donor.
Entry 2 in Table 1 shows either a mixture of α and β products, indicative of an SN1-like pathway (entry 1), or the stereospecific formation of the α anomer, an SN2-like pathway [19,20]. This apparent paradox can be explained by contact ion pairs. While the reaction of 3b may form the equatorial (eq) contact ion pair (CIP) (assuming that eq-CIP is more reactive than the axial (ax)-CIP) that then forms the exclusive α product, donor 3a may undergo departure of a dinitrophenolate ion and form an ax-CIP which may convert into the more reactive eq-CIP (Figure 2), forming a mixture of α and β products.
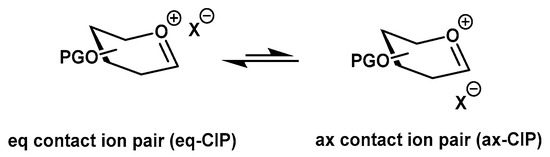
Figure 2.
Contact ion pairs.
After our initial success, we moved forward to probe the reaction scope with 2,4-DNP D-galactoside and complex acceptors using our microwave conditions. Our investigation commenced with treatment of benzylated 2,4-DNP donor 3 with benzylated 6-OH D-galactosyl acceptor (5) using various solvents under microwave conditions at different temperatures. (Table 2). Donor 3 was separated out into both the 3a and 3b anomers, which were treated individually to determine the effect of donor stereochemistry.

Table 2.
Preliminary reaction optimization under microwave conditions. a
A variety of solvents were tested; however, no glycosylation product was obtained when low boiling point and low microwave-absorbing solvents [18] (DCM, THF and ACN) were used (Table 2; entries 1–3). When the solvent was changed to a high boiling point, microwave-absorbing solvent [18] such as DMF, and N-methyl-2-pyrollidone (NMP), the glycosylation proceeded to afford the desired glycoside 6 (Table 2; entries 4–12). The reaction at 250 °C in DMF provided glycoside 6 in excellent yield and high α selectivity (Table 2; entries 10 and 11). Similarly, the reactions conducted at 200 °C in DMF gave higher yields for both 3a and 3b donors within 1 h. (Table 2; entry 8 and 9). However, reactions conducted at 150 °C required somewhat longer reaction time-courses for product formation (Table 2; entries 6 and 7). Reactions in which the temperature was noted to be below 150 °C yielded no product, as observed by NMR. Lower yields were observed in glycosylation reactions with NMP when comparing to DMF (Table 2; entry 12). In all cases, DMF as the solvent proved to produce the best results using our reaction conditions.
As noted in the literature, DMF is frequently used as a polar aprotic solvent for various transformations and can be degraded into many intermediates such as HCO2H, CO, HNMe2, HCONMe2, NH2COH, etc. [21]. To answer the question as to why DMF might be promoting efficient glycosylation at a high temperature under microwave irradiation, we performed a control experiment employing DCM in the presence of N,N-dimethylamine (10 eq with respect to donor). When compound 3a and 3b were subjected to microwave irradiation, product 6 was obtained. The reaction did require a longer reaction time for completion, most likely because the maximum microwave heating temperature for DCM is 150 °C [18]. When combined, the results confirm that the involvement of the N,N-dimethylamine can promote glycosylation; N,N-dimethylamine might be generated in situ from decomposition of DMF under our described reaction conditions [22]. The use of N,N-dimethylamine in DCM did not affect the yield nor the α/β ratio. Both 3a and 3b donors gave similar results with respect to yield and in all cases resulted in higher α selectivity. To analyze the role of dimethylamine as a base, we tested DMAP and triethylamine under the same conditions. However, no product formation was observed. This clearly indicates that dimethylamine does not only play a role as a base. The power (300 W) and pressure (250 psi) of our instrument were kept constant for all comparisons.
The reaction between donor 3 and 6-OH acceptor 5 at 250 °C in DMF gave a desired disaccharide 6 with an excellent yield of 80% (Table 2; entry 10). This is explained by the electron-withdrawing nitro group, which makes 2,4-DNP an excellent leaving group by stabilizing its conjugate base (compound (x), Figure 3).
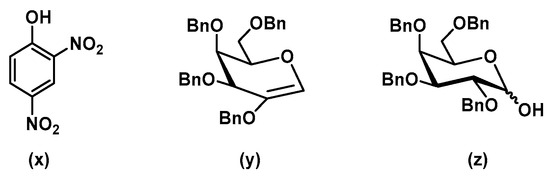
Figure 3.
By-products of glycosylation when using donor 3.
Having established that DMF could serve as the best solvent under our microwave conditions, we elected to utilize 0.03 M–0.04 M for acceptor concentration and 0.07 M–0.08 M donor concentration. This concentration ratio was based off our maximum yields obtained. In our studies, we noted that use of excess donor (1.5 eq. of donor and 1 eq. of acceptor) provided the best overall yields. However, use of excess acceptor reduced the number of by-products related to the donor (elimination product and hydrolysis product; (y) and (z) respectively). 2,4-dinitrophenol (x) was isolated as the sole by-product, which is derived from the donor. In addition, 2,3,4,6-tetra-O-benzyl-d-galactal (y), the elimination product (Figure 3), was formed when temperatures reached more than 150 °C. Furthermore, tetra-O-benzyl-d-galactopyranose (z), the hydrolysis product, could be formed as a result of H2O being added to the reaction mixture [9].
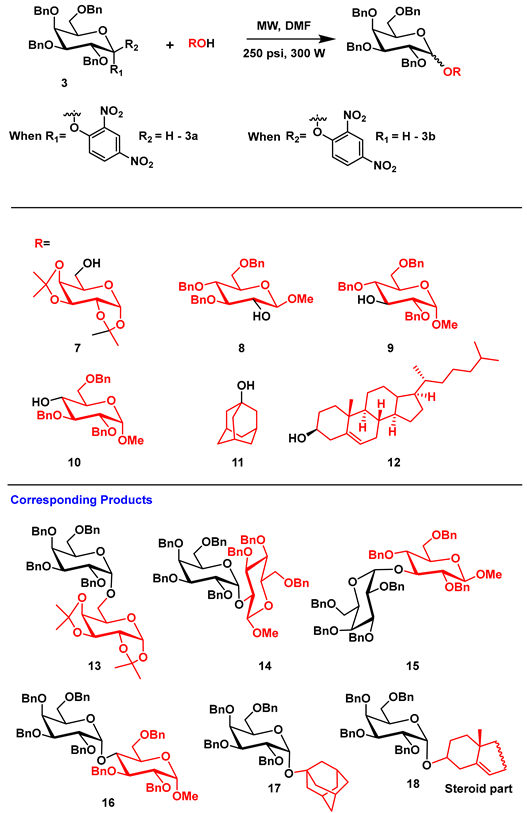
Using entries 8 and 9 (Table 2) as our optimized condition for the glycosylation, we turned our attention to increasing the substrate scope of the reaction by screening a range of acceptors (Table 3). We elected to use a number of sugar acceptors and examined the reaction of 3a and 3b with 6-OH acceptor 7. This is a primary acceptor which carries acid-sensitive isopropylidene groups, which proved to be a compatible substrate for this glycosylation protocol and afforded 13 in 60–68% (depending on α- or β-donor) with overall high α selectivity (Table 3; entries 1 and 2). Further experiments of monosaccharide acceptors showed that secondary hydroxyl groups at positions 2 or 3 also afforded good to moderate yields. Secondary 2-OH acceptor 8 produced disaccharide 14 in a 65% yield from 3a with high α-selectivity (Table 3; entry 3) and a 58% yield from 3b with 3:1 α/β ratio (Table 3; entry 4). When 3a and 3b were reacted with 3-OH acceptor 9, disaccharide 15 was obtained in 62–65% with high α-selectivity (Table 3, entry 5 and 6). Unsurprisingly, the 4-OH acceptor 10 is comparatively less reactive to other secondary acceptors and it was completely inert under our reaction conditions. It was gratifying to observe, however, that the reaction of 1-adamantanol 11, a tertiary alcohol, gave the resultant product 17 in 65–70% with exclusive α-selectivity (Table 3; entry 9 and 10). Cholesterol 12, as a secondary acceptor, gave high α-selectivity as well (Table 3; entry 11 and 12) from both 3a and 3b donors.

Table 3.
Glycosylation of different acceptors with per-benzylated galactosyl DNP donor. a
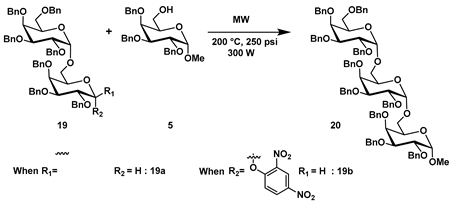
Based on our successful attempts with 2,4-DNP galactosyl donor 3a and 3b, we sought to investigate the synthesis of a trisaccharide under our microwave conditions (Table 4). To accomplish this, we synthesized disaccharide 2,4-DNP donor 19 using the double-base method [6], and then we examined the reactions of disaccharide 2,4-DNP donor 19 and 6-OH D-galactosyl acceptor 5. Trisaccharide 20 was obtained in a modest yield of 40% and 35% from 19a and 19b disaccharide donors, respectively (Table 4; entries 1 and 2) [23].

Table 4.
Synthesis of trisaccharide with DNP glycoside as a donor.
Although DMF is not very common in glycosylation, it has been utilized in glycosylation reactions as a solvent and as an additive to effect α-glycosylation by the Mong group, as noted in the literature [24,25]. In the course of our studies, we obtained 1,2-cis (α) as the major product. We believe this is the result of the addition of DMF to the oxocarbenium intermediate, forming both α and β glycosylimidates [25]. In addition to the modulating properties, DMF is a good microwave-absorbing solvent which may promote the thermal activation of glycosyl donors.
3. Conclusions
In conclusion, this microwave-assisted glycosylation method provides an important platform for the formation of glycosides under operationally simple conditions relative to the conventional glycosylation methods. Herein, we have introduced a new donor with a microwave-labile leaving group 2,4-DNP. Benzyl-protected (2,4-DNP donor) 3a and 3b donors showed a similar reactivity and inversion of configuration was observed under solvolysis conditions. O-glycosylations with the 2,4-DNP donor and various acceptors in DMF were promoted by microwave heating at elevated temperatures. Furthermore, we were able to synthesize a trisaccharide using optimized microwave reaction conditions. We believe that there is an effect of N,N-dimethylamine (base), which might form in situ during the reaction. Further investigations of compatibility of electron-withdrawing (disarming) acetate and benzoyl-protecting groups and the role of the N,N-dimethylamine are currently underway in our lab and will be reported in due course.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30183693/s1 [6,26,27,28,29,30,31,32,33,34].
Author Contributions
Conceptualization, S.M.P.G.; methodology, S.M.P.G., S.G. and P.E.; validation, P.E., S.G. and G.V.; investigation, S.M.P.G., R.S.B. and G.V.; resources, P.R.A.; data curation, S.M.P.G.; writing—original draft preparation, S.M.P.G.; writing—review and editing, P.R.A., D.C. and G.V.; supervision, S.G., P.E., P.R.A. and D.C.; project administration, P.R.A. and D.C.; funding acquisition, P.R.A. and D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the NIH under a U01 Research Project Cooperative Agreement: U01GM125271.
Data Availability Statement
The original contributions presented in this study are included in this article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We acknowledge Yong Wah Kim (University of Toledo) for assistance with NMR data and Jianglong Zhu (University of Toledo) for insightful discussion on the potentiality of microwave irradiation in glycosylation chemistry.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
References
- Adero, P.O.; Amarasekara, H.; Wen, P.; Bohé, L.; Crich, D. The Experimental Evidence in Support of Glycosylation Mechanisms at the SN1–SN2 Interface. Chem. Rev. 2018, 118, 8242–8284. [Google Scholar] [CrossRef]
- Levi, S.M.; Li, Q.; Rötheli, A.R.; Jacobsen, E.N. Catalytic activation of glycosyl phosphates for stereoselective coupling reactions. Proc. Natl. Acad. Sci. USA 2019, 116, 35. [Google Scholar] [CrossRef]
- Yu, F.; Li, J.; DeMent, P.M.; Tu, Y.-J.; Schlegel, H.B.; Nguyen, H.M. Phenanthroline-Catalyzed Stereoretentive Glycosylations. Angew. Chem. Int. Ed. 2019, 58, 6957–6961. [Google Scholar] [CrossRef]
- Wang, H.Y.; Simmons, C.J.; Blaszczyk, S.A.; Balzer, P.G.; Luo, R.; Duan, X.; Tang, W. Isoquinoline-1-Carboxylate as a Traceless Leaving Group for Chelation-Assisted Glycosylation under Mild and Neutral Reaction Conditions. Angew. Chem. Int. Ed. Engl. 2017, 56, 15698–15702. [Google Scholar] [CrossRef]
- Hadd, M.J.; Gervay, J. Glycosyl iodides are highly efficient donors under neutral conditions. Carbohydr. Res. 1999, 320, 61–69. [Google Scholar] [CrossRef]
- Petersen, L.; Jensen, K.J. A New, Efficient Glycosylation Method for Oligosaccharide Synthesis under Neutral Conditions: Preparation and Use of New DISAL Donors. J. Org. Chem. 2001, 66, 6268–6275. [Google Scholar] [CrossRef]
- Böhm, G.; Waldmann, H. O-Glycoside Synthesis under Neutral Conditions in Concentrated Solutions of LiClO4 in Organic Solvents Employing Benzyl-Protected Glycosyl Donors. Liebigs Ann. 1996, 1996, 613–619. [Google Scholar] [CrossRef]
- Jensen, K.J. O-Glycosylations under neutral or basic conditions. J. Chem. Soc. Perkin Trans. 1 2002, 2219–2233. [Google Scholar] [CrossRef]
- Larsen, K.; Worm-Leonhard, K.; Olsen, P.; Hoel, A.; Jensen, K.J. Reconsidering glycosylations at high temperature: Precise microwave heating. Org. Biomol. Chem. 2005, 3, 3966–3970. [Google Scholar] [CrossRef] [PubMed]
- Grathe, S.; Thygesen, M.B.; Larsen, K.; Petersen, L.; Jensen, K.J. Glucosamine derived DISAL donors for stereoselective glycosylations under neutral conditions. Tetrahedron Asymmetry 2005, 16, 1439–1448. [Google Scholar] [CrossRef]
- Mootoo, D.R.; Konradsson, P.; Udodong, U.; Fraser-Reid, B. Armed and disarmed n-pentenyl glycosides in saccharide couplings leading to oligosaccharides. J. Am. Chem. Soc. 1988, 110, 5583–5584. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. Engl. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Nigudkar, S.S.; Demchenko, A.V. Stereocontrolled 1,2-cis glycosylation as the driving force of progress in synthetic carbohydrate chemistry. Chem. Sci. 2015, 6, 2687–2704. [Google Scholar] [CrossRef]
- Gedye, R.; Smith, F.; Westaway, K.; Ali, H.; Baldisera, L.; Laberge, L.; Rousell, J. The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett. 1986, 27, 279–282. [Google Scholar] [CrossRef]
- Santra, S.; Andreana, P.R. Correction to A Rapid, One-Pot, Microwave-Influenced Synthesis of Spiro-2,5-diketopiperazines via a Cascade Ugi/6-Exo-Trig Aza-Michael Reaction. J. Org. Chem. 2011, 76, 7632. [Google Scholar] [CrossRef]
- Bornaghi, L.F.; Poulsen, S.-A. Microwave-accelerated Fischer glycosylation. Tetrahedron Lett. 2005, 46, 3485–3488. [Google Scholar] [CrossRef]
- Mathew, F.; Jayaprakash, K.N.; Fraser-Reid, B.; Mathew, J.; Scicinski, J. Microwave-assisted saccharide coupling with n-pentenyl glycosyl donors. Tetrahedron Lett. 2003, 44, 9051–9054. [Google Scholar] [CrossRef]
- Hayes, B.L. Microwave Synthesis: Chemistry at the Speed of Light; CEM Corp.: Matthews, NC, USA, 2002. [Google Scholar]
- Andreana, P.R.; Crich, D. Guidelines for O-Glycoside Formation from First Principles. ACS Cent. Sci. 2021, 7, 1454–1462. [Google Scholar] [CrossRef]
- Berven, L.A.; Dolphin, D.; Withers, S.G. The base-catalysed anomerization of dinitrophenyl glycosides: Evidence for a novel reaction mechanism. Can. J. Chem. 1990, 68, 1859–1866. [Google Scholar] [CrossRef]
- Heravi, M.M.; Ghavidel, M.; Mohammadkhani, L. Beyond a solvent: Triple roles of dimethylformamide in organic chemistry. RSC Adv. 2018, 8, 27832–27862. [Google Scholar] [CrossRef]
- Petersen, T.P.; Larsen, A.F.; Ritzén, A.; Ulven, T. Continuous Flow Nucleophilic Aromatic Substitution with Dimethylamine Generated in Situ by Decomposition of DMF. J. Org. Chem. 2013, 78, 4190–4195. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.; Laursen, J.B.; Larsen, K.; Motawia, M.S.; Jensen, K.J. DISAL Glycosyl Donors for the Synthesis of a Linear Hexasaccharide under Mild Conditions. Org. Lett. 2003, 5, 1309–1312. [Google Scholar] [CrossRef]
- Shingu, Y.; Miyachi, A.; Miura, Y.; Kobayashi, K.; Nishida, Y. One-pot α-glycosylation pathway via the generation in situ of α-glycopyranosyl imidates in N,N-dimethylformamide. Carbohydr. Res. 2005, 340, 2236–2244. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-R.; Lai, Y.-H.; Chen, J.-H.; Liu, C.-Y.; Mong, K.-K.T. Dimethylformamide: An Unusual Glycosylation Modulator. Angew. Chem. Int. Ed. 2011, 50, 7315–7320. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, S.; Matwiejuk, M.; Thiem, J. Acceptor-influenced and donor-tuned base-promoted glycosylation. Beilstein J. Org. Chem. 2012, 8, 413–420. [Google Scholar] [CrossRef]
- Lam, S.N.; Gervay-Hague, J. Solution- and solid-phase oligosaccharide synthesis using glucosyl iodides: A comparative study. Carbohydr. Res. 2002, 337, 1953–1965. [Google Scholar] [CrossRef]
- Kalikanda, J.; Li, Z. Study of the stereoselectivity of 2-azido-2-deoxygalactosyl donors: Relationship to the steric factors of glycosyl acceptors. Carbohydr. Res. 2011, 346, 2380–2383. [Google Scholar] [CrossRef]
- Levecque, P.; Gammon, D.W.; Kinfe, H.H.; Jacobs, P.; De Vos, D.; Sels, B. Tandem Epoxidation-Alcoholysis or Epoxidation-Hydrolysis of Glycals Catalyzed by Titanium(IV) Isopropoxide or Venturello’s. Adv. Synth. Catal. 2008, 350, 1557–1568. [Google Scholar] [CrossRef]
- Nokami, T.; Shibuya, A.; Tsuyama, H.; Suga, S.; Bowers, A.A.; Crich, D.; Yoshida, J.-I. Electrochemical Generation of Glycosyl Triflate Pools. J. Am. Chem. Soc. 2007, 129, 10922–10928. [Google Scholar] [CrossRef]
- Chu, A.-H.A.; Nguyen, S.H.; Sisel, J.A.; Minciunescu, A.; Bennett, C.S. Selective Synthesis of 1,2-cis-α-Glycosides without Directing Groups. Application to Iterative Oligosaccharide Synthesis. Org. Lett. 2013, 15, 2566–2569. [Google Scholar] [CrossRef]
- Sridhar, P.R.; Anjaneyulu, B.; Rao, B.U. Regioselective Anomeric O-Benzyl Deprotection in Carbohydrates. Eur. J. Org. Chem. 2021, 2021, 5665–5668. [Google Scholar] [CrossRef]
- Colombel, S.; Van Hijfte, N.; Poisson, T.; Leclerc, E.; Pannecoucke, X. Addition of Electrophilic Radicals to 2-Benzyloxyglycals: Synthesis and Functionalization of Fluorinated α-C-Glycosides and Derivatives. Chem. Eur. J. 2013, 19, 12778–12787. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, L.R.; Mach, L.; Heinrich, M.R. Nitrogen Oxides and Nitric Acid Enable the Sustainable Hydroxylation and Nitrohydroxylation of Benzenes under Visible Light Irradiation. J. Org. Chem. 2018, 83, 431–436. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).