From Apple Waste to Antimicrobial Solutions: A Review of Phenolics from PGI ‘Maçã de Alcobaça’ and Related Cultivars
Abstract
1. Introduction
2. Methodology Research
3. Phenolic Profile of ‘Maçã de Alcobaça’ and Related Apple Cultivars
4. Biological Activities of Phenolics from PGI ‘Maçã de Alcobaça’ and Related Cultivars
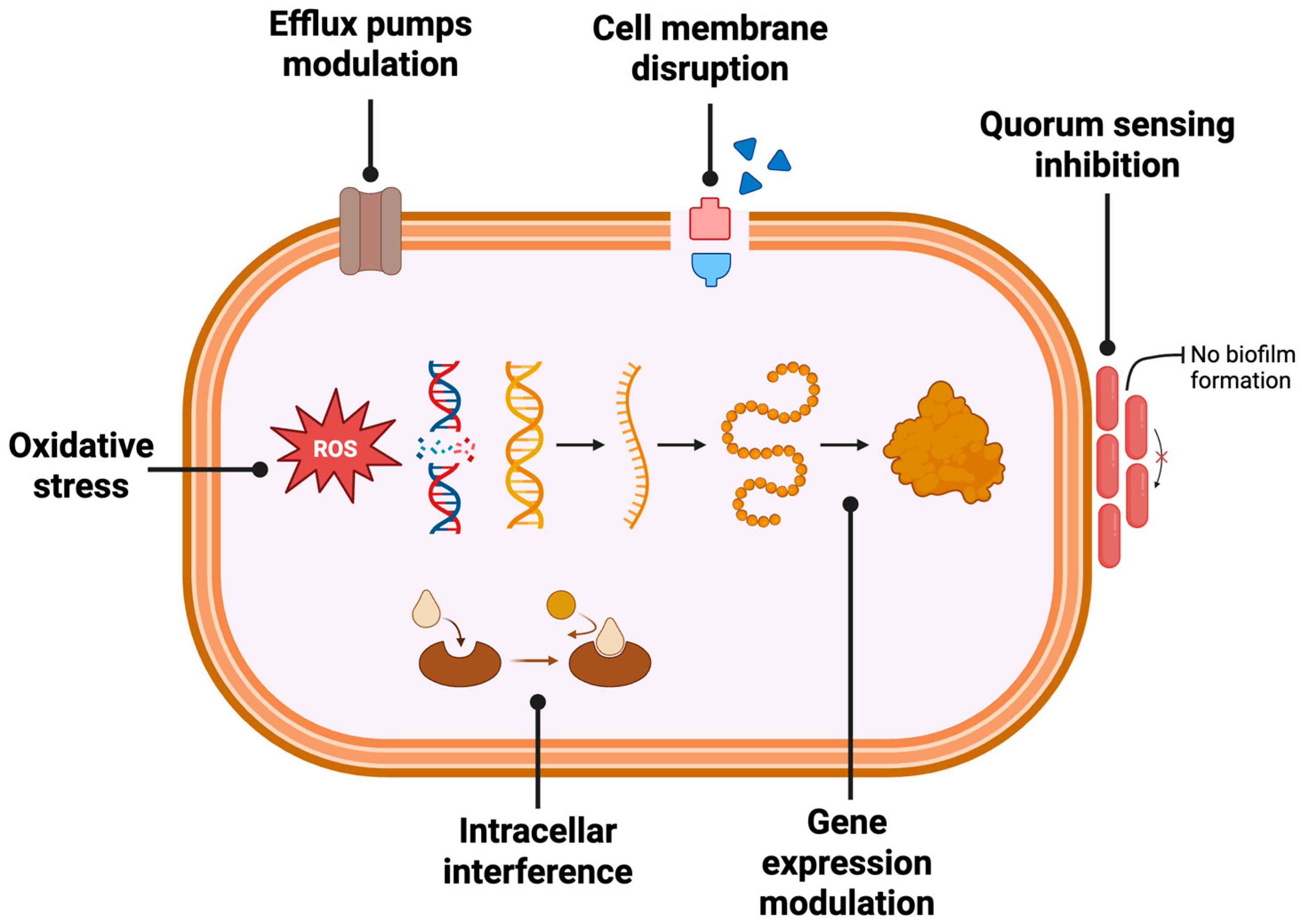
4.1. Antibacterial Activity
4.1.1. Mechanisms of Bacterial Inhibition
4.1.2. Structure-Activity Relationship
4.1.3. Extraction-Activity Relationship
4.2. Antioxidant Activity
4.3. Other Health Benefits
5. Potential Applications of Phenolics from PGI ‘Maçã de Alcobaça’ and Related Cultivars
5.1. Synergistic Effects with Antibiotics
5.2. Application in Food Systems
5.3. Medical and Therapeutic Applications
6. Challenges and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Agricultural Production Statistics 2010–2023; FAOSTAT Analytical Briefs: Rome, Italy, 2024. [Google Scholar]
- Arnold, M.; Gramza-Michalowska, A. Recent Development on the Chemical Composition and Phenolic Extraction Methods of Apple (Malus domestica)—A Review. Food Bioprocess Technol. 2024, 17, 2519–2560. [Google Scholar] [CrossRef]
- Balık, S.; Kaya, T.; Aslantaş, R. Fruit Quality Parameters, Sugars, Vitamin C, Antioxidant Activity, Organic Acids, and Phenolic Compounds for a New Endemic Apple Variety, “Long Apple”. Horticulturae 2023, 9, 1171. [Google Scholar] [CrossRef]
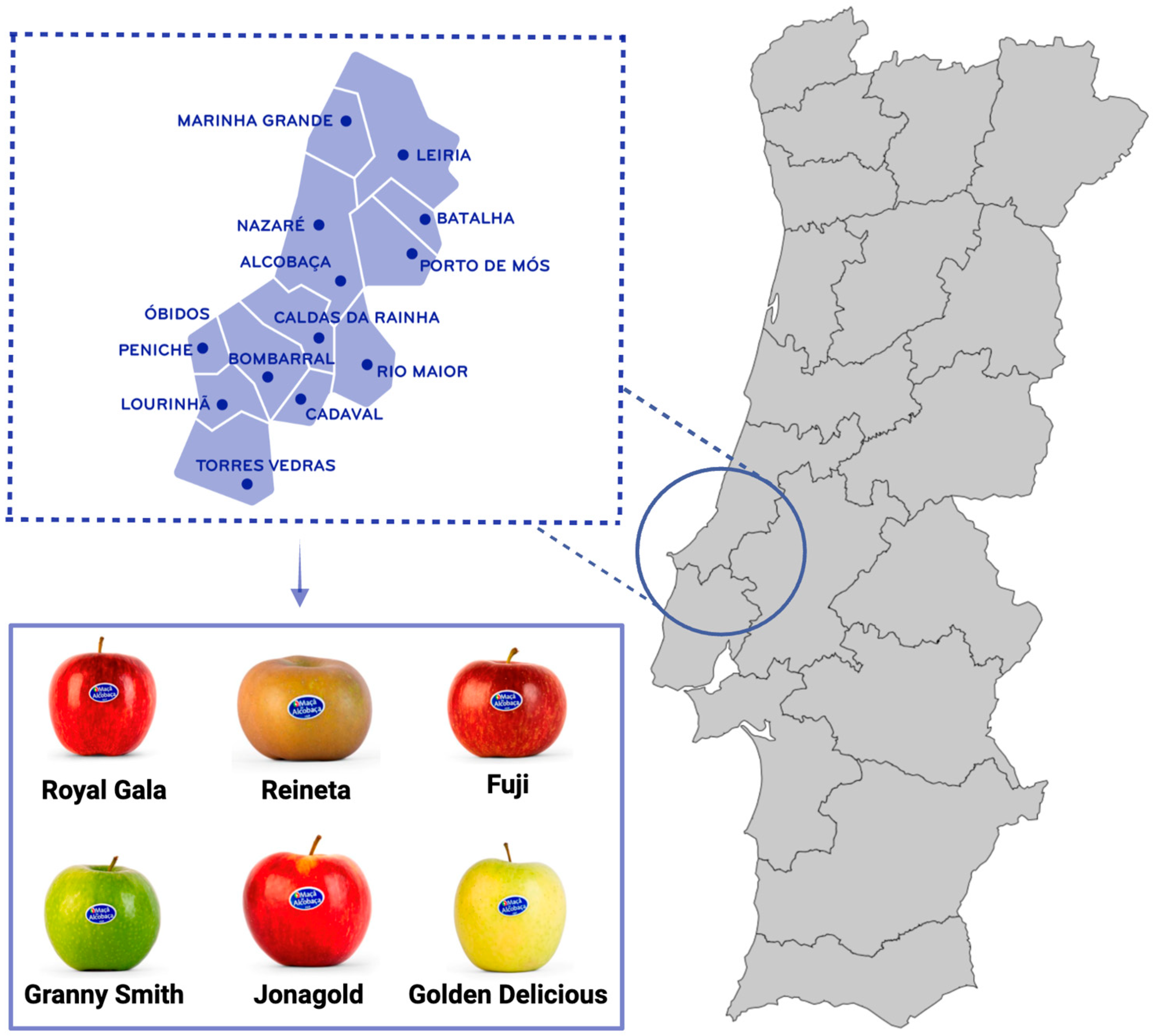
- APMA Maçã de Alcobaça. Available online: https://www.macadealcobaca.pt/macadealcobacaigp (accessed on 18 March 2025).
- Duarte, B.; Melo, J.; Mamede, R.; Carreiras, J.; Figueiredo, A.; Fonseca, V.F.; de Sousa, M.L.; Silva, A.B. In the Trail of “Maçã de Alcobaça” Protected Geographical Indication (PGI): Multielement Chemometrics as a Security and Anti-Fraud Tool to Depict Clones, Cultivars and Geographical Origins and Nutritional Value. J. Food Compos. Anal. 2023, 115, 104976. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Arraibi, A.A.; Ferreira, I.C.F.R. Bioactive and Functional Compounds in Apple Pomace from Juice and Cider Manufacturing: Potential Use in Dermal Formulations. Trends Food Sci. Technol. 2019, 90, 76–87. [Google Scholar] [CrossRef]
- Teixeira, J.D.; Soares Mateus, A.R.; Sanchez, C.; Parpot, P.; Almeida, C.; Sanches Silva, A. Antioxidant Capacity and Phenolics Profile of Portuguese Traditional Cultivars of Apples and Pears and Their By-Products: On the Way to Newer Applications. Foods 2023, 12, 1537. [Google Scholar] [CrossRef]
- Waldbauer, K.; McKinnon, R.; Kopp, B. Apple Pomace as Potential Source of Natural Active Compounds. Planta Med. 2017, 83, 994–1010. [Google Scholar] [CrossRef]
- Orozco-Flores, L.A.; Salas, E.; Rocha-Gutiérrez, B.; Peralta-Pérez, M.D.R.; González-Sánchez, G.; Ballinas-Casarrubias, L. Determination of Polyphenolic Profile of Apple Pomace (Malus domestica Golden Delicious Variety) by HPLC-MS. ACS Omega 2024, 9, 196–203. [Google Scholar] [CrossRef]
- Chen, X.; Lan, W.; Xie, J. Natural Phenolic Compounds: Antimicrobial Properties, Antimicrobial Mechanisms, and Potential Utilization in the Preservation of Aquatic Products. Food Chem. 2024, 440, 138198. [Google Scholar] [CrossRef]
- Almeida, D.P.F.; Gomes, M.H. Physicochemical Quality, Macronutrients, and Dietary Fiber in Apples from the Protected Geographical Indication ‘Maçã de Alcobaça’, Portugal. Eur. J. Hortic. Sci. 2017, 82, 239–243. [Google Scholar] [CrossRef]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and Their Applications: An Approach in Food Chemistry and Innovation Potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant and Antimicrobial Properties of Dried Portuguese Apple Variety (Malus domestica Borkh. Cv Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the Extraction Method on the Recovery of Bioactive Phenolic Compounds from Food Industry By-Products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.P.F.; Gião, M.S.; Pintado, M.; Gomes, M.H. Bioactive Phytochemicals in Apple Cultivars from the Portuguese Protected Geographical Indication “Maçã de Alcobaça:“ Basis for Market Segmentation. Int. J. Food Prop. 2017, 20, 2206–2214. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Gonzalez-Manzano, S.; Dueñas, M.; Gonzalez-Paramas, A.M. Extraction and Isolation of Phenolic Compounds. In Methods in Molecular Biology; John, M., Walker, Eds.; Springer Protocols: Berlin, Germany, 2012; Volume 864, pp. 427–464. ISBN 9781617796234. [Google Scholar]
- Wojdyło, A.; Oszmiański, J. Antioxidant Activity Modulated by Polyphenol Contents in Apple and Leaves during Fruit Development and Ripening. Antioxidants 2020, 9, 567. [Google Scholar] [CrossRef]
- Ferreira, C.; Ribeiro, C.; Nunes, F.M. Effect of Storage Conditions on Phenolic Composition, Vitamin C and Antioxidant Activity of “Golden Delicious” and “Red Delicious” Apples. Postharvest. Biol. Technol. 2024, 210, 112754. [Google Scholar] [CrossRef]
- Schmitzer, V.; Medic, A.; Bordon, A.; Hudina, M.; Veberic, R.; Jakopic, J.; Stampar, F. Metabolite Diversity in Pulp Segments, Peel, Leaves, and Bark of a Red-Fleshed ‘Baya Marisa’ Apple Cultivar. Agriculture 2023, 13, 1564. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Igrejas, G.; Aires, A.; Falco, V.; Valentão, P.; Poeta, P. Phenolic Compounds Classification and Their Distribution in Winemaking By-Products. Eur. Food Res. Technol. 2023, 249, 207–239. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Olšteine, A.; Krasnova, I.; Pugajeva, I.; Lacis, G.; Siger, A.; Michalak, M.; Soliven, A.; Segliņa, D. Phenolic Compounds in Different Fruit Parts of Crab Apple: Dihydrochalcones as Promising Quality Markers of Industrial Apple Pomace by-Products. Ind. Crops Prod. 2015, 74, 607–612. [Google Scholar] [CrossRef]
- Feng, S.; Yi, J.; Li, X.; Wu, X.; Zhao, Y.; Ma, Y.; Bi, J. Systematic Review of Phenolic Compounds in Apple Fruits: Compositions, Distribution, Absorption, Metabolism, and Processing Stability. J. Agric. Food Chem. 2021, 69, 7–27. [Google Scholar] [CrossRef]
- Napolitano, A.; Cascone, A.; Graziani, G.; Ferracane, R.; Scalfi, L.; Di Vaio, C.; Ritieni, A.; Fogliano, V. Influence of Variety and Storage on the Polyphenol Composition of Apple Flesh. J. Agric. Food Chem. 2004, 52, 6526–6531. [Google Scholar] [CrossRef]
- Giomaro, G.; Karioti, A.; Bilia, A.R.; Bucchini, A.; Giamperi, L.; Ricci, D.; Fraternale, D. Polyphenols Profile and Antioxidant Activity of Skin and Pulp of a Rare Apple from Marche Region (Italy). Chem. Cent. J. 2014, 8, 45. [Google Scholar] [CrossRef]
- Shehzadi, K.; Rubab, Q.; Asad, L.; Ishfaq, M.; Shafique, B.; Ranjha, M.M.A.N.; Mahmood, S.; Mueen-Ud-Din, G.; Javaid, T.; Sabtain, B.; et al. A Critical Review on Presence of Polyphenols in Commercial Varieties of Apple Peel, Their Extraction and Health Benefits. Open Access J. Biog. Sci. Res. 2020, 6, 18. [Google Scholar] [CrossRef]
- Lee, J.; Chan, B.L.S.; Mitchell, A.E. Identification/Quantification of Free and Bound Phenolic Acids in Peel and Pulp of Apples (Malus domestica) Using High Resolution Mass Spectrometry (HRMS). Food Chem. 2017, 215, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Manohar, S.; Kumari, P.; Krishnan, V.; Maheshwari, C.; Narwal, S.; Prakash Gupta, O.; Gowda, V.T.; Bansal, N.; Dahuja, A. The Role of Major Phenolics in Apple to Total Antioxidant Capacity. In Apple Cultivation—Recent Advances; IntechOpen: London, UK, 2023; ISBN 978-1-83969-801-9. [Google Scholar]
- De Paepe, D.; Valkenborg, D.; Noten, B.; Servaes, K.; Diels, L.; De Loose, M.; Van Droogenbroeck, B.; Voorspoels, S. Variability of the Phenolic Profiles in the Fruits from Old, Recent and New Apple Cultivars Cultivated in Belgium. Metabolomics 2015, 11, 739–752. [Google Scholar] [CrossRef]
- Cetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Savatović, S.; Mandić, A.; Tumbas, V. Assessment of Polyphenolic Content and in Vitro Antiradical Characteristics of Apple Pomace. Food Chem. 2008, 109, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.; Li, C.; Xu, H.; Sun, T.; Ge, Y. Melatonin Induces Resistance against Penicillium expansum in Apple Fruit through Enhancing Phenylpropanoid Metabolism. Physiol. Mol. Plant. Pathol. 2023, 127, 102082. [Google Scholar] [CrossRef]
- Rathee, P.; Sehrawat, R.; Rathee, P.; Khatkar, A.; Akkol, E.K.; Khatkar, S.; Redhu, N.; Türkcanoğlu, G.; Sobarzo-Sánchez, E. Polyphenols: Natural Preservatives with Promising Applications in Food, Cosmetics and Pharma Industries; Problems and Toxicity Associated with Synthetic Preservatives; Impact of Misleading Advertisements; Recent Trends in Preservation and Legislation. Materials 2023, 16, 4793. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Encinar, J.A.; Rodríguez-Díaz, J.C.; Micol, V. Antimicrobial Capacity of Plant Polyphenols against Gram-Positive Bacteria: A Comprehensive Review. Curr. Med. Chem. 2020, 27, 2576–2606. [Google Scholar] [CrossRef]
- Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing Microbial Infections with Natural Phenolic Compounds. Future Pharmacol. 2022, 2, 460–498. [Google Scholar] [CrossRef]
- Donadio, G.; Mensitieri, F.; Santoro, V.; Parisi, V.; Bellone, M.L.; De Tommasi, N.; Izzo, V.; Piaz, F.D. Interactions with Microbial Proteins Driving the Antibacterial Activity of Flavonoids. Pharmaceutics 2021, 13, 660. [Google Scholar] [CrossRef]
- Milutinović, M.; Dimitrijević-Branković, S.; Rajilić-Stojanović, M. Plant Extracts Rich in Polyphenols as Potent Modulators in the Growth of Probiotic and Pathogenic Intestinal Microorganisms. Front. Nutr. 2021, 8, 688843. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ali, A.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Identification of Phenolics Profile in Freeze-Dried Apple Peel and Their Bioactivities during In Vitro Digestion and Colonic Fermentation. Int. J. Mol. Sci. 2023, 24, 1514. [Google Scholar] [CrossRef]
- Gwiazdowska, D.; Juś, K.; Jasnowska-Małecka, J.; Kluczyńska, K. The Impact of Polyphenols on Bifidobacterium Growth. Acta Biochim. Pol. 2015, 62, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of Tea Phenolics and Their Aromatic Fecal Bacterial Metabolites on Intestinal Microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial Activity of Flavonoids and Their Structure–Activity Relationship: An Update Review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Laganà, G.; Ginestra, G.; Bisignano, C. Biochemical and Antimicrobial Activity of Phloretin and Its Glycosilated Derivatives Present in Apple and Kumquat. Food Chem. 2014, 160, 292–297. [Google Scholar] [CrossRef]
- Lee, J.H.; Regmi, S.C.; Kim, J.A.; Cho, M.H.; Yun, H.; Lee, C.S.; Lee, J. Apple Flavonoid Phloretin Inhibits Escherichia coli O157:H7 Biofilm Formation and Ameliorates Colon Inflammation in Rats. Infect. Immun. 2011, 79, 4819–4827. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Levin, C.E. Bactericidal Activities of Health-Promoting, Food-Derived Powders Against the Foodborne Pathogens Escherichia coli, Listeria monocytogenes, Salmonella enterica, and Staphylococcus aureus. J. Food Sci. 2013, 78, 270–275. [Google Scholar] [CrossRef]
- Almeida, D.P.F.; Cardoso, M.; Cerdeira, A.; Borges, A.; Gião, M.; Pintado, M. Antioxidant Activity of Pulp and Peel Apples Extracts Evaluated by Bacteriophage and DNA Protection Methods. In Proceedings of the XXVIIIth International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 939, Lisboa, Portugal, 22–27 August 2010; pp. 105–111. [Google Scholar]
- Wang, J.; Yang, R.; Xiao, Z.; Xu, Q.; Li, P.; Ma, F. Dihydrochalcones in Malus Inhibit Bacterial Growth by Reducing Cell Membrane Integrity. Food Funct. 2020, 11, 6517–6527. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, X.; Miao, Z.; Hassan, H.; Song, Y.; Fan, M. Screening for Antioxidant and Antibacterial Activities of Phenolics from Golden Delicious Apple Pomace. Chem. Cent. J. 2016, 10, 47. [Google Scholar] [CrossRef]
- Tian, L.; Wu, M.; Guo, W.; Li, H.; Gai, Z.; Gong, G. Evaluation of the Membrane Damage Mechanism of Chlorogenic Acid against Yersinia enterocolitica and Enterobacter sakazakii and Its Application in the Preservation of Raw Pork and Skim Milk. Molecules 2021, 26, 6748. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.J.; He, L.Y.; Li, S.; Xiong, C.J.; Lu, C.H.; Yang, X.Y. Chlorogenic Acid Exerts Antibacterial Effects by Affecting Lipid Metabolism and Scavenging ROS in Streptococcus pyogenes. FEMS Microbiol. Lett. 2022, 369, fnac061. [Google Scholar] [CrossRef] [PubMed]
- Veiko, A.G.; Olchowik-Grabarek, E.; Sekowski, S.; Roszkowska, A.; Lapshina, E.A.; Dobrzynska, I.; Zamaraeva, M.; Zavodnik, I.B. Antimicrobial Activity of Quercetin, Naringenin and Catechin: Flavonoids Inhibit Staphylococcus aureus-Induced Hemolysis and Modify Membranes of Bacteria and Erythrocytes. Molecules 2023, 28, 1252. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Barros, T.F.; Borges, J.S.; Silva, D.B.; Trentin, D.S. Glycosylated Flavonoids Have Fewer Antibacterial Activity than Corresponding Aglycone: Is It True for Antivirulence Activity? Rev. Bras. Farmacogn. 2024, 34, 910–926. [Google Scholar] [CrossRef]
- Amin, M.U.; Khurram, M.; Khattak, B.; Khan, J. Antibiotic Additive and Synergistic Action of Rutin, Morin and Quercetin against Methicillin Resistant Staphylococcus aureus. BMC Complement. Altern. Med. 2015, 15, 59. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New Insights of the Application of Water or Ethanol-Water Plant Extract Rich in Active Compounds in Food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef]
- Rasul Suleria, H.A.; Barrow, C. Bioactive Compounds from Plant Origin: Extraction, Applications, and Potential Health Benefits, 1st ed.; Apple Academic Press: New York, NY, USA, 2019; ISBN 9780429029288. [Google Scholar]
- Zaky, A.A.; Witrowa-Rajchert, D.; Nowacka, M. Revolution of Bioactive Compound Extraction: Impacts on Food Safety, Health, and Sustainability. Food Saf. Health 2025, 3, 315–333. [Google Scholar] [CrossRef]
- Rana, S.; Kumar, S.; Rana, A.; Sharma, V.; Katoch, P.; Padwad, Y.; Bhushan, S. Phenolic Constituents from Apple Tree Leaves and Their in Vitro Biological Activity. Ind. Crops Prod. 2016, 90, 118–125. [Google Scholar] [CrossRef]
- Raphaelli, C.O.; Dannenberg, G.; Dalmazo, G.O.; Pereira, E.S.; Radünz, M.; Vizzotto, M.; Fiorentini, A.M.; Gandra, E.A. Nora Antibacterial and Antioxidant Properties of Phenolic-Rich Extracts from Apple (Malus domestica Cv. Gala). Int. Food Res. J. 2019, 26, 1133–1142. [Google Scholar]
- EUCAST. Routine and Extended Internal Quality for MIC Determination and Disk Diffusion as Recommended by EUCAST, Version 13.1; 2023. Available online: https://szu.gov.cz/wp-content/uploads/2023/06/v_13.1_EUCAST_QC_tables_routine_and_extended_QC.pdf (accessed on 1 February 2025).
- Vieira, F.G.K.; Borges, G.D.S.C.; Copetti, C.; Di Pietro, P.F.; da Costa Nunes, E.; Fett, R. Phenolic Compounds and Antioxidant Activity of the Apple Flesh and Peel of Eleven Cultivars Grown in Brazil. Sci. Hortic. 2011, 128, 261–266. [Google Scholar] [CrossRef]
- Bottu, H.M.; Mero, A.; Husanu, E.; Tavernier, S.; Pomelli, C.S.; Dewaele, A.; Bernaert, N.; Guazzelli, L.; Brennan, L. The Ability of Deep Eutectic Solvent Systems to Extract Bioactive Compounds from Apple Pomace. Food Chem. 2022, 386, 132717. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Mitrea, L.; Călinoiu, L.F.; Teleky, B.E.; Martău, G.A.; Plamada, D.; Pascuta, M.S.; Nemeş, S.A.; Varvara, R.A.; Vodnar, D.C. Natural Polyphenol Recovery from Apple-, Cereal-, and Tomato-Processing By-Products and Related Health-Promoting Properties. Molecules 2022, 27, 7977. [Google Scholar] [CrossRef]
- Ren, Y.; Sun-Waterhouse, D.; Ouyang, F.; Tan, X.; Li, D.; Xu, L.; Li, B.; Wang, Y.; Li, F. Apple Phenolic Extracts Ameliorate Lead-Induced Cognitive Impairment and Depression- and Anxiety-like Behavior in Mice by Abating Oxidative Stress, Inflammation and Apoptosis via the MiR-22-3p/SIRT1 Axis. Food Funct. 2022, 13, 2647–2661. [Google Scholar] [CrossRef]
- Táborský, J.; Sus, J.; Lachman, J.; Šebková, B.; Adamcová, A.; Šatínský, D. Dynamics of Phloridzin and Related Compounds in Four Cultivars of Apple Trees during the Vegetation Period. Molecules 2021, 26, 3816. [Google Scholar] [CrossRef]
- Niederberger, K.E.; Tennant, D.R.; Bellion, P. Dietary Intake of Phloridzin from Natural Occurrence in Foods. Br. J. Nutr. 2020, 123, 942–950. [Google Scholar] [CrossRef]
- Li, D.; Yang, Y.; Sun, L.; Fang, Z.; Chen, L.; Zhao, P.; Wang, Z.; Guo, Y. Effect of Young Apple (Malus domestica Borkh. Cv. Red Fuji) Polyphenols on Alleviating Insulin Resistance. Food Biosci. 2020, 36, 100637. [Google Scholar] [CrossRef]
- Zielinska, D.; Laparra-Llopis, J.M.; Zielinski, H.; Szawara-Nowak, D.; Giménez-Bastida, J.A. Role of Apple Phytochemicals, Phloretin and Phloridzin, in Modulating Processes Related to Intestinal Inflammation. Nutrients 2019, 11, 1173. [Google Scholar] [CrossRef]
- Schaefer, S.; Baum, M.; Eisenbrand, G.; Dietrich, H.; Will, F.; Janzowski, C. Polyphenolic Apple Juice Extracts and Their Major Constituents Reduce Oxidative Damage in Human Colon Cell Lines. Mol. Nutr. Food Res. 2006, 50, 24–33. [Google Scholar] [CrossRef]
- Jung, M.; Triebel, S.; Anke, T.; Richling, E.; Erkel, G. Influence of Apple Polyphenols on Inflammatory Gene Expression. Mol. Nutr. Food Res. 2009, 53, 1263–1280. [Google Scholar] [CrossRef]
- Marks, S.C.; Mullen, W.; Borges, G.; Crozier, A. Absorption, Metabolism, and Excretion of Cider Dihydrochalcones in Healthy Humans and Subjects with an Ileostomy. J. Agric. Food Chem. 2009, 57, 2009–2015. [Google Scholar] [CrossRef]
- Kahle, K.; Huemmer, W.; Kempf, M.; Scheppach, W.; Erk, T.; Richling, E. Polyphenols Are Intensively Metabolized in the Human Gastrointestinal Tract after Apple Juice Consumption. J. Agric. Food Chem. 2007, 55, 10605–10614. [Google Scholar] [CrossRef]
- Choi, C.I. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors from Natural Products: Discovery of next-Generation Antihyperglycemic Agents. Molecules 2016, 21, 1136. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Q.; Shen, L.; Guo, K.; Zhou, X. Chlorogenic Acid Improves Glucose Tolerance, Lipid Metabolism, Inflammation and Microbiota Composition in Diabetic Db/Db Mice. Front. Endocrinol. 2022, 13, 1042044. [Google Scholar] [CrossRef]
- Tian, J.; Wu, X.; Zhang, M.; Zhou, Z.; Liu, Y. Comparative Study on the Effects of Apple Peel Polyphenols and Apple Flesh Polyphenols on Cardiovascular Risk Factors in Mice. Clin. Exp. Hypertens. 2017, 40, 65–72. [Google Scholar] [CrossRef]
- Kleemann, R.; Verschuren, L.; Morrison, M.; Zadelaar, S.; van Erk, M.J.; Wielinga, P.Y.; Kooistra, T. Anti-Inflammatory, Anti-Proliferative and Anti-Atherosclerotic Effects of Quercetin in Human in Vitro and in Vivo Models. Atherosclerosis 2011, 218, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Du, Y.; Qin, X.; Wu, H.; Huang, Y.; Cheng, Y.; Wei, Y. Comprehensive Evaluation of Effective Polyphenols in Apple Leaves and Their Combinatory Antioxidant and Neuroprotective Activities. Ind. Crops. Prod. 2019, 129, 242–252. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Thilakarathna, S.; Nair, S. Polyphenols: Chemistry, Dietary Sources and Health Benefits; Sun, J., Prasad, K.N., Ismail, A., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2013; ISBN 978-1-62081-809-1. [Google Scholar]
- Tsoupras, A.; Gkika, D.A.; Markopoulos, T.; Curran, R.; Scallon, C.; Karali, M.; Kyzas, G.Z. Apple Products (Apple Juice and Cider) and By-Products (Apple Pomace): Bioactive Compounds and Biological Properties; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–42. [Google Scholar]
- Pal, A.; Tripathi, A. Quercetin Inhibits Carbapenemase and Efflux Pump Activities among Carbapenem-resistant Gram-negative Bacteria. APMIS 2020, 128, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Embaby, M.A.; El-Raey, M.A.; Zaineldain, M.; Almaghrabi, O.; Marrez, D.A. Synergistic Effect and Efflux Pump Inhibitory Activity of Ficus nitida Phenolic Extract with Tetracycline against Some Pathogenic Bacteria. Toxin Rev. 2021, 40, 1187–1197. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; El-Sayed, H.S. Incorporating White Radish Extract and L. Plantarum to Ultra-Filtrated Soft Cheese for Boost Its Functional, Microbiological, and Sensory Qualities. Biocatal. Agric. Biotechnol. 2024, 58, 103188. [Google Scholar] [CrossRef]
- Kalogianni, A.I.; Lazou, T.; Bossis, I.; Gelasakis, A.I. Natural Phenolic Compounds for the Control of Oxidation, Bacterial Spoilage, and Foodborne Pathogens in Meat. Foods 2020, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, B.R.; Corrêa, R.C.G.; de Lima Sampaio, S.; Barros, L. Bioactive Compounds from Food and Its By-Products: Current Applications and Future Perspectives. In Food Waste Conversion; Springer: Berlin, Germany, 2023; pp. 3–41. [Google Scholar]
- Costa, M.; Sezgin-Bayindir, Z.; Losada-Barreiro, S.; Paiva-Martins, F.; Saso, L.; Bravo-Díaz, C. Polyphenols as Antioxidants for Extending Food Shelf-Life and in the Prevention of Health Diseases: Encapsulation and Interfacial Phenomena. Biomedicines 2021, 9, 1909. [Google Scholar] [CrossRef]
- Panzella, L. Natural Phenolic Compounds for Health, Food and Cosmetic Applications. Antioxidants 2020, 9, 427. [Google Scholar] [CrossRef]
- Vandorou, M.; Plakidis, C.; Tsompanidou, I.M.; Adamantidi, T.; Panagopoulou, E.A.; Tsoupras, A. A Review on Apple Pomace Bioactives for Natural Functional Food and Cosmetic Products with Therapeutic Health-Promoting Properties. Int. J. Mol. Sci. 2024, 25, 10856. [Google Scholar] [CrossRef]
- Santoso, S.P.; Angkawijaya, A.E.; Cheng, K.C.; Lin, S.P.; Hsu, H.Y.; Hsieh, C.W.; Rahmawati, A.; Shimomura, O.; Ismadji, S. Unlocking the Potential of Gallic Acid-Based Metal Phenolic Networks for Innovative Adsorbent Design. Molecules 2025, 30, 1218. [Google Scholar] [CrossRef]
- Habtemariam, S. The Molecular Pharmacology of Phloretin: Anti-Inflammatory Mechanisms of Action. Biomedicines 2023, 11, 143. [Google Scholar] [CrossRef]
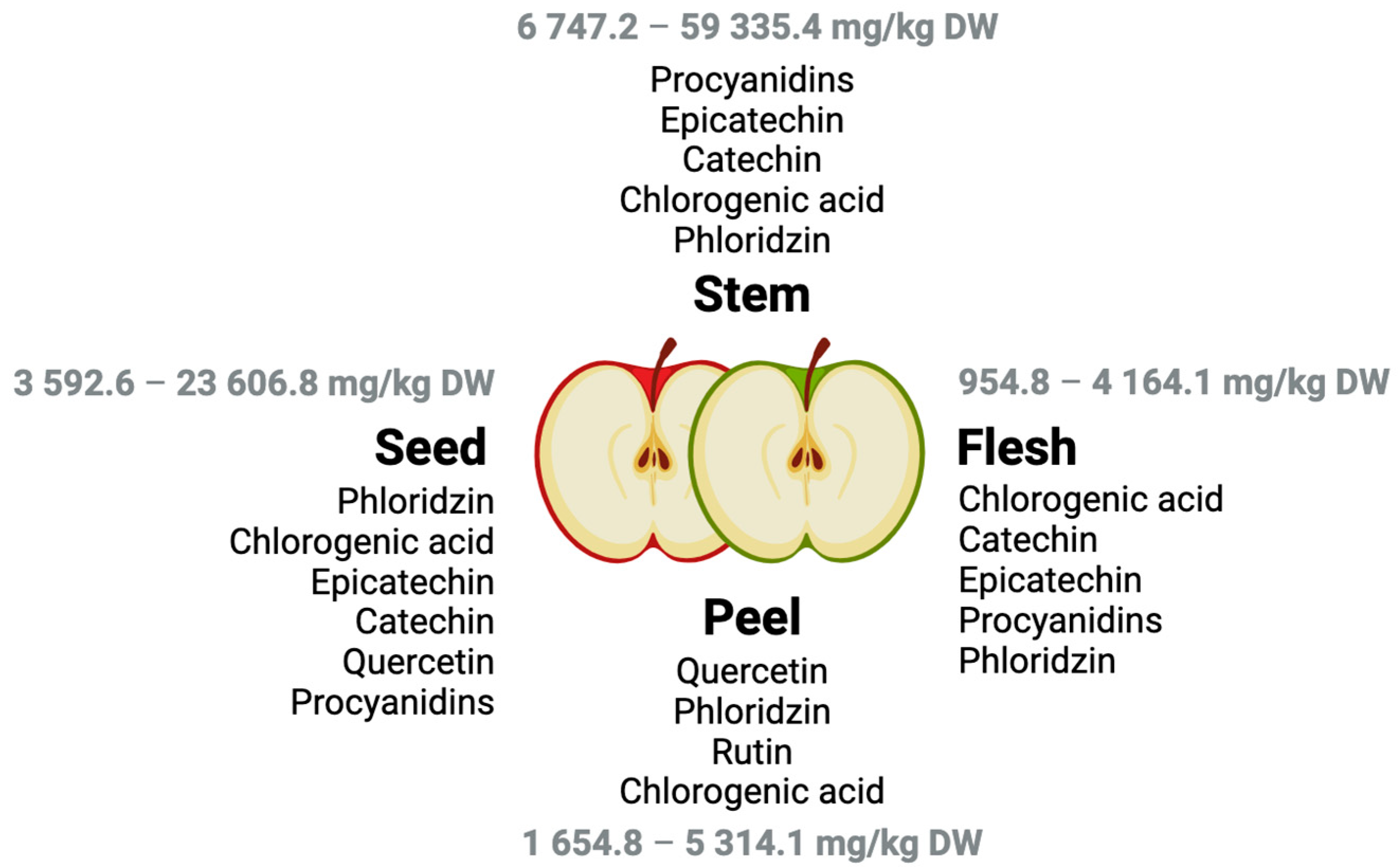
| Sample (Weight) | Solvent (Volume) | Extract Preparation | Phenolic Compounds | Yield (g GAE kg−1 FW) | Ref. |
|---|---|---|---|---|---|
| Peel (1 g) | MeOH:H2O:HCl (99:0:1 v/v/v) (-) | - | Epicatechin Hyperin Procyanidin B2 Phloridzin Quercitrin Catechin Isoquercetin Procyanidin B1 Quercetin Rutin | 14.7–65.1 | [15] |
| Flesh (1 g) | - | 5.2–14.4 | |||
| Peel (2 g) | MeOH: H2O: HCOOH (49.95:49.95:0.10 v/v/v) (10 mL) | The solution was agitated for 15 min in a horizontal shaker and centrifuged for 10 min at 20 °C. The supernatant was removed to another Falcon tube, and the extract was repeated with another 10 mL of the solvent. The second extract was merged with the first one. | Chlorogenic acidQuercetin-3-B-d-glucosideEpicatechin Phloridzin 4-Hydroxybenzoic acid Vanillic acid Gallic acid Rutin | 0.7–2.0 | [7] |
| Flesh (2 g) | Chlorogenic acid Gallic acid Vanillic acid | 0.1–0.4 | |||
| Seeds (2 g) | Chlorogenic acid Gallic acid Phloridzin Quercetin-3-B-d-glucoside Vanillic acid Quercetin | 0.3–0.9 |
| Phenolic Group | Chemical Structure | |
|---|---|---|
| Hydroxycinnamic acids Aromatic compounds with the framework of the C6-C3 structure [10]. | 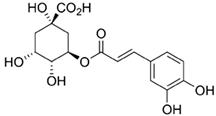 Chlorogenic acid | |
| Flavanols & Procyanidins A hydroxyl group is attached to position 3 of the ring C, and there is no double bond between positions 2 and 3, nor a carbonyl group at position 4 [20]. | 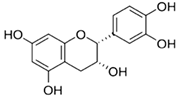 Epicatechin | |
| Flavonols A hydroxyl group is attached to position 3 of the ring C, and there is a double bond between positions 2 and 3, and a carbonyl group at position 4 [20]. | 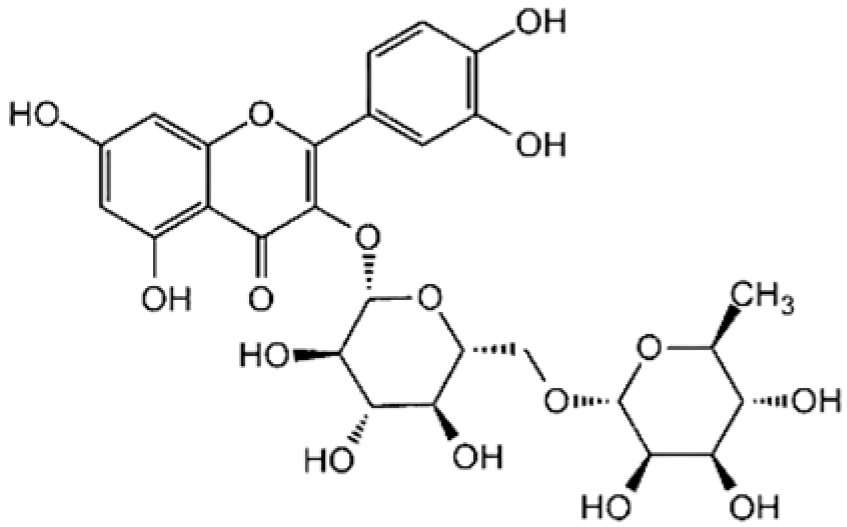 Rutin |  Quercetin |
| Dihydrochalcones Absence of ring C, consisting of two aromatic rings (ring A and B) linked by an aliphatic three-carbon chain [20]. | 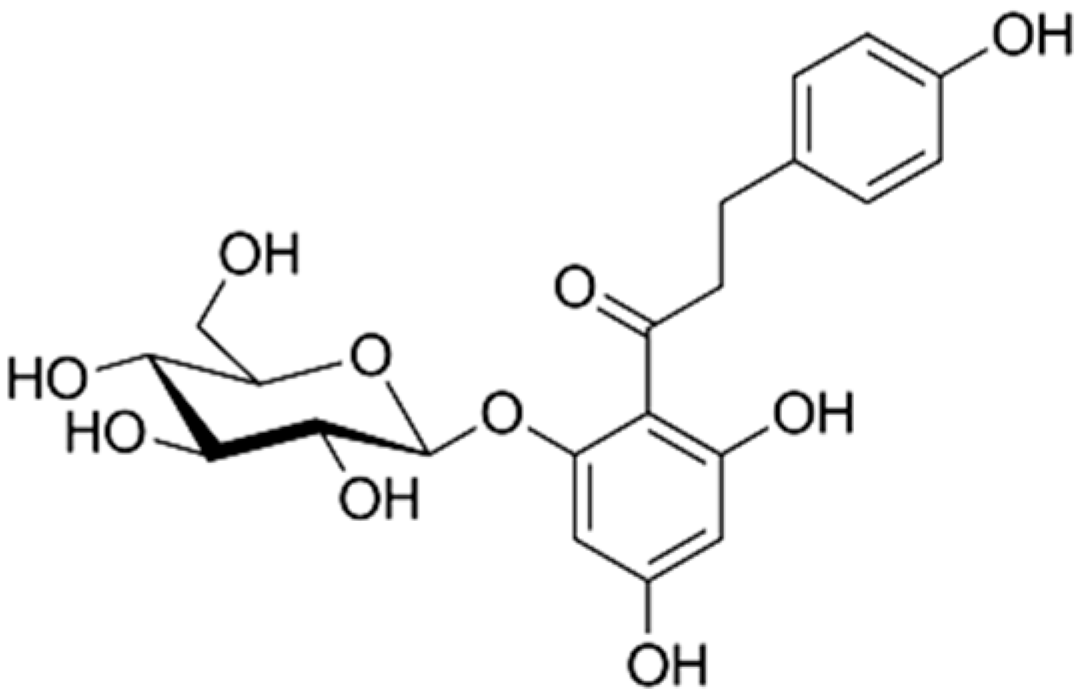 Phloridzin | |
| Compound | Phenolic Class | Key Structural Features | Main Antibacterial Mechanism(s) | Observations | Ref. |
|---|---|---|---|---|---|
| Phloridzin | Dihydrochalcone | Glucoside of phloretin (sugar at 2′-OH) | Enhances membrane permeability; promotes drug uptake | Less potent than aglycone; acts as penetration enhancer | [41] |
| Phloretin | Dihydrochalcone | Aglycone (no sugar moiety); lipophilic | Disrupts bacterial membrane; inhibits biofilm; increases fluidity | Stronger activity vs. Gram-positives and E. coli O157:H7 | [41,42] |
| Chlorogenic acid | Hydroxycinnamic acid | Caffeic acid esterified with quinic acid | Disrupts membrane; induces oxidative stress | Major phenolic in flesh and pomace | [2,10] |
| Catechin/Epicatechin | Flavanols | Multiple hydroxyls; flexible structure | Binds to membranes and proteins; causes oxidative stress | Present in both peel and flesh; activity varies by configuration | [11,34] |
| Procyanidins | Flavanol oligomers | Catechin dimers (e.g., B2); degree of polymerization critical | Membrane destabilization; enzyme inhibition | Abundant in skin; higher polymerization may enhance activity | [13,32,43] |
| Quercetin glycosides (e.g., quercetin-3-glucoside, rutin) | Flavonols | Glycosylated quercetin; planar structure | Enzyme inhibition; ROS generation; lower permeability than aglycone | Activity affected by glycosylation; quercetin more active than rutin | [15,44] |
| Method | Sample (Weight) | Solvent (Volume) | Extract Preparation | Yield | Tested Microorganisms | MIC (mg/mL) | Ref. |
|---|---|---|---|---|---|---|---|
| Ultrasound-assisted extraction | Pomace (100 g) | 100% ethyl acetate (500 mL) | The solution was placed in an ultrasonic bath at 37 °C for 40 min. | 2.51 g GAE kg−1 DW | S. aureus | 1.25 | [46] |
| E. coli | 2.50 | ||||||
| Maceration + liquid–liquid partition | Leaves (100 mg) | 70:30 ethanol:water (v/v) + hexane (2 mL) | The solution was vortexed for 2 min followed by centrifugation for 10 min at room temperature. The extraction process was repeated twice with 1.5 mL solvent and supernatant was collected and pooled to make final volume 5 mL with respective solvent. | - | Bacillus subtilis | 1.18 | [56] |
| Klebsiella pneumoniae | 1.18 | ||||||
| S. aureus | 1.18 | ||||||
| Micrococus luteus | 1.18 | ||||||
| E. coli | 1.18 | ||||||
| Listeria monocytogenes | 2.37 | ||||||
| Maceration | Dried apple without skin (1 g) | 80:20 methanol:water (v/v) (-) | The solution was placed under agitation at 25°C for 1h, followed by filtration. The residue was re-extracted with an additional portion of methanol:water mixture, and the combined extracts were evaporated under reduced pressure. | - | Acinetobacter baumannii | >20 | [13] |
| E. coli | 5 | ||||||
| ESBL-E. coli | 5 | ||||||
| K. pneumoniae | >20 | ||||||
| ESBL-K. pneumoniae | >20 | ||||||
| Morganella morganii | 5 | ||||||
| P. aeruginosa | >20 | ||||||
| Enterococcus faecalis | 5 | ||||||
| L. monocytogenes | 5 | ||||||
| MRSA | 5 | ||||||
| MSSA | 2.5 | ||||||
| Solvent extraction + solid-phase extraction | Apple slices (50 g) | 25:75 acetone:ethanol (v/v) (150 mL) | The solution was blended for 6 min and centrifuged at 0 °C for 25 min. The residue extract was then filtered and concentrated by evaporation under vacuum for 90 min at 40 °C. | - | S. aureus | 0.05 | [57] |
| E. coli | 0.05–0.5 | ||||||
| L. monocytogenes | 0.05–50 | ||||||
| Salmonella typhimurium | 0.05–0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, J.; Silva, V.; Dapkevicius, M.d.L.N.E.; Igrejas, G.; Barros, L.; Heleno, S.A.; Reis, F.S.; Poeta, P. From Apple Waste to Antimicrobial Solutions: A Review of Phenolics from PGI ‘Maçã de Alcobaça’ and Related Cultivars. Molecules 2025, 30, 3679. https://doi.org/10.3390/molecules30183679
Ribeiro J, Silva V, Dapkevicius MdLNE, Igrejas G, Barros L, Heleno SA, Reis FS, Poeta P. From Apple Waste to Antimicrobial Solutions: A Review of Phenolics from PGI ‘Maçã de Alcobaça’ and Related Cultivars. Molecules. 2025; 30(18):3679. https://doi.org/10.3390/molecules30183679
Chicago/Turabian StyleRibeiro, Jessica, Vanessa Silva, Maria de Lurdes N. E. Dapkevicius, Gilberto Igrejas, Lillian Barros, Sandrina A. Heleno, Filipa S. Reis, and Patrícia Poeta. 2025. "From Apple Waste to Antimicrobial Solutions: A Review of Phenolics from PGI ‘Maçã de Alcobaça’ and Related Cultivars" Molecules 30, no. 18: 3679. https://doi.org/10.3390/molecules30183679
APA StyleRibeiro, J., Silva, V., Dapkevicius, M. d. L. N. E., Igrejas, G., Barros, L., Heleno, S. A., Reis, F. S., & Poeta, P. (2025). From Apple Waste to Antimicrobial Solutions: A Review of Phenolics from PGI ‘Maçã de Alcobaça’ and Related Cultivars. Molecules, 30(18), 3679. https://doi.org/10.3390/molecules30183679















