Gemmological, Spectroscopic, and Origin Description Studies of Tourmaline from Yunnan, China
Abstract
1. Introduction
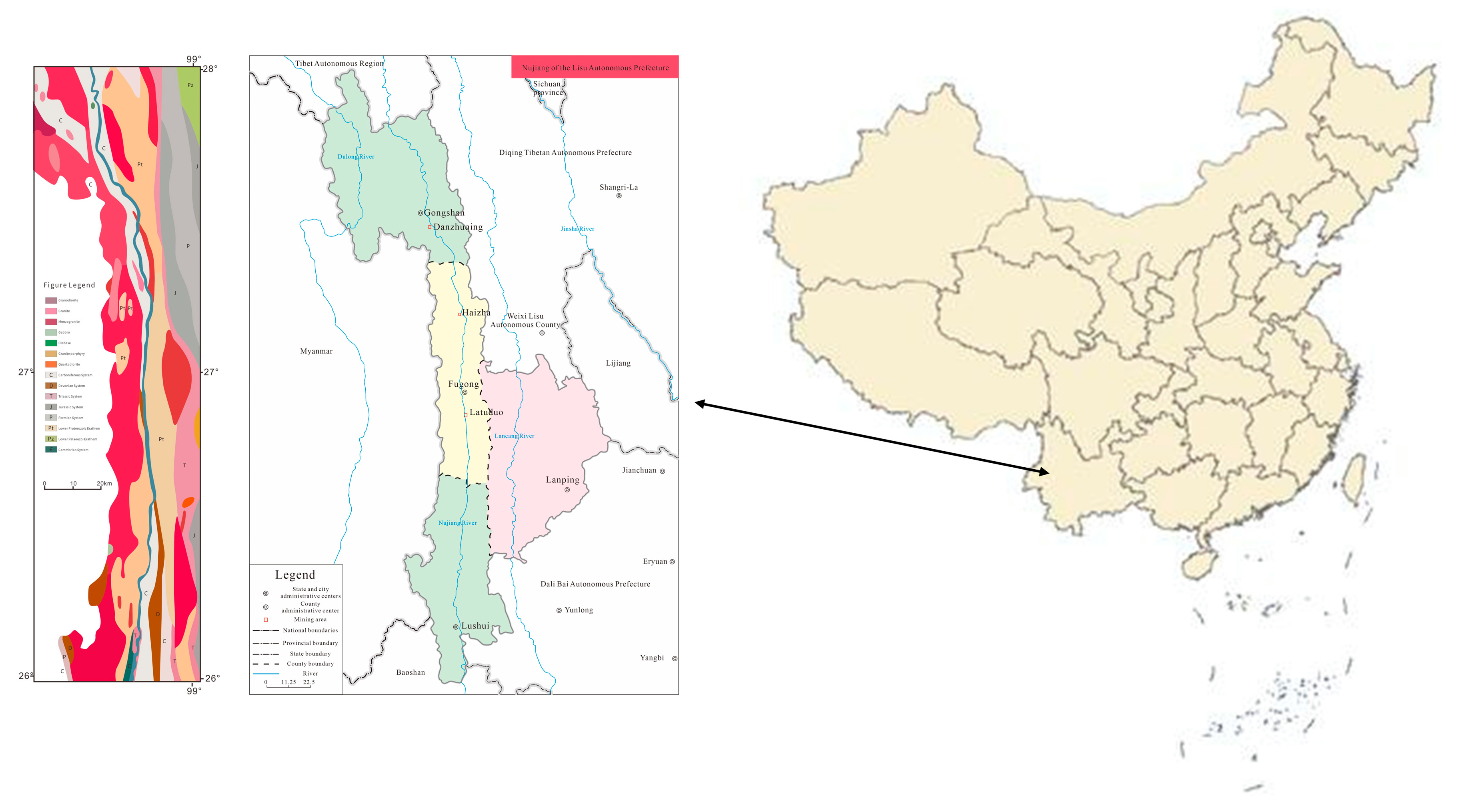
1.1. Location and Access
1.2. Geology
1.3. Mines and Production
2. Results and Discussion
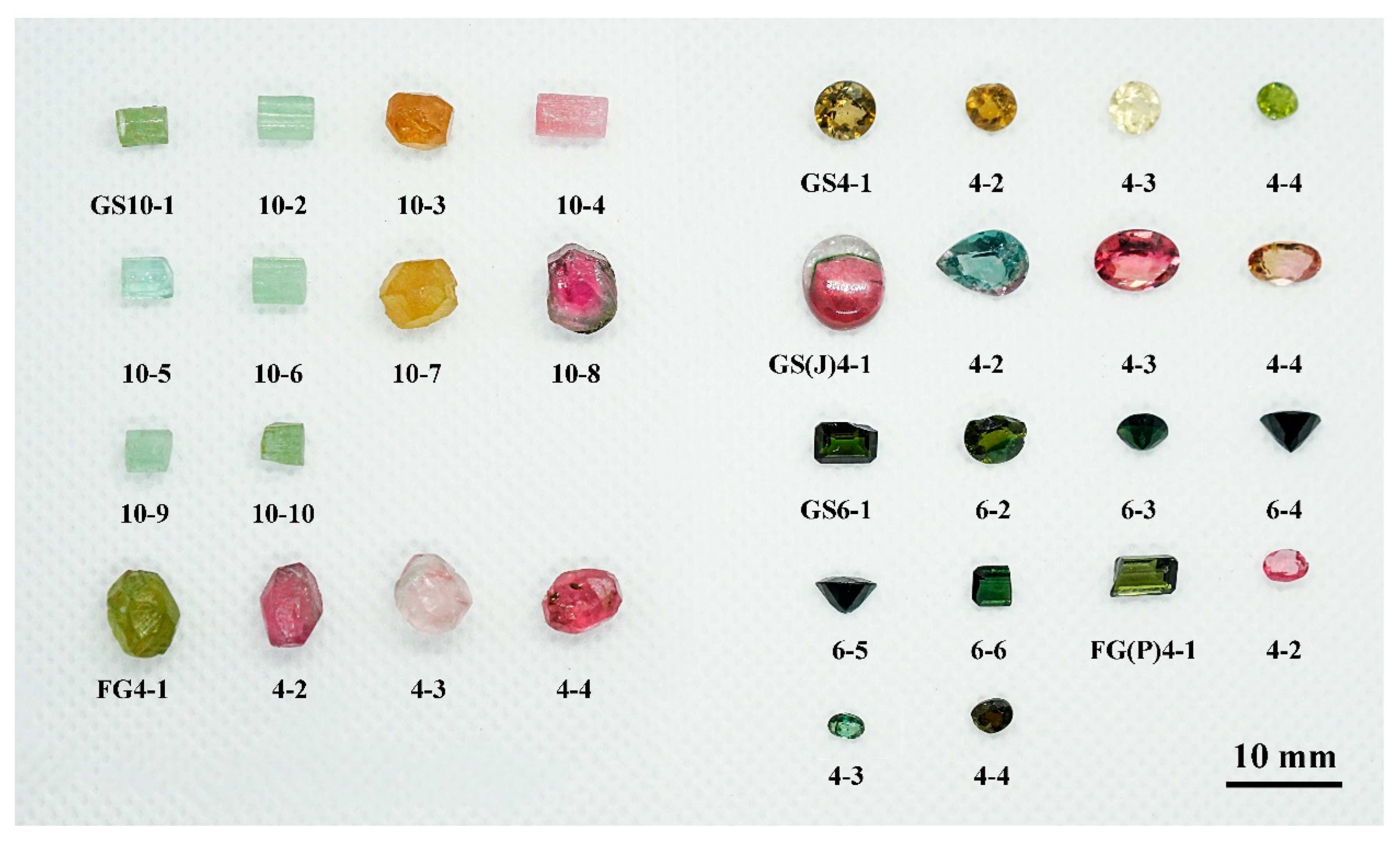
2.1. Basic Gemological Features
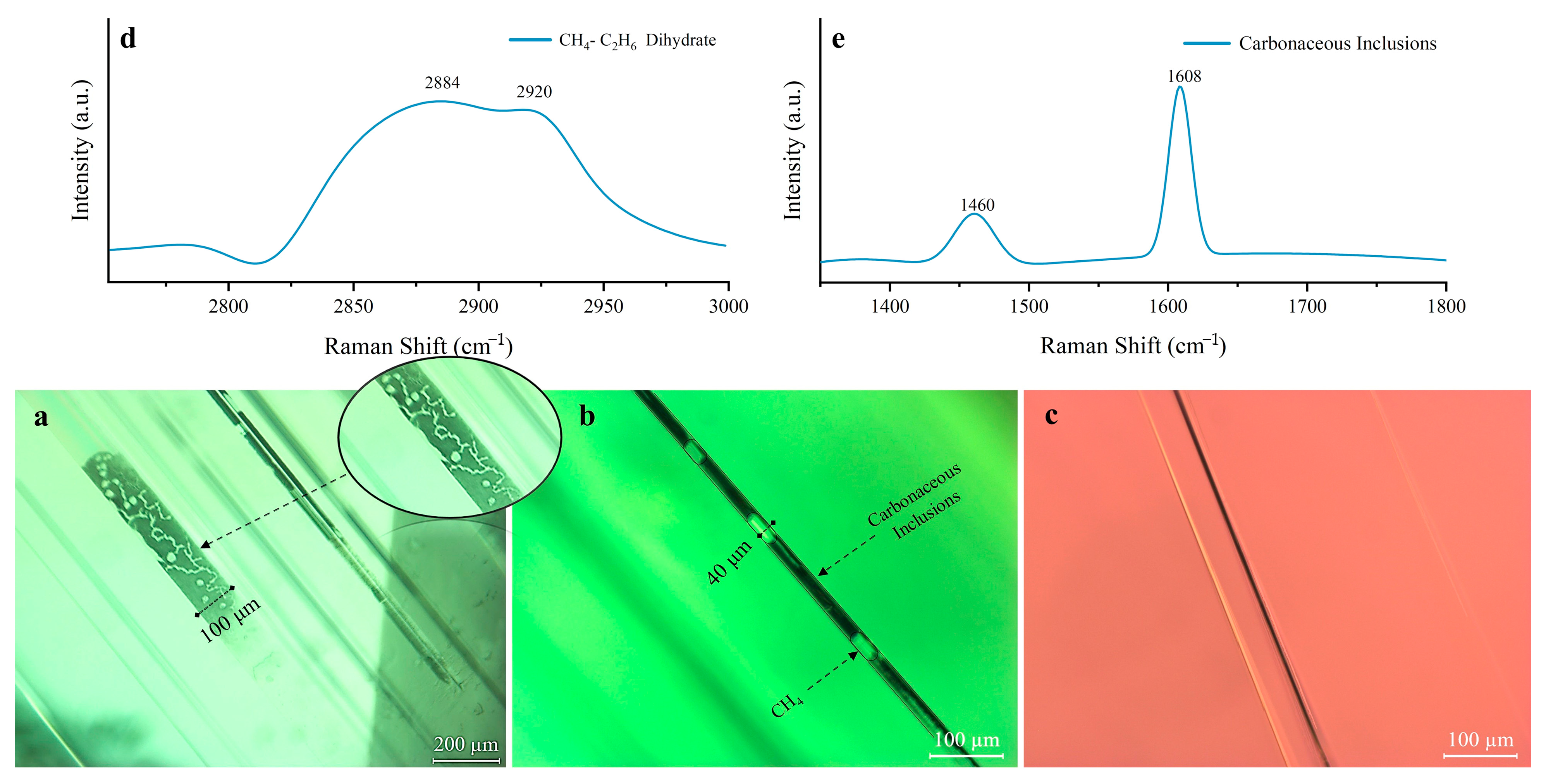
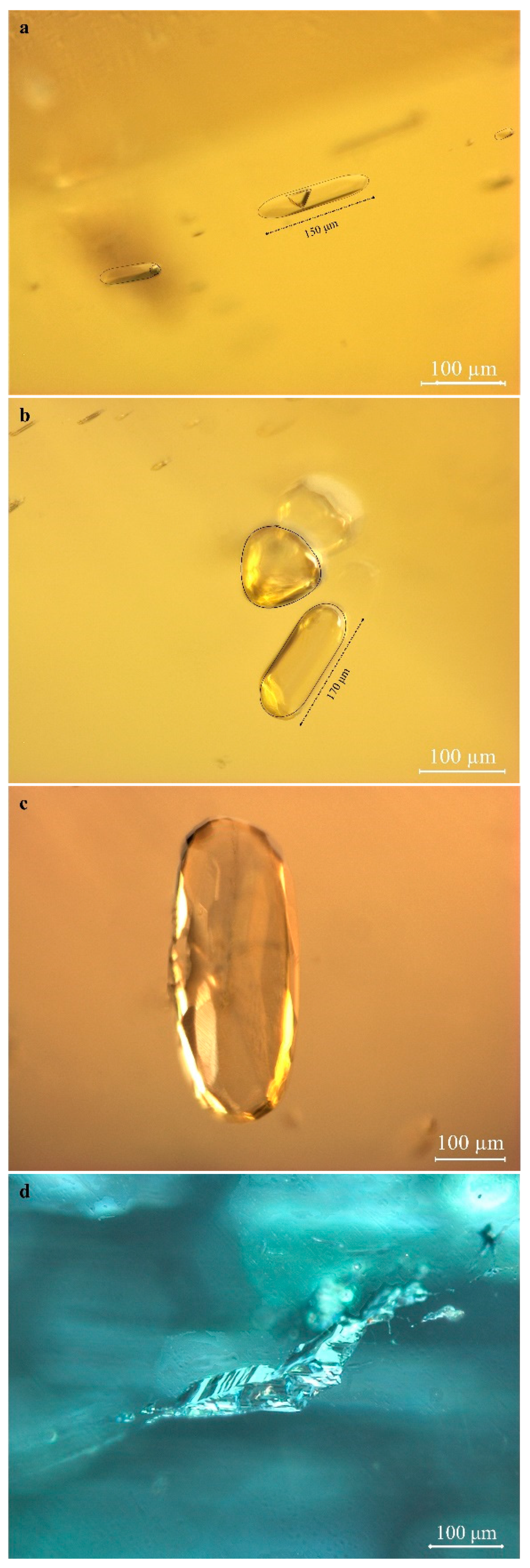
2.2. Microscopic Features
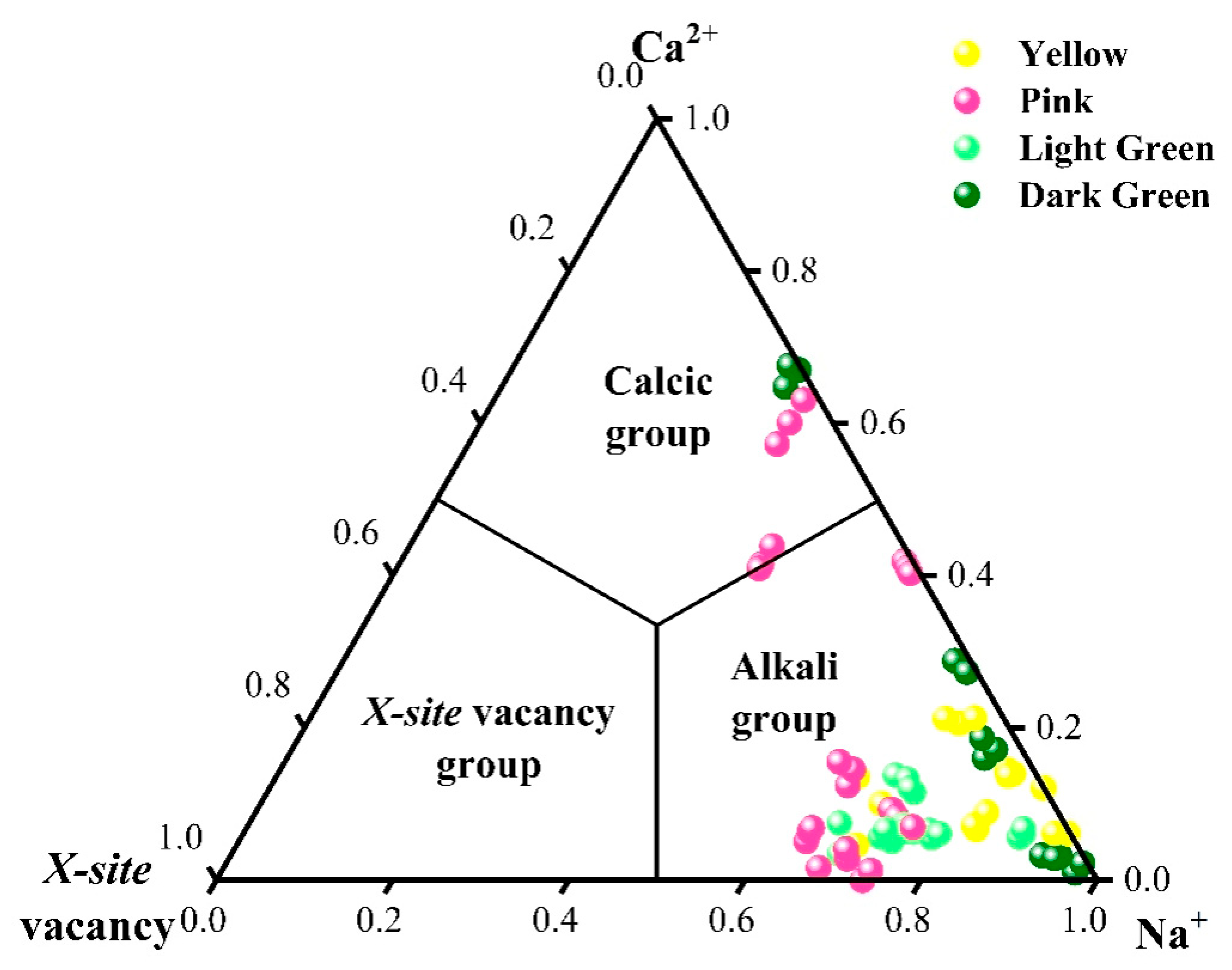
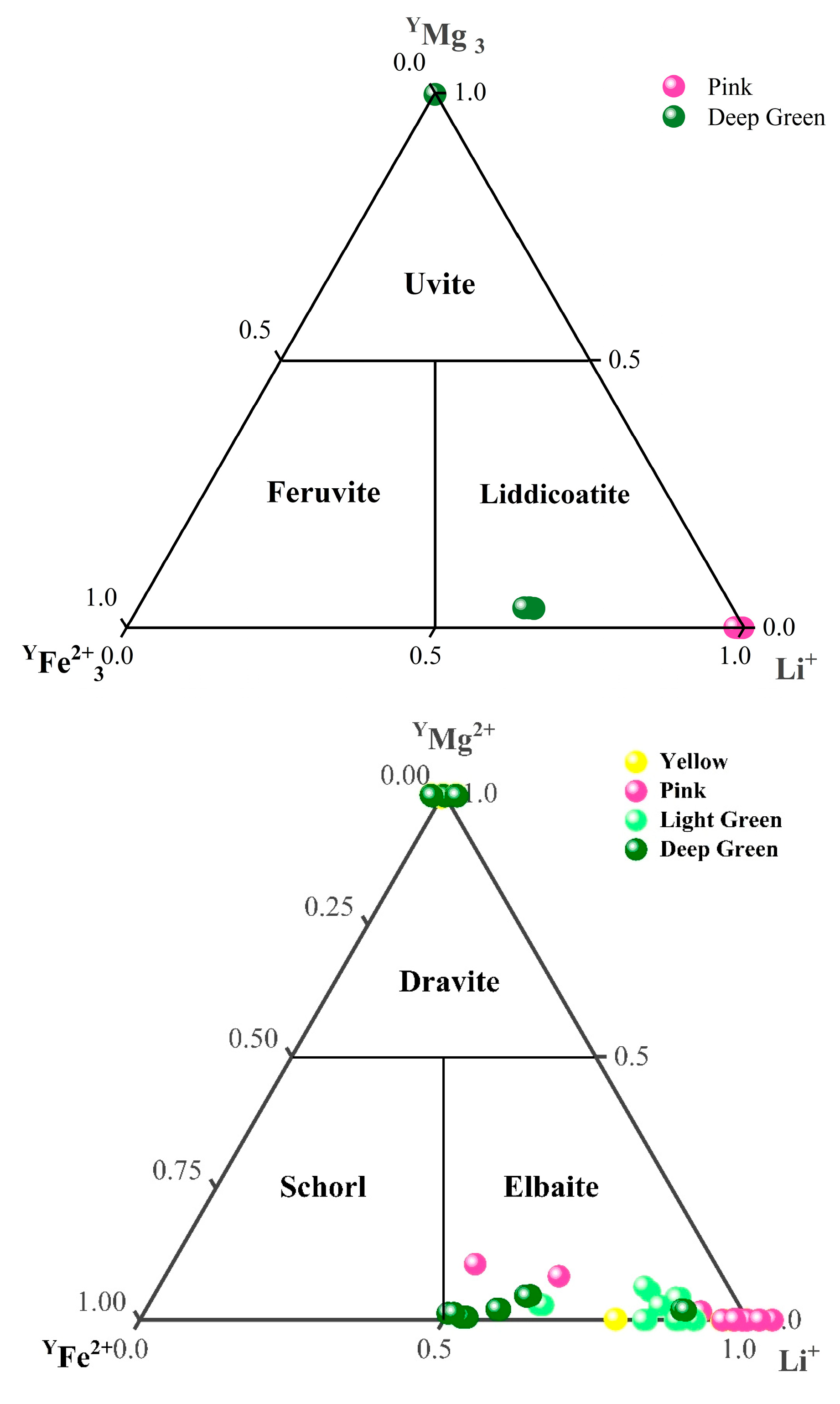
2.3. Chemical Element Analysis
- Yellow tourmalines
- 2.
- Green tourmalines
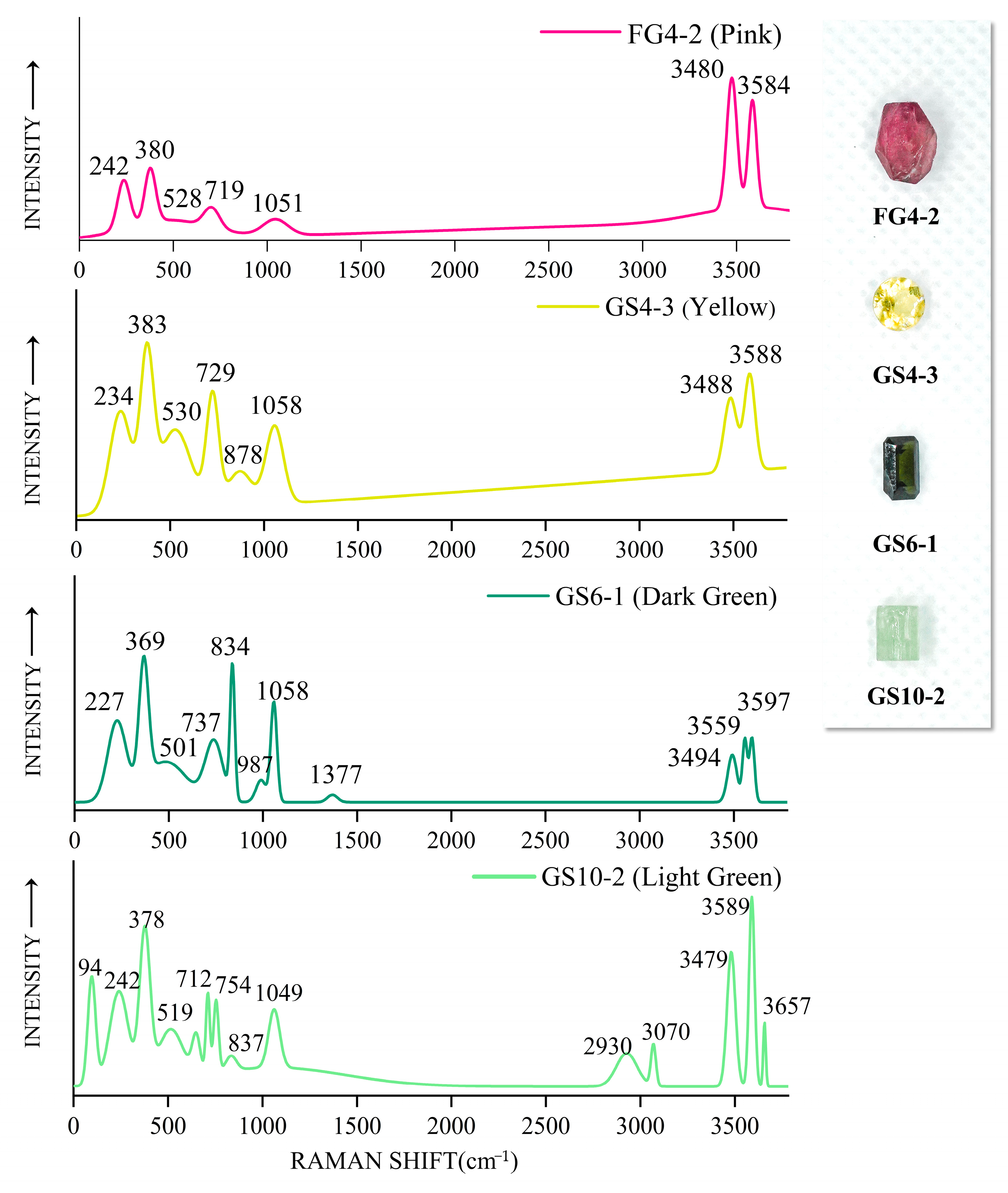
2.4. Raman Spectrum Analysis
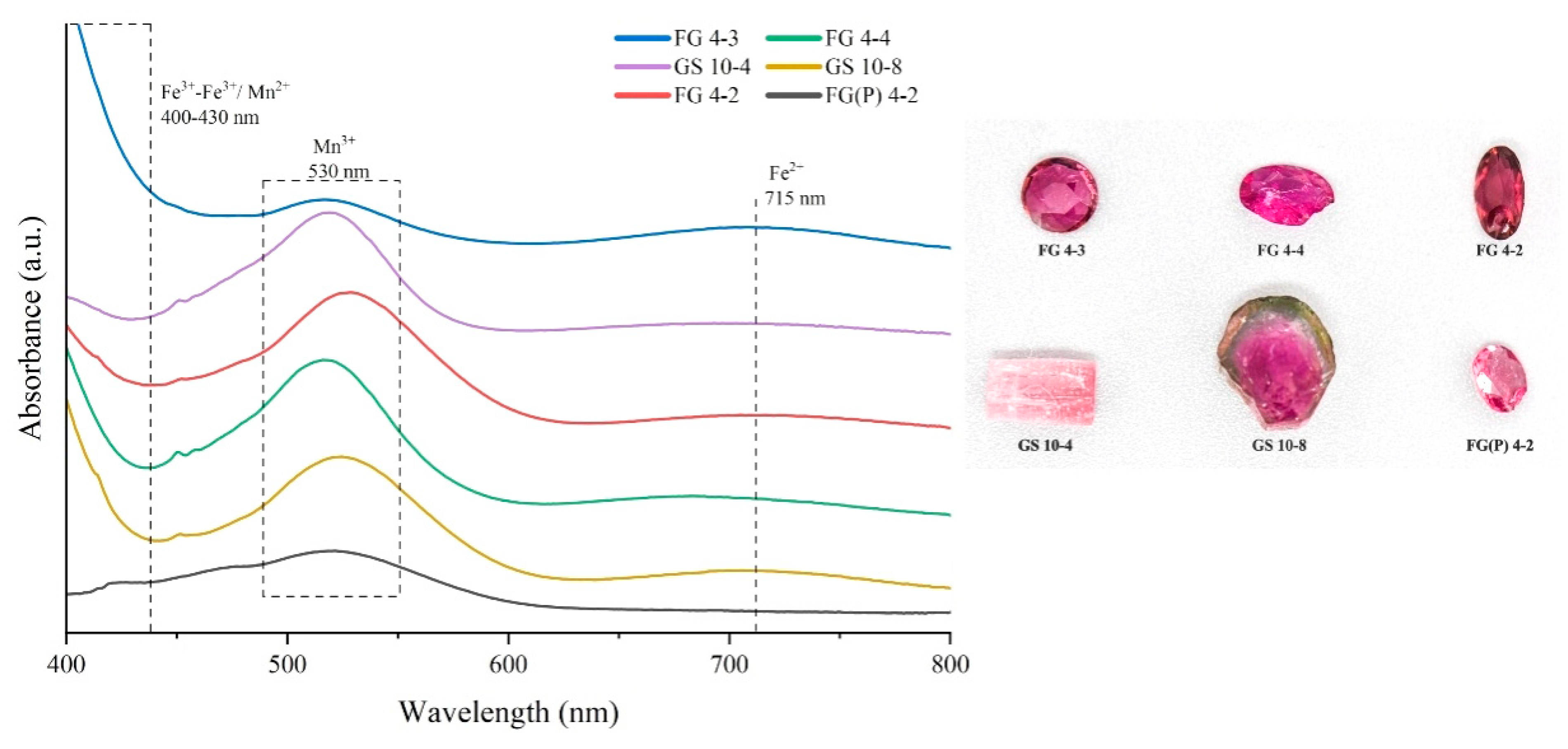
2.5. UV-Vis Absorption Spectroscopy
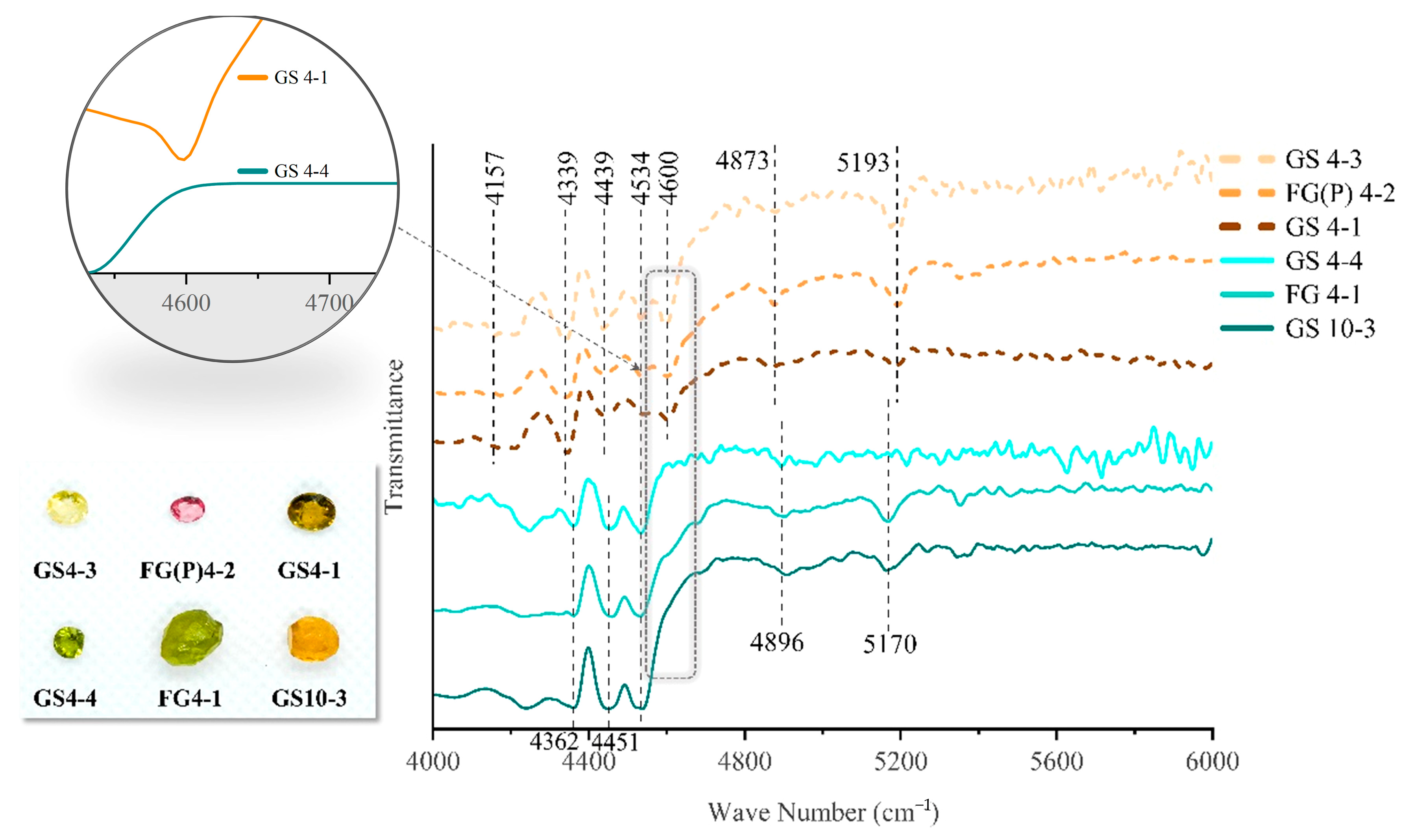
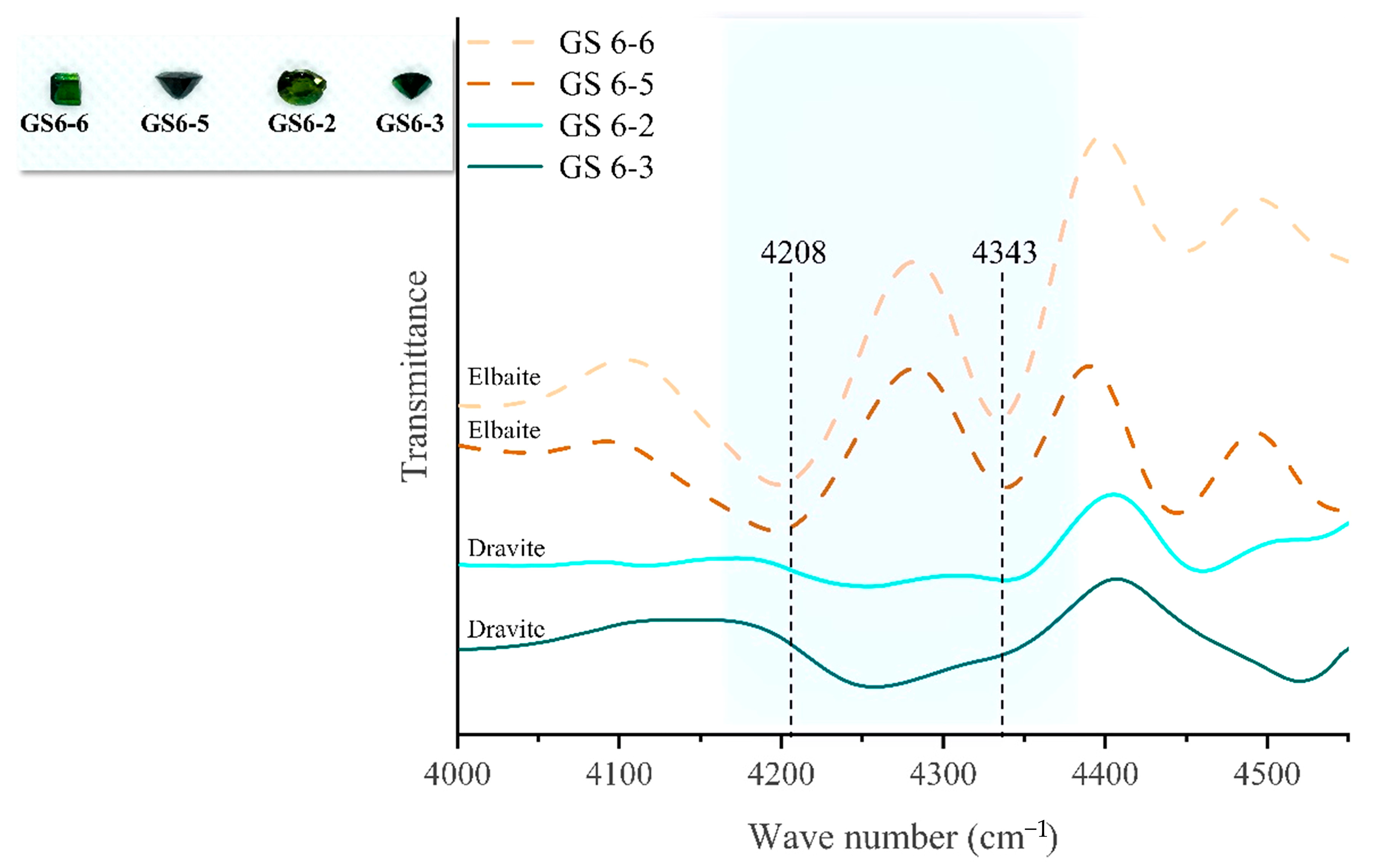
2.6. Infrared Spectroscopy Analysis
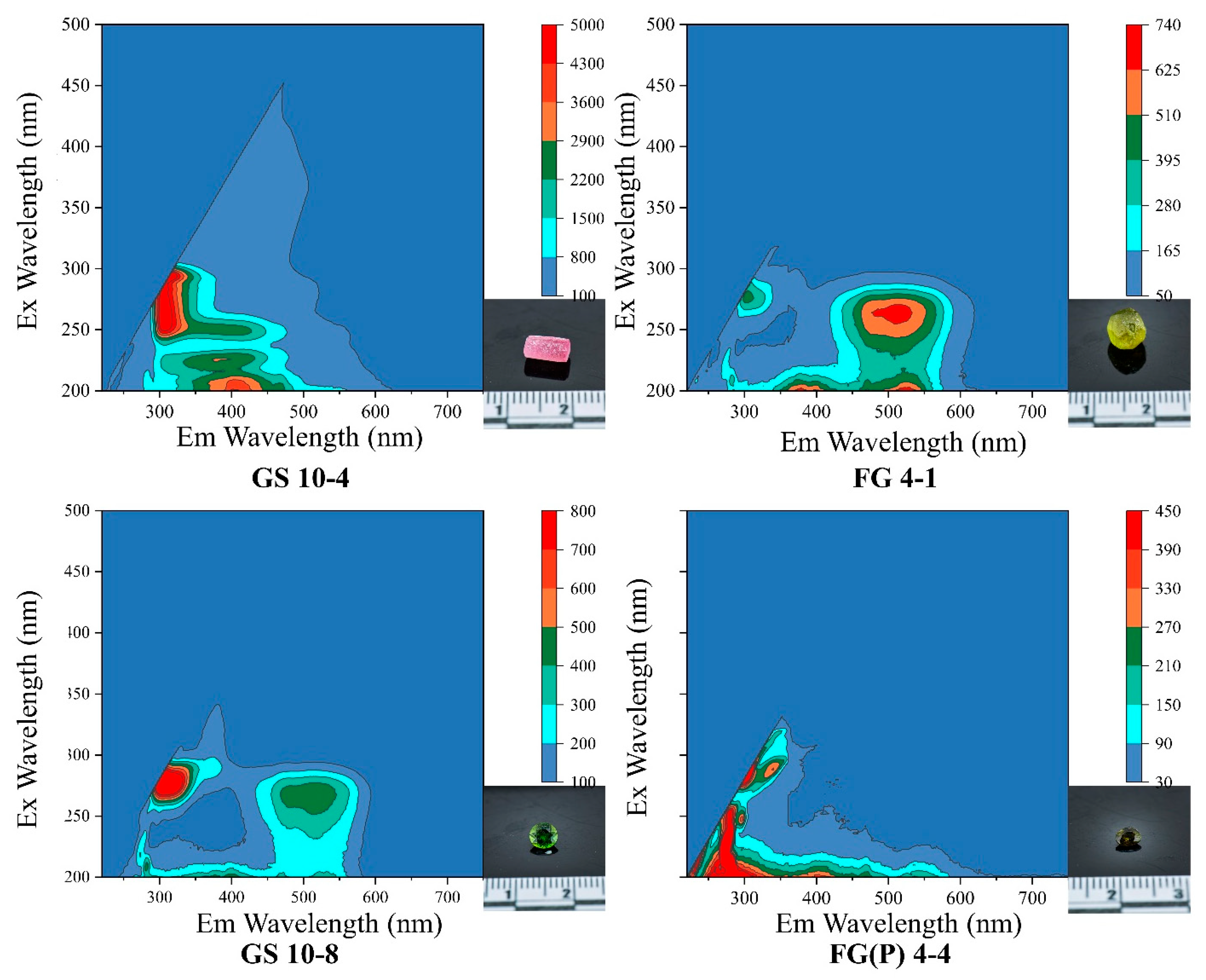
2.7. Three-Dimensional Fluorescence Spectrum Analysis
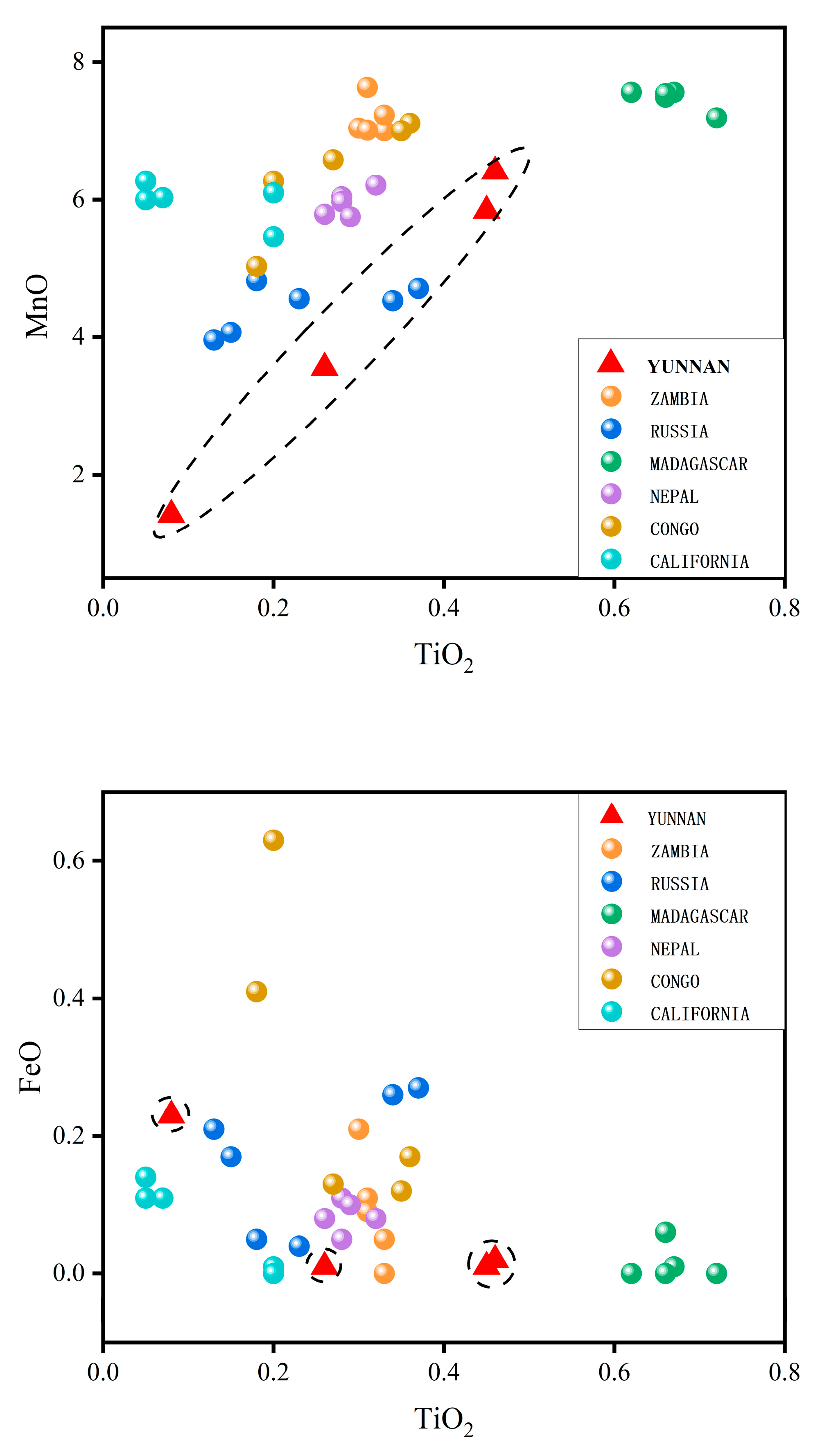
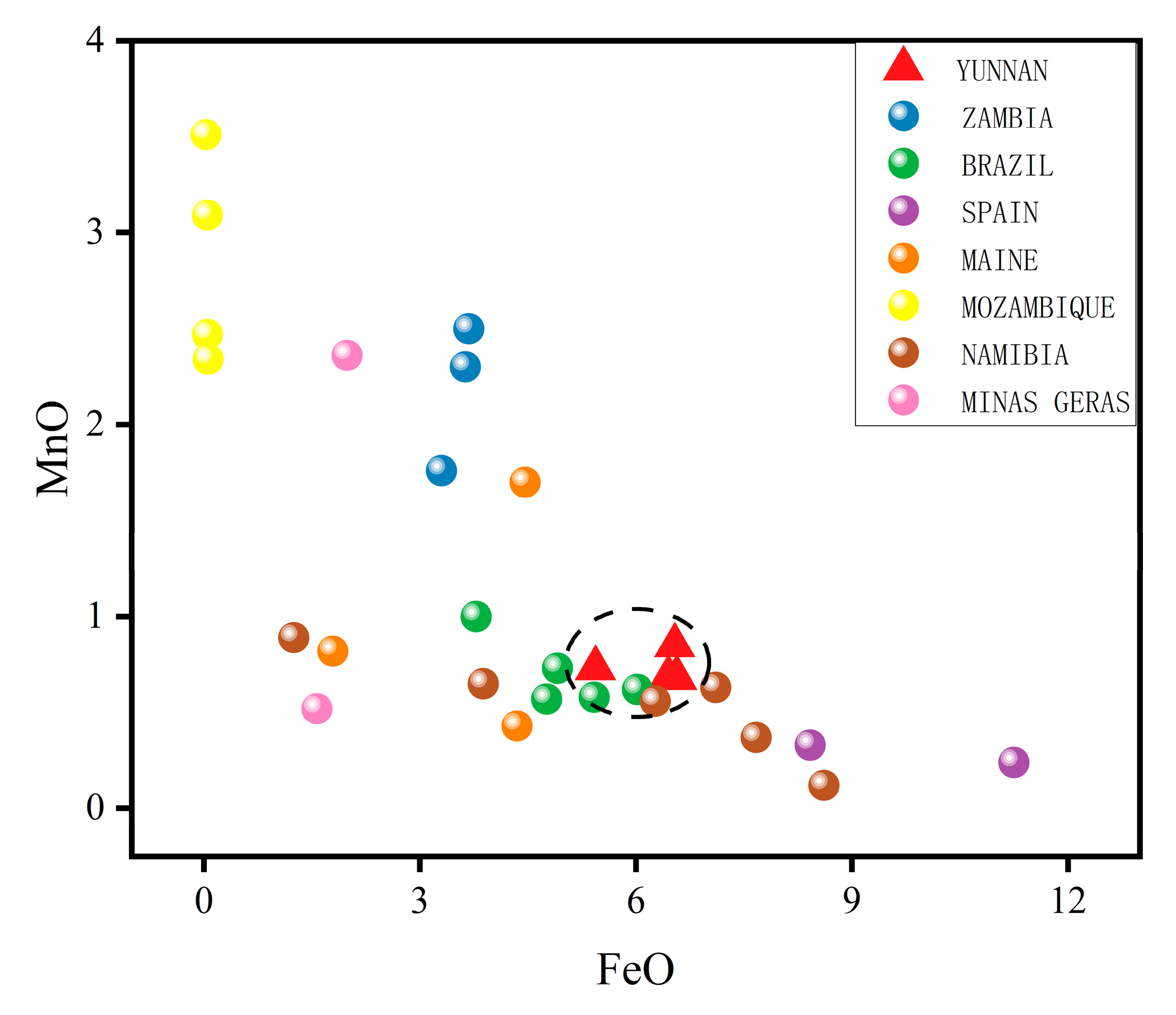
2.8. Chemical Characteristics of This Provenance
- Yellow tourmalines
- 2.
- Green tourmalines
3. Materials and Methods
3.1. The Samples Studied
3.2. Experimental Set-Up
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henry, D.J.; Dutrow, B.L. Tourmaline studies through time: Contributions to scientific advancements. J. Geosci. 2018, 63, 77. [Google Scholar] [CrossRef]
- Henry, D.J.; Novák, M.; Hawthorne, F.C.; Ertl, A.; Dutrow, B.; Uher, P.; Pezzotta, F. Nomenclature of the tourmaline supergroup minerals. Am. Min. 2011, 96, 895–913. [Google Scholar] [CrossRef]
- El-Hinnawi, E.; Hofmann, R. Optische und chemische Untersuchungen an neun Turmalinen (Elbaiten). Neues Jahrb. Mineral. 1966, 1966, 80–89. [Google Scholar]
- Katsurada, Y.; Sun, Z.; Breeding, C.M.; Dutrow, B.L. Geographic origin determination of paraíba tourmaline. Gems Gemol. 2019, 55, 648–659. [Google Scholar] [CrossRef]
- Okrusch, M.; Ertl, A.; Schüssler, U.; Tillmanns, E.; Brätz, H.; Bank, H. Major-and trace-element composition of paraíba-type tourmaline from brazil, mozambique and nigeria. J. Gemmol. 2016, 35, 120–140. [Google Scholar] [CrossRef]
- Abduriyim, A.; Kitawaki, H.; Furuya, M.; Schwarz, D. “Paraiba”-type copper-bearing tourmaline from Brazil, Nigeria, and Mozambique: Chemical fingerprinting by LA-ICP-MS. Gems Gemol. 2006, 42, 4. [Google Scholar] [CrossRef]
- Ertl, A.; Giester, G.; Schüssler, U.; Brätz, H.; Okrusch, M.; Tillmanns, E.; Bank, H. Cu-and Mn-bearing tourmalines from Brazil and Mozambique: Crystal structures, chemistry and Correlations. Mineral. Petrol. 2013, 107, 265–279. [Google Scholar] [CrossRef]
- Roda-Robles, E.; Pesquera, A.; Gil, P.; Torres-Ruiz, J.; Fontan, F. Tourmaline from the rare-element Pinilla pegmatite, (Central Iberian Zone, Zamora, Spain): Chemical variation and implications for pegmatitic evolution. Mineral. Petrol. 2004, 81, 249–263. [Google Scholar] [CrossRef]
- Simmons, W.B.; Laurs, B.M.; Falster, A.U.; Koivula, J.I.; Webber, K.L. Mt. Mica: A Renaissance in Maine’s Gem Tourmaline Production. Gems Gemol. 2005, 41, 150–163. [Google Scholar] [CrossRef]
- Zhong, R. Gem Mineralogical Characteristics of Blue-Green Tourmaline in Nurstan, Afghanistan. Master’s Thesis, China University of Geosciences, Beijing, China, 2014. [Google Scholar]
- Hoang, L.H.; Hien, N.T.M.; Chen, X.B.; Minh, N.V.; Yang, I.S. Raman spectroscopic study of various types of tourmalines. J. Raman Spectrosc. 2011, 42, 1442–1446. [Google Scholar] [CrossRef]
- Laurs, B.M.; Simmons, W.B.; Rossman, G.R.; Fritz, E.A.; Koivula, J.I.; Anckar, B.; Falster, A.U. Yellow Mn-rich tourmaline from the Canary mining area, Zambia. Gems Gemol. 2007, 43, 314–331. [Google Scholar] [CrossRef][Green Version]
- Prasad, P.S.R. Study of structural disorder in natural tourmalines by infrared spectroscopy. Gondwana Res. 2005, 8, 265–270. [Google Scholar] [CrossRef]
- Suwanmanee, W.; Wanthanachaisaeng, B.; Utapong, T.; Sutthirat, C. Colour Enhancement of Pink Tourmaline from Nigeria by Electron-Beam and Gamma Irradiation. J. Gemmol. 2021, 37, 514–526. [Google Scholar] [CrossRef]
- Watenphul, A.; Burgdorf, M.; Schluter, J.; Horn, I.; Malcherek, T.; Mihailova, B. Exploring the potential of Raman spectroscopy for crystallochemical analyses of complex hydrous silicates: II. Tourmalines. Am. Mineral. 2016, 101, 970–985. [Google Scholar] [CrossRef]
- Peng, Z.S. Geological background and main characteristics of tin ore in the Nu River area. Geol. Explor. 1992, 28, 12–16. [Google Scholar]
- Bu, X.F. Geochemistry, Chronology and Spatiotemporal Evolution of Granitoids in Nujiang Area, Northwest Yunnan Province. Master’s Thesis, China University of Geosciences, Wuhan, China, 2014. [Google Scholar]
- Liu, J. Gemological Characteristics of Tourmaline and Its Internal Inclusions. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2017. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201801&filename=1017217006.nh (accessed on 30 August 2025).
- Zeng, X.Y. CH4/CH4-C2H6 Hydrate Formation and Decomposition in Situ Raman Studies. Ph.D. Thesis, China University of Petroleum, Beijing, China, 2016. [Google Scholar]
- Zerda, T.W.; John, A.; Chmura, K. Raman studies of coals. Fuel 1981, 60, 375–378. [Google Scholar] [CrossRef]
- Zhang, N.; Tian, Z.J.; Mao, G.J.; Wu, S.H.; Liu, J.X.; De, Q. Raman spectral characteristics of asphalt inclusions. Geochemistry 2009, 38, 174–178. [Google Scholar] [CrossRef]
- Liu, G.B.; Lu, H.Z. Study on inclusions in gem-grade colored tourmaline in Xinjiang. Geochemistry 1985, 5, 264–268+294. [Google Scholar] [CrossRef]
- da Fonseca-Zang, W.A.; Zang, J.W.; Hofmeister, W. The Ti-influence on the tourmaline color. J. Braz. Chem. Soc. 2008, 19, 1186–1192. [Google Scholar] [CrossRef]
- Altieri, A.; Pezzotta, F.; Skogby, H.; Hålenius, U.; Bosi, F. Blue-growth zones caused by Fe2+ in tourmaline crystals from the San Piero in Campogem-bearing pegmatites, Elba Island, Italy. Mineral. Mag. 2022, 86, 910–919. [Google Scholar] [CrossRef]
- Altieri, A.; Pezzotta, F.; Skogby, H.; Hålenius, U.; Bosi, F. Dark-coloured Mn-rich overgrowths in an elbaitic tourmaline crystal from the Rosina pegmatite, San Piero in Campo, Elba Island, Italy: Witness of late-stage opening of the geochemical system. Mineral. Mag. 2023, 87, 130–147. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Henry, D.J. Classification of the minerals of the tourmaline group. Eur. J. Mineral. 1999, 11, 201–215. [Google Scholar] [CrossRef]
- Brethous, J.C.; Levasseur, A.; Villeneuve, G.; Echegut, P.; Hagenmuller, P.; Couzi, M. Etudes par spectroscopie Raman et par RMN des verres du système B2O3 SiO2 Li2O. J. Solid State Chem. 1981, 39, 199–208. [Google Scholar] [CrossRef]
- Fu, X.M. Polarized Raman spectroscopy of tourmaline. Miner. Geol. 1998, 12, 59–63. [Google Scholar]
- Wesełucha-Birczyńska, A.; Natkaniec-Nowak, L. A Raman microspectroscopic study of organic inclusions in “watermelon” tourmaline from the Paprok mine (Nuristan, Afghanistan). Vib. Spectrosc. 2011, 57, 248–253. [Google Scholar] [CrossRef]
- Reddy, B.J.; Frost, R.L.; Martens, W.N.; Wain, D.L.; Kloprogge, J.T. Spectroscopic characterization of Mn-rich tourmalines. Vib. Spectrosc. 2007, 44, 42–49. [Google Scholar] [CrossRef]
- Wang, J.J.; Tao, X.F.; Wang, W.J. Study on the color genesis of green tourmaline in Xinjiang. J. Rock Mineral. 2005, 24, 319–323. [Google Scholar]
- Mattson, S.M.; Rossman, G.R. Fe2+-Fe3+ interactions in tourmaline. Phys. Chem. Miner. 1987, 14, 163–171. [Google Scholar] [CrossRef]
- Pasetti, L.; Borromeo, L.; Bersani, D.; Andò, S.; Schnellrath, J.; Hennebois, U.; Karampelas, S. Identification of Some Gem Quality Blue to Green Li-Tourmalines. Minerals 2023, 14, 44. [Google Scholar] [CrossRef]
- Rondeau, B.; Fritsch, E.; Peucat, J.-J.; Nordrum, F.S.; Groat, L. Characterization of emeralds from a historical deposit: Byrud (Eidsvoll), Norway. Gems Gemol. 2008, 44, 108–122. [Google Scholar] [CrossRef]
- Fan, J.L.; Feng, X.Q.; Guo, S.G.; Liu, X.L. Optical absorption spectra of tourmaline crystals. J. Chin. Ceram. Soc. 2009, 37, 523–530. [Google Scholar]
- Surour, A.A.; Omar, S.M.A. Chemical and spectroscopic characterization of tourmaline from the ancient Roman mines in the Eastern Desert of Egypt. Environ. Earth Sci. 2022, 81, 78. [Google Scholar] [CrossRef]
- Li, X.J.; Zu, E.D. Near-infrared spectroscopy of cyclic silicate gem minerals. Bull. Silic. 2016, 35, 1318–1321. [Google Scholar]
- Liao, Q.J.; Huang, W.Z.; Zhang, Q.; Pei, J.C. Gemological and spectroscopic characterization of brown-yellow tourmaline in Mozambique. Spectrosc. Spectr. Anal. 2019, 39, 3844–3848. [Google Scholar]
- Liu, B.S.; He, X.; Zhao, J.J.; Zhao, Q.N. Effects of heat treatment on structure and spectral properties of TiO2 sputtered thin films. Rare Met. Mater. Eng. 2005, 34, 1451–1454. [Google Scholar]
- Simmons, W.B.; Falster, A.U.; Laurs, B.M. A survey of Mn-rich yellow tourmaline from worldwide localities and implications for the petrogenesis of granitic pegmatites. Can. Mineral. 2011, 49, 301–319. [Google Scholar] [CrossRef]
- Zhong, P.P.; Shen, X.T. A preliminary study on the color genesis of dark green tourmaline in Zambia. J. Gems Gemol. 2017, 19, 7–14. [Google Scholar] [CrossRef]
- Da Silva, S.F.; Moura, M.A.; Queiroz, H.d.A.; Ardisson, J.D. Chemical and spectroscopic characterization of tourmalines from the Mata Azul pegmatitic field, Central Brazil. J. Geosci. 2018, 63, 155–165. [Google Scholar] [CrossRef]







| Li2O | TiO2 | FeO | MnO | |
|---|---|---|---|---|
| Range | 1.72–2.41 | 0.08–0.46 | 0.01–0.23 | 1.42–6.41 |
| Average | 1.90 | 0.31 | 0.07 | 4.31 |
| Median | 1.74 | 0.355 | 0.02 | 4.52 |
| Detection limits (wt.%) | 0.0002–0.0004 | 0–0.0006 | 0.004–0.005 | 0.0002–0.0003 |
| Li2O | TiO2 | FeO | MnO | |
|---|---|---|---|---|
| Range | 1.40–1.62 | 0.03–0.13 | 6.44–6.57 | 0.68–0.85 |
| Average | 1.53 | 0.06 | 6.25 | 0.74 |
| Median | 1.54 | 0.03 | 6.495 | 0.71 |
| Detection limits (wt.%) | 0.0002–0.0005 | 0–0.0005 | 0.004–0.006 | 0.0002–0.0004 |
| Number/Element (wt.%) | LiO2 | Cr2O3 | V2O5 | FeO | MnO | TiO2 |
|---|---|---|---|---|---|---|
| GS 6-1 | 1.62 | 0.0002 | bdl | 5.44 | 0.73 | 0.13 |
| GS 6-2 | 0.004 | 0.15 | 0.06 | 0.04 | 0.004 | 0.62 |
| GS 6-3 | 0.021 | 0.37 | 1.02 | 0.02 | 0.007 | 0.54 |
| GS 6-4 | 1.54 | bdl | bdl | 6.35 | 0.67 | 0.03 |
| GS 6-5 | 1.54 | 0.0007 | bdl | 6.57 | 0.68 | 0.03 |
| FG 4-1 | 2.57 | bdl | bdl | 1.38 | 0.45 | 0.05 |
| Detection limits (wt.%) | 0–0.0002 | 0.0006–0.0012 | 0–0.00002 | 0.0033–0.0052 | 0.0001–0.0003 | 0–0.0004 |
| Sample Number | TiO2 (wt.%) | FeO (wt.%) |
|---|---|---|
| GS10-4 | 0.005 | 0.001 |
| FG4-1 | 0.57 | 0.01 |
| GS 10-8 | 0.002 | 0.10 |
| FG(P)4-4 | 0.09 | 4.10 |
| Detection limits (wt.%) | 0–0.0005 | 0.004–0.005 |
| Yunnan | Zambia * | Russia * | Madagascar * | Nepal * | Congo * | California * | |
|---|---|---|---|---|---|---|---|
| TiO2 | 0.31 | 0.32 | 0.23 | 0.67 | 0.29 | 0.27 | 0.11 |
| FeO | 0.07 | 0.09 | 0.17 | 0.01 | 0.08 | 0.29 | 0.07 |
| MnO | 4.31 | 7.18 | 4.44 | 7.47 | 5.95 | 6.40 | 5.97 |
| Li2O | 1.90 | 1.30 | 1.79 | 1.52 | 1.42 | 1.69 | 1.50 |
| Element (wt.%)/Origin | Yunnan | Zambia a | Brazil b | Maine c | Mozambique d | Namibia e | Minas Gerais f |
|---|---|---|---|---|---|---|---|
| TiO2 | 0.06 | 0.05 | 0.21 | 0.09 | 0.05 | 0.06 | 0.02 |
| FeO | 6.25 | 3.54 | 4.98 | 3.53 | 0.05 | 5.80 | 1.78 |
| MnO | 0.74 | 2.19 | 0.70 | 0.98 | 2.85 | 0.54 | 1.44 |
| Li2O | 1.53 | 1.77 | 1.53 | 1.54 | 1.22 | 1.29 | 2.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Zhan, F.; Yu, H.; Lu, Z.; Wan, X. Gemmological, Spectroscopic, and Origin Description Studies of Tourmaline from Yunnan, China. Molecules 2025, 30, 3680. https://doi.org/10.3390/molecules30183680
Zhou Q, Zhan F, Yu H, Lu Z, Wan X. Gemmological, Spectroscopic, and Origin Description Studies of Tourmaline from Yunnan, China. Molecules. 2025; 30(18):3680. https://doi.org/10.3390/molecules30183680
Chicago/Turabian StyleZhou, Qishen, Fangmin Zhan, Haochi Yu, Zhuo Lu, and Xin Wan. 2025. "Gemmological, Spectroscopic, and Origin Description Studies of Tourmaline from Yunnan, China" Molecules 30, no. 18: 3680. https://doi.org/10.3390/molecules30183680
APA StyleZhou, Q., Zhan, F., Yu, H., Lu, Z., & Wan, X. (2025). Gemmological, Spectroscopic, and Origin Description Studies of Tourmaline from Yunnan, China. Molecules, 30(18), 3680. https://doi.org/10.3390/molecules30183680






