Progress in Gliotoxin Research
Abstract
1. Introduction
2. Isolation
2.1. Gliotoxin Isolation
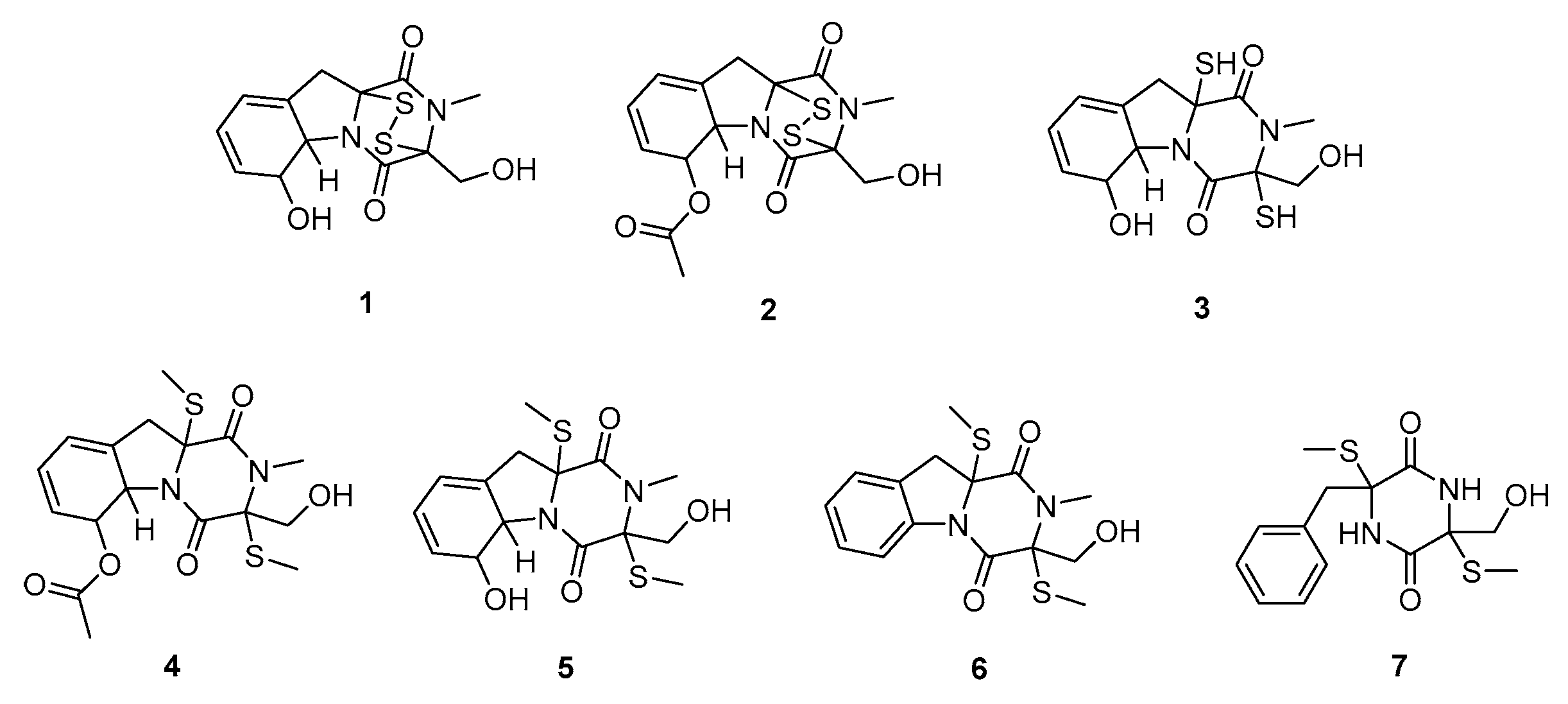
2.2. Gliotoxin Derivatives Isolation
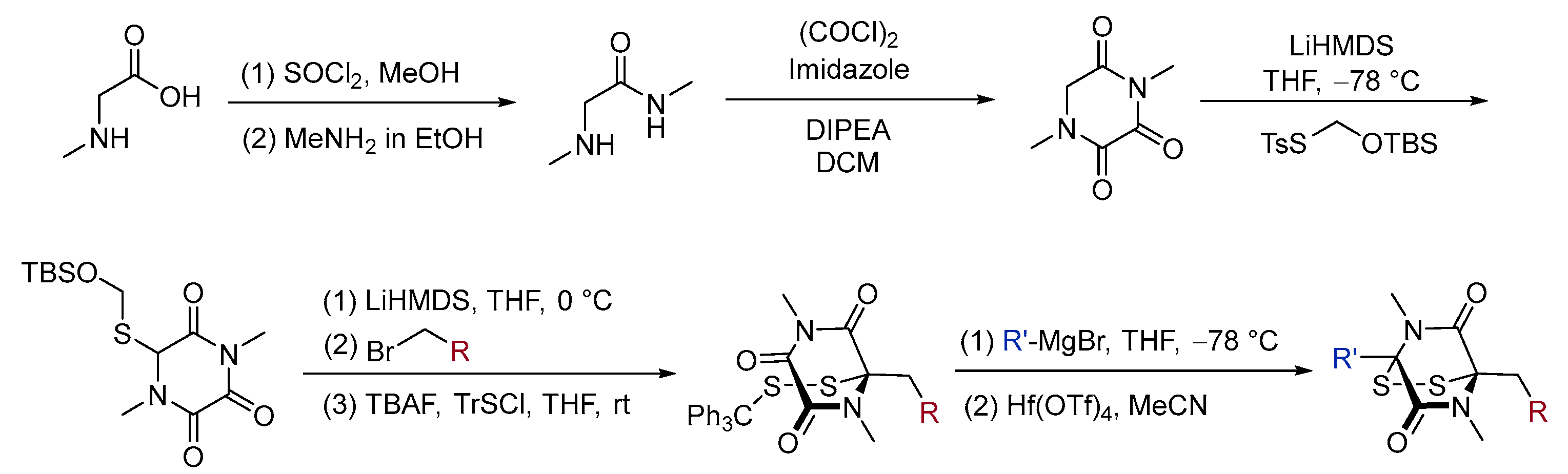
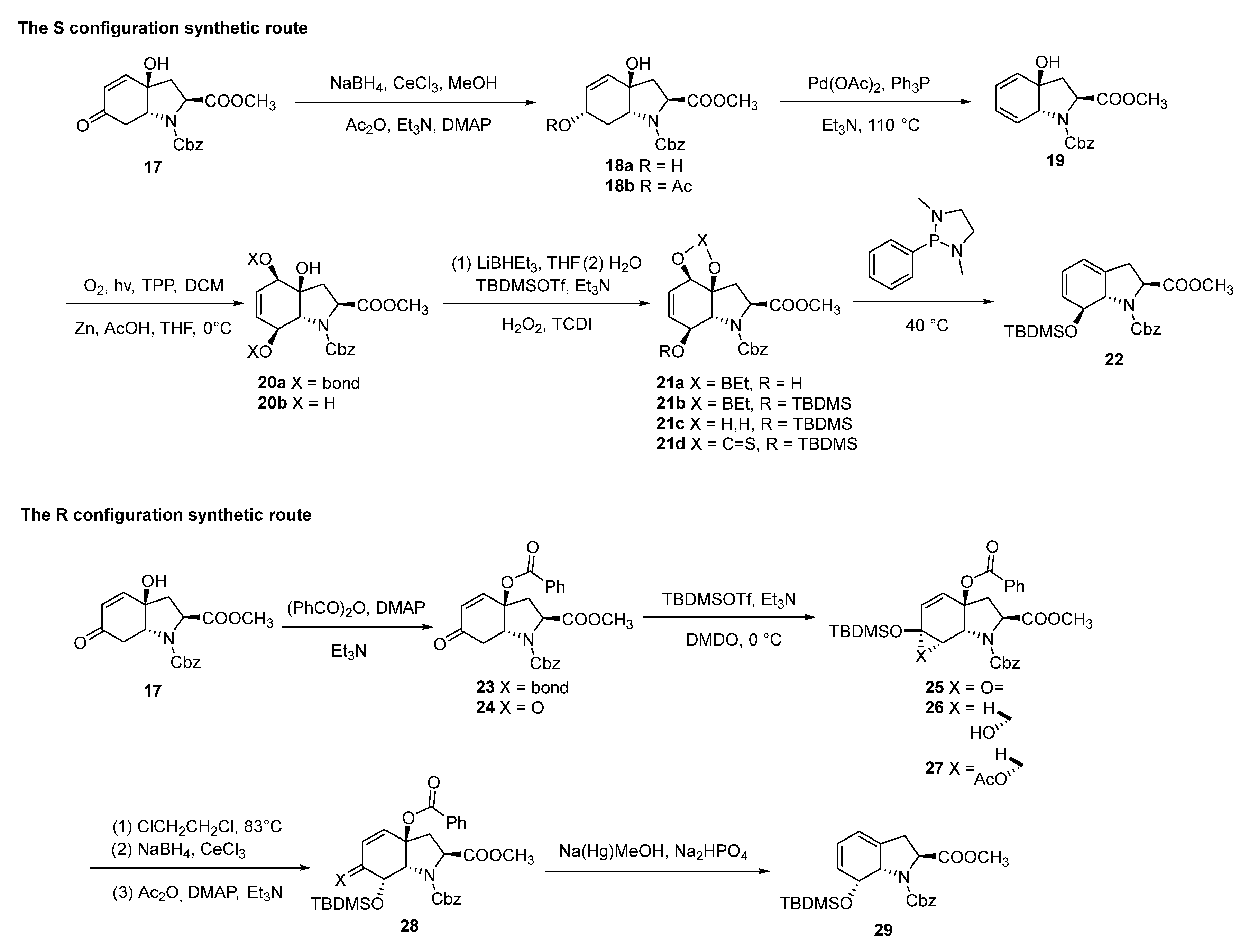
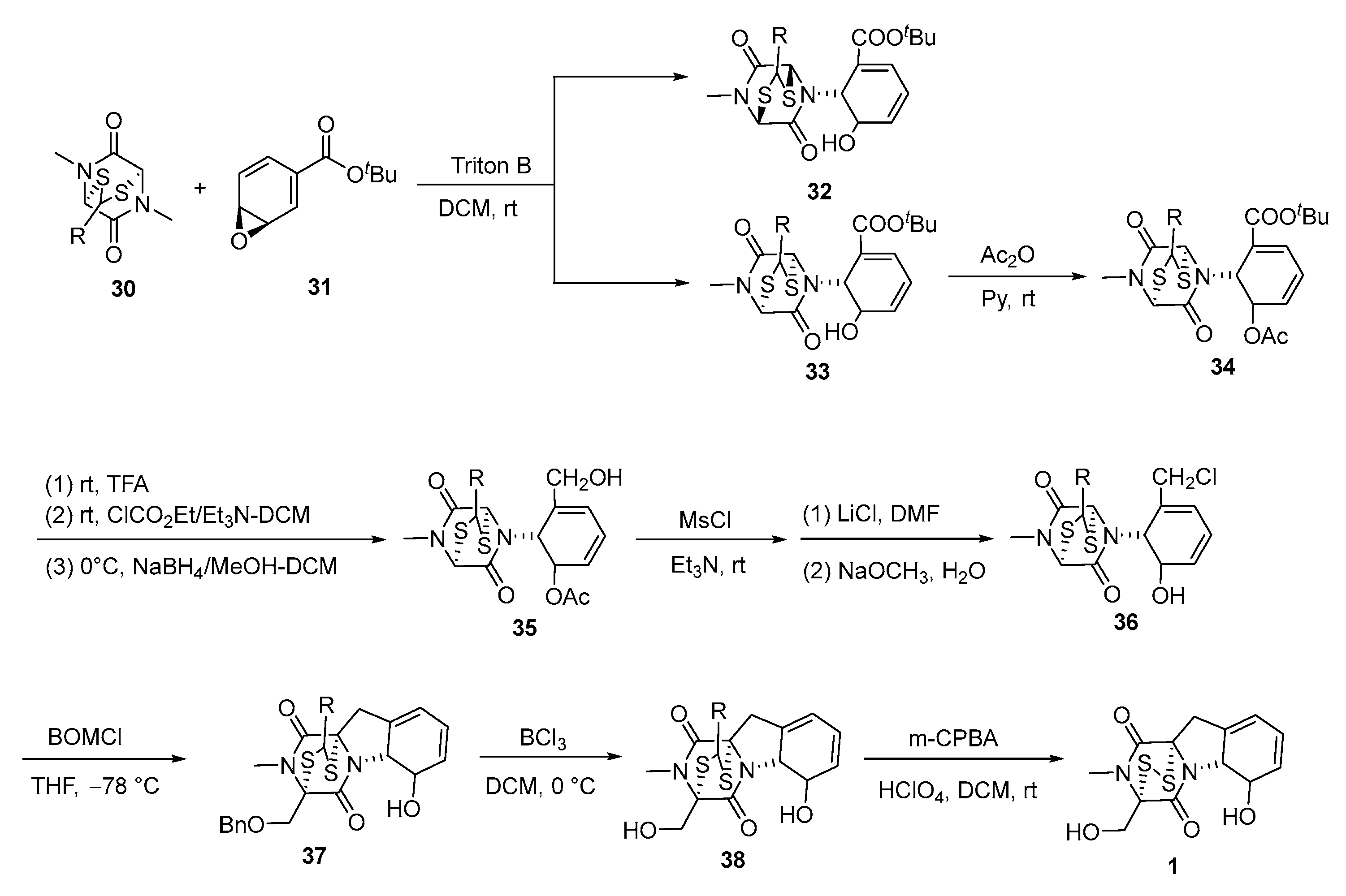
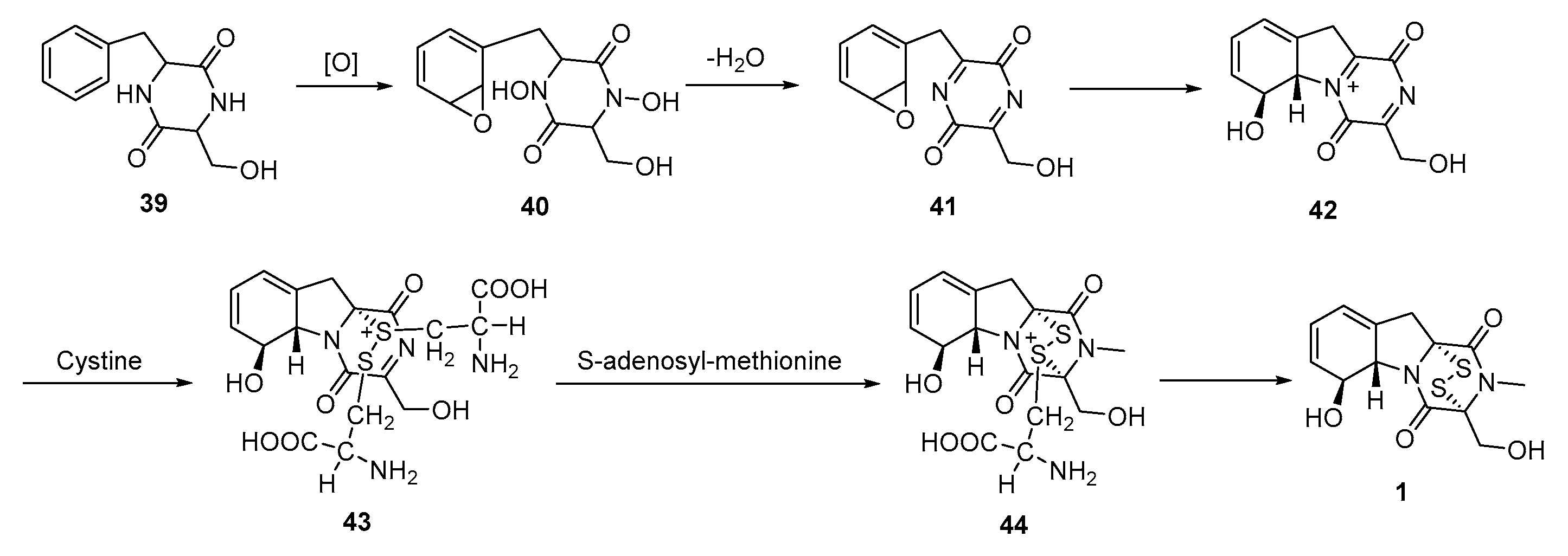
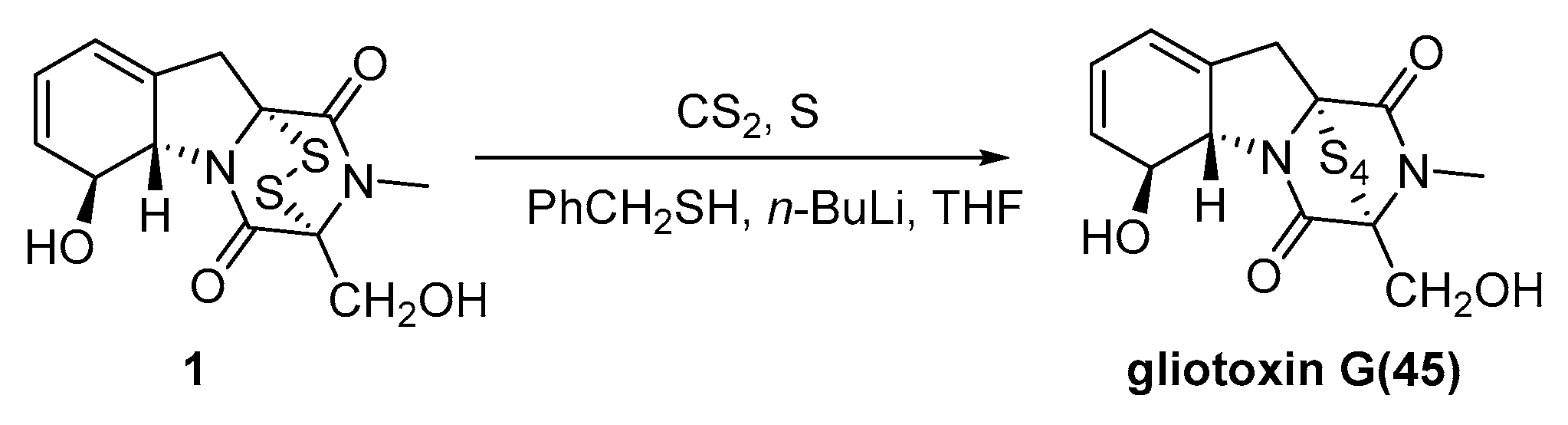
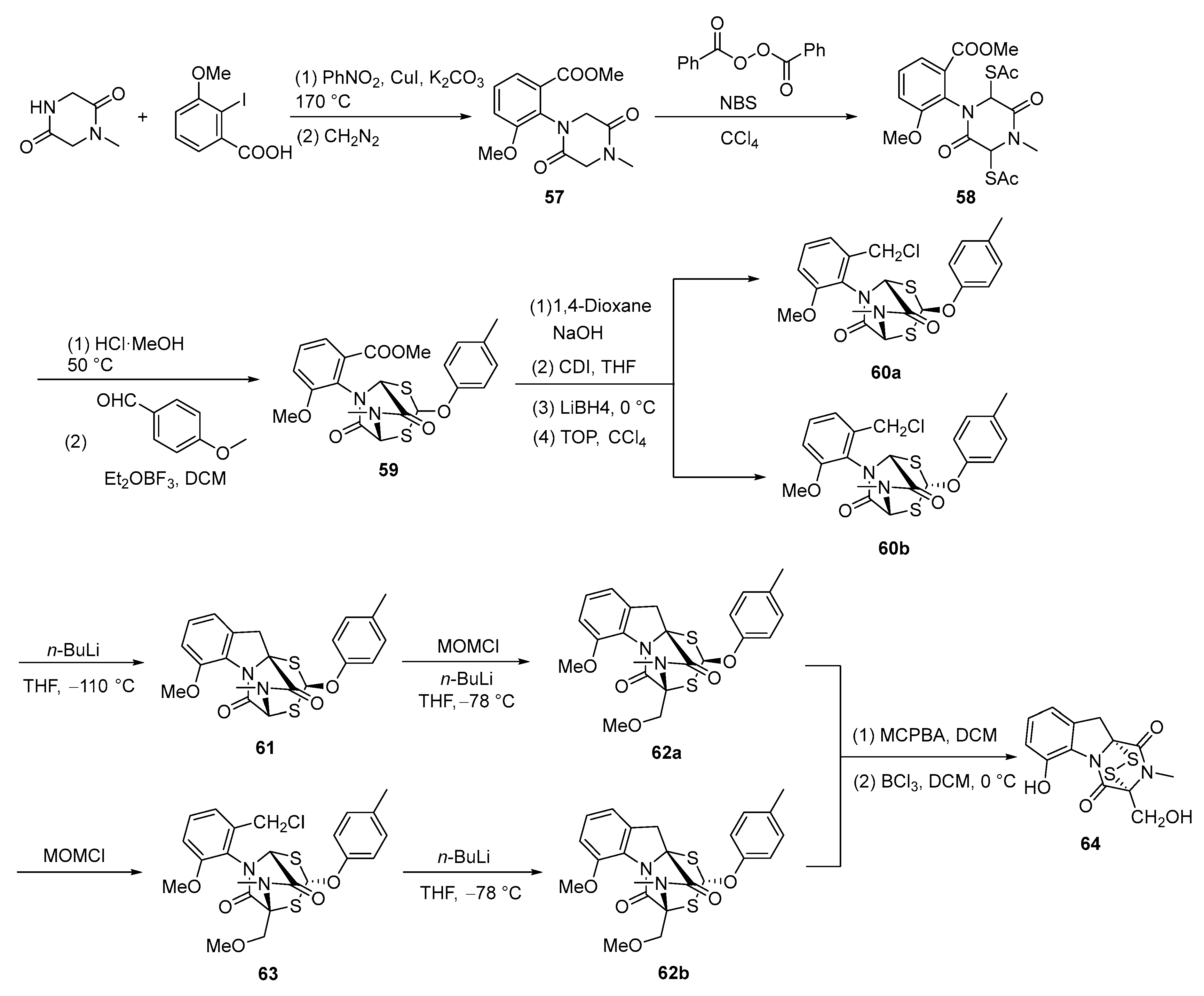
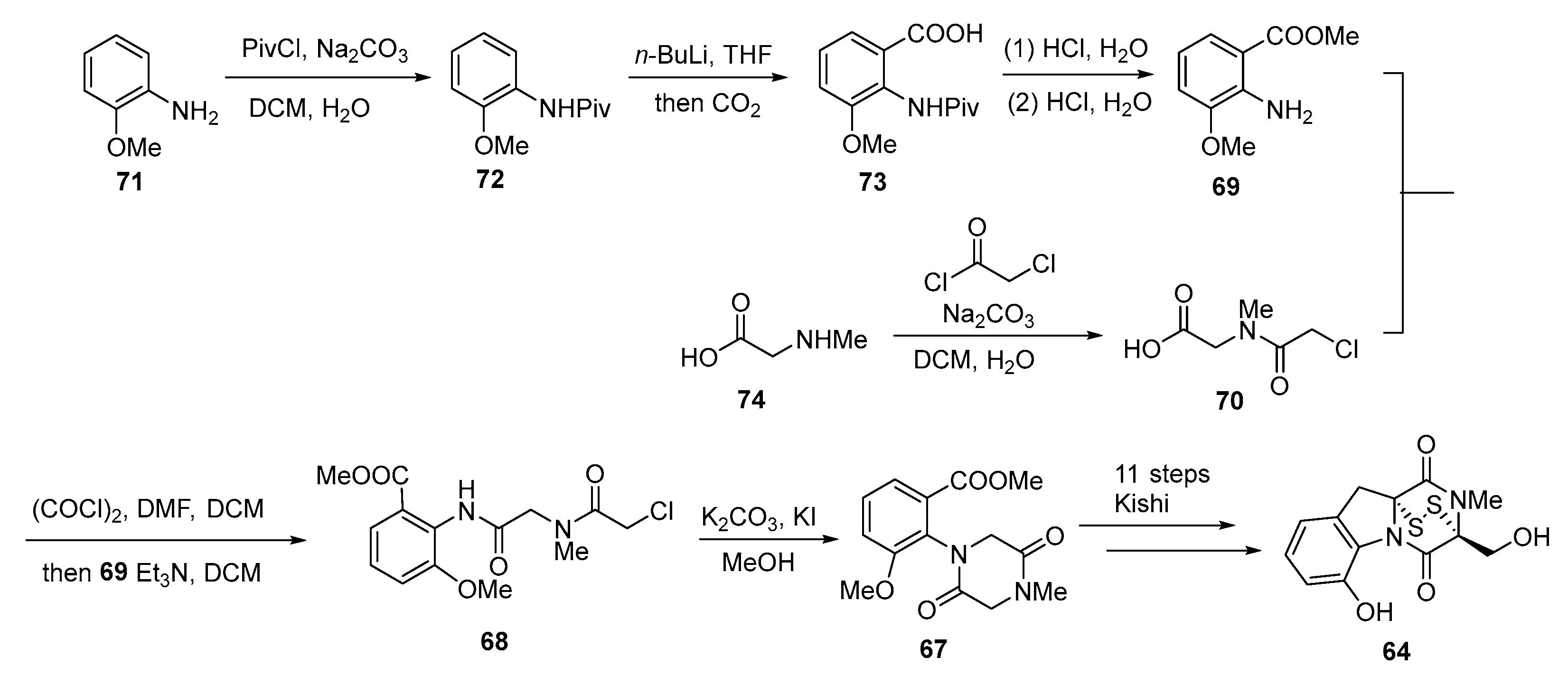
3. Synthesis
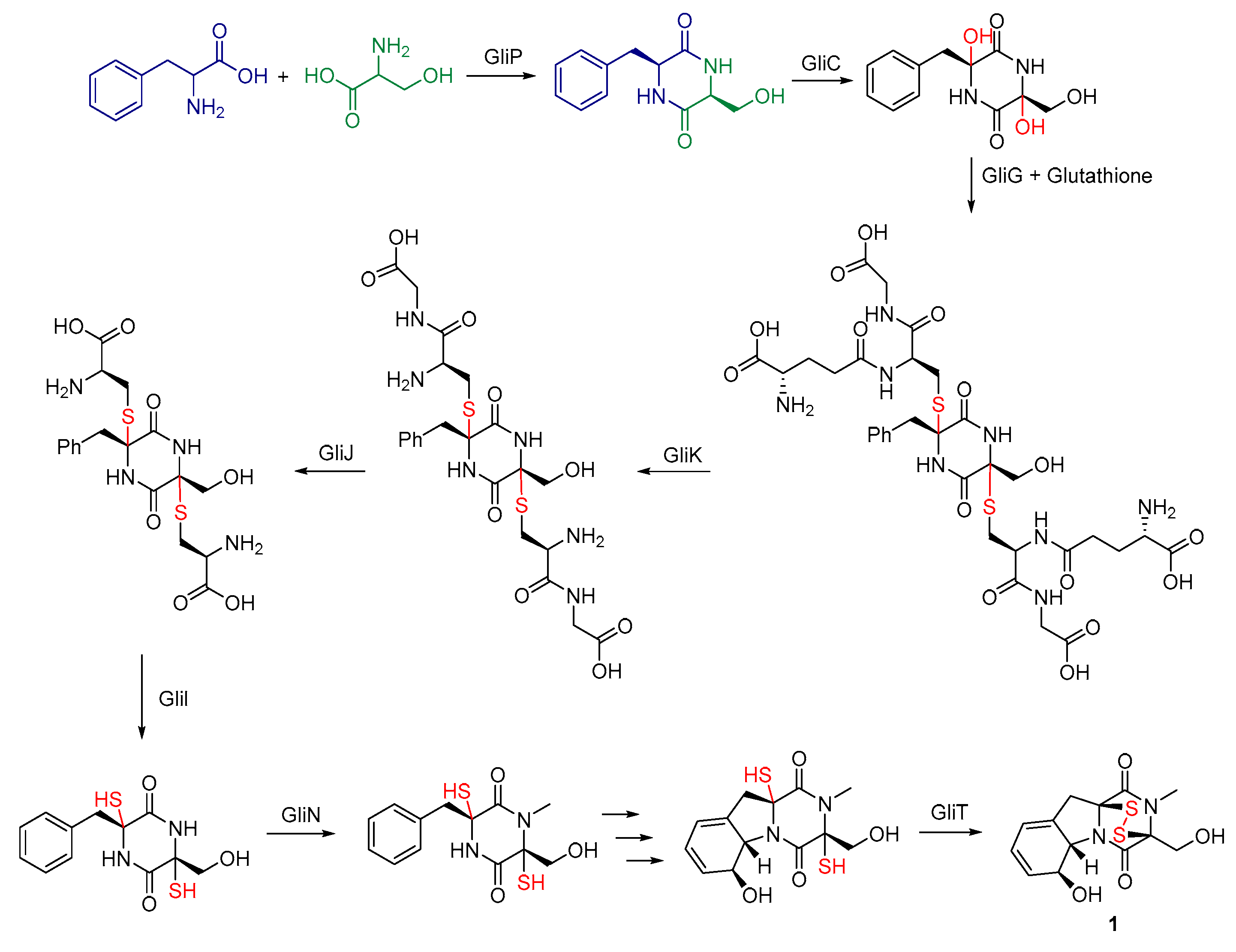
3.1. Biosynthesis
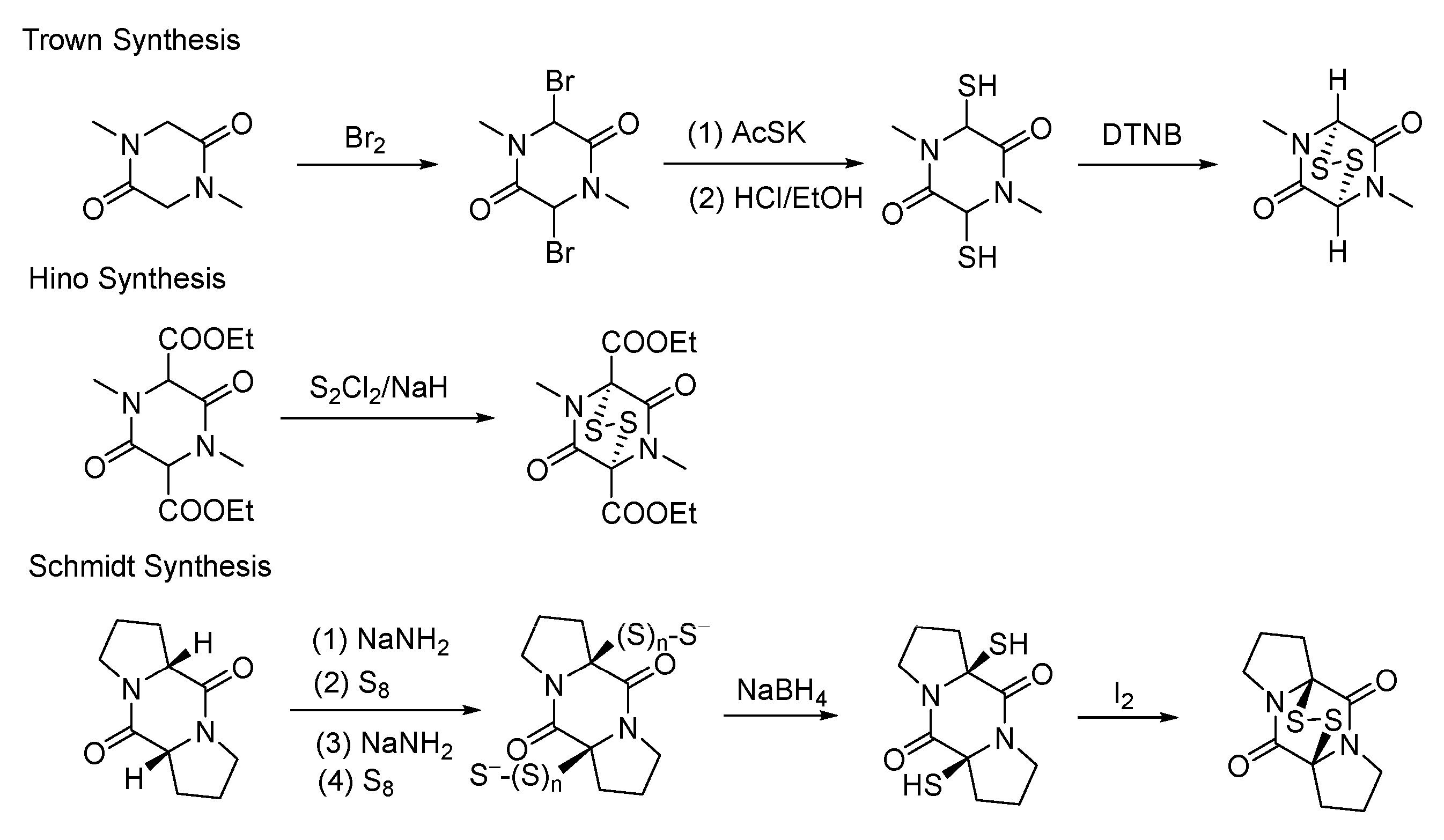
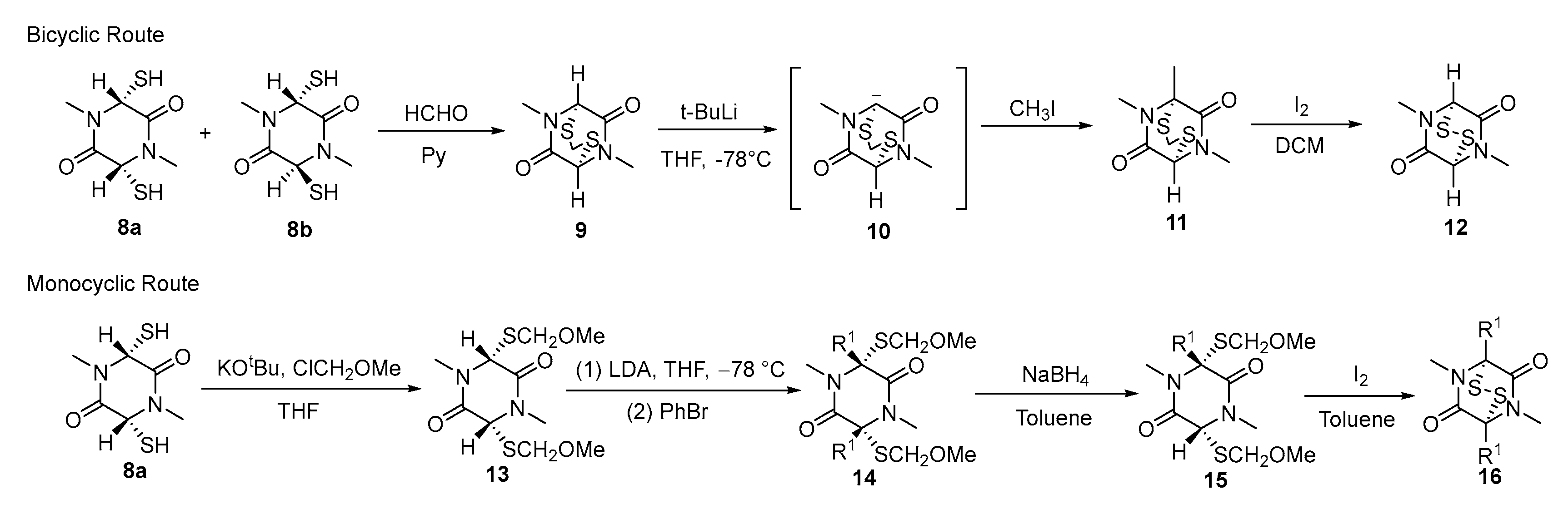
3.2. Chemical Synthesis
4. Bioactivity
4.1. Apoptosis
4.2. Inhibits Host Immune Responses
4.3. Antineoplastic Activity
4.4. Antiviral Activity
4.5. Antifungal and Antibacterial Activity
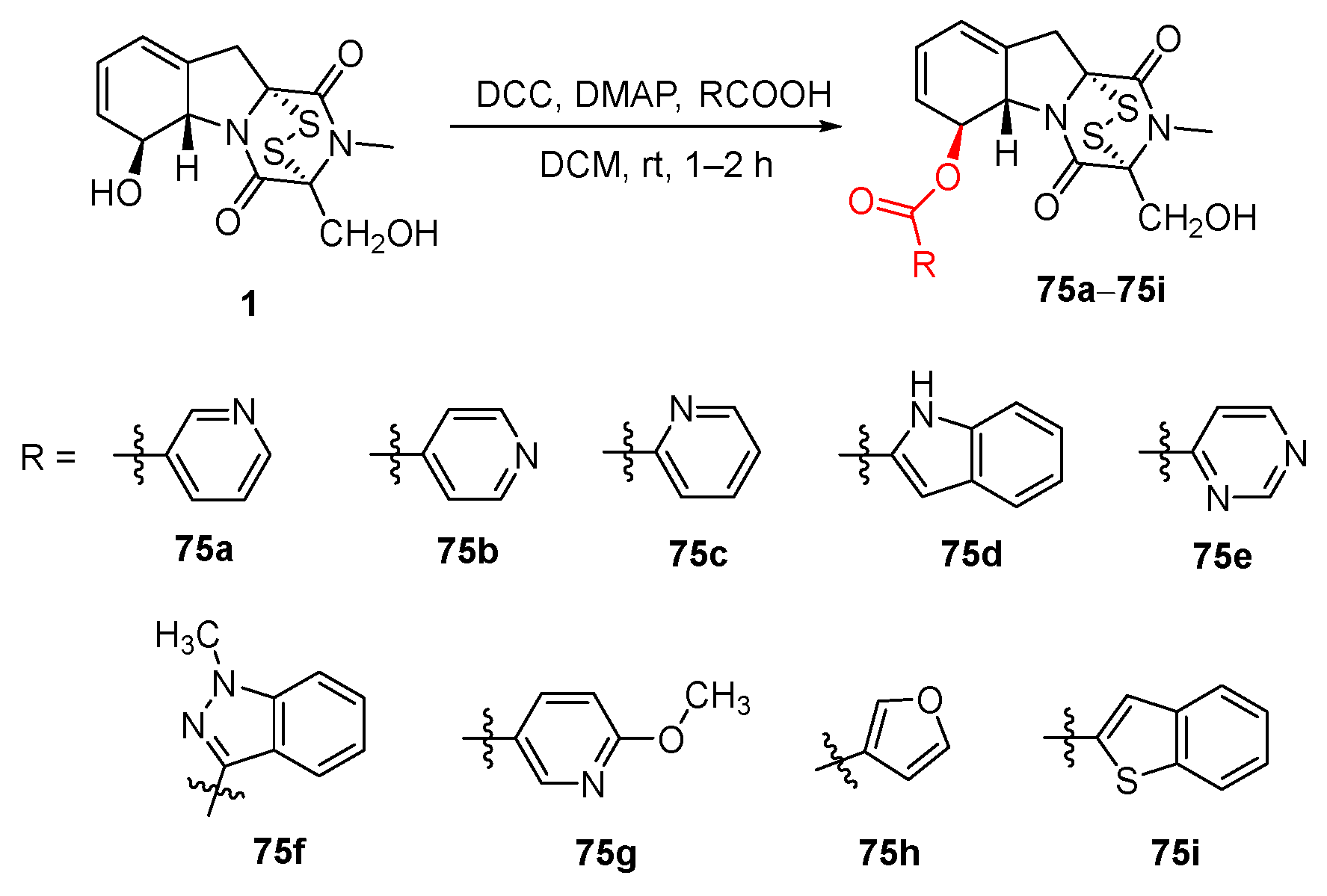
5. Structure–Activity Relationships
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GT | Gliotoxin |
| BmGT | Bisdethiobis(methylthio)gliotoxin |
| DKP | Diketopiperazine |
| ETPs | Epidithiodiketopiperazines |
| IA | invasive aspergillosis |
References
- Gardiner, D.M.; Waring, P.; Howlett, B.J. The epipolythiodioxopiperazine(ETP) class of fungal toxins: Distribution, mode of action, functions and biosynthesis. Microbiology 2005, 151, 1021–1032. [Google Scholar] [CrossRef]
- Hua, L.X.; Zeng, H.L.; He, L.; Jiang, Q.P.; Ye, P.S.; Liu, Y.; Sun, X.F.; Zhang, M. Gliotoxin Is an Important Secondary Metabolite Involved in Suppression of Sclerotium rolfsii of Trichoderma virens T23. Phytopathology 2021, 111, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Scharf, D.H.; Heinekamp, T.; Remme, N.; Hortschansky, P.; Brakhage, A.A.; Hertweck, C. Biosynthesis and Function of Gliotoxin in Aspergillus fumigatus. Appl. Microbiol. Biotechnol. 2012, 93, 467–472. [Google Scholar] [CrossRef]
- Ye, W.; Zhang, W.M.; Liu, T.M.; Huang, Z.L.; Zhu, M.Z.; Chen, Y.C.; Li, H.H.; Li, S.N. De novo Transcriptome Sequencing of the Deep-Sea-Derived Fungus Dichotomomyces cejpii and Analysis of Gliotoxin Biosynthesis Genes. Int. J. Mol. Sci. 2018, 19, 1910. [Google Scholar] [CrossRef]
- Beecham, A.F.; Fridrichsons, J.; Mathieson, A.M. The structure and absolute configuration of gliotoxin and the absolute configuration of sporidesmin. Tetrahedron Lett. 1966, 7, 3131–3138. [Google Scholar] [CrossRef]
- Fukuyama, T.; Kishi, Y. A total synthesis of gliotoxin. J. Am. Chem. Soc. 1976, 98, 6723–6724. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Lu, M.; Totokotsopoulos, S.; Heretsch, P.; Giguère, D.; Sun, Y.P.; Sarlah, D.; Nguyen, T.H.; Wolf, I.C.; Smee, D.F.; et al. Synthesis and biological evaluation of epidithio-, epitetrathio-, and bis-(methylthio)diketopiperazines: Synthetic methodology, enantioselective total synthesis of epicoccin G, 8,8′-epi-ent-rostratin B, gliotoxin, gliotoxin G, emethallicin E, and haematocin and discovery of new antiviral and antimalarial agents. J. Am. Chem. Soc. 2012, 134, 17320–17332. [Google Scholar]
- Dolan, S.K.; O’Keeffe, G.; Jones, G.W.; Doyle, S. Resistance is Not Futile: Gliotoxin Biosynthesis, Functionality and Utility. Trends Microbiol. 2015, 23, 419–428. [Google Scholar] [CrossRef]
- Jiang, C.S.; Müller, W.E.G.; Schröder, H.C.; Guo, Y.W. Disulfide- and Multisulfide-containing Metabolites from Marine Organisms. Chem. Rev. 2012, 112, 2179–2207. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Liu, T.M.; Zhang, W.Y.; Zhang, W.M. The Toxic Mechanism of Gliotoxins and Biosynthetic Strategies for Toxicity Prevention. Int. J. Mol. Sci. 2021, 22, 13510. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Kataoka, T.; Nagai, K. The Fungal Metabolite Gliotoxin: Immunosuppressive Activity on CTL-Mediated Cytotoxicity. Immunol. Lett. 2000, 71, 27–32. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.S. Gliotoxin from the marine fungus Aspergillus fumigatus induces apoptosis in HT1080 fibrosarcoma cells by downregulating NF-kB. Fish Aquatic Sci. 2016, 19, 27–32. [Google Scholar] [CrossRef]
- Geissler, A.; Haun, F.; Frank, D.O.; Wieland, K.; Simon, M.M.; Idzko, M.; Davis, R.J.; Maurer, U.; Borner, C. Apoptosis induced by the fungal pathogen gliotoxin requires a triple phosphorylation of Bim by JNK. Cell Death Differ. 2013, 20, 1317–1329. [Google Scholar] [CrossRef]
- Hubmann, R.; Hilgarth, M.; Schnabl, S.; Ponath, E.; Demirtas, D.; Reiter, M.; Zielinski, C.; Jäger, U.; Shehata, M. Inhibition of NOTCH Activity by GLIOTOXIN Induces Apoptosis in CLL Cells and Overcomes the Supportive Effect of Primary Bone Marrow Stromal Cells. Blood 2011, 118, 4609. [Google Scholar] [CrossRef]
- Dekel, R.; Zvibel, I.; Brill, S.; Brazovsky, E.; Halpern, Z.; Oren, R. Gliotoxin Ameliorates Development of Fibrosis and Cirrhosis in a Thioacetamide Rat Model. Digest. Dis. Sci. 2003, 48, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Song, K.W. The Function and Mechanism of GT in the Pathogenesis of Aspergillus fumigatus Keratitis; Qingdao University: Qingdao, China, 2021. [Google Scholar]
- Downes, S.G.; Owens, R.A.; Walshe, K.; Fitzpatrick, D.A.; Dorey, A.; Jones, G.W.; Doyle, S. Gliotoxin-mediated bacterial growth inhibition is caused by specific metal ion depletion. Sci. Rep. 2023, 13, 16156. [Google Scholar] [CrossRef]
- Tomah, A.A.; Alamer, I.S.A.; Li, B.; Zhang, J.Z. A new species of Trichoderma and gliotoxin role: A new observation in enhancing biocontrol potential of T. virens against Phytophthora capsici on chili pepper. Biol. Control 2020, 145, 104261. [Google Scholar] [CrossRef]
- Gan, L.; Yin, Y.L.; Niu, Q.C.; Yan, X.B.; Yin, S.X. New Insights into the Mechanism of Trichoderma virens-Induced Developmental Effects on Agrostis stolonifera Disease Resistance against Dollar Spot Infection. J. Fungi 2022, 8, 1186. [Google Scholar] [CrossRef]
- Gao, P.; Qi, K.; Han, Y.; Ma, L.; Zhang, B.; Zhang, Y.; Guan, X.; Qi, J. Effect of Trichoderma viride on rhizosphere microbial communities and biocontrol of soybean root rot. Front. Microbiol. 2023, 14, 1204688. [Google Scholar] [CrossRef]
- Schrettl, M.; Carberry, S.; Kavanagh, K.; Haas, H.; Jones, G.W.; O’Brien, J.; Nolan, A.; Stephens, J.; Fenelon, O.; Doyle, S. Self-protection against gliotoxin--a component of the gliotoxin biosynthetic cluster, GliT, completely protects Aspergillus fumigatus against exogenous gliotoxin. PLoS Pathog. 2010, 6, e1000952. [Google Scholar] [CrossRef]
- Orciuolo, E.; Stanzani, M.; Canestraro, M.; Galimberti, S.; Carulli, G.; Lewis, R.; Petrini, M.; Komanduri, K.V. Effects of Aspergillus fumigatus gliotoxin and methylprednisolone on human neutrophils: Implications for the pathogenesis of invasive aspergillosis. J. Leukoc. Biol. 2007, 82, 839–848. [Google Scholar] [CrossRef]
- Li, W.L.; Xia, J. Recent advances in diketopiperazines biosynthesis. Microbiol. China 2014, 41, 111–121. [Google Scholar]
- Li, L.Y.; Zhu, T.J.; Li, D.H.; Gu, Q.F. Progress in the research of epipolythiodioxopiperazines. Chin. J. Antibiot. 2013, 38, 161–174. [Google Scholar]
- Reece, K.M.; Richardson, E.D.; Cook, K.M.; Campbell, T.J.; Pisle, S.T.; Holly, A.J.; Venzon, D.J.; Liewehr, D.J.; Chau, C.H.; Price, D.K.; et al. Epidithiodiketopiperazines (ETPs) exhibit in vitro antiangiogenic and in vivo antitumor activity by disrupting the HIF-1α/p300 complex in a preclinical model of prostate cancer. Mol. Cancer 2014, 13, 91. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Lewis, R.E.; Kontoyiannis, D.P. Enemy of the (immunosuppressed) state: An update on the pathogenesis of Aspergillus fumigatus infection. Br. J. Haematol. 2010, 150, 406–417. [Google Scholar] [CrossRef]
- Zhou, W.Q.; Shen, H.; Zhang, Z.H.; Zhang, K. Research Progress of Aspergillus fumigatus Gliotoxin. Chin. J. Mycol. 2011, 6, 118–121. [Google Scholar]
- Kamei, K.; Watanabe, A. Aspergillus mycotoxins and their effect on the host. Med. Mycol. 2005, 43 (Suppl. 1), S95–S99. [Google Scholar] [CrossRef]
- Weindling, R. Trichoderma lignorum as a Parasite of Other Soil Fungi. Phytopathology 1932, 22, 837–845. [Google Scholar]
- Glister, G.A.; Williams, T.I. Production of Gliotoxin by Aspergillus fumigatus mut. helvola Yuill. Nature 1944, 153, 651. [Google Scholar] [CrossRef]
- Mull, R.P.; Townley, R.W.; Scholz, C.R. Production of gliotoxin and a second active isolate by penicillium obscurum biourge. J. Am. Chem. Soc. 1945, 67, 1626–1627. [Google Scholar] [CrossRef]
- Bracken, A.; Raistrick, H. Studies in the biochemistry of micro-organisms; dehydrocarolic acid, a metabolic product of Penicillium cinerascens Biourge. Biochem. J. 1947, 41, 569–575. [Google Scholar] [CrossRef]
- Johnson, J.R.; Kidwai, A.R.; Warner, J.S. Gliotoxin. XI. A Related Antibiotic from Penicillium terlikowski: Gliotoxin Monoacetate. J. Am. Chem. Soc. 1953, 75, 2110–2112. [Google Scholar] [CrossRef]
- Scharf, D.H.; Brakhage, A.A.; Mukherjee, P.K. Gliotoxin--bane or boon? Environ. Microbiol. 2016, 18, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E.; Wiederhold, N.P.; Lionakis, M.S.; Prince, R.A.; Kontoyiannis, D.P. Frequency and species distribution of gliotoxin-producing Aspergillus isolates recovered from patients at a tertiary-care cancer center. J. Clin. Microbiol. 2005, 43, 6120–6122. [Google Scholar] [CrossRef]
- Wilkinson, S.; Spilsbury, J.F. Gliotoxin from Aspergillus chevalieri (Mangin) Thom et Church. Nature 1965, 206, 619. [Google Scholar] [CrossRef]
- Anitha, R.; Murugesan, K. Production of gliotoxin on natural substrates by Trichoderma virens. J. Basic. Microbiol. 2005, 45, 12–19. [Google Scholar] [CrossRef]
- He, X.; Ding, L.; Yi, M.; Xu, J.; Zhou, X.; Zhang, W.; He, S. Separation of five diketopiperazines from the marine fungus Alternaria alternate HK-25 by high-speed counter-current chromatography. J. Sep. Sci. 2019, 42, 2510–2516. [Google Scholar] [CrossRef]
- Lowe, G.; Taylor, A.; Vining, L.C. Sporidesmins. Part VI. Isolation and structure of Dehydrogliotoxin a metabolite of Penicillium terlikowskii. J. Chem. Soc. C Org. 1966, 20, 1799–1803. [Google Scholar] [CrossRef]
- Okamoto, M.; Yoshida, K.; Uchida, I.; Nishikawa, M.; Kohsaka, M.; Aoki, H. Studies of platelet activating factor (PAF) antagonists from microbial products. I. Bisdethiobis(methylthio)gliotoxin and its derivatives. Chem. Pharm. Bull. 1986, 34, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.L.; Le, X.; Li, H.J.; Yang, X.L.; Chen, J.X.; Xu, J.; Liu, H.L.; Wang, L.Y.; Wang, K.T.; Hu, K.C.; et al. Exploring the Chemodiversity and Biological Activities of the Secondary Metabolites from the Marine Fungus Neosartorya pseudofischeri. Mar. Drugs. 2014, 12, 5657–5676. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, D.M.; Howlett, B.J. Bioinformatic and expression analysis of the putative gliotoxin biosynthetic gene cluster of Aspergillus fumigatus. Fems. Microbiol. Lett. 2010, 248, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Nierman, W.C.; Pain, A.; Anderson, M.J.; Wortman, J.R.; Kim, H.S.; Arroyo, J.; Berriman, M.; Abe, K.; Archer, D.B.; Bermejo, C.; et al. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 2005, 438, 1151–1156. [Google Scholar] [CrossRef]
- Gomez-Lopez, A.; Fernandez-Fernandez, C. Molecular characterization of gliotoxin synthesis in a biofilm model of Aspergillus fumigatus. Biofilm 2024, 8, 100238. [Google Scholar] [CrossRef]
- Balibar, C.J.; Walsh, C.T. GliP, a Multimodular Nonribosomal Peptide Synthetase in Aspergillus Fumigatus, Makes the Diketopiperazine Scaffold of gliotoxin. Biochemistry 2006, 45, 15029–15038. [Google Scholar] [CrossRef]
- Davis, C.; Carberry, S.; Schrettl, M.; Singh, I.; Stephens, J.C.; Barry, S.M.; Kavanagh, K.; Challis, G.L.; Brougham, D.; Doyle, S. The Role of Glutathione S-transferase GliG in Gliotoxin Biosynthesis in Aspergillus fumigatus. Chem. Biol. 2011, 18, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Kupfahl, C.; Heinekamp, T.; Geginat, G.; Ruppert, T.; Härtl, A.; Hof, H.; Brakhage, A.A. Deletion of the gliP Gene of Aspergillus fumigatus Results in Loss of Gliotoxin Production but Has no Effect on Virulence of the Fungus in a Low-dose Mouse Infection Model. Mol. Microbiol. 2006, 62, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.; Mokkawes, T.; Cao, Y.; de Visser, S.P. Mechanism of the Oxidative Ring-Closure Reaction during Gliotoxin Biosynthesis by Cytochrome P450 GliF. Int. J. Mol. Sci. 2024, 25, 8567. [Google Scholar] [CrossRef]
- Gallagher, L.; Owens, R.A.; Dolan, S.K.; O’Keeffe, G.; Schrettl, M.; Kavanagh, K.; Jones, G.W.; Doyle, S. The Aspergillus fumigatus protein GliK protects against oxidative stress and is essential for gliotoxin biosynthesis. Eukaryotic Cell 2012, 11, 1226–1238. [Google Scholar] [CrossRef]
- Marion, A.; Groll, M.; Scharf, D.H.; Scherlach, K.; Glaser, M.; Sievers, H.; Schuster, M.; Hertweck, C.; Brakhage, A.A.; Antes, I.; et al. Gliotoxin Biosynthesis: Structure, Mechanism, and Metal Promiscuity of Carboxypeptidase GliJ. ACS Chem. Biol. 2017, 12, 1874–1882. [Google Scholar] [CrossRef]
- Scharf, D.H.; Habel, A.; Heinekamp, T.; Brakhage, A.A.; Hertweck, C. Opposed Effects of Enzymatic Gliotoxin N-and S-methylations. J. Am. Chem. Soc. 2014, 136, 11674–11679. [Google Scholar] [CrossRef]
- Ye, W.; Li, S.; Liu, S.; Kong, Y.; Zhang, W.; Liu, S.; Liu, T.; Zhang, W. Characterization of Novel Gliotoxin Biosynthesis-related Genes from Deep-sea- derived Fungus Geosmithia pallida FS140. Biochimie 2021, 191, 1–10. [Google Scholar] [CrossRef]
- Dolan, S.K.; Owens, R.A.; O’Keeffe, G.; Hammel, S.; Fitzpatrick, D.A.; Jones, G.W.; Doyle, S. Regulation of Nonribosomal Peptide Synthesis: Bis-thiomethylation Attenuates Gliotoxin Biosynthesis in Aspergillus fumigatus. Chem. Biol. 2014, 21, 999–1012. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, Z.H.; Liu, Z.; Chen, Y.C.; Liu, H.X.; Li, H.H.; Zhang, W.M. Dichotocejpins A-C: New Diketopiperazines From a Deep-sea-derived Fungus Dichotomomyces cejpii FS110. Mar. Drugs 2016, 14, 164. [Google Scholar] [CrossRef]
- Bok, J.W.; Chung, D.; Balajee, S.A.; Marr, K.A.; Andes, D.; Nielsen, K.F.; Frisvad, J.C.; Kirby, K.A.; Keller, N.P. Gliz, a Transcriptional Regulator of Gliotoxin Biosynthesis, Contributes to Aspergillus fumigatus Virulence. Infect. Immun. 2006, 74, 6761–6768. [Google Scholar] [CrossRef]
- Huang, Z.L.; Ye, W.; Zhu, M.Z.; Kong, Y.L.; Li, S.N.; Liu, S.; Zhang, W.M. Interaction of a Novel Zn2cys6 Transcription Factor Dcgliz with Promoters in the Gliotoxin Biosynthetic Gene Cluster of the Deep-sea-derived Fungus Dichotomomyces cejpii. Biomolecules 2019, 10, 56. [Google Scholar] [CrossRef]
- Schoberle, T.J.; Nguyen-Coleman, C.K.; Herold, J.; Yang, A.; Weirauch, M.; Hughes, T.R.; McMurray, J.S.; May, G.S. A Novel C2H2 Transcription Factor that Regulates gliA Expression Interdependently with GliZ in Aspergillus fumigatus. PLoS Genet. 2014, 10, e1004336. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.D.; Calvo, A.M. The mtfA Transcription Factor Gene Controls Morphogenesis, Gliotoxin Production, and Virulence in the Opportunistic Human Pathogen Aspergillus fumigatus. Eukaryot. Cell 2014, 13, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Sugui, J.A.; Pardo, J.; Chang, Y.C.; Zarember, K.A.; Nardone, G.; Galvez, E.M.; Müllbacher, A.; Gallin, J.I.; Simon, M.M.; Kwon-Chung, K.J. Gliotoxin Is a Virulence Factor of Aspergillus fumigatus: gliP Deletion Attenuates Virulence in Mice Immunosuppressed with Hydrocortisone. Eukaryot. Cell 2007, 6, 1562–1569. [Google Scholar] [CrossRef]
- Ye, W.; Liu, T.; Liu, Y.; Li, M.; Wang, S.; Li, S.; Zhang, W. Enhancing gliotoxins production in deep-sea derived fungus Dichotomocyes cejpii by engineering the biosynthetic pathway. Bioresour. Technol. 2023, 377, 128905. [Google Scholar] [CrossRef] [PubMed]
- Trown, P.W. Antiviral activity of N, N′-dimethyl-epidithiapiperazinedione, a synthetic compound related to the gliotoxins, LL-S88alpha and beta, chetomin and the sporidesmins. Biochem. Biophys. Res. Commun. 1968, 33, 402–407. [Google Scholar] [CrossRef]
- Hino, T.; Sato, T. Synthesis of 3,6-diethoxycarbonyl-3,6-epipolythia-2,5-piperazinedione derivatives. Tetrahedron Lett. 1971, 12, 3127–3129. [Google Scholar] [CrossRef]
- Öhler, E.; Poisel, H.; Tataruch, F.; Schmidt, U. Syntheseversuche in der Reihe der 3.6-Epidithio-2.5-dioxo-piperazin-Antibiotika Gliotoxin, Sporidesmin, Aranotin und Chaetocin, IV. Synthese des Epidithio-L-prolyl-L-prolinanhydrids. Chem. Ber. 1972, 105, 635–641. [Google Scholar] [CrossRef]
- Fukuyama, T.; Nakatsuka, S.I.; Kishi, Y. Total synthesis of gliotoxin, Dehydrogliotoxin and hyalodendrin. Tetrahedron 1981, 37, 2045–2078. [Google Scholar] [CrossRef]
- Snaddon, T.N.; Scaggs, T.D.; Pearson, C.M.; Fyfe, J.W.B. Fyfe. A Modular Construction of Epidithiodiketopiperazines. Org. Lett. 2019, 21, 4873–4877. [Google Scholar] [CrossRef]
- Henninger, T.C.; Sabat, M.; Sundberg, R.J. Stereodivergent routes from tyrosine to the 7-(R) and 7-(S) diastereomers of the 7-hydroxy-2,3,7,7a-tetrahydroindole ring found in gliotoxin. Tetrahedron 1996, 52, 14403–14418. [Google Scholar] [CrossRef]
- Herscheid, J.D.M.; Nivard, R.J.F.; Tijhuis, M.W.; Ottenheijm, H.C.J. Biosynthesis of gliotoxin. Synthesis of sulfur-bridged dioxopiperazines from N-hydroxyamino acids. J. Org. Chem. 1980, 45, 1885–1888. [Google Scholar] [CrossRef]
- Kirby, G.W.; Rao, G.V.; Robins, D.J.; Stark, W.M. Partial synthesis of gliotoxin G, an epitetrathiodioxopiperazine. Tetrahedron Lett. 1986, 27, 5539–5540. [Google Scholar] [CrossRef]
- Kishi, Y.; Fukuyama, T.; Nakatsuka, S. A total synthesis of Dehydrogliotoxin. J. Am. Chem. Soc. 1973, 95, 6492–6493. [Google Scholar] [CrossRef] [PubMed]
- McMahon, T.C.; Stanley, S.; Kazyanskaya, E.; Hung, D.; Wood, J.L. A scaleable formal total synthesis of Dehydrogliotoxin. Tetrahedron Lett. 2011, 52, 2262–2264. [Google Scholar] [CrossRef]
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef]
- McDonagh, A.; Fedorova, N.D.; Crabtree, J.; Yu, Y.; Kim, S.; Chen, D.; Loss, O.; Cairns, T.; Goldman, G.; Armstrong-James, D.; et al. Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog. 2008, 4, e1000154. [Google Scholar] [CrossRef]
- Palmer, J.M.; Keller, N.P. Secondary metabolism in fungi: Does chromosomal location matter? Curr. Opin. Microbiol. 2010, 13, 431–436. [Google Scholar] [CrossRef]
- Blachowicz, A.; Raffa, N.; Bok, J.W.; Choera, T.; Knox, B.; Lim, F.Y.; Huttenlocher, A.; Wang, C.C.C.; Venkateswaran, K.; Keller, N.P. Contributions of Spore Secondary Metabolites to UV-C Protection and Virulence Vary in Different Aspergillus fumigatus Strains. mBio 2020, 11, e03415-19. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Keller, N.P. Translating biosynthetic gene clusters into fungal armor and weaponry. Nat. Chem. Biol. 2015, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.L.; Mead, M.E.; Silva, L.P.; Raja, H.A.; Steenwyk, J.L.; Goldman, G.H.; Oberlies, N.H.; Rokas, A. Gliotoxin, a Known Virulence Factor in the Major Human Pathogen Aspergillus fumigatus, Is Also Biosynthesized by Its Nonpathogenic Relative Aspergillus fischeri. mBio 2020, 11, e03361-19. [Google Scholar] [CrossRef] [PubMed]
- Knox, B.P.; Blachowicz, A.; Palmer, J.M.; Romsdahl, J.; Huttenlocher, A.; Wang, C.C.; Keller, N.P.; Venkateswaran, K. Characterization of Aspergillus fumigatus Isolates from Air and Surfaces of the International Space Station. mSphere 2016, 1, e00227-16. [Google Scholar] [CrossRef]
- Losada, L.; Ajayi, O.; Frisvad, J.C.; Yu, J.; Nierman, W.C. Effect of competition on the production and activity of secondary metabolites in Aspergillus species. Med. Mycol. 2009, 47 (Suppl. 1), S88–S96. [Google Scholar] [CrossRef]
- Raffa, N.; Keller, N.P. A call to arms: Mustering secondary metabolites for success and survival of an opportunistic pathogen. PLoS Pathog. 2019, 15, e1007606. [Google Scholar] [CrossRef] [PubMed]
- Scharf, H.D.; Heinekamp, T.; Brakhage, A.A. Human and plant fungal pathogens: The role of secondary metabolites. PLoS Pathog. 2014, 10, e1003859. [Google Scholar] [CrossRef]
- König, S.; Pace, S.; Pein, H.; Heinekamp, T.; Kramer, J.; Romp, E.; Straßburger, M.; Troisi, F.; Proschak, A.; Dworschak, J.; et al. Gliotoxin from Aspergillus fumigatus Abrogates Leukotriene B4 Formation through Inhibition of Leukotriene A4 Hydrolase. Cell Chem. Biol. 2019, 26, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.T.; Jackman, S.; Engle, J.; Larsen, B. Effect of gliotoxin on human polymorphonuclear neutrophils. Infect. Dis. Obstet. Gynecol. 1988, 6, 168–175. [Google Scholar] [CrossRef]
- Murayama, T.; Amitani, R.; Ikegami, Y.; Nawada, R.; Lee, W.J.; Kuze, F. Suppressive effects of Aspergillus fumigatus culture filtrates on human alveolar macrophages and polymorphonuclear leucocytes. Eur. Respir. J. 1996, 9, 293–300. [Google Scholar] [CrossRef]
- Waring, P.; Eichner, R.D.; Müllbacher, A. The chemistry and biology of the immunomodulating agent gliotoxin and related epipolythiodioxopiperazines. Med. Res. Rev. 1988, 8, 499–524. [Google Scholar] [CrossRef]
- Wichmann, G.; Herbarth, O.; Lehmann, I. The mycotoxins citrinin, gliotoxin, and patulin affect interferon-gamma rather than interleukin-4 production in human blood cells. Environ. Toxicol. 2002, 17, 211–218. [Google Scholar] [CrossRef]
- Waring, P.; Eichner, R.D.; Müllbacher, A.; Sjaarda, A. Gliotoxin induces apoptosis in macrophages unrelated to its antiphagocytic properties. J. Biol. Chem. 1988, 263, 18493–18499. [Google Scholar] [CrossRef]
- Sutton, P.; Newcombe, N.R.; Waring, P.; Müllbacher, A. In vivo immunosuppressive activity of gliotoxin, a metabolite produced by human pathogenic fungi. Infect. Immun. 1994, 62, 1192–1198. [Google Scholar] [CrossRef]
- Suen, Y.K.; Fung, K.P.; Lee, C.Y.; Kong, S.K. Gliotoxin induces apoptosis in cultured macrophages via production of reactive oxygen species and cytochrome c release without mitochondrial depolarization. Free Radic. Res. 2001, 35, 1–10. [Google Scholar] [CrossRef]
- Kweon, Y.O.; Paik, Y.H.; Schnabl, B.; Qian, T.; Lemasters, J.J.; Brenner, D.A. Gliotoxin-mediated apoptosis of activated human hepatic stellate cells. J. Hepatol. 2003, 39, 38–46. [Google Scholar] [CrossRef]
- Bernardo, P.H.; Brasch, N.; Chai, C.L.; Waring, P. A novel redox mechanism for the glutathione-dependent reversible uptake of a fungal toxin in cells. J. Biol. Chem. 2003, 278, 46549–46555. [Google Scholar] [CrossRef] [PubMed]
- Pardo, J.; Urban, C.; Galvez, E.M.; Ekert, P.G.; Müller, U.; Kwon-Chung, J.; Lobigs, M.; Müllbacher, A.; Wallich, R.; Borner, C.; et al. The mitochondrial protein Bak is pivotal for gliotoxin-induced apoptosis and a critical host factor of Aspergillus fumigatus virulence in mice. J. Cell Biol. 2006, 174, 509–519. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Wolter, K.G.; Youle, R.J. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. USA 1997, 94, 3668–3672. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, G.J.; Dubrez, L.; Morgan, C.P.; Jones, N.A.; Whitehouse, J.; Corfe, B.M.; Dive, C.; Hickman, J.A. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J. Cell Biol. 1999, 144, 903–914. [Google Scholar] [CrossRef]
- Nechushtan, A.; Smith, C.L.; Hsu, Y.T.; Youle, R.J. Conformation of theBax C-terminus regulates subcellular location and cell death. EMBO J. 1999, 18, 2330–2341. [Google Scholar]
- Anselmi, K.; Stolz, D.B.; Nalesnik, M.; Watkins, S.C.; Kamath, R.; Gandhi, C.R. Gliotoxin causes apoptosis and necrosis of rat Kupffer cells in vitro and in vivo in the absence of oxidative stress: Exacerbation by caspase and serine protease inhibition. J. Hepatol. 2007, 47, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.F. Effects of Gliotoxin in the Vitro Model of Human Bronchial Epithelial Cell Infected by Aspergillus Fumigatus; Fujian Medicine University: Fujian, China, 2013. [Google Scholar]
- Kroll, M.; Arenzana-Seisdedos, F.; Bachelerie, F.; Thomas, D.; Friguet, B.; Conconi, M. The secondary fungal metabolite gliotoxin targets proteolytic activities of the proteasome. Chem. Biol. 1999, 6, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, L.S.; Abe, S.; Tsunawaki, S. Fungal Gliotoxin Targets the Onset of Superoxide-Generating NADPH Oxidase of Human Neutrophils. Biochem. Bioph. Res. Comm. 2000, 268, 716–723. [Google Scholar] [CrossRef]
- Tsunawaki, S.; Yoshida, L.S.; Nishida, S.; Kobayashi, T.; Shimoyama, T. Fungal metabolite gliotoxin inhibits assembly of the human respiratory burst NADPH oxidase. Infect. Immun. 2004, 72, 3373–3382. [Google Scholar] [CrossRef]
- Nishida, S.; Yoshida, L.S.; Shimoyama, T.; Nunoi, H.; Kobayashi, T.; Tsunawaki, S. Fungal metabolite gliotoxin targets flavocytochrome b558 in the activation of the human neutrophil NADPH oxidase. Infect. Immun. 2005, 73, 235–244. [Google Scholar] [CrossRef]
- Spikes, S.; Xu, R.; Nguyen, C.K.; Chamilos, G.; Kontoyiannis, D.P.; Jacobson, R.H.; Ejzykowicz, D.E.; Chiang, L.Y.; Filler, S.G.; May, G.S. Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J. Infect. Dis. 2008, 197, 479–486. [Google Scholar] [CrossRef]
- Lemaitre, B.; Nicolas, E.; Michaut, L.; Reichhart, J.M.; Hoffmann, J.A. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 1996, 86, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Hao, X.Y.; Wang, Z.J.; Yao, T.L. Gliotoxin Inhibits the Growth of Esophageal Cancer Cells through PI3K/AKT/mTOR Axis. World J. Cancer Res. 2024, 14, 183–190. [Google Scholar] [CrossRef]
- Jiao, Y.N.; Chen, M. Research Progress on the Toxicity Mechanism of Gliotoxin. Chin. J. Public Health 2013, 29, 1656–1658. [Google Scholar]
- Duan, J.S.; Yu, Y.Q.; He, X.Y.; Kang, N. Advances in the mechanism of paraptosis induced by anti-tumor drugs. Chin. Bull. Life Sci. 2021, 33, 888–895. [Google Scholar]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef]
- Sharma, A.; Flora, S.J.S. Positive and Negative Regulation of Ferroptosis and Its Role in Maintaining Metabolic and Redox Homeostasis. Oxid. Med. Cell Longev. 2021, 2021, 9074206. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.B.; Zhao, R.Y.; Ge, M.; Sun, Y.; Li, Y.R.; Shan, L.H. Gliotoxin, a natural product with ferroptosis inducing properties. Bioorg. Chem. 2023, 133, 106415. [Google Scholar] [CrossRef]
- Jain, R.; Valiante, V.; Remme, N.; Docimo, T.; Heinekamp, T.; Hertweck, C.; Gershenzon, J.; Haas, H.; Brakhage, A.A. The MAP kinase MpkA controls cell wall integrity, oxidative stress response, gliotoxin production and iron adaptation in Aspergillus fumigatus. Mol. Microbiol. 2011, 82, 39–53. [Google Scholar] [CrossRef] [PubMed]
- de Castro, P.A.; Figueiredo Pinzan, C.; Dos Reis, T.F.; Valero, C.; Van Rhijn, N.; Menegatti, C.; de Freitas Migliorini, I.L.; Bromley, M.; Fleming, A.B.; Traynor, A.M.; et al. Aspergillus fumigatus mitogen-activated protein kinase MpkA is involved in gliotoxin production and self-protection. Nat. Commun. 2024, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Stoszko, M.; Al-Hatmi, A.M.S.; Skriba, A.; Roling, M.; Ne, E.; Crespo, R.; Mueller, Y.M.; Najafzadeh, M.J.; Kang, J.; Ptackova, R.; et al. Gliotoxin, identified from a screen of fungal metabolites, disrupts 7SK snRNP, releases P-TEFb, and reverses HIV-1 latency. Sci. Adv. 2020, 6, eaba6617. [Google Scholar] [CrossRef]
- Niwa, H.; Umehara, T. Structural insight into inhibitors of flavin adenine dinucleotide-dependent lysine demethylases. Epigenetics 2017, 12, 340–352. [Google Scholar] [CrossRef]
- Hino, S.; Kohrogi, K.; Nakao, M. Histone demethylase LSD1 controls the phenotypic plasticity of cancer cells. Cancer Sci. 2016, 107, 1187–1192. [Google Scholar] [CrossRef]
- Fu, D.J.; Li, J.; Yu, B. Annual review of LSD1/KDM1A inhibitors in 2020. Eur. J. Med. Chem. 2021, 214, 113254. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, P.; Yu, B. Advances toward LSD1 inhibitors for cancer therapy. Future. Med. Chem. 2017, 9, 1227–1242. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Zhang, H.Q.; Yang, X.K.; Shi, Y.T.; Yu, B. Annual review of lysine-specific demethylase 1 (LSD1/KDM1A) inhibitors in 2021. Eur. J. Med. Chem. 2022, 228, 114042. [Google Scholar] [CrossRef] [PubMed]
- Kaniskan, H.Ü.; Martini, M.L.; Jin, J. Inhibitors of Protein Methyltransferases and Demethylases. Chem. Rev. 2018, 118, 989–1068. [Google Scholar] [CrossRef]
- Li, Z.; Ding, L.; Li, Z.; Wang, Z.; Suo, F.; Shen, D.; Zhao, T.; Sun, X.; Wang, J.; Liu, Y.; et al. Development of the triazole-fused pyrimidine derivatives as highly potent and reversible inhibitors of histone lysine specific demethylase 1 (LSD1/KDM1A). Acta Pharm. Sin. B 2019, 9, 794–808. [Google Scholar] [CrossRef]
- Przespolewski, A.; Wang, E.S. Inhibitors of LSD1 as a potential therapy for acute myeloid leukemia. Expert. Opin. Inv. Drug. 2016, 25, 771–780. [Google Scholar] [CrossRef]
- Zheng, Y.C.; Yu, B.; Chen, Z.S.; Liu, Y.; Liu, H.M. TCPs: Privileged scaffolds for identifying potent LSD1 inhibitors for cancer therapy. Epigenomics 2016, 8, 651–666. [Google Scholar] [CrossRef]
- Fang, Y.; Liao, G.; Yu, B. LSD1/KDM1A inhibitors in clinical trials: Advances and prospects. J. Hematol. Oncol. 2019, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, H.M. Pharmacoepigenetics of LSD1 Inhibitors in Cancer. Pharmacoepigenetics 2019, 10, 523–530. [Google Scholar]
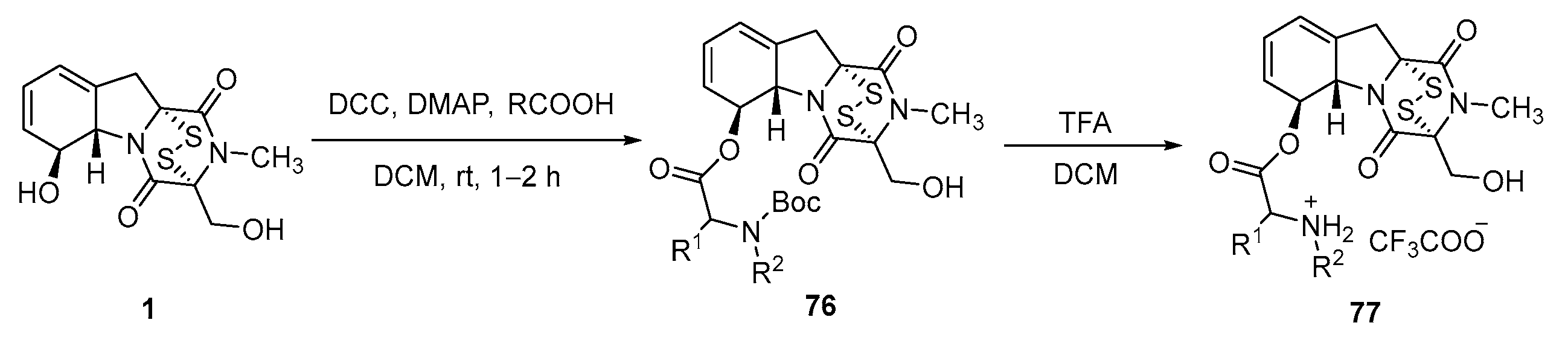
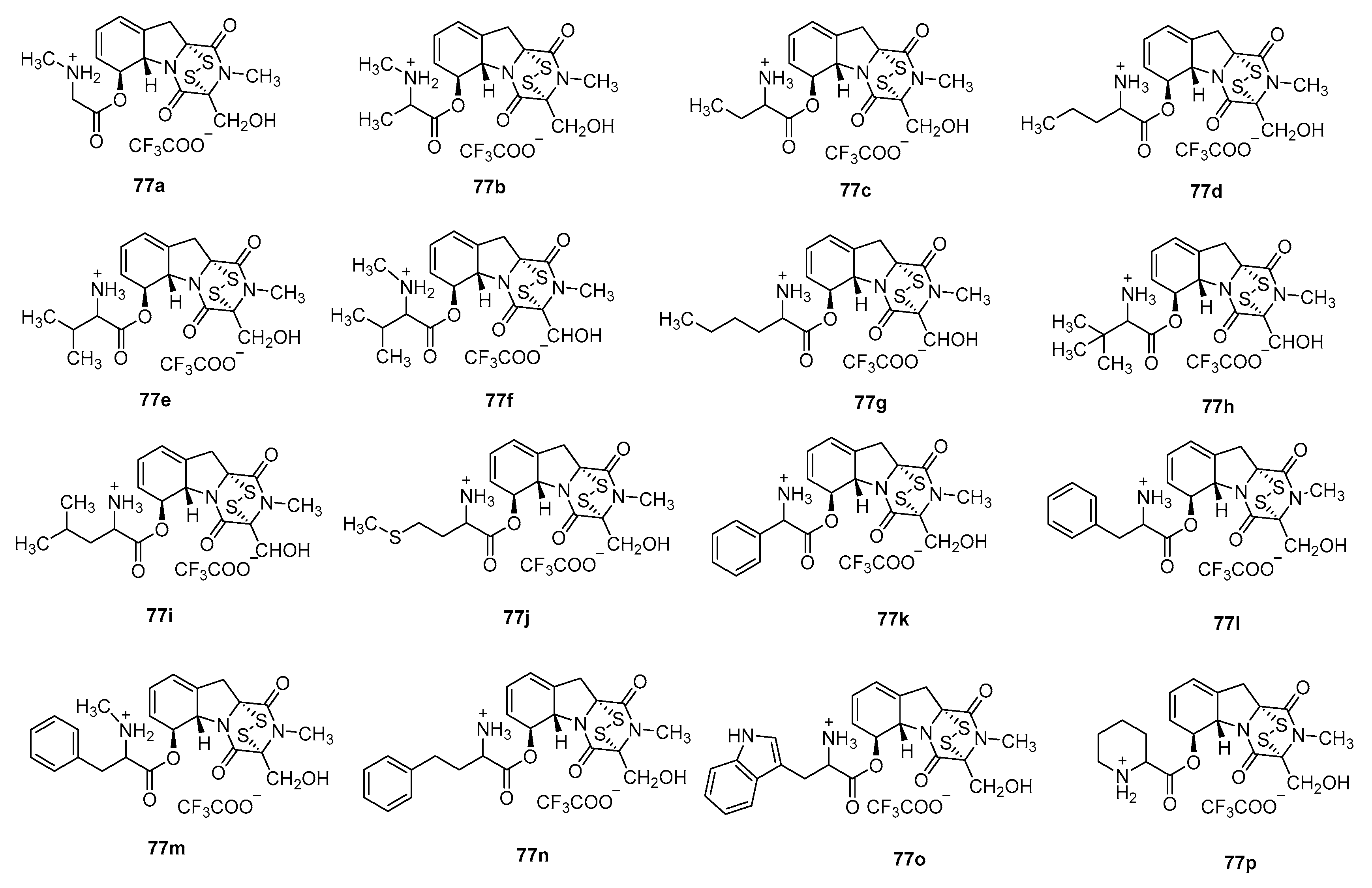
- Shan, L.H.; Li, Z.X.; Chen, H.B.; Ge, M.; Sun, Y.Y.; Sun, Y.; Li, Y.R.; Li, H.Y.; Fu, L.; Liu, H.M. 6-Heterocyclic carboxylic ester derivatives of gliotoxin lead to LSD1 inhibitors in gastric cancer cells. Bioorg. Chem. 2023, 131, 106150. [Google Scholar] [CrossRef]
- Shan, L.H.; Liu, H.M.; An, X.; Li, S.X.; Sun, Y.Y.; Zhao, R.Y. L-Amino Acid-6-Gliotoxin Ester Trifluoroacetate Derivatives and Their Preparation Methods. CN113105479A, 31 July 2021. [Google Scholar]
- Vidal-García, M.; Redrado, S.; Domingo, M.P.; Marquina, P.; Colmenarejo, C.; Meis, J.F.; Rezusta, A.; Pardo, J.; Galvez, E.M. Production of the Invasive Aspergillosis Biomarker Bis(methylthio)gliotoxin Within the Genus Aspergillus: In Vitro and in Vivo Metabolite Quantification and Genomic Analysis. Front. Microbiol. 2018, 9, 1246. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Liu, Y.; Wang, Q.; Song, H. Progress in Gliotoxin Research. Molecules 2025, 30, 3665. https://doi.org/10.3390/molecules30183665
Li L, Liu Y, Wang Q, Song H. Progress in Gliotoxin Research. Molecules. 2025; 30(18):3665. https://doi.org/10.3390/molecules30183665
Chicago/Turabian StyleLi, Longqi, Yuxiu Liu, Qingmin Wang, and Hongjian Song. 2025. "Progress in Gliotoxin Research" Molecules 30, no. 18: 3665. https://doi.org/10.3390/molecules30183665
APA StyleLi, L., Liu, Y., Wang, Q., & Song, H. (2025). Progress in Gliotoxin Research. Molecules, 30(18), 3665. https://doi.org/10.3390/molecules30183665





