Astaxanthin Alleviates Inflammatory Mechanical Hyperalgesia by Reducing Hyperexcitability of Trigeminal Nociceptive Secondary Neurons: Potential as an NSAID Alternative
Abstract
1. Introduction
2. Results
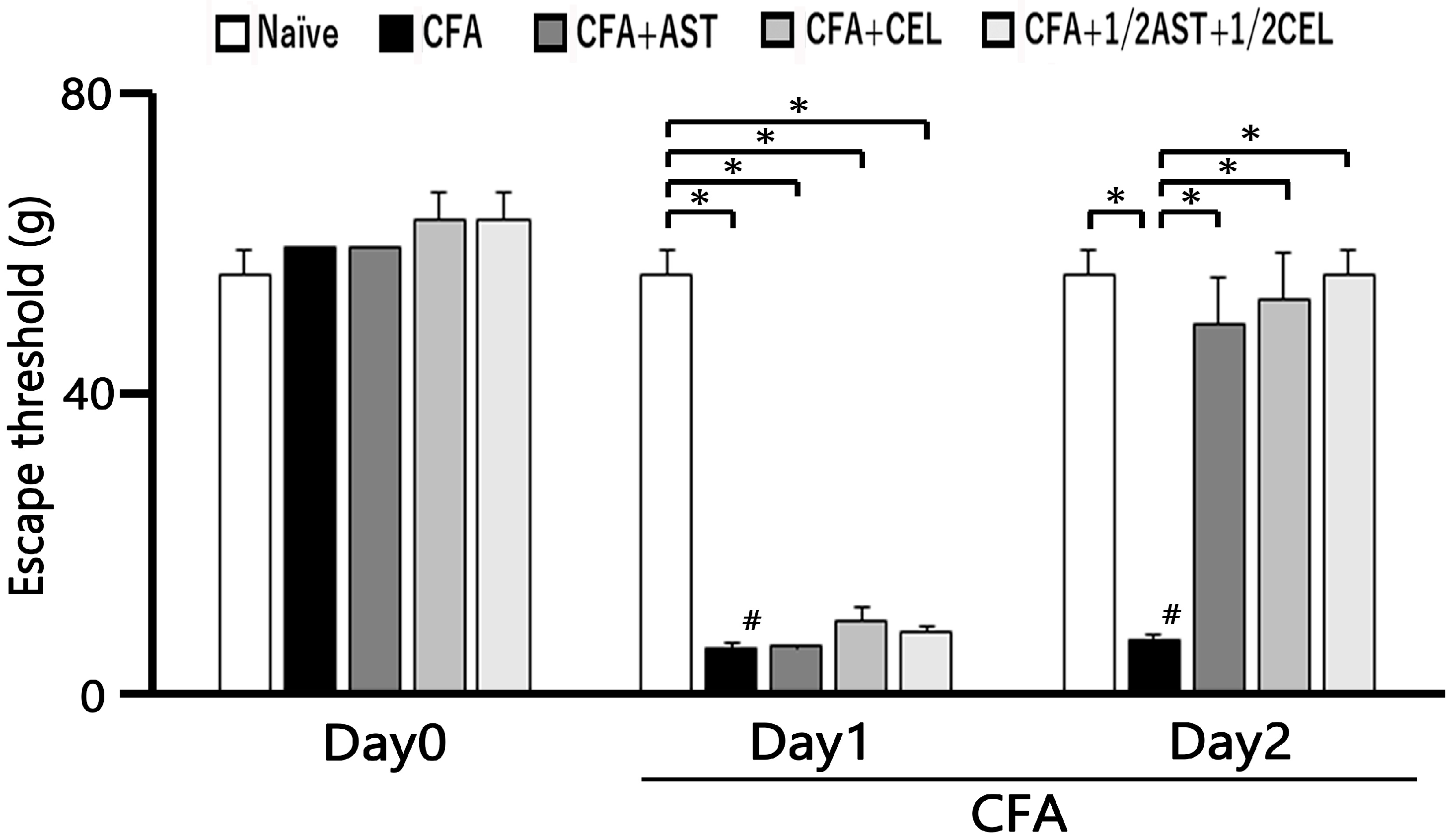
2.1. The Development of Inflammation-Induced Hyperalgesia
2.2. The Effect of Administration of AST, CEL and 1/2 CEL + 1/2 AST for Hyperalgesia
2.3. AST, CEL and 1/2 CEL + 1/2 AST Treatment Reduces Inflammatory Edema: Whisker Pad Thickness Measurements
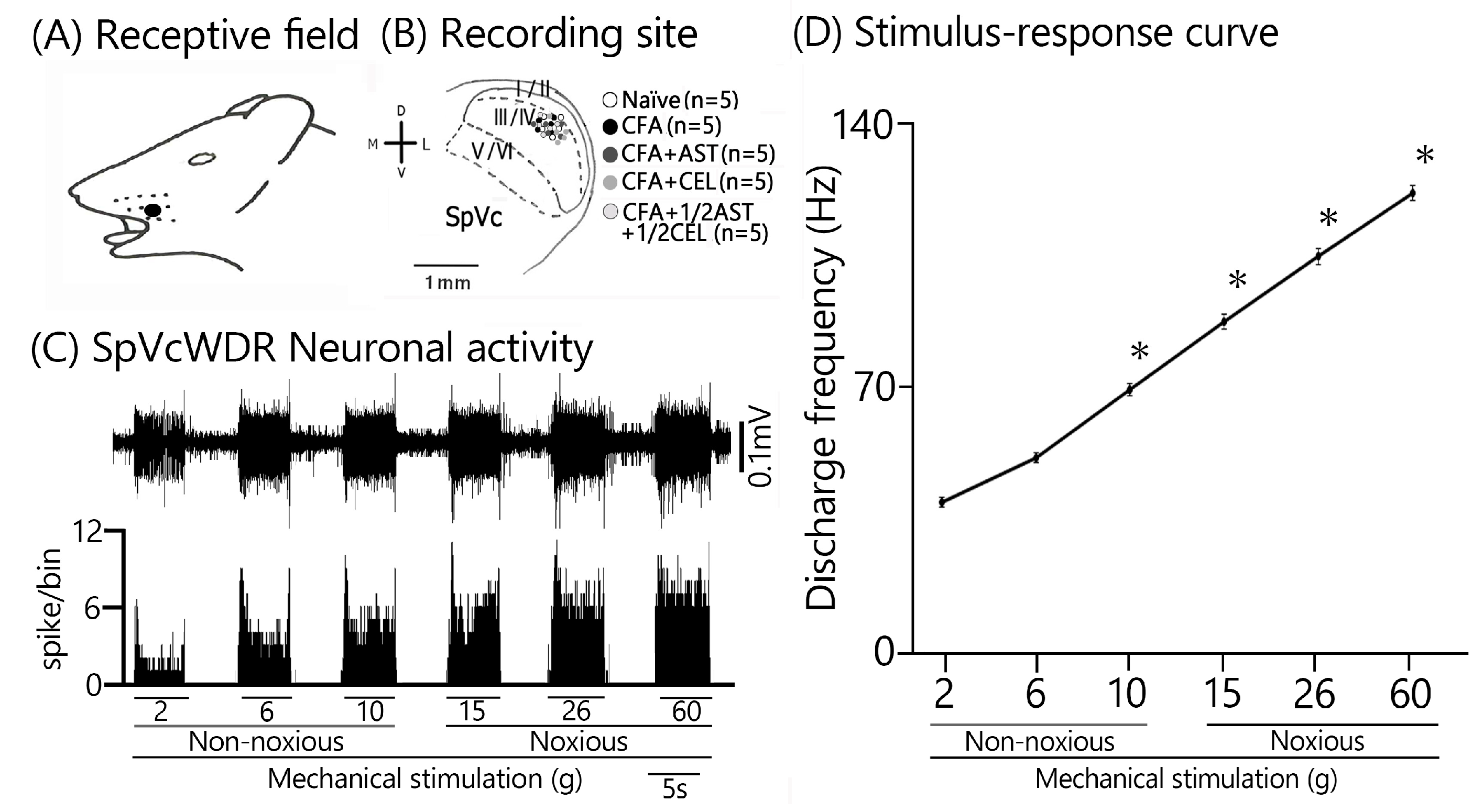
2.4. Inflammation-Induced Changes in SpVc WDR Neuron Excitability
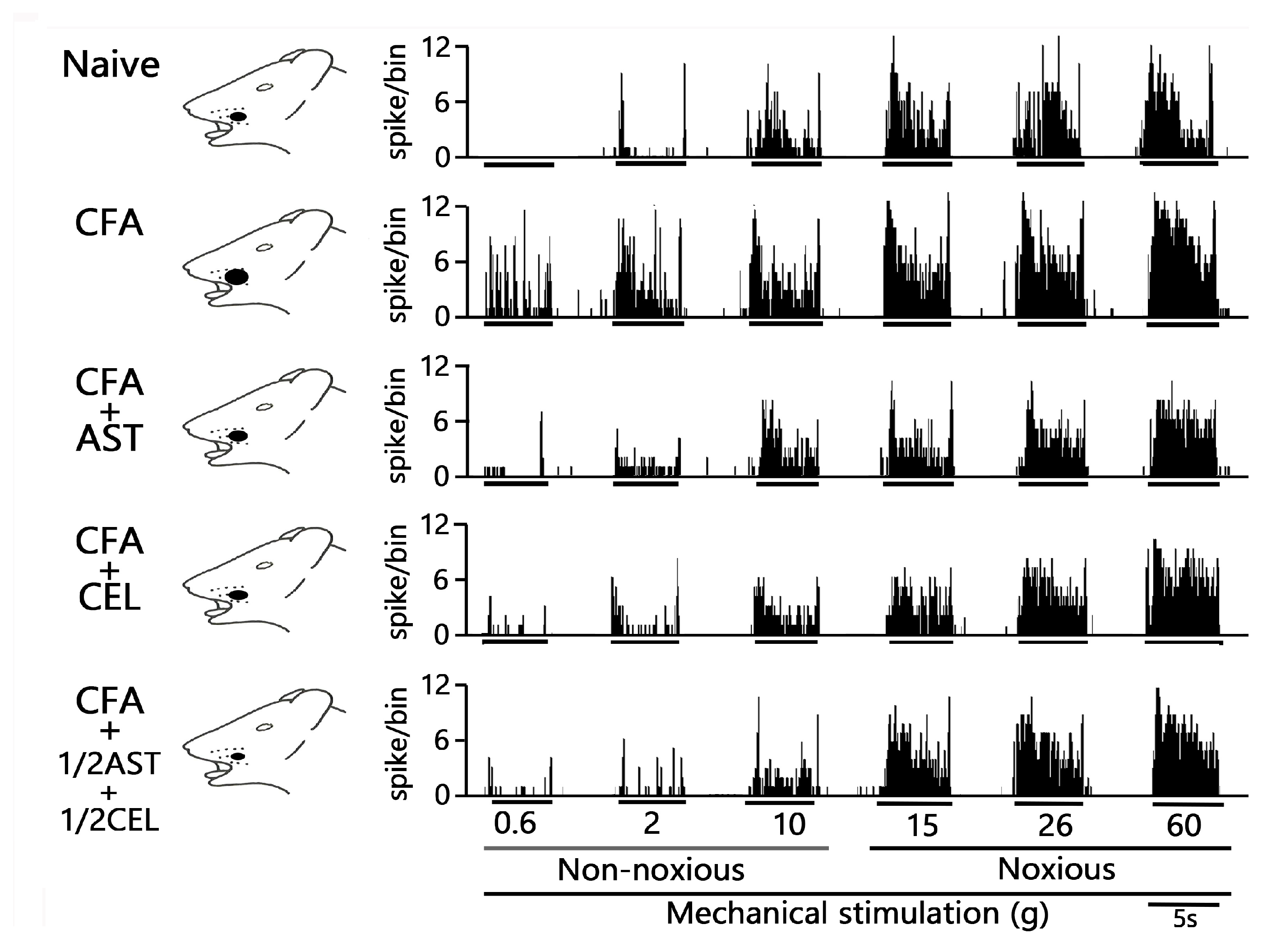
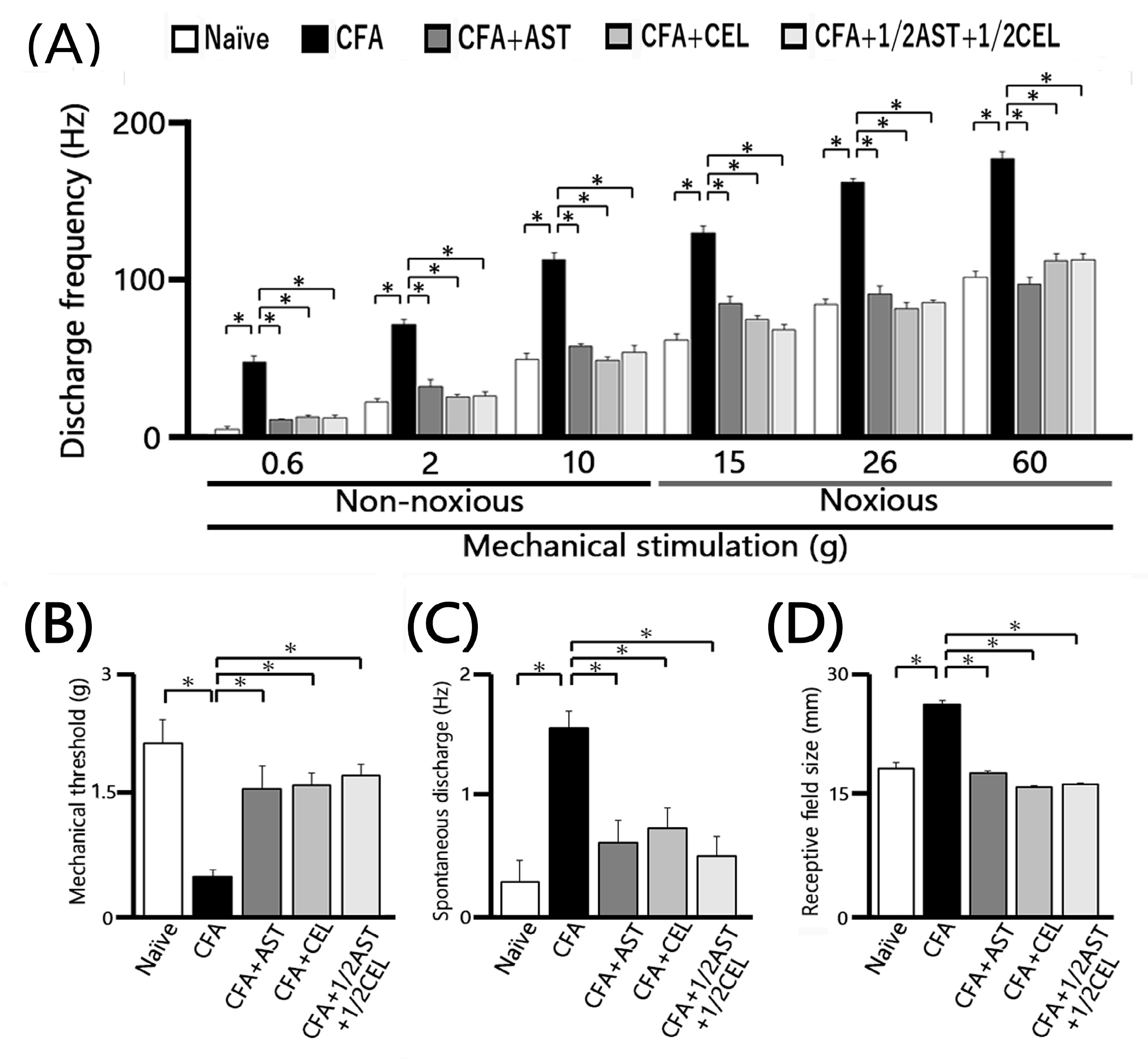
2.5. Astaxanthin Treatment Prevents the Development of Inflammation-Induced SpVc WDR Neuronal Hyperexcitability
2.6. Chronic Administration of CEL Inhibits Inflammation-Induced Hyperexcitability of SpVc WDR Neurons in Inflamed Rats
2.7. Chronic Administration of 1/2 AST + 1/2 CEL Inhibits Inflammation–Induced Hyperexcitability of SpVc WDR Neurons in Inflamed Rats
3. Discussion
3.1. AST Exerts a Potent Anti-Hyperalgesic Effect Against Trigeminal Inflammation
3.2. AST Effectively Ameliorates the Pathological Hyperexcitability of SpVc WDR Neurons That Underlies Inflammation-Induced Hyperalgesia
3.3. Contribution of AST to Hyperalgesia Alleviation Through SpVc Neuronal Hyperexcitability Suppression
4. Materials and Methods
4.1. Experimental Procedures: Inflammation Induction and Drug Administration (AST and NSAIDs)
4.2. Assessment of Mechanical Withdrawal Threshold
4.3. Electrophysiological Analysis of SpVc WDR Neurons: Single-Unit Recording
4.4. Experimental Design and Protocols
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AST | Astaxanthin |
| NSAID | Non-steroidal anti-inflammatory drug |
| CEL | Celecoxib |
| SpVc | Spinal Trigeminal nucleus caudalis |
| WDR | Wide-dynamic range |
| TG | Trigeminal ganglion |
| CFA | Complete Freund’s Adjuvant |
| CAM | Complementary Alternative Medicine |
| COX-2 | Cyclooxygenase-2 |
| PGE2 | Prostaglandine E2 |
| MAPK | Mitogen activated protein kinase |
| PKA | Protein Kinase A |
| PKC | Protein Kinase C |
| NMDA | N-methyl-D-aspartate |
| NR2B | NMDA receptor subtype 2B |
| Nav | Voltage-gated Na channels |
| Kv | Voltage-gated K channels |
| Cav | Voltage-gated Ca channels |
| TRPA1 | Transient receptor protein ankyrin 1 |
| ASIC3 | Acid sensing ion channels 3 |
| EPSP | Excitatory Post synaptic Potential |
| EP | Prostanoid E |
References
- Sessle, B.J. Chronic Orofacial Pain: Models, Mechanisms, and Genetic and Related Environmental Influences. Int. J. Mol. Sci. 2021, 22, 7112. [Google Scholar] [CrossRef]
- Sessle, B.J. Peripheral and Central Mechanisms of Orofacial Pain and Their Clinical Correlates. Minerva Anestesiol. 2005, 71, 117–136. [Google Scholar]
- Shinoda, M.; Suzuro, H.; Iwata, K.; Hayashi, Y. Plastic Changes in Nociceptive Pathways Contributing to Persistent Orofacial Pain. J. Oral Biosci. 2022, 64, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Takeda, M.; Oh, S.; Shinoda, M. Neurophysiology of Orofacial Pain. In Contemporary Oral Medicine; Farah, C.S., Balasubramaniam, R., McCullough, M.J., Eds.; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Iwata, K.; Tashiro, A.; Tsuboi, Y.; Imai, T.; Sumino, R.; Morimoto, T.; Dubner, R.; Ren, K. Medullary Dorsal Horn Neuronal Activity in Rats with Persistent Temporomandibular Joint and Perioral Inflammation. J. Neurophysiol. 1999, 82, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Imbe, H.; Iwata, K.; Zhou, Q.-Q.; Zou, S.; Dubner, R.; Ren, K. Orofacial Deep and Cutaneous Tissue Inflammation and Trigeminal Neuronal Activation. Cells Tissues Organs 2001, 169, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Matsumoto, S.; Sessle, B.J.; Shinoda, M.; Iwata, K. Peripheral and Central Mechanisms of Trigeminal Neuropathic Pain and Inflammatory Pain. J. Oral Biosci. 2011, 53, 318–328. [Google Scholar] [CrossRef]
- Konvicka, J.J.; Meyer, T.A.; McDavid, A.J.; Roberson, C.R. Complementary/Alternative Medicine Use among Chronic Pain Clinic Patients. J. PeriAnesth. Nurs. 2008, 23, 17–23. [Google Scholar] [CrossRef]
- Syoji, Y.; Kobayashi, R.; Miyamura, N.; Hirohara, T.; Kubota, Y.; Uotsu, N.; Yui, K.; Shimazu, Y.; Takeda, M. Suppression of Hyperexcitability of Trigeminal Nociceptive Neurons Associated with Inflammatory Hyperalgesia Following Systemic Administration of Lutein via Inhibition of Cyclooxygenase-2 Cascade Signaling. J. Inflamm. 2018, 15, 24. [Google Scholar] [CrossRef]
- Itou, H.; Toyota, R.; Takeda, M. Phytochemical Quercetin Alleviates Hyperexcitability of Trigeminal Nociceptive Neurons Associated with Inflammatory Hyperalgesia Comparable to NSAIDs. Mol. Pain 2022, 18, 17448069221108971. [Google Scholar] [CrossRef]
- Yajima, S.; Sakata, R.; Watanuki, Y.; Sashide, Y.; Takeda, M. Naringenin Suppresses the Hyperexcitability of Trigeminal Nociceptive Neurons Associated with Inflammatory Hyperalgesia: Replacement of NSAIDs with Phytochemicals. Nutrients 2024, 16, 389. [Google Scholar] [CrossRef]
- Kuedo, Z.; Sangsuriyawong, A.; Klaypradit, W.; Tipmanee, V.; Chonpathompikunlert, P. Effects of Astaxanthin from Litopenaeus vannamei on Carrageenan-Induced Edema and Pain Behavior in Mice. Molecules 2016, 21, 382. [Google Scholar] [CrossRef]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a Carotenoid with Potential in Human Health and Nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Naguib, T.M. Antioxidant Activities of Astaxanthin and Related Carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef]
- Liu, X.; Osawa, T. Astaxanthin Protects Neuronal Cells against Oxidative Damage and Is a Potent Candidate for Brain Food. Forum Nutr. 2009, 61, 129–135. [Google Scholar] [PubMed]
- Jyonouchi, H.; Sun, S.; Iijima, K.; Gross, M.D. Antitumor Activity of Astaxanthin and Its Mode of Action. Nutr. Cancer 2000, 36, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus Astaxanthin: Applications for Human Health and Nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Lee, S.J.; Bai, S.K.; Lee, K.S.; Namkoong, S.; Na, H.J.; Ha, K.S.; Han, J.A.; Yim, S.V.; Chang, K.; Kwon, Y.G.; et al. Astaxanthin Inhibits Nitric Oxide Production and Inflammatory Gene Expression by Suppressing IκB Kinase-Dependent NF-κB Activation. Mol. Cells 2003, 16, 97–105. [Google Scholar] [CrossRef]
- Chan, K.C.; Mong, M.C.; Yin, M.C. Antioxidative and Anti-Inflammatory Neuroprotective Effects of Astaxanthin and Canthaxanthin in Nerve Growth Factor Differentiated PC12 Cells. J. Food Sci. 2009, 74, 225–231. [Google Scholar] [CrossRef]
- Lin, T.Y.; Lu, C.W.; Wang, S.J. Astaxanthin Inhibits Glutamate Release in Rat Cerebral Cortex Nerve Terminals via Suppression of Voltage-Dependent Ca2+ Entry and Mitogen-Activated Protein Kinase Signaling Pathway. J. Agric. Food Chem. 2010, 58, 8271–8278. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, D.; Sharma, M.; Sharma, N.; Bidve, P.; Prajapati, P.; Kalia, K.; Tiwari, V. Astaxanthin Ameliorates Behavioral and Biochemical Alterations in In-Vitro and In-Vivo Model of Neuropathic Pain. Neurosci. Lett. 2018, 674, 162–170. [Google Scholar] [CrossRef]
- Manabe, Y.; Komatsu, T.; Seki, S.; Sugawara, T. Dietary Astaxanthin Can Accumulate in the Brain of Rats. Biosci. Biotechnol. Biochem. 2018, 82, 1433–1436. [Google Scholar] [CrossRef]
- Chida, R.; Yamaguchi, S.; Utugi, S.; Sashide, Y.; Takeda, M. Suppression of the Excitability of Rat Nociceptive Secondary Sensory Neurons Following Systemic Administration of Astaxanthin. Anesth. Res. 2024, 1, 117–127. [Google Scholar] [CrossRef]
- Ohgami, K.; Shiratori, K.; Kotake, S.; Nishida, T.; Mizuki, N.; Yazawa, K.; Ohno, S. Effect of Astaxanthin on Lipopolysaccharide-Induced Inflammation In Vitro and In Vivo. Investig. Ophthalmol. Visual Sci. 2003, 44, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-K.; Park, Y.-S.; Choi, D.-K.; Chang, H.-I. Effects of Astaxanthin on the Production of NO and the Expression of COX-2 and iNOS in LPS-Stimulated BV2 Microglial Cells. J. Microbiol. Biotechnol. 2008, 18, 1990–1996. [Google Scholar] [PubMed]
- Peng, J.-J.; Lu, J.-W.; Liu, F.-C.; Lee, C.-H.; Lee, H.-S.; Ho, Y.-J.; Hsieh, T.-H.; Wu, C.-C.; Wang, C.-C. Astaxanthin Attenuates Joint Inflammation Induced by Monosodium Urate Crystals. FASEB J. 2020, 34, 11205–11221. [Google Scholar] [CrossRef]
- Takeda, M.; Takehana, S.; Sekiguchi, K.; Kubota, Y.; Shimazu, Y. Modulatory Mechanism of Nociceptive Neuronal Activity by Dietary Constituents Resveratrol. Int. J. Mol. Sci. 2016, 17, 1702. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Lippross, S.; Neuhuber, W.L.; Zeilhofer, H.U. PGE2 Selectively Blocks Inhibitory Glycinergic Neurotransmission onto Rat Superficial Dorsal Horn Neurons. Nat. Neurosci. 2002, 5, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J. Diclofenac: An Update on Its Mechanism of Action and Safety Profile. Curr. Med. Res. Opin. 2010, 26, 1715–1731. [Google Scholar] [CrossRef] [PubMed]
- Craft, R.M.; Hewitt, K.A.; Britch, S.C. Antinociception Produced by Non-Steroidal Anti-Inflammatory Drugs in Female and Male Rats. Behav. Pharmacol. 2021, 32, 153–169. [Google Scholar] [CrossRef]
- Zhao, X.; Tao, T.; Song, T. Astaxanthin Alleviates Neuropathic Pain by Inhibiting the MAPKs and NF-κB Pathways. Eur. J. Pharmacol. 2021, 912, 174575. [Google Scholar] [CrossRef]
- Harriott, A.M.; Gold, M.S. Contribution of Primary Afferent Channels to Neuropathic Pain. Curr. Pain Headache Rep. 2009, 13, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, G.W.; Lewis, R.J.; Todorovic, S.M.; Arneric, S.P.; Snutch, T.P. Role of Voltage-Gated Calcium Channels in Ascending Pain Pathways. Brain Res. Rev. 2009, 60, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Schaible, H.G.; Richter, F. Pathophysiology of Pain. Langenbeck’s Arch. Surg. 2004, 389, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M.E.; Snutch, T.P. Contributions of T-Type Ca Channels to the Pathophysiology of Pain Signaling. Drug Discov. Today Dis. Mech. 2006, 3, 335–341. [Google Scholar] [CrossRef]
- Li, S.; Cao, J.; Yang, X.; Suo, Z.-W.; Shi, L.; Yang, H.-B.; Hu, X.-D. NR2B Phosphorylation at Tyrosine 1472 in Spinal Dorsal Horn Contributed to N-Methyl-D-Aspartate-Induced Pain Hypersensitivity in Mice. J. Neurosci. Res. 2011, 89, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.-X.; Cai, J.; Li, M.-J.; Chi, Y.-N.; Liao, F.-F.; Liu, F.-Y.; Wan, Y.; Han, J.-S.; Xing, G.-G. Role of Spinal Cord NR2B-Containing NMDA Receptors in the Development of Neuropathic Pain. Exp. Neurol. 2009, 215, 298–307. [Google Scholar] [CrossRef]
- Burstein, R.; Cutrer, M.F.; Yarnitsky, D. The Development of Cutaneous Allodynia during a Migraine Attack: Clinical Evidence for the Sequential Recruitment of Spinal and Supraspinal Nociceptive Neurons in Migraine. Brain 2009, 123, 1703–1709. [Google Scholar] [CrossRef]
- Roch, M.; Messlinger, K.; Kulchitsky, V.; Tichonovich, O.; Azev, O.; Koulchitsky, S. Ongoing Activity in Trigeminal Wide-Dynamic Range Neurons Is Driven from the Periphery. Neuroscience 2007, 150, 681–691. [Google Scholar] [CrossRef]
- Takeda, M.; Tanimoto, T.; Matsumoto, S. Change in Mechanical Receptive Field Properties Induced by GABAA Receptor Activation in the Trigeminal Spinal Nucleus Caudalis Neurons in Rats. Exp. Brain Res. 2000, 134, 409–416. [Google Scholar] [CrossRef]
- Stafford, G.I.; Pedersen, M.E.; van Staden, J.; Jager, A.K. Review on Plants with CNS-Effects Used in Traditional South African Medicine against Mental Diseases. J. Ethnopharmacol. 2008, 119, 513–637. [Google Scholar] [CrossRef]
- Copmans, D.; Orellana-Paucay, A.M.; Steurs, G.; Zhang, Y.; Ny, A.; Foubert, K.; Exarchou, V.; Siekierrska, A.; Kim, Y.; De Borggraeve, W.; et al. Methylated Flavonoids as Anti-Seizure Agents: Naringenin 4′,7-Dimethyl Ether Attenuates Epileptic Seizure in Zebrafish and Mouse Models. Neurochem. Int. 2018, 112, 124–133. [Google Scholar] [CrossRef]
- Balietti, M.; Giannubilo, S.R.; Giorgetti, B.; Solazzi, M.; Turi, A.; Casoli, T.; Ciavattini, A.; Fattoretti, P. The Effect of Astaxanthin on the Aging Rat Brain: Gender-Related Differences in Modulating Inflammation. J. Sci. Food Agric. 2016, 96, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.L.; Robinson, M.E.; Wise, E.A.; Price, D. A Meta-Analytic Review of Pain Perception Across the Menstrual Cycle. Pain 1999, 81, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Bartley, E.J.; Fillingim, R.B. Sex Differences in Pain: A Brief Review of Clinical and Experimental Findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Si, P.; Zhu, C. Biological and neurological activities of astaxanthin (review). Mol. Med. Rep. 2022, 26, 300. [Google Scholar] [CrossRef] [PubMed]
- Karthi, M.; Anbuslevan, G.J.; Senthilkumar, K.P.; Tamizharsi, S.; Raja, S.; Prabhakrr, K. NSAIDs in Orthodontic Tooth Movement. J. Pharm. Biol. Sci. 2012, 4 (Suppl. S2), S304–S306. [Google Scholar] [CrossRef]
- Shetty, N.; Patil, A.K.; Ganeshkar, S.V.; Hegde, S. Comparison of the Effects of Ibuprofen and Acetaminophen on PGE2 Levels in the GCF During Orthodontic Tooth Movement: A Human Study. Prog. Orthod. 2013, 14, 6. [Google Scholar] [CrossRef]
- Garlet, T.P.; Coelho, U.; Silva, J.S.; Garlet, G.P. Cytokine Expression Pattern in Compression and Tension Sides of Periodontal Ligament During Orthodontic Tooth Movement in Humans. Eur. J. Oral Sci. 2007, 115, 355–362. [Google Scholar] [CrossRef]
- Okubo, N.; Ishikawa, H.; Sano, R.; Shimazu, Y.; Takeda, M. Effect of Resveratrol on the Hyperexcitability of Nociceptive Neurons Associated with Ectopic Hyperalgesia Induced by Experimental Tooth Movement. Eur. J. Oral Biosci. 2020, 128, 275–283. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: New York, NY, USA, 1986. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chida, R.; Takeda, M. Astaxanthin Alleviates Inflammatory Mechanical Hyperalgesia by Reducing Hyperexcitability of Trigeminal Nociceptive Secondary Neurons: Potential as an NSAID Alternative. Molecules 2025, 30, 3664. https://doi.org/10.3390/molecules30183664
Chida R, Takeda M. Astaxanthin Alleviates Inflammatory Mechanical Hyperalgesia by Reducing Hyperexcitability of Trigeminal Nociceptive Secondary Neurons: Potential as an NSAID Alternative. Molecules. 2025; 30(18):3664. https://doi.org/10.3390/molecules30183664
Chicago/Turabian StyleChida, Risako, and Mamoru Takeda. 2025. "Astaxanthin Alleviates Inflammatory Mechanical Hyperalgesia by Reducing Hyperexcitability of Trigeminal Nociceptive Secondary Neurons: Potential as an NSAID Alternative" Molecules 30, no. 18: 3664. https://doi.org/10.3390/molecules30183664
APA StyleChida, R., & Takeda, M. (2025). Astaxanthin Alleviates Inflammatory Mechanical Hyperalgesia by Reducing Hyperexcitability of Trigeminal Nociceptive Secondary Neurons: Potential as an NSAID Alternative. Molecules, 30(18), 3664. https://doi.org/10.3390/molecules30183664





