Chiral Copper Catalysis in Enantioselective Domino Reactions
Abstract
1. Introduction
2. One- and Two-Component Domino Reactions
2.1. Michael-Type-Initiated Domino Reactions
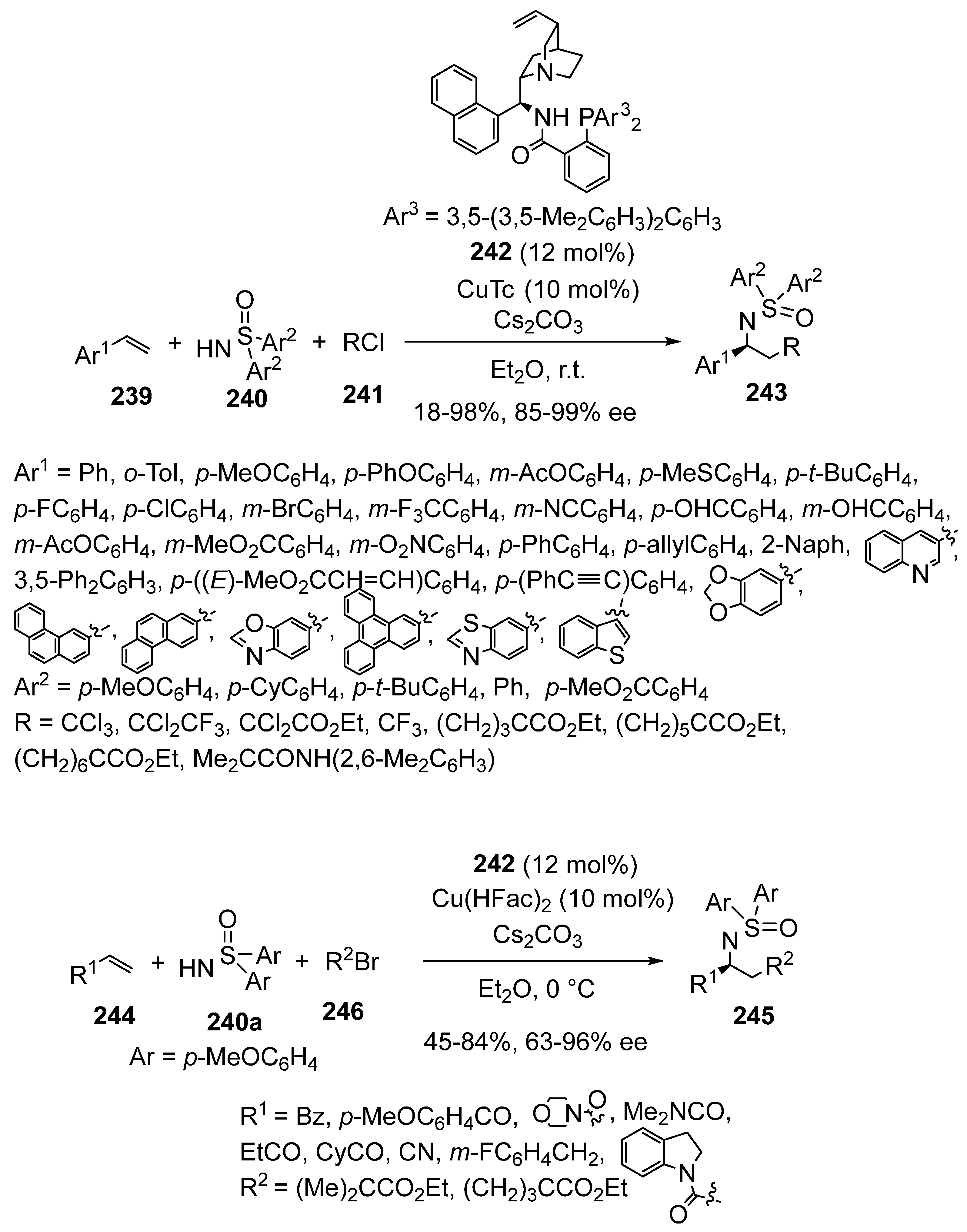
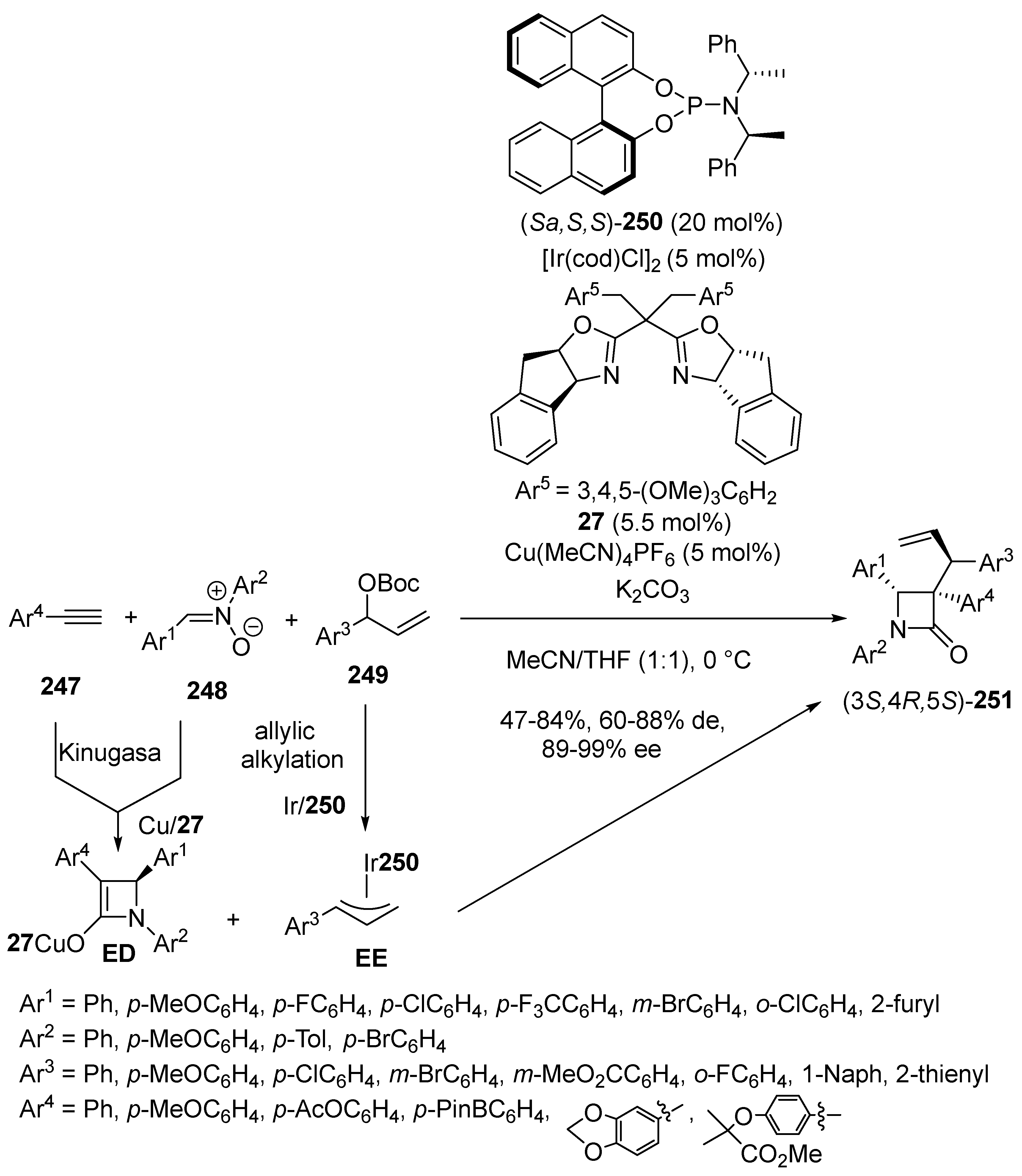
2.2. Kinugasa-Initiated Domino Reactions
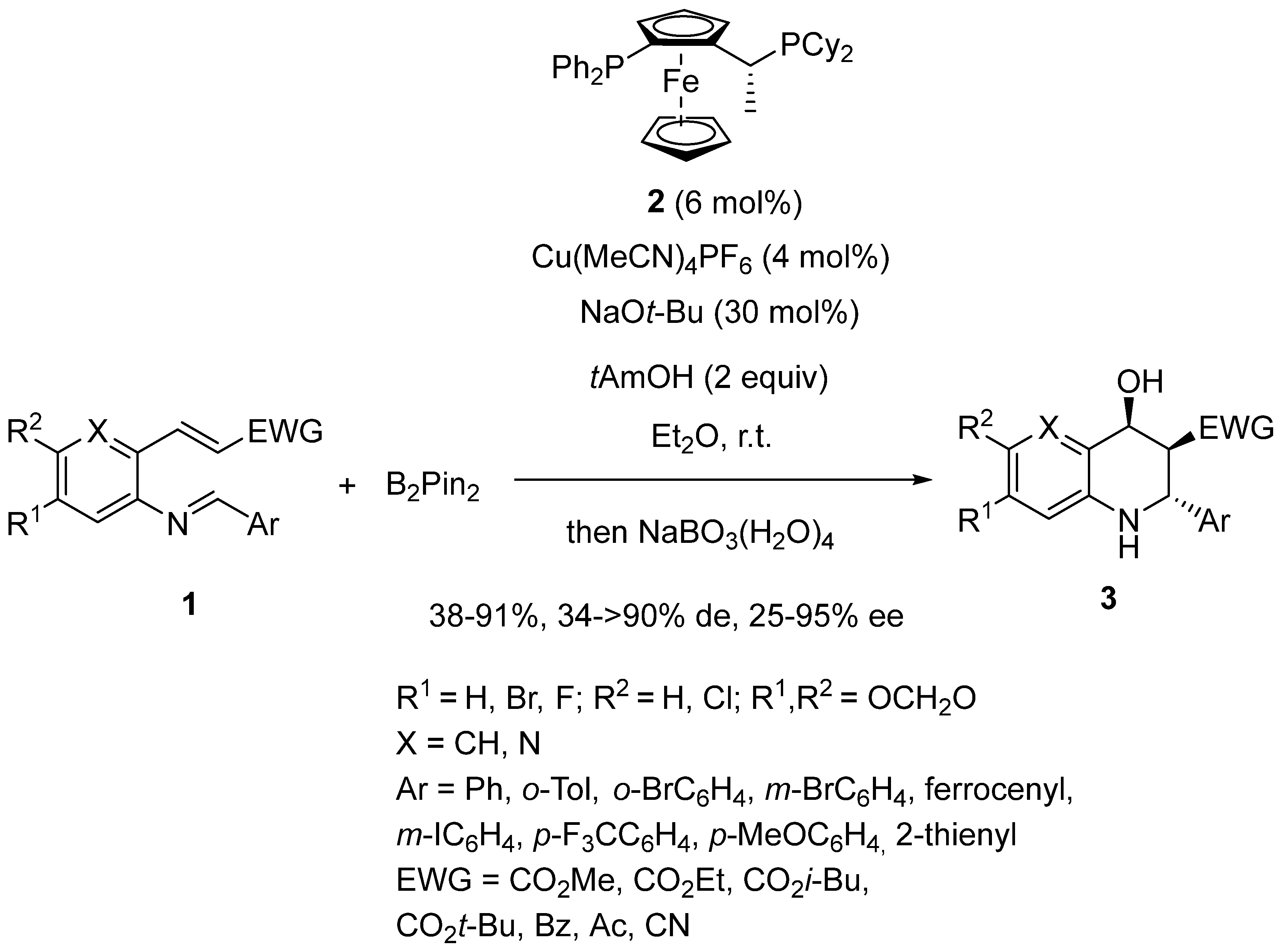
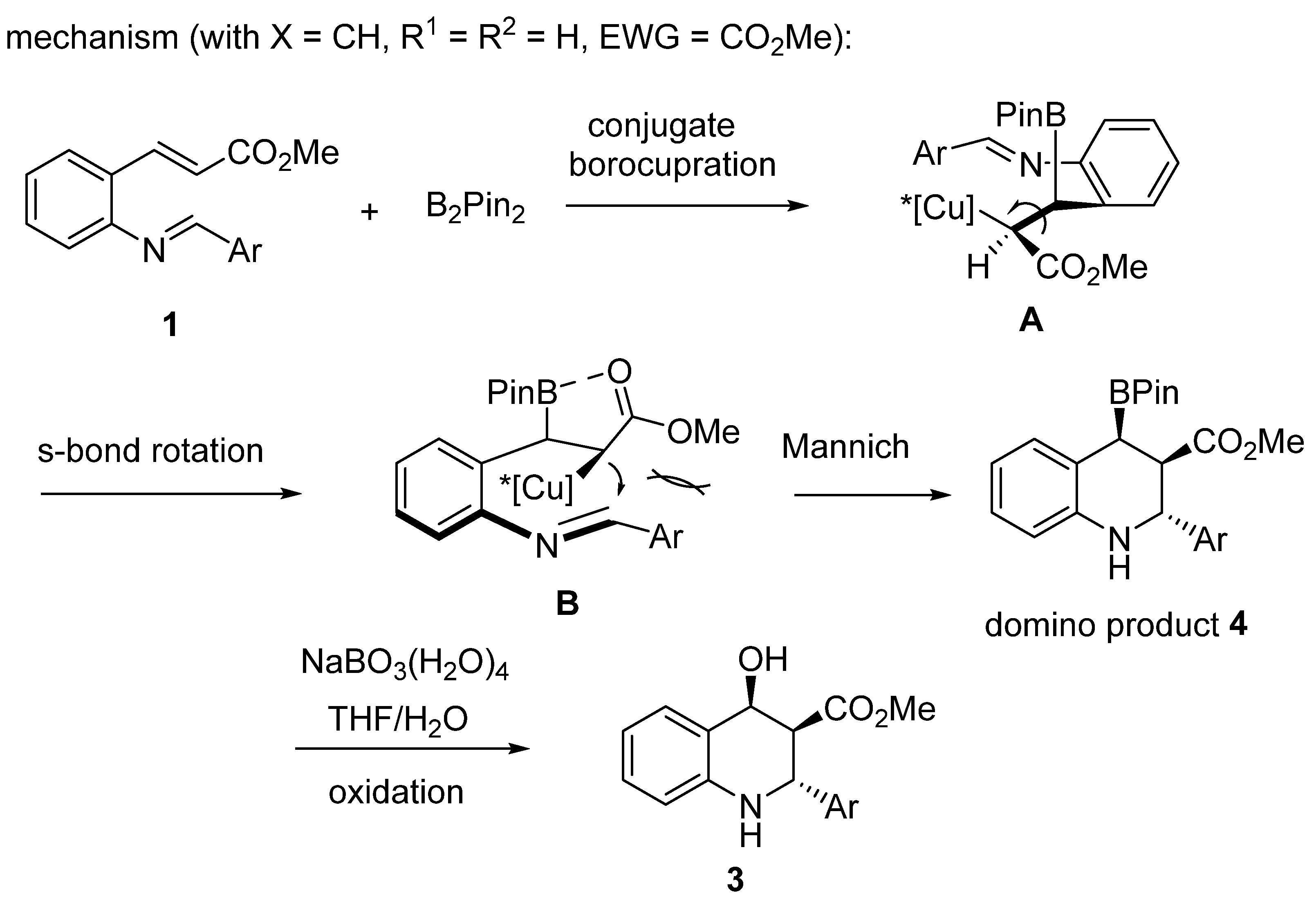
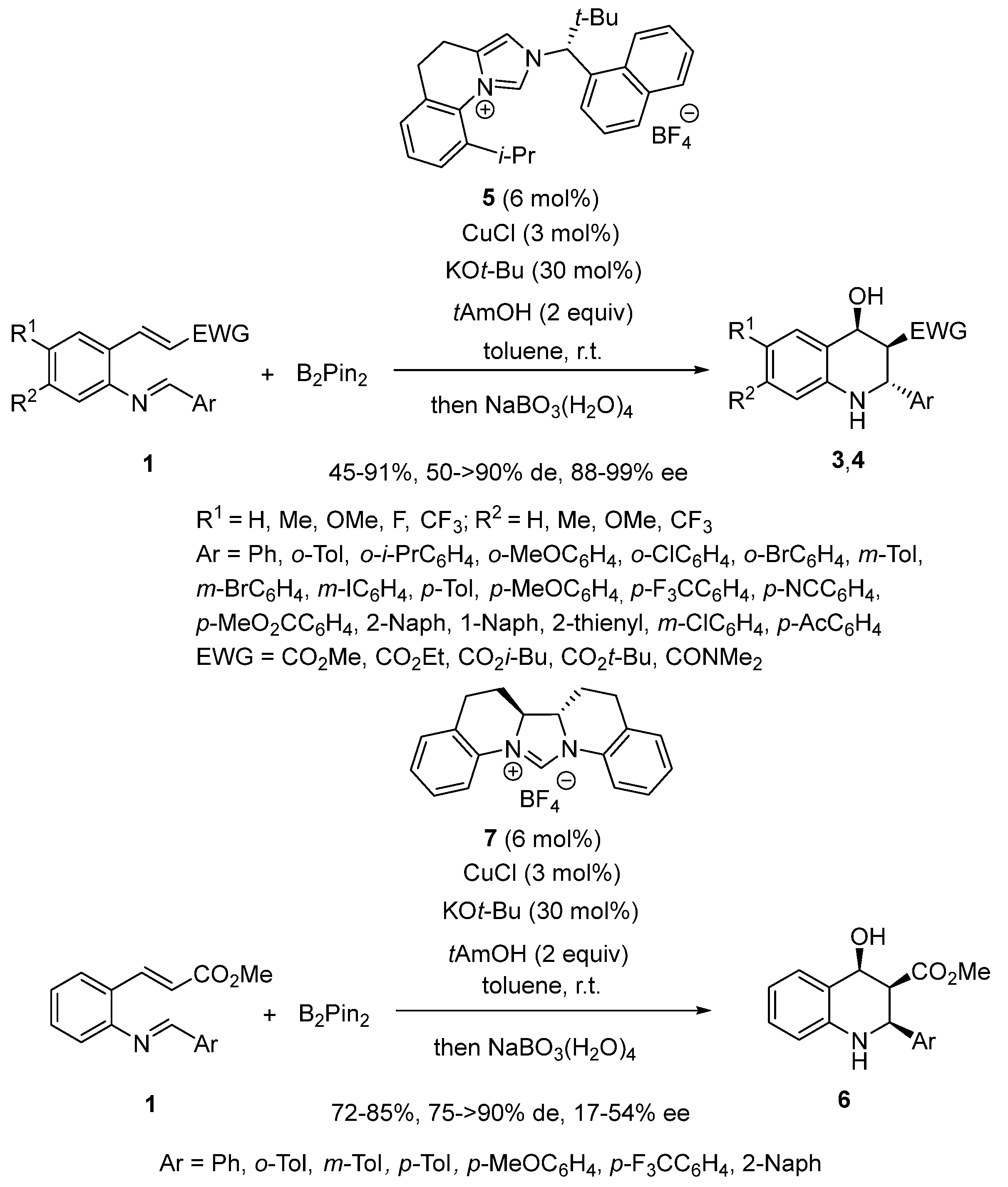
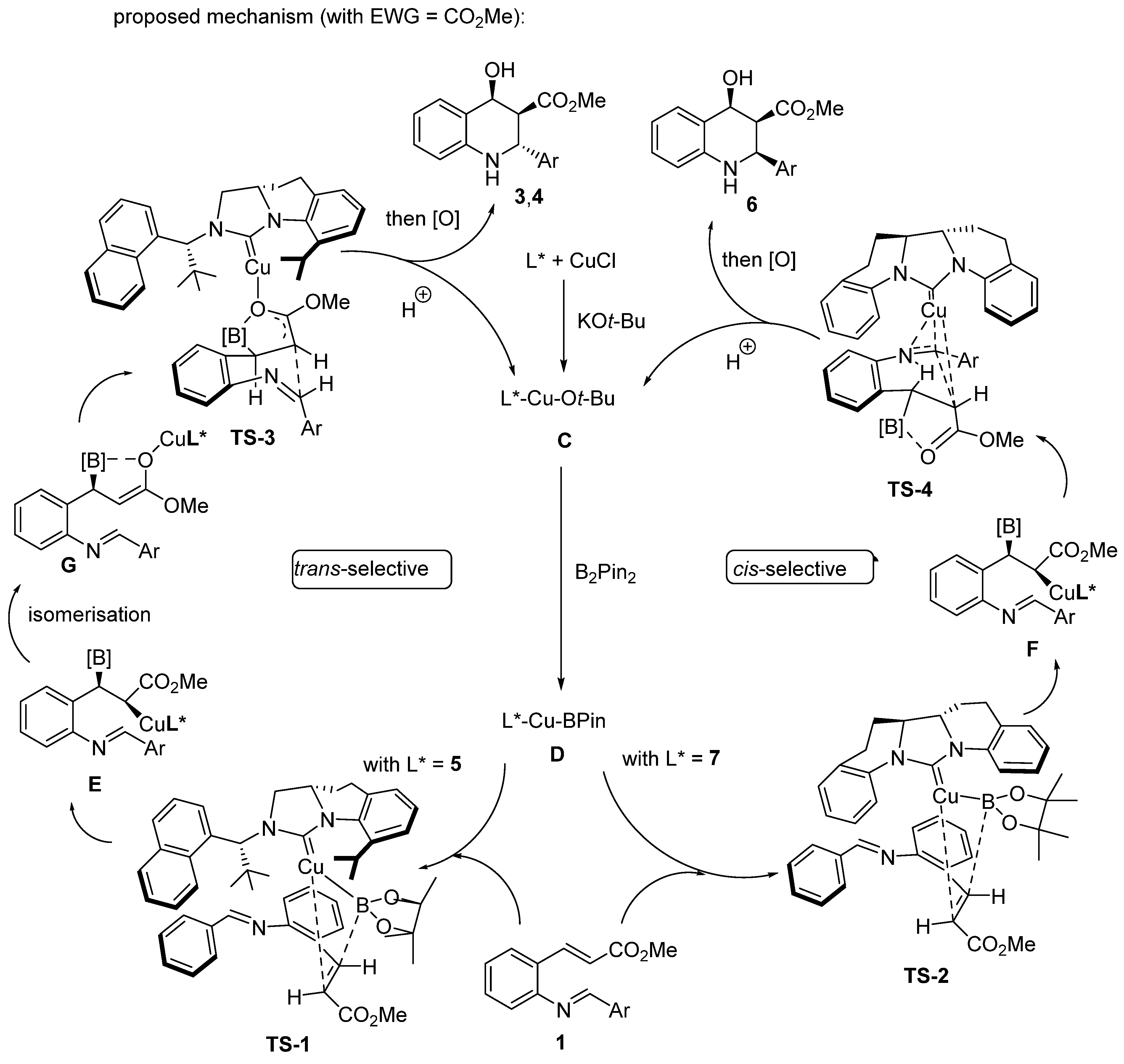
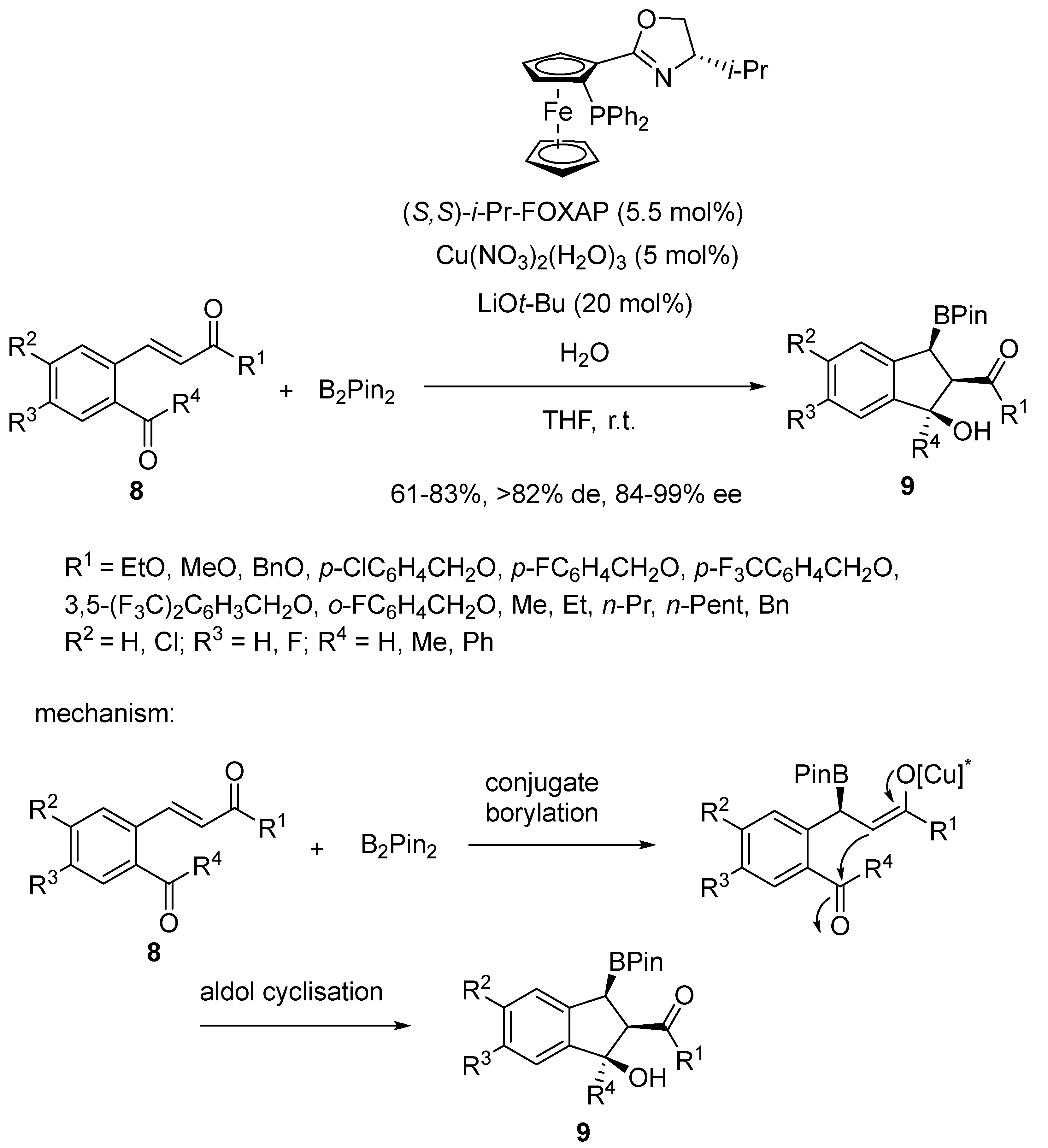
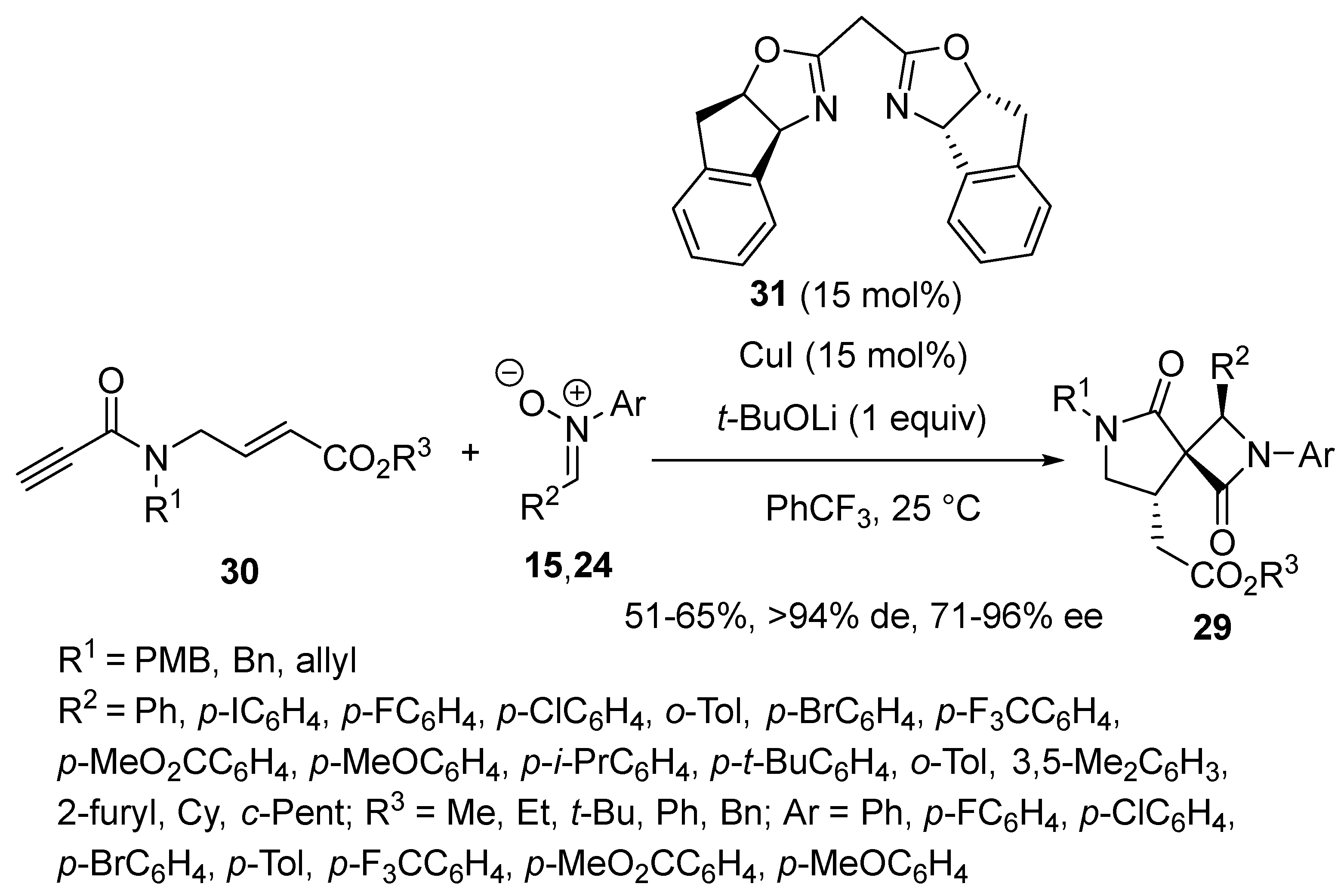
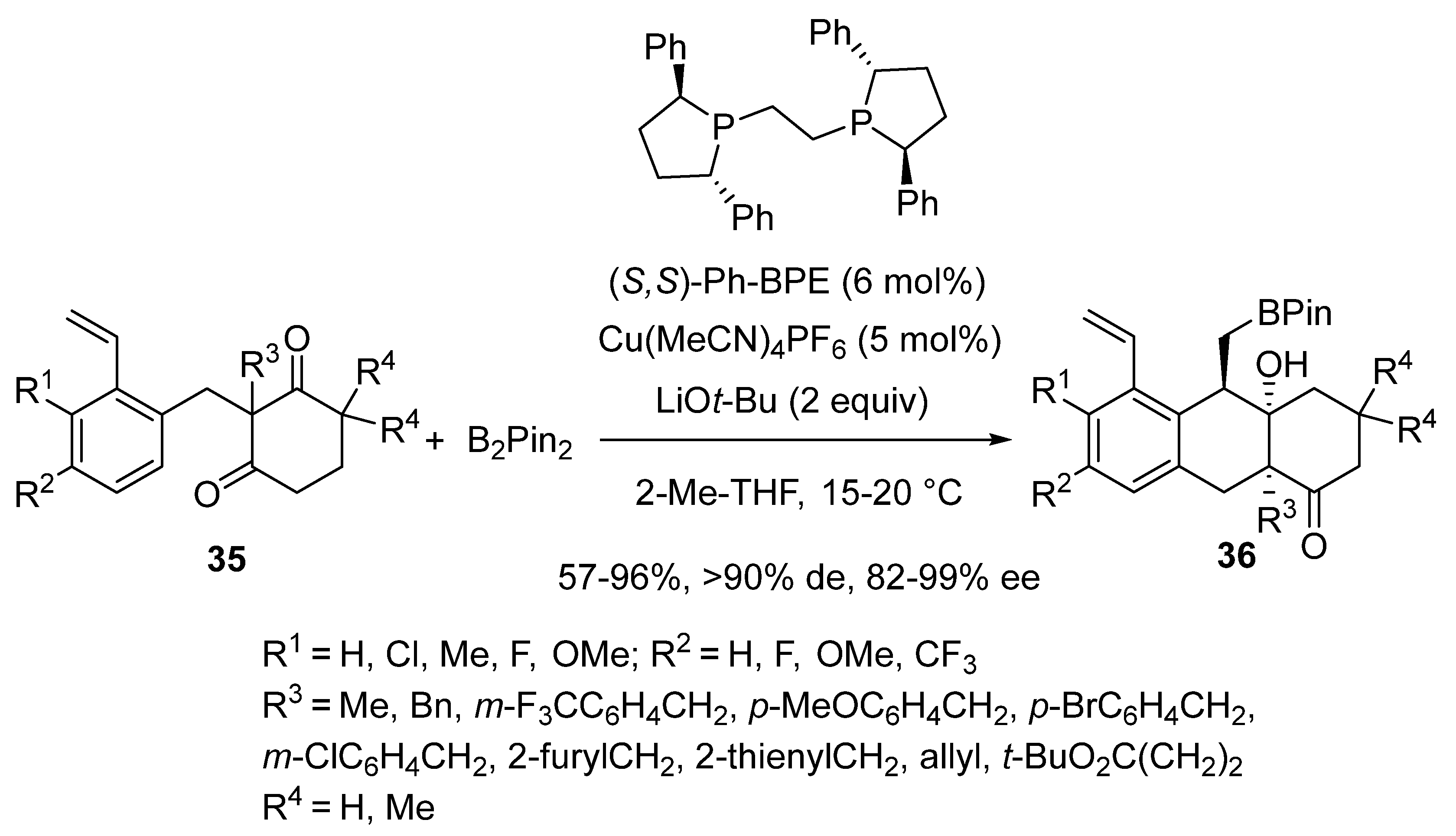
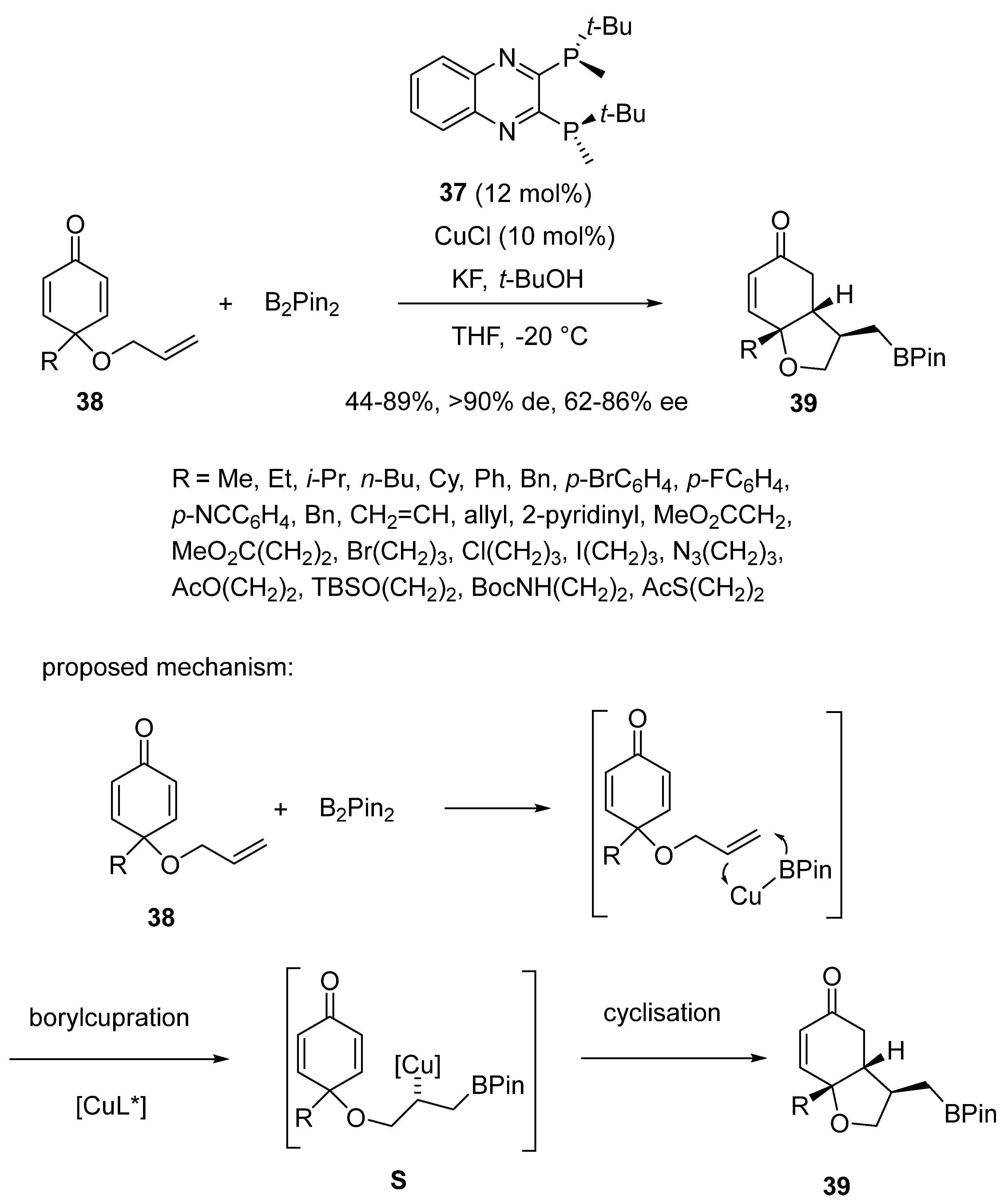
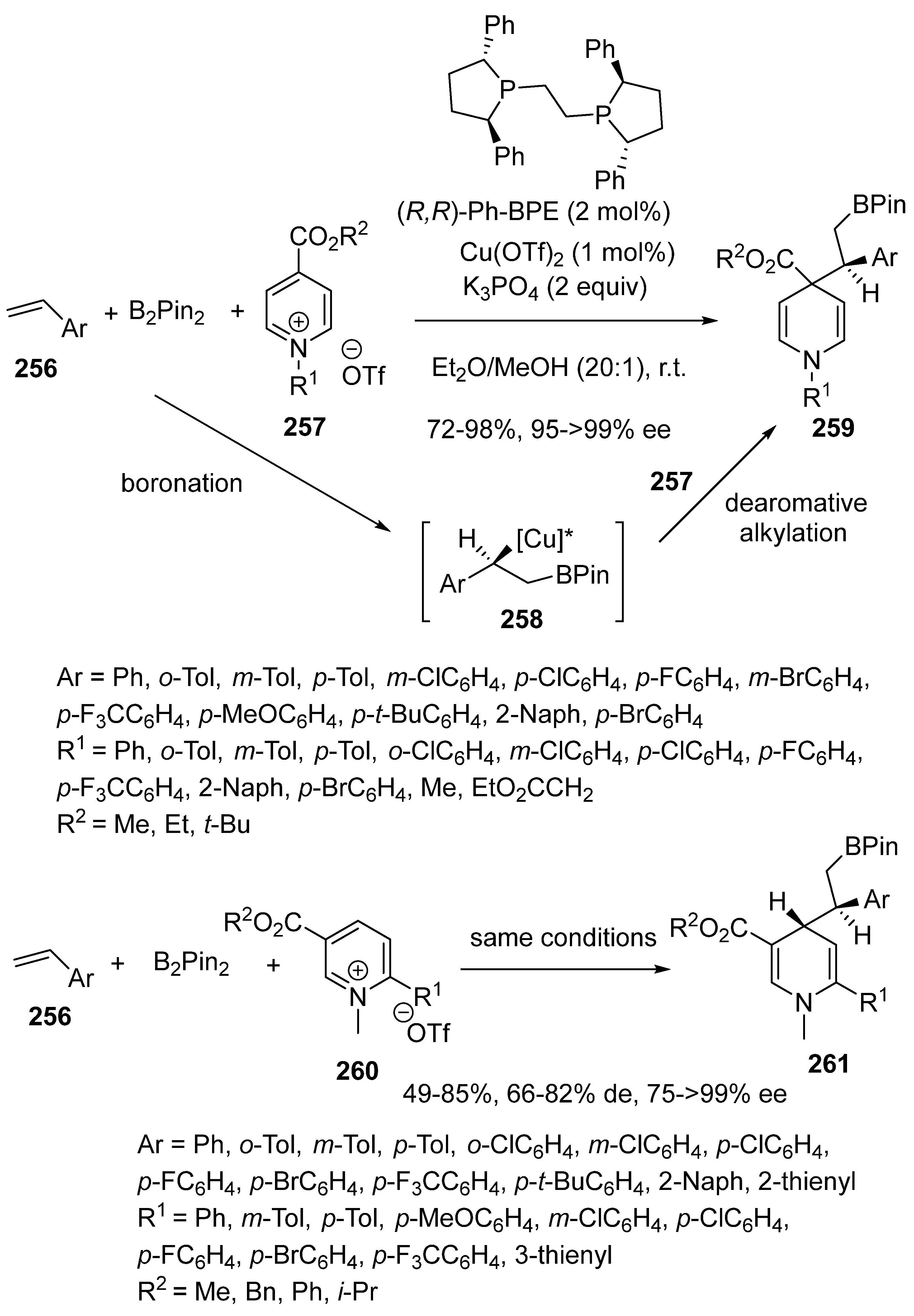
2.3. Borylcupration-Initiated Domino Reactions
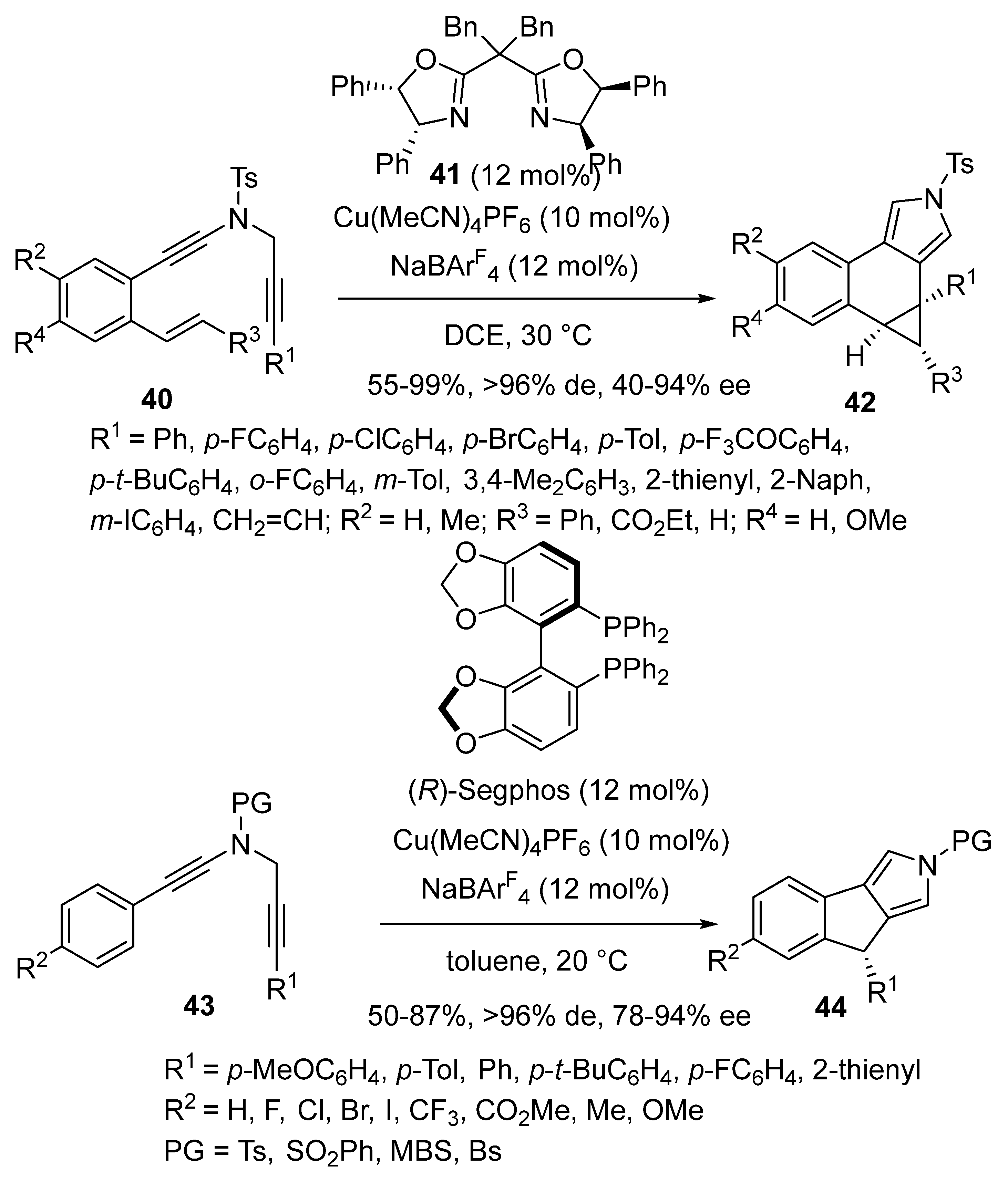
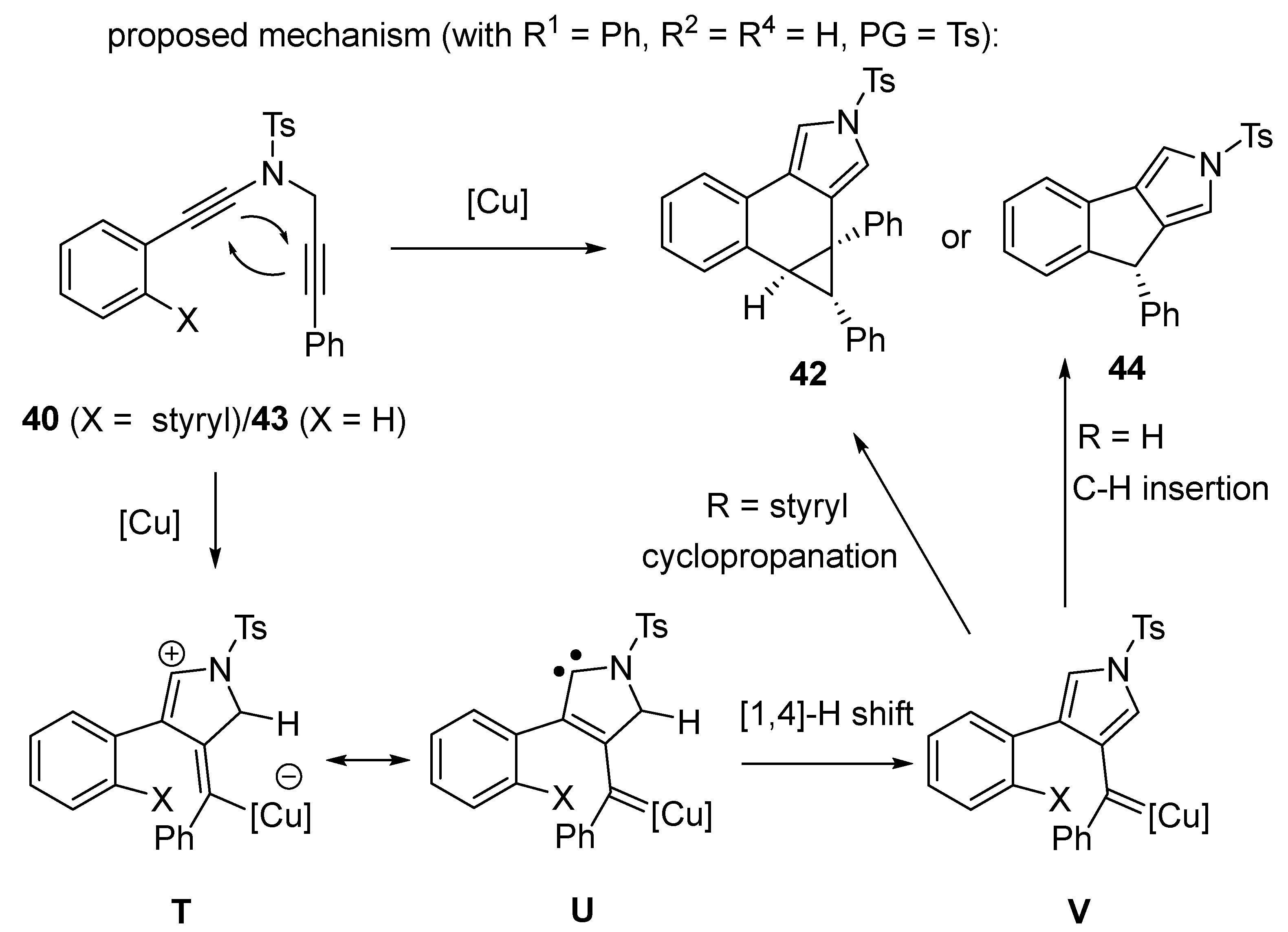
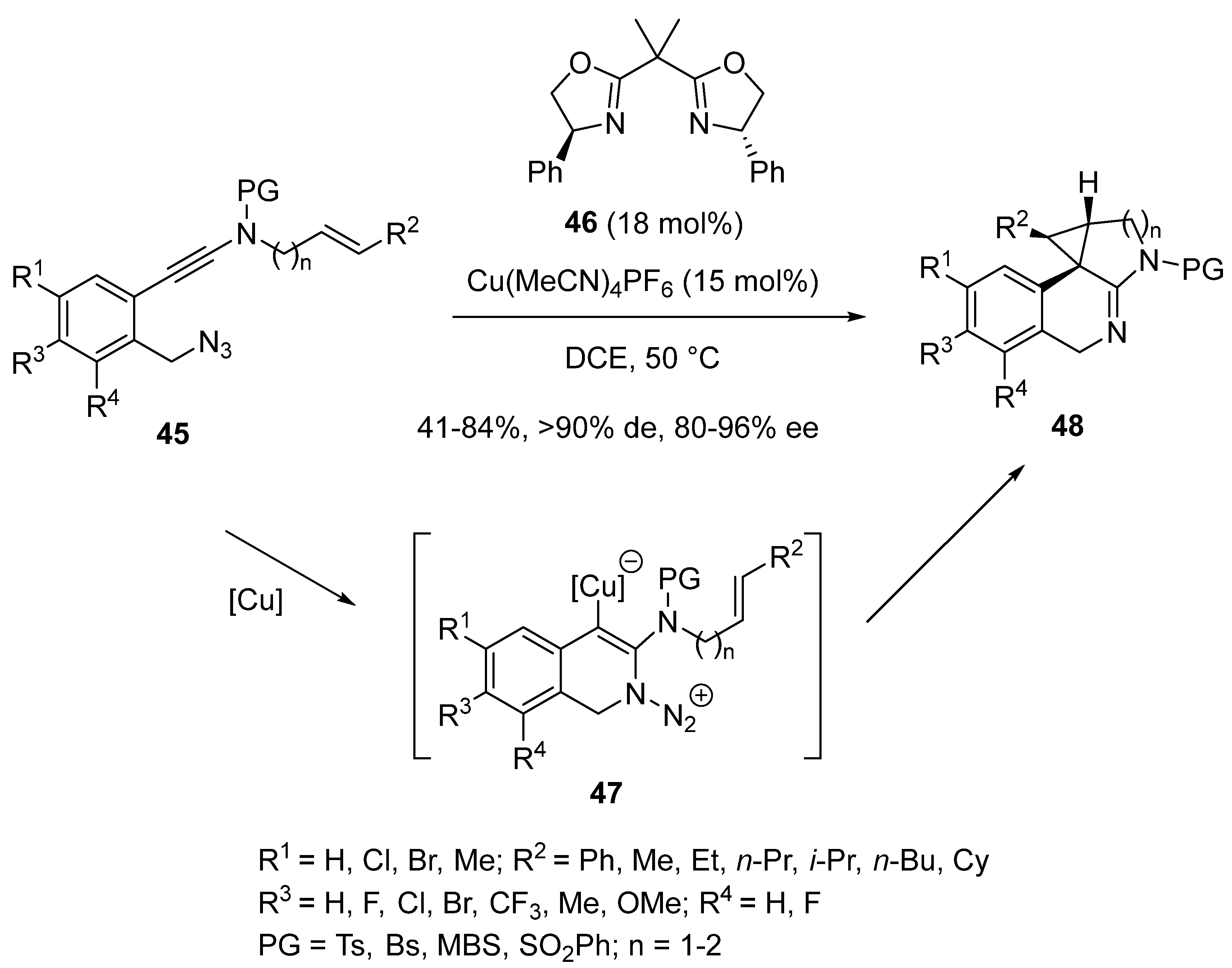
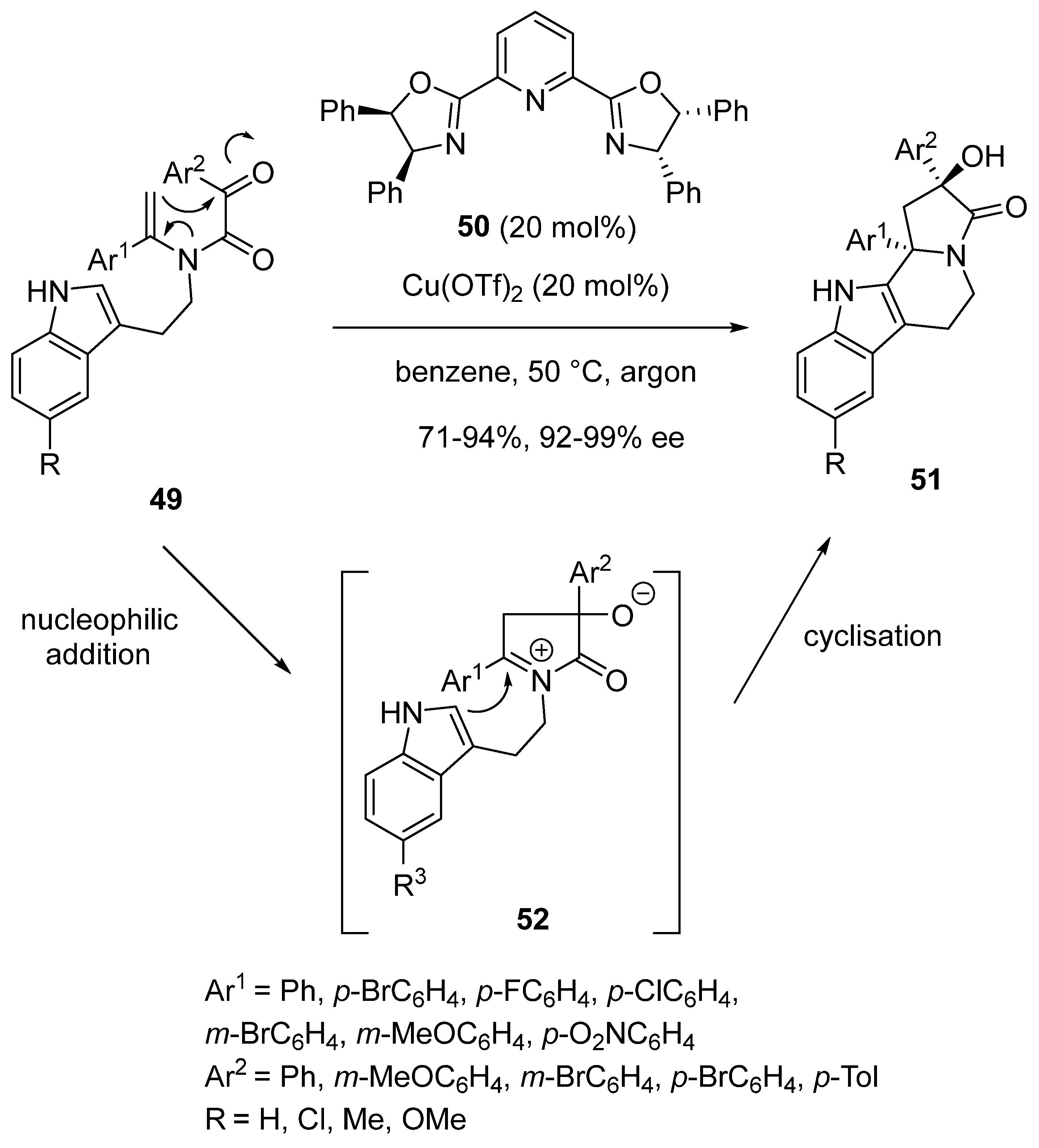
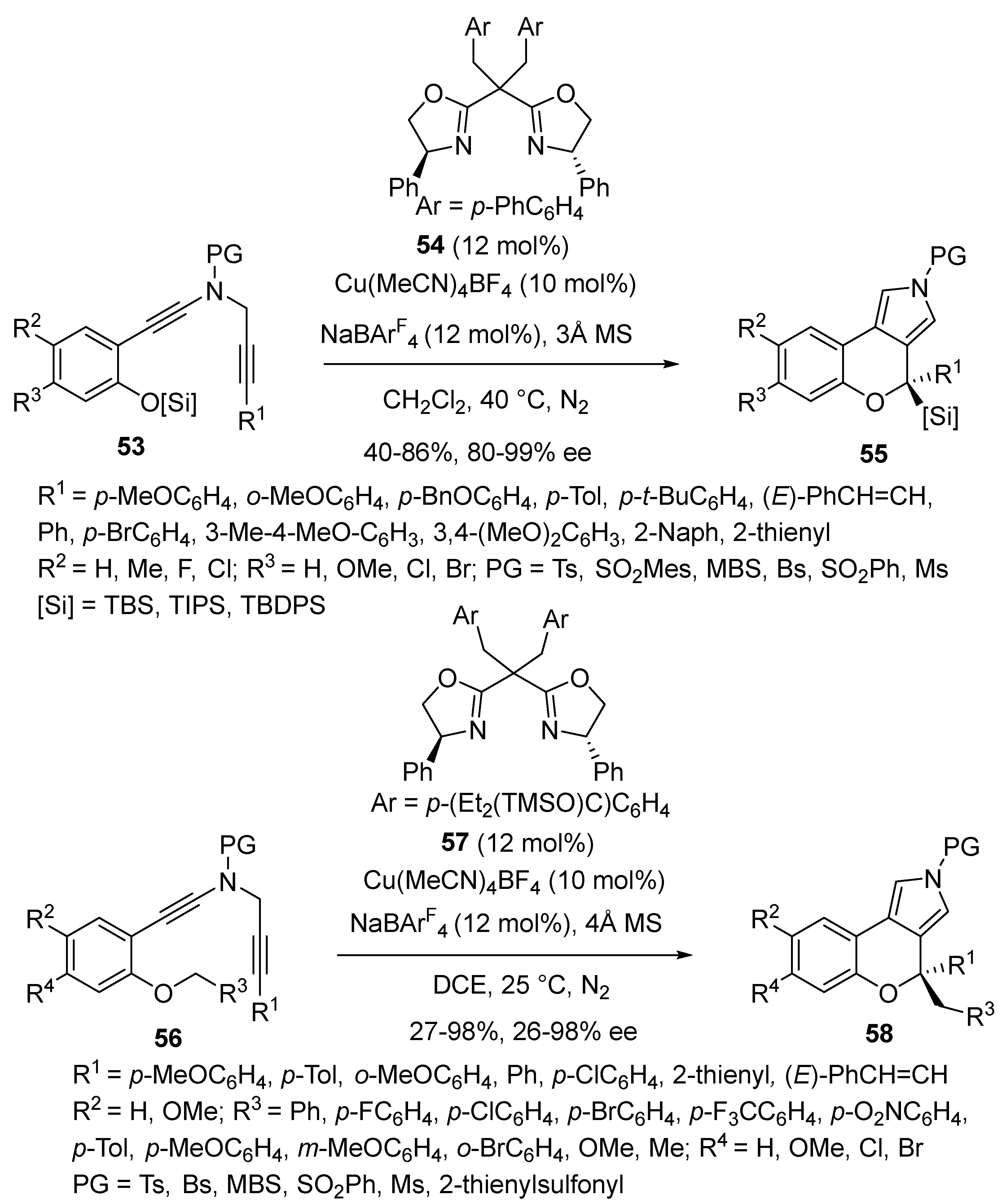
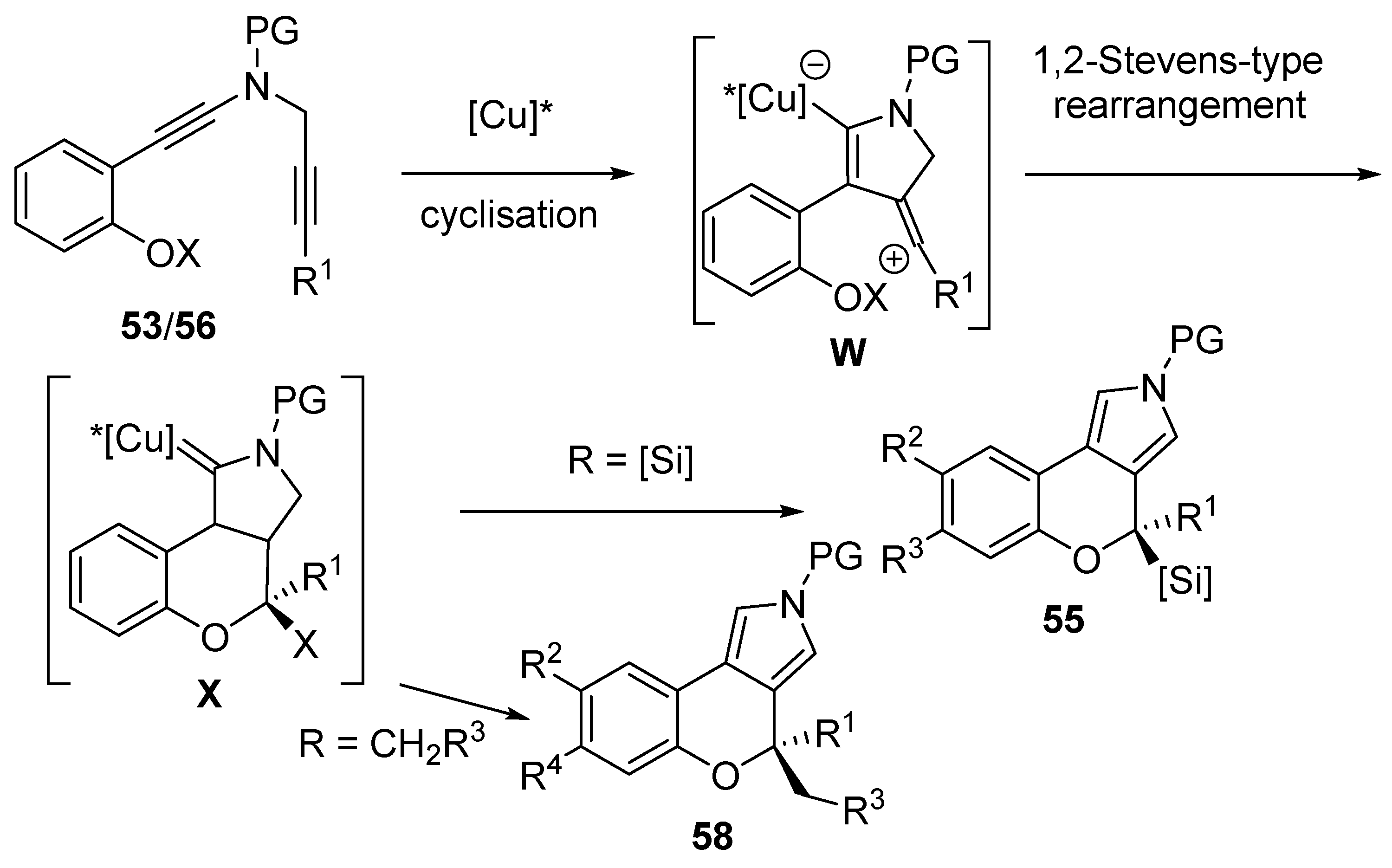
2.4. Domino Reactions Based on Intramolecular Cyclisation
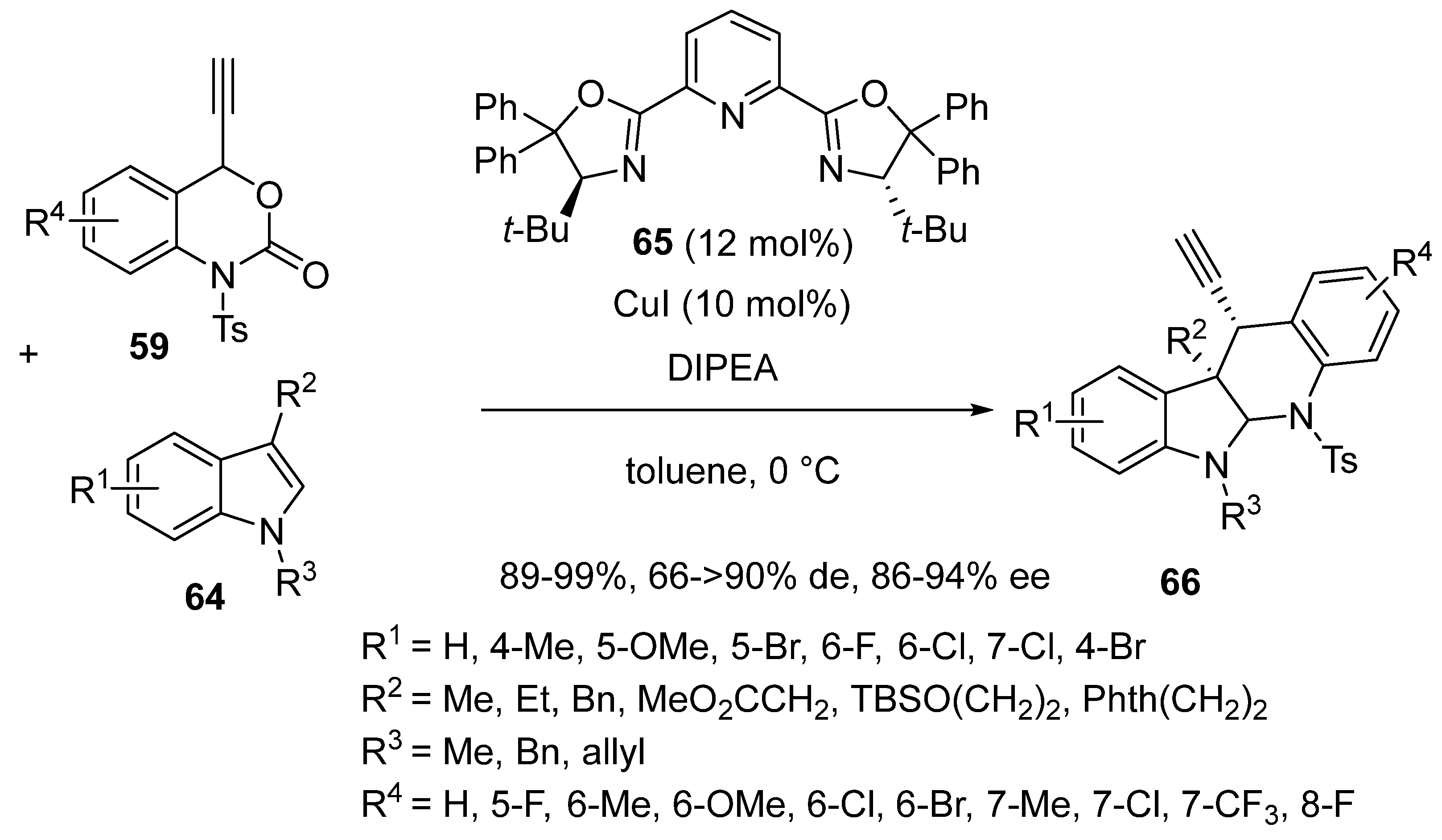
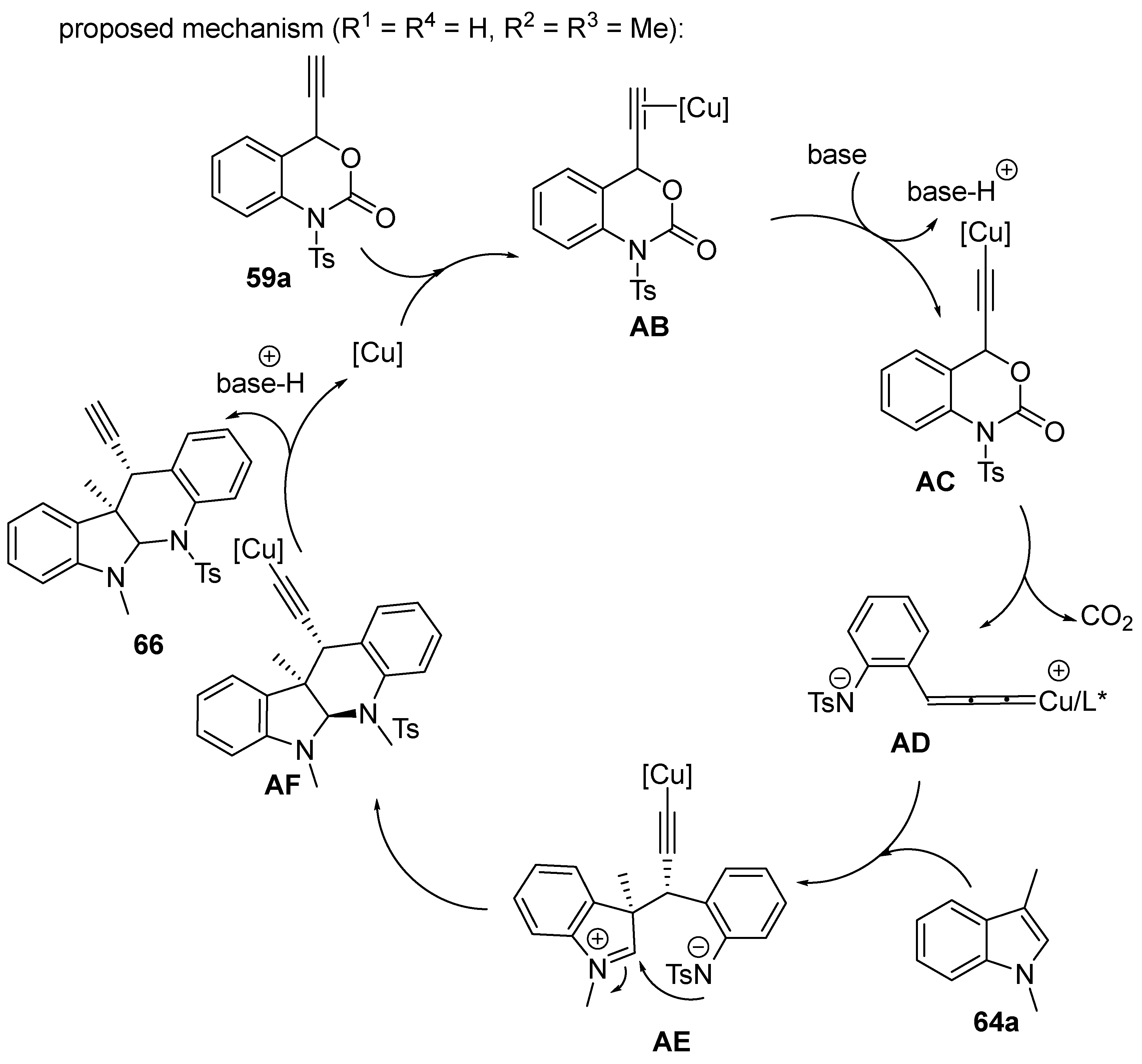
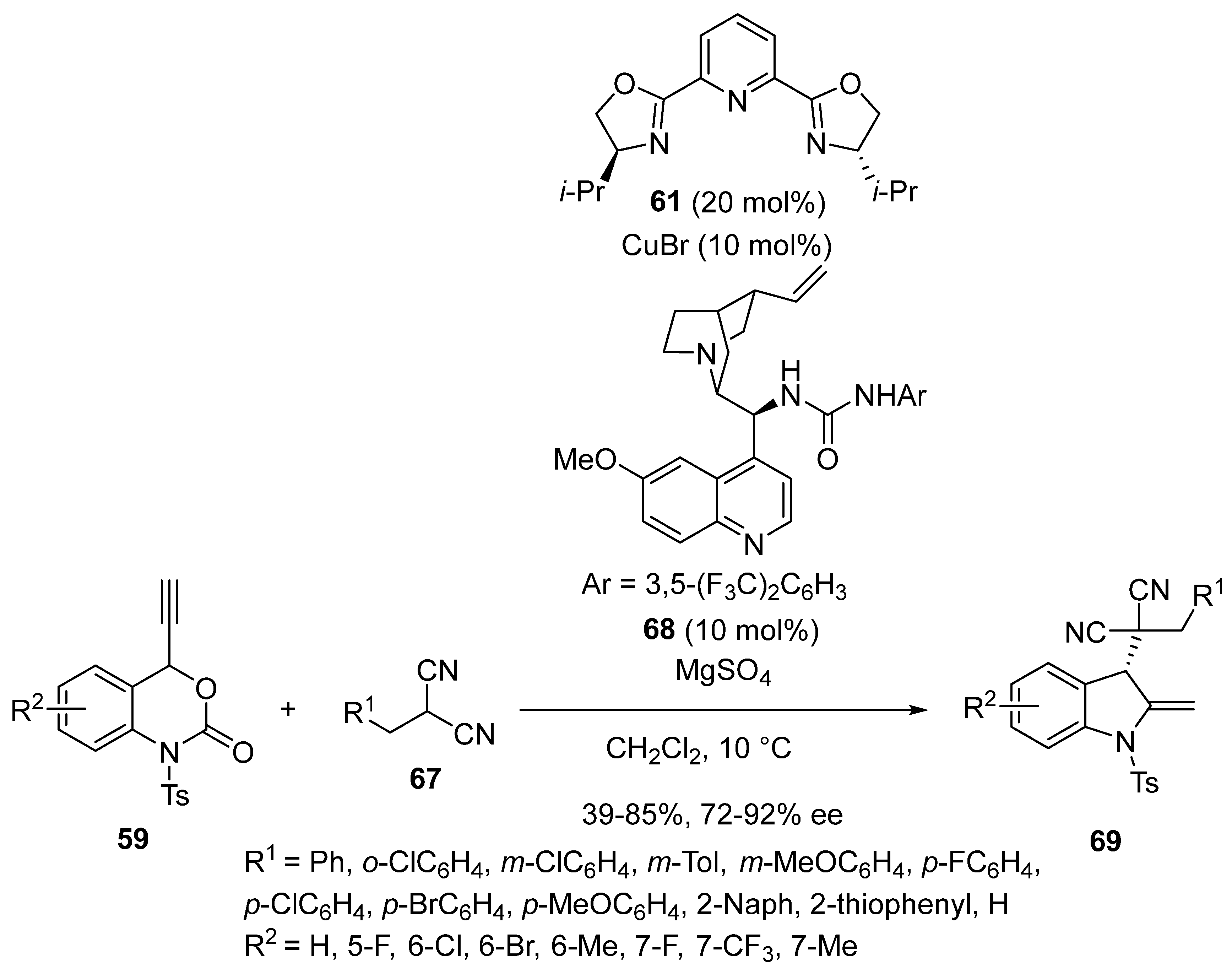
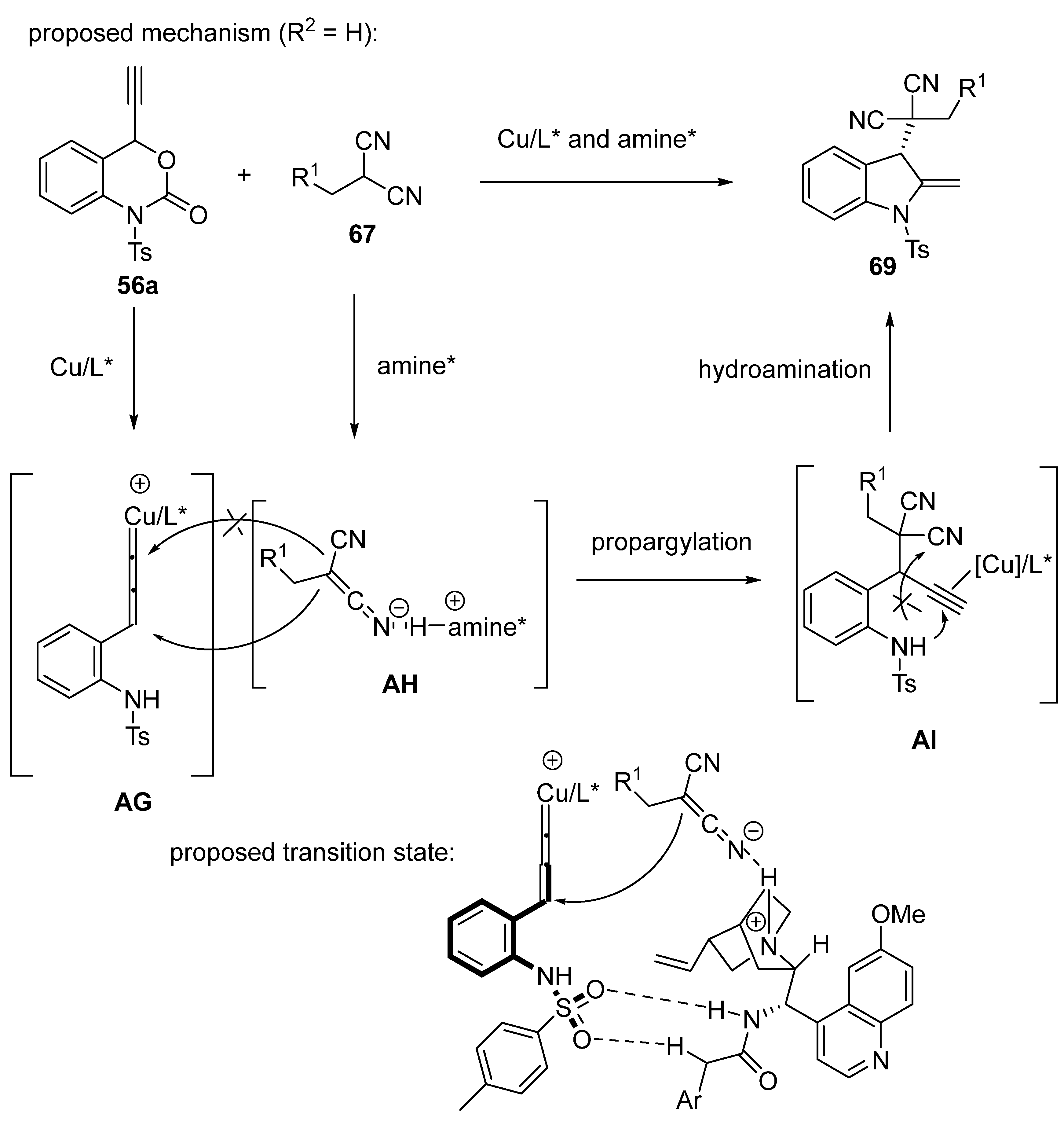
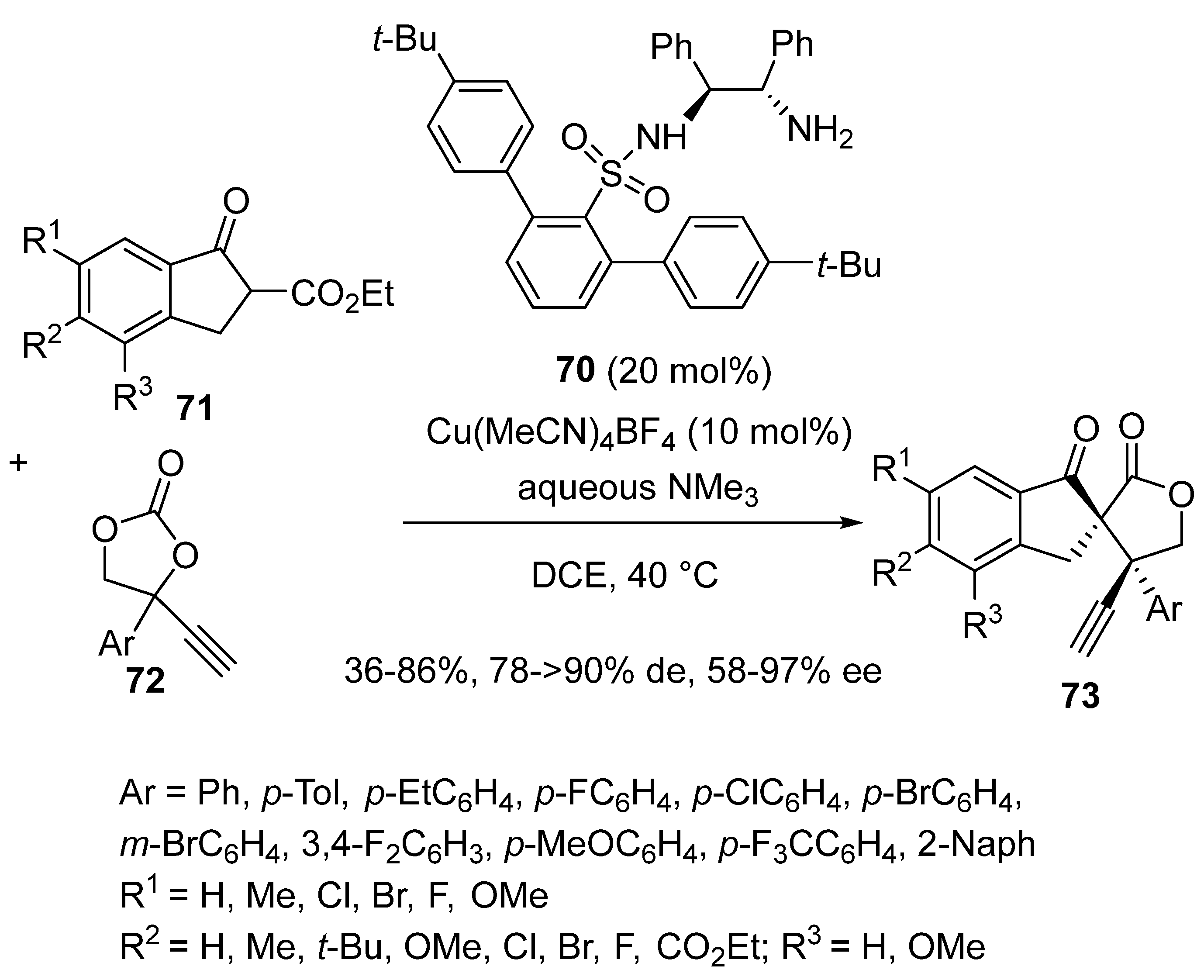
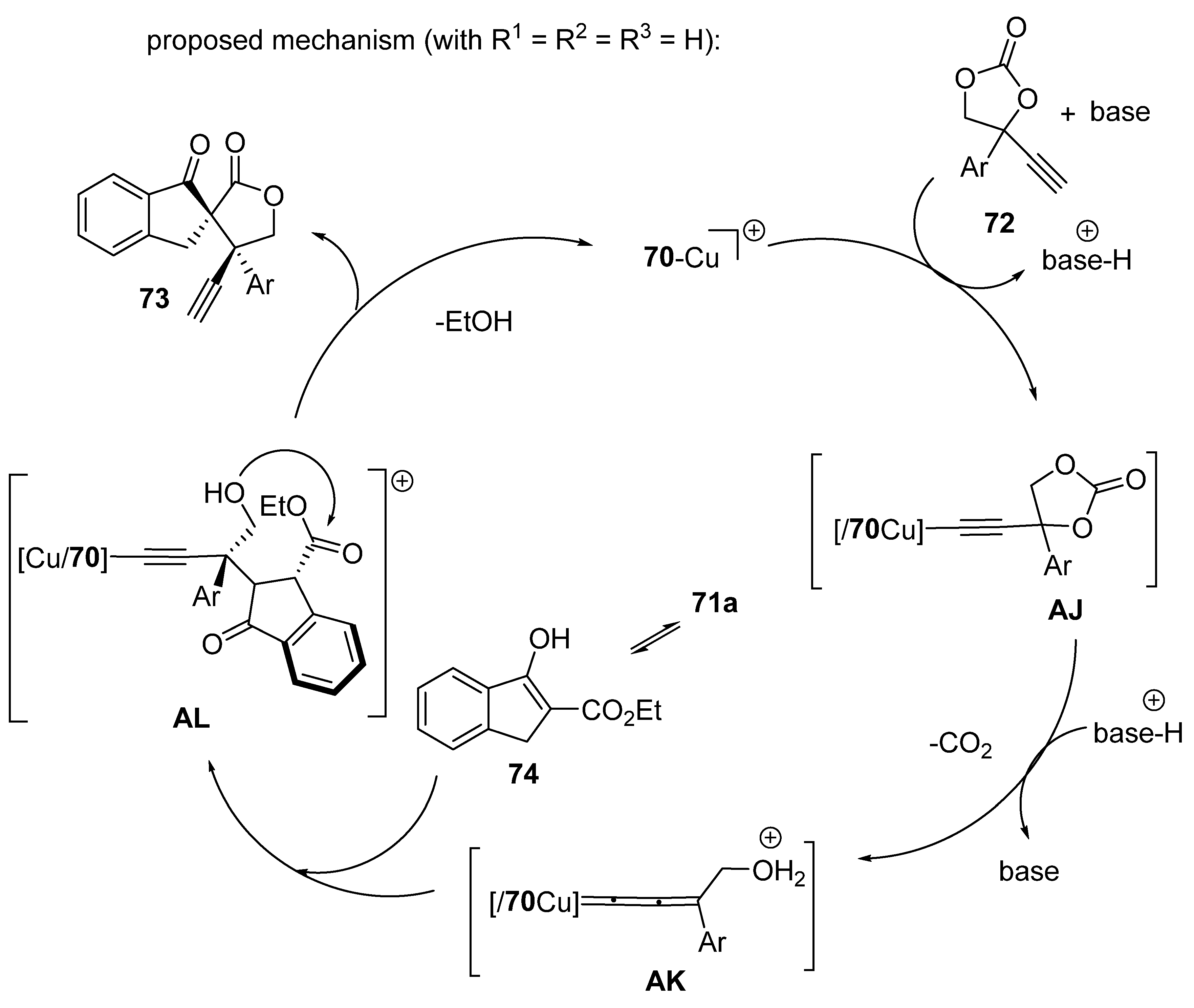
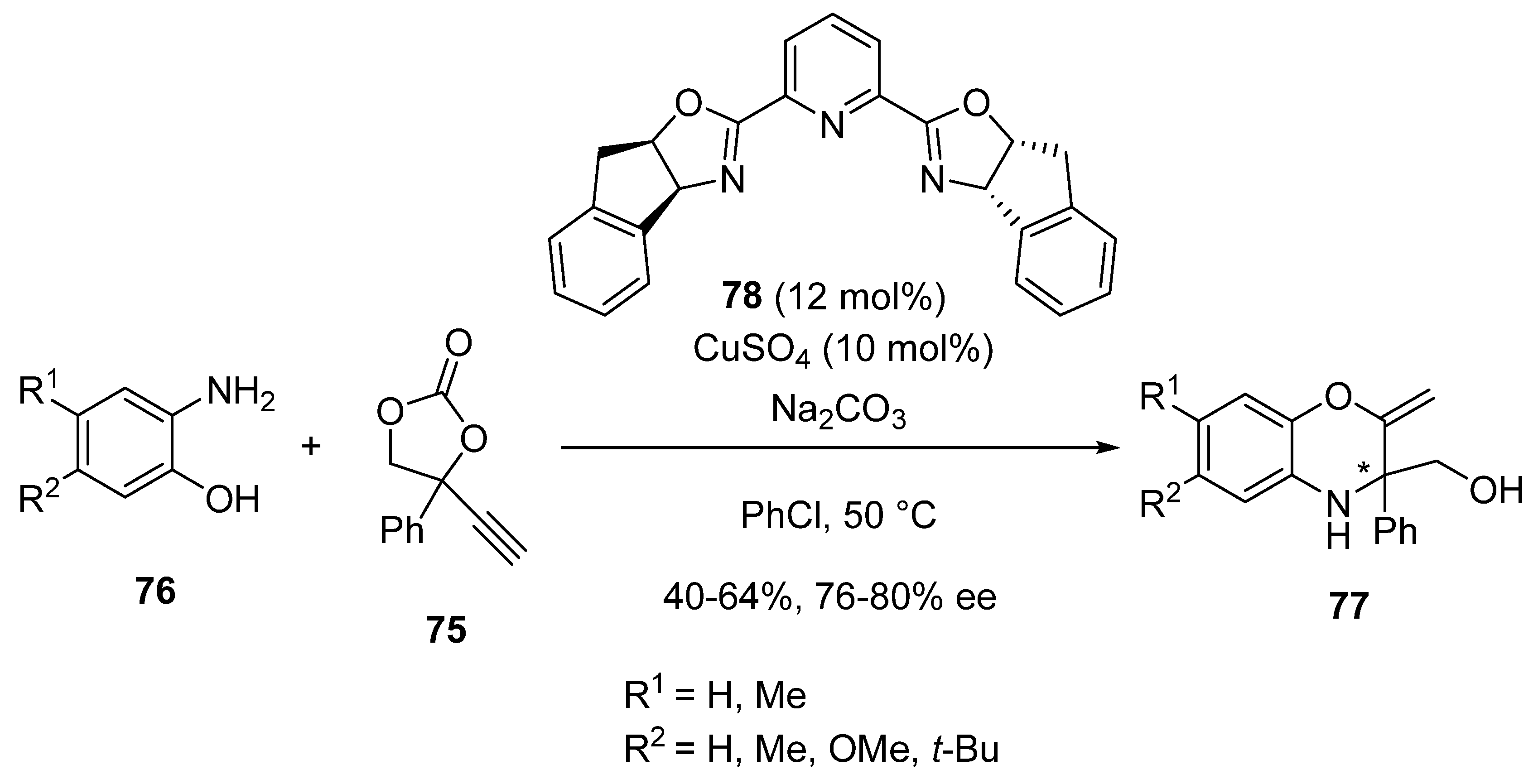
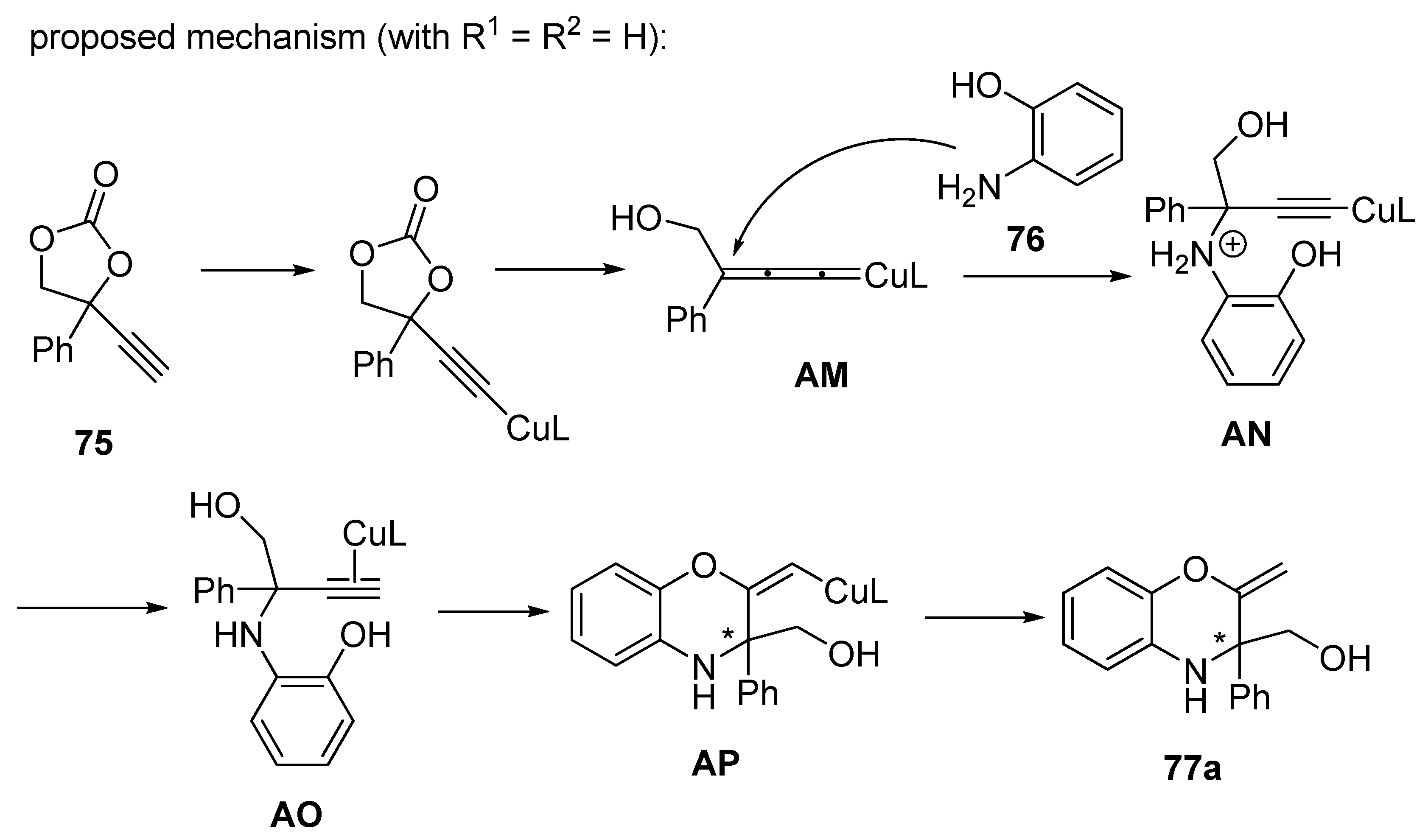
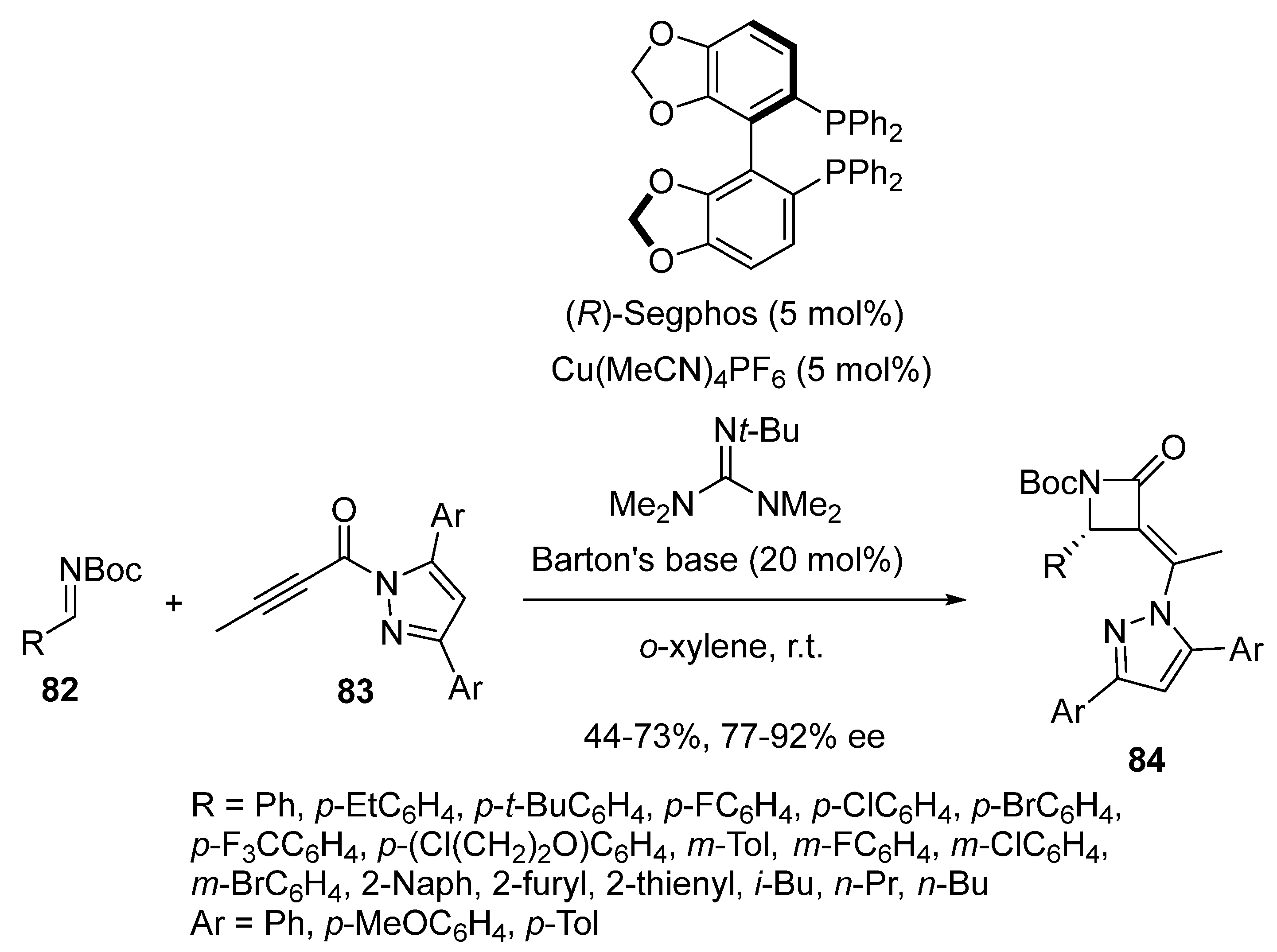
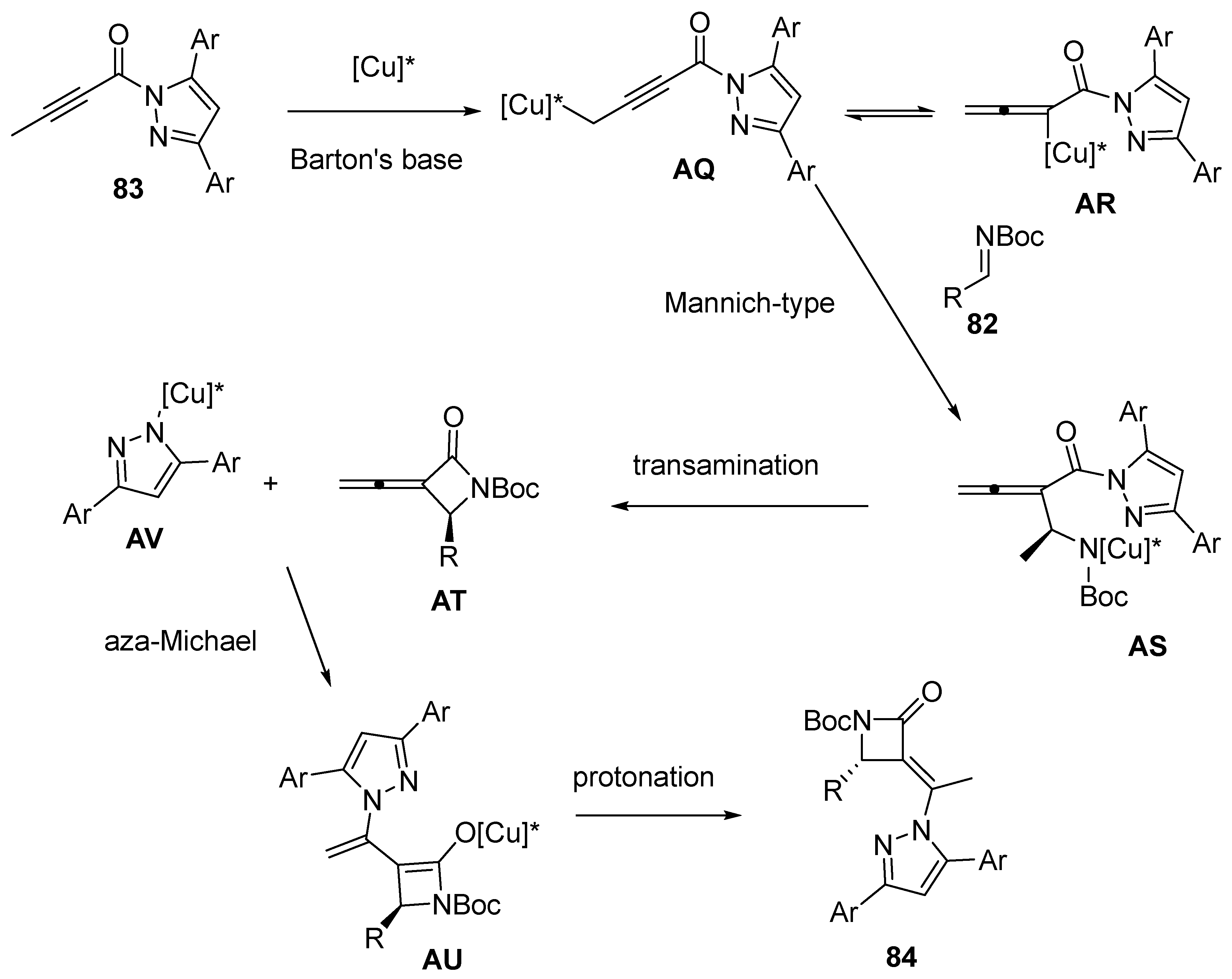
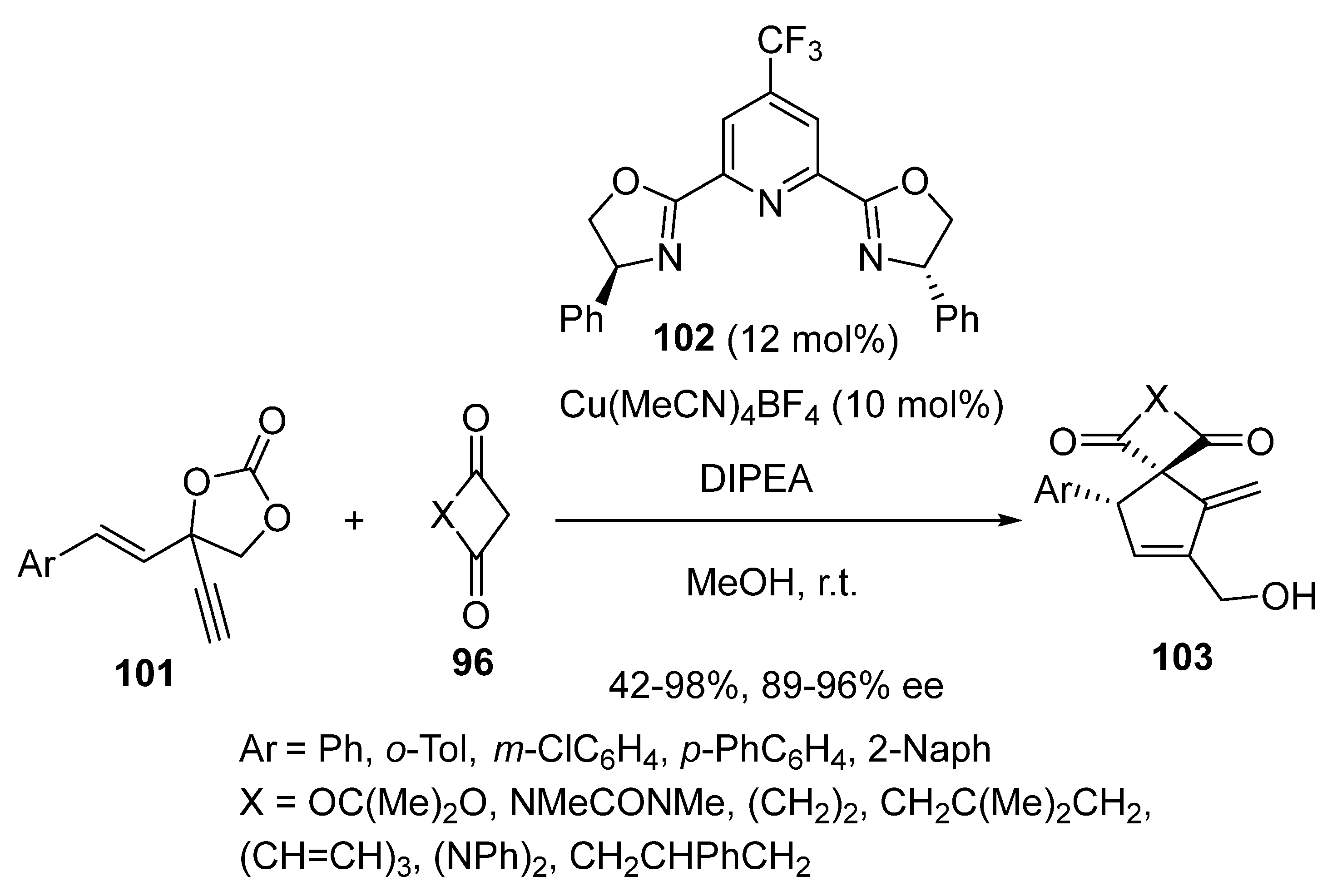
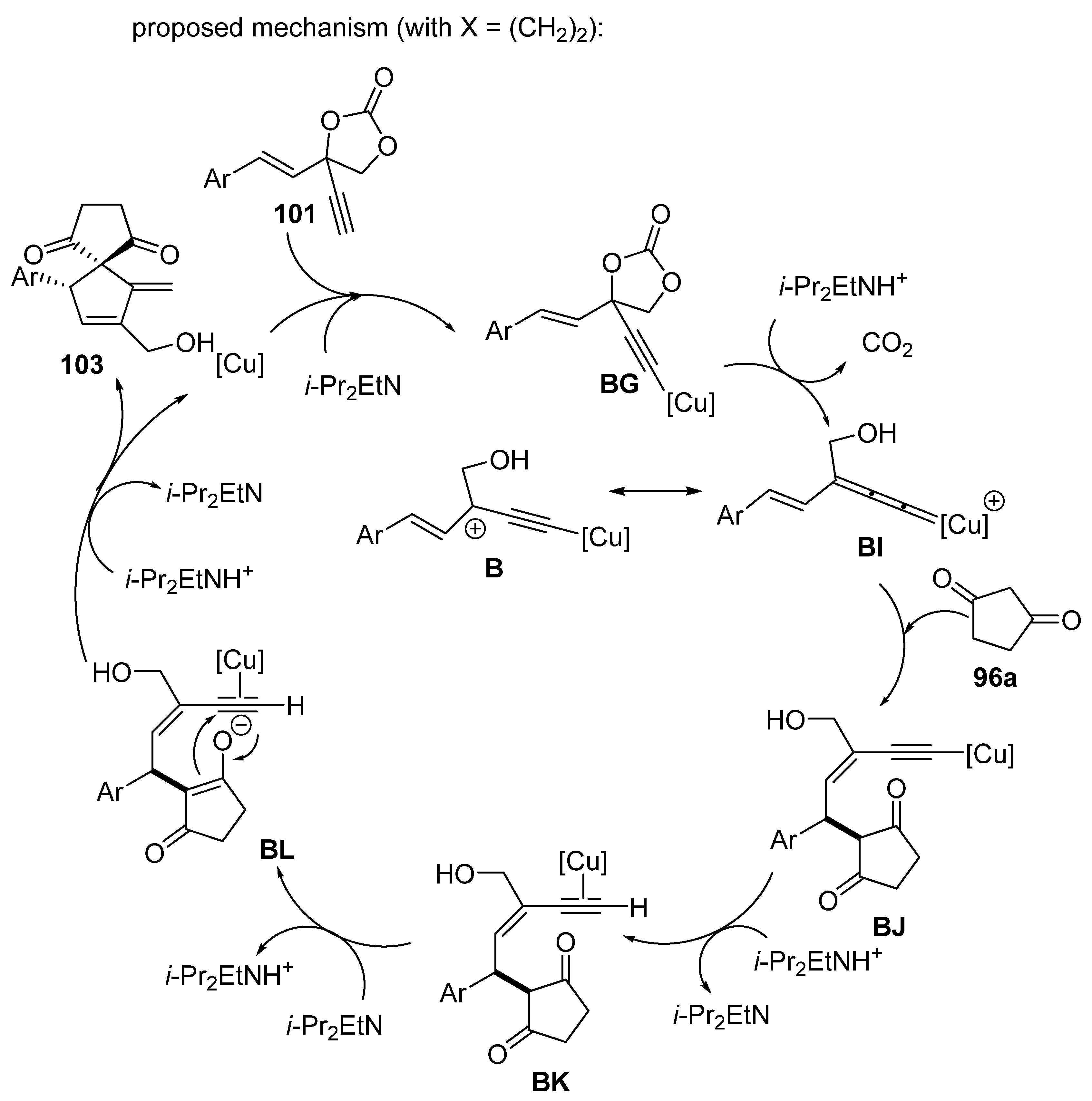
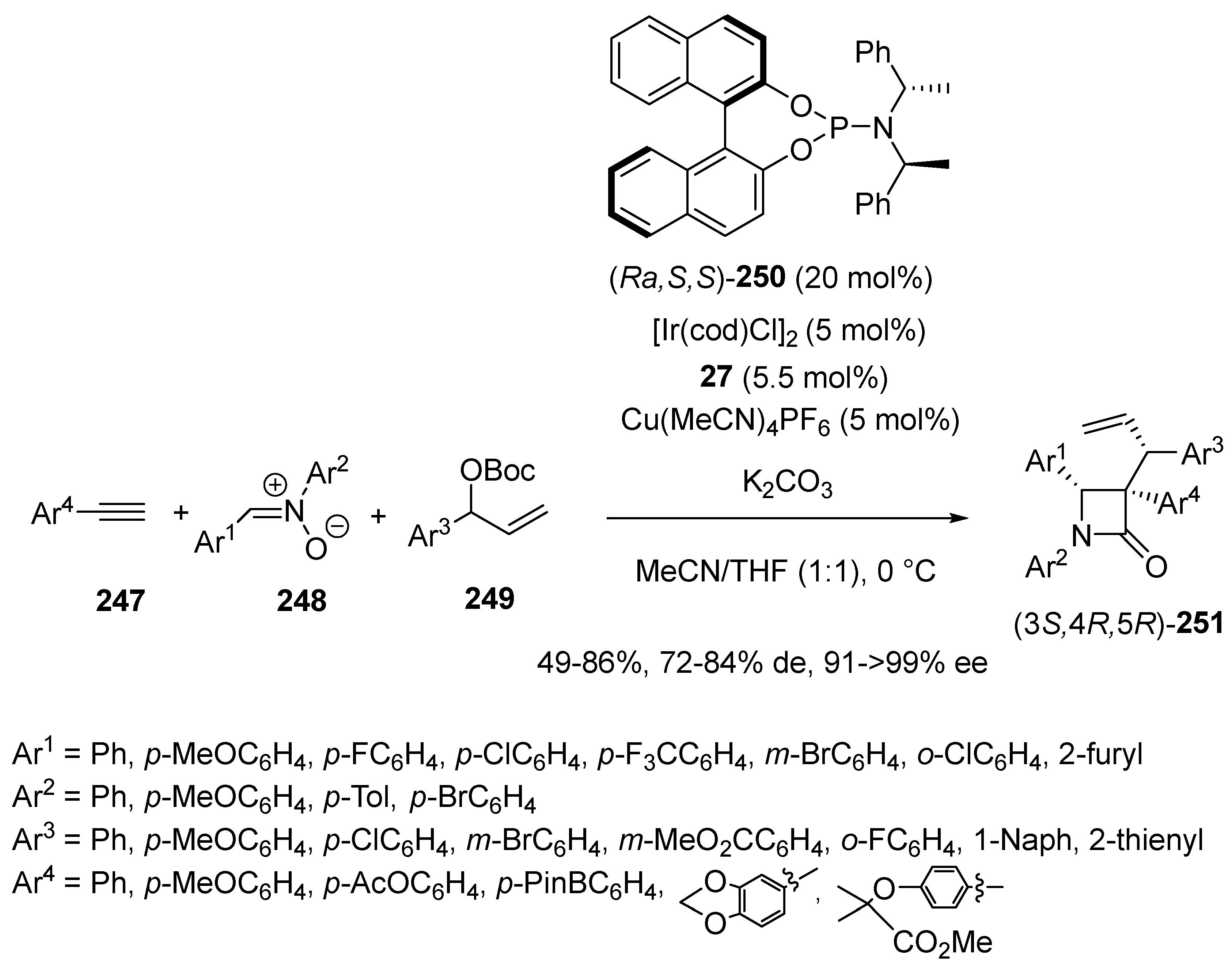
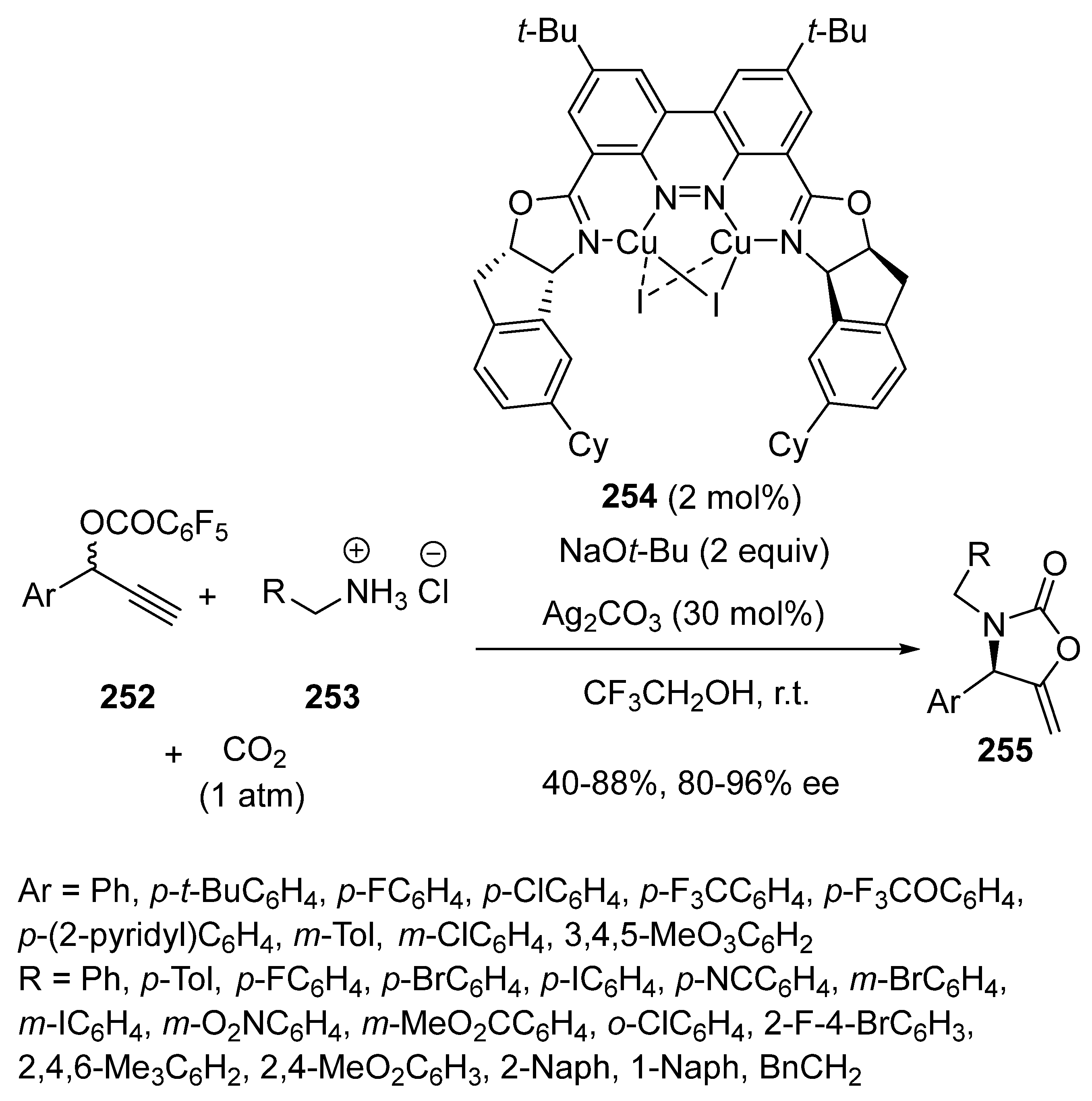
2.5. Domino Reactions Initiated by Decarboxylative Propargylation
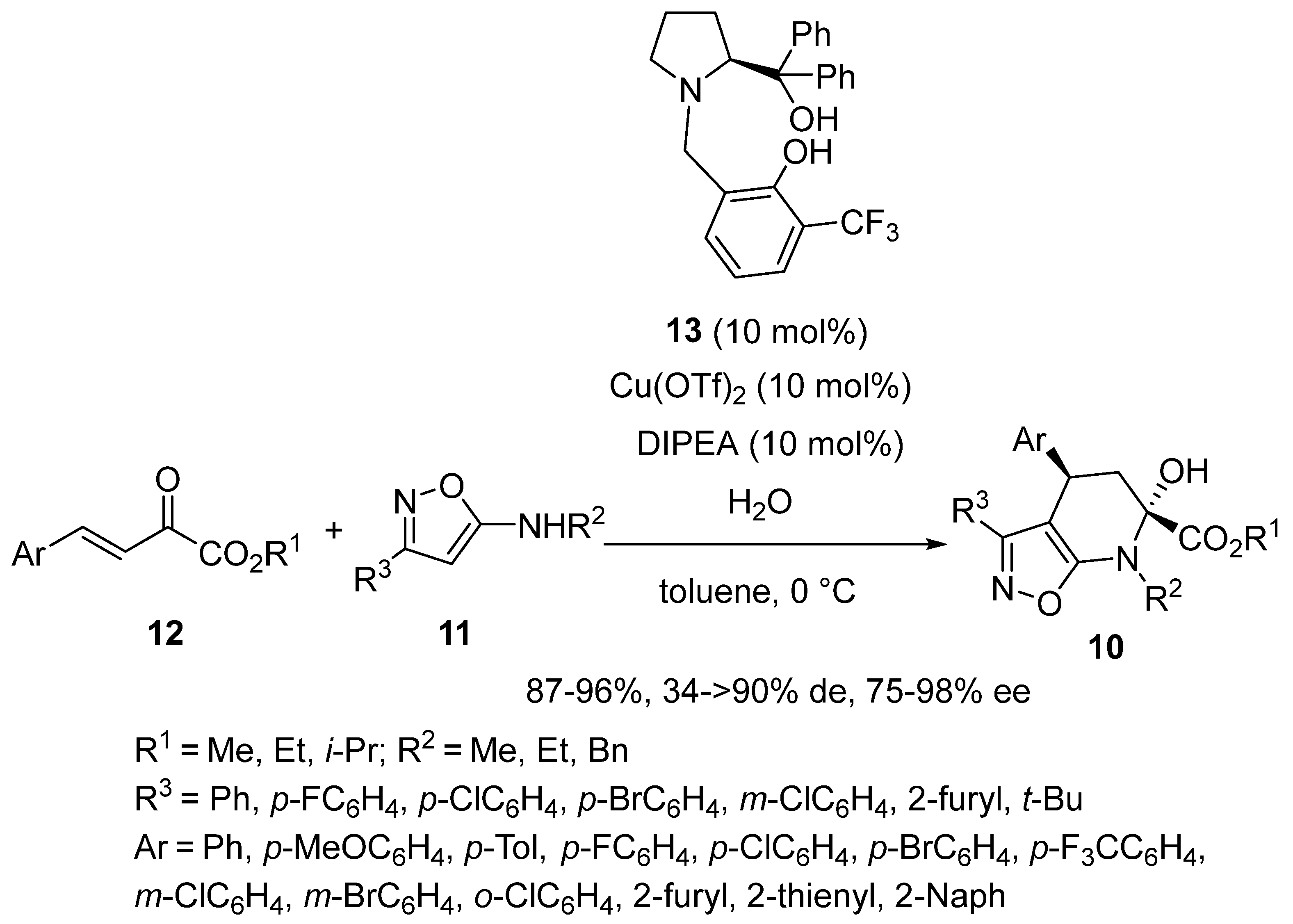
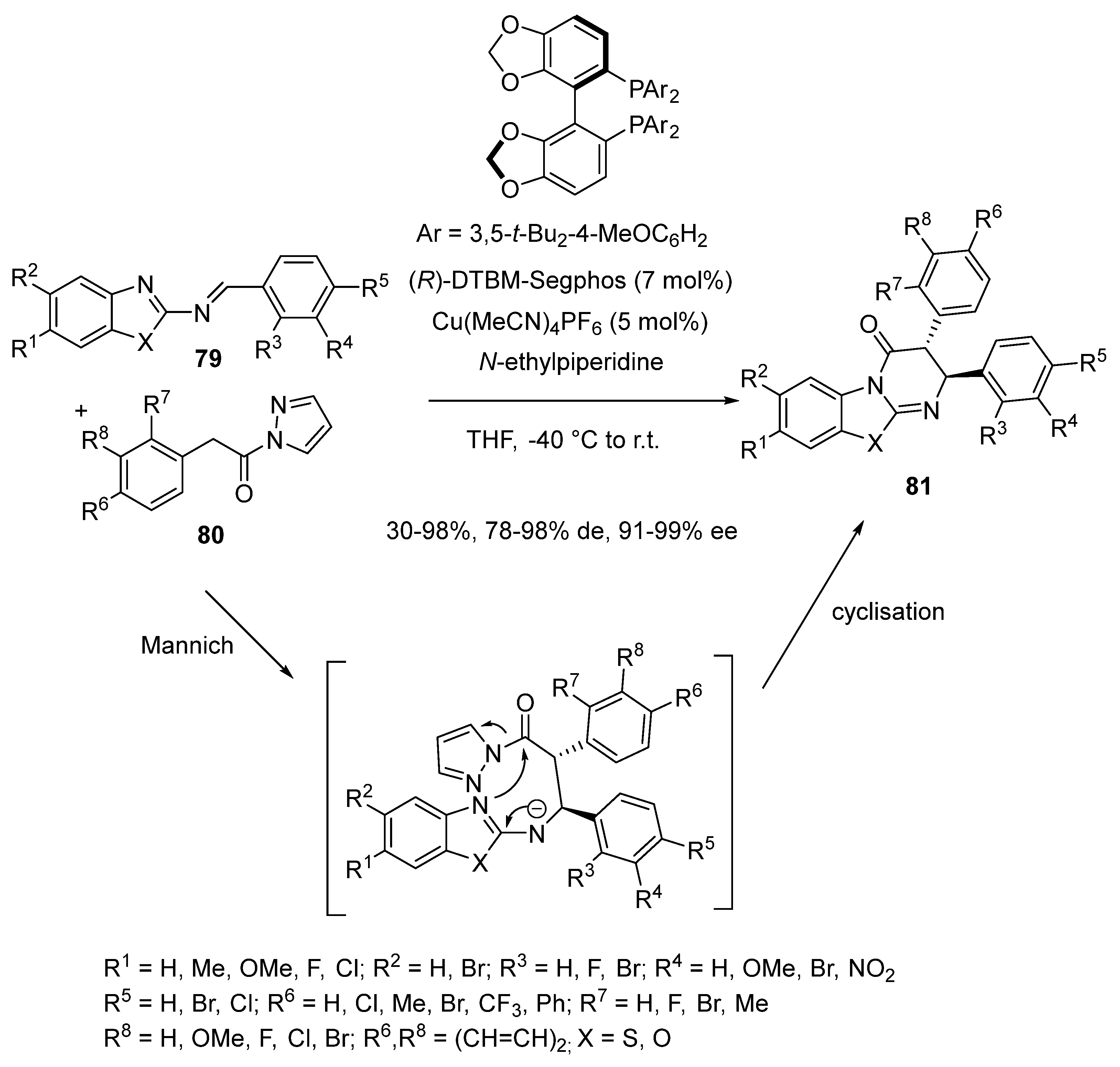
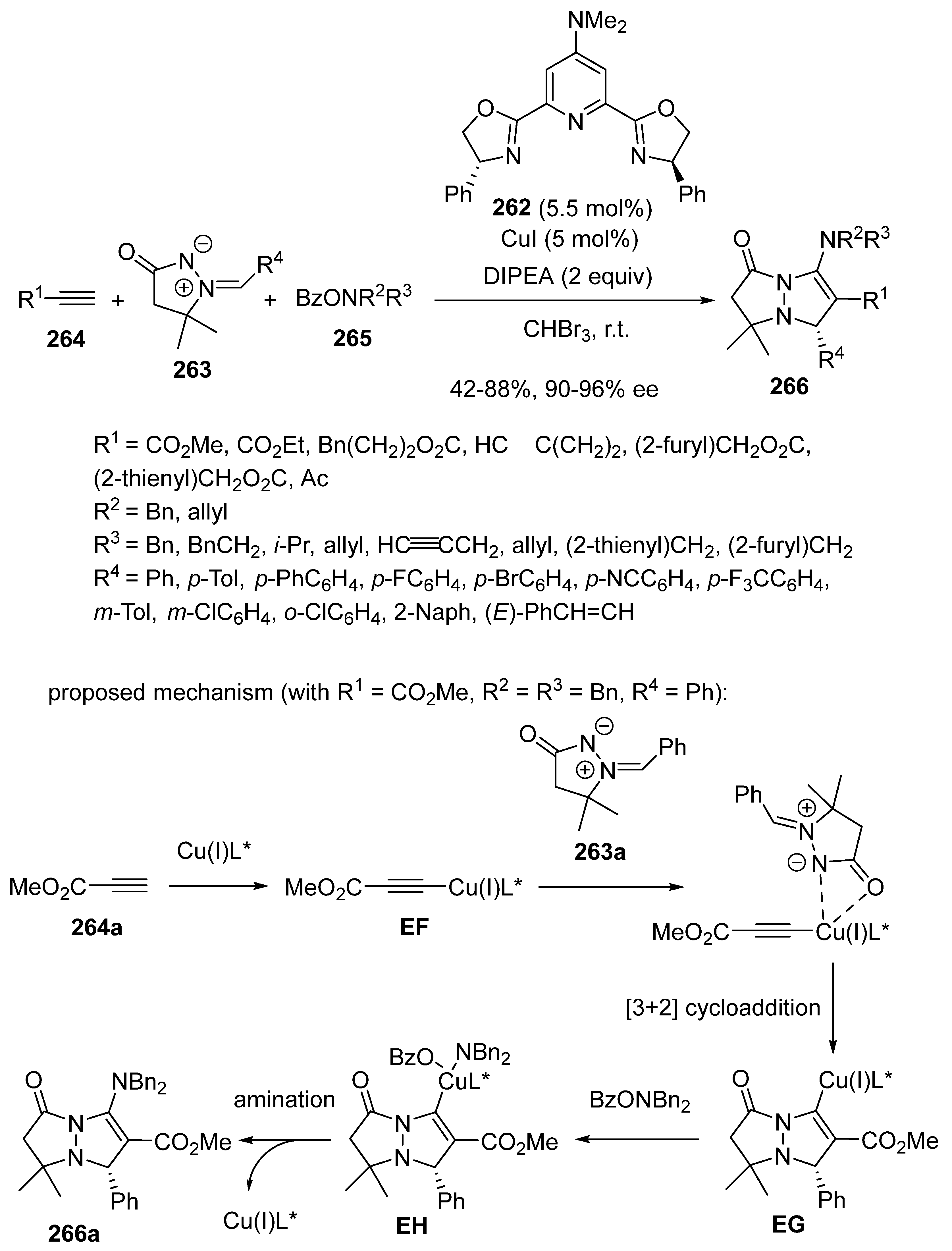
2.6. Mannich-Initiated Domino Reactions
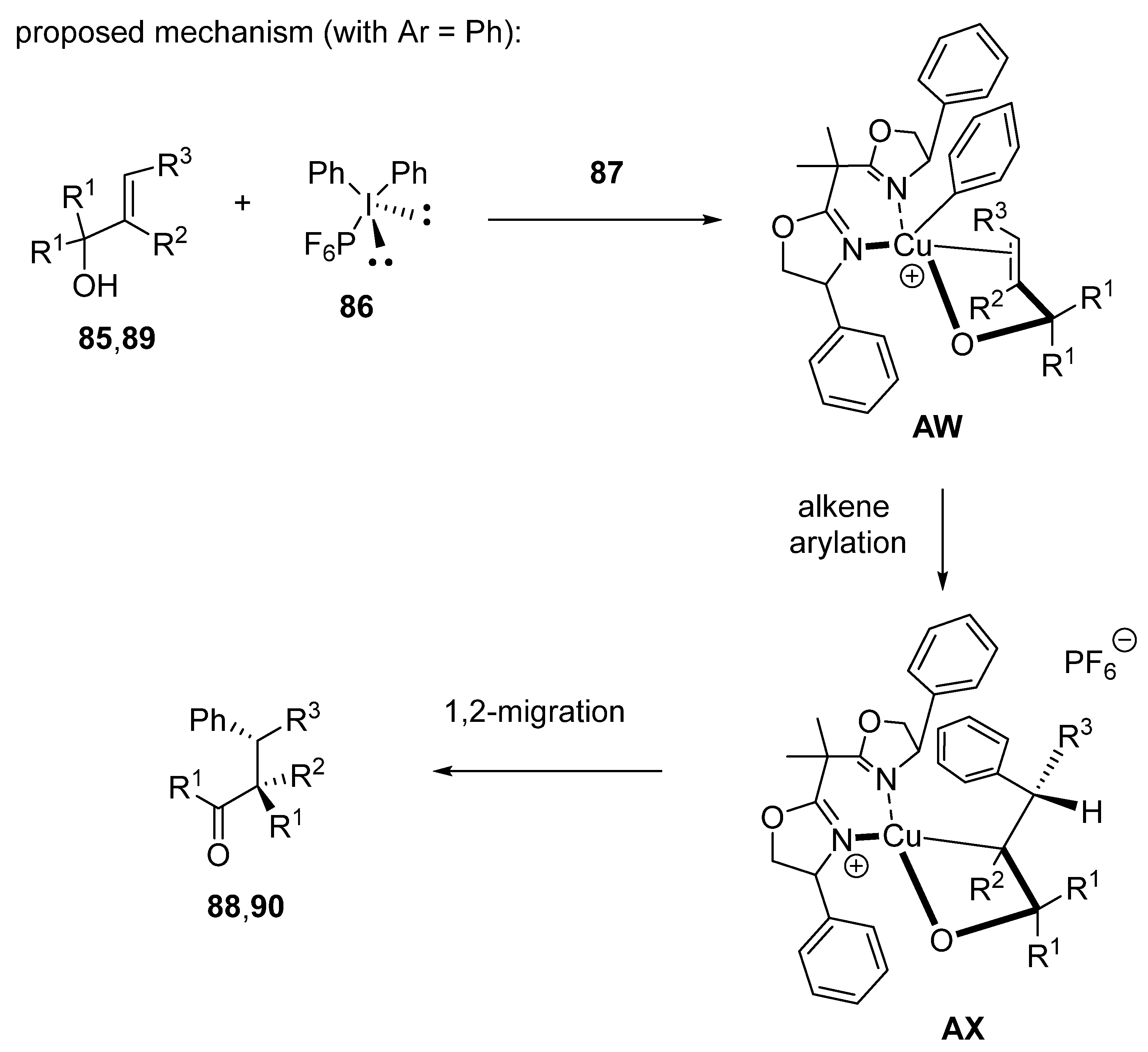
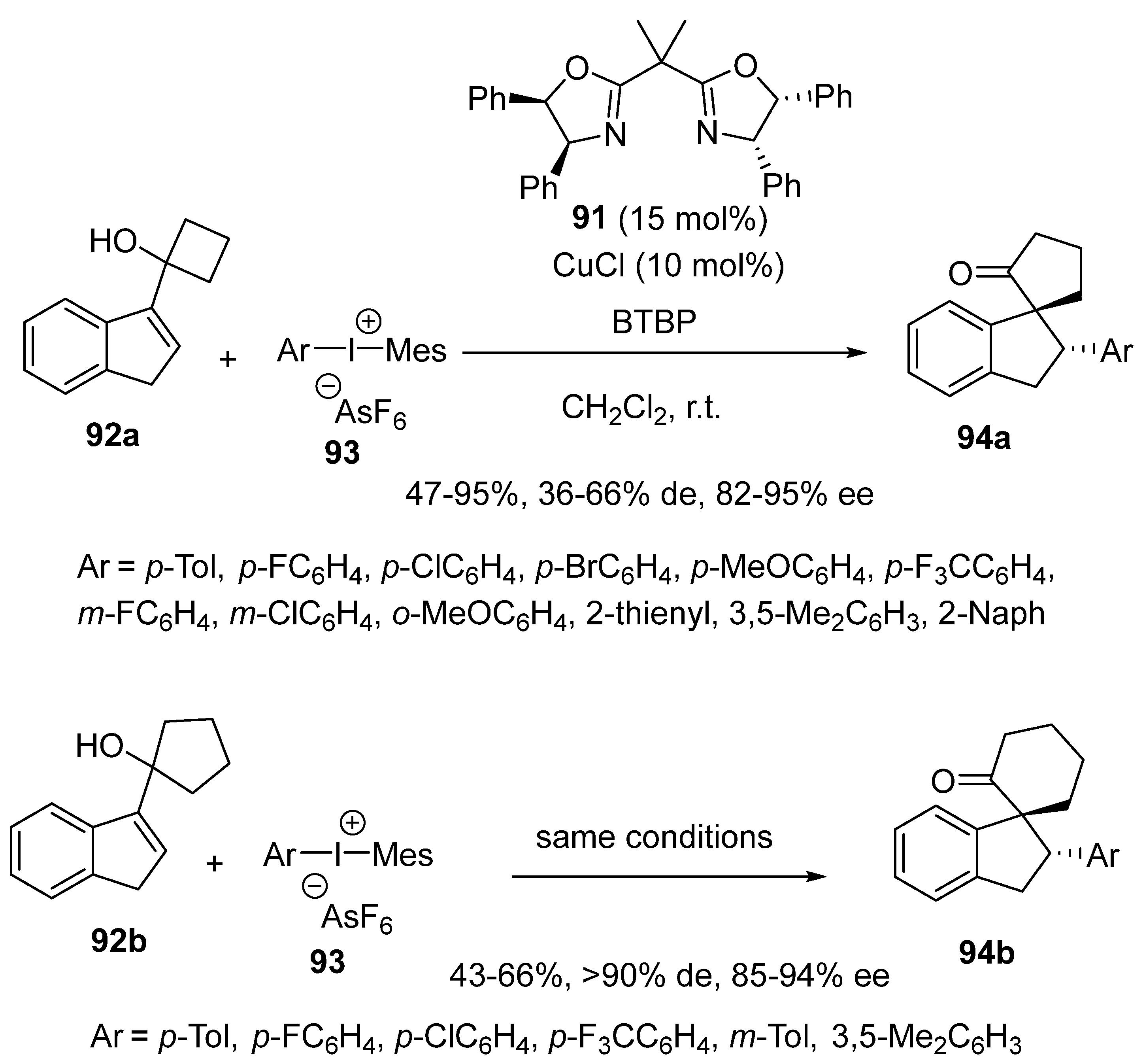
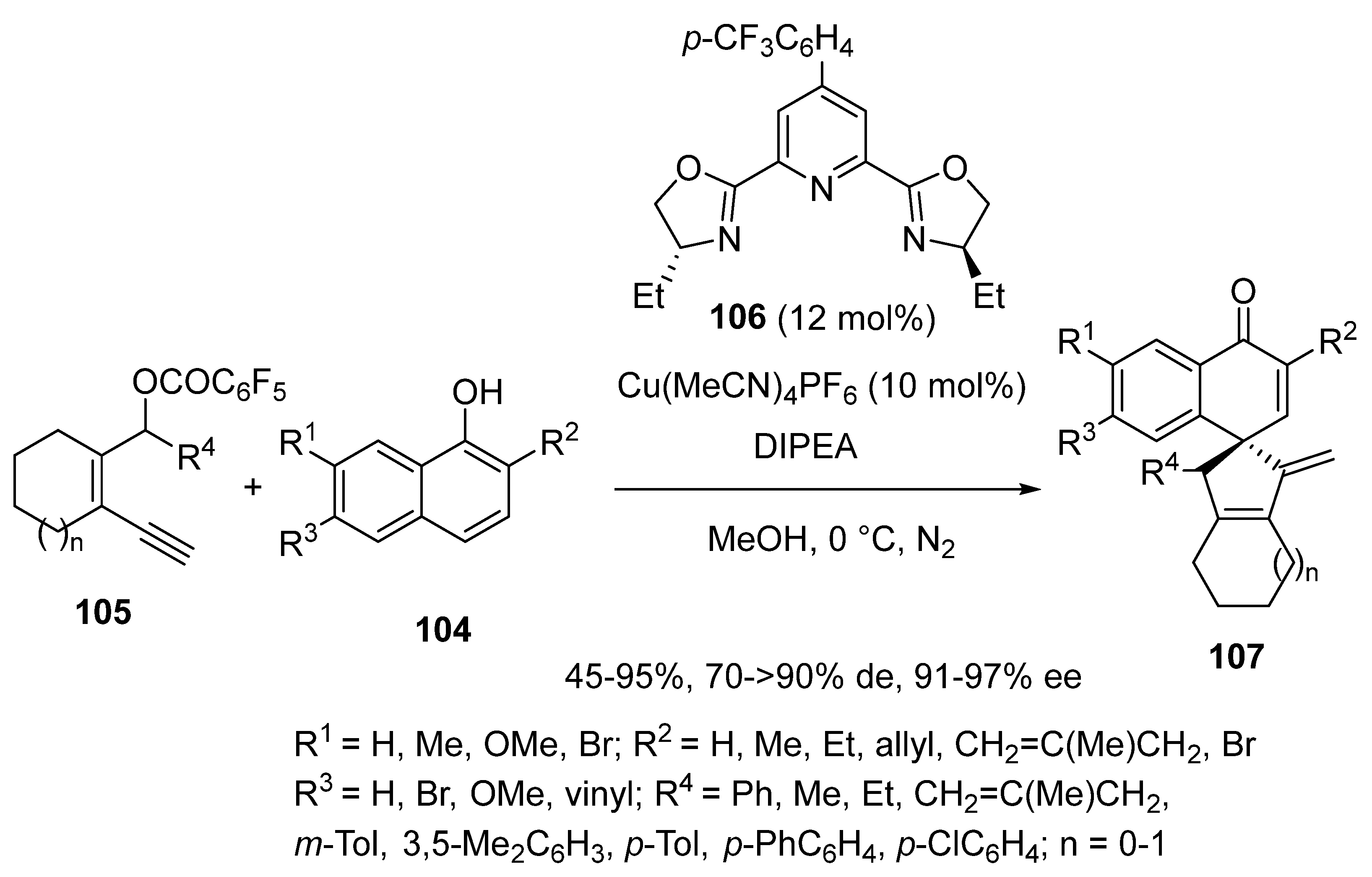
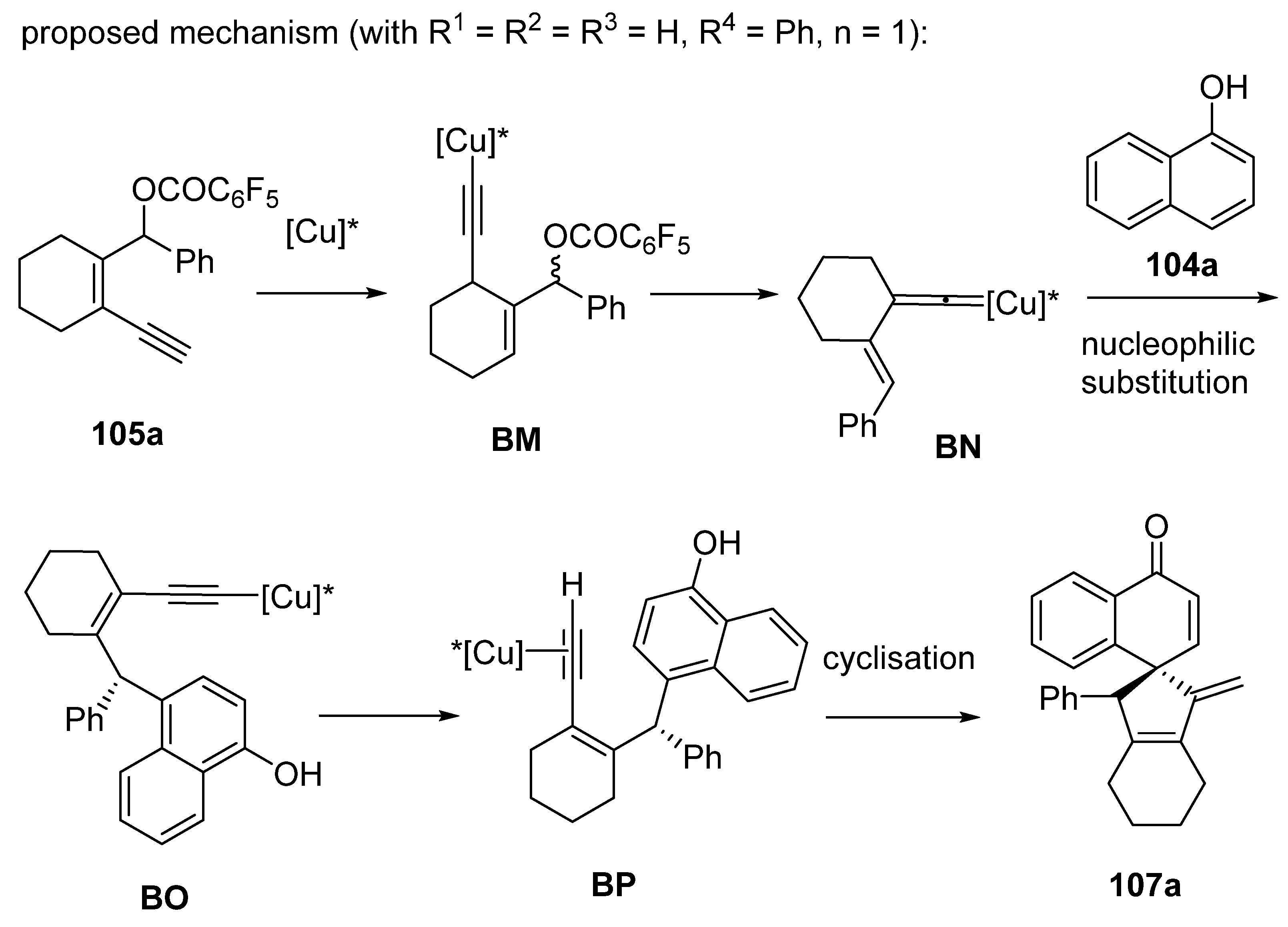
2.7. Arylation-Initiated Domino Reactions
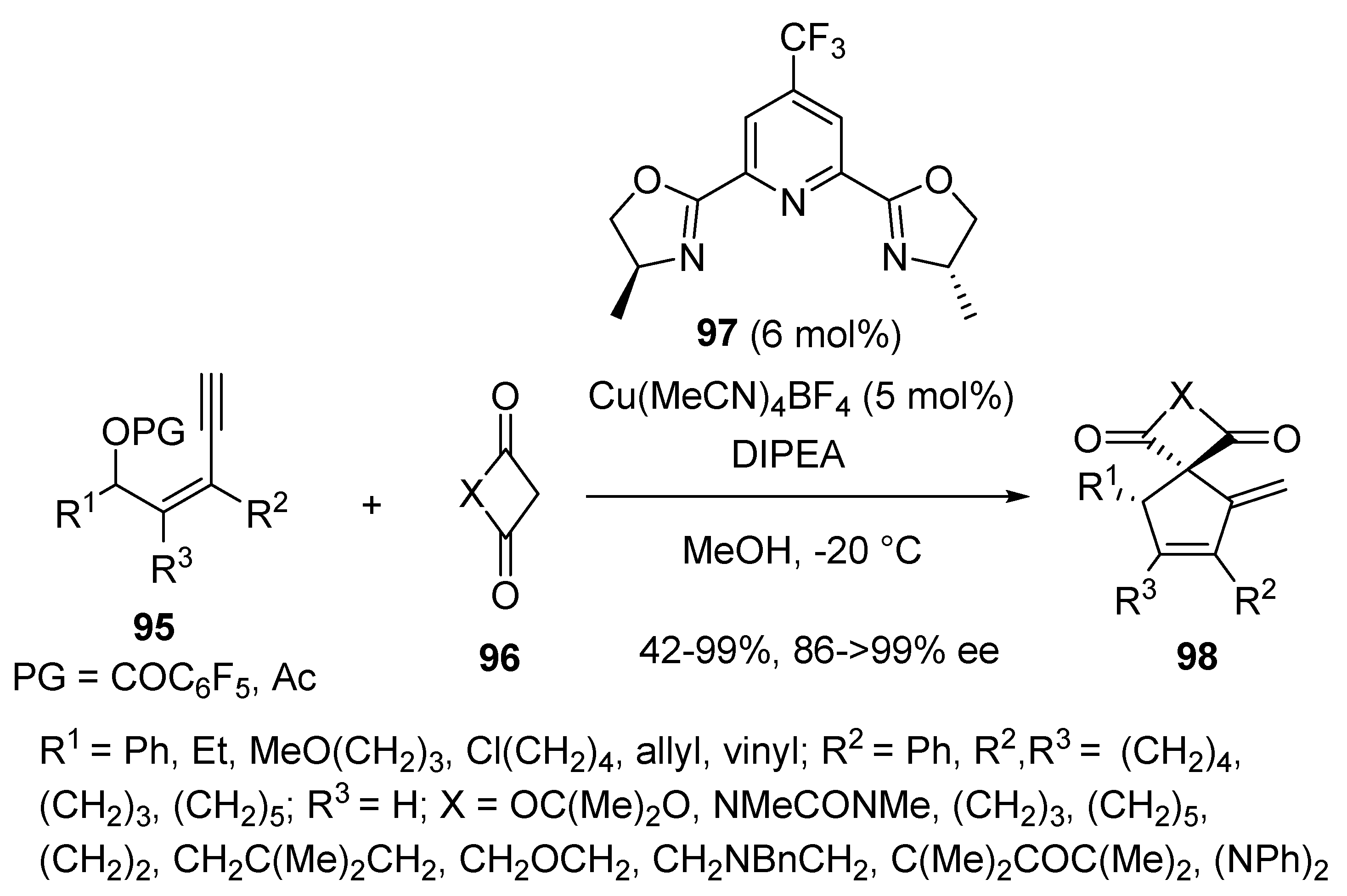
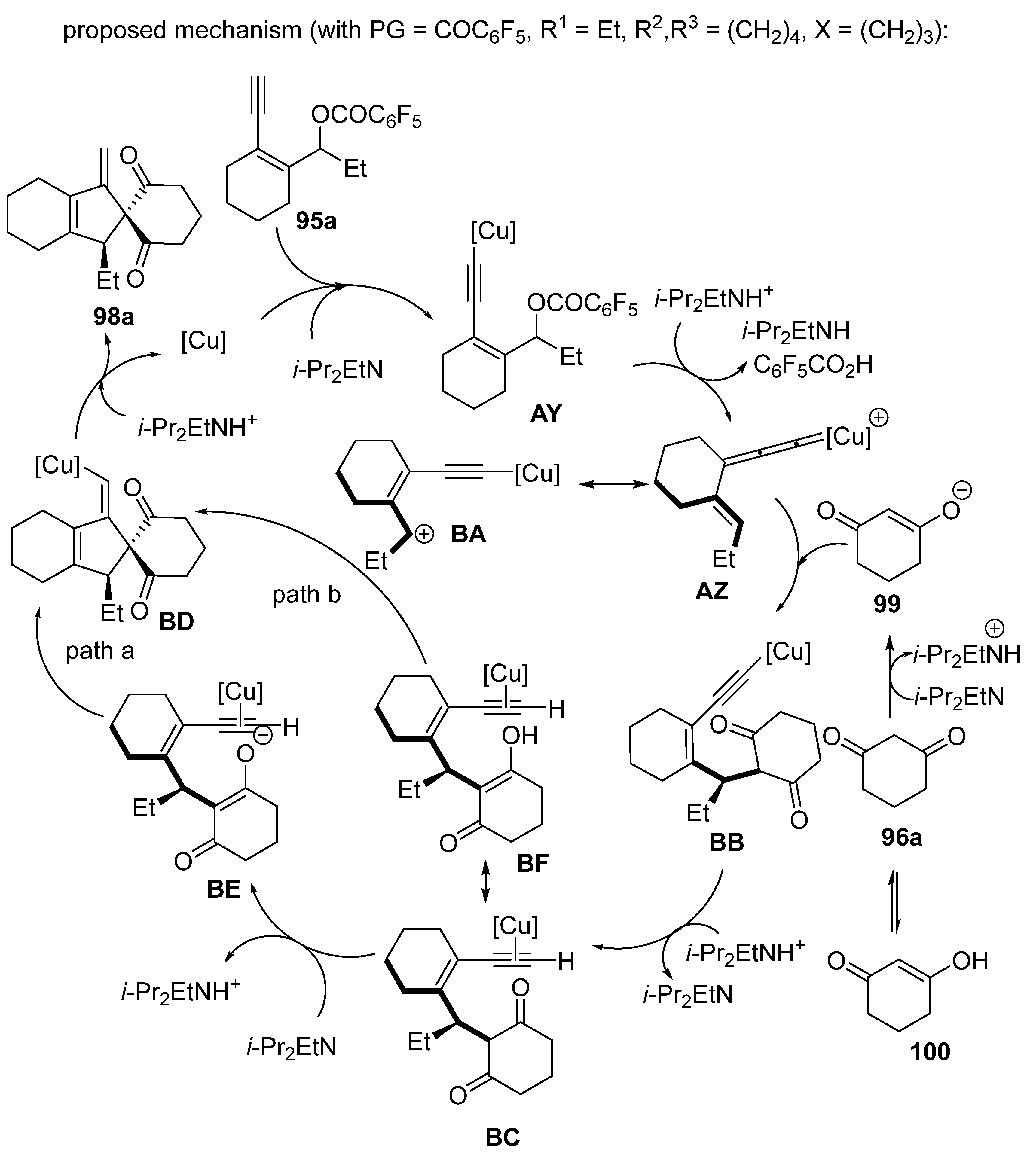
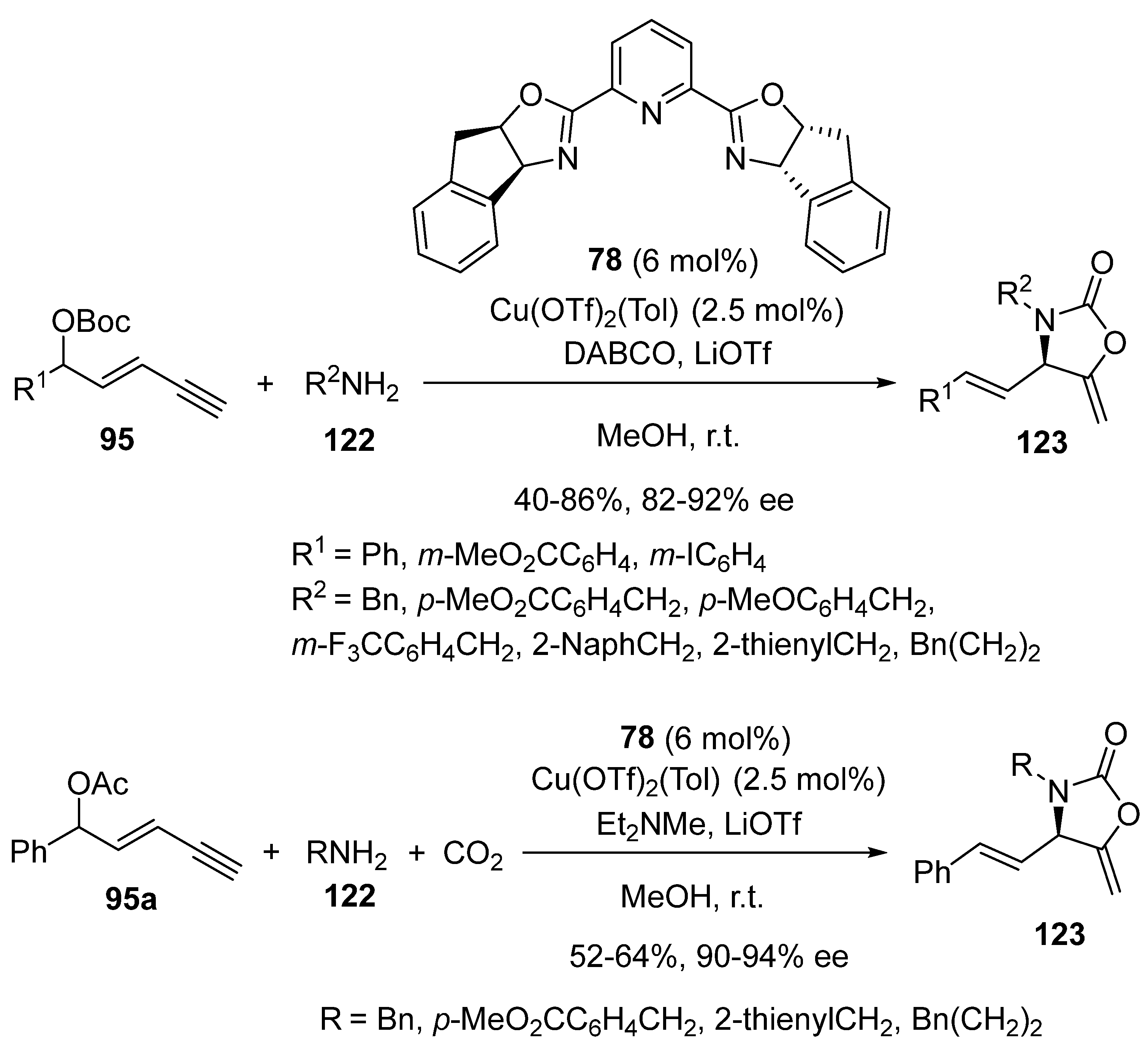
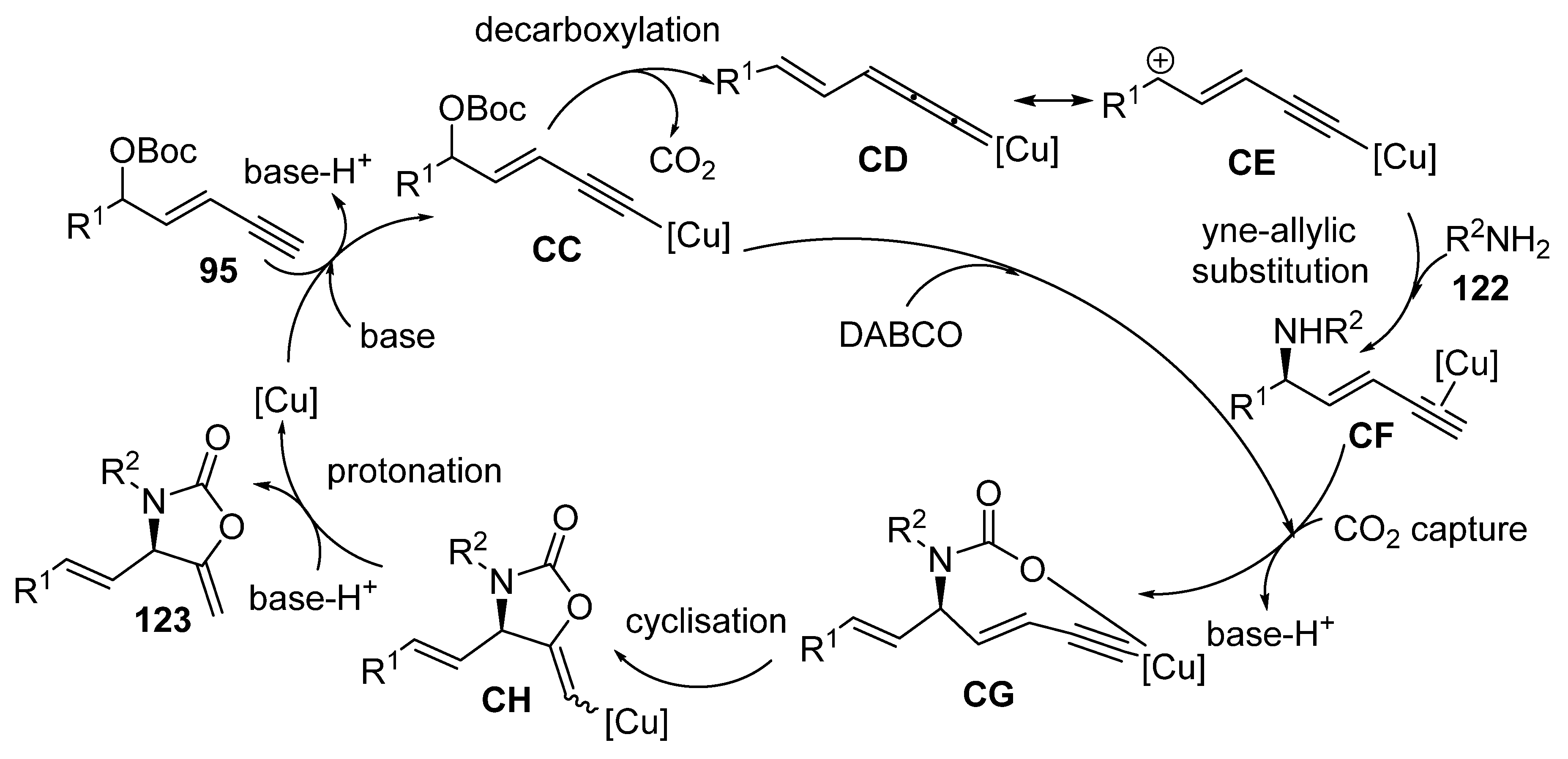
2.8. Domino Yne-Allylic Substitution/Cyclisation Reactions
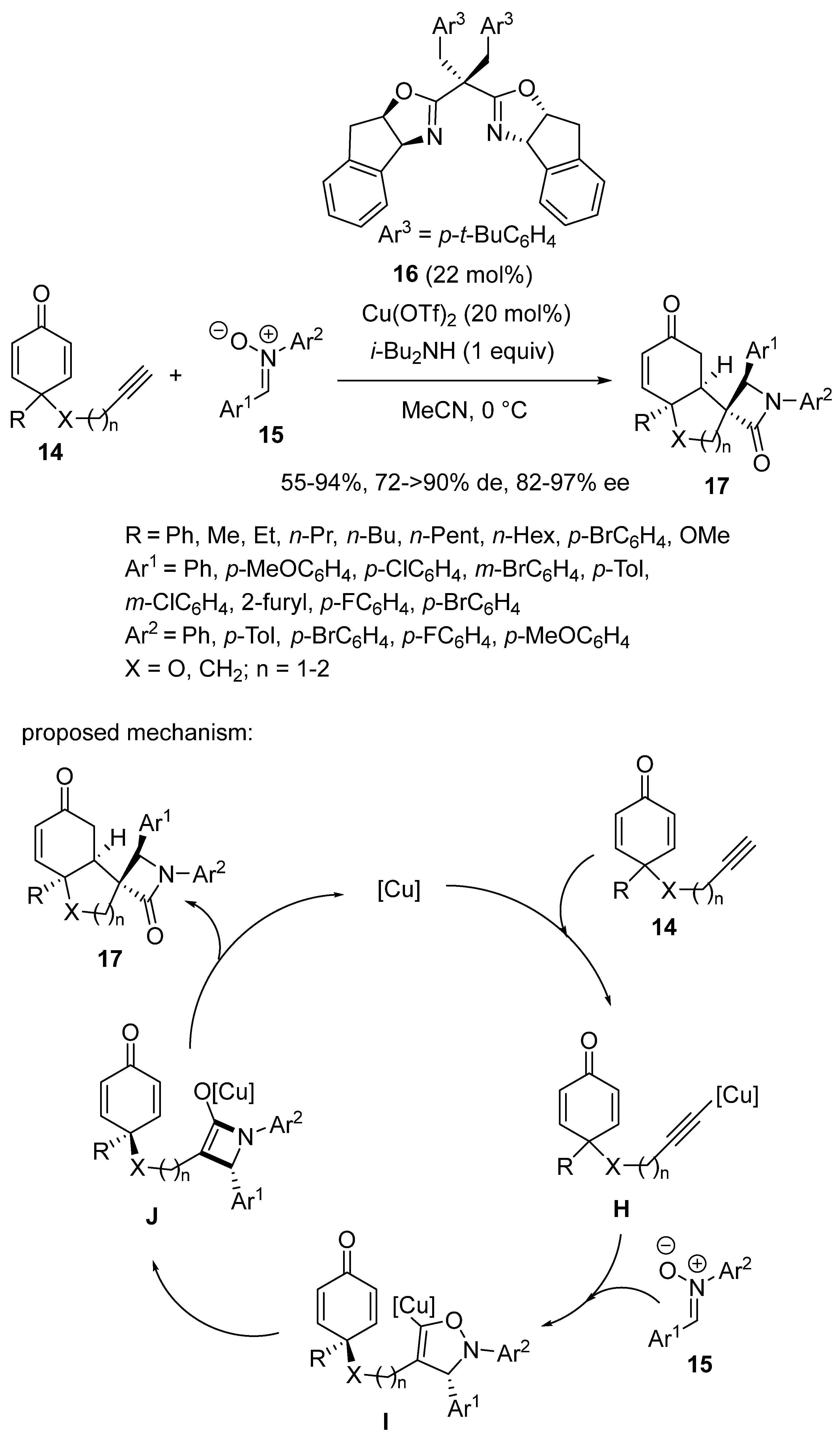
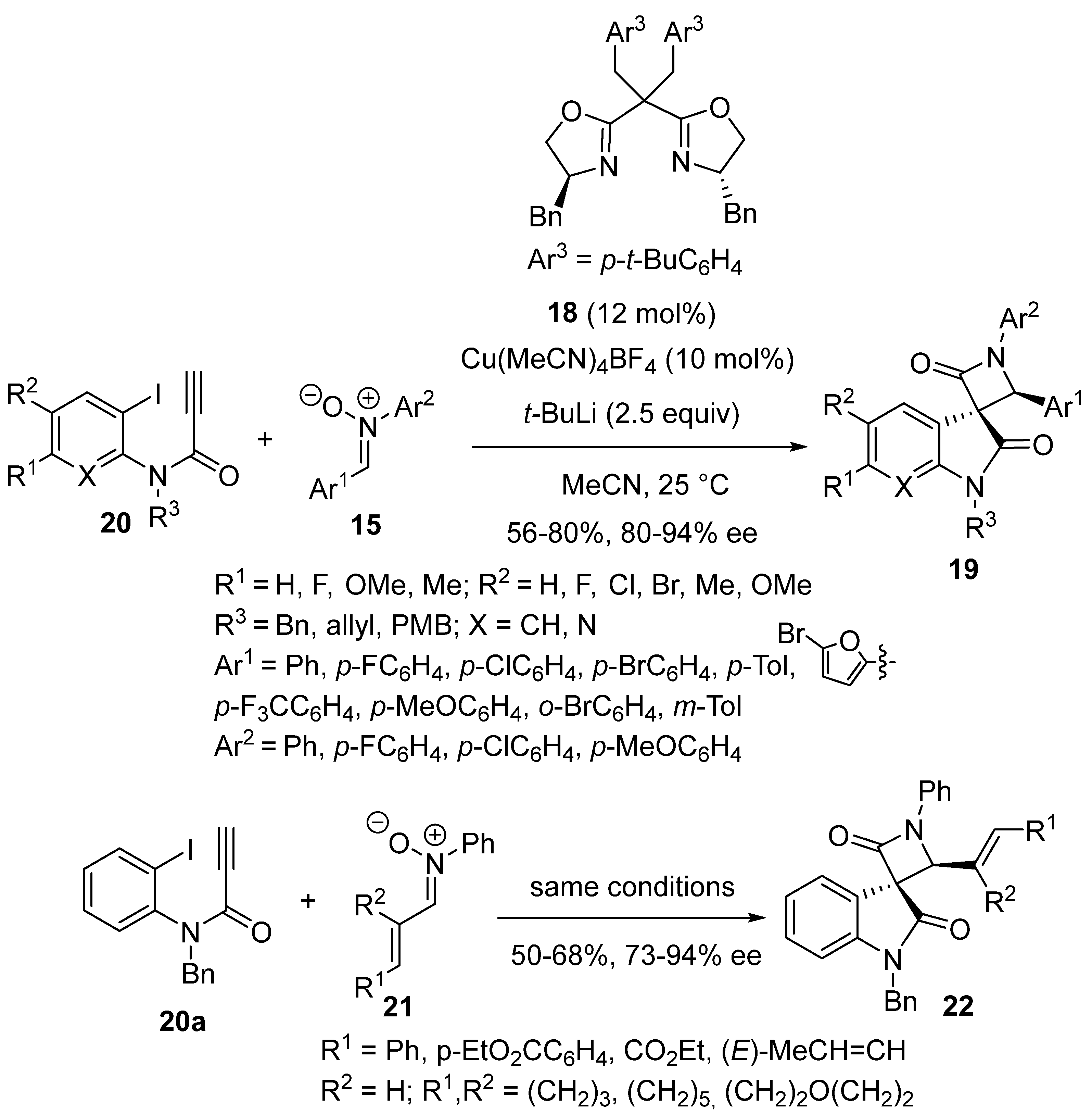
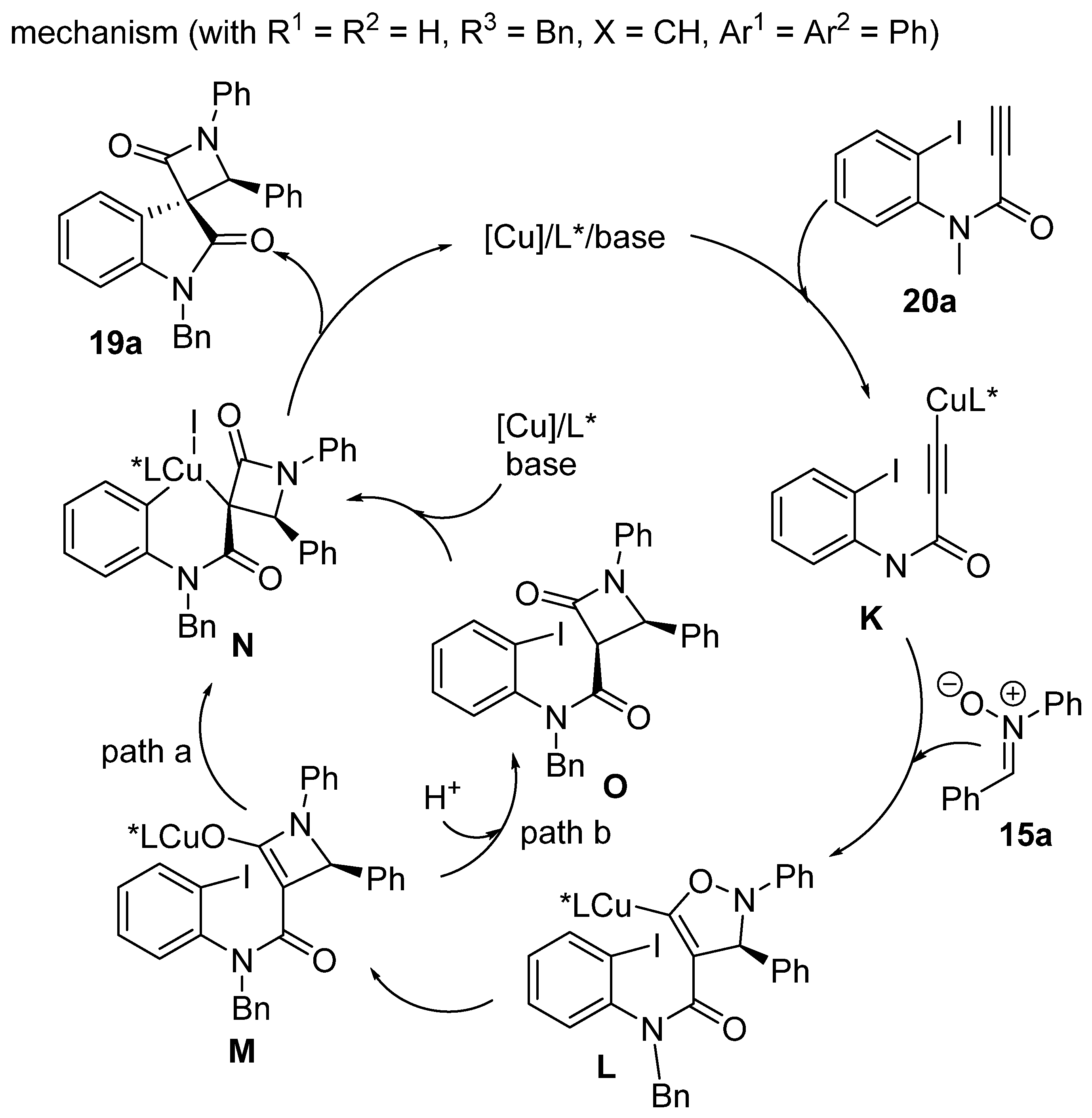
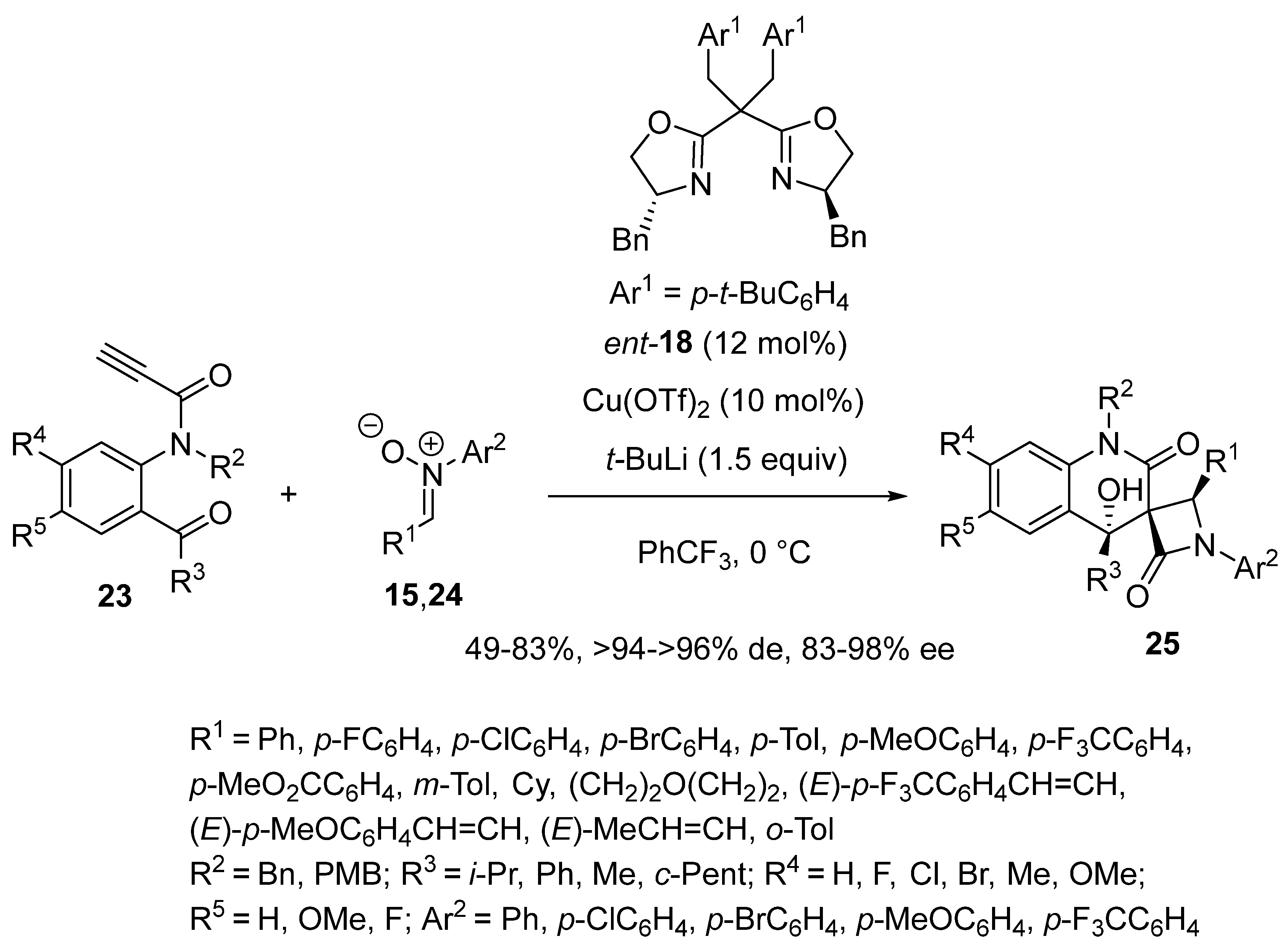
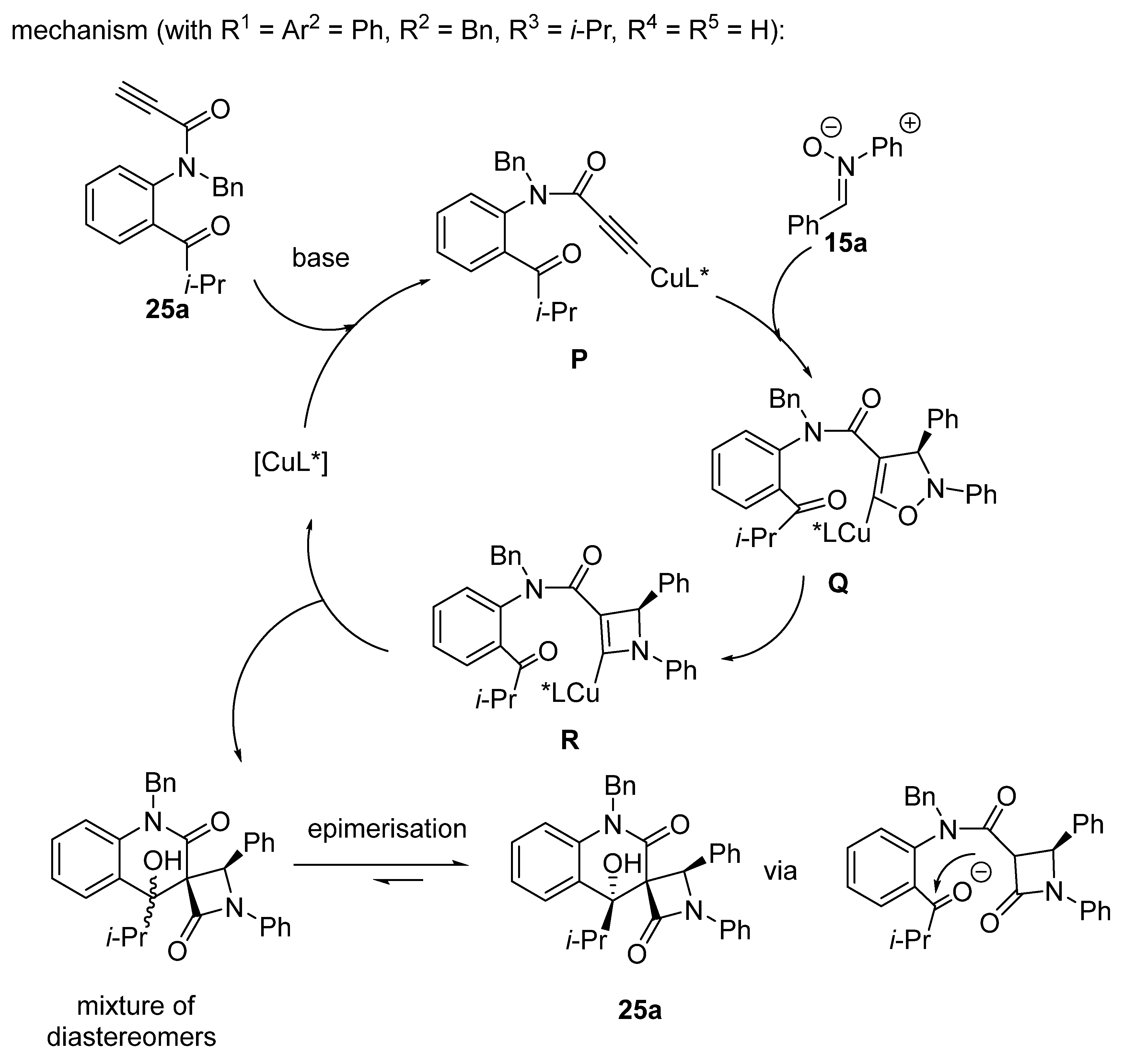
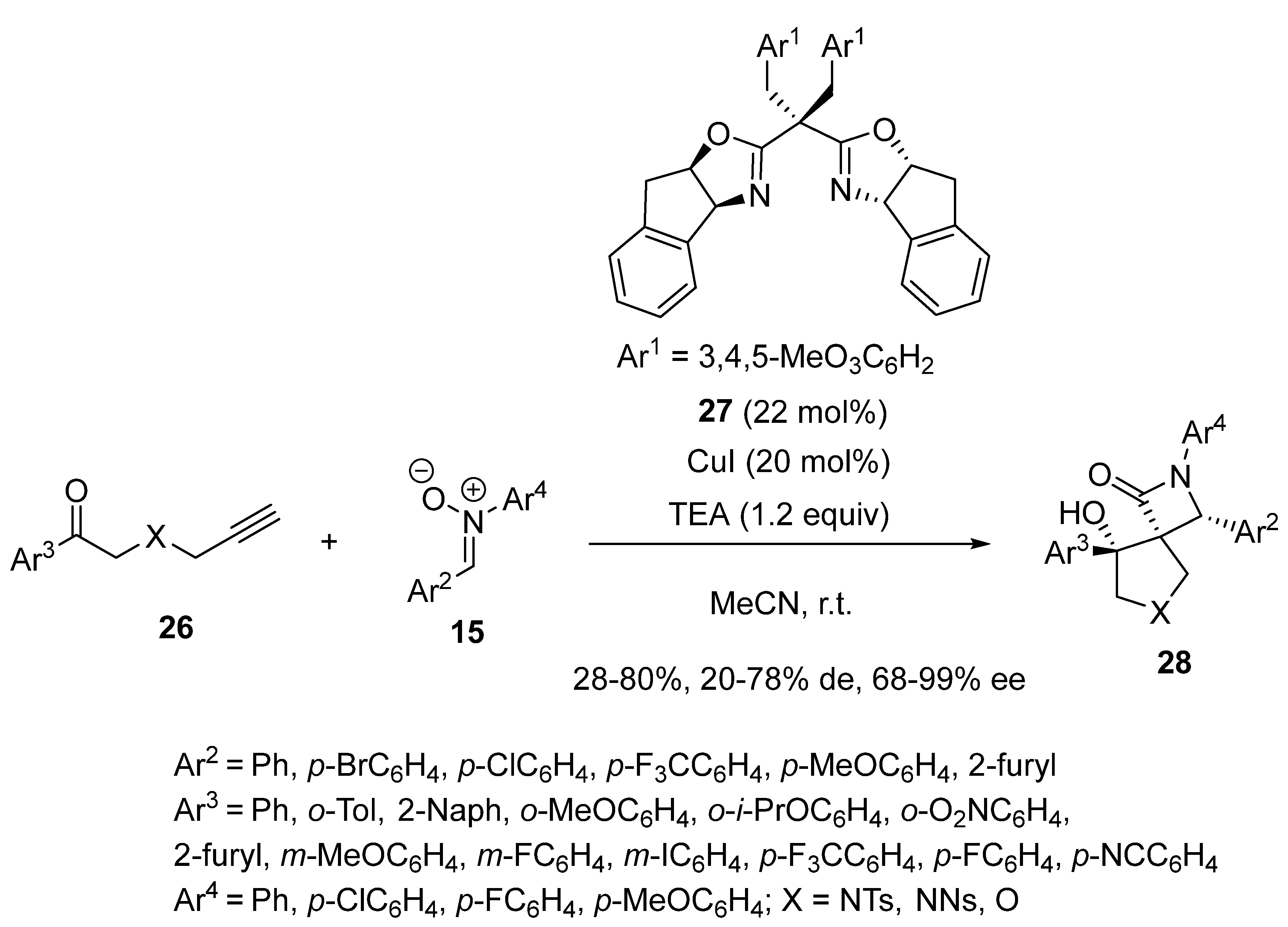
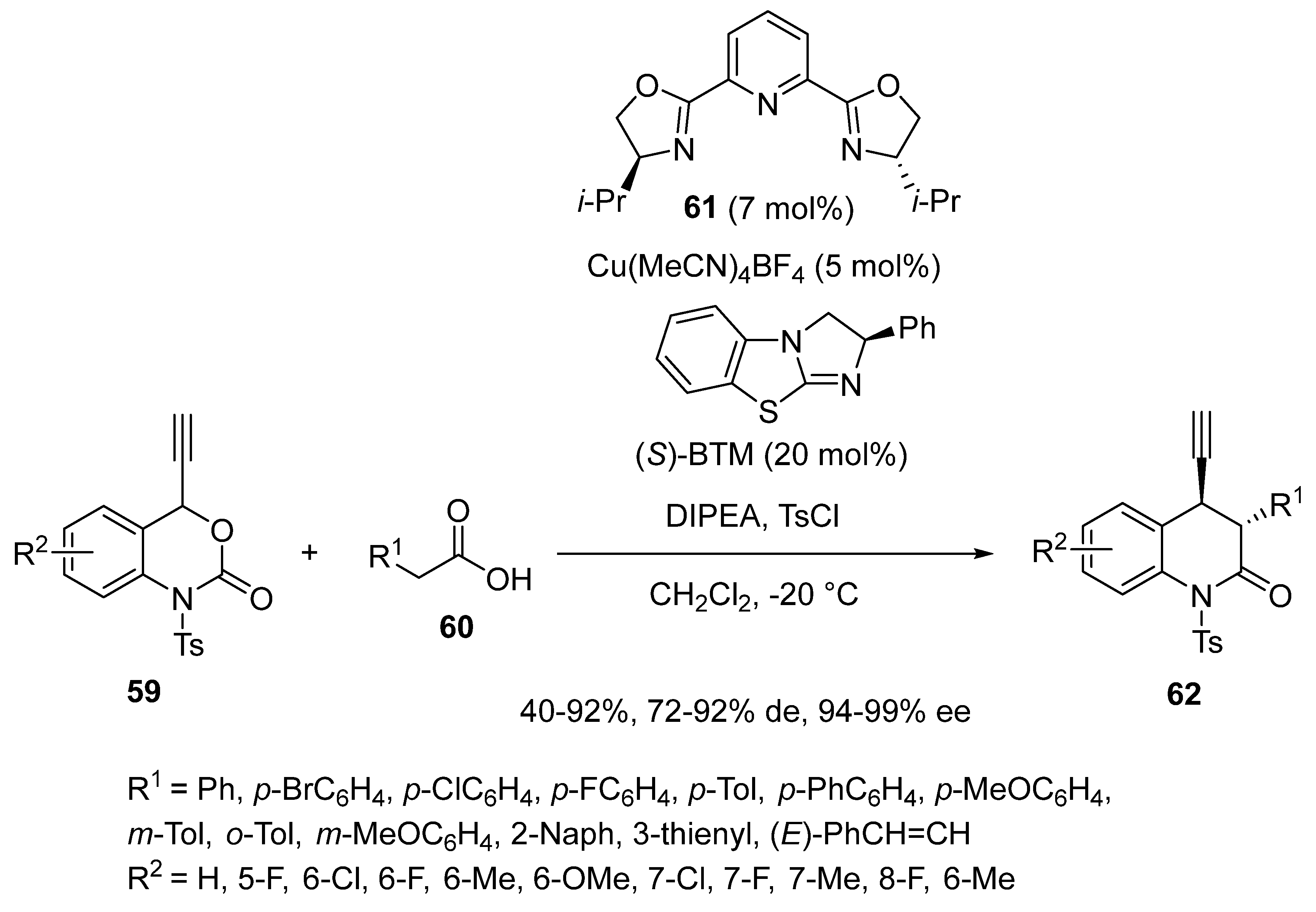
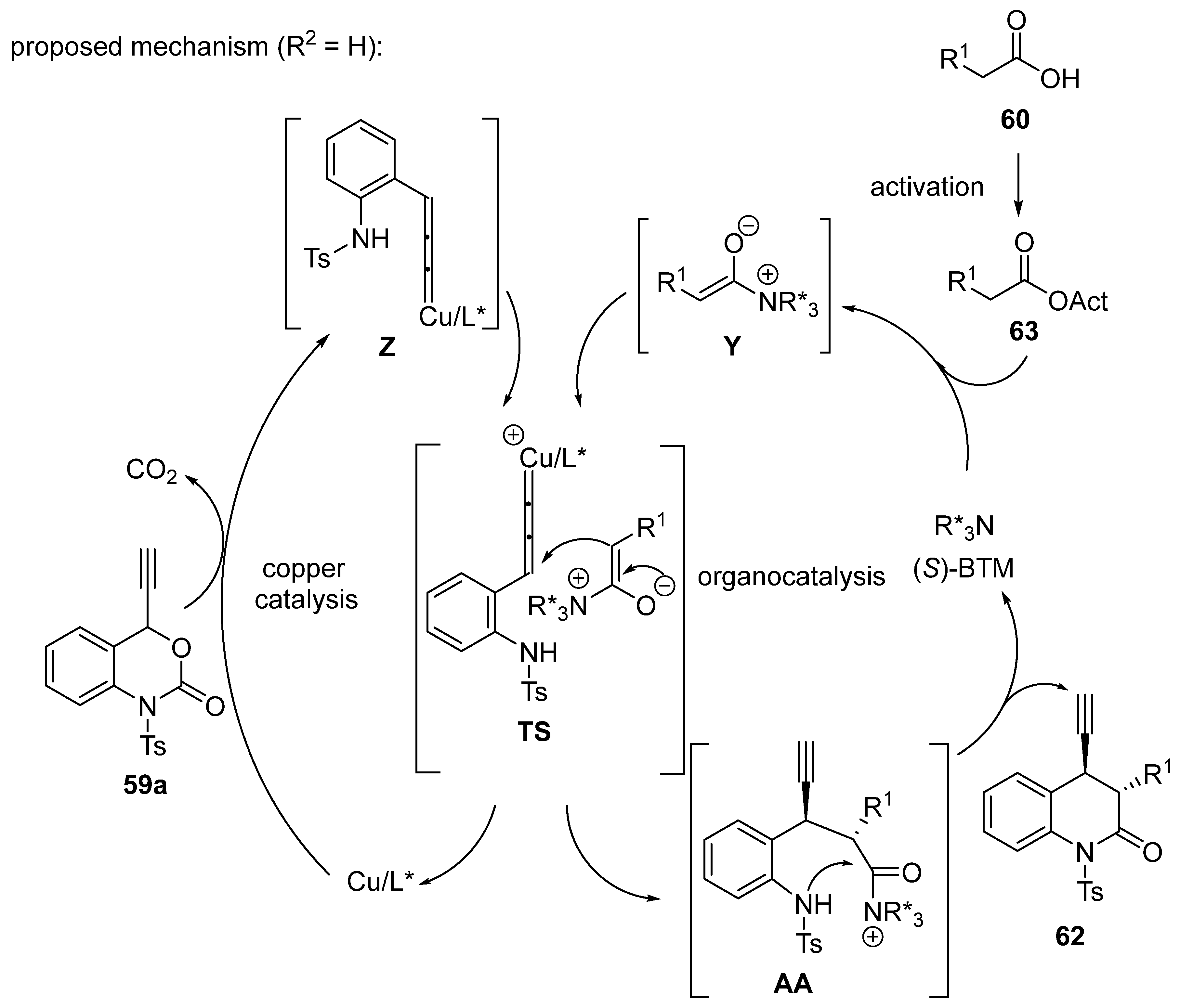
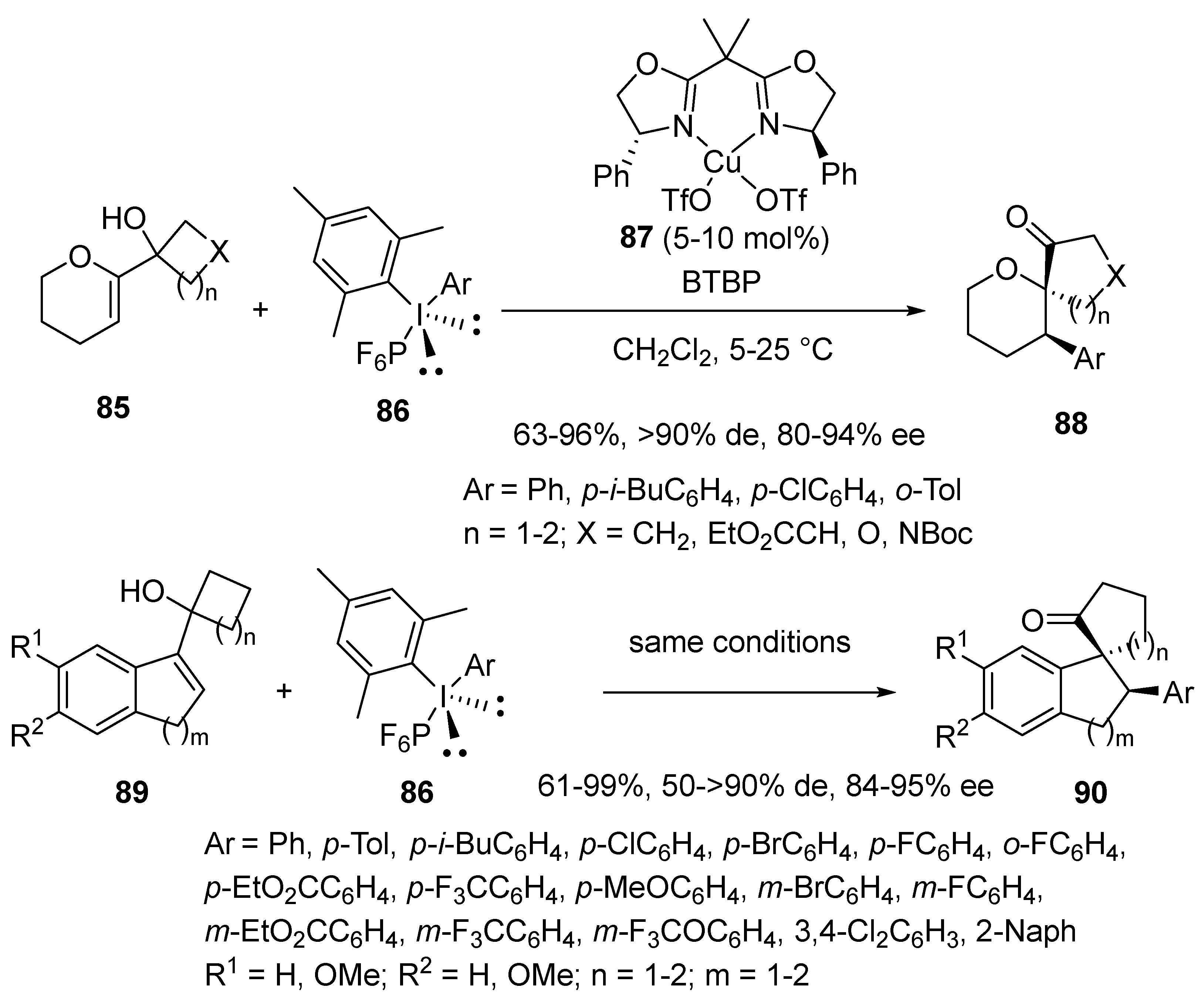
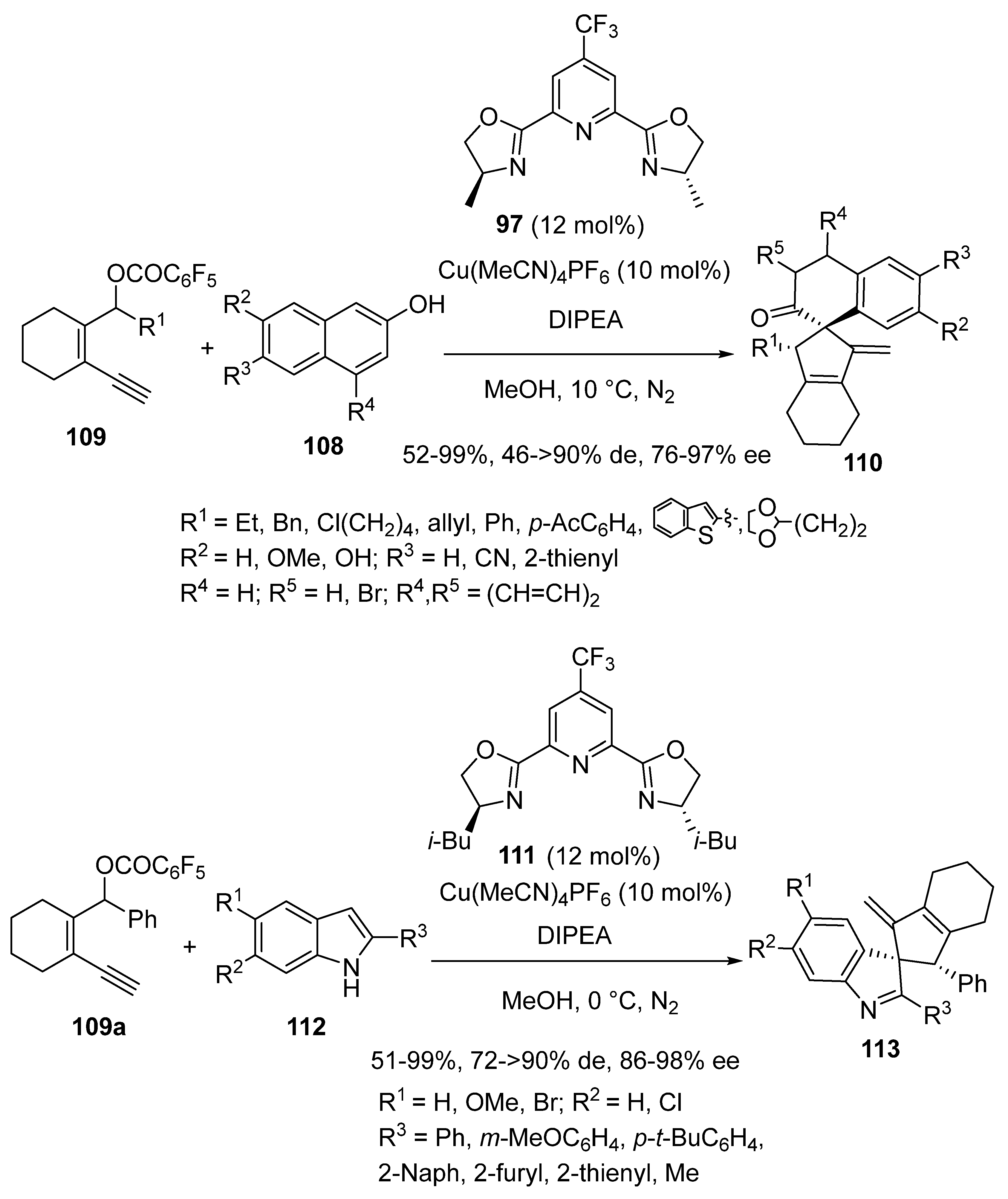
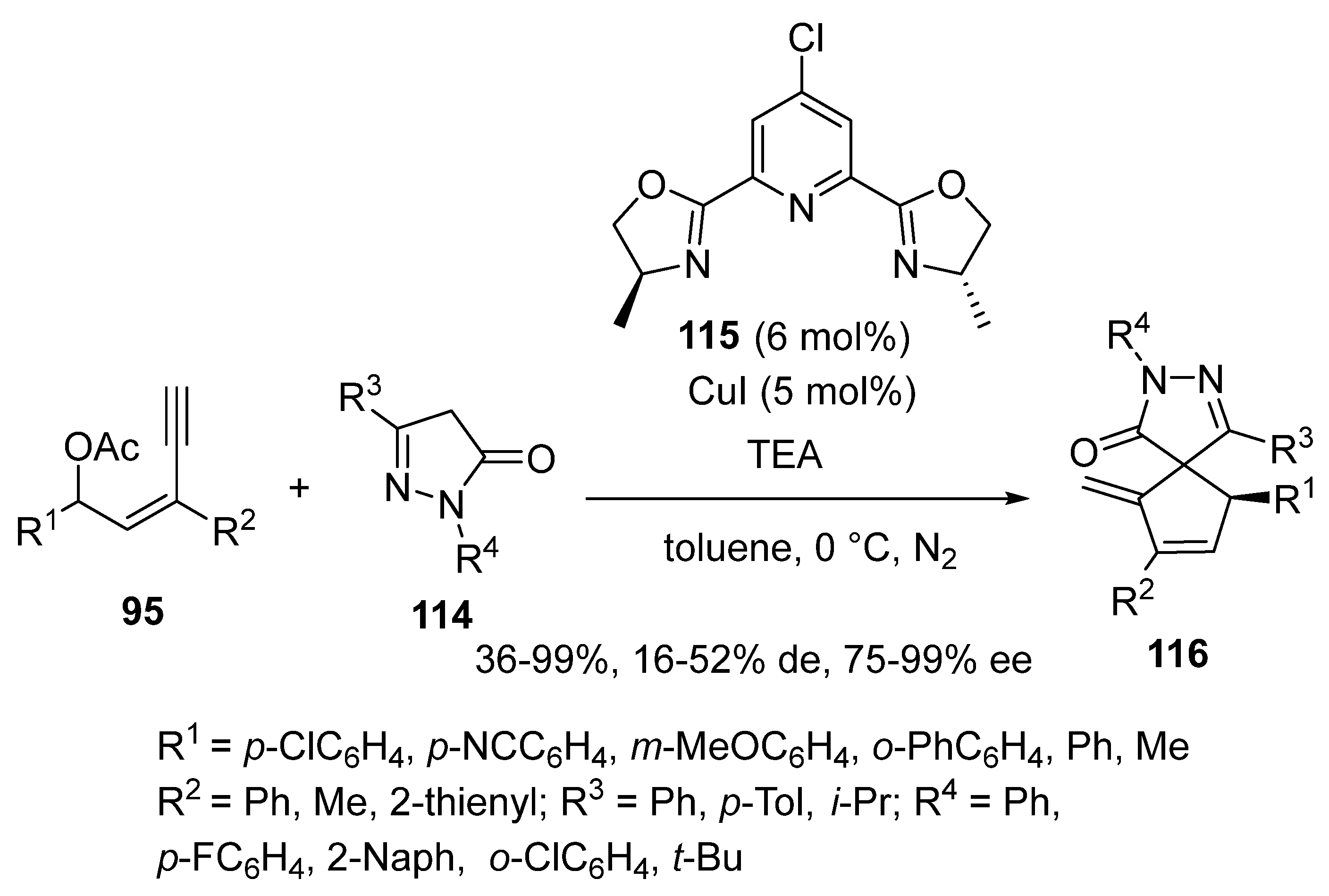
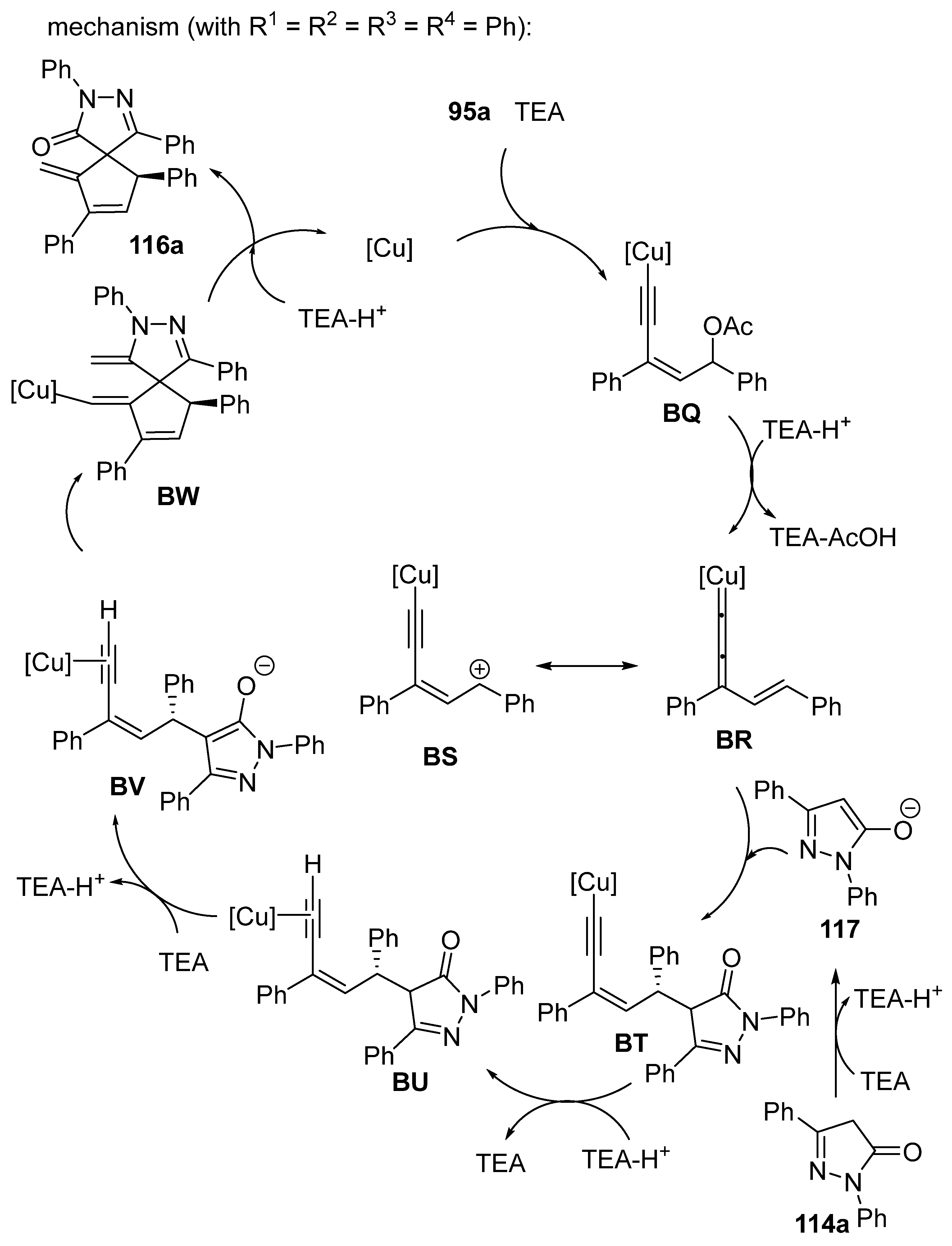
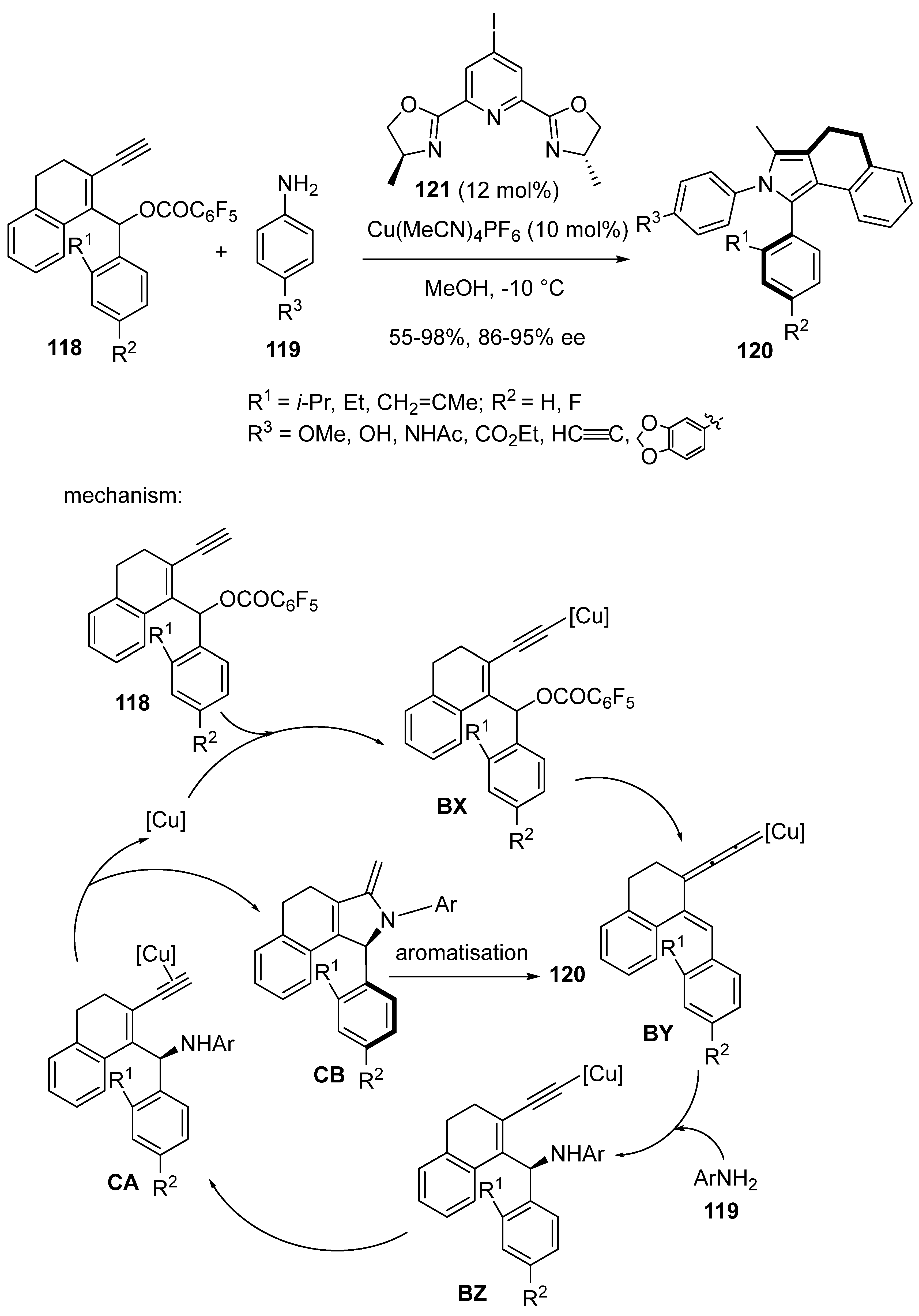
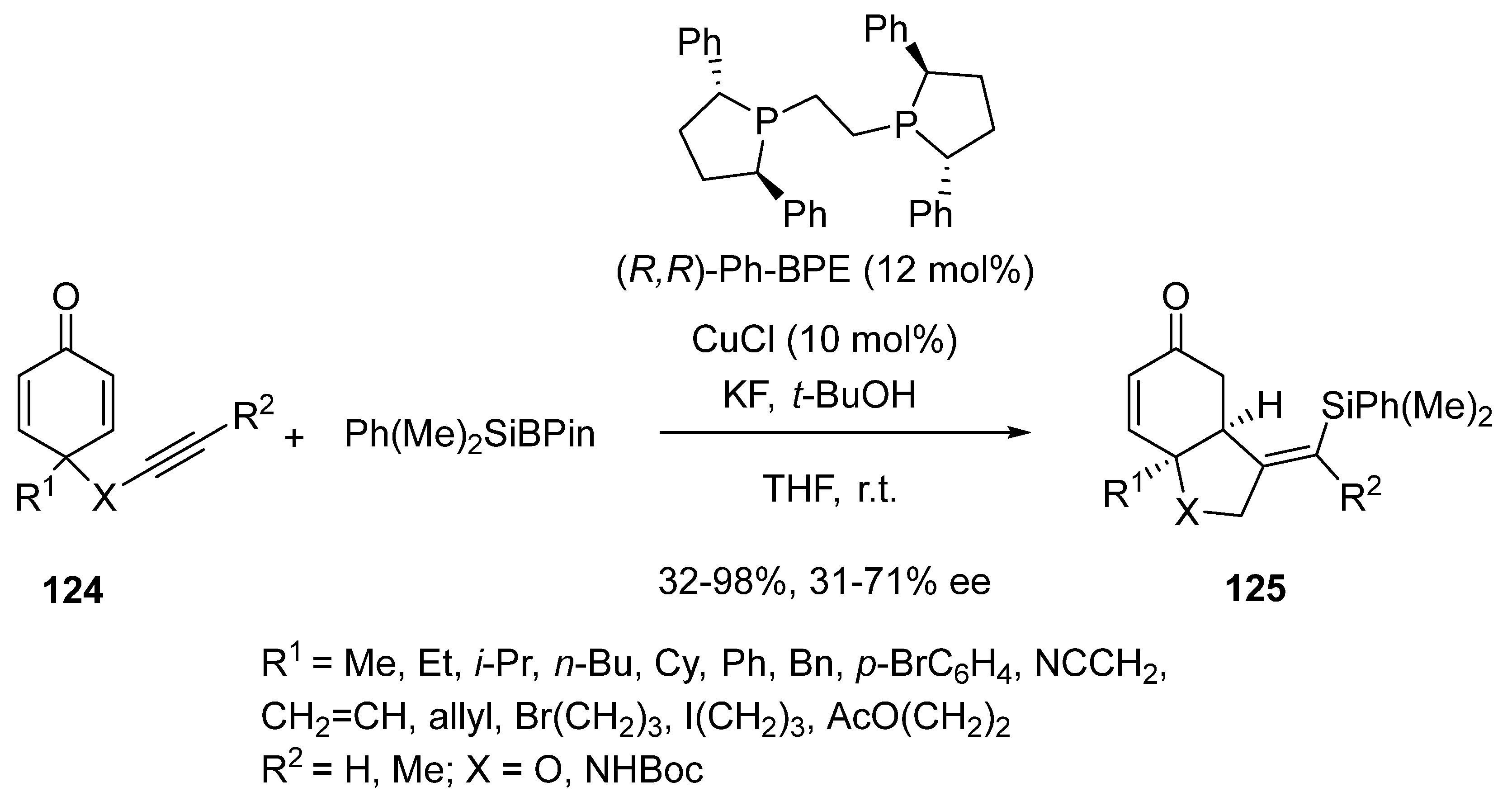
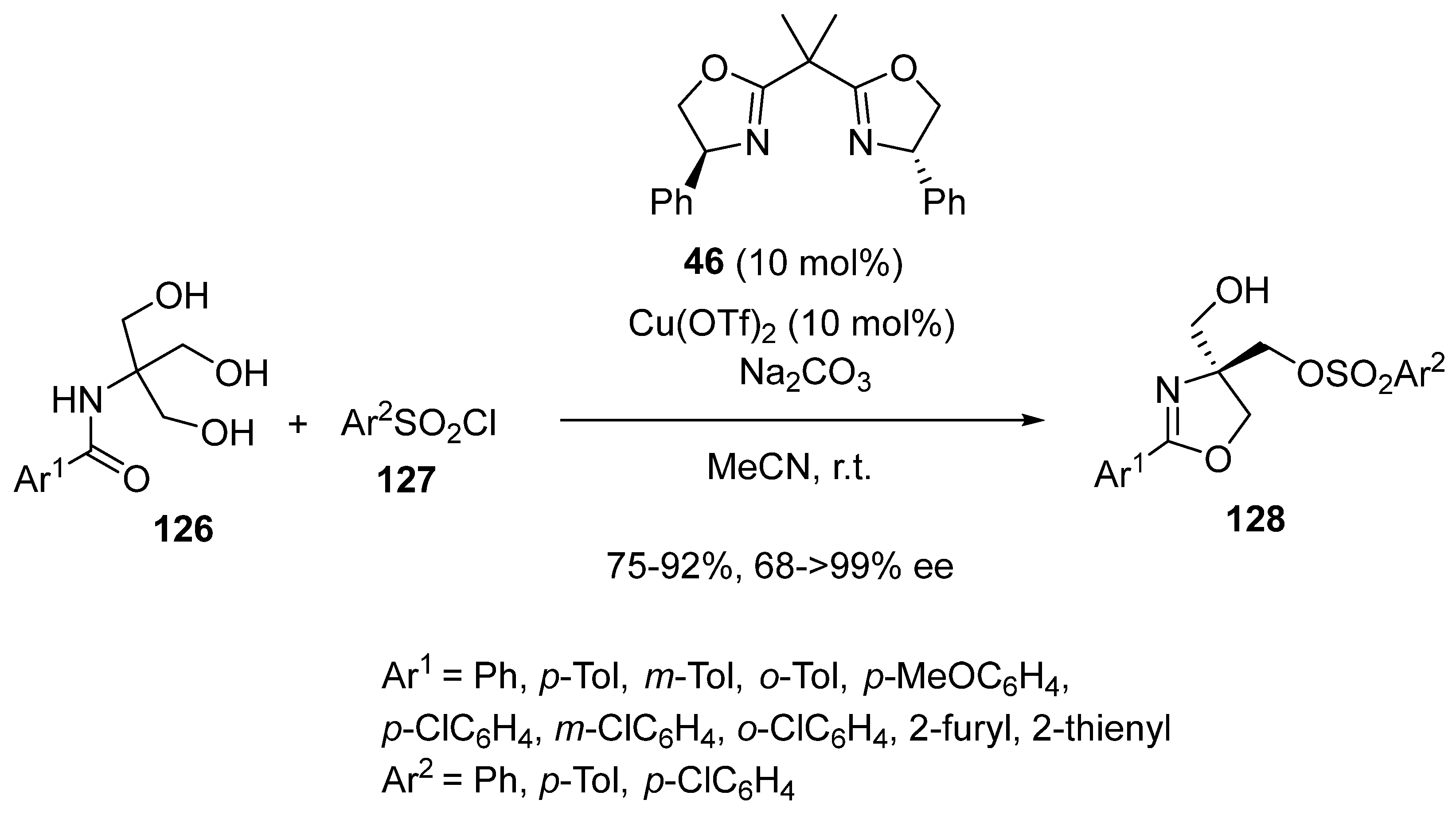
2.9. Miscellaneous Domino Reactions
2.9.1. Single Copper Catalysis
2.9.2. Multicatalysis
3. Multicomponent Domino Reactions
3.1. Michael-Initiated Multicomponent Reactions
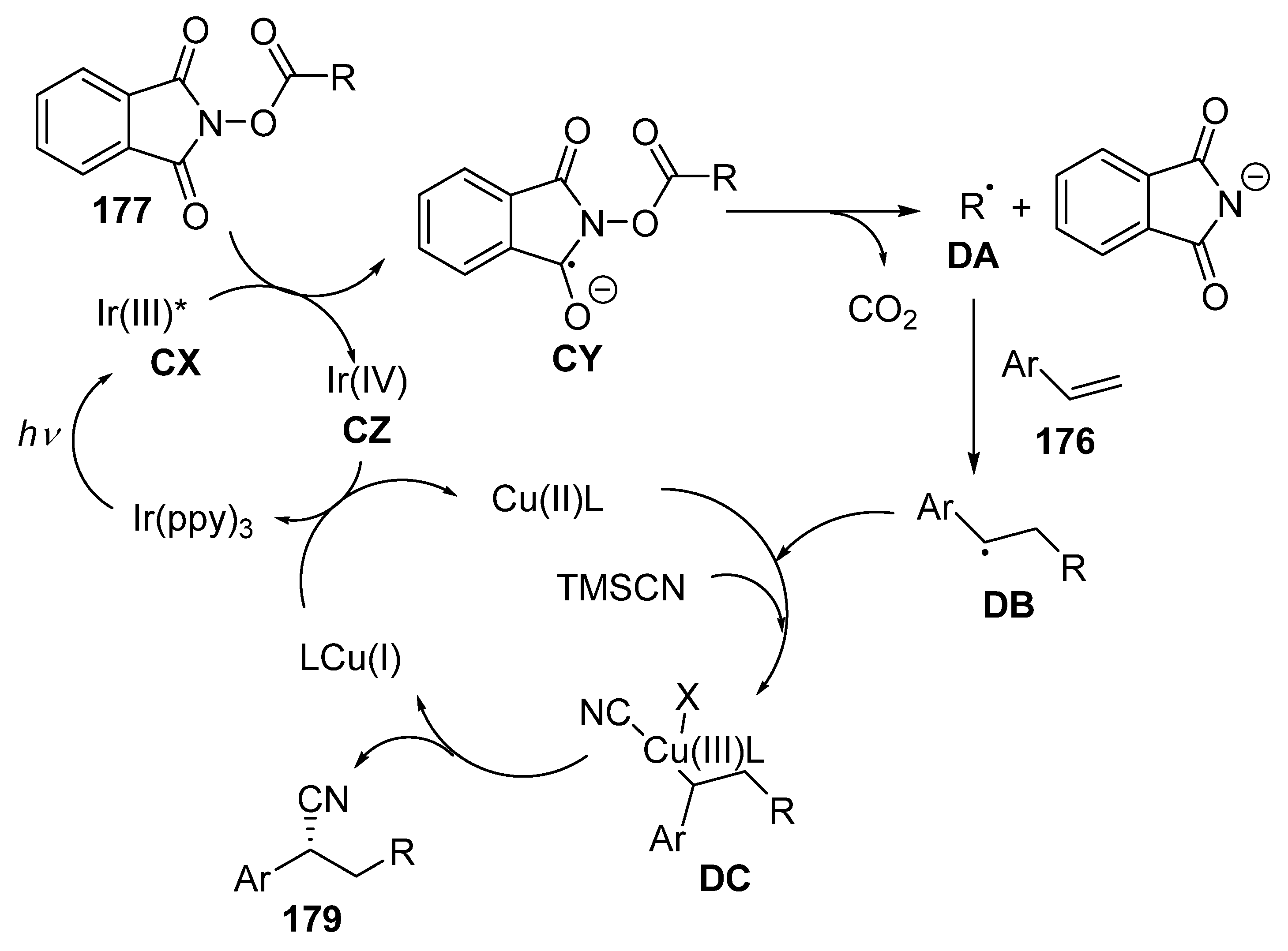
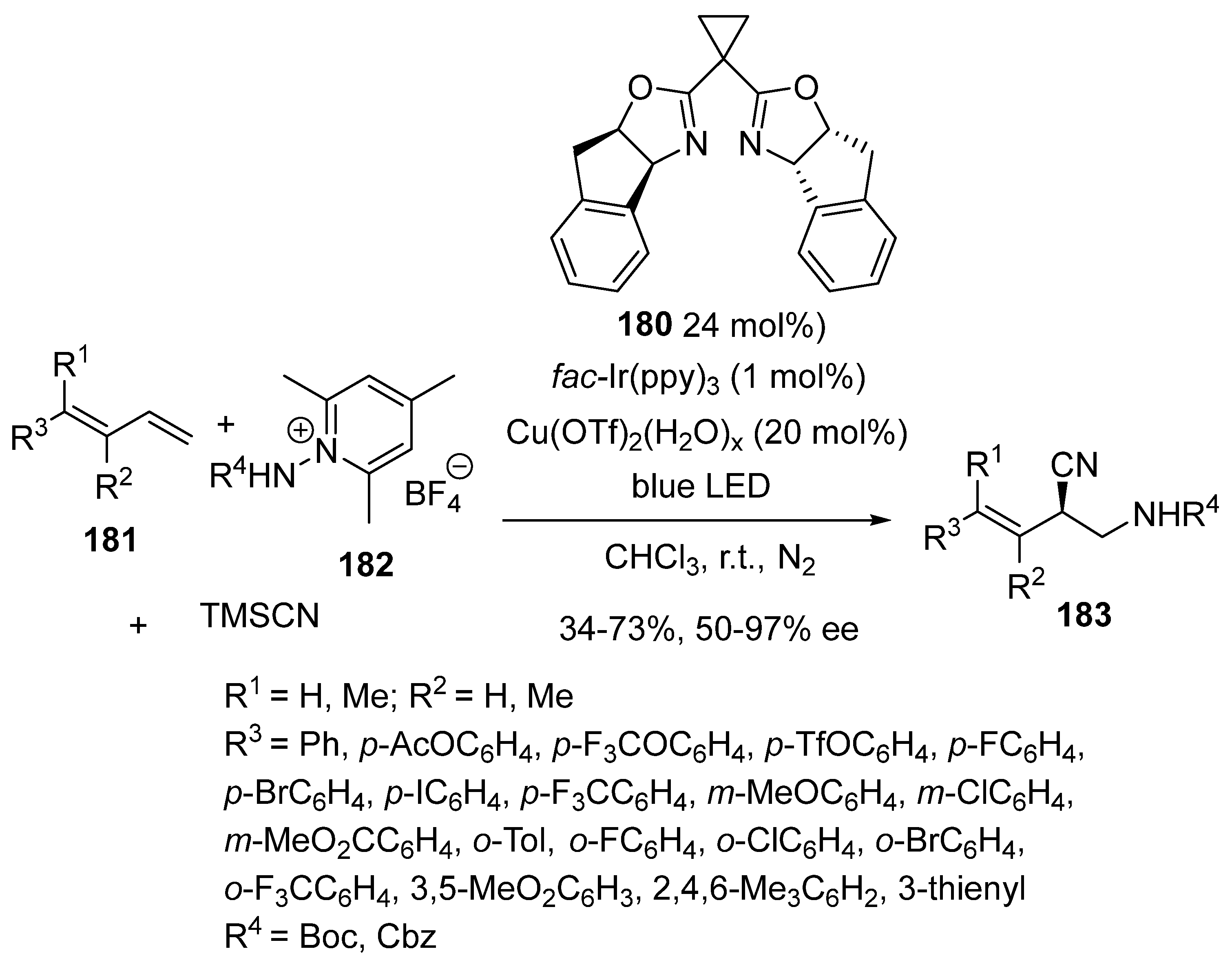
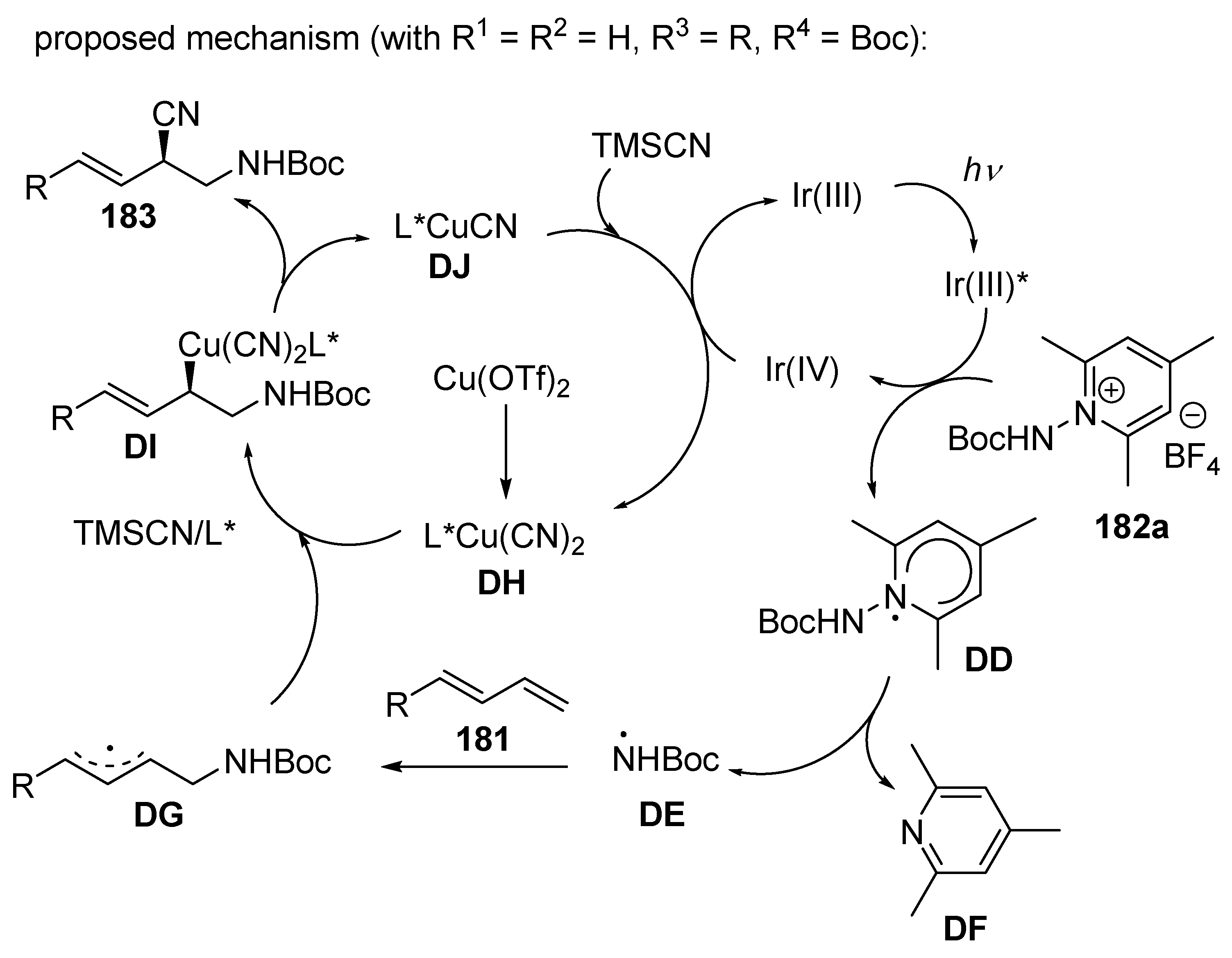
3.2. Photoredox-Catalysed Multicomponent Reactions
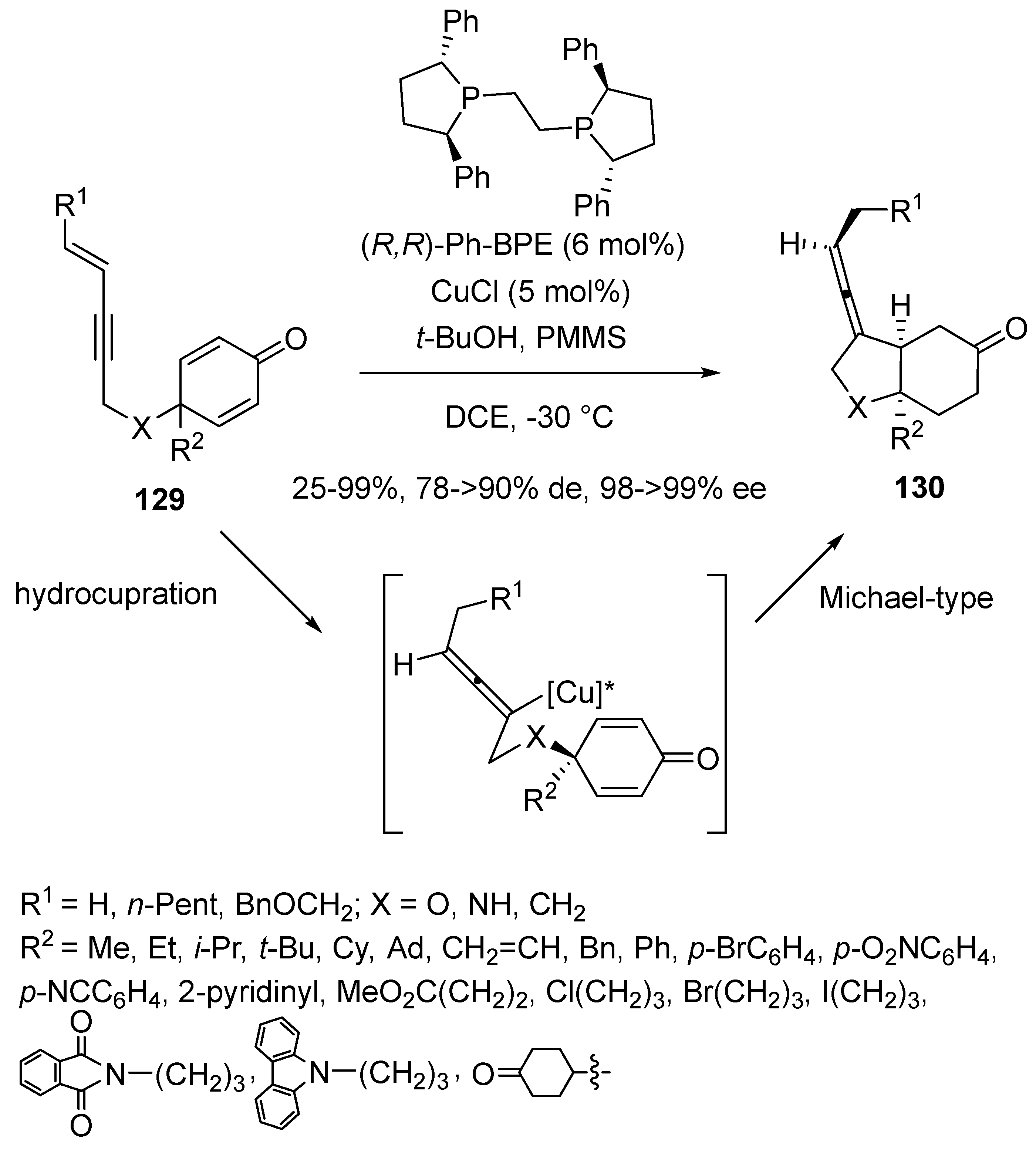
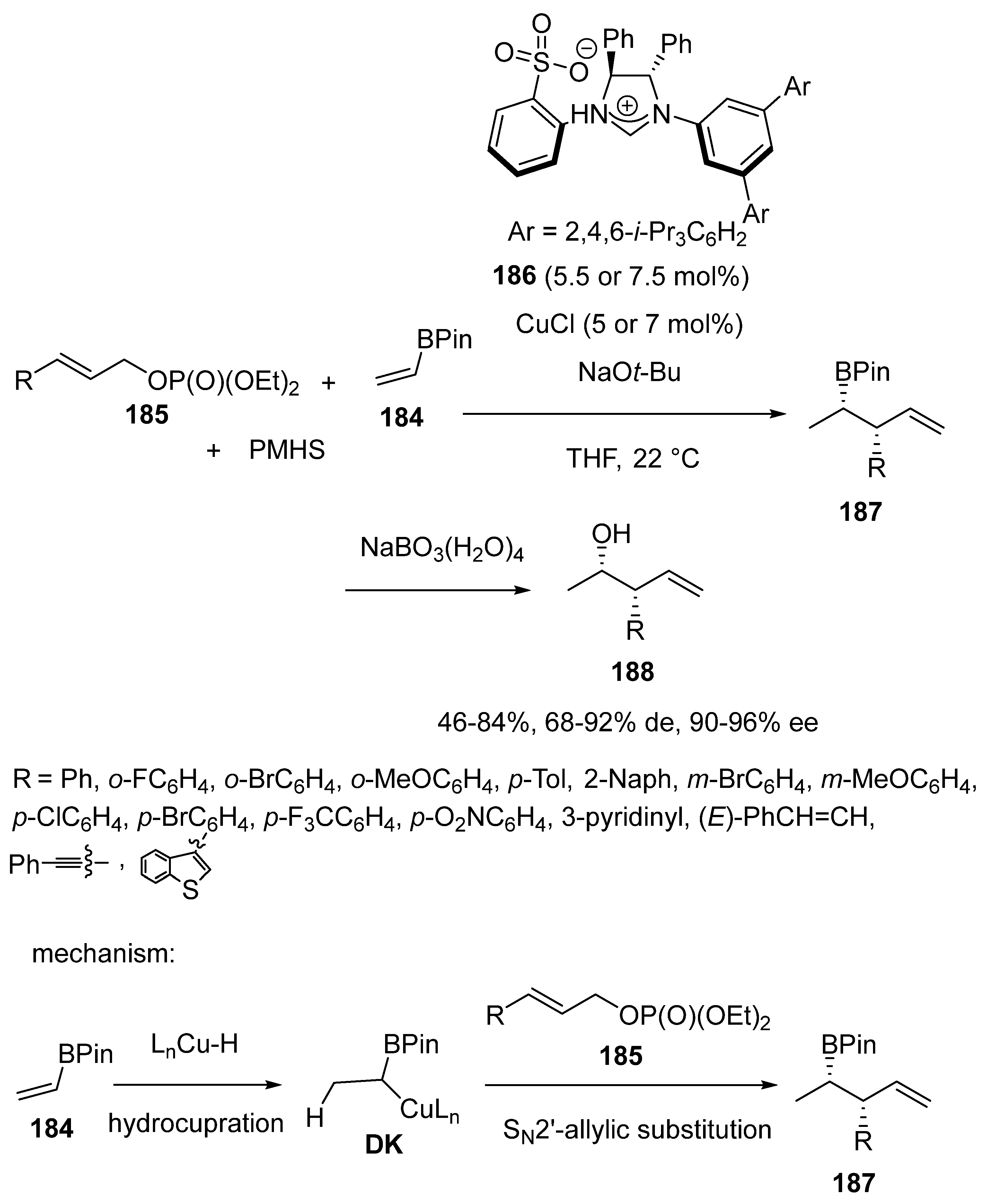
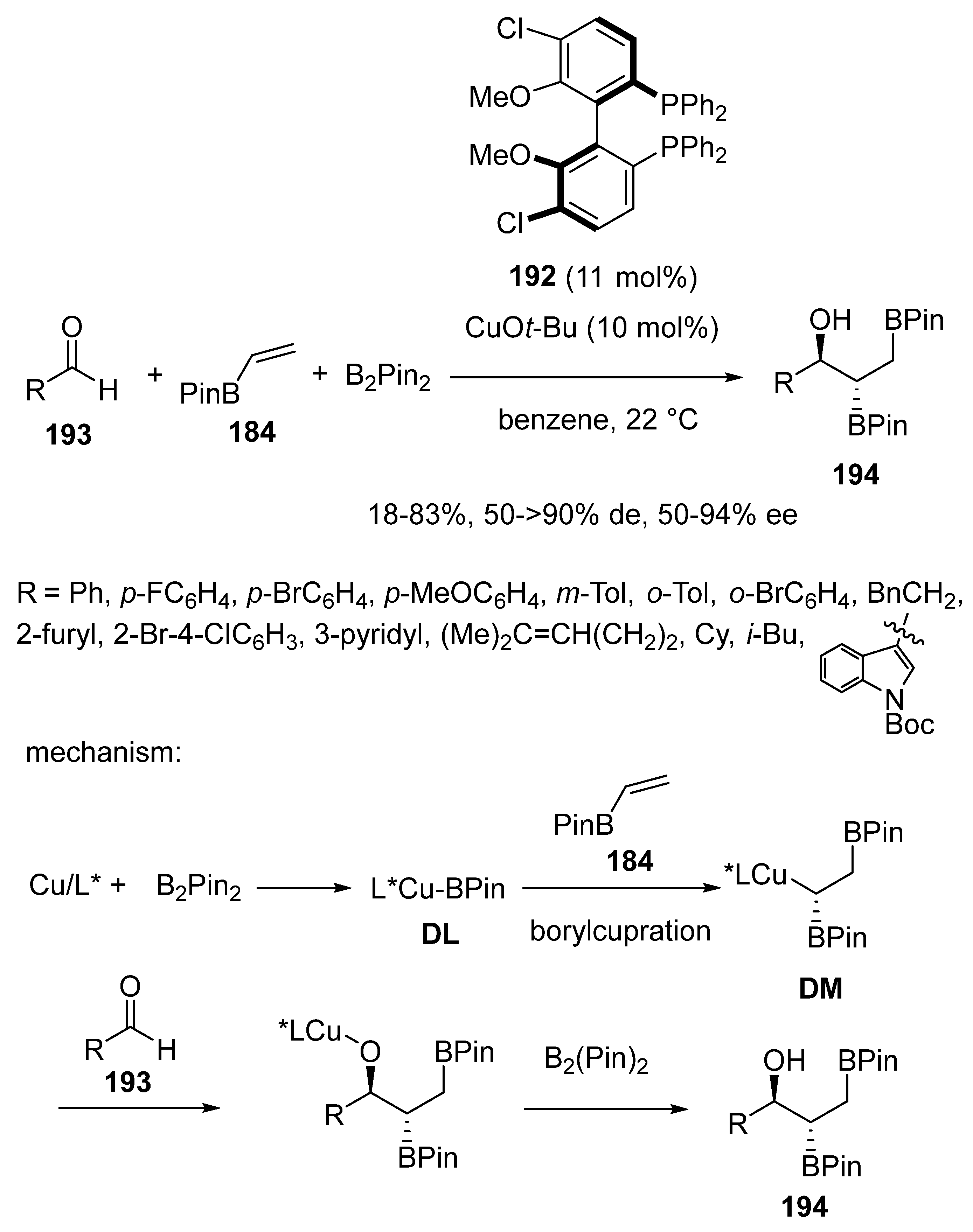
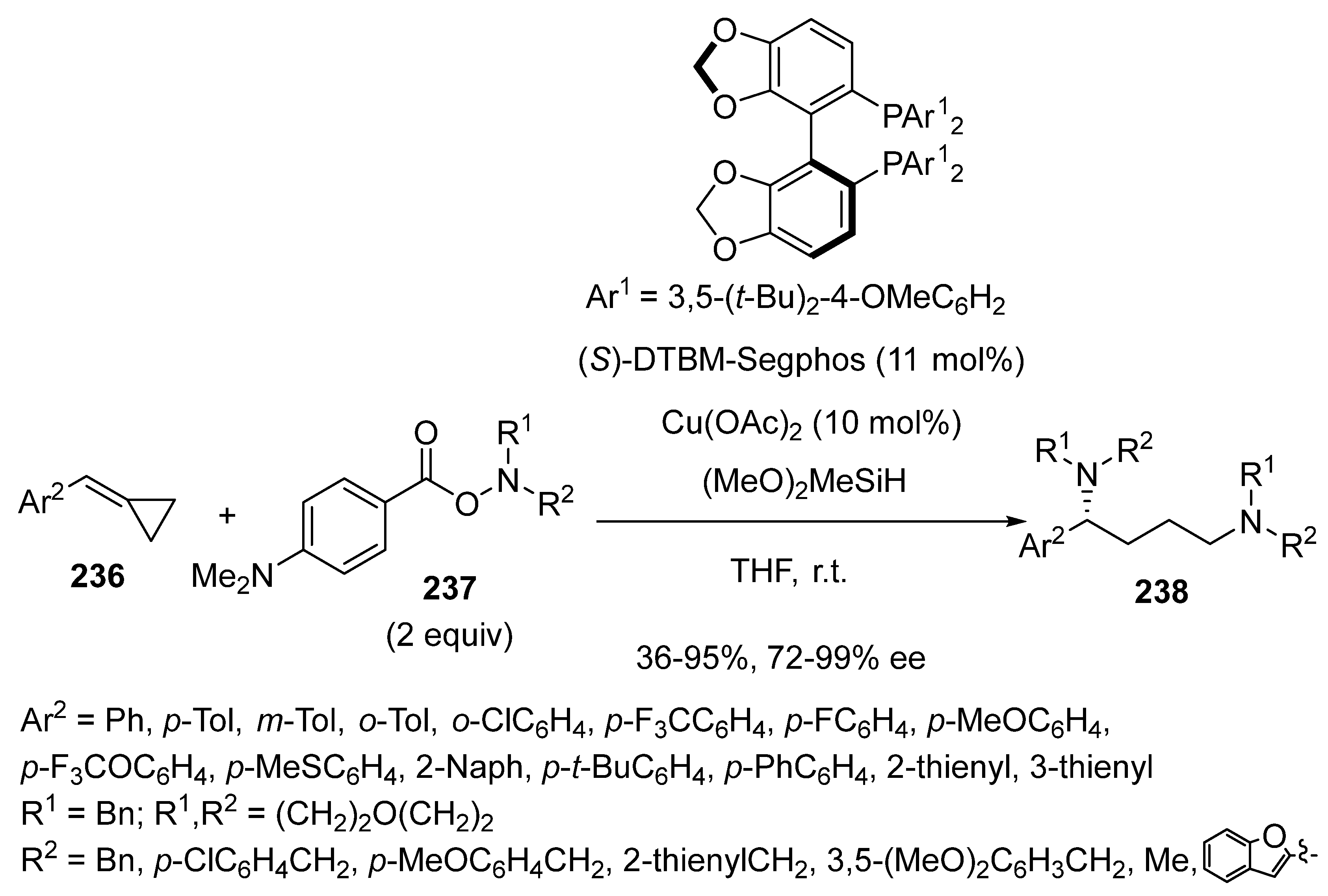
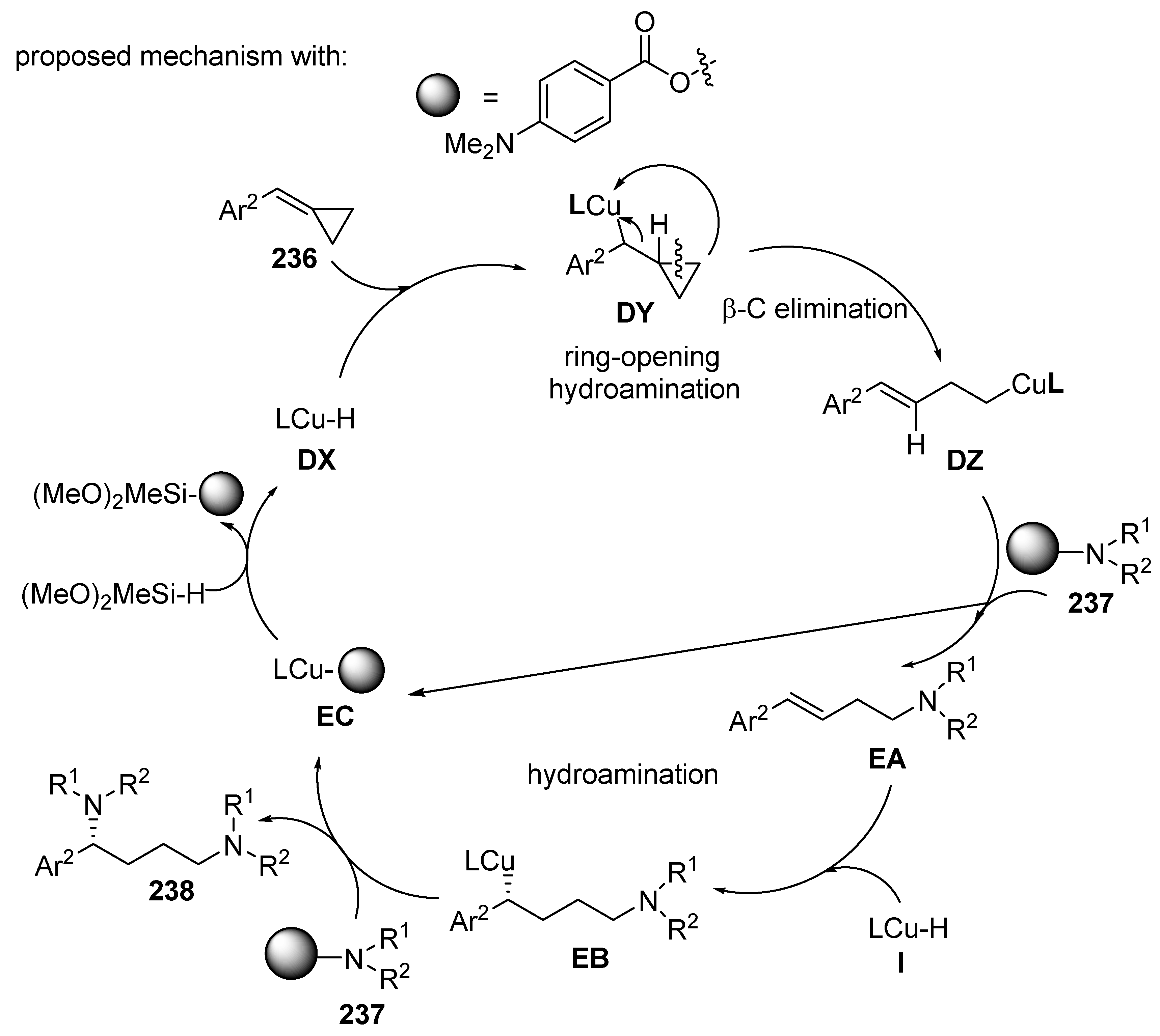
3.3. Multicomponent Reactions Initiated by Hydrocupration
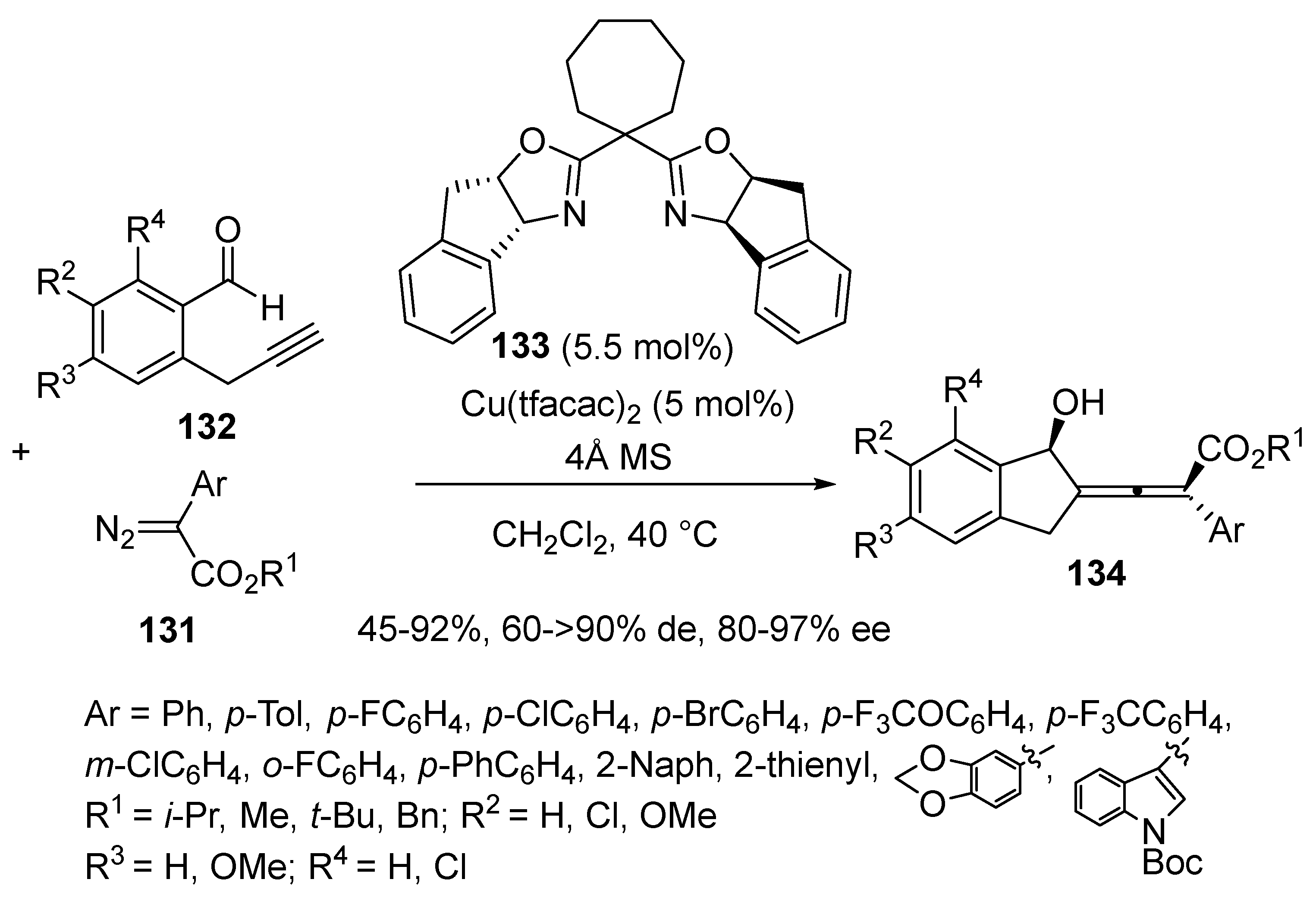
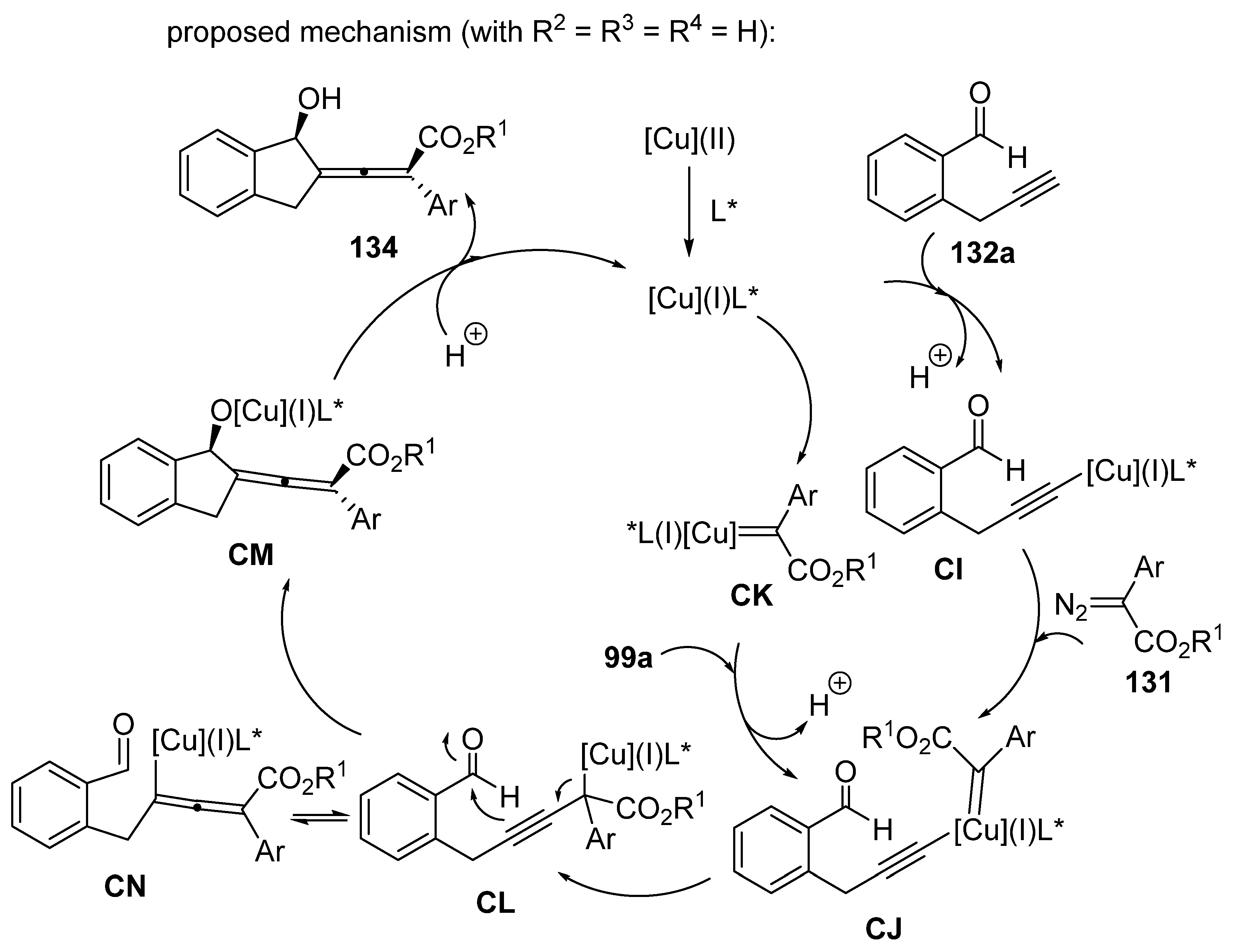
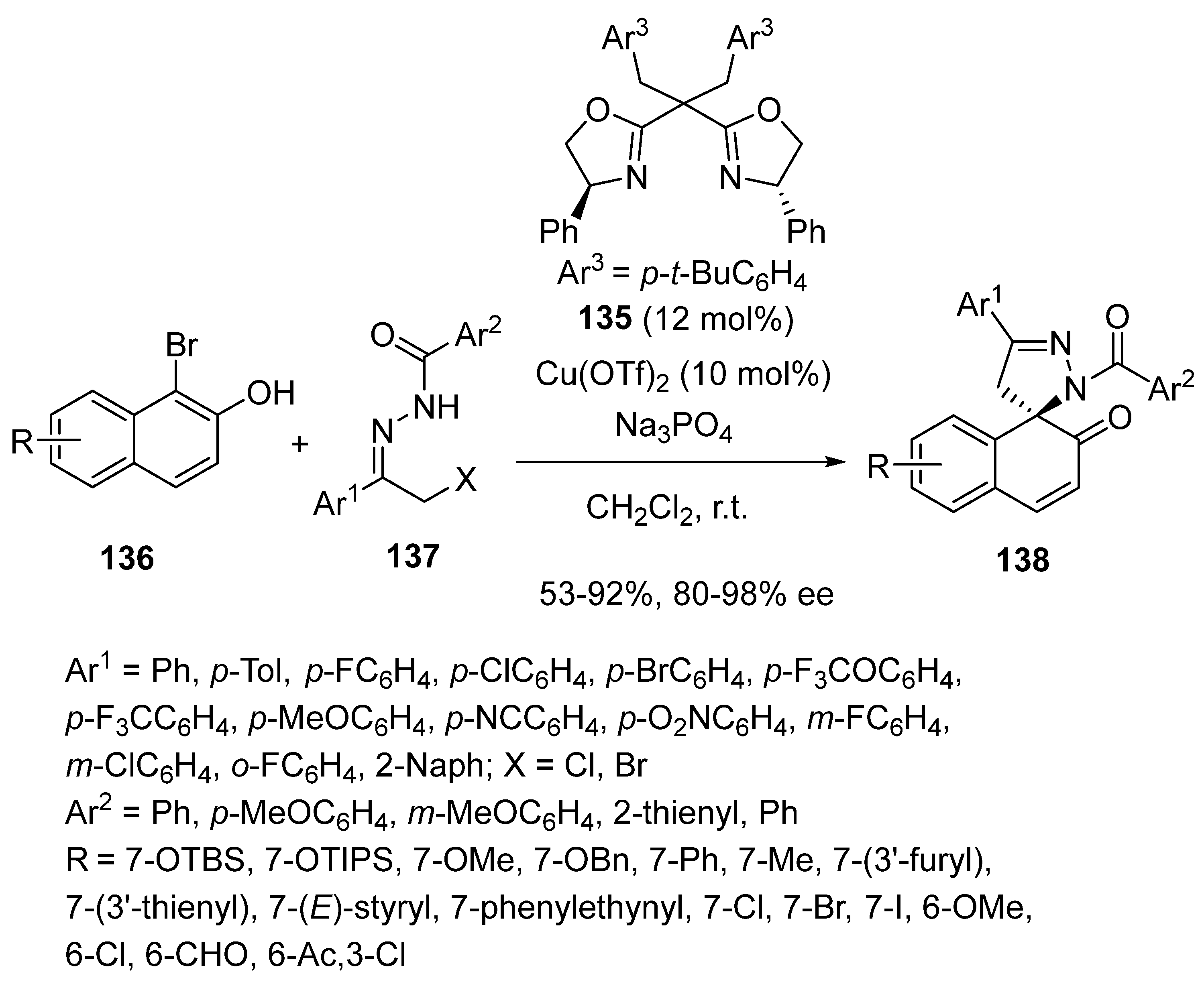
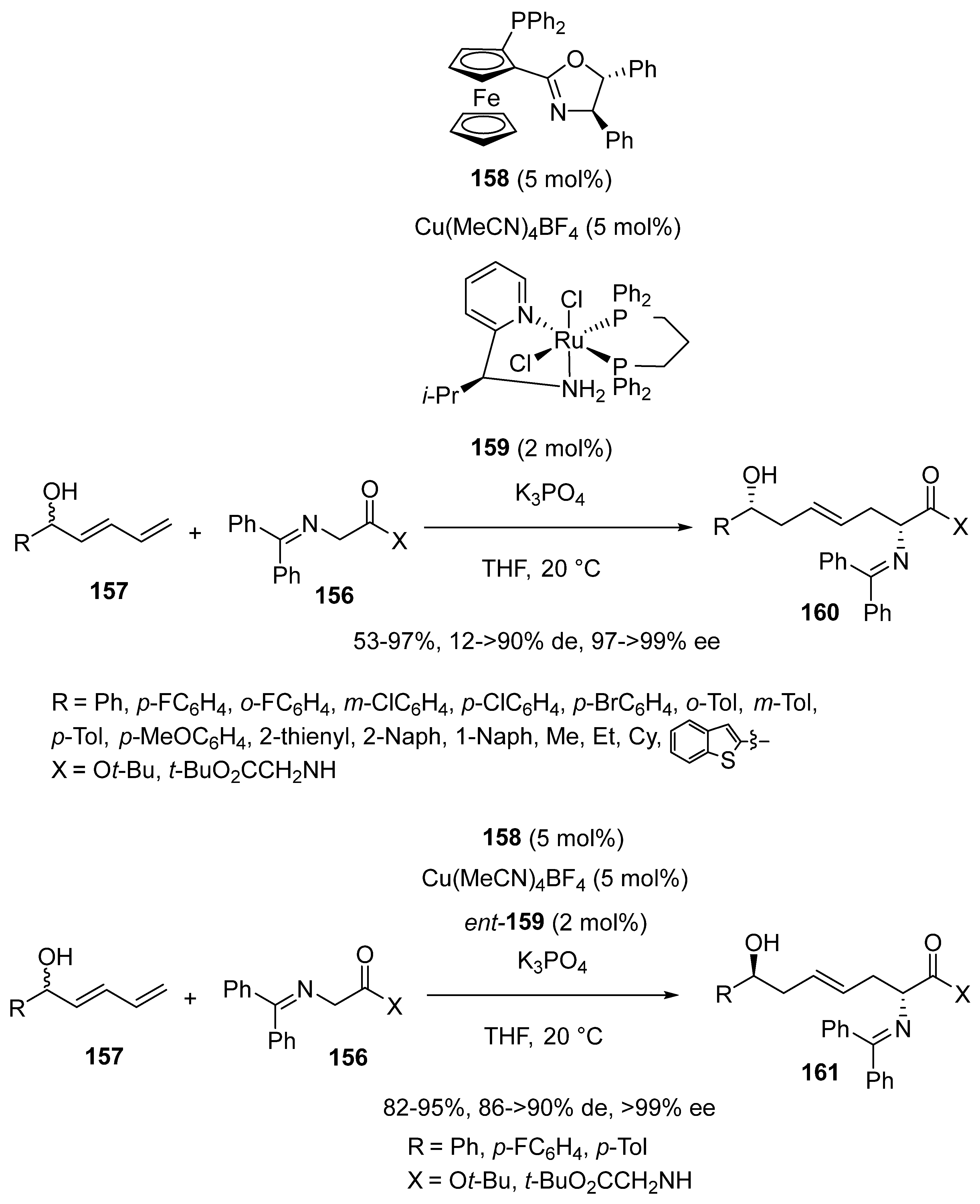
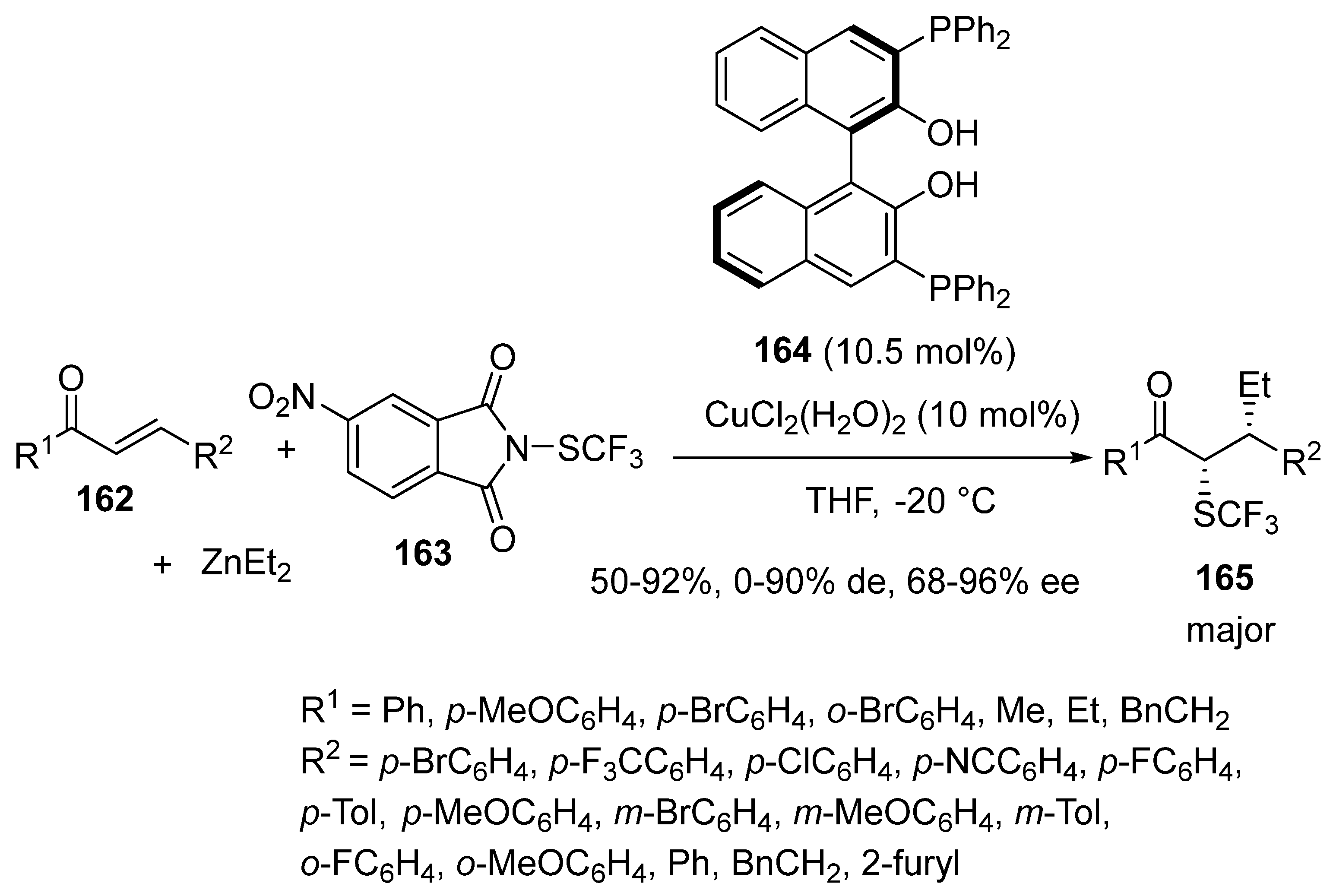
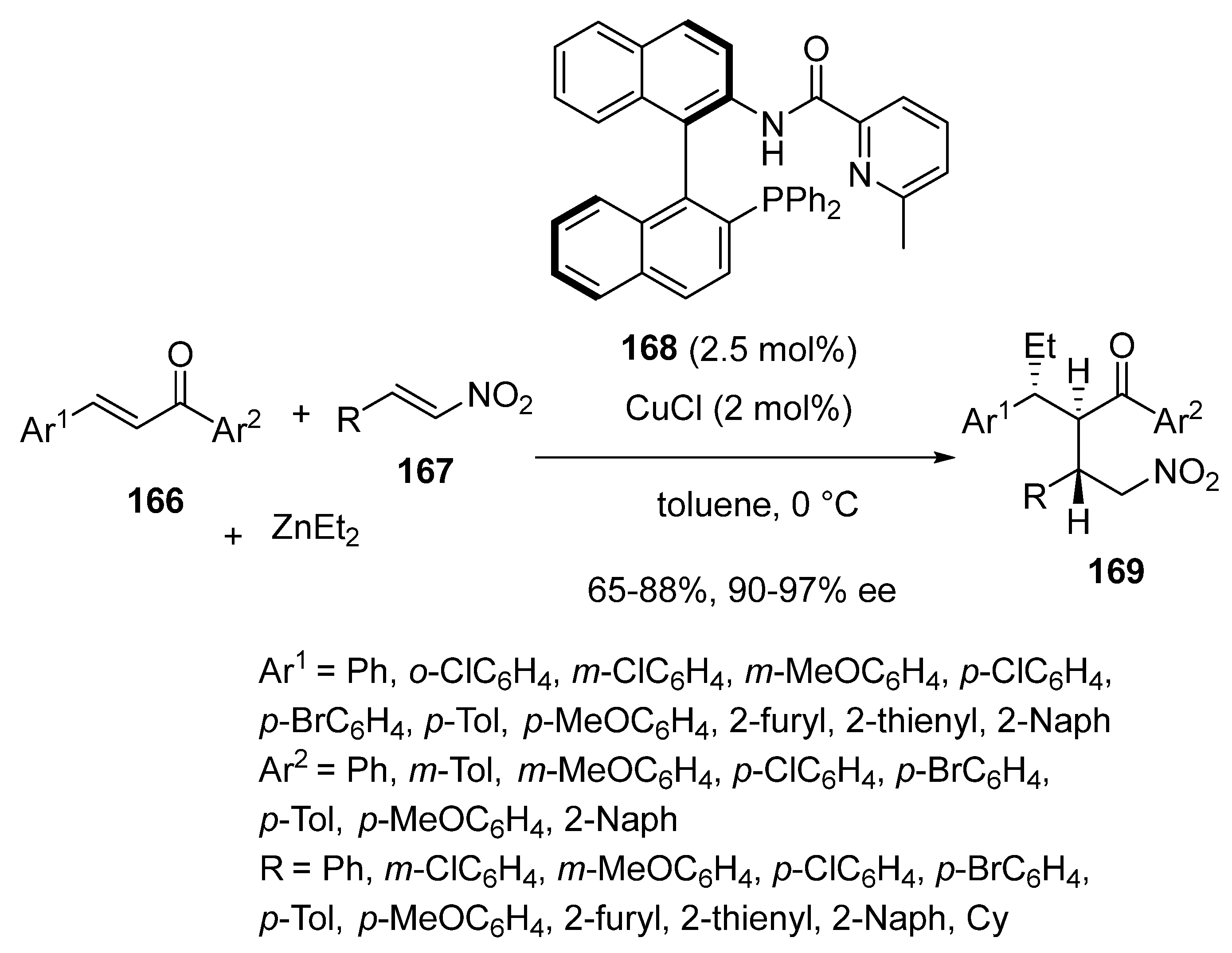
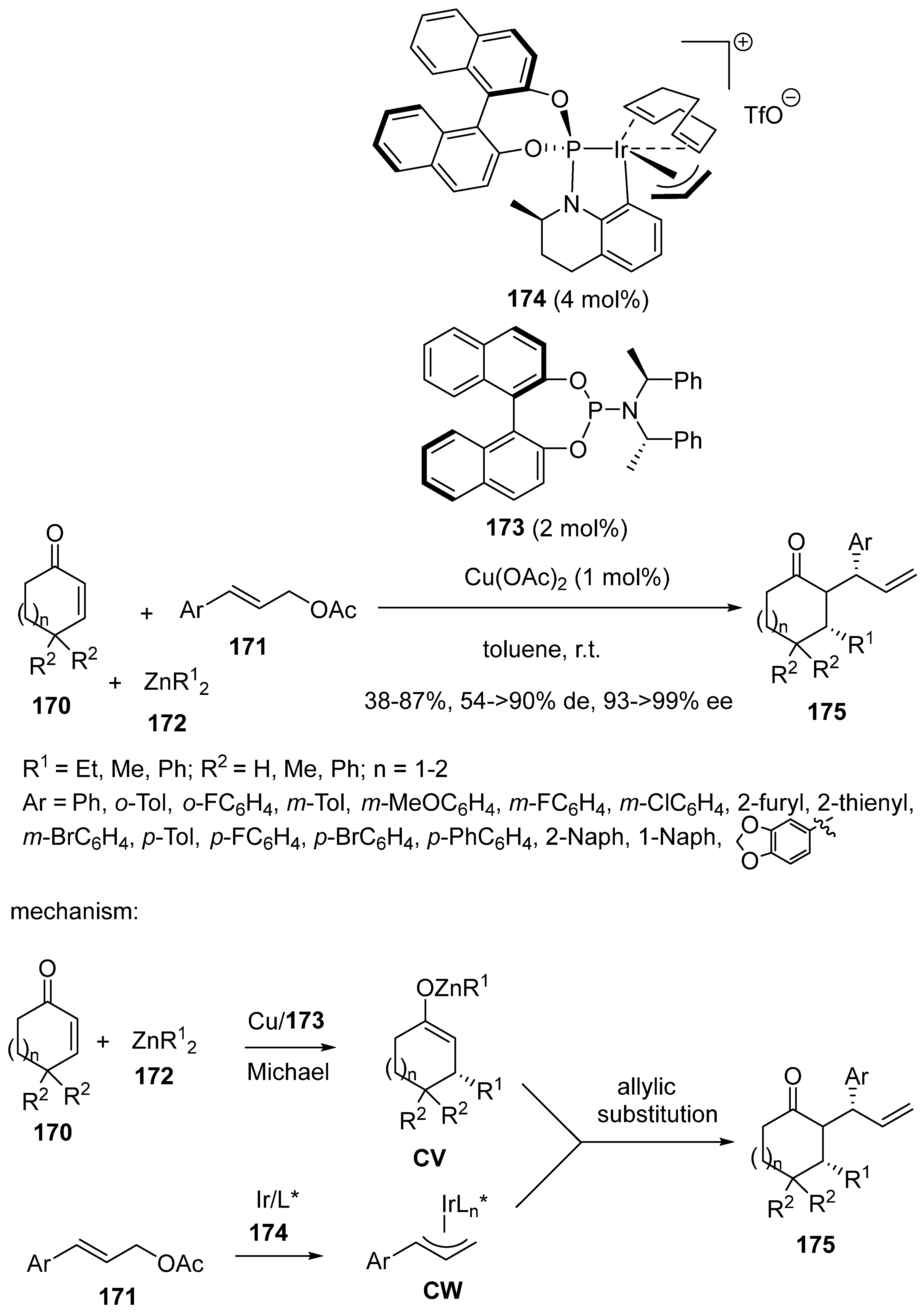
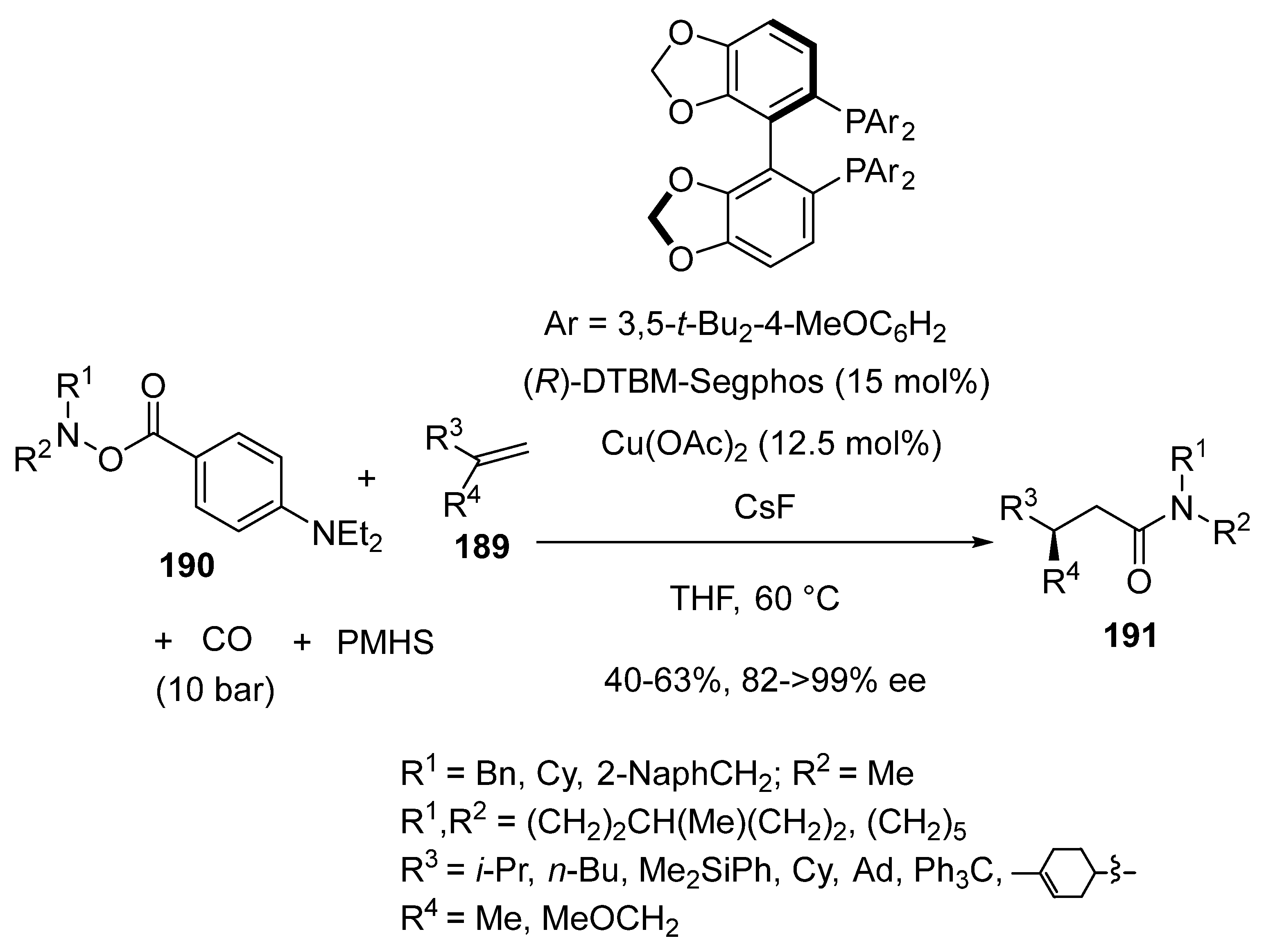
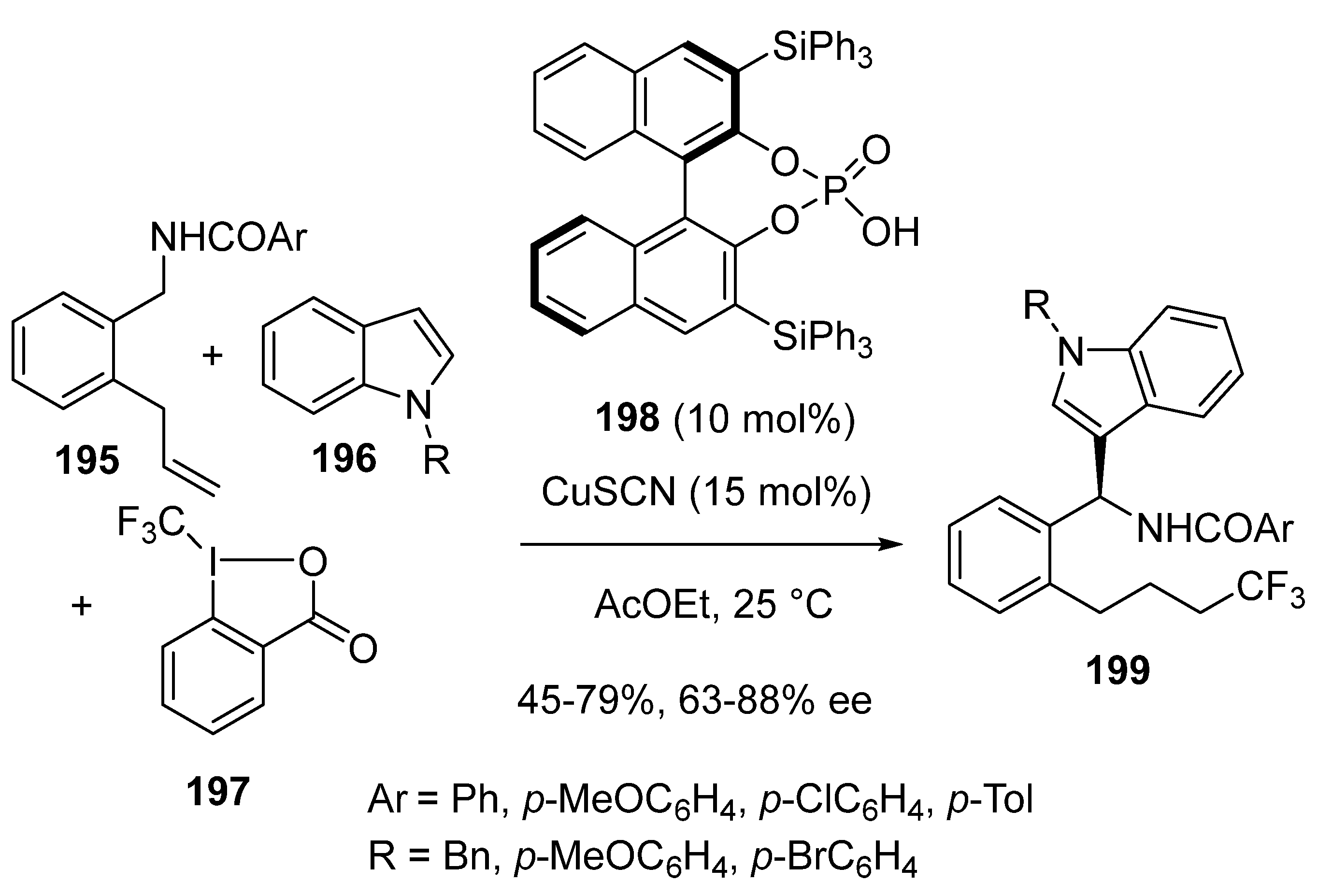
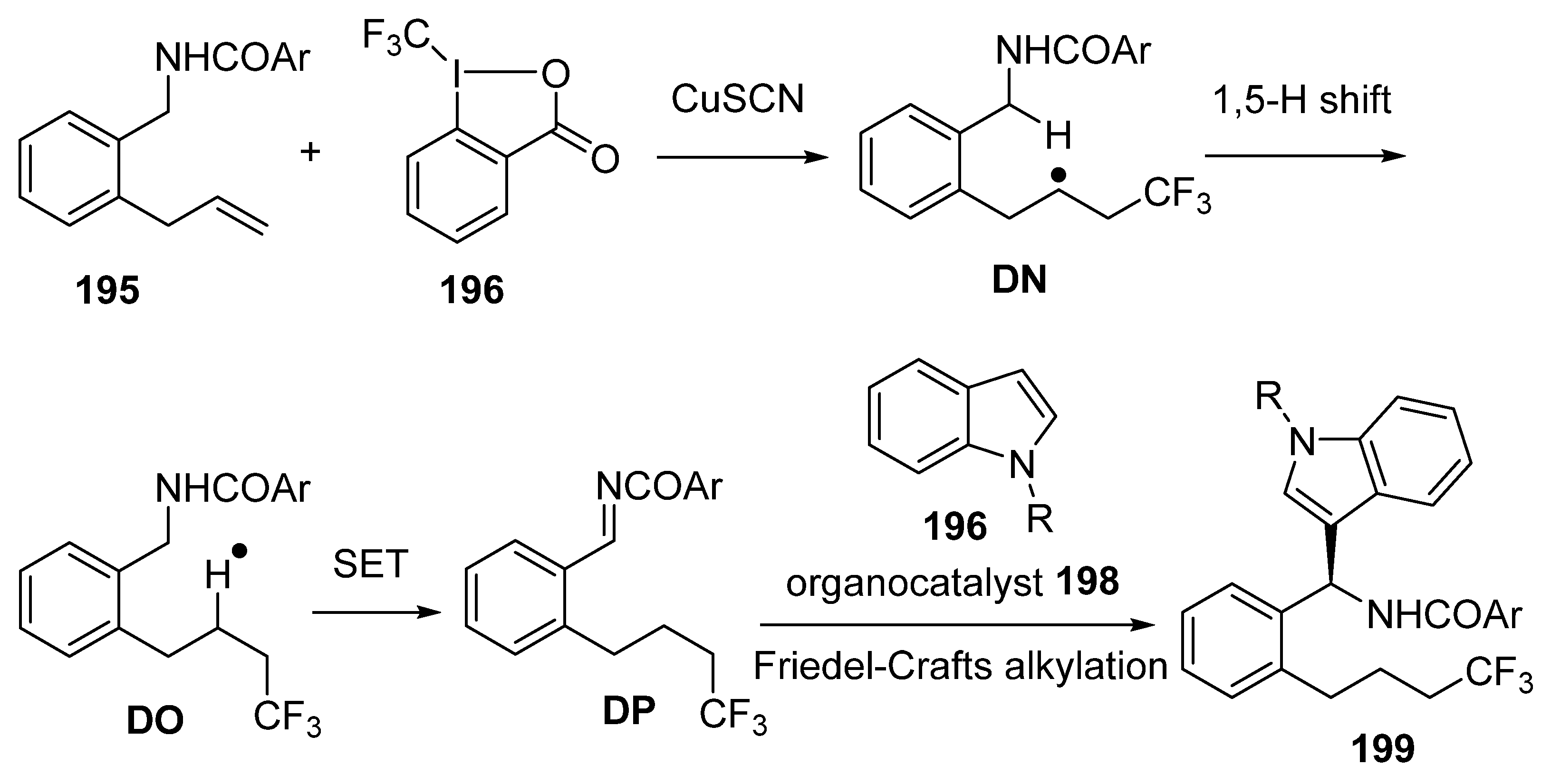
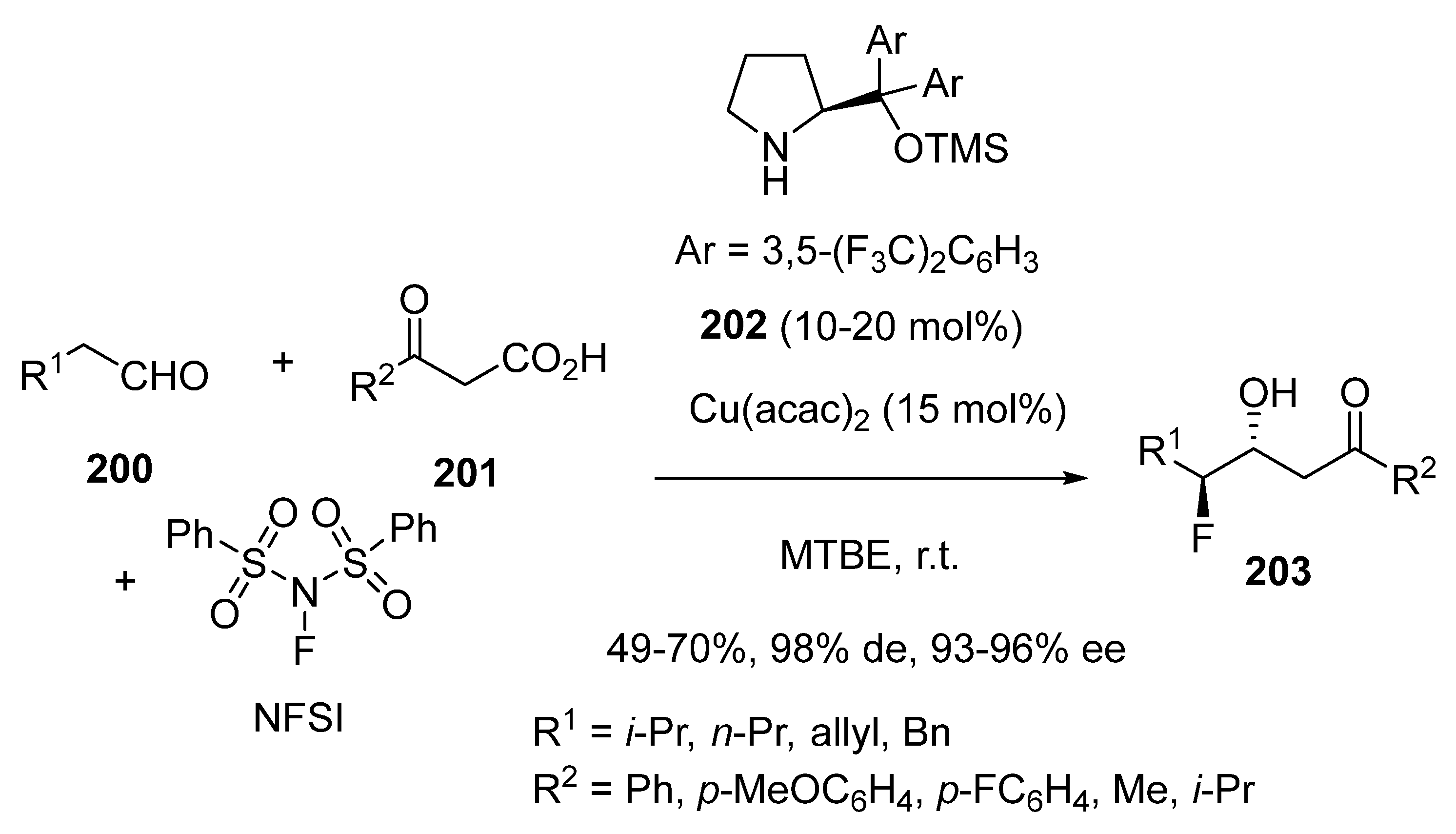
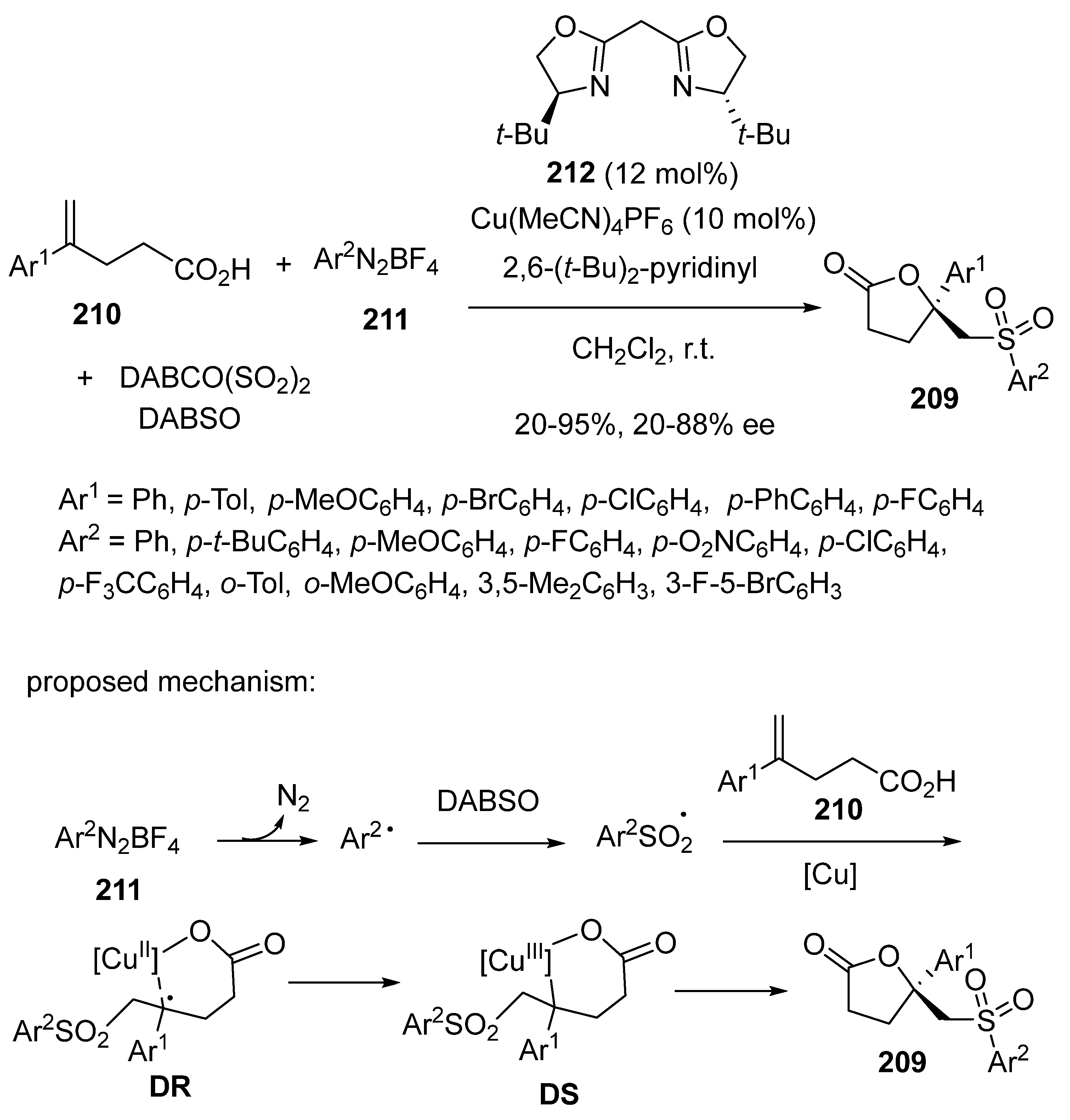
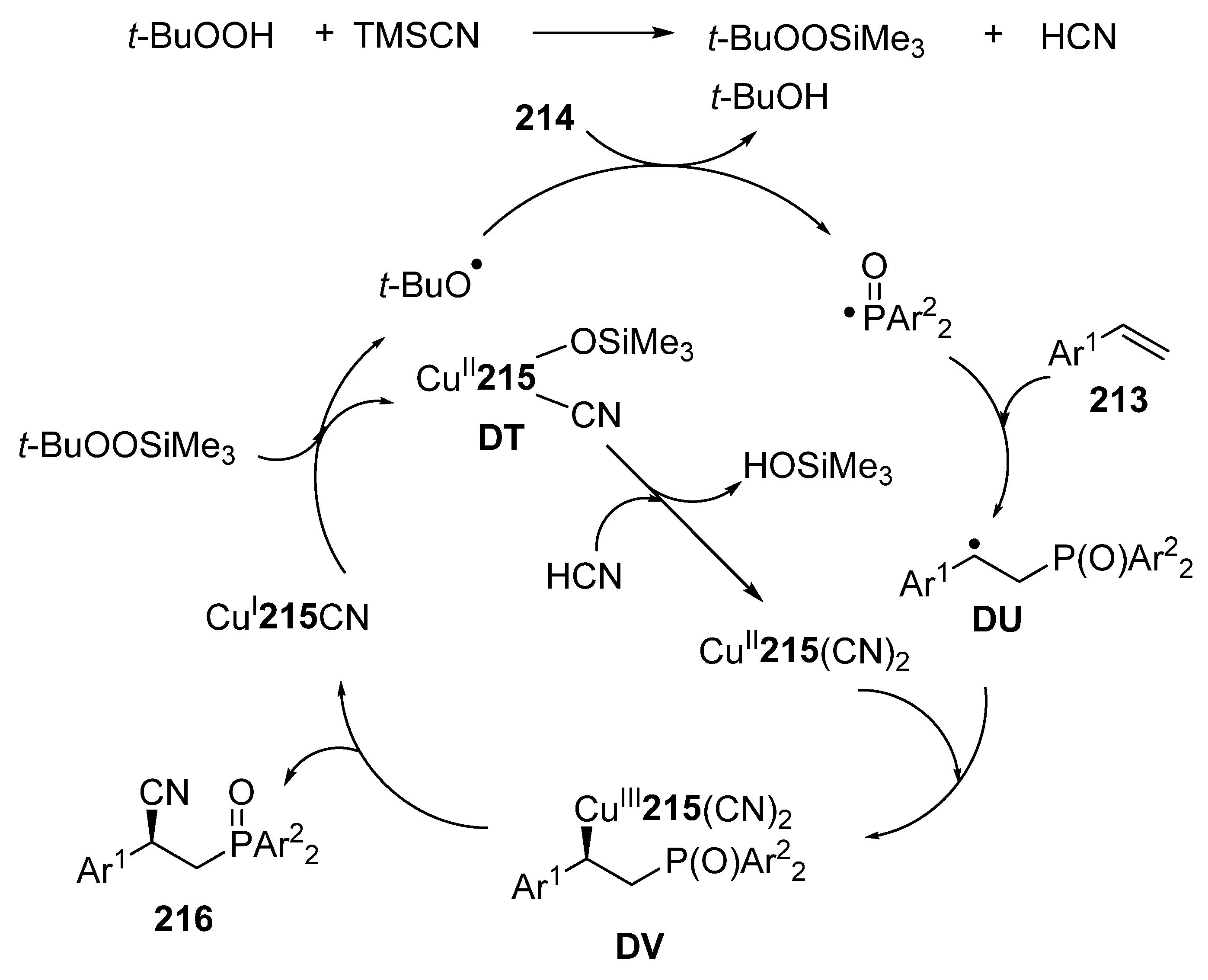
3.4. Miscellaneous Multicomponent Reactions
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ad | adamantyl |
| acac | acetylacetone |
| Ar | aryl |
| BArF | tetrakis(3,5-bis(trifluoromethyl)phenyl)borate |
| BINOL | 1,1′-bi-2-naphthol |
| BLED | blue light emitting diode |
| Bn | benzyl |
| Boc | tert-butoxycarbonyl |
| BPE | 1,2-bis(2-pyridyl)ethane |
| Bs | benzenesulfonyl |
| BTM | benzotetramizole |
| Bz | benzoyl |
| Cbz | benzyloxycarbonyl |
| cod | cyclooctadiene |
| Cy | cyclohexyl |
| DABCO | 1,4-diazabicyclo [2.2.2]octane |
| dan | 1,8-diamino-naphthalatoborane |
| DCE | dichloroethane |
| de | diastereomeric excess |
| DIPEA | diisopropylethylamine |
| DTBP | ditert-butylpyridine |
| DTBM | ditertbutylmethoxy |
| ee | enantiomeric excess |
| EWG | electron-withdrawing group |
| Fac | hexafluoroacetylacetonate |
| FOXAP | ferrocenyloxazolinylphosphine |
| GDME | ethylene glycol dimethyl ether |
| Hex | hexyl |
| L | ligand |
| LED | light emitting diode |
| LHMDS | lithium hexamethyldisilazide |
| MBS | p-methoxybenzenesulfonyl |
| Mes | mesityl |
| Ms | mesyl |
| MS | molecular sieves |
| MTBE | methyl tert-butyl ether |
| Naph | naphthyl |
| NHC | N-heterocyclic carbene |
| NFSI | N-fluorobenzenesulfonimide |
| NMP | N-methyl-2-pyrrolidone |
| Ns | nosyl (4-nitrobenzene sulfonyl) |
| Oct | octyl |
| Pent | pentyl |
| PG | protective group |
| Phth | phthaloyl |
| Pin | pinacolato |
| PMB | para-methoxybenzyl |
| PMHS | polymethylhydrosiloxane |
| PMMS | poly[(3-mercaptopropyl)methylsiloxane] |
| ppy | 2-phenylpyridine |
| Pybox | 2,6-bis(2-oxazolyl)pyridine |
| r.t. | room temperature |
| Segphos | 5,5′-Bis(diphenylphosphino)-4,4′-bi-1,3-benzodioxole |
| tAm | tert-amyl |
| TBDPS | tert-butyldiphenylsilyl |
| TBHP | tert-butyl hydroperoxide |
| TBS | tert-butyldimethylsilyl |
| Tc | thiophene carboxylate |
| TEA | trimethylamine |
| Tf | trifluoromethanesulfonyl |
| tfacac | trifluoroacetylacetonate |
| THF | tetrahydrofuran |
| TIPS | triisopropylsilyl |
| TMS | trimethylsilyl |
| Tol | tolyl |
| Ts | 4-toluenesulfonyl (tosyl) |
References
- Noyori, R. Asymmetric Catalysts in Organic Synthesis; Wiley-VCH: New-York, NY, USA, 1994. [Google Scholar]
- Jacobsen, E.N.; Pfaltz, A.; Yamamoto, H. Comprehensive Asymmetric Catalysis; Springer: New York, NY, USA, 1999. [Google Scholar]
- Ojima, I. Catalytic Asymmetric Synthesis, 2nd ed.; Wiley-VCH: New-York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Beller, M.; Bolm, C. Transition Metals for Organic Synthesis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar] [CrossRef]
- Tietze, L.F.; Hiriyakkanavar, I.; Bell, H.P. Enantioselective Palladium-Catalysed Transformations. Chem. Rev. 2004, 104, 3453–3516. [Google Scholar] [CrossRef] [PubMed]
- Ramon, D.J.; Yus, M. In the Arena of Enantioselective Synthesis, Titanium Complexes Wear the Laurel Wreath. Chem. Rev. 2006, 106, 2126–2208. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, H. Recent advances in enantioselective vanadium-catalysed transformations. Coord. Chem. Rev. 2015, 284, 93–110. [Google Scholar] [CrossRef]
- Pellissier, H. Enantioselective Indium-Catalysed Transformations. Synthesis 2021, 53, 1379–1395. [Google Scholar] [CrossRef]
- Pellissier, H. Recent developments in enantioselective zinc-catalysed transformations. Coord. Chem. Rev. 2021, 439, 213926. [Google Scholar] [CrossRef]
- Pellissier, H. Recent developments in enantioselective titanium-catalysed transformations. Coord. Chem. Rev. 2022, 463, 214537. [Google Scholar] [CrossRef]
- Pellissier, H. Recent developments in enantioselective scandium-catalyzed transformations. Chemistry 2024, 6, 98-152. [Google Scholar] [CrossRef]
- Pellissier, H. Enantioselective Zirconium-Catalyzed Transformations. Curr. Org. Chem. 2024, 28, 346–367. [Google Scholar] [CrossRef]
- Pellissier, H. Enantioselective iron-catalyzed transformations. An update. Tetrahedron 2024, 157, 133944. [Google Scholar] [CrossRef]
- Pellissier, H. Enantioselective Aluminum-Catalyzed Transformations. An Update. Curr. Org. Chem. 2025, 29, 1491–1507. [Google Scholar] [CrossRef]
- Pellissier, H. Recent developments in enantioselective silver-catalyzed transformations. Org. Prep. Proc Int. 2025. [Google Scholar] [CrossRef]
- Tietze, L.F. Domino Reactions; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Zhu, J.; Wang, Q.; Wang, M. Multicomponent Reactions in Organic Synthesis; Wiley: Weinheim, Germany, 2014. [Google Scholar]
- Herrera, R.P.; Marques-Lopez, E. Multicomponent Reactions: Concepts and Applications for Design and Synthesis; Wiley: Weinheim, Germany, 2015. [Google Scholar]
- Pellissier, H. Enantioselective Nickel-Catalysed Domino and Tandem Reactions. Enantioselective Nickel-Catalysed Domino and Tandem Processes. Curr. Org. Chem. 2016, 20, 234–265. [Google Scholar] [CrossRef]
- Snyder, S.A. Science of Synthesis. In Applications of Domino Transformations in Organic Synthesis; Thieme Verlag: Weinheim, Germany, 2016; Volume 1–2. [Google Scholar]
- Pellissier, H. Recent Developments in Enantioselective Metal-Catalysed Domino Reactions. Adv. Synth. Catal. 2016, 358, 2194–2259. [Google Scholar] [CrossRef]
- Pellissier, H. Synthesis of Chiral 3-Substituted 3-Amino-2-Oxindoles through Enantioselective Catalytic Domino and Tandem Reactions. Synthesis 2019, 51, 1311–1318. [Google Scholar] [CrossRef]
- Pellissier, H. Recent developments in enantioselective metal-catalysed domino reactions. Adv. Synth. Catal. 2019, 361, 1733–1755. [Google Scholar] [CrossRef]
- Pellissier, H. Asymmetric Metal Catalysis in Enantioselective Domino Reactions; Wiley: Weinheim, Germany, 2019. [Google Scholar]
- Pellissier, H. The Use of Domino Reactions for the Synthesis of Chiral Rings. Synthesis 2020, 52, 3837–3854. [Google Scholar] [CrossRef]
- Pellissier, H. Recent Developments in Enantioselective Domino Reactions. Part A: Noble Metal Catalysts. Adv. Synth. Catal. 2023, 365, 620–681. [Google Scholar] [CrossRef]
- Pellissier, H. Recent Developments in Enantioselective Domino Reactions. Part B: First Row Metal Catalysts. Adv. Synth. Catal. 2023, 365, 768–819. [Google Scholar] [CrossRef]
- Vargosa, D.; Nemethova, I.; Sebesta, R. Asymmetric copper-catalysed conjugate additions of organometallic reagents in the syntheses of natural compounds and pharmaceuticals. Org. Biomol. Chem. 2020, 18, 3780–3796. [Google Scholar] [CrossRef]
- Sadhna, S.; Braja, G.D.; Vinod, K.S. Recent advancement in copper-catalysed asymmetric reactions of alkynes. Tetrahedron 2021, 93, 132238. [Google Scholar] [CrossRef]
- Ahmad, H.; Bilal, M.; Maqbool, T.; Adnan, A.S.S.; Amiruddin, Z.Z. Recent advances on copper-catalysed asymmetric synthesis and their potential biological applications. J. Sausi Chem. Soc. 2023, 27, 101658. [Google Scholar] [CrossRef]
- Pellissier, H. Green Copper Catalysis in Enantioselective Domino Reactions. Curr. Org. Chem. 2018, 22, 2752–2779. [Google Scholar] [CrossRef]
- Colombo, S.; Loro, C.; Beccalli, E.M.; Broggini, G.; Papis, M. Cu(OTf)2-catalysed multicomponent reactions. Beilstein J. Org. Chem. 2025, 21, 122–145. [Google Scholar] [CrossRef] [PubMed]
- Galestokova, Z.; Sebesta, R. Domino Reactions Initiated by Enantioselective Cu-Catalysed Conjugate Addition. Eur. J. Org. Chem. 2012, 2012, 6688–6695. [Google Scholar] [CrossRef]
- Larin, E.M.; Loup, J.; Polishchuk, L.; Ross, R.J.; Whyte, A.; Lautens, M. Enantio- and diastereoselective conjugate borylation/Mannich cyclization. Chem. Sci. 2020, 11, 5716–5723. [Google Scholar] [CrossRef]
- Han, J.; He, Y.-M.; Pan, Y.; Li, F.; Li, D.; Hao, W.; Fan, Q.-H. Tunable Unsymmetrical Chiral N-Heterocyclic Carbene Ligands for Highly Diastereo- and Enantioselective Copper-Catalysed Tandem Borylative Cyclization: Ligand Controlled Diastereoselectivity Reversal. CCS Chem. 2023, 5, 2088–2100. [Google Scholar] [CrossRef]
- Liu, B.; Qiu, H.; Chen, X.; Li, W.; Zhang, J. Copper-catalysed asymmetric tandem borylative addition and aldol cyclization. Org. Chem. Front. 2020, 7, 2492–2498. [Google Scholar] [CrossRef]
- Li, K.; Gao, S.; Zha, Z.; Wang, Z. Construction of chiral N,O-hemiaminals via a copper-catalysed enantioselective Michael/N-hemiacetalization cascade reaction. Org. Biomol. Chem. 2023, 21, 4404–4408. [Google Scholar] [CrossRef]
- Kinugasa, M.; Hashimoto, S. The reactions of copper(I) phenylacetylide with nitrones. J. Chem. Soc. Chem. Commun. 1972, 466–467. [Google Scholar] [CrossRef]
- Pitts, C.R.; Lectka, T. Chemical Synthesis of β-Lactams: Asymmetric Catalysis and Other Recent Advances. Chem. Rev. 2014, 114, 7930–7953. [Google Scholar] [CrossRef]
- Shu, T.; Zhao, L.; Li, S.; Chen, X.-Y.; von Essen, C.; Rissanen, K.; Enders, D. Asymmetric Synthesis of Spirocyclic β-Lactams through Copper-Catalysed Kinugasa/Michael Domino Reactions. Angew. Chem. Int. Ed. 2018, 57, 10985–10988. [Google Scholar] [CrossRef]
- Zhong, X.; Huang, M.; Xiong, H.; Liang, Y.; Zhou, W.; Cai, Q. Asymmetric Synthesis of Spiro[Azetidine-3,3′-Indoline]-2,2′-Diones via Copper(I)-Catalysed Kinugasa/C–C Coupling Cascade Reaction. Angew. Chem. Int. Ed. 2022, 61, e202208323. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, H.; Zhong, X.; Li, S.; Zhang, J.; Ao, Y.; Zhou, W.; Cai, Q. Highly diastereo- and enantioselective synthesis of spiro β-lactams via copper-catalysed Kinugasa/aldol cascade reaction. Org. Chem. Front. 2023, 10, 5383–5388. [Google Scholar] [CrossRef]
- Torelli, A.; Choi, E.S.; Dupeux, A.; Perner, M.N.; Lautens, M. Stereoselective Kinugasa/Aldol Cyclization: Synthesis of Enantioenriched Spirocyclic β-Lactams. Org. Lett. 2023, 25, 8520–8525. [Google Scholar] [CrossRef] [PubMed]
- Ao, Y.; Ma, H.; Gan, B.; Wang, W.; Zhang, J.; Zhou, W.; Zhang, X.; Cai, Q. Copper-Catalysed Asymmetric Kinugasa/Michael Addition Cascade Reactions for the Synthesis of Chiral Spiro β-Lactams. Org. Lett. 2024, 26, 4761–4766. [Google Scholar] [CrossRef]
- Whyte, A.; Burton, K.I.; Zhang, J.; Lautens, M. Enantioselective Intramolecular Copper-Catalysed Borylacylation. Angew. Chem. Int. Ed. 2018, 57, 13927–13930. [Google Scholar] [CrossRef]
- Zheng, P.; Han, X.; Hu, J.; Zhao, X.; Xu, T. Enantioselective Copper-Catalysed Desymmetrization of 1,3-Diketones Involving Borylation of Styrenes. Org. Lett. 2019, 21, 6040–6044. [Google Scholar] [CrossRef]
- He, C.-Y.; Li, Q.-H.; Wang, X.; Wang, F.; Tian, P.; Lin, G.-Q. Copper-Catalysed Asymmetric Borylative Cyclization of Cyclohexadienone-Containing 1,6-Dienes. Adv. Synth. Catal. 2020, 362, 765–770. [Google Scholar] [CrossRef]
- Hong, F.-L.; Wang, Z.-S.; Wei, D.D.; Zhai, T.Y.; Deng, G.-C.; Lu, X.; Liu, R.S.; Ye, L.-W. Generation of Donor/Donor Copper Carbenes through Copper-Catalysed Diyne Cyclization: Enantioselective and Divergent Synthesis of Chiral Polycyclic Pyrroles. J. Am. Chem. Soc. 2019, 141, 16961–16970. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.-S.; Zhai, T.-Y.; Luo, C.; Zhang, Y.-P.; Chen, Y.-B.; Deng, C.; Liu, R.-S.; Ye, L.-W. Copper-Catalysed Azide–Ynamide Cyclization to Generate α-Imino Copper Carbenes: Divergent and Enantioselective Access to Polycyclic N-Heterocycles. Angew. Chem. Int. Ed. 2020, 59, 17984–17990. [Google Scholar] [CrossRef]
- Xu, X.-M.; Xie, M.; Li, J.; Wang, M.-X. Catalytic enantioselective synthesis of indolizino [8,7-b]indole alkaloid derivatives based on the tandem reaction of tertiary enamides. Org. Chem. Front. 2021, 8, 721–726. [Google Scholar] [CrossRef]
- Hong, F.-L.; Shi, C.-Y.; Hong, P.; Zhai, T.-Y.; Zhu, X.-Q.; Lu, X.; Ye, L.-W. Copper-Catalysed Asymmetric Diyne Cyclization via [1,2]-Stevens-Type Rearrangement for the Synthesis of Chiral Chromeno [3,4-c]pyrroles. Angew. Chem. Int. Ed. 2022, 61, e202115554. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, Z.-J.; Gong, L.-Z. Asymmetric [4 + 2] Annulation of C1 Ammonium Enolates with Copper-Allenylidenes. Angew. Chem. Int. Ed. 2017, 56, 5212–5216. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; You, S.-L. Highly Diastereo-and Enantioselective Synthesis of Tetrahydro-5H-Indolo [2,3-b]quinolinesthrough Copper-Catalysed Propargylic DearomatizationofIndoles. Chem. Eur. J. 2017, 23, 12489–12493. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Zhang, Z.-J.; Fan, L.-F.; Song, J. Enantioselective Decarboxylative Propargylation/Hydroamination Enabled by Organo/Metal Cooperative Catalysis. Org. Lett. 2018, 20, 2792–2795. [Google Scholar] [CrossRef]
- Zuo, S.; Tao, Y.; Liu, Z.; Zhang, K.; Zhang, L.; Ning, Y.; Chen, F.-E. Construction of Vicinal All-Carbon Stereogenic Centers via Copper-Catalysed Asymmetric Decarboxylative Propargylation: Enantio-and Diastereoselective Synthesis of Substituted Spirolactones. Org. Lett. 2023, 25, 410–415. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, H.; Li, X.; Han, Z.; Sun, J.; Jin, X.; Huang, H. Copper-Catalysed Formal [4 + 2] Cycloaddition of Ethynylethylene Carbonates for the Construction of 3,4-Dihydro-2H-benzo[b][1,4]oxazines. Eur. J. Org. Chem. 2024, 27, e202400306. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.-H.; Xia, Y.-Q.; Dong, L.; Chen, F.-E. Diastereo- and Enantioselective Mannich/Cyclization Cascade Reaction Access to Chiral Benzothiazolopyrimidine Derivatives. Chem. Eur. J. 2021, 27, 6183–6186. [Google Scholar] [CrossRef]
- Zhong, F.; Yue, W.-J.; Yin, X.-H.; Zhang, H.-M.; Yin, L. Copper(I)-Catalysed Asymmetric Synthesis of α-Allenylamines and β-Lactams through Regioselective Mannich-Type Reactions. ACS Catal. 2022, 12, 9181–9189. [Google Scholar] [CrossRef]
- Lukamto, D.H.; Gaunt, M.J. Enantioselective Copper-Catalysed Arylation-Driven Semipinacol Rearrangement of Tertiary Allylic Alcohols with Diaryliodonium Salts. J. Am. Chem. Soc. 2017, 139, 9160–9163. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Q.; Zhu, J. Copper-Catalysed Enantioselective Domino Arylation/Semipinacol Rearrangement of Allylic Alcohols with Diaryliodonium Salts. Chem. Eur. J. 2017, 23, 13037–13041. [Google Scholar] [CrossRef]
- Yang, S.; Fang, X. Copper-catalyzed yne-allylic substitutions: Concept and recent developments. Beilstein J. Org. Chem. 2024, 20, 2739–2775. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.-H.; Zhu, C.; Deng, S.; Xu, G.; Zhao, R.; Yao, C.; Xiang, H.-M.; Zhao, C.; Qi, X.; Xu, H. Remote Enantioselective [4 + 1] Annulation with Copper-Vinylvinylidene Intermediates. J. Am. Chem. Soc. 2022, 144, 21347–21355. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xu, L.; Zhu, B.; Liao, M.; Li, J.; Han, Z.; Sun, J.; Huang, H. Copper-Catalyzed Enantioselective Formal [4 + 1] and [3 + 3] Cycloaddition of Ethynylethylene Carbonates. Org. Lett. 2023, 25, 9213–9218. [Google Scholar] [CrossRef]
- Li, X.; Guo, J.-X.; Zhang, J.; Chen, Q.-Y.; He, Y.-J.; Sha, F.; Xiang, H.; Yu, P.; Liu, P.-N. Asymmetric Dearomatization of Nonfunctionalized 1-Naphthols via Copper-Catalysed Enantioselective [4 + 1] Spiroannulation. ACS Catal. 2024, 14, 9244–9253. [Google Scholar] [CrossRef]
- Zhao, R.; Deng, S.; Huang, R.; Kong, H.-H.; Lu, Y.; Yin, T.; Wang, J.; Li, Y.; Zhu, C.; Pan, F.; et al. Enantioselective Copper-Catalyzed Dearomative Spiroannulation of β-Naphthols or Indoles with Yne-Allylic Esters. ACS Catal. 2024, 14, 9254–9264. [Google Scholar] [CrossRef]
- Xu, G.; Zhu, C.; Li, X.; Zhu, K.; Xu, H. Copper-catalyzed asymmetric [4 + 1] annulation of yne-allylic esters with pyrazolones. Chin. Chem. Lett. 2024, 36, 110114. [Google Scholar] [CrossRef]
- Yao, C.; Li, D.-R.; Xiang, H.-M.; Li, S.-J.; Lu, Y.; Wang, Z.; Yin, T.; Wang, J.; Feng, K.; Zhu, C.; et al. Copper-catalysed asymmetric annulation of yne-allylic esters with amines to access axially chiral arylpyrroles. Nat. Commun. 2024, 15, 6848. [Google Scholar] [CrossRef]
- Li, Z.-H.; Ma, J.-S.; Lu, H.-Y.; Lin, G.-Q.; He, Z.-T. Asymmetric Multicomponent Propargylations via Carbon Dioxide Shuttling and Fixation. ACS Catal. 2024, 14, 11646–11656. [Google Scholar] [CrossRef]
- He, C.-Y.; Xie, L.-B.; Ding, R.; Tian, P.; Lin, G.-Q. Copper-catalysed asymmetric silylative cyclization of cyclohexadienone-containing 1,6-enynes. Tetrahedron 2019, 75, 1682–1685. [Google Scholar] [CrossRef]
- Yamamoto, K.; Tsuda, Y.; Kuriyama, M.; Demizu, Y.; Onomura, O. Copper-Catalysed Enantioselective Synthesis of Oxazolines from Aminotriols via Asymmetric Desymmetrization. Chem. Asian J. 2020, 15, 840–844. [Google Scholar] [CrossRef] [PubMed]
- He, C.-Y.; Tan, Y.-X.; Wang, X.; Ding, R.; Wang, Y.-F.; Wang, F.; Gao, D.; Tian, P.; Lin, G.-Q. Copper(I)-catalysed diastereo- and enantioselective construction of optically pure exocyclic allenes. Nature Com. 2020, 11, 4293–4300. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Wang, Z.; Shao, Y.; Sun, J. Copper-Catalysed Tandem Cross-Coupling and Alkynylogous Aldol Reaction: Access to Chiral Exocyclic α-Allenols. Org. Lett. 2021, 23, 5175–5179. [Google Scholar] [CrossRef] [PubMed]
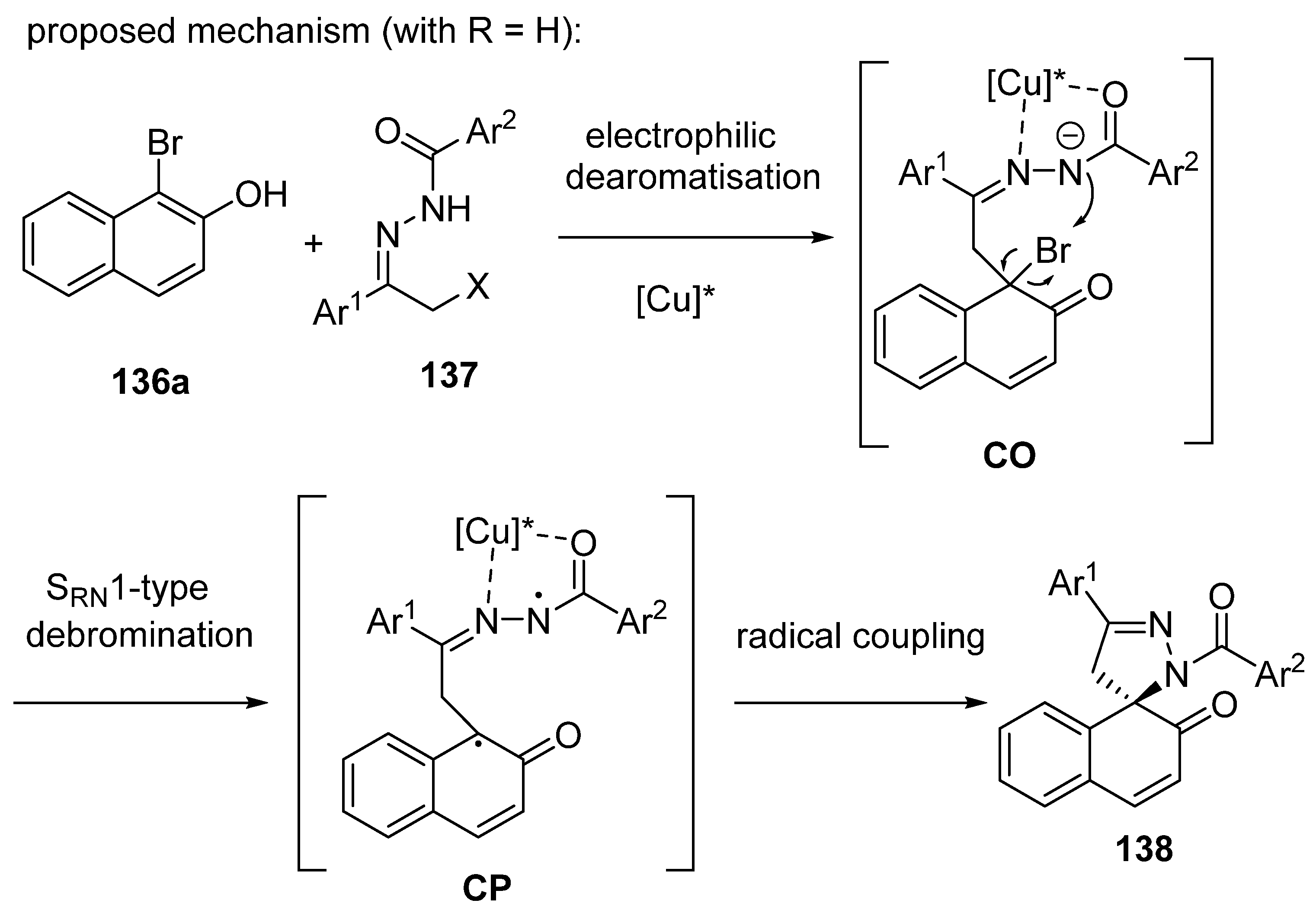
- Bai, L.; Luo, X.; Ge, Y.; Wang, H.; Liu, J.; Wang, Y.; Luan, X. Catalytic Asymmetric [4 + 1] Spiroannulation of α-Bromo-β-Naphthols with Azoalkenes by an Electrophilic Dearomatization/SRN1-Debromination Approach. CCS Chem. 2022, 4, 1054–1064. [Google Scholar] [CrossRef]
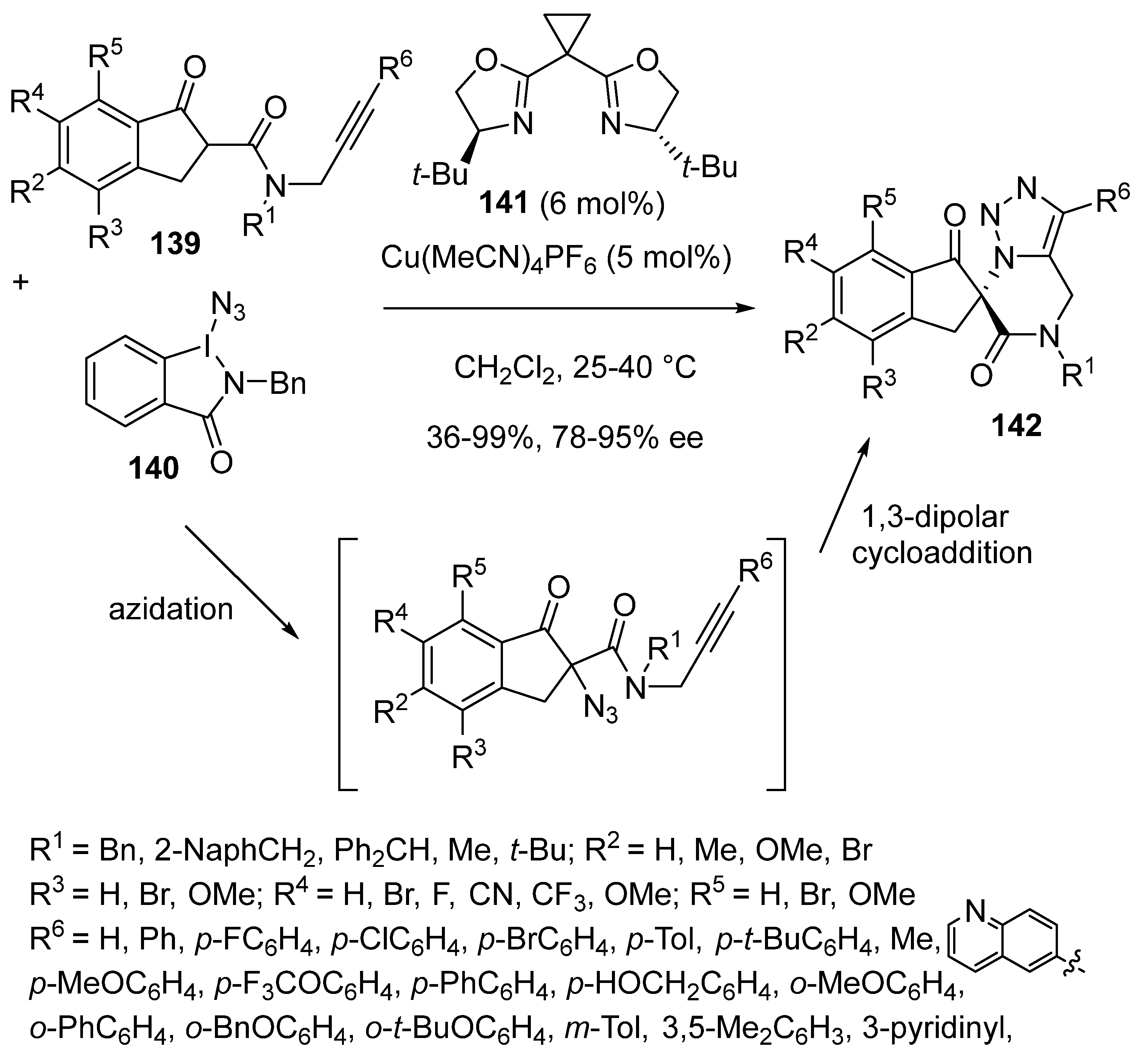
- Jiang, L.-F.; Wu, S.-H.; Jiang, Y.-X.; Ma, H.-X.; He, J.-J.; Bi, Y.-B.; Kong, D.-Y.; Cheng, Y.-F.; Cheng, X.; Deng, Q.-H. Enantioselective copper-catalysed azidation/click cascade reaction for access to chiral 1,2,3-triazoles. Nat. Commun. 2024, 15, 4919–4926. [Google Scholar] [CrossRef]
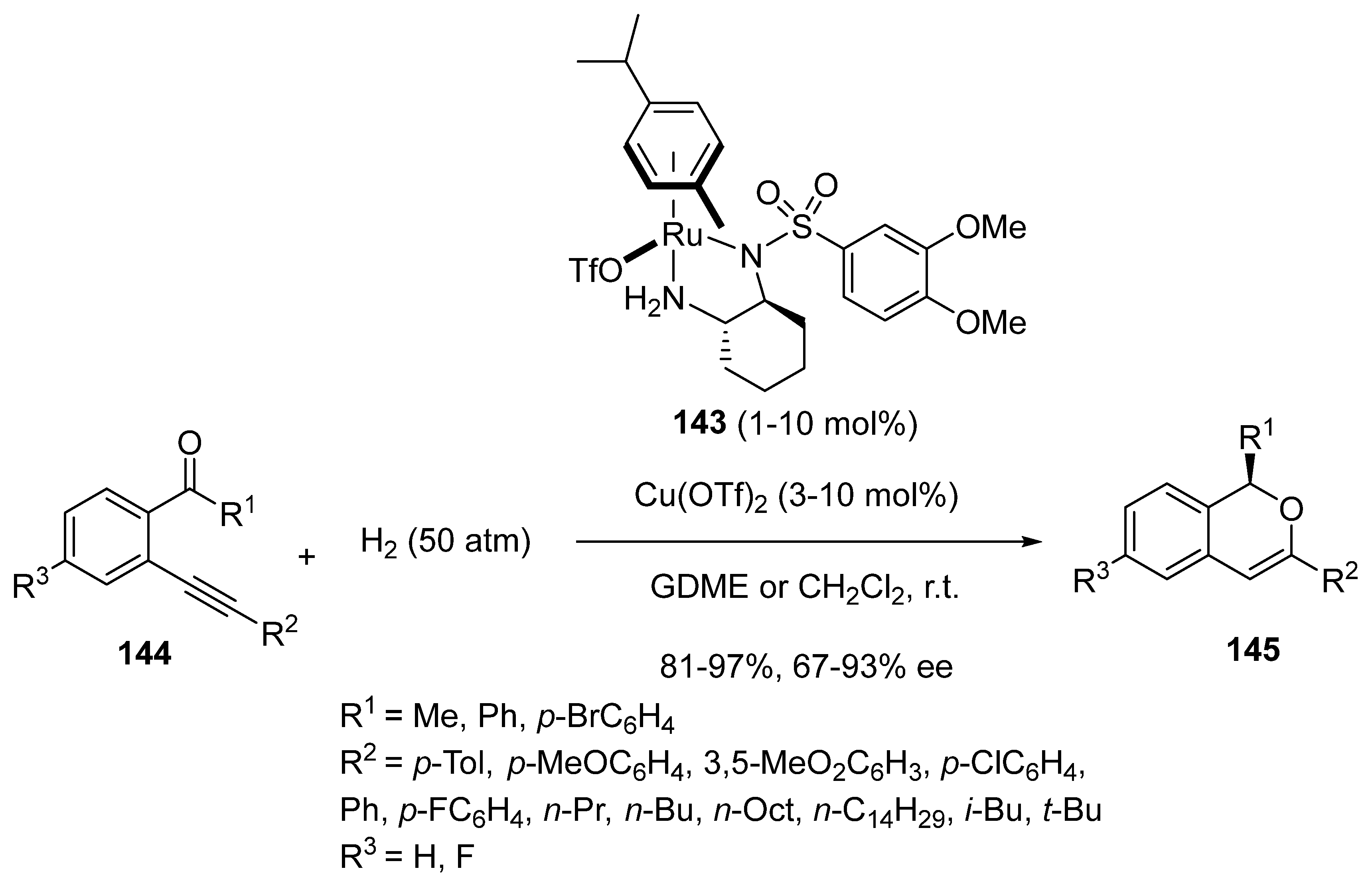
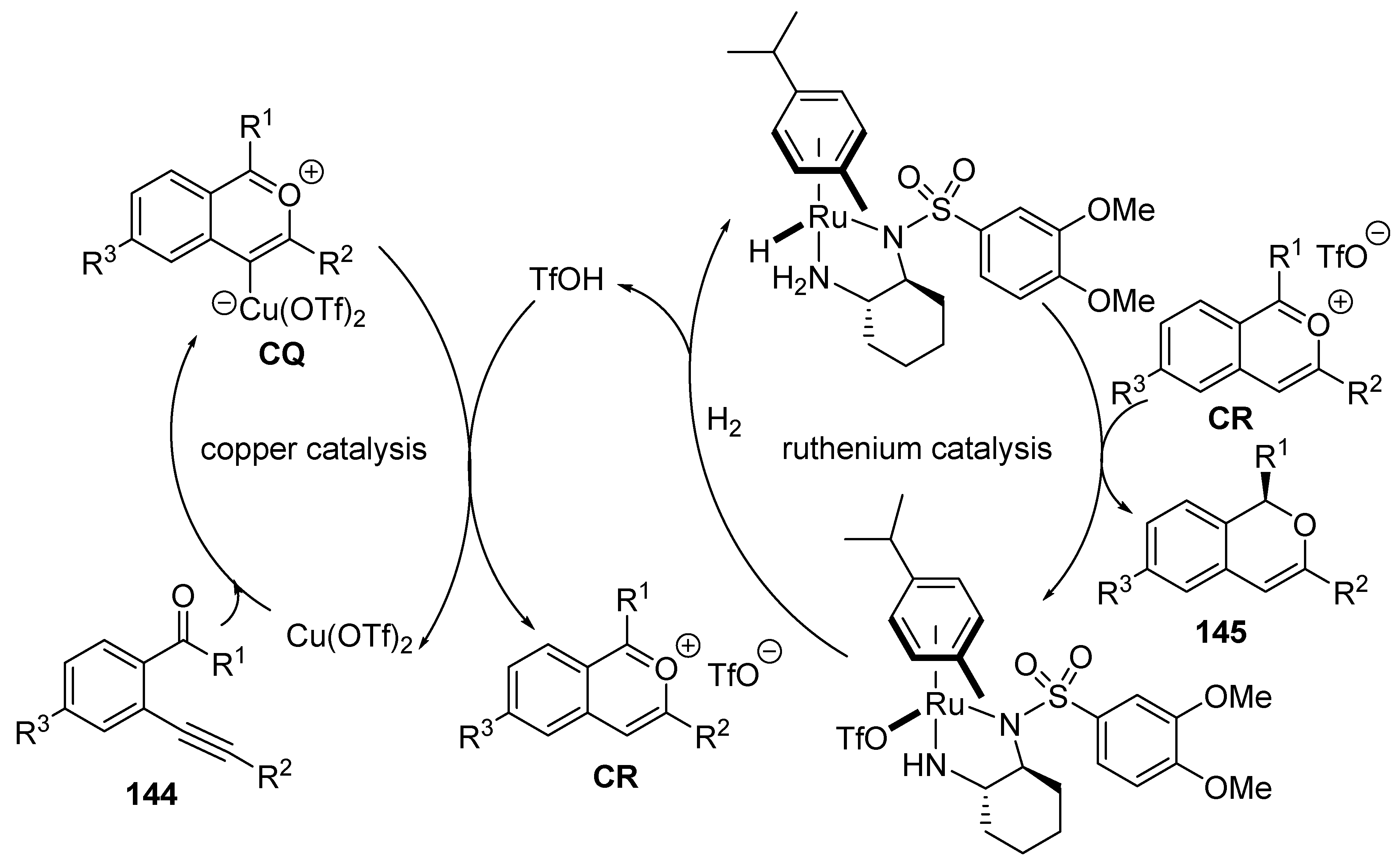
- Miao, T.; Tian, Z.-Y.; He, Y.-M.; Chen, F.; Chen, Y.; Yu, Z.-X.; Fan, Q.-H. Asymmetric Hydrogenation of In Situ Generated Isochromenylium Intermediates by Copper/Ruthenium Tandem Catalysis. Angew. Chem. Int. Ed. 2017, 56, 4135–4139. [Google Scholar] [CrossRef]
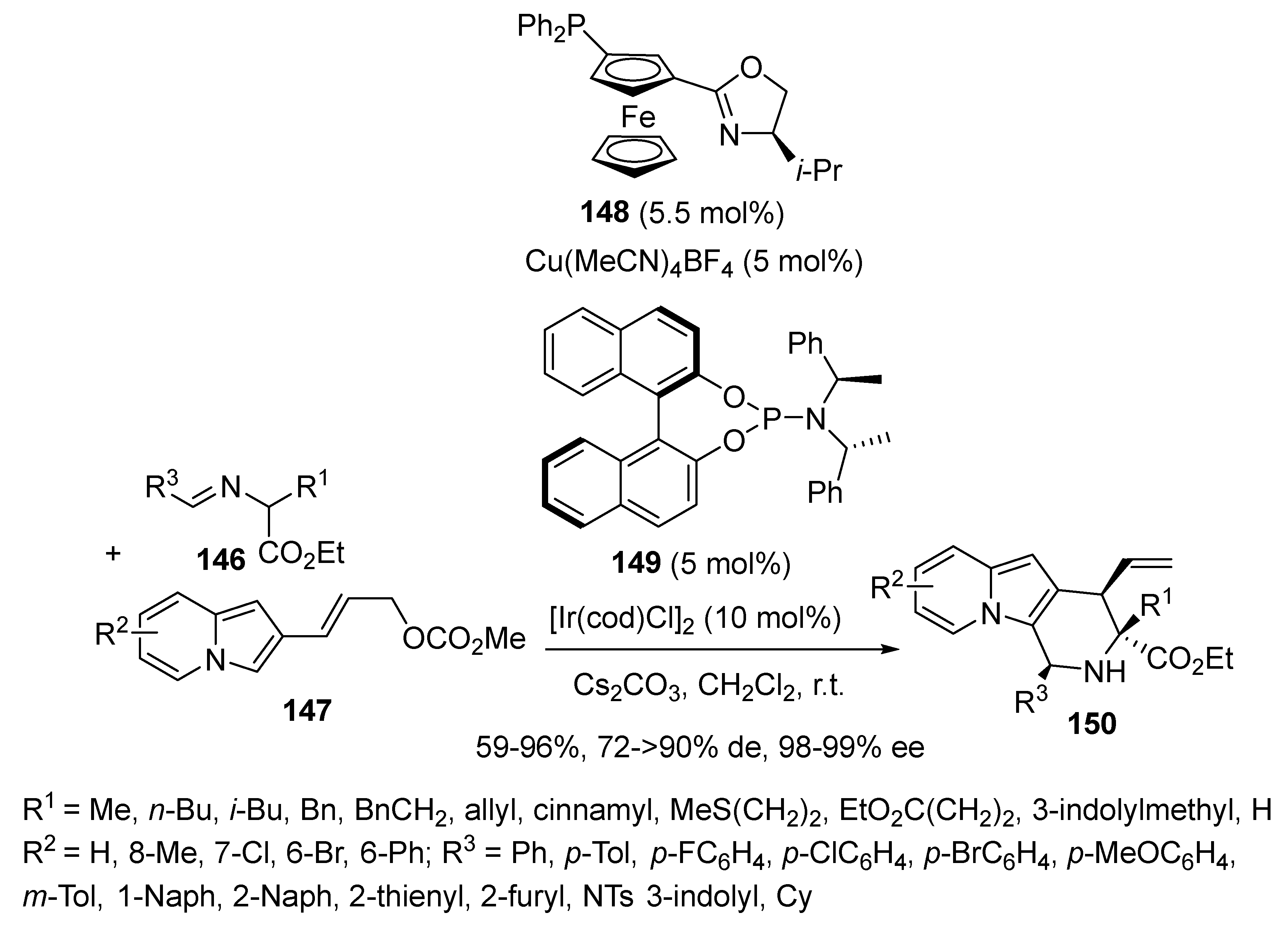
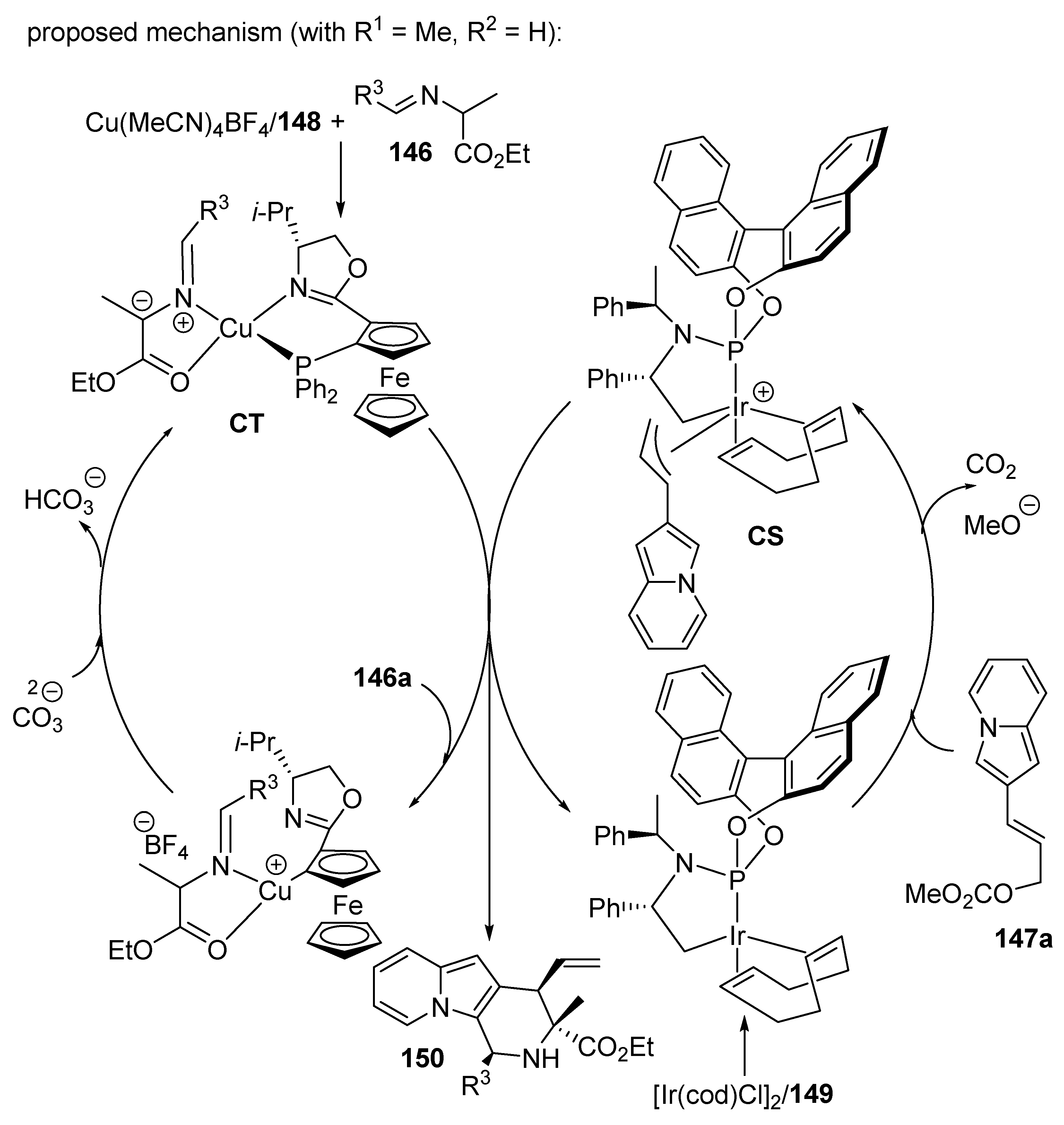
- Zhu, B.-K.; Xu, H.; Xiao, L.; Chang, X.; Wei, L.; Teng, H.; Dang, Y.; Dong, X.-Q.; Wang, C.-J. Enantio- and diastereodivergent synthesis of fused indolizines enabled by synergistic Cu/Ir catalysis. Chem. Sci. 2023, 14, 4134–4142. [Google Scholar] [CrossRef]
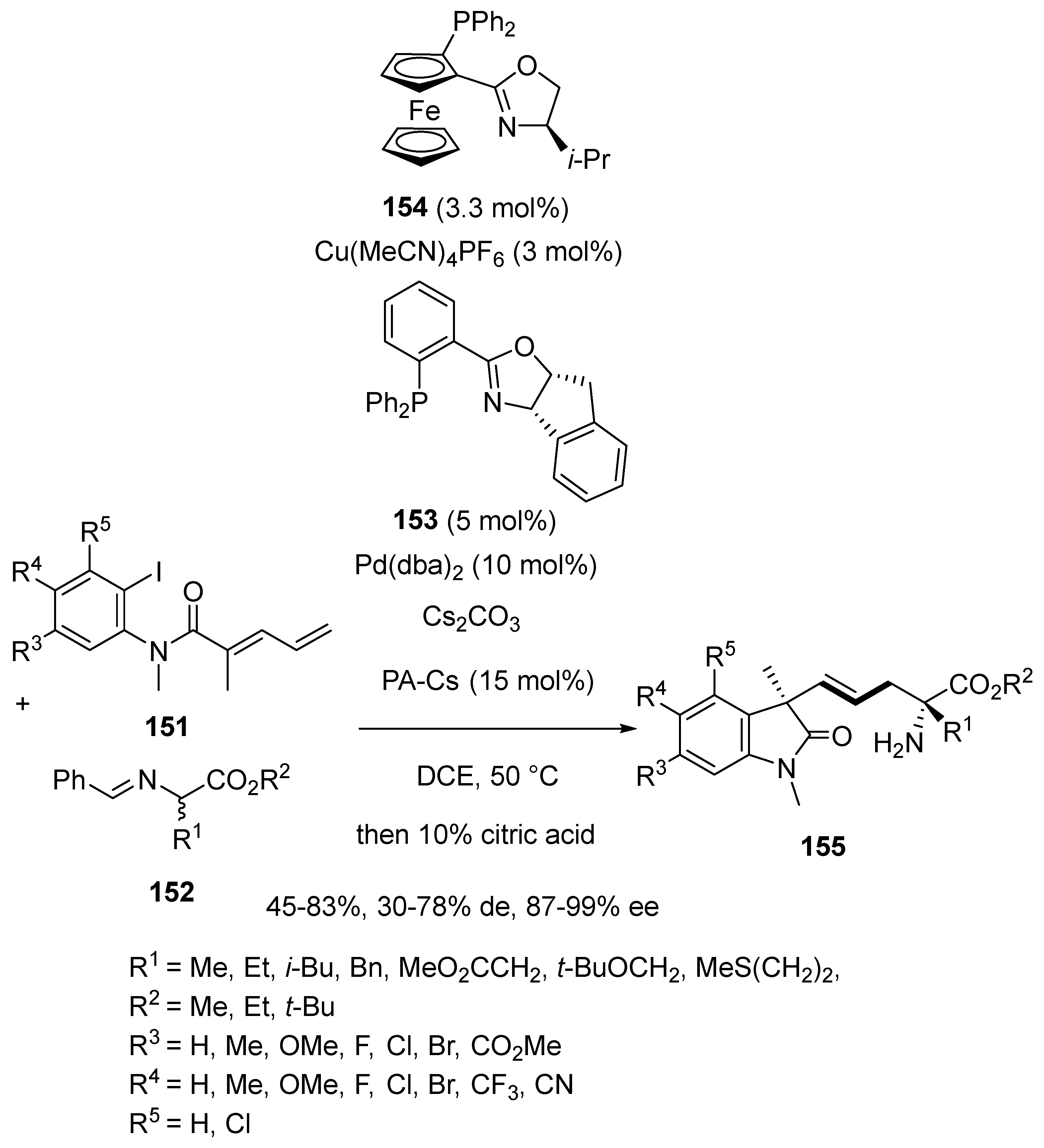
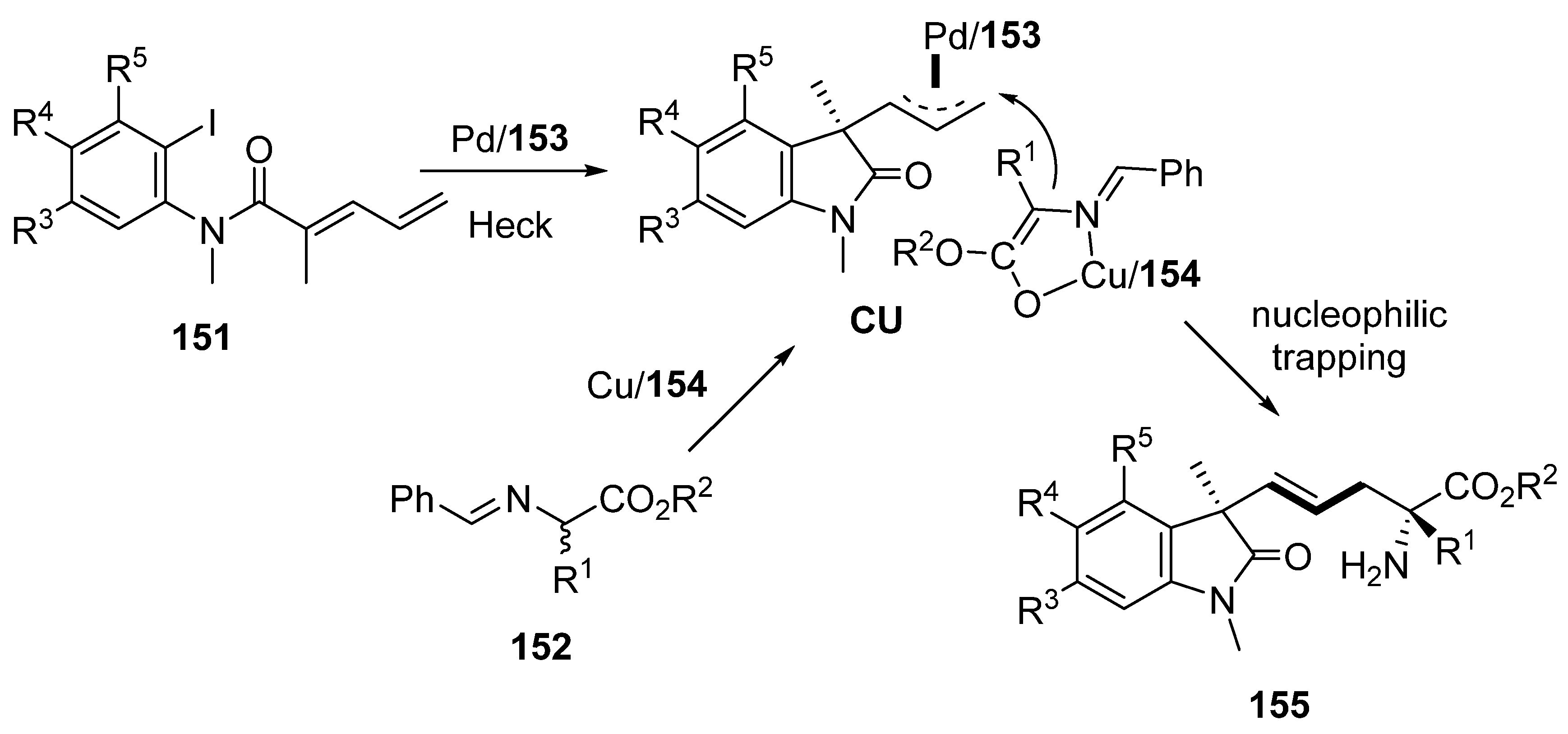
- Li, P.; Liu, Z.; Huo, X.; Zhang, W. Stereodivergent Construction of 1,5/1,7-Nonadjacent Tetrasubstituted Stereocenters Enabled by Pd/Cu-Cocatalysed Asymmetric Heck Cascade Reaction. Angew. Chem. Int. Ed. 2024, 63, e202407498. [Google Scholar] [CrossRef]
- Yang, H.-R.; Cheng, X.; Chang, X.; Wang, Z.-F.; Dong, X.-Q.; Wang, C.-J. Copper/ruthenium relay catalysis enables 1,6-double chiral inductions with stereodivergence. Chem. Sci. 2024, 15, 10135–10145. [Google Scholar] [CrossRef]
- Jin, M.Y.; Li, J.; Huang, R.; Zhou, Y.; Chung, L.W.; Wang, J. Catalytic asymmetric trifluoromethylthiolation of carbonyl compounds via a diastereo and enantioselective Cu-catalysed tandem reaction. Chem. Commun. 2018, 54, 4581–4584. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Hou, C.-J.; Chu, T.-T.; Hu, X.-P. Copper-catalysed asymmetric tandem double Michael reactions of diethylzinc to α,β-unsaturated ketones followed by trapping with nitroolefins. Tetrahedron 2019, 75, 3943–3950. [Google Scholar] [CrossRef]
- Xie, J.-H.; Hou, Y.-M.; Feng, Z.; You, S.-L. Stereodivergent Construction of 1,3-Chiral Centers via Tandem Asymmetric Conjugate Addition and Allylic Substitution Reaction. Angew. Chem. Int. Ed. 2023, 62, e202216396. [Google Scholar] [CrossRef]
- Twilton, J.; Zhang, P.; Shaw, M.H.; Evans, R.W.; MacMillan, D.W.C. The merger of transition metal and photocatalysis. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 2017, 1, 0052. [Google Scholar] [CrossRef]
- Skubi, K.L.; Blum, T.R.; Yoon, T.P. Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev. 2016, 116, 10035–10074. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-J.; Zhang, Y.-H.; Guib, Y.-Y.; Sunb, L.; Yu, D.-G. Merging Transition-Metal Catalysis with Photoredox Catalysis: An Environmentally Friendly Strategy for C–H Functionalization. Synthesis 2018, 50, 3359–3378. [Google Scholar] [CrossRef]
- Guillemard, L.; Wencel-Delord, J. When metal-catalysed C–H functionalization meets visible-light photocatalysis. Beilstein J. Org. Chem. 2020, 16, 1754–1804. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Chen, H.; Zhu, C.; Yu, S. A review of enantioselective dual transition metal/photoredox catalysis. Sci. China Chem. 2020, 63, 637–647. [Google Scholar] [CrossRef]
- Mastandrea, M.M.; Pericàs, M.A. Photoredox Dual Catalysis: A Fertile Playground for the Discovery of New Reactivities. Eur. J. Inorg. Chem. 2021, 2021, 3421–3431. [Google Scholar] [CrossRef]
- Pellissier, H. Domino Reactions through Metallaphotoredox Catalysis. Mini-Rev. Org. Chem. 2025, 22. [Google Scholar] [CrossRef]
- Hossain, A.; Bhattacharyya, A.; Reiser, O. Copper’s rapid ascent in visible-light photoredox catalysis. Science 2019, 364, eaav9713. [Google Scholar] [CrossRef]
- Sha, W.; Deng, L.; Ni, S.; Mei, H.; Han, J.; Pan, Y. Merging Photoredox and Copper Catalysis: Enantioselective Radical Cyanoalkylation of Styrenes. ACS Catal. 2018, 8, 7489–7494. [Google Scholar] [CrossRef]
- Forster, D.; Guo, W.; Wang, Q.; Zhu, J. Dual Photoredox and Copper Catalysis: Enantioselective 1,2-Amidocyanation of 1,3-Dienes. ACS Catal. 2023, 13, 7523–7528. [Google Scholar] [CrossRef]
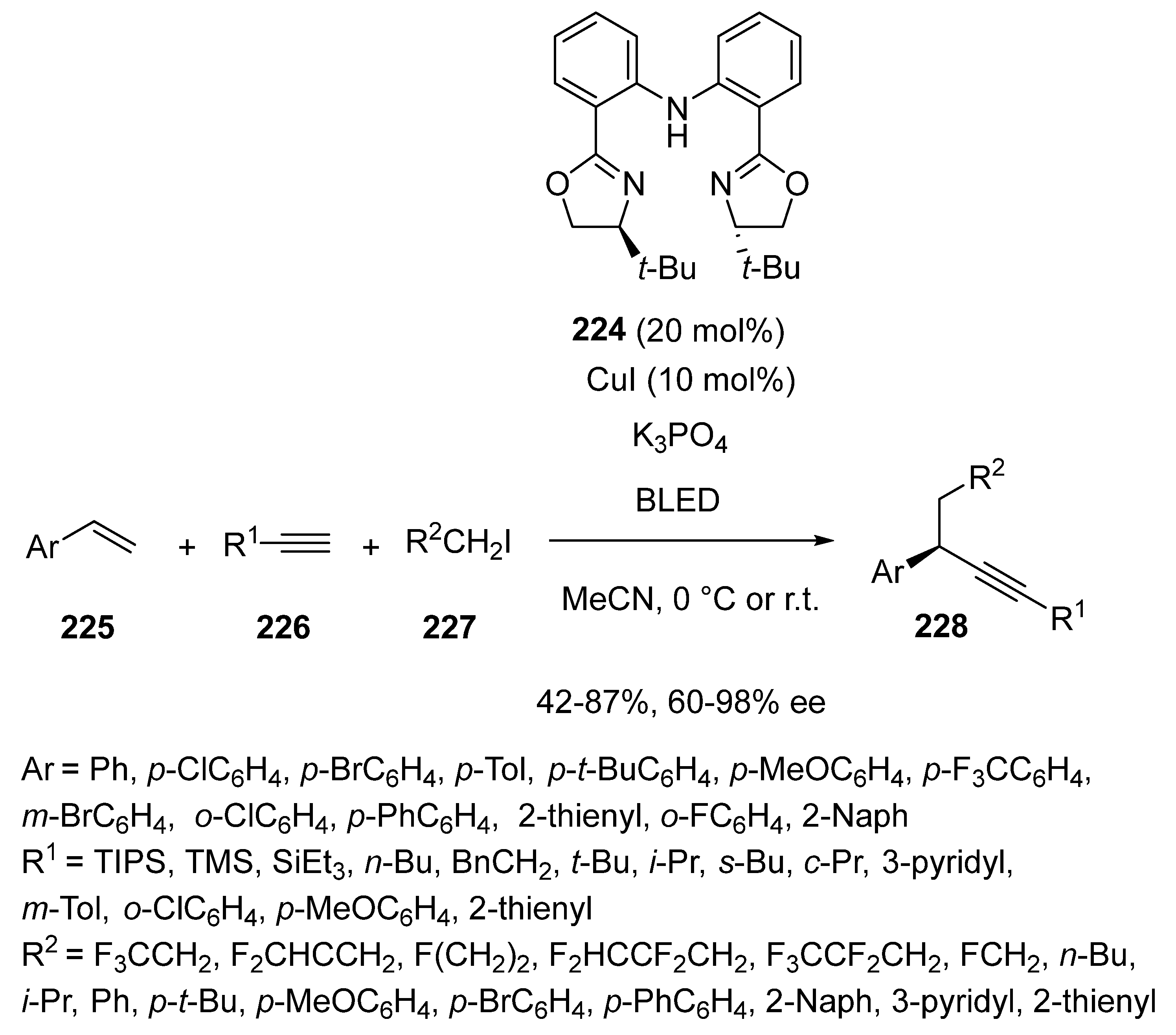
- Lee, J.; Torker, S.; Hoveyda, A.H. Versatile Homoallylic Boronates by Chemo-, SN2′-, Diastereo- and Enantioselective Catalytic Sequence of Cu–H Addition to Vinyl-B(pin)/Allylic Substitution. Angew. Chem. Int. Ed. 2017, 56, 821–826. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.; Li, W.; Zhao, Y.; Wu, X.-F. Regioselective and Enantioselective Copper-Catalysed Hydroaminocarbonylation of Unactivated Alkenes and Alkynes. Angew. Chem. Int. Ed. 2023, 62, e202309993. [Google Scholar] [CrossRef] [PubMed]
- Green, J.C.; Joannou, M.V.; Murray, S.A.; Zanghi, J.M.; Meek, S.J. Enantio- and Diastereoselective Synthesis of Hydroxy Bis(boronates) via Cu-Catalysed Tandem Borylation/1,2-Addition. ACS Catal. 2017, 7, 4441–4445. [Google Scholar] [CrossRef] [PubMed]
- Lia, T.; Yuc, P.; Duc, Y.-M.; Linc, J.-S.; Zhia, Y.; Liu, X.-Y. Enantioselective α-C-H functionalization of amides with indoles triggered by radical trifluoromethylation of alkenes: Highly selective formation of C-CF3 and C-C bonds. J. Fluorine Chem. 2017, 203, 210–214. [Google Scholar] [CrossRef]
- Quintard, A.; Rodriguez, J. Bicatalysed Three-Component Stereoselective Decarboxylative Fluoro-Aldolization for the Construction of Elongated Fluorohydrins. ACS Catal. 2017, 7, 5513–5517. [Google Scholar] [CrossRef]
- Guo, S.; Dong, P.; Chen, Y.; Feng, X.; Liu, X. Chiral Guanidine/Copper Catalysed Asymmetric Azide-Alkyne Cycloaddition/[2 + 2] Cascade Reaction. Angew. Chem. Int. Ed. 2018, 57, 16852–16856. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, L.; Zhou, J.; Wang, X.; Mei, H.; Han, J.; Pan, Y. Synthesis of Chiral Sulfonyl Lactones via Copper-Catalysed Asymmetric Radical Reaction of DABCO⋅(SO2). Adv. Synth. Catal. 2018, 360, 1060–1065. [Google Scholar] [CrossRef]
- Zhang, G.; Fu, L.; Chen, P.; Zou, J.; Liu, G. Proton-Coupled Electron Transfer Enables Tandem Radical Relay for Asymmetric Copper-Catalysed Phosphinoylcyanation of Styrenes. Org. Lett. 2019, 21, 5015–5020. [Google Scholar] [CrossRef]
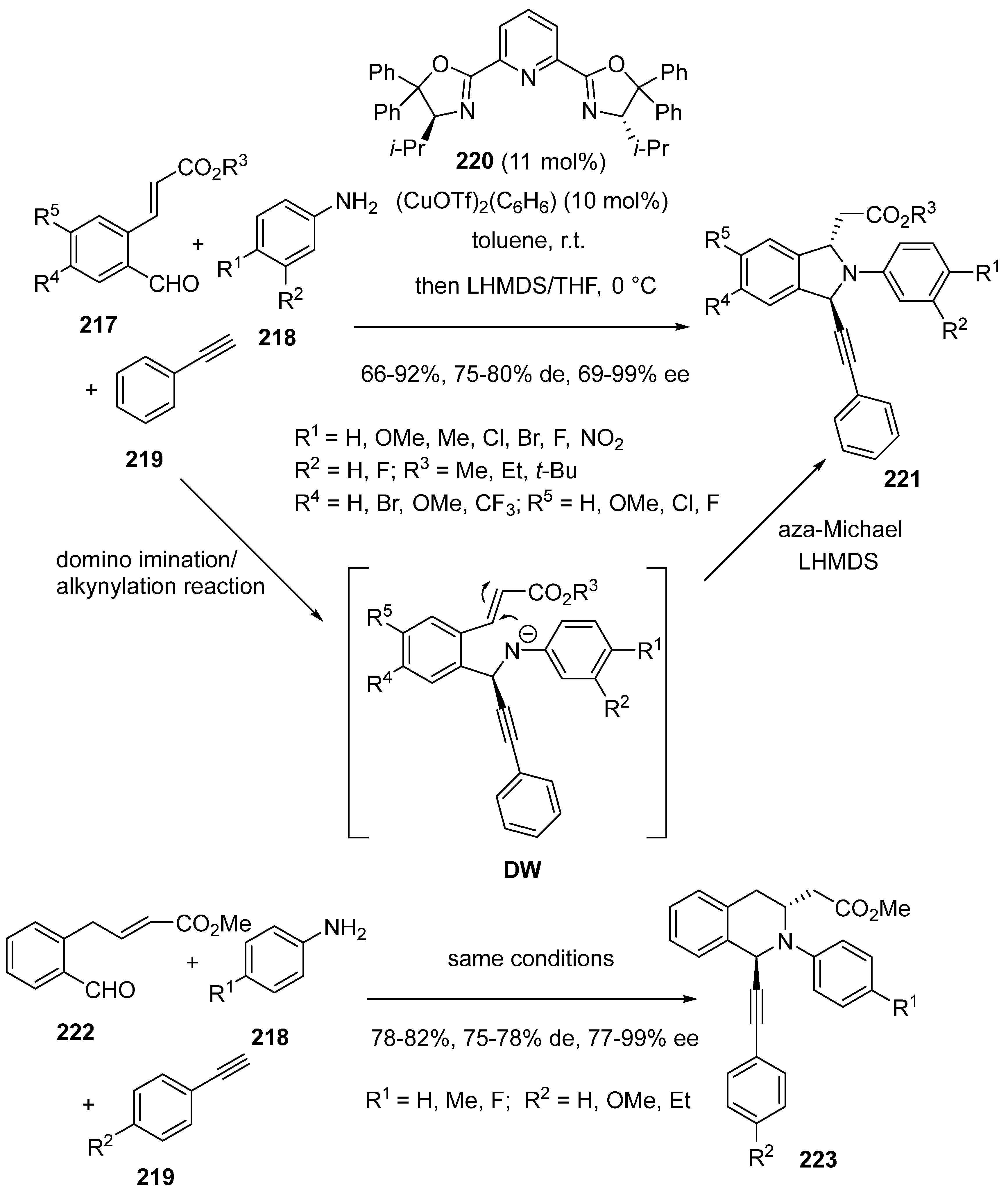
- Das, B.G.; Shah, S.; Singh, V.K. Copper Catalysed One-Pot Three-Component Imination-Alkynylation-aza-Michael Sequence: Enantio- and Diastereoselective Syntheses of 1,3-Disubstituted Isoindolines and Tetrahydroisoquinolines. Org. Lett. 2019, 21, 4981–4985. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Chen, B.; Xu, M.; Li, C.; Zhang, D.; Zhang, G. Copper-Catalysed Photoinduced Enantioselective Dual Carbofunctionalization of Alkenes. Org. Lett. 2020, 22, 1490–1494. [Google Scholar] [CrossRef] [PubMed]
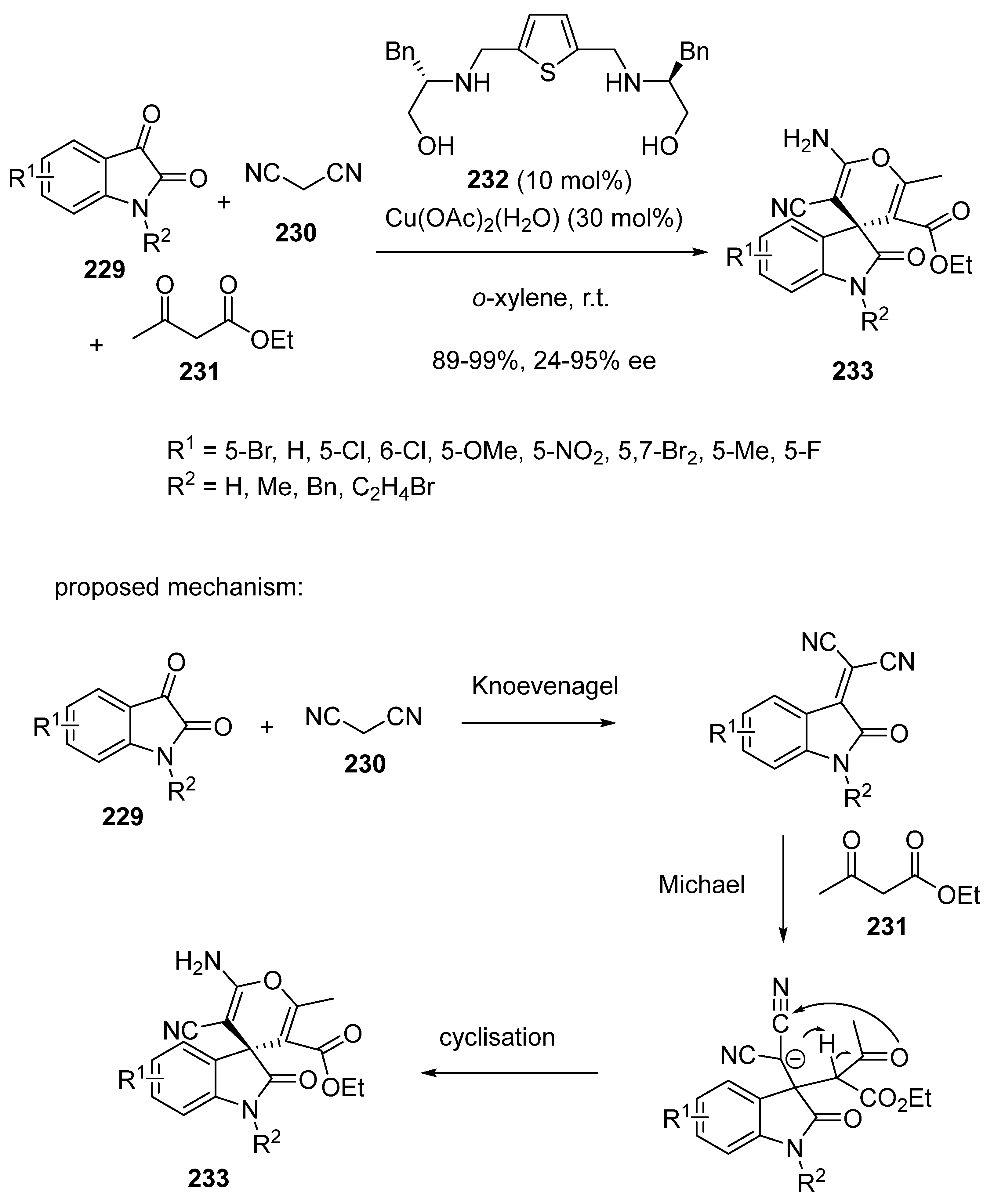
- Al-Majid, A.M.; Alammari, A.S.; Alshahrani, S.; Haukka, M.; Islam, M.S.; Barakat, A. Cu(ii)-thiophene-2,5-bis(amino-alcohol) mediated asymmetric Aldol reaction and Domino Knoevenagel Michael cyclization: A new highly efficient Lewis acid catalyst. RSC Adv. 2022, 12, 6149–6165. [Google Scholar] [CrossRef] [PubMed]
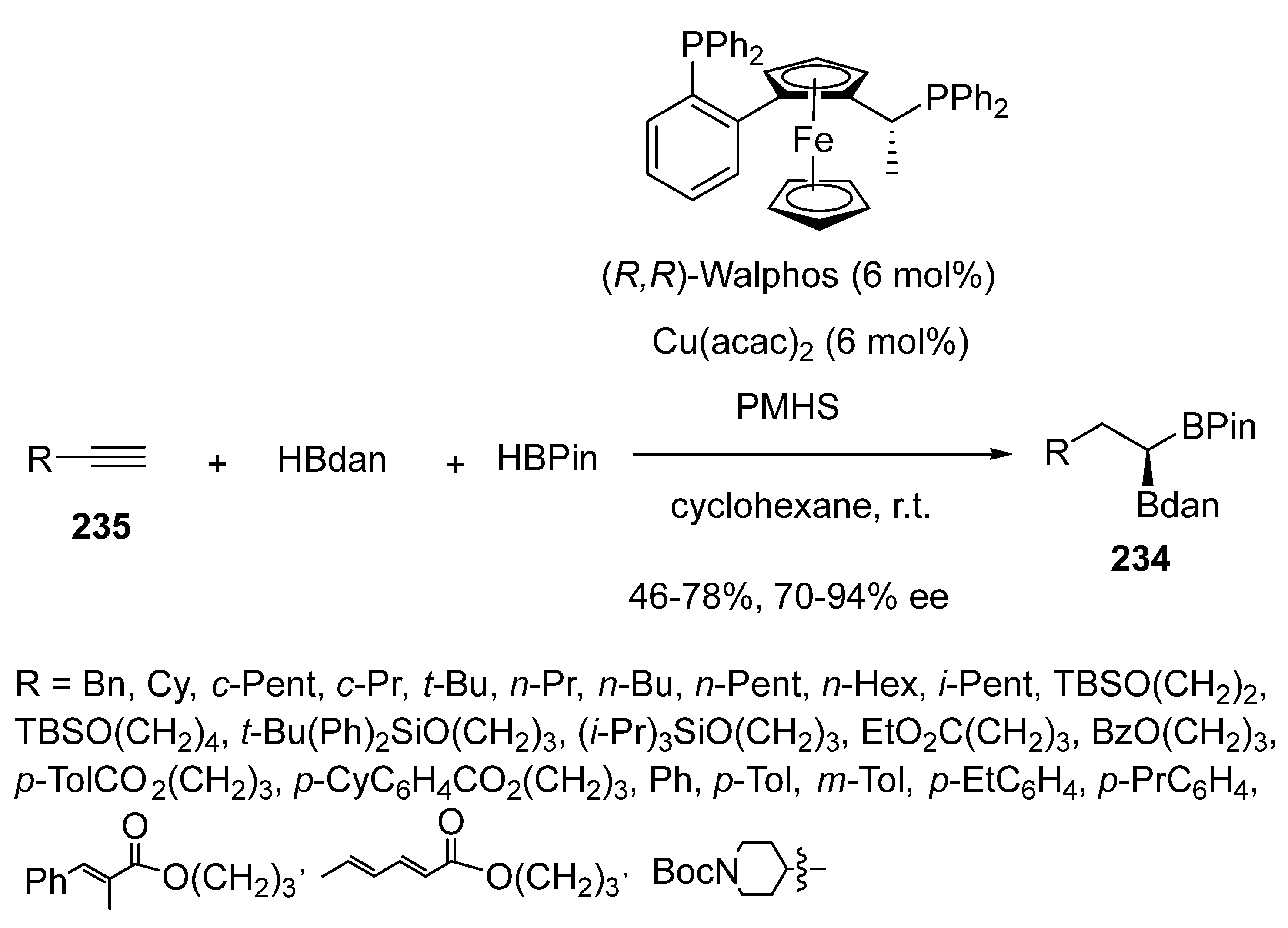
- Jin, S.; Li, J.; Liu, K.; Ding, W.-Y.; Wang, S.; Huang, X.; Li, X.; Yu, P.; Song, Q. Enantioselective Cu-catalysed double hydroboration of alkynes to access chiral gem-diborylalkanes. Nature Com. 2022, 13, 3524–3535. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, Q.; Lee, C.S.; Wang, J. Enantio- and Regioselective Construction of 1,4-Diamines via Cascade Hydroamination of Methylene Cyclopropanes. Angew. Chem. Int. Ed. 2022, 61, e202202160. [Google Scholar] [CrossRef]
- Cheng, X.-Y.; Zhang, Y.-F.; Wang, J.-H.; Gu, Q.-S.; Li, Z.-L.; Liu, X.-Y. A Counterion/Ligand-Tuned Chemo- and Enantioselective Copper-Catalysed Intermolecular Radical 1,2-Carboamination of Alkenes. J. Am. Chem. Soc. 2022, 144, 18081–18089. [Google Scholar] [CrossRef]
- Qi, J.; Song, T.; Yang, Z.; Sun, S.; Tung, C.-H.; Xu, Z. Simultaneous Dual Cu/Ir Catalysis: Stereodivergent Synthesis of Chiral β-Lactams with Adjacent Tertiary/Quaternary/Tertiary Stereocenters. ACS Catal. 2023, 13, 2555–2564. [Google Scholar] [CrossRef]
- Lan, Y.; Liu, P.; Fang, Z.; Shao, L.; Cai, Q.; Wang, X. Well-defined chiral dinuclear copper-catalysed tandem asymmetric propargylic amination–carboxylative cyclization sequence toward chiral 2-oxazolidinone derivatives. Org. Chem. Front. 2024, 11, 6319–6326. [Google Scholar] [CrossRef]
- Yang, F.; Dong, Y.; Wang, J.; Zhang, N.; Guo, H.; Zhang, C. Enantioselective Copper-Catalysed Three-Component Cascade Boronation–Dearomatization Reaction: Synthesis of Chiral Boron-Containing 1,4-Dihydropyridines. Org. Lett. 2025, 27, 857–862. [Google Scholar] [CrossRef]
- Fan, T.-G.; Sun, B.-X.; Jiang, W.; Hu, J.; Yang, L.-B.; Qu, Y.; Li, Y.-M. A copper-catalyzed enantioselective cycloaddition/amination cascade of azomethine imines, alkynes and O-benzoylhydroxylamines. Org. Chem. Front. 2025, 12, 3184–3190. [Google Scholar] [CrossRef]
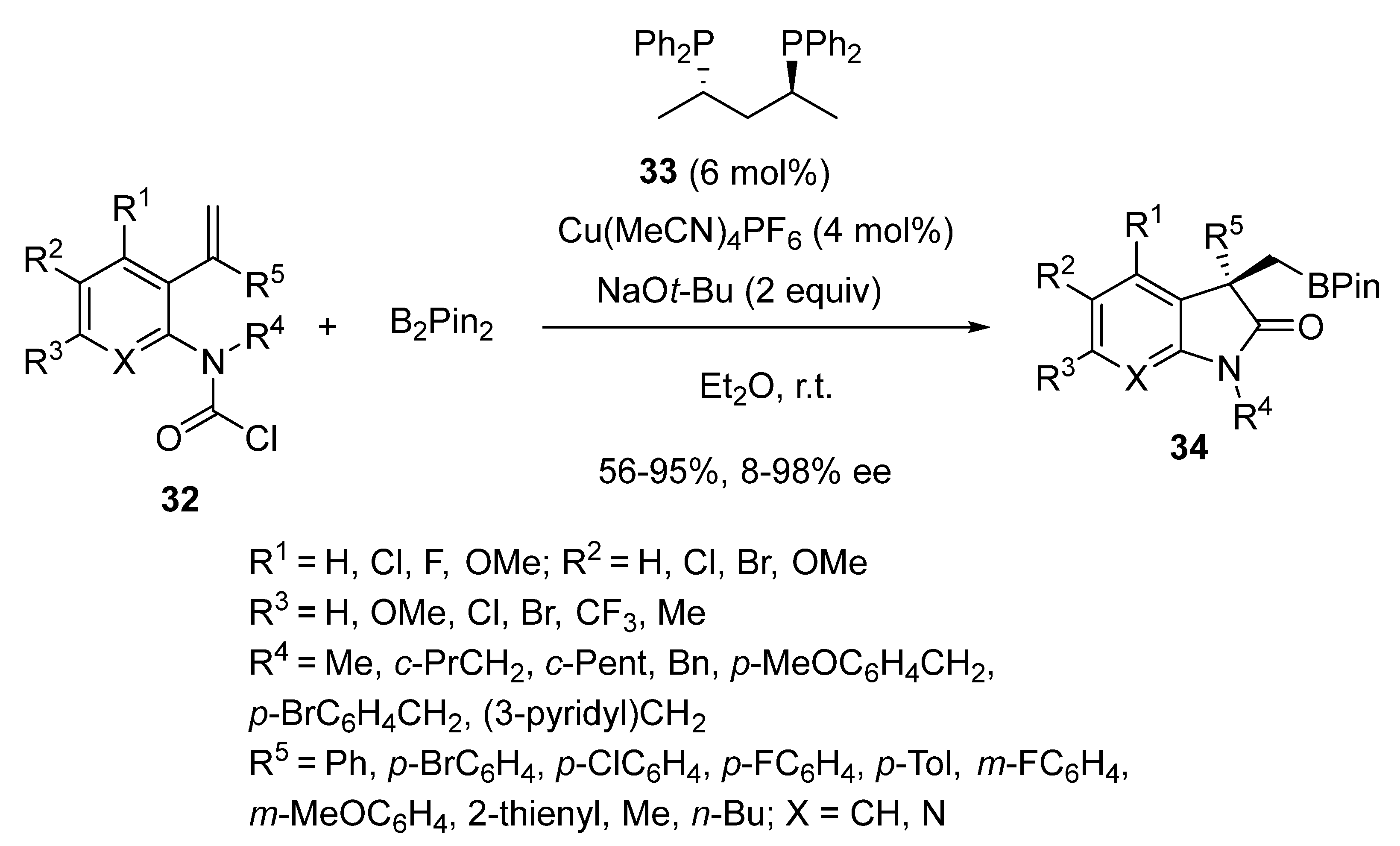


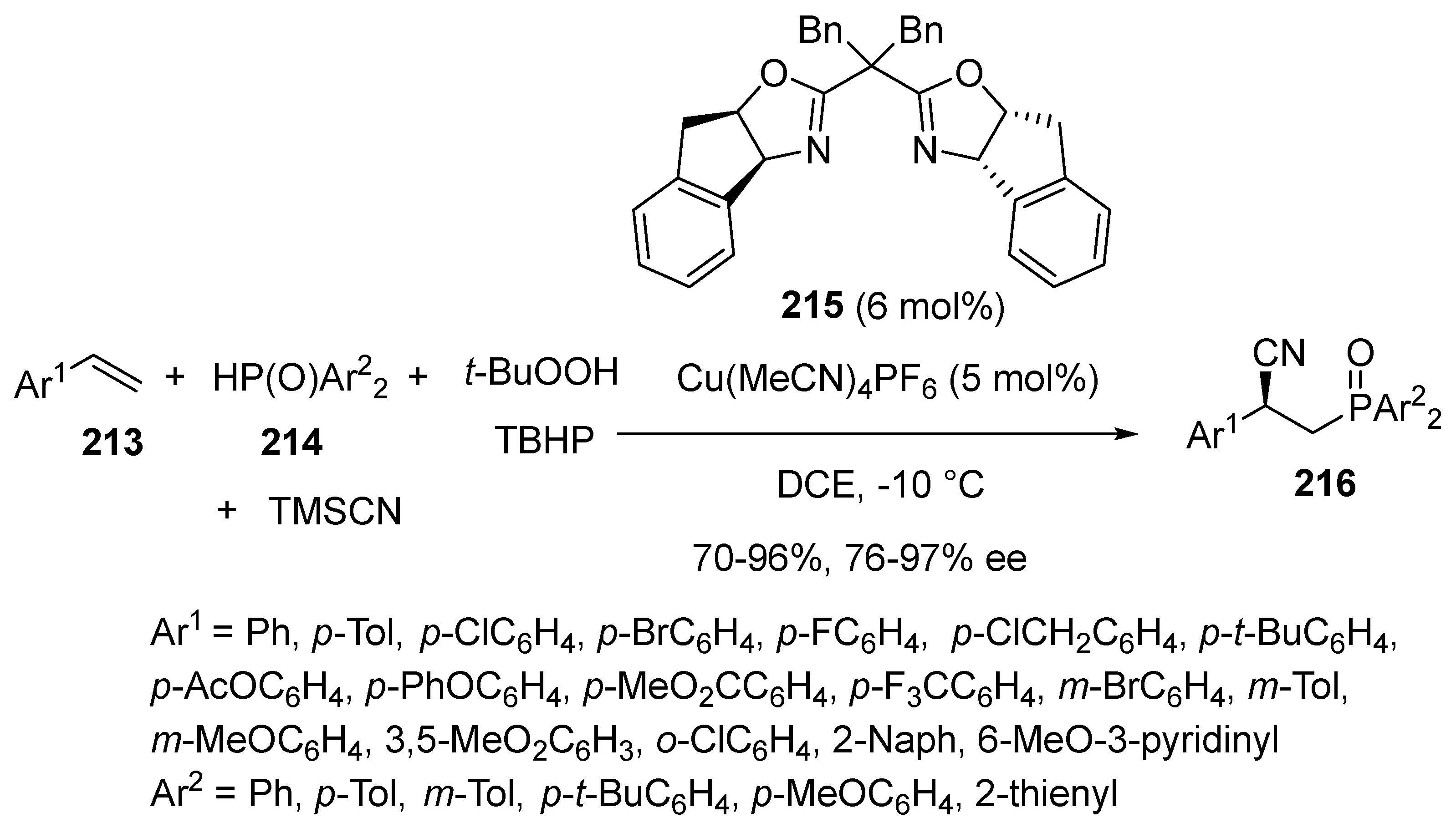
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellissier, H. Chiral Copper Catalysis in Enantioselective Domino Reactions. Molecules 2025, 30, 3654. https://doi.org/10.3390/molecules30173654
Pellissier H. Chiral Copper Catalysis in Enantioselective Domino Reactions. Molecules. 2025; 30(17):3654. https://doi.org/10.3390/molecules30173654
Chicago/Turabian StylePellissier, Hélène. 2025. "Chiral Copper Catalysis in Enantioselective Domino Reactions" Molecules 30, no. 17: 3654. https://doi.org/10.3390/molecules30173654
APA StylePellissier, H. (2025). Chiral Copper Catalysis in Enantioselective Domino Reactions. Molecules, 30(17), 3654. https://doi.org/10.3390/molecules30173654







