Recent Advances in the Synthesis and Applications of Nitrogen-Containing Macrocyclic Arenes
Abstract
1. Introduction
2. Synthesis of Nitrogen-Containing Macrocyclic Arenes
2.1. Macrocyclic Arenes with Non-Bridging Nitrogen
2.2. Macrocyclic Arenes with Nitrogen-Bridging Macrocycles
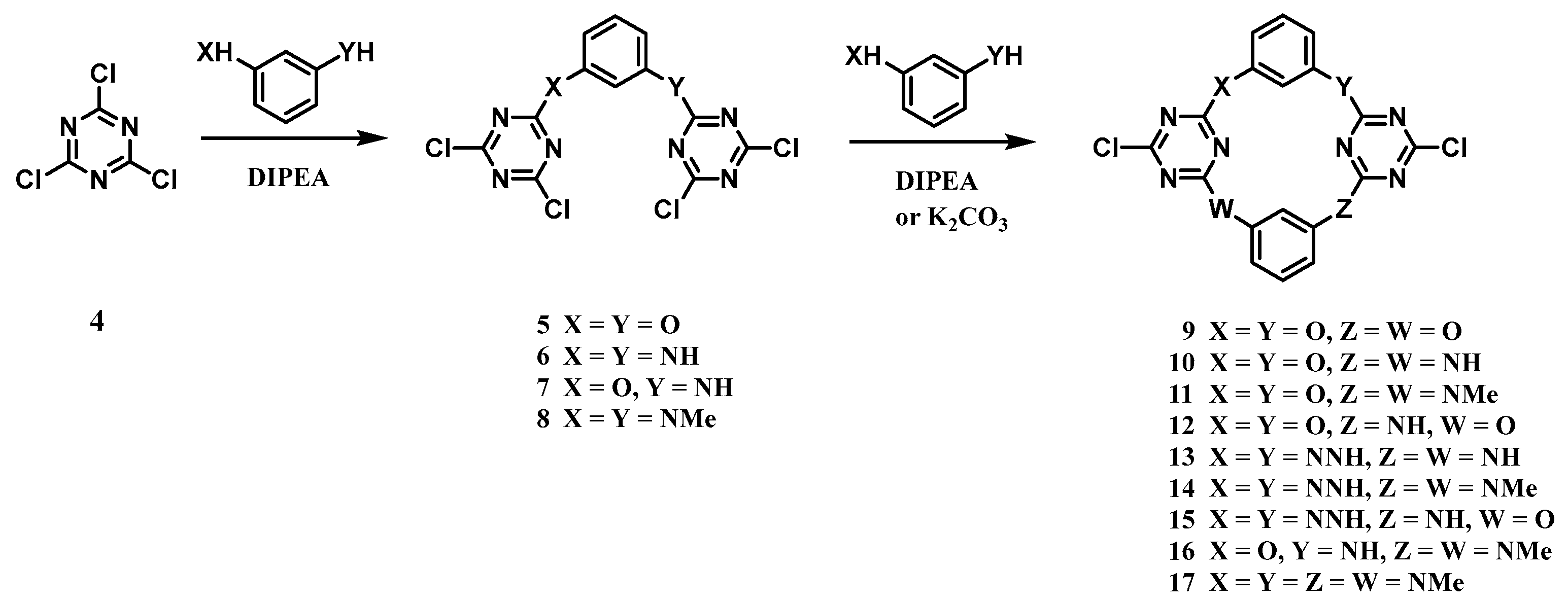
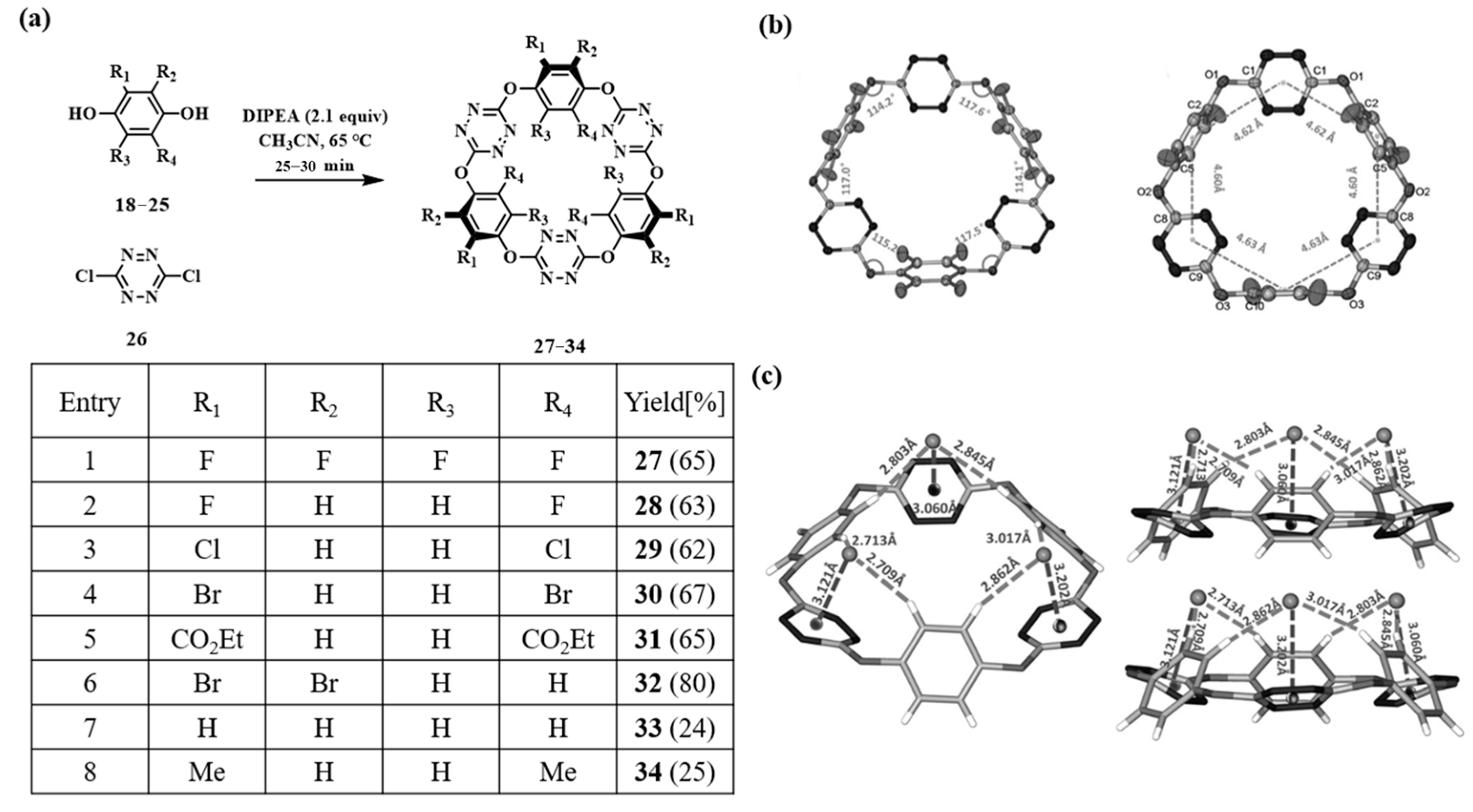
2.2.1. Nucleophilic Reaction
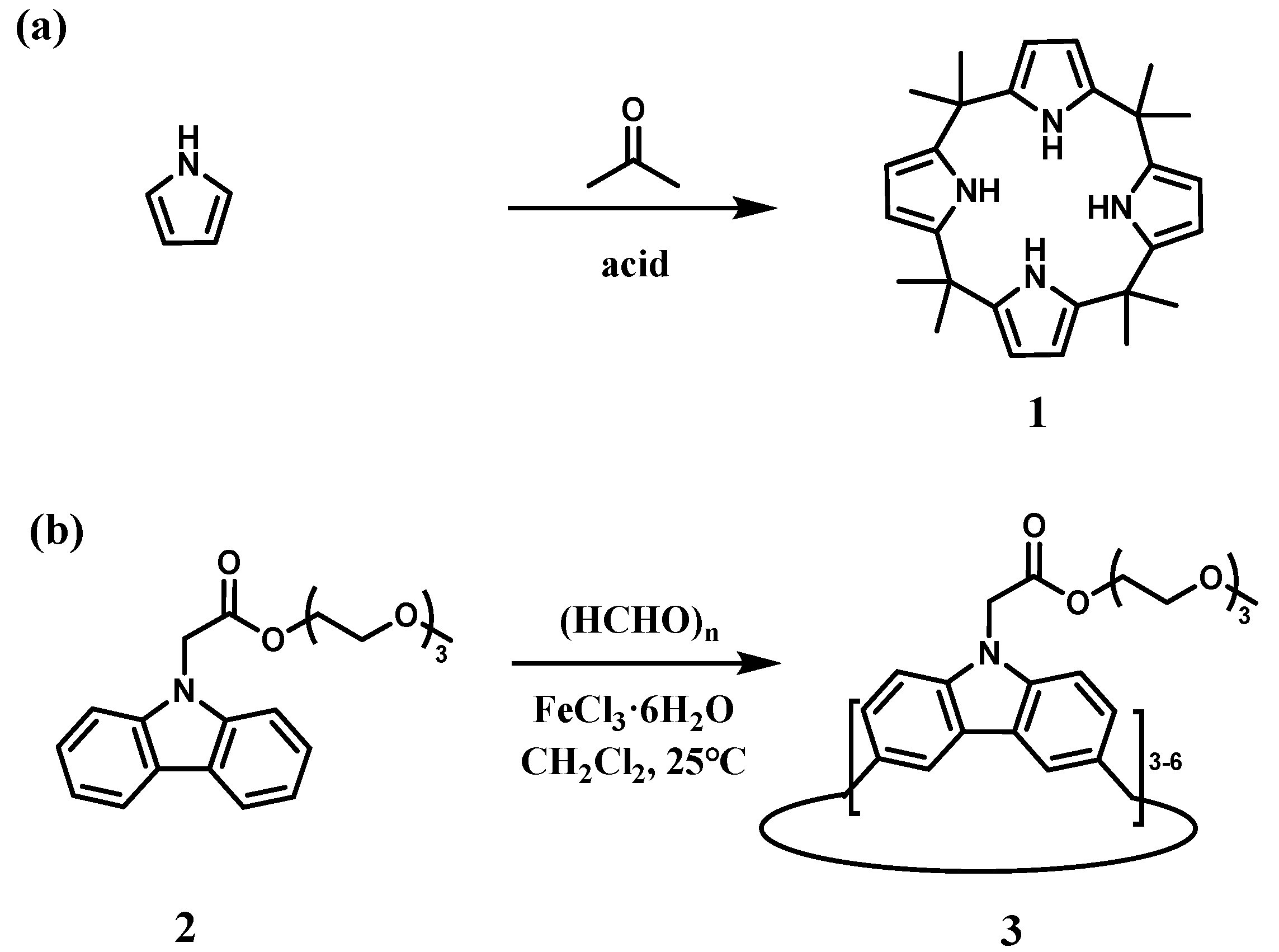
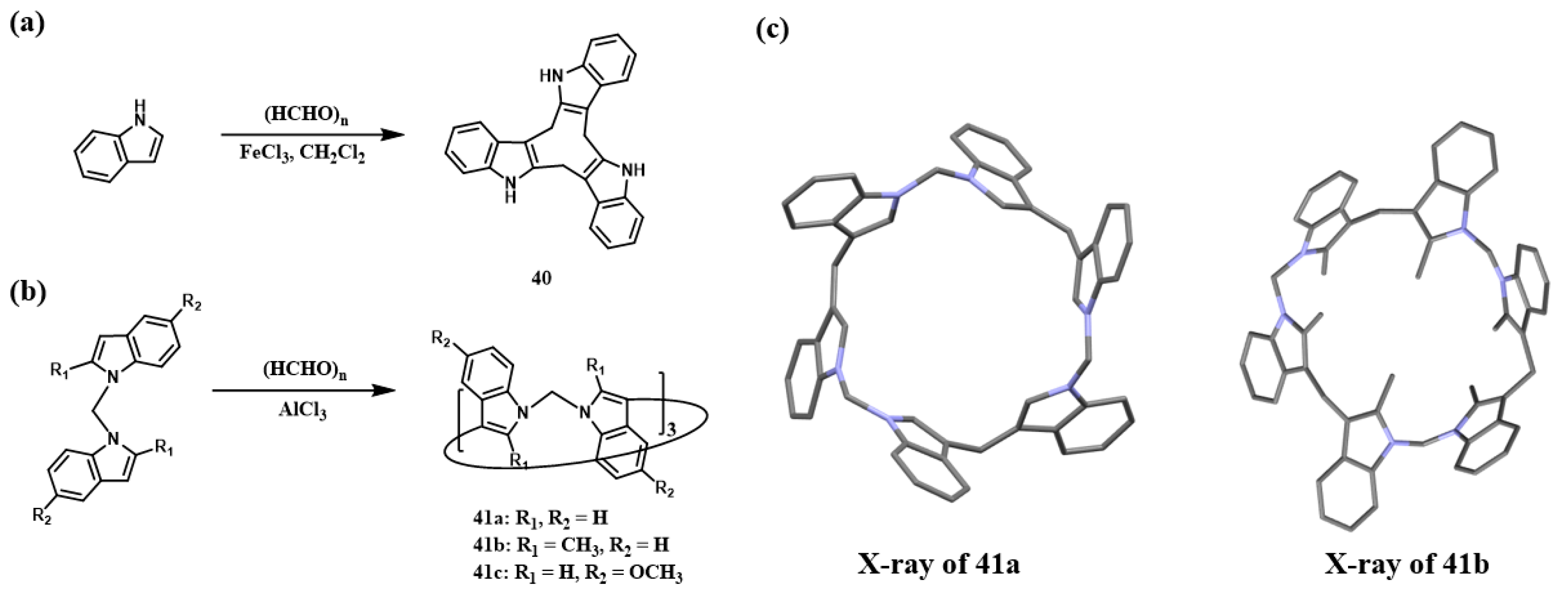
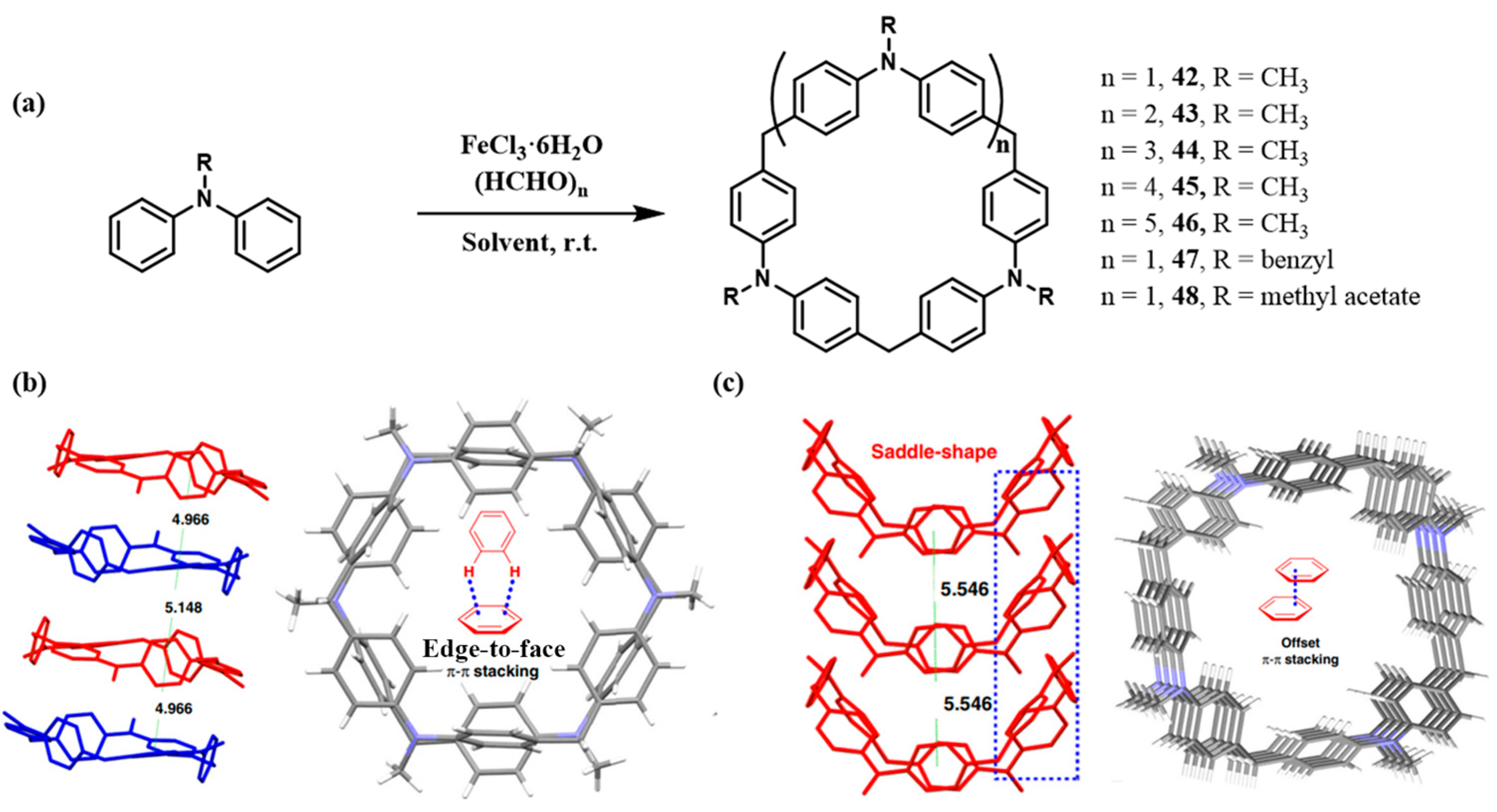
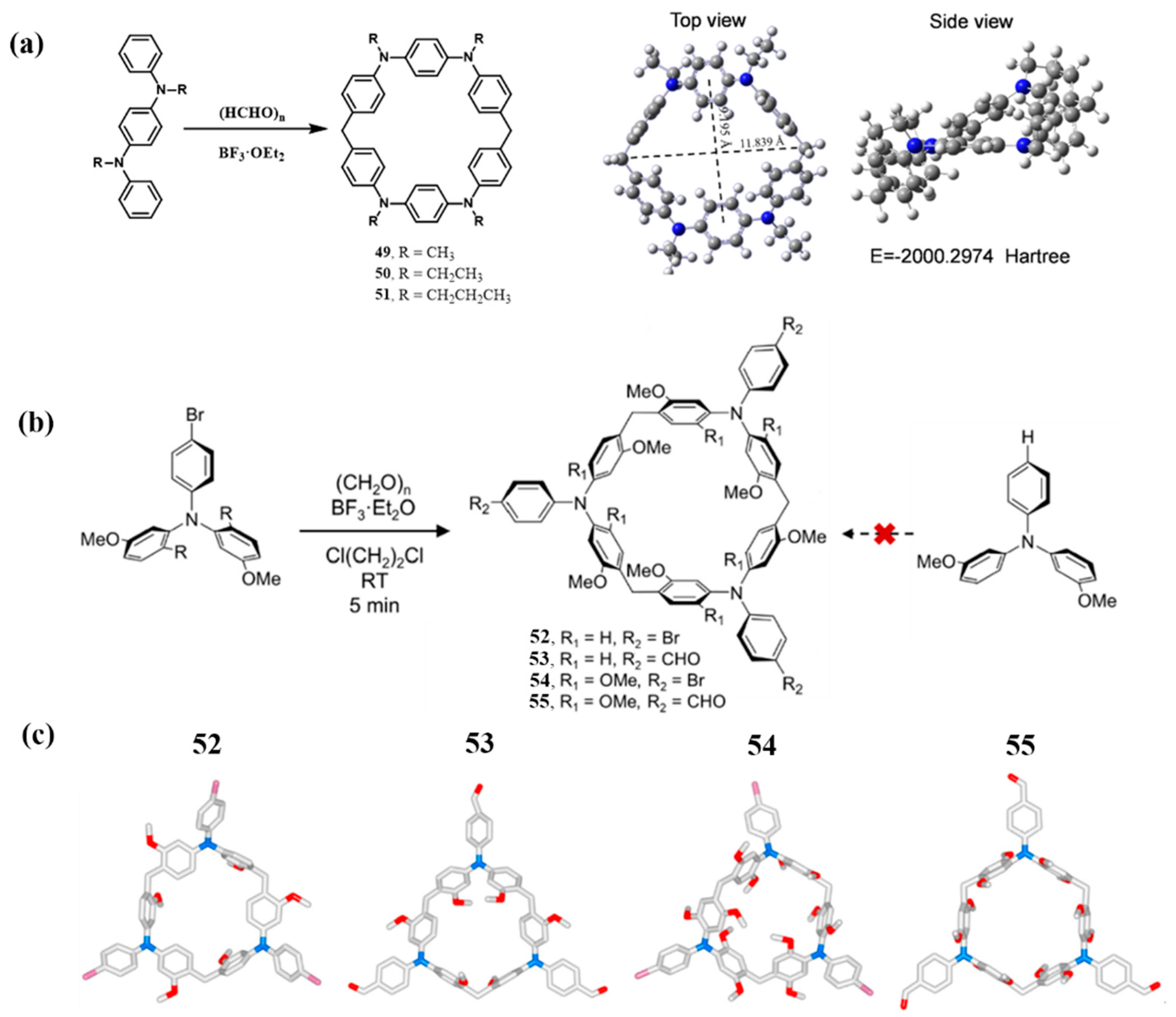
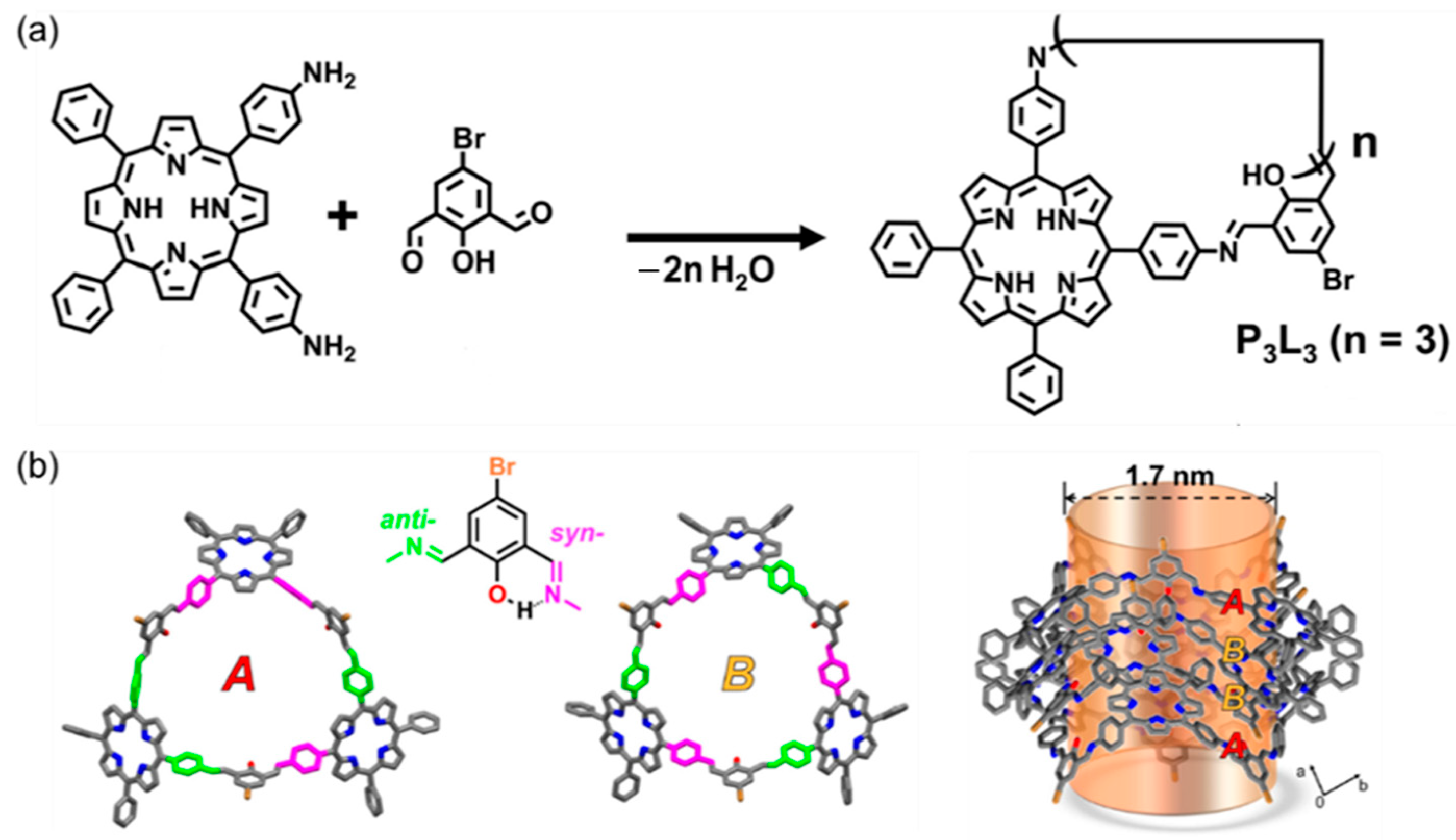
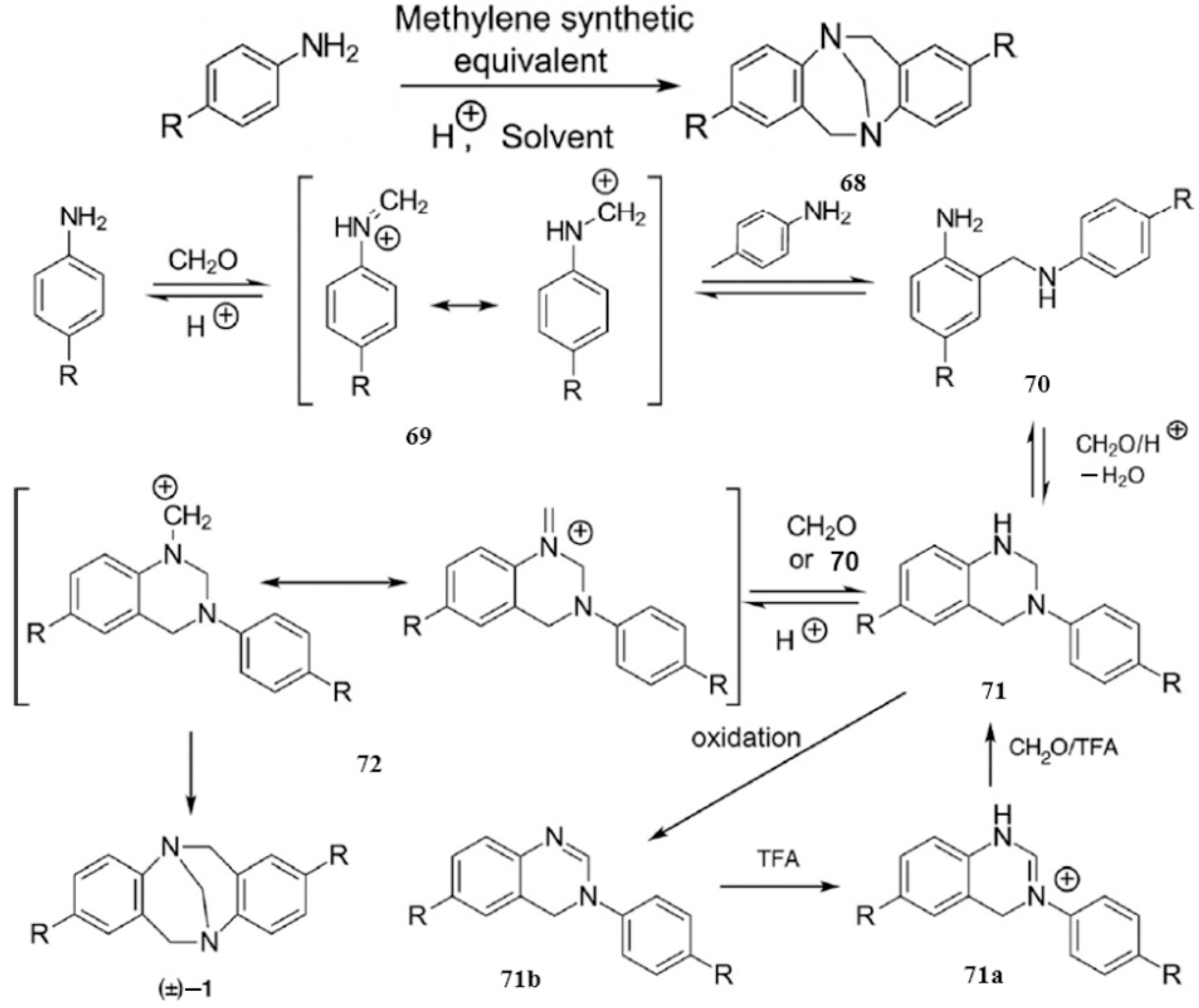
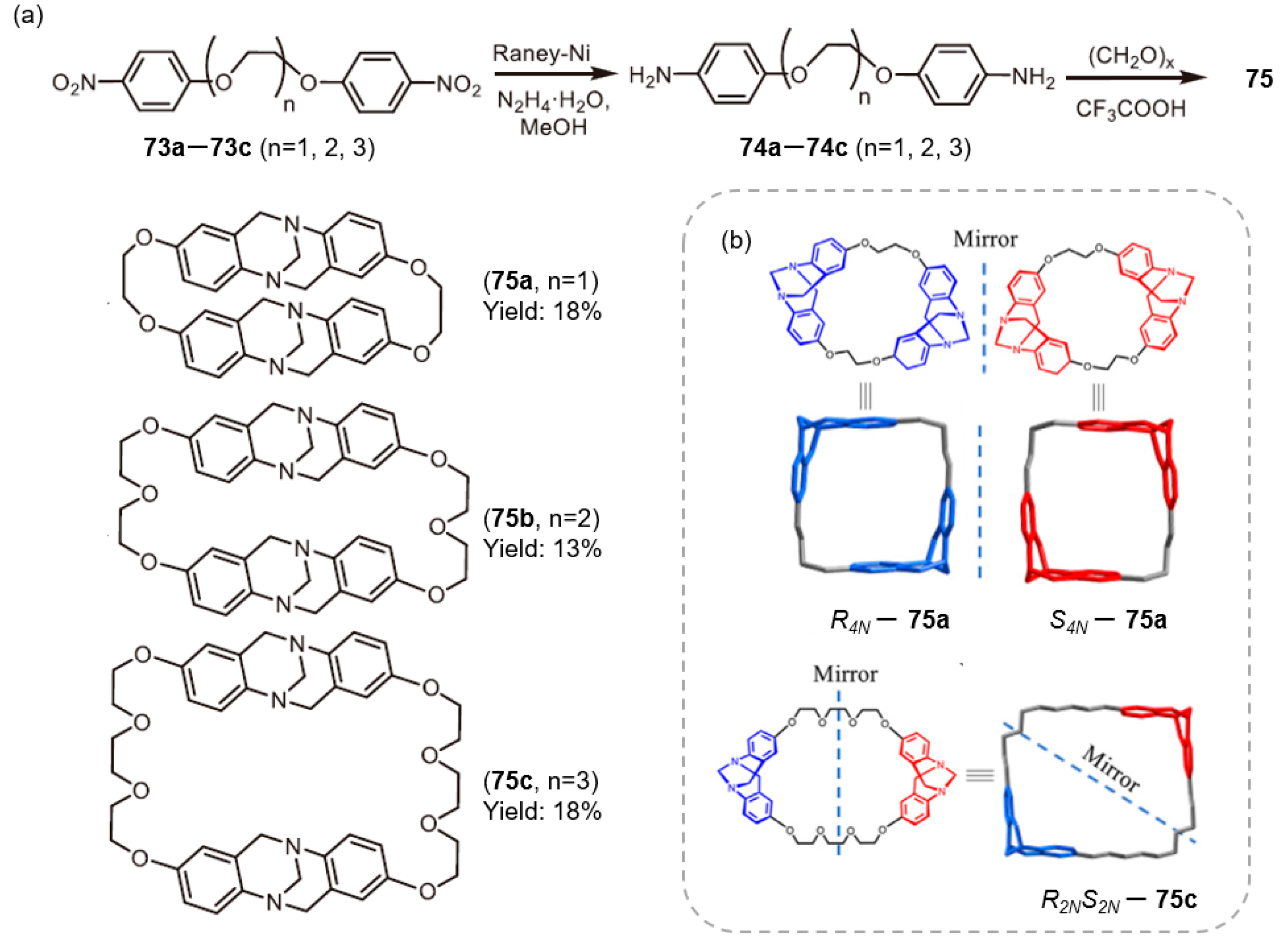
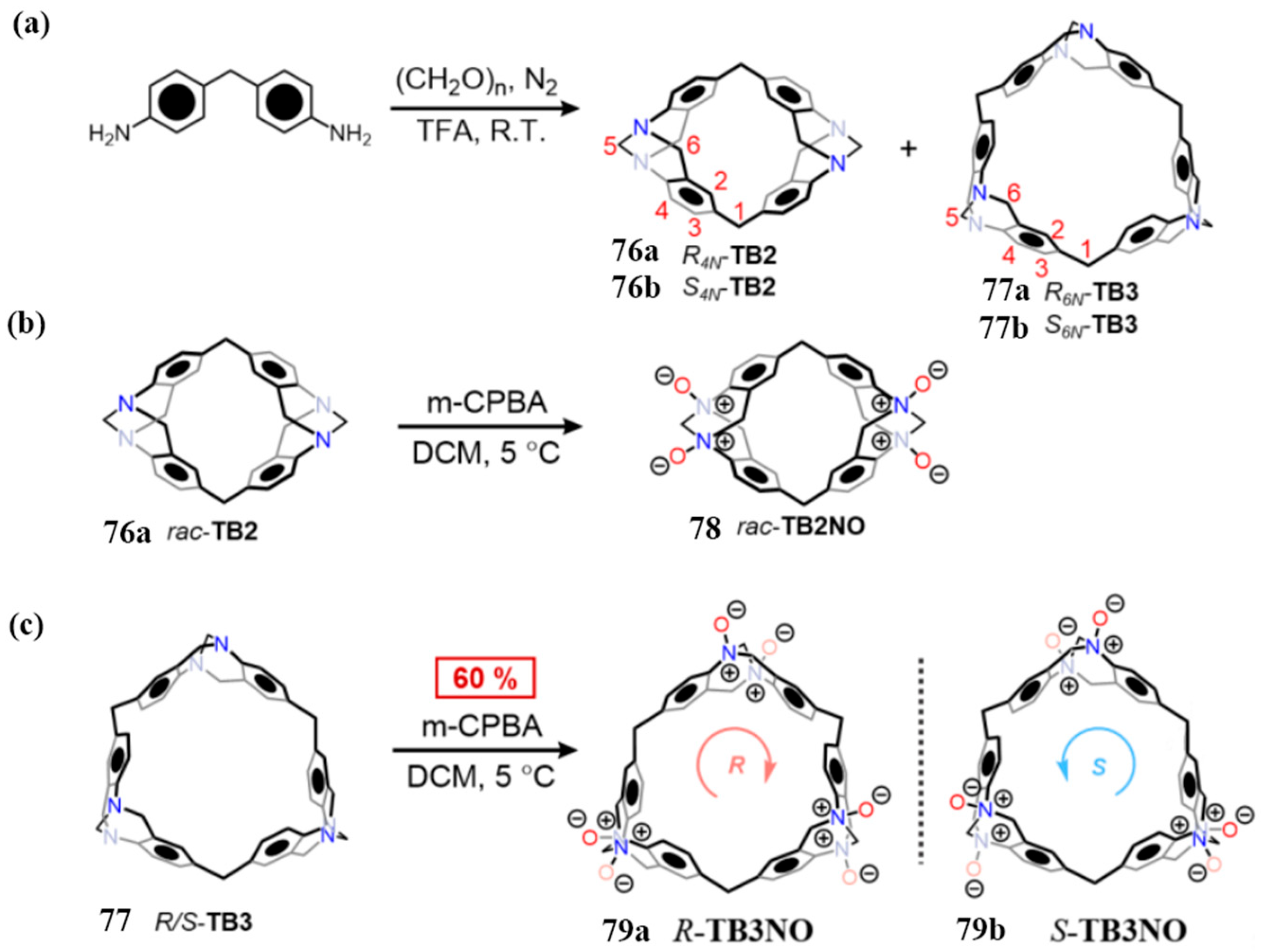
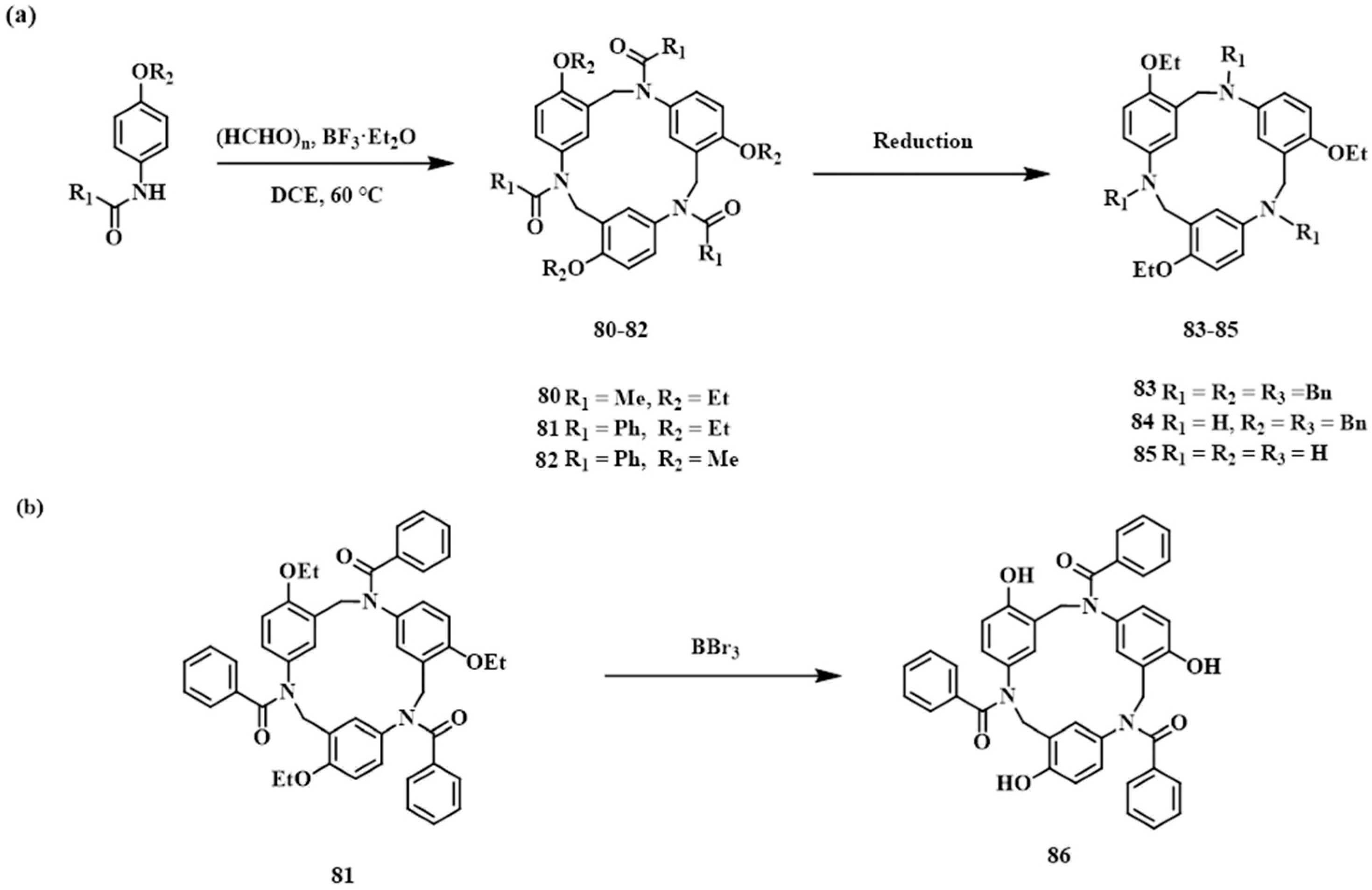
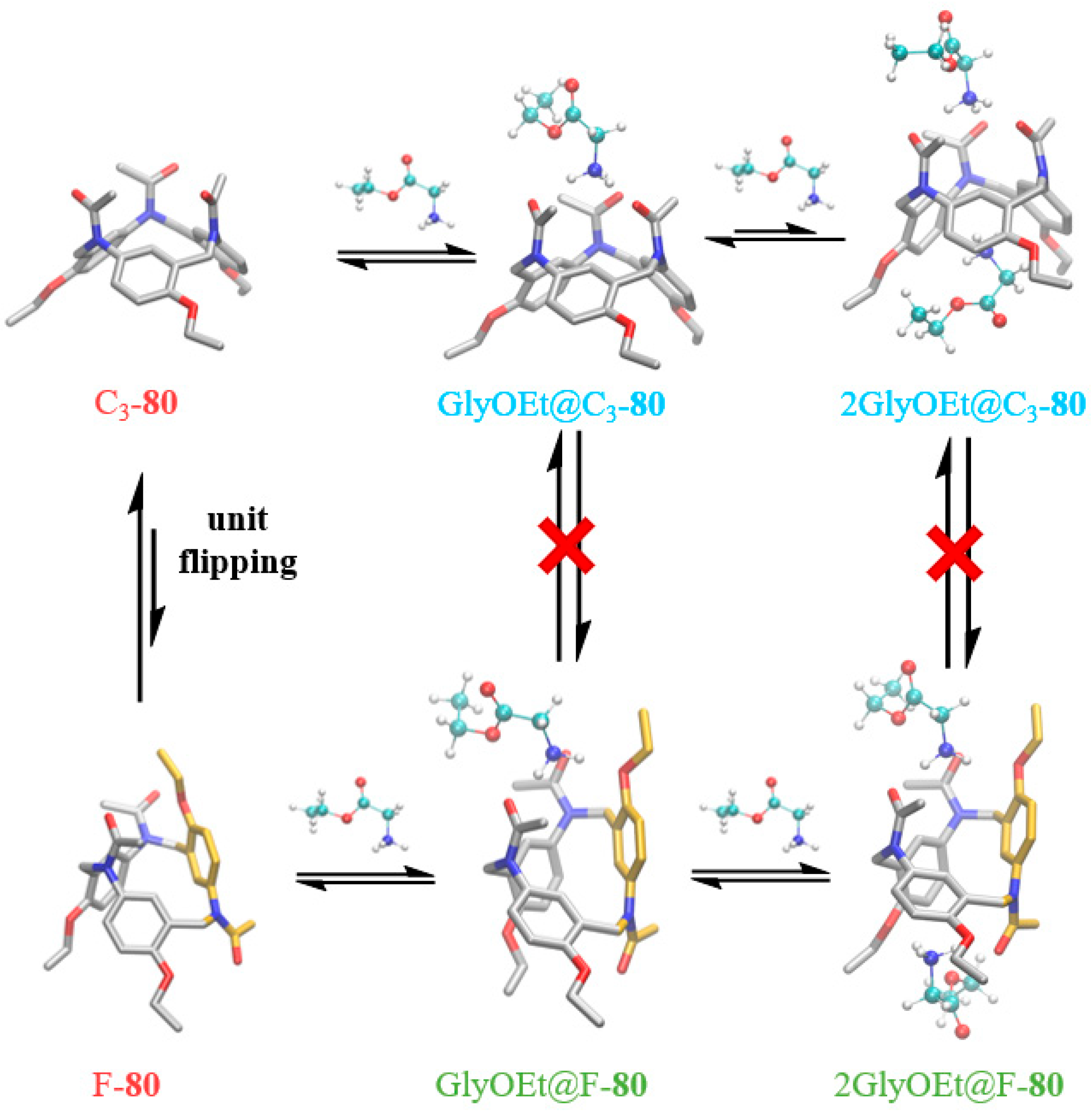
2.2.2. Mannich-Type Macrocyclization
3. Applications of N-Containing Macrocyclic Arenes
3.1. Molecular Recognition
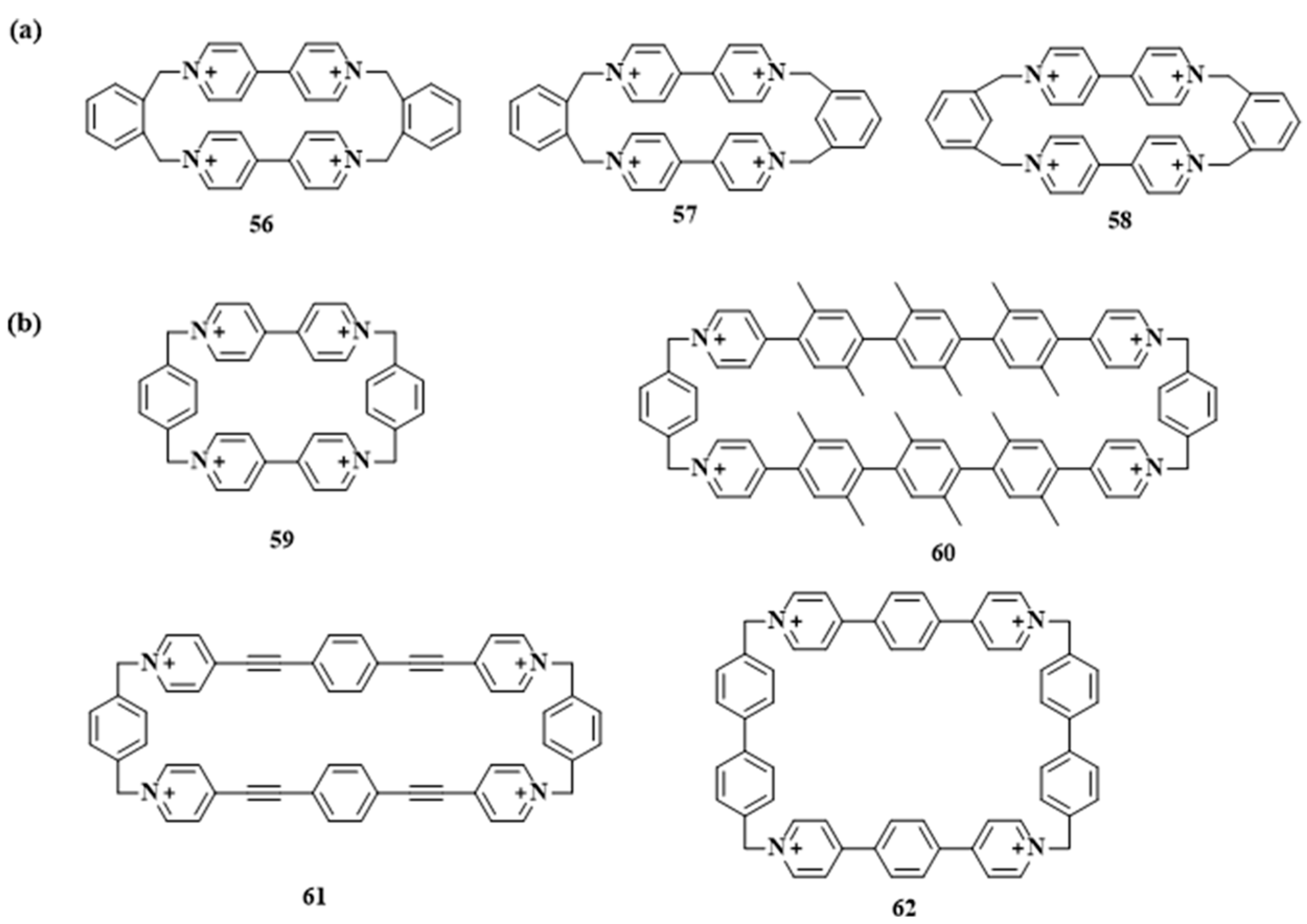
3.1.1. Recognition of Cationic Guests
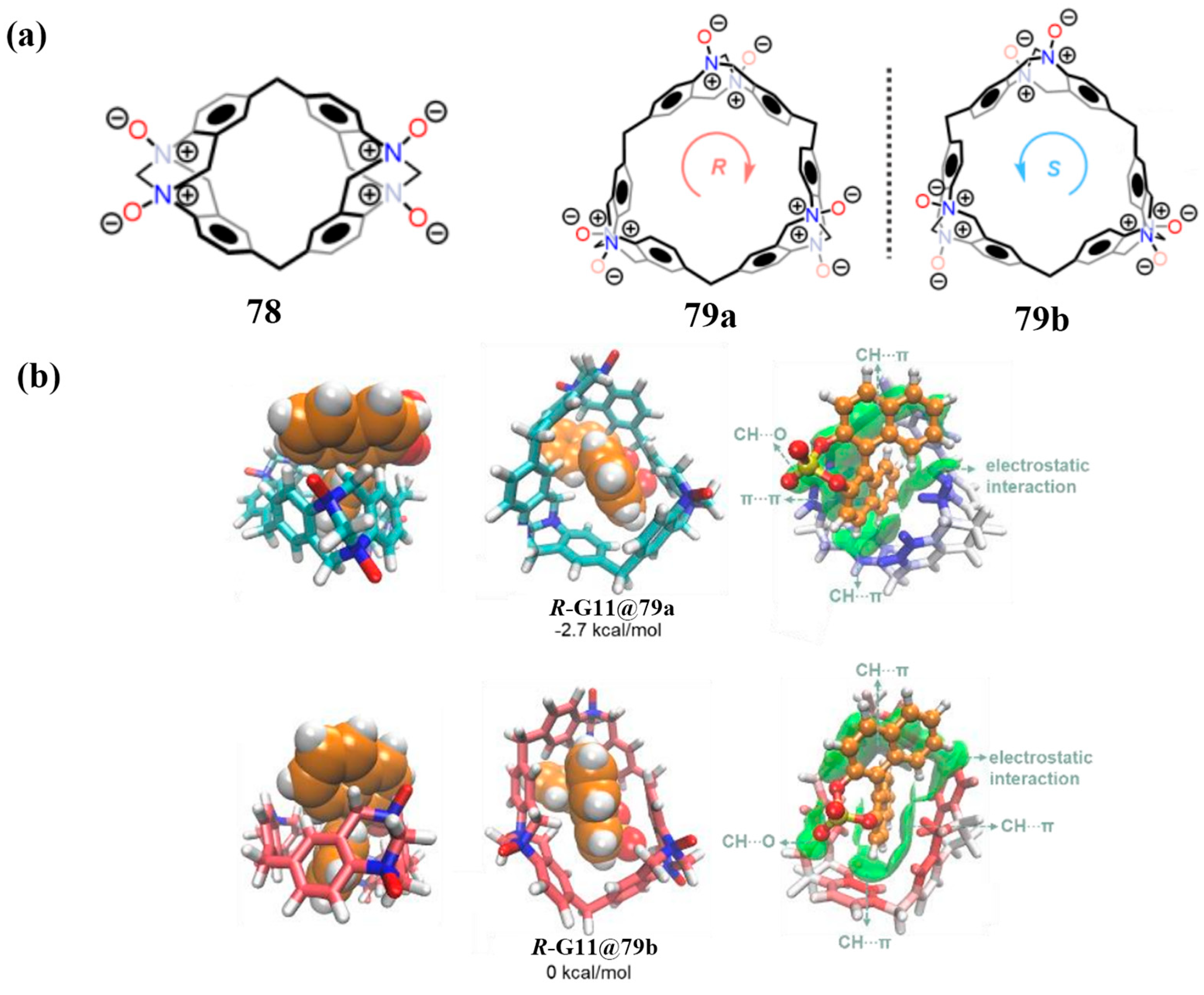
3.1.2. Enantioselective Recognition of Chiral Molecules
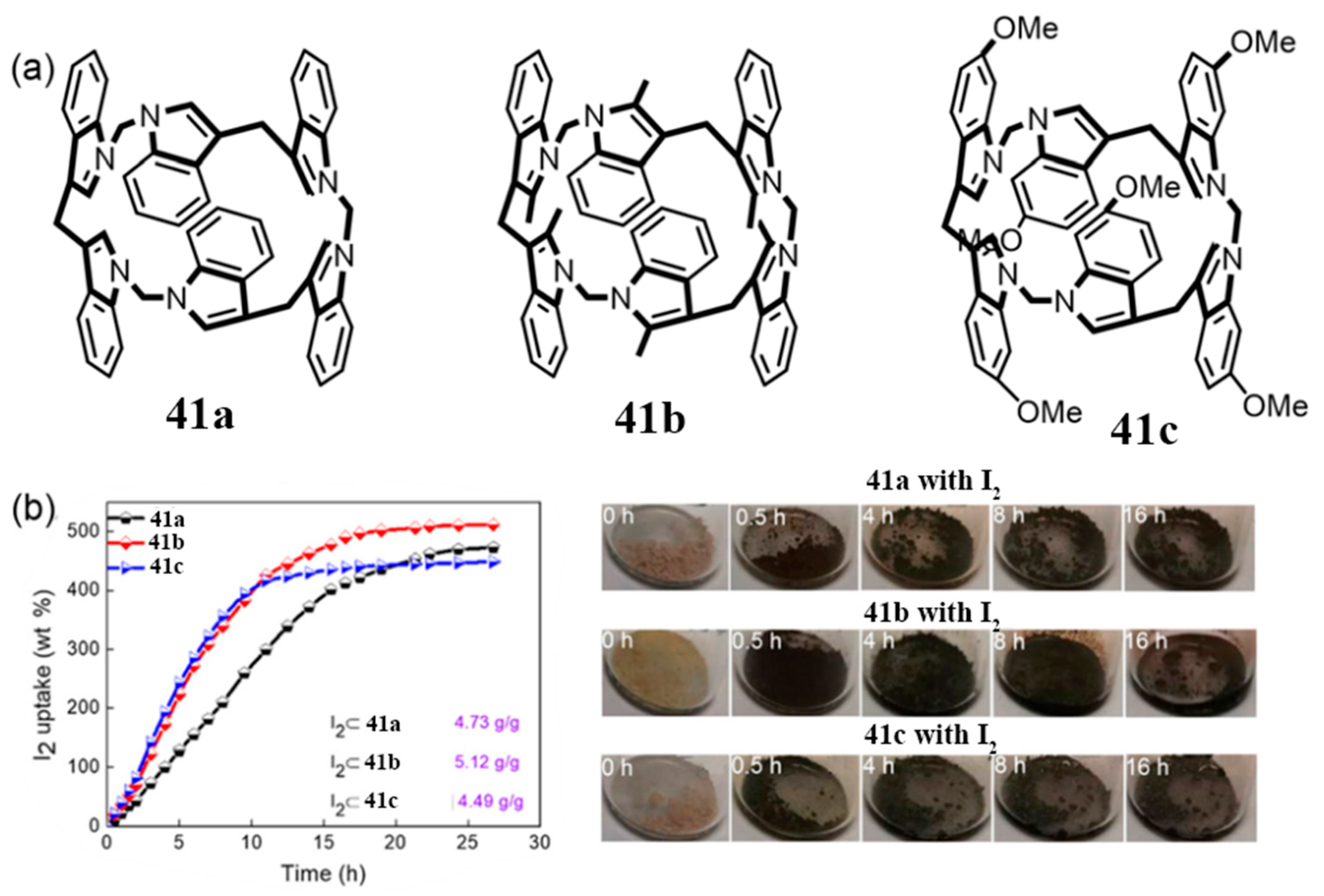
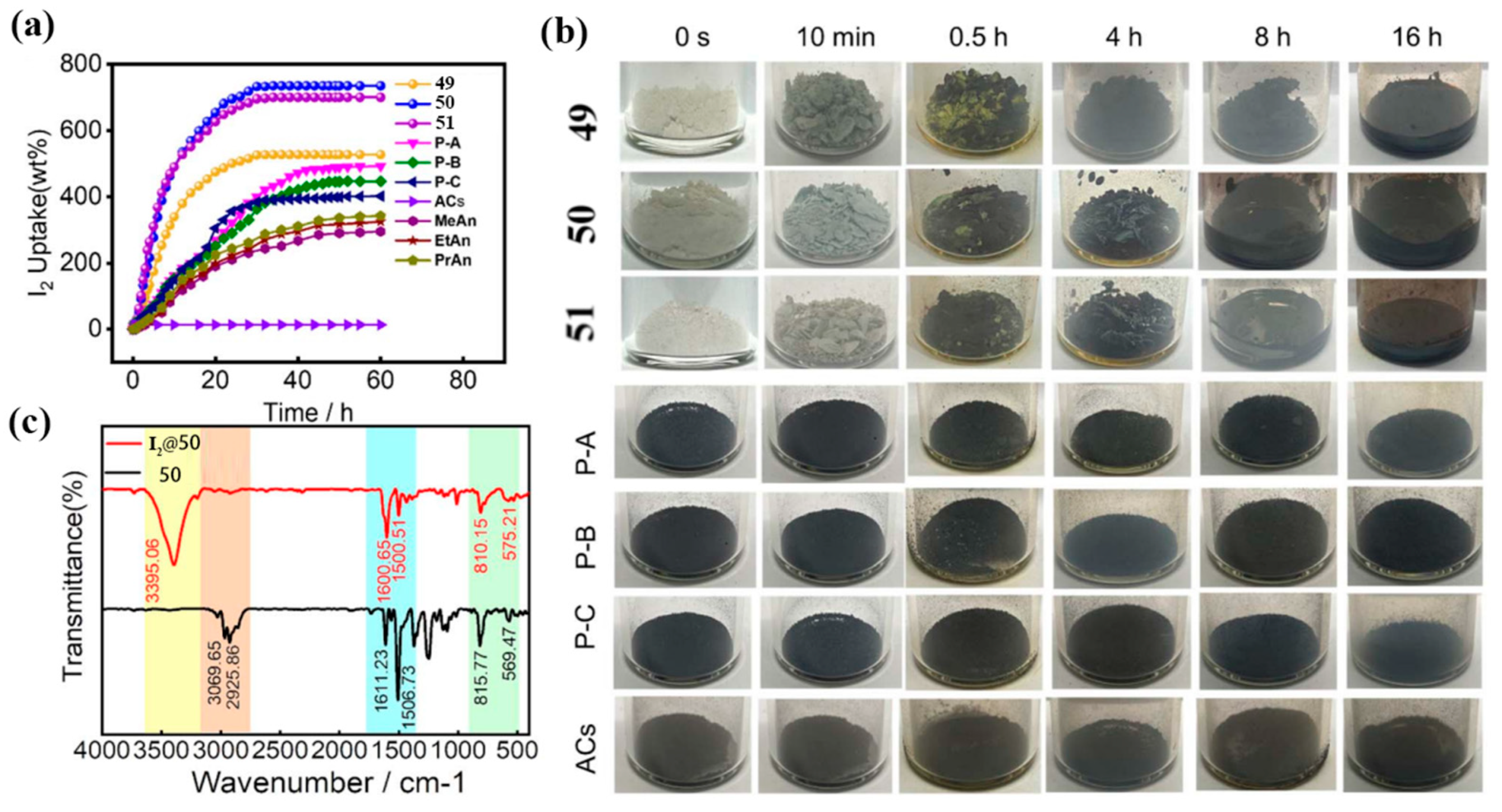
3.2. Adsorption and Separation
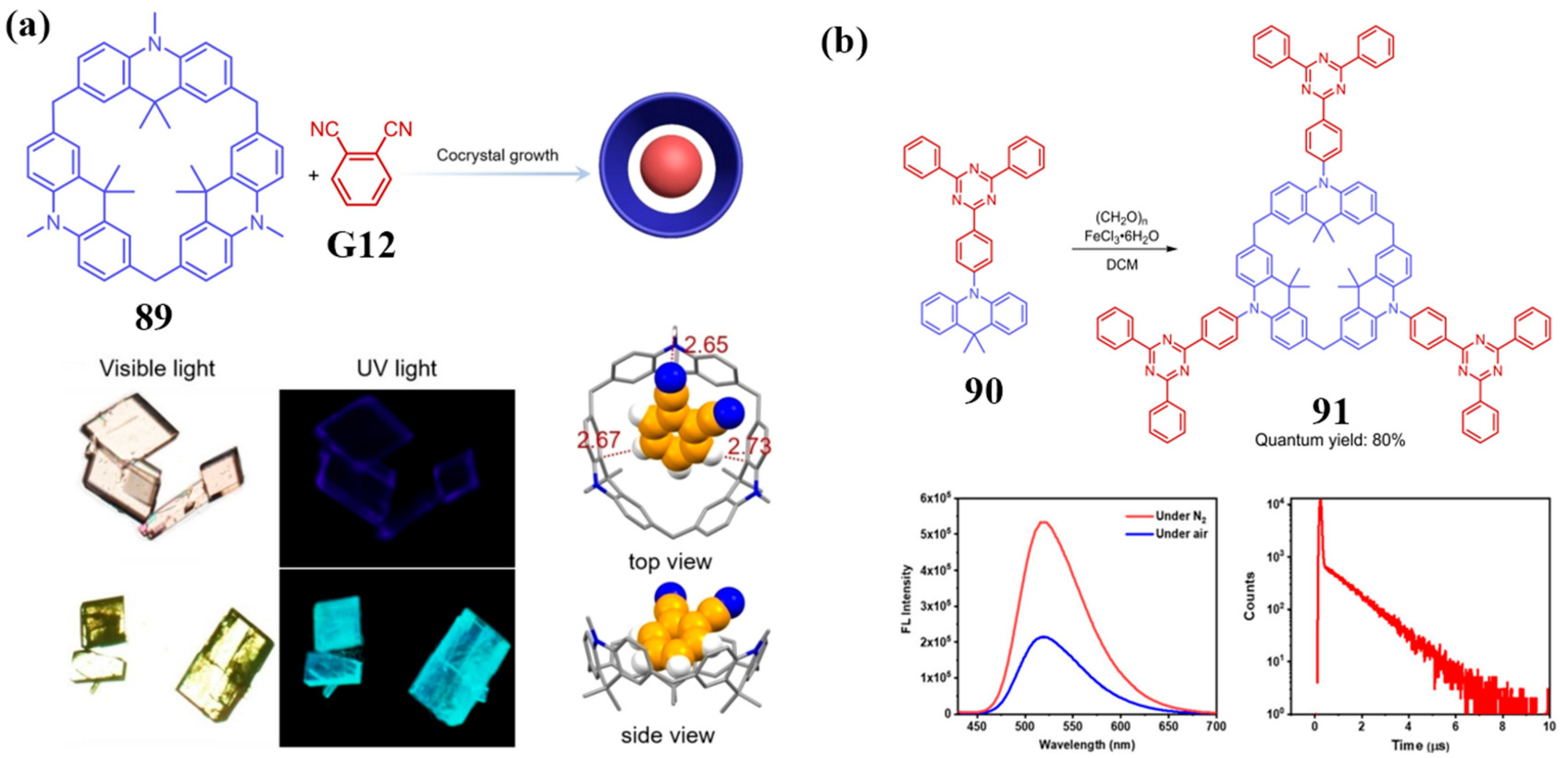
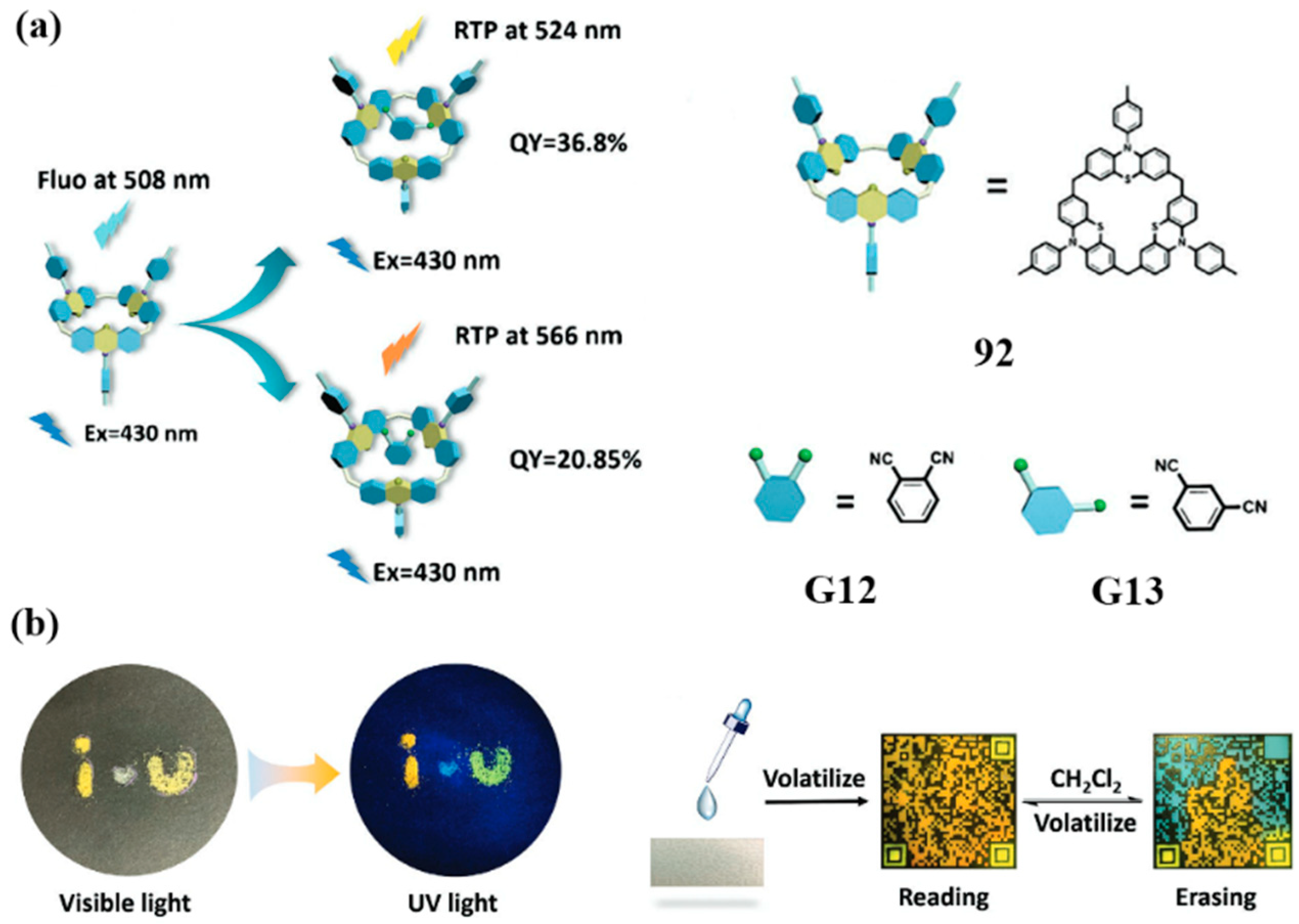
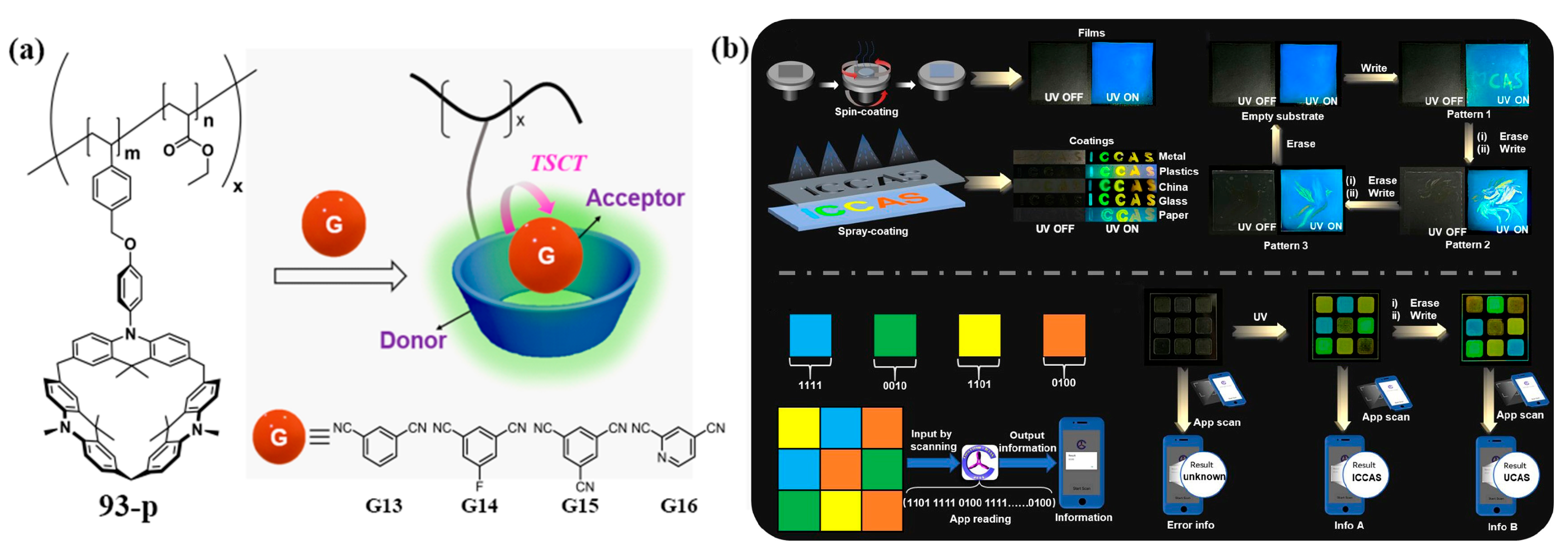
3.3. Novel Photophysical Properties
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cram, D.J. The Design of Molecular Hosts, Guests, and Their Complexes (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 1009–1020. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Chemistry—Scope and Perspectives Molecules, Supermolecules, and Molecular Devices (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Pedersen, C.J. The Discovery of Crown Ethers (Noble Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 1021–1027. [Google Scholar] [CrossRef]
- Feringa, B.L. The Art of Building Small: From Molecular Switches to Motors (Nobel Lecture). Angew. Chem. Int. Ed. 2017, 56, 11060–11078. [Google Scholar] [CrossRef]
- Sauvage, J.-P. From Chemical Topology to Molecular Machines (Nobel Lecture). Angew. Chem. Int. Ed. 2017, 56, 11080–11093. [Google Scholar] [CrossRef]
- Stoddart, J.F. Mechanically Interlocked Molecules (MIMs)—Molecular Shuttles, Switches, and Machines (Nobel Lecture). Angew. Chem. Int. Ed. 2017, 56, 11094–11125. [Google Scholar] [CrossRef]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. [Google Scholar] [CrossRef]
- Gutsche, C.D.; Muthukrishnan, R. Calixarenes. 1. Analysis of the product mixtures produced by the base-catalyzed condensation of formaldehyde with para-substituted phenols. J. Org. Chem. 1978, 43, 4905–4906. [Google Scholar] [CrossRef]
- Freeman, W.A.; Mock, W.L.; Shih, N.Y. Cucurbituril. J. Am. Chem. Soc. 1981, 103, 7367–7368. [Google Scholar] [CrossRef]
- Kim, K.; Selvapalam, N.; Ko, Y.H.; Park, K.M.; Kim, D.; Kim, J. Functionalized cucurbiturils and their applications. Chem. Soc. Rev. 2007, 36, 267–279. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef]
- Harada, A. Cyclodextrin-Based Molecular Machines. Acc. Chem. Res. 2001, 34, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.-A.; Nakamoto, Y. para-Bridged Symmetrical Pillar[5]arenes: Their Lewis Acid Catalyzed Synthesis and Host–Guest Property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef]
- Zhao, T.; Yi, J.; Liu, C.; Liang, X.; Shen, Y.; Wei, L.; Xie, X.; Wu, W.; Yang, C. “First Come, First Served” and Threshold Effects in a Central-to-Planar-to-Helical Hierarchical Chiral Induction. Angew. Chem. Int. Ed. 2023, 62, e202302232. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Wei, X.; Wu, W.; Fan, C.; Zhou, D.; Kanagaraj, K.; Cheng, G.; Luo, K.; Meng, X.-G.; Yang, C. The More the Slower: Self-Inhibition in Supramolecular Chirality Induction, Memory, Erasure, and Reversion. J. Am. Chem. Soc. 2022, 144, 1455–1463. [Google Scholar] [CrossRef]
- Han, X.-N.; Han, Y.; Chen, C.-F. Recent advances in the synthesis and applications of macrocyclic arenes. Chem. Soc. Rev. 2023, 52, 3265–3298. [Google Scholar] [CrossRef]
- Wang, Y.; Ping, G.; Li, C. Efficient complexation between pillar[5]arenes and neutral guests: From host–guest chemistry to functional materials. Chem. Commun. 2016, 52, 9858–9872. [Google Scholar] [CrossRef]
- Yang, L.-P.; Liu, W.-E.; Jiang, W. Naphthol-based macrocyclic receptors. Tetrahedron Lett. 2016, 57, 3978–3985. [Google Scholar] [CrossRef]
- Wu, J.-R.; Yang, Y.-W. New opportunities in synthetic macrocyclic arenes. Chem. Commun. 2019, 55, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-R.; Wu, G.; Yang, Y.-W. Pillararene-Inspired Macrocycles: From Extended Pillar[n]arenes to Geminiarenes. Acc. Chem. Res. 2022, 55, 3191–3204. [Google Scholar] [CrossRef]
- Dale, E.J.; Vermeulen, N.A.; Juríček, M.; Barnes, J.C.; Young, R.M.; Wasielewski, M.R.; Stoddart, J.F. Supramolecular Explorations: Exhibiting the Extent of Extended Cationic Cyclophanes. Acc. Chem. Res. 2016, 49, 262–273. [Google Scholar] [CrossRef]
- Chi, X.; Tian, J.; Luo, D.; Gong, H.-Y.; Huang, F.; Sessler, J.L. “Texas-Sized” Molecular Boxes: From Chemistry to Applications. Molecules 2021, 26, 2426. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Stoddart, J.F. Molecular Triangles: A New Class of Macrocycles. Acc. Chem. Res. 2021, 54, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Kanagaraj, K.; Rebek, J.; Yu, Y. Self-assembled hexameric capsule: A highly β-selective O-glycosylation reaction enabled via proton wire mechanism. Green Synth. Catal. 2023, 4, 7–9. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Li, C. Biphen[n]arenes: Modular Synthesis, Customizable Cavity Sizes, and Diverse Skeletons. Acc. Chem. Res. 2022, 55, 916–929. [Google Scholar] [CrossRef]
- Yang, W.; Samanta, K.; Wan, X.; Thikekar, T.U.; Chao, Y.; Li, S.; Du, K.; Xu, J.; Gao, Y.; Zuilhof, H.; et al. Tiara[5]arenes: Synthesis, Solid-State Conformational Studies, Host–Guest Properties, and Application as Nonporous Adaptive Crystals. Angew. Chem. Int. Ed. 2020, 59, 3994–3999. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-R.; Mu, A.U.; Li, B.; Wang, C.-Y.; Fang, L.; Yang, Y.-W. Desymmetrized Leaning Pillar[6]arene. Angew. Chem. Int. Ed. 2018, 57, 9853–9858. [Google Scholar] [CrossRef]
- Wu, J.-R.; Yang, Y.-W. Geminiarene: Molecular Scale Dual Selectivity for Chlorobenzene and Chlorocyclohexane Fractionation. J. Am. Chem. Soc. 2019, 141, 12280–12287. [Google Scholar] [CrossRef]
- Yao, J.; Wu, W.; Xiao, C.; Su, D.; Zhong, Z.; Mori, T.; Yang, C. Overtemperature-protection intelligent molecular chiroptical photoswitches. Nat. Commun. 2021, 12, 2600. [Google Scholar] [CrossRef]
- Yang, J.; Lou, X.-Y.; Dai, D.; Shi, J.; Yang, Y.-W. Desymmetrized pillar[8]arenes: High-yield synthesis, functionalization, and host-guest chemistry. Chin. Chem. Lett. 2025, 36, 109818. [Google Scholar] [CrossRef]
- Della Sala, P.; Del Regno, R.; Talotta, C.; Capobianco, A.; Hickey, N.; Geremia, S.; De Rosa, M.; Spinella, A.; Soriente, A.; Neri, P.; et al. Prismarenes: A New Class of Macrocyclic Hosts Obtained by Templation in a Thermodynamically Controlled Synthesis. J. Am. Chem. Soc. 2020, 142, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-N.; Han, Y.; Chen, C.-F. Pagoda[4]arene and i-Pagoda[4]arene. J. Am. Chem. Soc. 2020, 142, 8262–8269. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-S.; Zhang, D.-W.; Guo, Y.; Li, J.; Han, Y.; Chen, C.-F. Towards the Highly Efficient Synthesis and Selective Methylation of C(sp3)-Bridged[6]Cycloparaphenylenes from Fluoren[3]arenes. Angew. Chem. Int. Ed. 2021, 60, 13021–13028. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Han, Y.; Chen, C.-F. 3,6-Fluoren[5]arenes: Synthesis, structure and complexation with fullerenes C60 and C70. Chem. Commun. 2021, 57, 3987–3990. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, F.; Yang, L.-P.; Zhou, H.; Jiang, W. Conformationally adaptive macrocycles with flipping aromatic sidewalls. Chem. Soc. Rev. 2020, 49, 4176–4188. [Google Scholar] [CrossRef]
- Yang, L.-P.; Wang, X.; Yao, H.; Jiang, W. Naphthotubes: Macrocyclic Hosts with a Biomimetic Cavity Feature. Acc. Chem. Res. 2020, 53, 198–208. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, X.; Liu, B.; Qiao, P.; Li, J.; Wang, L. Macrocyclic host molecules with aromatic building blocks: The state of the art and progress. Chem. Commun. 2021, 57, 12379–12405. [Google Scholar] [CrossRef]
- Ma, C.; Chang, Y.; Yang, J.; Chen, L.; Wu, D.; Go, Y.; Wang, B.; Shi, L.; Li, B. Recent advances in photocatalytic C-H amination to nitrogenous structures. Green Synth. Catal. 2024. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Ouyang, K.; Zhang, W.-X. Transition-metal-catalyzed transformations of C–N single bonds: Advances in the last five years, challenges and prospects. Green Synth. Catal. 2021, 2, 87–122. [Google Scholar] [CrossRef]
- Han, X.-N.; Han, Y.; Chen, C.-F. Fluorescent Macrocyclic Arenes: Synthesis and Applications. Angew. Chem. Int. Ed. 2025, 64, e202424276. [Google Scholar] [CrossRef]
- Mao, Y.; Ma, J.; Ji, J.; Wang, Y.; Wu, W.; Yang, C. Crown aldoxime ethers: Their synthesis, structure, acid-catalyzed/photo-induced isomerization and adjustable guest binding. Chin. Chem. Lett. 2024, 35, 109927. [Google Scholar] [CrossRef]
- Smart, J.E.; Emerson-King, J.; Jeans, R.J.; Hood, T.M.; Lau, S.; Bara-Estaún, A.; Hintermair, U.; Pringle, P.G.; Chaplin, A.B. A Resorcin[4]arene-Based Phosphite-Phosphine Ligand for the Branched-Selective Hydroformylation of Alkyl Alkenes. ACS Catal. 2024, 14, 11803–11807. [Google Scholar] [CrossRef]
- Ayuso-Carrillo, J.; Bonifazi, D. Catalyst-Transfer Macrocyclization Protocol: Synthesis of π-Conjugated Azaparacyclophanes Made Easy. JACS Au 2025, 5, 1482–1498. [Google Scholar] [CrossRef]
- Espinosa, M.R.; Guerrero, F.; Kazmierczak, N.P.; Oyala, P.H.; Hong, A.; Hadt, R.G.; Agapie, T. Slow Electron Spin Relaxation at Ambient Temperatures with Copper Coordinated by a Rigid Macrocyclic Ligand. J. Am. Chem. Soc. 2025, 147, 14036–14042. [Google Scholar] [CrossRef]
- Lemport, P.S.; Petrov, V.S.; Matveev, P.I.; Leksina, U.M.; Roznyatovsky, V.A.; Gloriozov, I.P.; Yatsenko, A.V.; Tafeenko, V.A.; Dorovatovskii, P.V.; Khrustalev, V.N.; et al. First 24-Membered Macrocyclic 1,10-Phenanthroline-2,9-Diamides—An Efficient Switch from Acidic to Alkaline Extraction of f-Elements. Int. J. Mol. Sci. 2023, 24, 10261. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Zhu, W.; Wang, T.; Tang, Y.; Wang, J. N-Heterocyclic carbene-catalyzed enantioselective (dynamic) kinetic resolution for the assembly of inherently chiral macrocycles. Chem. Sci. 2025, 16, 11021–11026. [Google Scholar] [CrossRef] [PubMed]
- Ibragimova, R.; Lu, Q.-L.; Wang, M.-X.; Zhu, J.; Tong, S. Asymmetric Synthesis of Inherently Chiral Tetraoxacalix[2]arene[2]pyridines via SNAr-Based Cross-Cyclotetramerization. Chem. Asian J. 2025, 20, e202401621. [Google Scholar] [CrossRef]
- Flerin, M.; Duarte, F.; Langton, M.J. Chloride Selective, Nonprotonophoric Ion Transport with Macrocyclic Halogen Bonding Anionophores. Chem. A Eur. J. 2025, 31, e202502033. [Google Scholar] [CrossRef]
- Kurashov, I.A.; Kharlamova, A.D.; Abel, A.S.; Averin, A.D.; Beletskaya, I.P. Polyoxa- and Polyazamacrocycles Incorporating 6,7-Diaminoquinoxaline Moiety: Synthesis and Application as Tunable Optical pH-Indicators in Aqueous Solution. Molecules 2023, 28, 512. [Google Scholar] [CrossRef]
- Kharlamova, A.D.; Ermakova, E.V.; Abel, A.S.; Gontcharenko, V.E.; Cheprakov, A.V.; Averin, A.D.; Beletskaya, I.P.; Andraud, C.; Bretonnière, Y.; Bessmertnykh-Lemeune, A. Quinoxaline-based azamacrocycles: Synthesis, AIE behavior and acidochromism. Org. Biomol. Chem. 2024, 22, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Cheng, Z.P.; Luo, Y.; Lv, L.; Chen, S.; Li, Z. Pd/IPrBIDEA-Catalyzed Hydrodefluorination of gem-Difluorocyclopropanes: Regioselective Synthesis of Terminal Fluoroalkenes. J. Am. Chem. Soc. 2024, 146, 24–32. [Google Scholar] [CrossRef]
- Sahoo, D.; Bera, A.; Vennapusa, S.R.; De, S. Light-Triggered Reversible Helicity Switching of a Rotor by a Photo-Responsive Plier. Chem. A Eur. J. 2025, 31, e202404771. [Google Scholar] [CrossRef]
- Liang, X.; Zhao, T.; Shen, Y.; Fang, L.; Chen, L.; Zhou, D.; Wu, W.; Yang, C. Nitrogen-Oxidized Tröger’s Base Macrocyclic Arenes: Unprecedented Enantioselective Recognition in Water. Angew. Chem. Int. Ed. 2025, 64, e202416975. [Google Scholar] [CrossRef]
- Shen, Y.; Liang, X.; Ma, T.; Zhou, D.; Liu, W.; Ma, J.; Wu, W.; Yu, Z.; Yang, C. Phenacetin[3]Arenes: Mannich-Type Macrocyclization, Unique Structure, Versatile Functionalization, and Strong Allosteric Binding. Angew. Chem. Int. Ed. 2025, 137, e202504211. [Google Scholar] [CrossRef]
- Gale, P.A.; Anzenbacher, P., Jr.; Sessler, J.L. Calixpyrroles II. Coord. Chem. Rev. 2001, 222, 57–102. [Google Scholar] [CrossRef]
- Kim, S.K.; Sessler, J.L. Calix[4]pyrrole-Based Ion Pair Receptors. Acc. Chem. Res. 2014, 47, 2525–2536. [Google Scholar] [CrossRef]
- Yang, P.; Jian, Y.; Zhou, X.; Li, G.; Deng, T.; Shen, H.; Yang, Z.; Tian, Z. Calix[3]carbazole: One-Step Synthesis and Host–Guest Binding. J. Org. Chem. 2016, 81, 2974–2980. [Google Scholar] [CrossRef] [PubMed]
- Ostrauskaite, J.; Strohriegl, P. Formation of Macrocycles in the Synthesis of Poly(N-(2-ethylhexyl)carbazol-3,6-diyl). Macromol. Chem. Phys. 2003, 204, 1713–1718. [Google Scholar] [CrossRef]
- Coumont, L.S.; Veinot, J.G.C. Ni(COD)2 coupling of 3,6-dibromocarbazoles as a route to all-carbazole shape persistent macrocycles. Tetrahedron Lett. 2015, 56, 5595–5598. [Google Scholar] [CrossRef]
- Zhu, H.; Shi, B.; Chen, K.; Wei, P.; Xia, D.; Mondal, J.H.; Huang, F. Cyclo[4]carbazole, an Iodide Anion Macrocyclic Receptor. Org. Lett. 2016, 18, 5054–5057. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Badía-Domínguez, I.; Shi, B.; Li, Q.; Wei, P.; Xing, H.; Ruiz Delgado, M.C.; Huang, F. Cyclization-Promoted Ultralong Low-Temperature Phosphorescence via Boosting Intersystem Crossing. J. Am. Chem. Soc. 2021, 143, 2164–2169. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Hermann, M.; Otteny, F.; Esser, B. Calix[n]phenothiazines: Optoelectronic and Structural Properties and Host–Guest Chemistry. Org. Mater. 2020, 02, 235–239. [Google Scholar] [CrossRef]
- Chun, Y.; Jiten Singh, N.; Hwang, I.-C.; Woo Lee, J.; Yu, S.U.; Kim, K.S. Calix[n]imidazolium as a new class of positively charged homo-calix compounds. Nat. Commun. 2013, 4, 1797. [Google Scholar] [CrossRef]
- Baeyer, A. Ueber ein Condensationsproduct von Pyrrol mit Aceton. Berichte Der Dtsch. Chem. Ges. 1886, 19, 2184–2185. [Google Scholar] [CrossRef]
- Wang, M.-X.; Yang, H.-B. A General and High Yielding Fragment Coupling Synthesis of Heteroatom-Bridged Calixarenes and the Unprecedented Examples of Calixarene Cavity Fine-Tuned by Bridging Heteroatoms. J. Am. Chem. Soc. 2004, 126, 15412–15422. [Google Scholar] [CrossRef]
- Guo, Q.-H.; Fu, Z.-D.; Zhao, L.; Wang, M.-X. Synthesis, Structure, and Properties of O6-Corona[3]arene[3]tetrazines. Angew. Chem. Int. Ed. 2014, 53, 13548–13552. [Google Scholar] [CrossRef]
- Yu, X.; Wu, W.; Zhou, D.; Su, D.; Zhong, Z.; Yang, C. Bisindole[3]arenes—Indolyl Macrocyclic Arenes Having Significant Iodine Capture Capacity. CCS Chem. 2021, 4, 1806–1814. [Google Scholar] [CrossRef]
- Mao, L.; Hu, Y.; Tu, Q.; Jiang, W.-L.; Zhao, X.-L.; Wang, W.; Yuan, D.; Wen, J.; Shi, X. Highly efficient synthesis of non-planar macrocycles possessing intriguing self-assembling behaviors and ethene/ethyne capture properties. Nat. Commun. 2020, 11, 5806. [Google Scholar] [CrossRef]
- Jin, P.; Liang, W.; Rong, Y.; Wu, W.; Gou, M.; Tang, Y.; Yang, C. Remarkable iodine uptake by aniline-based macrocyclic arenes through a reverse dissolution mechanism. J. Mater. Chem. A 2023, 11, 11126–11132. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, J.; Guo, M.; Zhao, Y.; Sue, A.C.H. Triphenylamine[3]arenes: Streamlining Synthesis of a Versatile Macrocyclic Platform for Supramolecular Architectures and Functionalities. Angew. Chem. Int. Ed. 2024, 63, e202409120. [Google Scholar] [CrossRef] [PubMed]
- Geuder, W.; Hünig, S.; Suchy, A. Phanes with Two 4,4′-Bipyridinium Moieties—A New Class of Compounds. Angew. Chem. Int. Ed. Engl. 1983, 22, 489–490. [Google Scholar] [CrossRef]
- Geuder, W.; Hünig, S.; Suchy, A. Single and double bridged viologenes and intramolecular pimerization of their cation radicals. Tetrahedron 1986, 42, 1665–1677. [Google Scholar] [CrossRef]
- Kosiorek, S.; Rosa, B.; Boinski, T.; Butkiewicz, H.; Szymański, M.P.; Danylyuk, O.; Szumna, A.; Sashuk, V. Pillar[4]pyridinium: A square-shaped molecular box. Chem. Commun. 2017, 53, 13320–13323. [Google Scholar] [CrossRef]
- Kim, I.; Dhamija, A.; Hwang, I.-C.; Lee, H.; Ko, Y.H.; Kim, K. One-pot Synthesis of a Truncated Cone-shaped Porphyrin Macrocycle and Its Self-assembly into Permanent Porous Material. Chem. Asian J. 2021, 16, 3209–3212. [Google Scholar] [CrossRef]
- Sergeyev, S. Recent Developments in Synthetic Chemistry, Chiral Separations, and Applications of Tröger’s Base Analogues. Helv. Chim. Acta 2009, 92, 415–444. [Google Scholar] [CrossRef]
- Rúnarsson, Ö.V.; Artacho, J.; Wärnmark, K. The 125th Anniversary of the Tröger’s Base Molecule: Synthesis and Applications of Tröger’s Base Analogues. Eur. J. Org. Chem. 2012, 2012, 7015–7041. [Google Scholar] [CrossRef]
- Dolenský, B.; Havlík, M.; Král, V. Oligo Tröger’s bases—New molecular scaffolds. Chem. Soc. Rev. 2012, 41, 3839–3858. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Xu, G.; Qiu, H.; Li, Y.; Lu, X.; Jiang, J.; Wang, L. Tröger’s base-embedded macrocycles with chirality. Chem. Commun. 2025, 61, 2450–2467. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Chen, Y.; Wang, R.; Niu, P.; Shi, C.; Yang, Z.; Cheng, M.; Jiang, J.; Wang, L. Chiral selection of Tröger’s base-based macrocycles with different ethylene glycol chains length in crystallization. Chin. Chem. Lett. 2023, 34, 108038. [Google Scholar] [CrossRef]
- Mao, L.; Li, F.; Huang, L.; Qu, X.; Wang, K.; Zhang, Y.; Ma, D. Structurally Diversified Calix[3]phenoxazines: Synthesis, Solid-State Conformational Investigation, and Host–Guest Chemistry. Org. Lett. 2023, 25, 5597–5601. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, J.; Xia, G.-J.; Deng, J.; Sun, P.; Chruma, J.J.; Wu, W.; Yang, C.; Wang, Y.-G.; Huang, Z. Enantioselective photoinduced cyclodimerization of a prochiral anthracene derivative adsorbed on helical metal nanostructures. Nat. Chem. 2020, 12, 551–559. [Google Scholar] [CrossRef]
- Tu, C.; Wu, W.; Liang, W.; Zhang, D.; Xu, W.; Wan, S.; Lu, W.; Yang, C. Host–Guest Complexation-Induced Aggregation Based on Pyrene-Modified Cyclodextrins for Improved Electronic Circular Dichroism and Circularly Polarized Luminescence. Angew. Chem. Int. Ed. 2022, 61, e202203541. [Google Scholar] [CrossRef]
- Wei, X.; Ji, J.; Nie, Y.; Tang, L.; Rao, M.; Wang, X.; Wu, W.; Su, D.; Zhong, Z.; Yang, C. Synthesis of cyclodextrin derivatives for enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate. Nat. Protoc. 2022, 17, 2494–2516. [Google Scholar] [CrossRef]
- Ji, J.; Wei, X.; Wu, W.; Yang, C. Asymmetric Photoreactions in Supramolecular Assemblies. Acc. Chem. Res. 2023, 56, 1896–1907. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Guan, X.; Shen, Y.; Zhou, D.; Chen, L.; Chen, X.; Wu, W.; Wang, L.; Yang, C. Multi-step chirality transfer and racemization kinetics of pillar[5]arenes by tuning the halogen substituents on the rims. Org. Chem. Front. 2024, 11, 7229–7234. [Google Scholar] [CrossRef]
- Zhao, T.; Yi, J.; Liu, C.; Fang, L.; Chen, L.; Shen, Y.; Liang, X.; Li, K.; Wu, W.; Yang, C. Hierarchical chirality transfer of perylene diimide-tethered pillar[5]arenes for configuration and type differentiation of amino acid derivatives. New J. Chem. 2024, 48, 1856–1859. [Google Scholar] [CrossRef]
- Gao, F.; Yu, X.; Liu, L.; Chen, J.; Lv, Y.; Zhao, T.; Ji, J.; Yao, J.; Wu, W.; Yang, C. Chiroptical switching of molecular universal joint triggered by complexation/release of a cation: A stepwise synergistic complexation. Chin. Chem. Lett. 2023, 34, 107558. [Google Scholar] [CrossRef]
- Lv, Y.; Xiao, C.; Ma, J.; Zhou, D.; Wu, W.; Yang, C. Solvent and guest-binding-controlled chiroptical inversion of molecular devices based on pseudo[1]catenane-type pillar[5]arene derivatives. Chin. Chem. Lett. 2024, 35, 108757. [Google Scholar] [CrossRef]
- Huang, R.; Wei, X.; Wang, P.; Ma, J.; Mao, Y.; Zhou, D.; Wu, W.; Ji, J.; Yang, C. Chirality Induction and Memory of Pillar[4]arene[1]quinone Derivatives in Visible-Light Range. Org. Lett. 2024, 26, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Sivaguru, J.; Natarajan, A.; Kaanumalle, L.S.; Shailaja, J.; Uppili, S.; Joy, A.; Ramamurthy, V. Asymmetric Photoreactions within Zeolites: Role of Confinement and Alkali Metal Ions. Acc. Chem. Res. 2003, 36, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.; White, J.D. Asymmetric Catalysis Using Chiral Salen–Metal Complexes: Recent Advances. Chem. Rev. 2019, 119, 9381–9426. [Google Scholar] [CrossRef]
- Cabré, A.; Verdaguer, X.; Riera, A. Recent Advances in the Enantioselective Synthesis of Chiral Amines via Transition Metal-Catalyzed Asymmetric Hydrogenation. Chem. Rev. 2022, 122, 269–339. [Google Scholar] [CrossRef]
- Kuang, H.; Xu, C.; Tang, Z. Emerging Chiral Materials. Adv. Mater. 2020, 32, 2005110. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Sarma, D.; Malliakas, C.D.; Polychronopoulou, K.; Riley, B.J.; Pierce, D.A.; Chun, J.; Kanatzidis, M.G. Chalcogenide Aerogels as Sorbents for Radioactive Iodine. Chem. Mater. 2015, 27, 2619–2626. [Google Scholar] [CrossRef]
- Küpper, F.C.; Feiters, M.C.; Olofsson, B.; Kaiho, T.; Yanagida, S.; Zimmermann, M.B.; Carpenter, L.J.; Luther Iii, G.W.; Lu, Z.; Jonsson, M.; et al. Commemorating Two Centuries of Iodine Research: An Interdisciplinary Overview of Current Research. Angew. Chem. Int. Ed. 2011, 50, 11598–11620. [Google Scholar] [CrossRef]
- Saiz-Lopez, A.; Plane, J.M.C.; Baker, A.R.; Carpenter, L.J.; von Glasow, R.; Gómez Martín, J.C.; McFiggans, G.; Saunders, R.W. Atmospheric Chemistry of Iodine. Chem. Rev. 2012, 112, 1773–1804. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Xiao, X.-S.; Gong, S.-F.; Yuan, L.; Tang, L.-L. An electron-deficient supramolecular macrocyclic host for the selective separation of aromatics and cyclic aliphatics. Org. Chem. Front. 2022, 9, 4829–4833. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Zhang, D.-W.; Li, M.; Chen, C.-F. A Calix[3]acridan-Based Host–Guest Cocrystal Exhibiting Efficient Thermally Activated Delayed Fluorescence. Angew. Chem. Int. Ed. 2022, 61, e202117872. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Zhang, D.-W.; Han, X.-N.; Han, Y.; Chen, C.-F. A novel thermally activated delayed fluorescence macrocycle. Chem. Commun. 2022, 58, 12180–12183. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, L.; Liu, L.; Chen, Y.; Xu, W.-W.; Niu, J.; Qin, Y.; Xu, X.; Liu, Y. Two Calix[3]Phenothiazine-Based Amorphous Pure Organic Room-Temperature Phosphorescent Supramolecules Mediated by Guest. Adv. Opt. Mater. 2023, 11, 2300326. [Google Scholar] [CrossRef]
- Ma, J.-H.; Han, Y.; Guo, W.-C.; Lu, H.-Y.; Chen, C.-F. Color-Tunable Thermally Activated Delayed Fluorescence Polymeric Materials Constructed by Host–Guest Complexation Revealing Rewritable Advanced Information Encryption. CCS Chem. 2025, 7, 2623–2632. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Wu, W.; Yang, C. Recent Advances in the Synthesis and Applications of Nitrogen-Containing Macrocyclic Arenes. Molecules 2025, 30, 3646. https://doi.org/10.3390/molecules30173646
Hu J, Wu W, Yang C. Recent Advances in the Synthesis and Applications of Nitrogen-Containing Macrocyclic Arenes. Molecules. 2025; 30(17):3646. https://doi.org/10.3390/molecules30173646
Chicago/Turabian StyleHu, Jianhang, Wanhua Wu, and Cheng Yang. 2025. "Recent Advances in the Synthesis and Applications of Nitrogen-Containing Macrocyclic Arenes" Molecules 30, no. 17: 3646. https://doi.org/10.3390/molecules30173646
APA StyleHu, J., Wu, W., & Yang, C. (2025). Recent Advances in the Synthesis and Applications of Nitrogen-Containing Macrocyclic Arenes. Molecules, 30(17), 3646. https://doi.org/10.3390/molecules30173646






