Osteogenic Differentiation in Chitosan-Based Scaffolds via P28 and VEGF Delivery
Abstract
1. Introduction
2. Results
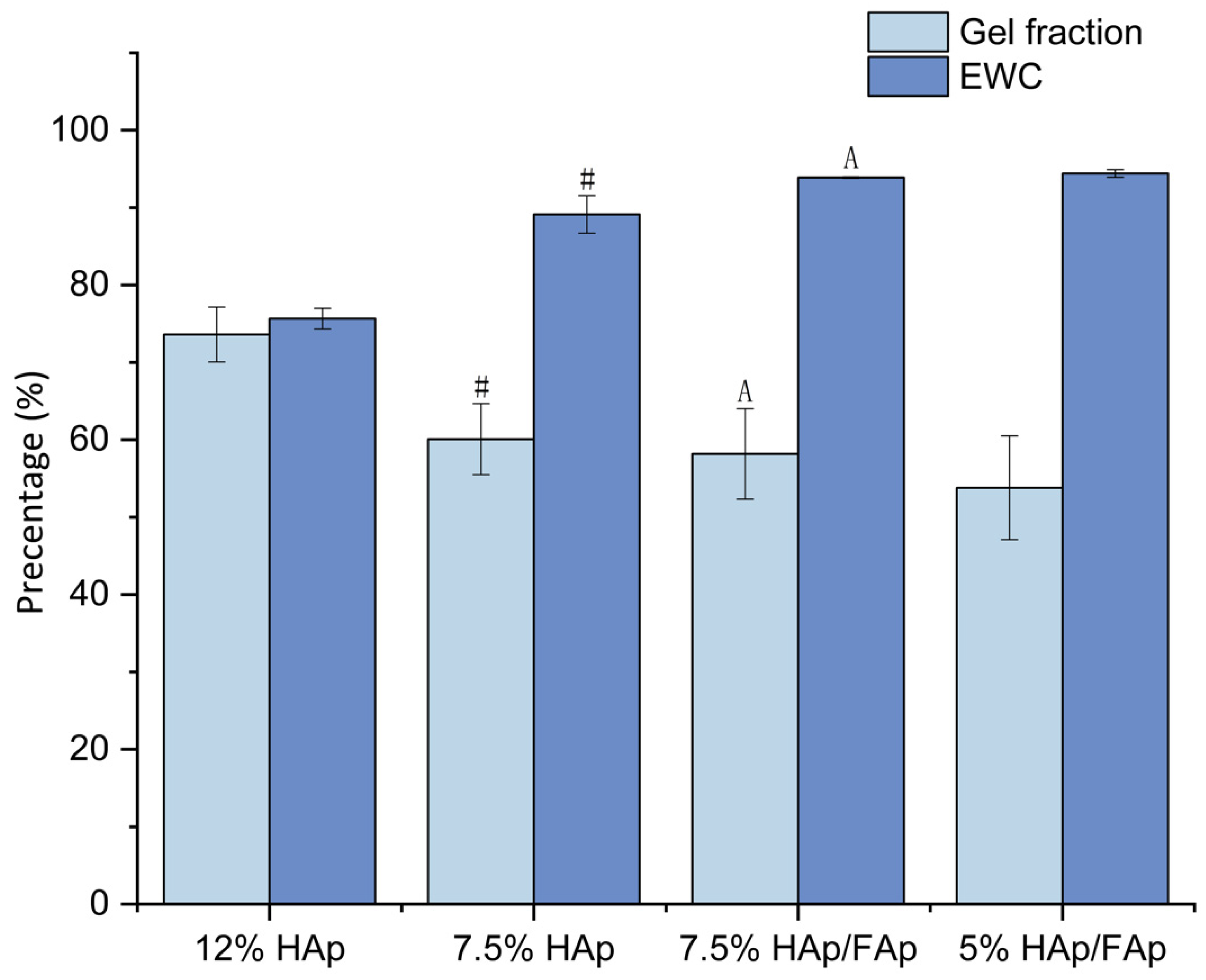
2.1. Swelling Behavior
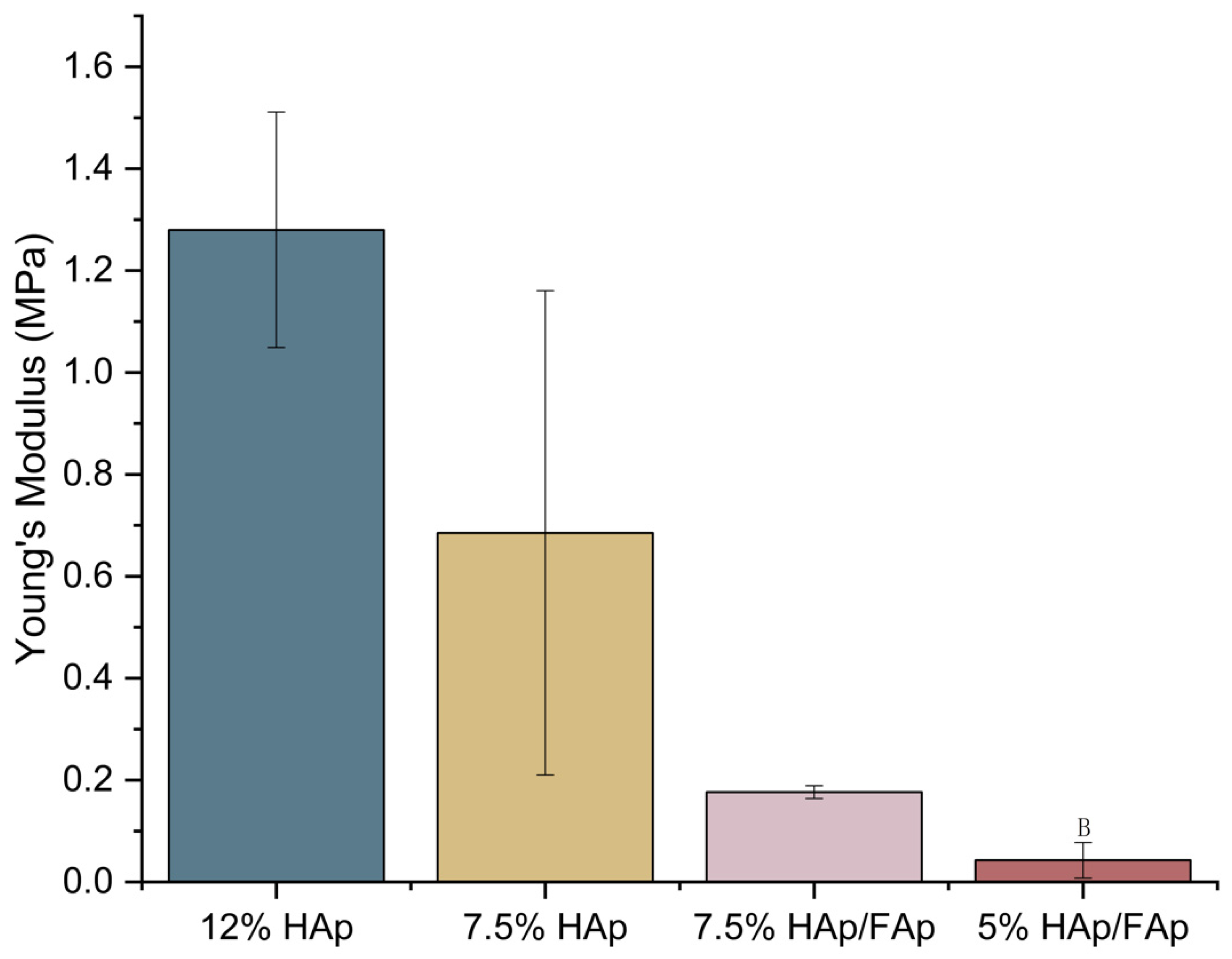
2.2. Mechanical Strength
2.3. Porosity
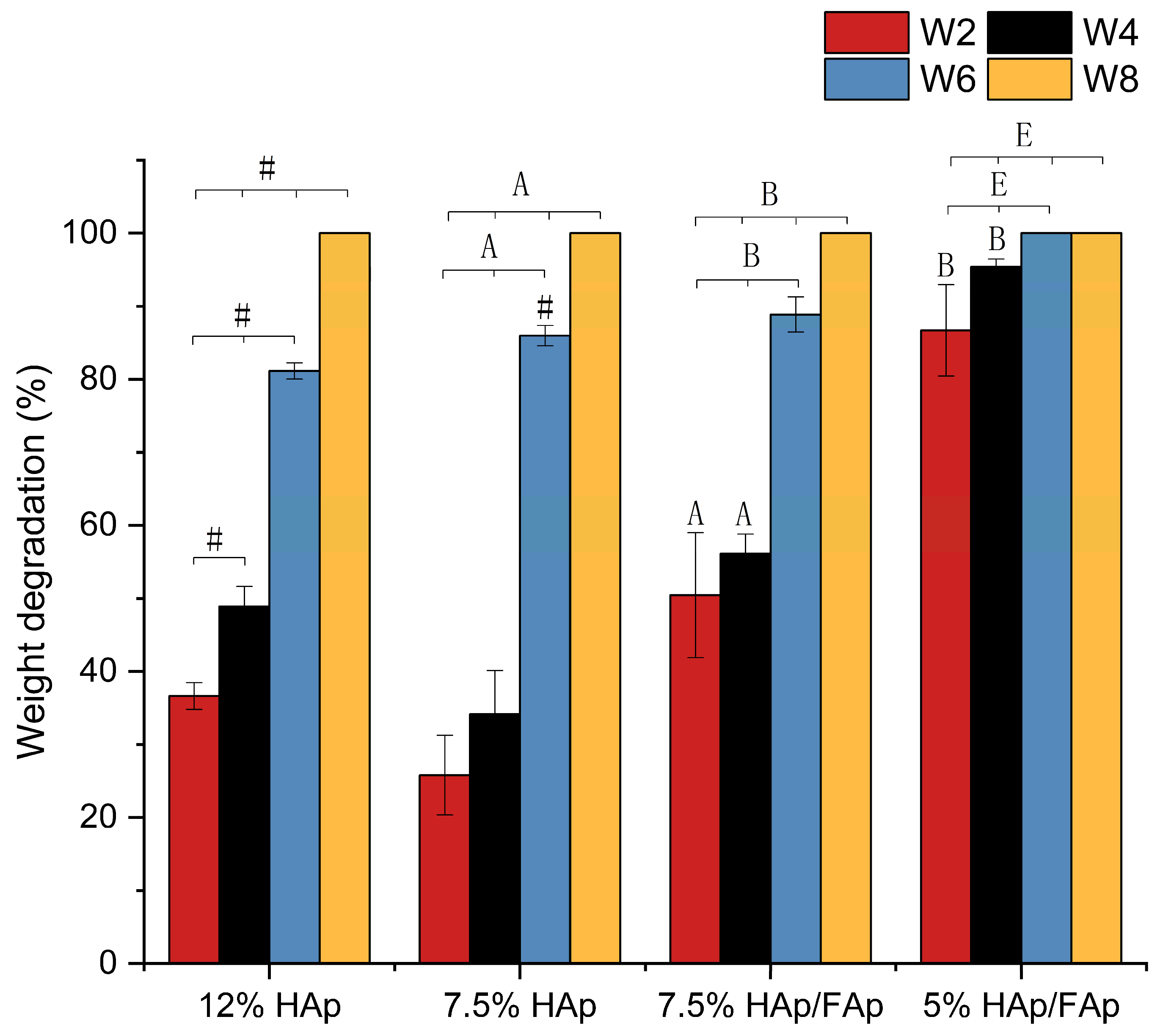
2.4. In Vitro Degradation Test
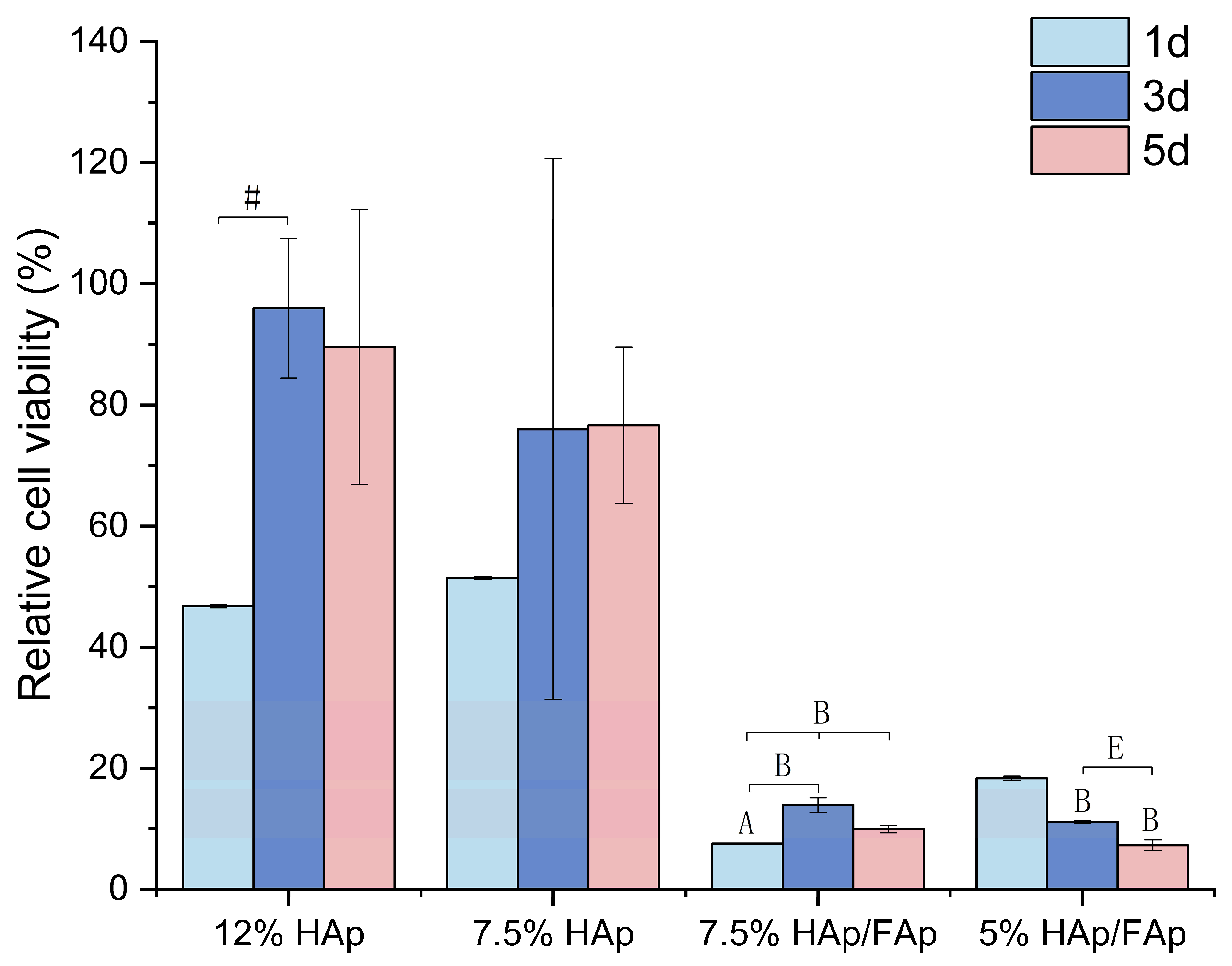
2.5. Cell Proliferation
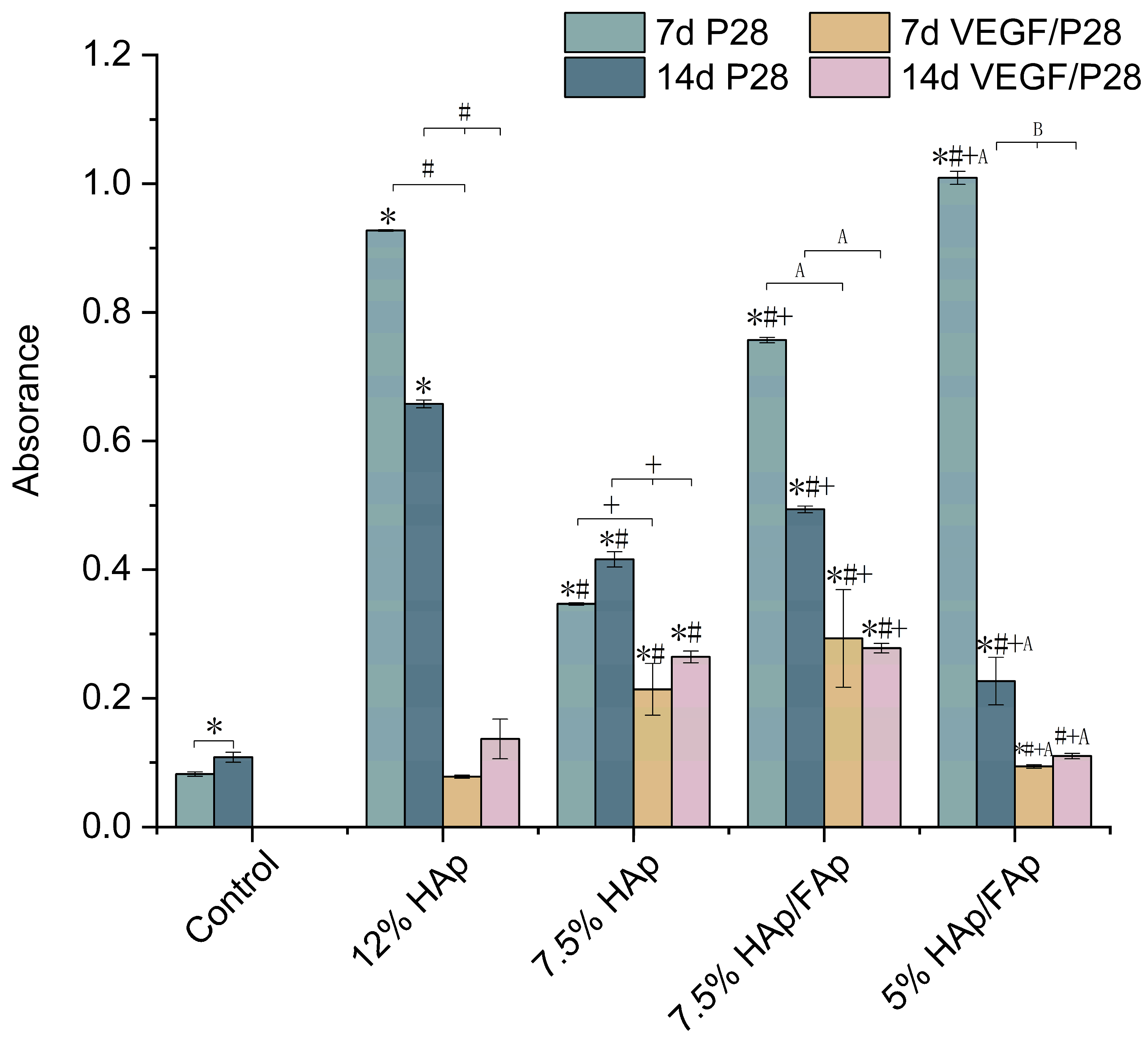
2.6. Alizarin Red S Stain
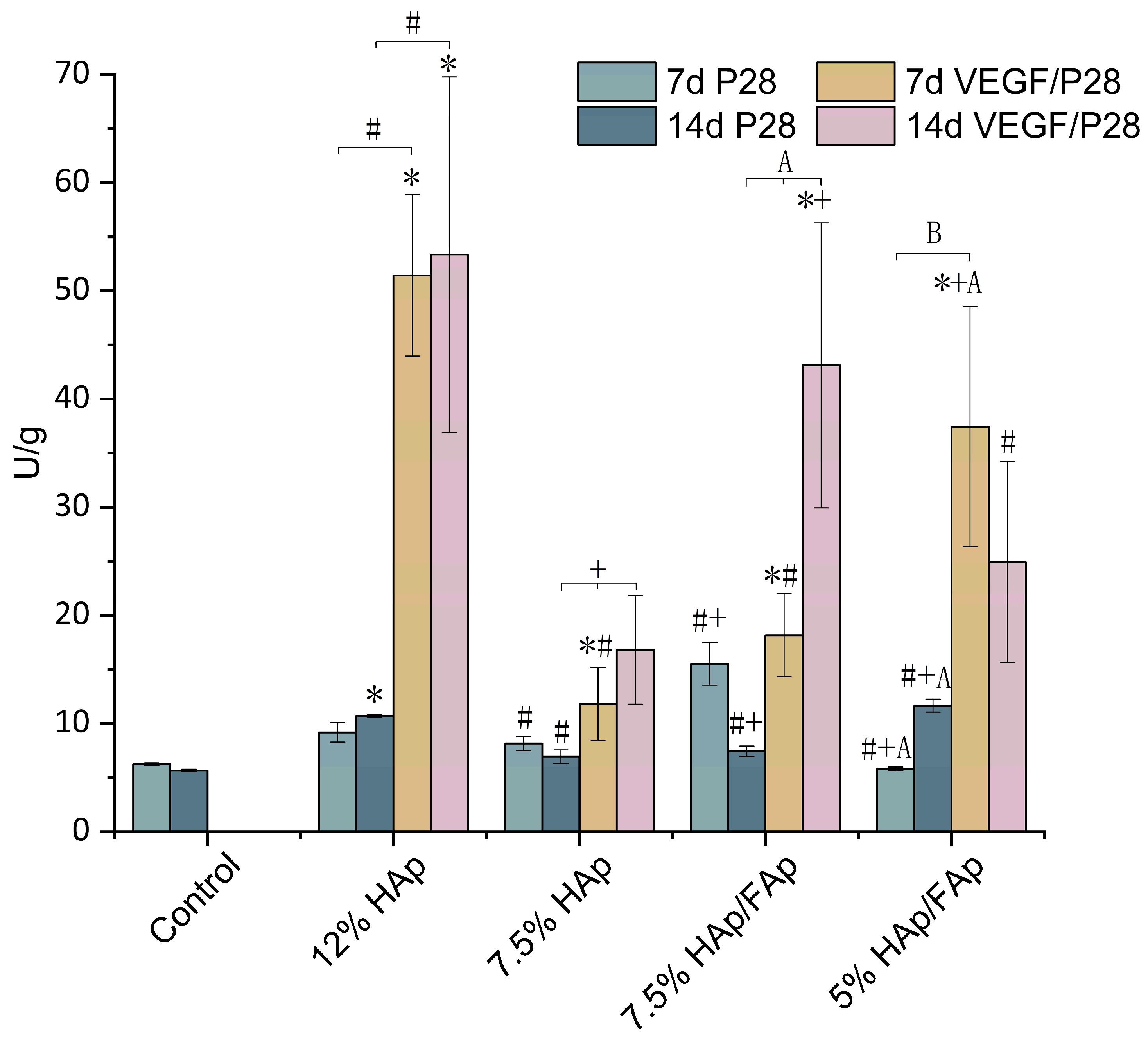
2.7. Alkaline Phosphatase Activity
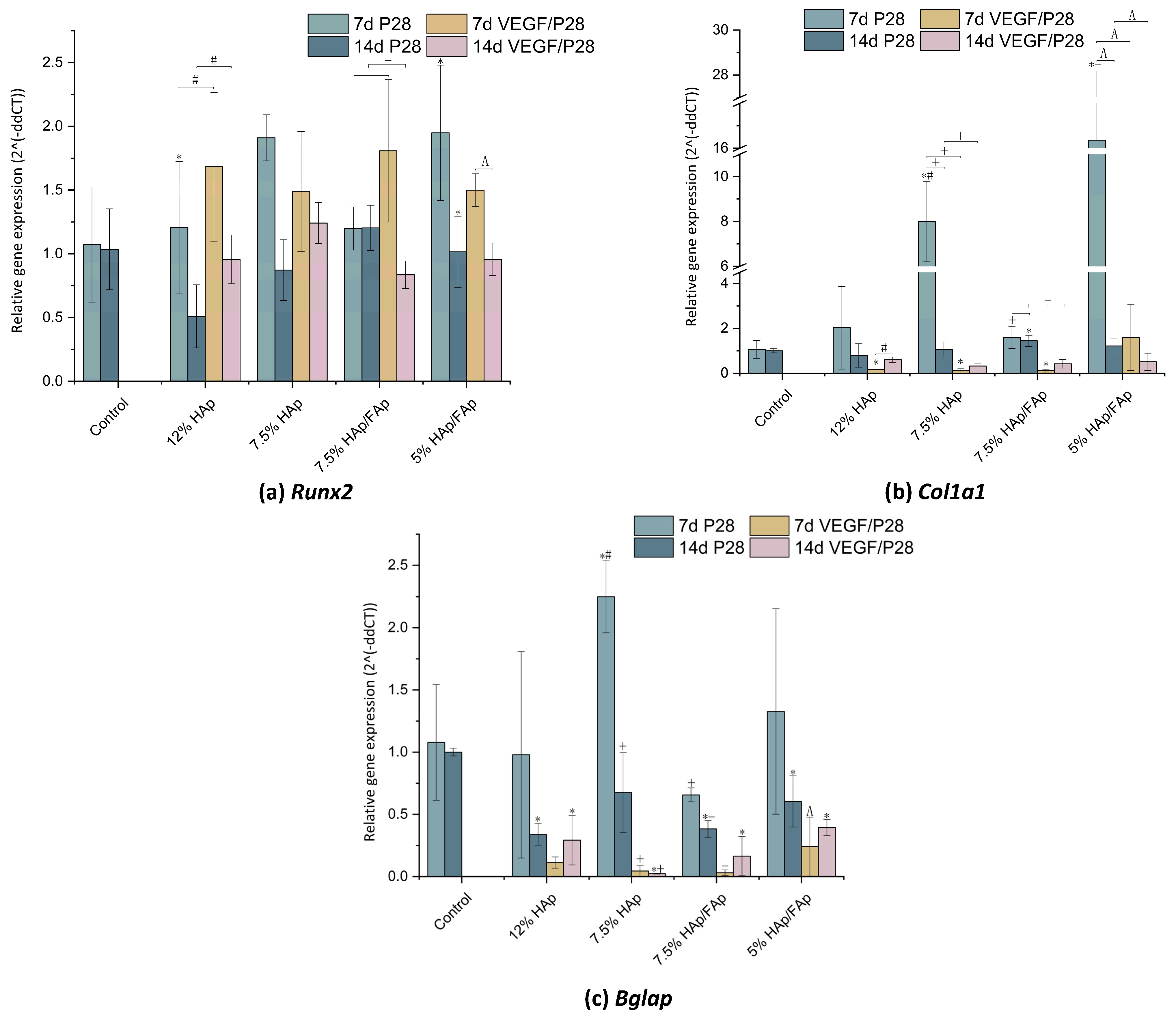
2.8. Gene-Expression Analysis
3. Discussion
4. Materials and Methods
4.1. Scaffold Preparation
4.2. Swelling Behavior
4.3. Compression Test
4.4. Porosity Test
4.5. In-Vitro Degradation Test in Simulated Body Fluid (SBF)
4.6. Cell Proliferation
4.7. Bioactive Factors Loading
4.8. Cell Seeding for Osteogenic Markers Analysis and Gene Expression
4.9. Alizarin Red S Staining (ARS)
4.10. Alkaline Phosphatase (ALP) Activity
4.11. Reverse Transcription Quantitative Polymerase Chain Reaction
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Acetic acid |
| ALP | Alkaline phosphatase activity |
| BMP-2 | Bone morphogenetic protein 2 |
| BTE | Bone tissue engineering |
| CCK–8 | Cell counting kit–8 |
| CS | Chitosan |
| ECM | Extracellular matrix |
| EWC | Equilibrium water content |
| FAp | Fluorapatite |
| FBS | Fetal Bovine Serum |
| GF | Gel fraction |
| HAp | Hydroxyapatite |
| Runx2 | Runt-related transcription factor 2 |
| RT-qPCR | Reverse transcription-quantitative polymerase chain reaction |
| SBF | Simulated body solution |
| VEGF | Vascular endothelial growth factor |
References
- Bolamperti, S.; Villa, I.; Rubinacci, A. Bone remodeling: An operational process ensuring survival and bone mechanical competence. Bone Res. 2022, 10, 48. [Google Scholar] [CrossRef]
- De Pace, R.; Molinari, S.; Mazzoni, E.; Perale, G. Bone Regeneration: A Review of Current Treatment Strategies. J. Clin. Med. 2025, 14, 1838. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.; Joseph, J.; Zhang, X.; Ferdows, B.E.; Patel, D.N.; Chen, W.; Banfi, G.; Molinaro, R.; Cosco, D.; et al. Biomaterials and nanomedicine for bone regeneration: Progress and future prospects. Exploration 2021, 1, 20210011. [Google Scholar] [CrossRef]
- Stahl, A.; Yang, Y.P. Regenerative Approaches for the Treatment of Large Bone Defects. Tissue Eng. Part B Rev. 2021, 27, 539–547. [Google Scholar] [CrossRef]
- Ashukina, N.; Vorontsov, P.; Maltseva, V.; Danyshchuk, Z.; Nikolchenko, O.; Samoylova, K.; Husak, V. Morphology of the repair of critical size bone defects which filling allogeneic bone implants in combination with mesenchymal stem cells depending on the recipient age in the experiment. Orthop. Traumatol. Prosthet. 2023, 3–4, 80–90. [Google Scholar] [CrossRef]
- Huang, E.E.; Zhang, N.; Shen, H.; Li, X.; Maruyama, M.; Utsunomiya, T.; Gao, Q.; Guzman, R.A.; Goodman, S.B. Novel Techniques and Future Perspective for Investigating Critical-Size Bone Defects. Bioengineering 2022, 9, 171. [Google Scholar] [CrossRef]
- Qi, J.; Yu, T.; Hu, B.; Wu, H.; Ouyang, H. Current Biomaterial-Based Bone Tissue Engineering and Translational Medicine. Int. J. Mol. Sci. 2021, 22, 10233. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Lu, Y.; Meng, L.; Wu, J.; Gong, T.; Zou, J.; Bosiakov, S.; Cheng, L. A Critical Review on the Design, Manufacturing and Assessment of the Bone Scaffold for Large Bone Defects. Front. Bioeng. Biotechnol. 2021, 9, 753715. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kim, J. Achieving the ideal balance between biological and mechanical requirements in composite bone scaffolds through a voxel-based approach. Comput. Methods Biomech. Biomed. Eng. 2024, 28, 923–936. [Google Scholar] [CrossRef]
- Soriente, A.; Fasolino, I.; Gomez-Sánchez, A.; Prokhorov, E.; Buonocore, G.G.; Luna-Barcenas, G.; Ambrosio, L.; Raucci, M.G. Chitosan/hydroxyapatite nanocomposite scaffolds to modulate osteogenic and inflammatory response. J. Biomed. Mater. Res. Part A 2022, 110, 266–272. [Google Scholar] [CrossRef]
- Kołakowska, A.; Gadomska-Gajadhur, A.; Ruśkowski, P. Biomimetic scaffolds based on chitosan in bone regeneration. A review. Chem. Process. Eng. 2023, 43, 305–330. [Google Scholar] [CrossRef]
- Yousefiasl, S.; Sharifi, E.; Salahinejad, E.; Makvandi, P.; Irani, S. Bioactive 3D-printed chitosan-based scaffolds for personalized craniofacial bone tissue engineering. Eng. Regen. 2023, 4, 1–11. [Google Scholar] [CrossRef]
- Adhikari, J.; Perwez, S.; Das, A.; Saha, P. Development of hydroxyapatite reinforced alginate–chitosan based printable biomaterial-ink. Nano-Struct. Nano-Objects 2021, 25, 100630. [Google Scholar] [CrossRef]
- Chatzipetros, E.; Damaskos, S.; Tosios, K.I.; Christopoulos, P.; Donta, C.; Kalogirou, E.-M.; Yfanti, Z.; Tsiourvas, D.; Papavasiliou, A.; Tsiklakis, K. The effect of nano-hydroxyapatite/chitosan scaffolds on rat calvarial defects for bone regeneration. Int. J. Implant. Dent. 2021, 7, 40. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Naghieh, S.; Yazdanpanah, Z.; Sardroud, H.A.; Sharma, N.; Wilson, L.D.; Chen, X. Fabrication of chitosan/alginate/hydroxyapatite hybrid scaffolds using 3D printing and impregnating techniques for potential cartilage regeneration. Int. J. Biol. Macromol. 2022, 204, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Chacon, E.L.; Bertolo, M.R.V.; de Guzzi Plepis, A.M.; da Conceição Amaro Martins, V.; dos Santos, G.R.; Pinto, C.A.L.; Pelegrine, A.A.; Teixeira, M.L.; Buchaim, D.V.; Nazari, F.M.; et al. Collagen-chitosan-hydroxyapatite composite scaffolds for bone repair in ovariectomized rats. Sci. Rep. 2023, 13, 28. [Google Scholar] [CrossRef]
- Ma, P.; Wu, W.; Wei, Y.; Ren, L.; Lin, S.; Wu, J. Biomimetic gelatin/chitosan/polyvinyl alcohol/nano-hydroxyapatite scaffolds for bone tissue engineering. Mater. Des. 2021, 207, 109865. [Google Scholar] [CrossRef]
- Borkowski, L.; Jojczuk, M.; Belcarz, A.; Pawlowska-Olszewska, M.; Kruk-Bachonko, J.; Radzki, R.; Bienko, M.; Slowik, T.; Lübek, T.; Nogalski, A.; et al. Comparing the Healing Abilities of Fluorapatite and Hydroxyapatite Ceramics in Regenerating Bone Tissue: An In Vivo Study. Materials 2023, 16, 5992. [Google Scholar] [CrossRef]
- Piard, C.; Luthcke, R.; Kamalitdinov, T.; Fisher, J. Sustained delivery of vascular endothelial growth factor from mesoporous calcium-deficient hydroxyapatite microparticles promotes in vitro angiogenesis and osteogenesis. J. Biomed. Mater. Res. Part A 2021, 109, 1080–1087. [Google Scholar] [CrossRef]
- Ding, T.; Kang, W.; Li, J.; Yu, L.; Ge, S. An in situ tissue engineering scaffold with growth factors combining angiogenesis and osteoimmunomodulatory functions for advanced periodontal bone regeneration. J. Nanobiotechnol. 2021, 19, 247. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yang, H.; Ni, X.; Deng, Y.; Li, Z.; Xing, X.; Du, M. Programmed release of vascular endothelial growth factor and exosome from injectable chitosan nanofibrous microsphere-based PLGA-PEG-PLGA hydrogel for enhanced bone regeneration. Int. J. Biol. Macromol. 2023, 253, 126721. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Zhang, Y.; Luo, G.; Li, D.; Ma, Y.; Xiao, Y.; Xiao, L.; Wang, X. The decisive early phase of biomaterial-induced bone regeneration. Appl. Mater. Today 2024, 38, 102236. [Google Scholar] [CrossRef]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for Bone Regeneration: From Graft to Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.; Wang, S.; Zhao, J.; Xu, L.; Zhu, C.; Zeng, D.; Chen, J.; Zhang, Z.; Kaplan, D.L.; et al. The use of injectable sonication-induced silk hydrogel for VEGF165 and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials 2011, 32, 9415–9424. [Google Scholar] [CrossRef]
- Alarcin, E.; Akguner, Z.P.; Ozturk, A.B.; Yasayan, G.; Ilhan-Ayisigi, E.; Kazan, A.; Yesil-Celiktas, O.; Akcora, D.S.; Akakin, D.; Kocaaga, B.; et al. Biomimetic 3D bioprinted bilayer GelMA scaffolds for the delivery of BMP-2 and VEGF exogenous growth factors to promote vascularized bone regeneration in a calvarial defect model in vivo. Int. J. Biol. Macromol. 2025, 306, 141440. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Wang, J.; Wang, L.; Gao, C.; Li, B.; Wang, Y.; Wu, J.; Quan, C. Bioactive gelatin cryogels with BMP-2 biomimetic peptide and VEGF: A potential scaffold for synergistically induced osteogenesis. Chin. Chem. Lett. 2022, 33, 1956–1962. [Google Scholar] [CrossRef]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Vallmajo-Martin, Q.; Millan, C.; Müller, R.; Weber, F.E.; Ehrbar, M.; Ghayor, C. Enhanced bone regeneration in rat calvarial defects through BMP2 release from engineered poly(ethylene glycol) hydrogels. Sci. Rep. 2024, 14, 4916. [Google Scholar] [CrossRef]
- Azaman, F.A.; Fournet, M.E.B.; Ab Hamid, S.S.; Zawawi, M.S.F.; da Silva Junior, V.A.; Devine, D.M. Enhancement of Scaffold In Vivo Biodegradability for Bone Regeneration Using P28 Peptide Formulations. Pharmaceuticals 2023, 16, 876. [Google Scholar] [CrossRef]
- Meng, C.; Su, W.; Liu, M.; Yao, S.; Ding, Q.; Yu, K.; Xiong, Z.; Chen, K.; Guo, X.; Bo, L.; et al. Controlled delivery of bone morphogenic protein-2-related peptide from mineralised extracellular matrix-based scaffold induces bone regeneration. Mater. Sci. Eng. C 2021, 126, 112182. [Google Scholar] [CrossRef]
- Zhou, K.; Simonassi-Paiva, B.; Fehrenbach, G.; Yan, G.; Portela, A.; Pogue, R.; Cao, Z.; Fournet, M.B.; Devine, D.M. Investigating the Promising P28 Peptide-Loaded Chitosan/Ceramic Bone Scaffolds for Bone Regeneration. Molecules 2024, 29, 4208. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Etxabide, A.; Seyfoddin, A.; Ramezani, M. Fabrication and characterisation of poly(vinyl alcohol)/chitosan scaffolds for tissue engineering applications. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Saatcioglu, E.; Ulag, S.; Sahin, A.; Yilmaz, B.K.; Ekren, N.; Inan, A.T.; Palaci, Y.; Ustundag, C.B.; Gunduz, O. Design and fabrication of electrospun polycaprolactone/chitosan scaffolds for ligament regeneration. Eur. Polym. J. 2021, 148, 110357. [Google Scholar] [CrossRef]
- Zhou, K.; Azaman, F.A.; Cao, Z.; Fournet, M.B.; Devine, D.M. Bone Tissue Engineering Scaffold Optimisation through Modification of Chitosan/Ceramic Composition. Macromol 2023, 3, 326–342. [Google Scholar] [CrossRef]
- Killion, J.A.; Geever, L.M.; Devine, D.M.; Grehan, L.; Kennedy, J.E.; Higginbotham, C.L. Modulating the mechanical properties of photopolymerised polyethylene glycol–polypropylene glycol hydrogels for bone regeneration. J. Mater. Sci. 2012, 47, 6577–6585. [Google Scholar] [CrossRef]
- Elakkiya, K.; Bargavi, P.; Balakumar, S. 3D interconnected porous PMMA scaffold integrating with advanced nanostructured CaP-based biomaterials for rapid bone repair and regeneration. J. Mech. Behav. Biomed. Mater. 2023, 147, 106106. [Google Scholar] [CrossRef]
- Jiao, J.; Hong, Q.; Zhang, D.; Wang, M.; Tang, H.; Yang, J.; Qu, X.; Yue, B. Influence of porosity on osteogenesis, bone growth and osteointegration in trabecular tantalum scaffolds fabricated by additive manufacturing. Front. Bioeng. Biotechnol. 2023, 11, 1117954. [Google Scholar] [CrossRef]
- Zhou, J.; Xiong, S.; Liu, M.; Yang, H.; Wei, P.; Yi, F.; Ouyang, M.; Xi, H.; Long, Z.; Liu, Y.; et al. Study on the influence of scaffold morphology and structure on osteogenic performance. Front. Bioeng. Biotechnol. 2023, 11, 1127162. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Park, S.; Choi, J.; Vu, T.T.H.; Doan, V.H.M.; Vo, T.T.; Lee, B.; Oh, J. Hydroxyapatite: A journey from biomaterials to advanced functional materials. Adv. Colloid Interface Sci. 2023, 321, 103013. [Google Scholar] [CrossRef]
- Harini, G.; Bharathi, R.; Sankaranarayanan, A.; Shanmugavadivu, A.; Selvamurugan, N. Nanoceramics-reinforced chitosan scaffolds in bone tissue engineering. Mater. Adv. 2023, 4, 3907–3928. [Google Scholar] [CrossRef]
- Xia, H.; Dong, L.; Hao, M.; Wei, Y.; Duan, J.; Chen, X.; Yu, L.; Li, H.; Sang, Y.; Liu, H. Osteogenic Property Regulation of Stem Cells by a Hydroxyapatite 3D-Hybrid Scaffold with Cancellous Bone Structure. Front. Chem. 2021, 9, 798299. [Google Scholar] [CrossRef]
- Tan, B.; Zhao, N.; Guo, W.; Huang, F.; Hu, H.; Chen, Y.; Li, J.; Ling, Z.; Zou, Z.; Hu, R.; et al. Biomimetic hydroxyapatite coating on the 3D-printed bioactive porous composite ceramic scaffolds promoted osteogenic differentiation via PI3K/AKT/mTOR signaling pathways and facilitated bone regeneration in vivo. J. Mater. Sci. Technol. 2022, 136, 54–64. [Google Scholar] [CrossRef]
- Piszko, P.J.; Piszko, A.; Kiryk, S.; Kiryk, J.; Horodniczy, T.; Struzik, N.; Wiśniewska, K.; Matys, J.; Dobrzyński, M. Bone Regeneration Capabilities of Scaffolds Containing Chitosan and Nanometric Hydroxyapatite—Systematic Review Based on In Vivo Examinations. Biomimetics 2024, 9, 503. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Dahlan, A.; Kamadjaja, M.J.K.; Subagyo, S.Y.; Talitha, P. Evaluation of swelling ability of scaffold combination of chitosan and hydroxypatite. Indones. J. Prosthodont. 2022, 3, 17–22. [Google Scholar] [CrossRef]
- Teimouri, R.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Surface modifications of scaffolds for bone regeneration. J. Mater. Res. Technol. 2023, 24, 7938–7973. [Google Scholar] [CrossRef]
- Ye, B.; Wu, B.; Su, Y.; Sun, T.; Guo, X. Recent Advances in the Application of Natural and Synthetic Polymer-Based Scaffolds in Musculoskeletal Regeneration. Polymers 2022, 14, 4566. [Google Scholar] [CrossRef]
- Elyaderani, A.K.; De Lama-Odría, M.D.C.; Valle, L.J.D.; Puiggalí, J. Multifunctional Scaffolds Based on Emulsion and Coaxial Electrospinning Incorporation of Hydroxyapatite for Bone Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 15016. [Google Scholar] [CrossRef]
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef]
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and Fluorapatite in Conservative Dentistry and Oral Implantology—A Review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef]
- Li, J.; Guo, D.; Li, J.; Wei, X.; Sun, Z.; Yang, B.; Lu, T.; Ouyang, P.; Chang, S.; Liu, W.; et al. Irregular pore size of degradable bioceramic Voronoi scaffolds prepared by stereolithography: Osteogenesis and computational fluid dynamics analysis. Mater. Des. 2022, 224, 111414. [Google Scholar] [CrossRef]
- Singh, G.; Soundarapandian, S. Effect of particle size and freezing conditions on freeze-casted scaffold. Ceram. Int. 2019, 45, 11633–11638. [Google Scholar] [CrossRef]
- Xie, J.; Du, H.; Chen, S.; Sun, X.; Xin, L. Chemical Modification Effect of Compound Solutions of Surfactants with Acetic Acid on Coal Pores. ACS Omega 2021, 6, 35342–35354. [Google Scholar] [CrossRef]
- Calore, A.R.; Srinivas, V.; Groenendijk, L.; Serafim, A.; Stancu, I.C.; Wilbers, A.; Leoné, N.; Sanchez, A.A.; Auhl, D.; Mota, C.; et al. Manufacturing of scaffolds with interconnected internal open porosity and surface roughness. Acta Biomater. 2023, 156, 158–176. [Google Scholar] [CrossRef] [PubMed]
- Ezeldeen, M.; Loos, J.; Nejad, Z.M.; Cristaldi, M.; Murgia, D.; Braem, A.; Jacobs, R. 3D-printing-assisted fabrication of chitosan scaffolds from different sources and cross-linkers for dental tissue engineering. Eur. Cell Mater. 2021, 41, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Heidari, F.; Razavi, M.; Bahrololoom, M.E.; Tahriri, M.; Tayebi, L. Investigation of the mechanical properties and degradability of a modified chitosan-based scaffold. Mater. Chem. Phys. 2018, 204, 187–194. [Google Scholar] [CrossRef]
- Azaman, F.A.; Zhou, K.; del Mar Blanes-Martínez, M.; Fournet, M.B.; Devine, D.M. Bioresorbable Chitosan-Based Bone Regeneration Scaffold Using Various Bioceramics and the Alteration of Photoinitiator Concentration in an Extended UV Photocrosslinking Reaction. Gels 2022, 8, 696. [Google Scholar] [CrossRef]
- Böhning, J.; Tarafder, A.K.; Bharat, T.A. The role of filamentous matrix molecules in shaping the architecture and emergent properties of bacterial biofilms. Biochem. J. 2024, 481, 245–263. [Google Scholar] [CrossRef]
- Dadou, S.M.; El-Barghouthi, M.I.; Alabdallah, S.K.; Badwan, A.A.; Antonijevic, M.D.; Chowdhry, B.Z. Effect of Protonation State and N-Acetylation of Chitosan on Its Interaction with Xanthan Gum: A Molecular Dynamics Simulation Study. Mar. Drugs 2017, 15, 298. [Google Scholar] [CrossRef]
- Pagani, S.; Liverani, E.; Giavaresi, G.; De Luca, A.; Belvedere, C.; Fortunato, A.; Leardini, A.; Fini, M.; Tomesani, L.; Caravaggi, P. Mechanical and in vitro biological properties of uniform and graded Cobalt-chrome lattice structures in orthopedic implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 2091–2103. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Liu, L.; Lv, L.; Gao, L.; Liu, N.; Wang, X.; Ye, J. Mechanical behavior of a titanium alloy scaffold mimicking trabecular structure. J. Orthop. Surg. Res. 2020, 15, 40. [Google Scholar] [CrossRef]
- Qiu, D.; Zhou, J.; Feng, Q.; Ren, K.; Zhang, H.; He, Y.; Li, C.; Liu, J.; Mai, N.T.T. Functionality, physicochemical properties, and applications of chitosan/nano-hydroxyapatite–tea polyphenol films. Food Chem. X 2024, 24, 101762. [Google Scholar] [CrossRef]
- Winayu, I.J.; Ekantari, N.; Puspita, I.D.; Ustadi; Budhijanto, W.; Nugraheni, P.S. The effect of reduced acetic acid concentration on nano-chitosan formulation as fish preservative. IOP Conf. Ser. Mater. Sci. Eng. 2019, 633, 012040. [Google Scholar] [CrossRef]
- Gui, X.; Zhang, B.; Qin, Y.; Lei, H.; Xia, X.; Li, Y.; Lei, H.; Zhou, X.; Tan, Y.; Dong, Z.; et al. Structural and material double mechanical enhancement of HAp scaffolds promote bone defect regeneration. Compos. Part A Appl. Sci. Manuf. 2024, 189, 108600. [Google Scholar] [CrossRef]
- Guo, C.; Wu, J.; Zeng, Y.; Li, H. Construction of 3D bioprinting of HAP/collagen scaffold in gelation bath for bone tissue engineering. Regen. Biomater. 2023, 10, rbad067. [Google Scholar] [CrossRef]
- Chen, Q.; Zou, B.; Zhao, Y.; Wang, X.; Zhou, X.; Lai, Q. 3D printing of PEGDA/bioceramic for guiding cell adhesion and migration. Surf. Interfaces 2024, 48, 104364. [Google Scholar] [CrossRef]
- Zoe, L.H.; David, S.R.; Rajabalaya, R. Chitosan nanoparticle toxicity: A comprehensive literature review of in vivo and in vitro assessments for medical applications. Toxicol. Rep. 2023, 11, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Mollentze, J.; Durandt, C.; Pepper, M.S.; De Girolamo, L. An In Vitro and In Vivo Comparison of Osteogenic Differentiation of Human Mesenchymal Stromal/Stem Cells. Stem Cells Int. 2021, 2021, 9919361. [Google Scholar] [CrossRef]
- Ye, F.; Li, J.; Xu, P.; Xie, Z.; Zheng, G.; Liu, W.; Ye, G.; Yu, W.; Lin, J.; Su, Z.; et al. Osteogenic differentiation of mesenchymal stem cells promotes c-Jun-dependent secretion of interleukin 8 and mediates the migration and differentiation of CD4+ T cells. Stem Cell Res. Ther. 2022, 13, 58. [Google Scholar] [CrossRef]
- Djouallah, S.; Belhouchet, H.; Kenzour, A.; Kherifi, D. Sintering behavior of fluorapatite-based composites produced from natural phosphate and alumina. Ceram. Int. 2021, 47, 3553–3564. [Google Scholar] [CrossRef]
- Zhou, J.; Xiong, Z.; Liu, M.; Yang, L.; Yao, S.; Chen, K.; Yu, K.; Qu, Y.; Sun, T.; Guo, X. Creation of Bony Microenvironment with Extracellular Matrix Doped-Bioactive Ceramics to Enhance Osteoblast Behavior and Delivery of Aspartic Acid-Modified BMP-2 Peptides. Int. J. Nanomed. 2020, 15, 8465–8478. [Google Scholar] [CrossRef]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W.-H. The roles of ions on bone regeneration. Drug Discov. Today 2018, 23, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, H.; Dong, X.; Geng, Z.; Xu, X.; Liu, Y. VEGF mitigates bisphosphonate-induced apoptosis and differentiation inhibition of MC3T3-E1 cells. Exp. Ther. Med. 2021, 23, 130. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Portolés-Gil, N.; López-Periago, A.M.; Domingo, C.; Hosta-Rigau, L. Immobilization of BMP-2 and VEGF Within Multilayered Polydopamine-Coated Scaffolds and the Resulting Osteogenic and Angiogenic Synergy of Co-Cultured Human Mesenchymal Stem Cells and Human Endothelial Progenitor Cells. Int. J. Mol. Sci. 2020, 21, 6418. [Google Scholar] [CrossRef]
- Kim, H.J.; Hong, S.J.; Lee, S.; Park, J.M.; Park, J.S.; Shim, S.H.; Park, K. Induction of Bone Formation by 3D Biologically Active Scaffolds Containing RGD-NPs, BMP2, and NtMPCs. Adv. Ther. 2021, 4, 2000245. [Google Scholar] [CrossRef]
- Sharma, S.; Sapkota, D.; Xue, Y.; Rajthala, S.; Yassin, M.A.; Finne-Wistrand, A.; Mustafa, K. Delivery of VEGFA in bone marrow stromal cells seeded in copolymer scaffold enhances angiogenesis, but is inadequate for osteogenesis as compared with the dual delivery of VEGFA and BMP2 in a subcutaneous mouse model. Stem Cell Res. Ther. 2018, 9, 23. [Google Scholar] [CrossRef]
- Kang, F.; Yi, Q.; Gu, P.; Dong, Y.; Zhang, Z.; Zhang, L.; Bai, Y. Controlled growth factor delivery system with osteogenic-angiogenic coupling effect for bone regeneration. J. Orthop. Transl. 2021, 31, 110–125. [Google Scholar] [CrossRef]
- Gan, Q.; Pan, H.; Zhang, W.; Yuan, Y.; Qian, J.; Liu, C. Fabrication and evaluation of a BMP-2/dexamethasone co-loaded gelatin sponge scaffold for rapid bone regeneration. Regen. Biomater. 2022, 9, rbac008. [Google Scholar] [CrossRef]
- Geng, Y.; Duan, H.; Xu, L.; Witman, N.; Yan, B.; Yu, Z.; Wang, H.; Tan, Y.; Lin, L.; Li, D.; et al. BMP-2 and VEGF-A modRNAs in collagen scaffold synergistically drive bone repair through osteogenic and angiogenic pathways. Commun. Biol. 2021, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.S.; Young, S.; Tabata, Y.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43, 931–940. [Google Scholar] [CrossRef]
- Martin-Iglesias, S.; Milian, L.; Sancho-Tello, M.; Salvador-Clavell, R.; de Llano, J.J.M.; Carda, C.; Mata, M.; Chimenti, I. BMP-2 Enhances Osteogenic Differentiation of Human Adipose-Derived and Dental Pulp Stem Cells in 2D and 3D In Vitro Models. Stem Cells Int. 2022, 2022, 4910399. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.-H.; Kim, Y.-H.; Hong, J.; Kim, W.K.; Jin, S.; Kang, B.-J. Sustained BMP-2 delivery via alginate microbeads and polydopamine-coated 3D-Printed PCL/β-TCP scaffold enhances bone regeneration in long bone segmental defects. J. Orthop. Transl. 2024, 49, 11–22. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, J.-S.; Lin, L.; Zhang, J.; Liu, Y.; Shuai, M.; Li, Q. Effects of BMP2 and VEGF165 on the osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. Exp. Ther. Med. 2013, 7, 625–629. [Google Scholar] [CrossRef]
- Kokubo, T. and Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Lei, Y.; Rai, B.; Ho, K.; Teoh, S. In vitro degradation of novel bioactive polycaprolactone—20% tricalcium phosphate composite scaffolds for bone engineering. Mater. Sci. Eng. C 2007, 27, 293–298. [Google Scholar] [CrossRef]



| Scaffold ID | HAp (%wt) | FAp (%wt) | Volume of 1% v/v AA/(mL) | PEGDMA (µL) |
|---|---|---|---|---|
| 5% HAp/FAp | 0.75 | 0.75 | 30 | 100 |
| 7.5% HAp | 1.5 | 0 | 20 | 100 |
| 7.5% HAp/FAp | 0.75 | 0.75 | 20 | 100 |
| 12% HAp | 1.5 | 0 | 12.5 | 100 |
| Ion Concentration (mM) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Mg2+ | Ca2+ | Cl− | HCO3− | HPO42− | SO42− | |
| SBF | 142.0 | 5.0 | 2.5 | 1.5 | 148.8 | 4.2 | 1.0 | 0.5 |
| Human plasma | 142.0 | 5.0 | 2.5 | 1.5 | 103.0 | 27.0 | 1.0 | 0.5 |
| Gene | Primer Sequences Forward/Reverse |
|---|---|
| Bglap | 5′-GACACCATGAGGACCATCTTTC–3′/5′-CATGAAGGCTTTGTCAGACTCA–3′ |
| Col1a1 | 5′–CCAATGGTGCTCCTGGTATT–3′/5′–GGTTCACCACTGTTACCCTT–3′ |
| Runx2 | 5′–CTCTGATCGCCTCAGTGATTT–3′/5′–CTGCCTGGGATCTGTAATCTG–3′ |
| Tbp | 5′–AGTGCCCAGCATCACTATTT–3′/5′–GGTCCATGATTCTCCCTTTCTT–3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, K.; Simonassi-Paiva, B.; Pogue, R.; Murphy, E.; Cao, Z.; Fournet, M.B.; Devine, D.M. Osteogenic Differentiation in Chitosan-Based Scaffolds via P28 and VEGF Delivery. Molecules 2025, 30, 3645. https://doi.org/10.3390/molecules30173645
Zhou K, Simonassi-Paiva B, Pogue R, Murphy E, Cao Z, Fournet MB, Devine DM. Osteogenic Differentiation in Chitosan-Based Scaffolds via P28 and VEGF Delivery. Molecules. 2025; 30(17):3645. https://doi.org/10.3390/molecules30173645
Chicago/Turabian StyleZhou, Keran, Bianca Simonassi-Paiva, Robert Pogue, Emma Murphy, Zhi Cao, Margaret Brennan Fournet, and Declan M. Devine. 2025. "Osteogenic Differentiation in Chitosan-Based Scaffolds via P28 and VEGF Delivery" Molecules 30, no. 17: 3645. https://doi.org/10.3390/molecules30173645
APA StyleZhou, K., Simonassi-Paiva, B., Pogue, R., Murphy, E., Cao, Z., Fournet, M. B., & Devine, D. M. (2025). Osteogenic Differentiation in Chitosan-Based Scaffolds via P28 and VEGF Delivery. Molecules, 30(17), 3645. https://doi.org/10.3390/molecules30173645







