Bacteriophage Power: Next-Gen Biocontrol Strategies for Safer Meat
Abstract
1. Introduction
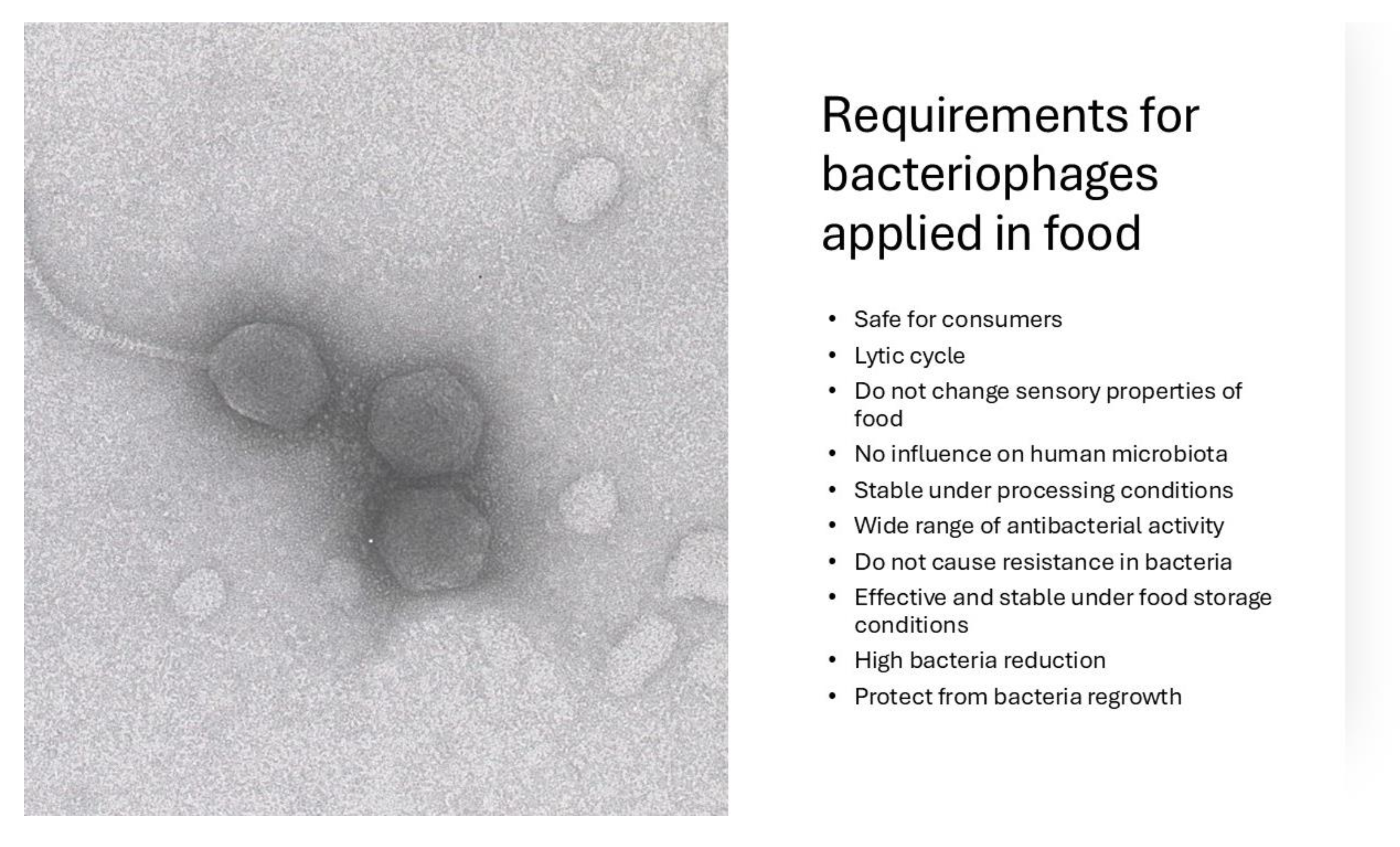
2. Requirements for Bacteriophages Used in Food
3. Law Barriers in European Union
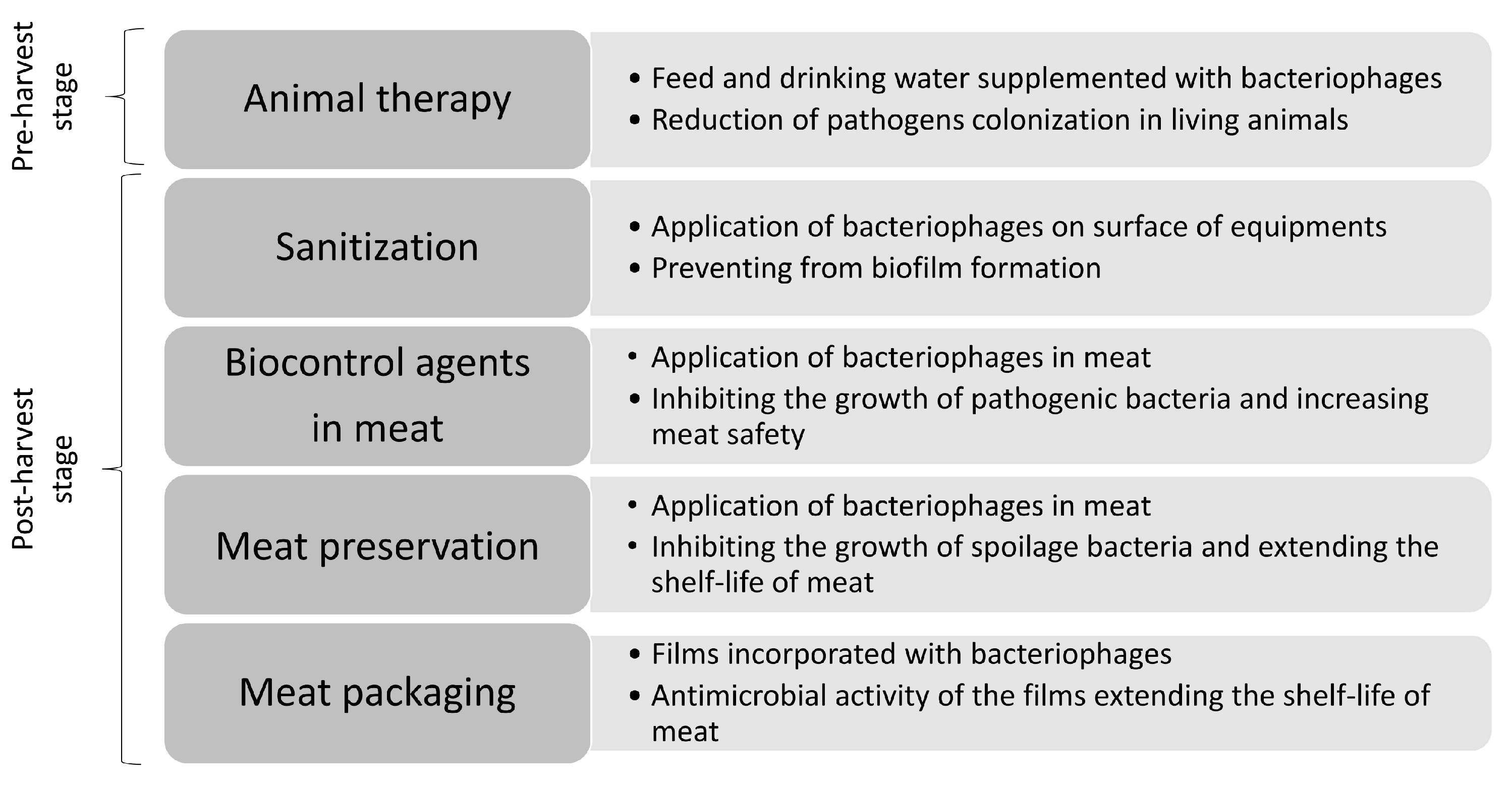
4. Application of Bacteriophages to Control the Growth of Pathogenic Bacteria in Meat
4.1. Salmonella sp.
4.2. Campylobacter sp.
4.3. Listeria monocytogenes
4.4. Escherichia coli
4.5. Yersinia enterocolitica
4.6. Shigella sp.
5. Application of Bacteriophages to Control the Growth of Meat Spoilage Bacteria
6. Synergistic Effects of Bacteriophages and Other Antimicrobial Agents
7. Application of Endolysins to Control Bacterial Growth in Meat
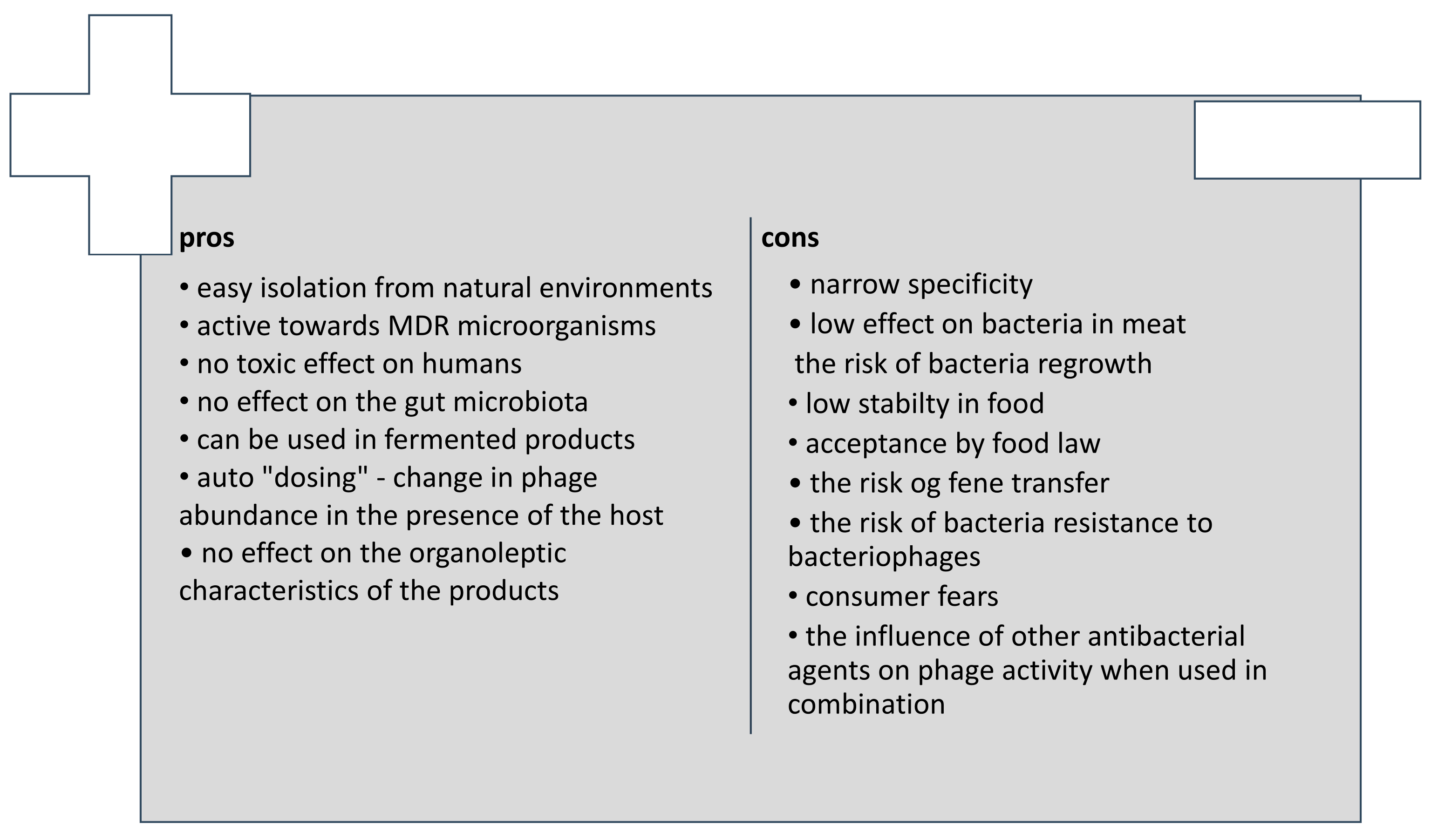
8. Advantages and Disadvantages
9. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European union one health 2023 zoonoses report. EFSA J. 2024, 22, e9106. [Google Scholar] [CrossRef]
- Oh, S.S.; Song, J.; Kim, J.; Shin, J. Increasing prevalence of multidrug-resistant mcr-1-positive Escherichia coli isolates from fresh vegetables and healthy food animals in South Korea. Int. J. Infect. Dis. 2020, 92, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Petternel, C.; Galler, H.; Zarfel, G.; Luxner, J.; Haas, D.; Grisold, A.J.; Reinthaler, F.F.; Feierl, G. Isolation and characterization of multidrug-resistant bacteria from minced meat in Austria. Food Microbiol. 2014, 44, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chatterjee, S.; Datta, S.; Prasad, R.; Dubey, D.; Prasad, R.K.; Vairale, M.G. Bacteriophages and its applications: An overview. Folia Microbiol. 2017, 62, 17–55. [Google Scholar] [CrossRef] [PubMed]
- Aranaga, C.; Pantoja, L.D.; Martínez, E.A.; Falco, A. Phage therapy in the era of multidrug resistance in bacteria: A systematic review. Int. J. Mol. Sci. 2022, 23, 4577. [Google Scholar] [CrossRef]
- Gourkhede, D.P.; Wankhade, P.R.; Prasastha, V.; Ram, S.K.; Sakhare, D.T.; Jagannath, A. Application of bacteriophages in food industry: A review. Int. J. Livest. Res. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Lee, C.; Kim, H.; Ryu, S. Bacteriophage and endolysin engineering for biocontrol of food pathogens/pathogens in the food: Recent advances and future trends. Crit. Rev. Food Sci. Nutr. 2022, 63, 8919–8938. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Zhan, W.; Li, Z.; Zou, L.; Zhao, Q. Challenges for the application of bacteriophages as effective antibacterial agents in the food industry. J. Sci. Food Agric. 2022, 102, 461–471. [Google Scholar] [CrossRef]
- Perez Pulido, R.; Grande Burgos, M.J.; Gálvez, A.; Lucas Lopez, R. Application of bacteriophages in post-harvest control of human pathogenic and food spoiling bacteria. Crit. Rev. Biotechnol. 2016, 36, 851–861. [Google Scholar] [CrossRef]
- Shannon, R.; Radford, D.R.; Balamurugan, S. Impacts of food matrix on bacteriophage and endolysin antimicrobial efficacy and performance. Crit. Rev. Food Sci. Nutr. 2020, 60, 1631–1640. [Google Scholar] [CrossRef]
- Żbikowska, K.; Michalczuk, M.; Dolka, B. The use of bacteriophages in the poultry industry. Animals 2020, 10, 872. [Google Scholar] [CrossRef]
- Han, S.; Byun, K.H.; Mizan, M.F.R.; Kang, I.; Ha, S.D. Bacteriophage and their lysins: A new era of biocontrol for inactivation of pathogenic bacteria in poultry processing and production—A review. Food Control 2022, 137, 108976. [Google Scholar] [CrossRef]
- García-Anaya, M.C.; Sepúlveda, D.R.; Zamudio-Flores, P.B.; Acosta-Muñiz, C.H. Bacteriophages as additives in edible films and coatings. Trends Food Sci. Technol. 2023, 132, 150–161. [Google Scholar] [CrossRef]
- Endersen, L.; Coffey, A. The use of bacteriophages for food safety. Curr. Opin. Food Sci. 2020, 36, 1–8. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Dawooud, A.; Rezk, N.; Makky, S.; Safwat, A.; Richards, P.J.; El-Shibiny, A. How to train your phage: The recent efforts in phage training. Biologics 2021, 1, 70–88. [Google Scholar] [CrossRef]
- Duc, H.M.; Son, H.M.; Honjoh, K.I.; Miyamoto, T. Isolation and application of bacteriophages to reduce Salmonella contamination in raw chicken meat. LWT 2018, 91, 353–360. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.J.; Jung, S.J.; Mizan, M.F.R.; Park, S.H.; Ha, S.D. Characterization of Salmonella spp.-specific bacteriophages and their biocontrol application in chicken breast meat. J. Food Sci. 2020, 85, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Z.; Shu, M.; Yang, W.Y.; Pan, H.; Tang, M.X.; Zhao, Y.Y.; Zhong, C.; Wu, G.P. Isolation and characterization of a novel Salmonella bacteriophage JNwz02 capable of lysing Escherichia coli O157: H7 and its antibacterial application in foods. LWT 2023, 173, 114251. [Google Scholar] [CrossRef]
- Colás-Medà, P.; Viñas, I.; Alegre, I. Evaluation of commercial anti-listerial products for improvement of food safety in ready-to-eat meat and dairy products. Antibiotics 2023, 12, 414. [Google Scholar] [CrossRef]
- Jun, J.W.; Park, S.C.; Wicklund, A.; Skurnik, M. Bacteriophages reduce Yersinia enterocolitica contamination of food and kitchenware. Int. J. Food Microbiol. 2018, 271, 33–47. [Google Scholar] [CrossRef]
- Ishaq, A.; Ebner, P.D.; Syed, Q.A.; ur Rahman, H.U. Employing list-shield bacteriophage as a bio-control intervention for Listeria monocytogenes from raw beef surface and maintain meat quality during refrigeration storage. LWT 2020, 132, 109784. [Google Scholar] [CrossRef]
- Demirarslan, Ö.A.; Alasalvar, H.; Yildirim, Z. Biocontrol of Salmonella Enteritidis on chicken meat and skin using lytic SE-P3, P16, P37, and P47 bacteriophages. LWT 2021, 137, 110469. [Google Scholar] [CrossRef]
- Thung, T.Y.; Premarathne, J.M.K.J.K.; San Chang, W.; Loo, Y.Y.; Chin, Y.Z.; Kuan, C.H.; Tan, C.W.; Basri, D.F.; Wan Mohamed Radzi, C.W.J.; Radu, S. Use of a lytic bacteriophage to control Salmonella Enteritidis in retail food. LWT 2017, 78, 222–225. [Google Scholar] [CrossRef]
- Thung, T.Y.; Lee, E.; Mahyudin, N.A.; Wan Mohamed Radzi, C.W.J.; Mazlan, N.; Tan, C.W.; Radu, S. Partial characterization and in vitro evaluation of a lytic bacteriophage for biocontrol of Campylobacter jejuni in mutton and chicken meat. J. Food Saf. 2020, 40, e12770. [Google Scholar] [CrossRef]
- Karaynir, A.; Salih, H.; Bozdoğan, B.; Güçlü, Ö.; Keskin, D. Isolation and characterization of Brochothrix phage ADU4. Virus Res. 2022, 321, 198902. [Google Scholar] [CrossRef]
- SalmoFresh™. Targets Contamination with Selected, Highly Pathogenic Salmonella-Serotypes in Foods and Food Processing Facilities. Available online: https://www.intralytix.com/product/3?e=SalmoFresh (accessed on 21 July 2025).
- Phage Technology for Salmonella Control. Available online: https://phageguard.com/solutions/salmonella?gad_source=1&gad_campaignid=21129864360&gbraid=0AAAAADha-M_YOkKAyfD75OH1gVJ2aO1lh&gclid=Cj0KCQjwyvfDBhDYARIsAItzbZG5yXyx-ZM0dsOwqEHG8yESDEsvCPR93PKXMo1rwmxjISCv3soy6HsaAli2EALw_wcB (accessed on 21 July 2025).
- Mangalea, M.R.; Duerkop, B.A. Fitness trade-offs resulting from bacteriophage resistance potentiate synergistic antibacterial strategies. Infect. Immun. 2020, 88, e00926-19. [Google Scholar] [CrossRef]
- Vaitekenas, A.; Tai, A.S.; Ramsay, J.P.; Stick, S.M.; Kicic, A. Pseudomonas aeruginosa resistance to bacteriophages and its prevention by strategic therapeutic cocktail formulation. Antibiotics 2021, 10, 145. [Google Scholar] [CrossRef]
- Sukumaran, A.T.; Nannapaneni, R.; Kiess, A.; Sharma, C.S. Reduction of Salmonella on chicken meat and chicken skin by combined or sequential application of lytic bacteriophage with chemical antimicrobials. Int. J. Food Microbiol. 2015, 207, 8–15. [Google Scholar] [CrossRef]
- Regulation EU no 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Off. J. Eur. Union. 2008, 354, 16–33.
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards). Scientific Opinion on the maintenance of the list of QPS microorganisms intentionally added to food or feed (2009 update). EFSA J. 2009, 7, 1431. [Google Scholar] [CrossRef]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards). Scientific Opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. EFSA J. 2017, 15, 4664. [Google Scholar] [CrossRef]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards). Statement on the update of the list of QPS-recommended microbiological agents intentionally added to food or feed as notified to EFSA 17: Suitability of taxonomic units notified to EFSA until September 2022. EFSA J. 2023, 21, 7746. [Google Scholar] [CrossRef]
- Vikram, A.; Woolston, J.; Sulakvelidze, A. Phage biocontrol applications in food production and processing. Curr. Issues Mol. Biol. 2021, 40, 267–302. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ). Evaluation of the safety and efficacy of Listex™ P100 for reduction of pathogens on different ready-to-eat (RTE) food products. EFSA J. 2016, 14, e04565. [Google Scholar] [CrossRef]
- Regulation EU no 1831/2003/EC. European Commission Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Union. 2003, 268, 29–43. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Safety and efficacy of a feed additive consisting on the bacteriophages PCM F/00069, PCM F/00070, PCM F/00071 and PCM F/00097 infecting Salmonella Gallinarum B/00111 (Bafasal®) for all avian species (Proteon Pharmaceuticals SA). EFSA J. 2021, 19, e06534. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; Bastos, M.D.L.; Christensen, H.; Dusemund, B.; Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; et al. Safety and efficacy of a feed additive consisting of the bacteriophages PCM F/00069, PCM F/00070, PCM F/00071 and PCM F/00097 (Bafasal®) for all avian species (Proteon Pharmaceuticals SA). EFSA J. 2023, 21, e07861. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Villa, R.E.; Azimonti, G.; Bonos, E.; Christensen, H.; Durjava, M.; Dusemund, B.; Gehring, R.; Glandorf, B.; Kouba, M.; et al. Safety and efficacy of a feed additive consisting of the bacteriophages PCM F/00069, PCM F/00070, PCM F/00071 and PCM F/00097 (Bafasal®) for all poultry (Proteon Pharmaceuticals SA). EFSA J. 2024, 22, e9132. [Google Scholar] [CrossRef]
- Regulation (EU) no 2025/1390 of 15 July 2025 concerning the authorisation of a preparation of the bacteriophages PCM F/00069, PCM F/00070, PCM F/00071 and PCM F/00097 as a feed additive for poultry (holder of authorisation: Proteon Pharmaceuticals S.A.). Off. J. Eur. Union. 2025. Available online: https://eur-lex.europa.eu/eli/reg_impl/2025/1390/oj/eng (accessed on 21 July 2025).
- Chang, Y.; Kim, M.; Ryu, S. Characterization of a novel endolysin LysSA11 and its utility as a potent biocontrol agent against Staphylococcus aureus on food and utensils. Food Microbiol. 2017, 68, 112–120. [Google Scholar] [CrossRef]
- Kumar, A.; Malik, H.; Dubal, Z.B.; Jaiswal, R.K.; Kumar, S.; Kumar, B.; Agarwal, R.K. Isolation and characterization of Salmonella phages and phage cocktail mediated biocontrol of Salmonella enterica serovar Typhimurium in chicken meat. LWT 2022, 155, 112957. [Google Scholar] [CrossRef]
- Aguilera, M.; Martínez, S.; Tello, M.; Gallardo, M.J.; García, V. Use of cocktail of bacteriophage for Salmonella Typhimurium control in chicken meat. Foods 2022, 11, 1164. [Google Scholar] [CrossRef]
- Galarce, N.; Escobar, B.; Rojas, V.; Navarro, C.; Turra, G.; Robeson, J.; Borie, C. Application of a virulent bacteriophage cocktail leads to reduction of Salmonella enterica serovar Enteritidis counts in processed meat products. Biocontrol Sci. Technol. 2016, 26, 462–475. [Google Scholar] [CrossRef]
- Carter, C.D.; Parks, A.; Abuladze, T.; Li, M.; Woolston, J.; Magnone, J.; Senecal, A.; Kropinski, A.M.; Sulakvelidze, A. Bacteriophage cocktail significantly reduces Escherichia coli O157:H7 contamination of lettuce and beef, but does not protect against recontamination. Bacteriophage 2012, 2, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Guenther, S.; Huwyler, D.; Richard, S.; Loessner, M.J. Virulent bacteriophage for efficientbiocontrol of Listeria monocytogenes in ready-to-eat foods. J. Appl. Environ. Microbiol. 2009, 75, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Spricigo, D.A.; Bardina, C.; Cortés, P.; Llagostera, M. Use of a bacteriophage cocktail to control Salmonella in food and the food industry. Int. J. Food Microbiol. 2013, 165, 169–174. [Google Scholar] [CrossRef]
- Sharma, C.S.; Dhakal, J.; Nannapaneni, R. Efficacy of lytic bacteriophage preparation in reducing Salmonella in vitro, on Turkey breast cutlets, and on ground Turkey. J. Food Prot. 2015, 78, 1357–1362. [Google Scholar] [CrossRef]
- Sukumaran, A.T.; Nannapaneni, R.; Kiess, A.; Sharma, C.S. Reduction of Salmonella on chicken breast fillets stored under aerobic or modified atmosphere packaging by the application of lytic bacteriophage preparation SalmoFreshTM. Poult. Sci. 2016, 95, 668–675.8. [Google Scholar] [CrossRef]
- Grant, A.; Parveen, S.; Schwarz, J.; Hashem, F.; Vimini, B. Reduction of Salmonella in ground chicken using a bacteriophage. Poult. Sci. 2017, 96, 2845–2852. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Q.; Zhang, C.; Yang, J.; Lu, Z.; Lu, F.; Bie, X. Characterization of a broad host-spectrum virulent Salmonella bacteriophage fmb-p1 and its application on duck meat. Virus Res. 2017, 236, 14–23. [Google Scholar] [CrossRef]
- Aykın-Dinçer, E.; Ergin, F.; Küçükçetin, A. Reduction of Salmonella enterica in Turkey breast slices kept under aerobic and vacuum conditions by application of lactic acid, a bacteriophage, and ultrasound. J. Food Saf. 2021, 43, e12923. [Google Scholar] [CrossRef]
- Shebs-Maurine, E.L.; Giotto, F.M.; Laidler, S.T.; de Mello, A.S. Effects of bacteriophages and peroxyacetic acid applications on beef contaminated with Salmonella during different grinding stages. Meat Sci. 2021, 173, 108407. [Google Scholar] [CrossRef]
- Yeh, Y.; Purushothaman, P.; Gupta, N.; Ragnone, M.; Verma, S.C.; De Mello, A.S. Bacteriophage application on red meats and poultry: Effects on Salmonella population in final ground products. Meat Sci. 2017, 127, 30–34. [Google Scholar] [CrossRef]
- Khan, M.A.S.; Islam, Z.; Barua, C.; Sarkar, M.M.H.; Ahmed, M.F.; Rahman, S.R. Phenotypic characterization and genomic analysis of a Salmonella phage L223 for biocontrol of Salmonella spp. in poultry. Sci. Rep. 2024, 14, 15347. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.F.; Tang, R.J.; Huang, J.; Luo, D. Isolation, characterization and application of a novel polyvalent bacteriophage SF02 for the control of Salmonella and Escherichia coli O157: H7 in foods. LWT 2024, 203, 116383. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shehata, A.M.; Arif, M.; Paswan, V.K.; Batiha, G.E.S.; Khafaga, A.F.; Elbestawy, A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: A review. Env. Sci. Pollut. Res. 2021, 28, 4989–5004. [Google Scholar] [CrossRef]
- Thames, H.T.; Sukumaran, A.T. A review of Salmonella and Campylobacter in broiler meat: Emerging challenges and food safety measures. Foods 2020, 9, 776. [Google Scholar] [CrossRef] [PubMed]
- Jäckel, C.; Hammerl, J.A.; Hertwig, S. Campylobacter phage isolation and characterization: What we have learned so far. Methods Protoc. 2019, 2, 18. [Google Scholar] [CrossRef]
- Zampara, A.; Ahern, S.J.; Briers, Y.; Brøndsted, L.; Sørensen, M.C.H. Two distinct modes of lysis regulation in Campylobacter Fletchervirus and Firehammervirus phages. Viruses 2020, 12, 1247. [Google Scholar] [CrossRef]
- Zampara, A.; Sørensen, M.C.H.; Elsser-Gravesen, A.; Brøndsted, L. Significance of phage-host interactions for biocontrol of Campylobacter jejuni in food. Food Control 2017, 73, 1169–1175. [Google Scholar] [CrossRef]
- Orquera, S.; Gölz, G.; Hertwig, S.; Hammerl, J.; Sparborth, D.; Joldic, A.; Alter, T. Control of Campylobacter spp. and Yersinia enterocolitica by virulent bacteriophages. J. Mol. Genet. Med. 2012, 6, 273–278. [Google Scholar] [CrossRef]
- Firlieyanti, A.S.; Connerton, P.L.; Connerton, I.F. Campylobacters and their bacteriophages from chicken liver: The prospect for phage biocontrol. Int. J. Food Microbiol. 2016, 237, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Pan, Q.; Wu, Y.; Ding, Y.; Wu, Q.; Zhang, J.; Wang, Z.; Liu, Z.; Wang, W.; Wang, J. Application of a novel phage vB_CjeM_WX1 to control Campylobacter jejuni in foods. Int. J. Food Microbiol. 2025, 427, 110975. [Google Scholar] [CrossRef] [PubMed]
- Soro, A.B.; Whyte, P.; Bolton, D.J.; Tiwari, B.K. Strategies and novel technologies to control Campylobacter in the poultry chain: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1353–1377. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.; Beauvais, W.; Guitian, J. Effect of enhanced biosecurity and selected on-farm factors on Campylobacter colonization of chicken broilers. Epidemiol. Infect. 2017, 145, 553–567. [Google Scholar] [CrossRef]
- Smith, S.; Messam, L.L.M.; Meade, J.; Gibbons, J.; McGill, K.; Bolton, D.; Whyte, P. The impact of biosecurity and partial depopulation on Campylobacter prevalence in Irish broiler flocks with differing levels of hygiene and economic performance. Infect. Ecol. Epidemiol. 2016, 6, 31454. [Google Scholar] [CrossRef]
- Kittler, S.; Fischer, A.; Abdulmawjood, A.; Glünder, G.; Klein, G. Effect of bacteriophage application on Campylobacter jejuni loads in commercial broiler flocks. Appl. Environ. Microbiol. 2013, 79, 7525–7533. [Google Scholar] [CrossRef]
- D’angelantonio, D.; Scattolini, S.; Boni, A.; Neri, D.; Di Serafino, G.; Connerton, P.; Connerton, I.; Pomilio, F.; Di Giantale, E.; Migliorati, G.; et al. Bacteriophage therapy to reduce colonization of Campylobacter jejuni in broiler chickens before slaughter. Viruses 2021, 13, 1428. [Google Scholar] [CrossRef]
- Chinivasagam, H.N.; Estella, W.; Maddock, L.; Mayer, D.G.; Weyand, C.; Connerton, P.L.; Connerton, I.F. Bacteriophages to control Campylobacter in commercially farmed broiler chickens, in Australia. Front. Microbiol. 2020, 11, 632. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Rodríguez-Rubio, L.; Fernández, L.; Martínez, B.; Rodríguez, A.; García, P. Applicability of commercial phage-based products against Listeria monocytogenes for improvement of food safety in Spanish dry-cured ham and food contact surfaces. Food Control 2017, 73, 1474–1482. [Google Scholar] [CrossRef]
- Ahmadi, H.; Barbut, S.; Lim, L.T.; Balamurugan, S. Examination of the use of bacteriophage as an additive and determining its best application method to control Listeria monocytogenes in a cooked-meat model system. Front. Microbiol. 2020, 11, 779. [Google Scholar] [CrossRef]
- Liu, L.; Mao, P.; Chen, J.; Li, L.; Wang, Y.; Song, J.; Chen, Z.; Ye, C. Isolation, characterization and genomic analysis of the novel Listeria bacteriophage LMLPA3 as a potential antimicrobial in foods. Food Microbiol. 2025, 128, 104720. [Google Scholar] [CrossRef]
- Newell, D.G.; La Ragione, R.M. Enterohaemorrhagic and other Shiga toxin-producing Escherichia coli (STEC): Where are we now regarding diagnostics and control strategies? Transbound. Emerg. Dis. 2018, 65, 49–71. [Google Scholar] [CrossRef] [PubMed]
- Begum, J.; Mir, N.A.; Dev, K.; Khan, I.A. Dynamics of antibiotic resistance with special reference to Shiga toxin-producing Escherichia coli infections. J. Appl. Microbiol. 2018, 125, 1228–1237. [Google Scholar] [CrossRef]
- Shebs, E.L.; Lukov, M.J.; Giotto, F.M.; Torres, E.S.; De Mello, A.S. Efficacy of bacteriophage and organic acids in decreasing STEC O157: H7 populations in beef kept under vacuum and aerobic conditions: A simulated High Event Period scenario. Meat Sci. 2020, 162, 108023. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Seo, D.J.; Oh, H.; Jeon, S.B.; Oh, M.H.; Choi, C. Inhibiting the growth of Escherichia coli O157: H7 in beef, pork, and chicken meat using a bacteriophage. Korean J. Food Sci. Anim. Resour. 2016, 36, 186. [Google Scholar] [CrossRef] [PubMed]
- Minh, D.H.; Minh, S.H.; Honjoh, K.I.; Miyamoto, T. Isolation and bio-control of Extended Spectrum Beta-Lactamase (ESBL)-producing Escherichia coli contamination in raw chicken meat by using lytic bacteriophages. LWT 2016, 71, 339–346. [Google Scholar] [CrossRef]
- Son, H.M.; Duc, H.M.; Masuda, Y.; Honjoh, K.I.; Miyamoto, T. Application of bacteriophages in simultaneously controlling Escherichia coli O157: H7 and extended-spectrum beta-lactamase producing Escherichia coli. Appl. Microbiol. Biotechnol. 2018, 102, 10259–10271. [Google Scholar] [CrossRef]
- Hudson, J.A.; Billington, C.; Cornelius, A.J.; Wilson, T.; On, S.L.W.; Premaratne, A.; King, N.J. Use of a bacteriophage to inactivate Escherichia coli O157: H7 on beef. Food Microbiol. 2013, 36, 14–21. [Google Scholar] [CrossRef]
- Shebs-Maurine, E.L.; Torres, E.S.; Yeh-Parker, Y.; de Mello, A.S. Application of MS bacteriophages on contaminated trimmings reduces Escherichia coli O157 and non-O157 in ground beef. Meat Sci. 2020, 170, 108243. [Google Scholar] [CrossRef]
- Witte, S.; Huijboom, L.; Klamert, S.; van de Straat, L.; Hagens, S.; Fieseler, L.; de Vegt, B.T.; van Mierlo, J.T. Application of bacteriophages EP75 and EP335 efficiently reduces viable cell counts of Escherichia coli O157 on beef and vegetables. Food Microbiol. 2022, 104, 103978. [Google Scholar] [CrossRef]
- Tomat, D.; Casabonne, C.; Aquili, V.; Balagué, C.; Quiberoni, A. Evaluation of a novel cocktail of six lytic bacteriophages against Shiga toxin-producing Escherichia coli in broth, milk and meat. Food Microbiol. 2018, 76, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Leon-Velarde, C.G.; Jun, J.W.; Skurnik, M. Yersinia phages and food safety. Viruses 2019, 11, 1105. [Google Scholar] [CrossRef]
- Bhunia, A.K. Shigella species. In Foodborne Microbial Pathogens; Springer: New York, NY, USA, 2018; pp. 331–341. [Google Scholar]
- Soffer, N.; Woolston, J.; Li, M.; Das, C.; Sulakvelidze, A. Bacteriophage preparation lytic for Shigella significantly reduces Shigella sonnei contamination in various foods. PLoS ONE 2017, 12, e0175256. [Google Scholar] [CrossRef]
- Shahin, K.; Bouzari, M. Bacteriophage application for biocontrolling Shigella flexneri in contaminated foods. J. Food Sci. Technol. 2018, 55, 550–559. [Google Scholar] [CrossRef]
- Shahin, K.; Zhang, L.; Delfan, A.S.; Komijani, M.; Hedayatkhah, A.; Bao, H.; Barazandeh, M.; Mansoorianfar, M.; Pang, M.; He, T.; et al. Effective control of Shigella contamination in different foods using a novel six-phage cocktail. LWT 2021, 144, 111137. [Google Scholar] [CrossRef]
- Shahin, K.; Bouzari, M.; Wang, R.; Yazdi, M. Prevalence and molecular characterization of multidrug-resistant Shigella species of food origins and their inactivation by specific lytic bacteriophages. Int. J. Food Microbiol. 2019, 305, 108252. [Google Scholar] [CrossRef]
- Greer, G.G. Homologous bacteriophage control of Pseudomonas growth and beef spoilage. J. Food Prot. 1986, 49, 104–109. [Google Scholar] [CrossRef]
- Greer, G.G.; Dilts, B.D. Control of Brochothrix thermosphacta spoilage of pork adipose tissue using bacteriophages. J. Food Prot. 2002, 65, 861–863. [Google Scholar] [CrossRef]
- Greer, G.G.; Dilts, B.D.; Ackermann, H.W. Characterization of a Leuconostoc gelidum bacteriophage from pork. Int. J. Food Microbiol. 2007, 114, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, C.; Yamada, K.; Takeuchi, H.; Inokuchi, Y.; Kashiwagi, A.; Toba, T. A lytic bacteriophage for controlling Pseudomonas lactis in raw cow’s milk. Appl. Environ. Microbiol. 2018, 84, e00111-18. [Google Scholar] [CrossRef] [PubMed]
- Deasy, T.; Mahony, J.; Neve, H.; Heller, K.J.; Van Sinderen, D. Isolation of a virulent Lactobacillus brevis phage and its application in the control of beer spoilage. J. Food Prot. 2011, 74, 2157–2161. [Google Scholar] [CrossRef]
- Rodríguez-Marca, C.; Domenech-Coca, C.; Nakamura, M.; Ortega-Olivé, N.; Puigbò, P. Use of live biopreservatives and bacteriophages to enhance the safety of meat products. Life 2025, 15, 197. [Google Scholar] [CrossRef]
- Moon, S.H.; Waite-Cusic, J.; Huang, E. Control of Salmonella in chicken meat using a combination of a commercial bacteriophage and plant-based essential oils. Food Control 2020, 110, 106984. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Zhu, X.; Lu, Y.; Xue, Y.; Lu, Z. Effects of Salmonella bacteriophage, nisin and potassium sorbate and their combination on safety and shelf life of fresh chilled pork. Food Control 2017, 73, 869–877. [Google Scholar] [CrossRef]
- Dykes, G.A.; Moorhead, S.M. Combined antimicrobial effect of nisin and a listeriophage against Listeria monocytogenes in broth but not in buffer or on raw beef. Int. J. Food Microbiol. 2002, 73, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Chibeu, A.; Agius, L.; Gao, A.; Sabour, P.M.; Kropinski, A.M.; Balamurugan, S. Efficacy of bacteriophage LISTEX™ P100 combined with chemical antimicrobials in reducing Listeria monocytogenes in cooked turkey and roast beef. Int. J. Food Microbiol. 2013, 167, 208–214. [Google Scholar] [CrossRef]
- Pelyuntha, W.; Vongkamjan, K. Control of Salmonella in chicken meat by a phage cocktail in combination with propionic acid and modified atmosphere packaging. Foods 2023, 12, 4181. [Google Scholar] [CrossRef]
- Yeh, Y.; de Moura, F.; Van Den Broek, K.; Fonseca, M.; De Mello, A.S. Effects of ultraviolet light, organic acids, and bacteriophage interventions on Salmonella populations in ground beef. Meat Muscle Biol. 2018, 149, 44–48. [Google Scholar] [CrossRef]
- Yang, S.; Sadekuzzaman, M.; Ha, S.D. Reduction of Listeria monocytogenes on chicken breasts by combined treatment with UV-C light and bacteriophage ListShield. LWT 2017, 86, 193–200. [Google Scholar] [CrossRef]
- Komora, N.; Maciel, C.; Amaral, R.A.; Fernandes, R.S.; Castro, M.; Saraiva, J.A.; Teixeira, P. Innovative hurdle system towards Listeria monocytogenes inactivation in a fermented meat sausage model-high pressure processing assisted by bacteriophage P100 and bacteriocinogenic Pediococcus acidilactici. Food Res. Int. 2021, 148, 110628. [Google Scholar] [CrossRef]
- Shebs, E.L.; Giotto, F.M.; de Mello, A.S. Effects of MS bacteriophages, ultraviolet light, and organic acid applications on beef trim contaminated with STEC O157: H7 and the “Big Six” serotypes after a simulated High Event Period Scenario. Meat Sci. 2022, 188, 108783. [Google Scholar] [CrossRef]
- Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Alqubaisy, M.; AlAli, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.-S.; Lim, S.H.E. An overview of the potential therapeutic applications of essential oils. Molecules 2021, 26, 628. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Jabbar, S.; Zhaoxin, L.; Jianhao, Z.; Abid, M.; Khan, K.U.R.; Korma, S.A.; Alghamdi, M.A.; El-Saadony, M.T.; Abd El-Hack, M.E.; et al. Probiotic-based bacteriocin: Immunity supplementation against viruses. An updated review. Front. Microbiol. 2022, 13, 1633. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.C.; Schmelcher, M.; Rodriguez-Rubio, L.; Klumpp, J.; Pritchard, D.G.; Dong, S.; Donovan, D.M. Endolysins as antimicrobials. Adv. Virus Res. 2012, 83, 299–365. [Google Scholar] [CrossRef] [PubMed]
- Aliakbarlu, J.; Manafi, L.; Mortazavi, N.; Lin, L.; Kaboudari, A. The antibacterial activity of endolysins against food-borne pathogenic bacteria in vitro and foods. Crit. Rev. Food Sci. Nutr. 2025, 1–15. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, X.; Wang, L.; Li, G.; Cong, C.; Li, R.; Cui, H.; Murtaza, B.; Xu, Y. The endolysin of the Acinetobacter baumannii phage vB_AbaP_D2 shows broad antibacterial activity. Microb. Biotechnol. 2021, 14, 403–418. [Google Scholar] [CrossRef]
- Kim, S.; Jin, J.S.; Choi, Y.J.; Kim, J. LysSAP26, a new recombinant phage endolysin with a broad spectrum antibacterial activity. Viruses 2020, 12, 1340. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.H.; Duc, H.M.; Masuda, Y.; Honjoh, K.I.; Miyamoto, T. Endolysin LysSTG2: Characterization and application to control Salmonella Typhimurium biofilm alone and in combination with slightly acidic hypochlorous water. Food Microbiol. 2021, 98, 103791. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, D.; Wang, L.; Qu, M.; Li, F.; Tan, Z.; Yao, L. Characterization of a broad-spectrum endolysin LysSP1 encoded by a Salmonella bacteriophage. Appl. Microbiol. Biotechnol. 2021, 105, 5461–5470. [Google Scholar] [CrossRef]
- Li, Y.; Dai, J.; Wu, S.; Rong, D.; Huang, J.; Zhao, M.; Zhang, J.; Ye, Q.; Gu, Q.; Zhang, Y.; et al. The food application of a novel Staphylococcus aureus bacteriophage vB_SA_STAP152 and its endolysin LysP152 with high enzymatic activity under cold temperature. Food Microbiol. 2025, 128, 104710. [Google Scholar] [CrossRef]
- Chang, Y.; Yoon, H.; Kang, D.H.; Chang, P.S.; Ryu, S. Endolysin LysSA97 is synergistic with carvacrol in controlling Staphylococcus aureus in foods. Int. J. Food Microbiol. 2017, 244, 19–26. [Google Scholar] [CrossRef]
- Chang, Y.; Ryu, S. Characterization of a novel cell wall binding domain-containing Staphylococcus aureus endolysin LysSA97. Appl. Microbiol. Biotechnol. 2017, 101, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Feng, Y.; Feng, X.; Sun, C.; Lei, L.; Ding, W.; Niu, F.; Jiao, L.; Yang, M.; Li, Y.; et al. Structural and biochemical characterization reveals LysGH15 as an unprecedented “EF-hand-like” calcium-binding phage lysin. PLoS Pathog. 2014, 10, e1004109. [Google Scholar] [CrossRef] [PubMed]
- Horgan, M.; O’Flynn, G.; Garry, J.; Cooney, J.; Coffey, A.; Fitzgerald, G.F.; Ross, R.P.; McAuliffe, O. Phage lysin LysK can be truncated to its CHAP domain and retain lytic activity against live antibiotic-resistant staphylococci. Appl. Environ. Microbiol. 2009, 75, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yang, R.; Yu, S.; Zhao, W. The strategy of biopreservation of meat product against MRSA using lytic domain of lysin from Staphylococcus aureus bacteriophage. Food Biosci. 2021, 41, 100967. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, M.; Lv, Q.; Tang, Z.; Zhang, Q.; Liu, Y.; Zhao, X.; Cai, Y. Application and potential therapeutic effect of endolysin Lys1472 against Clostridium perfringens in chicken meat. LWT 2025, 216, 117332. [Google Scholar] [CrossRef]
- Guan, P.; Ming, Z.; Liu, X.; Shao, Y.; Pan, H.; Ding, Y.; Wang, X. Expression and characterization of a novel endolysin LysPFX32 as potential biological antimicrobial agent against Pseudomonas fluorescens for pork preservation. Int. J. Biol. Macromol. 2025, 294, 139448. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Wang, J.; Ahn, J. Synergistic antimicrobial activity of essential oils in combination with phage endolysin against Salmonella Typhimurium in cooked ground beef. Food Control 2024, 157, 110187. [Google Scholar] [CrossRef]
- León, M.; Bastías, R. Virulence reduction in bacteriophage resistant bacteria. Front. Microbiol. 2015, 6, 343. [Google Scholar] [CrossRef]
| Target Microorganisms | Phage Preparation | Producer | Food Products Dedicated | Certification |
|---|---|---|---|---|
| Salmonella enterica (serotypes Typhimurium, Enteritidis, Heidelberg, Newport, Hadar, Kentucky, Thompson, Georgia, Agona, Grampian, Senftenberg, Alachua, Infantis, Reading, and Schwarzengrund) | SalmoFresh™ | Intralytix (Columbia, MD, USA) | poultry, red meat, fish and shellfish, and fresh and processed fruits and vegetables | Kosher; Halal; OMRI |
| raw and ready-to-eat poultry products, raw and ready-to-eat red meat products | ||||
| fish, shellfish, fresh and processed fruits and vegetables and poultry immediately before or after grinding, and on ready-to-eat products before slicing | ||||
| fish, shellfish, and fresh and processed fruits and vegetables, or on ready-to-eat poultry products prior to slicing and on raw poultry prior to grinding or after grinding | ||||
| Salmonella sp. | PhageGuard S™ (previously SalmonelexTM) | Phageguard Micreos Food Safety (Wageningen, The Netherlands) | Meat and poultry | Kosher; Halal; OMRI; SKAL |
| Salmonella sp. | SalmoPro® | Phagelux | poultry, red meat, fruits, vegetables, eggs, fish, and shellfish | |
| Listeria monocytogenes | ListShield™ | Intralytix (Columbia, MD, USA) | ready-to-eat meat and poultry products, smoked salmon, fresh and processed fruits and vegetables, dairy products (including cheese) | Kosher; Halal; OMRI |
| Listeria monocytogenes | Listex™ (Phage Guard LTM) | Phageguard Micreos Food Safety (Wageningen, The Netherlands) | ready-to-eat meat and poultry products, fresh salmon, fresh scallops and shrimp | Kosher; Halal; OMRI; SKAL |
| Escherichia coli STEC including E. coli O157:H7 | EcoShield PX™ | Intralytix (Columbia, MD, USA) | meat, poultry, fruits, vegetables, dairy products (including cheese), fish, and other seafood | Kosher and Halal |
| E. coli O157:H7 | PhageGuard ETM | Phageguard Micreos Food Safety (Wageningen, The Netherlands) | Beef carcass and parts, leafy green vegetables | - |
| Shigella sp. (S. flexneri, S. sonnei and S. dysenteriae) | ShigaShieldTM | Intralytix (Columbia, MD, USA) | ready-to-eat meat and poultry, fish (including smoked fish), shellfish, fresh and processed fruits and vegetables, and dairy products including cheese | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efenberger-Szmechtyk, M.; Nowak, A. Bacteriophage Power: Next-Gen Biocontrol Strategies for Safer Meat. Molecules 2025, 30, 3641. https://doi.org/10.3390/molecules30173641
Efenberger-Szmechtyk M, Nowak A. Bacteriophage Power: Next-Gen Biocontrol Strategies for Safer Meat. Molecules. 2025; 30(17):3641. https://doi.org/10.3390/molecules30173641
Chicago/Turabian StyleEfenberger-Szmechtyk, Magdalena, and Agnieszka Nowak. 2025. "Bacteriophage Power: Next-Gen Biocontrol Strategies for Safer Meat" Molecules 30, no. 17: 3641. https://doi.org/10.3390/molecules30173641
APA StyleEfenberger-Szmechtyk, M., & Nowak, A. (2025). Bacteriophage Power: Next-Gen Biocontrol Strategies for Safer Meat. Molecules, 30(17), 3641. https://doi.org/10.3390/molecules30173641