The Influence of NaClO on the Biocorrosion of Carbon Steel Induced by Chlorella vulgaris in Artificial Seawater
Abstract
1. Introduction
2. Results and Discussion
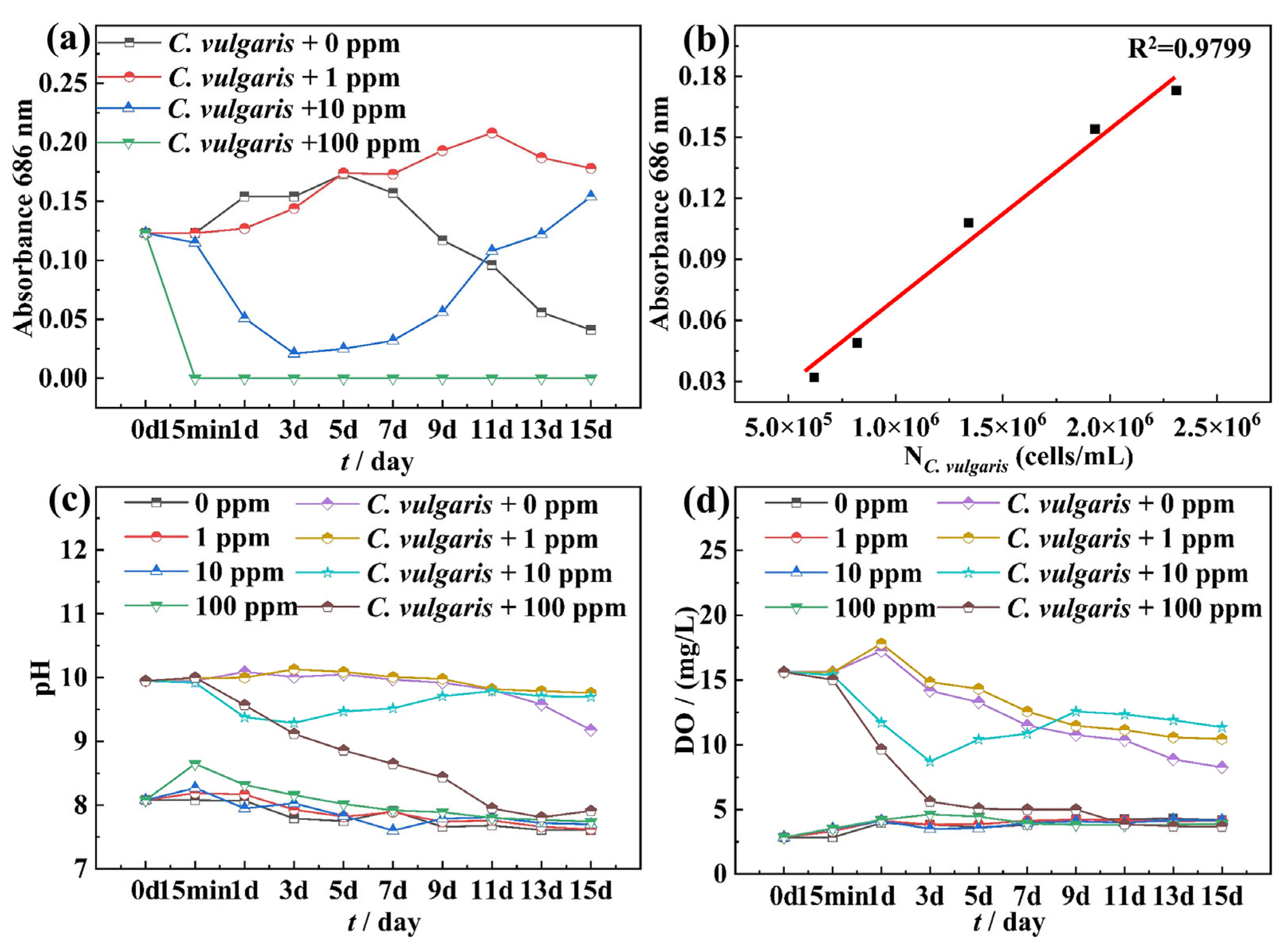
2.1. Growth Curve of C. vulgaris and Physicochemical Properties of Test Solutions
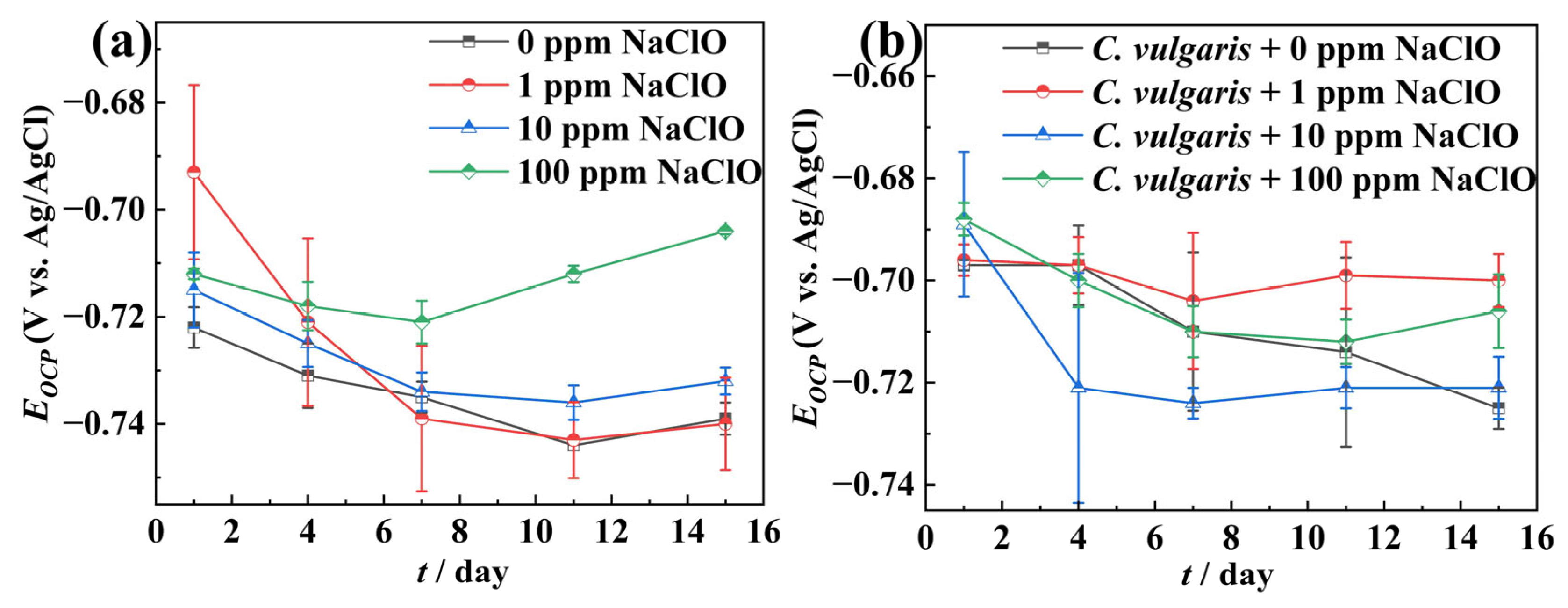
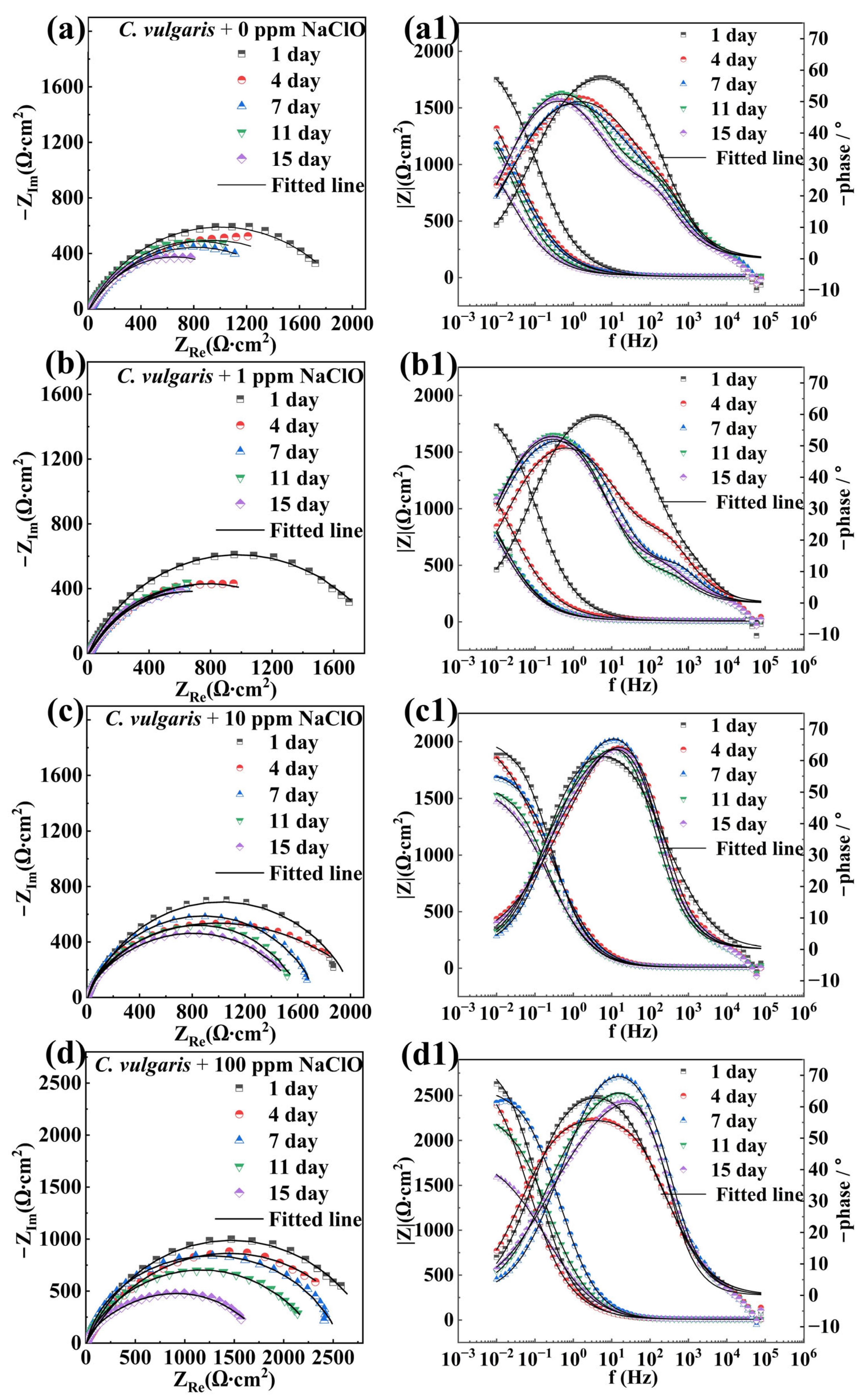
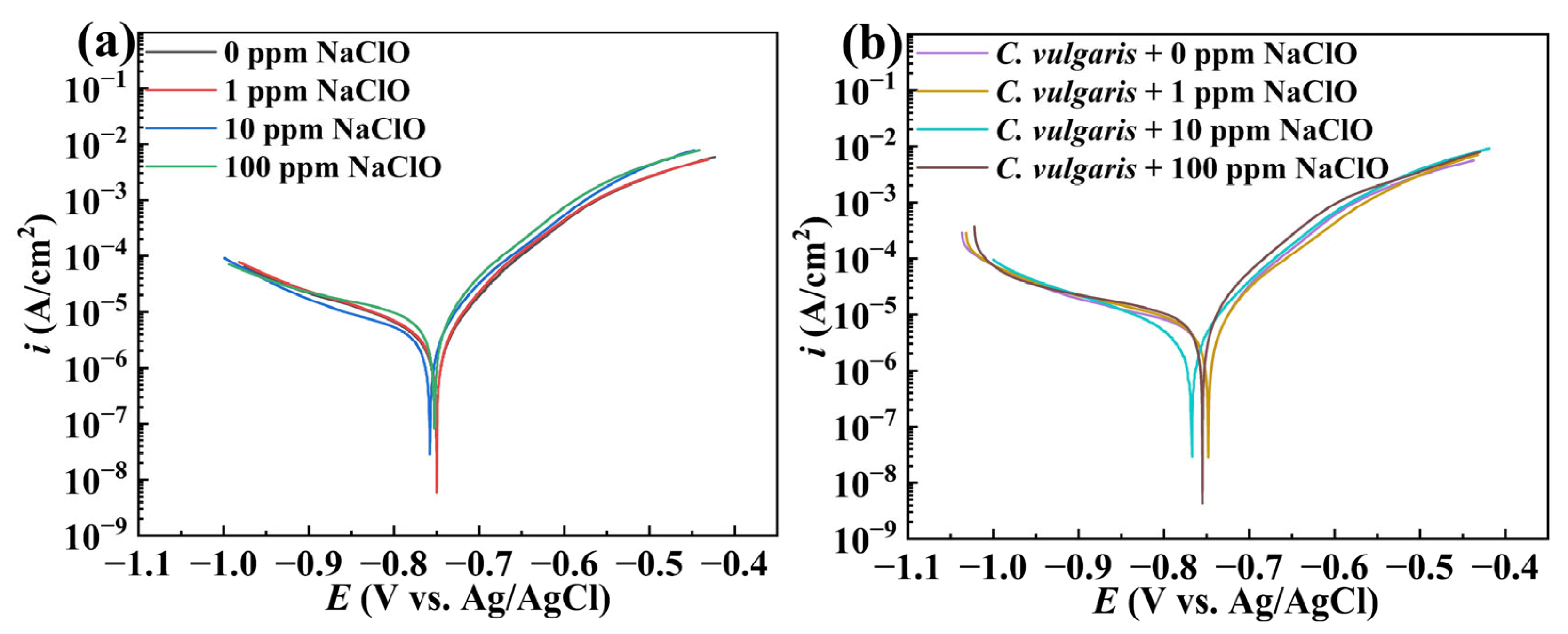
2.2. Electrochemical Measurements
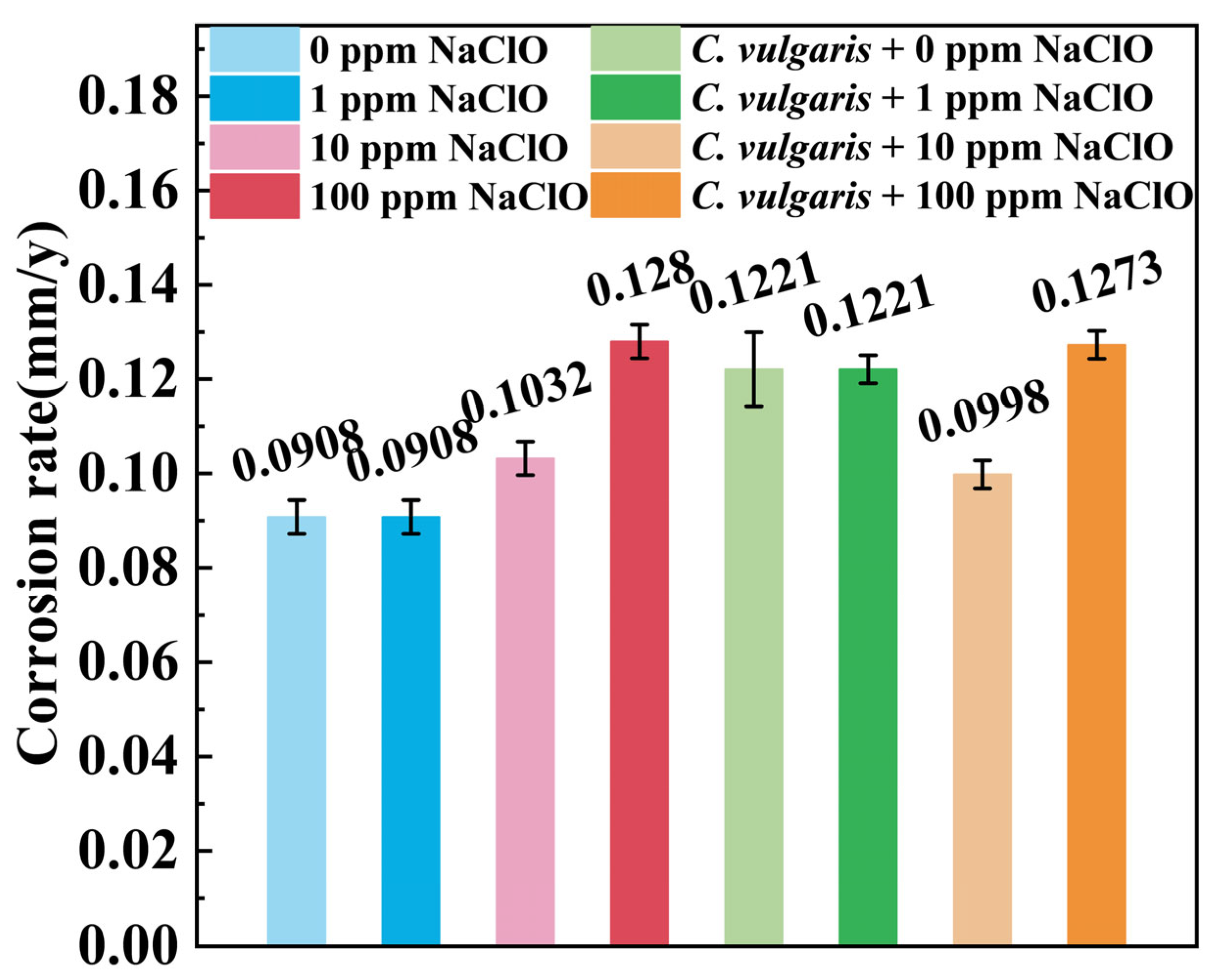
2.3. Weight Loss
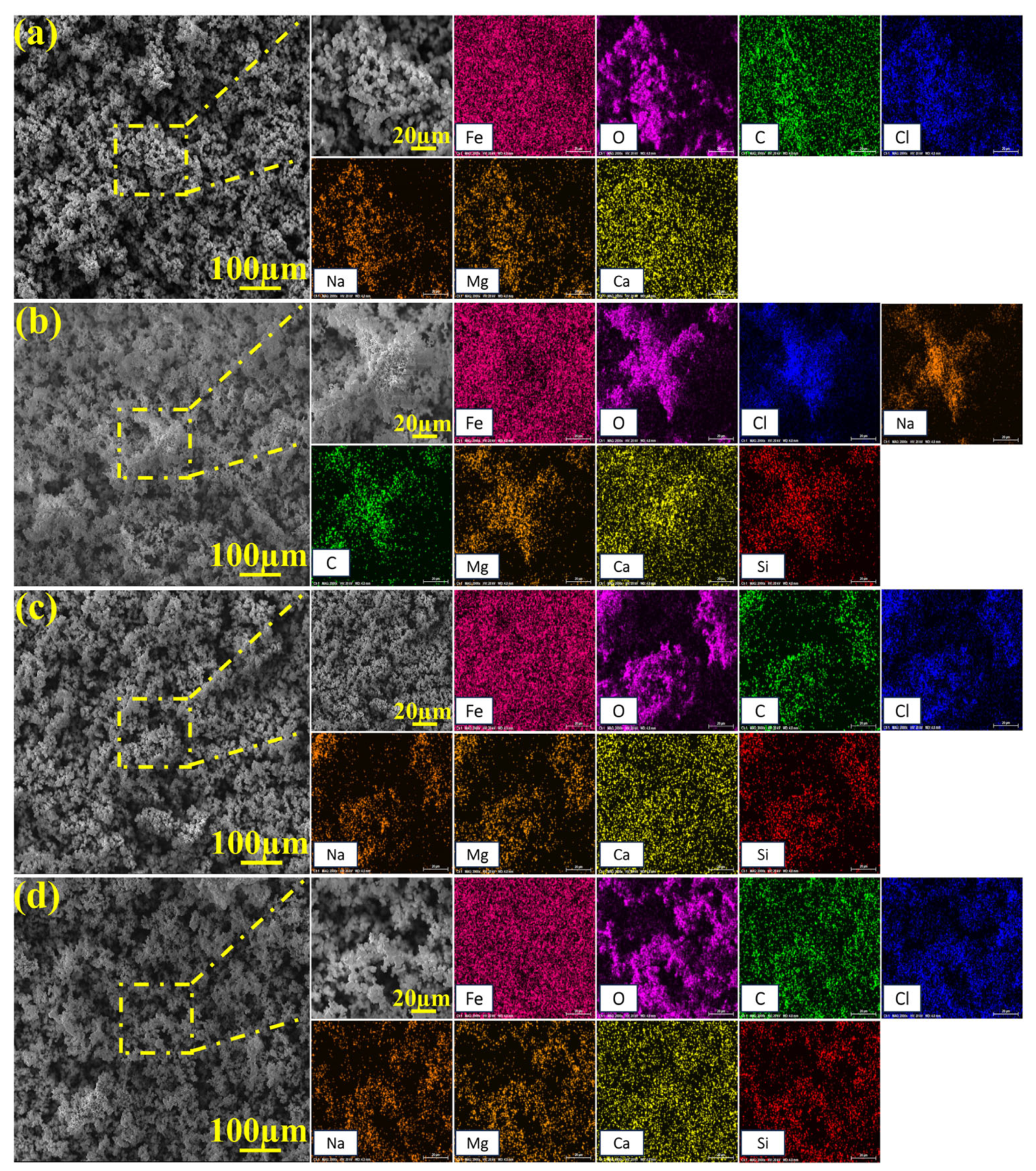
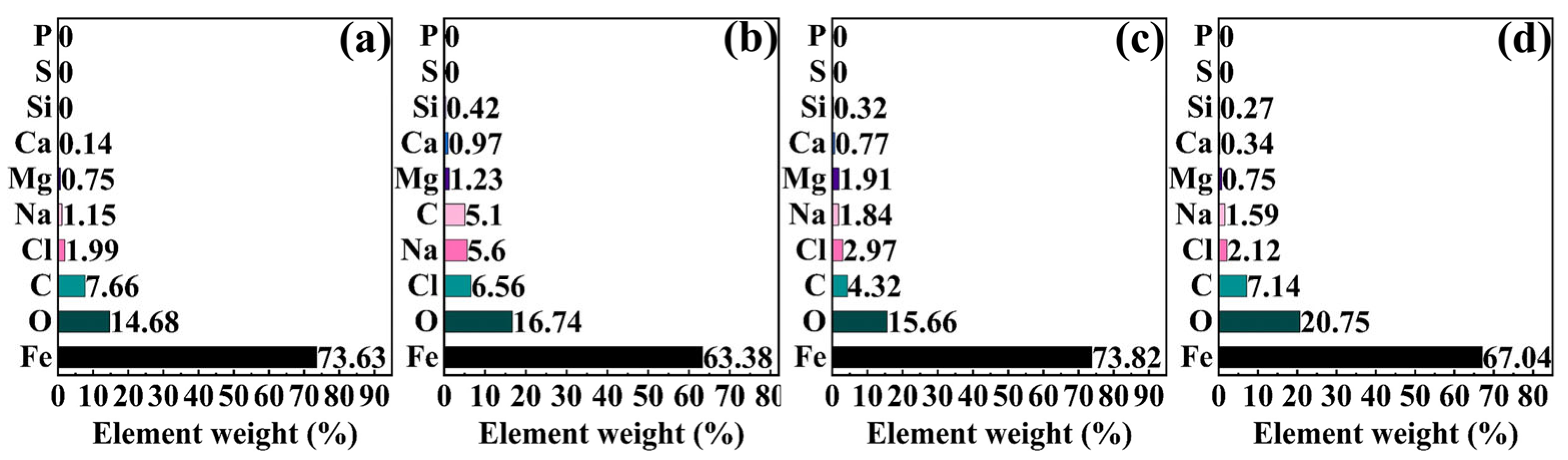
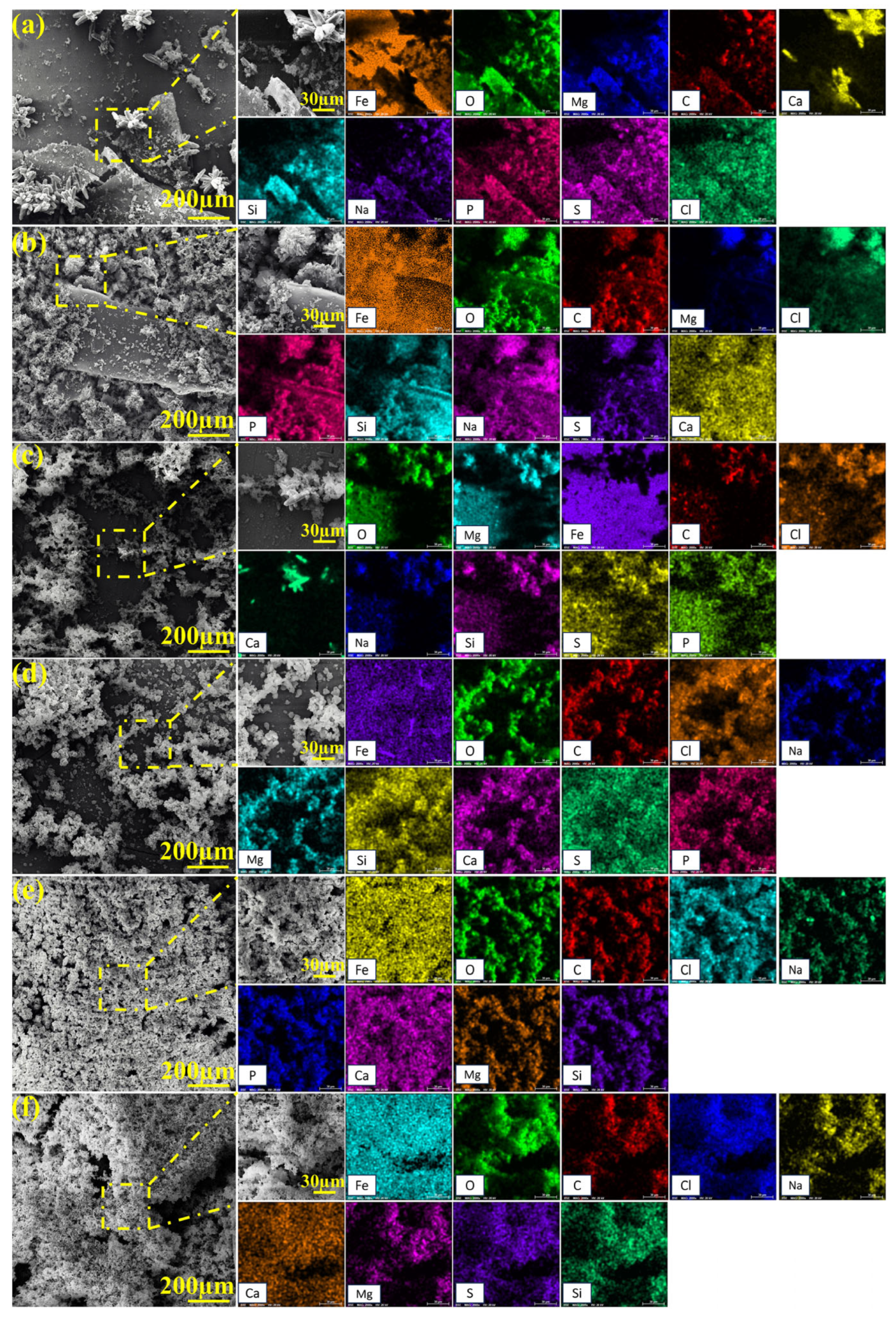
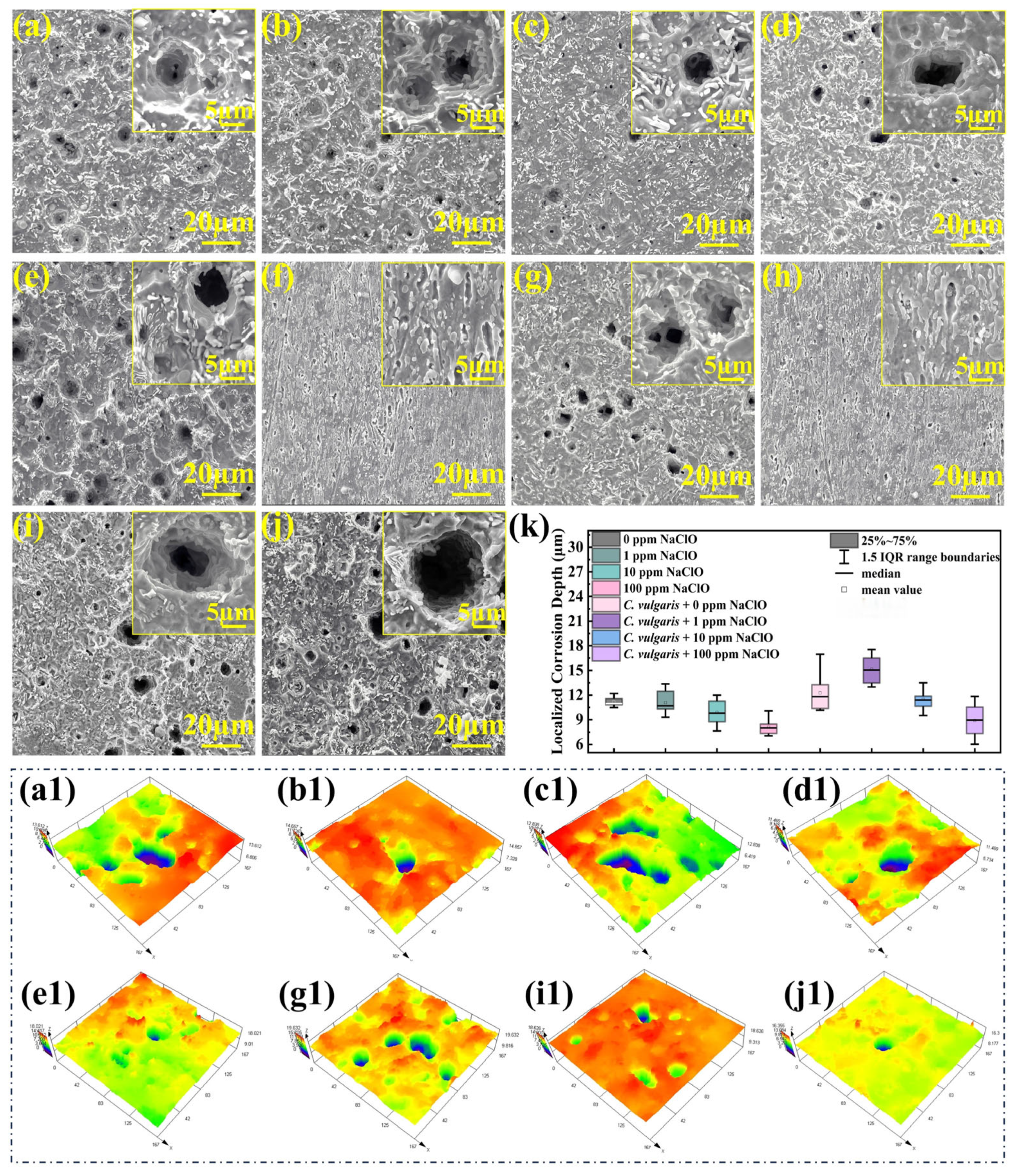
2.4. Macroscopic and Microscopic Morphologies of Specimen Surfaces
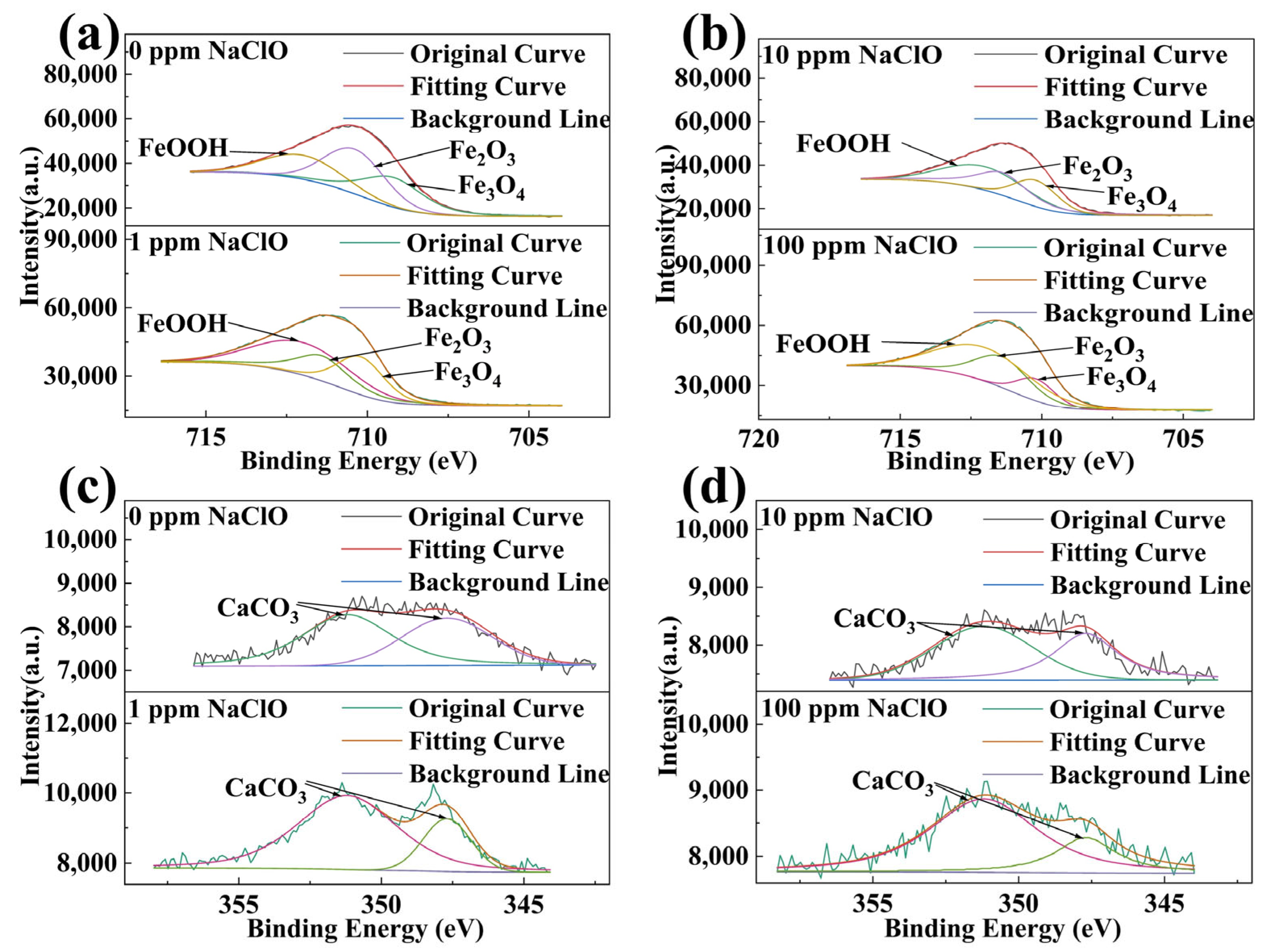
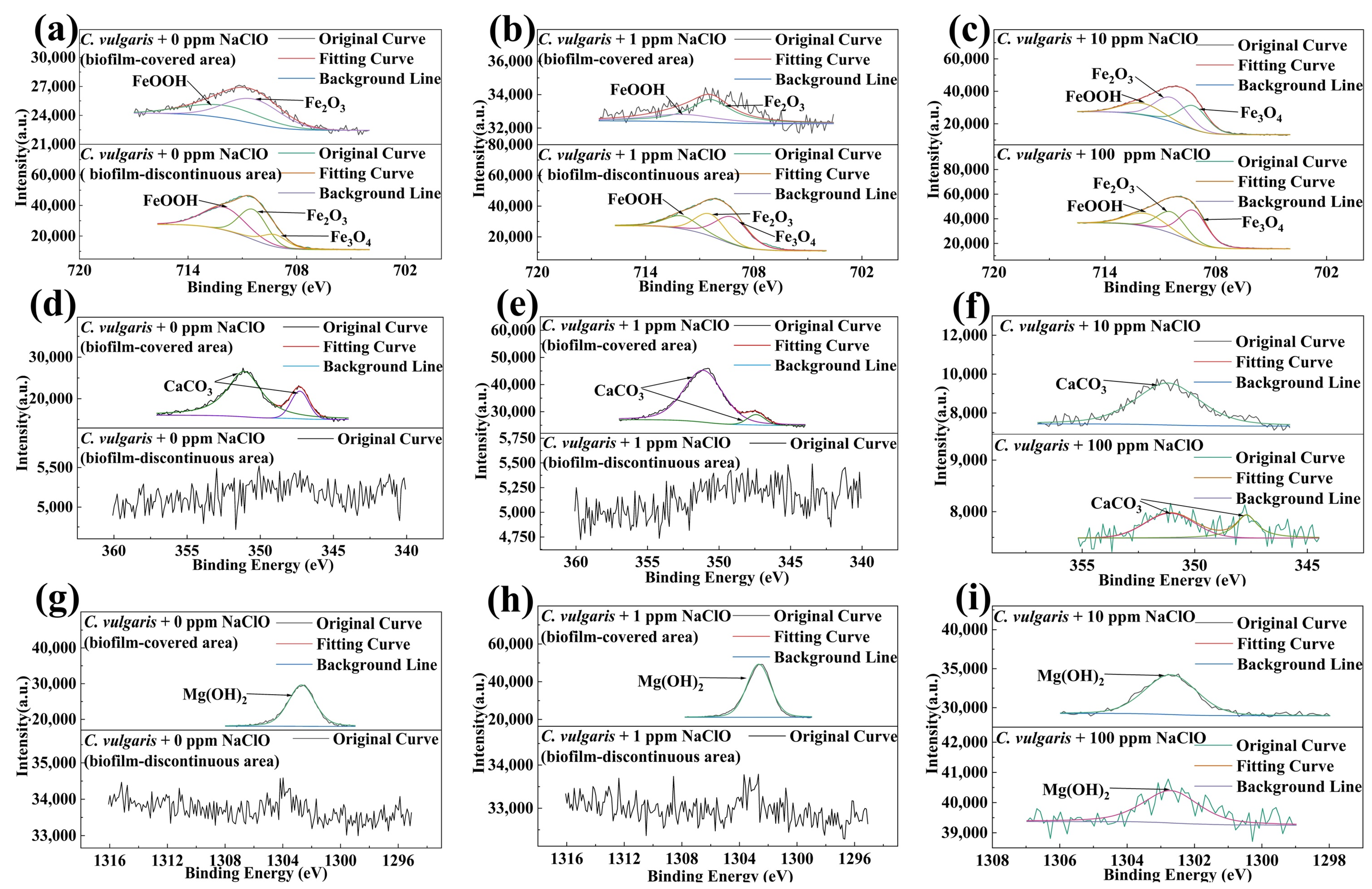
2.5. XPS Analysis Results
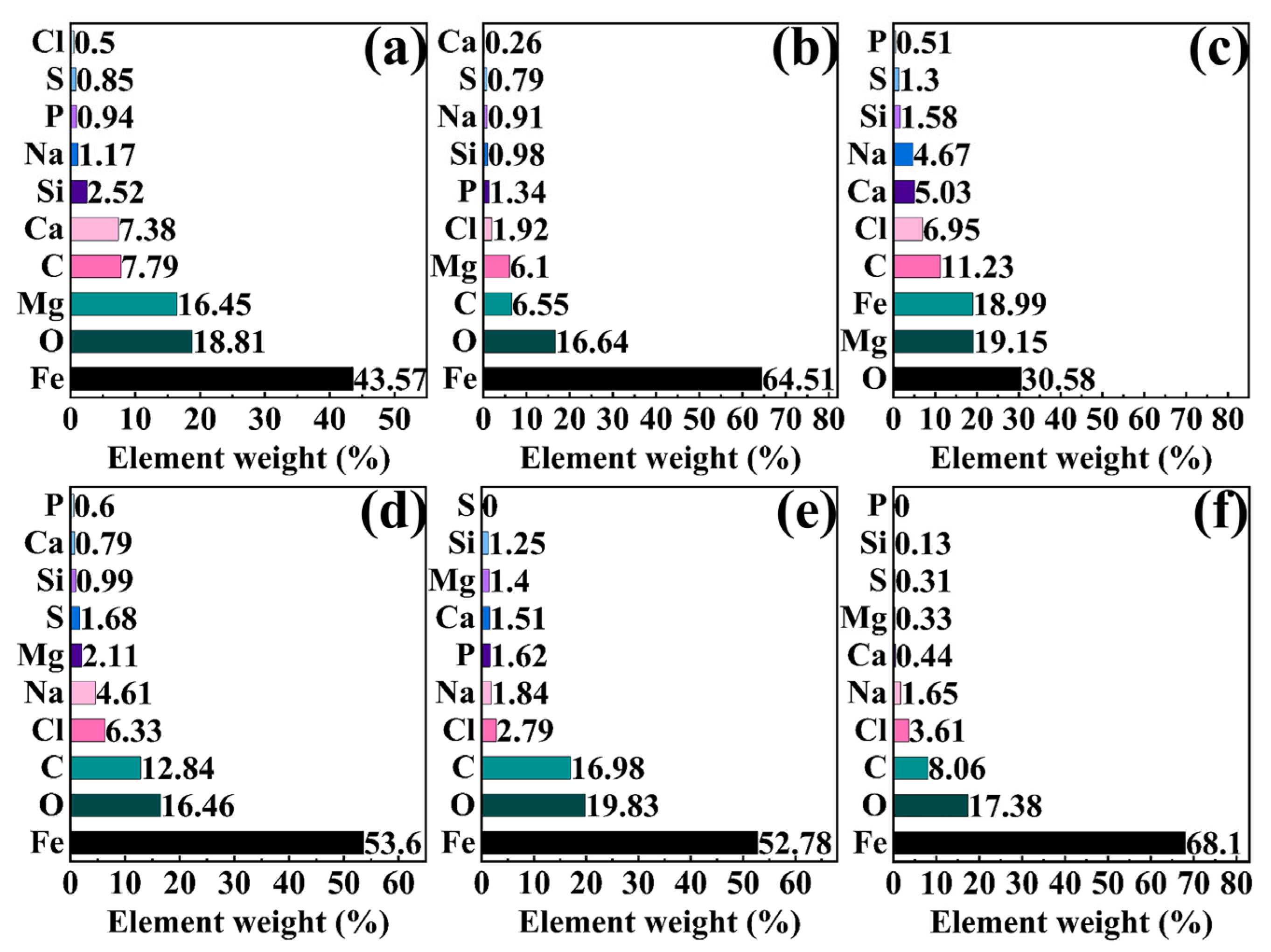
2.6. Corrosion Morphology After Removing Corrosion Products
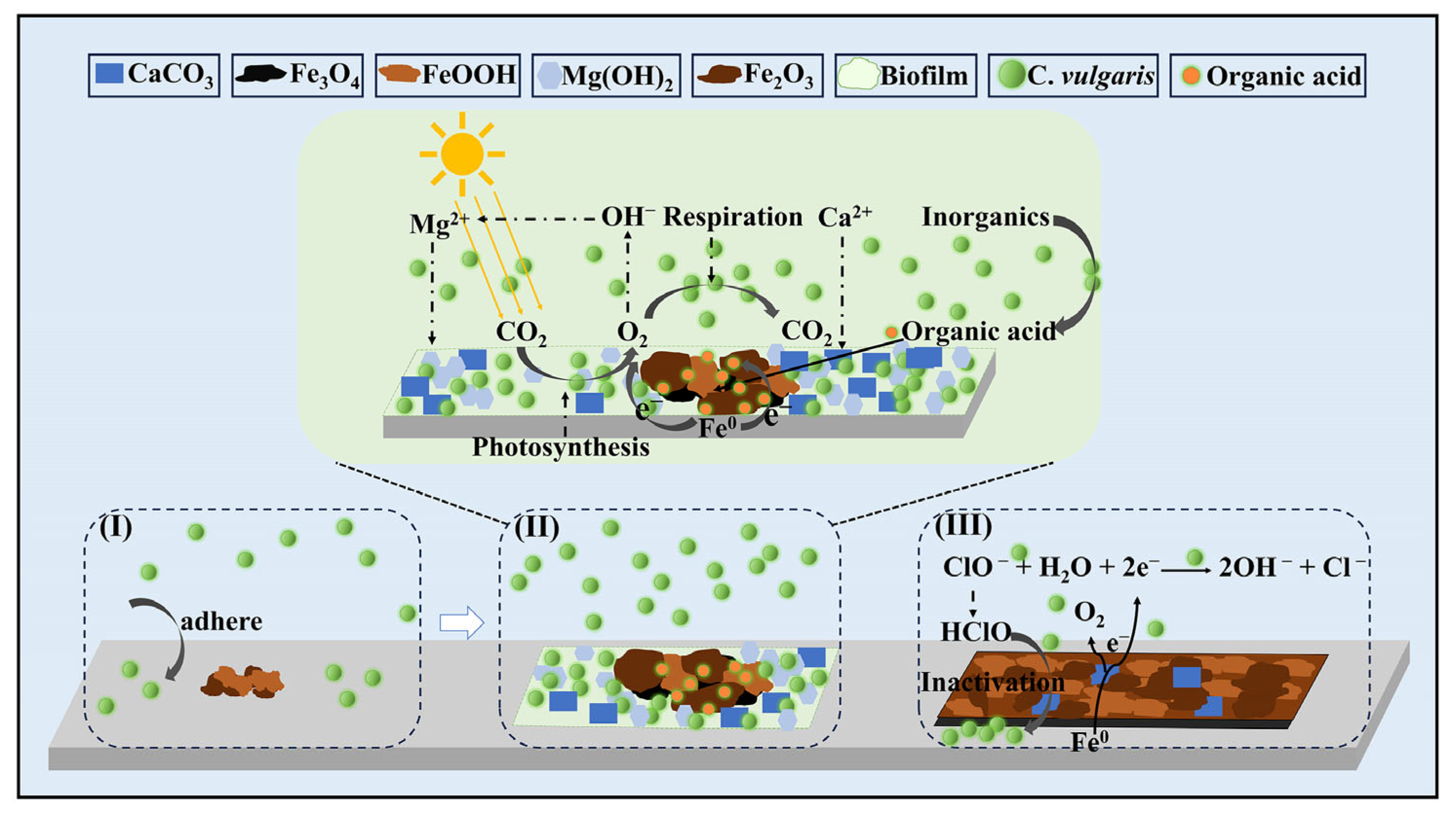
2.7. The Corrosion Mechanism of Carbon Steels Induced by C. vulgaris and NaClO
3. Materials and Methods
3.1. Experimental Materials
3.2. Algal Cultivation and Inoculation
3.3. Electrochemical Tests
3.4. Weight-Loss Measurements
3.5. Surface Characterization
4. Conclusions
- (1)
- In abiotic artificial seawater, bactericide NaClO elevates the overall corrosion rate of the steel but reduces the local corrosion penetration depth because it facilitates uniform oxidation across nearly the entire steel surface.
- (2)
- Algae C. vulgaris can adhere to the steel surface to form a biofilm in some areas, protecting the substrate steel from corrosion. However, in the area where the biofilm is discontinuous, localized corrosion is significantly aggravated by the organic acids and O2 generated from C. vulgaris.
- (3)
- When the concentration of NaClO is too low, e.g., 1 ppm, NaClO does not have a significant inhibitive effect on the growth of C. vulgaris, but after the concentration is sufficiently high, e.g., at 10 ppm, the C. vulgaris-induced corrosion, including the overall damage and local penetration, in the biotic artificial seawater is obviously inhibited.
- (4)
- If the concentration of NaClO is too high, e.g., at 100 ppm, the C. vulgaris in the biotic artificial seawater is completely eradicated. In this case, the corrosion of the steel is mainly governed by NaClO. Consequently, the corrosion rate and corrosion penetration depth in the biotic solution are similar to those in the abiotic solution at 100 ppm NaClO. However, in the abiotic artificial seawater, 100 ppm NaClO can significantly accelerate corrosion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Refait, P.; Paul, S. New frontiers in marine corrosion research. NPJ Mater. Degrad. 2025, 9, 11. [Google Scholar] [CrossRef]
- Pei, W.; Pei, X.; Xie, Z.; Wang, J. Research progress of marine anti-corrosion and wear-resistant coating. Tribol. Int. 2024, 198, 109864. [Google Scholar] [CrossRef]
- Li, Y.; Ning, C. Latest research progress of marine microbiological corrosion and bio-fouling, and new approaches of marine anti-corrosion and anti-fouling. Bioact. Mater. 2019, 4, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Hanif, N.; Miftah, J.A.; Yanti, H.D.; Oluwabusola, E.T.; Zahra, V.A.; Salleh, N.F.; Kundukad, B.; Tan, L.T.; de Voogd, N.J.; Rachmania, N.; et al. Integrated Biological and Chemical Investigation of Indonesian Marine Organisms Targeting Anti-Quorum-Sensing, Anti-Biofilm, Anti-Biofouling, and Anti-Biocorrosion Activities. Molecules 2025, 30, 1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wen, S.; Liu, H.; Liu, P.; Xiong, J.; Wu, Y.; Li, Z.; Tian, Z.; Liu, B.; Xu, D.; et al. Mitigation of biocorrosion of X80 carbon steel by a shale microbiome biofilm using a green biocide enhanced by d-amino acids. Bioelectrochemistry 2025, 161, 108831. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Ma, R.; Wang, C.; Li, R.; Huang, J.; Dong, J.; Xu, D. Influence of Shewanella algae and calcium-magnesium deposit layer on the corrosion mechanism of X80 carbon steel in marine environment. Mater. Des. 2025, 254, 114017. [Google Scholar] [CrossRef]
- Fu, Q.; Xu, J.; Wei, B.; Qin, Q.; Bai, Y.; Yu, C.; Sun, C. Mechanistic diversity between nitrate and nitrite on biocorrosion of X80 pipeline steel caused by Desulfovibrio desulfurican and Pseudomonas stutzeri. Corros. Sci. 2022, 207, 110573. [Google Scholar] [CrossRef]
- Dong, Y.; Li, J.; Xu, D.; Song, G.; Liu, D.; Wang, H.; Saleem Khan, M.; Yang, K.; Wang, F. Investigation of microbial corrosion inhibition of Cu-bearing 316L stainless steel in the presence of acid producing bacterium Acidithiobacillus caldus SM-1. J. Mater. Sci. Technol. 2021, 64, 176–186. [Google Scholar] [CrossRef]
- Cai, Z.; Wei, B.; Xu, J.; Yu, C.; Sun, C. The role of hydrogen gas in SRB-induced degradation of X80 pipeline steel in hydrogen-blending environments. Electrochim. Acta 2025, 509, 145301. [Google Scholar] [CrossRef]
- Cui, L.Y.; Liu, Z.Y.; Xu, D.K.; Hu, P.; Shao, J.M.; Du, C.W.; Li, X.G. The study of microbiologically influenced corrosion of 2205 duplex stainless steel based on high-resolution characterization. Corros. Sci. 2020, 174, 108842. [Google Scholar] [CrossRef]
- Fu, Q.; Song, G.-L.; Yao, X. Biofouling and corrosion of magnesium alloys WE43 and AM60 by Chlorella vulgaris in artificial seawater. Corros. Sci. 2025, 250, 112884. [Google Scholar] [CrossRef]
- Dai, Y.; Yang, S.; Zhao, D.; Hu, C.; Xu, W.; Anderson, D.M.; Li, Y.; Song, X.-P.; Boyce, D.G.; Gibson, L.; et al. Coastal phytoplankton blooms expand and intensify in the 21st century. Nature 2023, 615, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Mudge, M.C.; Riffle, M.; Chebli, G.; Plubell, D.L.; Rynearson, T.A.; Noble, W.S.; Timmins-Schiffman, E.; Kubanek, J.; Nunn, B.L. Harmful algal blooms are preceded by a predictable and quantifiable shift in the oceanic microbiome. Nat. Commun. 2025, 16, 3986. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Counillon, F.; Brajard, J.; Davy, R.; Outten, S.; Pettersson, L.H.; Keenlyside, N. Warming and freshening coastal waters impact harmful algal bloom frequency in high latitudes. Commun. Earth Environ. 2025, 6, 445. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Zhao, X.; Hu, X.; Yang, L.; Wang, N.; Li, Y.; Hou, B. Biomimetic one step fabrication of manganese stearate superhydrophobic surface as an efficient barrier against marine corrosion and Chlorella vulgaris-induced biofouling. Chem. Eng. J. 2016, 306, 441–451. [Google Scholar] [CrossRef]
- Jin, H.; Chuai, W.H.; Li, K.P.; Hou, G.L.; Wu, M.C.; Chen, J.P.; Wang, H.X.; Jia, J.; Han, D.X.; Hu, Q. Ultrahigh-cell-density heterotrophic cultivation of the unicellular green alga Chlorella sorokiniana for biomass production. Biotechnol. Bioeng. 2021, 118, 4138–4151. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, Z.; Liu, H.; Liu, R.; Zhang, Y.; Yin, Y.; Liu, H.; Yuan, X.; Fan, S.; Liu, H. Insights into the hydrophobic coating with integrated high-efficiency anti-corrosion, anti-biofouling and self-healing properties based on anti-bacterial nano LDH materials. Corros. Sci. 2024, 231, 111995. [Google Scholar] [CrossRef]
- Liu, H.; Xu, D.; Dao, A.Q.; Zhang, G.; Lv, Y.; Liu, H. Study of corrosion behavior and mechanism of carbon steel in the presence of Chlorella vulgaris. Corros. Sci. 2015, 101, 84–93. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, R.; Zhang, S.; Yuan, M.; Guo, H.; Meng, G.; Zhang, P. Effect of marine microalgae Synechococcus sp., Chlorella sp., Thalassiosira sp. on corrosion behavior of Q235 carbon steel in f/2 medium. Bioelectrochemistry 2023, 150, 108349. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, R.; Sand, W.; Mathivanan, K.; Zhang, Y.; Wang, N.; Duan, J.; Hou, B. Comprehensive Review on the Use of Biocides in Microbiologically Influenced Corrosion. Microorganisms 2023, 11, 2194. [Google Scholar] [CrossRef]
- Wu, S.; Shen, F.; Zhao, P.; Li, Y.; Shi, J.; Chen, T.; Ma, W. Designing a Bipolar Membrane Electrolyzer for NaCl Electrolysis to Produce High-Quality NaClO. J. Phys. Chem. C 2023, 127, 15177–15184. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, W.; Chen, X.; Zhou, Y.; He, B.; Tan, S. Analysis of the biodegradation performance and biofouling in a halophilic MBBR-MBR to improve the treatment of disinfected saline wastewater. Chemosphere 2021, 269, 128716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tian, Y.; Kang, M.; Chen, C.; Song, Y.; Li, H. Effects of chlorination/chlorine dioxide disinfection on biofilm bacterial community and corrosion process in a reclaimed water distribution system. Chemosphere 2019, 215, 62–73. [Google Scholar] [CrossRef]
- Liu, X.H.; Sui, Y.Q.; Zhou, J.Y.; Liu, Y.P.; Li, X.B.; Hou, J. Influence of available chlorine on corrosion behaviour of low alloy marine steel in natural seawater. Corros. Eng. Sci. Technol. 2023, 58, 475–481. [Google Scholar] [CrossRef]
- Su, W.; Tian, Y.; Peng, S. The influence of sodium hypochlorite biocide on the corrosion of carbon steel in reclaimed water used as circulating cooling water. Appl. Surf. Sci. 2014, 315, 95–103. [Google Scholar] [CrossRef]
- Oliveira, S.H.; Lima, M.A.G.A.; França, F.P.; Vieira, M.R.S.; Silva, P.; Urtiga Filho, S.L. Control of microbiological corrosion on carbon steel with sodium hypochlorite and biopolymer. Int. J. Biol. Macromol. 2016, 88, 27–35. [Google Scholar] [CrossRef]
- Peng, H.; Lyu, W.; Wu, C.; Wang, J.; Su, X.; Zhao, Y.; Xu, S.; Li, Z. Study on the corrosion failure mechanism of X80 pipeline steel by chloride ion at different concentrations. Eng. Fail. Anal. 2025, 179, 109784. [Google Scholar] [CrossRef]
- Yan, M.; Sun, C.; Xu, J.; Dong, J.; Ke, W. Role of Fe oxides in corrosion of pipeline steel in a red clay soil. Corros. Sci. 2014, 80, 309–317. [Google Scholar] [CrossRef]
- Song, X.; Yang, Y.; Yu, D.; Lan, G.; Wang, Z.; Mou, X. Studies on the impact of fluid flow on the microbial corrosion behavior of product oil pipelines. J. Pet. Sci. Eng. 2016, 146, 803–812. [Google Scholar] [CrossRef]
- Singh, A.; Dayu, X.; Ituen, E.; Ansari, K.; Quraishi, M.A.; Kaya, S.; Lin, Y. Tobacco extracted from the discarded cigarettes as an inhibitor of copper and zinc corrosion in an ASTM standard D1141-98(2013) artificial seawater solution. J. Mater. Res. Technol. 2020, 9, 5161–5173. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, Z.; Wan, H.; Wang, G.; Wu, H.; Chen, X.; Liu, H. Localized corrosion and brittle fracture of X80 carbon steel under tensile stress induced by sulfate reducing bacteria. Electrochim. Acta 2024, 497, 144598. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Sun, W.; Li, X.; Cui, M.; Zhou, E.; Wang, F.; Xu, D. Extracellular polysaccharides of Tenacibaculum mesophilum D-6 play a major role during its corrosion protection for X80 carbon steel in seawater. Corros. Sci. 2025, 249, 112811. [Google Scholar] [CrossRef]
- Wei, B.; Pang, J.; Xu, J.; Sun, C.; Zhang, H.; Wang, Z.; Yu, C.; Ke, W. Microbiologically influenced corrosion of TiZrNb medium-entropy alloys by Desulfovibrio desulfuricans. J. Alloys Compd. 2021, 875, 160020. [Google Scholar] [CrossRef]

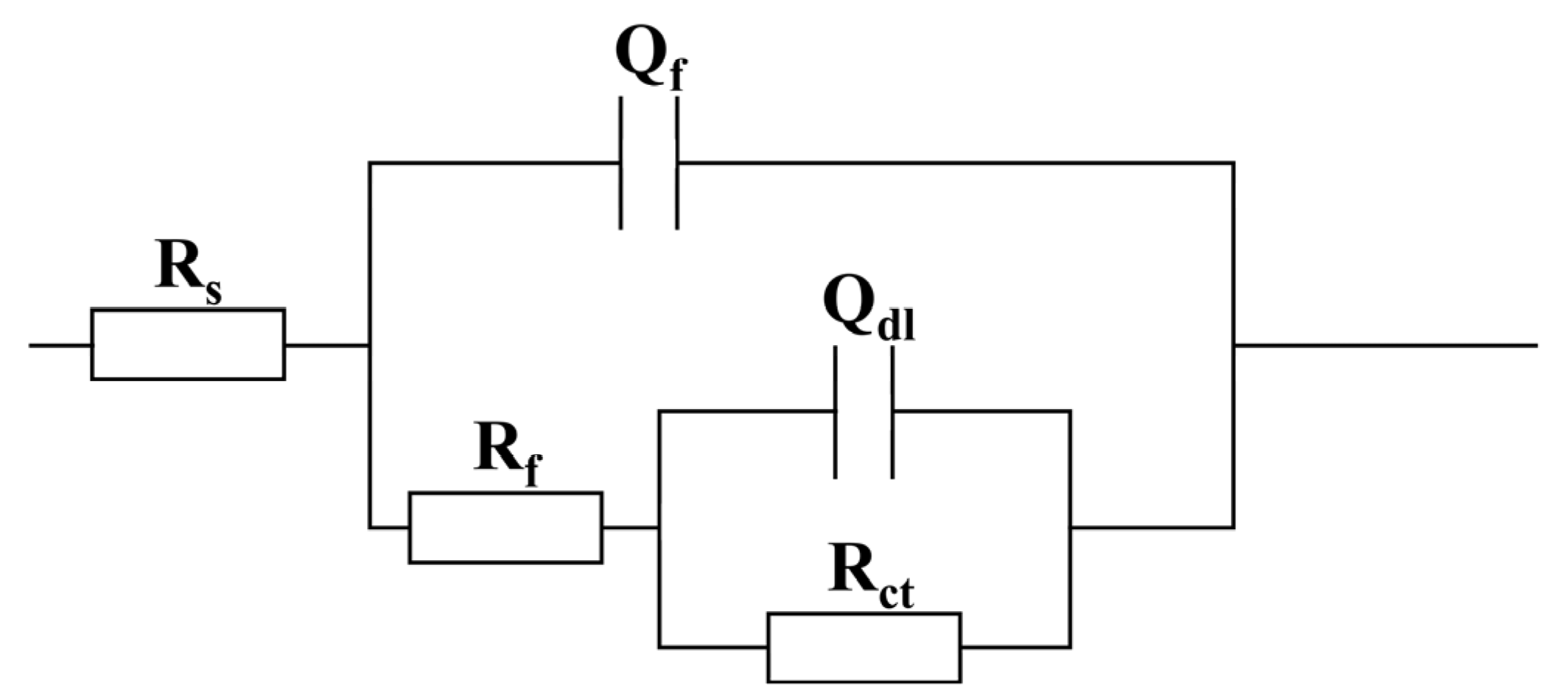
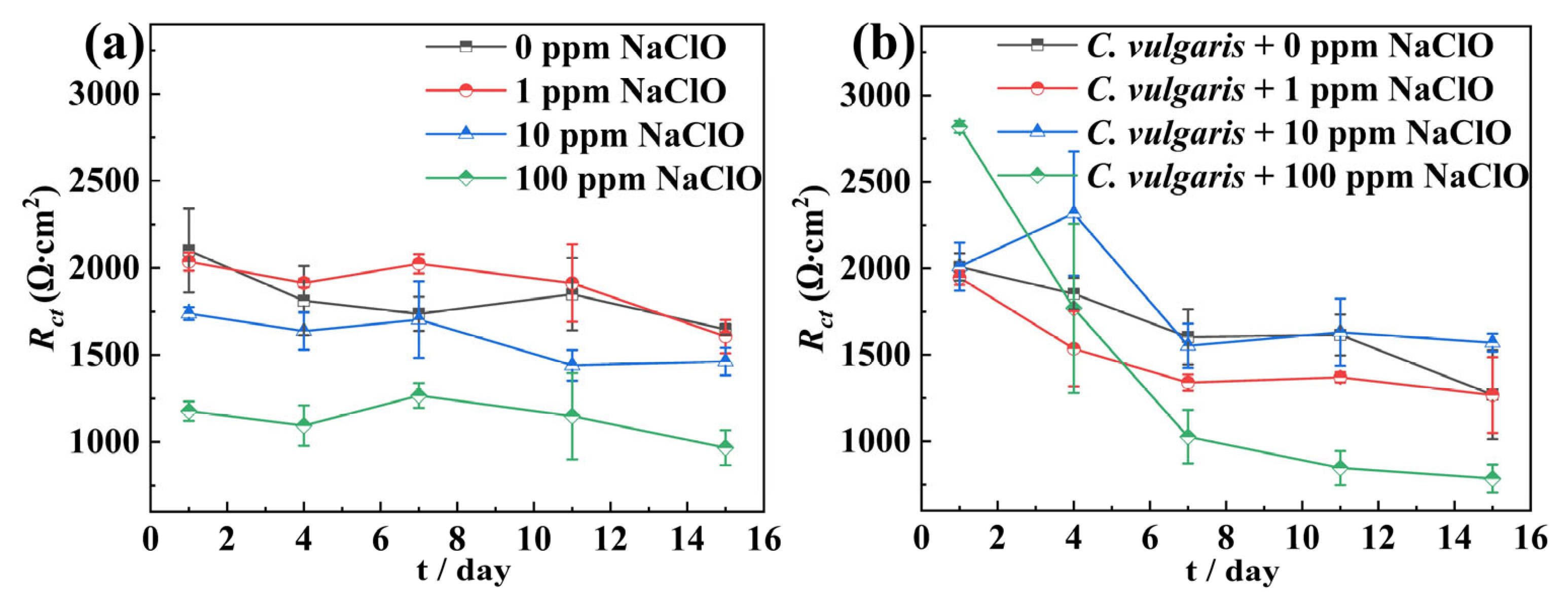

| Condition | t (d) | Rs (Ω·cm2) | Qf × 10−6 (S·cm−2·sn) | nf | Rf (Ω·cm2) | Qdl × 10−6 (S·cm−2·sn) | ndl | Rct (Ω·cm2) | χ2 (×10−3) |
|---|---|---|---|---|---|---|---|---|---|
| 0 ppm NaClO | 1 | 8.182 | 301.6 | 0.806 | 50.02 | 496.3 | 0.733 | 2100 | 1.14 |
| 4 | 8.904 | 197.7 | 0.792 | 672.3 | 231.8 | 0.681 | 1812 | 7.57 | |
| 7 | 7.241 | 219.1 | 0.862 | 550.3 | 527.5 | 0.522 | 1736 | 0.933 | |
| 11 | 10.36 | 226.4 | 0.871 | 315.3 | 367.1 | 0.591 | 1849 | 1.08 | |
| 15 | 8.325 | 218.7 | 0.889 | 270.2 | 478 | 0.469 | 1647 | 1.04 | |
| 1 ppm NaClO | 1 | 6.748 | 641 | 0.665 | 0.1308 | 19.38 | 1 | 2036 | 3.62 |
| 4 | 7.117 | 164.2 | 0.853 | 846 | 328.4 | 0.672 | 1914 | 2.84 | |
| 7 | 7.354 | 158.5 | 0.866 | 978.7 | 282.6 | 0.583 | 2024 | 4.60 | |
| 11 | 7.998 | 174.4 | 0.888 | 402 | 295.1 | 0.524 | 1913 | 1.62 | |
| 15 | 9.016 | 214.8 | 0.886 | 522.4 | 428.9 | 0.558 | 1606 | 8.60 | |
| 10 ppm NaClO | 1 | 5.384 | 567.9 | 0.739 | 67.03 | 1196 | 0.695 | 1738 | 2.31 |
| 4 | 8.634 | 178.4 | 0.804 | 373.3 | 325.3 | 0.543 | 1637 | 1.62 | |
| 7 | 8.539 | 145.3 | 0.794 | 17.37 | 45.02 | 0.644 | 1703 | 8.73 | |
| 11 | 8.589 | 277.2 | 0.887 | 572 | 441.6 | 0.486 | 1440 | 1.46 | |
| 15 | 9.859 | 327.4 | 0.832 | 674.3 | 452.5 | 0.624 | 1462 | 1.28 | |
| 100 ppm NaClO | 1 | 16.03 | 108.7 | 0.755 | 61.81 | 691.9 | 0.621 | 1177 | 1.41 |
| 4 | 8.69 | 47.85 | 0.809 | 608.599 | 582 | 0.574 | 1094 | 3.35 | |
| 7 | 9.827 | 261.9 | 0.790 | 433.5 | 890.3 | 0.604 | 1267 | 13.6 | |
| 11 | 13.15 | 137.2 | 0.795 | 762.2 | 1482 | 0.524 | 1148 | 86.7 | |
| 15 | 9.885 | 556.5 | 0.891 | 799.5 | 2280 | 0.629 | 967 | 16.8 |
| Condition | t (d) | Rs (Ω·cm2) | Qf × 10−6 (S·cm−2·sn) | nf | Rf (Ω·cm2) | Qdl × 10−6 (S·cm−2·sn) | ndl | Rct (Ω·cm2) | χ2 (×10−3) |
|---|---|---|---|---|---|---|---|---|---|
| C. vulgaris | 1 | 7.95 | 49.03 | 1 | 2.965 | 796.2 | 0.662 | 2008 | 2.1 |
| 4 | 8.518 | 194.7 | 0.868 | 10.85 | 1659 | 0.606 | 1853 | 1.83 | |
| 7 | 9.411 | 567.2 | 0.7511 | 22.25 | 1505 | 0.609 | 1603 | 0.39 | |
| 11 | 7.033 | 654.5 | 0.7452 | 19.92 | 2084 | 0.675 | 1615 | 1.62 | |
| 15 | 8.379 | 721 | 0.7628 | 17.48 | 2884 | 0.661 | 1269 | 1.52 | |
| C. vulgaris + 1 ppm NaClO | 1 | 6.085 | 40.79 | 1 | 3.233 | 839.8 | 0.701 | 1946 | 2.33 |
| 4 | 8.433 | 324.5 | 0.794 | 13.47 | 2359 | 0.635 | 1535 | 1.21 | |
| 7 | 7.566 | 171.7 | 0.909 | 4.147 | 4545 | 0.658 | 1339 | 0.68 | |
| 11 | 9.779 | 306.3 | 0.890 | 39.24 | 4776 | 0.678 | 1370 | 1.41 | |
| 15 | 8.519 | 745.9 | 0.782 | 26.636 | 4689 | 0.671 | 1267 | 1.20 | |
| C. vulgaris + 10 ppm NaClO | 1 | 7.828 | 275.3 | 0.777 | 2.066 | 169 | 0.733 | 2010 | 1.53 |
| 4 | 9.295 | 231.2 | 0.856 | 187.3 | 655.1 | 0.475 | 2040 | 2.29 | |
| 7 | 8.671 | 277.6 | 0.868 | 224 | 530.6 | 0.672 | 1521 | 0.69 | |
| 11 | 9.034 | 343.5 | 0.857 | 197 | 651.3 | 0.671 | 1431 | 1.25 | |
| 15 | 9.82 | 590 | 0.797 | 156.2 | 1531 | 0.661 | 1570 | 1.58 | |
| C. vulgaris + 100 ppm NaClO | 1 | 7.232 | 507.2 | 0.743 | 44.97 | 73.9 | 0.844 | 2888 | 1.67 |
| 4 | 7.876 | 634.7 | 0.718 | 448.4 | 265.1 | 0.638 | 2412 | 1.46 | |
| 7 | 7.929 | 176 | 0.859 | 1552 | 717.3 | 0.676 | 1026 | 1.62 | |
| 11 | 7.822 | 254.2 | 0.832 | 1357.2 | 429.3 | 0.577 | 1013 | 1.38 | |
| 15 | 7.329 | 268.4 | 0.829 | 942.2 | 763.3 | 0.576 | 869 | 2.07 |
| Condition | Ecorr (V vs. Ag/AgCl) | icorr (μA/cm2) | ba | bc |
|---|---|---|---|---|
| 0 ppm | −0.750 | 4.54 | 74.38 | 209.17 |
| 1 ppm | −0.750 | 4.93 | 73.67 | 218.91 |
| 10 ppm | −0.758 | 5.57 | 79.13 | 312.86 |
| 100 ppm | −0.752 | 9.34 | 77.88 | 375.39 |
| C. vulgaris + 0 ppm | −0.748 | 7.52 | 75.10 | 371.2 |
| C. vulgaris + 1 ppm | −0.748 | 8.65 | 84.97 | 263.93 |
| C. vulgaris + 10 ppm | −0.768 | 5.26 | 77.28 | 306.83 |
| C. vulgaris + 100 ppm | −0.755 | 11.55 | 77.34 | 464.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Fu, Q.; Song, G.-L. The Influence of NaClO on the Biocorrosion of Carbon Steel Induced by Chlorella vulgaris in Artificial Seawater. Molecules 2025, 30, 3636. https://doi.org/10.3390/molecules30173636
Zhang J, Fu Q, Song G-L. The Influence of NaClO on the Biocorrosion of Carbon Steel Induced by Chlorella vulgaris in Artificial Seawater. Molecules. 2025; 30(17):3636. https://doi.org/10.3390/molecules30173636
Chicago/Turabian StyleZhang, Junnan, Qi Fu, and Guang-Ling Song. 2025. "The Influence of NaClO on the Biocorrosion of Carbon Steel Induced by Chlorella vulgaris in Artificial Seawater" Molecules 30, no. 17: 3636. https://doi.org/10.3390/molecules30173636
APA StyleZhang, J., Fu, Q., & Song, G.-L. (2025). The Influence of NaClO on the Biocorrosion of Carbon Steel Induced by Chlorella vulgaris in Artificial Seawater. Molecules, 30(17), 3636. https://doi.org/10.3390/molecules30173636







