Abstract
Zinc-aluminum-magnesium (ZAM) steel, with its superior corrosion resistance and mechanical properties, is progressively supplanting traditional galvanized steel and zinc-aluminum steel. In this study, a solution containing sodium carbonate-only was employed as the treatment medium to form a vertically grown layered double hydroxide (LDH) pretreatment layer on the surface of ZAM steel via a simple immersion process at 50 °C. The temperature and salt solution not only provide the conditions for the dissolution of metal ions but also facilitate the formation of LDH products. The resulting LDH pretreatment layer exhibits excellent adhesion to the metal surface and enhances the adhesion of the top epoxy coatings. Furthermore, the “LDH/corrosion inhibitor/epoxy” coating system ensures ZAM steel remains rust-free in a 3.5 wt.% NaCl solution for a minimum of 120 days. This innovative approach offers a promising avenue for extending the durability and service life of ZAM steel in corrosive environments.
1. Introduction
Metallic materials, serving as widely utilized structural materials in human society, have a profound impact on public safety and social development through their susceptibility to corrosion. According to statistics, corrosion causes losses of at least 2.3 trillion US dollars annually [1,2]. As the most commonly employed method for metal protection, coating systems play a critical role in mitigating corrosion [3,4]. The pretreatment layer, together with organic resins, form the protective coating system [5,6]. Acting as the innermost protective structure adjacent to the substrate, the pretreatment layer not only provides highly effective active protection [7,8,9] but also enhances the adhesion between the topcoats and the metal substrate, thereby imparting greater durability and persistent barrier properties to the topcoat. Traditional pretreatment layers include phosphate coatings and chromate conversion coatings. These treatments offer commendable corrosion resistance to the substrate. However, the use of chromate conversion coatings releases hexavalent chromium ions, which are highly hazardous to human health when they enter the ecosystem. Meanwhile, phosphates used in phosphate coatings contribute to eutrophication in water bodies [10,11,12,13], thus limiting their practical applications. In light of these drawbacks, there is an urgent need within both the scientific community and industry for an environmentally friendly and high-performance pretreatment layer that can address these issues.
Layered double hydroxides (LDHs) have emerged as a novel class of layered materials in recent years. Their general chemical formula can be expressed as [M2+1−xM3+x(OH)2]x+An−x/n·mH2O, where M2+ and M3+ denote divalent and trivalent metal cations, respectively, which occupy the octahedral interstices of brucite-like hydroxide layers, thereby forming the metal hydroxide layer [14]. An− represents the anions intercalated within the LDH interlayers. These interlayer anions exhibit a certain degree of exchangeability, making LDHs suitable as nanocontainers for encapsulating corrosion inhibitors [15,16]. Additionally, the two-dimensional planar nature of LDHs confers them with effective ion-blocking capabilities against corrosive species [17,18]. As a rising star in the field of corrosion protection, LDHs find application primarily in two aspects: one is that, they can be incorporated directly into organic resins as “nano-containers” to serve as fillers [17,19]; the other is that, LDHs can be deposited on the substrate surface, functioning either as short-term protective films for metals [20,21] or as pretreatment layers for topcoats [22,23].
For the purpose of their use as a pretreatment layer, LDH films better exhibit a morphology that the (ab) plane can vertically to the metal substrate, so that the conversion coatings can provide improved adhesion to the top-coated coatings. The conventionally used hydrothermal method satisfies such requirements [24,25], however, it needs elevated temperatures and has prolonged reaction times [26,27,28]. This is detrimental to reactive elemental metals, as the metal substrate would undergo severe corrosion under such circumstances. The electrochemical deposition method needs a lower temperature and shorter preparation time, the as-prepared LDH coating is always flat and compact with the (ab) plane parallel to the substrate. Therefore, the electrodeposited LDH is hardly used as a pretreatment layer for organic coatings. Very recently, our laboratory [29] utilized a multi-potential-step electrodeposition approach to prepare a LDH pretreatment layer on galvanized steel, via the in situ generation of Zn2+ ions by anodic dissolution in the first step, and the formation of ZnAl-LDH with the participation of Al3+ ions that already existed in the solution by applying cathodic potentials in the second step. LDH coatings prepared by this two-step electrodeposition have a morphology with the (ab) plane perpendicular to the galvanized steel surface, but this technique needs additional energy input and equipment; therefore, proposing a mild, convenient, and rapid preparation method for the LDH pretreatment layer becomes particularly significant.
ZnAlMg (ZAM) coated steel combines the high strength of structural steel with the robust anti-corrosion performance provided by the coating, leading to its extensive use in automotive bodies and bridge frameworks, among other structures [30,31]. In spite of this, ZAM steels still require further improvement to their corrosion resistance by, for instance, applying polymeric coatings; this is urgent in harsh corrosive conditions, such as in marine environments. Here, a simple, efficient, and rapid method is introduced for the one-step preparation of an LDH pretreatment layer on ZAM steel using a precursor solution with the absence of any exogenous LDH-forming metal ions. The hot sodium carbonate solution not only provides a dissolution environment for Zn, Al, and Mg metals but also facilitates the formation of LDHs. Given that all metal ions originate from the ZAM steel itself, the in situ grown LDH coatings ensure excellent adhesion to the metal substrate. Moreover, the vertically oriented morphology (with ab-faces perpendicular to the substrates) of the as-prepared LDH conversion coatings ensures strong adhesion with the subsequent polymeric coatings and enables the pre-incorporation of the desired amount of corrosion inhibitors beneath the topcoat. The results demonstrate that the “LDH/corrosion inhibitor/epoxy” coating system prevents rust formation on ZAM steel in a 3.5 wt.% NaCl solution for at least 120 days. The synthesis of LDH requires only three min and does not necessitate the addition of any external film-forming metal ions.
2. Results
2.1. Characterization of LDH Pretreatment Layer
The surface morphology of ZAM steels after treatment in Na2CO3 solutions of various concentrations at 50 °C (a–d) and at a concentration of 0.16 M at different temperatures (e,f) is illustrated in Figure 1. At lower concentrations and temperatures, LDH formation is either partial (a,b) or does not occur on the ZAM substrate (e). Conversely, when the concentration or temperature of the Na2CO3 solution is high enough, the LDH exhibits dissolution behavior (d,f). The dissolved LDH transforms into oxides or hydroxides that cover the original LDH coatings. Optimal conditions, like 0.16 M Na2CO3 + 50 °C, lead to a uniform and high-coverage LDH coating. Unless otherwise mentioned, LDH conversion coatings in this work were prepared from 0.16 M Na2CO3 at 50 °C.
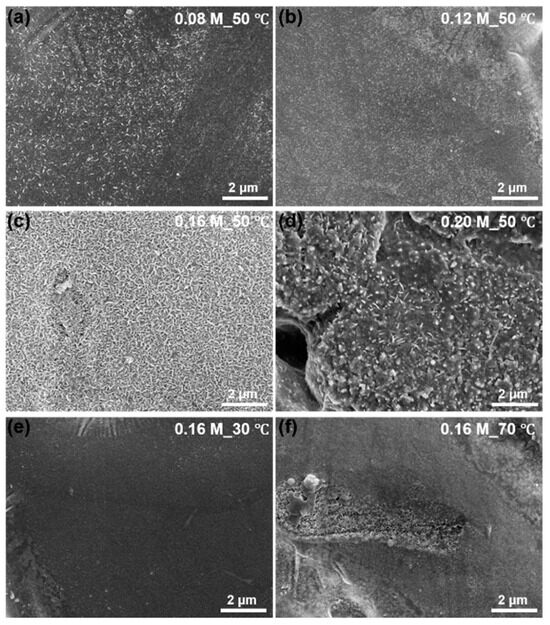
Figure 1.
SEM images of LDH conversion coatings prepared at a constant temperature of 50 °C with different Na2CO3 solution concentrations: 0.08 mol/L (a), 0.12 mol/L (b), 0.16 mol/L (c), and 0.20 mol/L (d); and at a constant concentration of 0.16 mol/L with different temperatures: 30 °C (e) and 70 °C (f).
The XRD patterns of bare ZAM steel and LDH-coated ZAM steel are presented in Figure 2a. In the untreated ZAM steel, no distinct diffraction peaks characteristic of LDHs, specifically the (003) and (006) peaks, were detected. The XRD pattern of this blank sample primarily consists of Zn peaks at 36.27°, 43.27°, and 77.46°, and Al peaks at 38.39°, 44.68°, 65.08°, and 77.88°. Additionally, there are some characteristic peaks of the intermetallic phase MgZn2 [32]. After treatment using the aforementioned method, characteristic LDH peaks appeared on the ZAM steel surface at 11.66° (003) and 24.22° (006) [33], indicating the formation of a well-crystallized LDH film. The Raman spectra align well with the XRD results (Figure 2b). For the bare ZAM steel, no significant signals were observed, resulting in a nearly flat baseline. After treatment, two prominent peaks attributed to LDH appear on the spectrum at 553 cm−1 and 1045 cm−1 [34].
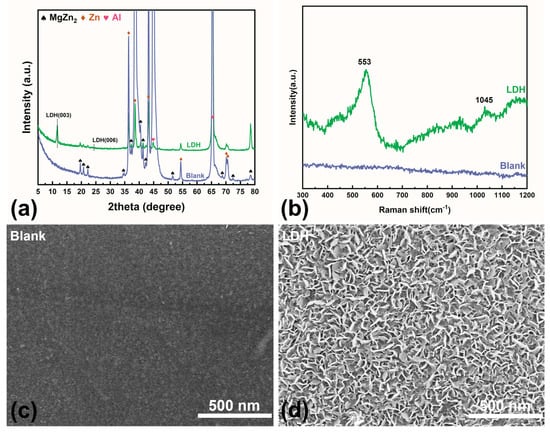
Figure 2.
(a) XRD patterns of bare ZAM steel and LDH-coated ZAM steel; (b) Raman spectra of bare ZAM steel and LDH-coated ZAM steel; (c,d) SEM images of LDH-coated ZAM steel and bare ZAM steel.
SEM images show that, compared to the blank sample (Figure 2c), the LDH sample (Figure 2d) exhibits a finely textured rough structure. The lamellar LDH grows vertically on the metal surface, endowing the ZAM steel with nanoscale pores and grooves. These nanostructures enable the pretreatment layer to absorb numerous corrosion inhibitors that are beneficial for enhancing the corrosion resistance of the metal. Additionally, they can interlock with subsequent polymeric resins, facilitating coating adhesion [9,29].
The samples were subjected to ultrasonic treatment, and the resulting LDH was examined using transmission electron microscopy (TEM). As shown in Figure 3a, Energy-Dispersive X-ray Spectroscopy (EDS) mapping results indicate that the LDH primarily consists of Zn, Al, C, and O, with trace amounts of Mg. Among these elements, C and O predominantly originate from the Na2CO3 solution used during preparation, which provides CO32− ions as intercalation ions for LDH formation. During the treatment of ZAM steel with a hot sodium carbonate solution, Zn, Al, and Mg undergo in situ transformation into hydroxides and soluble species such as Zn(OH)42− and Al(OH)4−. In the formation process of LDH, trivalent metal ions Al3+ partially substitute divalent metal ions Zn2+ within the layers, imparting an excess positive charge to the layer sheets. The carbonate ions enter the interlayer region via electrostatic interactions, neutralizing these positive charges and maintaining the overall charge neutrality of the LDH structure. A high-resolution TEM (HRTEM) image (Figure 3b) clearly displays the lattice fringes of the LDH. The inter-planar spacing of 0.24 nm corresponds to the (012) crystal plane of LDH. As shown in Figure 3c, the selected-area electron diffraction (SAED) pattern reveals the diffraction rings, indicating that the formed LDH possesses a highly ordered crystalline structure. These distinct diffraction rings correspond to different lattice planes within the crystal structure. Notably, the broadening at 2.4 Å and 5.5 Å corresponds to the (012) and (003) planes of the LDH. Figure S1 presents HRTEM images and EDS point scan results of LDH at lower magnification. From Figure S1a, it is evident that LDH exhibits a pronounced lamellar structure. The EDS analysis in Figure S1b indicates that the Zn/Al ratio of the formed LDH is approximately 2:1, which is the most favorable metal ratio for LDH formation on ZAM steel during natural corrosion [35].
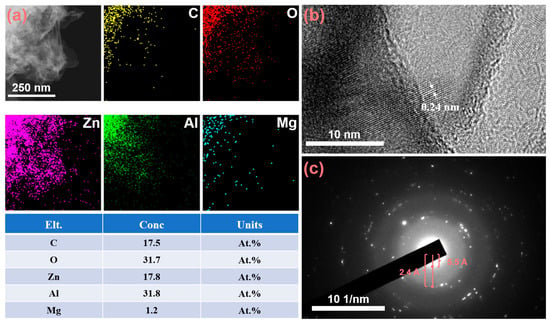
Figure 3.
(a) EDS mapping results of the LDH coating grown on ZAM steel, (b) High-resolution TEM (HRTEM) image (c) Electron diffraction pattern of LDH coating.
XPS spectra (Figure 4a) shows the presence of Zn, Al, Mg, C, and O elements in blank and LDH-coated ZAM coatings. In the results of the inhibitor-loaded samples, a distinct V signal is also observed. Figure 4b presents the Al 2p spectrum, which distinctly reflects the chemical state of Al on the substrate surface. For the blank sample, the Al 2p peak can be fitted into two components: one at approximately 72.3 eV corresponding to the Al-Al bond in metallic Al on the bare ZAM steel surface, and another around 74.8 eV attributed to the Al-O bond in Al2O3 [36]. Due to its high reactivity, an aluminum layer or alloy exposed to air typically develops a dense oxide layer that prevents further oxidation of the underlying metal. After treatment with a hot Na2CO3 solution (LDH sample), a new peak appears at 74.2 eV for Al-OH, completely replacing the 74.8 eV Al-O peak, indicating complete coverage of the ZAM surface by hydroxides. For the inhibitor-loaded sample (LDH/VOx sample), an additional peak at a higher binding energy of 75.2 eV is observed in the Al 2p spectrum, alongside the 74.2 eV Al-OH peak. This higher binding energy peak arises from the introduction of highly electronegative VO3− ions, which form complexes with metal ions, resulting in a blue shift of the binding energy. Figure 4c shows the V 2p peaks characteristic of the LDH/VOx sample, with two peaks at 524.3 eV for V 2p1/2 and 517.1 eV for V 2p3/2, confirming the successful loading of the VO3− inhibitor onto the LDH pretreatment layer. The XRD pattern of the LDH/VOx sample (Figure 4d) detected a broader (003) peak of LDH, compared with the LDH sample, indicating a decrease in crystallinity after inhibitor loading. The SEM image in Figure 4e corroborates this observation, showing a certain degree of collapse in the morphology of the LDH/VOx sample.
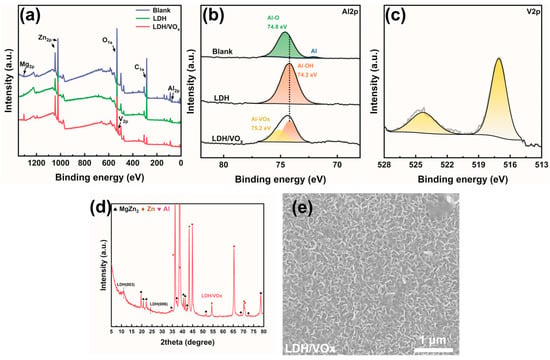
Figure 4.
(a) Full scan and (b) Al 2p XPS spectrum of bare ZAM steel, LDH-coated ZAM steel, and LDH-coated ZAM steel after the adsorption of corrosion inhibitors, (c) V 2p XPS spectrum, (d) XRD patterns, (e) SEM image. The corresponding phases in the XRD pattern have been labeled in the upper left corner.
2.2. Corrosion Performance of the LDH Pretreatment Layer and the Whole Coating System
The potentiodynamic polarization curves (Figure 5) show that, the blank ZAM steel exhibits the highest corrosion current density (Icorr, 1.60 × 10−5 A/cm−2, Table 1) among all samples, along with the most severe corrosion morphology (Figure 5b). After coated with a LDH conversion layer, Icorr slightly decreases to a value of 1.21 × 10−5 A/cm−2 (Table 1). The decrease is negligible, probably because of the vertical orientation of the LDH lamella; however, the corrosion morphology of the LDH sample showed significant improvement (Figure 5c). The lowest Icorr is observed in the LDH/VOx sample, which is approximately one order of magnitude lower than that of both the blank and LDH samples. The loading of the corrosion inhibitor VO3− significantly enhances the corrosion inhibition performance of the LDH coating, markedly improving the corrosion resistance of ZAM steel in a 3.5 wt.% NaCl aqueous solution (Figure 5d). It is quite evident that the corrosion rate ranking of the three samples is: Blank > LDH > LDH/VOx.
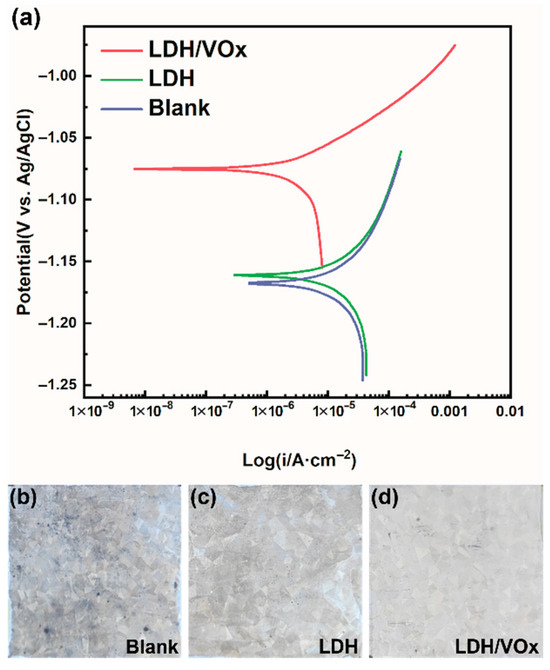
Figure 5.
(a) Potentiodynamic polarization curves of different samples after immersed in a 3.5 wt.% NaCl aqueous solution for 30 min; the surface morphology of (b) blank, (c) LDH, and (d) LDH/VOx sample after immersion in a 3.5 wt.% NaCl solution for 24 h.

Table 1.
The fitting results of potentiodynamic polarization curves.
Long-term immersion tests in a 3.5 wt.% NaCl solution were used to evaluate corrosion performance of epoxy coating samples (Figure 6). Noticeable rust appears beneath the coatings on blank ZAM substrates (Figure 6a). As the immersion time extended, these initial corrosion spots progressively expanded and covered the entire surface after 70 days. These results indicate that the pure epoxy coating alone cannot provide ZAM steel with durable and effective protection. In contrast, the LDH_EP sample (Figure 6b) did not show any corrosion phenomena until day 70, and even then, the extent of corrosion was significantly less severe compared to the Blank_EP sample. This suggests that the presence of the LDH pretreatment layer enhances the corrosion resistance of epoxy-coated ZAM steel. The superior corrosion resistance performance was most evident in the LDH/VOx_EP sample. Even after 120 days of immersion, its surface remained free from any visible corrosion. The LDH coatings loaded with VO3− further augmented the anti-corrosion performance of the steel. The long-term immersion test results demonstrate that while the pure epoxy coating provides limited protection, the incorporation of an LDH pretreatment layer markedly improves the corrosion resistance of ZAM steel. Moreover, the addition of the VO3− inhibitor within the LDH layer offers significant enhancement in protecting the steel substrate against corrosion, highlighting the effectiveness of this composite coating system.
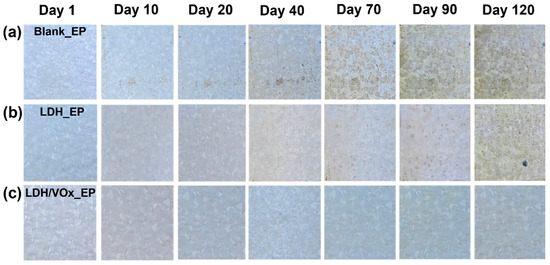
Figure 6.
Optical images of the intact coating samples immersed in a 3.5 wt.% NaCl solution for 120 days. (a) Blank_EP, (b) LDH_EP, (c) LDH/VOx_EP.
EIS experiments were employed to quantitatively evaluate the corrosion resistance of the coating systems and to further analyze the corrosion mechanisms of the coatings. The impedance modulus-Bode plots (left), phase angle-Bode plots (middle), and Nyquist diagrams (right) for intact coated samples immersed in a 3.5 wt.% NaCl solution are presented in Figure 7. The low-frequency impedance values of all samples (Figure 8a) continuously decreased throughout the immersion period, indicating a gradual deterioration of the barrier properties of the epoxy coatings. For the EP sample, even at the initial stages of immersion (Figure 7a), the impedance value was approximately 106 Ω·cm2; by the end of the immersion period (90–120 days), this value had declined to around 103 Ω·cm2. The relatively low initial impedance and the rapid decline suggest that, without the synergistic effect of an LDH pretreatment layer, the protection provided by epoxy resin to ZAM steel is quite limited. In contrast, the LDH_EP sample exhibited higher impedance values (Figure 7d) and larger semicircle diameters (Figure 7f) compared to the EP sample at the same immersion times, indicating that the formed LDH pretreatment layer can enhance the anti-corrosion performance of subsequent coatings to some extent. Consistent with previous results, the coating system prepared after loading the LDH with a corrosion inhibitor demonstrated the best anti-corrosion performance. The largest semicircle diameter (Figure 7i) and highest impedance values (Figure 7g) were observed for the LDH/VOx_EP samples. Throughout the entire immersion period, the impedance values remained above 106 Ω·cm2, providing sustained excellent protection for the ZAM steel substrate.
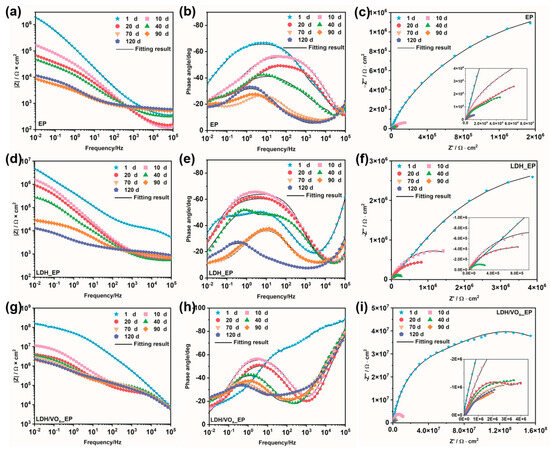
Figure 7.
Impedance module-Bode plots (left), phase angle-Bode plots (middle), and Nyquist diagram (right) of the intact coating samples immersed in a 3.5 wt.% NaCl solution. (a–c) EP; (d–f) LDH_EP; (g–i) LDH/VOx_EP.
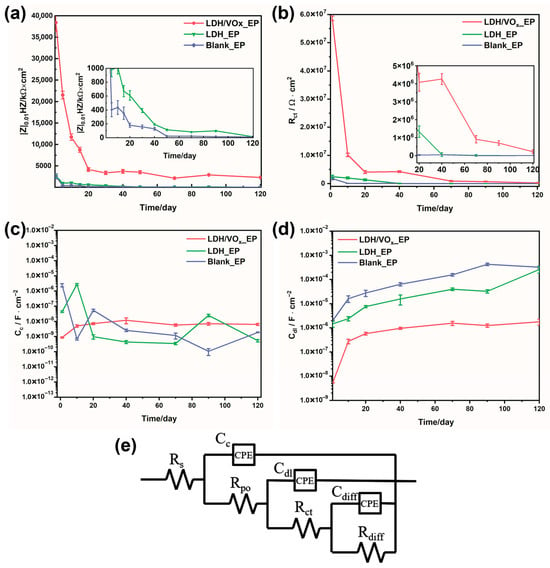
Figure 8.
(a) The variation in the low-frequency impedance modulus of the intact coating in a 3.5 wt.% NaCl solution; the variation in (b) Rct, (c) Cc, (d) Cdl over the entire immersion period; (e) the Equivalent electrical circuit model (EEC) used for fitting EIS data.
An appropriate Equivalent Electrical Circuit (EEC) was selected to fit the EIS data (Figure 8e). This EEC, which contains three time constants, was applicable for fitting throughout the entire immersion period. For the first time constant, Rpo and Cc represent the pore resistance and coating capacitance, respectively, after the electrolyte solution has penetrated the coating [37]. When the electrolyte reaches the substrate surface, electrochemical reactions occur at the metal/coating interface, introducing the second time constant. Rct and Cdl denote the charge transfer resistance and double-layer capacitance at the electrochemical reaction interface, respectively [38]. The third time constant is associated with the products of the electrochemical reactions. Its presence is linked to the accumulation of reaction products at the metal/coating interface. Rdiff and Cdiff are the diffusion resistance and diffusion capacitance of the coating [29]. It is noteworthy that the EEC employing Rdiff and Cdiff, characterized by Finite-Length Diffusion (FLD), has been widely adopted for fitting impedance data of coated samples after enhanced immersion [39]. All capacitive elements in the circuit were replaced with Constant Phase Elements (CPEs) to achieve accurate fitting results. Equation (1) defines the impedance of a CPE, where Y0 is the magnitude of the CPE, ω is the angular frequency, and n is a dimensionless parameter.
The values of Rct and Cdl are closely related to the degree to which metal corrosion is inhibited. Lower Cdl and higher Rct indicate better protection of the metal in the corrosive medium. Figure 8b,d shows that the LDH/VOx_EP sample maintained the highest Rct values and the lowest Cdl values throughout the entire immersion period, indicating superior protective performance provided by the inhibitor-loaded pretreatment layer. In contrast, the EP sample exhibited the highest Cdl values and the lowest Rct values. The continuously increasing trend of Cdl suggests a growing interface between the coating and the metal during immersion. This expansion is attributed to rapid corrosion, leading to the accumulation of significant amounts of corrosion products at the interface, which promotes delamination between the metal and the coating. The LDH_EP sample showed intermediate behavior between the two extremes, indicating that the LDH pretreatment layer can enhance the corrosion resistance of the coating system to some extent. The water absorption of the coating can typically be assessed by the coating capacitance Cc, lower values indicate greater diffusion resistance to water and, consequently, lower water absorption. Figure 8c presents the Cc values for all samples. During the 120-day immersion test, all samples exhibited similar Cc values. This similarity in Cc suggests that, despite differences in their protective performance against corrosion, the coatings have comparable resistance to water diffusion. However, it is important to note that while Cc provides insight into water absorption, it does not fully capture the complex interactions between the coating and the corrosive environment, which are also influenced by factors such as the presence of protective layers and inhibitors. Overall, the corrosion rate ranking of the coated samples is as follows: Blank_EP > LDH_EP > LDH/VOx_EP.
Artificial defects were introduced on intact coated samples, which were then immersed in a 3.5 wt.% NaCl solution. Optical photographs of all samples at various immersion durations are shown in Figure 9a–c. On the first day of immersion, rust began to appear along the scratch on the blank sample (Figure 9a). As the immersion time extended, corrosion spots appeared by day seven, with extensive corrosion marks evident by day thirty-five. By the end of the immersion period, corrosion products had accumulated extensively at the metal/coating interface, indicating that the epoxy coating (EP) offered limited protection to ZAM steel. For the LDH_EP sample (Figure 9b), corrosion spots also appeared by day seven but did not expand further with prolonged immersion. Even after 90 days, only sporadic corrosion products were observed. After mechanical damage to the coating, the LDH pretreatment layer effectively inhibited the corrosive medium from attacking the metal/coating interface. The lamellar structure of LDHs significantly increased the lateral diffusion resistance of the medium [40]. When loaded with a corrosion inhibitor, the protective performance of the pretreatment layer was further enhanced. The synergistic effect of active protection (inhibitor action) and suppression of lateral electrolyte diffusion resulted in the LDH/VOx_EP sample showing corrosion traces only by day 65 (Figure 9c). Subsequent immersion did not lead to an expansion of corrosion spots or additional corrosion. The coating system composed of LDH/VOx and subsequent epoxy resin significantly improved the corrosion resistance of ZAM steel. Figure 9d,e shows the amounts of Zn and Al ions leached due to corrosion after 90 days of immersion. The results indicate that the LDH/VOx_EP sample has the lowest levels of Zn and Al ion release, followed by the LDH_EP sample. The presence of the LDH pretreatment layer greatly restricted the lateral spread of the electrolyte solution, preventing corrosion products from spreading outward from the defect site even when the EP coating was damaged. In contrast, the EP sample exhibited the highest ion release, consistent with the previously summarized corrosion resistance ranking.
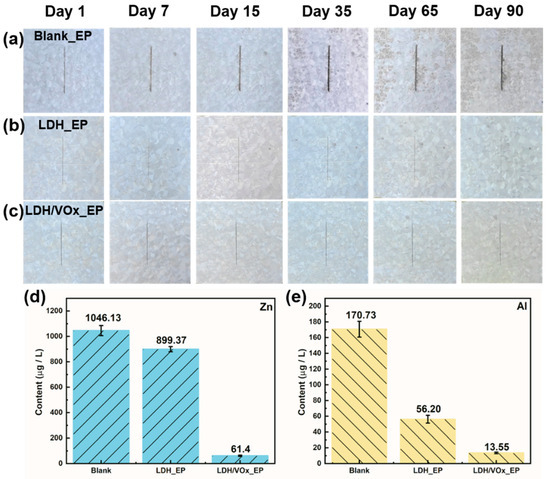
Figure 9.
Optical images of the scratched Blank_EP sample (a), LDH_EP sample (b), and LDH/VOx_EP sample (c) immersed in a 3.5 wt.% NaCl solution for 90 days. (d) Zn and (e) Al content in the solution after 90 days of immersion of scratched coating samples.
The pull-off test results for all samples are presented in Figure 10a. On the bare ZAM steel substrate, the adhesion strength of the epoxy (EP) coating was only 1.93 MPa. When a pretreatment layer was incorporated into the coating system, the adhesion strength of the EP significantly increased to 10.27 MPa for the LDH_EP sample and 9.32 MPa for the LDH/VOx_EP sample. Figure 10b–d show the SEM images of different samples after the pull-off tests. On substrates with LDH pretreatment layers, the lamellar LDH structures interlocked with the EP coating, forming a “concave-filled structure” that substantially enhanced the adhesion strength of the EP. In contrast, the EP sample exhibited extensive delamination due to its lower adhesion strength. The interlocking mechanism between the LDH pretreatment layer and the EP coating is evident from the SEM images, where the lamellar LDHs are observed to be tightly integrated with the EP matrix. This structural integration not only increases the mechanical interlocking but also improves the chemical bonding at the interface, contributing to the significant enhancement in adhesion strength.
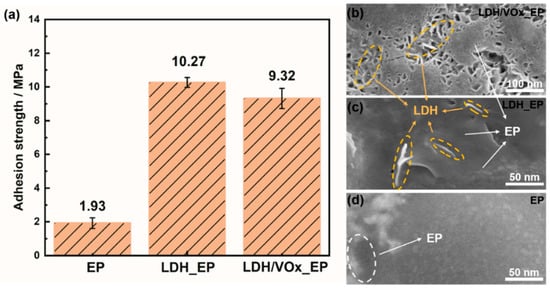
Figure 10.
(a) Pull-off test results of different samples; (b–d) SEM images of different samples after pull-off test.
3. Discussion
3.1. Formation Mechanism of LDH Pretreatment Layer
The ZAM coating contains substantial amounts of zinc (Zn) and aluminum (Al), along with trace amounts of magnesium (Mg) [41]. Both Zn and Al are amphoteric metals, which can react with either acids or bases. Leveraging this unique property, a method is devised in this paper that utilizes heat and alkalinity in concert, to dissolve the metals from the coating and deposit them to form LDH conversion coatings. This approach allows for the in situ preparation of ZnAlMg-LDH coatings on the surface of ZAM steel using a hot sodium carbonate solution without the addition of external metal ions. The reaction equations that occurred during the preparation process are summarized in Table 2. Sodium carbonate hydrolyses in water to create an alkaline environment; moreover, the existing CO32− ions can also serve as intercalation ions for LDH formation, aiding in the stabilization of the LDH structure [42]. The deposition process of LDHs, as illustrated in Figure 11, proceeds as follows: initially, CO32− ions in the solution react with H2O, generating OH− ions (Equations (2) and (3)), thereby providing an alkaline environment for the dissolution of Al and Zn from the ZAM steel coating (Equations (4) and (5)). Concurrently, under heating conditions, Al, Zn, and Mg react with water, transforming these metals into hydroxide deposits (Equations (6)–(8)), which anchor onto the metal surface and provide nucleation sites for the in situ growth of LDHs. Alternatively, the soluble aluminate and zincate species in the solution transformed into their corresponding hydroxides under cooling conditions (Equations (9) and (10)) promoting the growth of LDH nuclei and resulting in the formation of a uniformly coated LDH pretreatment layer on the metal surface.

Table 2.
Reaction equations involved in the preparation process.
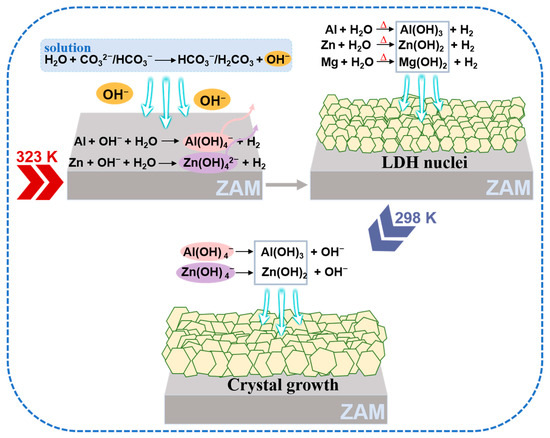
Figure 11.
Schematic diagram of the in situ deposition of the ZnAlMg-LDH coating on the surface of ZAM steel without the addition of external film-forming metal salts.
The hydrolysis of carbonate ions (Equations (2) and (3)) is crucial for LDH formation, governed by their hydrolysis equilibrium constants Ka (Equations (11) and (12)). According to the van’t Hoff equation (Equation (13)), Ka increases with temperature (T), as the reactions (2) and (3) are endothermic (∆H0 > 0), elevating OH− concentration. Conversely, lower temperatures reduce pH. Moreover, the OH− concentration further depends on the Na2CO3 concentration. At a fixed temperature (constant Ka), a higher Na2CO3 concentration drives Equations (2) and (3) toward the forward direction. As indicated by Equations (11) and (12), this results in an increased OH− concentration and a corresponding rise in pH. Conversely, reducing the Na2CO3 concentration leads to a decrease in the system’s pH; therefore, when the temperature or concentration of the solution is low, the metals in the ZAM steel coating do not receive sufficient OH− and thermal energy to dissolve, and the LDH lacks the appropriate OH− concentration necessary for its growth. At excessively high temperatures or concentrations, the amphoteric metals (Zn, Al) forming the LDH can undergo dissolution due to the elevated OH− content.
3.2. Mechanism of Corrosion Protection of the Coating Systems
For LDH conversion layers employed as pretreatment layers, several essential criteria must be met. Primarily, a robust interfacial adhesion between the pretreatment layer and the metal substrate is required. Furthermore, strong bonding between the pretreatment layer and subsequent coatings is necessary. Ultimately, the pretreatment layer must exhibit effective corrosion inhibitor loading capacity and resistance to corrosive ions. Herein, a facile and mild alkaline treatment was employed on ZAM steel, where a short 30 s immersion in a Na2CO3 solution at merely 50 °C enabled the in situ growth of a vertically aligned LDH pretreatment layer. Figure S3 shows the surface morphology of the LDH and LDH/VOx samples after cross-cut testing and tape peeling. The LDH pretreatment layer remained tightly adhered to the metal substrate. Notably, since no external metal ions were supplied during the process, the ZAM coating itself served as the exclusive source of metallic species. This complete in situ metal ion supply resulted in an unprecedented interfacial bonding strength, thereby establishing an exceptional primary interface for the coating system.
The LDH layers are rich in hydroxyl groups [43], which endows them with excellent affinity toward epoxy resin [44]. Furthermore, as demonstrated by Karami et al. [45], the strong interaction between epoxy resin and Zn/Al-LDH hydroxide layers enables Zn atoms to attack the lone pair electrons of oxygen in epoxide rings, participating in ring-opening reactions during resin curing and thereby enhancing the curing performance of epoxy resin. High affinity combined with the ability to promote resin curing results in firm anchoring of the epoxy resin onto the LDH pretreatment layer. In coating technology, the interaction between the resin and the substrate can be categorized into two mechanisms. The first is adhesion, whereby the resin directly adheres to the metal substrate through van der Waals forces, hydrogen bonding, or chemical bonding. The second mechanism involves the presence of an intermediate “adhesive” layer between the resin and the substrate, which forms strong bonds with both materials and thereby enhances the overall adhesion of the coating system. Figure 12 presents the SEM and EDS results of the coating system after resin delamination. On the exposed pretreatment layer surface following outer resin removal, uniformly distributed signals of C and O (from epoxy resin) as well as Zn and Al confirm the tight interlocking between the resin and LDH, forming a “concave-filled structure.” The LDH pretreatment layer serves as an effective adhesive interface, greatly enhancing the bonding between the resin and the substrate and thereby improving its resistance to delamination. The pull-off test results demonstrate a significant increase in adhesion strength (Figure 10). Consequently, a second high-performance interfacial layer is successfully established.
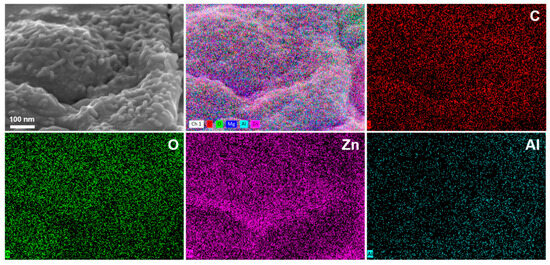
Figure 12.
SEM and EDS results of the LDH pretreatment layers after a pull-out test.
Vanadate ions exhibit excellent corrosion inhibition performance for Al alloys [46,47], which enables their application as high-efficiency inhibitors for ZAM steel. Figure S2 presents the Tafel curves of ZAM steel in a 3.5 wt.% NaCl solution and a 3.5 wt.% NaCl + 0.01 M NaVO3 solution, along with corresponding surface morphologies after 24 h immersion. In the NaCl solution, ZAM steel showed a corrosion current density (Icorr) of 3.67 × 10−6 A/cm2, while in the NaVO3 containing solution, the Icorr dramatically decreased by two orders of magnitude to merely 6.81 × 10−8 A/cm2. A comparison of optical images (c) and (d) after immersion clearly demonstrates that NaVO3 significantly enhances the corrosion resistance of ZAM steel in a 3.5 wt.% NaCl solution.
In the anion exchange sequence of LDH, the planar structure and strong electronegativity of CO32− prevents conventional Cl−-for-CO32− interlayer anion exchange [48,49], which implies that the LDH pretreatment layer prepared in this study cannot capture Cl− during natural corrosion processes. Figure 13a,b present XRD patterns of bare LDH and LDH/VOx samples before and after immersion in a 3.5 wt.% NaCl solution. After 24 h of immersion, the (003) diffraction peaks of both samples show no significant shift, indicating that Cl− did not intercalate into the LDH interlayers and the crystal structure remained unchanged. However, the decreased (003) peak intensity suggests partial LDH dissolution due to exposure to high Cl− concentrations.
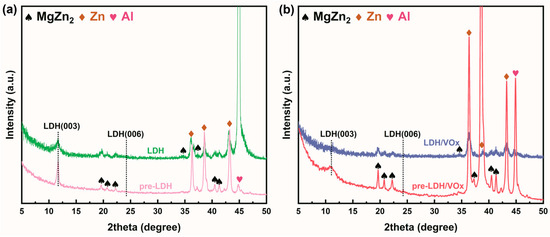
Figure 13.
XRD results of (a) LDH and (b) LDH/VOx samples after 24 h of immersion in a 3.5 wt.% NaCl solution.
3.3. Commercial Resin Applications
The LDH pretreatment layer was compared with a commercial phosphating process, over which a commercial epoxy resin (CEP) was subsequently coated. Optical images of the intact commercial EP coating immersed in a 3.5 wt.% NaCl solution are provided in Figure S4. Corrosion spots started to appear on the Blank_CEP sample on the fifteenth day of immersion and continued to expand during further soaking. The P_CEP sample began to show slight corrosion only after the fiftieth day. Both LDH_CEP and LDH/VOx_CEP samples exhibited excellent corrosion resistance throughout the entire 90-day period. This indicates that the LDH pretreatment layer outperforms the commercial phosphating process. The pull-off test results for the commercial resin system (Figure S5) demonstrated that the adhesion strength of the LDH_CEP sample (9.88 MPa) and the LDH/VOx_CEP sample (7.76 MPa) were higher than that of the P_CEP sample (5.78 MPa), while the Blank_CEP sample showed the lowest adhesion strength (1.75 MPa). Therefore, the LDH pretreatment layer can still be effectively utilized in commercial epoxy resin systems, providing them with superior corrosion resistance and coating adhesion.
4. Experimental
4.1. Materials and Chemicals
The ZAM steels were kindly supplied by Baosteel Co., Ltd. (Shanghai, China) and were cut into a size of 40 mm × 30 mm × 0.5 mm. The ZAM coating consists of 55 wt.% Al, 43 wt.% Zn, 1 wt.% Mg and balance Si. Sodium carbonate (Na2CO3, AR), sodium chloride (NaCl, AR) and sodium metavanadate (NaVO3, AR) were purchased from Aladding Industrial Corporation (Shanghai, China). Isopropyl alcohol ((CH3)2CHOH, AR) was purchased from Sinopharm Group (Beijing, China). Commercial phosphating solution (DR-378) was purchased from Dingrun Chemical Co., Ltd. (Dongguan, China). Pure epoxy resin (Ep, bis-phenol A, type E-44) and the curing agent polyamide (650) were purchased from Macklin (Shanghai, China). Commercial epoxy resin (9000-B001), curing agent (9000-B001H), and diluter (9000-B001T) were purchased from AA-sund Co., Ltd. (Yangzhou, China).
4.2. Synthesis of LDH Conversion Coatings on ZAM Steel
Prior to use, the ZAM steel substrates were ultrasonically cleaned in acetone and ethanol sequentially, each for 5 min, to remove surface contaminants. A series of isopropanol/water (with volume ratio of 3:7) solutions containing sodium carbonate at concentrations of 0.08, 0.12, 0.16, and 0.20 mol/L were prepared. These solutions were subsequently heated to different temperatures (30, 50, and 70 °C). ZnAlMg steel sheets were immersed statically into the pre-heated solutions and allowed to react for 30 s. Then, the obtained samples were alternately rinsed with deionized water and ethanol, followed by drying at 70 °C for 1 h.
4.3. Encapsulation of Corrosion Inhibitor (NaVO3) on LDH Conversion Coatings
As a commonly used corrosion inhibitor for Al alloys, NaVO3 was selected as the corrosion inhibitor for ZAM steel and loaded into the LDH pretreatment layer. A 0.1 mol/L NaVO3 solution with pH 8.5 ± 0.1 was adjusted by using a 0.1 mol/L NaOH aqueous solution. The LDH-coated ZAM steel sheets were then immersed in this solution at 25 ± 1 °C for 3 h. The resulting samples were designated as LDH/VOx.
4.4. Preparation of Epoxy Coatings
The polymeric coating was prepared using epoxy resin (E44) and a polyamide curing agent. The epoxy resin, curing agent, and solvent were ultrasonically mixed. The mass ratio of epoxy resin to curing agent was 5:4 and the solvent was composed of xylene and n-butanol with a volume ratio of 7:3. The epoxy resin was applied onto bare ZAM steel, LDH-coated ZAM steel, and LDH/VOx-coated ZAM steel using a film applicator (OSP-80/250, Qigong, Shanghai, China). The coated samples were initially cured at 25 °C for 12 h, followed by post-curing at 45 °C for 7 days. The thickness of the epoxy coatings, measured using a coating thickness gauge (Qnix 8500, Cologne, Germany), was maintained at 40 ± 2 μm. The epoxy-coated bare ZAM steel, LDH-coated ZAM steel, and LDH/VOx-coated ZAM steel were designated as EP, LDH_EP, and LDH/VOx_EP, respectively.
4.5. Characterization
The X-pert Powder diffractometer (40 kV, 40 mA, CuKα radiation, X’celerator detector, Malvern, UK) was used to examine the phase structure of the LDH conversion coatings. The surface morphology was characterized by scanning electron microscopy (SEM, SU8010, Hitachi, Tokyo, Japan). Laser Confocal Raman spectrometer (LabRAM-HR-evolution, HORIBA Jobin Yvon, Paris, France) was conducted to analyze the composition of the LDH coatings. The elemental composition was characterized by a Field Emission Analytical Transmission Electron Microscope (2100F, Tokyo, Japan). X-ray Photoelectron Spectroscopy (Axis-Supra+, Kratos, Manchester, UK) was conducted to determine the chemical compositions of the LDH pretreatment layer.
The adhesion strength of the coatings was measured by the pull-off adhesion test (Defelsko, positest AT-A) according to ISO 4624:2023 [50]. Zn and Al ion dissolution tests after intensive corrosion of artificially scratched coating samples were carried out on an Inductively Coupled Plasma Mass Spectrometer (NexION-2000, Perkin-Elmer, Waltham, MA, USA).
4.6. Corrosion Performance Evaluation
The corrosion evaluation of LDH conversion coatings alone and epoxy-coated samples was accelerated using a 3.5 wt.% NaCl solution. Epoxy-coated samples were assessed in both intact (coated) and artificially defective conditions, followed by immersion in the aforementioned NaCl solution. Artificial defects in the coating were introduced according to ISO 17872:2019 [51], with a scratch length of 10 mm and depth reaching the substrate. Optical photographs of the samples were taken at various times throughout the immersion process.
To quantitatively evaluate the corrosion rate of the LDH-filmed samples, potentiodynamic polarization curves were employed. The polarization curves were measured in a 3.5 wt.% NaCl solution using an M273 potentiostat. The potential scan rate and the overpotential were 0.2 mV/s and 100 mV. Electrochemical impedance spectroscopy (EIS) was utilized to monitor the electrochemical corrosion processes of the epoxy-coated samples during the immersion period. EIS tests were conducted in a 3.5 wt.% NaCl solution on a VersaSTAT 3 (Princeton Applied Research, Oak Ridge, TN, USA) with a three-electrode system, where the intact coating samples served as the working electrode, a platinum sheet acted as the counter electrode, and a Ag/AgCl electrode functioned as the reference electrode. The frequency range for the tests was selected from 100 kHz to 10 mHz with a sine wave voltage amplitude of 25 mV.
5. Conclusions
A rapid, mild, and environmentally friendly thermal carbonate solution treatment method was employed in this study to prepare the LDH pretreatment layer. The thin film and its formation process exhibit the following characteristics:
- All metal ions required for the formation of the thin film are derived from the substrate itself, eliminating the need for any external addition of film-forming metal ions. This approach significantly reduces preparation costs. The Na2CO3 solution used not only provides an alkaline environment but also facilitates the formation of LDH with CO32− intercalation. By sourcing all necessary metal ions for LDH formation from the substrate, excellent adhesion between the LDH layer and the substrate is ensured.
- The pretreatment layer obtained via the thermal carbonate solution treatment possesses a distinctive upright-growth morphology of LDH, which greatly enhances the adhesion strength of subsequent coatings.
- The upright growth morphology also imparts a porous structure to the pretreatment layer, ensuring a high loading capacity for corrosion inhibitors.
- The formation process of the LDH conversion coating is remarkably fast; a well-formed coating can be achieved within just a few minutes.
These features collectively contribute to the superior performance of the LDH conversion coating, laying a solid foundation for constructing a highly corrosion-resistant and strongly adherent complete coating system. The unique combination of rapid processing, excellent adhesion, enhanced porosity for inhibitor loading, and upright-growth morphology makes the LDH pretreatment layer particularly suitable for applications requiring high-performance protective coatings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30173491/s1, Figure S1: (a) TEM image and (b) EDS point scan results of LDH coating; Figure S2: (a) Tafel plots and (b) fitting results of ZAM steel in 3.5 wt.% NaCl solution (with and without NaVO3); (c) Optical images of ZAM steel after immersion in 3.5 wt.% NaCl + 0.01 M NaVO3 solution for 24 h; (d) Optical images of ZAM steel after immersion in 3.5 wt.% NaCl solution for 24 h; Figure S3: The surface topography of LDH and LDH/VOx samples following cross-hatch adhesion testing with tape removal; Figure S4: Optical images of the intact commercial coating samples immersed in 3.5 wt.% NaCl solution during 90 days; Figure S5: Pull-off test results of different commercial coating samples.
Author Contributions
J.-M.H.: project administration, investigation, funding acquisition. L.Y.: writing—original draft, methodology, formal analysis, data curation, conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Zhejiang Provincial Natural Science Foundation of China (LZ24E010001) and Zhejiang Province High-level Talent Supporting Program (2022R52001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank Fang Chen and Jin Zhuang from the Chemistry Instrumentation Centre, Zhejiang University, for their support with electron microscopy.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ZAM | Zn-Al-Mg |
| LDH | Layered double hydroxides |
References
- Cai, Y.; Xu, Y.; Zhao, Y.; Ma, X. Atmospheric corrosion prediction: A review. Corros. Rev. 2020, 38, 299–321. [Google Scholar] [CrossRef]
- Brown, R.K.; Schmidt, U.C.; Harnisch, F.; Schröder, U. Combining hydrogen evolution and corrosion data—A case study on the economic viability of selected metal cathodes in microbial electrolysis cells. J. Power Sources 2017, 356, 473–483. [Google Scholar] [CrossRef]
- Sen, S.; Chatterjee, A.; Ramakanth, D.; Singh, S.; Maji, P.K. Recent advances in cathodic electrodeposition coatings with special reference to resin materials: A comprehensive review. Prog. Org. Coat. 2024, 190, 108387. [Google Scholar] [CrossRef]
- Olajire, A.A. Recent advances on organic coating system technologies for corrosion protection of offshore metallic structures. J. Mol. Liq. 2018, 269, 572–606. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, J.-B.; Hu, J.-M. Anticorrosive painting system constructed on a porous silica pretreatment layer prepared with the aid of alkaline catalysis. Prog. Org. Coat. 2021, 154, 106178. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.-H.; Hu, J.-M. A novel and facile method for constructing micro-nano porous phytic acid pretreatment layer on metal surface. Corros. Sci. 2021, 186, 109464. [Google Scholar] [CrossRef]
- Wang, J.; Wu, L.-K.; Zhou, J.-H.; Hu, J.-M.; Zhang, J.-Q.; Cao, C.-N. Construction of a novel painting system using electrodeposited SiO2 film as the pretreatment layer. Corros. Sci. 2013, 68, 57–65. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Jin, X.-H.; Hu, J.-M. Electrodeposited silica films post-treated with organosilane coupling agent as the pretreatment layers of organic coating system. Corros. Sci. 2016, 106, 127–136. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Kuznetsova, A.; Maltanava, H.M.; Poznyak, S.K.; Ferreira, M.G.S.; Zheludkevich, M.L. Corrosion protection of zinc by LDH conversion coatings. Corros. Sci. 2024, 229, 111889. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Liu, L.; You, L.; Wang, J.; Xiang, R. Effect of cement-based composite pellets on phosphorus removal and microbial community structure in eutrophic water. Environ. Technol. Innov. 2024, 34, 103631. [Google Scholar] [CrossRef]
- Ponomareva, M.; Duchoslav, J.; Stifter, D.; Kogler, M.; Miano, D.; Akbari, E.; Bleij, A.; Luckeneder, G.; Singewald, T.; Valtiner, M. Effect of Surface Pretreatments on the Formation of Zr-Based Conversion Layers on Zn–Al–Mg Alloy-Coated Steel. Mater. Corros. 2024, 76, 510–518. [Google Scholar] [CrossRef]
- Maeda, S. Steel surface chemistry affecting the performance of organic coatings. Prog. Org. Coat. 1983, 11, 1–18. [Google Scholar] [CrossRef]
- Wang, X.; Jain, A.; Chen, B.; Wang, Y.; Jin, Q.; Yugandhar, P.; Xu, Y.; Sun, S.; Hu, F. Differential efficacy of water lily cultivars in phytoremediation of eutrophic water contaminated with phosphorus and nitrogen. Plant Physiol. Biochem. 2022, 171, 139–146. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, Y.; Wei, Y.; Zhang, Z.; Zhang, Y. LDH-Based “Smart” Films for Corrosion Sensing and Protection. Materials 2023, 16, 3483. [Google Scholar] [CrossRef] [PubMed]
- Tedim, J.; Bastos, A.C.; Kallip, S.; Zheludkevich, M.L.; Ferreira, M.G.S. Corrosion protection of AA2024-T3 by LDH conversion films. Analysis of SVET results. Electrochim. Acta 2016, 210, 215–224. [Google Scholar] [CrossRef]
- Tan, J.K.E.; Balan, P.; Birbilis, N. Advances in LDH coatings on Mg alloys for biomedical applications: A corrosion perspective. Appl. Clay Sci. 2021, 202, 105948. [Google Scholar] [CrossRef]
- Imanieh, I.; Afshar, A. Corrosion protection of aluminum by smart coatings containing layered double hydroxide (LDH) nanocontainers. J. Mater. Res. Technol. 2019, 8, 3004–3023. [Google Scholar] [CrossRef]
- Aminifazl, A.; Karunarathne, D.J.; Golden, T.D. Synthesis of Silane Functionalized LDH-Modified Nanopowders to Improve Compatibility and Enhance Corrosion Protection for Epoxy Coatings. Materials 2024, 29, 819. [Google Scholar] [CrossRef]
- Ismail, N.A.; Shakoor, R.A.; Al-Qahtani, N.; Kahraman, R. Multilayered LDH/Microcapsule Smart Epoxy Coating for Corrosion Protection. ACS Omega 2023, 8, 30838–30849. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Lu, L.; Chowwanonthapunya, T.; Chen, W.; Li, Z.; Ma, L.; Zhang, D. Corrosion-oriented self-healing coating based on ZnMg MOF for efficient corrosion protection of aluminum alloy via in-situ LDH film formation. Chem. Eng. J. 2024, 500, 156898. [Google Scholar] [CrossRef]
- Stephan, J.; Kasneryk, V.; Serdechnova, M.; Scharnagl, N.; Gazenbiller, E.; Vaghefinazari, B.; Volovitch, P.; Starykevich, M.; Blawert, C.; Zheludkevich, M.L. Formation of Li-Al LDH conversion layer on AA2024 alloy for corrosion protection. Appl. Surf. Sci. 2024, 659, 159919. [Google Scholar] [CrossRef]
- Song, Z.; Xie, Z.; Ding, L.; Zhang, Y.; Hu, X. Preparation of corrosion-resistant MgAl-LDH/Ni composite coating on Mg alloy AZ31B. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127699. [Google Scholar] [CrossRef]
- Mohammadi, I.; Shahrabi, T.; Mahdavian, M.; Izadi, M. Construction of an epoxy coating with excellent protection performance on the AA 2024-T3 using ion-exchange materials loaded with eco-friendly corrosion inhibitors. Prog. Org. Coat. 2022, 166, 106786. [Google Scholar] [CrossRef]
- Wu, J.; Peng, D.; He, Y.; Du, X.; Zhang, Z.; Zhang, B.; Li, X.; Huang, Y. In Situ Formation of Decavanadate-Intercalated Layered Double Hydroxide Films on AA2024 and their Anti-Corrosive Properties when Combined with Hybrid Sol Gel Films. Materials 2017, 10, 426. [Google Scholar] [CrossRef]
- Yan, T.; Xu, S.; Peng, Q.; Zhao, L.; Zhao, X.; Lei, X.; Zhang, F. Self-Healing of Layered Double Hydroxide Film by Dissolution/Recrystallization for Corrosion Protection of Aluminum. J. Electrochem. Soc. 2013, 160, C480. [Google Scholar] [CrossRef]
- Farghali, M.A.; Selim, A.M.; Khater, H.F.; Bagato, N.; Alharbi, W.; Alharbi, K.H.; Taha Radwan, I. Optimized adsorption and effective disposal of Congo red dye from wastewater: Hydrothermal fabrication of MgAl-LDH nanohydrotalcite-like materials. Arabian J. Chem. 2022, 15, 104171. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, W. Removal of borate by layered double hydroxides prepared through microwave-hydrothermal method. Water Process Eng. 2017, 17, 271–276. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, H.; Peng, S.; Li, D.; Gao, H.; Wang, D. Performance and mechanisms of wastewater sludge conditioning with slag-based hydrotalcite-like minerals (Ca/Mg/Al-LDH). Water Res. 2020, 169, 115265. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Yu, L.; Hu, J.-M. In-situ Zn-Al layered double hydroxide conversion coatings prepared on galvanized steels by a two-step electrochemical method. Corros. Sci. 2024, 233, 112057. [Google Scholar] [CrossRef]
- Thierry, D.; Persson, D.; Luckeneder, G.; Stellnberger, K.-H. Atmospheric corrosion of ZnAlMg coated steel during long term atmospheric weathering at different worldwide exposure sites. Corros. Sci. 2019, 148, 338–354. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Song, S.; Tang, L.; Zhang, H.; Zhou, H.; Fang, F. Effect of Coating on Stress Corrosion Performance of Bridge Cable Steel Wire. Coatings 2023, 13, 1339. [Google Scholar] [CrossRef]
- Persson, D.; Prosek, T.; LeBozec, N.; Thierry, D.; Luckeneder, G. Initial SO2-induced atmospheric corrosion of ZnAlMg coated steel studied with in situ Infrared Reflection Absorption Spectroscopy. Corros. Sci. 2015, 90, 276–283. [Google Scholar] [CrossRef]
- Salgueiro Azevedo, M.; Allély, C.; Ogle, K.; Volovitch, P. Corrosion mechanisms of Zn(Mg,Al) coated steel: 2. The effect of Mg and Al alloying on the formation and properties of corrosion products in different electrolytes. Corros. Sci. 2015, 90, 482–490. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Hu, L.; Huang, S.; Jin, Z.; Zhang, M.; Huang, X.; Lu, J.; Ruan, S.; Zeng, Y.-J. Multifunctional Zn–Al layered double hydroxides for surface-enhanced Raman scattering and surface-enhanced infrared absorption. Dalton Trans. 2019, 48, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Salak, A.; Lisenkov, A.D.; Zheludkevich, M.; Ferreira, M. Carbonate-Free Zn-Al (1:1) Layered Double Hydroxide Film Directly Grown on Zinc-Aluminum Alloy Coating. ECS Electrochem. Lett. 2014, 3, C9–C11. [Google Scholar] [CrossRef]
- Uan, J.-Y.; Lin, J.-K.; Tung, Y.-S. Direct growth of oriented Mg–Al layered double hydroxide film on Mg alloy in aqueous HCO3−/CO32− solution. J. Mater. Chem. 2010, 20, 761–766. [Google Scholar] [CrossRef]
- Zhou, H.; Li, J.; Li, J.; Ruan, Q.; Jin, W.; Yu, Z.; Li, W.; Chu, P.K. Calcium phosphate coating on biomedical WE43 magnesium alloy pretreated with a magnesium phosphate layer for corrosion protection. Surf. Coat. Technol. 2020, 401, 126248. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, W.; Ma, K.; Dai, C.; Wang, D.; Wang, J. In-situ growth and anticorrosion mechanism of a bilayer CaCO3/MgO coating via rapid electrochemical deposition on AZ41 Mg alloy concrete formwork. J. Mater. Res. Technol. 2023, 25, 6628–6643. [Google Scholar] [CrossRef]
- Hu, J.-M.; Zhang, J.-T.; Zhang, J.-Q.; Cao, C.-N. Corrosion electrochemical characteristics of red iron oxide pigmented epoxy coatings on aluminum alloys. Corros. Sci. 2005, 47, 2607–2618. [Google Scholar] [CrossRef]
- Li, L.-X.; Xie, Z.-H.; Fernandez, C.; Wu, L.; Cheng, D.; Jiang, X.-H.; Zhong, C.-J. Development of a thiophene derivative modified LDH coating for Mg alloy corrosion protection. Electrochim. Acta 2020, 330, 135186. [Google Scholar] [CrossRef]
- Ahmadi, M.; Salgın, B.; Kooi, B.J.; Pei, Y. Genesis and mechanism of microstructural scale deformation and cracking in ZnAlMg coatings. Mater. Des. 2020, 186, 108364. [Google Scholar] [CrossRef]
- Amanian, S.; Naderi, R.; Mahdavian, M. The Role of an In-Situ Grown Zn-Al Layered Double Hydroxide Conversion Coating in the Protective Properties of Epoxy Coating on Galvanized Steel. J. Electrochem. Soc. 2022, 169, 031511. [Google Scholar] [CrossRef]
- Rybka, K.; Matusik, J.; Kuligiewicz, A.; Leiviskä, T.; Cempura, G. Surface chemistry and structure evaluation of Mg/Al and Mg/Fe LDH derived from magnesite and dolomite in comparison to LDH obtained from chemicals. Appl. Surf. Sci. 2021, 538, 147923. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Hamad, S.M.; Ganjali, M.R.; Aghazadeh, M.; Torre, L.; Puglia, D.; Saeb, M.R. Curing epoxy with Mg-Al LDH nanoplatelets intercalated with carbonate ion. Prog. Org. Coat. 2019, 136, 105278. [Google Scholar] [CrossRef]
- Karami, Z.; Aghazadeh, M.; Jouyandeh, M.; Zarrintaj, P.; Vahabi, H.; Ganjali, M.R.; Torre, L.; Puglia, D.; Saeb, M.R. Epoxy/Zn-Al-CO3 LDH nanocomposites: Curability assessment. Prog. Org. Coat. 2020, 138, 105355. [Google Scholar] [CrossRef]
- Farid, R.; Sarkar, D.K.; Das, S. Studies of Corrosion Inhibition Performance of Inorganic Inhibitors for Aluminum Alloy. Materials 2025, 18, 595. [Google Scholar] [CrossRef] [PubMed]
- Kharitonov, D.S.; Örnek, C.; Claesson, P.M.; Sommertune, J.; Zharskii, I.M.; Kurilo, I.I.; Pan, J. Corrosion Inhibition of Aluminum Alloy AA6063-T5 by Vanadates: Microstructure Characterization and Corrosion Analysis. J. Electrochem. Soc. 2018, 165, C116. [Google Scholar] [CrossRef]
- Kutlu, B.; Leuteritz, A.; Boldt, R.; Jehnichen, D.; Heinrich, G. Effects of LDH synthesis and modification on the exfoliation and introduction of a robust anion-exchange procedure. Chem. Eng. J. 2014, 243, 394–404. [Google Scholar] [CrossRef]
- Zhao, X.-J.; Zhu, Y.-Q.; Xu, S.-M.; Liu, H.-M.; Yin, P.; Feng, Y.-L.; Yan, H. Anion exchange behavior of MIIAl layered double hydroxides: A molecular dynamics and DFT study. Phys. Chem. Chem. Phys. 2020, 22, 19758–19768. [Google Scholar] [CrossRef] [PubMed]
- ISO 4624:2023; Paints and Varnishes—Pull-Off Test for Adhesion. International Organization for Standardization: Geneva, Switzerland, 2023.
- ISO 17872:2019; Paints and Varnishes—Guidelines for the Introduction of Scribe Marks Through Coatings on Metallic Panels for Corrosion Testing. International Organization for Standardization: Geneva, Switzerland, 2019.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).