Behavioral Selectivity: Species-Specific Effects of Nutmeg, Cinnamon, and Clove Essential Oils on Sitophilus oryzae and Its Parasitoid Lariophagus distinguendus
Abstract
1. Introduction
2. Results
2.1. Chemical Profiles of Nutmeg, Cinnamon, and Clove Essential Oils
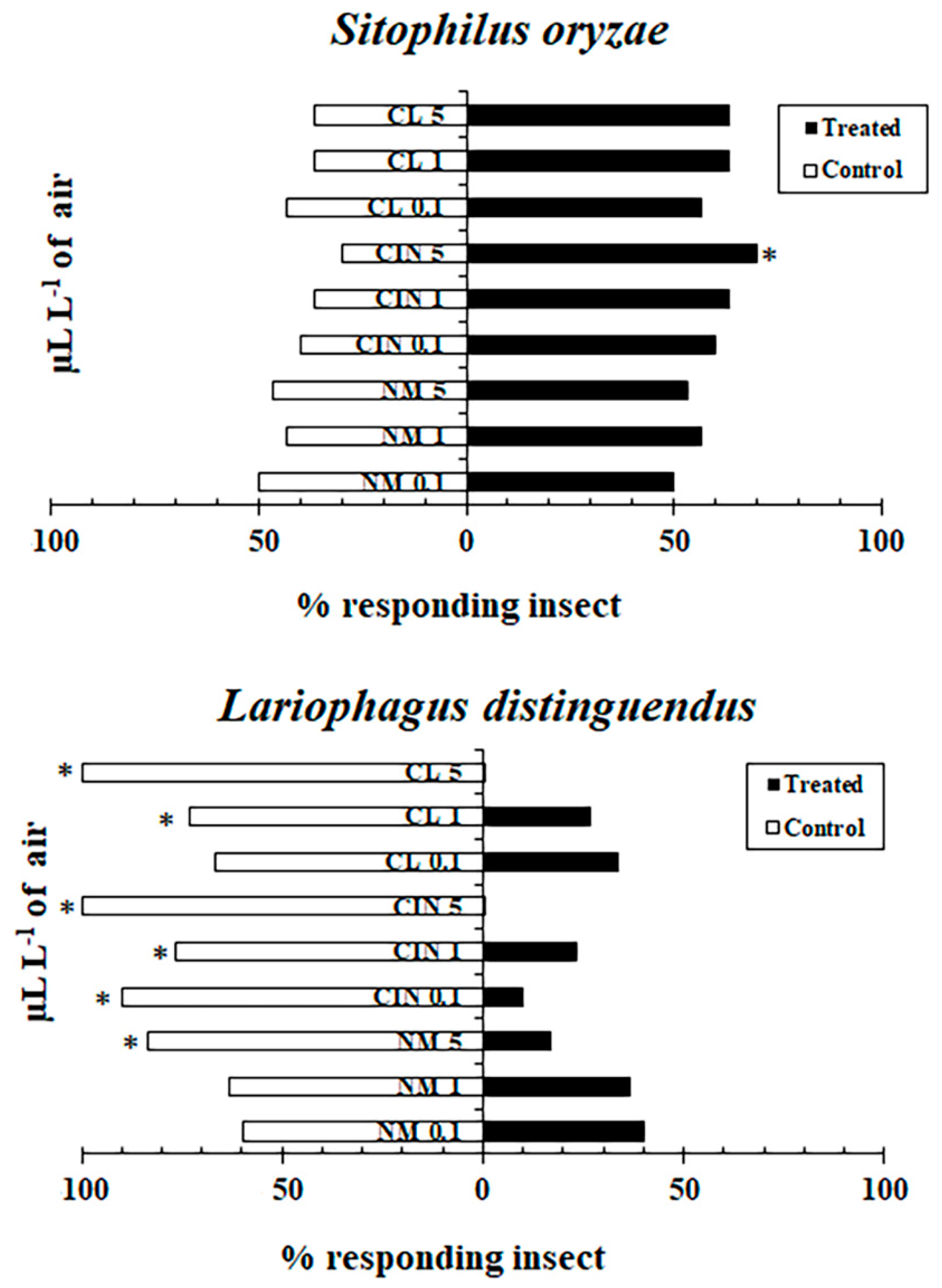
2.2. Behavioral Responses of S. oryzae and L. distinguendus to Essential Oils in a Two-Choice Olfactometer
2.3. Behavioral Responses of S. oryzae and L. distinguendus to Essential Oils in the Area Preference Method
2.4. Bioactivity of Major EO Constituents Against S. oryzae Using the Area Preference Method
3. Discussion
4. Materials and Methods
4.1. Insect Rearing Conditions
4.1.1. Sitophilus oryzae
4.1.2. Lariophagus distinguendus
4.2. Essential Oils and Chemical Standards
4.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
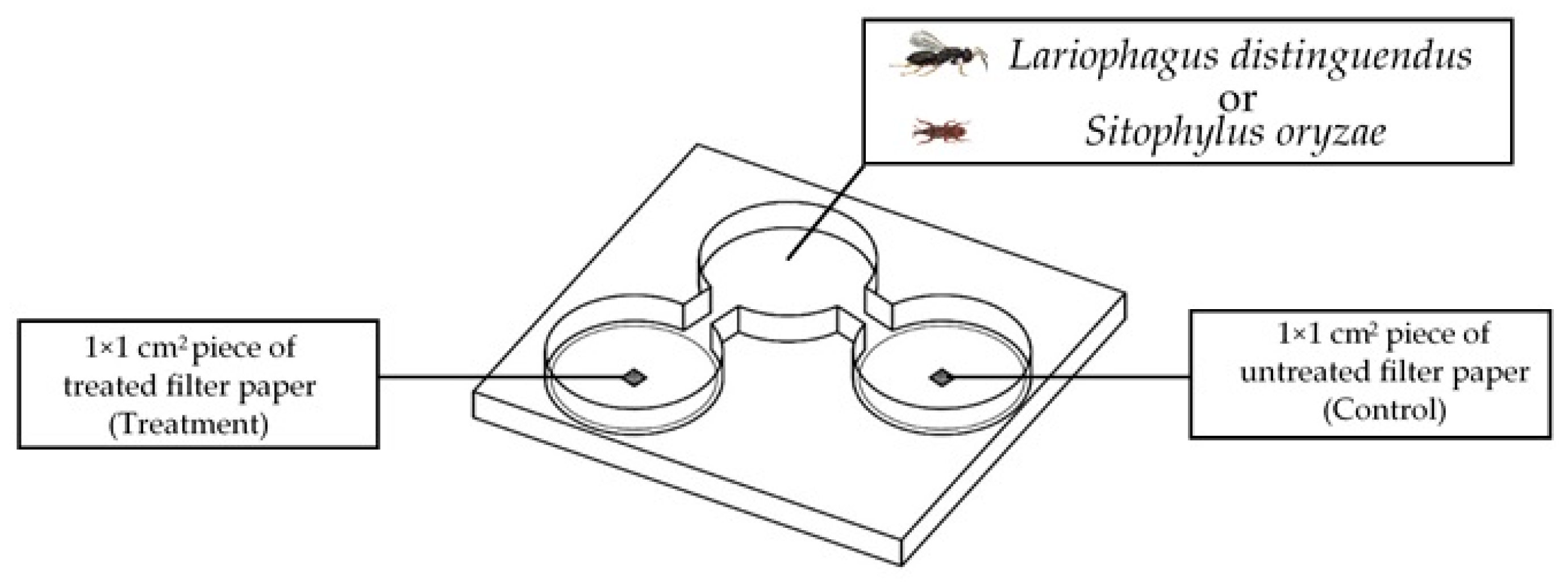
4.4. Two-Choice Olfactometer Tests
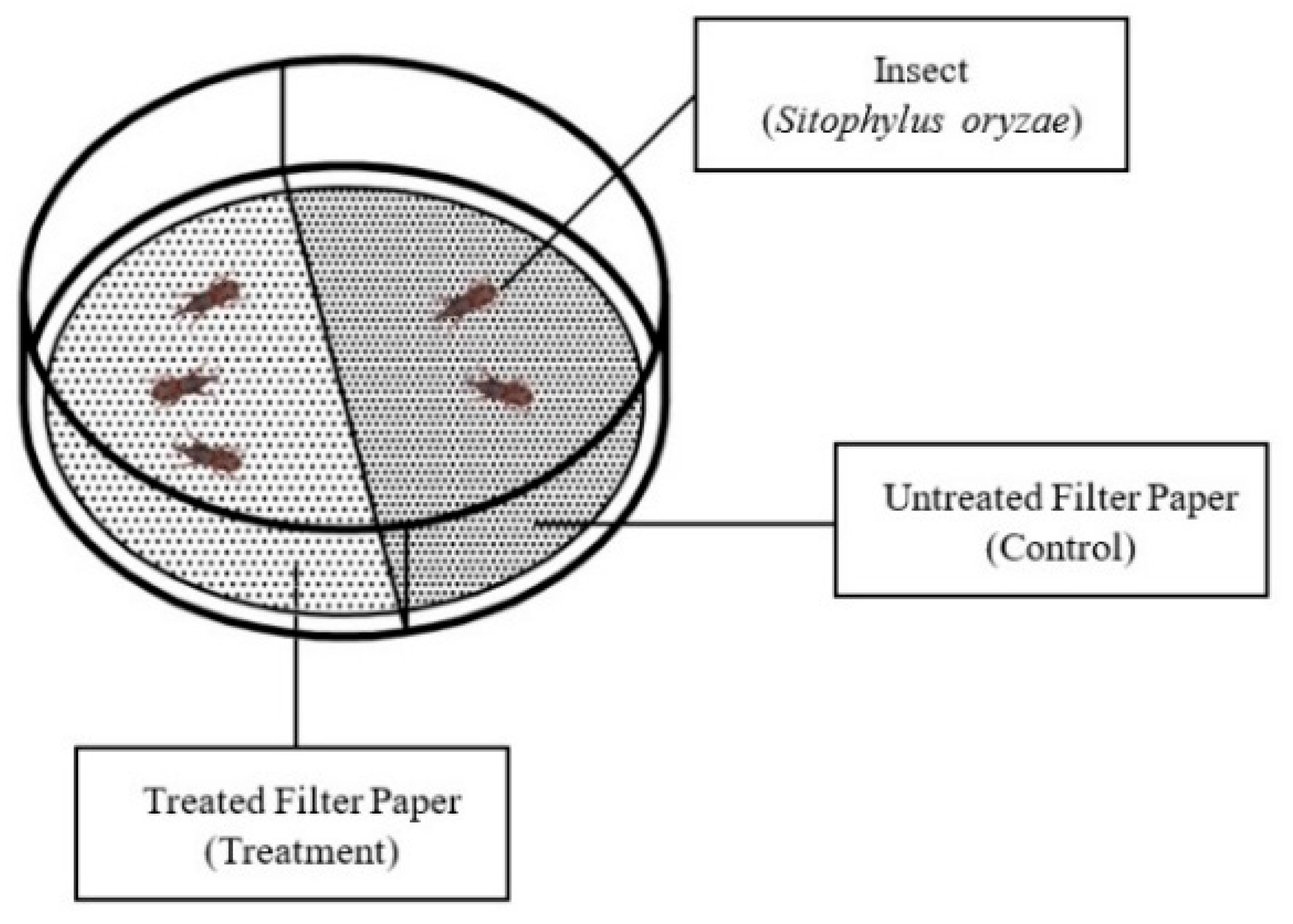
4.5. Bioactivity Assessment of EOs and EO Components Against S. oryzae Using the Area Preference Method
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APM | Area Preference Method |
| CIN | Cinnamon oil (Cinnamomum verum) |
| CL | Clove oil (Syzygium aromaticum) |
| EOs | Essential oils |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| LRI | Linear Retention Index |
| NM | Nutmeg oil (Myristica fragrans) |
| PI | Preference Index |
| RH | Relative Humidity |
| SD | Standard Deviation |
| TCB | Two-Choice Behavioral Bioassay |
References
- Food and Agriculture Organization of the United Nations. World Food and Agriculture–Statistical Yearbook 2023; FAO: Rome, Italy, 2023; p. 384. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef]
- Berhe, M.; Subramanyam, B.; Chichaybelu, M.; Demissie, G.; Abay, F.; Harvey, J. Post-harvest insect pests and their management practices for major food and export crops in East Africa: An Ethiopian case study. Insects 2022, 13, 1068. [Google Scholar] [CrossRef]
- Stathas, I.G.; Sakellaridis, A.C.; Papadelli, M.; Kapolos, J.; Papadimitriou, K.; Stathas, G.J. The effects of insect infestation on stored agricultural products and the quality of food. Foods 2023, 12, 2046. [Google Scholar] [CrossRef]
- Daglish, G.J.; Nayak, M.K.; Arthur, F.H.; Athanassiou, C.G. Insect pest management in stored grain. In Recent Advances in Stored Product Protection; Athanassiou, C.G., Arthur, F.H., Eds.; Springer: Heidelberg, Germany, 2018; pp. 45–63. [Google Scholar] [CrossRef]
- Swamy, K.C.N.; Mutthuraju, G.P.; Jagadeesh, E.; Thirumalaraju, G.T. Biology of Sitophilus oryzae (L.) (Coleoptera: Curculionidae) on stored maize grains. Curr. Biotica 2014, 8, 76–81. [Google Scholar]
- Phillips, T.W.; Jiang, X.L.; Burkholder, W.E.; Phillips, J.K.; Tran, H.Q. Behavioral responses to food volatiles by two species of stored-product coleoptera, Sitophilus oryzae (curculionidae) and Tribolium castaneum (tenebrionidae). J. Chem. Ecol. 1993, 19, 723–734. [Google Scholar] [CrossRef]
- Sharifi, S.; Mills, R.B. Developmental activities and behavior of the rice weevil inside wheat kernels. J. Econ. Entomol. 1971, 64, 1114–1118. [Google Scholar] [CrossRef]
- Soujanya, P.L.; Sekhar, J.C.; Karjagi, C.G.; Vidhyadhari, V.; Suby, S.B.; Sunil, N.; Sreelatha, D.; Chaudhary, D. Impact of harvesting time on field carry over infestation of Sitophilus oryzae (L.) (Coleoptera: Curculionidae) in different maize genotypes. Phytoparasitica 2017, 45, 485–499. [Google Scholar] [CrossRef]
- Agha, M.K.; Lee, W.; Wang, C.; Mankin, R.; Blount, A.; Bucklin, R.; Bliznyuk, N. Detection and prediction of Sitophilus oryzae infestations in triticale via visible and near-infrared spectral signatures. J. Stored Prod. Res. 2017, 72, 1–10. [Google Scholar] [CrossRef]
- Imura, O.; Sinha, R.N. Effect of infestation by Sitotroga cerealella (Lepidoptera: Gelechiidae) and Sitophilus oryzae (Coleoptera: Curculionidae) on the deterioration of bagged wheat. Environ. Entomol. 1984, 13, 1471–1477. [Google Scholar] [CrossRef]
- DiBartolomeis, M.; Kegley, S.; Mineau, P.; Radford, R.; Klein, K. An assessment of acute insecticide toxicity loading (AITL) of chemical pesticides used on agricultural land in the United States. PLoS ONE 2019, 14, e0220029. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Adarkwah, C.; Obeng-Ofori, D.; Büttner, C.; Reichmuth, C.; Schöller, M. Potential of Lariophagus distinguendus (Förster) (Hymenoptera: Pteromalidae) to suppress the maize weevil Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) in bagged and bulk stored maize. Biol. Control 2011, 60, 175–181. [Google Scholar] [CrossRef]
- Benelli, G.; Pacini, N.; Conti, B.; Canale, A. Following a scented beetle: Larval faeces as a key olfactory cue in host location of Stegobium paniceum (Coleoptera: Anobiidae) by Lariophagus distinguendus (Hymenoptera: Pteromalidae). Chemoecology 2013, 23, 129–136. [Google Scholar] [CrossRef]
- Niedermayer, S.; Pollmann, M.; Steidle, J. Lariophagus distinguendus (Hymenoptera: Pteromalidae) (Förster)—Past, present, and future: The history of a biological control method using L. distinguendus against different storage pests. Insects 2016, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Fields, P.G.; White, N.D.G. Alternatives to methyl bromide treatments for stored-product and quarantine insects. Annu. Rev. Entomol. 2002, 47, 331–359. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, J.; Chen, Z.; Chang, C.; Ma, Y.; Li, N.; Deng, M.; Mao, G.; Bao, Q.; Deng, S.; et al. Exploration of clove bud (Syzygium aromaticum) essential oil as a novel attractant against Bactrocera dorsalis (Hendel) and Its safety evaluation. Insects 2022, 13, 918. [Google Scholar] [CrossRef]
- Tian, Y.; Hogsette, J.A.; Norris, E.J.; Hu, X.P. Topical toxicity and repellency profiles of 17 essential oil components against insecticide-resistant and susceptible strains of adult Musca domestica (Diptera: Muscidae). Insects 2024, 15, 384. [Google Scholar] [CrossRef]
- Gupta, I.; Singh, R.; Muthusamy, S.; Sharma, M.; Grewal, K.; Singh, H.P.; Batish, D.R. Plant essential oils as biopesticides: Applications, mechanisms, innovations, and constraints. Plants 2023, 12, 2916. [Google Scholar] [CrossRef]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential oils and their application in food safety. Front. Sustain. Food Syst. 2021, 5. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Parichanon, P.; Ascrizzi, R.; Echeverría, M.C.; Farina, P.; Pieracci, Y.; Flamini, G.; Semprucci, F.; Guidi, L.; Grassi, E.; Gogou, T.I.; et al. The aromatic plant essential oils and their hormetic effect on Rhyzopertha dominica (Coleoptera: Bostrichidae). Crop Prot. 2025, 194, 107235. [Google Scholar] [CrossRef]
- Bedini, S.; Djebbi, T.; Ascrizzi, R.; Farina, P.; Pieracci, Y.; Echeverría, M.C.; Flamini, G.; Trusendi, F.; Ortega, S.; Chiliquinga, A.; et al. Repellence and attractiveness: The hormetic effect of aromatic plant essential oils on insect behavior. Ind. Crops Prod. 2024, 210, 118122. [Google Scholar] [CrossRef]
- Zeni, V.; Benelli, G.; Campolo, O.; Giunti, G.; Palmeri, V.; Maggi, F.; Rizzo, R.; Lo Verde, G.; Lucchi, A.; Canale, A. Toxics or lures? Biological and behavioral effects of plant essential oils on tephritidae fruit flies. Molecules 2021, 26, 5898. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Li, L.; Li, F.; Zang, L. Identification and comparative expression profiles of candidate olfactory receptors in the transcriptomes of the important egg parasitoid wasp Anastatus japonicus Ashmead (Hymenoptera: Eupelmidae). Plants 2023, 12, 915. [Google Scholar] [CrossRef]
- Ruther, J.; Schmitt, T.; Stökl, J. Editorial: Recent advances in the chemical ecology of parasitic Hymenoptera. Front. Ecol. Evol. 2023, 11, 1310233. [Google Scholar] [CrossRef]
- Cai, P.; Song, Y.; Huo, D.; Lin, J.; Zhang, H.; Zhang, Z.; Xiao, C.; Huang, F.; Ji, Q. Chemical cues induced from fly-oviposition mediate the host-seeking behaviour of Fopius arisanus (Hymenoptera: Braconidae), an effective egg parasitoid of Bactrocera dorsalis (Diptera: Tephritidae), within a Tritrophic Context. Insects 2020, 11, 231. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Lacotte, V.; Rey, M.; Peignier, S.; Mercier, P.; Rahioui, I.; Sivignon, C.; Razy, L.; Benhamou, S.; Livi, S.; Da Silva, P. Bioactivity and chemical composition of forty plant essential oils against the pea aphid Acyrthosiphon pisum revealed peppermint oil as a promising biorepellent. Ind. Crops Prod. 2023, 197, 116610. [Google Scholar] [CrossRef]
- Abenaim, L.; Mandoli, A.; Venturi, F.; Bedini, S.; Conti, B. Evaluation of a quasi-dimeric eugenol derivative as repellent against the stored grain insect pest Sitophilus oryzae (Coleoptera: Curculionidae). Pest Manag. Sci. 2022, 78, 2588–2595. [Google Scholar] [CrossRef]
- Ninkovic, V.; Markovic, D.; Rensing, M. Plant volatiles as cues and signals in plant communication. Plant Cell Environ. 2020, 44, 1030–1043. [Google Scholar] [CrossRef]
- Deletre, E.; Chandre, F.; Williams, L.; Duménil, C.; Menut, C.; Martin, T. Electrophysiological and behavioral characterization of bioactive compounds of the Thymus vulgaris, Cymbopogon winterianus, Cuminum cyminum and Cinnamomum zeylanicum essential oils against Anopheles gambiae and prospects for their use as bednet treatments. Parasites Vectors 2015, 8, 316. [Google Scholar] [CrossRef]
- Sarma, R.; Adhikari, K.; Mahanta, S.; Khanikor, B. Combinations of plant essential oil based terpene compounds as larvicidal and adulticidal agent against Aedes aegypti (Diptera: Culicidae). Sci. Rep. 2019, 9, 9471. [Google Scholar] [CrossRef] [PubMed]
- De Brito Sanchez, M.G.; Lorenzo, E.; Su, S.; Liu, F.; Zhan, Y.; Giurfa, M. The tarsal taste of honey bees: Behavioral and electrophysiological analyses. Front. Behav. Neurosci. 2014, 8, 25. [Google Scholar] [CrossRef]
- Galizia, C.G.; Rössler, W. Parallel olfactory systems in insects: Anatomy and function. Annu. Rev. Entomol. 2009, 55, 399–420. [Google Scholar] [CrossRef]
- Akotsen-Mensah, C.; Blaauw, B.R.; Rivera, M.J.; Rodriguez-Saona, C.; Nielsen, A.L. Behavioral response of Halyomorpha halys (Hemiptera: Pentatomidae) and its egg parasitoid Trissolcus japonicus (Hymenoptera: Scelionidae) to host plant odors. Front. Ecol. Evol. 2021, 9, 696814. [Google Scholar] [CrossRef]
- Ahmed, Q.; Agarwal, M.; Al-Obaidi, R.; Wang, P.; Ren, Y. Evaluation of aphicidal effect of essential oils and their synergistic effect against Myzus persicae Sulzer (Hemiptera: Aphididae). Molecules 2021, 26, 3055. [Google Scholar] [CrossRef]
- Filomeno, C.A.; Barbosa, L.C.A.; Teixeira, R.R.; Pinheiro, A.L.; De Sá Farias, E.; Ferreira, J.S.; Picanço, M.C. Chemical diversity of essential oils of Myrtaceae species and their insecticidal activity against Rhyzopertha dominica. Crop Prot. 2020, 137, 105309. [Google Scholar] [CrossRef]
- Giunti, G.; Benelli, G.; Palmeri, V.; Laudani, F.; Ricupero, M.; Ricciardi, R.; Maggi, F.; Lucchi, A.; Guedes, R.N.C.; Desneux, N.; et al. Non-target effects of essential oil-based biopesticides for crop protection: Impact on natural enemies, pollinators, and soil invertebrates. Biol. Control 2022, 176, 105071. [Google Scholar] [CrossRef]
- Sulg, S.; Kaasik, R.; Kallavus, T.; Veromann, E. Toxicity of essential oils on cabbage seedpod weevil (Ceutorhynchus obstrictus) and a model parasitoid (Nasonia vitripennis). Front. Agron. 2023, 5, 1107201. [Google Scholar] [CrossRef]
- Yuan, J.S.; Köllner, T.G.; Wiggins, G.; Grant, J.; Degenhardt, J.; Chen, F. Molecular and genomic basis of volatile-mediated indirect defense against insects in rice. Plant J. 2008, 55, 491–503. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, Q.; Huang, L.; Athanassiou, C.G.; Maggi, F.; D’Isita, I.; Liu, Y.; Pistillo, O.M.; Miao, M.; Germinara, G.S.; et al. Attraction of Sitophilus oryzae (L.) (Coleoptera: Curculionidae) to the semiochemical volatiles of stored rice materials. J. Pest Sci. 2023, 97, 73–85. [Google Scholar] [CrossRef]
- Tang, Q.; Yang, T.; Jiang, J. Herbivore-induced rice grain volatiles affect attraction behavior of herbivore enemies. Interciencia 2016, 41, 319–324. [Google Scholar]
- Wright, G.A. Different thresholds for detection and discrimination of odors in the honey bee (Apis mellifera). Chem. Senses 2004, 29, 127–135. [Google Scholar] [CrossRef]
- Ayllón-Gutiérrez, R.; Díaz-Rubio, L.; Montaño-Soto, M.; Del Pilar Haro-Vázquez, M.; Córdova-Guerrero, I. Applications of plant essential oils in pest control and their encapsulation for controlled release: A review. Agriculture 2024, 14, 1766. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2006, 52, 375–400. [Google Scholar] [CrossRef]
- Navarro-Llopis, V.; Alfaro, F.; Domínguez, J.; Sanchis, J.; Primo, J. Evaluation of traps and lures for mass trapping of Mediterranean fruit fly in citrus groves. J. Econ. Entomol. 2008, 101, 126–131. [Google Scholar] [CrossRef]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex pheromones and their impact on pest management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides in the twenty-first century—Fulfilling their promise? Annu. Rev. Entomol. 2019, 65, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Bell, C. A review of insect responses to variations encountered in the managed storage environment. J. Stored Prod. Res. 2014, 59, 260–274. [Google Scholar] [CrossRef]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Venthur, H.; Zhou, J. Odorant receptors and odorant-binding proteins as insect pest control Targets: A comparative analysis. Front. Physiol. 2018, 9, 1163. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Standards and Technology (NIST). NIST/EPA/NIH Mass Spectral Library (NIST Standard Reference Database Number 69); U.S. Department of Commerce: Gaithersburg, MD, USA, 2014. [CrossRef]
- Zimmermann, R.C.; de Carvalho Aragão, C.E.; de Araújo, P.J.P.; Benatto, A.; Chaaban, A.; Martins, C.E.N.; Zawadneak, M.A.C. Insecticide activity and toxicity of essential oils against two stored-product insects. Crop Prot. 2021, 144, 105575. [Google Scholar] [CrossRef]
- Lee, H.; Hong, S.J.; Hasan, N.; Baek, E.J.; Kim, J.T.; Kim, Y.; Park, M. Repellent efficacy of essential oils and plant extracts against Tribolium castaneum and Plodia interpunctella. Entomol. Res. 2020, 50, 450–459. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Fuentes-Lopez, K.; Stashenko, E.E.; Olivero-Verbel, J. Chemical composition, repellent action, and toxicity of essential oils from Lippia origanoides, Lippia alba chemotypes, and Pogostemon cablin on adults of Ulomoides dermestoides (Coleoptera: Tenebrionidae). Insects 2023, 14, 41. [Google Scholar] [CrossRef] [PubMed]
| Compounds | LRI 1 | LRI 2 | Class | Relative Abundance ± Standard Deviation | ||
|---|---|---|---|---|---|---|
| Nutmeg | Cinnamon | Clove | ||||
| α-thujene | 922 | 933 | mh | 1.1 ± 0.02 | - 3 | - |
| α-pinene | 933 | 941 | mh | 17.0 ± 0.60 | 0.1 ± 0.02 | - |
| camphene | 955 | 955 | mh | 0.3 ± 0.01 | - | - |
| benzaldehyde | 959 | 962 | nt | - | 0.1 ± 0.01 | - |
| sabinene | 974 | 976 | mh | 14.1 ± 0.45 | - | - |
| β-pinene | 977 | 975 | mh | 13.4 ± 0.42 | - | - |
| myrcene | 991 | 991 | mh | 1.0 ± 0.03 | - | - |
| α-phellandrene | 1006 | 1004 | mh | 0.2 ± 0.00 | 0.3 ± 0.06 | - |
| δ-3-carene | 1011 | 1012 | mh | 1.0 ± 0.03 | - | - |
| α-terpinene | 1017 | 1020 | mh | 0.4 ± 0.01 | 0.2 ± 0.03 | - |
| p-cymene | 1024 | 1027 | mh | 4.0 ± 0.10 | 0.7 ± 0.12 | - |
| limonene | 1029 | 1031 | mh | 4.2 ± 0.09 | - | |
| β-phellandrene | 1029 | 1032 | mh | - | 0.9 ± 0.15 | - |
| 1,8-cineole | 1032 | 1035 | om | 0.2 ± 0.01 | - | - |
| 2-heptyl acetate | 1045 | 1043 | nt | - | - | 0.1 ± 0.02 |
| γ-terpinene | 1058 | 1062 | mh | 0.8 ± 0.04 | - | - |
| cis-sabinene hydrate | 1066 | 1066 | om | 0.3 ± 0.00 | - | - |
| terpinolene | 1089 | 1088 | mh | 0.5 ± 0.01 | - | - |
| trans-sabinene hydrate | 1098 | 1099 | om | 0.3 ± 0.02 | - | - |
| linalool | 1101 | 1101 | om | 0.2 ± 0.01 | 1.4 ± 0.22 | - |
| hydroxycinnamaldehyde | 1162 | - | pp | - | 0.3 ± 0.03 | - |
| 4-terpineol | 1177 | 1179 | om | 5.0 ± 0.02 | 0.1 ± 0.02 | - |
| p-cymen-8-ol | 1185 | 1185 | om | 0.3 ± 0.01 | - | - |
| α-terpineol | 1191 | 1191 | om | - | 0.3 ± 0.04 | - |
| methyl salicylate | 1194 | 1192 | nt | - | - | 0.3 ± 0.02 |
| (Z)-cinnamaldehyde | 1219 | 1219 | pp | - | 0.3 ± 0.01 | - |
| (E)-cinnamaldehyde | 1270 | 1270 | pp | - | 78.5 ± 1.58 | - |
| trans-ascaridol glycol | 1271 | 1273 | om | 0.2 ± 0.00 | - | - |
| carvacrol | 1302 | 1300 | om | 0.2 ± 0.01 | - | - |
| eugenol | 1356 | 1359 | pp | - | 5.2 ± 0.43 | 89.2 ± 0.2 |
| α-copaene | 1376 | 1377 | sh | - | 0.6 ± 0.13 | - |
| methyl eugenol | 1405 | 1402 | pp | 0.2 ± 0.02 | - | - |
| β-caryophyllene | 1419 | 1420 | sh | - | 4.3 ± 0.97 | 3.9 ± 0.04 |
| (E)-cinnamyl acetate | 1449 | 1448 | pp | - | 3.8 ± 0.05 | |
| α-humulene | 1453 | 1455 | sh | - | 0.8 ± 0.18 | 0.5 ± 0.01 |
| (E)-methyl isoeugenol | 1492 | 1495 | pp | 0.1 ± 0.00 | - | - |
| (E)-o-methoxycinnamaldehyde | 1505 | 1505 | pp | - | 0.4 ± 0.14 | - |
| myristicin | 1521 | 1520 | pp | 33.0 ± 1.59 | - | - |
| eugenol acetate | 1528 | 1525 | pp | - | - | 5.8 ± 0.14 |
| elemicin | 1558 | 1559 | pp | 1.8 ± 0.10 | - | - |
| caryophyllene oxide | 1581 | 1581 | os | - | 0.5 ± 0.12 | 0.2 ± 0.02 |
| tetradecanal | 1613 | 1611 | nt | - | 0.2 ± 0.04 | - |
| benzyl benzoate | 1763 | 1762 | nt | - | 1.1 ± 0.04 | - |
| Monoterpene hydrocarbons (mh) | 58.0 ± 1.78 | 2.2 ± 0.37 | - | |||
| Oxygenated monoterpenes (om) | 6.7 ± 0.04 | 1.8 ± 0.28 | - | |||
| Sesquiterpene hydrocarbons (sh) | - | 5.7 ± 1.28 | 4.4 ± 0.05 | |||
| Oxygenated sesquiterpenes (os) | - | 0.5 ± 0.12 | 0.2 ± 0.02 | |||
| Phenylpropanoids (pp) | 35.1 ± 1.71 | 88.5 ± 2.11 | 95.0 ± 0.06 | |||
| Other non-terpene derivatives (nt) | - | 1.4 ± 0.08 | 0.4 ± 0.00 | |||
| Total identified (%) | 99.8 ± 0.04 | 100.1 ± 0.01 | 100.0 ± 0.00 | |||
| EO | Preference Index (PI; Mean ± SD) | ||
| Concentration of EO (µL L−1 of Air Equivalent) | |||
| 0.94 | 1.89 | 3.77 | |
| Cinnamon | 0.38 ± 0.16 A | 0.40 ± 0.23 A | 0.50 ± 0.16 A |
| Clove | 0.40 ± 0.21 A | 0.48 ± 0.16 A | 0.56 ± 0.11 A |
| Nutmeg | 0.14 ± 0.11 B | 0.24 ± 0.18 B | 0.22 ± 0.19 B |
| Pure Component | Originating Essential Oil | Preference Index (PI; Mean ± SD) | ||
| Concentration of Pure Components (µL L−1 Air Equivalent) | ||||
| 0.94 | 1.89 | 3.77 | ||
| Myristicin | NM | 0.73 ± 0.12 | 0.10 ± 0.26 | −0.33 ± 0.25 |
| β-pinene | NM | 0.40 ± 0.34 | −0.02 ± 0.18 | −0.18 ± 0.43 |
| α-pinene | CIN, NM | 0.38 ± 0.18 | 0.04 ± 0.62 | −0.26 ± 0.15 |
| Limonene | NM | 0.20 ± 0.10 | 0.08 ± 0.23 | −0.20 ± 0.07 |
| Cinnamaldehyde | CIN | 0.16 ± 0.24 | −0.16 ± 0.27 | −0.20 ± 0.16 |
| β-caryophyllene | CIN, CL | 0.02 ± 0.50 | −0.16 ± 0.23 | −0.24 ± 0.17 |
| 1,8-cineole | NM | −0.02 ± 0.13 | 0.04 ± 0.19 | −0.14 ± 0.11 |
| Sabinene | NM | −0.06 ± 0.30 | −0.16 ± 0.18 | −0.22 ± 0.43 |
| Terpinolene | NM | −0.14 ± 0.09 | −0.26 ± 0.17 | −0.36 ± 0.21 |
| Linalool | NM, CIN | −0.18 ± 0.08 | −0.30 ± 0.22 | −0.24 ± 0.23 |
| Methyl eugenol | NM | −0.26 ± 0.05 | −0.26 ± 0.11 | −0.36 ± 0.11 |
| α-terpineol | CIN | −0.48 ± 0.31 | −0.48 ± 0.27 | −0.50 ± 0.07 |
| Eugenol | CIN, CL | −0.58 ± 0.33 | −0.66 ± 0.18 | −0.70 ± 0.10 |
| Positive Control | ||||
| MR-08 | - | −0.18 ± 0.26 | −0.26 ± 0.09 | −0.42 ± 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parichanon, P.; Ascrizzi, R.; Flamini, G.; Pieracci, Y.; Echeverría, M.C.; Ortega-Andrade, S.; Conti, B. Behavioral Selectivity: Species-Specific Effects of Nutmeg, Cinnamon, and Clove Essential Oils on Sitophilus oryzae and Its Parasitoid Lariophagus distinguendus. Molecules 2025, 30, 3627. https://doi.org/10.3390/molecules30173627
Parichanon P, Ascrizzi R, Flamini G, Pieracci Y, Echeverría MC, Ortega-Andrade S, Conti B. Behavioral Selectivity: Species-Specific Effects of Nutmeg, Cinnamon, and Clove Essential Oils on Sitophilus oryzae and Its Parasitoid Lariophagus distinguendus. Molecules. 2025; 30(17):3627. https://doi.org/10.3390/molecules30173627
Chicago/Turabian StyleParichanon, Prangthip, Roberta Ascrizzi, Guido Flamini, Ylenia Pieracci, Maria Cristina Echeverría, Sania Ortega-Andrade, and Barbara Conti. 2025. "Behavioral Selectivity: Species-Specific Effects of Nutmeg, Cinnamon, and Clove Essential Oils on Sitophilus oryzae and Its Parasitoid Lariophagus distinguendus" Molecules 30, no. 17: 3627. https://doi.org/10.3390/molecules30173627
APA StyleParichanon, P., Ascrizzi, R., Flamini, G., Pieracci, Y., Echeverría, M. C., Ortega-Andrade, S., & Conti, B. (2025). Behavioral Selectivity: Species-Specific Effects of Nutmeg, Cinnamon, and Clove Essential Oils on Sitophilus oryzae and Its Parasitoid Lariophagus distinguendus. Molecules, 30(17), 3627. https://doi.org/10.3390/molecules30173627











