Exploring Germination to Unlock the Nutritional Potential of Sorghum (Sorghum bicolor)
Abstract
1. Introduction
2. Results and Discussion
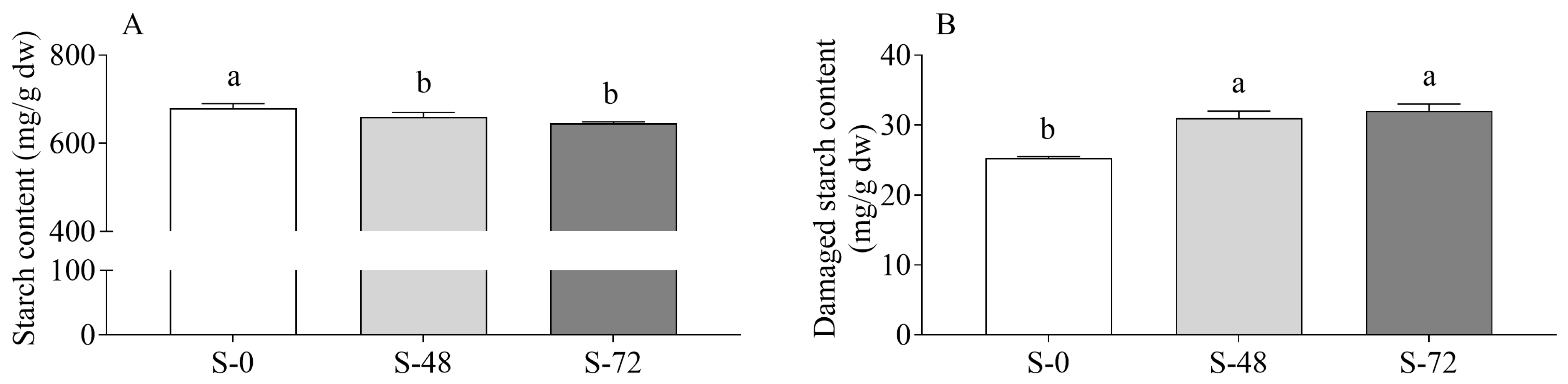
2.1. Effects of Sprouting on Sorghum Starch
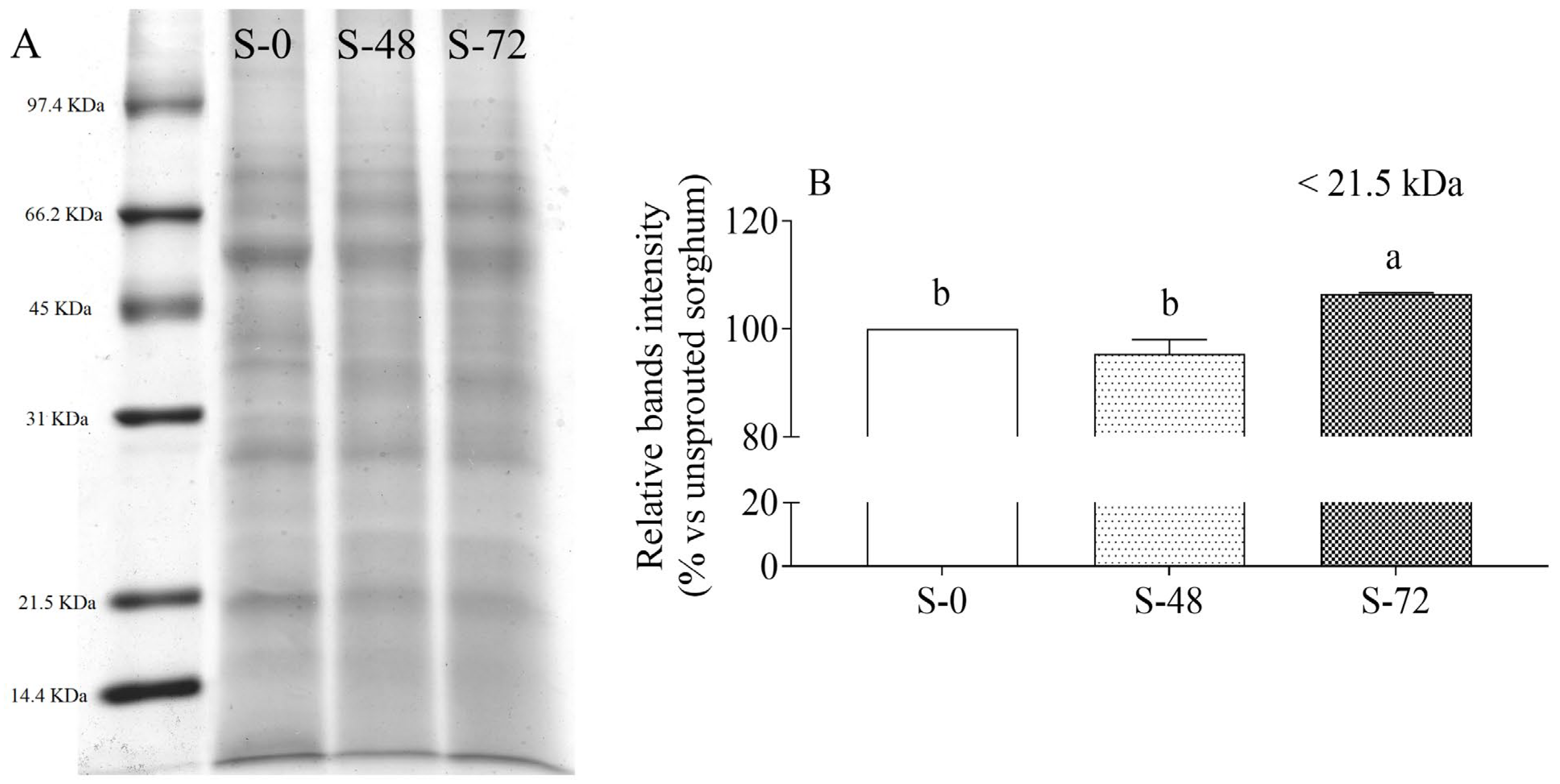
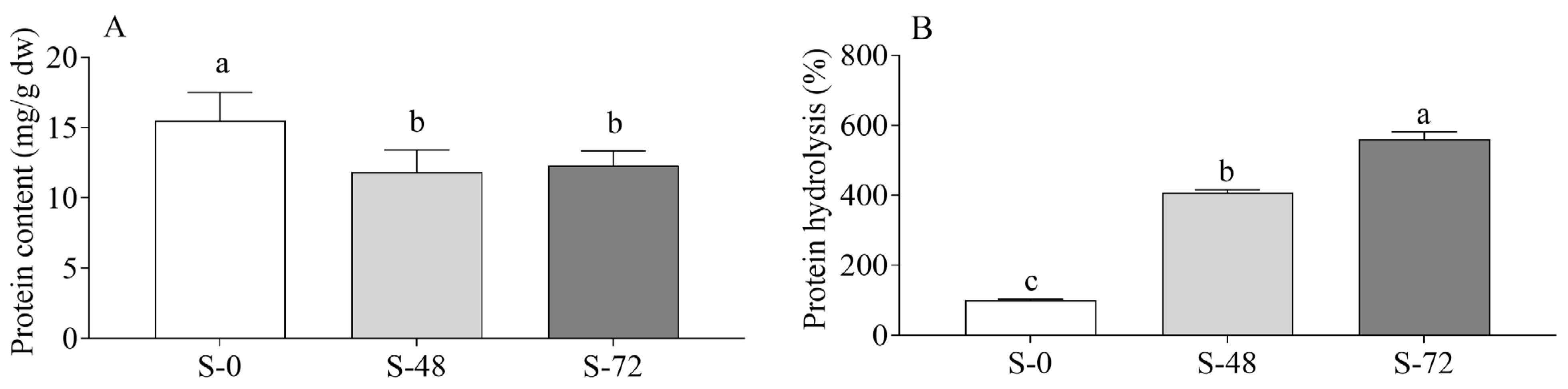
2.2. Effects of Sprouting on Sorghum Proteins
2.3. Effects of Sprouting on Sorghum Lipids and Lipid Oxidative Status
2.4. Effects of Sprouting on Sorghum Antioxidants
2.5. Effects of Sprouting on Sorghum Anti-Nutritional Factors
3. Materials and Methods
3.1. Materials
3.2. Sprouting Process
3.3. Total Starch Content
3.4. Damaged Starch Content
3.5. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) of Total Proteins
3.6. Protein Content and Hydrolysis Degree
3.7. Fatty Acid Content and Composition
3.8. Lipid Peroxidation
3.9. Phytic Acid Content
3.10. Pepsin Activity
3.11. Tocol Extraction and Determination via HPLC–FLD
3.12. Extraction and Determination of Free and Bound Phenolic Compounds
3.13. Total Antioxidant Capacity (TAC)
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AACC | American Association of Cereal Chemists |
| HPLC | High-Performance Liquid Chromatography |
| Da | Dalton |
| TRIS | Tris(hydroxymethyl)aminomethane |
References
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Palavecino, P.M.; Curti, M.I.; Bustos, M.C.; Penci, M.C.; Ribotta, P.D. Sorghum Pasta and Noodles: Technological and Nutritional Aspects. Plant Foods Hum. Nutr. 2020, 75, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.L.; Awika, J.M. Sorghum polyphenols and other bioactive components as functional and health promoting food ingredients. J. Cereal Sci. 2018, 84, 112–124. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Fang, Z. Sorghum Grain: From Genotype, Nutrition, and Phenolic Profile to Its Health Benefits and Food Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2025–2046. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Yones, H.A.; Karim, N.; Taha, E.M.; Chen, W. Potential processing technologies for developing sorghum-based food products: An update and comprehensive review. Trends Food Sci. Technol. 2021, 110, 168–182. [Google Scholar] [CrossRef]
- Rodríguez-España, M.; Figueroa-Hernández, C.Y.; Figueroa-Cárdenas, J.D.; Rayas-Duarte, P.; Hernández-Estrada, Z.J. Effects of germination and lactic acid fermentation on nutritional and rheological properties of sorghum: A graphical review. Curr. Res. Food Sci. 2022, 5, 807–812. [Google Scholar] [CrossRef]
- Adebo, O.A. African Sorghum-Based Fermented Foods: Past, Current and Future Prospects. Nutrients 2020, 12, 1111. [Google Scholar] [CrossRef]
- Khoddami, A.; Messina, V.; Vadabalija Venkata, K.; Farahnaky, A.; Blanchard, C.L.; Roberts, T.H. Sorghum in foods: Functionality and potential in innovative products. Crit. Rev. Food Sci. Nutr. 2023, 63, 1170–1186. [Google Scholar] [CrossRef]
- Ari Akin, P.; Demirkesen, I.; Bean, S.R.; Aramouni, F.; Boyaci, I.H. Sorghum Flour Application in Bread: Technological Challenges and Opportunities. Foods 2022, 11, 2466. [Google Scholar] [CrossRef]
- Cardone, G.; Rumler, R.; Speranza, S.; Marti, A.; Schönlechner, R. Sprouting Time Affects Sorghum (Sorghum bicolor [L.] Moench) Functionality and Bread-Baking Performance. Foods 2021, 10, 2285. [Google Scholar] [CrossRef]
- Hugo, L.F.; Rooney, L.W.; Taylor, J.R.N. Fermented Sorghum as a Functional Ingredient in Composite Breads. Cereal Chem. 2003, 80, 495–499. [Google Scholar] [CrossRef]
- Keyata, E.O.; Tola, Y.B.; Bultosa, G.; Forsido, S.F. Premilling treatments effects on nutritional composition, antinutritional factors, and in vitro mineral bioavailability of the improved Assosa I sorghum variety (Sorghum bicolor L.). Food Sci. Nutr. 2021, 9, 1929–1938. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Bernhardt, R. Influence of grain germination on functional properties of sorghum flour. Food Chem. 2010, 121, 387–392. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Bernhardt, R. Combination Effect of Germination and Fermentation on Functional Properties of Sorghum Flour. Curr. J. Appl. Sci. Technol. 2018, 30, 1–12. [Google Scholar] [CrossRef]
- Hassan, S.; Imran, M.; Ahmad, M.H.; Khan, M.I.; Xu, C.; Khan, M.K.; Muhammad, N. Phytochemical characterization of ultrasound-processed sorghum sprouts for the use in functional foods. Int. J. Food Prop. 2020, 23, 853–863. [Google Scholar] [CrossRef]
- Marchini, M.; Marti, A.; Folli, C.; Prandi, B.; Ganino, T.; Conte, P.; Fadda, C.; Mattarozzi, M.; Carini, E. Sprouting of Sorghum (Sorghum bicolor [L.] Moench): Effect of Drying Treatment on Protein and Starch Features. Foods 2021, 10, 407. [Google Scholar] [CrossRef]
- Marengo, M.; Bonomi, F.; Marti, A.; Pagani, M.A.; Elkhalifa, A.E.O.; Iametti, S. Molecular features of fermented and sprouted sorghum flours relate to their suitability as components of enriched gluten-free pasta. LWT—Food Sci. Technol. 2015, 63, 511–518. [Google Scholar] [CrossRef]
- Osman, M.A. Changes in sorghum enzyme inhibitors, phytic acid, tannins and in vitro protein digestibility occurring during Khamir (local bread) fermentation. Food Chem. 2004, 88, 129–134. [Google Scholar] [CrossRef]
- Saithalavi, K.M.; Bhasin, A.; Yaqoob, M. Impact of sprouting on physicochemical and nutritional properties of sorghum: A review. J. Food Meas. Charact. 2021, 15, 4190–4204. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Wang, G.; Zhang, J.; Wang, G. Analysis of gene expression in early seed germination of rice: Landscape and genetic regulation. BMC Plant Biol. 2022, 22, 70. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef]
- Setia, R.; Dai, Z.; Nickerson, M.T.; Sopiwnyk, E.; Malcolmson, L.; Ai, Y. Impacts of short-term germination on the chemical compositions, technological characteristics and nutritional quality of yellow pea and faba bean flours. Food Res. Int. 2019, 122, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Oh, S.G.; Lee, D.H.; Baik, H.W.; Chung, H.J. Effect of germination on the structures and physicochemical properties of starches from brown rice, oat, sorghum, and millet. Int. J. Biol. Macromol. 2017, 105, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Teobaldi, A.G.; Carrillo Parra, E.J.; Barrera, G.N.; Ribotta, P.D. The Properties of Damaged Starch Granules: The Relationship Between Granule Structure and Water-Starch Polymer Interactions. Foods 2024, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Salvati, D.; Paschoalinotto, B.H.; Mandim, F.; Ferreira, I.; Steinmacher, N.C.; Pereira, C.; Dias, M.I. Exploring the Impacts of Sorghum (Sorghum bicolor L. Moench) Germination on the Flour’s Nutritional, Chemical, Bioactive, and Technological Properties. Foods 2024, 13, 491. [Google Scholar] [CrossRef]
- Sharma, B.; Gujral, H.S. Modifying the dough mixing behavior, protein & starch digestibility and antinutritional profile of minor millets by sprouting. Int. J. Biol. Macromol. 2020, 153, 962–970. [Google Scholar] [CrossRef]
- Shah, U.; Dwivedi, D.; Hackett, M.; Al-Salami, H.; Utikar, R.P.; Blanchard, C.; Gani, A.; Rowles, M.R.; Johnson, S.K. Physicochemical characterisation of kafirins extracted from sorghum grain and dried distillers grain with solubles related to their biomaterial functionality. Sci. Rep. 2021, 11, 15204. [Google Scholar] [CrossRef]
- Abdelbost, L.; Morel, M.H.; Nascimento, T.P.D.; Cameron, L.C.; Bonicel, J.; Larraz, M.F.S.; Mameri, H. Sorghum grain germination as a route to improve kafirin digestibility: Biochemical and label free proteomics insights. Food Chem. 2023, 424, 136407. [Google Scholar] [CrossRef]
- Egger, L.; Schlegel, P.; Baumann, C.; Stoffers, H.; Guggisberg, D.; Brügger, C.; Dürr, D.; Stoll, P.; Vergères, G.; Portmann, R. Physiological comparability of the harmonized INFOGEST in vitro digestion method to in vivo pig digestion. Food Res. Int. 2017, 102, 567–574. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Loffi, C.; Montalbano, S.; Chiarello, E.; Dellafiora, L.; Picone, G.; Antonelli, G.; Tedeschi, T.; Buschini, A.; Capozzi, F.; et al. Cleaning the Label of Cured Meat; Effect of the Replacement of Nitrates/Nitrites on Nutrients Bioaccessibility, Peptides Formation, and Cellular Toxicity of In Vitro Digested Salami. Int. J. Mol. Sci. 2022, 23, 12555. [Google Scholar] [CrossRef]
- Kehinde, B.A.; Majid, I.; Hussain, S. Isolation of bioactive peptides and multiple nutraceuticals of antidiabetic and antioxidant functionalities through sprouting: Recent advances. J. Food Biochem. 2022, 46, e14317. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Gómez-Cabellos, S.; Giménez, M.J.; Barro, F.; Diaz, I.; Diaz-Mendoza, M. Plant Proteases: From Key Enzymes in Germination to Allies for Fighting Human Gluten-Related Disorders. Front. Plant Sci. 2019, 10, 721. [Google Scholar] [CrossRef]
- Ogbonna, A.C.; Obi, S.K.C.; Okolo, B.N.; Odibo, F.J.C. Purification and some properties of a cysteine proteinase from sorghum malt variety SK5912. J. Sci. Food Agric. 2004, 84, 113–120. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, M.; Shang, P.; Liu, J.; Zhao, R. Effect of Microwave Treatment on Protease Activity, Dough Properties and Protein Quality in Sprouted Wheat. Foods 2024, 13, 1277. [Google Scholar] [CrossRef] [PubMed]
- Bera, I.; O’Sullivan, M.; Flynn, D.; Shields, D.C. Relationship between Protein Digestibility and the Proteolysis of Legume Proteins during Seed Germination. Molecules 2023, 28, 3204. [Google Scholar] [CrossRef]
- Hassan, S.; Imran, M.; Ahmad, N.; Khan, M.K. Lipids characterization of ultrasound and microwave processed germinated sorghum. Lipids Health Dis. 2017, 16, 125. [Google Scholar] [CrossRef]
- Al-Taher, F.; Nemzer, B. Effect of Germination on Fatty Acid Composition in Cereal Grains. Foods 2023, 12, 3306. [Google Scholar] [CrossRef]
- Kumar, R.R.; Bhargava, D.V.; Pandit, K.; Goswami, S.; Mukesh Shankar, S.; Singh, S.P.; Rai, G.K.; Tara Satyavathi, C.; Praveen, S. Lipase—The fascinating dynamics of enzyme in seed storage and germination—A real challenge to pearl millet. Food Chem. 2021, 361, 130031. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Peng, J.; Li, F.; Ali, F.; Wang, Z. Regulation of seed germination: ROS, epigenetic, and hormonal aspects. J. Adv. Res. 2025, 71, 107–125. [Google Scholar] [CrossRef]
- Rai-Kalal, P.; Tomar, R.S.; Jajoo, A. H2O2 signaling regulates seed germination in ZnO nanoprimed wheat (Triticum aestivum L.) seeds for improving plant performance under drought stress. Environ. Exp. Bot. 2021, 189, 104561. [Google Scholar] [CrossRef]
- Gonçalves, J.P.; Gasparini, K.; Picoli, E.A.T.; Costa, M.D.L.; Araujo, W.L.; Zsögön, A.; Ribeiro, D.M. Metabolic control of seed germination in legumes. J. Plant Physiol. 2024, 295, 154206. [Google Scholar] [CrossRef]
- Amft, J.; Meissner, P.M.; Steffen-Heins, A.; Hasler, M.; Stöckmann, H.; Meynier, A.; Birault, L.; Velasco, J.; Vermoesen, A.; Perez-Portabella, I.; et al. Interlaboratory study on lipid oxidation during accelerated storage trials with rapeseed and sunflower oil analyzed by conjugated dienes as primary oxidation products. Eur. J. Lipid Sci. Technol. 2023, 125, 2300067. [Google Scholar] [CrossRef]
- Goswami, S.; Asrani, P.; Ansheef Ali, T.P.; Kumar, R.D.; Vinutha, T.; Veda, K.; Kumari, S.; Sachdev, A.; Singh, S.P.; Satyavathi, C.T.; et al. Rancidity Matrix: Development of Biochemical Indicators for Analysing the Keeping Quality of Pearl Millet Flour. Food Anal. Methods 2020, 13, 2147–2164. [Google Scholar] [CrossRef]
- Niro, S.; D’Agostino, A.; Fratianni, A.; Cinquanta, L.; Panfili, G. Gluten-Free Alternative Grains: Nutritional Evaluation and Bioactive Compounds. Foods 2019, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, D.; Liu, T.; Liao, M.; Li, Y.; Zhang, W.; Liu, Z.; Chen, M. Effect of Overexpression of γ-Tocopherol Methyltransferase on α-Tocopherol and Fatty Acid Accumulation and Tolerance to Salt Stress during Seed Germination in Brassica napus L. Int. J. Mol. Sci. 2022, 23, 15933. [Google Scholar] [CrossRef] [PubMed]
- Tarasevičienė, Ž.; Viršilė, A.; Danilčenko, H.; Duchovskis, P.; Paulauskienė, A.; Gajewski, M. Effects of germination time on the antioxidant properties of edible seeds. CyTA—J. Food 2019, 17, 447–454. [Google Scholar] [CrossRef]
- Azzi, A.; Breyer, I.; Feher, M.; Pastori, M.; Ricciarelli, R.; Spycher, S.; Staffieri, M.; Stocker, A.; Zimmer, S.; Zingg, J.M. Specific cellular responses to alpha-tocopherol. J. Nutr. 2000, 130, 1649–1652. [Google Scholar] [CrossRef]
- Delgado, A.; Al-Hamimi, S.; Ramadan, M.F.; Wit, M.D.; Durazzo, A.; Nyam, K.L.; Issaoui, M. Contribution of Tocols to Food Sensorial Properties, Stability, and Overall Quality. J. Food Qual. 2020, 2020, 8885865. [Google Scholar] [CrossRef]
- Nagy, R.; Kun-Nemes, A.; Szőllősi, E.; Bíróné Molnár, P.; Cziáky, Z.; Murányi, E.; Sipos, P.; Remenyik, J. Physiological potential of different Sorghum bicolor varieties depending on their bioactive characteristics and antioxidant potential as well as different extraction methods. Heliyon 2024, 10, e35807. [Google Scholar] [CrossRef]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From Theory to Practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef]
- Borgonovi, S.M.; Chiarello, E.; Pasini, F.; Picone, G.; Marzocchi, S.; Capozzi, F.; Bordoni, A.; Barbiroli, A.; Marti, A.; Iametti, S.; et al. Effect of Sprouting on Biomolecular and Antioxidant Features of Common Buckwheat (Fagopyrum esculentum). Foods 2023, 12, 2047. [Google Scholar] [CrossRef]
- Ti, H.; Zhang, R.; Zhang, M.; Li, Q.; Wei, Z.; Zhang, Y.; Tang, X.; Deng, Y.; Liu, L.; Ma, Y. Dynamic changes in the free and bound phenolic compounds and antioxidant activity of brown rice at different germination stages. Food Chem. 2014, 161, 337–344. [Google Scholar] [CrossRef]
- Tomé-Sánchez, I.; Martín-Diana, A.B.; Peñas, E.; Bautista-Expósito, S.; Frias, J.; Rico, D.; González-Maillo, L.; Martinez-Villaluenga, C. Soluble Phenolic Composition Tailored by Germination Conditions Accompany Antioxidant and Anti-inflammatory Properties of Wheat. Antioxidants 2020, 9, 426. [Google Scholar] [CrossRef]
- Liu, A.-L.; Wang, Y.-H.; Wang, T.-Y.; Zhu, Y.; Wu, P.; Li, L.-J. Comparative metabolomic profiling of secondary metabolites in different tissues of Euryale ferox and functional characterization of phenylalanine ammonia-lyase. Ind. Crops Prod. 2023, 195, 116450. [Google Scholar] [CrossRef]
- Kaur, K.; Asthir, B. Regulation of polyphenol catabolism in amelioration of high-temperature stress vis-a-vis antioxidant defense system in wheat. Cereal Res. Commun. 2022, 50, 987–998. [Google Scholar] [CrossRef]
- Dicko, M.H.; Gruppen, H.; Zouzouho, O.C.; Traoré, A.S.; van Berkel, W.J.H.; Voragen, A.G.J. Effects of germination on the activities of amylases and phenolic enzymes in sorghum varieties grouped according to food end-use properties. J. Sci. Food Agric. 2006, 86, 953–963. [Google Scholar] [CrossRef]
- Punia, H.; Tokas, J.; Malik, A.; Bajguz, A.; El-Sheikh, M.A.; Ahmad, P. Ascorbate-Glutathione Oxidant Scavengers, Metabolome Analysis and Adaptation Mechanisms of Ion Exclusion in Sorghum under Salt Stress. Int. J. Mol. Sci. 2021, 22, 13249. [Google Scholar] [CrossRef] [PubMed]
- Modgil, R.; Sood, P. Effect of Roasting and Germination on Carbohydrates and Anti-nutritional Constituents of Indigenous and Exotic Cultivars of Pseudo-cereal (Chenopodium). J. Life Sci. 2017, 9, 64–70. [Google Scholar] [CrossRef]
- Santos, S.C.; Fortes, G.A.C.; Camargo, L.T.F.M.; Camargo, A.J.; Ferri, P.H. Antioxidant effects of polyphenolic compounds and structure-activity relationship predicted by multivariate regression tree. LWT 2021, 137, 110366. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef]
- Feizollahi, E.; Mirmahdi, R.S.; Zoghi, A.; Zijlstra, R.T.; Roopesh, M.S.; Vasanthan, T. Review of the beneficial and anti-nutritional qualities of phytic acid, and procedures for removing it from food products. Food Res. Int. 2021, 143, 110284. [Google Scholar] [CrossRef]
- Brouns, F. Phytic Acid and Whole Grains for Health Controversy. Nutrients 2021, 14, 25. [Google Scholar] [CrossRef]
- Elliott, H.; Woods, P.; Green, B.D.; Nugent, A.P. Can sprouting reduce phytate and improve the nutritional composition and nutrient bioaccessibility in cereals and legumes? Nutr. Bull. 2022, 47, 138–156. [Google Scholar] [CrossRef]
- Maldonado-Alvarado, P.; Pavón-Vargas, D.J.; Abarca-Robles, J.; Valencia-Chamorro, S.; Haros, C.M. Effect of Germination on the Nutritional Properties, Phytic Acid Content, and Phytase Activity of Quinoa (Chenopodium quinoa Willd). Foods 2023, 12, 389. [Google Scholar] [CrossRef]
- Silva, V.M.; Putti, F.F.; White, P.J.; Reis, A.R.D. Phytic acid accumulation in plants: Biosynthesis pathway regulation and role in human diet. Plant Physiol. Biochem. 2021, 164, 132–146. [Google Scholar] [CrossRef]
- Kårlund, A.; Paukkonen, I.; Gómez-Gallego, C.; Kolehmainen, M. Intestinal Exposure to Food-Derived Protease Inhibitors: Digestion Physiology- and Gut Health-Related Effects. Healthcare 2021, 9, 1002. [Google Scholar] [CrossRef] [PubMed]
- Stanforth, K.; Wilcox, M.; Chater, P.; Brownlee, I.; Zakhour, M.; Banecki, K.; Pearson, J. Pepsin properties, structure, and its accurate measurement: A narrative review. Ann. Esophagus 2021, 5, 9. [Google Scholar] [CrossRef]
- Gomez-Urios, C.; Siroli, L.; Grassi, S.; Patrignani, F.; Blesa, J.; Lanciotti, R.; Frígola, A.; Iametti, S.; Esteve, M.J.; Di Nunzio, M. Sustainable valorization of citrus by-products: Natural deep eutectic solvents for bioactive extraction and biological applications of Citrus sinensis peel. Eur. Food Res. Technol. 2025, 251, 1965–1980. [Google Scholar] [CrossRef]
- Borgonovi, S.M.; Perugino, F.; Dellafiora, L.; Annunziata, F.; Pedroni, L.; Galaverna, G.; Pinto, A.; Dallavalle, S.; Iametti, S.; Di Nunzio, M. Assessing the impact of food-derived bioactives on digestive proteases by in vitro and in silico approaches. Food Funct. 2025, 16, 2959–2971. [Google Scholar] [CrossRef]
- Budhwar, S.; Sethi, K.; Chakraborty, M. Efficacy of germination and probiotic fermentation on underutilized cereal and millet grains. Food Prod. Process. Nutr. 2020, 2, 12. [Google Scholar] [CrossRef]
- Dia, V.P.; Gomez, T.; Vernaza, G.; Berhow, M.; Chang, Y.K.; de Mejia, E.G. Bowman-Birk and Kunitz protease inhibitors among antinutrients and bioactives modified by germination and hydrolysis in Brazilian soybean cultivar BRS 133. J. Agric. Food Chem. 2012, 60, 7886–7894. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.A. The proteolysis of trypsin inhibitors in legume seeds. Crit. Rev. Biotechnol. 1988, 8, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bobade, H.; Sharma, S.; Singh, B.; Gupta, A. Enhancement of Digestibility of Nutrients (In vitro), Antioxidant Potential and Functional Attributes of Wheat Flour Through Grain Germination. Plant Foods Hum. Nutr. 2021, 76, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Azeez, S.O.; Chinma, C.E.; Bassey, S.O.; Eze, U.R.; Makinde, A.F.; Sakariyah, A.A.; Okubanjo, S.S.; Danbaba, N.; Adebo, O.A. Impact of germination alone or in combination with solid-state fermentation on the physicochemical, antioxidant, in vitro digestibility, functional and thermal properties of brown finger millet flours. LWT 2022, 154, 112734. [Google Scholar] [CrossRef]
- Yang, F.; Basu, T.K.; Ooraikul, B. Studies on germination conditions and antioxidant contents of wheat grain. Int. J. Food Sci. Nutr. 2001, 52, 319–330. [Google Scholar] [CrossRef]
- Setia, R.; Dai, Z.; Nickerson, M.T.; Sopiwnyk, E.; Malcolmson, L.; Ai, Y. Properties and bread-baking performance of wheat flour composited with germinated pulse flours. Cereal Chem. 2020, 97, 459–471. [Google Scholar] [CrossRef]
- Sharanagat, V.S.; Nema, P.K. Bread preparation by partial replacement of wheat by germinated sorghum. Food Sci. Technol. Int. 2023, 29, 13–24. [Google Scholar] [CrossRef]
- Nkurikiye, E.; Xiao, R.; Tilley, M.; Siliveru, K.; Li, Y. Bread-making properties of different pulse flours in composites with refined wheat flour. J. Texture Stud. 2023, 54, 311–322. [Google Scholar] [CrossRef]
- Tagliasco, M.; Font, G.; Renzetti, S.; Capuano, E.; Pellegrini, N. Role of particle size in modulating starch digestibility and textural properties in a rye bread model system. Food Res. Int. 2024, 190, 114565. [Google Scholar] [CrossRef]
- Soglia, F.; Mazzoni, M.; Zappaterra, M.; Di Nunzio, M.; Babini, E.; Bordini, M.; Sirri, F.; Clavenzani, P.; Davoli, R.; Petracci, M. Distribution and Expression of Vimentin and Desmin in Broiler Pectoralis major Affected by the Growth-Related Muscular Abnormalities. Front. Physiol. 2019, 10, 1581. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Betoret, E.; Taccari, A.; Dalla Rosa, M.; Bordoni, A. Impact of processing on the nutritional and functional value of mandarin juice. J. Sci. Food Agric. 2020, 100, 4558–4564. [Google Scholar] [CrossRef] [PubMed]
- Di Nunzio, M.; Loffi, C.; Chiarello, E.; Dellafiora, L.; Picone, G.; Antonelli, G.; Di Gregorio, C.; Capozzi, F.; Tedeschi, T.; Galaverna, G.; et al. Impact of a Shorter Brine Soaking Time on Nutrient Bioaccessibility and Peptide Formation in 30-Months-Ripened Parmigiano Reggiano Cheese. Molecules 2022, 27, 664. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.S. Advances in Lipid Extraction Methods-A Review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef] [PubMed]
- Ghini, V.; Di Nunzio, M.; Tenori, L.; Valli, V.; Danesi, F.; Capozzi, F.; Luchinat, C.; Bordoni, A. Evidence of a DHA Signature in the Lipidome and Metabolome of Human Hepatocytes. Int. J. Mol. Sci. 2017, 18, 359. [Google Scholar] [CrossRef]
- Bub, A.; Malpuech-Brugère, C.; Orfila, C.; Amat, J.; Arianna, A.; Blot, A.; Di Nunzio, M.; Holmes, M.; Kertész, Z.; Marshall, L.; et al. A Dietary Intervention of Bioactive Enriched Foods Aimed at Adults at Risk of Metabolic Syndrome: Protocol and Results from PATHWAY-27 Pilot Study. Nutrients 2019, 11, 1814. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Valli, V.; Bordoni, A. Pro- and anti-oxidant effects of polyunsaturated fatty acid supplementation in HepG2 cells. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 121–127. [Google Scholar] [CrossRef]
- Abeyrathne, E.; Nam, K.; Ahn, D.U. Analytical Methods for Lipid Oxidation and Antioxidant Capacity in Food Systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Verma, A.; Singh, S.; Thawait, L.K.; Mahatma, M.K.; Singh, A.L. An expedient ion chromatography based method for high-throughput analysis of phytic acid in groundnut kernels. J. Food Sci. Technol. 2022, 59, 4479–4486. [Google Scholar] [CrossRef]
- Urbinati, E.; Di Nunzio, M.; Picone, G.; Chiarello, E.; Bordoni, A.; Capozzi, F. The Effect of Balsamic Vinegar Dressing on Protein and Carbohydrate Digestibility is Dependent on the Food Matrix. Foods 2021, 10, 411. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Chiarello, E.; Caboni, M.F.; Capozzi, F.; Gianotti, A.; Bordoni, A. Olive oil by-product as functional ingredient in bakery products. Influence of processing and evaluation of biological effects. Food Res. Int. 2020, 131, 108940. [Google Scholar] [CrossRef]
| FAME | S-0 | S-48 | S-72 |
|---|---|---|---|
| C14:0 | n.d. a | 0.08 ± 0.04 a | n.d. a |
| C16:0 | 3.95 ± 0.06 a | 3.91 ± 0.47 a | 3.23 ± 0.01 a |
| C16:1 n-7 | 0.06 ± 0.09 a | 0.12 ± 0.18 a | 0.05 ± 0.07 a |
| C18:0 | n.d. a | 0.35 ± 0.14 a | 0.66 ± 0.29 a |
| C18:1 n-9 | 8.04 ± 0.05 a | 7.73 ± 1.30 a | 5.88 ± 0.04 a |
| C18:2 n-6 | 12.13 ± 1.01 a | 10.75 ± 0.27 ab | 9.29 ± 0.12 b |
| C18:3 n-3 | 0.36 ± 0.05 a | 0.46 ± 0.00 a | 0.43 ± 0.02 a |
| ΣSFAs | 3.95 ± 0.06 a | 4.34 ± 0.37 a | 3.89 ± 0.28 a |
| ΣMUFAs | 8.11 ± 0.14 a | 7.85 ± 1.48 a | 5.93 ± 0.03 a |
| ΣPUFAs | 12.49 ± 1.06 a | 11.20 ± 0.27 ab | 9.72 ± 0.10 b |
| Σn-6/Σn-3 | 33.64 ± 1.75 a | 23.49 ± 0.47 b | 21.71 ± 1.18 b |
| Total | 24.55 ± 0.98 a | 23.40 ± 1.58 ab | 19.53 ± 0.35 b |
| UI | 136.21 ± 2.83 a | 131.45 ± 4.85 a | 132.04 ± 1.59 a |
| PI | 60.57 ± 1.96 a | 58.35 ± 3.51 a | 59.53 ± 0.68 a |
| Tocols | S-0 | S-48 | S-72 |
|---|---|---|---|
| α-tocopherol | 3.47 ± 0.00 b | 11.53 ± 0.43 a | 10.44 ± 0.18 a |
| α-tocotrienol | 1.98 ± 0.10 b | 3.56 ± 0.21 a | 3.20 ± 0.18 a |
| γ-tocopherol | 14.77 ± 0.24 a | 5.05 ± 0.64 b | 3.03 ± 0.00 c |
| δ-tocopherol | 1.78 ± 0.27 b | 2.63 ± 0.17 a | 2.54 ± 0.00 ab |
| Total | 22.00 ± 0.61 a | 22.77 ± 1.45 a | 19.21 ± 0.36 a |
| Compounds | [M/H]− | MS Fragments | Q. T. | Free Phenolic Compounds | ANOVA | ||
|---|---|---|---|---|---|---|---|
| S-0 | S-48 | S-72 | |||||
| Phenolic acids | |||||||
| 1-3-O-dicaffeoylglycerol | 415 | 161, 253, 135 | 415 → 135 | 270.10 ± 8.46 a | 72.41 ± 0.63 b | 23.83 ± 0.64 c | p < 0.05 |
| Caffeic acid | 179 | 135 | 179 → 135 | 35.92 ± 1.58 a | 16.02 ± 0.05 b | 3.90 ± 0.35 c | p < 0.05 |
| Total phenolic acid | 306.02 ± 10.04 a | 88.43 ± 0.68 b | 27.73 ± 0.99 c | p < 0.05 | |||
| Flavan-3-ols | |||||||
| Catechin | 289 | 123, 109, 203 | 289 → 123 | n.d. b | 33.99 ± 1.70 a | n.d. b | p < 0.05 |
| Total flavan-3-ol | n.d. b | 33.99 ± 1.70 a | n.d. b | p < 0.05 | |||
| Flavonols | |||||||
| Taxifolin | 303 | 125, 175, 217 | 303 → 125 | 1327.69 ± 45.66 c | 2233.50 ± 9.50 a | 1803.68 ± 42.04 b | p < 0.05 |
| Total flavonol | 1327.69 ± 45.66 c | 2233.50 ± 9.50 a | 1803.68 ± 42.04 b | p < 0.05 | |||
| Flavones | |||||||
| Apigenin | 269 | 117, 149, 227 | 269 → 117 | 23.86 ± 2.27 b | 21.72 ± 0.59 b | 29.79 ± 0.30 a | p < 0.05 |
| Hispidulin isomer 1 | 299 | 284, 256, 136 | 299 → 284 | 58.23 ± 8.18 c | 122.05 ± 5.37 b | 179.52 ± 0.67 a | p < 0.05 |
| Hispidulin isomer 2 | 299 | 284, 256, 136 | 299 → 284 | 188.27 ± 13.19 b | 150.60 ± 7.90 b | 281.74 ± 23.64 a | p < 0.05 |
| Luteolin | 285 | 133, 151, 217 | 285 → 133 | 373.47 ± 18.47 a | 348.81 ± 4.69 a | 435.66 ± 33.45 a | p < 0.05 |
| Total flavones | 643.83 ± 42.11 b | 643.18 ± 18.55 b | 926.71 ± 58.06 a | p < 0.05 | |||
| Proanthocyanidins | |||||||
| Procyanidin dimer | 577 | 289, 407, 425 | 577 → 289 | n.d. c | 36.08 ± 3.54 a | 26.96 ± 3.44 b | p < 0.05 |
| Total proanthocyanidins | n.d. c | 36.08 ± 3.54 a | 26.96 ± 3.44 b | p < 0.05 | |||
| Flavanones | |||||||
| Eriodictyol | 287 | 125, 151, 193 | 287 → 125 | 40.51 ± 3.29 c | 219.26 ± 3.19 a | 202.01 ± 2.77 b | p < 0.05 |
| Naringenin | 271 | 119, 107, 151 | 271 → 119 | 5.83 ± 0.49 b | 2.02 ± 0.03 b | 37.82 ± 2.09 a | p < 0.05 |
| Kaempferol | 285 | 199, 175, 217 | 285 → 199 | 4.48 ± 0.56 c | 18.71 ± 0.14 a | 10.41 ± 1.25 b | p < 0.05 |
| Total flavanones | 50.82 ± 4.34 b | 239.99 ± 3.36 a | 250.24 ± 6.11 a | p < 0.05 | |||
| Flavanonols | |||||||
| Dihydromyricetin 3-O-Rhamnoside | 465 | 285, 275, 303 | 465 → 285 | 31.98 ± 1.31 a | 34.30 ± 1.38 a | 41.13 ± 5.13 a | p < 0.05 |
| Total flavanonols | 31.98 ± 1.31 a | 34.30 ± 1.38 a | 41.13 ± 5.13 a | p < 0.05 | |||
| 3-Deoxyanthocyanidins | |||||||
| Luteolinidin | 269 | 201, 227, 241 | 269 → 201 | 2.23 ± 0.16 c | 4.96 ± 0.62 b | 7.64 ± 0.54 a | p < 0.05 |
| Apigeninidin | 253 | 209, 117, 181 | 253 → 209 | 6.51 ± 0.59 a | 5.04 ± 0.50 a | 1.29 ± 0.10 b | p < 0.05 |
| 5-methoxy-luteolinidin | 283 | 268, 196, 240 | 283 → 268 | 6.02 ± 0.10 a | 7.08 ± 0.22 a | 3.68 ± 0.50 b | p < 0.05 |
| 7-methoxy-apigeninidin | 267 | 252, 224, 180 | 267 → 252 | 1.58 ± 0.31 c | 3.10 ± 0.43 b | 5.25 ± 0.30 a | p < 0.05 |
| Total 3-deoxyanthocyanidins | 16.34 ± 1.16 b | 20.18 ± 1.77 a | 17.86 ± 1.44 b | p < 0.05 | |||
| Total phenol compounds | 2376.68 ± 104.62 c | 3329.65 ± 40.48 a | 3104.31 ± 3117.21 b | p < 0.05 | |||
| Compounds | [M/H]− | MS Fragments | Q. T. | Bound Phenolic Compounds | ANOVA | ||
|---|---|---|---|---|---|---|---|
| S-0 | S-48 | S-72 | |||||
| Phenolic acids | |||||||
| 1-3-O-dicaffeoylglycerol | 415 | 161, 253, 135 | 415 → 135 | n.d. | n.d. | n.d. | |
| Caffeic acid | 179 | 135 | 179 → 135 | n.d. | n.d. | n.d. | |
| Total phenolic acid | n.d. | n.d. | n.d. | ||||
| Flavan-3-ols | |||||||
| Catechin | 289 | 123, 109, 203 | 289 → 123 | n.d. | n.d. | n.d. | |
| Total flavan-3-ol | n.d. | n.d. | n.d. | ||||
| Flavonols | |||||||
| Taxifolin | 303 | 125, 175, 217 | 303 → 125 | n.d. b | 13.02 ± 1.23 a | 9.46 ± 1.33 a | p < 0.05 |
| Total flavonol | n.d. b | 13.02 ± 1.23 a | 9.46 ± 1.33 a | p < 0.05 | |||
| Flavones | |||||||
| Apigenin | 269 | 117, 149, 227 | 269 → 117 | n.d. | n.d. | n.d. | |
| Hispidulin isomer 1 | 299 | 284, 256, 136 | 299 → 284 | n.d. c | 17.06 ± 0.72 b | 62.32 ± 2.97 a | p < 0.05 |
| Hispidulin isomer 2 | 299 | 284, 256, 136 | 299 → 284 | 7.49 ± 0.17 c | 24.34 ± 0.54 b | 34.65 ± 1.23 a | p < 0.05 |
| Luteolin | 285 | 133, 151, 217 | 285 → 133 | 67.65 ± 4.66 c | 84.21 ± 0.10 b | 103.23 ± 3.65 a | p < 0.05 |
| Total flavones | 75.14 ± 4.83 c | 125.61 ± 1.36 b | 200.20 ± 7.85 a | p < 0.05 | |||
| Proanthocyanidins | |||||||
| Procyanidin dimer | 577 | 289, 407, 425 | 577 → 289 | n.d. | n.d. | n.d. | |
| Total proanthocyanidins | n.d. | n.d. | n.d. | ||||
| Flavanones | |||||||
| Eriodictyol | 287 | 125, 151, 193 | 287 → 125 | n.d. b | 21.64 ± 0.18 a | n.d. b | p < 0.05 |
| Naringenin | 271 | 119, 107, 151 | 271 → 119 | 11.17 ± 0.15 c | 34.41 ± 2.65 b | 49.70 ± 1.18 a | p < 0.05 |
| Kaempferol | 285 | 199, 175, 217 | 285 → 199 | n.d. | n.d. | n.d. | |
| Total flavanones | 11.17 ± 0.15 b | 56.05 ± 2.83 a | 49.70 ± 1.18 a | p < 0.05 | |||
| Flavanonols | |||||||
| Dihydromyricetin 3-O-Rhamnoside | 465 | 285, 275, 303 | 465 → 285 | n.d. | n.d. | n.d. | |
| Total flavanonols | n.d. | n.d. | n.d. | ||||
| 3-Deoxyanthocyanidins | |||||||
| Luteolinidin | 269 | 201, 227, 241 | 269 → 201 | n.d. | n.d. | n.d. | |
| Apigeninidin | 253 | 209, 117, 181 | 253 → 209 | n.d. | n.d. | n.d. | |
| 5-methoxy-luteolinidin | 283 | 268, 196, 240 | 283 → 268 | n.d. | n.d. | n.d. | |
| 7-methoxy-apigeninidin | 267 | 252, 224, 180 | 267 → 252 | n.d. | n.d. | n.d. | |
| Total 3-deoxyanthocyanidins | n.d. | n.d. | n.d. | ||||
| Total phenol compounds | 86.31 ± 4.98 c | 194.68 ± 5.42 b | 259.36 ± 10.36 a | p < 0.05 | |||
| w/o Extract | S-0 | S-48 | S-72 | |
|---|---|---|---|---|
| Pepsin activity | 89.44 ± 4.81 b | 115.00 ± 17.40 ab | 139.44 ± 18.43 a | 148.89 ± 20.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgonovi, S.M.; Marzocchi, S.; Pasini, F.; Bordoni, A.; Barbiroli, A.; Marti, A.; Iametti, S.; Di Nunzio, M. Exploring Germination to Unlock the Nutritional Potential of Sorghum (Sorghum bicolor). Molecules 2025, 30, 3622. https://doi.org/10.3390/molecules30173622
Borgonovi SM, Marzocchi S, Pasini F, Bordoni A, Barbiroli A, Marti A, Iametti S, Di Nunzio M. Exploring Germination to Unlock the Nutritional Potential of Sorghum (Sorghum bicolor). Molecules. 2025; 30(17):3622. https://doi.org/10.3390/molecules30173622
Chicago/Turabian StyleBorgonovi, Sara Margherita, Silvia Marzocchi, Federica Pasini, Alessandra Bordoni, Alberto Barbiroli, Alessandra Marti, Stefania Iametti, and Mattia Di Nunzio. 2025. "Exploring Germination to Unlock the Nutritional Potential of Sorghum (Sorghum bicolor)" Molecules 30, no. 17: 3622. https://doi.org/10.3390/molecules30173622
APA StyleBorgonovi, S. M., Marzocchi, S., Pasini, F., Bordoni, A., Barbiroli, A., Marti, A., Iametti, S., & Di Nunzio, M. (2025). Exploring Germination to Unlock the Nutritional Potential of Sorghum (Sorghum bicolor). Molecules, 30(17), 3622. https://doi.org/10.3390/molecules30173622











