Abstract
An efficient access to the novel representatives of α,α-disubstituted α-amino acids with 1,3-enyne unit located at their side chain has been elaborated. The method is based on Pd(II)-catalyzed hydroalkynylation of α-allenyl-α-dimethylamino esters with terminal acetylenes. The developed strategy is the first example of the metal-catalyzed allene-alkyne coupling to provide a convenient route to new unsaturated α-amino acid derivatives in good yields and high selectivity.
1. Introduction
The development of facile and selective methods enabling rapid access to value complex molecules from readily available starting materials remains a major focus in modern organic synthesis [1,2,3,4,5]. In this context, conjugated 1,3-enynes have been identified as important synthetic blocks and essential units found in a variety of biologically active compounds [6,7,8,9,10,11,12]. As a consequence, the elaboration of efficient and atom-economic pathways to 1,3-enynes has been a subject of considerable interest during the past decade. Transition metal-catalyzed cross-coupling of allenes with terminal alkynes [13,14,15] has emerged as one of the most flexible synthetic routes to a variety of functionally substituted 1,3-enynes via the formation of a new C-C bond. Although several efficient protocols have been developed [16,17,18,19], control of the regio- and stereoselectivity of this transformation is often difficult to achieve due to the different reactivity modes of the unique allene system consisting two 1,2-comulative double bonds. The outcome of these reactions is greatly affected by the nature of metal species and ligand, the steric and electronic properties of the substituents, as well as their location in the allene system.
While Rh- [20,21], Ru- [22] and Co- [23] catalyzed hydroalkynylation of allenes typically yields exo-1,3-enynes, Pd-catalyzed reactions result in the formation of (E)-endo-1,3-enynes [13,15,16,17,18,19] in a majority of published examples (Scheme 1a). However, in the case of some particular substrates, such as allenylamides and allenylphosphine oxides, capable of specific coordination with catalytically active Pd-complexes, the corresponding exo-1,3-enynes [24], (Z)-endo-1,3-enynes or linear 1,4-enynes [25] can also be selectively obtained depending on the choice of palladium complex (Scheme 1b) or ligand (Scheme 1c).
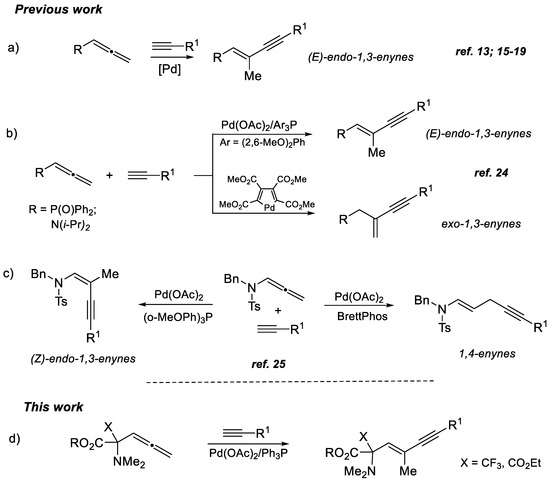
Scheme 1.
Pd-catalyzed hydroalkynylation of allenes: previous (a–c) and present work (d) [13,15,16,17,18,19,24,25].
On the other hand, the incorporation of latent (bio)orthogonal reactive unsaturated functionalities into α-amino acid side chains not only enriches their biological properties, but also creates a universal platform for the implementation of numerous useful transformations leading to structurally diverse non-proteinogenic α-amino acids [26,27,28]. In this regard, the development of efficient synthetic protocols that would provide straightforward access to novel α-amino acids with 1,3-enyne fragments in their structure is of great importance for the sustainable drug discovery process.
Our current research program connects with the elaboration of efficient synthetic approaches to new α-amino acid derivatives through the functionalization of their unsaturated precursors under metal catalysis [29]. Recently we have revealed that readily available α-amino acid derivatives with terminal allenyl group at the side chain are unique substrates for a variety of transition metal-catalyzed transformations [30], such as hydroamination [31], C-H activation/annulation [32], and hydroarylation [33] reactions under Cu-, Rh-, and Pd-catalysis, respectively. In continuation of this study, herein we want to disclose a palladium-catalyzed regioselective hydroalkynylation of allenyl α-amino acid derivatives with different terminal alkynes as a simple and robust route to a new family of α,α-disubstituted α-amino acids bearing a 1,3-enyne moiety at their side chains (Scheme 1d). To the best of our knowledge allenyl-containing α-amino acids have not been applied in this catalytic process before.
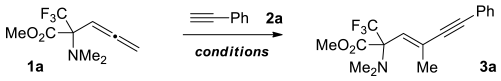
2. Results
Given the fact that many α-amino acids bearing fluoroalkyl groups at the α-position can act as selective inhibitors of pyridoxal phosphate-dependent enzymes, often exhibiting a number of interesting pharmacological properties [34,35,36], we commenced our study with the model reaction of readily available CF3-containing α-allenyl-α-amino carboxylate 1a [37] with phenyl acetylene. Palladium acetate/phosphine mixture was selected as the most competent catalytic system to initiate this process. Initially, we found that the reaction takes place at the presence of 5 mol% Pd(OAc)2 and 10 mol% of PPh3 under heating in toluene at 60 °C, affording the corresponding α-amino acid derivative 3a in 80% NMR yield after 4 h, along with noticeable amount of starting allene 1a (Table 1, entry 1). No reaction occurs at ambient temperature.

Table 1.
Optimization of the reaction conditions.
The isolated product was identified as (E)-endo-1,3-enyne-containing N,N-dimethyl-α-amino ester 3a using standard physicochemical methods including 2D 1H-19F HOESY NMR spectroscopy (see Supporting Info). The utilization of 1,4-dioxane instead of toluene as a solvent slightly increased the conversion of 1a. The further improvement was achieved by changing PPh3 ligand for dppf (entry 3). Other screened phosphines, such as Xantphos and (4-CF3-Ph)3P, did not improve the conversion of starting allene (entries 4 and 5). It is noteworthy that no products were detected in the reaction mixture with the presence of BrettPhos ligand (entry 6) as well as under catalysis with PdCl2(Ph3P)2 complex (entry 7). Then, it was revealed that the decrease in Pd(OAc)2 loading to 2 mol% almost did not affect the outcome of the process. Consequently, the following optimal conditions for the reaction were established: the heating of the allene with a small excess of acetylene in dioxane in the presence of 2 mol% Pd(OAc)2 and 4 mol% PPh3 at 55 °C for 4 h. The preference for PPh3 is due to its low cost and small difference in activity compared to the dppf ligand. It should be noted that the reaction conditions found require the use of 2.5 times less palladium catalyst compared to a similar process described earlier for the alkynylation of allene derivatives [18]. The further reduction in catalyst loading (to 1 mol%) significantly increased the reaction time (entries 9 and 11). In addition, the last experiment was performed in 2-MeTHF, affording the product in good yield for 4 h (entry 13), demonstrating a possibility to use green solvent for the process.
With these conditions in hand, the scope of the alkynylation of allene 1a with respect to different aryl and alkyl acetylene substrates 2a-p was investigated (Scheme 2). As a result, it was found that the electronic and steric nature of substituents in terminal aryl(alkyl) acetylene does not significantly affect the yield of the corresponding (E)-endo-1,3-enyne-containing α-amino acid derivatives 3a-p, which were the sole products of allene β-alkynylation reaction. However, in the case of bulky t-Bu acetylene, the conversion of the reaction with allene 1a did not exceed 50% even under heating a mixture of the reagents at 80 °C for 8 h. This problem was successfully solved by replacing the PPh3 ligand with dppf and maintaining the reaction mixture at an elevated temperature (80 °C) for 4 h, which allowed achieving almost full conversion of 1a and a good yield of 3p (79%). However, in this case, insignificant amount of 1,4-enyne 3p’ was isolated from the reaction mixture as a result of γ-alkynylation by-process.
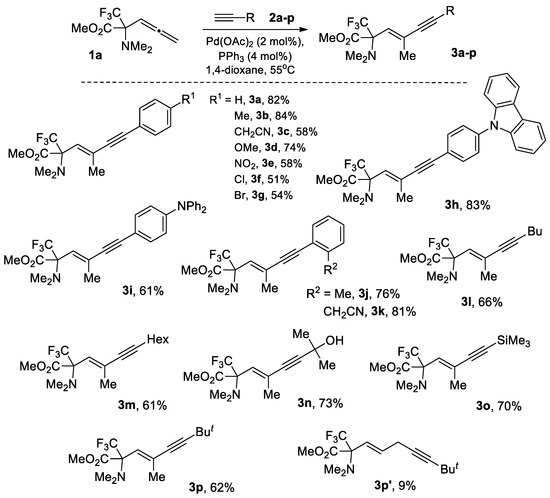
Scheme 2.
Synthesis of α-CF3-α-amino acid derivatives 3.
To expand the scope of the hydroalkynylation reaction, we investigated the reactivity of allene 1b [30] using the same conditions found for CF3-allene 1a. As it turned out, these conditions are also well-suited for the reactions of 1b with terminal alkynes. Thus, a series of new α-amino acid derivatives 4a-n (Scheme 3) decorated with 1,3-enyne moiety were obtained in moderate to good yields with high degree of regio- and stereoselectivity. No alternative isomers were detected in all studied reactions, except the only example with bulky t-Bu acetylene. As it was for fluorinated allene 1a, the reaction of 1b with this alkyne was accomplished under heating at 80 °C for 6 h to reach an acceptable conversion of 1b, yielding the desired product 4n along with insignificant admixture of 1,4-enyne 4n’ (see Supplementary Materials).
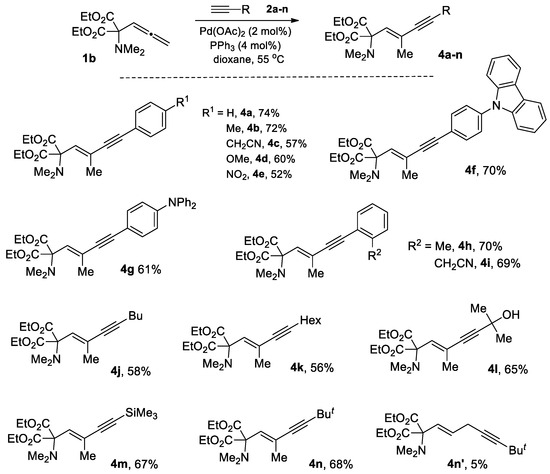
Scheme 3.
Hydroalkynylation of allene 1b.
Based on the literature examples described for Pd-catalyzed hydroalkynylation of allenes [13,14,15,16,17,18,19,25] and the experimental data obtained, plausible mechanism for the reaction of allene 1 with terminal acetylene is depicted in Scheme 4. After oxidative addition (OA) step to obtain intermediate complex A, the coordination allene 1 with A occurs to form B. The latter undergoes hydropalladation leading to complex C. Finally, the reductive elimination (RE) of C gives the 1,3-enyne product, liberating an active Pd species for the next catalytic cycle. The formation of minor 1,4-enyne in the case of t-Bu acetylene can likely be rationalized by the realization of a less favorable carbopalladation process via intermediate D (Scheme 4).
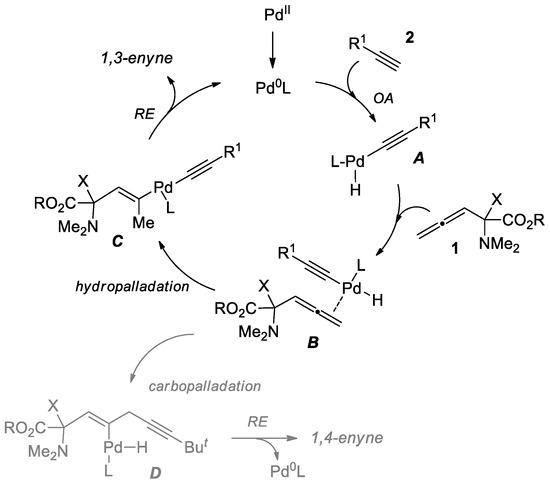
Scheme 4.
Plausible mechanism of hydroalkynylation of allene 1.
Furthermore, 1,3-enyne α-amino esters 3k and 4c containing CH2-CN group can be successfully utilized for the installation of an additional pharmacophore unit via well-established nitrile–azide coupling [38] to afford the corresponding 5-amino-1,2,3-triazole derivatives 5a,b in acceptable yields (Scheme 5).
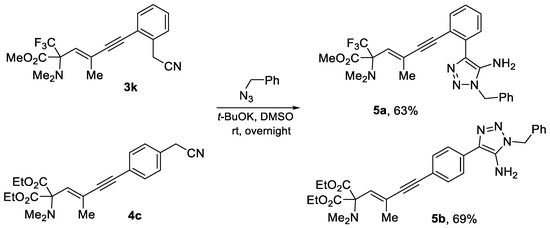
Scheme 5.
Synthesis of 5-amino-1,2,3-triazoles 5a,b by nitrile–azide coupling.
3. Materials and Methods
3.1. General Information
All the reactions were carried out under argon atmosphere, and the solvents were distilled from appropriate drying agents prior to use. All reagents were used as purchased from Sigma-Aldrich (Munich, Germany). Analytical TLC was performed with Merck silica gel 60 F 254 plates (Darmstadt, Germany); visualization was accomplished with UV light, iodine vapors or Ce(SO4)2 solution in 5% H2SO4. Chromatography was carried out using Merck silica gel (Kieselgel 60, 0.063–0.200 mm, Darmstadt, Germany) and petroleum ether/ethyl acetate as an eluent. NMR spectra were obtained with Bruker AV-300 (Karlsruhe, Germany) and Inova-400 (Varian, Palo Alto, CA, USA) spectrometers operating at 300 and 400 MHz, respectively, for 1H (TMS reference), at 101 MHz for 13C, and at 282 and 376 MHz for 19F (CCl3F reference). High-resolution mass spectra were recorded on a LCMS-9030 device (Shimadzu, Japan) by electrospray ionization mass spectrometry (ESI-MS). Measurements were carried out in positive ion mode; samples were dissolved in acetonitrile and injected into the mass-spectrometer chamber from an HPLC system LC-40 Nexera (Shimadzu, Japan).
3.2. General Procedure for Hydroalkynylation of Allenes with Terminal Alkynes
Under argon in a Schlenk tube with a magnetic stirring bar, corresponding allene (100 mg, 0.44 mmol, 1.0 equiv.) and corresponding alkyne (0.54 mmol, 1.2 equiv.) were dissolved in dry 1,4-dioxane (2 mL). Then, Pd(OAc)2 (2 mg, 9 μmol, 2 mol%) and PPh3 (4.7 mg, 18 μmol, 4 mol%) were added, and the reaction mixture was stirred at 55 °C for 4–8 h until the completion of the reaction monitored by TLC and 19F-NMR. The reaction mixture was cooled to room temperature and concentrated under reduced pressure. Purification by chromatography (gradient elution: petroleum ether/dichloromethane = 1:1, petroleum ether/ethyl acetate = 15:1) gave analytically pure desired product.
3.3. General Procedure for Preparation of 5-Amino-1,2,3-Triazole Derivatives 5a and 5b via Dipolar Azide–Nitrile Cycloaddition (DCR)
5-amino-1,2,3-triazole derivatives 5a and 5b were synthesized according to literature procedure [39] with minor modifications. A screw-cap vial equipped with a magnetic stir bar was charged with 0.2 mmol of 3k or 4c, 3.0 equiv. of benzyl azide, DMSO (4 mL), and 0.5 equiv. of powdered potassium tert-butoxide. The reaction mixture was allowed to stir for 24 h at room temperature. Upon completion, the mixture was poured into water and extracted with dichloromethane. The combined organic phases were washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure. Purification by chromatography (eluent–hexane: ethyl acetate 1:1) gave analytically pure desired product.
3.4. Characterization Data of New Compounds
(E)-Methyl 2-(dimethylamino)-4-methyl-6-phenyl-2-(trifluoromethyl)hex-3-en-5-ynoate (3a). Yield 82% (110.7 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 7.45–7.42 (m, 2H, Ar), 7.31–7.30 (m, 3H, Ar), 5.97 (s, 1H, CH), 3.80 (s, 3H, OCH3), 2.52 (s, 6H, N(CH3)2), 2.11 (s, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 167.3, 131.6, 128.5, 128.4, 128.3, 127.5, 125.8 (q, J = 297.0 Hz, CF3), 122.8, 91.5, 88.7, 73.8 (q, J = 24.0 Hz, >C<), 52.7, 39.9, 18.6. 19F-NMR (376 MHz, CDCl3) δ -66.70 (s, 3F, CF3). HRMS (ESI): m/z calcd. for C17H19F3NO2 [M + H]+ 326.1362, found 326.1362.
(E)-Methyl 2-(dimethylamino)-4-methyl-6-p-tolyl-2-(trifluoromethyl)hex-3-en-5-ynoate (3b). Yield 84% (83.7 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 7.32 (d, J = 8.0 Hz, 2H, Ar), 7.11 (d, J = 7.8 Hz, 2H, Ar), 5.93 (s, 1H, CH), 3.79 (s, 3H, OCH3), 2.51 (s, 6H, N(CH3)2), 2.33 (s, 3H, CH3), 2.08 (s, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 167.3, 138.6, 131.5, 129.1, 128.6, 127.1, 125.8 (q, J = 297.1 Hz, CF3), 119.7, 90.9, 88.9, 73.8 (q, J = 24.3 Hz, >C<), 52.7, 40.0, 21.4, 18.7. 19F-NMR (376 MHz, CDCl3) δ -66.72 (s, 3F, CF3). HRMS (ESI): m/z calcd. for C18H21F3NO2 [M + H]+ 340.1519, found 340.1522.
(E)-Methyl 6-(4-(cyanomethyl)phenyl)-2-(dimethylamino)-4-methyl-2-(trifluoromethyl) hex-3-en-5-ynoate (3c). Yield 58% (57.5 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 7.43 (d, J = 8.1 Hz, 2H, Ar), 7.27 (d, J = 8.1 Hz, 2H, Ar), 5.96 (s, 1H, CH), 3.79 (s, 3H, OCH3), 3.73 (s, 2H, CH2), 2.50 (s, 6H, N(CH3)2), 2.08 (s, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 167.2, 132.2, 130.1, 128.3, 128.0, 127.9, 125.7 (q, J = 296.9 Hz, CF3), 122.8, 117.3, 92.3, 87.7, 73.8 (q, J = 24.0 Hz, >C<), 52.7, 40.0, 23.5, 18.6. 19F-NMR (376 MHz, CDCl3) δ -66.72 (s, 3F, CF3). HRMS (ESI): m/z calcd. for C19H20F3N2O2 [M + H]+ 365.1471, found 365.1477.
(E)-Methyl 2-(dimethylamino)-6-(4-methoxyphenyl)-4-methyl-2-(trifluoromethyl)hex-3-en- 5-ynoate (3d). Yield 74% (91.2 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 7.36 (d, J = 8.8 Hz, 2H, Ar), 6.82 (d, J = 8.8 Hz, 2H, Ar), 5.91 (s, 1H, CH), 3.79 (s, 6H, 2OCH3), 2.50 (s, 6H, N(CH3)2), 2.08 (s, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 167.3, 159.7, 133.0, 128.7, 126.7, 125.8 (q, J = 297.0 Hz, CF3), 114.8, 113.9, 90.3, 88.7, 73.8 (q, J = 24.1 Hz, >C<), 55.2, 52.6, 39.9, 18.7. 19F-NMR (376 MHz, CDCl3) δ -66.73 (s, 3F, CF3). HRMS (ESI): m/z calcd. for C18H21F3NO3 [M + H]+ 356.1468, found 356.1471.
(E)-Methyl 2-(dimethylamino)-4-methyl-6-(4-nitrophenyl)-2-(trifluoromethyl)hex-3-en-5- ynoate (3e). Yield 58% (67.3 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 8.17 (d, J = 7.2 Hz, 2H, Ar), 7.56 (d, J = 8.0 Hz, 2H, Ar), 6.03 (s, 1H, CH), 3.81 (s, 3H, OCH3), 2.50 (s, 6H, N(CH3)2), 2.11 (s, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 167.0, 147.1, 132.3, 129.7, 129.6, 127.8, 125.6 (q, J = 296.8 Hz, CF3), 123.6, 96.4, 86.6, 73.8 (q, J = 23.9 Hz, >C<), 52.8, 40.0, 18.4. 19F-NMR (376 MHz, CDCl3) δ -66.68 (s, 3F, CF3). HRMS (ESI): m/z calcd. for C17H18F3N2O4 [M + H]+ 371.1213, found 371.1224.
(E)-Methyl 6-(4-chlorophenyl)-2-(dimethylamino)-4-methyl-2-(trifluoromethyl)hex-3-en-5- ynoate (3f). Yield 51% (52.6 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 7.35 (d, J = 8.5 Hz, 2H, Ar), 7.28 (d, J = 8.4 Hz, 2H, Ar), 5.95 (s, 1H, CH), 3.80 (s, 3H, OCH3), 2.50 (s, 6H, N(CH3)2), 2.08 (s, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 167.2, 134.5, 132.8, 128.6, 128.3, 127.9, 125.7 (q, J = 297.0 Hz, CF3), 121.3, 92.4, 87.5, 73.8 (q, J = 24.2 Hz, >C<), 52.7, 40.0, 18.5. 19F-NMR (376 MHz, CDCl3) δ -66.71 (s, 3F, CF3). HRMS (ESI): m/z calcd. for C17H18ClF3NO2 [M + H]+ 360.0973, found 360.0971.
(E)-Methyl 6-(4-bromophenyl)-2-(dimethylamino)-4-methyl-2-(trifluoromethyl) hex-3-en-5- ynoate (3g). Yield 54% (99.2 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 7.43 (d, J = 8.5 Hz, 2H, Ar), 7.28 (d, J = 8.5 Hz, 2H, Ar), 5.96 (s, 1H, CH), 3.80 (s, 3H, OCH3), 2.50 (s, 6H, N(CH3)2), 2.08 (s, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 167.2, 132.9, 131.6, 128.3, 127.9, 125.7 (q, J = 296.9 Hz, CF3), 122.7, 121.7, 92.5, 87.5, 73.8 (q, J = 24.1 Hz, >C<), 52.7, 40.0, 18.5. 19F-NMR (376 MHz, CDCl3) δ -66.70 (s, 3F, CF3). HRMS (ESI): m/z calcd. for C17H17BrF3NO2 [M]+ 404.0468, found 404.0473.
(E)-Methyl 6-(4-(9H-carbazol-9-yl)phenyl)-2-(dimethylamino)-4-methyl-2-(trifluoro methyl)hex-3-en-5-ynoate (3h). Yield 83% (118.7 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 8.13 (d, J = 7.7 Hz, 2H, Ar), 7.66 (d, J = 8.3 Hz, 2H, Ar), 7.54 (d, J = 8.3 Hz, 2H, Ar), 7.44–7.39 (m, 4H, Ar), 7.31–7.27 (m, 2H, Ar), 6.03 (s, 1H, CH), 3.84 (s, 3H, OCH3), 2.55 (s, 6H, N(CH3)2), 2.15 (s, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 167.3, 140.5, 137.7, 133.1, 128.4, 127.9, 126.8, 126.0, 125.8 (q, J = 297.1 Hz, CF3), 123.5, 121.7, 120.3, 120.2, 109.7, 92.4, 87.9, 73.7, 52.8, 40.1, 18.7. 19F-NMR (376 MHz, CDCl3) δ -66.66 (s, 3F, CF3). HRMS (ESI): m/z calcd. for C29H26F3N2O2 [M + H]+ 491.1936, found 491.1941.
(E)-Methyl 2-(dimethylamino)-6-(4-(diphenylamino)phenyl)-4-methyl-2-(trifluoromethyl) hex-3-en-5-ynoate (3i). Yield 61% (66.5 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 7.28–7.24 (m, 6H, Ar), 7.10–7.03 (m, 6H, Ar), 6.96 (d, J = 8.5 Hz, 2H, Ar), 5.91 (s, 1H, CH), 3.80 (s, 3H, OCH3), 2.52 (s, 6H, N(CH3)2), 2.08 (s, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 167.3, 148.1, 147.1, 132.5, 129.4, 128.7, 126.7, 125.8 (q, J = 297.1 Hz, CF3), 125.0, 123.6, 122.0, 115.4, 90.9, 89.0, 73.8 (q, J = 24.7 Hz, >C<), 52.7, 40.0, 18.7. 19F-NMR (376 MHz, CDCl3) δ -66.69 (s, 3F, CF3). HRMS (ESI): m/z calcd. for C29H27F3N2O2 [M]+ 492.2019, found 492.2021.
(E)-Methyl 2-(dimethylamino)-4-methyl-6-o-tolyl-2-(trifluoromethyl)hex-3-en-5-ynoate (3j). Yield 76% (86.1 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 7.40 (d, J = 7.5 Hz, 1H, Ar), 7.23–7.18 (m, 2H, Ar), 7.15–7.11 (m, 1H, Ar), 5.95 (s, 1H, CH), 3.81 (s, 3H, OCH3), 2.53 (s, 6H, N(CH3)2), 2.43 (s, 3H, CH3), 2.12 (s, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 167.3, 140.2, 131.9, 129.4, 128.7, 128.5, 126.9, 125.8 (q, J = 297.0 Hz, CF3), 125.5, 122.5, 95.5, 87.7, 73.8 (q, J = 24.1 Hz, >C<), 52.7, 40.0, 20.6, 18.8. 19F-NMR (376 MHz, CDCl3) δ -66.71 (s, 3F, CF3). HRMS (ESI): m/z calcd. for C18H21F3NO2 [M + H]+ 340.1519, found 340.1518.
(E)-Methyl 6-(2-(cyanomethyl)phenyl)-2-(dimethylamino)-4-methyl-2-(trifluoromethyl) hex-3-en-5-ynoate (3k). Yield 81% (78.7 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 7.47–7.45 (m, 2H, Ar), 7.36–7.27 (m, 2H, Ar), 5.98 (s, 1H, CH), 3.86 (s, 2H, CH2), 3.81 (s, 3H, OCH3), 2.51 (s, 6H, N(CH3)2), 2.12 (s, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 167.1, 132.4, 131.7, 129.2, 128.4, 128.1, 127.9, 125.6 (q, J = 296.7 Hz, CF3), 122.3, 117.2, 97.6, 85.3, 73.8 (q, J = 24.3 Hz, >C<), 52.8, 40.0, 22.7, 18.6. 19F-NMR (376 MHz, CDCl3) δ -66.70 (s, 3F, CF3). HRMS (ESI): m/z calcd. for C19H20F3N2O2 [M + H]+ 365.1471, found 365.1472.
(E)-Methyl 2-(dimethylamino)-4-methyl-2-(trifluoromethyl)dec-3-en-5-ynoate (3l). Yield 66% (72.2 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 5.72 (s, 1H, CH), 3.75 (s, 3H, OCH3), 2.45 (s, 6H, N(CH3)2), 2.26 (t, J = 7.0 Hz, 2H, CH2), 1.93 (s, 3H, CH3), 1.51–1.44 (m, 2H, CH2), 1.42–1.33 (m, 2H, CH2), 0.88 (t, J = 7.2 Hz, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 167.4, 128.9, 125.9, 125.8 (q, J = 297.0 Hz, CF3), 89.8, 82.9, 73.6 (q, J = 24.0 Hz, >C<), 52.5, 39.9, 30.6, 21.9, 18.9, 18.8, 13.5. 19F-NMR (376 MHz, CDCl3) δ -66.91 (s, 3F, CF3). HRMS (ESI): m/z calcd. for C15H23F3NO2 [M + H]+ 306.1676, found: 306.1682.
(E)-Methyl 2-(dimethylamino)-4-methyl-2-(trifluoromethyl)dodec-3-en-5-ynoate (3m). Yield 61% (63.8 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 5.74 (s, 1H, CH), 3.77 (s, 3H, OCH3), 2.46 (s, 6H, N(CH3)2), 2.26 (t, J = 7.1 Hz, 2H, CH2), 1.95 (s, 3H, CH3), 1.54–1.47 (m, 2H, CH2), 1.40–1.33 (m, 2H, CH2), 1.30–1.24 (m, 4H, CH2), 0.87 (t, J = 6.8 Hz, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 167.4, 128.9, 125.9, 125.8 (q, J = 297.1 Hz, CF3), 89.9, 82.9, 73.6 (q, J = 23.9 Hz, >C<), 52.6, 39.9, 31.3, 28.5, 22.5, 19.2, 18.9, 13.9. 19F-NMR (376 MHz, CDCl3) δ -66.87 (s, 3F, CF3). HRMS (ESI): m/z calcd. for C17H27F3NO2 [M + H]+ 334.1988, found 334.1988.
(E)-Methyl 2-(dimethylamino)-7-hydroxy-4,7-dimethyl-2-(trifluoromethyl)oct-3-en-5- ynoate (3n). Yield 73% (81.4 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 5.77 (s, 1H, CH), 3.75 (s, 3H, OCH3), 2.44 (s, 6H, N(CH3)2), 2.29 (s, 1H, OH), 1.94 (s, 3H, CH3), 1.49 (s, 6H, 2CH3). 13C-NMR (101 MHz, CDCl3) δ 167.2, 128.0, 127.4, 125.7 (q, J = 296.9 Hz, CF3), 93.0, 84.2, 73.6 (q, J = 24.0 Hz, >C<), 65.3, 52.6, 39.9, 31.3, 18.6. 19F-NMR (376 MHz, CDCl3) δ -66.83 (s, 3F, CF3). HRMS (ESI): m/z calcd. for C14H21F3NO3 [M + H]+ 308.1468, found: 308.1468.
(E)-Methyl 2-(dimethylamino)-4-methyl-2-(trifluoromethyl)-6-(trimethylsilyl)hex-3-en-5- ynoate (3o). Yield 70% (58.6 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 5.87 (s, 1H, CH), 3.76 (s, 3H, OCH3), 2.46 (s, 6H, N(CH3)2), 1.97 (s, 3H, CH3), 0.16 (s, 9H, 3CH3). 13C-NMR (101 MHz, CDCl3) δ 167.1, 128.5, 128.2, 125.7 (q, J = 297.0 Hz, CF3), 107.0, 93.3, 73.7 (q, J = 24.0 Hz, >C<), 52.6, 39.9, 18.5, -0.2. 19F-NMR (376 MHz, CDCl3) δ -66.76 (s, 3F, CF3). HRMS (ESI): m/z calcd. for C14H23F3NO2Si [M + H]+ 322.1445, found: 322.1443.
(E)-Methyl 2-(dimethylamino)-4,7,7-trimethyl-2-(trifluoromethyl)oct-3-en-5-ynoate (3p). Yield 62% (52.4 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 5.70 (s, 1H, CH), 3.77 (s, 3H, OCH3), 2.46 (s, 6H, N(CH3)2), 1.94 (s, 3H, CH3), 1.21 (s, 9H, CH3). 13C-NMR (101 MHz, CDCl3) δ 167.4, 129.0, 125.8 (q, J = 297.1 Hz, CF3), 125.5, 97.8, 81.4, 73.6 (q, J = 24.2 Hz, >C<), 52.5, 39.9, 30.9, 27.7, 19.1. 19F-NMR (376 MHz, CDCl3) δ -66.80 (s, 3F, CF3). HRMS (ESI): m/z calcd. for C15H23F3NO2 [M + H]+ 306.1675, found: 306.1673.
Methyl 2-(dimethylamino)-7,7-dimethyl-4-methylene-2-(trifluoromethyl)oct-5-ynoate (3p’). Yield 9% (7.5 mg) as a pale yellow thick oil. 1H-NMR (300 MHz, CDCl3) δ 5.96–5.93 (m, 2H, 2CH), 3.85 (s, 3H, OCH3), 3.01 (d, J = 3.1 Hz, 2H, CH2), 2.52 (s, 6H, N(CH3)2), 1.23 (s, 9H, 3CH3). 19F-NMR (282 MHz, CDCl3) δ -66.65 (s, 3F, CF3). HRMS (ESI): m/z calcd. for C15H23F3NO2 [M + H]+ 306.1675, found: 306.1674.
(E)-Diethyl 2-(dimethylamino)-2-(2-methyl-4-phenylbut-1-en-3-ynyl)malonate (4a). Yield 74% (76.5 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 7.42–7.40 (m, 2H, Ar), 7.29–7.27 (m, 3H, Ar), 6.29 (s, 1H, CH), 4.26–4.20 (m, 4H, 2OCH2), 2.37 (s, 6H, N(CH3)2), 2.02 (s, 3H, CH3), 1.26 (t, J = 7.1 Hz, 6H, 2CH3). 13C-NMR (101 MHz, CDCl3) δ 167.5, 131.9, 131.5, 128.2, 128.1, 125.7, 123.1, 92.1, 87.8, 75.1, 61.3, 40.5, 18.6, 14.2. HRMS (ESI): m/z calcd. for C20H26NO4 [M + H]+ 344.1856, found 344.1854.
(E)-Diethyl 2-(dimethylamino)-2-(2-methyl-4-p-tolylbut-1-en-3-ynyl)malonate (4b). Yield 72% (72 mg) as a pale yellow thick oil. 1H-NMR (300 MHz, CDCl3) δ 7.35 (d, J = 8.1 Hz, 2H, Ar), 7.13 (d, J = 7.9 Hz, 2H, Ar), 6.30 (s, 1H, CH), 4.31–4.24 (m, 4H, 2OCH2), 2.41 (s, 6H, N(CH3)2), 2.36 (s, 3H, CH3), 2.05 (s, 3H, CH3), 1.31 (t, J = 7.1 Hz, 6H, 2CH3). 13C-NMR (101 MHz, CDCl3) δ 167.5, 138.2, 131.5, 131.4, 128.9, 125.8, 120.0, 91.5, 88.0, 75.1, 61.3, 40.4, 21.4, 18.6, 14.2. HRMS (ESI): m/z calcd. for C21H28NO4 [M + H]+ 358.2013, found 358.2007.
(E)-Diethyl 2-(4-(4-(cyanomethyl)phenyl)-2-methylbut-1-en-3-ynyl)-2-(dimethylamino)malonate (4c). Yield 57% (72.3 mg) as a pale yellow thick oil. 1H-NMR (300 MHz, CDCl3) δ 7.47 (d, J = 8.3 Hz, 2H, Ar), 7.31–7.28 (m, 2H, Ar), 6.35 (s, 1H, CH), 4.32–4.24 (m, 4H, 2OCH2), 3.77 (s, 2H, CH2), 2.42 (s, 6H, N(CH3)2), 2.06 (s, 3H, CH3), 1.31 (t, J = 7.1 Hz, 6H, 2CH3). 13C-NMR (101 MHz, CDCl3) δ 167.4, 132.4, 132.1, 129.8, 127.9, 125.4, 123.1, 117.4, 92.9, 86.9, 75.1, 61.3, 40.4, 23.4, 18.5, 14.2. HRMS (ESI): m/z calcd. for C22H27N2O4 [M + H]+ 383.1965, found 383.1966.
(E)-Diethyl 2-(dimethylamino)-2-(4-(4-methoxyphenyl)-2-methylbut-1-en-3-ynyl)malonate (4d). Yield 60% (65.7 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 7.35 (d, J = 8.9 Hz, 2H, Ar), 6.81 (d, J = 8.9 Hz, 2H, Ar), 6.24 (s, 1H, CH), 4.26–4.20 (m, 4H, 2OCH2), 3.78 (s, 3H, OCH3), 2.37 (s, 6H, N(CH3)2), 2.00 (s, 3H, CH3), 1.26 (t, J = 7.1 Hz, 6H, 2CH3). 13C-NMR (101 MHz, CDCl3) δ 167.5, 159.5, 132.9, 131.1, 125.9, 115.2, 113.9, 90.9, 87.8, 75.0, 61.3, 55.2, 40.5, 18.7, 14.2. HRMS (ESI): m/z calcd. for C21H28NO5 [M + H]+ 374.1962, found 374.1963.
(E)-Diethyl 2-(dimethylamino)-2-(2-methyl-4-(4-nitrophenyl)but-1-en-3-ynyl)malonate (4e). Yield 52% (53.1 mg) as a pale yellow thick oil. 1H-NMR (300 MHz, CDCl3) δ 8.20 (d, J = 8.9 Hz, 2H, Ar), 7.59 (d, J = 8.9 Hz, 2H, Ar), 6.43 (s, 1H, CH), 4.33–4.25 (m, 4H, 2OCH2), 2.42 (s, 6H, N(CH3)2), 2.09 (s, 3H, CH3), 1.32 (t, J = 7.1 Hz, 6H, 2CH3). 13C-NMR (101 MHz, CDCl3) δ 167.3, 146.9, 134.1, 132.2, 130.1, 125.0, 123.5, 97.2, 85.9, 75.1, 61.4, 40.4, 18.2, 14.2. HRMS (ESI): m/z calcd. for C20H25N2O6 [M + H]+ 389.1707, found 389.1709.
(E)-Diethyl 2-(4-(4-(9H-carbazol-9-yl)phenyl)-2-methylbut-1-en-3-ynyl)-2-(dimethylamino)malonate (4f). Yield 70% (75.1 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 8.13 (d, J = 7.7 Hz, 2H, Ar), 7.66 (d, J = 8.2 Hz, 2H, Ar), 7.52 (d, J = 8.1 Hz, 2H, Ar), 7.41–7.38 (m, 4H, Ar), 7.31–7.27 (m, 2H, Ar), 6.41 (s, 1H, CH), 4.29 (q, J = 7.0 Hz, 4H, 2OCH2), 2.44 (s, 6H, N(CH3)2), 2.11 (s, 3H, CH3), 1.31 (t, J = 7.1 Hz, 6H, 2CH3). 13C-NMR (101 MHz, CDCl3) δ 167.5, 140.5, 137.5, 133.1, 132.4, 126.7, 126.0, 125.6, 123.5, 122.1, 120.3, 120.2, 109.7, 93.0, 87.2, 75.2, 61.4, 40.5, 18.6, 14.3. HRMS (ESI): m/z calcd. for C32H33N2O4 [M + H]+ 509.2435, found 509.2430.
(E)-Diethyl 2-(dimethylamino)-2-(4-(4-(diphenylamino)phenyl)-2-methylbut-1-en-3-ynyl) malonate (4g). Yield 61% (63.6 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 7.27–7.23 (m, 6H, Ar), 7.09–7.07 (m, 4H, Ar), 7.05–7.01 (m, 2H, Ar), 6.95 (d, J = 8.7 Hz, 2H, Ar), 6.25 (s, 1H, CH), 4.24 (q, J = 7.1 Hz, 4H, 2OCH2), 2.38 (s, 6H, N(CH3)2), 2.02 (s, 3H, CH3), 1.28 (t, J = 7.1 Hz, 6H, 2CH3). 13C-NMR (101 MHz, CDCl3) δ 167.6, 147.8, 147.1, 132.5, 129.4, 124.9, 123.5, 122.2, 115.9, 91.4, 88.1, 75.1, 61.3, 40.6, 18.8, 14.2. HRMS (ESI): m/z calcd. for C32H35N2O4 [M + H]+ 511.2591, found 511.2581.
(E)-Diethyl 2-(dimethylamino)-2-(2-methyl-4-o-tolylbut-1-en-3-ynyl)malonate (4h). Yield 70% (76.4 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 7.37 (d, J = 7.6 Hz, 1H, Ar), 7.19–7.16 (m, 2H, Ar), 7.13–7.08 (m, 1H, Ar), 6.27 (s, 1H, CH), 4.24 (q, J = 7.1 Hz, 4H, 2OCH2), 2.41 (s, 3H, CH3), 2.38 (s, 6H, N(CH3)2), 2.04 (s, 3H, CH3), 1.27 (t, J = 7.1 Hz, 6H, 2CH3). 13C-NMR (101 MHz, CDCl3) δ 167.5, 140.1, 131.8, 131.4, 129.3, 128.2, 125.9, 125.5, 122.8, 96.1, 86.9, 75.1, 61.3, 40.4, 20.6, 18.7, 14.2. HRMS (ESI): m/z calcd. for C21H28NO4 [M + H]+ 358.2013, found 358.2015.
(E)-Diethyl 2-(4-(2-(cyanomethyl)phenyl)-2-methylbut-1-en-3-ynyl)-2-(dimethylamino) malonate (4i). Yield 69% (82.6 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 7.44–7.43 (m, 2H, Ar), 7.33–7.24 (m, 2H, Ar), 6.31 (s, 1H, CH), 4.23 (q, J = 6.8 Hz, 4H, 2OCH2), 3.85 (s, 2H, CH2), 2.36 (s, 6H, N(CH3)2), 2.04 (s, 3H, CH3), 1.26 (t, J = 6.9 Hz, 6H, 2CH3). 13C-NMR (101 MHz, CDCl3) δ 167.3, 132.9, 132.3, 131.6, 128.9, 128.1, 128.0, 125.2, 122.6, 117.3, 98.2, 84.5, 75.1, 61.4, 40.4, 22.6, 18.5, 14.2. HRMS (ESI): m/z calcd. for C22H27N2O4 [M + H]+ 383.1965, found 383.1966.
(E)-Diethyl 2-(dimethylamino)-2-(2-methyloct-1-en-3-ynyl)malonate (4j). Yield 58% (52.1 mg) as a pale yellow thick oil. 1H-NMR (300 MHz, CDCl3) δ 6.11 (s, 1H, CH), 4.25 (q, J = 7.1 Hz, 4H, 2OCH2), 2.38 (s, 6H, N(CH3)2), 2.31 (t, J = 6.9 Hz, 2H, CH2), 1.92 (s, 3H, CH3), 1.57–1.37 (m, 4H, 2CH2), 1.29 (t, J = 7.1 Hz, 6H, 2CH3), 0.93 (t, J = 7.1 Hz, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 167.6, 130.1, 126.1, 88.8, 83.3, 74.9, 61.2, 40.4, 30.7, 21.9, 18.9, 18.9, 14.1, 13.5. HRMS (ESI): m/z calcd. for C18H30NO4 [M + H]+ 324.2169, found 324.2170.
(E)-Diethyl 2-(dimethylamino)-2-(2-methyldec-1-en-3-ynyl)malonate (4k). Yield 56% (61.6 mg) as a pale yellow thick oil. 1H-NMR (400 MHz, CDCl3) δ 6.05 (s, 1H, CH), 4.20 (q, J = 7.2 Hz, 4H, 2OCH2), 2.32 (s, 6H, N(CH3)2), 2.25 (t, J = 7.1 Hz, 2H, CH2), 1.87 (s, 3H, CH3), 1.48 (p, J = 7.1 Hz, 2H, CH2), 1.38–1.31 (m, 2H, CH2), 1.28–1.22 (m, 10H, 2CH2, 2CH3), 0.85 (t, J = 6.7 Hz, 3H, CH3). 13C-NMR (101 MHz, CDCl3) δ 167.6, 130.0, 126.2, 88.9, 83.3, 74.9, 61.2, 40.4, 31.3, 28.6, 28.5, 22.5, 19.2, 18.9, 14.2, 13.9. HRMS (ESI): m/z calcd. for C20H34NO4 [M + H]+ 352.2482, found 352.2477.
(E)-Diethyl 2-(dimethylamino)-2-(5-hydroxy-2,5-dimethylhex-1-en-3-ynyl)malonate (4l). Yield 65% (63.5 mg) as a pale yellow thick oil. 1H-NMR (300 MHz, CDCl3) δ 6.17 (s, 1H, CH), 4.29–4.21 (m, 4H, 2OCH2), 2.36 (s, 6H, N(CH3)2), 2.04 (br s, 1H, OH), 1.93 (s, 3H, CH3), 1.54 (s, 6H, 2CH3), 1.28 (t, J = 7.1 Hz, 6H, 2CH3). 13C-NMR (101 MHz, CDCl3) δ 167.4, 131.6, 125.2, 92.1, 84.6, 74.9, 65.3, 61.3, 40.4, 31.4, 18.5, 14.1. HRMS (ESI): m/z calcd. for C17H28NO5 [M + H]+ 326.1962, found 326.1968.
(E)-Diethyl 2-(dimethylamino)-2-(2-methyl-4-(trimethylsilyl)but-1-en-3-ynyl)malonate (4m). Yield 67% (68.8 mg) as a pale yellow thick oil. 1H-NMR (300 MHz, CDCl3) δ 6.27 (s, 1H, CH), 4.30–4.22 (m, 4H, 2OCH2), 2.38 (s, 6H, N(CH3)2), 1.96 (s, 3H, CH3), 1.30 (t, J = 7.1 Hz, 6H, 2CH3), 0.20 (s, 9H, 3CH3). 13C-NMR (101 MHz, CDCl3) δ 167.4, 132.6, 125.7, 107.7, 92.1, 74.9, 61.3, 40.4, 18.4, 14.1, -0.1. HRMS (ESI): m/z calcd. for C17H30NO4Si [M + H]+ 340.1939, found 340.1941.
(E)-Diethyl 2-(dimethylamino)-2-(2,5,5-trimethylhex-1-en-3-ynyl)malonate (4n). Yield 68% (85.6 mg) as a pale yellow thick oil. 1H-NMR (300 MHz, CDCl3) δ 6.08 (s, 1H, CH), 4.30–4.22 (m, 4H, 2OCH2), 2.38 (s, 6H, N(CH3)2), 1.91 (s, 3H, CH3), 1.29 (t, J = 7.1 Hz, 6H, 2CH3), 1.24 (s, 9H, 3CH3). 13C-NMR (101 MHz, CDCl3) δ 167.7, 129.7, 126.2, 96.8, 81.7, 74.9, 61.2, 40.4, 30.9, 27.7, 19.0, 14.2. HRMS (ESI): m/z calcd. for C18H30NO4 [M + H]+ 324.2169, found 324.2173.
(E)-Diethyl 2-(dimethylamino)-2-(6,6-dimethylhept-1-en-4-ynyl)malonate (4n’). Yield 5% (6.2 mg) as a pale yellow thick oil. 1H-NMR (300 MHz, CDCl3) δ 6.17 (dt, J = 15.7, 1.8 Hz, 1H, CH), 5.92 (dt, J = 15.8, 5.2 Hz, 1H, CH), 4.27 (q, J = 7.1 Hz, 4H, 2OCH2), 2.99 (dd, J = 5.2, 1.8 Hz, 2H, CH2), 2.42 (s, 6H, N(CH3)2), 1.30 (t, J = 7.1 Hz, 6H, 2CH3), 1.23 (s, 9H, 3CH3). 13C-NMR (101 MHz, CDCl3) δ 168.3, 130.8, 126.7, 74.4, 62.0, 61.3, 51.4, 40.5, 31.2, 27.3, 22.0, 14.1. HRMS (ESI): m/z calcd. for C18H30NO4 [M + H]+ 324.2169, found: 324.2173.
(E)-methyl 6-(2-(5-amino-1-benzyl-1H-1,2,3-triazol-4-yl)phenyl)-2-(dimethylamino)-4- methyl-2-(trifluoromethyl)hex-3-en-5-ynoate (5a). Yield 63% (49.0 mg) as a pale yellow oil. 1H-NMR (400 MHz, Chloroform-d) δ 7.70 (d, J = 7.7 Hz, 1H), 7.50 (d, J = 7.5 Hz, 1H), 7.40 (t, J = 8.6 Hz, 1H), 7.37–7.32 (m, 3H), 7.31–7.27 (m, 3H), 5.96 (s, 1H), 5.44 (s, 2H), 3.80 (s, 3H), 3.72 (s, 2H), 2.48 (s, 6H), 1.88 (s, 3H). 19F-NMR (376 MHz, Chloroform-d) δ -66.66 (s, 3F, CF3). 13C-NMR (101 MHz, Chloroform-d) δ 167.3, 138.1, 134.5, 133.9, 132.9, 132.1, 130.3, 129.4, 129.3, 128.7, 128.3, 128.2, 127.7, 127.5, 127.3, 125.9 (q, J = 295.8 Hz, CF3), 120.1, 95.4, 87.9, 73.9 (q, J = 24.0 Hz, >C<), 52.9, 50.9, 40.2, 18.3. HRMS (ESI): m/z calcd. for C26H27F3N5O2 [M + H]+ 498.2111, found 498.2114.
(E)-diethyl 2-(4-(4-(5-amino-1-benzyl-1H-1,2,3-triazol-4-yl)phenyl)-2-methylbut-1-en- 3-yn-1-yl)-2-(dimethylamino)malonate (5b). Yield 69% (54.0 mg) as a pale yellow oil. 1H-NMR (400 MHz, Chloroform-d) δ 7.59 (d, J = 8.0 Hz, 2H), 7.46 (d, J = 8.2 Hz, 2H), 7.36–7.31 (m, 3H), 7.22 (d, J = 6.6 Hz, 2H), 6.30 (s, 1H), 5.41 (s, 2H), 4.24 (dd, J = 7.0, 2.1 Hz, 4H), 3.81 (s, 2H), 2.38 (s, 6H), 2.03 (s, 3H), 1.28 (t, J = 7.1 Hz, 6H). 13C-NMR (101 MHz, Chloroform-d) δ 167.6, 137.7, 134.1, 132.3, 132.1, 131.6, 130.7, 129.4, 128.7, 127.4, 125.8, 125.3, 121.6, 92.8, 88.0, 75.2, 61.5, 50.8, 40.6, 29.8, 18.7, 14.3. HRMS (ESI+) of C29H33N5O4, m/z: calcd for [M + H]+ 516.2606, found 516.2601; for [M-NMe2]+ 471.2032, found 471.2024.
4. Conclusions
In summary, we have elaborated an efficient synthetic approach to the novel representative of α,α-disubstituted α-amino acid derivatives comprising 1,3-enyne moiety at their side chain. The method is based on Pd-catalyzed hydroalkynylation of functional allenes with various terminal acetylenes. The maximum conversion of starting compounds and the best yields of the target products have been achieved under moderate heating of reaction mixture in 1,4-dioxane in the presence of Pd(OAc)2/PPh3 (2/4 mol%) catalytic system for 4–8 h. The developed strategy is the first example of the metal-catalyzed allene–alkyne coupling to provide a convenient access to new unsaturated derivatives of α-amino acids in good yields and high regio- and stereoselectivity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30173623/s1. The following are available online: copies of 1H, 19F and 13C-NMR spectra for all novel compounds as well as copies of 2D 1H19F-HOESY NMR Spectrum for 3a.
Author Contributions
Conceptualization, D.V.V. and S.N.O.; methodology, S.N.O.; investigation, D.V.V., A.S.B., I.A.G., A.N.P. and P.S.G. (synthesis and NMR investigations); E.P.A. (HRMS spectra registering and characterization); writing—original draft preparation, S.N.O.; writing—review and editing, S.N.O.; supervision, S.N.O.; project administration, S.N.O.; funding acquisition, S.N.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the RSF (grant No. 25-13-00209, https://rscf.ru/en/project/25-13-00209/, accessed on 30 August 2025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
Spectral characterization and NMR study were performed using the equipment of the Center for Collective Use of INEOS RAS with support from the Ministry of Science and Higher Education of the Russian Federation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nicolaou, K.C.; Edmonds, D.J.; Bulger, P.G. Cascade reactions in total synthesis. Angew. Chem. Int. Ed. 2006, 45, 7134–7186. [Google Scholar] [CrossRef]
- Enders, D.; Grondal, C.; Huttl, M.R. Asymmetric organocatalytic domino reactions. Angew. Chem. Int. Ed. 2007, 46, 1570–1581. [Google Scholar] [CrossRef]
- Moyano, A.; Rios, R. Asymmetric organocatalytic cyclization and cycloaddition reactions. Chem. Rev. 2011, 111, 4703–4832. [Google Scholar] [CrossRef] [PubMed]
- Volla, C.M.; Atodiresei, I.; Rueping, M. Catalytic C-C bond-forming multi-component cascade or domino reactions: Pushing the boundaries of complexity in asymmetric organocatalysis. Chem. Rev. 2014, 114, 2390–2431. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.M.L.; Sorensen, E.J. Rapid complexity generation in natural product total synthesis. Chem. Soc. Rev. 2009, 38, 2981–2982. [Google Scholar] [CrossRef]
- Trost, B.M. Atom Economy—A Challenge for Organic Synthesis: Homogeneous Catalysis Leads the Way. Angew. Chem. Int. Ed. Engl. 2003, 34, 259–281. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Dai, W.M.; Tsay, S.C.; Estevez, V.A.; Wrasidlo, W. Designed enediynes: A new class of DNA-cleaving molecules with potent and selective anticancer activity. Science 1992, 256, 1172–1178. [Google Scholar] [CrossRef]
- Trost, B.M. The atom economy—A search for synthetic efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef]
- Fontana, A.; d’Ippolito, G.; D’Souza, L.; Mollo, E.; Parameswaram, P.S.; Cimino, G. New acetogenin peroxides from the Indian sponge Acarnus bicladotylota. J. Nat. Prod. 2001, 64, 131–133. [Google Scholar] [CrossRef]
- Campbell, K.; Kuehl, C.J.; Ferguson, M.J.; Stang, P.J.; Tykwinski, R.R. Coordination-driven self-assembly: Solids with bidirectional porosity. J. Am. Chem. Soc. 2002, 124, 7266–7267. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.; Lee, D.; Kim, S.; Kim, D. Asymmetric total syntheses of (+)-3-(z)-laureatin and (+)-3-(z)-isolaureatin by “lone pair-lone pair interaction-controlled” isomerization. J. Am. Chem. Soc. 2007, 129, 2269–2274. [Google Scholar] [CrossRef]
- Wessig, P.; Muller, G. The dehydro-Diels-Alder reaction. Chem. Rev. 2008, 108, 2051–2063. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Kottirsch, G. Novel allene-acetylene cross-condensation catalyzed by palladium complexes. J. Am. Chem. Soc. 2002, 112, 2816–2818. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Omata, K.; Hirama, M. Rhodium-catalyzed cross coupling of unactivated allenes and 1-alkynes. Tetrahedron Lett. 1994, 35, 5689–5692. [Google Scholar] [CrossRef]
- Bruyere, D.; Grigg, R.; Hinsley, J.; Hussain, R.K.; Korn, S.; Orgaz De La Cierva, C.; Sridharan, V.; Wang, J. Highly regioselective palladium/copper-catalysed cross-coupling reactions of terminal alkynes and allenes. Tetrahedron Lett. 2003, 44, 8669–8672. [Google Scholar] [CrossRef]
- Jeanne-Julien, L.; Masson, G.; Kouoi, R.; Regazzetti, A.; Genta-Jouve, G.; Gandon, V.; Roulland, E. Stereoselective Access to (E)-1,3-Enynes through Pd/Cu-Catalyzed Alkyne Hydrocarbation of Allenes. Org. Lett. 2019, 21, 3136–3141. [Google Scholar] [CrossRef]
- Blieck, R.; Taillefer, M.; Monnier, F. Metal-Catalyzed Intermolecular Hydrofunctionalization of Allenes: Easy Access to Allylic Structures via the Selective Formation of C-N, C-C, and C-O Bonds. Chem. Rev. 2020, 120, 13545–13598. [Google Scholar] [CrossRef]
- Liu, Z.K.; Yang, Y.; Zhan, Z.P. Preparation of (E)-1,3-Enyne Derivatives through Palladium Catalyzed Hydroalkynylation of Allenes. J. Org. Chem. 2022, 87, 1589–1597. [Google Scholar] [CrossRef]
- Pradhan, T.R.; Park, J.K. Intermolecular Allene–Alkyne Coupling: A Significantly Useful Synthetic Transformation. ACS Catal. 2024, 14, 7814–7845. [Google Scholar] [CrossRef]
- Guo, X.-X.; Sawano, T.; Nishimura, T.; Hayashi, T. Rhodium-catalyzed enantioselective alkynylative cyclization of allenyl aldehydes with terminal alkynes. Tetrahedron Asymmetry 2010, 21, 1730–1736. [Google Scholar] [CrossRef]
- Nishimura, T.; Guo, X.X.; Hayashi, T. Rhodium-catalyzed asymmetric addition of terminal alkynes to diarylphosphinylallenes. Chem. Asian J. 2008, 3, 1505–1510. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Kido, Y.; Omata, K.; Hirama, M. Synthesis of Exo-enynes by Ruthenium-Catalyzed Cross Coupling Reaction of Hydroxy Allenes and 1-Alkynes. Synlett 1995, 1995, 1181–1182. [Google Scholar] [CrossRef]
- Sawano, T.; Ou, K.; Nishimura, T.; Hayashi, T. Cobalt-catalyzed asymmetric addition of silylacetylenes to 1,1-disubstituted allenes. J. Org. Chem. 2013, 78, 8986–8993. [Google Scholar] [CrossRef]
- Rubin, M.; Markov, J.; Chuprakov, S.; Wink, D.J.; Gevorgyan, V. Highly regiocontrolled Pd-catalyzed cross-coupling reaction of terminal alkynes and allenylphosphine oxides. J. Org. Chem. 2003, 68, 6251–6256. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, T.R.; Kim, H.W.; Park, J.K. Regiodivergent Synthesis of 1,3- and 1,4-Enynes through Kinetically Favored Hydropalladation and Ligand-Enforced Carbopalladation. Angew. Chem. Int. Ed. 2018, 57, 9930–9935. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zheng, E.; Li, G.; Luo, Y.; Huo, X.; Ma, S.; Zhang, W. Stereodivergent access to non-natural alpha-amino acids via enantio- and Z/E-selective catalysis. Science 2024, 385, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.; Kinderman, S.S.; van Esseveldt, B.C.; van Delft, F.L.; Schoemaker, H.E.; Blaauw, R.H.; Rutjes, F.P. Synthetic applications of aliphatic unsaturated alpha-H-alpha-amino acids. Org. Biomol. Chem. 2005, 3, 3435–3467. [Google Scholar] [CrossRef]
- Rutjes, F.P.J.T.; Wolf, L.B.; Schoemaker, H.E. Applications of aliphatic unsaturated non-proteinogenic a-H-a-amino acids. J. Chem. Soc. Perkin Trans. 2000, 1, 4197–4212. [Google Scholar] [CrossRef]
- Vorobyeva, D.V.; Petropavlovskikh, D.A.; Godovikov, I.A.; Nefedov, S.E.; Osipov, S.N. Rh(III)-Catalyzed C−H Activation/Annulation of Aryl Hydroxamates with CF3-Containing α-Propargyl α-Amino Acid Derivatives. Eur. J. Org. Chem. 2021, 2021, 1883–1890. [Google Scholar] [CrossRef]
- Mailyan, A.K.; Peregudov, A.S.; Dixneuf, P.H.; Bruneau, C.; Osipov, S.N. Cyclobutene ring-opening of bicyclo [4.2.0]octa-1,6-dienes: Access to CF3-substituted 5,6,7,8-tetrahydro-1,7-naphthyridines. J. Org. Chem. 2012, 77, 8518–8526. [Google Scholar] [CrossRef]
- Philippova, A.N.; Vorobyeva, D.V.; Monnier, F.; Osipov, S.N. Synthesis of alpha-CF(3)-substituted E-dehydroornithine derivatives via copper(i)-catalyzed hydroamination of allenes. Org. Biomol. Chem. 2020, 18, 3274–3280. [Google Scholar] [CrossRef] [PubMed]
- Vorobyeva, D.V.; Bubnova, A.S.; Godovikov, I.A.; Smol’yakov, A.F.; Osipov, S.N. Rh(III)-Catalyzed Annulation of (Het)aryl Amides with α-Allenyl-Containing α-Amino Acid Derivatives. Asian J. Org. Chem. 2024, 14, e202400476. [Google Scholar] [CrossRef]
- Bubnova, A.S.; Philippova, A.N.; Gribanov, P.S.; Smol’yakov, A.F.; Osipov, S.N.; Vorobyeva, D.V. Pd(II)-catalyzed regioselective hydroarylation of allenyl-containing alpha-amino acid derivatives with aryl boronic acids. Org. Biomol. Chem. 2025, 23, 5396–5400. [Google Scholar] [CrossRef]
- Smits, R.; Cadicamo, C.D.; Burger, K.; Koksch, B. Synthetic strategies to alpha-trifluoromethyl and alpha-difluoromethyl substituted alpha-amino acids. Chem. Soc. Rev. 2008, 37, 1727–1739. [Google Scholar] [CrossRef]
- Moschner, J.; Stulberg, V.; Fernandes, R.; Huhmann, S.; Leppkes, J.; Koksch, B. Approaches to Obtaining Fluorinated alpha-Amino Acids. Chem. Rev. 2019, 119, 10718–10801. [Google Scholar] [CrossRef]
- Mei, H.; Han, J.; White, S.; Graham, D.J.; Izawa, K.; Sato, T.; Fustero, S.; Meanwell, N.A.; Soloshonok, V.A. Tailor-Made Amino Acids and Fluorinated Motifs as Prominent Traits in Modern Pharmaceuticals. Chem. Eur. J. 2020, 26, 11349–11390. [Google Scholar] [CrossRef]
- Vorobyeva, D.V.; Mailyan, A.K.; Peregudov, A.S.; Karimova, N.M.; Vasilyeva, T.P.; Bushmarinov, I.S.; Bruneau, C.; Dixneuf, P.H.; Osipov, S.N. Synthesis of functionalized CF3-containing heterocycles via [2,3]-sigmatropic rearrangement and sequential catalytic carbocyclization. Tetrahedron 2011, 67, 3524–3532. [Google Scholar] [CrossRef]
- Krishna, P.M.; Ramachary, D.B.; Peesapati, S. Azide–acetonitrile “click” reaction triggered by Cs2CO3: The atom-economic, high-yielding synthesis of 5-amino-1,2,3-triazoles. RSC Adv. 2015, 5, 62062–62066. [Google Scholar] [CrossRef]
- Gribanov, P.S.; Philippova, A.N.; Topchiy, M.A.; Lypenko, D.A.; Dmitriev, A.V.; Tokarev, S.D.; Smol’yakov, A.F.; Rodionov, A.N.; Asachenko, A.F.; Osipov, S.N. Synthesis of 5-(Aryl)amino-1,2,3-triazole-containing 2,1,3-Benzothiadiazoles via Azide-Nitrile Cycloaddition Followed by Buchwald-Hartwig Reaction. Molecules 2024, 29, 2151. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).