Managing Vibrio parahaemolyticus and Vibrio alginolyticus Infections in the Whiteleg Shrimp (Penaeus vannamei): A Systematic Review
Abstract
1. Introduction
2. Results
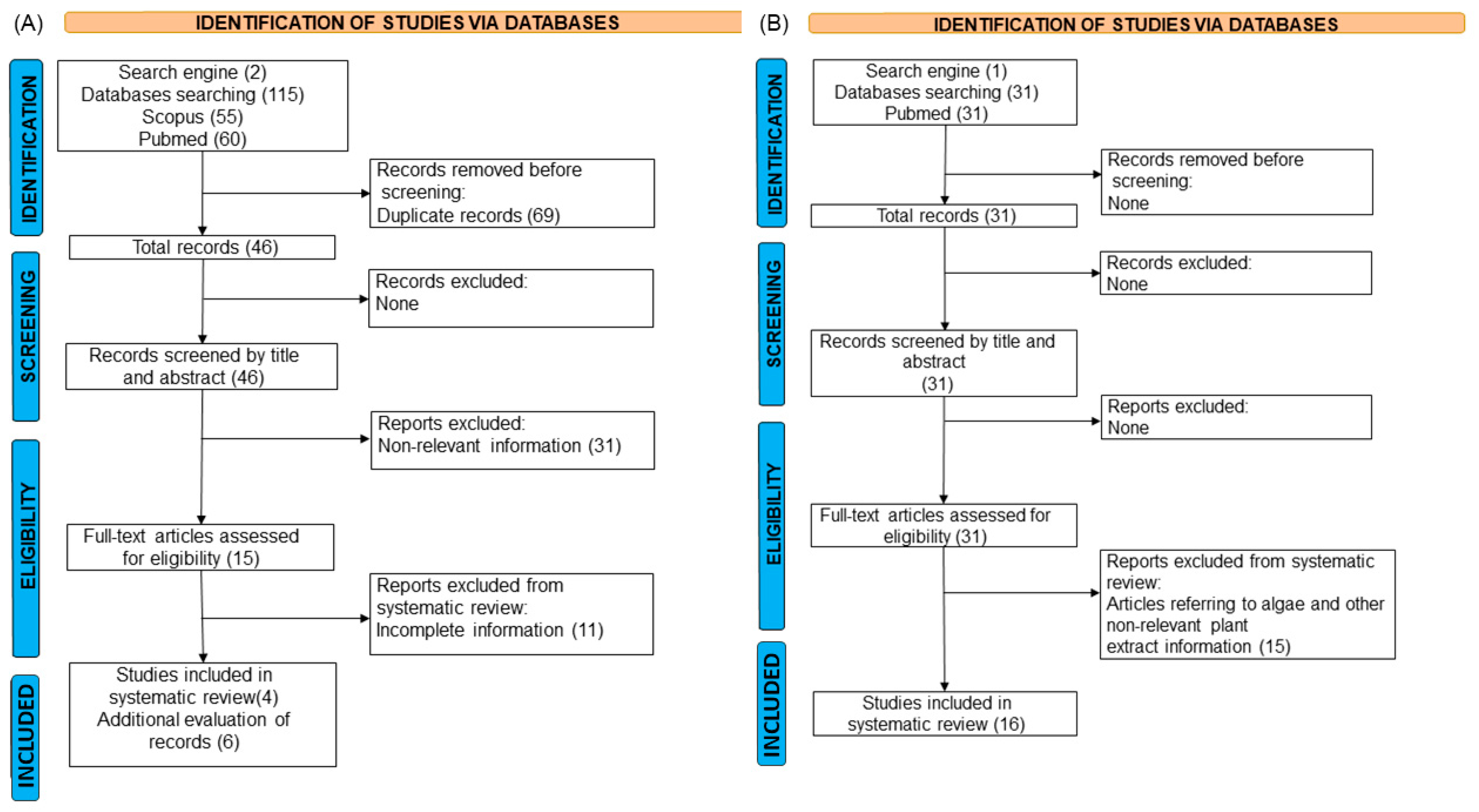
2.1. Study Inclusion Criteria and Characteristics of the Eligible Studies
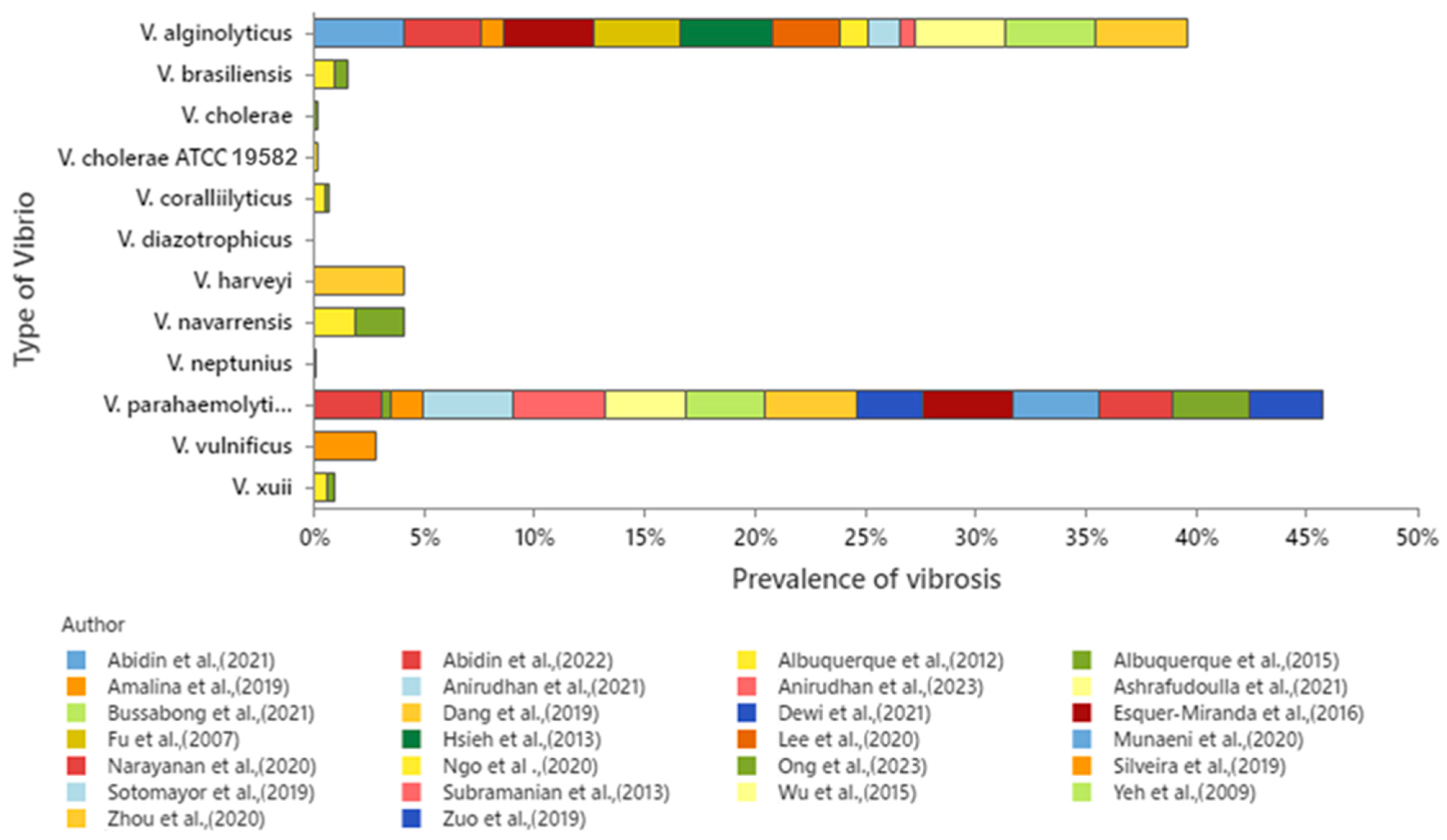
2.2. General Effects of Vibrio Species in Penaeus vannamei
| Study | Region | Country | Vibrio Species | Antibiotics | Families | Susceptibility |
|---|---|---|---|---|---|---|
| [27,28] | South America | Brazil | V. parahaemolyticus | Oxytetracycline | Tetracyclines | Intermediate |
| V. parahaemolyticus | Ampicillin, Colistin, Streptomycin | Penicillins, Aminoglycosides | Resistant | |||
| V. parahaemolyticus | Penicillin, Tetracycline | Penicillins, Tetracyclines | Resistant | |||
| Other Vibrio species: V. brasiliensis, V. cholerae, V. coralliilyticus, V. navarrensis, V. vulnificus, V. xuii, and V. cholerae ATCC 19582 | Ampicillin, Aztreonam, Ceftriaxone, Cephalothin, Chloramphenicol, Ciprofloxacin, Gentamicin, Imipenem, Nalidixic acid, Nitrofurantoin, Penicillin, Streptomycin, Sulfamethoxazole-trimethoprim, Tetracycline | Penicillins, Cephalosporins, Aminoglycosides, Fluoroquinolones, Tetracyclines, Sulfonamides, Carbapenems, Monobactams, Amphenicols, Nitrofuranos | Sensitive | |||
| [23] | South America | Ecuador | V. alginolyticus | Nalidixic acid, Chloramphenicol, Ciprofloxacin, Enrofloxacin, Florfenicol, Fosfomycin, Furazolidone, Norfloxacin | Quinolone, Amphenicols, Fluoroquinolones Phosphonic acids, Nitrofurans | Sensitive |
| [1] | Asia | India | V. parahaemolyticus | Chloramphenicol, Gentamicin, Meropenem, Tetracycline, Trimethoprim/sulfamethoxazole | Amphenicols, Aminoglycosides, Carbapenems, Tetracyclines, Sulfonamide/Trimethoprim | Sensitive |
| Other Studies | ||||||
| [29] | South America | Brazil | Other Vibrio species: V. vulnificus | Chloramphenicol, Ciprofloxacin, Norfloxacin | Amphenicols, Fluoroquinolones | Intermediate |
| Other Vibrio species: V. vulnificus | Amikacin, Ampicillin, Enrofloxacin, Gentamicin, Neomycin, Penicillin, Tetracycline | Aminoglycosides, Penicillins, Fluoroquinolones, Tetracyclines | Resistant | |||
| [30] | Asia | Malaysia | V. cholerae | Streptomycin, Erythromycin, Tetracycline | Aminoglycosides, Macrolides, Tetracyclines | Sensitive |
| V. parahaemolyticus | Bacitracin | Polypeptides | Intermediate | |||
| V. parahaemolyticus Other Vibrio species: V. cholerae | Penicillins, Glycopeptides | Penicillins, Glycopeptides | Resistant | |||
| [5] | Asia | Korea | V. parahaemolyticus | Vancomycin, Amikacin, Cefepime, Cefotaxime, Ceftriaxone, Erythromycin, Imipenem, Streptomycin, Ticarcillin-clavulanic acid | Glycopeptides, Aminoglycosides, Cephalosporins, Macrolides, Carbapenems, Penicillin/Beta-lactamase | Sensitive |
| V. parahaemolyticus | Amikacin, Ampicillin, Cefepime, Ceftazidime, Chloramphenicol, Polymyxin B, Tazobactam-piperacillin | Glycopeptides, Aminoglycosides, Cephalosporins, Macrolides, Penicillin/Beta-lactamase | Intermediate | |||
| V. parahaemolyticus | Amikacin, Ampicillin, Cefepime, Ciprofloxacin, Clarithromycin, Doxycycline, Gentamicin, Kanamycin, Levofloxacin, Minocycline, Nalidixic acid, Penicillin, Sulfamethoxazole- trimethoprim, Tetracycline, Tobramycin | Aminoglycosides, Penicillins, Cephalosporins, Fluoroquinolones, Macrolides, Tetracyclines, Sulfonamides/Trimethoprim | Resistant | |||
| [31] | Asia | Singapore | V. parahaemolyticus | Ampicillin/sulbactam, Cefotaxime, Chloramphenicol, Ciprofloxacin, Sulfamethoxazole- trimethoprim, Tetracycline | Penicillin/Beta-lactamase, Cephalosporins, Chloramphenicols, Fluoroquinolones, Sulfonamides/Trimethoprim, Tetracyclines | Sensitive |
| V. parahaemolyticus | Ampicillin, Penicillin | Penicillins | Resistant | |||
| [32] | Asia | Vietnam | V. parahaemolyticus | Chloramphenicol, Gentamicin, Kanamycin, Nalidixic acid, Oxacillin, Tebipenem | Amphenicols, Aminoglycosides, Quinolones, Penicillins, Carbapenems | Sensitive |
| [33] | Asia | China | V. alginolyticus | Chloramphenicol, Doxycycline, Minocycline | Chloramphenicols, Tetracyclines | Sensitive |
| V. alginolyticus | Amikacin, Cefperazone, Gentamicin, Kanamycin, Tetracycline | Aminoglycosides, Cephalosporins, Tetracyclines | Intermediate | |||
| V. alginolyticus | Ampicillin, Carbenicillin, Cefazolin, Cefperazone, Ceftazidime, Ceftriaxone, Cefuroxime, Cephalexin, Cephradine, Ciprofloxacin, Clarithromycin, Erythromycin, Furazolidone, Nalidixic acid, Ofloxacin, Oxacillin, Penicillin, Piperacillin, Polymyxin B, SMZ/TMP, Vancomycin | Penicillins, Cephalosporins, Fluoroquinolones, Macrolides, Nitrofurans, Quinolones, Polymyxins, Sulfonamides/Trimethoprim, Glycopeptides | Resistant | |||
| Study | Region | Country | Vibrio Species | Plant | Effectiveness * | Type of Extract |
|---|---|---|---|---|---|---|
| [34] | North America | Mexico | V. parahaemolyticus, V. alginolyticus | Caulerpa sertularioides | High | Methanolic extract |
| [35] | Asia | Indonesia | V. parahaemolyticus | Eleutherine bulbosa | Intermediate/High | Ethanolic extract |
| [36] | Asia | Korea | V. alginolyticus | Rubus coreanus | High | Ethanolic extract |
| [37,38] | Asia | Malaysia | V. parahaemolyticus | Pandanus tectorius | Intermediate/High | Methanolic extract |
| [39] | Asia | Taiwan | V. alginolyticus | Cinnamomum kanehirae | Intermediate/High | Water extract |
| [40] | Asia | Taiwan | V. alginolyticus | Gelidium amansii | High | Water extract |
| [41] | Asia | Taiwan | V. alginolyticus | Gynura bicolor | Intermediate/High | Water extract |
| [42] | Asia | Taiwan | V. alginolyticus | Gynura bicolor | Intermediate | Water extract |
| [43] | Asia | Taiwan | V. alginolyticus | Phyllanthus amarus | Intermediate/High | Water extract |
| [44] | Asia | Taiwan | V. parahaemolyticus | Psidium guajava | Intermediate/High | Water extract |
| [45,46] | Asia | Taiwan | V. parahaemolyticus and V. alginolyticus | Moringa oleifera | Intermediate/High | Water extract |
| [47] | Asia | Taiwan | V. alginolyticus | Theobroma cacao | High | Extract from fresh peels |
| [48] | Asia | Thailand | V. parahaemolyticus | Macleaya cordata | High | Water extract |
| [49] | Asia | Vietnam | V. parahaemolyticus Other Vibrio species: V. harveyi | Rhodomyrtus tomentosa | Intermediate | Seed extract |
2.3. Prevalence of Vibrio-Related Infections in P. vannamei and Their Geographical Distribution
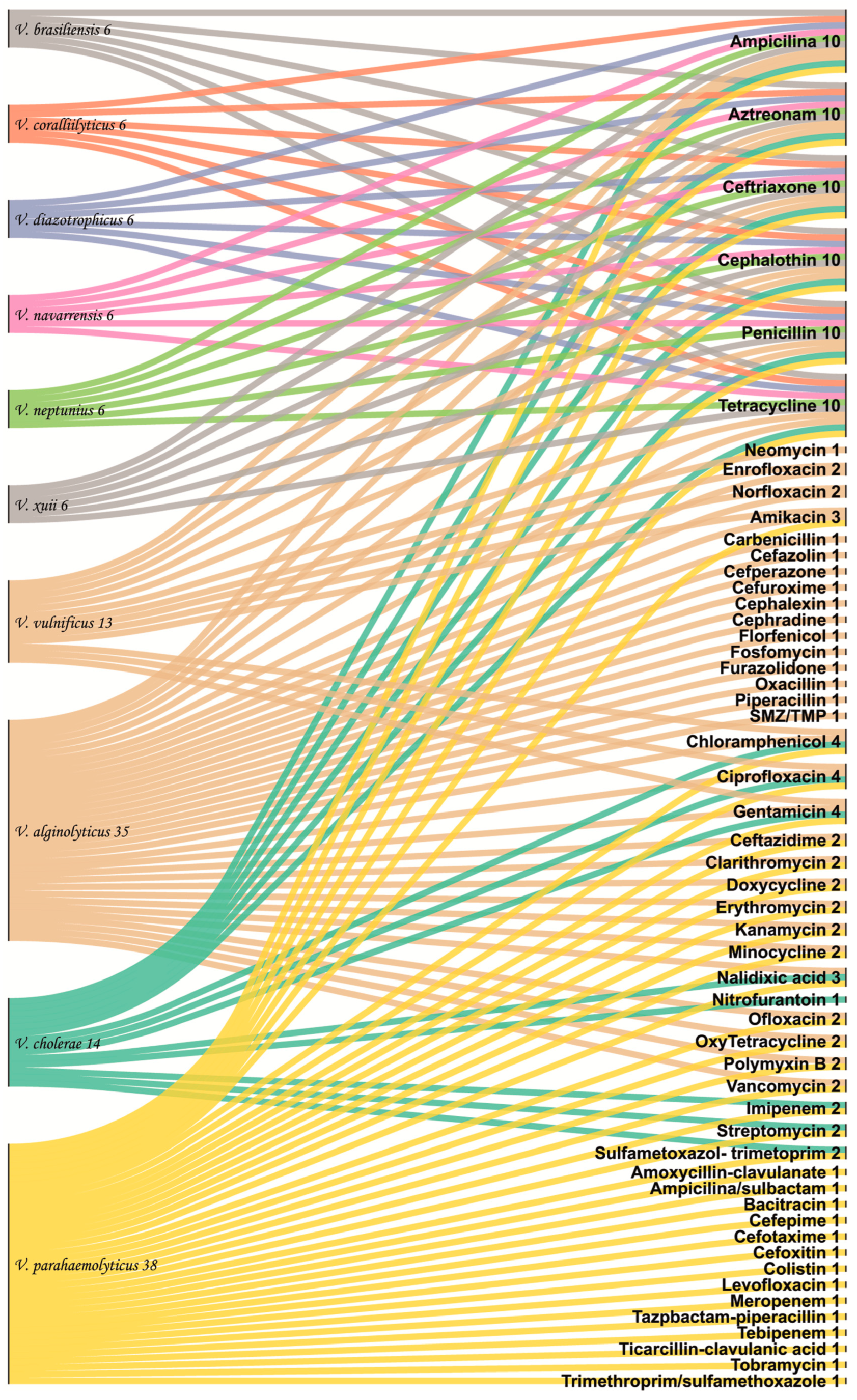
2.4. Antibiotics Studies
| Vibrio Species | Antibiotics Families | Concentration (μg) | Conditions | N° Strains Susceptibility | Studies |
|---|---|---|---|---|---|
| V. alginolyticus | Aminoglycosides | 30 | 28 °C-24 h | 3 Intermediate | [23,27,33] |
| Carbapenems | 30 | 28 °C-24 h | 1 Resistant | ||
| Cephalosporins | 30–75 | 28/35 °C-24 h | 3 Sensitive 1 Intermediate 6 Resistant | ||
| Fluoroquinolones | 5–10 | 30 °C-24 h 28 °C-24 h | 4 Sensitive 3 Resistant | ||
| Glycopeptides | 30 | 28 °C-24 h | 1 Resistant | ||
| Macrolides | 15 | 28 °C-24 h | 2 Resistant | ||
| Monobactam | 30 | 35 °C-24 h | 1 Sensitive | ||
| Nitrofuran | 100 | 30 °C-24 h | 1 Sensitive 1 Resistant | ||
| Penicillins | 1–100 10 U | 28/35 °C-24 h | 2 Sensitive 6 Resistant | ||
| Peptide | 200 (l U) | 28 °C-24 h | 1 Resistant | ||
| Phenicole | 30 | 28/30 °C-24 h | 3 Sensitive | ||
| Phosphonate | 10 | 30 °C-24 h | 1 Sensitive | ||
| Sulfonamide | 23.75/1.25 | 28 °C-24 h | 1 Resistant | ||
| Tetracyclines | 30 | 28/35 °C-24 h | 4 Sensitive 1 Intermediate 2 Resistant | ||
| V. parahaemolyticus | Aminoglycosides | 10–30 | 37 °C-24 h | 69 Sensitive 63 Intermediate 202 Resistant | [1,5,27,30,31,32] |
| Beta-lactamase | 110 | 37 °C-24 h | 16 Sensitive 44 Intermediate 2 Resistant | ||
| Carbapenems | 10–30 | 37 °C-24 h | 73 Sensitive 16 Intermediate 1 Resistant | ||
| Cephalosporins | 30 | 35 °C-24 h 37 °C-24 h | 165 Sensitive 129 Intermediate 65 Resistant | ||
| Fluoroquinolones | 5–30 | 37 °C-24 h | 19 Sensitive 53 Intermediate 144 Resistant | ||
| Glycopeptides | 30 | 37 °C-24 h | 62 Resistant | ||
| Macrolides | 2–15 | 37 °C-24 h | 40 Sensitive 22 Intermediate 62 Resistant | ||
| Monobactam | 30 | 35 °C-24 h | 1 Sensitive | ||
| Penicillins | 5–20 (10 U) | 35/37 °C-24 h | 62 Sensitive 42 Intermediate 158 Resistant | ||
| Peptide | 10–300 | 37 °C-24 h | 8 Sensitive 50 Intermediate 18 Resistant | ||
| Phenicole | 30 | 37 °C-24 h | 59 Sensitive 30 Intermediate 2 Resistant | ||
| Sulfonamide | 1.24–25 | 37 °C-24 h | 28 Sensitive 26 Intermediate 36 Resistant | ||
| Tetracyclines | 30 | 35/37 °C-24 h | 32 Sensitive 9 Intermediate 174 Resistant | ||
| Other Types of Vibrio Analyzed | |||||
| V. cholerae | Aminoglycosides | 10 | 37 °C-24 h | 11 Sensitive 8 Intermediate 4 Resistant | [27,28,30] |
| Cephalosporins | 30 | 35 °C-24 h | 28 Sensitive 1 Intermediate | ||
| Glycopeptides | 30 | 37 °C-24 h | 8 Sensitive 3 Intermediate 2 Resistant | ||
| Macrolides | 15 | 37 °C-24 h | 2 Sensitive 1 Intermediate 10 Resistant | ||
| Monobactam | 30 | 35/37 °C-24 h | 22 Sensitive 6 Intermediate | ||
| Penicillins | 10 (10 U) | 35/37 °C-24 h | 28 Sensitive 7 Intermediate 8 Resistant | ||
| Thetracyclines | 30 | 35/37 °C-24 h | 1 Sensitive 2 Intermediate 11 Resistant | ||
| V. cholerae ATCC 19582 | Aminoglycosides | 10 | NA | 26 Sensitive | [28] |
| Carbapenems | 10 | NA | 26 Sensitive | ||
| Cephalosporins | 30 | NA | 10 Sensitive 3 Intermediate 3 Resistant | ||
| Fluoroquinolones | 5 30 | NA | 25 Sensitive 1 Intermediate | ||
| Nitrofurans | 300 | NA | 26 Sensitive | ||
| Penicillins | 10 | NA | 17 Sensitive 9 Resistant | ||
| Phenicole | 30 | NA | 26 Sensitive | ||
| Sulfonamide | 25 | NA | 26 Sensitive | ||
| Tetracyclines | 30 | NA | 26 Sensitive | ||
| V. brasiliensis | Cephalosporins | 30 | 35 °C-24 h | 2 Sensitive 1 Intermediate | [27] |
| Monobactam | 30 | 35 °C-24 h | 1 Sensitive | ||
| Penicillins | 10 (10 U) | 35 °C-24 h | 1 Sensitive 1 Intermediate 1 Resistant | ||
| Tetracyclines | 30 | 35 °C-24 h | 1 Sensitive | ||
| V. coralliilyticus | Cephalosporins | 30 | 35 °C-24 h | 2 Sensitive | [27] |
| Monobactam | 30 | 35 °C-24 h | 1 Sensitive | ||
| Penicillins | 10 (10 U) | 35 °C-24 h | 1 Sensitive 1 Resistant | ||
| Tetracyclines | 30 | 35 °C-24 h | 1 Sensitive | ||
| V. diazotrophicus | Cephalosporins | 30 | 35 °C-24 h | 2 Sensitive | [27] |
| Monobactam | 30 | 35 °C-24 h | 1 Sensitive | ||
| Penicillins | 10 (10 U) | 35 °C-24 h | 2 Sensitive | ||
| Tetracyclines | 30 | 35 °C-24 h | 1 Sensitive | ||
| V. navarrensis | Cephalosporins | 30 | 35 °C-24 h | 2 Sensitive 2 Intermediate | [27] |
| Monobactam | 30 | 35 °C-24 h | 1 Sensitive 1 Intermediate | ||
| Penicillins | 10 (10 U) | 35 °C-24 h | 1 Sensitive 1 Intermediate 1 Resistant | ||
| Tetracyclines | 30 | 35 °C-24 h | 1 Sensitive | ||
| V. vulnificus | Aminoglycosides | 10–30 | 37 °C–24 h | 2 Sensitive 2 Intermediate 1 Resistant | [27,29] |
| Cephalosporins | 30 | 35 °C-24 h | 3 Sensitive | ||
| Fluoroquinolones | 5 | 37 °C–24 h | 3 Resistant | ||
| Monobactam | 30 | 35 °C-24 h | 2 Sensitive | ||
| Penicillins | 10 (10 U) | 35 °C-24 h 37 °C–24 h | 4 Sensitive 1 Intermediate 2 Resistant | ||
| Phenicole | 30 | 37 °C–24 h | 1 Resistant | ||
| Tetracyclines | 30 | 35 °C-24 h 37 °C–24 h | 1 Sensitive 2 Resistant | ||
| V. neptunius | Cephalosporins | 30 | 35 °C-24 h | 2 Sensitive | [27] |
| Monobactam | 30 | 35 °C-24 h | 1 Sensitive | ||
| Penicillins | 10 (10 U) | 35 °C-24 h | 2 Sensitive | ||
| Tetracyclines | 30 | 35 °C-24 h | 1 Sensitive | ||
| V. xuii | Cephalosporins | 30 | 35 °C-24 h | 2 Sensitive 1 Intermediate | [27] |
| Monobactam | 30 | 35 °C-24 h | 1 Sensitive | ||
| Penicillins | 10 (10 U) | 35 °C-24 h | 1 Sensitive 1 Intermediate 1 Resistant | ||
| Tetracyclines | 30 | 35 °C-24 h | 1 Sensitive | ||
2.5. Plant Extract Evaluation
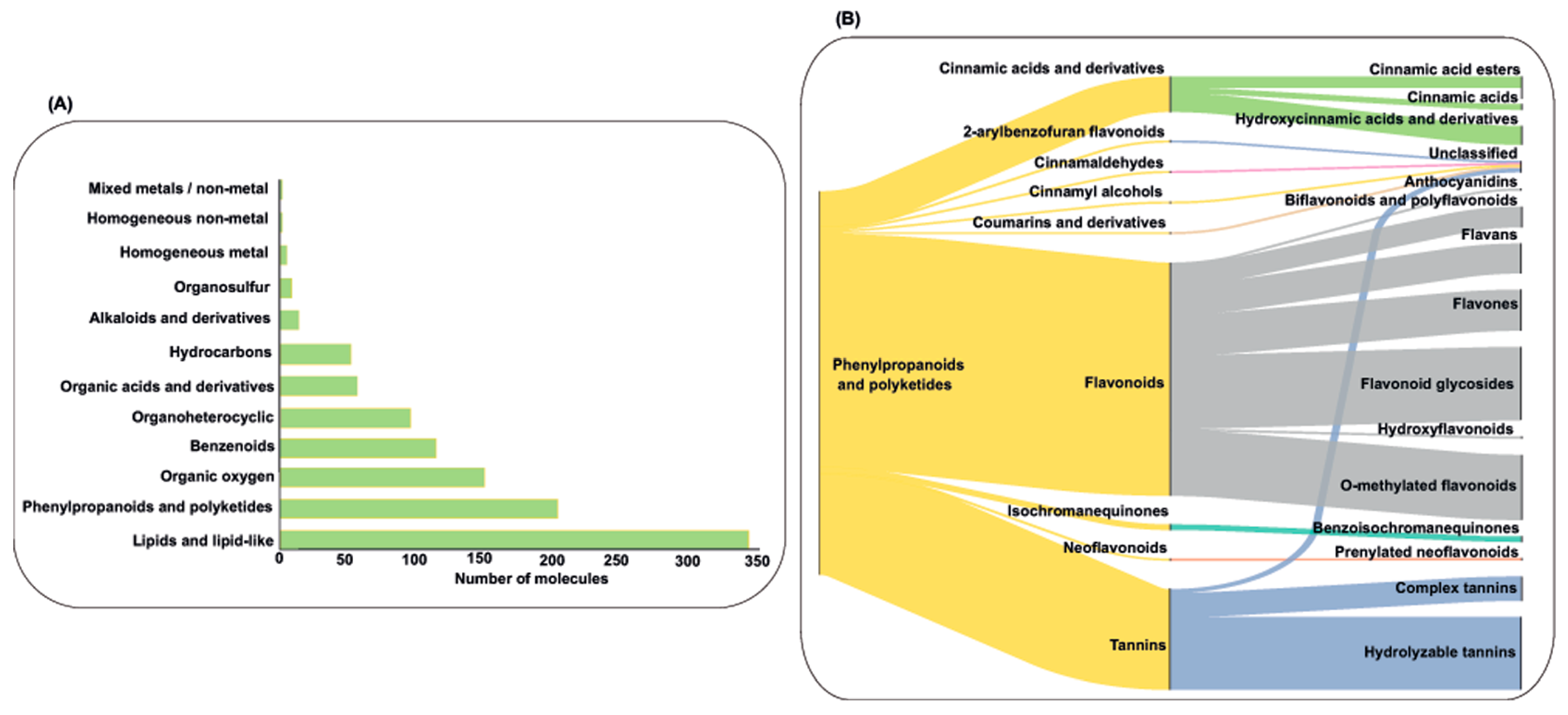
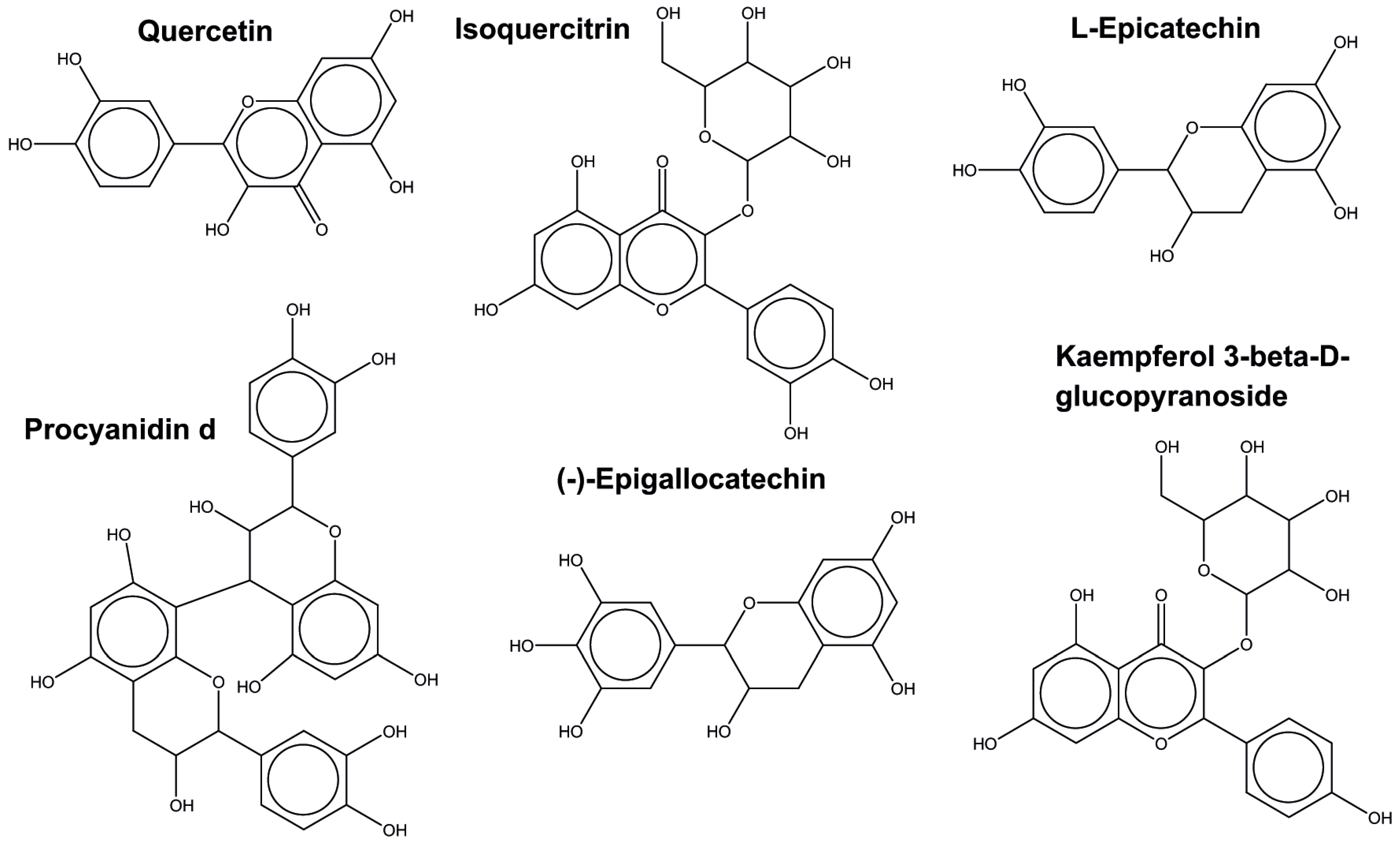
2.5.1. Plant-Related Compounds Analysis
2.5.2. Antibacterial Activity by Plant Extracts
| Vibrio Species | Plant Extracts | Concentration | Conditions | N° Strains Susceptibility | Studies |
|---|---|---|---|---|---|
| V. alginolyticus | Caulerpa sertularioides | 300 mg/L | 25 ± 5 °C-7 days | 1 sensitive | [34,36,39,40,41,42,43,45,47,51] |
| Cinnamomum kanehirae | 2 μg/shrimp | 28 °C-4 days | 1 intermediate 1 sensitive | ||
| Gelidium amansii. | 0.5–2 g/kg | 28 ± 1 °C-6 days | 1 sensitive | ||
| Gynura bicolor | 0.5–2 g/kg (diet) 2–8 μg/g | 27 ± 1 °C-28 days 27 ± 1 °C-2 days | 1 intermediate 1 sensitive | ||
| Rubus coreanus | 0.25–0.5% RcEE diet | 25 ± 0.5 °C-7 days | 1 sensitive | ||
| Theobroma cacao | 10–40 μg/shrimp | 28 ± 1 °C-6 days | 1 sensitive | ||
| Moringa oleifera | 1.25–5 g/kg | 35 °C-24 h | 1 sensitive | ||
| Phyllanthus amarus | 10–40 g/kg | 27 ± 1 °C-56 days | 3 sensitive | ||
| V. parahaemolyticus | Caulerpa sertularioides | 300 mg/L | 25 ± 5 °C-7 days | 1 sensitive | [34,35,37,38,44,45,48,51] |
| Eleutherine bulbosa | 1.25–25 g/kg | 28 ± 1 °C-7 days | 1 intermediate 1 sensitive | ||
| Macleaya cordata | 100–300 mg/kg | 26 ± 1 °C-30 days | sensitive | ||
| Moringa oleifera | 1.25 g/kg 2.5 g/kg 5.0 g/kg | 35 °C-24 h | sensitive | ||
| Pandanus tectorius | 0.5–6 g/L | 28 °C-24 h | 1 sensitive | ||
| Psidium guajava | 1–10 g/kg | 27 ± 2 °C-7 days | sensitive | ||
| Rhodomyrtus tomentosa | 3500 μg/disc | 25 ± 1 °C-7 days | 1 sensitive | ||
| Other Types of Vibrio Analyzed | |||||
| V. harveyi | Rhodomyrtus tomentosa | 3500 μg/disc | 25 ± 1 °C-7 days | 2 sensitive | [49] |
2.6. Strategies for Vibrio-Related Infections in Penaeus vannamei
2.6.1. Strengths and Limitations of Current Strategies
| Strategies | Description | Strengths | Limitations | References |
|---|---|---|---|---|
| Antibiotics | Antibiotics are commonly used in aquaculture both for prophylactic purposes and to treat bacterial infections. The most used are tetracyclines, quinolones, phenicols, macrolides, and sulfonamides among others. They are usually administered directly in balanced food or water. Frequent and indiscriminate use is related to the development of bacterial resistance. | Establish adequate doses and administration times according to shrimp species and production stage. Rotate different families of antibiotics to avoid resistance. Use responsibly, only when necessary, based on laboratory diagnosis. Improve farming conditions to reduce stressful factors that promote infections. | Different concentrations of antibiotics in the aquaculture industry depending on the country-quality requirements and antibiotic residues in the final product The emergence of antibiotic-resistant Vibrio strains that persist in the aquaculture environment. Risk of transfer of resistance genes to human pathogenic bacteria through the food chain. Effects on other organisms in the aquatic ecosystem such as fish, mollusks, and plankton. Generation of antibiotic residues in shrimp tissue that reach the consumer. | [27] |
| Combined therapy (antibiotics plus plant extracts) | The most effective were furazolidone, ciprofloxacin, chloramphenicol, norfloxacin, nalidixic acid, florfenicol, and fosfomycin (inhibited the growth of most or all isolated strains). The effectiveness of essential oil EO1 (oregano oil extract, inhibited 100% of strains). | Furazolidone, ciprofloxacin, chloramphenicol, norfloxacin, nalidixic acid, florfenicol, and fosfomycin, enrofloxacin, inhibited most or all Vibrio strains. Oregano oil extract inhibited all Vibrio strains at low concentrations. Plant essential oils (EOs) combined with antibiotics: EOs can enhance the efficacy of antibiotics against multidrug-resistant pathogens. | Many antibiotics are not approved for use in aquaculture. Also, antimicrobial resistance was found in some strains. Some plant extracts exhibit toxicity, and their mechanisms of action are not well studied. Further, in vivo studies are required to confirm effectiveness. Effective in vitro doses may not correlate with the response in shrimp. More research is needed on specific antimicrobial action mechanisms and synergies with other compounds. | [23,53,54] |
| Plant extracts | Many medicinal plants have antibacterial activity against Vibrio species. Single extracts or a mixture of extracted compounds are used to treat vibriosis in shrimp. There are different types of plant extracts, among them the most used are aqueous, ethanolic, and methanolic. | According to the bibliography, the use of different types of plant extracts has the benefit of shrimp, improving growth and promoting immune activity. Additionally, it is a strategy that provides sustainable, ecological, and safe compounds, such as alkaloids, saponins, terpenoids, and flavonoids, to replace chemical compounds and antibiotics in aquaculture, being a solution for the antibiotic resistance that is currently observed in the industry. | The greatest limitation of this technique is the lack of more studies that apply it in vivo, to be able to generalize the results of efficacy and safety for the host (shrimp) because it has been reported that various plant extracts exhibit toxicity, so their mechanisms of action must be studied in greater depth. In addition, further research must be carried out to be able to make a protocol depending on the type of extract, and the larval stage of the shrimp. | [35,55] |
| Probiotics | This technique consists of administering probiotics, prebiotics, postbiotics, and synbiotics to the shrimp through the diet to combat or prevent Vibrio species. | The use of pro-, post-, pre-, and synbiotics in aquaculture has been considered effective food supplements that are prescribed to improve growth, antioxidant status, immunity, and resistance capacity to diseases such as vibriosis in different species of shrimp and other seafood. | The most significant disadvantage to using pro-, post-, pre-, and synbiotics is that they are often unable to maintain themselves and require supplementation regularly, which results in making this strategy less cost-effective. | [51,56,57] |
2.6.2. Comparative Cost and Efficiency
| Strategy | Representative Agent(s)/Study | Dose/Usage (as Reported) | Reported Efficiency vs. Vibrio | Cost Category * | Notes/Caveats | References |
|---|---|---|---|---|---|---|
| Antibiotics | Oxolinic acid + oxytetracycline | 50 mg OA/kg + 100 mg OTC/kg feed for 5 days; challenged with V. parahaemolyticus | Survival higher than control; combination therapy most effective | Medium–High | Effective in reducing mortality but associated with antimicrobial resistance (AMR), residue concerns, and monitoring costs | [58] |
| Combined therapy (antibiotics plus plant extracts) | Enrofloxacin (ENR) + San-Huang-San (SHS) | 10–40 mg ENR/kg + 250–1000 mg SHS/kg feed for 5 days; challenged with AHPND-causing V. parahaemolyticus | Survival higher than with ENR or SHS alone; combination therapy significantly reduced mortality and improved immune response. | Medium. | Combination improves survival and immune response while allowing a reduction in antibiotic dose; still consider risks of AMR, standardization of herbal extract, dosing regimen, and short duration (5 days) → longer-term effects not assessed. | [59] |
| Plant extracts | Psidium guajava leaf extract | 5 g/kg feed for 28–56 days; challenge with V. parahaemolyticus | Survival ~72% at optimal dose; improved growth and immune markers | Low–Medium | Promising alternative with immune-stimulating and eco-friendly properties; however, dose optimization and in vivo safety studies are needed | [44] |
| Probiotics | Lactobacillus paracasei or Bifidobacterium longum | Fed for 28 days; challenge with V. parahaemolyticus | Survival 67–73% depending on species | Low–Medium | Enhance immune responses and growth; generally cost-effective but require continuous supplementation | [60] |
2.6.3. Mechanistic Comparison of Antibiotics, Plant-Derived Agents, and Other Alternatives
3. Discussion
4. Materials and Methods
4.1. Literature Search
4.1.1. Literature Search Vibrio Treatment with Antibiotics
4.1.2. Literature Search Vibrio Treatment with Plant Extracts
4.2. Screening Process
4.3. Eligibility Criteria, Data Extraction, and Quality Assessment
4.4. Plant-Compound Relationship Identification and Molecular Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHPND | Acute hepatopancreatic necrosis disease |
| WFD | White feces disease |
| SGS | Seagull syndrome |
| HGT | Horizontal gene transfer |
| PRISMA | Preferred Reporting Items for Systematic Reviews and systematic review |
| MESH | Boolean terms and Medical Subject Heading |
| KTKP | Korean Traditional knowledge portal |
| IMPPAT | Indian Medicinal Plants |
References
- Narayanan, S.V.; Joseph, T.C.; Peeralil, S.; Koombankallil, R.; Vaiyapuri, M.; Mothadaka, M.P.; Lalitha, K.V. Tropical Shrimp Aquaculture Farms Harbour Pathogenic Vibrio parahaemolyticus with High Genetic Diversity and Carbapenam Resistance. Mar. Pollut. Bull. 2020, 160, 111551. [Google Scholar] [CrossRef] [PubMed]
- Zermeño-Cervantes, L.A.; Barraza, A.; González-Ponce, H.A.; Martinez-Diaz, S.F.; Cardona-Félix, C. Penaeus vannamei Challenged with a Vibrio parahaemolyticus AHPND Strain Shows Hepatopancreatic Microbiota Imbalance. Cienc. Mar. 2023, 49, e3234. [Google Scholar] [CrossRef]
- Amoah, K.; Dong, X.; Tan, B.; Zhang, S.; Kuebutornye, F.K.A.; Chi, S.; Yang, Q.; Liu, H.; Zhang, H.; Yang, Y. In Vitro Assessment of the Safety and Potential Probiotic Characteristics of Three Bacillus Strains Isolated from the Intestine of Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Front. Vet. Sci. 2021, 8, 675962. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, F.; Chaieb, K.; Zmantar, T.; Kallel, H.; Bakhrouf, A. Adherence Assays and Slime Production of Vibrio alginolyticus and Vibrio parahaemolyticus. Braz. J. Microbiol. 2009, 40, 394–398. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Na, K.W.; Hossain, M.I.; Mizan, M.F.R.; Nahar, S.; Toushik, S.H.; Roy, P.K.; Park, S.H.; Ha, S.-D. Molecular and Pathogenic Characterization of Vibrio parahaemolyticus Isolated from Seafood. Mar. Pollut. Bull. 2021, 172, 112927. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Mizan, M.F.R.; Park, H.; Byun, K.-H.; Lee, N.; Park, S.H.; Ha, S.-D. Genetic Relationship, Virulence Factors, Drug Resistance Profile and Biofilm Formation Ability of Vibrio parahaemolyticus Isolated From Mussel. Front. Microbiol. 2019, 10, 513. [Google Scholar] [CrossRef]
- Sasikala, D.; Srinivasan, P. Characterization of Potential Lytic Bacteriophage against Vibrio alginolyticus and Its Therapeutic Implications on Biofilm Dispersal. Microb. Pathog. 2016, 101, 24–35. [Google Scholar] [CrossRef]
- Butt, U.D.; Lin, N.; Akhter, N.; Siddiqui, T.; Li, S.; Wu, B. Overview of the Latest Developments in the Role of Probiotics, Prebiotics and Synbiotics in Shrimp Aquaculture. Fish Shellfish Immunol. 2021, 114, 263–281. [Google Scholar] [CrossRef]
- Yang, F.; Xu, L.; Huang, W.; Li, F. Highly Lethal Vibrio parahaemolyticus Strains Cause Acute Mortality in Penaeus vannamei Post-Larvae. Aquaculture 2022, 548, 737605. [Google Scholar] [CrossRef]
- de Souza Valente, C.; Wan, A.H.L. Vibrio and Major Commercially Important Vibriosis Diseases in Decapod Crustaceans. J. Invertebr. Pathol. 2021, 181, 107527. [Google Scholar] [CrossRef]
- Thangaraj, S.K.; Nathamuni, S.P.; Katneni, V.K.; Jangam, A.K.; Avunje, S.; Thulasi, D.N.; Grover, M.; Angel, J.R.J.; Shekhar, M.S. Microbial Communities Associated with Zoea-2 Syndrome and White Feces Syndrome in P. vannamei Farming. Front. Mar. Sci. 2023, 10, 1120004. [Google Scholar] [CrossRef]
- Vandenberghe, J.; Verdonck, L.; Robles-Arozarena, R.; Rivera, G.; Bolland, A.; Balladares, M.; Gomez-Gil, B.; Calderon, J.; Sorgeloos, P.; Swings, J. Vibrios Associated with Litopenaeus vannamei Larvae, Postlarvae, Broodstock, and Hatchery Probionts. Appl. Environ. Microbiol. 1999, 65, 2592–2597. [Google Scholar] [CrossRef] [PubMed]
- Lien, H.H.; de Mey, Y.; Nhan, D.K.; Bush, S.; Meuwissen, M.P.M. Can Cooperation Reduce Yield Risks Associated with Infectious Diseases in Shrimp Aquaculture in Vietnam? Aquac. Econ. Manag. 2024, 28, 660–680. [Google Scholar] [CrossRef]
- Widjaja, L.; Indrawati, A.; Nurhidayat, N.; Yulinery, T.; Triana, E.; Soeka, Y.S.; Emilia, Q.; Ariyanti, D.; Ghozali, A.A.; Manalu, J. Quantitative in Vitro Assessment of Lytic Vibriophages Isolated from Acute Hepatopancreatic Necrosis Disease-Affected Shrimp Cultures. Aquaculture 2026, 611, 743025. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, J.; Zhan, P.; Xiong, J. Postlarval Shrimp-Associated Microbiota and Underlying Ecological Processes over AHPND Progression. Microorganisms 2025, 13, 720. [Google Scholar] [CrossRef]
- Fadel, A.; Khafage, A.; Abdelsalam, M.; Abdel-Rahim, M.M. Comparative Evaluation of Three Herbal Extracts on Growth Performance, Immune Response, and Resistance against Vibrio parahaemolyticus in Litopenaeus vannamei. BMC Vet. Res. 2025, 21, 166. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Li, P.; Chen, T.; Ren, C.; Hu, C.; Luo, P. Identification of Candidate Genes Associated with Resistance against Vibrio parahaemolyticus Causing Acute Hepatopancreatic Necrosis Disease in Litopenaeus vannamei via Bulked Segregant Analysis. Fish Shellfish Immunol. 2025, 163, 110405. [Google Scholar] [CrossRef]
- Liao, J.; Cai, Y.; Wang, X.; Shang, C.; Zhang, Q.; Shi, H.; Wang, S.; Zhang, D.; Zhou, Y. Effects of a Potential Host Gut-Derived Probiotic, Bacillus Subtilis 6-3-1, on the Growth, Non-Specific Immune Response and Disease Resistance of Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Probiotics Antimicrob. Proteins 2021, 13, 1119–1137. [Google Scholar] [CrossRef]
- Srinivas, D.; Ch, V. Prevalence of Vibriosis in Penaeus (Litopenaeus) Vannamei in Three Different Locations of Nellore District of Coastal Andhra Pradesh. Int. J. Adv. Res. Biol. Sci. 2019, 6, 28–33. [Google Scholar] [CrossRef]
- Zhao, S.; Ma, L.; Wang, Y.; Fu, G.; Zhou, J.; Li, X.; Fang, W. Antimicrobial Resistance and Pulsed-Field Gel Electrophoresis Typing of Vibrio parahaemolyticus Isolated from Shrimp Mariculture Environment along the East Coast of China. Mar. Pollut. Bull. 2018, 136, 164–170. [Google Scholar] [CrossRef]
- Piedrahita, Y. Evolución Histórica, Mejora Genética, Reforestación de Manglares, Barreras Sanitarias y Otros Desarrollos. Available online: https://www.globalseafood.org/advocate/la-industria-de-cultivo-de-camaron-en-ecuador-parte-1/ (accessed on 19 August 2025).
- Jara-Medina, N.R.; Cueva, D.F.; Cedeño-Pinargote, A.C.; Gualle, A.; Aguilera-Pesantes, D.; Méndez, M.Á.; Orejuela-Escobar, L.; Cisneros-Heredia, D.F.; Cortez-Zambrano, R.; Miranda-Moyano, N.; et al. Eco-Alternative Treatments for Vibrio parahaemolyticus and V. cholerae Biofilms from Shrimp Industry through Eucalyptus (Eucalyptus globulus) and Guava (Psidium guajava) Extracts: A Road for an Ecuadorian Sustainable Economy. PLoS ONE 2024, 19, e0304126. [Google Scholar] [CrossRef]
- Sotomayor, M.A.; Reyes, J.K.; Restrepo, L.; Domínguez-Borbor, C.; Maldonado, M.; Bayot, B. Efficacy Assessment of Commercially Available Natural Products and Antibiotics, Commonly Used for Mitigation of Pathogenic Vibrio Outbreaks in Ecuadorian Penaeus (Litopenaeus) Vannamei Hatcheries. PLoS ONE 2019, 14, e0210478. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, L.; Liu, S.; Wang, Q.; Guo, Z.; Chen, C.; Feng, J. What Drives Changes in the Virulence and Antibiotic Resistance of Vibrio harveyi in the South China Sea? J. Fish Dis. 2020, 43, 853–862. [Google Scholar] [CrossRef]
- Cangui-Panchi, S.P.; Ñacato-Toapanta, A.L.; Enríquez-Martínez, L.J.; Salinas-Delgado, G.A.; Reyes, J.A.; Garzón-Chavez, D.R.; Machado, A. Battle Royale: Immune Response on Biofilms—Host-Pathogen Interactions. Curr. Res. Immunol. 2023, 4, 100057. [Google Scholar] [CrossRef] [PubMed]
- Cangui-Panchi, S.P.; Ñacato-Toapanta, A.L.; Enríquez-Martínez, L.J.; Reyes, J.A.; Garzón-Chavez, D.R.; Machado, A. Biofilm-Forming Microorganisms Causing Hospital-Acquired Infections from Intravenous Catheter: A Systematic Review. Curr. Res. Microb. Sci. 2022, 3, 100175. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque Costa, R.; Araújo, R.L.; Souza, O.V.; Vieira, R.H.S. dos F. Antibiotic-Resistant Vibrios in Farmed Shrimp. BioMed Res. Int. 2015, 2015, 505914. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.A.; Colares, L.P.; Lima, R.A.; Vieira, R.H.S.d.F.; de Sousa, O.V. Effect of Seawater on the Activity of Antibiotics Against Vibrios Isolated from the Hemolymph of Cultured Pacific White Shrimp. J. World Aquac. Soc. 2012, 43, 727–732. [Google Scholar] [CrossRef]
- Silveira, D.R.; da Rosa, J.V.; Kaefer, K.; Bach, L.G.; Barbosa, A.d.O.; Timm, C.D. Ability of Vibrio vulnificus Isolated from Fish of the Lagoa Dos Patos Estuary in South Brazil to Form Biofilms after Sublethal Stress and Bacterial Resistance to Antibiotics and Sanitizers. Int. J. Food Microbiol. 2019, 303, 19–25. [Google Scholar] [CrossRef]
- Amalina, N.Z.; Santha, S.; Zulperi, D.; Amal, M.N.A.; Yusof, M.T.; Zamri-Saad, M.; Ina-Salwany, M.Y. Prevalence, Antimicrobial Susceptibility and Plasmid Profiling of Vibrio spp. Isolated from Cultured Groupers in Peninsular Malaysia. BMC Microbiol. 2019, 19, 251. [Google Scholar] [CrossRef]
- Ong, H.M.G.; Zhong, Y.; Hu, C.C.; Ong, K.H.; Khor, W.C.; Schlundt, J.; Aung, K.T. Quantitative Risk Evaluation of Antimicrobial-Resistant Vibrio parahaemolyticus Isolated from Farmed Grey Mullets in Singapore. Pathogens 2023, 12, 93. [Google Scholar] [CrossRef]
- Zuo, Z.; Shang, B.; Shao, Y.; Li, W.; Sun, J. Screening of Intestinal Probiotics and the Effects of Feeding Probiotics on the Growth, Immune, Digestive Enzyme Activity and Intestinal Flora of Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 86, 160–168. [Google Scholar] [CrossRef]
- Zhou, S.; Tu, X.; Pang, H.; Hoare, R.; Monaghan, S.J.; Luo, J.; Jian, J. A T3SS Regulator Mutant of Vibrio alginolyticus Affects Antibiotic Susceptibilities and Provides Significant Protection to Danio Rerio as a Live Attenuated Vaccine. Front. Cell Infect. Microbiol. 2020, 10, 183. [Google Scholar] [CrossRef]
- Esquer-Miranda, E.; Nieves-Soto, M.; Rivas-Vega, M.E.; Miranda-Baeza, A.; Piña-Valdez, P. Effects of Methanolic Macroalgae Extracts from Caulerpa sertularioides and Ulva lactuca on Litopenaeus vannamei Survival in the Presence of Vibrio Bacteria. Fish Shellfish Immunol. 2016, 51, 346–350. [Google Scholar] [CrossRef]
- Munaeni, W.; Widanarni; Yuhana, M.; Setiawati, M.; Wahyudi, A.T. Effect in White Shrimp Litopenaeus vannamei of Eleutherine Bulbosa (Mill.) Urb. Powder on Immune Genes Expression and Resistance against Vibrio parahaemolyticus Infection. Fish Shellfish Immunol. 2020, 102, 218–227. [Google Scholar] [CrossRef]
- Subramanian, D.; Jang, Y.-H.; Kim, D.-H.; Kang, B.-J.; Heo, M.-S. Dietary Effect of Rubus Coreanus Ethanolic Extract on Immune Gene Expression in White Leg Shrimp, Penaeus vannamei. Fish Shellfish Immunol. 2013, 35, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, A.; Iryani, M.T.M.; Andriani, Y.; Sorgeloos, P.; Tan, M.P.; Wong, L.L.; Mok, W.J.; Ming, W.; Yantao, L.; Lau, C.C.; et al. The Effects of Pandanus tectorius Leaf Extract on the Resistance of White-Leg Shrimp Penaeus vannamei towards Pathogenic Vibrio parahaemolyticus. Fish Shellfish Immunol. Rep. 2023, 4, 100101. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, A.; Okomoda, V.T.; Mimi Iryani, M.T.; Andriani, Y.; Abd Wahid, M.E.; Tan, M.P.; Danish-Daniel, M.; Wong, L.L.; Tengku-Muhammad, T.S.; Mok, W.J.; et al. Pandanus Tectorius Fruit Extract Promotes Hsp70 Accumulation, Immune-Related Genes Expression and Vibrio parahaemolyticus Tolerance in the White-Leg Shrimp Penaeus vannamei. Fish Shellfish Immunol. 2021, 109, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Yeh, R.-Y.; Shiu, Y.-L.; Shei, S.-C.; Cheng, S.-C.; Huang, S.-Y.; Lin, J.-C.; Liu, C.-H. Evaluation of the Antibacterial Activity of Leaf and Twig Extracts of Stout Camphor Tree, Cinnamomum Kanehirae, and the Effects on Immunity and Disease Resistance of White Shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2009, 27, 26–32. [Google Scholar] [CrossRef]
- Fu, Y.-W.; Hou, W.-Y.; Yeh, S.-T.; Li, C.-H.; Chen, J.-C. The Immunostimulatory Effects of Hot-Water Extract of Gelidium amansii via Immersion, Injection and Dietary Administrations on White Shrimp Litopenaeus vannamei and Its Resistance against Vibrio alginolyticus. Fish Shellfish Immunol. 2007, 22, 673–685. [Google Scholar] [CrossRef]
- Wu, C.-C.; Chang, Y.-P.; Wang, J.-J.; Liu, C.-H.; Wong, S.-L.; Jiang, C.-M.; Hsieh, S.-L. Dietary Administration of Gynura bicolor (Roxb. Willd.) DC Water Extract Enhances Immune Response and Survival Rate against Vibrio alginolyticus and White Spot Syndrome Virus in White Shrimp Litopeneaus vannamei. Fish Shellfish Immunol. 2015, 42, 25–33. [Google Scholar] [CrossRef]
- Hsieh, S.-L.; Wu, C.-C.; Liu, C.-H.; Lian, J.-L. Effects of the Water Extract of Gynura bicolor (Roxb. & Willd.) DC on Physiological and Immune Responses to Vibrio alginolyticus Infection in White Shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2013, 35, 18–25. [Google Scholar] [CrossRef]
- Ngo, H.-V.-T.; Huang, H.-T.; Lee, P.-T.; Liao, Z.-H.; Chen, H.-Y.; Nan, F.-H. Effects of Phyllanthus Amarus Extract on Nonspecific Immune Responses, Growth, and Resistance to Vibrio alginolyticus in White Shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2020, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dewi, N.R.; Huang, H.-T.; Wu, Y.-S.; Liao, Z.-H.; Lin, Y.-J.; Lee, P.-T.; Nan, F.-H. Guava (Psidium guajava) Leaf Extract Enhances Immunity, Growth, and Resistance against Vibrio parahaemolyticus in White Shrimp Penaeus vannamei. Fish Shellfish Immunol. 2021, 118, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abidin, Z.; Huang, H.-T.; Liao, Z.-H.; Chen, B.-Y.; Wu, Y.-S.; Lin, Y.-J.; Nan, F.-H. Moringa Oleifera Leaves’ Extract Enhances Nonspecific Immune Responses, Resistance against Vibrio alginolyticus, and Growth in Whiteleg Shrimp (Penaeus vannamei). Animals 2021, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Sallam, G.R.; Abdel-Rahim, M.M.; Lotfy, A.M.; Fayed, W.M.; Shehata, A.I.; El Basuini, M.F.; Elwan, R.I.; Al-absawey, M.A.; Elhetawy, A.I.G. Long Term Dietary Moringa Oleifera Leaf Extract to Florida Red Tilapia oreochromis sp. Improves Performance Immunity Maturation and Reproduction in Saltwater. Sci. Rep. 2025, 15, 20261. [Google Scholar] [CrossRef]
- Lee, C.-L.; Kuo, H.-W.; Chang, C.-C.; Cheng, W. Injection of an Extract of Fresh Cacao Pod Husks into Litopenaeus vannamei Upregulates Immune Responses via Innate Immune Signaling Pathways. Fish Shellfish Immunol. 2020, 104, 545–556. [Google Scholar] [CrossRef]
- Bussabong, P.; Rairat, T.; Chuchird, N.; Keetanon, A.; Phansawat, P.; Cherdkeattipol, K.; Pichitkul, P.; Kraitavin, W. Effects of Isoquinoline Alkaloids from Macleaya Cordata on Growth Performance, Survival, Immune Response, and Resistance to Vibrio parahaemolyticus Infection of Pacific White Shrimp (Litopenaeus vannamei). PLoS ONE 2021, 16, e0251343. [Google Scholar] [CrossRef]
- Dang, L.T.; Nguyen, H.T.; Hoang, H.H.; Lai, H.N.T.; Nguyen, H.T. Efficacy of Rose Myrtle Rhodomyrtus tomentosa Seed Extract against Acute Hepatopancreatic Necrosis Disease in Pacific Whiteleg Shrimp Penaeus vannamei. J. Aquat. Anim. Health 2019, 31, 311–319. [Google Scholar] [CrossRef]
- Mougin, J.; Copin, S.; Bojolly, D.; Raguenet, V.; Robert-Pillot, A.; Quilici, M.-L.; Midelet-Bourdin, G.; Grard, T.; Bonnin-Jusserand, M. Adhesion to Stainless Steel Surfaces and Detection of Viable but Non Cultivable Cells of Vibrio parahaemolyticus and Vibrio Cholerae Isolated from Shrimps in Seafood Processing Environments: Stayin’ Alive? Food Control 2019, 102, 122–130. [Google Scholar] [CrossRef]
- Abidin, Z.; Huang, H.-T.; Hu, Y.-F.; Chang, J.-J.; Huang, C.-Y.; Wu, Y.-S.; Nan, F.-H. Effect of Dietary Supplementation with Moringa Oleifera Leaf Extract and Lactobacillus Acidophilus on Growth Performance, Intestinal Microbiota, Immune Response, and Disease Resistance in Whiteleg Shrimp (Penaeus vannamei). Fish Shellfish Immunol. 2022, 127, 876–890. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Das, B.; Verma, J.; Kumar, P.; Ghosh, A.; Ramamurthy, T. Antibiotic Resistance in Vibrio Cholerae: Understanding the Ecology of Resistance Genes and Mechanisms. Vaccine 2020, 38, A83–A92. [Google Scholar] [CrossRef]
- Huang, X.; Lao, Y.; Pan, Y.; Chen, Y.; Zhao, H.; Gong, L.; Xie, N.; Mo, C.-H. Synergistic Antimicrobial Effectiveness of Plant Essential Oil and Its Application in Seafood Preservation: A Review. Molecules 2021, 26, 307. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Panda, S.K.; Luyten, W. Anti-Vibrio and Immune-Enhancing Activity of Medicinal Plants in Shrimp: A Comprehensive Review. Fish Shellfish Immunol. 2021, 117, 192–210. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Yilmaz, E.; Dawood, M.A.O.; Ringø, E.; Ahmadifar, E.; Yilmaz, S. Shrimp Vibriosis and Possible Control Measures Using Probiotics, Postbiotics, Prebiotics, and Synbiotics: A Review. Aquaculture 2022, 551, 737951. [Google Scholar] [CrossRef]
- Kah Sem, N.A.D.; Abd Gani, S.; Chong, C.M.; Natrah, I.; Shamsi, S. Management and Mitigation of Vibriosis in Aquaculture: Nanoparticles as Promising Alternatives. Int. J. Mol. Sci. 2023, 24, 12542. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-T.; Chang, J.-J.; Lin, Y.-R.; Chen, Y.-Y.; Wu Chang, Y.-H.; Chen, B.-Y.; Nan, F.-H. Synergistic Effects of Dietary Oxolinic Acid Combined with Oxytetracycline on Nonspecific Immune Responses and Resistance against Vibrio parahaemolyticus Infection of White Shrimp (Penaeus vannamei). Fish Shellfish Immunol. 2022, 127, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Li, J. Effectiveness of Traditional Chinese Herbal Medicine, San-Huang-San, in Combination with Enrofloxacin to Treat AHPND-Causing Strain of Vibrio parahaemolyticus Infection in Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 87, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-T.; Hu, Y.-F.; Lee, B.-H.; Huang, C.-Y.; Lin, Y.-R.; Huang, S.-N.; Chen, Y.-Y.; Chang, J.-J.; Nan, F.-H. Dietary of Lactobacillus Paracasei and Bifidobacterium Longum Improve Nonspecific Immune Responses, Growth Performance, and Resistance against Vibrio parahaemolyticus in Penaeus vannamei. Fish Shellfish Immunol. 2022, 128, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xie, S.; Wang, M.; Chen, L.; Yu, H. Distribution and Management of Residual Antibiotics in the Litopenaeus vannamei Shrimp Farming Environment: Recommendations for Effective Control. Fishes 2024, 9, 84. [Google Scholar] [CrossRef]
- Kumar, V.; Roy, S.; Meena, D.K.; Sarkar, U.K. Application of Probiotics in Shrimp Aquaculture: Importance, Mechanisms of Action, and Methods of Administration. Rev. Fish. Sci. Aquac. 2016, 24, 342–368. [Google Scholar] [CrossRef]
- Başaran, S.N.; Öksüz, L. Newly Developed Antibiotics against Multidrug-Resistant and Carbapenem-Resistant Gram-Negative Bacteria: Action and Resistance Mechanisms. Arch. Microbiol. 2025, 207, 110. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Kazi, R.N.A.; Aatif, M.; Azhar, A.; Oirdi, M.E.; Farhan, M. Antimicrobial Resistance: Linking Molecular Mechanisms to Public Health Impact. SLAS Discov. 2025, 33, 100232. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahian, S.; Graham, J.P.; Halaji, M. A Review of the Mechanisms That Confer Antibiotic Resistance in Pathotypes of E. coli. Front. Cell Infect. Microbiol. 2024, 14, 1387497. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Zamora-Mendoza, L.; Alexis, F.; Álvarez-Suarez, J.M. Use of Plant Extracts, Bee-Derived Products, and Probiotic-Related Applications to Fight Multidrug-Resistant Pathogens in the Post-Antibiotic Era. Future Pharmacol. 2023, 3, 535–567. [Google Scholar] [CrossRef]
- Fernandez-Soto, P.; Celi, D.; Tejera, E.; Alvarez-Suarez, J.M.; Machado, A. Cinnamomum sp. and Pelargonium Odoratissimum as the Main Contributors to the Antibacterial Activity of the Medicinal Drink Horchata: A Study Based on the Antibacterial and Chemical Analysis of 21 Plants. Molecules 2023, 28, 693. [Google Scholar] [CrossRef]
- Zin, H.; Ham, I.; Shin, S.; Yu, H.; Choi, T.-J.; Ha, K.; Mok, J.S. Distribution, Antibiotic Resistance, and Virulence Factors of Vibrio parahaemolyticus in the Southern Coastal Waters of Republic of Korea. Antibiotics 2025, 14, 435. [Google Scholar] [CrossRef]
- Sadighara, P.; Rostami, S.; Shafaroodi, H.; Sarshogi, A.; Mazaheri, Y.; Sadighara, M. The Effect of Residual Antibiotics in Food on Intestinal Microbiota: A Systematic Review. Front. Sustain. Food Syst. 2023, 7, 1163885. [Google Scholar] [CrossRef]
- Machado, A.; Toubarro, D.; Baptista, J.; Tejera, E.; Álvarez-Suárez, J.M. Selected Honey as a Multifaceted Antimicrobial Agent: Review of Compounds, Mechanisms, and Research Challenges. Future Microbiol. 2025, 20, 589–610. [Google Scholar] [CrossRef]
- Celi, D.; Jimenes-Vargas, K.; Machado, A.; Álvarez-Suárez, J.M.; Tejera, E. Chemical Composition and Biological Activities of Pelargonium sp.: A Review with In Silico Insights into Potential Anti-Inflammatory Mechanism. Molecules 2025, 30, 3198. [Google Scholar] [CrossRef]
- Cabezas-Mera, F.S.; Atiencia-Carrera, M.B.; Villacrés-Granda, I.; Proaño, A.A.; Debut, A.; Vizuete, K.; Herrero-Bayo, L.; Gonzalez-Paramás, A.M.; Giampieri, F.; Abreu-Naranjo, R.; et al. Evaluation of the Polyphenolic Profile of Native Ecuadorian Stingless Bee Honeys (Tribe: Meliponini) and Their Antibiofilm Activity on Susceptible and Multidrug-Resistant Pathogens: An Exploratory Analysis. Curr. Res. Food Sci. 2023, 7, 100543. [Google Scholar] [CrossRef]
- Cabezas-Mera, F.S.; Cedeño-Pinargote, A.C.; Tejera, E.; Alvarez-Suarez, J.M.; Machado, A. Antimicrobial Activity of Stingless Bee Honey (Tribe: Meliponini) on Clinical and Foodborne Pathogens: A Systematic Review and Meta-Analysis. Food Front. 2024, 5, 964–993. [Google Scholar] [CrossRef]
- Majtan, J.; Machado, A.; Giampieri, F.; Álvarez-Suárez, J.M. Health Benefits and Uses of Honey in Medicine. In Bee Products—Chemical and Biological Properties; Springer Nature: Cham, Switzerland, 2025; pp. 105–140. [Google Scholar]
- Yatip, P.; Nitin Chandra Teja, D.; Flegel, T.W.; Soowannayan, C. Extract from the Fermented Soybean Product Natto Inhibits Vibrio Biofilm Formation and Reduces Shrimp Mortality from Vibrio harveyi Infection. Fish Shellfish Immunol. 2018, 72, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, W.; Zou, Y.; Xia, X. Antimicrobial Activity and Mechanisms of Punicalagin against Vibrio parahaemolyticus. Foods 2024, 13, 1366. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Labbe, R.G.; Shetty, K. Inhibition of Vibrio parahaemolyticus in Seafood Systems Using Oregano and Cranberry Phytochemical Synergies and Lactic Acid. Innov. Food Sci. Emerg. Technol. 2005, 6, 453–458. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Feyaerts, A.F.; Van Dijck, P.; Bossier, P. Inhibitory Activity of Essential Oils against Vibrio Campbellii and Vibrio parahaemolyticus. Microorganisms 2020, 8, 1946. [Google Scholar] [CrossRef]
- Touati, A.; Mairi, A.; Ibrahim, N.A.; Idres, T. Essential Oils for Biofilm Control: Mechanisms, Synergies, and Translational Challenges in the Era of Antimicrobial Resistance. Antibiotics 2025, 14, 503. [Google Scholar] [CrossRef]
- Vazquez-Armenta, F.J.; Aros-Corrales, M.O.; Alvarez-Ainza, M.L.; Bernal-Mercado, A.T.; Ayala-Zavala, J.F.; Ochoa-Leyva, A.; Lopez-Zavala, A.A. Antibacterial and Anti-Virulence Potential of Plant Phenolic Compounds against Vibrio parahaemolyticus. F1000Research 2023, 12, 1256. [Google Scholar] [CrossRef]
- Restrepo, L.; Domínguez-Borbor, C.; Bajaña, L.; Betancourt, I.; Rodríguez, J.; Bayot, B.; Reyes, A. Microbial Community Characterization of Shrimp Survivors to AHPND Challenge Test Treated with an Effective Shrimp Probiotic (Vibrio diabolicus). Microbiome 2021, 9, 88. [Google Scholar] [CrossRef]
- Tamilselvan, M.; Raja, S. Exploring the Role and Mechanism of Potential Probiotics in Mitigating the Shrimp Pathogens. Saudi J. Biol. Sci. 2024, 31, 103938. [Google Scholar] [CrossRef]
- Xie, G.; Chen, X.; Feng, Y.; Yu, Z.; Lu, Q.; Li, M.; Ye, Z.; Lin, H.; Yu, W.; Shu, H. Effects of Dietary Multi-Strain Probiotics on Growth Performance, Antioxidant Status, Immune Response, and Intestinal Microbiota of Hybrid Groupers (Epinephelus fuscoguttatus ♀ × E. Lanceolatus ♂). Microorganisms 2024, 12, 1358. [Google Scholar] [CrossRef] [PubMed]
- Amobonye, A.; Pillay, B.; Hlope, F.; Asong, S.T.; Pillai, S. Postbiotics: An Insightful Review of the Latest Category in Functional Biotics. World J. Microbiol. Biotechnol. 2025, 41, 293. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, R.B.; Perumal, A.B.; Shittu, T.; Sadiku, E.R.; Sellamuthu, P.S. Editorial: Probiotics, Prebiotics, Synbiotics, Postbiotics, & Paraprobiotics—New Perspective for Functional Foods and Nutraceuticals. Front. Nutr. 2023, 10, 1164676. [Google Scholar] [CrossRef]
- Guo, H.; Fu, X.; He, J.; Wang, R.; Yan, M.; Wang, J.; Dong, P.; Huang, L.; Zhang, D. Gut Bacterial Consortium Enriched in a Biofloc System Protects Shrimp against Vibrio parahaemolyticus Infection. Microbiome 2023, 11, 230. [Google Scholar] [CrossRef]
- Fan, S.; Qin, P.; Lu, J.; Wang, S.; Zhang, J.; Wang, Y.; Cheng, A.; Cao, Y.; Ding, W.; Zhang, W. Bioprospecting of Culturable Marine Biofilm Bacteria for Novel Antimicrobial Peptides. iMeta 2024, 3, e244. [Google Scholar] [CrossRef]
- Chraniuk, P.; Bzducha-Wróbel, A. Functional Properties of Yeast Mannoproteins—Current Knowledge and Future Perspectives. Fermentation 2025, 11, 374. [Google Scholar] [CrossRef]
- Barroso, R.A.; Agüero-Chapin, G.; Sousa, R.; Marrero-Ponce, Y.; Antunes, A. Unlocking Antimicrobial Peptides: In Silico Proteolysis and Artificial Intelligence-Driven Discovery from Cnidarian Omics. Molecules 2025, 30, 550. [Google Scholar] [CrossRef]
- Guan, F.; Yu, C.; Yang, L.; Yuan, Y. Broad-Spectrum Antimicrobial Peptides Suppress Vibrio parahaemolyticus Based on Lactobacillus paracasei A1 Fermentation. Foodborne Pathog. Dis. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Taheri-Araghi, S. Synergistic Action of Antimicrobial Peptides and Antibiotics: Current Understanding and Future Directions. Front. Microbiol. 2024, 15, 1390765. [Google Scholar] [CrossRef]
- Rattanadilog Na Phuket, T.; Charoensapsri, W.; Amparyup, P.; Imjongjirak, C. Antibacterial Activity and Immunomodulatory Role of a Proline-Rich Antimicrobial Peptide SpPR-AMP1 against Vibrio campbellii Infection in Shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2023, 132, 108479. [Google Scholar] [CrossRef]
- Zeng, L.; Sun, Y.; Zhang, H.; Yi, X.; Du, R.; Chen, Z.; Wang, Q. Scorpion Venom Peptides Enhance Immunity and Survival in Litopenaeus vannamei through Antibacterial Action against Vibrio parahaemolyticus. Front. Immunol. 2025, 16, 1551816. [Google Scholar] [CrossRef]
- Lv, X.; Li, S.; Yu, Y.; Zhang, X.; Li, F. Crustin Defense against Vibrio parahaemolyticus Infection by Regulating Intestinal Microbial Balance in Litopenaeus vannamei. Mar. Drugs 2023, 21, 130. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, E.R.; Quiroga, E. Farmed Shrimp Aquaculture in Coastal Wetlands of Latin America—A Review of Environmental Issues. Mar. Pollut. Bull. 2022, 183, 113956. [Google Scholar] [CrossRef]
- López-Landavery, E.A.; Urquizo-Rosado, Á.; Saavedra-Flores, A.; Tapia-Morales, S.; Fernandino, J.I.; Zelada-Mázmela, E. Cellular and Transcriptomic Response to Pathogenic and Non-Pathogenic Vibrio parahaemolyticus Strains Causing Acute Hepatopancreatic Necrosis Disease (AHPND) in Litopenaeus vannamei. Fish Shellfish Immunol. 2024, 148, 109472. [Google Scholar] [CrossRef] [PubMed]
- Helena Rebouças, R.; Viana de Sousa, O.; Sousa Lima, A.; Roger Vasconcelos, F.; de Carvalho, P.B.; dos Fernandes Vieira, R.H.S. Antimicrobial Resistance Profile of Vibrio Species Isolated from Marine Shrimp Farming Environments (Litopenaeus vannamei) at Ceará, Brazil. Environ. Res. 2011, 111, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.; Sathiyaraj, G.; Mandal, A.; Kandan, S.; Biju, N.; Palanisamy, S.; You, S.; Nisha, R.G.; Prabhu, N.M. Surveillance of Disease Incidence in Shrimp Farms Located in the East Coastal Region of India and in Vitro Antibacterial Efficacy of Probiotics against Vibrio parahaemolyticus. J. Invertebr. Pathol. 2021, 179, 107536. [Google Scholar] [CrossRef]
- Liao, G.; Wu, Q.; Mo, B.; Zhou, J.; Li, J.; Zou, J.; Fan, L. Intestinal Morphology and Microflora to Vibrio alginolyticus in Pacific White Shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2022, 121, 437–445. [Google Scholar] [CrossRef]
- Haque, Z.F.; Islam, M.S.; Sabuj, A.A.M.; Pondit, A.; Sarkar, A.K.; Hossain, M.G.; Saha, S. Molecular Detection and Antibiotic Resistance of Vibrio Cholerae, Vibrio parahaemolyticus, and Vibrio alginolyticus from Shrimp (Penaeus monodon) and Shrimp Environments in Bangladesh. Aquac. Res. 2023, 2023, 5436552. [Google Scholar] [CrossRef]
- Li, Y.; Xie, T.; Pang, R.; Wu, Q.; Zhang, J.; Lei, T.; Xue, L.; Wu, H.; Wang, J.; Ding, Y.; et al. Food-Borne Vibrio parahaemolyticus in China: Prevalence, Antibiotic Susceptibility, and Genetic Characterization. Front. Microbiol. 2020, 11, 1670. [Google Scholar] [CrossRef]
- Hirshfeld, B.; Lavelle, K.; Lee, K.Y.; Atwill, E.R.; Kiang, D.; Bolkenov, B.; Gaa, M.; Li, Z.; Yu, A.; Li, X.; et al. Prevalence and Antimicrobial Resistance Profiles of Vibrio spp. and Enterococcus spp. in Retail Shrimp in Northern California. Front. Microbiol. 2023, 14, 1192769. [Google Scholar] [CrossRef]
- Loo, K.; Letchumanan, V.; Law, J.W.; Pusparajah, P.; Goh, B.; Ab Mutalib, N.; He, Y.; Lee, L. Incidence of Antibiotic Resistance in Vibrio spp. Rev. Aquac. 2020, 12, 2590–2608. [Google Scholar] [CrossRef]
- Reverter, M.; Tapissier-Bontemps, N.; Sarter, S.; Sasal, P.; Caruso, D. Moving towards More Sustainable Aquaculture Practices: A Meta-analysis on the Potential of Plant-enriched Diets to Improve Fish Growth, Immunity and Disease Resistance. Rev. Aquac. 2021, 13, 537–555. [Google Scholar] [CrossRef]
- Albini, E.; Orso, M.; Cozzolino, F.; Sacchini, L.; Leoni, F.; Magistrali, C.F. A Systematic Review and Meta-Analysis on Antimicrobial Resistance in Marine Bivalves. Front. Microbiol. 2022, 13, 1040568. [Google Scholar] [CrossRef]
- Loo, K.-Y.; Tan, L.T.-H.; Law, J.W.-F.; Pusparajah, P.; Lee, L.-H.; Letchumanan, V. Detection of Multidrug Resistant Vibrio parahaemolyticus and Anti-Vibrio Streptomyces sp. MUM 178J. Prog. Microbes Mol. Biol. 2023, 6, a0000347. [Google Scholar] [CrossRef]
- Onohuean, H.; Agwu, E.; Nwodo, U.U. Systematic Review and Meta-Analysis of Environmental Vibrio Species—Antibiotic Resistance. Heliyon 2022, 8, e08845. [Google Scholar] [CrossRef] [PubMed]
- Murtala, R.; Bashir, A.; Abubakar Aliyu, I.; Abdulhadi Sale, K. Systematic Review on the Antibacterial Resistance of Vibrio Cholerae. UMYU Sci. 2022, 1, 60–66. [Google Scholar] [CrossRef]
- Yousefi, A.; Vaez, H.; Sahebkar, A.; Khademi, F. A Systematic Review and Meta-Analysis on the Epidemiology of Antibiotic Resistance of Vibrio Cholerae in Iran. Ann. Ig. 2019, 31, 279–290. [Google Scholar] [CrossRef]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A Systematic Review of Plants with Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Front. Pharmacol. 2021, 11, 586548. [Google Scholar] [CrossRef]
- Ajitha, S.; Sridhar, M.; Sridhar, N.; Singh, I.S.B.; Varghese, V. Probiotic Effects of Lactic Acid Bacteria Against Vibrio alginolyticus in Penaeus (Fenneropenaeus) Indicus (H.Milne Edwards). Asian Fish. Sci. 2004, 17, 71–80. [Google Scholar] [CrossRef]
- Selcuk, A.A. A Guide for Systematic Reviews: PRISMA. Turk. Arch. Otorhinolaryngol. 2019, 57, 57–58. [Google Scholar] [CrossRef]
- Rutz, A.; Sorokina, M.; Galgonek, J.; Mietchen, D.; Willighagen, E.; Gaudry, A.; Graham, J.G.; Stephan, R.; Page, R.; Vondrášek, J.; et al. The LOTUS Initiative for Open Knowledge Management in Natural Products Research. eLife 2022, 11, e70780. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, M.; Merseburger, P.; Rajan, K.; Yirik, M.A.; Steinbeck, C. COCONUT Online: Collection of Open Natural Products Database. J. Cheminform. 2021, 13, 2. [Google Scholar] [CrossRef]
- Vivek-Ananth, R.P.; Mohanraj, K.; Sahoo, A.K.; Samal, A. IMPPAT 2.0: An Enhanced and Expanded Phytochemical Atlas of Indian Medicinal Plants. ACS Omega 2023, 8, 8827–8845. [Google Scholar] [CrossRef]
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated Chemical Classification with a Comprehensive, Computable Taxonomy. J. Cheminform. 2016, 8, 61. [Google Scholar] [CrossRef]
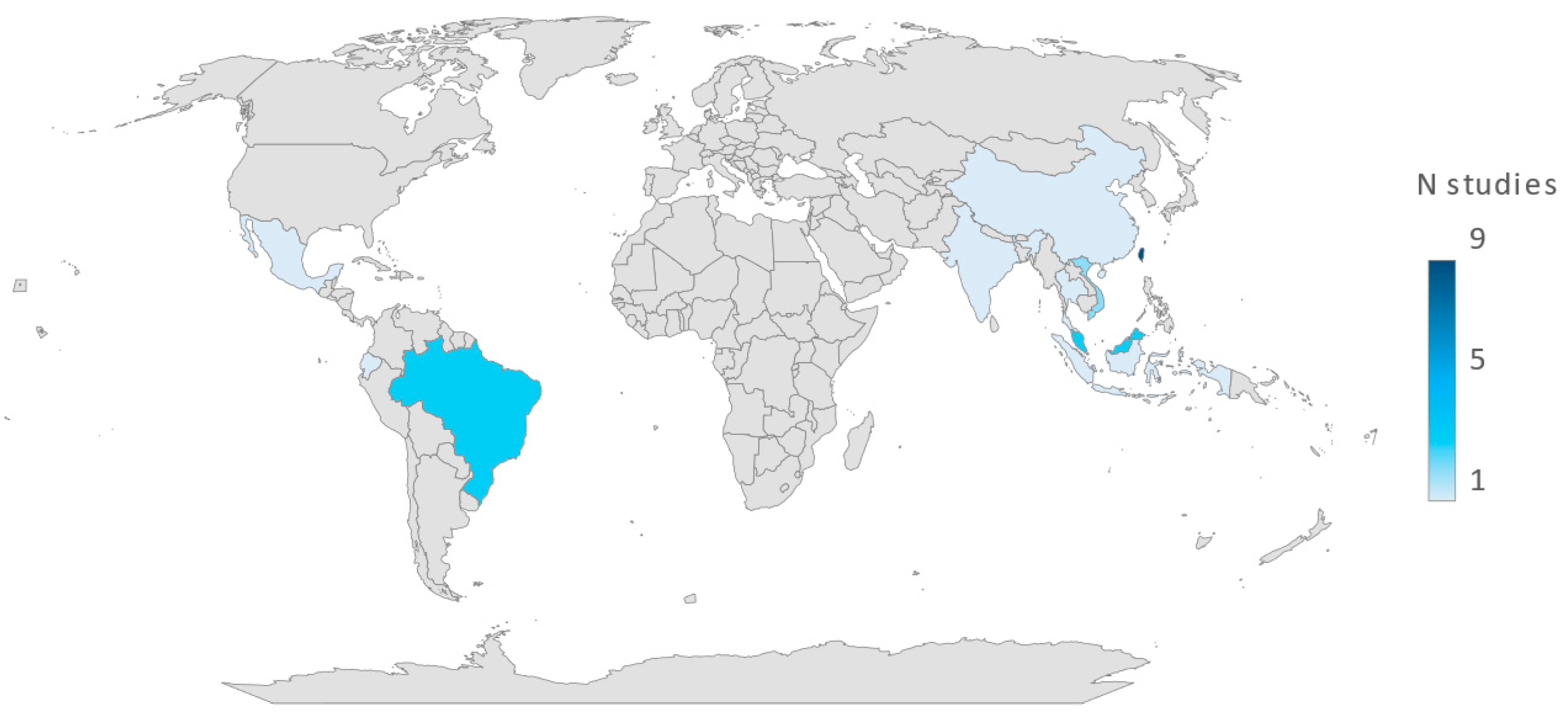
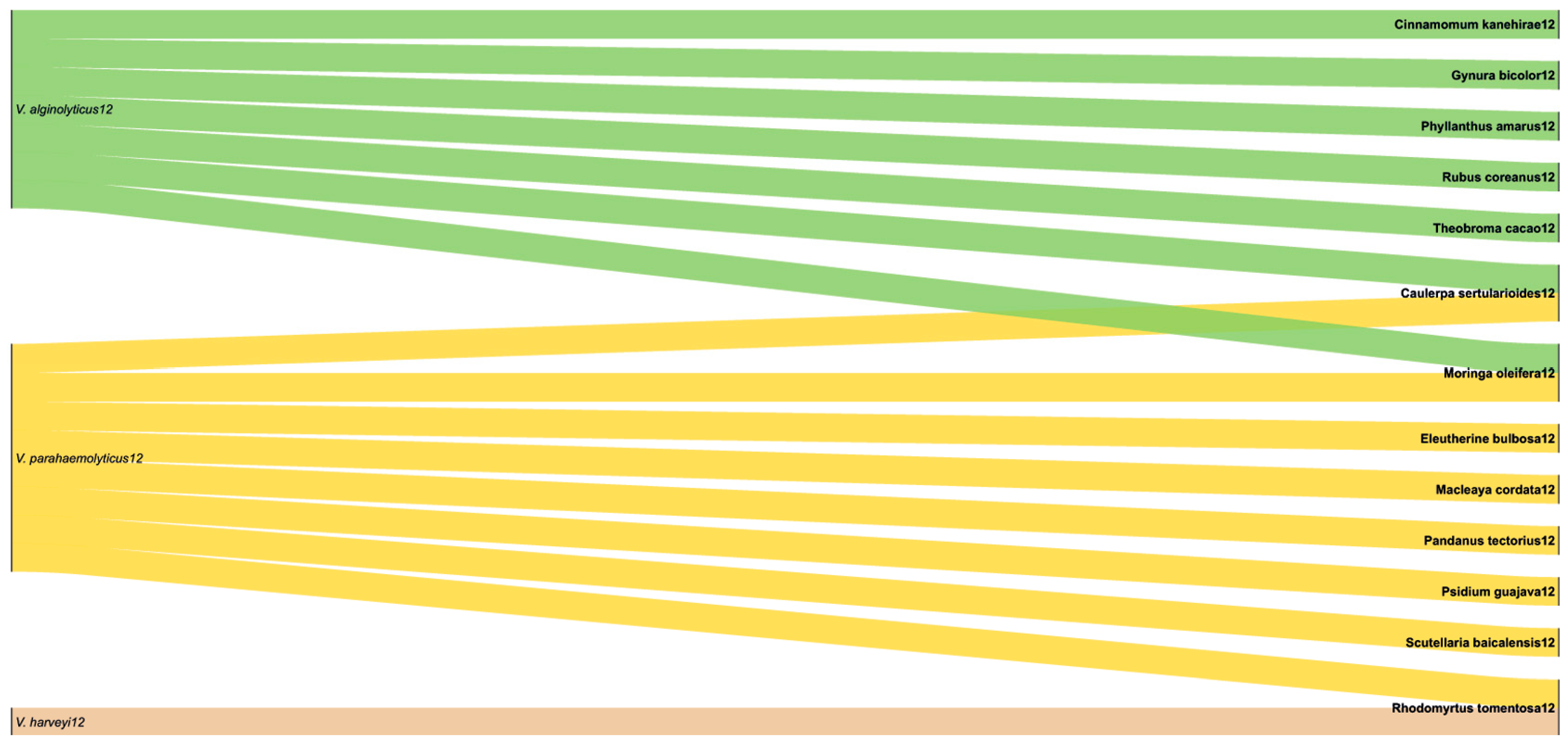
| Region | Country | Number of Studies | Vibrio Presence Rate | Prevalence of Vibrio (%) |
|---|---|---|---|---|
| Asia | Thailand Vietnam Taiwan Indonesia Korea Malaysia India Singapore China | 21 | 6038/7112 | 84.89 |
| South America | Brazil Ecuador | 4 | 353/474 | 74.47 |
| North America | Mexico | 1 | 180/180 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jara-Medina, N.R.; Cedeño-Pinargote, A.C.; Beltrán-Noboa, A.; Tejera, E.; Machado, A. Managing Vibrio parahaemolyticus and Vibrio alginolyticus Infections in the Whiteleg Shrimp (Penaeus vannamei): A Systematic Review. Molecules 2025, 30, 3620. https://doi.org/10.3390/molecules30173620
Jara-Medina NR, Cedeño-Pinargote AC, Beltrán-Noboa A, Tejera E, Machado A. Managing Vibrio parahaemolyticus and Vibrio alginolyticus Infections in the Whiteleg Shrimp (Penaeus vannamei): A Systematic Review. Molecules. 2025; 30(17):3620. https://doi.org/10.3390/molecules30173620
Chicago/Turabian StyleJara-Medina, Nicolás Renato, Ariana Cecibel Cedeño-Pinargote, Andrea Beltrán-Noboa, Eduardo Tejera, and António Machado. 2025. "Managing Vibrio parahaemolyticus and Vibrio alginolyticus Infections in the Whiteleg Shrimp (Penaeus vannamei): A Systematic Review" Molecules 30, no. 17: 3620. https://doi.org/10.3390/molecules30173620
APA StyleJara-Medina, N. R., Cedeño-Pinargote, A. C., Beltrán-Noboa, A., Tejera, E., & Machado, A. (2025). Managing Vibrio parahaemolyticus and Vibrio alginolyticus Infections in the Whiteleg Shrimp (Penaeus vannamei): A Systematic Review. Molecules, 30(17), 3620. https://doi.org/10.3390/molecules30173620







