Abstract
Background: This systematic review aims to evaluate the effectiveness of various treatments and strategies for managing infections caused by Vibrio parahaemolyticus and Vibrio alginolyticus in whiteleg shrimp (Penaeus vannamei). Shrimp aquaculture faces significant challenges from these pathogens, resulting in substantial economic losses. Vibrio species are known for their ability to form biofilms, enhancing their resistance to conventional treatments. Methods: The review follows the PRISMA guidelines, searching Scopus and PubMed databases for relevant studies on antibiotics and plant extracts used against these pathogens. Data were extracted and analysed to assess the effectiveness of different treatments, including antibiotics, plant extracts, and combined therapies. Results: The review found that while antibiotics remain widely used, the emergence of antibiotic-resistant strains necessitates alternative strategies. Plant extracts, rich in bioactive compounds such as flavonoids, showed promising antimicrobial activity. Combined therapies involving antibiotics and plant extracts were also explored for their potential to enhance treatment efficacy and reduce resistance. Conclusions: The findings underscore the importance of addressing biofilm formation in managing Vibrio-related infections and highlight the need for further research to develop sustainable and effective treatment protocols for shrimp aquaculture.
1. Introduction
Shrimp farming represents one of the most important aquaculture activities and has been established as an important socioeconomic promoter worldwide, especially in countries like Ecuador. Whiteleg shrimp (Penaeus vannamei) aquaculture constantly faces the challenge of diseases thanks to the conditions that promote the rapid growth of opportunistic bacteria such as many Vibrio species, with Vibrio parahaemolyticus and Vibrio alginolyticus being among the main pathogens associated with mortality and significant economic losses in shrimp and mollusk exporting countries [1,2]. These diseases caused by V. parahaemolyticus and V. alginolyticus species can result in losses ranging from 20% to 80% of the total production [3]. In addition, it is well known that Vibrio spp. can form biofilms. In this case, the species of interest demonstrate a high capacity to adhere to biotic and abiotic surfaces for biofilm formation, which makes them more resistant to conventional treatments and control [4].
Vibrio is a genus of motile, curved, Gram-negative bacteria found naturally in aquatic environments primarily forming multi-species biofilms [5]. V. parahaemolyticus is a halophilic marine bacterium, considered an emerging pathogen with a global distribution. It is the most prevalent shrimp pathogen in aquaculture, associated with recurrent outbreaks and substantial economic impact. V. alginolyticus is a moderately halophilic and mesophilic bacterium, being recognized as an epizootic pathogen responsible for severe outbreaks of diseases such as vibriosis in aquatic and marine animals [6,7]. Although some species of Vibrio are harmless or even beneficial to aquatic organisms, certain pathogenic strains can cause serious diseases in farmed shrimp. As previously mentioned, the culture disease that affects the greatest proportion is vibriosis, whose mortality can vary between 70 and 100%, and it damages the oral cavity and appendages of the shrimp [8]. Additionally, it is recorded that farmed shrimp also acquire other diseases due to the previously mentioned Vibrio species, such as acute hepatopancreatic necrosis disease (AHPND), zoea 2 syndrome, white feces disease (WFD), seagull syndrome (SGS), mysis mold syndrome, and bolitas syndrome, which are the most common reported in the literature [1,9,10,11,12]. Among these conditions, AHPND is probably the most impactful Vibrio-related infection in shrimp aquaculture, being responsible for severe economic losses worldwide, with estimates approaching USD 43 billion and up to 60% production losses in affected regions [13,14]. Clinically, AHPND (also known as early mortality syndrome) is characterized by reduced feeding, erratic swimming followed by lethargy, empty or pale/atrophic hepatopancreas, and rapid progression to high or even mass mortalities in cultured P. vannamei [15,16,17]. Vibrio species cause infection at all life stages (from eggs to brood stock). The adaptability of these bacteria to stress conditions, their rapid reproduction, their ability to generate resistance to antibiotics, and their ability to form biofilms pose a significant challenge for the aquaculture industry [18,19].
The presence of bacterial diseases caused by V. parahaemolyticus and V. alginolyticus represents a constant threat to the sustainability and profitability of whiteleg shrimp aquaculture [20]. These losses include direct mortality of infected shrimp, decreased growth and meat quality, as well as reduced feed conversion ratio, without considering the economic impact of these losses. Historically, Ecuador has had different massive outbreaks of systemic vibriosis that have harmed the economy and the production of P. vannamei shrimp, with mortality rates of 5 to 90%. Consequently, the economic problems in exports were severe and the Ecuadorian economy suffered a contraction, which led to an acute economic crisis within the shrimp industry worldwide [10,21]. Therefore, to control Vibrio infections in shrimp farms worldwide, various treatments and strategies have been implemented. Among the most common methods are the use of antibiotics, plant extracts, probiotics, disinfectants, immunotherapy, and improvements in management practices and biosecurity in production [22]. However, V. parahaemolyticus and V. alginolyticus can form biofilms and generate resistance to these treatments, which leads to persistence in aquaculture systems. These biofilms are microbial communities attached to solid surfaces, such as farm structures and the shrimp themselves [23]. Biofilms protect Vibrio species from disinfectants, shrimp immune systems, and other control factors, making their eradication and efficient control difficult to achieve nowadays [24,25,26].
This systematic review aims to collect and analyse relevant scientific studies that evaluate the effectiveness of the different treatments and strategies used to control diseases caused by Vibrio species in whiteleg shrimp farms. Additionally, the effectiveness of measures aimed at destabilizing and eliminating biofilms formed by these bacteria was examined, considering their influence on the persistence and resistance of Vibrio species in aquaculture systems.
This systematic review aims to synthesize current knowledge on the effectiveness of treatments used to control diseases caused by Vibrio spp. in whiteleg shrimp aquaculture. Particular attention is given to the role of biofilm formation, which represents a major challenge for therapeutic success. The overall objective is to identify which strategies, including antibiotics, plant-derived compounds, and combined approaches, are most effective, while also clarifying their limitations and outlining promising alternatives for sustainable disease management.
2. Results
2.1. Study Inclusion Criteria and Characteristics of the Eligible Studies
A total of 46 studies with antibiotics were retrieved and, after the screening and eligibility steps, 4 full-length articles were selected, but the additional evaluation of records (references) of the initial set of 4 studies allowed us to select 6 studies more evaluating antibiotics against Vibrio species (see Figure 1A). Moreover, these additional studies also reported data of other Vibrio species besides the species of interest (i.e., V. parahaemolyticus and V. alginolyticus). These studies were included to provide complementary insights, given the limited availability of information on the target species. Their inclusion was justified by their use of comparable methodologies and their relevance to understanding broader Vibrio-related dynamics. On the other hand, a total of 31 studies with plant extracts were collected and 16 full-length articles were used, where no additional records were found. These studies met the inclusion criteria for the systematic review (see Figure 1B). The final dataset included studies from different global regions. All available and relevant data from each study were extracted, specifically focusing on Vibrio species, antibiotics, and plant extracts, applied concentrations, methodologies, and quantitative parameters (such as replicates, survival rate, mean, and standard deviation).
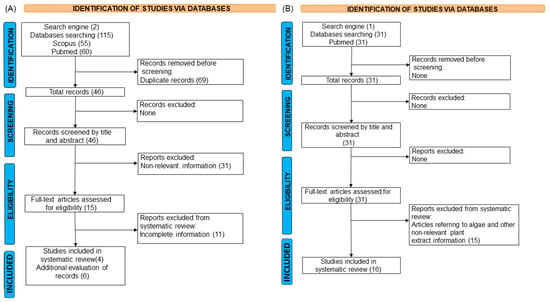
Figure 1.
Prisma flow chart of the selection process for Vibrio treatments with antibiotics (A) and plant extracts (B).
After applying the screening, eligibility, and quality assessment criteria, 26 studies addressing antibiotics and plant extracts were included in this systematic review (Figure 1).
2.2. General Effects of Vibrio Species in Penaeus vannamei
The general data of the selected antibiotic studies are shown in Table 1, which comprises studies conducted between 2012 and 2020 in several countries around the world, primarily in Asia (1/4) and South America (3/4). Furthermore, after checking additional records in the initial four studies, the other six studies were found between 2019 and 2023, also from Asia (5/6) and South America (1/6). Additionally, the disk diffusion assay was used to evaluate the effectiveness of antibiotics against Vibrio isolates. The general data of the selected plant extract studies are shown in Table 2, which comprises studies conducted between 2007 and 2021 in several countries around the world, mainly in Asia (15/16) and North America (1/16). Vibrio survival assays were used to evaluate the effectiveness of plant extracts against Vibrio. The tested strains of different Vibrio species were isolated from shrimp farming areas in different countries.

Table 1.
General antibiotic information extracted from the dataset selected for the present systematic review.
Table 1.
General antibiotic information extracted from the dataset selected for the present systematic review.
| Study | Region | Country | Vibrio Species | Antibiotics | Families | Susceptibility |
|---|---|---|---|---|---|---|
| [27,28] | South America | Brazil | V. parahaemolyticus | Oxytetracycline | Tetracyclines | Intermediate |
| V. parahaemolyticus | Ampicillin, Colistin, Streptomycin | Penicillins, Aminoglycosides | Resistant | |||
| V. parahaemolyticus | Penicillin, Tetracycline | Penicillins, Tetracyclines | Resistant | |||
| Other Vibrio species: V. brasiliensis, V. cholerae, V. coralliilyticus, V. navarrensis, V. vulnificus, V. xuii, and V. cholerae ATCC 19582 | Ampicillin, Aztreonam, Ceftriaxone, Cephalothin, Chloramphenicol, Ciprofloxacin, Gentamicin, Imipenem, Nalidixic acid, Nitrofurantoin, Penicillin, Streptomycin, Sulfamethoxazole-trimethoprim, Tetracycline | Penicillins, Cephalosporins, Aminoglycosides, Fluoroquinolones, Tetracyclines, Sulfonamides, Carbapenems, Monobactams, Amphenicols, Nitrofuranos | Sensitive | |||
| [23] | South America | Ecuador | V. alginolyticus | Nalidixic acid, Chloramphenicol, Ciprofloxacin, Enrofloxacin, Florfenicol, Fosfomycin, Furazolidone, Norfloxacin | Quinolone, Amphenicols, Fluoroquinolones Phosphonic acids, Nitrofurans | Sensitive |
| [1] | Asia | India | V. parahaemolyticus | Chloramphenicol, Gentamicin, Meropenem, Tetracycline, Trimethoprim/sulfamethoxazole | Amphenicols, Aminoglycosides, Carbapenems, Tetracyclines, Sulfonamide/Trimethoprim | Sensitive |
| Other Studies | ||||||
| [29] | South America | Brazil | Other Vibrio species: V. vulnificus | Chloramphenicol, Ciprofloxacin, Norfloxacin | Amphenicols, Fluoroquinolones | Intermediate |
| Other Vibrio species: V. vulnificus | Amikacin, Ampicillin, Enrofloxacin, Gentamicin, Neomycin, Penicillin, Tetracycline | Aminoglycosides, Penicillins, Fluoroquinolones, Tetracyclines | Resistant | |||
| [30] | Asia | Malaysia | V. cholerae | Streptomycin, Erythromycin, Tetracycline | Aminoglycosides, Macrolides, Tetracyclines | Sensitive |
| V. parahaemolyticus | Bacitracin | Polypeptides | Intermediate | |||
| V. parahaemolyticus Other Vibrio species: V. cholerae | Penicillins, Glycopeptides | Penicillins, Glycopeptides | Resistant | |||
| [5] | Asia | Korea | V. parahaemolyticus | Vancomycin, Amikacin, Cefepime, Cefotaxime, Ceftriaxone, Erythromycin, Imipenem, Streptomycin, Ticarcillin-clavulanic acid | Glycopeptides, Aminoglycosides, Cephalosporins, Macrolides, Carbapenems, Penicillin/Beta-lactamase | Sensitive |
| V. parahaemolyticus | Amikacin, Ampicillin, Cefepime, Ceftazidime, Chloramphenicol, Polymyxin B, Tazobactam-piperacillin | Glycopeptides, Aminoglycosides, Cephalosporins, Macrolides, Penicillin/Beta-lactamase | Intermediate | |||
| V. parahaemolyticus | Amikacin, Ampicillin, Cefepime, Ciprofloxacin, Clarithromycin, Doxycycline, Gentamicin, Kanamycin, Levofloxacin, Minocycline, Nalidixic acid, Penicillin, Sulfamethoxazole- trimethoprim, Tetracycline, Tobramycin | Aminoglycosides, Penicillins, Cephalosporins, Fluoroquinolones, Macrolides, Tetracyclines, Sulfonamides/Trimethoprim | Resistant | |||
| [31] | Asia | Singapore | V. parahaemolyticus | Ampicillin/sulbactam, Cefotaxime, Chloramphenicol, Ciprofloxacin, Sulfamethoxazole- trimethoprim, Tetracycline | Penicillin/Beta-lactamase, Cephalosporins, Chloramphenicols, Fluoroquinolones, Sulfonamides/Trimethoprim, Tetracyclines | Sensitive |
| V. parahaemolyticus | Ampicillin, Penicillin | Penicillins | Resistant | |||
| [32] | Asia | Vietnam | V. parahaemolyticus | Chloramphenicol, Gentamicin, Kanamycin, Nalidixic acid, Oxacillin, Tebipenem | Amphenicols, Aminoglycosides, Quinolones, Penicillins, Carbapenems | Sensitive |
| [33] | Asia | China | V. alginolyticus | Chloramphenicol, Doxycycline, Minocycline | Chloramphenicols, Tetracyclines | Sensitive |
| V. alginolyticus | Amikacin, Cefperazone, Gentamicin, Kanamycin, Tetracycline | Aminoglycosides, Cephalosporins, Tetracyclines | Intermediate | |||
| V. alginolyticus | Ampicillin, Carbenicillin, Cefazolin, Cefperazone, Ceftazidime, Ceftriaxone, Cefuroxime, Cephalexin, Cephradine, Ciprofloxacin, Clarithromycin, Erythromycin, Furazolidone, Nalidixic acid, Ofloxacin, Oxacillin, Penicillin, Piperacillin, Polymyxin B, SMZ/TMP, Vancomycin | Penicillins, Cephalosporins, Fluoroquinolones, Macrolides, Nitrofurans, Quinolones, Polymyxins, Sulfonamides/Trimethoprim, Glycopeptides | Resistant | |||
All tests were carried out using the disk diffusion method.

Table 2.
General plant extract information extracted from the dataset of the present systematic review.
Table 2.
General plant extract information extracted from the dataset of the present systematic review.
| Study | Region | Country | Vibrio Species | Plant | Effectiveness * | Type of Extract |
|---|---|---|---|---|---|---|
| [34] | North America | Mexico | V. parahaemolyticus, V. alginolyticus | Caulerpa sertularioides | High | Methanolic extract |
| [35] | Asia | Indonesia | V. parahaemolyticus | Eleutherine bulbosa | Intermediate/High | Ethanolic extract |
| [36] | Asia | Korea | V. alginolyticus | Rubus coreanus | High | Ethanolic extract |
| [37,38] | Asia | Malaysia | V. parahaemolyticus | Pandanus tectorius | Intermediate/High | Methanolic extract |
| [39] | Asia | Taiwan | V. alginolyticus | Cinnamomum kanehirae | Intermediate/High | Water extract |
| [40] | Asia | Taiwan | V. alginolyticus | Gelidium amansii | High | Water extract |
| [41] | Asia | Taiwan | V. alginolyticus | Gynura bicolor | Intermediate/High | Water extract |
| [42] | Asia | Taiwan | V. alginolyticus | Gynura bicolor | Intermediate | Water extract |
| [43] | Asia | Taiwan | V. alginolyticus | Phyllanthus amarus | Intermediate/High | Water extract |
| [44] | Asia | Taiwan | V. parahaemolyticus | Psidium guajava | Intermediate/High | Water extract |
| [45,46] | Asia | Taiwan | V. parahaemolyticus and V. alginolyticus | Moringa oleifera | Intermediate/High | Water extract |
| [47] | Asia | Taiwan | V. alginolyticus | Theobroma cacao | High | Extract from fresh peels |
| [48] | Asia | Thailand | V. parahaemolyticus | Macleaya cordata | High | Water extract |
| [49] | Asia | Vietnam | V. parahaemolyticus Other Vibrio species: V. harveyi | Rhodomyrtus tomentosa | Intermediate | Seed extract |
* Effectiveness (survival rate of shrimps): Intermediate (Between 50 and 70%), Intermediate/High (Between 70 and 80%), and High (Up to 90%).
In this systematic review, four studies focused on V. parahaemolyticus and V. alginolyticus, while six additional studies involving other Vibrio species were included to complement information on antibiotic use within the genus. Table 1 summarizes the 55 antibiotics reported across the 10 studies, which were grouped into 16 antibiotic families and tested against 11 Vibrio species. The primary species analyzed were V. alginolyticus and V. parahaemolyticus, although other species such as V. brasiliensis, V. cholerae, V. coralliilyticus, V. neptunius, V. vulnificus, and V. xuii were also evaluated, providing broader context without diminishing the relevance of the two focal species. Regarding susceptibility, V. alginolyticus generally exhibited greater sensitivity to antibiotics, whereas V. parahaemolyticus showed comparatively higher resistance.
Plant extracts were also included in this systematic review, with 16 studies selected for analysis. Table 2 summarizes the 13 plant extracts tested against V. parahaemolyticus, V. alginolyticus, and, in one case, V. harveyi. Survival ranges of shrimp infected with these Vibrio species were evaluated to assess the in vivo efficacy of the extracts. Overall, the plant extracts demonstrated good protective effects, with most water-, methanol-, and ethanol-based preparations yielding intermediate to high shrimp survival (70–90%). Among the tested extracts, only the seed extract produced an intermediate shrimp survival rate (up to 50%). The dataset encompassed studies from diverse global regions, and relevant information was systematically extracted, including Vibrio species, treatments (antibiotics and plant extracts), applied concentrations, methodologies, and quantitative parameters such as replicates, survival rate, mean, and standard deviation.
2.3. Prevalence of Vibrio-Related Infections in P. vannamei and Their Geographical Distribution
Pacific white shrimp (P. vannamei), also known as whiteleg shrimp, is one of the most farmed and traded shrimp species worldwide. The contamination of this type of shrimp with V. parahaemolyticus and V. alginolyticus as well as other Vibrio species has been a problem for a long-time and currently has serious repercussions on the industry. It is well-known that Vibrio species can form mature and mostly resistant biofilms [22,50]. Table 3 shows how the prevalence of Vibrio is distributed in the shrimp samples at a geographic level. In our database previously explained in Table 1 and Table 2, of the 10 and 16 articles on the use of antibiotics and plant extracts against Vibrio strains in this review, respectively, all samples originated from shrimp farming areas with the presence of different species of Vibrio, with V. parahaemolyticus and V. alginolyticus being the ones that occurred in the greatest proportion, and regarding infections in the P. vannamei shrimp, it was observed that for the most part, the most common condition present was vibriosis. Studies in Asia and South America showed infection rates relatively close in numbers. However, the four studies in South America (Brazil and Ecuador) revealed a lower number of Vibrio strains for antibiotic efficacy evaluation [23,27,28,29].

Table 3.
Analysis of different types of Vibrio observed in various geographical regions.
The prevalence of Vibrio types in shrimp varied significantly among studies from different regions and countries, especially between South America and North America. On the other hand, Asia reported an intermediate prevalence of Vibrio presence of around 74%. However, the limited number of studies may lead to biased conclusions, underscoring the need for additional data. Figure 2 shows that the dataset included few countries and most of them merely reported one study, except for Taiwan (9 studies), Malaysia (3 studies), Brazil (3 studies), Vietnam (2 studies), and Korea (2 studies).
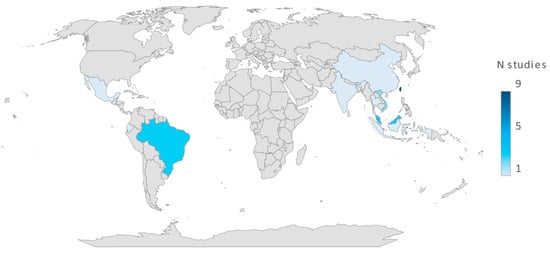
Figure 2.
Summary of global studies reporting the prevalence of Vibrio species and related infections.
Although it is established that the review focuses on V. parahaemolyticus and V. alginolyticus, the analysis of the database indicates that a total set of 12 Vibrio species is presented, of which 11 Vibrio species were used for studies of antibiotics, while only 3 Vibrio species were used in plant extract studies. The prevalence of Vibrio types for each Vibrio species in the dataset (26 articles) was mainly attributed to V. parahaemolyticus (46%), followed by V. alginolyticus (43%), and a minority of 11% to the remaining species (Figure 3).
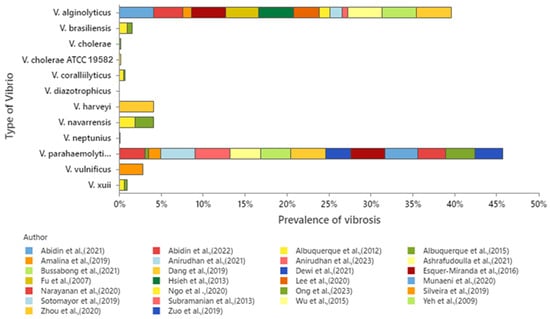
Figure 3.
Percentage of Vibrio types present in the dataset. Vibrio cholerae ATCC 19582 is included as a reference strain used for comparison in antimicrobial susceptibility testing [1,5,23,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,47,48,49,51].
The data collected showed that numerous Vibrio species exhibited resistance to different antibiotics, whereas most plant extracts demonstrated consistent antimicrobial activity. Although all samples were obtained from isolates associated with shrimp culture, one study employed V. cholerae ATCC 19582 as a reference strain [28], while other isolates originated from previously published reports [37,45]. This strain is a standard laboratory reference used in antimicrobial susceptibility testing and does not represent a distinct subspecies. Reported prevalence of Vibrio types, particularly V. parahaemolyticus and V. alginolyticus, varied considerably among studies, underscoring the need for broader and more standardized investigations to establish their true global prevalence.
2.4. Antibiotics Studies
In current aquaculture practices, the most common strategy to combat Vibrio infections is the use of broad-spectrum antibiotics. According to the literature, the antibiotics most frequently applied in shrimp farming include cephalothin (5.8%), ampicillin (9.9%), penicillin (8.9%), ceftriaxone (6.3%), aztreonam (5.8%), and tetracycline (9.4%). In our dataset of 10 articles, a wide variety of antibiotics were identified across different studies. To facilitate interpretation, the analysis was grouped by antibiotic families, since members of the same family share similar mechanisms of action against Vibrio. Table 4 provides an overview of the conditions, concentrations, and susceptibility profiles reported among Vibrio species. For example, 14 antibiotic families were tested against V. alginolyticus, using varying concentrations under culture temperatures between 28 and 35 °C for 24 h. This species showed resistance in 8 families, sensitivity in 5 families, and an intermediate response in 1 family. V. parahaemolyticus was tested against 13 antibiotic families under similar conditions (35–37 °C for 24 h) and exhibited resistance, intermediate responses, and sensitivity across all families. Notably, the proportion of sensitive strains in V. parahaemolyticus was higher, with 47.1% of isolates responding to most antibiotic families.

Table 4.
Antibiotic treatments and conditions against Vibrio species.
Table 4.
Antibiotic treatments and conditions against Vibrio species.
| Vibrio Species | Antibiotics Families | Concentration (μg) | Conditions | N° Strains Susceptibility | Studies |
|---|---|---|---|---|---|
| V. alginolyticus | Aminoglycosides | 30 | 28 °C-24 h | 3 Intermediate | [23,27,33] |
| Carbapenems | 30 | 28 °C-24 h | 1 Resistant | ||
| Cephalosporins | 30–75 | 28/35 °C-24 h | 3 Sensitive 1 Intermediate 6 Resistant | ||
| Fluoroquinolones | 5–10 | 30 °C-24 h 28 °C-24 h | 4 Sensitive 3 Resistant | ||
| Glycopeptides | 30 | 28 °C-24 h | 1 Resistant | ||
| Macrolides | 15 | 28 °C-24 h | 2 Resistant | ||
| Monobactam | 30 | 35 °C-24 h | 1 Sensitive | ||
| Nitrofuran | 100 | 30 °C-24 h | 1 Sensitive 1 Resistant | ||
| Penicillins | 1–100 10 U | 28/35 °C-24 h | 2 Sensitive 6 Resistant | ||
| Peptide | 200 (l U) | 28 °C-24 h | 1 Resistant | ||
| Phenicole | 30 | 28/30 °C-24 h | 3 Sensitive | ||
| Phosphonate | 10 | 30 °C-24 h | 1 Sensitive | ||
| Sulfonamide | 23.75/1.25 | 28 °C-24 h | 1 Resistant | ||
| Tetracyclines | 30 | 28/35 °C-24 h | 4 Sensitive 1 Intermediate 2 Resistant | ||
| V. parahaemolyticus | Aminoglycosides | 10–30 | 37 °C-24 h | 69 Sensitive 63 Intermediate 202 Resistant | [1,5,27,30,31,32] |
| Beta-lactamase | 110 | 37 °C-24 h | 16 Sensitive 44 Intermediate 2 Resistant | ||
| Carbapenems | 10–30 | 37 °C-24 h | 73 Sensitive 16 Intermediate 1 Resistant | ||
| Cephalosporins | 30 | 35 °C-24 h 37 °C-24 h | 165 Sensitive 129 Intermediate 65 Resistant | ||
| Fluoroquinolones | 5–30 | 37 °C-24 h | 19 Sensitive 53 Intermediate 144 Resistant | ||
| Glycopeptides | 30 | 37 °C-24 h | 62 Resistant | ||
| Macrolides | 2–15 | 37 °C-24 h | 40 Sensitive 22 Intermediate 62 Resistant | ||
| Monobactam | 30 | 35 °C-24 h | 1 Sensitive | ||
| Penicillins | 5–20 (10 U) | 35/37 °C-24 h | 62 Sensitive 42 Intermediate 158 Resistant | ||
| Peptide | 10–300 | 37 °C-24 h | 8 Sensitive 50 Intermediate 18 Resistant | ||
| Phenicole | 30 | 37 °C-24 h | 59 Sensitive 30 Intermediate 2 Resistant | ||
| Sulfonamide | 1.24–25 | 37 °C-24 h | 28 Sensitive 26 Intermediate 36 Resistant | ||
| Tetracyclines | 30 | 35/37 °C-24 h | 32 Sensitive 9 Intermediate 174 Resistant | ||
| Other Types of Vibrio Analyzed | |||||
| V. cholerae | Aminoglycosides | 10 | 37 °C-24 h | 11 Sensitive 8 Intermediate 4 Resistant | [27,28,30] |
| Cephalosporins | 30 | 35 °C-24 h | 28 Sensitive 1 Intermediate | ||
| Glycopeptides | 30 | 37 °C-24 h | 8 Sensitive 3 Intermediate 2 Resistant | ||
| Macrolides | 15 | 37 °C-24 h | 2 Sensitive 1 Intermediate 10 Resistant | ||
| Monobactam | 30 | 35/37 °C-24 h | 22 Sensitive 6 Intermediate | ||
| Penicillins | 10 (10 U) | 35/37 °C-24 h | 28 Sensitive 7 Intermediate 8 Resistant | ||
| Thetracyclines | 30 | 35/37 °C-24 h | 1 Sensitive 2 Intermediate 11 Resistant | ||
| V. cholerae ATCC 19582 | Aminoglycosides | 10 | NA | 26 Sensitive | [28] |
| Carbapenems | 10 | NA | 26 Sensitive | ||
| Cephalosporins | 30 | NA | 10 Sensitive 3 Intermediate 3 Resistant | ||
| Fluoroquinolones | 5 30 | NA | 25 Sensitive 1 Intermediate | ||
| Nitrofurans | 300 | NA | 26 Sensitive | ||
| Penicillins | 10 | NA | 17 Sensitive 9 Resistant | ||
| Phenicole | 30 | NA | 26 Sensitive | ||
| Sulfonamide | 25 | NA | 26 Sensitive | ||
| Tetracyclines | 30 | NA | 26 Sensitive | ||
| V. brasiliensis | Cephalosporins | 30 | 35 °C-24 h | 2 Sensitive 1 Intermediate | [27] |
| Monobactam | 30 | 35 °C-24 h | 1 Sensitive | ||
| Penicillins | 10 (10 U) | 35 °C-24 h | 1 Sensitive 1 Intermediate 1 Resistant | ||
| Tetracyclines | 30 | 35 °C-24 h | 1 Sensitive | ||
| V. coralliilyticus | Cephalosporins | 30 | 35 °C-24 h | 2 Sensitive | [27] |
| Monobactam | 30 | 35 °C-24 h | 1 Sensitive | ||
| Penicillins | 10 (10 U) | 35 °C-24 h | 1 Sensitive 1 Resistant | ||
| Tetracyclines | 30 | 35 °C-24 h | 1 Sensitive | ||
| V. diazotrophicus | Cephalosporins | 30 | 35 °C-24 h | 2 Sensitive | [27] |
| Monobactam | 30 | 35 °C-24 h | 1 Sensitive | ||
| Penicillins | 10 (10 U) | 35 °C-24 h | 2 Sensitive | ||
| Tetracyclines | 30 | 35 °C-24 h | 1 Sensitive | ||
| V. navarrensis | Cephalosporins | 30 | 35 °C-24 h | 2 Sensitive 2 Intermediate | [27] |
| Monobactam | 30 | 35 °C-24 h | 1 Sensitive 1 Intermediate | ||
| Penicillins | 10 (10 U) | 35 °C-24 h | 1 Sensitive 1 Intermediate 1 Resistant | ||
| Tetracyclines | 30 | 35 °C-24 h | 1 Sensitive | ||
| V. vulnificus | Aminoglycosides | 10–30 | 37 °C–24 h | 2 Sensitive 2 Intermediate 1 Resistant | [27,29] |
| Cephalosporins | 30 | 35 °C-24 h | 3 Sensitive | ||
| Fluoroquinolones | 5 | 37 °C–24 h | 3 Resistant | ||
| Monobactam | 30 | 35 °C-24 h | 2 Sensitive | ||
| Penicillins | 10 (10 U) | 35 °C-24 h 37 °C–24 h | 4 Sensitive 1 Intermediate 2 Resistant | ||
| Phenicole | 30 | 37 °C–24 h | 1 Resistant | ||
| Tetracyclines | 30 | 35 °C-24 h 37 °C–24 h | 1 Sensitive 2 Resistant | ||
| V. neptunius | Cephalosporins | 30 | 35 °C-24 h | 2 Sensitive | [27] |
| Monobactam | 30 | 35 °C-24 h | 1 Sensitive | ||
| Penicillins | 10 (10 U) | 35 °C-24 h | 2 Sensitive | ||
| Tetracyclines | 30 | 35 °C-24 h | 1 Sensitive | ||
| V. xuii | Cephalosporins | 30 | 35 °C-24 h | 2 Sensitive 1 Intermediate | [27] |
| Monobactam | 30 | 35 °C-24 h | 1 Sensitive | ||
| Penicillins | 10 (10 U) | 35 °C-24 h | 1 Sensitive 1 Intermediate 1 Resistant | ||
| Tetracyclines | 30 | 35 °C-24 h | 1 Sensitive | ||
Figure 4 shows the frequency of antibiotic families used against Vibrio species. The interactions of antibiotics used against Vibrio species demonstrate a greater number of antibiotics used against V. parahaemolyticus, V. alginolyticus, and V. cholerae. More accurately, 10 antibiotic families were found to be more effective against V. alginolyticus. However, only 3 antibiotic families (carbapenems, cephalosporins, and phenicols) were more efficacious against V. parahaemolyticus. Furthermore, phenicol, monobactam, and cephalosporins families demonstrated treatment effectiveness against both Vibrio species. In contrast, eight antibiotic families proved to be ineffective against Vibrio species, specifically peptide, aminoglycosides, fluoroquinolones, glycopeptides, macrolides, penicillins, sulphonamides, and tetracyclines.
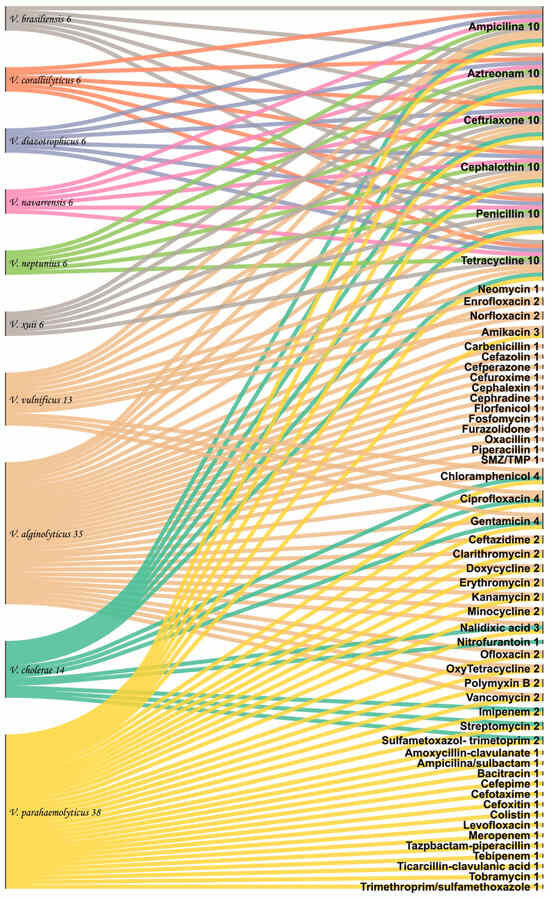
Figure 4.
Interaction of antibiotic use in Vibrio species on the total dataset of the present study.
2.5. Plant Extract Evaluation
Dietary supplementation with herbs, oils, and plant extracts is a good strategy to combat different bacteria in the food industry nowadays. This strategy is one of the techniques that has the greatest use and research since it can improve the resistance of shrimps to infections of different types of Vibrio (such as vibriosis), the healing status, and growth performance. Plants are rich in a wide variety of secondary metabolites and bioactive compounds such as flavonoids, phenolic acid, alkaloids, tannins, and terpenoids [51,52]. Supplementation with plant extracts in shrimp diet improves their health status, and it is well known that herbal medicines can regulate gut microbial composition and its secretion, as well as activate the immune system of shrimp.
2.5.1. Plant-Related Compounds Analysis
In the initial literature search, 31 articles were found evaluating the effects of plant-related compounds on pathogens, of which 16 full articles were analyzed. The compounds belonged to numerous plants, more exactly Psidium guajava, Pandanus tectorius, Phyllanthus amarus, Moringa oleifera, Eleutherine bulbosa, Scutellaria baicalensis, Salvinia cucullata, Macleaya cordata, Gynura bicolor, Theobroma cacao, Rhodomyrtus tomentosa, Rubus coreanus, and Cinnamomum kanehirae.
After consulting five databases for chemical compounds present in these plants, no information was found for S. cucullata and C. kanehirae. Across the remaining 11 plants, however, a total of 1082 unique molecules were identified. Among these, 11 were inorganic compounds, while 1071 were organic. The distribution of organic compounds was as follows: E. bulbosa (n = 58), G. bicolor (n = 41), M. cordata (n = 24), M. oleifera (n = 289), P. tectorius (n = 63), P. amarus (n = 154), P. guajava (n = 688), R. tomentosa (n = 55), R. coreanus (n = 61), S. baicalensis (n = 240), and T. cacao (n = 186). Figure 5 illustrates that most of the identified compounds belong to the phenylpropanoids and polyketides, along with the lipids and lipid-like classes. The abundance of lipids reflects the emphasis of many plant chemical characterizations on essential oils. The phenylpropanoids and polyketides include diverse flavonoids, which are of particular interest due to their antimicrobial properties.
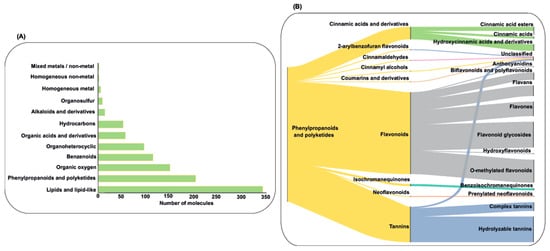
Figure 5.
Illustration of the number of compounds in each chemical class (A) and representative diagram of the abundance of molecule subclasses into the phenylpropanoids and polyketides chemical class (B).
In the flavonoid subclass, 210 unique compounds are distributed across 8 of the 11 plants in which chemical compounds were identified, more exactly E. bulbosa, M. oleifera, P. tectorius, P. amarus, P. guajava, R. tomentosa, R. coreanus, S. baicalensis, and T. cacao. Among these 210 compounds, the most commonly found molecules across the plants are presented in Figure 6.
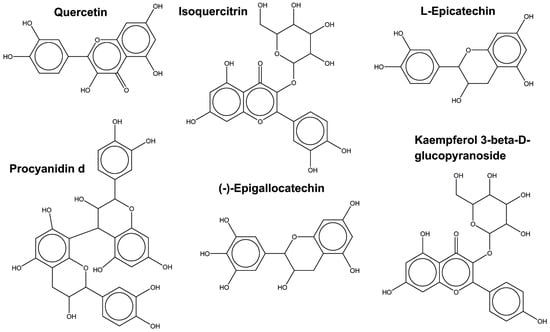
Figure 6.
Structural representation of the most common flavonoids found across the plants under our study set.
Moreover, quercetin and isoquercitrin are regularly found in 6 and 5 plants, respectively, being structurally similar to kaempferol and its derivatives. A similar chemical scaffold is also found between epicatechin and epigallocatechin, while procyanidin (from the polyflavonoids subclass) is the most structurally different. Interestingly, all these molecules have a well-known antimicrobial effect.
2.5.2. Antibacterial Activity by Plant Extracts
In our dataset (16 studies; Figure 1B), most extracts were aqueous (10/16), followed by methanol (3/16), ethanol (2/16), and a single seed extract (1/16). These preparations were obtained from different plant species, each contributing distinct properties and bioactive compounds. Table 5 summarizes the range of plants tested at varying concentrations and their antibacterial activity against the Vibrio species of interest.

Table 5.
Efficiency of plant extract treatments on shrimp infected with different Vibrio species.
Table 5.
Efficiency of plant extract treatments on shrimp infected with different Vibrio species.
| Vibrio Species | Plant Extracts | Concentration | Conditions | N° Strains Susceptibility | Studies |
|---|---|---|---|---|---|
| V. alginolyticus | Caulerpa sertularioides | 300 mg/L | 25 ± 5 °C-7 days | 1 sensitive | [34,36,39,40,41,42,43,45,47,51] |
| Cinnamomum kanehirae | 2 μg/shrimp | 28 °C-4 days | 1 intermediate 1 sensitive | ||
| Gelidium amansii. | 0.5–2 g/kg | 28 ± 1 °C-6 days | 1 sensitive | ||
| Gynura bicolor | 0.5–2 g/kg (diet) 2–8 μg/g | 27 ± 1 °C-28 days 27 ± 1 °C-2 days | 1 intermediate 1 sensitive | ||
| Rubus coreanus | 0.25–0.5% RcEE diet | 25 ± 0.5 °C-7 days | 1 sensitive | ||
| Theobroma cacao | 10–40 μg/shrimp | 28 ± 1 °C-6 days | 1 sensitive | ||
| Moringa oleifera | 1.25–5 g/kg | 35 °C-24 h | 1 sensitive | ||
| Phyllanthus amarus | 10–40 g/kg | 27 ± 1 °C-56 days | 3 sensitive | ||
| V. parahaemolyticus | Caulerpa sertularioides | 300 mg/L | 25 ± 5 °C-7 days | 1 sensitive | [34,35,37,38,44,45,48,51] |
| Eleutherine bulbosa | 1.25–25 g/kg | 28 ± 1 °C-7 days | 1 intermediate 1 sensitive | ||
| Macleaya cordata | 100–300 mg/kg | 26 ± 1 °C-30 days | sensitive | ||
| Moringa oleifera | 1.25 g/kg 2.5 g/kg 5.0 g/kg | 35 °C-24 h | sensitive | ||
| Pandanus tectorius | 0.5–6 g/L | 28 °C-24 h | 1 sensitive | ||
| Psidium guajava | 1–10 g/kg | 27 ± 2 °C-7 days | sensitive | ||
| Rhodomyrtus tomentosa | 3500 μg/disc | 25 ± 1 °C-7 days | 1 sensitive | ||
| Other Types of Vibrio Analyzed | |||||
| V. harveyi | Rhodomyrtus tomentosa | 3500 μg/disc | 25 ± 1 °C-7 days | 2 sensitive | [49] |
RcEE diet–Rubus coreanus ethanolic extract.
For V. alginolyticus, eight different extracts were tested across varied concentration ranges and units, underscoring the lack of standardization in experimental protocols. Nevertheless, most studies were performed under comparable conditions (25–28 °C, 24 h up to 56 days) and consistently reported 1–3 sensitive strains per study, with occasional intermediate responses. For V. parahaemolyticus, seven extracts from different plants were evaluated under similar conditions (25–35 °C, 24 h up to 7 days). Here, sensitivity was again the dominant outcome, with only one intermediate response reported for E. bulbosa extract [35]. In addition, R. tomentosa extract was tested against V. harveyi at 25 °C for 7 days, yielding two sensitive strains. Figure 7 illustrates the frequency of each plant extract tested against the different Vibrio species. Notably, only two extracts, C. sertularioides [34] and M. oleifera [45,51], were evaluated against both V. parahaemolyticus and V. alginolyticus, while R. tomentosa was simultaneously tested against V. parahaemolyticus and V. harveyi [49]. This limited overlap in plant selection highlights a research gap; despite encouraging activity, reproducibility remains hindered by heterogeneous dosing and reporting. Future work should prioritize standardized protocols and cross-species testing to identify extracts with consistent and scalable efficacy in aquaculture.
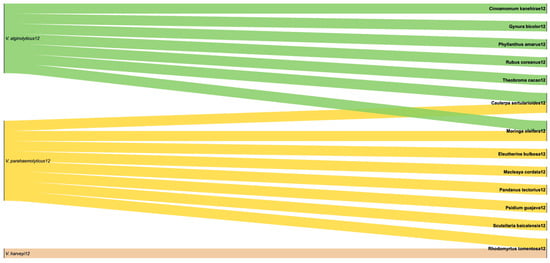
Figure 7.
Representative illustration of the interactions of plant extracts used against Vibrio species in our study set.
2.6. Strategies for Vibrio-Related Infections in Penaeus vannamei
It is well established that each strategy for controlling Vibrio-related infections has its own advantages and drawbacks. When evaluating the activity of different approaches against V. parahaemolyticus, V. alginolyticus, and other Vibrio species affecting whiteleg shrimp (P. vannamei), it is essential to consider this diversity and its practical implications (see Table 5).
2.6.1. Strengths and Limitations of Current Strategies
Several studies have analyzed the benefits and drawbacks of the main strategies applied to whiteleg shrimp (P. vannamei) for the management of Vibrio-related infections. Among the 26 articles evaluated in this review, the most commonly reported approaches include antibiotics, combined therapies, plant-based or natural treatments, and probiotics.
Table 6 summarizes the current strategies used to mitigate and prevent infections by V. parahaemolyticus, V. alginolyticus, and other Vibrio species in aquaculture, highlighting both their advantages and limitations. Depending on shrimp density and larval stage, different approaches may be selected. At present, antibiotics remain the most common method due to the availability of diverse compounds suitable for large tanks and early larval stages. Their main limitation, however, is the variation in effective concentrations depending on the antibiotic type and country. Moreover, different Vibrio strains can adapt to environmental conditions and harbor resistance genes, contributing to the widespread problem of resistance to multiple antibiotic families [53]. This challenge has driven the development of combined strategies, such as pairing effective antibiotics with plant extracts or incorporating probiotics with plant-derived compounds, to reduce resistance and improve protection. Although further studies are needed to fully characterize their antimicrobial benefits in shrimp, promising results have already been reported for controlling Vibrio-related infections in small tanks, larval stages, and adult shrimp [35,51].

Table 6.
Evaluation of strengths and limitations of the main strategies to mitigate and prevent Vibrio-related infections in shrimp P. vannamei.
Table 6.
Evaluation of strengths and limitations of the main strategies to mitigate and prevent Vibrio-related infections in shrimp P. vannamei.
| Strategies | Description | Strengths | Limitations | References |
|---|---|---|---|---|
| Antibiotics | Antibiotics are commonly used in aquaculture both for prophylactic purposes and to treat bacterial infections. The most used are tetracyclines, quinolones, phenicols, macrolides, and sulfonamides among others. They are usually administered directly in balanced food or water. Frequent and indiscriminate use is related to the development of bacterial resistance. | Establish adequate doses and administration times according to shrimp species and production stage. Rotate different families of antibiotics to avoid resistance. Use responsibly, only when necessary, based on laboratory diagnosis. Improve farming conditions to reduce stressful factors that promote infections. | Different concentrations of antibiotics in the aquaculture industry depending on the country-quality requirements and antibiotic residues in the final product The emergence of antibiotic-resistant Vibrio strains that persist in the aquaculture environment. Risk of transfer of resistance genes to human pathogenic bacteria through the food chain. Effects on other organisms in the aquatic ecosystem such as fish, mollusks, and plankton. Generation of antibiotic residues in shrimp tissue that reach the consumer. | [27] |
| Combined therapy (antibiotics plus plant extracts) | The most effective were furazolidone, ciprofloxacin, chloramphenicol, norfloxacin, nalidixic acid, florfenicol, and fosfomycin (inhibited the growth of most or all isolated strains). The effectiveness of essential oil EO1 (oregano oil extract, inhibited 100% of strains). | Furazolidone, ciprofloxacin, chloramphenicol, norfloxacin, nalidixic acid, florfenicol, and fosfomycin, enrofloxacin, inhibited most or all Vibrio strains. Oregano oil extract inhibited all Vibrio strains at low concentrations. Plant essential oils (EOs) combined with antibiotics: EOs can enhance the efficacy of antibiotics against multidrug-resistant pathogens. | Many antibiotics are not approved for use in aquaculture. Also, antimicrobial resistance was found in some strains. Some plant extracts exhibit toxicity, and their mechanisms of action are not well studied. Further, in vivo studies are required to confirm effectiveness. Effective in vitro doses may not correlate with the response in shrimp. More research is needed on specific antimicrobial action mechanisms and synergies with other compounds. | [23,53,54] |
| Plant extracts | Many medicinal plants have antibacterial activity against Vibrio species. Single extracts or a mixture of extracted compounds are used to treat vibriosis in shrimp. There are different types of plant extracts, among them the most used are aqueous, ethanolic, and methanolic. | According to the bibliography, the use of different types of plant extracts has the benefit of shrimp, improving growth and promoting immune activity. Additionally, it is a strategy that provides sustainable, ecological, and safe compounds, such as alkaloids, saponins, terpenoids, and flavonoids, to replace chemical compounds and antibiotics in aquaculture, being a solution for the antibiotic resistance that is currently observed in the industry. | The greatest limitation of this technique is the lack of more studies that apply it in vivo, to be able to generalize the results of efficacy and safety for the host (shrimp) because it has been reported that various plant extracts exhibit toxicity, so their mechanisms of action must be studied in greater depth. In addition, further research must be carried out to be able to make a protocol depending on the type of extract, and the larval stage of the shrimp. | [35,55] |
| Probiotics | This technique consists of administering probiotics, prebiotics, postbiotics, and synbiotics to the shrimp through the diet to combat or prevent Vibrio species. | The use of pro-, post-, pre-, and synbiotics in aquaculture has been considered effective food supplements that are prescribed to improve growth, antioxidant status, immunity, and resistance capacity to diseases such as vibriosis in different species of shrimp and other seafood. | The most significant disadvantage to using pro-, post-, pre-, and synbiotics is that they are often unable to maintain themselves and require supplementation regularly, which results in making this strategy less cost-effective. | [51,56,57] |
2.6.2. Comparative Cost and Efficiency
Beyond their biological activity, the choice of treatment strategies in aquaculture also depends on cost and efficiency, both of which determine their feasibility for large-scale application. A comparative analysis of these parameters highlights important differences among antibiotics, plant-derived treatments, probiotics, and emerging alternatives (see Table 7).

Table 7.
Comparative cost and efficiency of main strategies to mitigate Vibrio-related infections in P. vannamei.
Table 7.
Comparative cost and efficiency of main strategies to mitigate Vibrio-related infections in P. vannamei.
| Strategy | Representative Agent(s)/Study | Dose/Usage (as Reported) | Reported Efficiency vs. Vibrio | Cost Category * | Notes/Caveats | References |
|---|---|---|---|---|---|---|
| Antibiotics | Oxolinic acid + oxytetracycline | 50 mg OA/kg + 100 mg OTC/kg feed for 5 days; challenged with V. parahaemolyticus | Survival higher than control; combination therapy most effective | Medium–High | Effective in reducing mortality but associated with antimicrobial resistance (AMR), residue concerns, and monitoring costs | [58] |
| Combined therapy (antibiotics plus plant extracts) | Enrofloxacin (ENR) + San-Huang-San (SHS) | 10–40 mg ENR/kg + 250–1000 mg SHS/kg feed for 5 days; challenged with AHPND-causing V. parahaemolyticus | Survival higher than with ENR or SHS alone; combination therapy significantly reduced mortality and improved immune response. | Medium. | Combination improves survival and immune response while allowing a reduction in antibiotic dose; still consider risks of AMR, standardization of herbal extract, dosing regimen, and short duration (5 days) → longer-term effects not assessed. | [59] |
| Plant extracts | Psidium guajava leaf extract | 5 g/kg feed for 28–56 days; challenge with V. parahaemolyticus | Survival ~72% at optimal dose; improved growth and immune markers | Low–Medium | Promising alternative with immune-stimulating and eco-friendly properties; however, dose optimization and in vivo safety studies are needed | [44] |
| Probiotics | Lactobacillus paracasei or Bifidobacterium longum | Fed for 28 days; challenge with V. parahaemolyticus | Survival 67–73% depending on species | Low–Medium | Enhance immune responses and growth; generally cost-effective but require continuous supplementation | [60] |
* Cost categories are qualitative, derived from peer-reviewed literature on input prices, application requirements, and biosecurity needs, not vendor pricing. Antibiotics are considered medium–high cost due to drug expenses, residue monitoring, and antimicrobial resistance (AMR) mitigation. Combined therapies (antibiotics + plant extracts) are classed as medium, since herbal supplements may reduce antibiotic dosage but still involve additional formulation costs and monitoring. Plant extracts are generally low–medium cost, described as eco-friendly and practical but requiring optimization for dosing and sourcing. Probiotics are also low–medium cost: they are widely regarded as cost-effective and scalable, but continuous supplementation and quality assurance are needed to maintain efficacy.
Antibiotics remain highly effective in controlling Vibrio-related infections but are associated with medium–high costs, antimicrobial resistance, and residue concerns in aquaculture products [58,60,61]. In contrast, plant extracts such as guava leaf extract represent cost-effective and environmentally sustainable alternatives, offering immunostimulatory benefits alongside moderate protection against Vibrio species [22,44]. Probiotics also demonstrate encouraging results in reducing mortality and improving shrimp health, often at lower costs, though they require continuous supplementation to maintain their benefits [51,60,62]. Likewise, combined therapies, such as enrofloxacin with San-Huang-San, show strong potential by significantly reducing mortality compared to single agents and allowing for lower antibiotic doses, but still carry considerations related to antimicrobial resistance, herbal standardization, and formulation costs [59]. Taken together, the comparison underscores that while antibiotics remain the dominant tool, plant extracts, probiotics, and integrated antibiotic–plant extract therapies are emerging as promising, cost-effective, and sustainable alternatives that could help reduce reliance on antibiotics in shrimp aquaculture.
2.6.3. Mechanistic Comparison of Antibiotics, Plant-Derived Agents, and Other Alternatives
In aquaculture, antibiotics remain the most widely applied approach against Vibrio spp., targeting essential cellular processes. β-lactams and cephalosporins act by inhibiting cell wall synthesis; while tetracyclines, macrolides, and phenicols block protein synthesis, and fluoroquinolones inhibit DNA replication and sulfonamides interfere with folate metabolism [63,64,65]. While effective against planktonic cells, these agents face limitations in biofilm contexts, where reduced penetration and altered metabolic states confer biofilm tolerance, often requiring substantially higher concentrations for eradication than for inhibition [22,66,67]. Moreover, the frequent use of antibiotics in aquaculture has accelerated the emergence of multidrug-resistant Vibrio strains, complicating treatment outcomes and raising concerns about the dissemination of resistance genes through the aquatic environment [68]. Residual antibiotics in shrimp tissues also represent a risk for food safety and international trade compliance, which further underscores the need for alternative or complementary strategies [61,69].
By contrast, many phytochemicals and plant-derived extracts exert multi-target mechanisms that make them less likely to induce resistance [66,70,71]. Flavonoids, tannins, and phenolic acids can cause membrane disruption and depolarization, quorum-sensing (QS) inhibition, and down-regulation of virulence factors, thereby limiting bacterial colonization and pathogenicity [70,72]. Other compounds interfere with extracellular polymeric substance (EPS) matrix formation, impairing adhesion and biofilm stability, or modulate oxidative stress pathways via reactive oxygen species (ROS) [73,74]. Recent studies confirm that extracts of guava, moringa, and pomegranate derivatives reduce Vibrio virulence gene expression, biofilm biomass, and shrimp mortality at sub-MIC levels [22,75]. Essential oils, such as those from oregano and thyme, have demonstrated strong antibiofilm and bactericidal activity, and several phenolic compounds (e.g., quercetin, punicalagin) have shown synergy with antibiotics [76,77,78]. These features not only broaden their antimicrobial spectrum but also highlight their potential in integrated therapy frameworks, particularly when combined with antibiotics to overcome resistance and improve shrimp survival.
Together, these findings suggest that while antibiotics continue to serve as the backbone of Vibrio management, the integration of plant-derived agents provides additional mechanisms that can weaken bacterial defenses and reduce virulence. This complementary nature has motivated increasing attention to combined therapies, where antibiotics and phytochemicals are applied in synergy to enhance treatment efficacy while reducing selective pressures for resistance and lowering the doses required of each agent [59]. This dose-sparing effect is particularly valuable in aquaculture because it reduces both toxicological risks to shrimp and the residues accumulating in shrimp tissues destined for human consumption [23,53,54,59]. Mechanistically, many plant-derived compounds act as adjuvants: essential oils and flavonoids increase membrane permeability, facilitating antibiotic entry; tannins and alkaloids can inhibit efflux pumps, thereby restoring antibiotic susceptibility in resistant Vibrio isolates, and phenolic compounds can interfere with quorum sensing, attenuating virulence and making bacteria more vulnerable to antibiotics [44,79,80]. Several studies have confirmed that combinations such as enrofloxacin with herbal mixtures or ciprofloxacin with essential oils produce superior survival outcomes in shrimp challenged with V. parahaemolyticus compared to either agent alone [59,81]. Moreover, synergistic combinations not only enhance antimicrobial activity but also limit the selective pressure for resistance emergence, since the bacteria are attacked through multiple targets simultaneously [66,80]. While most evidence still comes from laboratory studies, these findings strongly support the idea that antibiotic–plant extract combinations could form the basis of sustainable integrated management frameworks in shrimp aquaculture, provided that more standardized in vivo trials and cost-effectiveness analyses are performed.
In recent years, probiotics, prebiotics, postbiotics, and synbiotics have emerged as promising alternatives to antibiotics and phytochemicals in shrimp aquaculture. Their protective mechanisms are multifactorial: they compete with pathogenic Vibrio for adhesion sites and nutrients, produce antimicrobial metabolites such as bacteriocins, organic acids, and hydrogen peroxide, and stimulate host immunity by activating innate defense mechanisms (e.g., phenoloxidase activity and antimicrobial peptide production). Probiotics can also promote gut microbiota resilience, maintaining a balanced community that indirectly limits Vibrio colonization and biofilm formation [82,83,84]. Prebiotics, by providing substrates for beneficial bacteria, further enhance this effect, while postbiotics (non-viable bacterial products) supply bioactive metabolites without the risks of live inoculation. Synbiotics, combining probiotics and prebiotics, are being increasingly studied for their additive benefits [56,85,86]. Recent in vivo studies have shown that Lactobacillus spp., Bacillus spp., and Bifidobacterium spp. supplementation improves shrimp survival against V. parahaemolyticus and V. alginolyticus challenges, while also enhancing growth performance and antioxidant capacity [60,83,87]. Other promising candidates include yeast-derived products and marine actinobacteria, which have demonstrated immunostimulatory and antivirulence properties [88,89]. Importantly, some probiotics not only reduce mortality but also decrease biofilm formation and virulence factor expression, complementing the antibiofilm role of plant extracts. Nevertheless, as indicated in Table 7, the main limitation of these strategies is the need for continuous supplementation to maintain their beneficial effects, which may raise operational costs and affect scalability [60]. In this context, attention has recently shifted to antimicrobial peptides (AMPs) as another biologically derived tool with the potential to complement existing probiotic and phytochemical strategies. AMPs, particularly those derived from marine bacteria and aquaculture-associated microbiomes, represent a novel mechanism-distinct option. They act primarily by disrupting bacterial membranes, destabilizing biofilm structure, and in some cases interfering with quorum sensing [88,90,91]. Importantly, AMPs can be integrated with existing strategies: they have shown synergy with antibiotics, plant extracts, and even probiotics, offering dose-sparing and enhanced antibiofilm activity [90,92]. While challenges remain in production cost and stability, recent studies demonstrate their efficacy against multidrug-resistant V. parahaemolyticus, V. alginolyticus and other Vibrio species in experimental shrimp models [93,94,95]. This highlights AMPs as promising candidates for inclusion in combined therapy frameworks, complementing antibiotics, phytochemicals, and probiotics as part of sustainable Vibrio management.
3. Discussion
Shrimp farming is one of the fastest-growing industries and represents significant economic importance worldwide. In Ecuador, it is the main aquaculture activity, as this is the main country producing shrimp worldwide. This industry principally involves the production of P. vannamei or whiteleg shrimp, which is cultured in 21 countries and is the primary species reported in the Americas [96]. However, shrimps are highly susceptible to diseases, and in recent years, various problems have significantly affected shrimp production and health, with vibriosis being one of the major issues [97]. Vibrio-related infections are caused by multiple species of the facultatively anaerobic, Gram-negative genus Vibrio. V. parahaemolyticus, V. alginolyticus, and V. harveyi are among the Vibrio species most isolated and identified in diseased shrimp, as reported in the literature [56]. Therefore, this systematic review was conducted to analyze the strategies currently used to treat different Vibrio-related infections, identify the most prevalent species, and highlight important points for the culture of P. vannamei shrimp.
Through the analysis of our data, various strategies were identified to mitigate infections related to V. parahaemolyticus and V. alginolyticus in addition to other species of Vibrio, especially for vibriosis in Pacific white shrimp (P. vannamei) were identified in different geographic regions. The analysis indicated a high prevalence of types of Vibrio in shrimp, with the highest prevalence reported in North America (100%), followed by Asia (84.89%) and South America (74.14%). The most common Vibrio species were V. parahaemolyticus and V. alginolyticus, although other species were identified. In comparison with other studies, it is observed that the prevalence of Vibrio and their species varies in Asia, ranging from 10.47% to 47.6% according to certain reports, but other studies stated almost 100%, differing also to the American continent studies, which report a prevalence of 60% in most articles [98,99]. In the majority of the worldwide studies, the predominant species causing vibriosis in shrimp are V. parahaemolyticus and V. alginolyticus, followed by other Vibrio species such as V. cholerae and V. vulnificus [100,101,102,103]. However, the variation in prevalence among continents, with a higher incidence in Asia compared to America, could be attributed to environmental factors, farming practices, and disease control strategies implemented in each region, leading to varying levels of antibiotic resistance [104].
This review highlights that while multiple strategies have been investigated to control Vibrio-related infections in P. vannamei, their effectiveness varies substantially depending on whether pathogens are in planktonic or biofilm states, the compound applied, and the farming context. Antibiotics remain the backbone of disease control because of their broad availability, established protocols, and rapid action. However, across the studies reviewed, their consistent limitations were reduced efficacy against biofilms, the rapid emergence of resistant strains, and the persistence of resistance genes in aquatic environments [105,106,107,108,109,110]. This not only undermines treatment success but also creates a direct link between aquaculture and global antimicrobial resistance challenges, reinforcing that antibiotic-only approaches are unsustainable in the long term.
In contrast, plant-derived agents and phytochemicals provide multi-target activities that include membrane disruption, quorum-sensing inhibition, and biofilm destabilization [22,55,66,70,71,72,73,74,75,111]. Their ecological origin makes them attractive alternatives, yet reproducibility across studies remains inconsistent due to variation in plant sources, extraction methods, and compound concentrations. Without standardized protocols for extraction, dosing, and testing, the translation of promising laboratory results into reliable field applications will remain limited. Still, the observation that some extracts not only reduce Vibrio virulence but also enhance shrimp survival suggests that these agents could serve as effective adjuvants in integrated strategies rather than as stand-alone treatments [22,55,77].
Combined therapies, particularly the use of antibiotics together with plant extracts or isolated phytochemicals, emerge as one of the most promising strategies identified in this review [59,76,77,78,80,81,82]. These combinations can enhance antimicrobial efficacy, reduce the required antibiotic dose, and slow the development of resistance by targeting bacteria through complementary mechanisms. Beyond these synergies, an important research opportunity lies in expanding the scope of combination strategies to include not only antibiotics but also emerging alternatives such as postbiotics and antimicrobial peptides (AMPs) [56,85,86,87,88,89,90,91,92,93,94,95]. Plant compounds with antivirulence and antibiofilm properties could potentiate the effects of these newer biological tools, providing a multi-layered approach that integrates conventional treatments with ecological and sustainable innovations. The challenge ahead is to validate these combinations under standardized in vivo conditions in shrimp, ensuring reproducibility, scalability, and cost-effectiveness for practical aquaculture application [51,55,77,112].
Taken together, these patterns highlight that the sustainable control of Vibrio-related infections in shrimp aquaculture will likely depend less on single agents and more on rationally designed combinations that balance efficacy, safety, sustainability, and cost. Importantly, this review also revealed a key limitation: most of the available evidence comes from Asia and South America, with very limited data from Africa, Europe, and North America [98,99,100,101,102,103,104]. This geographic imbalance constrains the generalizability of findings and underscores the need for research efforts in underrepresented regions to capture a broader diversity of farming practices and environmental conditions.
Future work should focus on (i) establishing standardized procedures for in vivo testing of antibiotics, plant extracts, probiotics, postbiotics, and AMPs in shrimp models, (ii) integrating cost–benefit analyses into efficacy studies to ensure practical scalability, (iii) exploring regional differences in Vibrio prevalence and resistance dynamics, and (iv) prioritizing combination therapy designs that exploit synergies while minimizing resistance development. Addressing these gaps will be essential not only for improving shrimp health and productivity but also for ensuring that aquaculture aligns with One Health goals of reducing antimicrobial resistance and promoting sustainable food systems.
4. Materials and Methods
4.1. Literature Search
4.1.1. Literature Search Vibrio Treatment with Antibiotics
This study was conducted following Preferred Reporting Items for Systematic Reviews and systematic review (PRISMA) guidelines [113]. The Scopus and PubMed databases were searched for journal articles, in English, using combinations of Boolean terms and Medical Subject Headings (MESH). Specifically, the searched terms and combinations were as follows: “Vibrio parahaemolyticus”, “Vibrio alginolyticus”, “Litopenaeus vannamei”, “Penaeus vannamei”, and “treatment”. Articles that reported results on antimicrobial tests of different antibiotics against Vibrio pathogens, specifically the two species V. parahaemolyticus and V. alginolyticus of interest in the agricultural and shrimp industry, were positively selected. The references to these articles were also checked to find additional records. All references were compiled into a Zotero Library database (www.zotero.org) and then managed using Excel software.
4.1.2. Literature Search Vibrio Treatment with Plant Extracts
The PubMed database was searched for journal articles, in English, using combinations of Boolean terms and MESH terms. The searched terms and combinations were as follows: “Vibrio”, “V. parahaemolyticus”, “V. alginolyticus”, and “extract”. Articles reporting results on antimicrobial tests using different plant extracts against the two Vibrio species of interest were considered. The references to these articles were also checked to find additional records. All references were compiled into a Zotero Library database (www.zotero.org) and then managed using Excel software.
4.2. Screening Process
Duplicates were initially identified and removed in Zotero after entering all the recognized studies into a self-created Excel database (see File S1). All articles were assessed by three reviewers (AB-N, NRJ-M, and ACC-P) by screening titles, abstracts, topics, and finally full-length texts. At each level, the reviewers independently screened the articles and then merged their conclusions. An additional examination of the selected articles was conducted by two other reviewers (ET and AM) to ensure the homogeneity of the eligibility criteria applied by the previous reviewers in the initial dataset. Discrepancies were resolved by discussion before finalizing the records for the evaluation of eligibility criteria (see next subsection). In case of disagreements, a third assessor (AM or ET) was assigned to make a final decision.
4.3. Eligibility Criteria, Data Extraction, and Quality Assessment
The main inclusion criteria were the results of antimicrobial assays with different types of antibiotics and plant extracts. Data on the most used antibiotics and different types of plant extracts, Vibrio species, and methodologies were extracted if available. Duplicate reports from different databases were excluded from the final dataset for the systematic review. Finally, articles without full-length text available and studies with missing or incomplete data were also omitted. The extracted information included the first authors’ names, year of publication, location, Vibrio species, antibiotic names, methodologies, plants, types of extract, and quantifiable parameters (effectiveness, survival rate, and number of replicates). The last three parameters were the most critical methodological criteria in the initial screening. Additionally, studies involving assays with methodologies other than agar diffusion method were identified for further evaluation. The initial three authors (AB-N, NRJ-M, and ACC-P) extracted all data, and further confirmation and evaluation were conducted by the lead authors (ET and AM). The final document with the collected data is available upon request from the authors.
4.4. Plant-Compound Relationship Identification and Molecular Analysis
For the identification of chemical compounds present in the plants obtained from the literature review, we explored the following publicly available databases (accessed on 1 February 2024): LOTUS [114], COCONUT [115], Korean Traditional Knowledge Portal (KTKP) (https://www.koreantk.com/ktkp2014/), IMPPAT: Indian Medicinal Plants [116], and ETM-DB (http://biosoft.kaist.ac.kr/etm).
The compounds extracted from these databases are presented in several formats. These formats include SMILES representation, SDF data information, or only the molecule names. The RDKit (RDKit: Open-source cheminformatics, https://www.rdkit.org, accessed on 1 March 2024) and PubChemPy (https://github.com/mcs07/PubChemPy, accessed on 1 March 2024) python packages were used to homogenize representation to SMILES strings and to remove duplicated molecules. Additionally, all molecules were classified using NP classifier version 1.5 (https://npclassifier.ucsd.edu/, accessed on 1 March 2024) [117], according to their chemical classes and subclasses.
5. Conclusions
This systematic review provides comprehensive insights into strategies for managing V. parahaemolyticus and V. alginolyticus infections in whiteleg shrimp. While antibiotics remain the most widely used intervention, the analysis shows that only certain families, particularly phenicols, cephalosporins, and carbapenems, retain consistent activity against these pathogens. Their use, however, must be restricted and guided by laboratory diagnosis to avoid further resistance development.
Among natural alternatives, plant extracts rich in flavonoids, tannins, and phenolic acids (such as guava (Psidium guajava), moringa (Moringa oleifera), and pomegranate derivatives) emerged as the most promising candidates, with evidence of reducing virulence gene expression, biofilm biomass, and shrimp mortality. These agents, if standardized for dosage and extraction protocols, could be implemented as sustainable feed additives in shrimp farming.
Combined therapies represent the most immediately applicable innovation. Integrating antibiotics with phytochemicals allows dose reduction, improved survival outcomes, and lower selective pressure for resistance. Practical examples include the combination of enrofloxacin with herbal formulations or ciprofloxacin with essential oils, which have shown superior protection in experimental infections. Such combinations could be adapted into farm protocols with proper cost–benefit evaluation and safety validation.
Looking ahead, stakeholders should prioritize (i) adopting combined strategies that integrate antibiotics with validated plant-based agents, (ii) supporting the development of standardized protocols for extract preparation and in vivo testing, and (iii) monitoring regional prevalence and resistance trends to guide antibiotic family selection. This tiered approach offers the most feasible path toward sustainable Vibrio management in shrimp aquaculture, reducing reliance on antibiotics while ensuring shrimp health, farm productivity, and food safety.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30173620/s1, File S1. Seleccionador, which constitutes the Excel database containing the data collected in this study.
Author Contributions
A.M. was responsible for modelling and analysis design. The screening process, data collection, and quality assessment were performed by A.B.-N., N.R.J.-M. and A.C.C.-P. Eligibility criteria were performed by N.R.J.-M., A.C.C.-P., E.T. and A.M. Data analysis was developed by E.T., A.B.-N., N.R.J.-M., A.C.C.-P. and A.M. The writing manuscript was elaborated by N.R.J.-M., A.C.C.-P. and A.M. Reviewing the manuscript was realized by E.T. and A.M. Research project supervision was conducted by A.M. in the Microbiology Institute at USFQ. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by COCIBA Research Grants under Project ID: 17357 entitled “Alternative approaches for eliminating Biofilms”, Project ID: 16801 entitled “Characterization of single and mixed Biofilms”, and Project ID: 20173 entitled “Preparation and Characterization of Bacteriophage Cocktail to treat Vibrio-related Biofilms” to António Machado.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that supports the findings of this study are available in the main text of the manuscript and Supplementary Material. Further information can also be given on request from the corresponding authors.
Acknowledgments
Special recognition deserves all colleagues of the Biofilm Research Group (BRG) at the Microbiology Institute of COCIBA USFQ, as well as the Research Office of Universidad San Francisco de Quito and COCIBA for their financial support in this study. Also, we want to thank Dario F. Cueva and Danilo Endara for their assistance in pitching the initial idea and literature search of the present study, respectively.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| AHPND | Acute hepatopancreatic necrosis disease |
| WFD | White feces disease |
| SGS | Seagull syndrome |
| HGT | Horizontal gene transfer |
| PRISMA | Preferred Reporting Items for Systematic Reviews and systematic review |
| MESH | Boolean terms and Medical Subject Heading |
| KTKP | Korean Traditional knowledge portal |
| IMPPAT | Indian Medicinal Plants |
References
- Narayanan, S.V.; Joseph, T.C.; Peeralil, S.; Koombankallil, R.; Vaiyapuri, M.; Mothadaka, M.P.; Lalitha, K.V. Tropical Shrimp Aquaculture Farms Harbour Pathogenic Vibrio parahaemolyticus with High Genetic Diversity and Carbapenam Resistance. Mar. Pollut. Bull. 2020, 160, 111551. [Google Scholar] [CrossRef] [PubMed]
- Zermeño-Cervantes, L.A.; Barraza, A.; González-Ponce, H.A.; Martinez-Diaz, S.F.; Cardona-Félix, C. Penaeus vannamei Challenged with a Vibrio parahaemolyticus AHPND Strain Shows Hepatopancreatic Microbiota Imbalance. Cienc. Mar. 2023, 49, e3234. [Google Scholar] [CrossRef]
- Amoah, K.; Dong, X.; Tan, B.; Zhang, S.; Kuebutornye, F.K.A.; Chi, S.; Yang, Q.; Liu, H.; Zhang, H.; Yang, Y. In Vitro Assessment of the Safety and Potential Probiotic Characteristics of Three Bacillus Strains Isolated from the Intestine of Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Front. Vet. Sci. 2021, 8, 675962. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, F.; Chaieb, K.; Zmantar, T.; Kallel, H.; Bakhrouf, A. Adherence Assays and Slime Production of Vibrio alginolyticus and Vibrio parahaemolyticus. Braz. J. Microbiol. 2009, 40, 394–398. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Na, K.W.; Hossain, M.I.; Mizan, M.F.R.; Nahar, S.; Toushik, S.H.; Roy, P.K.; Park, S.H.; Ha, S.-D. Molecular and Pathogenic Characterization of Vibrio parahaemolyticus Isolated from Seafood. Mar. Pollut. Bull. 2021, 172, 112927. [Google Scholar] [CrossRef]
- Ashrafudoulla, M.; Mizan, M.F.R.; Park, H.; Byun, K.-H.; Lee, N.; Park, S.H.; Ha, S.-D. Genetic Relationship, Virulence Factors, Drug Resistance Profile and Biofilm Formation Ability of Vibrio parahaemolyticus Isolated From Mussel. Front. Microbiol. 2019, 10, 513. [Google Scholar] [CrossRef]
- Sasikala, D.; Srinivasan, P. Characterization of Potential Lytic Bacteriophage against Vibrio alginolyticus and Its Therapeutic Implications on Biofilm Dispersal. Microb. Pathog. 2016, 101, 24–35. [Google Scholar] [CrossRef]
- Butt, U.D.; Lin, N.; Akhter, N.; Siddiqui, T.; Li, S.; Wu, B. Overview of the Latest Developments in the Role of Probiotics, Prebiotics and Synbiotics in Shrimp Aquaculture. Fish Shellfish Immunol. 2021, 114, 263–281. [Google Scholar] [CrossRef]
- Yang, F.; Xu, L.; Huang, W.; Li, F. Highly Lethal Vibrio parahaemolyticus Strains Cause Acute Mortality in Penaeus vannamei Post-Larvae. Aquaculture 2022, 548, 737605. [Google Scholar] [CrossRef]
- de Souza Valente, C.; Wan, A.H.L. Vibrio and Major Commercially Important Vibriosis Diseases in Decapod Crustaceans. J. Invertebr. Pathol. 2021, 181, 107527. [Google Scholar] [CrossRef]
- Thangaraj, S.K.; Nathamuni, S.P.; Katneni, V.K.; Jangam, A.K.; Avunje, S.; Thulasi, D.N.; Grover, M.; Angel, J.R.J.; Shekhar, M.S. Microbial Communities Associated with Zoea-2 Syndrome and White Feces Syndrome in P. vannamei Farming. Front. Mar. Sci. 2023, 10, 1120004. [Google Scholar] [CrossRef]
- Vandenberghe, J.; Verdonck, L.; Robles-Arozarena, R.; Rivera, G.; Bolland, A.; Balladares, M.; Gomez-Gil, B.; Calderon, J.; Sorgeloos, P.; Swings, J. Vibrios Associated with Litopenaeus vannamei Larvae, Postlarvae, Broodstock, and Hatchery Probionts. Appl. Environ. Microbiol. 1999, 65, 2592–2597. [Google Scholar] [CrossRef] [PubMed]
- Lien, H.H.; de Mey, Y.; Nhan, D.K.; Bush, S.; Meuwissen, M.P.M. Can Cooperation Reduce Yield Risks Associated with Infectious Diseases in Shrimp Aquaculture in Vietnam? Aquac. Econ. Manag. 2024, 28, 660–680. [Google Scholar] [CrossRef]
- Widjaja, L.; Indrawati, A.; Nurhidayat, N.; Yulinery, T.; Triana, E.; Soeka, Y.S.; Emilia, Q.; Ariyanti, D.; Ghozali, A.A.; Manalu, J. Quantitative in Vitro Assessment of Lytic Vibriophages Isolated from Acute Hepatopancreatic Necrosis Disease-Affected Shrimp Cultures. Aquaculture 2026, 611, 743025. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, J.; Zhan, P.; Xiong, J. Postlarval Shrimp-Associated Microbiota and Underlying Ecological Processes over AHPND Progression. Microorganisms 2025, 13, 720. [Google Scholar] [CrossRef]
- Fadel, A.; Khafage, A.; Abdelsalam, M.; Abdel-Rahim, M.M. Comparative Evaluation of Three Herbal Extracts on Growth Performance, Immune Response, and Resistance against Vibrio parahaemolyticus in Litopenaeus vannamei. BMC Vet. Res. 2025, 21, 166. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Li, P.; Chen, T.; Ren, C.; Hu, C.; Luo, P. Identification of Candidate Genes Associated with Resistance against Vibrio parahaemolyticus Causing Acute Hepatopancreatic Necrosis Disease in Litopenaeus vannamei via Bulked Segregant Analysis. Fish Shellfish Immunol. 2025, 163, 110405. [Google Scholar] [CrossRef]
- Liao, J.; Cai, Y.; Wang, X.; Shang, C.; Zhang, Q.; Shi, H.; Wang, S.; Zhang, D.; Zhou, Y. Effects of a Potential Host Gut-Derived Probiotic, Bacillus Subtilis 6-3-1, on the Growth, Non-Specific Immune Response and Disease Resistance of Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Probiotics Antimicrob. Proteins 2021, 13, 1119–1137. [Google Scholar] [CrossRef]
- Srinivas, D.; Ch, V. Prevalence of Vibriosis in Penaeus (Litopenaeus) Vannamei in Three Different Locations of Nellore District of Coastal Andhra Pradesh. Int. J. Adv. Res. Biol. Sci. 2019, 6, 28–33. [Google Scholar] [CrossRef]
- Zhao, S.; Ma, L.; Wang, Y.; Fu, G.; Zhou, J.; Li, X.; Fang, W. Antimicrobial Resistance and Pulsed-Field Gel Electrophoresis Typing of Vibrio parahaemolyticus Isolated from Shrimp Mariculture Environment along the East Coast of China. Mar. Pollut. Bull. 2018, 136, 164–170. [Google Scholar] [CrossRef]
- Piedrahita, Y. Evolución Histórica, Mejora Genética, Reforestación de Manglares, Barreras Sanitarias y Otros Desarrollos. Available online: https://www.globalseafood.org/advocate/la-industria-de-cultivo-de-camaron-en-ecuador-parte-1/ (accessed on 19 August 2025).
- Jara-Medina, N.R.; Cueva, D.F.; Cedeño-Pinargote, A.C.; Gualle, A.; Aguilera-Pesantes, D.; Méndez, M.Á.; Orejuela-Escobar, L.; Cisneros-Heredia, D.F.; Cortez-Zambrano, R.; Miranda-Moyano, N.; et al. Eco-Alternative Treatments for Vibrio parahaemolyticus and V. cholerae Biofilms from Shrimp Industry through Eucalyptus (Eucalyptus globulus) and Guava (Psidium guajava) Extracts: A Road for an Ecuadorian Sustainable Economy. PLoS ONE 2024, 19, e0304126. [Google Scholar] [CrossRef]
- Sotomayor, M.A.; Reyes, J.K.; Restrepo, L.; Domínguez-Borbor, C.; Maldonado, M.; Bayot, B. Efficacy Assessment of Commercially Available Natural Products and Antibiotics, Commonly Used for Mitigation of Pathogenic Vibrio Outbreaks in Ecuadorian Penaeus (Litopenaeus) Vannamei Hatcheries. PLoS ONE 2019, 14, e0210478. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, L.; Liu, S.; Wang, Q.; Guo, Z.; Chen, C.; Feng, J. What Drives Changes in the Virulence and Antibiotic Resistance of Vibrio harveyi in the South China Sea? J. Fish Dis. 2020, 43, 853–862. [Google Scholar] [CrossRef]
- Cangui-Panchi, S.P.; Ñacato-Toapanta, A.L.; Enríquez-Martínez, L.J.; Salinas-Delgado, G.A.; Reyes, J.A.; Garzón-Chavez, D.R.; Machado, A. Battle Royale: Immune Response on Biofilms—Host-Pathogen Interactions. Curr. Res. Immunol. 2023, 4, 100057. [Google Scholar] [CrossRef] [PubMed]
- Cangui-Panchi, S.P.; Ñacato-Toapanta, A.L.; Enríquez-Martínez, L.J.; Reyes, J.A.; Garzón-Chavez, D.R.; Machado, A. Biofilm-Forming Microorganisms Causing Hospital-Acquired Infections from Intravenous Catheter: A Systematic Review. Curr. Res. Microb. Sci. 2022, 3, 100175. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque Costa, R.; Araújo, R.L.; Souza, O.V.; Vieira, R.H.S. dos F. Antibiotic-Resistant Vibrios in Farmed Shrimp. BioMed Res. Int. 2015, 2015, 505914. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.A.; Colares, L.P.; Lima, R.A.; Vieira, R.H.S.d.F.; de Sousa, O.V. Effect of Seawater on the Activity of Antibiotics Against Vibrios Isolated from the Hemolymph of Cultured Pacific White Shrimp. J. World Aquac. Soc. 2012, 43, 727–732. [Google Scholar] [CrossRef]
- Silveira, D.R.; da Rosa, J.V.; Kaefer, K.; Bach, L.G.; Barbosa, A.d.O.; Timm, C.D. Ability of Vibrio vulnificus Isolated from Fish of the Lagoa Dos Patos Estuary in South Brazil to Form Biofilms after Sublethal Stress and Bacterial Resistance to Antibiotics and Sanitizers. Int. J. Food Microbiol. 2019, 303, 19–25. [Google Scholar] [CrossRef]
- Amalina, N.Z.; Santha, S.; Zulperi, D.; Amal, M.N.A.; Yusof, M.T.; Zamri-Saad, M.; Ina-Salwany, M.Y. Prevalence, Antimicrobial Susceptibility and Plasmid Profiling of Vibrio spp. Isolated from Cultured Groupers in Peninsular Malaysia. BMC Microbiol. 2019, 19, 251. [Google Scholar] [CrossRef]
- Ong, H.M.G.; Zhong, Y.; Hu, C.C.; Ong, K.H.; Khor, W.C.; Schlundt, J.; Aung, K.T. Quantitative Risk Evaluation of Antimicrobial-Resistant Vibrio parahaemolyticus Isolated from Farmed Grey Mullets in Singapore. Pathogens 2023, 12, 93. [Google Scholar] [CrossRef]
- Zuo, Z.; Shang, B.; Shao, Y.; Li, W.; Sun, J. Screening of Intestinal Probiotics and the Effects of Feeding Probiotics on the Growth, Immune, Digestive Enzyme Activity and Intestinal Flora of Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 86, 160–168. [Google Scholar] [CrossRef]
- Zhou, S.; Tu, X.; Pang, H.; Hoare, R.; Monaghan, S.J.; Luo, J.; Jian, J. A T3SS Regulator Mutant of Vibrio alginolyticus Affects Antibiotic Susceptibilities and Provides Significant Protection to Danio Rerio as a Live Attenuated Vaccine. Front. Cell Infect. Microbiol. 2020, 10, 183. [Google Scholar] [CrossRef]
- Esquer-Miranda, E.; Nieves-Soto, M.; Rivas-Vega, M.E.; Miranda-Baeza, A.; Piña-Valdez, P. Effects of Methanolic Macroalgae Extracts from Caulerpa sertularioides and Ulva lactuca on Litopenaeus vannamei Survival in the Presence of Vibrio Bacteria. Fish Shellfish Immunol. 2016, 51, 346–350. [Google Scholar] [CrossRef]
- Munaeni, W.; Widanarni; Yuhana, M.; Setiawati, M.; Wahyudi, A.T. Effect in White Shrimp Litopenaeus vannamei of Eleutherine Bulbosa (Mill.) Urb. Powder on Immune Genes Expression and Resistance against Vibrio parahaemolyticus Infection. Fish Shellfish Immunol. 2020, 102, 218–227. [Google Scholar] [CrossRef]
- Subramanian, D.; Jang, Y.-H.; Kim, D.-H.; Kang, B.-J.; Heo, M.-S. Dietary Effect of Rubus Coreanus Ethanolic Extract on Immune Gene Expression in White Leg Shrimp, Penaeus vannamei. Fish Shellfish Immunol. 2013, 35, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, A.; Iryani, M.T.M.; Andriani, Y.; Sorgeloos, P.; Tan, M.P.; Wong, L.L.; Mok, W.J.; Ming, W.; Yantao, L.; Lau, C.C.; et al. The Effects of Pandanus tectorius Leaf Extract on the Resistance of White-Leg Shrimp Penaeus vannamei towards Pathogenic Vibrio parahaemolyticus. Fish Shellfish Immunol. Rep. 2023, 4, 100101. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, A.; Okomoda, V.T.; Mimi Iryani, M.T.; Andriani, Y.; Abd Wahid, M.E.; Tan, M.P.; Danish-Daniel, M.; Wong, L.L.; Tengku-Muhammad, T.S.; Mok, W.J.; et al. Pandanus Tectorius Fruit Extract Promotes Hsp70 Accumulation, Immune-Related Genes Expression and Vibrio parahaemolyticus Tolerance in the White-Leg Shrimp Penaeus vannamei. Fish Shellfish Immunol. 2021, 109, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Yeh, R.-Y.; Shiu, Y.-L.; Shei, S.-C.; Cheng, S.-C.; Huang, S.-Y.; Lin, J.-C.; Liu, C.-H. Evaluation of the Antibacterial Activity of Leaf and Twig Extracts of Stout Camphor Tree, Cinnamomum Kanehirae, and the Effects on Immunity and Disease Resistance of White Shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2009, 27, 26–32. [Google Scholar] [CrossRef]
- Fu, Y.-W.; Hou, W.-Y.; Yeh, S.-T.; Li, C.-H.; Chen, J.-C. The Immunostimulatory Effects of Hot-Water Extract of Gelidium amansii via Immersion, Injection and Dietary Administrations on White Shrimp Litopenaeus vannamei and Its Resistance against Vibrio alginolyticus. Fish Shellfish Immunol. 2007, 22, 673–685. [Google Scholar] [CrossRef]
- Wu, C.-C.; Chang, Y.-P.; Wang, J.-J.; Liu, C.-H.; Wong, S.-L.; Jiang, C.-M.; Hsieh, S.-L. Dietary Administration of Gynura bicolor (Roxb. Willd.) DC Water Extract Enhances Immune Response and Survival Rate against Vibrio alginolyticus and White Spot Syndrome Virus in White Shrimp Litopeneaus vannamei. Fish Shellfish Immunol. 2015, 42, 25–33. [Google Scholar] [CrossRef]
- Hsieh, S.-L.; Wu, C.-C.; Liu, C.-H.; Lian, J.-L. Effects of the Water Extract of Gynura bicolor (Roxb. & Willd.) DC on Physiological and Immune Responses to Vibrio alginolyticus Infection in White Shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2013, 35, 18–25. [Google Scholar] [CrossRef]
- Ngo, H.-V.-T.; Huang, H.-T.; Lee, P.-T.; Liao, Z.-H.; Chen, H.-Y.; Nan, F.-H. Effects of Phyllanthus Amarus Extract on Nonspecific Immune Responses, Growth, and Resistance to Vibrio alginolyticus in White Shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2020, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dewi, N.R.; Huang, H.-T.; Wu, Y.-S.; Liao, Z.-H.; Lin, Y.-J.; Lee, P.-T.; Nan, F.-H. Guava (Psidium guajava) Leaf Extract Enhances Immunity, Growth, and Resistance against Vibrio parahaemolyticus in White Shrimp Penaeus vannamei. Fish Shellfish Immunol. 2021, 118, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abidin, Z.; Huang, H.-T.; Liao, Z.-H.; Chen, B.-Y.; Wu, Y.-S.; Lin, Y.-J.; Nan, F.-H. Moringa Oleifera Leaves’ Extract Enhances Nonspecific Immune Responses, Resistance against Vibrio alginolyticus, and Growth in Whiteleg Shrimp (Penaeus vannamei). Animals 2021, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Sallam, G.R.; Abdel-Rahim, M.M.; Lotfy, A.M.; Fayed, W.M.; Shehata, A.I.; El Basuini, M.F.; Elwan, R.I.; Al-absawey, M.A.; Elhetawy, A.I.G. Long Term Dietary Moringa Oleifera Leaf Extract to Florida Red Tilapia oreochromis sp. Improves Performance Immunity Maturation and Reproduction in Saltwater. Sci. Rep. 2025, 15, 20261. [Google Scholar] [CrossRef]
- Lee, C.-L.; Kuo, H.-W.; Chang, C.-C.; Cheng, W. Injection of an Extract of Fresh Cacao Pod Husks into Litopenaeus vannamei Upregulates Immune Responses via Innate Immune Signaling Pathways. Fish Shellfish Immunol. 2020, 104, 545–556. [Google Scholar] [CrossRef]
- Bussabong, P.; Rairat, T.; Chuchird, N.; Keetanon, A.; Phansawat, P.; Cherdkeattipol, K.; Pichitkul, P.; Kraitavin, W. Effects of Isoquinoline Alkaloids from Macleaya Cordata on Growth Performance, Survival, Immune Response, and Resistance to Vibrio parahaemolyticus Infection of Pacific White Shrimp (Litopenaeus vannamei). PLoS ONE 2021, 16, e0251343. [Google Scholar] [CrossRef]
- Dang, L.T.; Nguyen, H.T.; Hoang, H.H.; Lai, H.N.T.; Nguyen, H.T. Efficacy of Rose Myrtle Rhodomyrtus tomentosa Seed Extract against Acute Hepatopancreatic Necrosis Disease in Pacific Whiteleg Shrimp Penaeus vannamei. J. Aquat. Anim. Health 2019, 31, 311–319. [Google Scholar] [CrossRef]
- Mougin, J.; Copin, S.; Bojolly, D.; Raguenet, V.; Robert-Pillot, A.; Quilici, M.-L.; Midelet-Bourdin, G.; Grard, T.; Bonnin-Jusserand, M. Adhesion to Stainless Steel Surfaces and Detection of Viable but Non Cultivable Cells of Vibrio parahaemolyticus and Vibrio Cholerae Isolated from Shrimps in Seafood Processing Environments: Stayin’ Alive? Food Control 2019, 102, 122–130. [Google Scholar] [CrossRef]
- Abidin, Z.; Huang, H.-T.; Hu, Y.-F.; Chang, J.-J.; Huang, C.-Y.; Wu, Y.-S.; Nan, F.-H. Effect of Dietary Supplementation with Moringa Oleifera Leaf Extract and Lactobacillus Acidophilus on Growth Performance, Intestinal Microbiota, Immune Response, and Disease Resistance in Whiteleg Shrimp (Penaeus vannamei). Fish Shellfish Immunol. 2022, 127, 876–890. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Das, B.; Verma, J.; Kumar, P.; Ghosh, A.; Ramamurthy, T. Antibiotic Resistance in Vibrio Cholerae: Understanding the Ecology of Resistance Genes and Mechanisms. Vaccine 2020, 38, A83–A92. [Google Scholar] [CrossRef]
- Huang, X.; Lao, Y.; Pan, Y.; Chen, Y.; Zhao, H.; Gong, L.; Xie, N.; Mo, C.-H. Synergistic Antimicrobial Effectiveness of Plant Essential Oil and Its Application in Seafood Preservation: A Review. Molecules 2021, 26, 307. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Panda, S.K.; Luyten, W. Anti-Vibrio and Immune-Enhancing Activity of Medicinal Plants in Shrimp: A Comprehensive Review. Fish Shellfish Immunol. 2021, 117, 192–210. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Yilmaz, E.; Dawood, M.A.O.; Ringø, E.; Ahmadifar, E.; Yilmaz, S. Shrimp Vibriosis and Possible Control Measures Using Probiotics, Postbiotics, Prebiotics, and Synbiotics: A Review. Aquaculture 2022, 551, 737951. [Google Scholar] [CrossRef]
- Kah Sem, N.A.D.; Abd Gani, S.; Chong, C.M.; Natrah, I.; Shamsi, S. Management and Mitigation of Vibriosis in Aquaculture: Nanoparticles as Promising Alternatives. Int. J. Mol. Sci. 2023, 24, 12542. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-T.; Chang, J.-J.; Lin, Y.-R.; Chen, Y.-Y.; Wu Chang, Y.-H.; Chen, B.-Y.; Nan, F.-H. Synergistic Effects of Dietary Oxolinic Acid Combined with Oxytetracycline on Nonspecific Immune Responses and Resistance against Vibrio parahaemolyticus Infection of White Shrimp (Penaeus vannamei). Fish Shellfish Immunol. 2022, 127, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Li, J. Effectiveness of Traditional Chinese Herbal Medicine, San-Huang-San, in Combination with Enrofloxacin to Treat AHPND-Causing Strain of Vibrio parahaemolyticus Infection in Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 87, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-T.; Hu, Y.-F.; Lee, B.-H.; Huang, C.-Y.; Lin, Y.-R.; Huang, S.-N.; Chen, Y.-Y.; Chang, J.-J.; Nan, F.-H. Dietary of Lactobacillus Paracasei and Bifidobacterium Longum Improve Nonspecific Immune Responses, Growth Performance, and Resistance against Vibrio parahaemolyticus in Penaeus vannamei. Fish Shellfish Immunol. 2022, 128, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xie, S.; Wang, M.; Chen, L.; Yu, H. Distribution and Management of Residual Antibiotics in the Litopenaeus vannamei Shrimp Farming Environment: Recommendations for Effective Control. Fishes 2024, 9, 84. [Google Scholar] [CrossRef]
- Kumar, V.; Roy, S.; Meena, D.K.; Sarkar, U.K. Application of Probiotics in Shrimp Aquaculture: Importance, Mechanisms of Action, and Methods of Administration. Rev. Fish. Sci. Aquac. 2016, 24, 342–368. [Google Scholar] [CrossRef]
- Başaran, S.N.; Öksüz, L. Newly Developed Antibiotics against Multidrug-Resistant and Carbapenem-Resistant Gram-Negative Bacteria: Action and Resistance Mechanisms. Arch. Microbiol. 2025, 207, 110. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Kazi, R.N.A.; Aatif, M.; Azhar, A.; Oirdi, M.E.; Farhan, M. Antimicrobial Resistance: Linking Molecular Mechanisms to Public Health Impact. SLAS Discov. 2025, 33, 100232. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahian, S.; Graham, J.P.; Halaji, M. A Review of the Mechanisms That Confer Antibiotic Resistance in Pathotypes of E. coli. Front. Cell Infect. Microbiol. 2024, 14, 1387497. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Zamora-Mendoza, L.; Alexis, F.; Álvarez-Suarez, J.M. Use of Plant Extracts, Bee-Derived Products, and Probiotic-Related Applications to Fight Multidrug-Resistant Pathogens in the Post-Antibiotic Era. Future Pharmacol. 2023, 3, 535–567. [Google Scholar] [CrossRef]
- Fernandez-Soto, P.; Celi, D.; Tejera, E.; Alvarez-Suarez, J.M.; Machado, A. Cinnamomum sp. and Pelargonium Odoratissimum as the Main Contributors to the Antibacterial Activity of the Medicinal Drink Horchata: A Study Based on the Antibacterial and Chemical Analysis of 21 Plants. Molecules 2023, 28, 693. [Google Scholar] [CrossRef]
- Zin, H.; Ham, I.; Shin, S.; Yu, H.; Choi, T.-J.; Ha, K.; Mok, J.S. Distribution, Antibiotic Resistance, and Virulence Factors of Vibrio parahaemolyticus in the Southern Coastal Waters of Republic of Korea. Antibiotics 2025, 14, 435. [Google Scholar] [CrossRef]
- Sadighara, P.; Rostami, S.; Shafaroodi, H.; Sarshogi, A.; Mazaheri, Y.; Sadighara, M. The Effect of Residual Antibiotics in Food on Intestinal Microbiota: A Systematic Review. Front. Sustain. Food Syst. 2023, 7, 1163885. [Google Scholar] [CrossRef]
- Machado, A.; Toubarro, D.; Baptista, J.; Tejera, E.; Álvarez-Suárez, J.M. Selected Honey as a Multifaceted Antimicrobial Agent: Review of Compounds, Mechanisms, and Research Challenges. Future Microbiol. 2025, 20, 589–610. [Google Scholar] [CrossRef]
- Celi, D.; Jimenes-Vargas, K.; Machado, A.; Álvarez-Suárez, J.M.; Tejera, E. Chemical Composition and Biological Activities of Pelargonium sp.: A Review with In Silico Insights into Potential Anti-Inflammatory Mechanism. Molecules 2025, 30, 3198. [Google Scholar] [CrossRef]
- Cabezas-Mera, F.S.; Atiencia-Carrera, M.B.; Villacrés-Granda, I.; Proaño, A.A.; Debut, A.; Vizuete, K.; Herrero-Bayo, L.; Gonzalez-Paramás, A.M.; Giampieri, F.; Abreu-Naranjo, R.; et al. Evaluation of the Polyphenolic Profile of Native Ecuadorian Stingless Bee Honeys (Tribe: Meliponini) and Their Antibiofilm Activity on Susceptible and Multidrug-Resistant Pathogens: An Exploratory Analysis. Curr. Res. Food Sci. 2023, 7, 100543. [Google Scholar] [CrossRef]
- Cabezas-Mera, F.S.; Cedeño-Pinargote, A.C.; Tejera, E.; Alvarez-Suarez, J.M.; Machado, A. Antimicrobial Activity of Stingless Bee Honey (Tribe: Meliponini) on Clinical and Foodborne Pathogens: A Systematic Review and Meta-Analysis. Food Front. 2024, 5, 964–993. [Google Scholar] [CrossRef]
- Majtan, J.; Machado, A.; Giampieri, F.; Álvarez-Suárez, J.M. Health Benefits and Uses of Honey in Medicine. In Bee Products—Chemical and Biological Properties; Springer Nature: Cham, Switzerland, 2025; pp. 105–140. [Google Scholar]
- Yatip, P.; Nitin Chandra Teja, D.; Flegel, T.W.; Soowannayan, C. Extract from the Fermented Soybean Product Natto Inhibits Vibrio Biofilm Formation and Reduces Shrimp Mortality from Vibrio harveyi Infection. Fish Shellfish Immunol. 2018, 72, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, W.; Zou, Y.; Xia, X. Antimicrobial Activity and Mechanisms of Punicalagin against Vibrio parahaemolyticus. Foods 2024, 13, 1366. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Labbe, R.G.; Shetty, K. Inhibition of Vibrio parahaemolyticus in Seafood Systems Using Oregano and Cranberry Phytochemical Synergies and Lactic Acid. Innov. Food Sci. Emerg. Technol. 2005, 6, 453–458. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Feyaerts, A.F.; Van Dijck, P.; Bossier, P. Inhibitory Activity of Essential Oils against Vibrio Campbellii and Vibrio parahaemolyticus. Microorganisms 2020, 8, 1946. [Google Scholar] [CrossRef]
- Touati, A.; Mairi, A.; Ibrahim, N.A.; Idres, T. Essential Oils for Biofilm Control: Mechanisms, Synergies, and Translational Challenges in the Era of Antimicrobial Resistance. Antibiotics 2025, 14, 503. [Google Scholar] [CrossRef]
- Vazquez-Armenta, F.J.; Aros-Corrales, M.O.; Alvarez-Ainza, M.L.; Bernal-Mercado, A.T.; Ayala-Zavala, J.F.; Ochoa-Leyva, A.; Lopez-Zavala, A.A. Antibacterial and Anti-Virulence Potential of Plant Phenolic Compounds against Vibrio parahaemolyticus. F1000Research 2023, 12, 1256. [Google Scholar] [CrossRef]
- Restrepo, L.; Domínguez-Borbor, C.; Bajaña, L.; Betancourt, I.; Rodríguez, J.; Bayot, B.; Reyes, A. Microbial Community Characterization of Shrimp Survivors to AHPND Challenge Test Treated with an Effective Shrimp Probiotic (Vibrio diabolicus). Microbiome 2021, 9, 88. [Google Scholar] [CrossRef]
- Tamilselvan, M.; Raja, S. Exploring the Role and Mechanism of Potential Probiotics in Mitigating the Shrimp Pathogens. Saudi J. Biol. Sci. 2024, 31, 103938. [Google Scholar] [CrossRef]
- Xie, G.; Chen, X.; Feng, Y.; Yu, Z.; Lu, Q.; Li, M.; Ye, Z.; Lin, H.; Yu, W.; Shu, H. Effects of Dietary Multi-Strain Probiotics on Growth Performance, Antioxidant Status, Immune Response, and Intestinal Microbiota of Hybrid Groupers (Epinephelus fuscoguttatus ♀ × E. Lanceolatus ♂). Microorganisms 2024, 12, 1358. [Google Scholar] [CrossRef] [PubMed]
- Amobonye, A.; Pillay, B.; Hlope, F.; Asong, S.T.; Pillai, S. Postbiotics: An Insightful Review of the Latest Category in Functional Biotics. World J. Microbiol. Biotechnol. 2025, 41, 293. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, R.B.; Perumal, A.B.; Shittu, T.; Sadiku, E.R.; Sellamuthu, P.S. Editorial: Probiotics, Prebiotics, Synbiotics, Postbiotics, & Paraprobiotics—New Perspective for Functional Foods and Nutraceuticals. Front. Nutr. 2023, 10, 1164676. [Google Scholar] [CrossRef]
- Guo, H.; Fu, X.; He, J.; Wang, R.; Yan, M.; Wang, J.; Dong, P.; Huang, L.; Zhang, D. Gut Bacterial Consortium Enriched in a Biofloc System Protects Shrimp against Vibrio parahaemolyticus Infection. Microbiome 2023, 11, 230. [Google Scholar] [CrossRef]
- Fan, S.; Qin, P.; Lu, J.; Wang, S.; Zhang, J.; Wang, Y.; Cheng, A.; Cao, Y.; Ding, W.; Zhang, W. Bioprospecting of Culturable Marine Biofilm Bacteria for Novel Antimicrobial Peptides. iMeta 2024, 3, e244. [Google Scholar] [CrossRef]
- Chraniuk, P.; Bzducha-Wróbel, A. Functional Properties of Yeast Mannoproteins—Current Knowledge and Future Perspectives. Fermentation 2025, 11, 374. [Google Scholar] [CrossRef]
- Barroso, R.A.; Agüero-Chapin, G.; Sousa, R.; Marrero-Ponce, Y.; Antunes, A. Unlocking Antimicrobial Peptides: In Silico Proteolysis and Artificial Intelligence-Driven Discovery from Cnidarian Omics. Molecules 2025, 30, 550. [Google Scholar] [CrossRef]
- Guan, F.; Yu, C.; Yang, L.; Yuan, Y. Broad-Spectrum Antimicrobial Peptides Suppress Vibrio parahaemolyticus Based on Lactobacillus paracasei A1 Fermentation. Foodborne Pathog. Dis. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Taheri-Araghi, S. Synergistic Action of Antimicrobial Peptides and Antibiotics: Current Understanding and Future Directions. Front. Microbiol. 2024, 15, 1390765. [Google Scholar] [CrossRef]
- Rattanadilog Na Phuket, T.; Charoensapsri, W.; Amparyup, P.; Imjongjirak, C. Antibacterial Activity and Immunomodulatory Role of a Proline-Rich Antimicrobial Peptide SpPR-AMP1 against Vibrio campbellii Infection in Shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2023, 132, 108479. [Google Scholar] [CrossRef]
- Zeng, L.; Sun, Y.; Zhang, H.; Yi, X.; Du, R.; Chen, Z.; Wang, Q. Scorpion Venom Peptides Enhance Immunity and Survival in Litopenaeus vannamei through Antibacterial Action against Vibrio parahaemolyticus. Front. Immunol. 2025, 16, 1551816. [Google Scholar] [CrossRef]
- Lv, X.; Li, S.; Yu, Y.; Zhang, X.; Li, F. Crustin Defense against Vibrio parahaemolyticus Infection by Regulating Intestinal Microbial Balance in Litopenaeus vannamei. Mar. Drugs 2023, 21, 130. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, E.R.; Quiroga, E. Farmed Shrimp Aquaculture in Coastal Wetlands of Latin America—A Review of Environmental Issues. Mar. Pollut. Bull. 2022, 183, 113956. [Google Scholar] [CrossRef]
- López-Landavery, E.A.; Urquizo-Rosado, Á.; Saavedra-Flores, A.; Tapia-Morales, S.; Fernandino, J.I.; Zelada-Mázmela, E. Cellular and Transcriptomic Response to Pathogenic and Non-Pathogenic Vibrio parahaemolyticus Strains Causing Acute Hepatopancreatic Necrosis Disease (AHPND) in Litopenaeus vannamei. Fish Shellfish Immunol. 2024, 148, 109472. [Google Scholar] [CrossRef] [PubMed]
- Helena Rebouças, R.; Viana de Sousa, O.; Sousa Lima, A.; Roger Vasconcelos, F.; de Carvalho, P.B.; dos Fernandes Vieira, R.H.S. Antimicrobial Resistance Profile of Vibrio Species Isolated from Marine Shrimp Farming Environments (Litopenaeus vannamei) at Ceará, Brazil. Environ. Res. 2011, 111, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.; Sathiyaraj, G.; Mandal, A.; Kandan, S.; Biju, N.; Palanisamy, S.; You, S.; Nisha, R.G.; Prabhu, N.M. Surveillance of Disease Incidence in Shrimp Farms Located in the East Coastal Region of India and in Vitro Antibacterial Efficacy of Probiotics against Vibrio parahaemolyticus. J. Invertebr. Pathol. 2021, 179, 107536. [Google Scholar] [CrossRef]
- Liao, G.; Wu, Q.; Mo, B.; Zhou, J.; Li, J.; Zou, J.; Fan, L. Intestinal Morphology and Microflora to Vibrio alginolyticus in Pacific White Shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2022, 121, 437–445. [Google Scholar] [CrossRef]
- Haque, Z.F.; Islam, M.S.; Sabuj, A.A.M.; Pondit, A.; Sarkar, A.K.; Hossain, M.G.; Saha, S. Molecular Detection and Antibiotic Resistance of Vibrio Cholerae, Vibrio parahaemolyticus, and Vibrio alginolyticus from Shrimp (Penaeus monodon) and Shrimp Environments in Bangladesh. Aquac. Res. 2023, 2023, 5436552. [Google Scholar] [CrossRef]
- Li, Y.; Xie, T.; Pang, R.; Wu, Q.; Zhang, J.; Lei, T.; Xue, L.; Wu, H.; Wang, J.; Ding, Y.; et al. Food-Borne Vibrio parahaemolyticus in China: Prevalence, Antibiotic Susceptibility, and Genetic Characterization. Front. Microbiol. 2020, 11, 1670. [Google Scholar] [CrossRef]
- Hirshfeld, B.; Lavelle, K.; Lee, K.Y.; Atwill, E.R.; Kiang, D.; Bolkenov, B.; Gaa, M.; Li, Z.; Yu, A.; Li, X.; et al. Prevalence and Antimicrobial Resistance Profiles of Vibrio spp. and Enterococcus spp. in Retail Shrimp in Northern California. Front. Microbiol. 2023, 14, 1192769. [Google Scholar] [CrossRef]
- Loo, K.; Letchumanan, V.; Law, J.W.; Pusparajah, P.; Goh, B.; Ab Mutalib, N.; He, Y.; Lee, L. Incidence of Antibiotic Resistance in Vibrio spp. Rev. Aquac. 2020, 12, 2590–2608. [Google Scholar] [CrossRef]
- Reverter, M.; Tapissier-Bontemps, N.; Sarter, S.; Sasal, P.; Caruso, D. Moving towards More Sustainable Aquaculture Practices: A Meta-analysis on the Potential of Plant-enriched Diets to Improve Fish Growth, Immunity and Disease Resistance. Rev. Aquac. 2021, 13, 537–555. [Google Scholar] [CrossRef]
- Albini, E.; Orso, M.; Cozzolino, F.; Sacchini, L.; Leoni, F.; Magistrali, C.F. A Systematic Review and Meta-Analysis on Antimicrobial Resistance in Marine Bivalves. Front. Microbiol. 2022, 13, 1040568. [Google Scholar] [CrossRef]
- Loo, K.-Y.; Tan, L.T.-H.; Law, J.W.-F.; Pusparajah, P.; Lee, L.-H.; Letchumanan, V. Detection of Multidrug Resistant Vibrio parahaemolyticus and Anti-Vibrio Streptomyces sp. MUM 178J. Prog. Microbes Mol. Biol. 2023, 6, a0000347. [Google Scholar] [CrossRef]
- Onohuean, H.; Agwu, E.; Nwodo, U.U. Systematic Review and Meta-Analysis of Environmental Vibrio Species—Antibiotic Resistance. Heliyon 2022, 8, e08845. [Google Scholar] [CrossRef] [PubMed]
- Murtala, R.; Bashir, A.; Abubakar Aliyu, I.; Abdulhadi Sale, K. Systematic Review on the Antibacterial Resistance of Vibrio Cholerae. UMYU Sci. 2022, 1, 60–66. [Google Scholar] [CrossRef]
- Yousefi, A.; Vaez, H.; Sahebkar, A.; Khademi, F. A Systematic Review and Meta-Analysis on the Epidemiology of Antibiotic Resistance of Vibrio Cholerae in Iran. Ann. Ig. 2019, 31, 279–290. [Google Scholar] [CrossRef]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A Systematic Review of Plants with Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Front. Pharmacol. 2021, 11, 586548. [Google Scholar] [CrossRef]
- Ajitha, S.; Sridhar, M.; Sridhar, N.; Singh, I.S.B.; Varghese, V. Probiotic Effects of Lactic Acid Bacteria Against Vibrio alginolyticus in Penaeus (Fenneropenaeus) Indicus (H.Milne Edwards). Asian Fish. Sci. 2004, 17, 71–80. [Google Scholar] [CrossRef]
- Selcuk, A.A. A Guide for Systematic Reviews: PRISMA. Turk. Arch. Otorhinolaryngol. 2019, 57, 57–58. [Google Scholar] [CrossRef]
- Rutz, A.; Sorokina, M.; Galgonek, J.; Mietchen, D.; Willighagen, E.; Gaudry, A.; Graham, J.G.; Stephan, R.; Page, R.; Vondrášek, J.; et al. The LOTUS Initiative for Open Knowledge Management in Natural Products Research. eLife 2022, 11, e70780. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, M.; Merseburger, P.; Rajan, K.; Yirik, M.A.; Steinbeck, C. COCONUT Online: Collection of Open Natural Products Database. J. Cheminform. 2021, 13, 2. [Google Scholar] [CrossRef]
- Vivek-Ananth, R.P.; Mohanraj, K.; Sahoo, A.K.; Samal, A. IMPPAT 2.0: An Enhanced and Expanded Phytochemical Atlas of Indian Medicinal Plants. ACS Omega 2023, 8, 8827–8845. [Google Scholar] [CrossRef]
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated Chemical Classification with a Comprehensive, Computable Taxonomy. J. Cheminform. 2016, 8, 61. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).