Eco-Friendly Polysaccharides as Moisture Retainers: Influence on Humic Acid Colloidal Stability in Model and Natural Systems
Abstract
1. Introduction
2. Results and Discussions
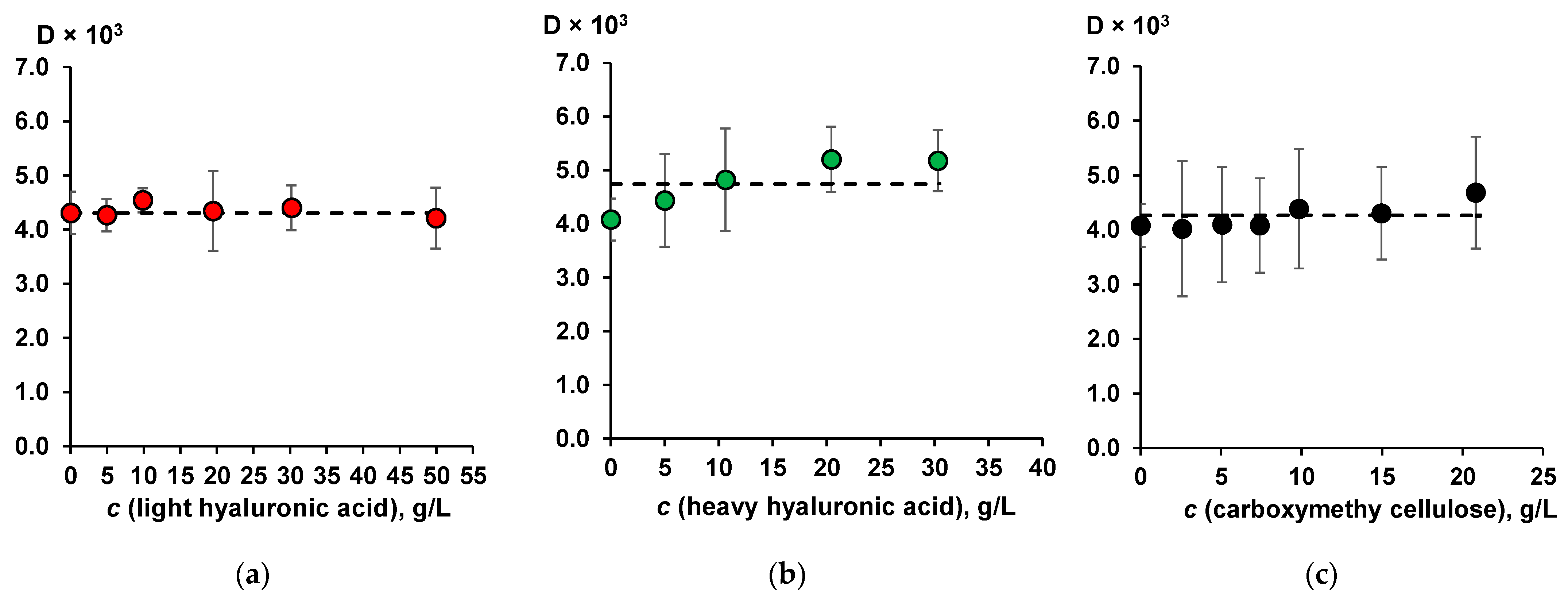
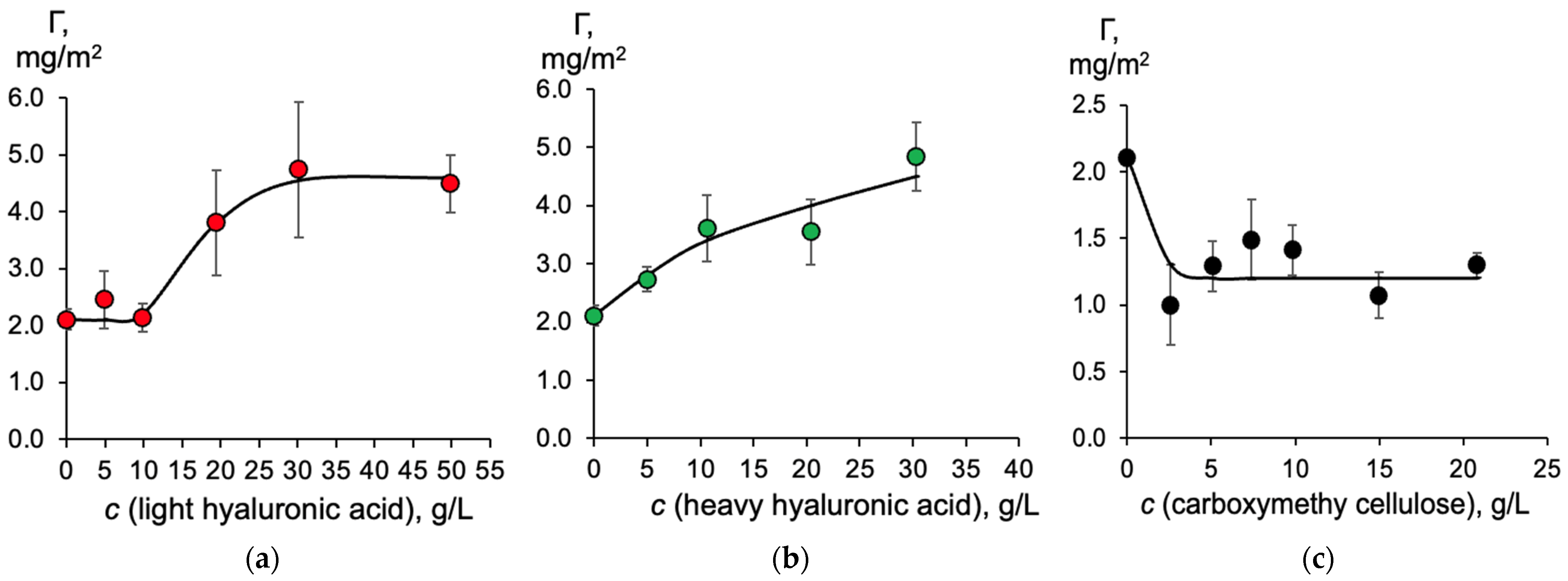
2.1. The Effect of Moisture-Retaining Compounds on the Hydrophobic and Surface-Active Properties of Humic Acids
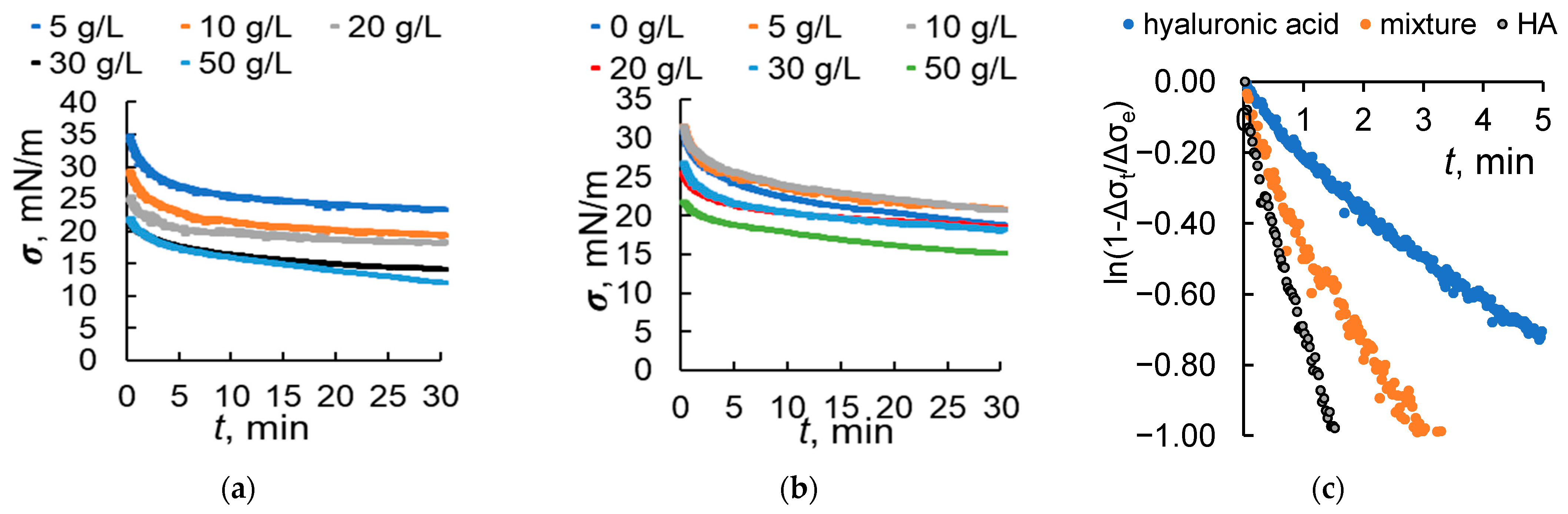
2.2. The Effect of Humic Acids on the Hydrophobic and Surface-Active Properties of Hyaluronic Acid
2.3. Peculiarities of the Interfacial Changes at the Aqueous–Toluene Interface When Hyaluronic Acid and HA Are Present
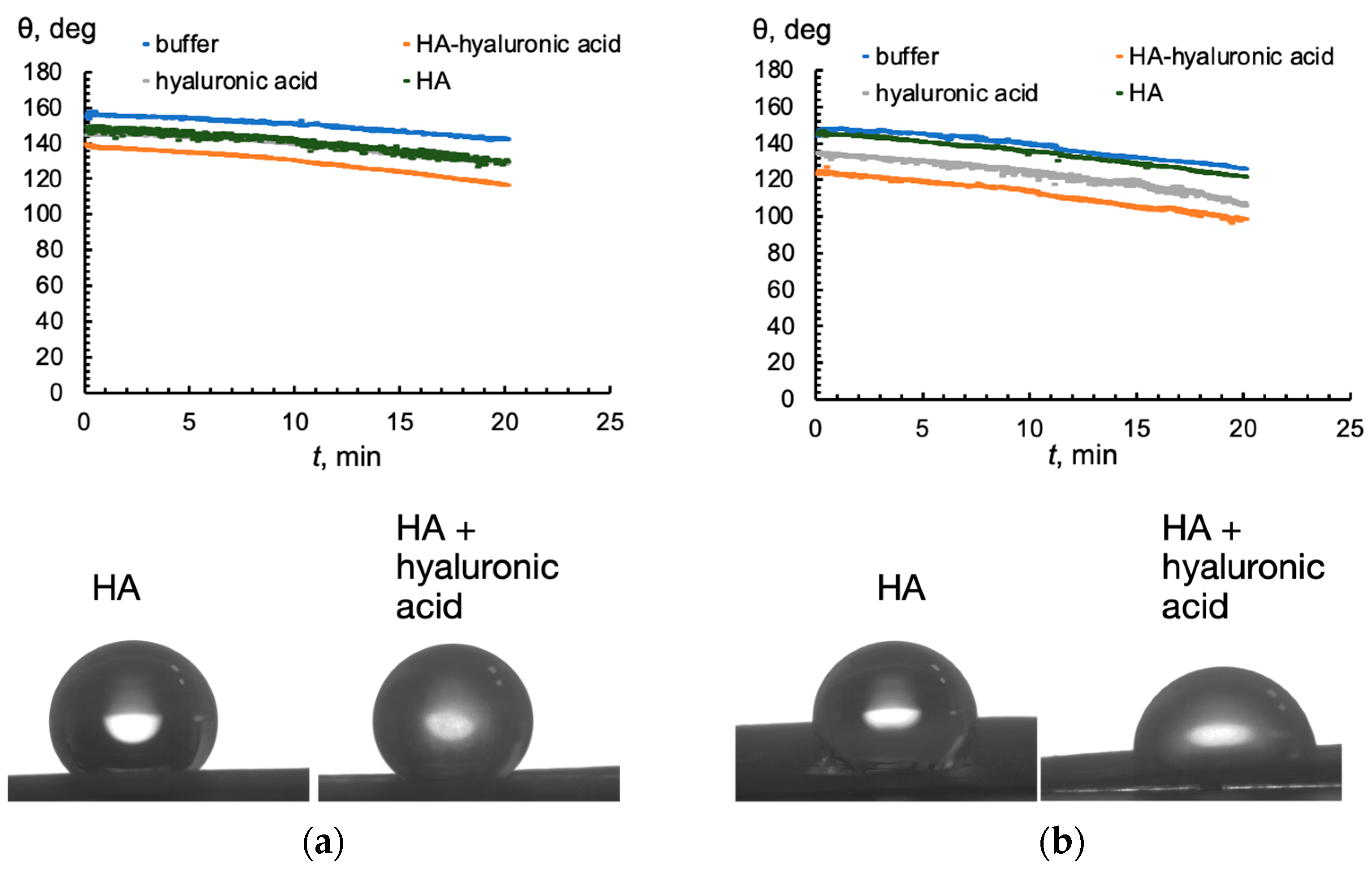
2.4. Wetting the Surface of a Wheat Leaf with Solutions of Hyaluronic Acid and Humic Acid
2.5. The Role of HA in Hyaluronic Acid Uptake by Leaves
3. Materials and Methods
3.1. Materials
3.2. Radiotracer Used in Studying Polymers’ Behavior at the Interfaces: Liquid–Liquid and the Leaf of a Wheat Seedling
3.3. Measuring Interfacial Tension and Contact Angle
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HA | Humic acids |
| CMC | Carboxymethyl cellulose |
| CA | Contact angle |
References
- Saucedo-Pompa, S.; Martínez Ávila, G.C.G.; Cortés, J.S.; Loredo-Treviño, A.; Ventura-Sobrevilla, J.M. Biocontrol Systems and Plant Physiology in Modern Agriculture, 1st ed.; Apple Academic Press: New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of Foliar Fertilization: A review of current status and future perspectives. J. Soil Sci. Plant Nutr. 2021, 21, 104–118. [Google Scholar] [CrossRef]
- Fernández, V.; Eichert, T. Uptake of hydrophilic solutes through plant leaves: Current state of knowledge and perspectives of foliar fertilization. CRC Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef]
- Zimbovskaya, M.M.; Polyakov, A.Y.; Volkov, D.S.; Kulikova, N.A.; Lebedev, V.A.; Pankratov, D.A.; Konstantinov, A.I.; Parfenova, A.M.; Zhilkibaev, O.T.; Perminova, I.V. Foliar Application of humic-stabilized nanoferrihydrite resulted in an increase in the content of iron in wheat leaves. Agronomy 2020, 10, 1891. [Google Scholar] [CrossRef]
- Maffia, A.; Oliva, M.; Marra, F.; Mallamaci, C.; Nardi, S.; Muscolo, A. Humic substances: Bridging ecology and agriculture for a greener future. Agronomy 2025, 15, 410. [Google Scholar] [CrossRef]
- Mazloom, N.; Khorassani, R.; Zohury, G.H.; Emami, H.; Whalen, J. Lignin-based hydrogel alleviates drought stress in maize. Environ. Exp. Bot. 2020, 175, 104055. [Google Scholar] [CrossRef]
- Niu, C.; Lin, Z.; Fu, Q.; Xu, Y.; Chen, Y.; Lu, L. An eco-friendly versatile superabsorbent hydrogel based on sodium alginate and urea for soil improvement with a synchronous chemical loading strategy. Carbohydr. Polym. 2024, 327, 121676. [Google Scholar] [CrossRef]
- Ribeiro, A.B.; Moreira, H.; Pereira, S.I.A.; Godinho, M.; Da, S.; Sousa, A.S.; Castro, P.; Pereira, C.F.; Casanova, F.; Freixo, R.; et al. Bio-based superabsorbent hydrogels for nutrient release. J. Environ. Chem. Eng. 2024, 12, 112031. [Google Scholar] [CrossRef]
- Zhang, S.; He, F.; Fang, X.; Zhao, X.; Liu, Y.; Yu, G.; Zhou, Y.; Feng, Y.; Li, J. Enhancing soil aggregation and acetamiprid adsorption by ecofriendly polysaccharides hydrogel based on Ca2+-amphiphilic sodium alginate. J. Environ. Sci. 2022, 113, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, X.; Jiang, X.; Liu, S.; Lin, J.; Lin, X.; Zhang, Y.; Shi, C.; Zhao, C.; Yang, J. Application of polysaccharide-rich solution derived from waste macroalgae Enteromorpha prolifera in cherry tomato preservation and utilizing post-extraction residue for crude bio-oil production. Food Chem. 2023, 409, 135301. [Google Scholar] [CrossRef]
- Sun, X.; Wang, L.; Fu, R.; Yang, Y.; Cheng, R.; Li, J.; Wang, S.; Zhang, J. The chemical properties and hygroscopic activity of the exopolysaccharide lubcan from Paenibacillus sp. ZX1905. Int. J. Biol. Macromol. 2020, 164, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Guo, Q.; Xin, Y.; Liu, Y. Comprehensive review in moisture retention mechanism of polysaccharides from algae, plants, bacteria and fungus. Arab. J. Chem. 2022, 15, 104163. [Google Scholar] [CrossRef]
- Wu, S.; Lu, M.; Wang, S. Effect of oligosaccharides derived from Laminaria japonica-incorporated pullulan coatings on preservation of cherry tomatoes. Food Chem. 2016, 199, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Torti, M.J.; Sims, C.A.; Adams, C.M.; Sarnoski, P.J. Polysaccharides as alternative moisture retention agents for shrimp. J. Food Sci. 2016, 81, S728–S735. [Google Scholar] [CrossRef] [PubMed]
- Tavallali, V.; Rahmati, S.; Bahmanzadegan, A.; Lasibi, M.J.M. Variation in developmental and phytochemical characteristics of Calendula officinalis L. using foliar application of iron nanoparticles formed on hyaluronic acid. Sci. Hortic. 2024, 323, 112539. [Google Scholar] [CrossRef]
- Antunes, D.R.; Forini, M.M.L.H.; Coqueiro, Y.A.; Pontes, M.S.; Lima, P.H.C.; Cavalcante, L.A.F.; Sanches, A.O.; Caires, A.R.L.; Santiago, E.F.; Grillo, R. Effect of hyaluronic acid-stabilized silver nanoparticles on lettuce (Lactuca sativa L.) seed germination. Chemosphere 2024, 364, 143080. [Google Scholar] [CrossRef]
- Changseong, H. Control of Plant Pathogen Using Hyaluronic Acid. KR20020078155A, 4 April 2001. Available online: https://patents.google.com/patent/KR20020078155A/en (accessed on 21 July 2025).
- Szekeres, G.P.; Pagel, K.; Heiner, Z. Analytical challenges of glycosaminoglycans at biological interfaces. Anal. Bioanal. Chem. 2022, 414, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, Z.; Yin, K.; Li, L.; Zhang, Z.; Xu, X.; Liu, H.; Qing, Y.; Li, X.; Wu, Y. Biodegradable liquid slow-release mulch film based on bamboo residue for selenium-enriched crop cultivation. Research 2025, 8, 1–15. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Li, C.; Jiang, C.; Hu, J.; Lv, Y.; Tao, Y.; Lu, J.; Pan, G.; Du, J.; et al. Biodegradable and multifunctional black mulch film decorated with darkened lignin induced by iron ions for “green” agriculture. Int. J. Biol. Macromol. 2024, 265, 130981. [Google Scholar] [CrossRef]
- Badun, G.A.; Chernysheva, M.G.; Tyasto, Z.A.; Kulikova, N.A.; Kudryavtsev, A.V.; Perminova, I.V. A new technique for tritium labeling of humic substances. Radiochimica Acta 2010, 98, 161–166. [Google Scholar] [CrossRef]
- Sinolits, A.V.; Chernysheva, M.G.; Badun, G.A. Preparation of tritium-labeled hyaluronic acid by tritium thermal activation method. Radiochemistry 2021, 63, 507–511. [Google Scholar] [CrossRef]
- Chernysheva, M.G.; Badun, G.A.; Kulikova, N.A.; Perminova, I.V. Behavior of humic substances in the liquid-liquid system directly measured using tritium label. Chemosphere 2020, 238, 124646. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, J.; Jiang, Y.; Meng, Y.; Zhang, K.; Ban, Q.; Wang, X. Emulsions stabilised by casein and hyaluronic acid: Effects of high intensity ultrasound on the stability and vitamin E digestive characteristics. Ultrason Sonochem. 2023, 94, 106314. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Sun, J.; Zhang, Y. The physicochemical properties and stability of myofibrillar protein oil-in-water emulsions as affected by the structure of sugar. Food Chem. X 2023, 18, 100677. [Google Scholar] [CrossRef]
- Švecová, E.; Ostatná, V.; Fojt, L.; Hermannová, M.; Velebný, V.; Ondreáš, F. Adsorption/desorption behavior of hyaluronic acid fragments at charged hydrophobic surface. Carbohydr. Polym. 2022, 277, 118831. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.F.; Kobayashi, Y.; Ina, T.; Nitta, K.; Uruga, T. Hofmeister anion effects on protein adsorption at an air–water interface. Langmuir 2016, 32, 9892–9898. [Google Scholar] [CrossRef] [PubMed]
- Chernysheva, M.G.; Trishkin, G.N.; Savel’ev, D.E.; Kulikova, N.A.; Badun, G.A. Colloidal properties of humic substances: Influence of pH, ionic strength and carbamide. Polym. Sci. Ser. A 2024, 66, 274–280. (In Russian) [Google Scholar]
- Tian, R.; Liu, W.; Wang, Y.; Wang, W. Cuticular wax in wheat: Biosynthesis, genetics, and the stress response. Front. Plant Sci. 2024, 15, 1498505. [Google Scholar] [CrossRef]
- Chachalis, D.; Reddy, K.N.; Elmore, C.D. Characterization of leaf surface, wax composition, and control of redvine and trumpetcreeper with glyphosate. Weed Sci. 2001, 49, 156–163. [Google Scholar] [CrossRef]
- Taylor, P. The wetting of leaf surfaces. Curr. Opin. Colloid Interface Sci. 2011, 16, 326–334. [Google Scholar] [CrossRef]
- de Moura, O.V.T.; Berbara, R.L.L.; de Oliveira Torchia, D.F.; Silva, H.F.O.D.; de Castro, T.A.T.; Tavares, O.C.H.; Rodrigues, N.F.; Zonta, E.; Santos, L.A.; García, A.C. Humic foliar application as sustainable technology for improving the growth, yield, and abiotic stress protection of agricultural crops. A review. J. Saudi Soc. Agric. Sci. 2023, 22, 493–513. [Google Scholar] [CrossRef]
- Ali, Z.; Merrium, S.; Habib-ur-Rahman, M.; Hakeem, S.; Saddique, M.A.B.; Sher, M.A. Wetting mechanism and morphological adaptation; leaf rolling enhancing atmospheric water acquisition in wheat crop—A review. Environ. Sci. Pollut. Res. 2022, 29, 30967–30985. [Google Scholar] [CrossRef]
- Yang, C.; Zeng, Q.; Huang, J.; Guo, Z. Droplet manipulation on superhydrophobic surfaces based on external stimulation: A review. Adv Colloid Interface Sci. 2022, 306, 102724. [Google Scholar] [CrossRef]
- Bormashenko, E.Y. Wetting of Real Surfaces; De Gruyter: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progess in superhydrophobic surface development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef]
- Jung, Y.C.; Bhushan, B. Wetting behaviour during evaporation and condensation of water microdroplets on superhydrophobic patterned surfaces. J. Microsc. 2008, 229, 127–140. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, F.; Niu, J.; Jiang, Y.; Wang, Z. Superhydrophobic surfaces: From structural control to functional application. J. Mater. Chem. 2008, 18, 621–633. [Google Scholar] [CrossRef]
- Barthlott, W.; Mail, M.; Bhushan, B.; Koch, K. Plant surfaces: Structures and functions for biomimetic innovations. Nanomicro Lett. 2017, 9, 23–63. [Google Scholar] [CrossRef] [PubMed]
- Orbovic, V.; Jifon, J.L.; Syvertsen, J.P. Foliar-applied surfactants and urea temporarily reduce carbon assimilation of grapefruit leaves. J. Am. Soc. Horticultural Sci. 2001, 126, 486–490. [Google Scholar] [CrossRef]

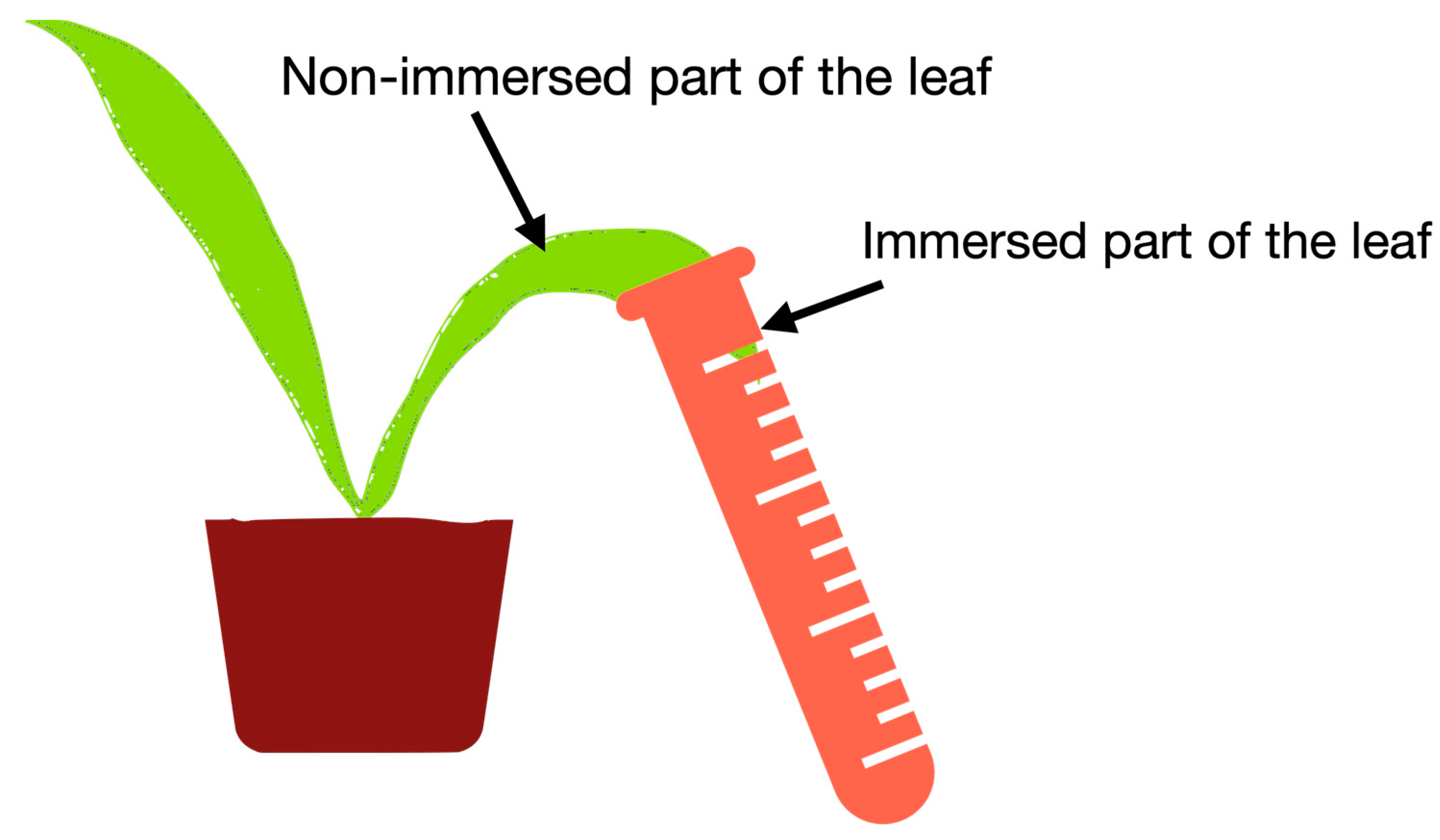
| Solution Composition | Part of Leaf | Mass Content of Hyaluronic Acid, mg per 1 g of Leaf |
|---|---|---|
| [3H]Hyaluronic acid | immersed | 82 ± 40 |
| non-immersed | 2.3 ± 1.5 | |
| [3H]Hyaluronic acid + HA | immersed | 112 ± 30 |
| non-immersed | 1.8 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trishkin, G.N.; Chernysheva, M.G.; Kulikova, N.A.; Badun, G.A. Eco-Friendly Polysaccharides as Moisture Retainers: Influence on Humic Acid Colloidal Stability in Model and Natural Systems. Molecules 2025, 30, 3618. https://doi.org/10.3390/molecules30173618
Trishkin GN, Chernysheva MG, Kulikova NA, Badun GA. Eco-Friendly Polysaccharides as Moisture Retainers: Influence on Humic Acid Colloidal Stability in Model and Natural Systems. Molecules. 2025; 30(17):3618. https://doi.org/10.3390/molecules30173618
Chicago/Turabian StyleTrishkin, Gleb N., Maria G. Chernysheva, Natalia A. Kulikova, and Gennadii A. Badun. 2025. "Eco-Friendly Polysaccharides as Moisture Retainers: Influence on Humic Acid Colloidal Stability in Model and Natural Systems" Molecules 30, no. 17: 3618. https://doi.org/10.3390/molecules30173618
APA StyleTrishkin, G. N., Chernysheva, M. G., Kulikova, N. A., & Badun, G. A. (2025). Eco-Friendly Polysaccharides as Moisture Retainers: Influence on Humic Acid Colloidal Stability in Model and Natural Systems. Molecules, 30(17), 3618. https://doi.org/10.3390/molecules30173618







