Experimental Study on Rapeseed Drying Characteristics with Magnesium Sulfate as Solid Desiccant
Abstract
1. Introduction
2. Results and Discussion
- Magnesium sulfate is a widely used inexpensive fertilizer in agriculture that does not pollute the soil. It provides essential nutrients such as sulfur and magnesium to plants and supports crop seed storage [40].
- In an anhydrous state, magnesium sulfate is widely used as a desiccant [41]. It forms compounds with the formula MgSO4·nH2O, where n can range from 1 to 11 [42]. The predominant and most stable natural magnesium sulfates include epsomite MgSO4∙7H2O, hexahydrate MgSO4∙6H2O, and kieserite MgSO4∙H2O [43]. Previous studies have shown that contact sorption drying of wheat seeds using granular kieserite as a post-harvest treatment is an effective method that reduces moisture content in the seeds while maintaining high germination rates [35,37].
- Magnesium sulfate is considered safe, and is used in the food industry as an additive E518 that has various functions. It can be used as a firming agent in canned vegetables and fruits, as a flavor enhancer in certain dairy products, and as a salt substitute. It also serves as a desiccant to help maintains product dryness. Magnesium sulfate is acceptable in foods conforming to the following commodity standards [44,45].
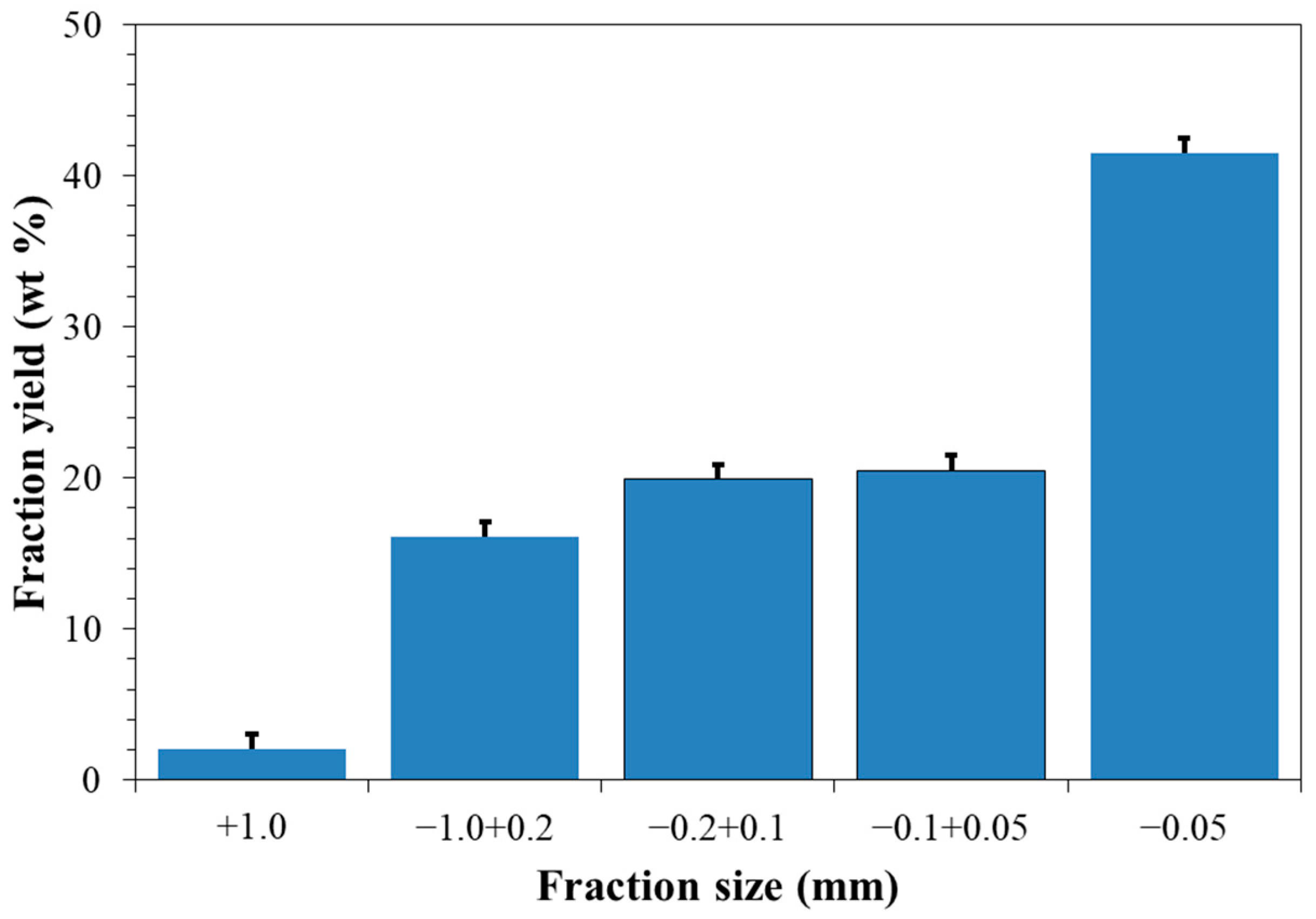
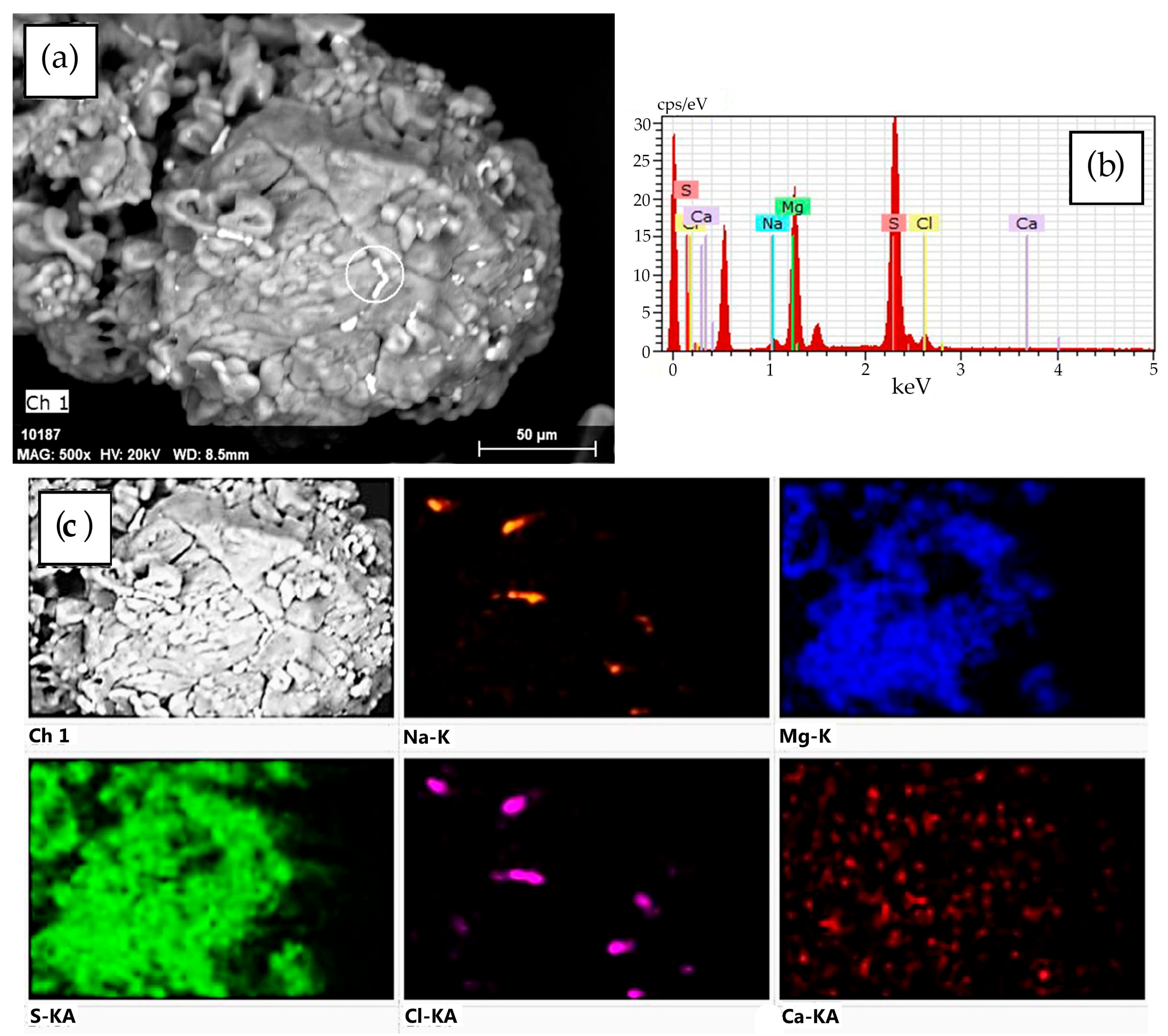
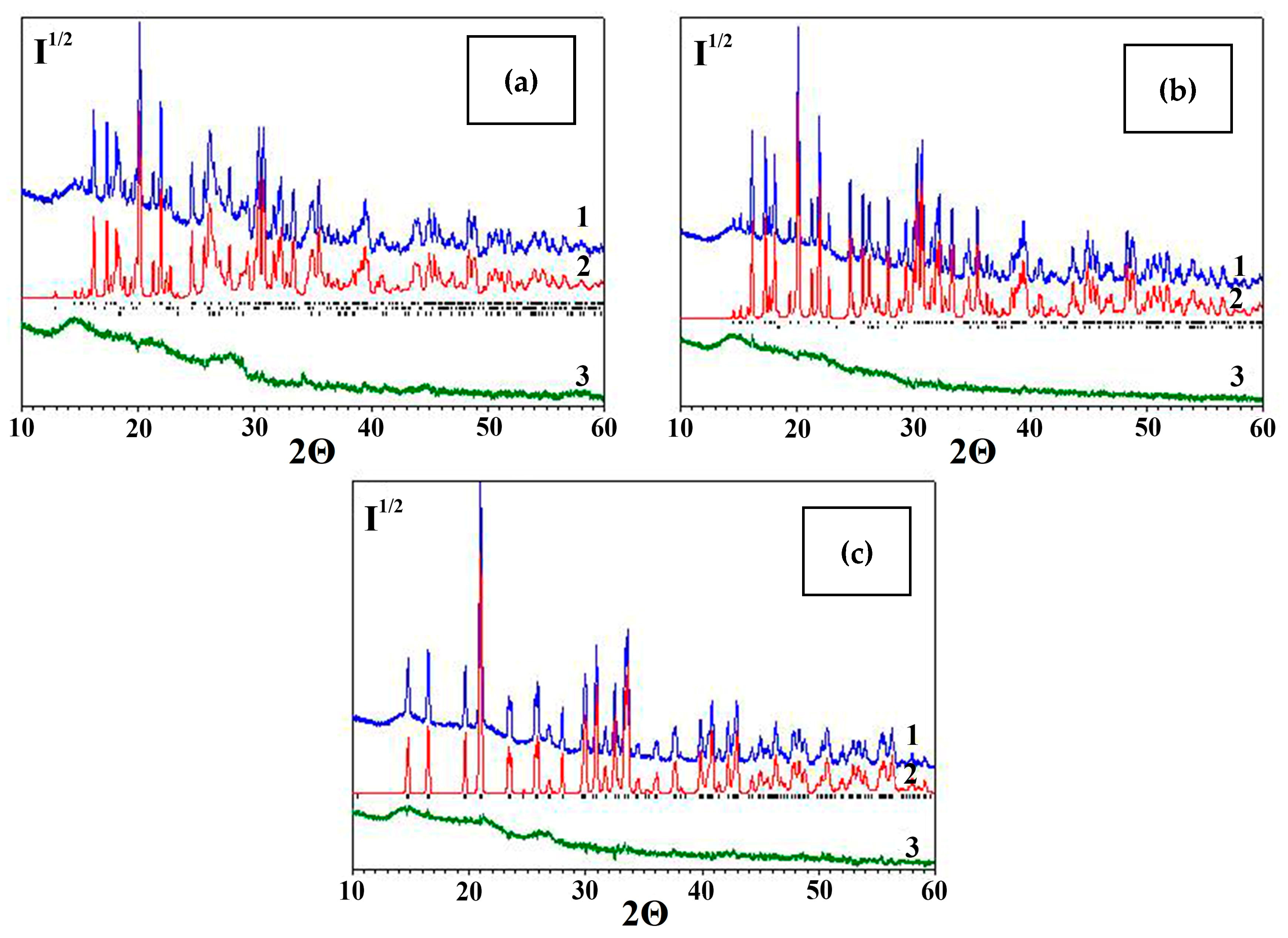
2.1. Characterization of the Initial Desiccant
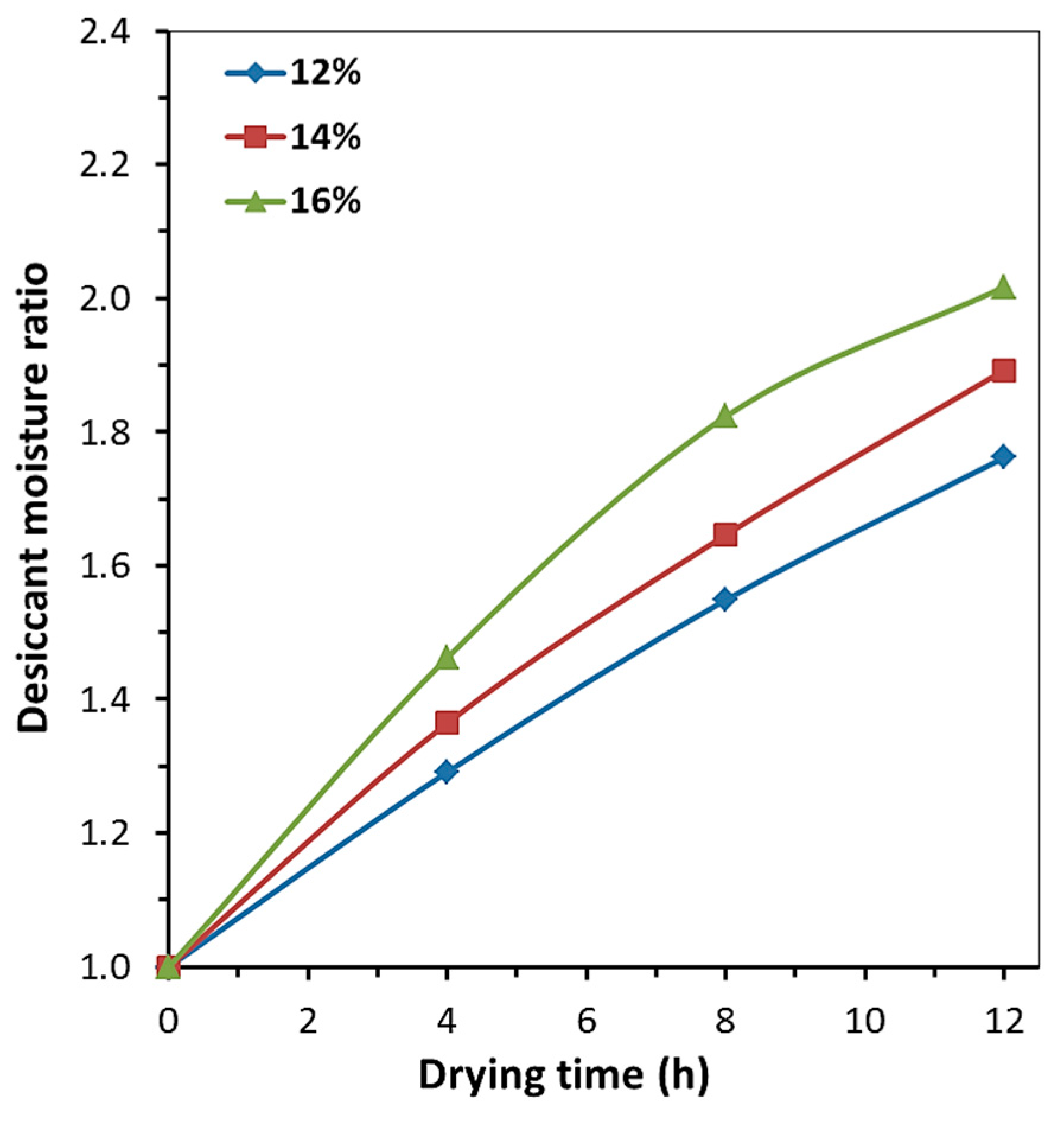
2.2. Drying Kinetics of Rapeseed
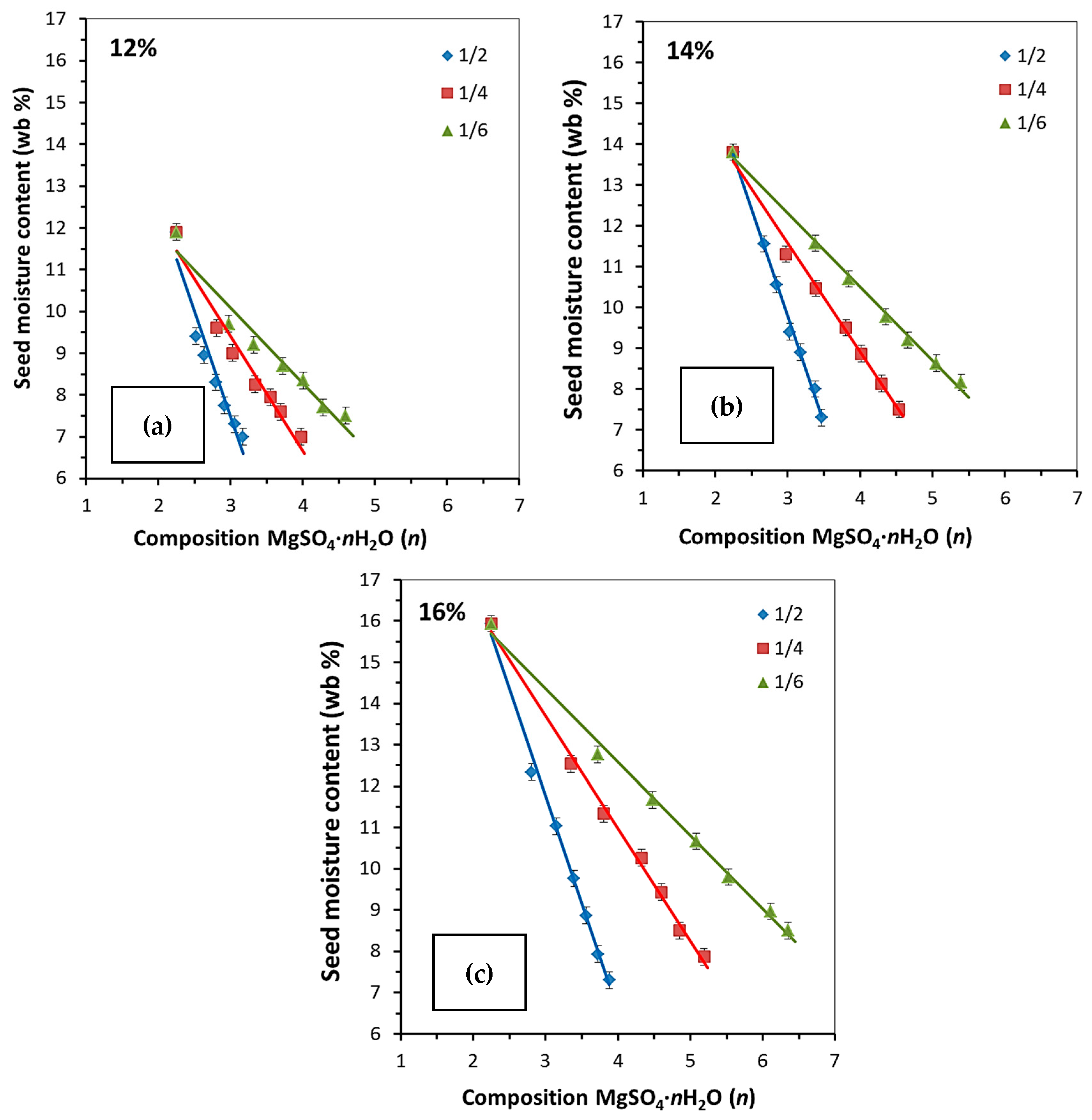
2.2.1. Single-Stage Sorption Drying
2.2.2. Three-Stage Sorption Drying
2.3. Rapeseed Germination
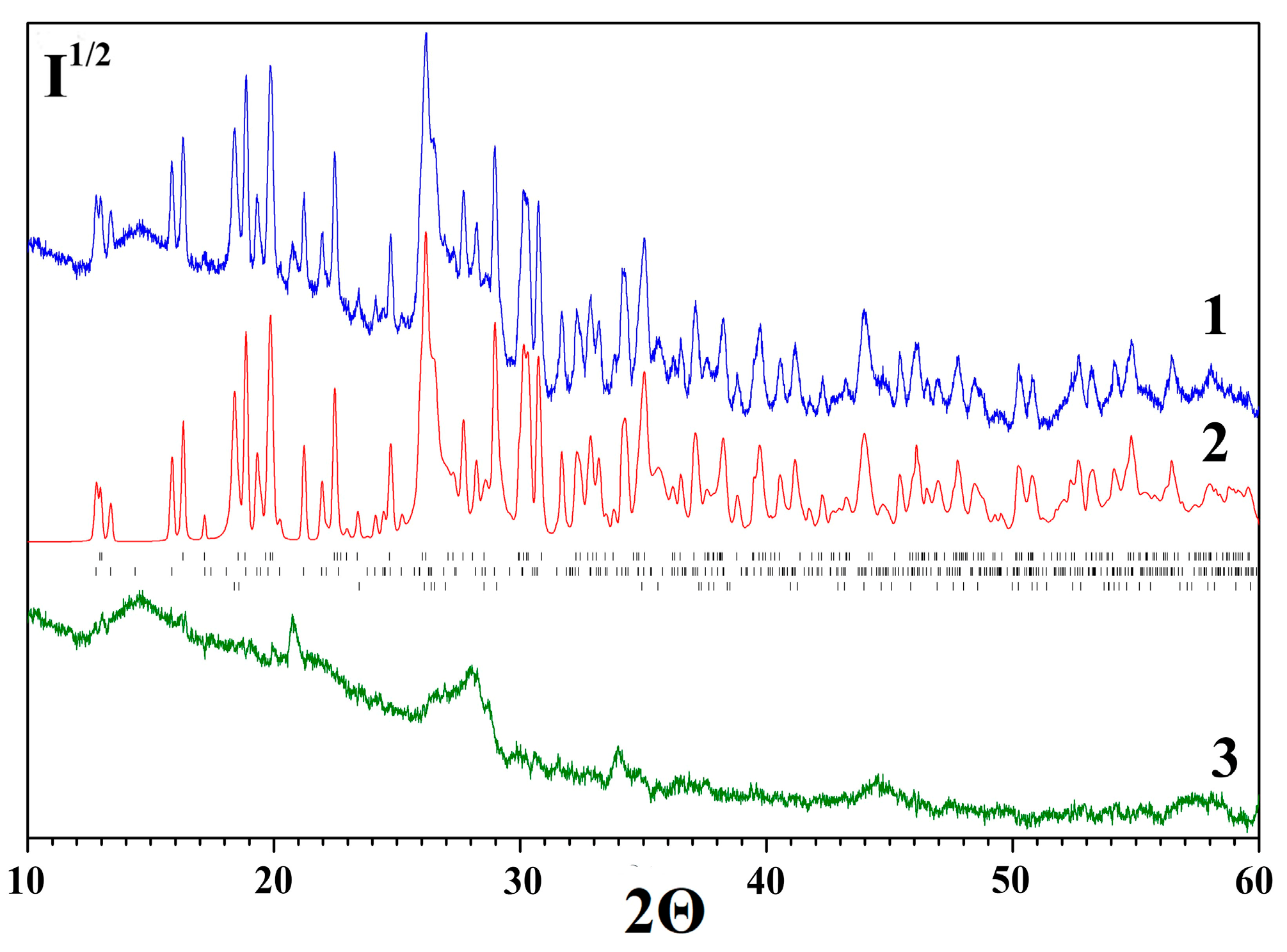
2.4. Characterization of Desiccant After Sorption Drying of Rapeseed
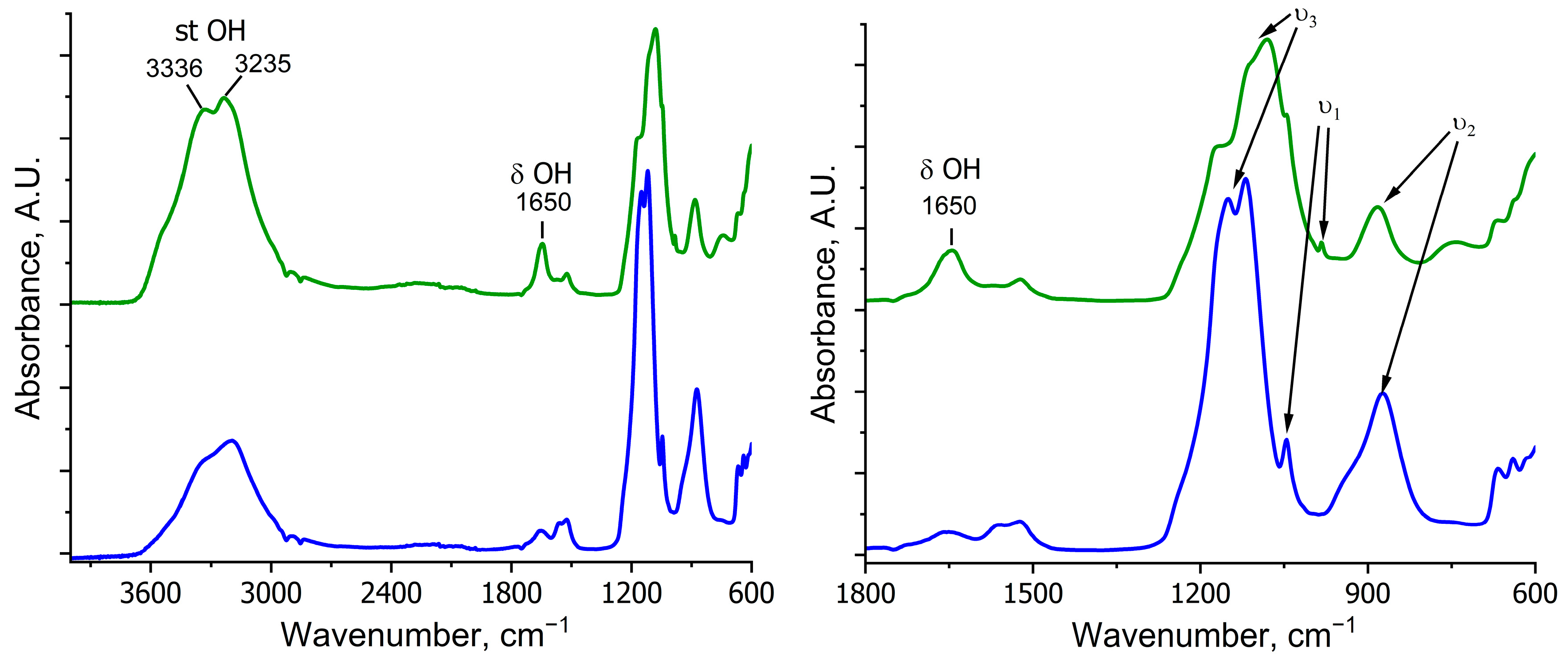
- in the crystal structure of kieserite, water molecules can occupy non-equivalent positions such as coordinated water (Mg–OH2), with the band peaking at 3336 cm−1, and free or weakly bound water peaking at 3235 cm−1 [56];
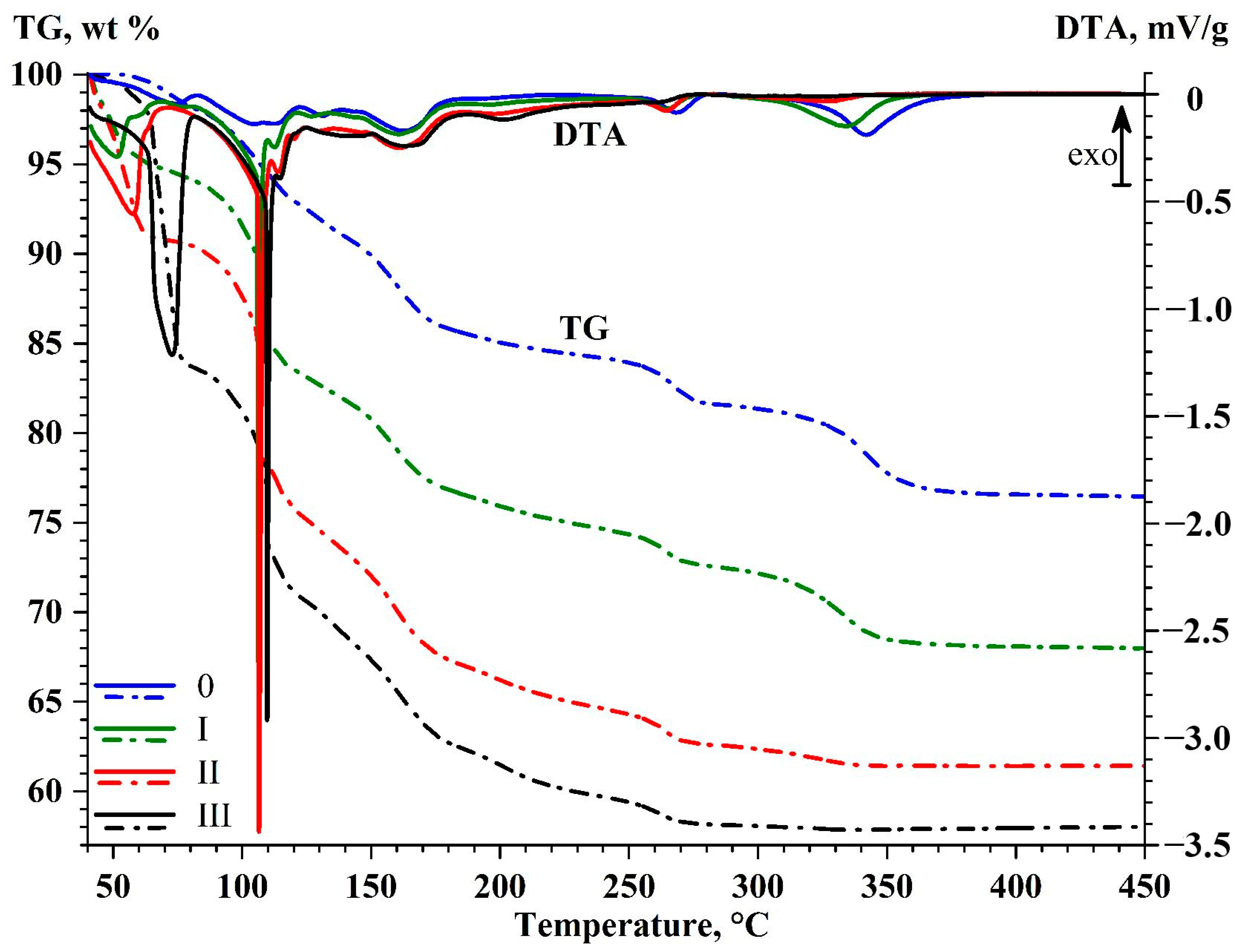
- The low-temperature endothermic peaks with the extrema at 51–75 °C correspond to dehydration of MgSO4·nH2O crystalline hydrates (n ≥ 3) to a state approaching the metastable trihydrate MgSO4·3H2O.
- The low-temperature peaks with extrema at 105–114 °C correspond to dehydration to a metastable state corresponding to sanderite MgSO4·2H2O [59,60]. There are abrupt endothermic peaks at 106, 107, and 110 °C, corresponding to the endothermic process of boiling saturated solution surrounding crystalline hydrate [59]. This crystalline hydrate was formed at the early stages of dehydration at 51–75 °C, when evaporation of free water not included in the crystalloid structure was thermodynamically constrained. Further loss of crystallization water (the weak peaks at 112–114 °C) gave rise to a mixture of metastable crystalline hydrates MgSO4·2H2O+ MgSO4·H2O [60].
- The groups of peaks at 131–140 °C and 161–162 °C are associated with dehydration to a state approaching magnesium sulfate monohydrate MgSO4·H2O (kieserite). Notably, the formation of a stoichiometric monohydrate upon dehydration of crystalline hydrates MgSO4·nH2O (where n > 2) is extremely challenging [61] because of formation of an amorphous state with variable content of crystallization water (1.18–1.30 mole H2O per mole MgSO4), which is significantly affected by partial pressure of water vapor.
- In the high-temperature region (above 185 °C), three groups of endothermic peaks are observed (°C): at 196–202, 263–268, and 325–342, being accompanied by mass loss equivalent to the loss of the final structural water molecule, resulting in formation of anhydrous MgSO4.
3. Materials and Methods
3.1. Experimental Materials
3.2. Experimental Procedures
3.2.1. Desiccant Characterization Methods
3.2.2. Determination of Moisture Content
3.2.3. Sorption Drying Experiments
3.2.4. Drying Kinetics
3.2.5. Rapeseed Germination Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR-MIR | Attenuated total reflectance infrared spectroscopy |
| DSC-TG | Differential scanning calorimeter—Thermogravimetry |
| EDS | Energy-dispersive spectroscopy |
| MC | Moisture content |
| MR | Moisture ratio |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
References
- Raboanatahiry, N.; Li, H.; Yu, L.; Li, M. Rapeseed (Brassica napus): Processing, Utilization, and Genetic Improvement. Agronomy 2021, 11, 1776. [Google Scholar] [CrossRef]
- Ayadi, J.; Debouba, M.; Rahmani, R.; Bouajila, J. Brassica Genus Seeds: A Review on Phytochemical Screening and Pharmacological Properties. Molecules 2022, 27, 6008. [Google Scholar] [CrossRef]
- Vlasenkov, A.N.; Perekopskiy, A.N.; Dementiev, A.M. Optimal timing for harvesting rapeseed for biofuel. IOP Conf. Ser. Earth Environ. Sci. 2022, 1045, 012023. [Google Scholar] [CrossRef]
- Khan, A.A.; Siddiqui, Y.; Siddique, K.H.M.; Bobo, J.A.; Ali, A. Minimizing postharvest food losses: A vital strategy to alleviate food insecurity and malnutrition in developing nations: A review. Discov. Food 2024, 4, 145. [Google Scholar] [CrossRef]
- Hashim, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef]
- Aemu, T.T. Review on the role of improved storage technologies in post-harvest loss reduction of perishable crops and enhancing food and nutrition security. J. Nutr. Food Sci. 2023, 13, 1000895. Available online: https://www.longdom.org/author/tolcha-techane-aemu-56439 (accessed on 27 August 2025).
- Kraiem, A.; Madiouli, J.; Shigidi, I.; Sghaier, J. Experimental analysis of drying conditions’ effect on the drying kinetics and moisture desorption isotherms at several temperatures on food materials: Corn case study. Processes 2023, 11, 184. [Google Scholar] [CrossRef]
- Ariyo, D.O.; Ahmed, T.; Olowolaju, E.D.; Onyegbula, A.F.; Solana, O.I.; Adediran, B.I.; Atanda, S.A. Post-harvest management in Africa: A review of innovative technologies and multidisciplinary approaches for reducing food losses. J. Appl. Sci. Inf. Comput. (JASIC) 2025, 6, 148–157. [Google Scholar] [CrossRef]
- Nath, B.; Chen, G.; O’Sullivan, C.M.; Zare, D. Research and technologies to reduce grain postharvest losses: A review. Foods 2024, 13, 1875. [Google Scholar] [CrossRef]
- Schmidt, M.; Zannini, E.; Arendt, E.K. Recent advances in physical post-harvest treatments for shelf-life extension of cereal crops. Foods 2018, 7, 45. [Google Scholar] [CrossRef]
- Sawicka, B. Post-harvest Losses of Agricultural Produce. In Zero Hunger. Encyclopedia of the UN Sustainable Development Goals; Filho, W.L., Azul, A., Brandli, L., Özuyar, P., Wall, T., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Pilyaeva, O.V. Problems and Prospects of Post-Harvest Grain Processing; Achinsk Branch, Krasnoyarsk State Agrarian University: Achinsk, Russia, 2017; p. 74. (In Russian) [Google Scholar]
- GOST 10583-76; Rape Seed. Industrial Raw Material. State Committee for Standards of the Council of Ministers of the USSR: Moscow, Russia, 1976. (In Russian)
- Fridrihsone, A.; Romagnoli, F.; Cabulis, U. Environmental Life Cycle Assessment of Rapeseed and Rapeseed Oil Produced in Northern Europe: A Latvian Case Study. Sustainability 2020, 12, 5699. [Google Scholar] [CrossRef]
- Kudra, T.; Mujumdar, A.S. Advanced Drying Technologies, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Sharma, H.K.; Kumar, N. Agro-Processing and Food Engineering Operational and Application Aspects; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Barrozo, M.A.S. Air-Drying of Seeds: A Review. Dry. Technol. 2014, 32, 1127–1141. [Google Scholar] [CrossRef]
- Sundareswaran, S.; Choudhury, P.R.; Vanitha, C.; Yadava, D.K. Seed Quality: Variety Development to Planting—An Overview. In Seed Science and Technology; Dadlani, M., Yadava, D.K., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Hashim, N.; Onwude, D.I.; Maringgal, B. Chapter 15—Technological advances in postharvest management of food grains. In Research and Technological Advances in Food Science; Academic Press: Cambridge, MA, USA, 2022; pp. 371–406. [Google Scholar] [CrossRef]
- Defraeye, T. Advanced computational modelling for drying processes—A review. Appl. Energy 2014, 131, 323–344. [Google Scholar] [CrossRef]
- Kudra, T. Energy Aspects in Drying. Dry. Technol. 2004, 22, 917–932. [Google Scholar] [CrossRef]
- Lamidi, R.O.; Jiang, L.; Pathare, P.B.; Wang, Y.D.; Roskilly, A.P. Recent advances in sustainable drying of agricultural produce: A review. Appl. Energy 2019, 233–234, 367–385. [Google Scholar] [CrossRef]
- Jimoh, K.A.; Hashim, N.; Shamsudin, R.; Man, H.C.; Jahari, M.; Onwude, D.I. Recent Advances in the Drying Process of Grains. Food Eng. Rev. 2023, 15, 548–576. [Google Scholar] [CrossRef]
- Batukray, J.D. Progressive Review on Use of Desiccant Drying in Agricultural Applications. J. Agric. Sci. Eng. 2019, 5, 24–31. Available online: http://www.aiscience.org/journal/paperInfo/jase?paperId=4388 (accessed on 27 August 2025).
- Yu, P.; Zhu, W.; Shen, C.; Qiao, Y.; Zhang, W.; Zhu, Y.; Gong, J.; Cai, J. Current Status of Grain Drying Technology and Equipment Development: A Review. Foods 2025, 14, 2426. [Google Scholar] [CrossRef] [PubMed]
- Łupińska, A.; Kozioł, A.; Araszkiewicz, M.; Łupiński, M. The Changes of Quality in Rapeseeds during Microwave Drying. Dry. Technol. 2009, 27, 857–862. [Google Scholar] [CrossRef]
- Bandura, V.; Bezbah, I.; Kupchuk, I.; Fialkovska, L. Innovative methods of drying rapeseeds using microwave energy. Polityka Energetyczna Energy Policy J. 2023, 26, 217–230. [Google Scholar] [CrossRef]
- Ren, X.; Wang, L.; Xu, B.; Wei, B.; Liu, Y.; Zhou, C.; Ma, H.; Wang, Z. Influence of microwave pretreatment on the flavor attributes and oxidative stability of cold-pressed rapeseed oil. Dry. Technol. 2018, 37, 397–408. [Google Scholar] [CrossRef]
- Ganeev, I.; Karimov, K.; Fayzrakhmanov, S.; Masalimov, I.; Permyakov, V. Intensification of the drying process of small seed oilseeds using microwave electromagnetic radiation. Acta Agric. Slov. 2020, 115, 261–271. [Google Scholar] [CrossRef]
- Bulgakov, V.; Bandura, V.; Arak, M.; Olt, J. Intensification of rapeseed drying process through the use of infrared emitters. Agron. Res. 2018, 16, 349–356. [Google Scholar] [CrossRef]
- Xu, B.; Wei, B.; Ren, X.; Liu, Y.; Jiang, H.; Zhou, C.; Ma, H.; Chalamaiah, M.; Liang, Q.; Wang, Z. Dielectric pretreatment of rapeseed 1: Influence on the drying characteristics of the seeds and physico-chemical properties of cold-pressed oil. Food Bioprocess Technol. 2018, 11, 1236–1247. [Google Scholar] [CrossRef]
- Sniezhkin, Y.F.; Paziuk, V.M.; Petrova, Z.O.; Samoilenko, K.M.; Petrov, A.I.; Biriukov, S.O. Energy-efficient low-temperature unit of condensation type for drying seed grain. Energy Technol. Resour. Sav. 2025, 82, 127–137. [Google Scholar] [CrossRef]
- Tiwari, A.K. Advances in Seed Production and Management; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Donkers, P.A.J.; Beckert, S.; Pel, L.; Stallmach, F.; Steiger, M.; Adan, O.C.G. Water transport in MgSO4·7H2O during dehydration in view of thermal storage. J. Phys. Chem. C 2015, 119, 28711–28720. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Shabanov, V.F.; Anshits, A.G. Sorption drying of wheat seeds using kieserite as a solid desiccant. AgriEngineering 2024, 6, 2023–2042. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Solovyov, L.A.; Shabanov, V.F.; Anshits, A.G. The Preparation and Contact Drying Performance of Encapsulated Microspherical Composite Sorbents Based on Fly Ash Cenospheres. Molecules 2024, 29, 2391. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Surin, N.A.; Akimochkina, G.V.; Rabchevskiy, E.V.; Rogovenko, E.S.; Butkovskaya, L.K.; Kozulina, N.S.; Lipshin, A.G.; Spedt, A.A.; et al. Method of Contact Drying of Grain. Patent RU 2788857 C1, 25 January 2023. Available online: https://patents.google.com/patent/RU2788857C1/en (accessed on 27 August 2025).
- Fomenko, E.V.; Anshits, N.N.; Akimochkina, G.V.; Rabchevsky, E.V.; Rogovenko, E.S.; Shabanov, V.F.; Anshits, A.G. Method of Obtaining Microspherical Composite Desiccant for Bulk Materials. Patent RU 2789376 C1, 2 February 2023. Available online: https://patents.google.com/patent/RU2789376C1/en (accessed on 27 August 2025).
- TU 20.13.41-001-23877036-2017; Agrochemical Magnesium Sulfate Grades: Fine-Crystalline Epsomite, Granulated Epsomite, Fine-Crystalline Kieserite, Granulated Kieserite. South Ural Magnesium Compounds Plant: Kuvandyk, Russia, 2017. (In Russian)
- Ministry of Agriculture of Russia. State Catalogue of Pesticides and Agrochemicals Authorized for Use in the Russian Federation as of November 19, 2024; Ministry of Agriculture of Russia: Moscow, Russia, 2024. (In Russian)
- Gordon, A.J.; Ford, R.A. The Chemist’s Companion: A Handbook of Practical Data, Techniques, and References; Wiley: New York, NY, USA, 1972; p. 560. [Google Scholar]
- Minerals Database. Available online: https://www.mindat.org/ (accessed on 27 August 2025).
- Scheidema, M.N.; Taskinen, P. Decomposition thermodynamics of magnesium sulfate. Ind. Eng. Chem. Res. 2011, 50, 9550–9556. [Google Scholar] [CrossRef]
- General Standard for Food Additives (GSFA) Provisions for Magnesium Sulfate. Available online: https://www.fao.org/gsfaonline/additives/details.html?id=400 (accessed on 27 August 2025).
- The Food Chemicals Codex (FCC). Available online: https://www.foodchemicalscodex.org (accessed on 27 August 2025).
- Powder Diffraction File Database. Available online: https://www.icdd.com/pdf-2 (accessed on 13 February 2025).
- GOST 12038-84; Seeds of Agricultural Crops. Germination Methods. Publishing of Standards. Standardinform: Moscow, Russia, 2004. (In Russian)
- ISTA. International Rules for Seed Testing (Bassersdorf: International Seed Testing Association). 2005. Available online: https://www.seedtest.org/en/publications/international-rules-seed-testing.html (accessed on 13 February 2025).
- GOST 52325-2005; Seeds of Agricultural Plants. Varietal and Sowing Characteristics. General Specifications. Standardinform: Moscow, Russia, 2005. (In Russian)
- Boulard, C.; Bardet, M.; Chardot, T.; Dubreucq, B.; Gromova, M.; Guillermo, A.; Miquel, M.; Nesi, N.; Yen-Nicolaÿ, S.; Jolivet, P. The structural organization of seed oil bodies could explain the contrasted oil extractability observed in two rapeseed genotypes. Planta 2015, 242, 53–68. [Google Scholar] [CrossRef]
- Silverstein, R.; Webster, F.; Kiemle, D. Spectrometric Identification of Organic Compounds; John Wiley & Sons, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Lewinska, A.; Zebrowski, J.; Duda, M.; Gorka, A.; Wnuk, M. Fatty Acid Profile and Biological Activities of Linseed and Rapeseed Oils. Molecules 2015, 20, 22872–22880. [Google Scholar] [CrossRef]
- Dhandapani, M.; Thyagu, L.; Prakash, P.A.; Amirthaganesan, G.; Kandhaswamy, M.A.; Srinivasan, V. Synthesis and characterization of potassium magnesium sulphate hexahydrate crystals. Cryst. Res. Technol. 2006, 41, 328–331. [Google Scholar] [CrossRef]
- Zhao, L.-J.; Zhang, Y.-H.; Wei, Z.-F.; Cheng, H.; Li, X.-H. Magnesium Sulfate Aerosols Studied by FTIR Spectroscopy: Hygroscopic Properties, Supersaturated Structures, and Implications for Seawater Aerosols. J. Phys. Chem. A 2006, 110, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Y.; Ding, F.; Zhang, Y.-H.; Zhao, L.-J.; Hu, Y.-A. Anomalous hygroscopic growth of fine particles of MgSO4 aerosols investigated by FTIR/ATR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Cao, Q.; Guan, Y.; Cheng, H.; Wang, X.; Miller, J.D. FTIR analysis of water structure and its influence on the flotation of arcanite (K2SO4) and epsomite (MgSO4·7H2O). Int. J. Miner. Process. 2013, 122, 36–42. [Google Scholar] [CrossRef]
- Bordiga, S.; Regli, L.; Lamberti, C.; Zecchina, A.; Bjørgen, M.; Lillerud, K.P. FTIR Adsorption Studies of H2O and CH3OH in the Isostructural H-SSZ-13 and H-SAPO-34: Formation of H-Bonded Adducts and Protonated Clusters. J. Phys. Chem. B 2005, 109, 7724–7732. [Google Scholar] [CrossRef]
- Chaban, G.M.; Huo, W.M.; Lee, T.J. Theoretical study of infrared and Raman spectra of hydrated magnesium sulfate salts. J. Chem. Phys. 2002, 117, 2532–2537. [Google Scholar] [CrossRef]
- Paulik, J.; Paulik, F.; Arnold, M. Dehydration of magnesium sulphate heptahydrate investigated by quasi isothermal-quasi isobaric TG. Thermochim. Acta 1981, 50, 105–110. [Google Scholar] [CrossRef]
- Grevel, K.-D.; Majzlan, J. Internally consistent thermodynamic data for magnesium sulfate hydrates. Geochim. Cosmochim. Acta 2009, 73, 6805–6815. [Google Scholar] [CrossRef]
- Okhrimenko, L.; Favergeon, L.; Johannes, K.; Kuznik, K. New kinetic model of the dehydration reaction of magnesium sulfate hexahydrate: Application for heat storage. Thermochim. Acta 2020, 687, 178569. [Google Scholar] [CrossRef]
- Solovyov, L.A. Full-profile refinement by derivative difference minimization. J. Appl. Crystallogr. 2004, 37, 743–749. [Google Scholar] [CrossRef]
- DIN 51007:1994-06; Thermal Analysis; Differential Thermal Analysis; Principles. Deutsche Institut für Normung e.V. (DIN): Berlin, Germany, 1994.
- GOST 10856-96; Oilseeds. Method for Determining Moisture Content. Standartinform: Moscow, Russia, 2010. (In Russian)
- ISO 665:2020; Oilseeds—Determination of Moisture and Volatile Matter Content. ISO: Geneva, Switzerland, 2020.
- Corbineau, F. The Effects of Storage Conditions on Seed Deterioration and Ageing: How to Improve Seed Longevity. Seeds 2024, 3, 56–75. [Google Scholar] [CrossRef]
- GOST R ISO 5725-2-2002; Accuracy (Trueness and Precision) of Measurement Methods and Results. Part 2. Basic Method for the Determination of Repeatability and Reproducibility of a Standard Measurement Method. Standartinform: Moscow, Russia, 2002. (In Russian)



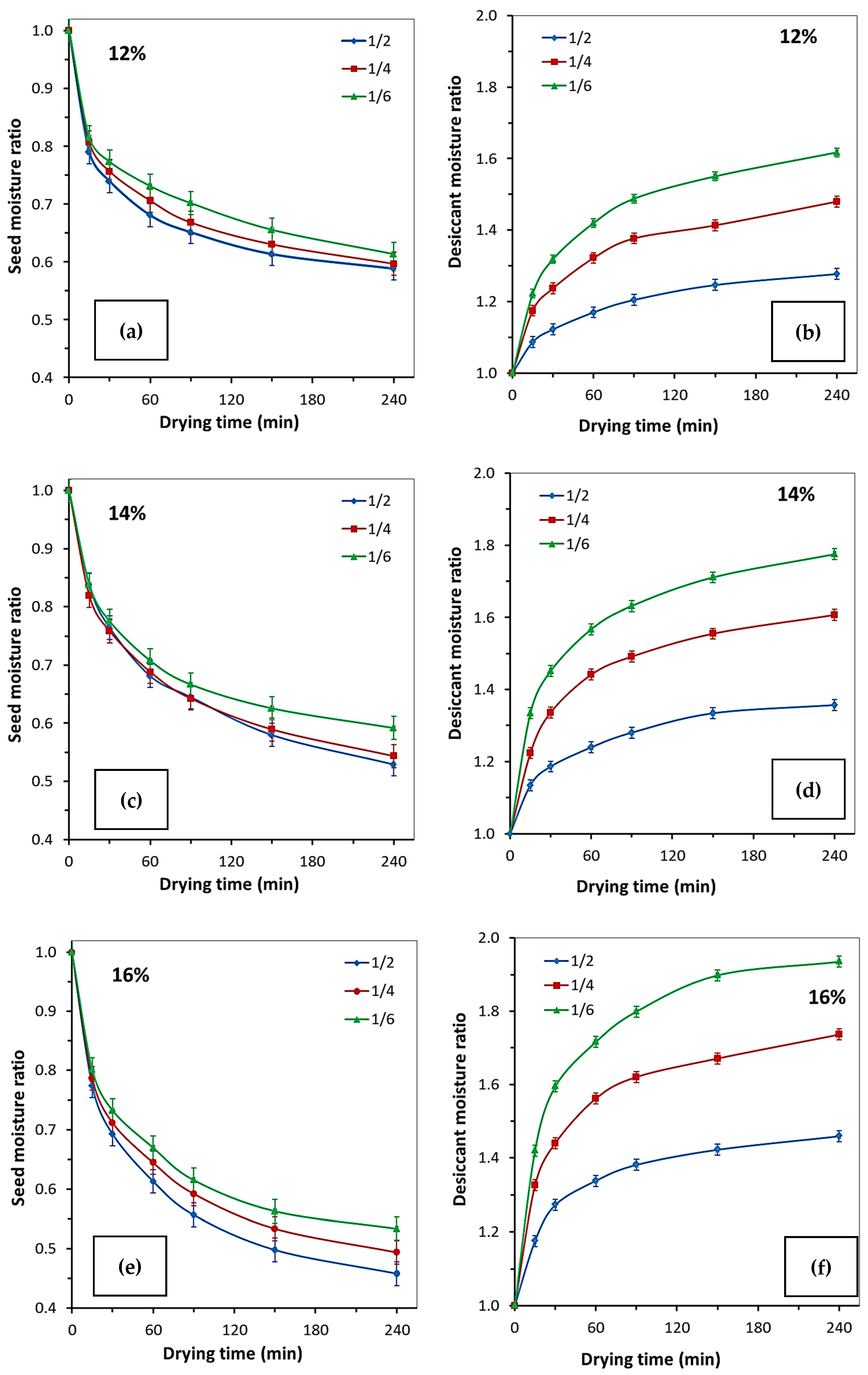

| Initial Rapeseed MC | Desiccant-to-Rapeseed Mass Ratio | Time (Min) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 60 | 90 | 150 | 240 | ||
| 12% | Rapeseed moisture content (% wb) ± 0.1 (p = 0.95) | |||||||
| 1:2 | 11.90 | 9.1 | 8.8 | 8.1 | 7.8 | 7.3 | 7.0 | |
| 1:4 | 11.90 | 9.6 | 9.0 | 8.4 | 8.0 | 7.5 | 7.1 | |
| 1:6 | 11.90 | 9.7 | 9.2 | 8.7 | 8.4 | 7.8 | 7.3 | |
| Desiccant moisture content (wt%) ± 0.02 (p = 0.95) | ||||||||
| 1:2 | 25.19 | 27.38 | 28.28 | 29.48 | 30.35 | 31.40 | 32.18 | |
| 1:4 | 25.19 | 29.60 | 31.17 | 33.31 | 34.69 | 35.61 | 37.28 | |
| 1:6 | 25.19 | 30.79 | 33.22 | 35.77 | 37.49 | 39.06 | 40.75 | |
| 14% | Rapeseed moisture content (% wb) ± 0.1 (p = 0.95) | |||||||
| 1:2 | 13.8 | 11.6 | 10.6 | 9.4 | 8.9 | 8.0 | 7.3 | |
| 1:4 | 13.8 | 11.3 | 10.5 | 9.5 | 8.9 | 8.1 | 7.5 | |
| 1:6 | 13.8 | 11.6 | 10.7 | 9.8 | 9.2 | 8.6 | 8.2 | |
| Desiccant moisture content (wt%) ± 0.02 (p = 0.95) | ||||||||
| 1:2 | 25.16 | 28.56 | 29.87 | 31.21 | 32.24 | 33.59 | 34.17 | |
| 1:4 | 25.16 | 30.81 | 33.65 | 36.29 | 37.55 | 39.15 | 40.46 | |
| 1:6 | 25.16 | 33.60 | 36.54 | 39.46 | 41.07 | 43.06 | 44.69 | |
| 16% | Rapeseed moisture content (% wb) ± 0.1 (p = 0.95) | |||||||
| 1:2 | 15.9 | 12.3 | 11.0 | 9.8 | 8.9 | 7.9 | 7.3 | |
| 1:4 | 15.9 | 12.5 | 11.3 | 10.3 | 9.4 | 8.5 | 7.9 | |
| 1:6 | 15.9 | 12.8 | 11.7 | 10.7 | 9.8 | 9.0 | 8.5 | |
| Desiccant moisture content (wt%) ±0.02 (p = 0.95) | ||||||||
| 1:2 | 25.17 | 29.59 | 32.04 | 33.69 | 34.77 | 35.80 | 36.73 | |
| 1:4 | 25.17 | 33.38 | 36.26 | 39.31 | 40.79 | 42.06 | 43.71 | |
| 1:6 | 25.17 | 35.75 | 40.13 | 43.20 | 45.28 | 47.78 | 48.71 | |
| Initial Rapeseed MC | Desiccant-to-Rapeseed Grain Mass Ratio | Time (Min) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 60 | 90 | 150 | 240 | ||
| 12% | Number of H2O molecules (n) | |||||||
| 1:2 | 2.25 | 2.52 | 2.63 | 2.79 | 2.91 | 3.06 | 3.17 | |
| 1:4 | 2.25 | 2.81 | 3.03 | 3.34 | 3.55 | 3.69 | 3.97 | |
| 1:6 | 2.25 | 2.97 | 3.32 | 3.72 | 4.01 | 4.28 | 4.59 | |
| 14% | 1:2 | 2.25 | 2.67 | 2.85 | 3.03 | 3.18 | 3.38 | 3.47 |
| 1:4 | 2.25 | 2.97 | 3.39 | 3.81 | 4.02 | 4.30 | 4.54 | |
| 1:6 | 2.25 | 3.38 | 3.85 | 4.35 | 4.66 | 5.05 | 5.40 | |
| 16% | 1:2 | 2.25 | 2.81 | 3.15 | 3.39 | 3.56 | 3.73 | 3.88 |
| 1:4 | 2.25 | 3.35 | 3.80 | 4.33 | 4.60 | 4.85 | 5.19 | |
| 1:6 | 2.25 | 3.72 | 4.48 | 5.08 | 5.53 | 6.11 | 6.35 | |
| Stage | Rapeseed MC, wt% ±0.1 (p = 0.95) | Desiccant MC, wt% ±0.02 (p = 0.95) | Crystalline Hydrate Composition |
|---|---|---|---|
| MC0 of rapeseed~12% | |||
| 0 | 12.3 | 25.09 | MgSO4·2.24H2O |
| I | 6.9 | 32.40 | MgSO4·3.20H2O |
| II | 7.4 | 38.86 | MgSO4·4.25H2O |
| III | 7.9 | 44.20 | MgSO4·5.29H2O |
| MC0 of rapeseed~14% | |||
| 0 | 13.8 | 25.09 | MgSO4·2.24H2O |
| I | 7.2 | 34.24 | MgSO4·3.48H2O |
| II | 7.6 | 41.30 | MgSO4·4.70H2O |
| III | 8.0 | 47.48 | MgSO4·6.04H2O |
| MC0 of rapeseed~16% | |||
| 0 | 15.9 | 25.12 | MgSO4·2.24H2O |
| I | 7.2 | 36.74 | MgSO4·3.88H2O |
| II | 8.0 | 45.80 | MgSO4·5.65H2O |
| III | 10.2 | 50.66 | MgSO4·6.86H2O |
| Phase | Desiccant Sample | |||
|---|---|---|---|---|
| 0 | I | II | III | |
| MgSO4·H2O | 28(1) | 21(1) | 4.2(2) | – |
| MgSO4·2.5H2O | 20.0(5) | – * | – | – |
| MgSO4·4H2O | 28.2(7) | 9.4(5) | – | – |
| MgSO4·6H2O | – | 60.0(8) | 94.7(8) | – |
| MgSO4·7H2O | – | – | – | 83(1) |
| NaCl | 0.8(1) | 0.9(1) | 0.7(1) | 0.8(1) |
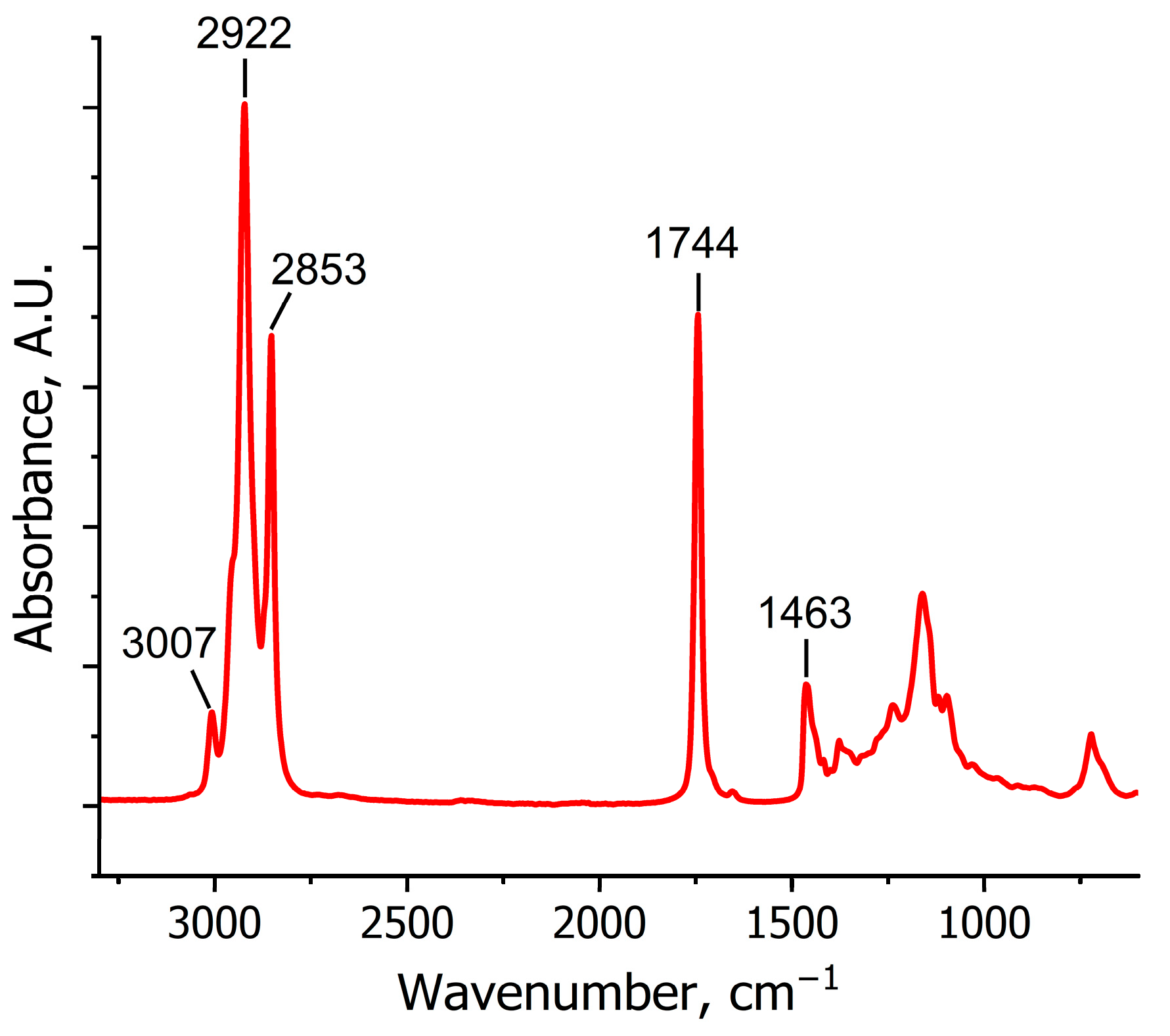
| Wavenumber, cm−1 | Functional Group | Vibration Mode |
|---|---|---|
| 3007 | =CH | stretching |
| 2922 | C-H (CH2) | stretching asymmetrical |
| 2853 | C-H (CH2) | stretching symmetrical |
| 1744 | C=O | stretching |
| 1463 | C-H (CH2) | scissoring |
| 1420 | C-H | bending |
| 1380 | C-H (CH3) | symmetrical bending |
| 1250–1100 | C-O | stretching |
| 873 | CH2 | rocking |
| 690 | HC=CH | bending out of plane |
| Desiccant Sample | Temperature Range, °C | (H2O)/(MgSO4), mol | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 40–100 | 40–150 | 40–200 | 40–250 | 40–300 | 40–350 | 40–400 | 40–450 | |||
| ∆m, wt% | ||||||||||
| 0 | 3.78 | 10.07 | 14.93 | 16.06 | 18.62 | 22.21 | 23.42 | 23.53 | 2.06 | |
| I | 8.25 | 19.11 | 23.95 | 25.53 | 27.71 | 31.39 | 31.78 | 31.89 | 3.13 | |
| II | 12.28 | 27.87 | 33.72 | 35.64 | 37.58 | 38.51 | 38.52 | 38.52 | 4.20 | |
| III | 18.77 | 32.64 | 38.52 | 40.61 | 41.94 | 42.14 | 42.05 | 41.99 | 4.84 | |
| Water capacity, mg/g anhydrous * | ∆m, wt% ** | T, °C *** | ||||||||
| 0 | 49 | 132 | 195 | 210 | 243 | 290 | 306 | 308 | ||
| I | 121 | 281 | 352 | 375 | 407 | 461 | 467 | 468 | 21.70 | 165.5 |
| II | 200 | 453 | 548 | 580 | 611 | 626 | 627 | 627 | 29.32 | 157.6 |
| III | 324 | 563 | 664 | 700 | 723 | 726 | 725 | 724 | 33.31 | 154.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fomenko, E.V.; Anshits, N.N.; Akimochkina, G.V.; Ivanenko, T.Y.; Morozov, E.V.; Yumashev, V.V.; Solovyov, L.A.; Shestakov, N.P.; Shabanov, V.F. Experimental Study on Rapeseed Drying Characteristics with Magnesium Sulfate as Solid Desiccant. Molecules 2025, 30, 3604. https://doi.org/10.3390/molecules30173604
Fomenko EV, Anshits NN, Akimochkina GV, Ivanenko TY, Morozov EV, Yumashev VV, Solovyov LA, Shestakov NP, Shabanov VF. Experimental Study on Rapeseed Drying Characteristics with Magnesium Sulfate as Solid Desiccant. Molecules. 2025; 30(17):3604. https://doi.org/10.3390/molecules30173604
Chicago/Turabian StyleFomenko, Elena V., Natalia N. Anshits, Galina V. Akimochkina, Timur Yu. Ivanenko, Evgeny V. Morozov, Vladimir V. Yumashev, Leonid A. Solovyov, Nikolay P. Shestakov, and Vasily F. Shabanov. 2025. "Experimental Study on Rapeseed Drying Characteristics with Magnesium Sulfate as Solid Desiccant" Molecules 30, no. 17: 3604. https://doi.org/10.3390/molecules30173604
APA StyleFomenko, E. V., Anshits, N. N., Akimochkina, G. V., Ivanenko, T. Y., Morozov, E. V., Yumashev, V. V., Solovyov, L. A., Shestakov, N. P., & Shabanov, V. F. (2025). Experimental Study on Rapeseed Drying Characteristics with Magnesium Sulfate as Solid Desiccant. Molecules, 30(17), 3604. https://doi.org/10.3390/molecules30173604






