Dihexyl (2-(Hydroxyamino)-2-Oxoethyl) Phosphonate as a Novel Collector for Flotation Separation of Scheelite and Quartz
Abstract
1. Introduction
2. Experimental Sections
2.1. Materials
2.2. Methods
2.2.1. Micro-Flotation Tests
2.2.2. Zeta Potential and Contact Angle Tests
2.2.3. FTIR and XPS Tests
2.2.4. DFT Calculation
3. Results and Discussion
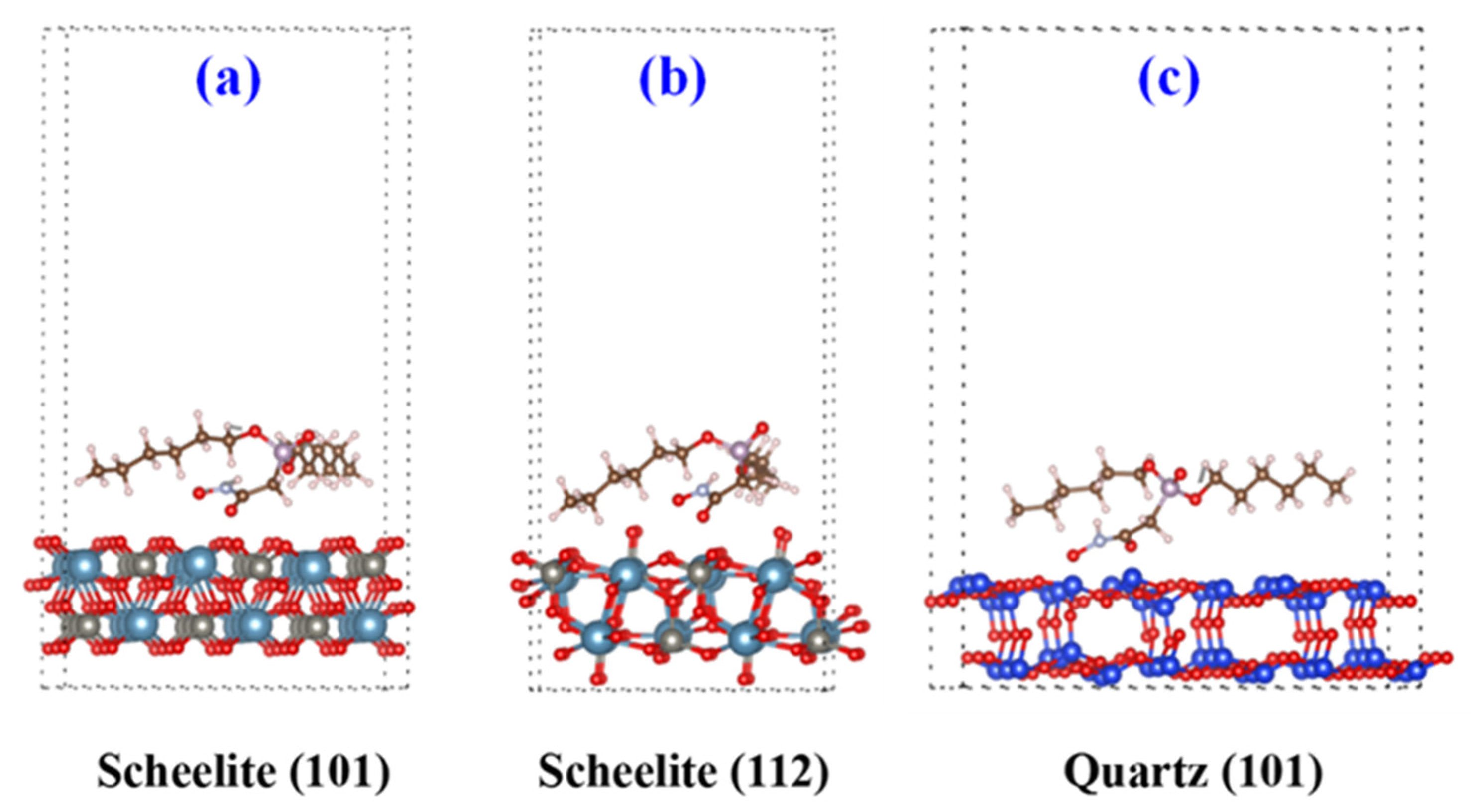
3.1. DFT Calculation Analyses
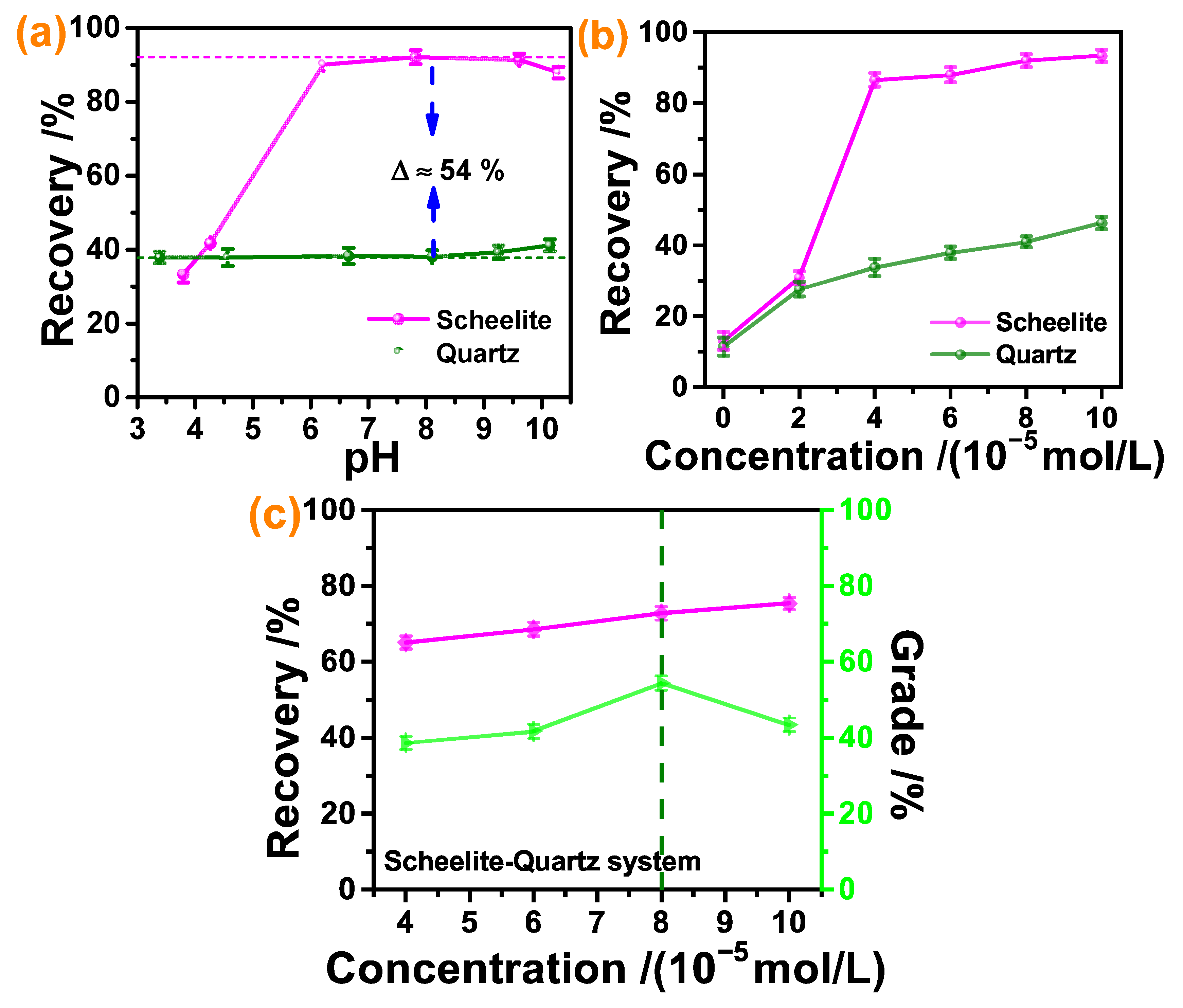
3.2. Micro-Flotation Results
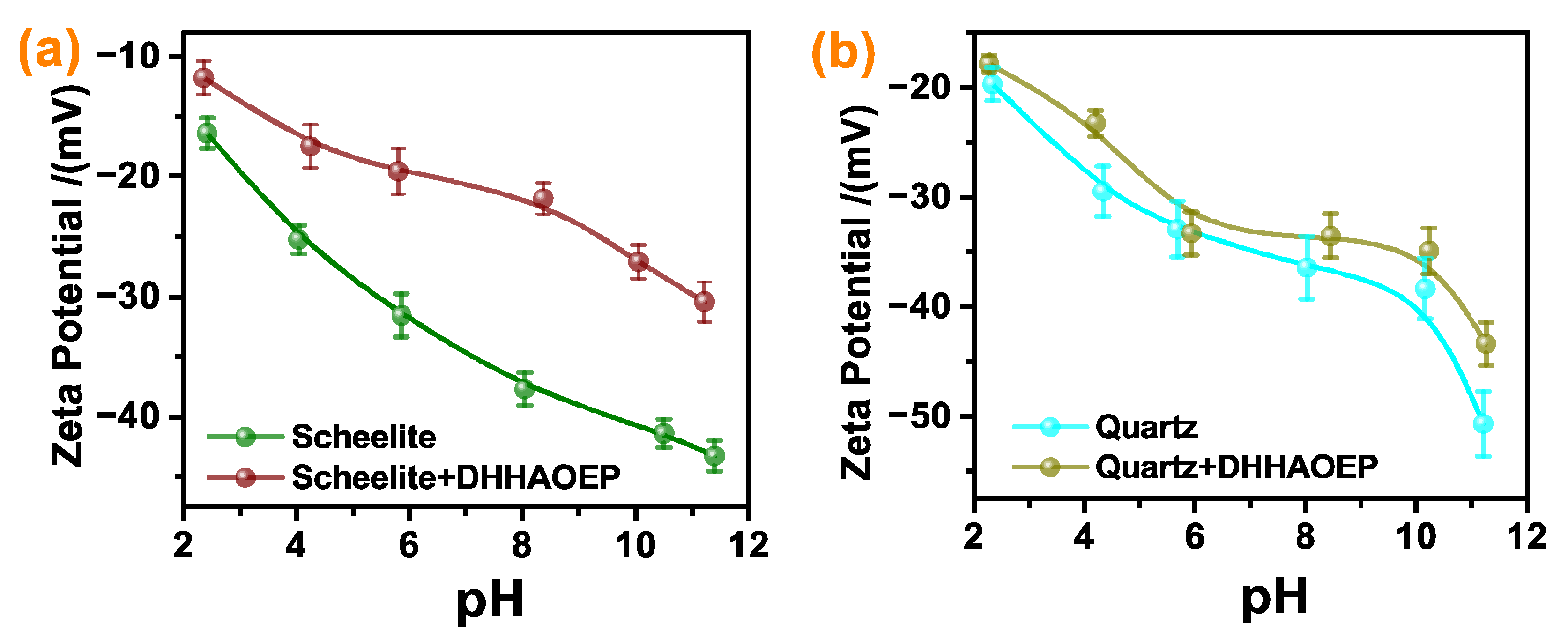
3.3. Zeta Potential Analysis
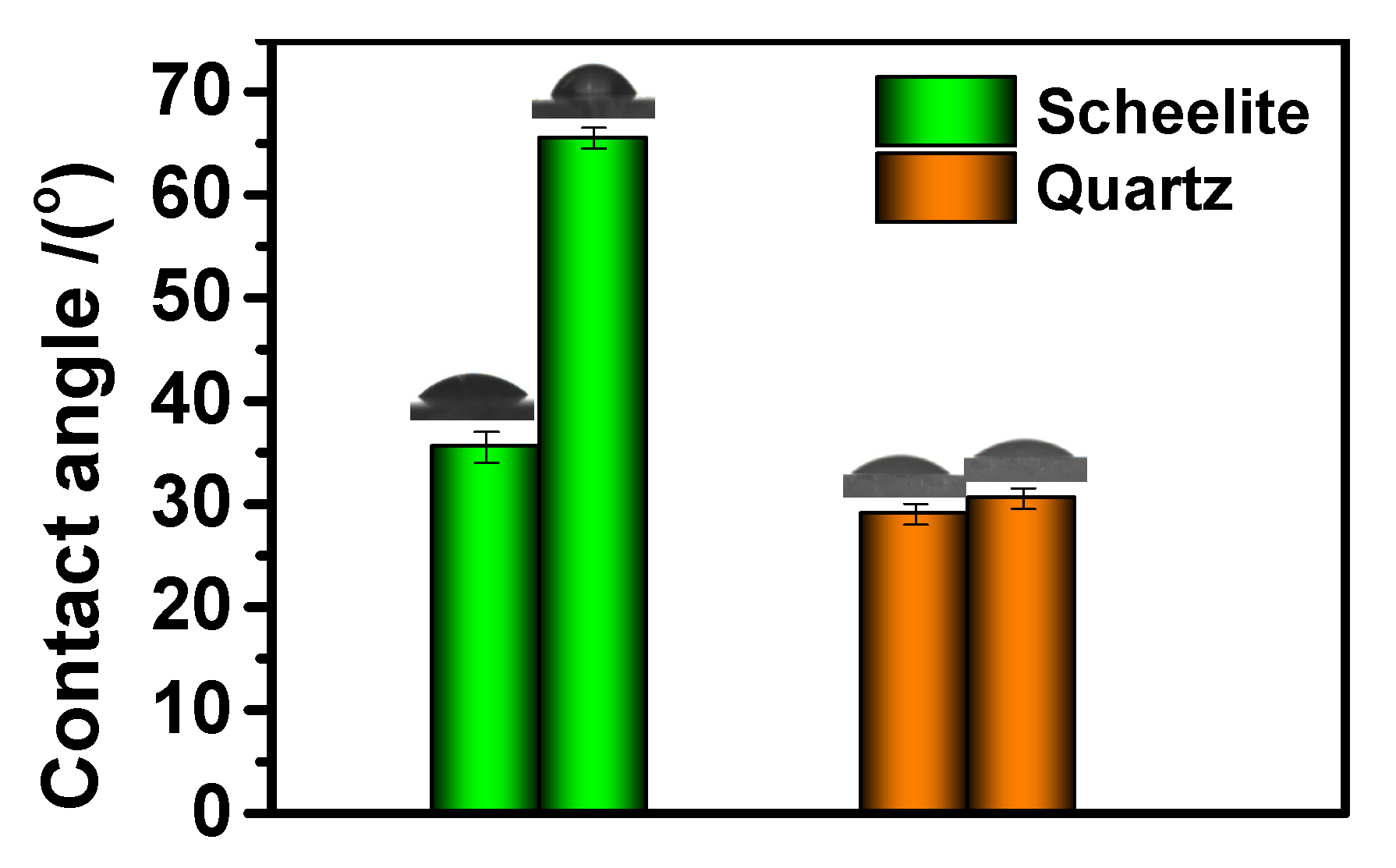
3.4. Contact Angle Analysis
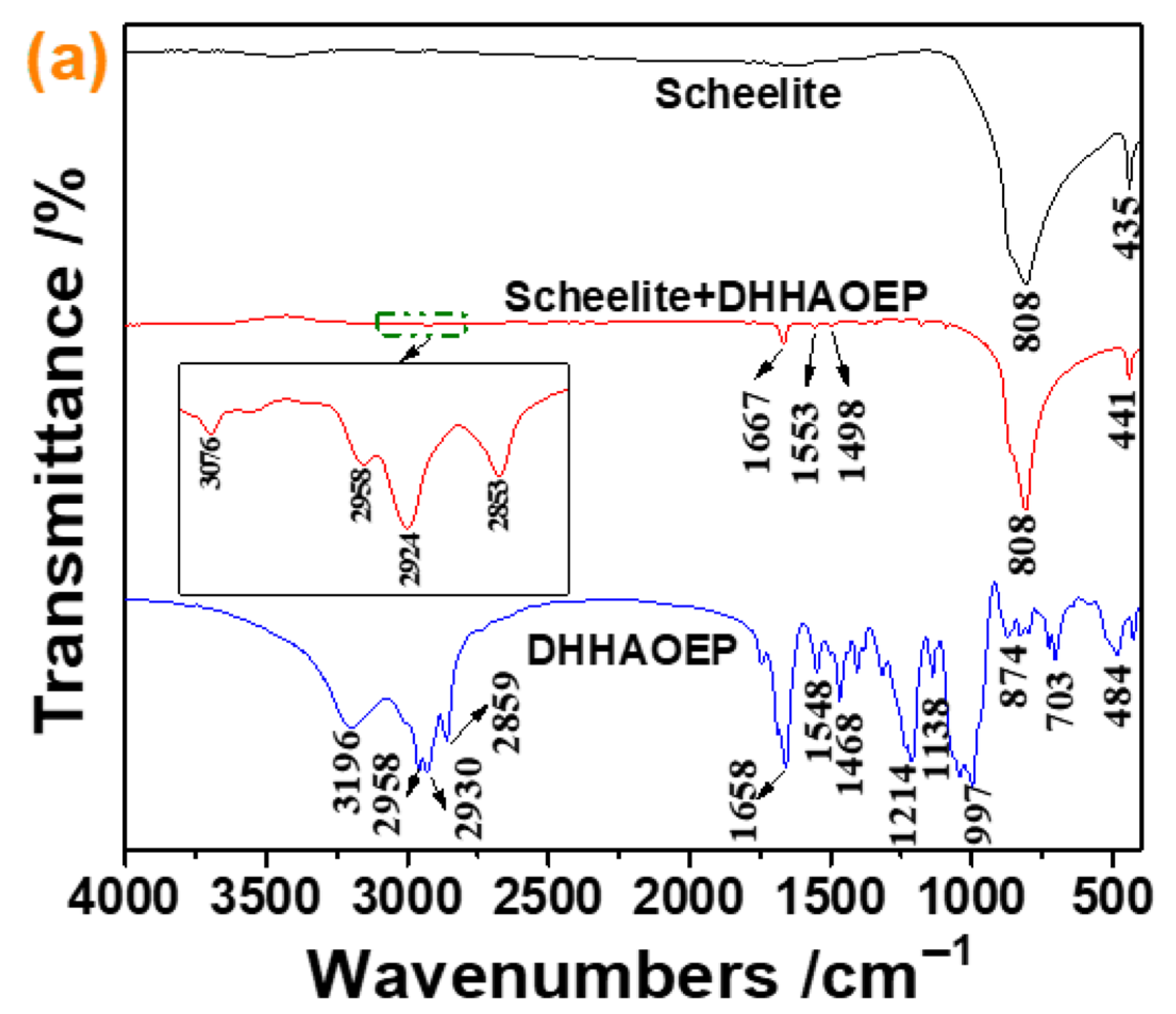
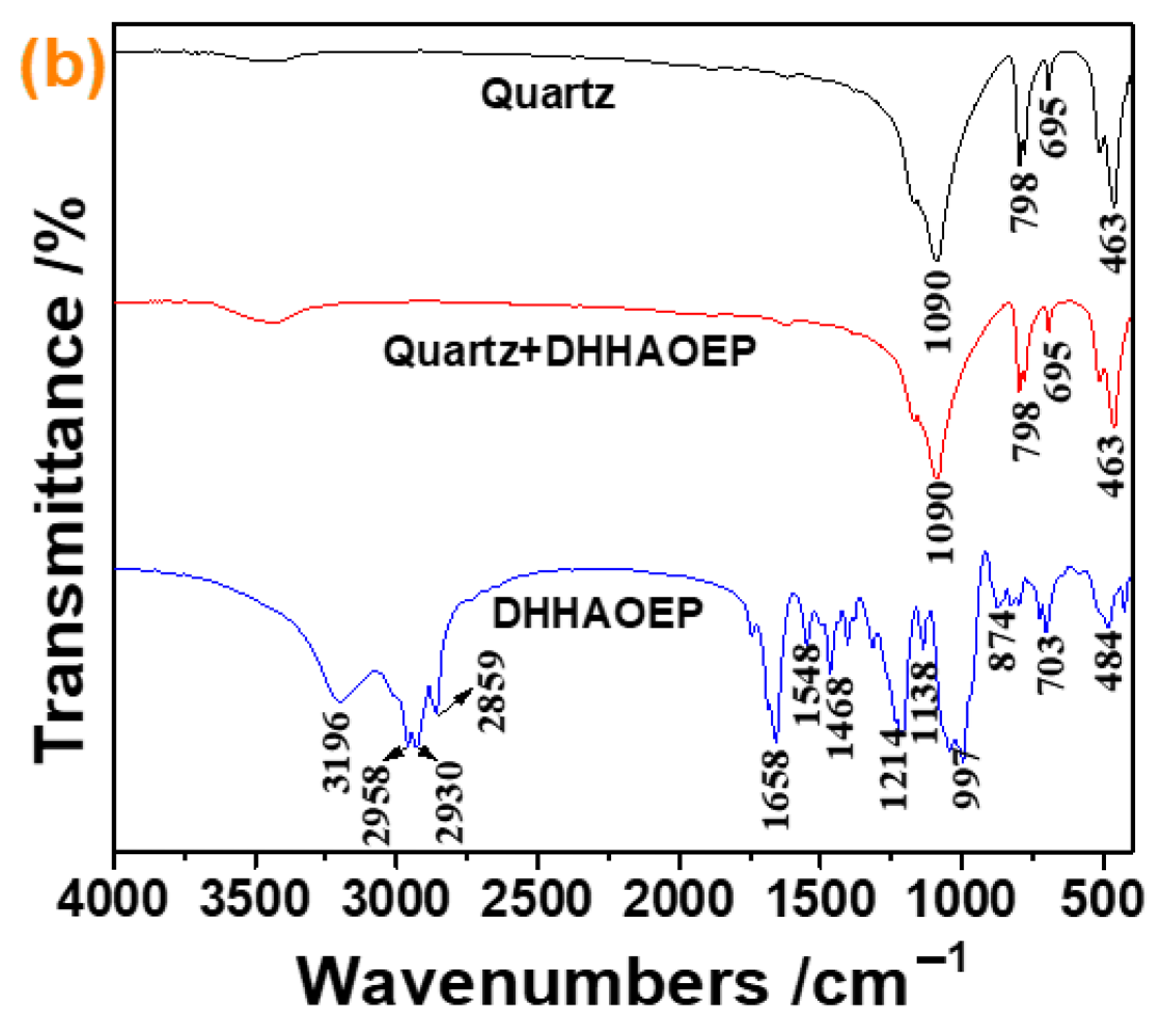
3.5. FTIR Analysis
3.6. XPS Analysis
3.7. Discussion
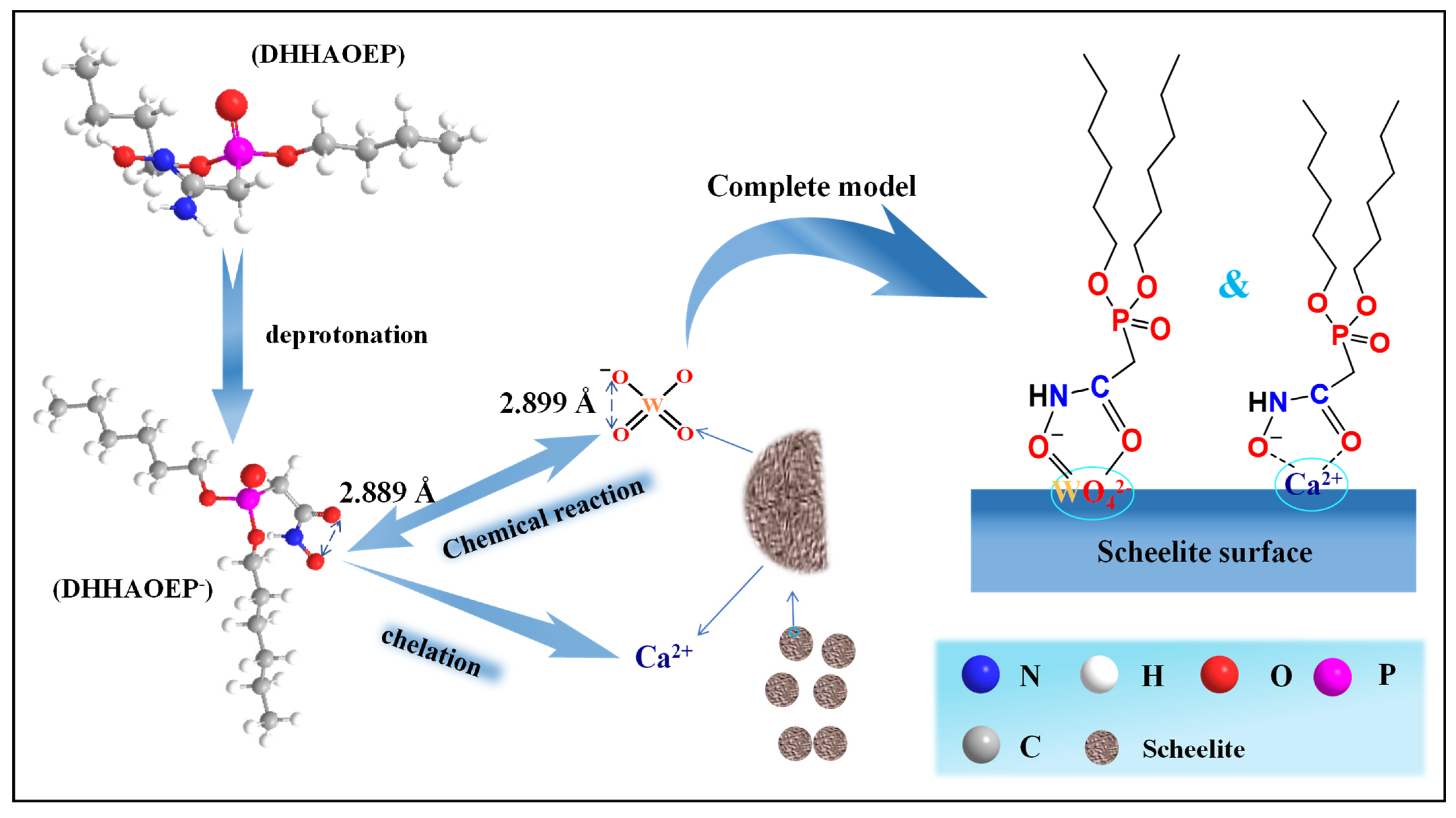
 configuration with W in WO42−, as shown in Figure 8. The results are in agreement with those calculated by FTIR, XPS, and DFT. At the same time, the binding form of such hydroxamic acid with minerals is similar to the N-((hydroxyamino)-alkyl) alkyl amide surfactants (NHOD−) studied by Deng [28]. However, the bond length between two adjacent oxygen atoms in NHOD− is 2.842 Å, while that in DHHAOEP− is 2.889 Å, which is closer to the 2.899 Å between two adjacent oxygen atoms in scheelite. Therefore, it can be inferred that the presence of the P=O group in DHHAOEP increases the distance between adjacent oxygen atoms in C(=O)-NHO−, which can better react with the active sites on the surface of scheelite.
configuration with W in WO42−, as shown in Figure 8. The results are in agreement with those calculated by FTIR, XPS, and DFT. At the same time, the binding form of such hydroxamic acid with minerals is similar to the N-((hydroxyamino)-alkyl) alkyl amide surfactants (NHOD−) studied by Deng [28]. However, the bond length between two adjacent oxygen atoms in NHOD− is 2.842 Å, while that in DHHAOEP− is 2.889 Å, which is closer to the 2.899 Å between two adjacent oxygen atoms in scheelite. Therefore, it can be inferred that the presence of the P=O group in DHHAOEP increases the distance between adjacent oxygen atoms in C(=O)-NHO−, which can better react with the active sites on the surface of scheelite.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- Yao, X.; Yu, X.; Wang, L.; Zeng, Y.; Mao, L.; Liu, S.; Xie, H.; He, G.; Huang, Z.; Zhang, S. Preparation of cinnamon hydroxamic acid and its flotation characteristics and mechanism to fine-grained wolframite. J. Mol. Liq. 2022, 362, 119721. [Google Scholar] [CrossRef]
- Shuai, S.; Huang, Z.; Burov, V.E.; Poilov, V.Z.; Li, F.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Li, W.; et al. Selective separation of wolframite from calcite by froth flotation using a novel amidoxime surfactant: Adsorption mechanism and DFT calculation. Miner. Eng. 2022, 185, 107716. [Google Scholar] [CrossRef]
- Huang, Z.; Shuai, S.; Burov, V.E.; Poilov, V.Z.; Li, F.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Li, W.; et al. Application of a new amidoxime surfactant in flotation separation of scheelite and calcite: Adsorption mechanism and DFT calculation. J. Mol. Liq. 2022, 364, 120036. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, W.; Han, H.; Gui, X.; Xing, Y. Flotation chemistry of scheelite and its practice: A comprehensive review. Miner. Eng. 2023, 204, 108404. [Google Scholar] [CrossRef]
- Li, C.; Gao, Z. Effect of grinding media on the surface property and flotation behavior of scheelite particles. Powder Technol. 2017, 322, 386–392. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q. Investigations on flotation separation of scheelite from calcite by using a novel depressant: Sodium phytate. Miner. Eng. 2018, 126, 116–122. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Yao, X.; Yu, X.; Wang, L.; Zeng, Y.; Mao, L.; Liu, S.; Xie, H.; He, G.; Huang, Z.; Liu, Z. Preparation of cinnamic hydroxamic acid collector and study on flotation characteristics and mechanism of scheelite. Int. J. Min. Sci. Technol. 2023, 33, 773–781. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Tan, J.; Hu, F. Flotation separation of pyrite from chalcopyrite by tetrazinan-thione collectors. J. Cent. South Univ. 2023, 30, 2587–2598. [Google Scholar] [CrossRef]
- Foucaud, Y.; Filippov, L.; Filippova, I.; Badawi, M. The Challenge of Tungsten Skarn Processing by Froth Flotation: A Review. Front. Chem. 2020, 8, 230. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, S.; Wang, H.; Liu, R.; Cheng, C.; Shuai, S.; Hu, Y.; Zeng, Y.; Yu, X.; He, G.; et al. Recovery of wolframite from tungsten mine tailings by the combination of shaking table and flotation with a novel “crab” structure sebacoyl hydroxamic acid. J. Environ. Manag. 2022, 317, 115372. [Google Scholar] [CrossRef]
- Qi, J.; Zhao, G.; Liu, S.; Chen, W.; Liu, G. Strengthen flotation enrichment of Pb(II)-activated scheelite with N-[(3-hydroxyamino)-propoxyl]-N-hexyl dithiocarbamate. J. Ind. Eng. Chem. 2022, 114, 338–346. [Google Scholar] [CrossRef]
- Zhao, G.; Zhong, H.; Qiu, X.; Wang, S.; Gao, Y.; Dai, Z.; Huang, J.; Liu, G. The DFT study of cyclohexyl hydroxamic acid as a collector in scheelite flotation. Miner. Eng. 2013, 49, 54–60. [Google Scholar] [CrossRef]
- Xiao, J.; Wu, J.; Liu, S.; Tu, J.; Liu, R.; Li, C.; Zhao, G. Preparation of a novel surfactant dibutyl (2-(hydroxyamino)-2-oxoethyl) phosphonate and its adsorption mechanism in cassiterite flotation. J. Cent. South Univ. 2023, 30, 1569–1580. [Google Scholar] [CrossRef]
- Chassagne, C.; Mietta, F.; Winterwerp, J.C. Electrokinetic study of kaolinite suspensions. J. Colloid Interface Sci. 2009, 336, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xiao, J.; Liu, J.; Qu, X.; Liu, Q.; Zeng, H.; Yang, X.; Xie, L.; Zhong, H.; Liu, Q.; et al. In situ probing the self-assembly of 3-hexyl-4-amino-1,2,4-triazole-5-thione on chalcopyrite surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2016, 511, 285–293. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab Initiomolecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-Dynamics Simulation of the Liquid-Metal-Amorphous-Semiconductor Transition in Germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Cao, Z.; Wang, S.; Yang, J.; Zhong, H. Flotation performance and adsorption mechanism of styryl phosphonate mono-iso-octyl ester to malachite. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123698. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, S.; Qi, J.; Yang, L.; Liu, G. Tungsten minerals flotation with 4-alkoxy benzohydroxamic acid: The structure-performance relationship of its C3 derivatives. Miner. Eng. 2023, 203, 108361. [Google Scholar] [CrossRef]
- Fukui, K. The role of frontier orbitals in chemical reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhao, G.; Zhong, H.; Wang, S.; Liu, G. Investigation on the selectivity of N-((hydroxyamino)-alkyl) alkylamide surfactants for scheelite/calcite flotation separation. J. Ind. Eng. Chem. 2016, 33, 131–141. [Google Scholar] [CrossRef]
- Qi, J.; Dong, Y.; Liu, S.; Liu, G. A selective flotation of cassiterite with a dithiocarbamate-hydroxamate molecule and its adsorption mechanism. Appl. Surf. Sci. 2021, 538, 147996. [Google Scholar] [CrossRef]
- Deng, L.; Wang, S.; Zhong, H.; Liu, G. N-(6-(hydroxyamino)-6-oxohexyl) decanamide collector: Flotation performance and adsorption mechanism to diaspore. Appl. Surf. Sci. 2015, 347, 79–87. [Google Scholar] [CrossRef]
- Liu, S.; Zhong, H.; Liu, G.; Xu, Z. Cu(I)/Cu(II) mixed-valence surface complexes of S-[(2-hydroxyamino)-2-oxoethyl]-N,N-dibutyldithiocarbamate: Hydrophobic mechanism to malachite flotation. J. Colloid Interface Sci. 2018, 512, 701–712. [Google Scholar] [CrossRef]
- Liu, S.; Xie, L.; Liu, J.; Liu, G.; Zhong, H.; Wang, Y.; Zeng, H. Probing the interactions of hydroxamic acid and mineral surfaces: Molecular mechanism underlying the selective separation. Chem. Eng. J. 2019, 374, 123–132. [Google Scholar] [CrossRef]
- Kim, S.; Baek, S.-H.; Han, Y.; Jeon, H.-S. Laboratory Testing of Scheelite Flotation from Raw Ore in Sangdong Mine for Process Development. Minerals 2020, 10, 971. [Google Scholar] [CrossRef]
- Pecheur, O.; Guillaumont, D.; Dourdain, S.; Berthon, L.; Turgis, R.; Fillaux, C.; Arrachart, G.; Testard, F. Uranium Extraction by a Bifunctional Amido-Phosphonic Acid: Coordination Structure and Aggregation. Solvent Extr. Ion Exch. 2016, 34, 260–273. [Google Scholar] [CrossRef]
- Daasch, L.W.; Smith, D.C. Infrared Spectra of Phosphorus Compounds. Anal. Chem. 1951, 23, 853–868. [Google Scholar] [CrossRef]
- Yang, S.; Hua, M.; Shen, L.; Han, X.; Xu, M.; Kuang, L.; Hua, D. Phosphonate and carboxylic acid co-functionalized MoS2 sheets for efficient sorption of uranium and europium: Multiple groups for broad-spectrum adsorption. J. Hazard. Mater. 2018, 354, 191–197. [Google Scholar] [CrossRef]
- Fan, H.; Qin, J.; Liu, J.; Liu, G.; Yang, X. Investigation into the flotation of malachite, calcite and quartz with three phosphate surfactants. J. Mater. Res. Technol. 2019, 8, 5140–5148. [Google Scholar] [CrossRef]
- Sun, Q.; Ma, X.; Lu, Y.; Wang, S.; Zhong, H. Insights into the selective adsorption mechanism of a multifunctional thioether-containing hydroxamic acid on separation of wolframite from fluorite. Powder Technol. 2021, 380, 421–429. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, L.; Ye, G.; Liu, Q.; Li, J.; Wang, X.; Chen, J.; Xu, S.; Ma, S. De novo synthesis of bifunctional conjugated microporous polymers for synergistic coordination mediated uranium entrapment. Nano Res. 2021, 14, 788–796. [Google Scholar] [CrossRef]
- Xie, R.; Zhao, Z.; Wang, X.; Tong, X.; Xie, X. Flotation separation of scheelite from fluorite by new depressant nitrilotriacetic acid and its mechanism. J. Taiwan Inst. Chem. Eng. 2023, 152, 105153. [Google Scholar] [CrossRef]
- Qiao, L.; Dong, L.; Zhang, T.; Shen, P.; Liu, D. Metal ions modified small molecule organic inhibitor to achieve efficient flotation separation of scheelite from calcite. Adv. Powder Technol. 2024, 35, 104557. [Google Scholar] [CrossRef]
- Kay, M.I.; Frazer, B.C.; Almodovar, I. Neutron Diffraction Refinement of CaWO4. J. Chem. Phys. 1964, 40, 504. [Google Scholar] [CrossRef]
- Le Page, Y.; Donnay, G. Refinement of the Crystal Structure of Low-Quartz. Struct. Sci. 1976, 32, 2456–2459. [Google Scholar] [CrossRef]
- Sun, H.; Yang, B.; Zhu, Z.; Yin, W.; Sheng, Q.; Hou, Y.; Yao, J. New insights into selective-depression mechanism of novel depressant EDTMPS on magnesite and quartz surfaces: Adsorption mechanism, DFT calculations, and adsorption model. Miner. Eng. 2021, 160, 106660. [Google Scholar] [CrossRef]


| Sample | WO3 | Al2O3 | CaO | Au | SiO2 |
|---|---|---|---|---|---|
| Scheelite | 77.12 | 0.780 | 0.107 | 0.306 | 1.11 |
| Quartz | 0.0146 | 0.923 | <0.042 | <0.02 | 98.54 |
| Substance | Atomic Concentration/% | |||||
|---|---|---|---|---|---|---|
| C 1s | N 1s | O 1s | P 2p | W 4f | Ca 2p | |
| DHHAOEP | 66.99 | 4.45 | 23.44 | 5.12 | - | - |
| Scheelite | 25.29 | - | 51.05 | - | 11.43 | 12.24 |
| DHHAOEP-modified Scheelite | 25.59 | 0.91 | 49.14 | 1.66 | 10.92 | 11.78 |
| Reaction Conditions | EMineral (kJ/mol) | EReagents (kJ/mol) | ETotal (kJ/mol) | ΔE (kJ/mol) |
|---|---|---|---|---|
| Scheelite (101) + DHHAOEP | −86,003.52 | −26,933.76 | −113,214.72 | −277.44 |
| Scheelite (112) + DHHAOEP | −76,201.92 | −26,933.76 | −103,390.08 | −254.40 |
| Quartz (101) + DHHAOEP | −99,743.04 | −26,933.76 | −126,876.48 | −199.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Xiao, P.; Miao, Y.; Liu, S.; Tu, J.; Tang, Q.; Li, C.; Xiao, Z.; Liu, R. Dihexyl (2-(Hydroxyamino)-2-Oxoethyl) Phosphonate as a Novel Collector for Flotation Separation of Scheelite and Quartz. Molecules 2025, 30, 3607. https://doi.org/10.3390/molecules30173607
Xiao J, Xiao P, Miao Y, Liu S, Tu J, Tang Q, Li C, Xiao Z, Liu R. Dihexyl (2-(Hydroxyamino)-2-Oxoethyl) Phosphonate as a Novel Collector for Flotation Separation of Scheelite and Quartz. Molecules. 2025; 30(17):3607. https://doi.org/10.3390/molecules30173607
Chicago/Turabian StyleXiao, Jingjing, Pan Xiao, Yongjun Miao, Sisi Liu, Jia Tu, Qing Tang, Changzhu Li, Zhihong Xiao, and Rukuan Liu. 2025. "Dihexyl (2-(Hydroxyamino)-2-Oxoethyl) Phosphonate as a Novel Collector for Flotation Separation of Scheelite and Quartz" Molecules 30, no. 17: 3607. https://doi.org/10.3390/molecules30173607
APA StyleXiao, J., Xiao, P., Miao, Y., Liu, S., Tu, J., Tang, Q., Li, C., Xiao, Z., & Liu, R. (2025). Dihexyl (2-(Hydroxyamino)-2-Oxoethyl) Phosphonate as a Novel Collector for Flotation Separation of Scheelite and Quartz. Molecules, 30(17), 3607. https://doi.org/10.3390/molecules30173607





