Thermal Decomposition and Prebiotic Formation of Adenosine Phosphates in Simulated Early-Earth Evaporative Settings
Abstract
1. Introduction
2. Results
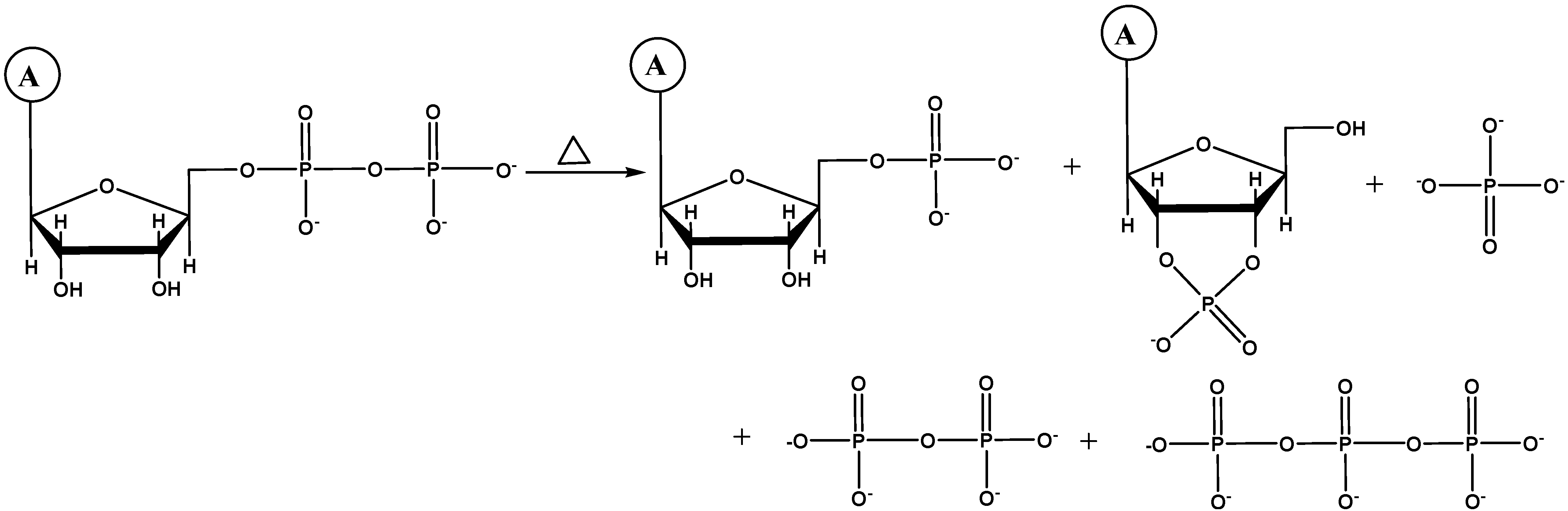
2.1. 31P-NMR Analyses of Adenosine Phosphates
2.1.1. AMP
2.1.2. ADP
2.1.3. ATP
2.1.4. Comparison of Various P Products in Decomposition Reactions of AMP, ADP and ATP
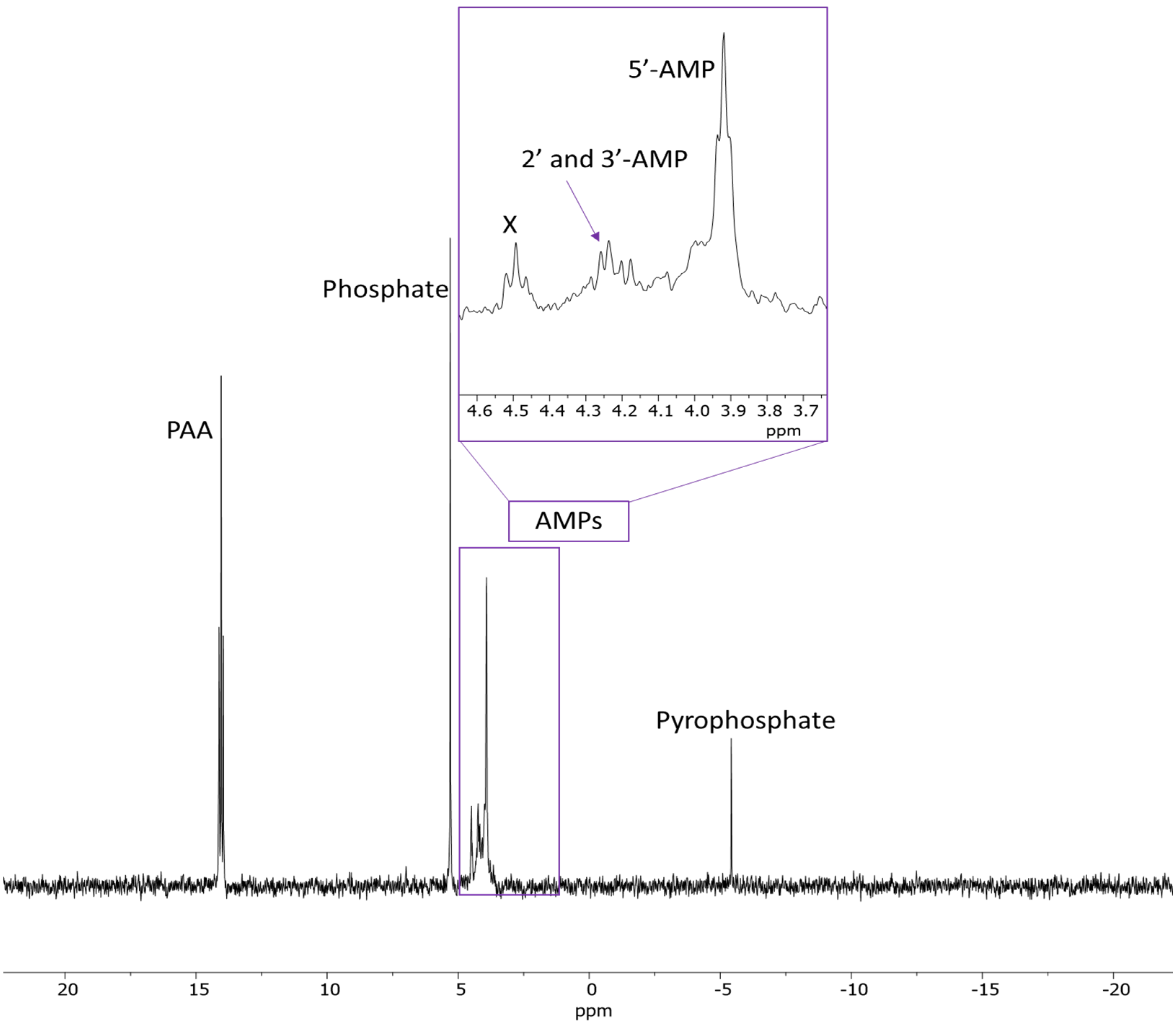
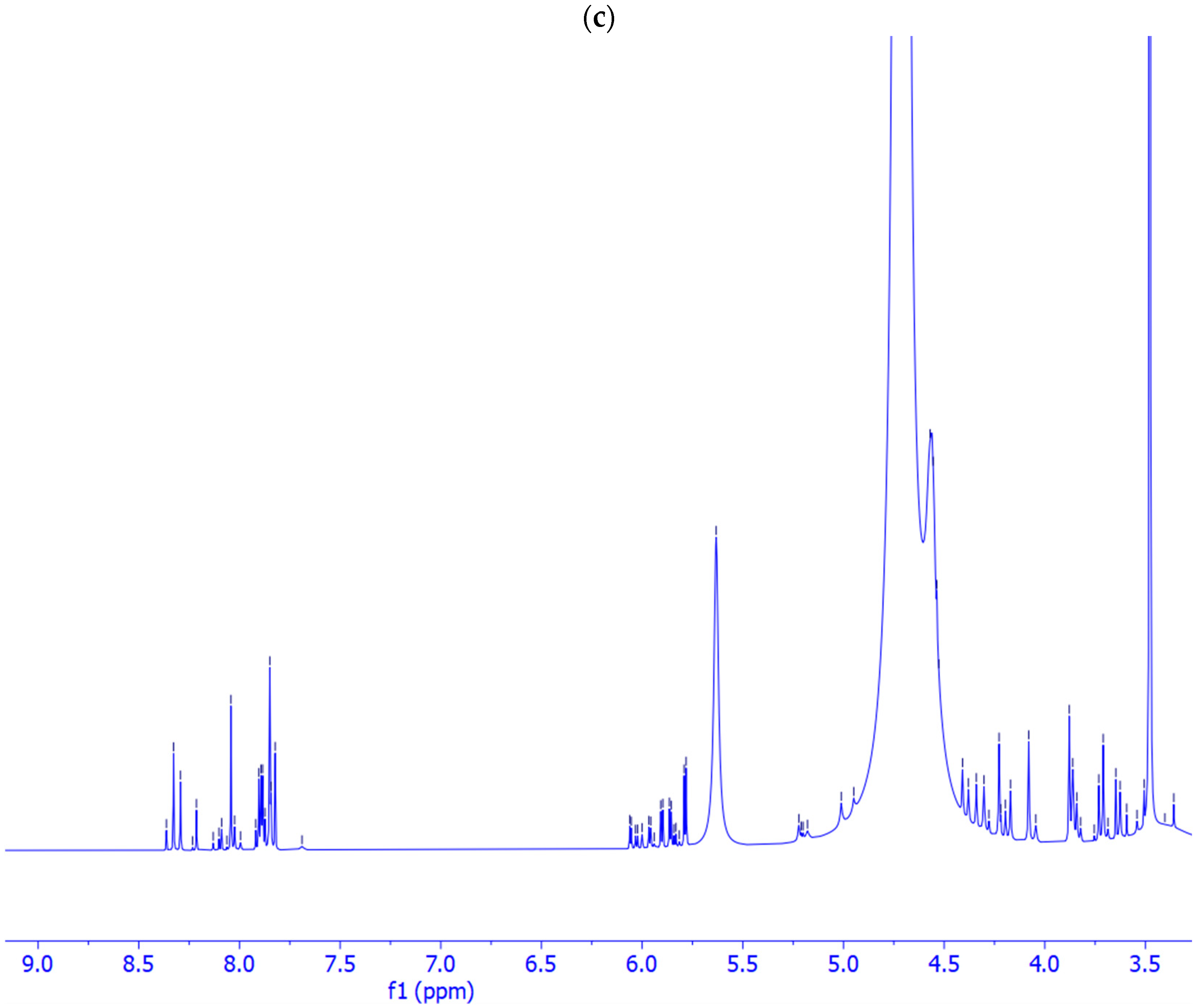
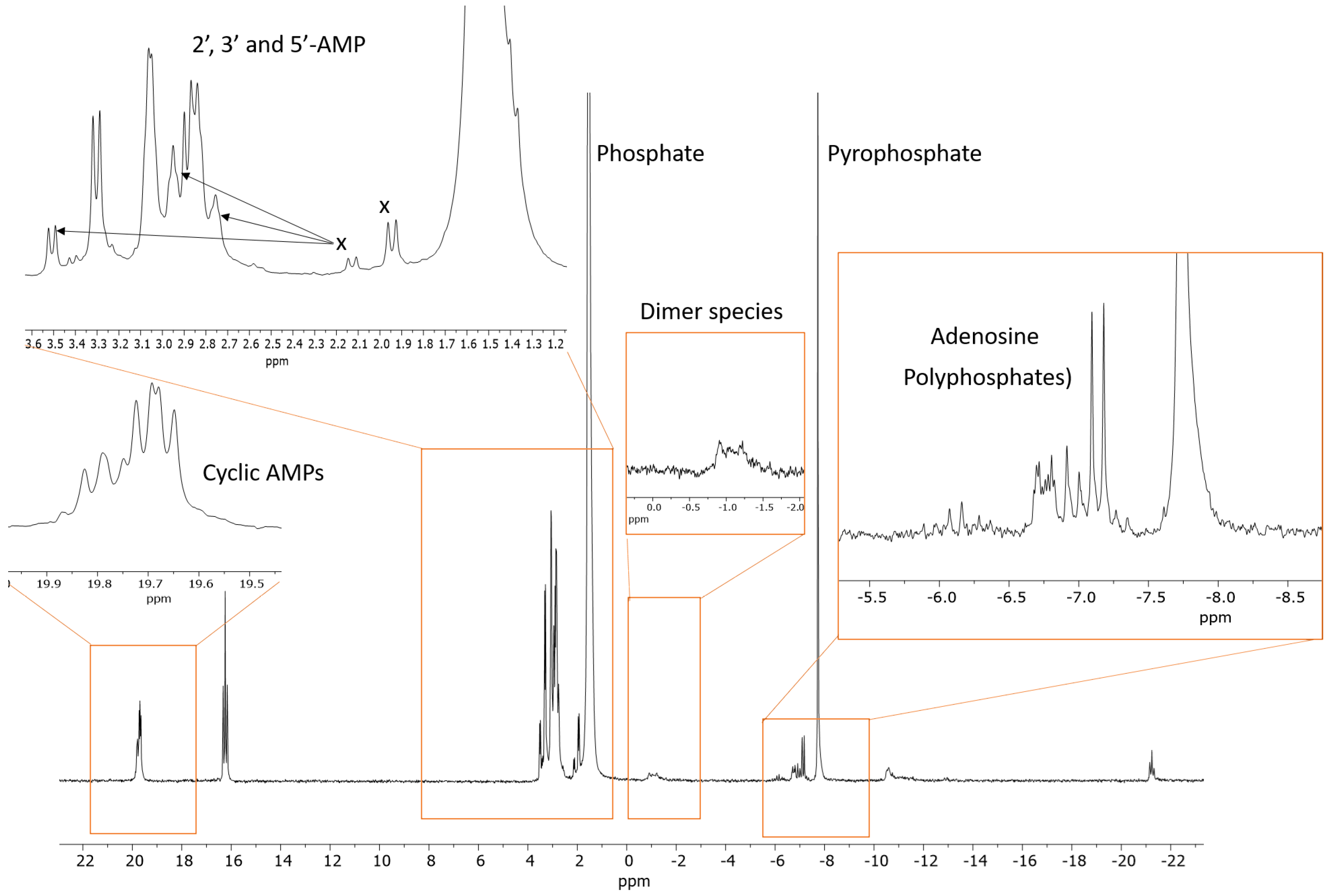
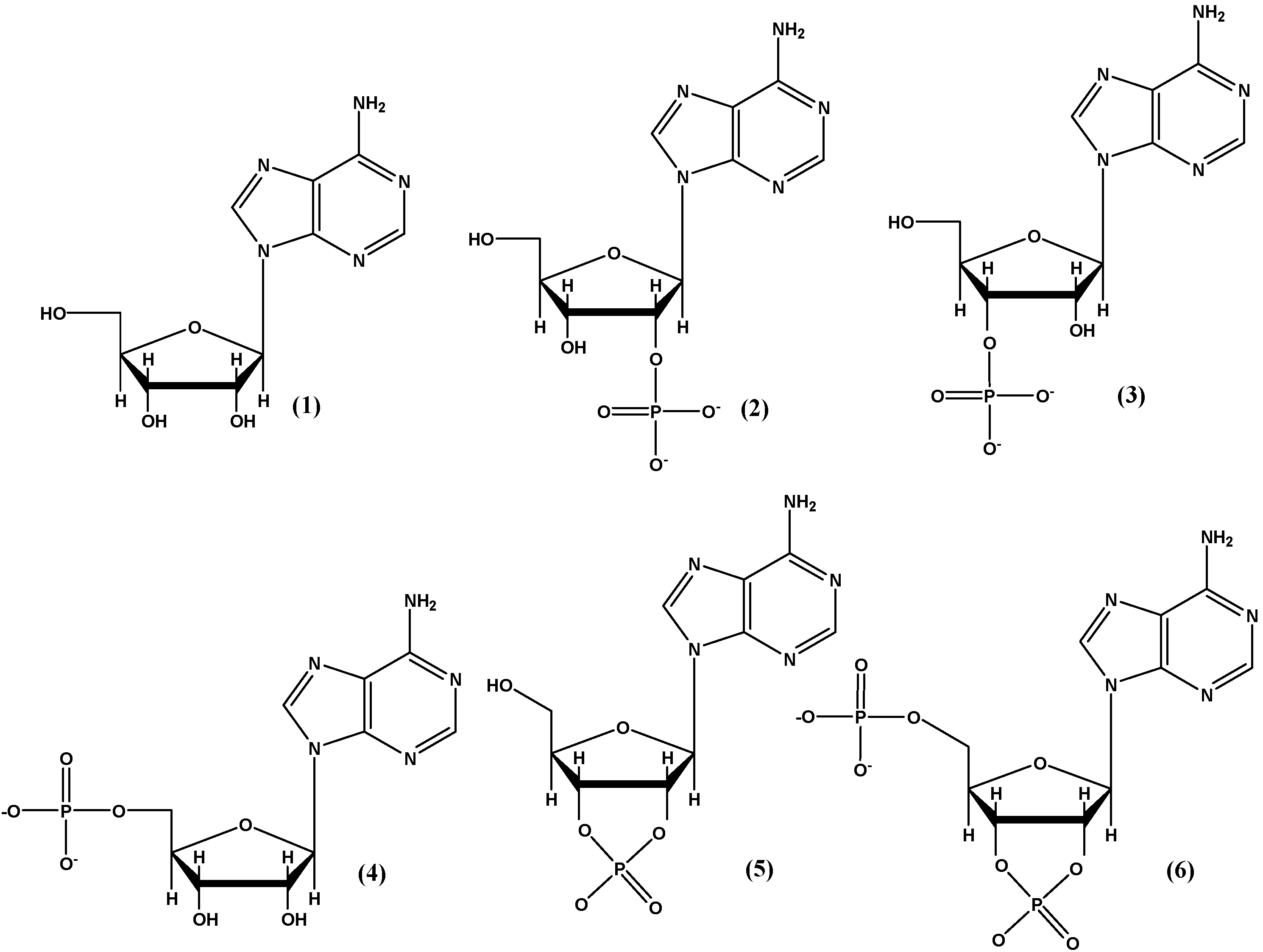
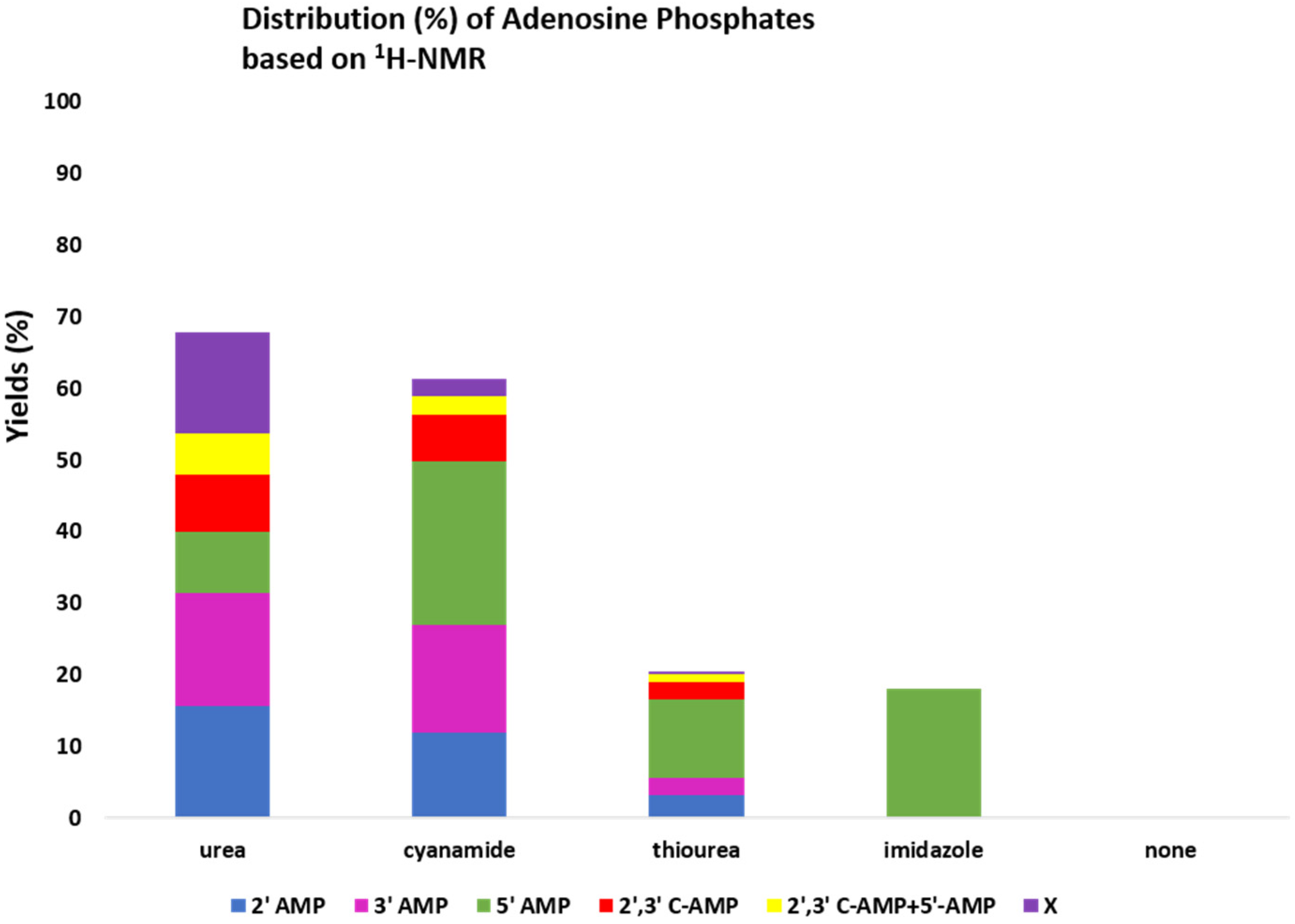
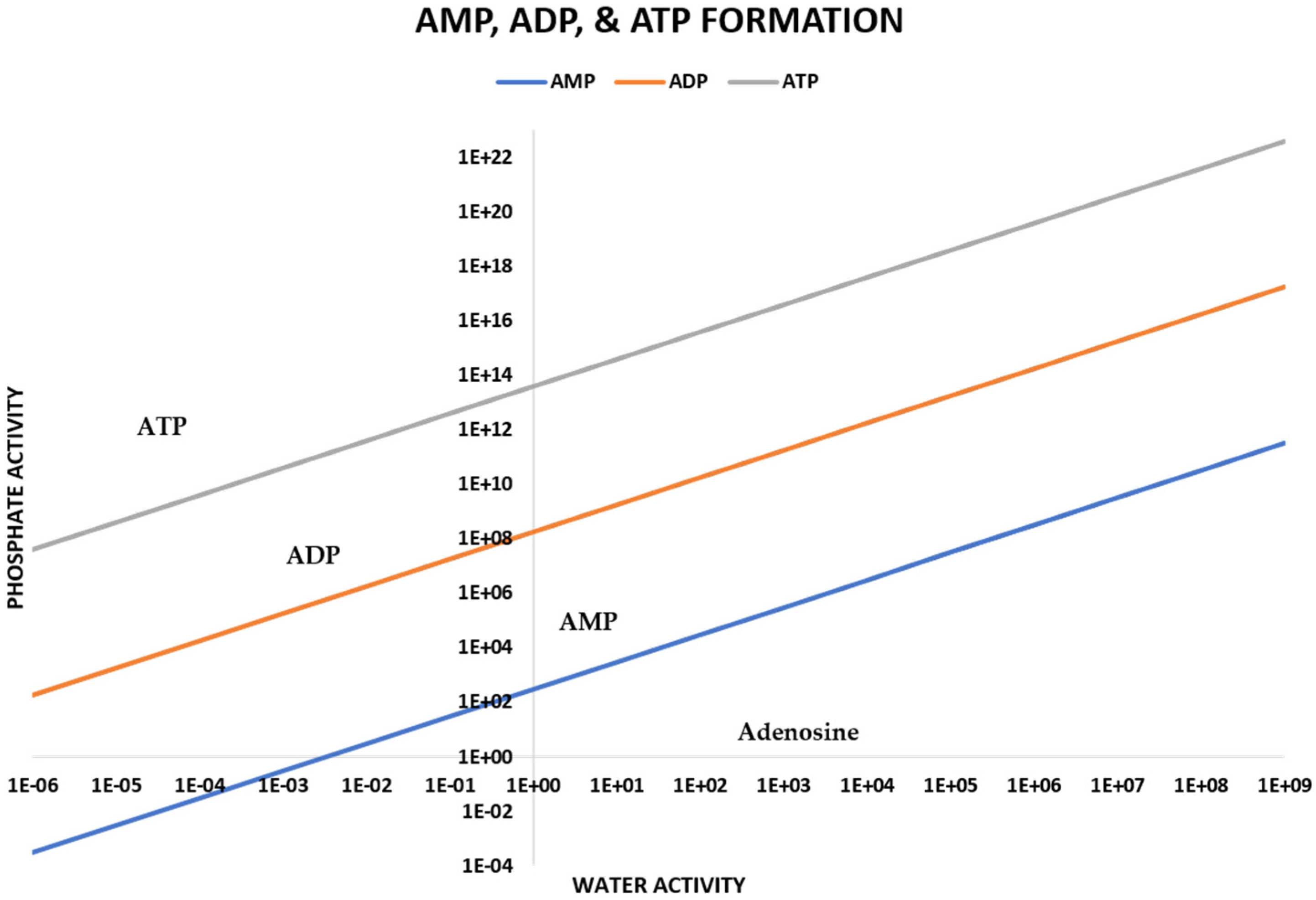
2.2. Prebiotic Synthesis Reactions of Adenosine Phosphates
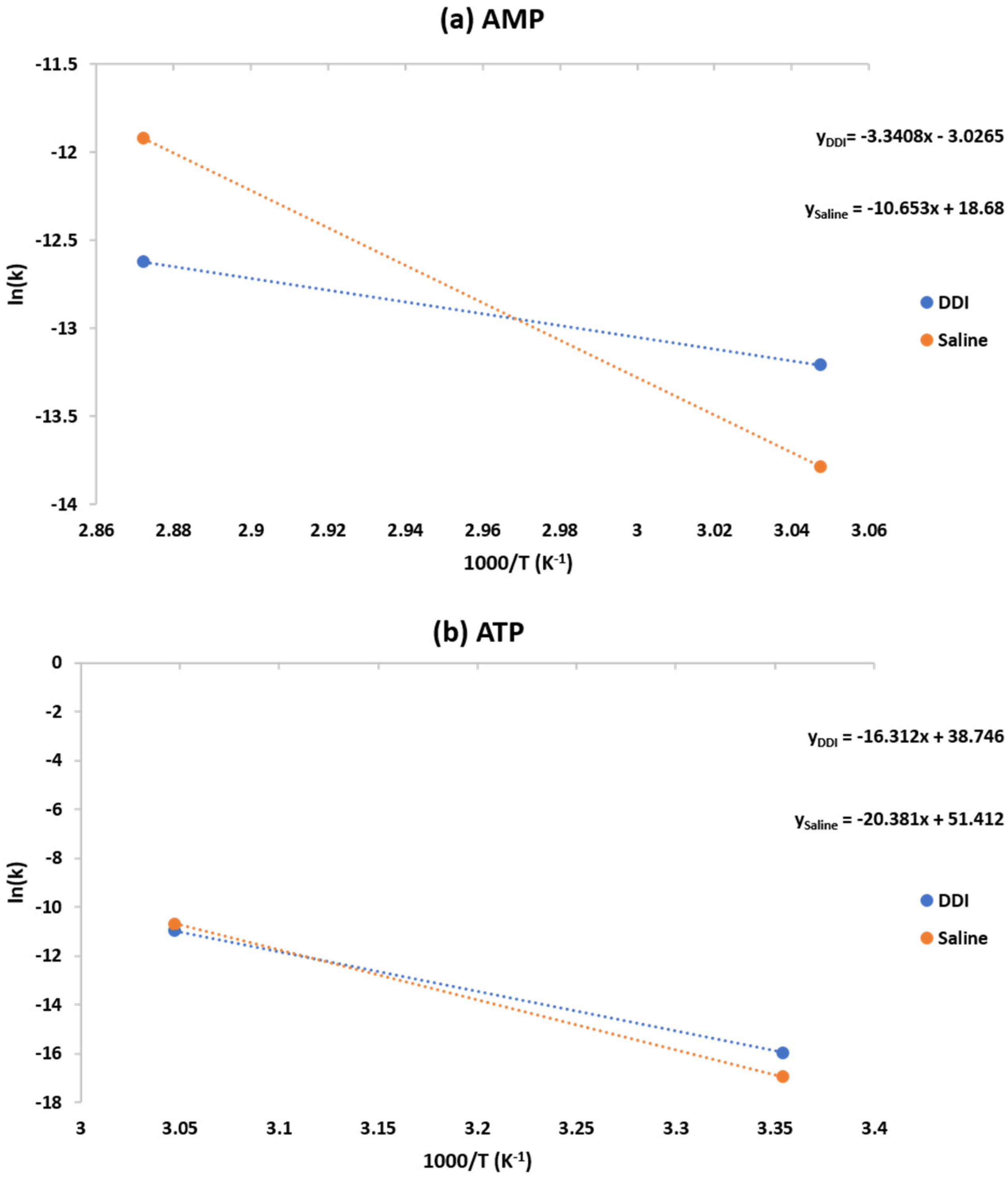
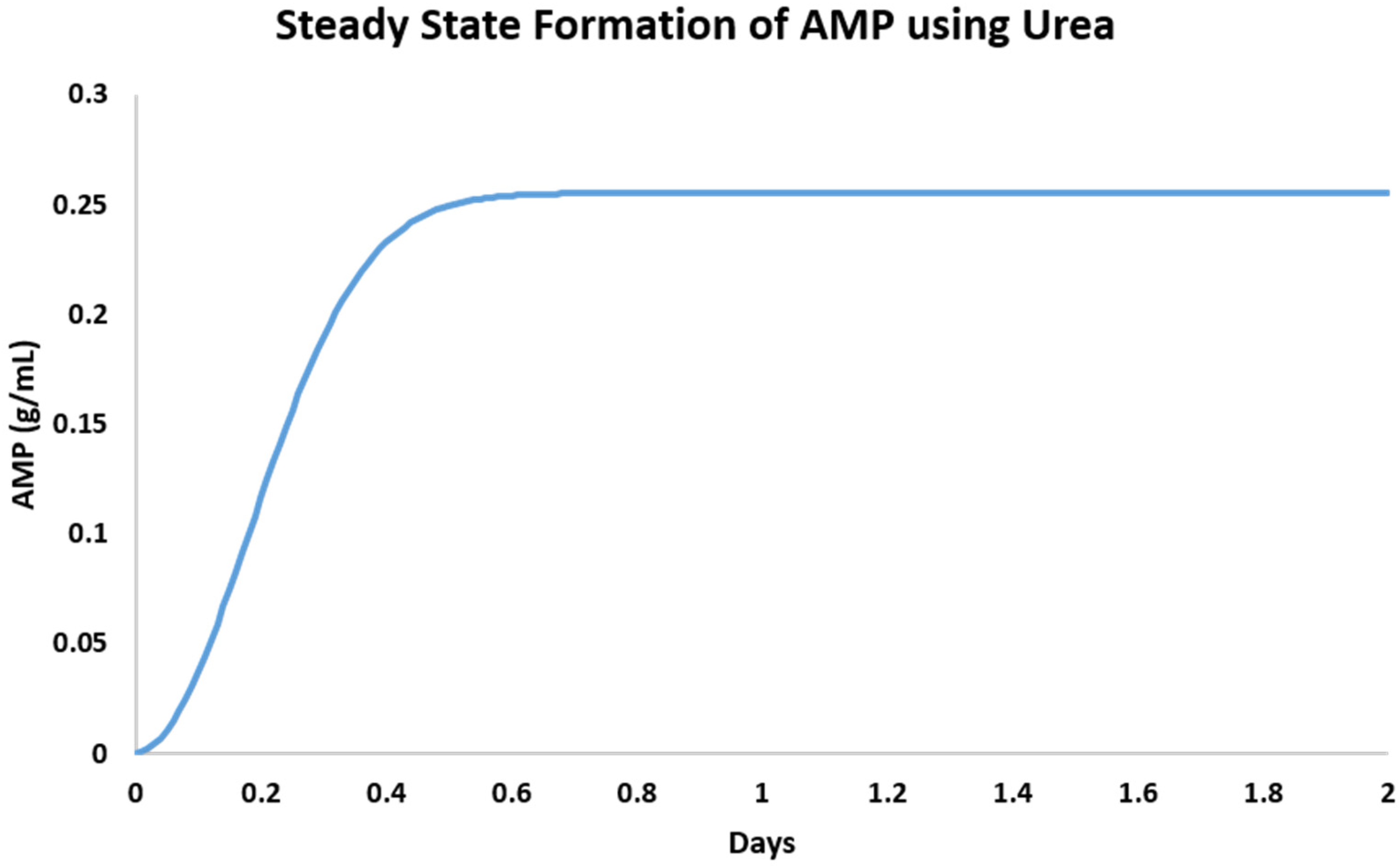
2.3. Numerical Modeling of Degradation and Rate of Production
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyer, P.D. Energy, Life, and ATP (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1998, 37, 2296–2307. [Google Scholar] [CrossRef]
- Fontecilla-Camps, J.C. The Complex Roles of Adenosine Triphosphate in Bioenergetics. ChemBioChem. 2022, 23, 202200064. [Google Scholar] [CrossRef] [PubMed]
- Pasek, M.A.; Kee, T.P. On the Origin of Phosphorylated Biomolecules. In Origins of Life: The Primal Self-Organization; Egel, R., Lankenau, D.-H., Mulkidjanian, A.Y., Eds.; Springer Berlin Heidelberg: Berlin, Germany, 2011; pp. 57–84. [Google Scholar]
- Toppozini, L.; Dies, H.; Deamer, D.W.; Rheinstädter, M.C. Adenosine monophosphate forms ordered arrays in multilamellar lipid matrices: Insights into assembly of nucleic acid for primitive life. PLoS ONE 2013, 8, 62810. [Google Scholar] [CrossRef]
- Hud, N.V. Searching for lost nucleotides of the pre-RNA World with a self-refining model of early Earth. Nat. Commun. 2018, 9, 5171. [Google Scholar] [CrossRef]
- Robertson, M.P.; Joyce, G.F. The origins of the RNA world. Cold Spring Harb. Perspect. Biol. 2012, 4, 003608. [Google Scholar] [CrossRef]
- Pressman, A.; Blanco, C.; Chen, I.A. The RNA World as a Model System to Study the Origin of Life. Curr. Biol. 2015, 25, 953–963. [Google Scholar] [CrossRef]
- Kim, H.J.; Ricardo, A.; Illangkoon, H.I.; Kim, M.J.; Carrigan, M.A.; Frye, F.; Benner, S.A. Synthesis of carbohydrates in mineral-guided prebiotic cycles. J. Am. Chem. Soc. 2011, 133, 9457–9468. [Google Scholar] [CrossRef]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef]
- Orgel, L.E. Prebiotic chemistry and the origin of the RNA world. Crit. Rev. Biochem. Mol. Biol. 2004, 39, 99–123. [Google Scholar] [CrossRef] [PubMed]
- Fialho, D.M.; Clarke, K.C.; Moore, M.K.; Schuster, G.B.; Krishnamurthy, R.; Hud, N. Glycosylation of a model proto-RNA nucleobase with non-ribose sugars: Implications for the prebiotic synthesis of nucleosides. Org. Biomol. Chem. 2018, 16, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Cafferty, B.J.; Fialho, D.M.; Khanam, J.; Krishnamurthy, R.; Hud, N.V. Spontaneous formation and base pairing of plausible prebiotic nucleotides in water. Nat. Commun. 2016, 7, 11328. [Google Scholar] [CrossRef]
- Chen, M.C.; Cafferty, B.J.; Mamajanov, I.; Gállego, I.; Khanam, J.; Krishnamurthy, R.; Hud, N.V. Spontaneous prebiotic formation of a β-ribofuranoside that self-assembles with a complementary heterocycle. J. Am. Chem. Soc. 2014, 136, 5640–5646. [Google Scholar] [CrossRef]
- Eschenmoser, A. Chemical etiology of nucleic acid structure. Science 1999, 284, 2118–2124. [Google Scholar] [CrossRef]
- Pasek, M.A. Thermodynamics of Prebiotic Phosphorylation. Chem. Rev. 2020, 120, 4690–4706. [Google Scholar] [CrossRef]
- Peretó, J.; Bada, J.L.; Lazcano, A. Charles Darwin and the origin of life. Orig. Life Evol. Biosph. 2009, 39, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Damer, B. A Field Trip to the Archaean in Search of Darwin’s Warm Little Pond. Life 2016, 6, 21. [Google Scholar] [CrossRef]
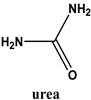
- Burcar, B.; Pasek, M.; Gull, M.; Cafferty, B.J.; Velasco, F.; Hud, N.V.; Menor-Salván, C. Darwin’s warm little pond: A one-pot reaction for prebiotic phosphorylation and the mobilization of phosphate from minerals in a urea-based solvent. Angew. Chem. Int. Ed. Engl. 2016, 55, 13249–13253. [Google Scholar] [CrossRef] [PubMed]
- Gull, M.; Omran, A.; Feng, T.; Pasek, M.A. Silicate-, Magnesium Ion-, and Urea-Induced Prebiotic Phosphorylation of Uridine via Pyrophosphate; Revisiting the Hot Drying Water Pool Scenario. Life 2020, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Lohrmann, L.; Orgel, L.E. Urea-inorganic phosphate mixtures as prebiotic phosphorylating agents. Science 1971, 171, 490–494. [Google Scholar] [CrossRef]
- Cheng, C.; Fan, C.; Wan, R.; Tong, C.; Miao, Z.; Chen, J.; Zhao, Y. Phosphorylation of adenosine with trimetaphosphate under simulated prebiotic conditions. Origins Life Evol. Biosph. 2002, 32, 219–224. [Google Scholar] [CrossRef]
- Schwartz, A.W.; Ponnamperuma, C. Phosphorylation of adenosine with linear polyphosphate salts in aqueous solution. Nature 1968, 218, 443. [Google Scholar] [CrossRef]
- Schoffstall, A.M.; Laing, E.M. Phosphorylation mechanisms in chemical evolution. Origins Life Evol. Biosph. 1985, 15, 141–150. [Google Scholar] [CrossRef]
- Costanzo, G.; Saladino, R.; Crestini, C.; Ciciriello, F.; Di Mauro, E. Nucleoside phosphorylation by phosphate minerals. J. Biol. Chem. 2007, 282, 16729–16735. [Google Scholar] [CrossRef]
- Schoffstall, A.M.; Barto, R.J.; Ramos, D.L. Nucleoside and deoxynucleoside phosphorylation in formamide solutions. Origins Life Evol. Biosph. 1982, 12, 143–151. [Google Scholar] [CrossRef]
- Schoffstall, A.M. Prebiotic phosphorylation of nucleosides in formamide. Origins Life Evol. Biosph. 1976, 4, 399–412. [Google Scholar] [CrossRef]
- Bechtel, M.; Hümmer, E.; Trapp, O. Selective Phosphorylation of RNA- and DNA-Nucleosides under Prebiotically Plausible Conditions. Chem.Systems.Chem. 2022, 4, e2200020. [Google Scholar] [CrossRef]
- Maguire, O.R.; Smokers, I.B.A.; Huck, W.T.S. A physicochemical orthophosphate cycle via a kinetically stable thermodynamically activated intermediate enables mild prebiotic phosphorylations. Nat. Commun. 2021, 12, 5517. [Google Scholar] [CrossRef]
- Kim, H.J.; Benner, S.A. Abiotic Synthesis of Nucleoside 5′-Triphosphates with Nickel Borate and Cyclic Trimetaphosphate (CTMP). Astrobiology 2021, 21, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Kim, H.J.; Hutter, D.; Benner, S.A. Abiotic regioselective phosphorylation of adenosine with borate in formamide. Astrobiology 2015, 15, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Furukawa, Y.; Kakegawa, T.; Bita, A.; Scorei, R.; Benner, S.A. Evaporite Borate-Containing Mineral Ensembles Make Phosphate Available and Regiospecifically Phosphorylate Ribonucleosides: Borate as a Multifaceted Problem Solver in Prebiotic Chemistry. Angew. Chem. Int. Ed. Engl. 2016, 55, 15816–15820. [Google Scholar] [CrossRef] [PubMed]
- Gull, M.; Zhou, M.; Fernández, F.M.; Pasek, M.A. Prebiotic phosphate ester syntheses in a deep eutectic solvent. J. Mol. Evol. 2014, 78, 109–117. [Google Scholar] [CrossRef]
- Gull, M.; Mojica, M.A.; Fernández, F.M.; Gaul, D.A.; Orlando, T.M.; Liotta, C.L.; Pasek, M.A. Nucleoside phosphorylation by the mineral schreibersite. Sci. Rep. 2015, 5, 17198. [Google Scholar] [CrossRef] [PubMed]
- Gull, M.; Feng, T.; Bracegirdle, J.; Abbott-Lyon, H.; Pasek, M.A. Organophosphorus Compound Formation Through the Oxidation of Reduced Oxidation State Phosphorus Compounds on the Hadean Earth. J. Mol. Evol. 2023, 91, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Gibard, C.; Bhowmik, S.; Karki, M.; Kim, E.K.; Krishnamurthy, R. Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions. Nat. Chem. 2018, 10, 212–217. [Google Scholar] [CrossRef]
- Cruz, H.A.; Jiménez, E.I.; Krishnamurthy, R. Enhancing Prebiotic Phosphorylation and Modulating the Regioselectivity of Nucleosides with Diamidophosphate. J. Am. Chem. Soc. 2023, 145, 23781–23793. [Google Scholar] [CrossRef]
- Kuzicheva, E.A.; Gontareva, N.B. Prebiotic synthesis of nucleotides at the Earth orbit in presence of Lunar soil. Adv. Space Res. 2002, 30, 1525–1531. [Google Scholar] [CrossRef]
- Guo, X.; Fu, S.; Ying, J.; Zhao, Y. Prebiotic chemistry: A review of nucleoside phosphorylation and polymerization. Open Biol. 2023, 13, 220234. [Google Scholar] [CrossRef] [PubMed]
- Hulett, H.R. Non-enzymatic hydrolysis of adenosine phosphates. Nature 1970, 225, 1248–1249. [Google Scholar] [CrossRef]
- Moeller, C.; Schmidt, C.; Guyot, F.; Wilke, M. Hydrolysis rate constants of ATP determined in situ at elevated temperatures. Biophys. Chem. 2022, 290, 106878. [Google Scholar] [CrossRef]
- Damer, B.; Deamer, D. The Hot Spring Hypothesis for an Origin of Life. Astrobiology 2020, 20, 429–452. [Google Scholar] [CrossRef]
- Corliss, J.B.; Baross, J.A.; Hoffman, S.E. An hypothesis concerning the relationship between submarine hot springs and the origin of life on Earth. Oceanol. Acta 1981, 4, 59–69. [Google Scholar]
- Darwin Correspondence Project, “Letter No. 7471”. Available online: www.darwinproject.ac.uk/DCP-LETT-7471 (accessed on 19 July 2025).
- Pasek, M.A.; Omran, A.; Feng, T.; Gull, M.; Lang, C.; Abbatiello, J.; Garong, L.; Johnston, R.; Ryan, J.; Abbott-Lyon, H. Serpentinization as a route to liberating phosphorus on habitable worlds. Geochim. Cosmochim. Acta 2022, 336, 332–340. [Google Scholar] [CrossRef]
- Lian, Y.; Jiang, H.; Feng, J.; Wang, X.; Hou, X.; Deng, P. Direct and simultaneous quantification of ATP, ADP and AMP by (1)H and (31)P Nuclear Magnetic Resonance spectroscopy. Talanta 2016, 150, 485–492. [Google Scholar] [CrossRef]
- Gull, M.; Cruz, H.A.; Krishnamurthy, R.; Pasek, M.A. Phosphorylation of nucleosides by P-N bond species generated from prebiotic reduced phosphorus sources. Commun. Chem. 2025, 8, 187. [Google Scholar] [CrossRef]
- Coulon, R.; Papoušková, B.; Mohammadi, E.; Otyepka, M.; Wunnava, S.; Šponer, J.; Šponer, J.E. Prebiotic Synthesis of 3′,5′-Cyclic Adenosine and Guanosine Monophosphates through Carbodiimide-Assisted Cyclization. Chem. Bio. Chem. 2023, 24, e202300510. [Google Scholar] [CrossRef]
- Kadoya, S.; Krissansen-Totton, J.; Catling, D.C. Probable Cold and Alkaline Surface Environment of the Hadean Earth Caused by Impact Ejecta Weathering. Geochem. Geophys. Geosyst. 2020, 21, e2019GC008734. [Google Scholar] [CrossRef]
- Trail, D.; Mojzsis, S.J.; Harrison, T.M.; Schmitt, A.K.; Watson, E.B.; Young, E.D. Constraints on Hadean zircon protoliths from oxygen isotopes, Ti-thermometry, and rare earth elements. Geochem. Geophys. Geosyst. 2007, 8, Q06014. [Google Scholar] [CrossRef]
- Garcia, A.K.; Schopf, J.W.; Yokobori, S.I.; Akanuma, S.; Yamagishi, A. Reconstructed ancestral enzymes suggest long-term cooling of Earth’s photic zone since the Archean. Proc. Natl. Acad. Sci. USA 2017, 114, 4619–4624. [Google Scholar] [CrossRef]
- Catling, D.C.; Zahnle, K.J. The Archean atmosphere. Sci. Adv. 2020, 6, eaax1420. [Google Scholar] [CrossRef]
- Lehninger, A.L.; Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry; Macmillan: New York, NY, USA, 2005. [Google Scholar]
- Jay, Z.J.; Beam, J.P.; Dlakić, M.; Rusch, D.B.; Kozubal, M.A.; Inskeep, W.P. Marsarchaeota are an aerobic archaeal lineage abundant in geothermal iron oxide microbial mats. Nat. Microbiol. 2018, 3, 732–740. [Google Scholar] [CrossRef]
- Kotopoulou, E.; Delgado Huertas, A.; Garcia-Ruiz, J.M.; Dominguez-Vera, J.M.; Lopez-Garcia, J.M.; Guerra-Tschuschke, I.; Rull, F. A Polyextreme Hydrothermal System Controlled by Iron: The Case of Dallol at the Afar Triangle. ACS Earth Space Chem. 2019, 3, 90–99. [Google Scholar] [CrossRef]
- Landrum, J.T.; Bennett, P.C.; Engel, A.S.; Alsina, M.A.; Pasten, P.A.; Milliken, K. Partitioning geochemistry of arsenic and antimony, El Tatio Geyser Field, Chile. J. Appl. Geochem. 2009, 24, 664–676. [Google Scholar] [CrossRef]
- Semenkov, I.N.; Klink, G.V.; Lebedeva, M.P.; Krupskaya, V.V.; Chernov, M.S.; Dorzhieva, O.V.; Kazinskiy, M.T.; Sokolov, V.N.; Zavadskaya, A.V. The variability of soils and vegetation of hydrothermal fields in the Valley of Geysers at Kamchatka Peninsula. Sci. Rep. 2021, 11, 11077. [Google Scholar] [CrossRef]
- Wood, C.P. Geology of the rotorua geothermal system. Geothermics 1992, 21, 25–41. [Google Scholar] [CrossRef]
- Cleaves, H.J. Prebiotic Chemistry: What We Know, What We Don’t. Evo. Edu. Outreach 2012, 5, 342–360. [Google Scholar] [CrossRef]
- Lohrmann, R.; Orgel, L.E. Prebiotic synthesis: Phosphorylation in aqueous solution. Science 1968, 161, 64–66. [Google Scholar] [CrossRef]
- Robertson, M.P.; Levy, M.; Miller, S.L. Prebiotic synthesis of diaminopyrimidine and thiocytosine. J. Mol. Evol. 1996, 43, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, J.; Ponnamperuma, C. Prebiotic condensation reactions in an aqueous medium: A review of condensing agents. Origins Life Evol. Biosph. 1976, 7, 197–224. [Google Scholar] [CrossRef] [PubMed]
- Ritson, D.J.; Poplawski, M.W.; Bond, A.D.; Sutherland, J.D. Azoles as Auxiliaries and Intermediates in Prebiotic Nucleoside Synthesis. J. Am. Chem. Soc. 2022, 144, 19447–19455. [Google Scholar] [CrossRef]
- Clark, B.C.; Kolb, V.M. Macrobiont: Cradle for the Origin of Life and Creation of a Biosphere. Life 2020, 10, 278. [Google Scholar] [CrossRef]
- Sutherland, J.D. The Origin of Life—Out of the Blue. Angew. Chem. Int. Ed. Engl. 2016, 55, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Faure, G. Principles and Applications of Geochemistry, 2nd ed.; Pearson: London, UK, 1997. [Google Scholar]
- 31P NMR of Adenosine Phosphates. Available online: https://www.aiinmr.com/wp-content/uploads/2020/06/31P-of-Adenosine-Phoshates.pdf (accessed on 30 August 2025).

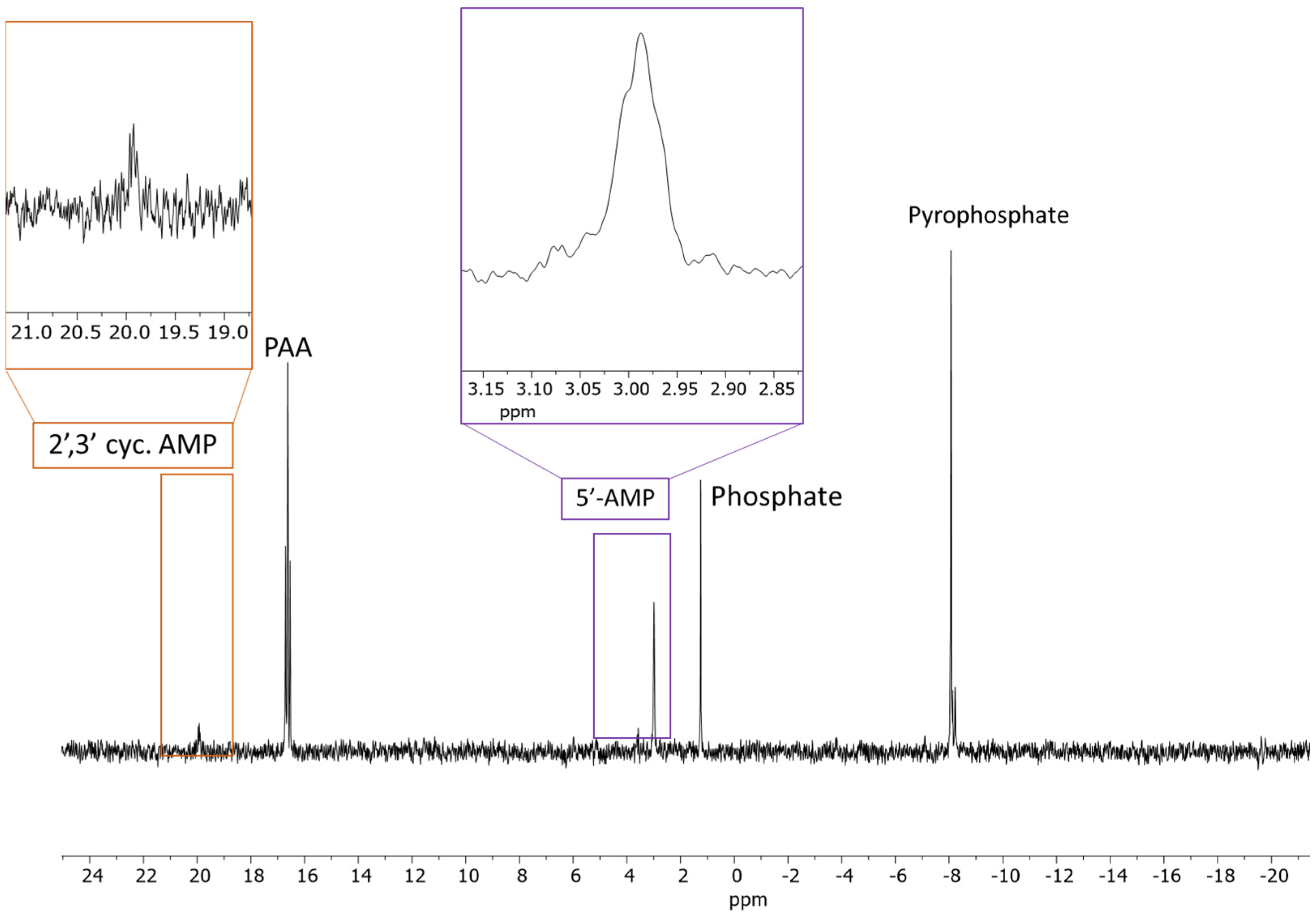

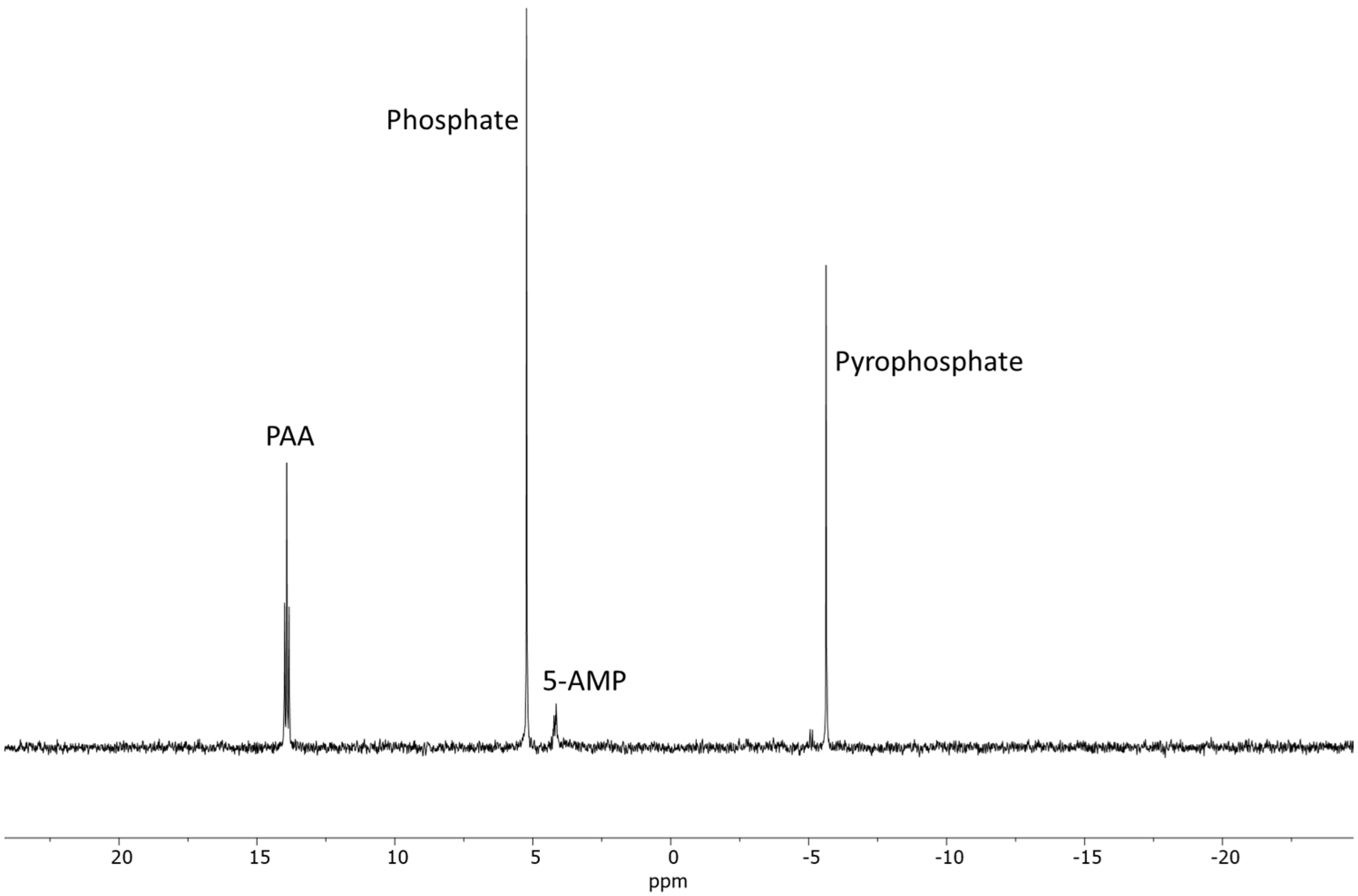
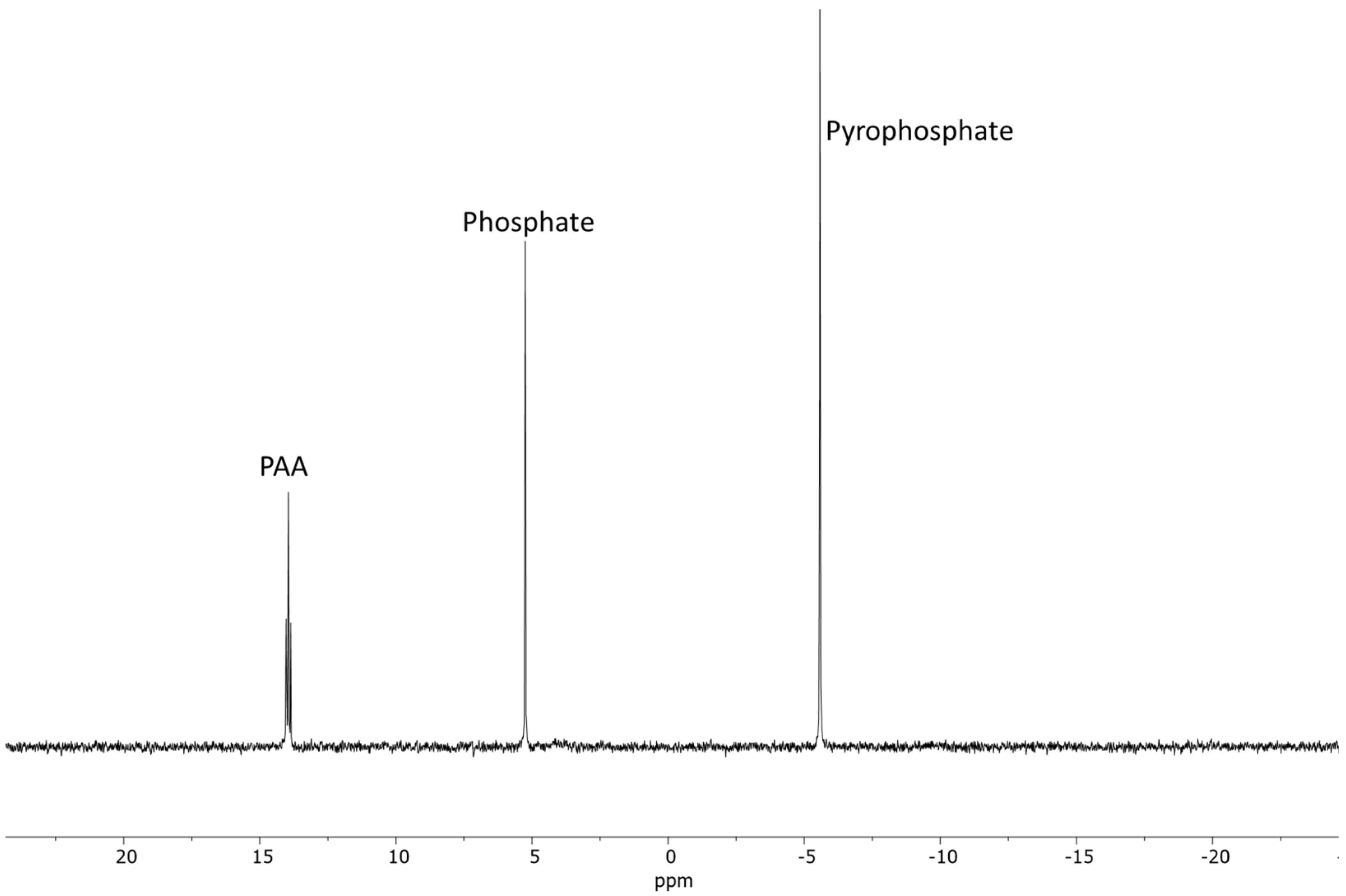
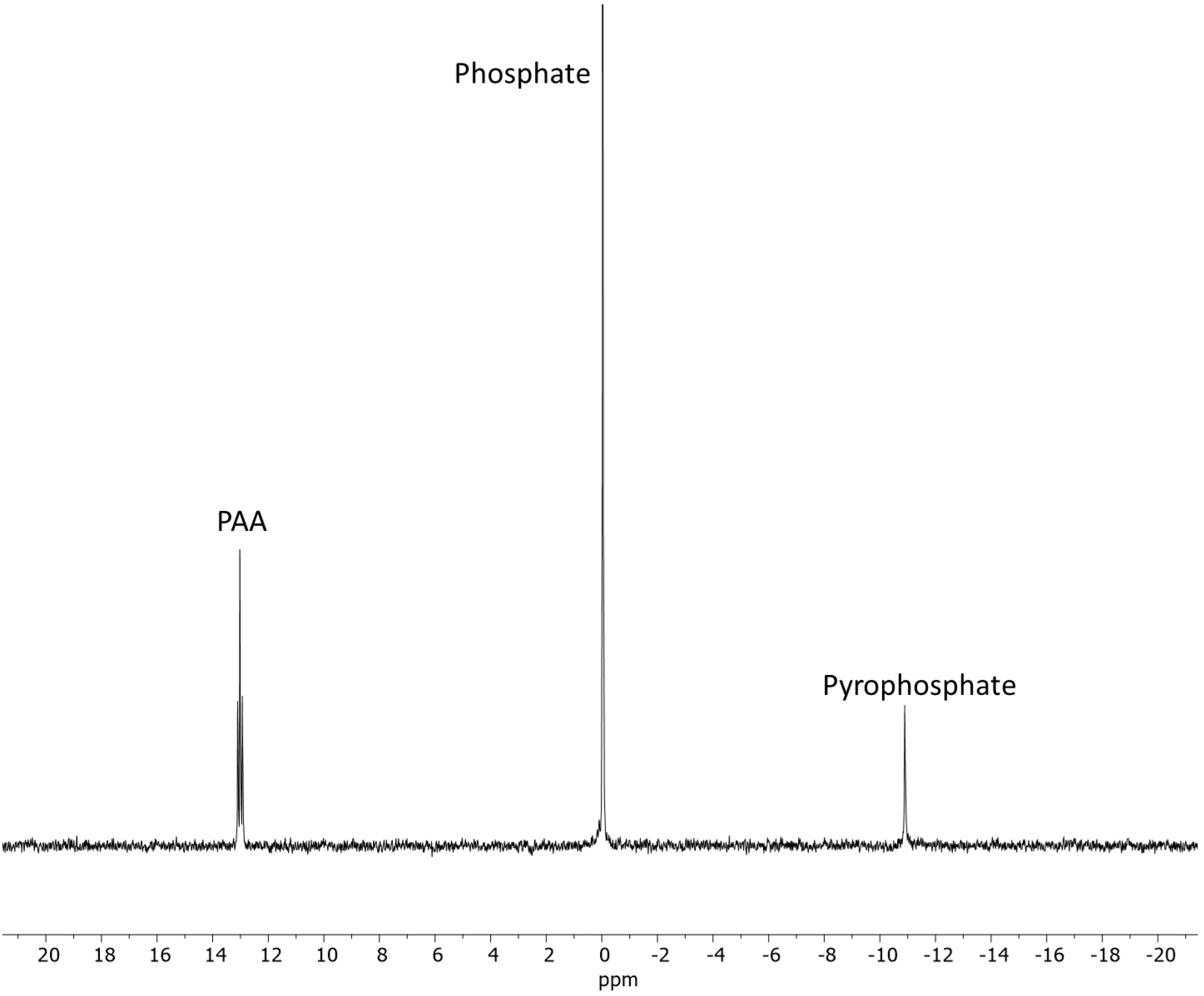
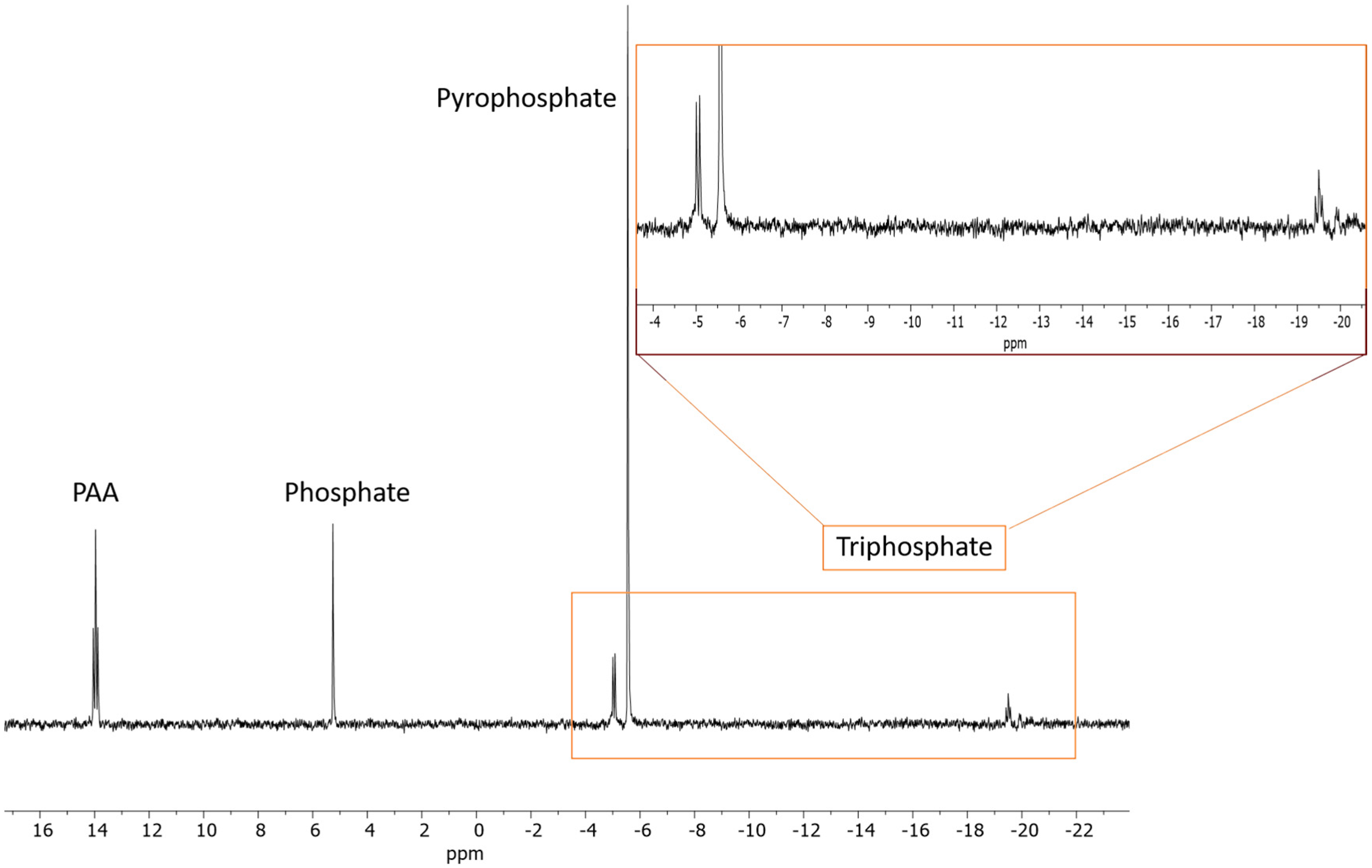

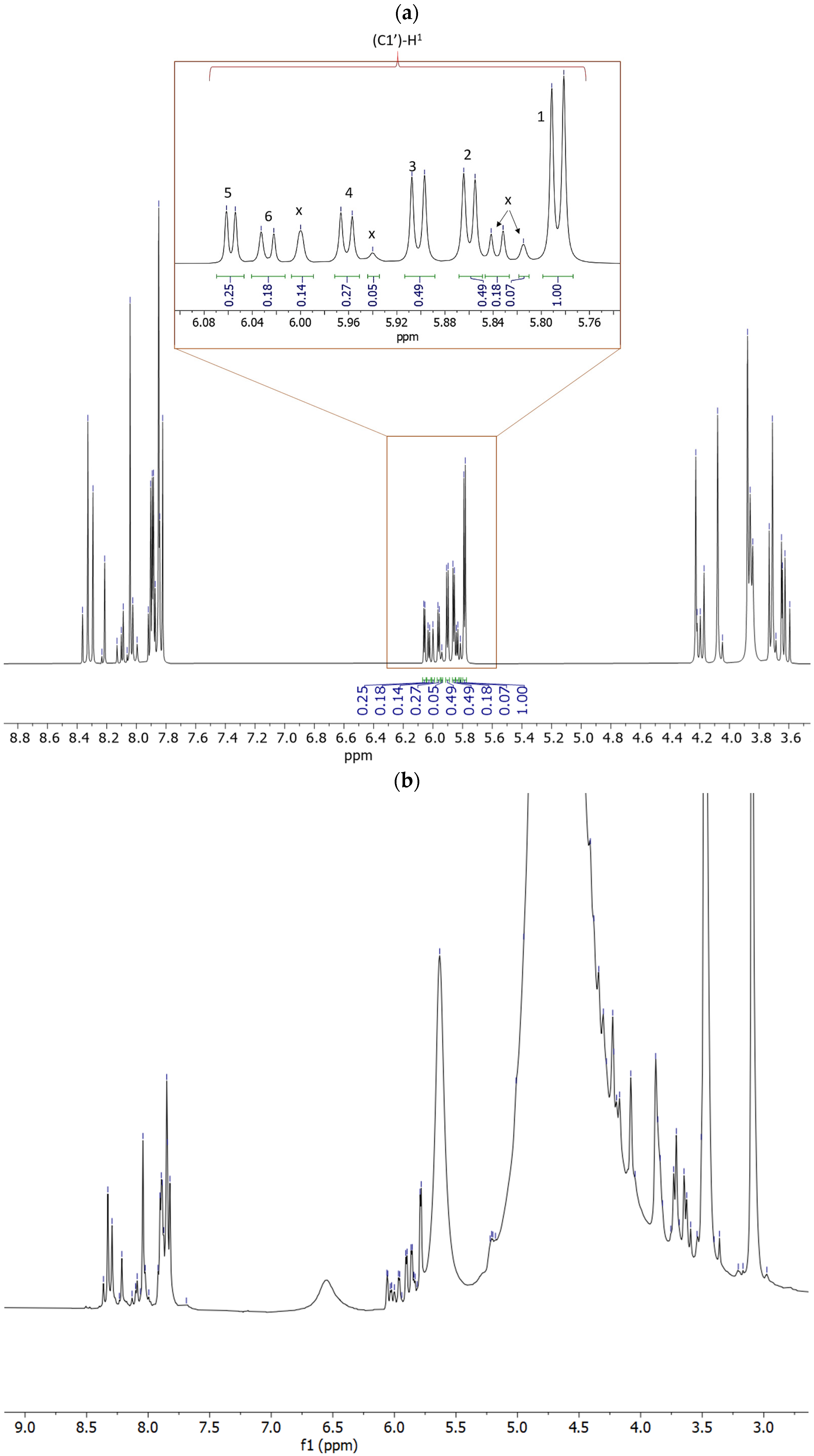
| Sample | Reaction Set No | Time | Temp. | AMP | PO43− | P2O74− | * Other P |
|---|---|---|---|---|---|---|---|
| (Days) | °C | ||||||
| AD-1 | AMP-DDI | 2 | 22–25 | 100 | ** BDL | BDL | BDL |
| AD-2 | AMP-DDI | 2 | 50–55 | 100 | BDL | BDL | BDL |
| AD-3 | AMP-DDI | 2 | 70–75 | 86 | 10 | 4 | BDL |
| AD-4 | AMP-DDI | 2 | 85–90 | BDL | 61 | 39 | BDL |
| AD-5 | AMP-DDI | 4 | 22–25 | 100 | BDL | BDL | BDL |
| AD-6 | AMP-DDI | 4 | 50–55 | 53 | 37 | 10 | BDL |
| AD-7 | AMP-DDI | 4 | 70–75 | 32 | 20 | 41 | 7 |
| AD-8 | AMP-DDI | 4 | 85–90 | BDL | 51 | 49 | BDL |
| AD-9 | AMP-SW | 2 | 22–25 | 100 | BDL | BDL | BDL |
| AD-10 | AMP-SW | 2 | 50–55 | 75 | 18 | 7 | BDL |
| AD-11 | AMP-SW | 2 | 70–75 | 42 | 26.5 | 7 | 24.5 |
| AD-12 | AMP-SW | 2 | 85–90 | BDL | 100 | BDL | BDL |
| AD-13 | AMP-SW | 4 | 22–25 | 100 | BDL | BDL | BDL |
| AD-14 | AMP-SW | 4 | 50–55 | 70 | 30 | BDL | BDL |
| AD-15 | AMP-SW | 4 | 70–75 | 11 | 51 | 38 | BDL |
| AD-16 | AMP-SW | 4 | 85–90 | BDL | 69.5 | 30.5 | BDL |
| Sample | Reaction Set No | Time | Temp. | ADP | AMP | PO43− | P2O74− | * Other P |
|---|---|---|---|---|---|---|---|---|
| (Days) | °C | |||||||
| AD-17 | ADP-DDI | 2 | 22–25 | 100 | ** BDL | BDL | BDL | BDL |
| AD-18 | ADP-DDI | 2 | 50–55 | BDL | 11.5 | 65 | 23.5 | BDL |
| AD-19 | ADP-DDI | 2 | 70–75 | BDL | 5 | 37 | 48 | 10 |
| AD-20 | ADP-DDI | 2 | 85–90 | BDL | BDL | 27 | 49 | 24 |
| AD-21 | ADP-DDI | 4 | 22–25 | 100 | BDL | BDL | BDL | BDL |
| AD-22 | ADP-DDI | 4 | 50–55 | BDL | 19 | 49 | 32 | BDL |
| AD-23 | ADP-DDI | 4 | 70–75 | BDL | 6 | 66 | 28 | BDL |
| AD-24 | ADP-DDI | 4 | 85–90 | BDL | 4 | 35 | 46.5 | 14.5 |
| AD-25 | ADP-SW | 2 | 22–25 | 100 | BDL | BDL | BDL | BDL |
| AD-26 | ADP-SW | 2 | 50–55 | BDL | 5 | 54 | 41 | BDL |
| AD-27 | ADP-SW | 2 | 70–75 | BDL | BDL | 29 | 71 | BDL |
| AD-28 | ADP-SW | 2 | 85–90 | BDL | BDL | 42 | 58 | BDL |
| AD-29 | ADP-SW | 4 | 22–25 | 100 | BDL | 0 | BDL | BDL |
| AD-30 | ADP-SW | 4 | 50–55 | BDL | BDL | 40.5 | 59.5 | BDL |
| AD-31 | ADP-SW | 4 | 70–75 | BDL | BDL | 29 | 61 | 10 |
| AD-32 | ADP-SW | 4 | 85–90 | BDL | BDL | 30 | 63 | 7 |
| Sample | Reaction Set No | Time | Temp. | ATP | ADP | AMP | PO43− | P2O74− | * Other P |
|---|---|---|---|---|---|---|---|---|---|
| (Days) | °C | ||||||||
| AD-33 | ATP-DDI | 2 | 22–25 | 98 | ** BDL | BDL | 2 | BDL | BDL |
| AD-34 | ATP-DDI | 2 | 50–55 | 5 | 10 | 4 | 26 | 29 | 26 |
| AD-35 | ATP-DDI | 2 | 70–75 | BDL | 10 | BDL | 23 | 43 | 24 |
| AD-36 | ATP-DDI | 2 | 85–90 | BDL | BDL | BDL | 83 | 17 | BDL |
| AD-37 | ATP-DDI | 4 | 22–25 | 96 | 2 | BDL | 2 | BDL | BDL |
| AD-38 | ATP-DDI | 4 | 50–55 | BDL | BDL | 6 | 40.5 | 45.5 | 8 |
| AD-39 | ATP-DDI | 4 | 70–75 | BDL | BDL | BDL | 76 | 24 | BDL |
| AD-40 | ATP-DDI | 4 | 85–90 | BDL | BDL | BDL | 30 | 70 | BDL |
| AD-41 | ATP-SW | 2 | 22–25 | 99 | BDL | BDL | 1 | BDL | BDL |
| AD-42 | ATP-SW | 2 | 50–55 | 2 | 5 | BDL | 31 | 62 | BDL |
| AD-43 | ATP-SW | 2 | 70–75 | BDL | BDL | BDL | 40 | 60 | BDL |
| AD-44 | ATP-SW | 2 | 85–90 | BDL | BDL | BDL | 17 | 65 | 18 |
| AD-45 | ATP-SW | 4 | 22–25 | 98.5 | BDL | BDL | 1.5 | BDL | BDL |
| AD-46 | ATP-SW | 4 | 50–55 | BDL | BDL | BDL | 31.5 | 68.50 | BDL |
| AD-47 | ATP-SW | 4 | 70–75 | BDL | BDL | BDL | 45 | 55 | BDL |
| AD-48 | ATP-SW | 4 | 85–90 | BDL | BDL | BDL | 24 | 67 | 9 |
| Additive | 2′-AMP | 3′-AMP | 5′-AMP | 2′,3′-AMP | 2′,3′+5′-AMP | c X | b Total Yields |
|---|---|---|---|---|---|---|---|
| 1H-NMR based a yields (%) of various adenosine P compounds | |||||||
 (3.33 mmoles) | 15.70 | 15.70 | 8.65 | 8 | 5.7 | 14 | 68 |
 (4.75 mmoles) | 12 | 15 | 22.77 | 6.5 | 2.7 | 2.31 | 61.2 |
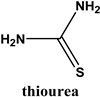 2.62 mmoles | 3.17 | 2.38 | 11.11 | 2.38 | 1 | 0.5 | 20.54 |
 2.93 mmoles | BDL | BDL | 18 | BDL | BDL | BDL | 18 |
| None | BDL | BDL | BDL | BDL | BDL | BDL | BDL |
| Compound | Conditions | Half-Life (Days) |
|---|---|---|
| AMP | 50–55 °C, DDI | 4.37 |
| AMP | 70–75 °C, DDI | 2.43 |
| AMP | 50–55 °C, SW | 7.78 |
| AMP | 70–75 °C, SW | 1.2 |
| ATP | 22–25 °C, DDI | 54 |
| ATP | 50–55 °C, DDI | 0.5 |
| ATP | 22–25 °C SW | 183 |
| Medium | AMP Activation Energy (J/mol) | ATP Activation Energy (J/mol) |
|---|---|---|
| DDI | 27.8 | 135.6 |
| Saline | 88.6 | 169.4 |
| Additive | Rate of Synthesis (g/days) | Steady State Maxima (g) | Concentration (g/mL) |
|---|---|---|---|
| Urea | 0.073 | 0.26 | 0.051 |
| Cyanamide | 0.065 | 0.23 | 0.046 |
| Thiourea | 0.022 | 0.08 | 0.015 |
| Imidazole | 0.019 | 0.07 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gull, M.; Mehta, C.; Perez, M.J.H.; Seeley, A.; Rogers, K.L.; Pasek, M.A. Thermal Decomposition and Prebiotic Formation of Adenosine Phosphates in Simulated Early-Earth Evaporative Settings. Molecules 2025, 30, 3587. https://doi.org/10.3390/molecules30173587
Gull M, Mehta C, Perez MJH, Seeley A, Rogers KL, Pasek MA. Thermal Decomposition and Prebiotic Formation of Adenosine Phosphates in Simulated Early-Earth Evaporative Settings. Molecules. 2025; 30(17):3587. https://doi.org/10.3390/molecules30173587
Chicago/Turabian StyleGull, Maheen, Christopher Mehta, Maria Jesus Herrero Perez, Annika Seeley, Karyn L. Rogers, and Matthew A. Pasek. 2025. "Thermal Decomposition and Prebiotic Formation of Adenosine Phosphates in Simulated Early-Earth Evaporative Settings" Molecules 30, no. 17: 3587. https://doi.org/10.3390/molecules30173587
APA StyleGull, M., Mehta, C., Perez, M. J. H., Seeley, A., Rogers, K. L., & Pasek, M. A. (2025). Thermal Decomposition and Prebiotic Formation of Adenosine Phosphates in Simulated Early-Earth Evaporative Settings. Molecules, 30(17), 3587. https://doi.org/10.3390/molecules30173587







