Unlocking New Potential in the Functionalization of Chlorinated Silsesquioxanes: A Rapid and Chemoselective Thiolation Method
Abstract
1. Introduction
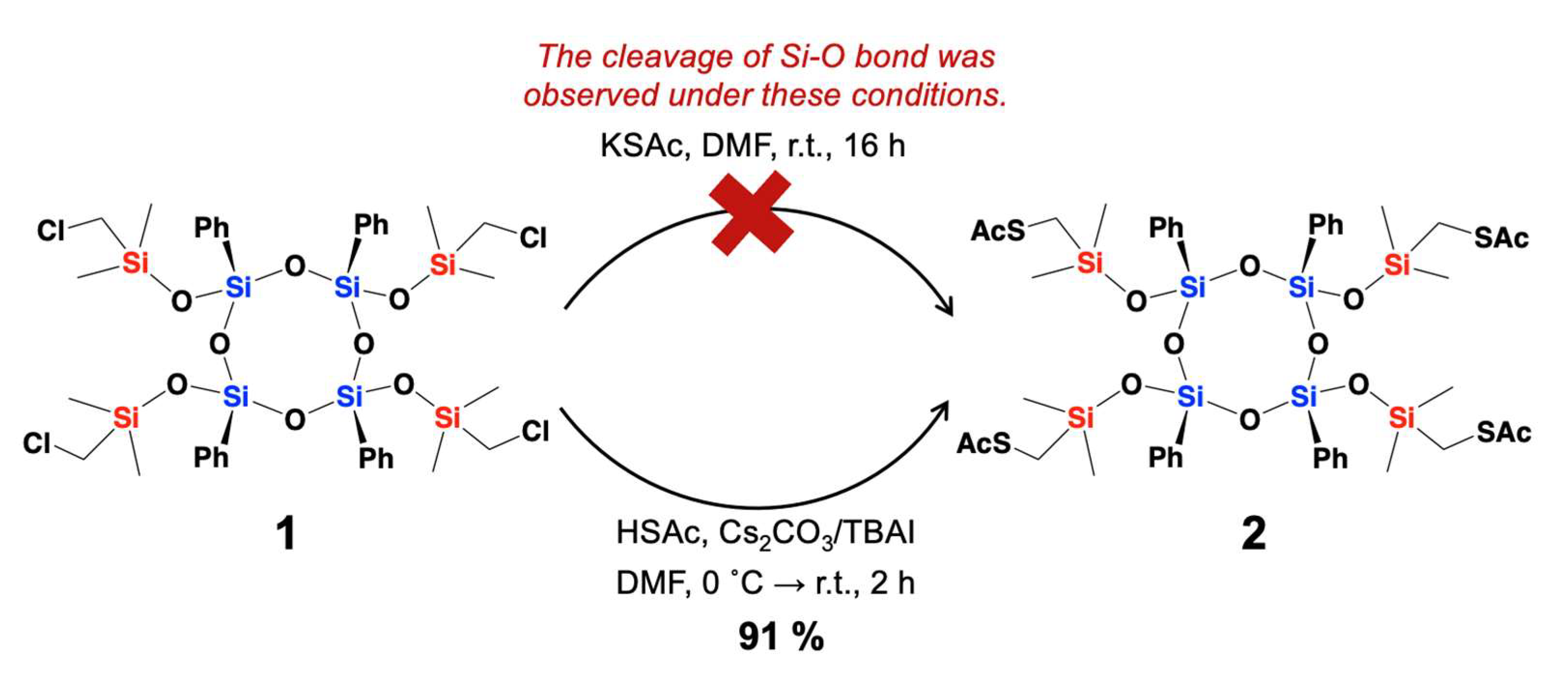
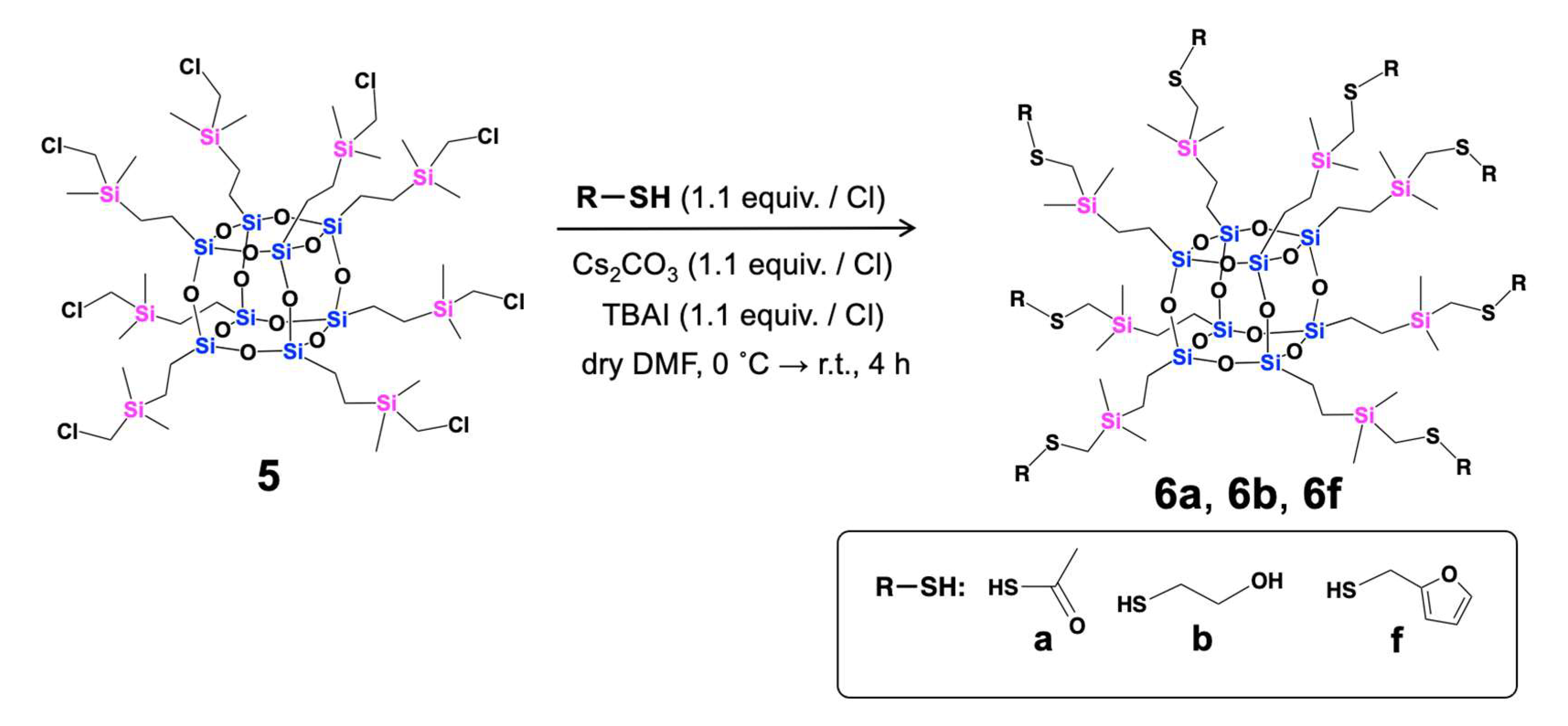
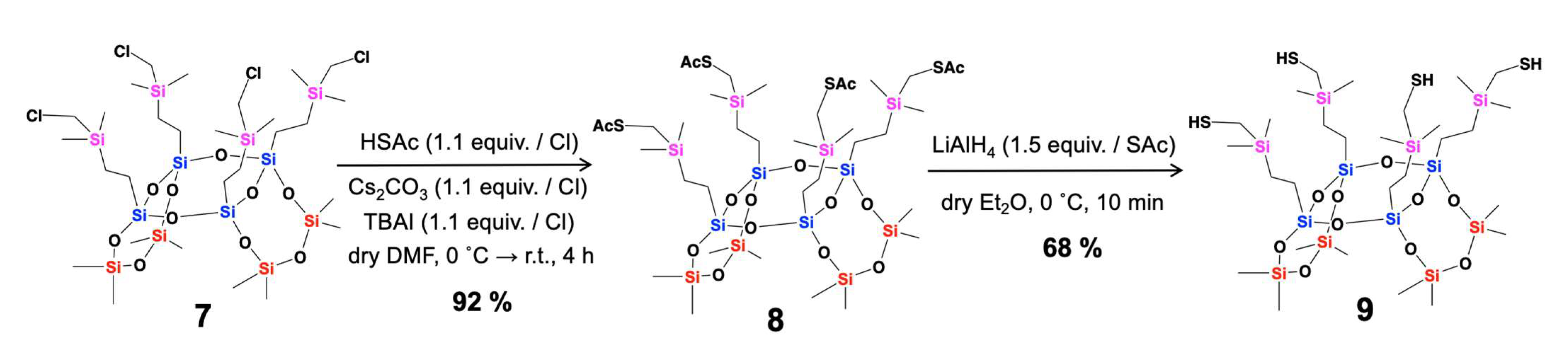
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations
3.2. Experimental Procedures for Compounds 2, 4a–4f, 6a, 6b, 6f, 8, and 9
- a.
- Synthetic Procedure for Compound 2
- b.
- General Synthetic Procedure for Compounds 4a–4f
- c.
- General Synthetic Procedure for Compounds 6a, 6b, and 6f
- d.
- Synthetic Procedure for Compound 8
- e.
- Synthetic Procedure for Compound 9
3.3. Characterization Data for Compounds 2, 4a–4f, 6a, 6b, 6f, 8, and 9
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzuki, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Kickelbick, G. Silsesquioxanes. In Functional Molecular Silicon Compounds I; Scheschkewitz, D., Ed.; Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2014; Volume 155, pp. 1–28. [Google Scholar]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Laine, R.M.; Roll, M.F. Polyhedral Phenylsilsesquioxanes. Macromolecules 2011, 44, 1073–1109. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Chen, F.; Lin, F.; Zhang, Q.; Cai, R.; Wu, Y.; Ma, X. Polyhedral Oligomeric Silsesquioxane Hybrid Polymers: Well-Defined Architectural Design and Potential Functional Applications. Macromol. Rapid Commun. 2019, 40, 1900101. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H. Cage-like silsesquioxanes-based hybrid materials. Dalton Trans. 2020, 49, 5396–5405. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Aprile, C.; Gruttadauria, M.; Giacalone, F. POSS nanostructures in catalysis. Catal. Sci. Technol. 2020, 10, 7415–7447. [Google Scholar] [CrossRef]
- Unno, M.; Alias, S.B.; Saito, H.; Matsumoto, H. Synthesis of Hexasilsesquioxanes Bearing Bulky Substituents: Hexakis((1,1,2-trimethylpropyl)silsesquioxane) and Hexakis(tert-butylsilsesquioxane). Organometallics 1996, 15, 2413–2414. [Google Scholar] [CrossRef]
- Chimjarn, S.; Kunthom, R.; Chancharone, P.; Sodkhomkhum, R.; Sangtrirutnugul, P.; Ervithayasuporn, V. Synthesis of aromatic functionalized cage-rearranged silsesquioxanes (T8, T10, and T12) via nucleophilic substitution reactions. Dalton Trans. 2015, 44, 916–919. [Google Scholar] [CrossRef]
- Hanprasit, S.; Tungkijanansin, N.; Prompawilai, A.; Eangpayung, S.; Ervithayasuporn, V. Synthesis and isolation of non-chromophore cage-rearranged silsesquioxanes from base-catalyzed reactions. Dalton Trans. 2016, 45, 16117–16120. [Google Scholar] [CrossRef]
- Furgal, J.C.; Goodson, T., III; Laine, R.M. D5h[PhSiO1.5]10 synthesis via F− catalyzed rearrangement of [PhSiO1.5]n. An experimental/computational analysis of likely reaction pathways. Dalton Trans. 2016, 45, 1025–1039. [Google Scholar] [CrossRef]
- Wang, H.; Nie, M.-X.; Lin, X.; Li, X.-Q.; Liu, H.; Guo, Q.-Y.; Han, D.; Fu, Q. Cage-rearranged and cage-intact syntheses of azido-functionalized larger T10 and T12 POSSs. Dalton Trans. 2024, 53, 9467–9472. [Google Scholar] [CrossRef] [PubMed]
- Dudziec, B.; Marciniec, B. Double-decker Silsesquioxanes: Current Chemistry and Applications. Curr. Org. Chem. 2017, 21, 2794–2813. [Google Scholar] [CrossRef]
- Unno, M.; Suto, A.; Matsumoto, T. Laddersiloxanes—Silsesquioxanes with defined ladder structure. Russ. Chem. Rev. 2013, 82, 289–302. [Google Scholar] [CrossRef]
- Liu, Y.; Chaiprasert, T.; Ouali, A.; Unno, M. Well-defined cyclic silanol derivatives. Dalton Trans. 2022, 51, 4227–4245. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Nie, M.-X.; Liu, H.; Zhou, D.-L.; Fu, S.-R.; Zhang, Q.; Han, D.; Fu, Q. Topology-Enabled Simultaneous Enhancement of Mechanical and Healable Properties in Glassy Polymeric Materials Using Larger POSS. Chem. Mater. 2024, 36, 575–584. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Z.; Zhang, M.; Yang, F.; Zhang, W.; Wu, X.; Yang, R. Facile Synthesis of Alkali Metal Polyhedral Oligomeric Silsesquioxane Salt and Its Application in Flame-Retardant Epoxy Resins. ACS Appl. Polym. Mater. 2023, 5, 3848–3857. [Google Scholar] [CrossRef]
- Chaudhuri, H.; Lim, C.-R.; Yun, Y.-S. Single-step synthesis of prominently selective and easily regenerable POSS functionalized with high loadings of sulfur and carboxylic acids. J. Mater. Chem. A 2023, 11, 23463–23478. [Google Scholar] [CrossRef]
- Dascalu, M.; Stoica, A.-C.; Bele, A.; Macslm, A.-M.; Bargan, A.; Varganici, C.-D.; Stiubianu, G.-T.; Racles, C.; Shova, S.; Cazacu, M. Octakis(Carboxyalkyl-Thioethyl)Silsesquioxanes and Derived Metal Complexes: Synthesis, Characterization and Catalytic Activity Assessements. J. Inorg. Organomet. Polym. 2022, 32, 3955–3970. [Google Scholar] [CrossRef]
- Zhou, D.-L.; Yang, D.; Han, D.; Zhang, Q.; Chen, F.; Fu, Q. Fabrication of superhydrophilic and underwater superoleophobic membranes for fast and effective oil/water separation with excellent durability. J. Membr. Sci. 2021, 620, 118898. [Google Scholar] [CrossRef]
- Dare, E.O.; Vendrell-Criado, V.; Jiménez, M.C.; Pérez-Ruiz, R.; Díaz Díaz, D. Highly efficient latent fingerprint detection by eight-dansyl-functionalized octasilsesquioxane nanohybrids. Dyes Pigments 2021, 184, 108841. [Google Scholar] [CrossRef]
- Ren, J.; Feng, J.; Wang, L.; Chen, G.; Zhou, Z.; Li, Q. High specific surface area hybrid silica aerogel containing POSS. Microporous Mesoporous Mater. 2021, 310, 110456. [Google Scholar] [CrossRef]
- Gan, Z.; Kong, D.; Yu, Q.; Jia, Y.; Dong, X.-H.; Wang, L. Fabrication superhydrophobic composite membranes with hierarchical geometries and low-surface-energy modifications. Polymer 2020, 211, 123097. [Google Scholar] [CrossRef]
- An, Z.; Chen, S.; Tong, X.; He, H.; Han, J.; Ma, M.; Shi, Y.; Wang, X. Widely applicable AIE Chemosensor for On-Site Fast Detection of Drugs Based on the POSS-Core Dendrimer with the Controlled Self-Assembly Mechanism. Langmuir 2019, 35, 2649–2654. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fu, Y.; Li, Z.; Nan, N.; Zhu, Y.; Li, Y. Froth flotation giant surfactants. Polymer 2019, 162, 58–62. [Google Scholar] [CrossRef]
- Zheng, P.; Zhang, Z.; Jiang, X.; Rui, L.; Gao, Y.; Zhang, W. Unimolecular micelles from POSS-based star-shaped block copolymers for photodynamic therapy. Polymer 2017, 118, 268–279. [Google Scholar] [CrossRef]
- Strauch, H.; Engelmann, J.; Scheffler, K.; Mayer, H.A. A simple approach to a new T8-POSS based MRI contrast agent. Dalton Trans. 2016, 45, 15104–15113. [Google Scholar] [CrossRef]
- Piorecka, K.; Radzikowska, E.; Kurjata, J.; Rozga-Wijas, K.; Stanczyk, W.A.; Wielgus, E. Synthesis of the first POSS cage-anthracycline conjugates via amide bonds. New J. Chem. 2016, 40, 5997–6000. [Google Scholar] [CrossRef]
- Han, S.-Y.; Wang, X.-M.; Shao, Y.; Guo, Q.-Y.; Li, Y.; Zhang, W.-B. Janus POSS Based on Mixed [2:6] Octakis-Adduct Regioisomers. Chem. Eur. J. 2016, 22, 6397–6403. [Google Scholar] [CrossRef]
- Wang, K.; Peng, H.; Thurecht, K.J.; Whittaker, A.K. Fluorinated POSS-Star Polymers for 19F MRI. Macromol. Chem. Phys. 2016, 217, 2262–2274. [Google Scholar] [CrossRef]
- Li, L.; Lu, B.; Fan, Q.; Wu, J.; Wei, L.; Hou, J.; Guo, X.; Liu, Z. Synthesis and self-assembly behavior of pH-responsive star-shaped POSS-(PCL-P(DMAEMA-co-PEGMA))16 inorganic/organic hybrid block copolymer for the controlled intracellular delivery of doxorubicin. RSC Adv. 2016, 6, 61630–61640. [Google Scholar] [CrossRef]
- Bivona, L.A.; Fichera, O.; Fusaro, L.; Giacalone, F.; Buaki-Sogo, M.; Gruttadauria, M.; Aprile, C. A polyhedral oligomeric silsesquioxane-based catalyst for the efficient synthesis of cyclic carbonates. Catal. Sci. Technol. 2015, 5, 5000–5007. [Google Scholar] [CrossRef]
- Wang, X.; Chin, J.M.; He, C.; Xu, J. Highly Thermally Resistant Polyhedral Oligomeric Silsesquioxanes Lubricating Oil Prepared via a Thiol-Ene Click Reaction. Sci. Adv. Mater. 2014, 6, 1553–1561. [Google Scholar] [CrossRef]
- Liu, Y.; Kigure, M.; Okawa, R.; Takeda, N.; Unno, M.; Ouali, A. Synthesis and characterization of tetrathiol-substituted double-decker or ladder silsesquioxane nano-cores. Dalton Trans. 2021, 50, 3473–3478. [Google Scholar] [CrossRef]
- Krizhanovskiy, I.N.; Frank, I.V.; Shkinev, P.D.; Khanin, D.A.; Malakhova, Y.N.; Temnikov, M.N.; Anisimov, A. Janus star-shaped siloxane polymers with oriented alkoxy functional groups. Synthesis and properties. J. Organomet. Chem. 2024, 1022, 123374. [Google Scholar] [CrossRef]
- Krizhanovskiy, I.; Temnikov, M.; Drozdov, F.; Peregudov, A.; Anisimov, A. Sequential Hydrothiolation-hydrosilylation: A route to the creation of new organosilicon compounds with preset structures. React. Chem. Eng. 2023, 8, 1005–1014. [Google Scholar] [CrossRef]
- Vysochinskaya, Y.; Anisimov, A.; Krylov, F.; Buzin, M.; Buzin, A.; Peregudov, A.; Shchegolikhina, O.; Muzafarov, A. Synthesis of functional derivatives of stereoregular organocyclosilsesquioxanes by thiol-ene addition. J. Organomet. Chem. 2021, 954–955, 122072. [Google Scholar] [CrossRef]
- Zhang, X.; Haxton, K.J.; Ropartz, L.; Cole-Hamilton, D.J.; Morris, R.E. Synthesis and computer modelling of hydroxy-derivatised carbosilane dendrimers based on polyhedral silsesquioxane cores. J. Chem. Soc. Dalton Trans. 2001, 3261–3268. [Google Scholar] [CrossRef]
- Tang, P.; Xiang, K.; Wei, H.; Li, S.; Xu, C. A high-emissive and stable luminescent silafluorene hybrid constructed by hydrosilylation. J. Chem. Res. 2019, 43, 144–148. [Google Scholar] [CrossRef]
- Zhan, X.; Lu, J.; Xu, H.; Liu, J.; Liu, X.; Cao, X.; Li, J. Enhanced pervaporation performance of PDMS membranes based on nano-sized Octa[(trimethoxysilyl)ethyl]-POSS as macro-crosslinker. Appl. Surf. Sci. 2019, 473, 785–798. [Google Scholar] [CrossRef]
- Herrero, M.; Alonso, B.; Losada, J.; García-Armada, P.; Casado, C.M. Ferrocenyl Dendrimers Based on Octasilsesquioxane Cores. Organometallics 2012, 31, 6344–6350. [Google Scholar] [CrossRef]
- Mrzygłód, A.; Januszewski, R.; Duszczak, J.; Dutkiewicz, M.; Kubicki, M.; Dudziec, B. Tricky but repeatable synthetic approach to branched, multifunctional silsesquioxane dendrimer derivatives. Inorg. Chem. Front. 2023, 10, 4587–4596. [Google Scholar] [CrossRef]
- Liu, Y.; Koizumi, K.; Takeda, N.; Unno, M.; Ouali, A. Synthesis of Octachloro- and Octaazido-Functionalized T8-Cages and Application to Recyclable Palladium Catalyst. Inorg. Chem. 2022, 61, 1495–1503. [Google Scholar] [CrossRef]
- Liu, Y.; Kigure, M.; Koizumi, K.; Takeda, N.; Unno, M.; Ouali, A. Synthesis of Tetrachloro, Tetraiodo, and Tetraazido Double-Decker Siloxanes. Inorg. Chem. 2020, 59, 15478–15486. [Google Scholar] [CrossRef]
- Liu, Y.; Endo, A.; Zhang, P.; Takizawa, A.; Takeda, N.; Ouali, A.; Unno, M. Synthesis, Characterization, and Reaction of Divinyl-substituted Laddersiloxanes. Silicon 2022, 14, 2723–2730. [Google Scholar] [CrossRef]
- Liu, Y.; Katano, M.; Yingsukkamol, P.; Takeda, N.; Unno, M.; Ouali, A. Tricyclic 6-8-6 laddersiloxanes derived from all-cis-tetravinylcyclotetrasiloxanolate: Synthesis, characterization and reactivity. J. Organomet. Chem. 2022, 959, 122213. [Google Scholar] [CrossRef]
- Liu, Y.; Tokuda, M.; Takeda, N.; Ouali, A.; Unno, M. New Janus Tricyclic Laddersiloxanes: Synthesis, Characterization, and reactivity. Molecules 2023, 28, 5699. [Google Scholar] [CrossRef]
- Zheng, Z.; Yagafarov, N.; Xu, Z.; Ouali, A.; Takeda, N.; Liu, Y.; Unno, M. BINOL and triazole-containing Janus rings and 29-8-29-membered tricyclic ladder-type hybridized siloxane: Application in the fluorescence sensing of anions. Dalton Trans. 2023, 52, 10298–10304. [Google Scholar] [CrossRef]
- Cheng, G.; Vautravers, N.R.; Morris, R.E.; Cole-Hamilton, D.J. Synthesis of functional cubes from octavinylsilsesquioxane (OVS). Org. Biomol. Chem. 2008, 6, 4662–4667. [Google Scholar] [CrossRef] [PubMed]
- André, P.; Cheng, G.; Ruseckas, A.; van Mourik, T.; Früchtl, H.; Crayston, J.A.; Morris, R.E.; Cole-Hamilton, D.; Samuel, I.D. Hybrid Dendritic Molecules with Confined Chromophore Architecture to Tune Fluorescence Efficiency. J. Phys. Chem. B 2008, 112, 16382–16392. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.; Bhaskar, A.; Zhang, J.; Guda, R.; Goodson, T., III; Laine, R.M. Molecules with Perfect Cubic Symmetry as Nanobuilding Blocks for 3-D Assemblies. Elaboration of Octavinylsilsesquioxane. Unusual Luminescence Shifts May Indicate Extended Conjugation Involving the Silsesquioxane Core. Chem. Mater. 2008, 20, 5563–5573. [Google Scholar] [CrossRef]
- Kawakami, Y.; Sakuma, Y.; Wakuda, T.; Nakai, T.; Shirasaka, M.; Kabe, Y. Hydrogen-Bonding 3D Networks by Polyhedral Organosilanols: Selective Inclusion of Hydrocarbons in Open Frameworks. Organometallics 2010, 29, 3281–3288. [Google Scholar] [CrossRef]
- Furgal, J.C.; Jung, J.H.; Goodson, T., III; Laine, R.M. Analyzing Structure-Photophysical Property Relationships for Isolated T8, T10, and T12 Stilbenevinylsilsesquioxanes. J. Am. Chem. Soc. 2013, 135, 12259–12269. [Google Scholar] [CrossRef]
- Zak, P.; Dudziec, B.; Kubicki, M.; Marciniec, B. Silylative Coupling versus Metathesis-Efficient Methods for the Synthesis of Difunctionalized Double-Decker Silsesquioxane Derivatives. Chem. Eur. J. 2014, 20, 9387–9393. [Google Scholar] [CrossRef]
- Zak, P.; Dudziec, B.; Dutkiewicz, M.; Ludwiczak, M.; Marciniec, B.; Nowicki, M. A New Class of Stereoregular Vinylene-Arylene Copolymers with Double-Decker Silsesquioxane in the Main Chain. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1044–1055. [Google Scholar] [CrossRef]
- Zak, P.; Majchrzak, M.; Wilkowski, G.; Dudziec, B.; Dutkiewicz, M.; Marciniec, B. Synthesis and characterization of functionalized molecular and macromolecular double-decker silsesquioxane systems. RSC Adv. 2016, 6, 10054–10063. [Google Scholar] [CrossRef]
- Lo, M.Y.; Zhen, C.; Lauters, M.; Jabbour, G.E.; Sellinger, A. Organic-Inorganic Hybrids Based on Pyrene Functionalized Octavinylsilsesquioxane Cores for Application in OLEDs. J. Am. Chem. Soc. 2007, 129, 5808–5809. [Google Scholar] [CrossRef]
- Chanmungkalakul, S.; Ervithayasuporn, V.; Hanprasit, S.; Masik, M.; Prigyai, N.; Kiatkamjornwong, S. Silsesquioxane cages as fluoride sensors. Chem. Commun. 2017, 53, 12108–12111. [Google Scholar] [CrossRef] [PubMed]
- Chanmungkalakul, S.; Ervithayasuporn, V.; Boonkitti, P.; Phuekphong, A.; Prigyai, N.; Kladsomboon, S.; Kiatkamjornwong, S. Anion identification using silsesquioxane cages. Chem. Sci. 2018, 9, 7753–7765. [Google Scholar] [CrossRef]
- Liu, N.; Wei, K.; Wang, L.; Zheng, S. Organic-inorganic polyimides with double decker silsesquioxane in the main chains. Polym. Chem. 2016, 7, 1158–1167. [Google Scholar] [CrossRef]
- Liu, N.; Li, L.; Wang, L.; Zheng, S. Organic-inorganic polybenzoxazine copolymers with double decker silsesquioxanes in the main chains: Synthesis and thermally activated ring-opening polymerization behavior. Polymer 2017, 109, 254–265. [Google Scholar] [CrossRef]
- Guan, J.; Tomobe, K.; Madu, I.; Goodson, T., III; Makhal, K.; Trinh, M.T.; Rand, S.C.; Yodsin, N.; Jungsuttiwong, S.; Laine, R.M. Photophysical Properties of Functionalized Double Decker Phenylsilsesquioxane Macromonomers: [PhSiO1.5]8[OSiMe2]2 and [PhSiO1.5]8[O0.5SiMe3]4. Cage-Centered Lowest Unoccupied Molecular Orbitals Form Even When Two Cage Edge Bridges Are Removed, Verified by Modeling and Ultrafast Magnetic Light Scattering Experiments. Macromolecules 2019, 52, 7413–7422. [Google Scholar]
- Guan, J.; Arias, J.J.R.; Tomobe, K.; Ansari, R.; Marques, M.d.F.V.; Rebane, A.; Mahbub, S.; Furgal, J.C.; Yodsin, N.; Jungsuttiwong, S.; et al. Unconventional Conjugation via vinylMeSi(O-)2 Siloxane Bridges May Imbue Semiconducting properties in [vinyl(Me)SiO(PhSiO1.5)8OSi(Me)vinyl-Ar] Double-Decker Copolymers. ACS Appl. Polym. Mater. 2020, 2, 3894–3907. [Google Scholar] [CrossRef]
- Guan, J.; Sun, Z.; Ansari, R.; Liu, Y.; Endo, A.; Unno, M.; Ouali, A.; Mahbub, S.; Furgal, J.C.; Yodsin, N.; et al. Conjugated Copolymers That Shouldn’t Be. Angew. Chem. Int. Ed. 2021, 60, 11115–11119. [Google Scholar] [CrossRef] [PubMed]
- Panisch, R.; Bassindale, A.R.; Korlyukov, A.A.; Pitak, M.B.; Coles, S.J.; Tayler, P.G. Selective Derivatization and Characterization of Bifunctional “Janus-Type” Cyclotetrasiloxanes. Organometallics 2013, 32, 1732–1742. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H. Triazine-Functionalized Silsesquioxane-Based Hybrid Porous Polymers for Efficient Photocatalytic Degradation of Both Acidic and Basic Dyes under Visible Light. ChemCatChem 2021, 13, 5178–5190. [Google Scholar] [CrossRef]
- Wu, Y.; Li, L.; Feng, S.; Liu, H. Hybrid nanocomposites based on novolac resin and octa(phenethyl)polyhedral oligomeric silsesquioxanes (POSS): Miscibility, specific interactions and thermomechanical properties. Polym. Bull. 2013, 70, 3261–3277. [Google Scholar] [CrossRef]
- Yang, X.; Liu, H. Ferrocene-Functionalized Silsesquioxane-Based Porous Polymer for Efficient Removal of Dyes and Heavy Metal Ions. Chem. Eur. J. 2018, 24, 13504–13511. [Google Scholar] [CrossRef]
- Meng, X.; Liu, Y.; Wang, S.; Du, J.; Ye, Y.; Song, X.; Liang, Z. Silsesquioxane-Carbazole-Corbelled Hybrid Porous Polymers with Flexible Nanopores for Efficient CO2 Conversion and Luminescence Sensing. ACS Appl. Polym. Mater. 2020, 2, 189–197. [Google Scholar] [CrossRef]
- Ito, K.; Liu, Y.; Thai, A.N.; Takahashi, M.; Koizumi, K.; Yagafarov, N.; Petit, E.; Sene, S.; Larionova, J.; Guari, Y.; et al. Structural effects of thiolated silsesquioxane ligands on the stabilization of gold nanoparticles: Implications for the catalytic dehydrogenation of alcohols. J. Catal. 2025, 450, 116342. [Google Scholar] [CrossRef]
- Sun, C.; Liu, H. Room temperature route to silsesquioxane-based porphyrin functional NIR porous polymer for efficient photodegradation of azo-dyes under sunlight. Next Mater. 2024, 2, 100123. [Google Scholar] [CrossRef]
- Hao, B.; Luo, Y.; Chan, W.; Cai, L.; Lyu, S.; Luo, Z. Fabrication of a multiple-self-healing and self-cleaning polymer coating for mechanical-damaged optical glass surface. Chem. Eng. J. 2024, 496, 153750. [Google Scholar] [CrossRef]
- Li, W.; Liu, H. Novel organic-inorganic hybrid polymer based on fluorinated polyhedral oligomeric silsesquioxanes for stable superamphiphobic fabrics and aluminum corrosion protection. Mater. Today Chem. 2023, 29, 101390. [Google Scholar] [CrossRef]
- He, Y.; Jiang, T.; Li, C.; Zhou, C.; Yang, G.; Nie, J.; Wang, F.; Lu, C.; Yin, D.; Yang, X.; et al. Thiol-ene-mediated degradable POSS-PEG/PEG hybrid hydrogels as potential cell scaffolds in tissue engineering. Polym. Degrad. Stab. 2023, 211, 110316. [Google Scholar] [CrossRef]
- Li, C.; Jiang, T.; Zhou, C.; Jiang, A.; Lu, C.; Yang, G.; Nie, J.; Wang, F.; Yang, X.; Chen, Z. Injectable self-healing chitosan-based POSS-PEG hybrid hydrogel as wound dressing to promote diabetic wound healing. Carbohydr. Polym. 2023, 299, 120198. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dai, J.; Xu, Q.; Lu, C.; Yang, G.; Wang, F.; Nie, J.; Hu, X.; Dong, N.; Shi, J. Synthesis of thiol-terminated PEG-functionalized POSS cross-linkers and fabrication of high-strength and hydrolytic degradable hybrid hydrogels in aqueous phase. Eur. Polym. J. 2019, 116, 74–83. [Google Scholar] [CrossRef]
- Xia, Y.; Ding, S.; Liu, Y.; Qi, Z. Facile synthesis and self-assembly of amphiphilic polyether-octafunctionalized polyhedral oligomeric silsesquioxane via thiol-ene click reaction. Polymers 2017, 9, 251. [Google Scholar] [CrossRef]
- Li, W.; Feng, S. New functionalized ionic liquids based on POSS for the detection of Fe3+ ion. Polymers 2021, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Feng, S. New sensors for the detection of picric acid: Ionic liquids based on polyhedral oligomeric silsesquioxanes prepared via a thiol-ene click reaction. J. Mol. Liq. 2018, 265, 269–275. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Han, D.; Sun, R.; Zhang, J.; Feng, S. New polyhedral oligomeric silsesquioxanes-based fluorescent ionic liquids: Synthesis, self-assembly and application in sensors for detecting nitroaromatic explosives. Polymers 2018, 10, 917. [Google Scholar] [CrossRef]
- Kaneshiro, T.L.; Wang, X.; Lu, Z.-R. Synthesis, Characterization, and Gene Delivery of Poly-L-lysine Octa(3-aminopropyl)silsesquioxane Dendrimers: Nanoglobular Drug Carriers with Precisely Defined Molecular Architectures. Mol. Pharm. 2007, 4, 759–768. [Google Scholar] [CrossRef]
- Tang, G.; Chen, S.; Ye, F.; Xu, X.; Fang, J.; Wang, X. Loofah-like gel network formed by the self-assembly of a 3D radially symmetrical organic-inorganic hybrid gelator. Chem. Commun. 2014, 50, 7180–7183. [Google Scholar] [CrossRef]
- Siano, P.; Johnston, A.; Loman-Cortes, P.; Zhin, Z.; Vivero-Escoto, J.L. Evaluation of Polyhedral Oligomeric Silsesquioxane Porphyrin Derivatives on Photodynamic Therapy. Molecules 2020, 25, 4965. [Google Scholar] [CrossRef]
- Huang, D.; Yang, F.; Wang, X.; Shen, H.; You, Y.; Wu, D. Facile synthesis and self-assembly behaviour of pH-responsive degradable polyacetal dendrimers. Polym. Chem. 2016, 7, 6154–6158. [Google Scholar] [CrossRef]
- Ervithayasuporn, V.; Kwanplod, K.; Boonmak, J.; Youngme, S.; Sangtrirutnugul, P. Homogeneous and heterogeneous catalysts of organopalladium functionalized-polyhedral oligomeric silsesquioxanes for Suzuki–Miyaura reaction. J. Catal. 2015, 332, 62–69. [Google Scholar] [CrossRef]
- Rahimifard, M.; Ziarani, G.Z.; Badiei, A.; Yazdian, F. Synthesis of Polyhedral Oligomeric Silsesquioxane (POSS) with Multifunctional Sulfonamide Groups Through Click Chemistry. J. Inorg. Organomet. Polym. 2017, 27, 1037–1044. [Google Scholar] [CrossRef]
- Pérez-Ojeda, M.E.; Trastoy, B.; Rol, Á.; Chiara, M.D.; García-Moreno, I.; Chiara, J.L. Controlled Click-Assembly of Well-Defined Hetero-Bifunctional Cubic Silsesquioxanes and Their Application in Targeted Bioimaging. Chem. Eur. J. 2013, 19, 6630–6640. [Google Scholar] [CrossRef]
- Trastoy, B.; Bonsor, D.A.; Pérez-Ojeda, M.E.; Jimeno, M.L.; Méndez-Ardoy, A.; García Fernández, J.M.; Sundberg, E.J.; Chiara, J.L. Synthesis and Biophysical Study of Disassembling Nanohybrid Bioconjugates with a Cubic Octasilsesquioxane Core. Adv. Funct. Mater. 2012, 22, 3191–3201. [Google Scholar] [CrossRef]
- Fabritz, S.; Heyl, D.; Bagutski, V.; Empting, M.; Rikowski, E.; Frauendorf, H.; Balog, I.; Fessner, W.-D.; Schneider, J.J.; Avrutina, O.; et al. Towards Click Bioconjugations on Cube-Octameric Silsesquioxane Scaffolds. Org. Biomol. Chem. 2010, 8, 2212–2218. [Google Scholar] [CrossRef]
- Heyl, D.; Rikowski, E.; Hoffmann, R.C.; Schneider, J.J.; Fessner, W.-D. A “Clickable” Hybrid Nanocluster of Cubic Symmetry. Chem. Eur. J. 2010, 16, 5544–5548. [Google Scholar] [CrossRef]
- Pérez-Ojeda, M.E.; Trastoy, B.; López-Arbeloa, Í.; Bañuelos, J.; Costela, Á.; García-Moreno, I.; Chiara, J.L. Click Assembly of Dye-Functionalized Octasilsesquioxanes for Highly Efficient and Photostable Photonic Systems. Chem. Eur. J. 2011, 17, 13258–13268. [Google Scholar] [CrossRef]
- Ak, M.; Gacal, B.; Kiskan, B.; Yagci, Y.; Toppare, L. Enhancing Electrochromic Properties of Polypyrrole by Silsesquioxane Nanocages. Polymer 2008, 49, 2202–2210. [Google Scholar] [CrossRef]
- Ervithayasuporn, V.; Thapakorn, T.; Takeda, N.; Unno, M.; Chalyanurakkul, A.; Hamkool, R.; Osothan, T. Synthesis and Characterization of Octakis(3-propyl ethanethioate)octasilsesquioxane. Organometallics 2011, 30, 4475–4478. [Google Scholar] [CrossRef]
- Soares, L.A.; Serantoni de Silveira, T.F.; Silvestrini, D.R.; Bicalho, U.; Ribeiro do Carmo, D. Use of a silsesquioxane organically modified with 4-amino-5-(4-pyridyl)-4H-1,2,4-triazole-3-thiol(APTT) for adsorption of metal ions. Int. J. Chem. 2013, 5, 39–48. [Google Scholar] [CrossRef]
- Soares, L.A.; Serantoni de Silveira, T.F.; Silvestrini, D.R.; Bicalho, U.; Dias Filho, N.L.; Ribeiro do Carmo, D. A new hybrid polyhedral cubic silsesquioxane chemically modified with 4-amino-5-(4-pyridyl)-4H-1,2,4-triazole-3-thiol(APTT) and copper hexacyanoferrate (III) for voltametric determination of nitrite. Int. J. Electrochem. Sci. 2013, 8, 4654–4669. [Google Scholar] [CrossRef]
- Raghuvanshi, A.; Strohmann, C.; Tissot, J.; Clement, S.; Mehdi, A.; Richeter, S.; Biau, L.; Knorr, M. Assembly of Coordination Polymers Using Thioether-Functionalized Octasilsesquioxanes: Occurrence of (CuX)n Clusters (X = Br and I) within 3D-POSS Networks. Chem. Eur. J. 2017, 23, 16479–16483. [Google Scholar] [CrossRef]
- Ervithayasuporn, V.; Wang, X.; Kawakami, Y. Synthesis and characterization of highly pure azido-functionalized polyhedral oligomeric silsesquioxanes (POSS). Chem. Commun. 2009, 5130–5132. [Google Scholar] [CrossRef]
- Shchegolikhina, O.I.; Pozdnyakova, Y.A.; Molodtsova, Y.A.; Korkin, S.D.; Bukalov, S.S.; Leites, L.A.; Lyssenko, K.A.; Peregudov, A.S.; Auner, N.; Katsoulis, D. Synthesis and Properties of Stereoregular Cyclic Polysilanols: cis-[PhSi(O)OH]4, cis-[PhSi(O)OH]6, and Tris-cis-tris-trans-[PhSi(O)OH]12. Inorg. Chem. 2002, 41, 6892–6904. [Google Scholar] [CrossRef]
- Salvatore, R.N.; Smith, R.A.; Nischwitz, A.K.; Gavin, T. A mild and highly convenient chemoselective alkylation of thiols using Cs2CO3–TBAI. Tetrahedron Lett. 2005, 46, 8931–8935. [Google Scholar] [CrossRef]
- Anestopoulos, I.; Kiousi, D.E.; Klavaris, A.; Galanis, A.; Salek, K.; Euston, S.R.; Pappa, A.; Panayiotidis, M.I. Surface Active Agents and Their Health-Promoting Properties: Molecules of Multifunctional Significance. Pharmaceutics 2020, 12, 688. [Google Scholar] [CrossRef]
- Abula, A.; Xu, Z.; Zhu, Z.; Peng, C.; Chen, Z.; Zhu, W.; Aisa, H.A. Substitution Effect of the Trifluoromethyl Group on the Bioactivity in Medicinal Chemistry: Statistical Analysis and Energy Calculations. J. Chem. Inf. Model. 2020, 60, 6242–6250. [Google Scholar] [CrossRef] [PubMed]
- Chohan, Z.H.; Shaikh, A.U.; Supuran, C.T. In-vitro Antibacterial, Antifungal and cytotoxic activity of cobalt (II), copper (II), nickel (II) and zinc (II) complexes with furanylmethyl- and thienylmethyl-dithiolenes: [1, 3-dithiole- 2-one and 1,3-dithiole-2-thione]. J. Enzyme Inhib. Med. Chem. 2006, 21, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yagafarov, N.; Shimamura, K.; Takeda, N.; Unno, M.; Ouali, A. Synthesis of an Azido-Substituted 8-Membered Ring Laddersiloxane and Its Application in Catalysis. Molecules 2025, 30, 373. [Google Scholar] [CrossRef] [PubMed]


| Compounds | Isolated Yield (%) |
|---|---|
| 4a | 91 |
| 4b | 64 |
| 4c | 81 |
| 4d | 77 |
| 4e | 65 |
| 4f | 83 |
| Compounds | Isolated Yield (%) |
|---|---|
| 6a | 92 |
| 6b | 70 |
| 6f | 60 |
| Compounds | Td5 (°C) | Tmax (°C) | Residue at 1000 °C (%) |
|---|---|---|---|
| 2 | 335 | 384 | 23 |
| 4a | 249 | 403 | 6 |
| 4b | 219 | 424 | 3 |
| 4c | 207 | 427 | 27 |
| 4d | 231 | 426 | 15 |
| 4e | 236 | 411 | 2 |
| 4f | 253 | 323 | 8 |
| 6a | 373 | 384 | 48 |
| 6b | 238 | 430 | 12 |
| 6f | 243 | 315 | 39 |
| 8 | 308 | 390 | 18 |
| 9 | 305 | 350 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagafarov, N.; Liu, Y.; Adachi, N.; Takeda, N.; Unno, M.; Ouali, A. Unlocking New Potential in the Functionalization of Chlorinated Silsesquioxanes: A Rapid and Chemoselective Thiolation Method. Molecules 2025, 30, 3583. https://doi.org/10.3390/molecules30173583
Yagafarov N, Liu Y, Adachi N, Takeda N, Unno M, Ouali A. Unlocking New Potential in the Functionalization of Chlorinated Silsesquioxanes: A Rapid and Chemoselective Thiolation Method. Molecules. 2025; 30(17):3583. https://doi.org/10.3390/molecules30173583
Chicago/Turabian StyleYagafarov, Niyaz, Yujia Liu, Naoto Adachi, Nobuhiro Takeda, Masafumi Unno, and Armelle Ouali. 2025. "Unlocking New Potential in the Functionalization of Chlorinated Silsesquioxanes: A Rapid and Chemoselective Thiolation Method" Molecules 30, no. 17: 3583. https://doi.org/10.3390/molecules30173583
APA StyleYagafarov, N., Liu, Y., Adachi, N., Takeda, N., Unno, M., & Ouali, A. (2025). Unlocking New Potential in the Functionalization of Chlorinated Silsesquioxanes: A Rapid and Chemoselective Thiolation Method. Molecules, 30(17), 3583. https://doi.org/10.3390/molecules30173583








