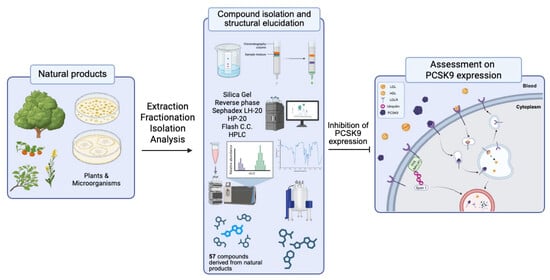
Naturally Occurring PCSK9 Inhibitors: An Updated Review
Abstract
1. Introduction
2. Results and Discussion
2.1. The Naturally Occurring PCSK9 Inhibitors Reported in 2020
2.2. The Naturally Occurring PCSK9 Inhibitors Reported in 2021
2.3. The Naturally Occurring PCSK9 Inhibitors Reported in 2022
2.4. The Naturally Occurring PCSK9 Inhibitors Reported in 2023 and 2024
2.5. The Naturally Occurring PCSK9 Inhibitors Reported in 2025
3. Methodology
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Huff, T.; Boyd, B.; Jialal, I. Physiology, Cholesterol. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- High Cholesterol. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/high-cholesterol (accessed on 9 May 2025).
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- LDL and HDL Cholesterol and Triglycerides. Available online: https://www.cdc.gov/cholesterol/about/ldl-and-hdl-cholesterol-and-triglycerides.html (accessed on 9 May 2025).
- Liu, Y.; Neogi, A.; Mani, A. The Role of Wnt Signalling in Development of Coronary Artery Disease and Its Risk Factors. Open Biol. 2020, 10, 200128. [Google Scholar] [CrossRef]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2019. [Google Scholar]
- Stein, E.A.; Mellis, S.; Yancopoulos, G.D.; Stahl, N.; Logan, D.; Smith, W.B.; Lisbon, E.; Gutierrez, M.; Webb, C.; Wu, R.; et al. Effect of a Monoclonal Antibody to PCSK9 on LDL Cholesterol. N. Engl. J. Med. 2012, 366, 1108–1118. [Google Scholar] [CrossRef]
- Cheng, J.M.; Oemrawsingh, R.M.; Garcia-Garcia, H.M.; Boersma, E.; van Geuns, R.-J.; Serruys, P.W.; Kardys, I.; Akkerhuis, K.M. PCSK9 in Relation to Coronary Plaque Inflammation: Results of the ATHEROREMO-IVUS Study. Atherosclerosis 2016, 248, 117–122. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. The LDL Receptor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 431–438. [Google Scholar] [CrossRef]
- Medina, M.W.; Krauss, R.M. The Role of HMGCR Alternative Splicing in Statin Efficacy. Trends Cardiovasc. Med. 2009, 19, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Maslub, M.G.; Radwan, M.A.; Daud, N.A.A.; Sha’aban, A. Association between CYP3A4/CYP3A5 Genetic Polymorphisms and Treatment Outcomes of Atorvastatin Worldwide: Is There Enough Research on the Egyptian Population? Eur. J. Med. Res. 2023, 28, 381. [Google Scholar] [CrossRef]
- Maron, D.J.; Fazio, S.; Linton, M.F. Current Perspectives on Statins. Circulation 2000, 101, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Chan, D.C.; Watts, G.F. The Knowns and Unknowns of Contemporary Statin Therapy for Familial Hypercholesterolemia. Curr. Atheroscler. Rep. 2020, 22, 64. [Google Scholar] [CrossRef]
- Jang, A.Y.; Lim, S.; Jo, S.-H.; Han, S.H.; Koh, K.K. New Trends in Dyslipidemia Treatment. Circ. J. 2021, 85, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Seidah, N.G.; Benjannet, S.; Wickham, L.; Marcinkiewicz, J.; Jasmin, S.B.; Stifani, S.; Basak, A.; Prat, A.; Chretien, M. The Secretory Proprotein Convertase Neural Apoptosis-Regulated Convertase 1 (NARC-1): Liver Regeneration and Neuronal Differentiation. Proc. Natl. Acad. Sci. USA 2003, 100, 928–933. [Google Scholar] [CrossRef]
- Bao, X.; Liang, Y.; Chang, H.; Cai, T.; Feng, B.; Gordon, K.; Zhu, Y.; Shi, H.; He, Y.; Xie, L. Targeting Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9): From Bench to Bedside. Signal Transduct. Target. Ther. 2024, 9, 13. [Google Scholar] [CrossRef]
- Adorni, M.P.; Zimetti, F.; Lupo, M.G.; Ruscica, M.; Ferri, N. Naturally Occurring PCSK9 Inhibitors. Nutrients 2020, 12, 1440. [Google Scholar] [CrossRef]
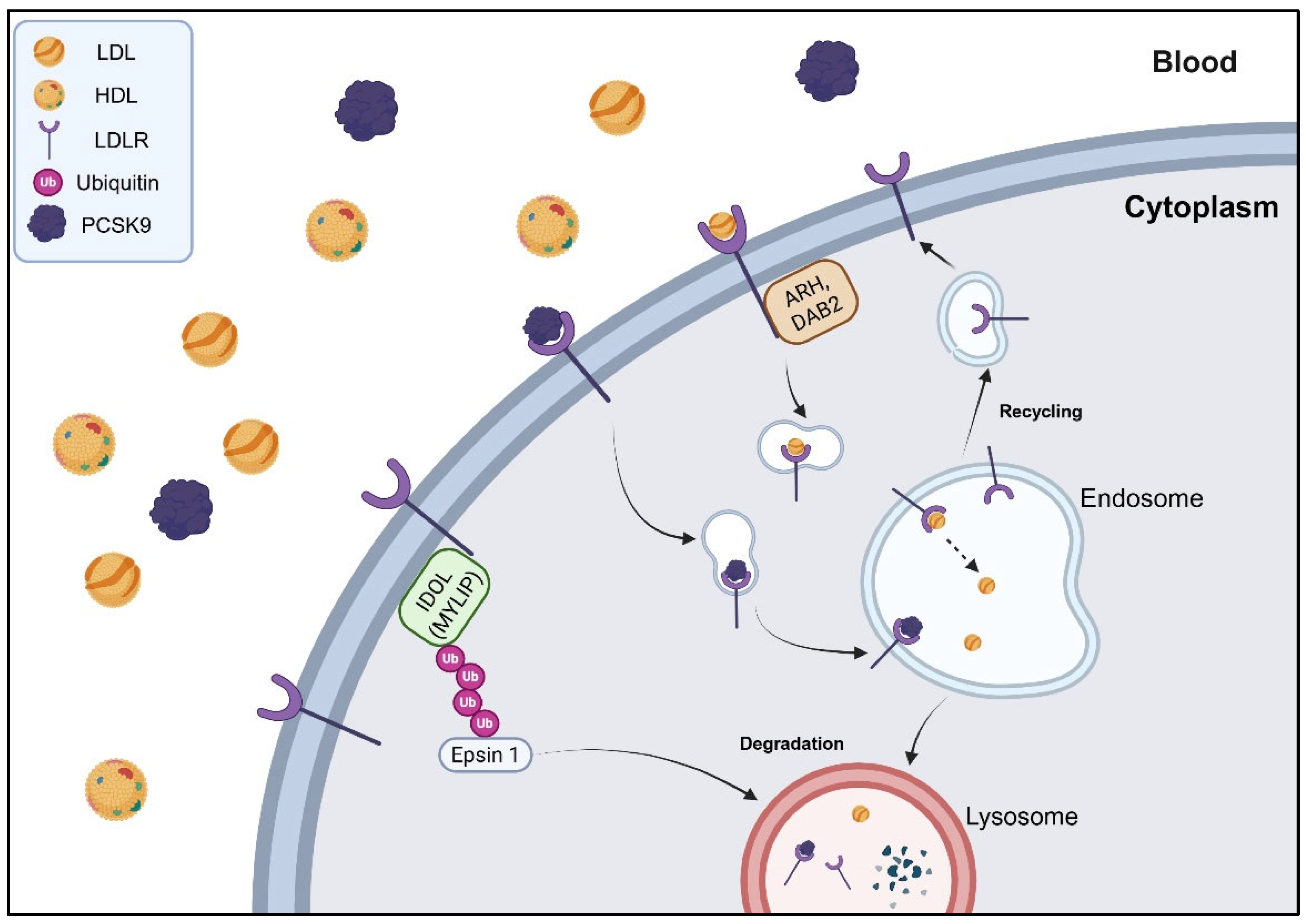
- Nassoury, N.; Blasiole, D.A.; Oler, A.T.; Benjannet, S.; Hamelin, J.; Poupon, V.; McPherson, P.S.; Attie, A.D.; Prat, A.; Seidah, N.G. The Cellular Trafficking of the Secretory Proprotein Convertase PCSK9 and Its Dependence on the LDLR. Traffic 2007, 8, 718–732. [Google Scholar] [CrossRef]
- Qian, Y.-W.; Schmidt, R.J.; Zhang, Y.; Chu, S.; Lin, A.; Wang, H.; Wang, X.; Beyer, T.P.; Bensch, W.R.; Li, W.; et al. Secreted PCSK9 Downregulates Low Density Lipoprotein Receptor through Receptor-Mediated Endocytosis. J. Lipid Res. 2007, 48, 1488–1498. [Google Scholar] [CrossRef]
- Denis, M.; Marcinkiewicz, J.; Zaid, A.; Gauthier, D.; Poirier, S.; Lazure, C.; Seidah, N.G.; Prat, A. Gene Inactivation of Proprotein Convertase Subtilisin/Kexin Type 9 Reduces Atherosclerosis in Mice. Circulation 2012, 125, 894–901. [Google Scholar] [CrossRef]
- Tavori, H.; Melone, M.; Rashid, S. Alirocumab: PCSK9 Inhibitor for LDL Cholesterol Reduction. Expert Rev. Cardiovasc. Ther. 2014, 12, 1137–1144. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Tardif, J.-C.; Amarenco, P.; Duggan, W.; Glynn, R.J.; Jukema, J.W.; Kastelein, J.J.P.; Kim, A.M.; Koenig, W.; Nissen, S.; et al. Lipid-Reduction Variability and Antidrug-Antibody Formation with Bococizumab. N. Engl. J. Med. 2017, 376, 1517–1526. [Google Scholar] [CrossRef]
- Kastelein, J.J.P.; Nissen, S.E.; Rader, D.J.; Hovingh, G.K.; Wang, M.-D.; Shen, T.; Krueger, K.A. Safety and Efficacy of LY3015014, a Monoclonal Antibody to Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9): A Randomized, Placebo-Controlled Phase 2 Study. Eur. Heart J. 2016, 37, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ji, Y.; Wang, G.; Ma, X.; Yao, Z.; Han, X.; Chen, J.; Chen, J.; Huang, W.; Xu, G.; et al. Efficacy and Safety of Ongericimab in Chinese Patients with Heterozygous Familial Hypercholesterolemia: A Randomized, Double-Blind, Placebo-Controlled Phase 3 Trial. Atherosclerosis 2025, 403, 119120. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhao, X.; Xie, Q.; Du, W.; Ma, Q.; Zhu, T.; Deng, H.; Qian, L.; Zheng, S.; Cui, Y. Pharmacokinetic/LDL-C and Exposure-Response Analysis of Tafolecimab in Chinese Hypercholesterolemia Patients: Results from Phase I, II, and III Studies. Clin. Transl. Sci. 2023, 16, 2791–2803. [Google Scholar] [CrossRef]
- Zhang, Y.; Pei, Z.; Chen, B.; Qu, Y.; Dong, X.; Yu, B.; Wang, G.; Xu, F.; Lu, D.; He, Z.; et al. Ebronucimab in Chinese Patients with Hypercholesterolemia—A Randomized Double-Blind Placebo-Controlled Phase 3 Trial to Evaluate the Efficacy and Safety of Ebronucimab. Pharmacol. Res. 2024, 207, 107340. [Google Scholar] [CrossRef]
- Sun, Y.; Lv, Q.; Guo, Y.; Wang, Z.; Huang, R.; Gao, X.; Han, Y.; Yao, Z.; Zheng, M.; Luo, S.; et al. Recaticimab as Add-on Therapy to Statins for Nonfamilial Hypercholesterolemia: The Randomized, Phase 3 REMAIN-2 Trial. J. Am. Coll. Cardiol. 2024, 84, 2037–2047. [Google Scholar] [CrossRef]
- Turner, T.A.; Butcher, B.; Mangu, P.; Kereiakes, D.; Fu, R.; Bakker-Arkema, R.; Stein, E.A. Results of a 52 Week Open-Label Phase 2B Study to Assess Long-Term Safety, Immunogenicity and LDL-C Efficacy of Monthly Dosing with LIB003 a Novel Anti-PCSK9 Recombinant Fusion Protein. Atherosclerosis 2020, 315, e9. [Google Scholar] [CrossRef]
- Burnett, J.R.; Hooper, A.J. MK-0616: An Oral PCSK9 Inhibitor for Hypercholesterolemia Treatment. Expert Opin. Investig. Drugs 2023, 32, 873–878. [Google Scholar] [CrossRef]
- Koren, M.J.; Descamps, O.; Hata, Y.; Hengeveld, E.M.; Hovingh, G.K.; Ikonomidis, I.; Radu Juul Jensen, M.D.; Langbakke, I.H.; Martens, F.M.A.C.; Søndergaard, A.L.; et al. PCSK9 Inhibition with Orally Administered NNC0385-0434 in Hypercholesterolaemia: A Randomised, Double-Blind, Placebo-Controlled and Active-Controlled Phase 2 Trial. Lancet Diabetes Endocrinol. 2024, 12, 174–183. [Google Scholar] [CrossRef]
- Raal, F.J.; Mehta, V.; Kayikcioglu, M.; Blom, D.; Gupta, P.; Elis, A.; Turner, T.; Daniels, C.; Vest, J.; Mitchell, T.; et al. Lerodalcibep and Evolocumab for the Treatment of Homozygous Familial Hypercholesterolaemia with PCSK9 Inhibition (LIBerate-HoFH): A Phase 3, Randomised, Open-Label, Crossover, Non-Inferiority Trial. Lancet Diabetes Endocrinol. 2025, 13, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, T.; Rao, B.H.; Gupta, K.; Parikh, S.S.; Abramov, D.; Mehta, A.; Al Rifai, M.; Virani, S.S.; Nambi, V.; Minhas, A.M.K.; et al. Inclisiran as a SiRNA Inhibitor of Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9); Past, Present, and Future. Am. J. Cardiovasc. Drugs 2025, 25, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Vega, R.; Garkaviy, P.; Knöchel, J.; Barbour, A.; Rudvik, A.; Laru, J.; Twaddle, L.; Mccarthy, M.C.; Rosenmeier, J.B. AZD0780, the First Oral Small Molecule PCSK9 Inhibitor for the Treatment of Hypercholesterolemia: Results from a Randomized, Single-Blind, Placebo-Controlled Phase 1 Trial. Atherosclerosis 2024, 395, 118514. [Google Scholar] [CrossRef]
- Mitchell, T.; Chao, G.; Sitkoff, D.; Lo, F.; Monshizadegan, H.; Meyers, D.; Low, S.; Russo, K.; DiBella, R.; Denhez, F.; et al. Pharmacologic Profile of the Adnectin BMS-962476, a Small Protein Biologic Alternative to PCSK9 Antibodies for Low-Density Lipoprotein Lowering. J. Pharmacol. Exp. Ther. 2014, 350, 412–424. [Google Scholar] [CrossRef]
- Aguchem, R.N.; Okagu, I.U.; Okorigwe, E.M.; Uzoechina, J.O.; Nnemolisa, S.C.; Ezeorba, T.P.C. Role of CETP, PCSK-9, and CYP7-Alpha in Cholesterol Metabolism: Potential Targets for Natural Products in Managing Hypercholesterolemia. Life Sci. 2024, 351, 122823. [Google Scholar] [CrossRef]
- Barale, C.; Melchionda, E.; Morotti, A.; Russo, I. PCSK9 Biology and Its Role in Atherothrombosis. Int. J. Mol. Sci. 2021, 22, 5880. [Google Scholar] [CrossRef] [PubMed]
- Hummelgaard, S.; Vilstrup, J.P.; Gustafsen, C.; Glerup, S.; Weyer, K. Targeting PCSK9 to Tackle Cardiovascular Disease. Pharmacol. Ther. 2023, 249, 108480. [Google Scholar] [CrossRef]
- Waiz, M.; Alvi, S.S.; Khan, M.S. Potential Dual Inhibitors of PCSK-9 and HMG-R from Natural Sources in Cardiovascular Risk Management. EXCLI J. 2022, 21, 47–76. [Google Scholar] [PubMed]
- Liu, C.; Chen, J.; Chen, H.; Zhang, T.; He, D.; Luo, Q.; Chi, J.; Hong, Z.; Liao, Y.-J.; Zhang, S.; et al. PCSK9 Inhibition: From Current Advances to Evolving Future. Cells 2022, 11, 2972. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, H.; Ramankutty, R.; Ramaswamy, S.; Agrawal, N. Natural Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors: A Review. Comb. Chem. High Throughput Screen. 2023, 26, 2668–2678. [Google Scholar] [CrossRef]
- Liou, J.-W.; Chen, P.-Y.; Gao, W.-Y.; Yen, J.-H. Natural Phytochemicals as Small-Molecule Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors. Tzu Chi Med. J. 2024, 36, 360–369. [Google Scholar] [CrossRef]
- Li, H.-H.; Li, J.; Zhang, X.-J.; Li, J.-M.; Xi, C.; Wang, W.-Q.; Lu, Y.-L.; Xuan, L.-J. 23,24-Dihydrocucurbitacin B Promotes Lipid Clearance by Dual Transcriptional Regulation of LDLR and PCSK9. Acta Pharmacol. Sin. 2020, 41, 327–335. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Wang, W.; Chen, T.; Xuan, L. Lipid-Lowering Activities of Cucurbitacins Isolated from Trichosanthes Cucumeroides and Their Synthetic Derivatives. J. Nat. Prod. 2020, 83, 3536–3544. [Google Scholar] [CrossRef]
- Pel, P.; Chae, H.-S.; Nhoek, P.; Kim, Y.-M.; Khiev, P.; Kim, G.J.; Nam, J.-W.; Choi, H.; Choi, Y.H.; Chin, Y.-W. A Stilbene Dimer and Flavonoids from the Aerial Parts of Chromolaena Odorata with Proprotein Convertase Subtilisin/Kexin Type 9 Expression Inhibitory Activity. Bioorg. Chem. 2020, 99, 103869. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, B.; Park, S.W.; Lee, H.-S.; Chen, W.; Liu, J. Hepatocyte Nuclear Factor 1alpha Plays a Critical Role in PCSK9 Gene Transcription and Regulation by the Natural Hypocholesterolemic Compound Berberine. J. Biol. Chem. 2009, 284, 28885–28895. [Google Scholar] [CrossRef] [PubMed]
- Nhoek, P.; Chae, H.-S.; Kim, Y.-M.; Pel, P.; Huh, J.; Kim, H.W.; Choi, Y.H.; Lee, K.; Chin, Y.-W. Sesquiterpenoids from the Aerial Parts of Salvia Plebeia with Inhibitory Activities on Proprotein Convertase Subtilisin/Kexin Type 9 Expression. J. Nat. Prod. 2021, 84, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Lou, Y.-Y.; Wang, Y.-S.; Huang, Y.-P.; Zhang, J.; Yin, Z.-Q.; Pan, K. New Dammarane-Type Glycosides from Gynostemma Pentaphyllum and Their Lipid-Lowering Activity. Bioorg. Chem. 2021, 111, 104843. [Google Scholar] [CrossRef]
- Kim, E.; Kim, Y.-M.; Ahn, J.; Chae, H.-S.; Chin, Y.-W.; Kim, J. Prenylated Flavonoid Glycosides with PCSK9 MRNA Expression Inhibitory Activity from the Aerial Parts of Epimedium Koreanum. Molecules 2021, 26, 3590. [Google Scholar] [CrossRef]
- Woo, S.; Chae, H.-S.; Kim, J.; Chin, Y.-W. Selaginellin Derivatives from Selaginella Tamariscina and Their Upregulating Effects on Low-Density Lipoprotein Receptor Expression. J. Nat. Prod. 2021, 84, 857–864. [Google Scholar] [CrossRef]
- Ahn, J.; Chae, H.-S.; Pel, P.; Kim, Y.-M.; Choi, Y.H.; Kim, J.; Chin, Y.-W. Dilignans with a Chromanol Motif Discovered by Molecular Networking from the Stem Barks of Magnolia Obovata and Their Proprotein Convertase Subtilisin/Kexin Type 9 Expression Inhibitory Activity. Biomolecules 2021, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-P.; Wang, Y.-S.; Liu, B.-W.; Song, Z.; Liang, X.-S.; Teng, Y.; Zhang, J.; Yin, Z.-Q.; Pan, K. Dammarane-Type Saponins with Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitory Activity from Gynostemma Pentaphyllum. Phytochemistry 2022, 194, 113005. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, X.; Jin, Q.; Chen, J.; Yang, L.; Wei, W.; Qu, H.; Yao, C.; Hou, J.; Gong, L.; et al. Novel Triterpenoids from Alisma Plantago-Aquatica with Influence on LDL Uptake in HepG2 Cells by Inhibiting PCSK9. Phytomedicine 2022, 105, 154342. [Google Scholar] [CrossRef]
- Huh, J.; Park, T.K.; Chae, H.-S.; Nhoek, P.; Kim, Y.-M.; An, C.-Y.; Lee, S.; Kim, J.; Chin, Y.-W. Acylated Saponins and Flavonoid Glycosides from the Fruits of Stewartia Koreana. Phytochemistry 2022, 193, 112980. [Google Scholar] [CrossRef]
- Pel, P.; Kim, Y.-M.; Kim, H.J.; Nhoek, P.; An, C.-Y.; Son, M.-G.; Won, H.; Lee, S.E.; Lee, J.; Kim, H.W.; et al. Isocoumarins and Benzoquinones with Their Proprotein Convertase Subtilisin/Kexin Type 9 Expression Inhibitory Activities from Dried Roots of Lysimachia Vulgaris. ACS Omega 2022, 7, 47296–47305. [Google Scholar] [CrossRef]
- Pel, P.; Chae, H.-S.; Nhoek, P.; Kim, Y.-M.; An, C.-Y.; Yoo, H.; Kang, M.; Kim, H.W.; Choi, Y.H.; Chin, Y.-W. Chemical Constituents from the Roots and Rhizomes of Sophora Tonkinensis and Their Effects on Proprotein Convertase Substilisin/Kexin Type 9 Expression. ACS Omega 2022, 7, 20952–20958. [Google Scholar] [CrossRef]
- Nhoek, P.; An, C.-Y.; Son, M.-G.; Chae, H.-S.; Pel, P.; Kim, Y.-M.; Khiev, P.; Choi, W.J.; Choi, Y.H.; Chin, Y.-W. Stereochemical Assignment of Clerodane-Type Diterpenes from the Fruits of Casearia Grewiifolia and Their Ability to Inhibit PCSK9 Expression. Phytochemistry 2023, 216, 113864. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Lin, Z.-C.; Li, L.-L.; Zhang, S.-F.; Li, W.-H.; Liu, W.; Song, B.-L.; Luo, J. SUMOylation of the Ubiquitin Ligase IDOL Decreases LDL Receptor Levels and Is Reversed by SENP1. J. Biol. Chem. 2021, 296, 100032. [Google Scholar] [CrossRef]
- Martinelli, L.; Adamopoulos, A.; Johansson, P.; Wan, P.T.; Gunnarsson, J.; Guo, H.; Boyd, H.; Zelcer, N.; Sixma, T.K. Structural Analysis of the LDL Receptor-Interacting FERM Domain in the E3 Ubiquitin Ligase IDOL Reveals an Obscured Substrate-Binding Site. J. Biol. Chem. 2020, 295, 13570–13583. [Google Scholar] [CrossRef]
- An, C.-Y.; Son, M.-G.; Chin, Y.-W. Acyclic Triterpenoids from Alpinia Katsumadai Seeds with Proprotein Convertase Subtilisin/Kexin Type 9 Expression and Secretion Inhibitory Activity. ACS Omega 2023, 8, 32804–32816. [Google Scholar] [CrossRef]
- Hu, X.; Chen, T.; Guo, P.; Wang, Q.; Ding, A.; Qin, G.; Wang, W.; Xuan, L. Amide Alkaloids and Neolignans from Piper Hongkongense and Their Inhibitory Activities of PCSK9 Expression. Fitoterapia 2024, 175, 105951. [Google Scholar] [CrossRef]
- Son, M.-G.; Pel, P.; An, C.-Y.; Park, C.-W.; Lee, S.H.; Yang, T.-J.; Chin, Y.-W. Chemical Constituents from the Roots of Cynanchum Wilfordii with PCSK9 Secretion Inhibitory Activities. Phytochemistry 2024, 226, 114205. [Google Scholar] [CrossRef] [PubMed]
- Won, H.; Son, M.-G.; Pel, P.; Nhoek, P.; An, C.-Y.; Kim, Y.-M.; Chae, H.-S.; Chin, Y.-W. Chemical Constituents from Morus Alba with Proprotein Convertase Subtilisin/Kexin Type 9 Expression and Secretion Inhibitory Activity. Org. Biomol. Chem. 2023, 21, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Wu, M.; Li, H.; Kraemer, F.B.; Adeli, K.; Seidah, N.G.; Park, S.W.; Liu, J. Strong Induction of PCSK9 Gene Expression through HNF1alpha and SREBP2: Mechanism for the Resistance to LDL-Cholesterol Lowering Effect of Statins in Dyslipidemic Hamsters. J. Lipid Res. 2010, 51, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Ataei, S.; Kesharwani, P.; Sahebkar, A. Berberine: Ins and Outs of a Nature-Made PCSK9 Inhibitor. EXCLI J. 2022, 21, 1099–1110. [Google Scholar]
- Wang, X.; Chen, X.; Zhang, X.; Su, C.; Yang, M.; He, W.; Du, Y.; Si, S.; Wang, L.; Hong, B. A Small-Molecule Inhibitor of PCSK9 Transcription Ameliorates Atherosclerosis through the Modulation of FoxO1/3 and HNF1α. EBioMedicine 2020, 52, 102650. [Google Scholar] [CrossRef]
- Wei, Y.; Wen, H.; Yang, L.; Zhang, B.; Li, X.; Li, S.; Dong, J.; Liang, Z.; Zhang, Y. Hypecotumines A-D, New Isoquinoline Alkaloids with Potential PCSK9 Inhibition Activity from Hypecoum erectum L. Nat. Prod. Bioprospect. 2024, 14, 57. [Google Scholar]
- Lee, S.; Son, M.-G.; Kim, Y.-M.; An, C.-Y.; Kim, H.J.; Nhoek, P.; Pel, P.; Won, H.; Lee, Y.; Yun, N.; et al. Dihydrostilbenes and Flavonoids from Whole Plants of Jacobaea Vulgaris. Phytochemistry 2024, 222, 114107. [Google Scholar] [CrossRef] [PubMed]
- An, C.-Y.; Pel, P.; Bae, M.; Park, C.-W.; Kwon, H.; Lee, H.S.; Van Dung, L.; Kim, C.; Lee, D.; Choi, Y.H.; et al. Cycloartane-Type Triterpenoids from Combretum Quadrangulare Kurz with PCSK9 Secretion Inhibitory Activities. Phytochemistry 2025, 230, 114330. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, A.A.; Dileep, S.A.; Sj, A.R.; Singam, S.S.R.; Martin, A. Saffron and Its Active Constituents Ameliorate Hypercholesterolemia by Inhibiting PCSK9 and Modulating Sortilin, LDLR, and SREBP-2 Signaling in High Fat Diet Induced Hypercholesterolemic C57BL/6 Mice. J. Ethnopharmacol. 2025, 346, 119697. [Google Scholar] [CrossRef] [PubMed]
- Rho, H.; Terry, A.R.; Chronis, C.; Hay, N. Hexokinase 2-Mediated Gene Expression via Histone Lactylation Is Required for Hepatic Stellate Cell Activation and Liver Fibrosis. Cell Metab. 2023, 35, 1406–1423.e8. [Google Scholar] [CrossRef]

| No. | Compound Name | Compound Class | Origin/Source | Study Model | Activity Level | Mechanism | References |
|---|---|---|---|---|---|---|---|
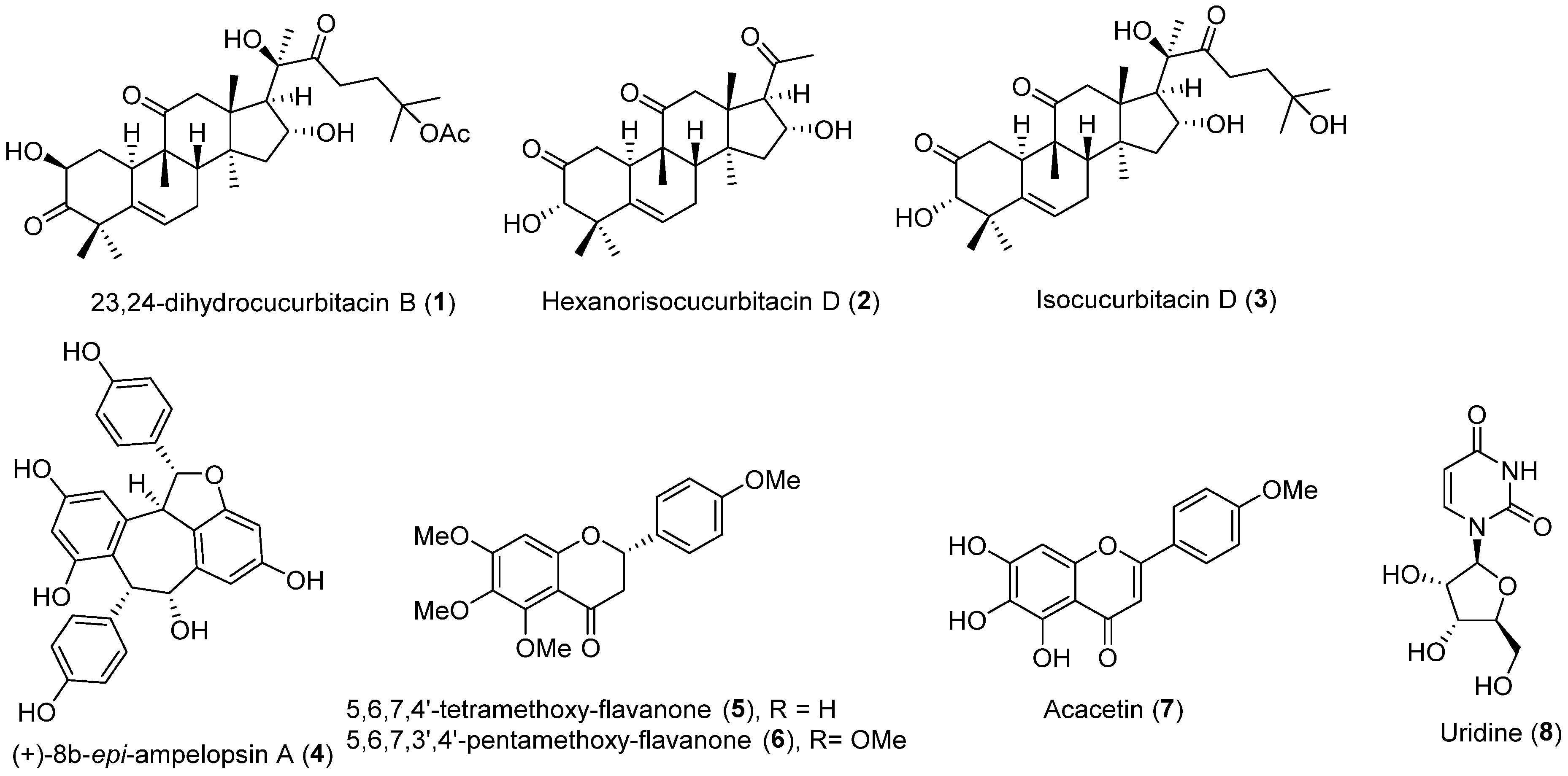
| 1 | 23,24-dihydrocucurbitacin B | Triterpenoid | Trichosanthes cucumeroides roots | HepG2 cells | Inhibits PCSK9 and HNF-1α level; increases LDLR and SREBP2 levels | [43] | |
| HFD-fed hamsters | 30 mg/kg; 50% downregulated; 80% and 70% increased | ||||||
| 2 | Hexanorisocucurtitacin D | HepG2 cells | 5 μM; LDL uptake rate of 2.53 | Suppresses PCSK9 mRNA; enhances LDLR mRNA | [44] | ||
| 3 | Isocucurbitacin D | 5 μM; LDL uptake rate of 2.47 | [44] | ||||
| 4 | (+)-8b-epi-ampelopsin A | Stilbene | Chromolaena odorata aerial parts | HepG2 cells | IC50 20.6 μM | Inhibits PCSK9 mRNA expression | [45] |
| 5 | 5,6,7,4′-tetramethoxyflavanone | Flavonoid | IC50 21.4 μM | ||||
| 6 | 5,6,7,3′,4′-pentamethoxyflavanone | IC50 31.7 μM | |||||
| 7 | Acacetin | IC50 15.0 μM | |||||
| 8 | Uridine | Nucleic acid | IC50 13.7 μM |
| No. | Compound Name | Compound Class | Origin/Source | Study Model | Activity Level | Mechanism | References |
|---|---|---|---|---|---|---|---|
| 9 | Plebeic acid A | Sesquiterpene | Salvia plebeian roots | HepG2 cells | IC50 24.4 μM | Inhibitory effects on PCSK9 mRNA expression; upregulates LDLR mRNA expression | [47] |
| 10 | (1S,5S,8S,10R)-1-acetoxy-8-methoxy-2-oxoeudesman-3,7(11)-dien-8,12-olide | IC50 25.2 μM | |||||
| 11 | Eudebeiolide B | IC50 27.8 μM | |||||
| 12 | Gypenoside LXXXIX | Triterpenoidal saponin | Whole herb of Gynostema pentaphyllum | HepG2 cells | 20 μM | Inhibition against simvastatin-induced PCSK9 expression | [48] |
| 13 | Gypenoside XC | 10 and 20 μM | |||||
| 14 | Gypenoside XCI | 10 and 20 μM | |||||
| 15 | Ginsenoside Rg5 | 10 and 20 μM | |||||
| 16 | Icariside I | Flavonoid glycoside | Epimedium koreanum aerial parts | HepG2 cells | 10 μM | Inhibits PCSK9 mRNA expression | [49] |
| 17 | Ikarisoside A | Inhibits PCSK9 mRNA expression; increases LDLR mRNA expression | |||||
| 18 | Icariin | Inhibits PCSK9 mRNA expression | |||||
| 19 | Anhydroicaritin 3-O-β-d-fucopyranosyl(1→2)-rhamnopyranoside-7-O-β-d-glucoside | ||||||
| 20 | Korepimedoside A | ||||||
| 21 | Epimedokoreanoside I | ||||||
| 22 | Korepimeoside C | ||||||
| 23 | Epimedin L | ||||||
| 24 | Caohuoside B | ||||||
| 25 | Epimedoicarisoside A | ||||||
| 26 | Selaginpulvilin U | Selaginellin derivative | Selaginella tamariscina roots and rhizophores | HepG2 cells | 50 μM | Increases LDLR expression | [50] |
| 27 | Obovatalin A | Lignan | Dried bark of Magnolia obovate | HepG2 cells | IC50 12.0 μM | Inhibitory effects on PCSK9 protein levels and increases LDLR expression | [51] |
| 28 | Obovatalin B | IC50 45.4 μM | |||||
| 29 | Magnolol | IC50 22.9 μM |
| No. | Compound Name | Compound Class | Origin/Source | Study Model | Activity Level | Mechanism | References |
|---|---|---|---|---|---|---|---|
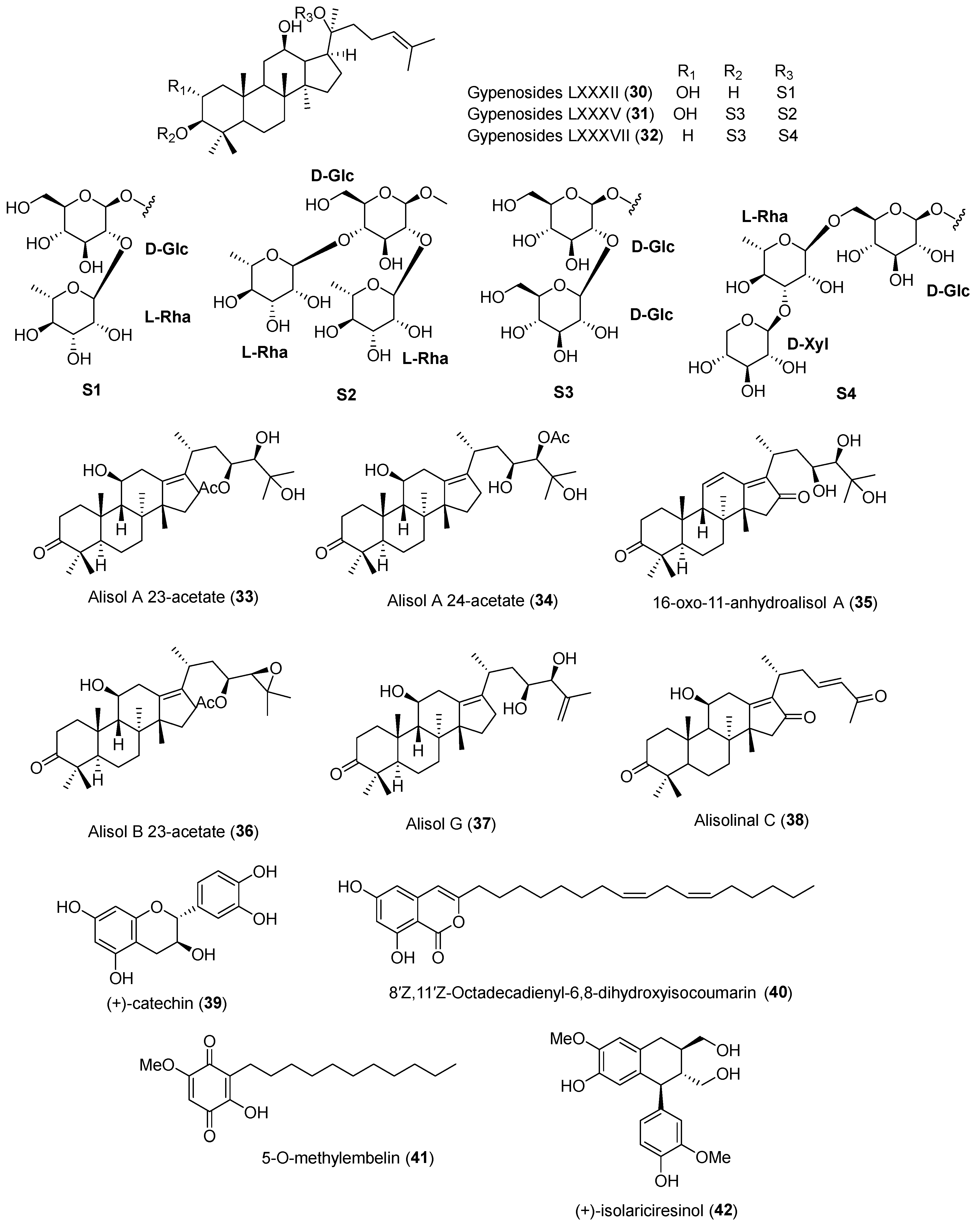
| 30 | Gypenoside LXXXII | Triterpenoidal saponin | Whole herb of Gynostema pentaphyllum | HepG2 cells | 5, 10 and 20 μM | Inhibition against LPDS-induced PCSK9 expression | [52] |
| 31 | Gypenoside LXXXV | 10 and 20 μM | |||||
| 32 | Gypenoside LXXXVII | 20 μM | |||||
| 33 | Alisol A 23-acetate | Triterpene | Alisma plantago-aquatica rhizomes | HepG2 cells | 10 μM | Inhibits PCSK9 mRNA expression | [53] |
| 34 | Alisol A 24-acetate | Triterpene | |||||
| 35 | 16-oxo-11-anhydroalisol A | Triterpene | |||||
| 36 | Alisol B 23-acetate | Triterpene | |||||
| 37 | Alisol G | Triterpene | 58%, 10 μM | ||||
| 38 | Alisolinal C | Triterpene | 46%, 10 μM | ||||
| 39 | (+)-Catechin | Flavonoid | Stewartia koreana fruits | HepG2 cells | 50 μM | Suppresses PCSK9 protein levels and increases LDLR levels | [54] |
| 40 | 8′Z,11′Z-octadecadienyl-6,8-dihydroxyisocoumarin | Isocoumarin | Lysimachia vulgaris roots | HepG2 cells | IC50 11.9 μM | Inhibits PCSK9 mRNA expression | [55] |
| 41 | 5-O-methylembelin | Benzofuran | IC50 4.9 μM | ||||
| 42 | (+)-Isolariciresinol | Lignan | Sophora tonkinensis rhizomes | HepG2 cells | 10 and 50 μM | Downregulates HNF1α and SREBP mRNA expression; reduces expression of PCSK9 and LDLR protein | [56] |
| No. | Compound Name | Compound Class | Origin/Source | Study Model | Activity Level | Mechanism | References |
|---|---|---|---|---|---|---|---|
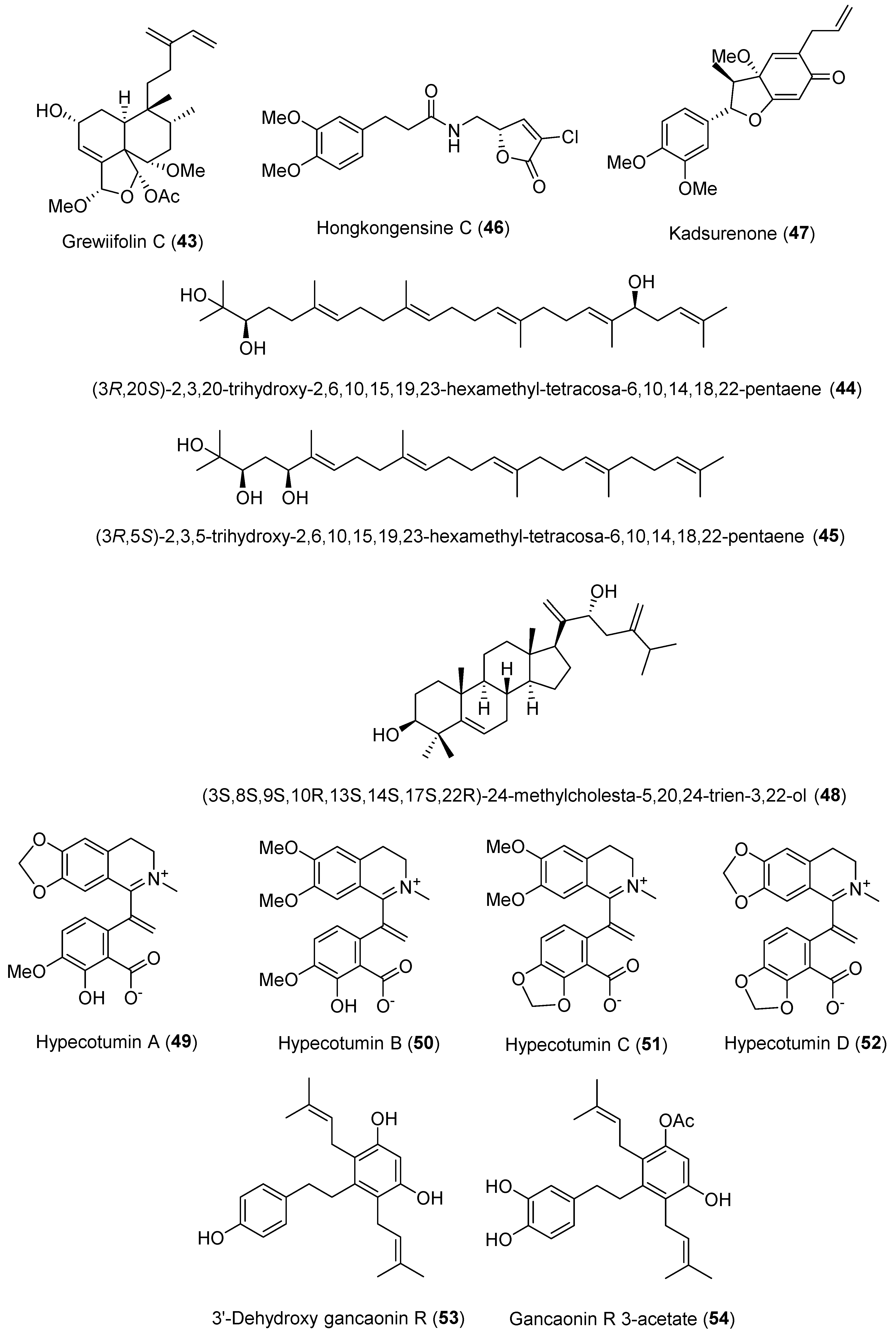
| 43 | Grewiifolin C | Diterpene | Casearia grewiifolia fruits | HepG2 cells | 20 μM | Inhibits PCSK9 and IDOL mRNA expression | [57] |
| 44 | (3R,20S)-2,3,20-trihydroxy-2,6,10,15,19,23-hexamethyl-tetracosa-6,10,14,18,22-pentaene | Acyclic triterpenoid | Dried seeds of Alpinia katsumadai | HepG2 cells | IC50 2.94 μM | Inhibition of PCSK9 mRNA expression | [60] |
| 45 | (3R,5S)-2,3,5-trihydroxy-2,6,10,15,19,23-hexamethyl-tetracosa-6,10,14,18,22-pentaene | IC50 15.08 μM | |||||
| 46 | Hongkongensine C | Amide alkaloid | Aerial part of Piper hongkongense | HepG2 cells | 5 μM, 38.4% | Inhibitory activity against PCSK9 expression | [61] |
| 47 | Kadsurenone | Lignan | 5 μM, 52.0% | ||||
| 48 | (3S,8S,9S,10R,13S,14S,17S,22R)-24-methylcholesta-5,20,24-trien-3,22-ol | Triterpene | Cynanchum wilfordii roots | HepG2 cells | 2.5 (63%), 5 (27%), and 10 (27%) μM | Suppresses PCSK9 expression | [63] |
| 49 | Hypecotumine A | Isoquinoline alkaloids | Whole herb of Hypecoum erectum | Affinity with PCSK9 protein by SPR analysis | KD 306.0 μM | Downregulates PCSK9 protein levels; upregulates LDLR protein levels | [67] |
| 50 | Hypecotumine B | KD 248.0 μM | |||||
| 51 | Hypecotumine C | KD 95.1 μM | |||||
| 52 | Hypecotumine D | KD 59.9 μM | |||||
| 53 | 3′-dehydroxy gancaonin R | Stilbenes | Whole herb of Jacobaea vulgaris | HepG2 cells | IC50 16.1 μM | Inhibition of PCSK9 mRNA expression; upregulation of LDLR protein levels | [68] |
| 54 | Gancaonin R 3-acetate | IC50 20.6 μM |
| No. | Compound Name | Compound Class | Origin/Source | Study Model | Activity Level | Mechanism | References |
|---|---|---|---|---|---|---|---|
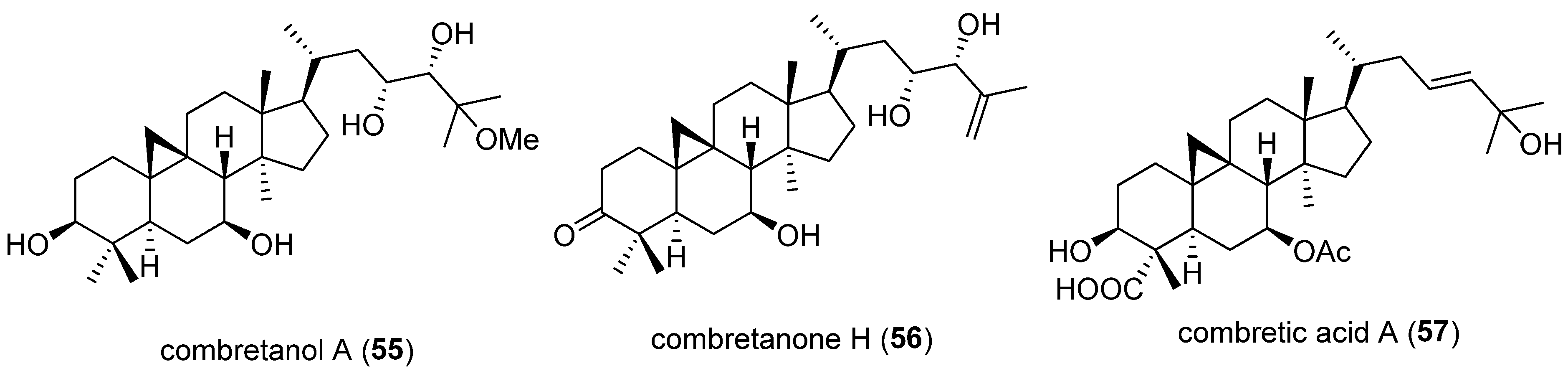
| 55 | Combretanol A | Triterpenoid | Combretum quadrangulare twigs | HepG2 cells | 20 μM | Suppresses PCSK9 mRNA expression; promotes LDLR mRNA expression | [69] |
| 56 | Combretanone H | 5 and 10 μM | |||||
| 57 | Combretic acid A | 2.5, 5, 10, and 20 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huh, J.; Kim, H. Naturally Occurring PCSK9 Inhibitors: An Updated Review. Molecules 2025, 30, 3582. https://doi.org/10.3390/molecules30173582
Huh J, Kim H. Naturally Occurring PCSK9 Inhibitors: An Updated Review. Molecules. 2025; 30(17):3582. https://doi.org/10.3390/molecules30173582
Chicago/Turabian StyleHuh, Jungmoo, and Hyunwoo Kim. 2025. "Naturally Occurring PCSK9 Inhibitors: An Updated Review" Molecules 30, no. 17: 3582. https://doi.org/10.3390/molecules30173582
APA StyleHuh, J., & Kim, H. (2025). Naturally Occurring PCSK9 Inhibitors: An Updated Review. Molecules, 30(17), 3582. https://doi.org/10.3390/molecules30173582