Stable Gold@Polydopamine@ssDNA Bioconjugates for Highly Efficient Detection of Tumor-Related mRNA in Living Cells
Abstract
1. Introduction
2. Results and Discussion
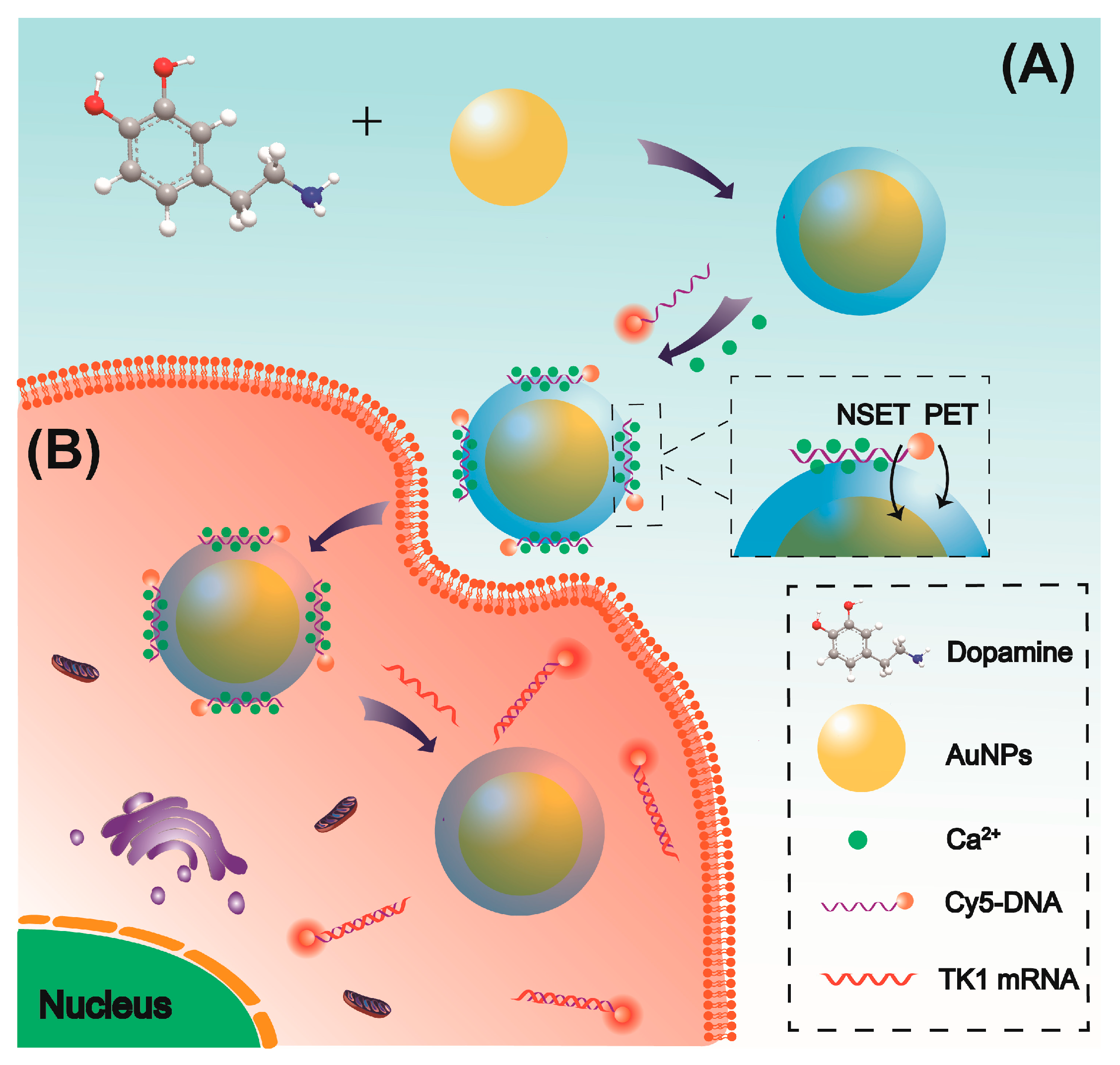
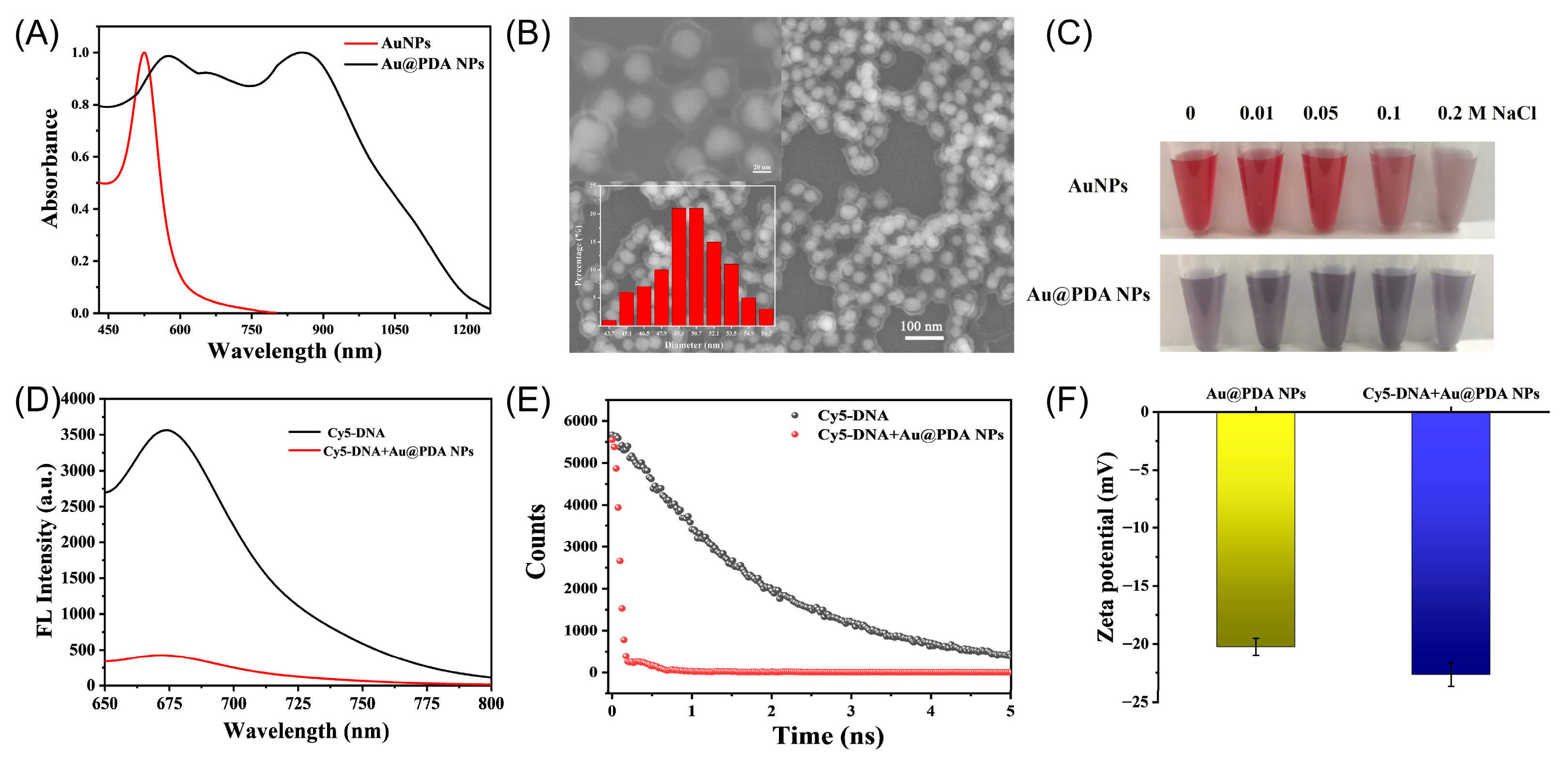
2.1. Characterization of AuNP and Au@PDA Bioconjugates
2.2. Interaction Between DNA Probe and Au@PDA Bioconjugates
2.3. Coexistence of Charge and Energy Transfer Process
2.4. Optimization of the Experimental Conditions
2.5. Analytical Performance of AuNP@PDA-Based Method
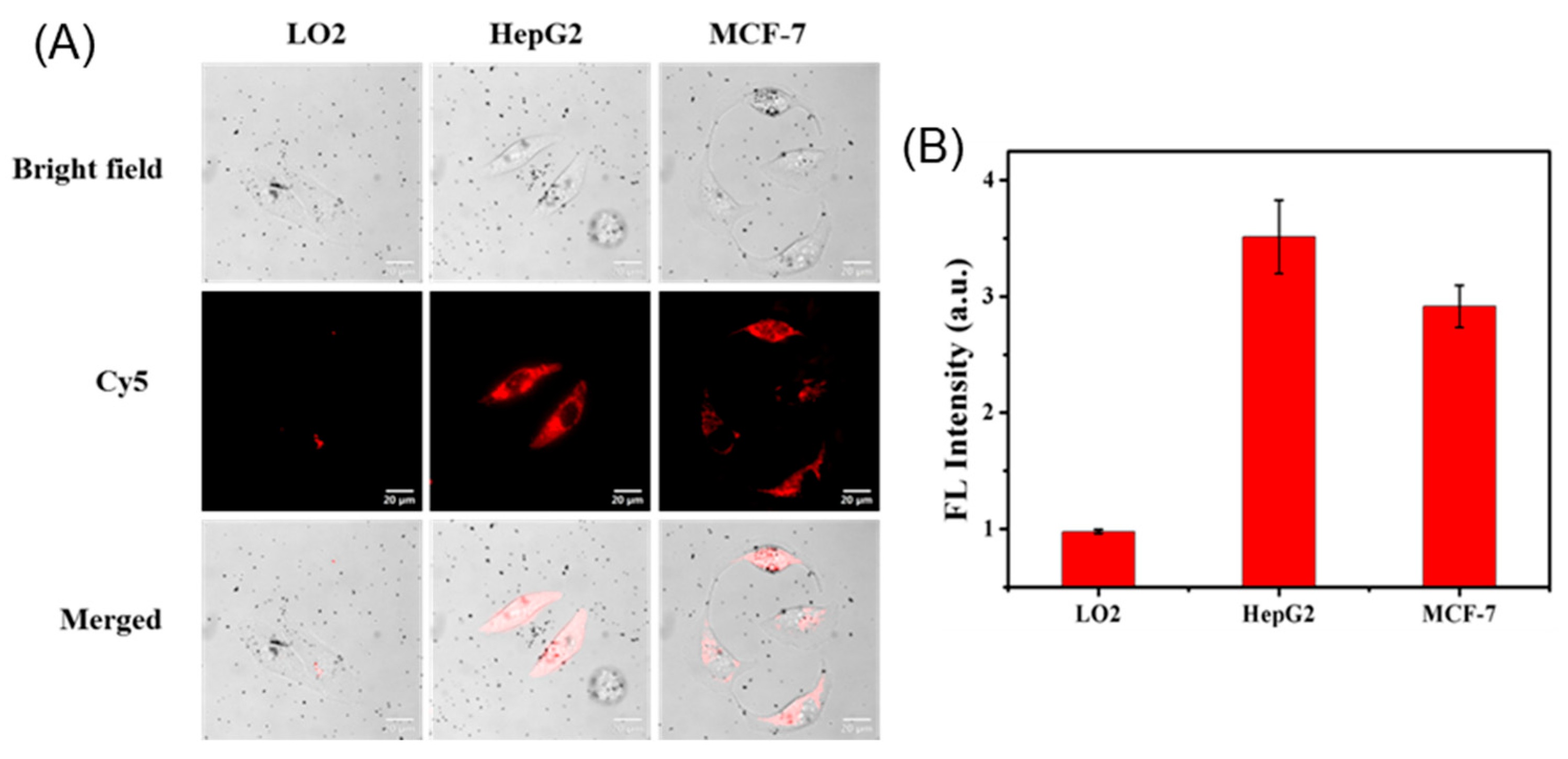
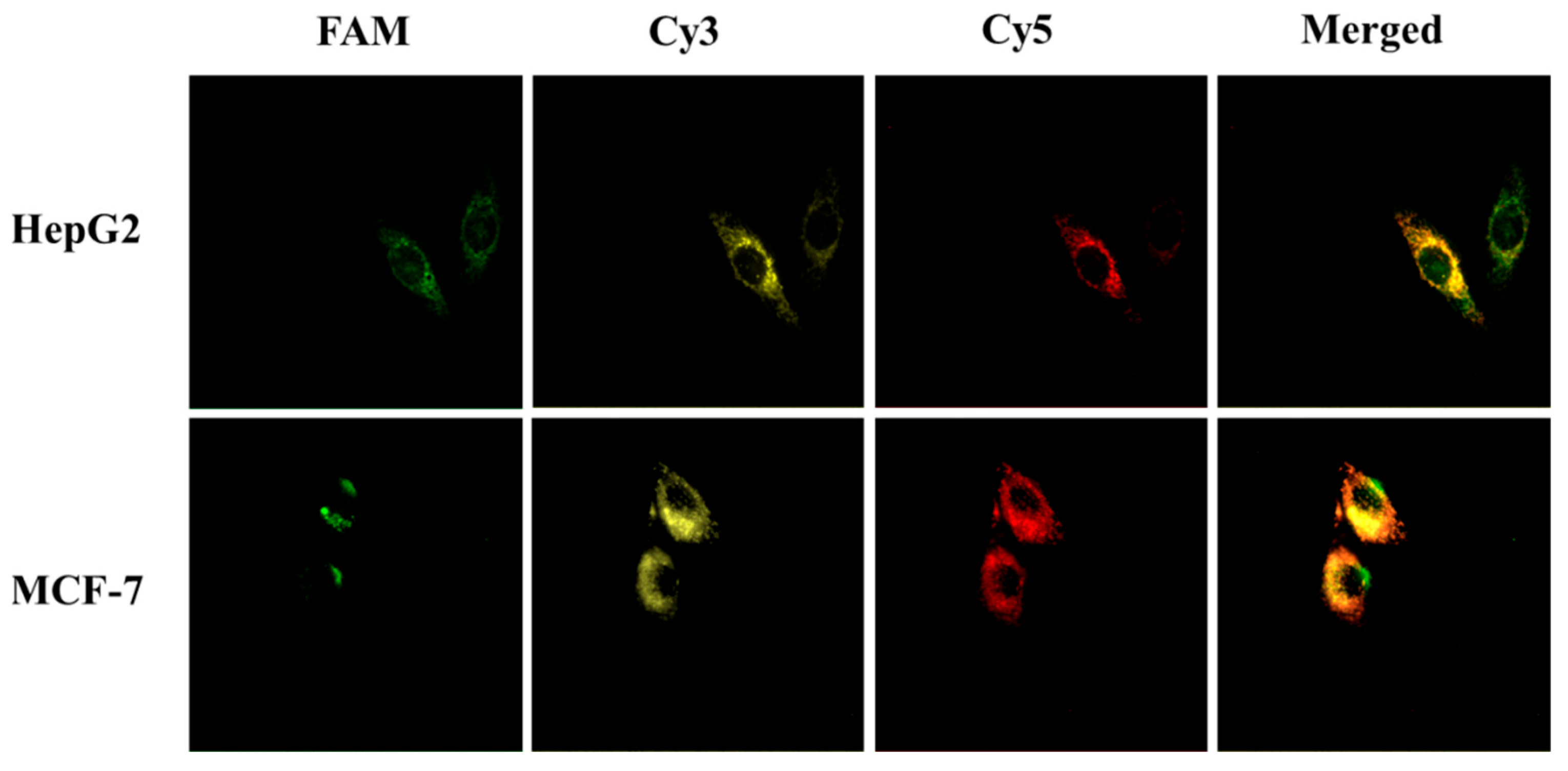
2.6. Intracellular Operation of the Au@PDA-ssDNA
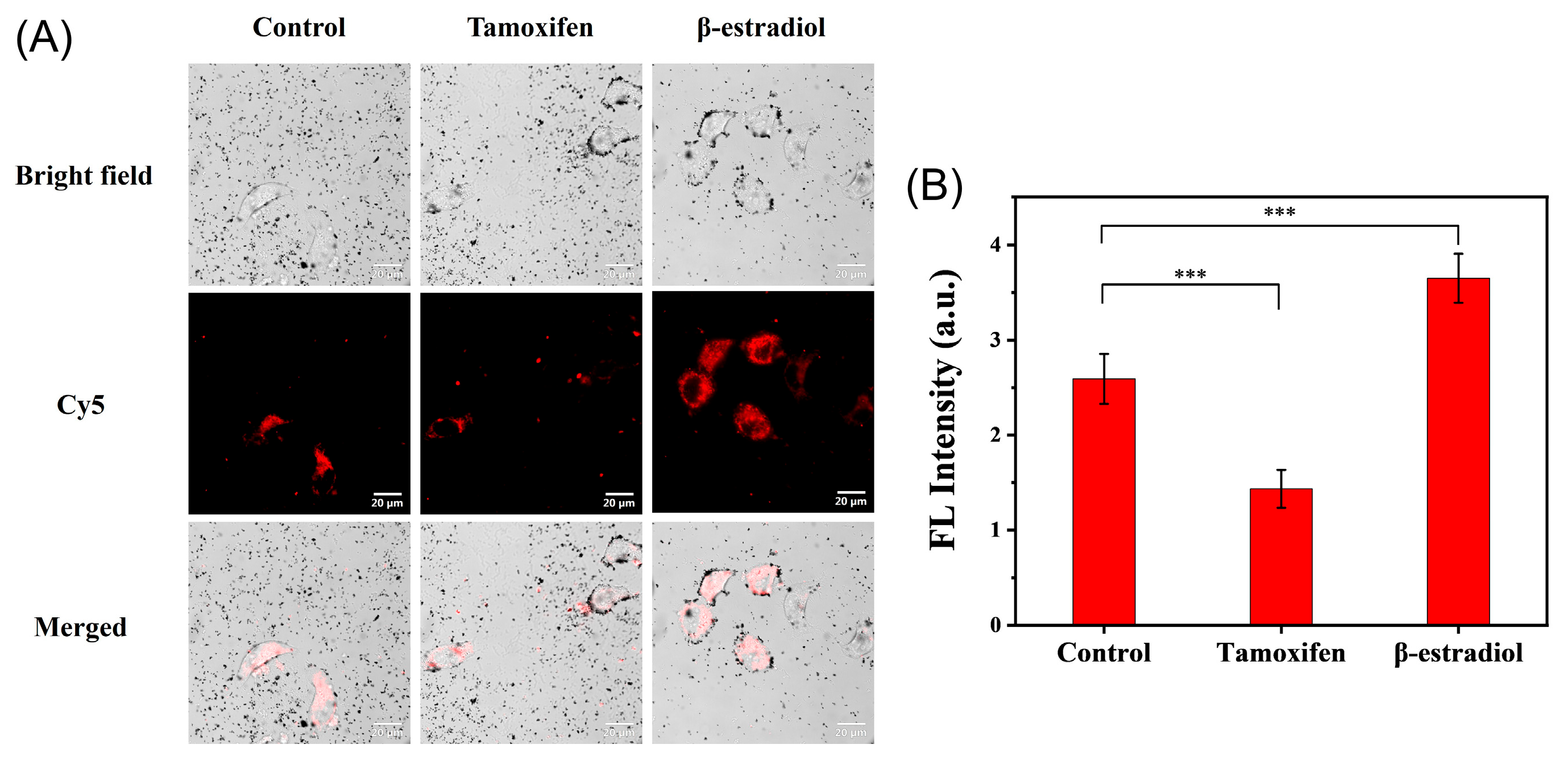
2.7. Generality Evaluation of the Bioconjugates
3. Experimental Section
3.1. Materials and Reagents
3.2. Apparatus
3.3. Preparation of AuNPs and Au@PDA NPs
3.4. Au@PDA Bioconjugate Preparation
3.5. Cell Culture and Cytotoxicity Assay
3.6. mRNA Determination
3.7. Imaging of mRNA in Living Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Passaro, A.; Al Bakir, M.; Hamilton, E.G.; Diehn, M.; André, F.; Roy-Chowdhuri, S.; Mountzios, G.; Wistuba, I.I.; Swanton, C.; Peters, S. Cancer biomarkers: Emerging trends and clinical implications for personalized treatment. Cell 2024, 187, 1617–1635. [Google Scholar] [CrossRef]
- Wu, L.; Qu, X. Cancer biomarker detection: Recent achievements and challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.R.; Morris, R.M.; Velazquez, E.J.; Bellini, D.M.; O’Neill, K. Abstract 1902: Elevated thymidine kinase 1 (TK1) in cancer serum may suppress immune function. Cancer Res. 2021, 81 (Suppl. S13), 1902. [Google Scholar] [CrossRef]
- Kataoka, Y.; Iimori, M.; Niimi, S.; Tsukihara, H.; Wakasa, T.; Saeki, H.; Oki, E.; Maehara, Y.; Kitao, H. Cytotoxicity of trifluridine correlates with the thymidine kinase 1 expression level. Sci. Rep. 2019, 9, 7964. [Google Scholar] [CrossRef]
- Chen, C.; Hildebrandt, N. Resonance energy transfer to gold nanoparticles: NSET defeats FRET. TrAC Trends Anal. Chem. 2020, 123, 115748. [Google Scholar] [CrossRef]
- Gao, P.F.; Li, Y.F.; Huang, C.Z. Plasmonics-attended NSET and PRET for analytical applications. TrAC Trends Anal. Chem. 2020, 124, 115805. [Google Scholar] [CrossRef]
- Li, H.; Warden, A.R.; Su, W.; He, J.; Zhi, X.; Wang, K.; Zhu, L.; Shen, G.; Ding, X. Highly sensitive and portable mRNA detection platform for early cancer detection. J. Nanobiotechnol. 2021, 19, 287. [Google Scholar] [CrossRef]
- Uddin, M.D.I.; Kilburn, T.C.; Yang, R.; McCollum, G.W.; Wright, D.W.; Penn, J.S. Targeted Imaging of VCAM-1 mRNA in a Mouse Model of Laser-Induced Choroidal Neovascularization Using Antisense Hairpin-DNA-Functionalized Gold-Nanoparticles. Mol. Pharm. 2018, 15, 5514–5520. [Google Scholar] [CrossRef]
- Zhu, D.; Li, X.; Zhu, Y.; Wei, Q.; Hu, Y.; Su, S.; Chao, J.; Wang, L.; Weng, L. Spatiotemporal Monitoring of Subcellular mRNAs in Situ via Polyadenine-Mediated Dual-Color Sticky Flares. ACS Appl. Mater. Interfaces 2023, 15, 15250–15259. [Google Scholar] [CrossRef]
- Yang, M.; Wang, R.; Xie, Y.; Zhu, L.; Huang, J.; Xu, W. Applications of DNA functionalized gold nanozymes in biosensing. Biosens. Bioelectron. 2025, 271, 116987. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Yan, A.-M.; Guo, W.Y.-Z.; Fang, Y.-Y.; Dong, Q.-J.; Li, R.-R.; Ni, S.-N.; Sun, Y.; Yang, W.-C.; Yang, G.-F. Human Neutrophil Elastase Activated Fluorescent Probe for Pulmonary Diseases Based on Fluorescence Resonance Energy Transfer Using CdSe/ZnS Quantum Dots. ACS Nano 2020, 14, 4244–4254. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, C.; Luo, S.; Wu, Z.-S. Spherical Nucleic Acid-Mediated Spatial Matching-Guided Nonenzymatic DNA Circuits for the Prediction and Prevention of Malignant Tumor Invasion. Anal. Chem. 2024, 96, 7091–7100. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, G.; Wang, K.; Wan, Q.; Tao, L.; Zhang, X.; Wei, Y. Recent developments in polydopamine: An emerging soft matter for surface modification and biomedical applications. Nanoscale 2016, 8, 16819–16840. [Google Scholar] [CrossRef]
- Li, H.; Jia, Y.; Bai, S.; Peng, H.; Li, J. Metal-chelated polydopamine nanomaterials: Nanoarchitectonics and applications in biomedicine, catalysis, and energy storage. Adv. Colloid Interface Sci. 2024, 334, 103316. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, A.; Chang, P.; Shi, Y.; Li, Z. Sensitivity enhanced SPR/LSPR biosensor based on Au/PDA/AuNps co-modified PCF for rabbit IgG detection. Opt. Fiber Technol. 2025, 91, 104148. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, S.; Chao, M.; Wang, Y.; Liu, P.; Li, P.; Fang, X.; Routledge, M.N.; Peng, C.; Zhang, C. Highly resistant and sensitive colorimetric immunochromatographic assay for sibutramine (SBT) illegally adulterated into diet food based on PDA/AuNP labelling. Analyst 2023, 148, 5094–5104. [Google Scholar] [CrossRef]
- Bailey, C.G.; Nothling, M.D.; Fillbrook, L.L.; Vo, Y.; Beves, J.E.; McCamey, D.R.; Stenzel, M.H. Polydopamine as a Visible-Light Photosensitiser for Photoinitiated Polymerisation. Angew. Chem. Int. Ed. 2023, 62, e202301678. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Z.; Ding, T.; Tang, J.; Xie, X.; Xing, Y.; Wang, L.; Zhang, J.; Cai, K. Interfacial Engineering of Hybrid Polydopamine/Polypyrrole Nanosheets with Narrow Band Gaps for Fluorescence Sensing of MicroRNA. ACS Appl. Mater. Interfaces 2021, 13, 42183–42194. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, P.; Zhou, W.; Ding, J.; Liu, J. Bioorthogonal DNA Adsorption on Polydopamine Nanoparticles Mediated by Metal Coordination for Highly Robust Sensing in Serum and Living Cells. ACS Nano 2018, 12, 9070–9080. [Google Scholar] [CrossRef]
- Zandieh, M.; Liu, J. Transition Metal-Mediated DNA Adsorption on Polydopamine Nanoparticles. Langmuir 2020, 36, 3260–3267. [Google Scholar] [CrossRef]
- Craciun, A.-M.; Astilean, S.; Focsan, M.; Lamy de la Chapelle, M. Gold nanoparticles conjugated with fluorophore-labeled DNA: Overview of sensing and imaging applications. TrAC Trends Anal. Chem. 2024, 180, 117913. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hirao, G.; Fukuzumi, N.; Asahi, T.; Maeda, M.; Ogawa, A.; Zako, T. Effect of DNA Density on Nucleic Acid Detection Using Cross-Linking Aggregation of DNA-Modified Gold Nanoparticles. Langmuir 2025, 41, 4560–4568. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.Y.; Gao, P.F.; Gao, M.X.; Huang, C.Z. Polydopamine-embedded Cu2−xSe nanoparticles as a sensitive biosensing platform through the coupling of nanometal surface energy transfer and photo-induced electron transfer. Analyst 2015, 140, 4121–4129. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, R. Nanomaterial-enhanced fluorescence sensors for dopamine neurotransmitters: A photophysical perspective. Anal. Methods 2025, 17, 4251–4292. [Google Scholar] [CrossRef]
- Epanchintseva, A.V.; Gorbunova, E.A.; Ryabchikova, E.I.; Pyshnaya, I.A.; Pyshnyi, D.V. Effect of Fluorescent Labels on DNA Affinity for Gold Nanoparticles. Nanomaterials 2021, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lu, D.; Liang, H.; Xie, S.; Zhang, X.; Liu, Q.; Yuan, Q.; Tan, W. mRNA-Initiated, Three-Dimensional DNA Amplifier Able to Function inside Living Cells. J. Am. Chem. Soc. 2018, 140, 258–263. [Google Scholar] [CrossRef]
- Jung, H.-S.; Cho, K.-J.; Joo, S.; Lee, M.; Kim, M.Y.; Kwon, I.H.; Song, N.W.; Shim, J.H.; Neuman, K.C. Mesoporous Polydopamine-Encapsulated Fluorescent Nanodiamonds: A Versatile Platform for Biomedical Applications. ACS Appl. Mater. Interfaces 2023, 15, 33425–33436. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, D.; Pedrero, M.; Guo, Z.; Pingarrón, J.M.; Campuzano, S.; Zou, X. Advances and opportunities of polydopamine coating in biosensing: Preparation, functionality, and applications. Coord. Chem. Rev. 2024, 501, 215564. [Google Scholar] [CrossRef]
- Bitter, E.E.; Townsend, M.H.; Erickson, R.; Allen, C.; O’Neill, K.L. Thymidine kinase 1 through the ages: A comprehensive review. Cell Biosci. 2020, 10, 138. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, N.; Zhou, F.; Qiao, R.; Wan, Y.; Liu, R.; Yang, S.; Gu, M.; Xu, H.; Dong, X.; et al. Orthogonally Sequential Activation of Self-Powered DNAzymes Cascade for Reliable Monitoring of mRNA in Living Cells. Adv. Healthc. Mater. 2024, 13, 2303074. [Google Scholar] [CrossRef]
- Zhong, X.; Hua, J.; Shi, M.; He, Y.; Huang, Y.; Wang, B.; Zhang, L.; Zhao, S.; Hou, L.; Liang, H. Self-Feedback DNAzyme Motor for Cascade-Amplified Imaging of mRNA in Live Cells and In Vivo. ACS Sens. 2024, 9, 1280–1289. [Google Scholar] [CrossRef]
- Wang, Q.; Du, Y.; Zheng, J.; Shi, L.; Li, T. G-Quadruplex-Programmed Versatile Nanorobot Combined with Chemotherapy and Gene Therapy for Synergistic Targeted Therapy. Small 2024, 20, 2400267. [Google Scholar] [CrossRef] [PubMed]
- Steigmeyer, A.D.; Lowery, S.C.; Rangel-Angarita, V.; Malaker, S.A. Decoding Extracellular Protein Glycosylation in Human Health and Disease. Annu. Rev. Anal. Chem. 2025, 18, 241–264. [Google Scholar] [CrossRef] [PubMed]
- Bastús, N.G.; Comenge, J.; Puntes, V. Kinetically Controlled Seeded Growth Synthesis of Citrate-Stabilized Gold Nanoparticles of up to 200 nm: Size Focusing versus Ostwald Ripening. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Choi, C.K.K.; Hong, H.; Xiao, Y.; Kwok, M.L.; Liu, H.; Tian, X.Y.; Choi, C.H.J. Dopamine Receptor-Mediated Binding and Cellular Uptake of Polydopamine-Coated Nanoparticles. ACS Nano 2021, 15, 13871–13890. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, Y.; Dai, P.; Cheng, G.; He, P.; Fang, Y. The Split Primer Ligation-triggered 8-17 DNAzyme Assisted Cascade Rolling Circle Amplification for High Specific Detection of Liver Cancer-involved mRNAs: TK1 and c-myc. Electroanalysis 2020, 32, 554–560. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, Y.; Cheng, G.; He, P.; Fang, Y. Dual-signal electrochemical sensor for detection of cancer cells by the split primer ligation-triggered catalyzed hairpin assembly. Talanta 2020, 217, 121079. [Google Scholar] [CrossRef]
- He, M.; He, M.; Nie, C.; Yi, J.; Zhang, J.; Chen, T.; Chu, X. mRNA-Activated Multifunctional DNAzyme Nanotweezer for Intracellular mRNA Sensing and Gene Therapy. ACS Appl. Mater. Interfaces 2021, 13, 8015–8025. [Google Scholar] [CrossRef]
- Li, T.; Sun, M.; Xia, S.; Huang, T.; Li, R.-T.; Li, C.; Dai, Z.; Chen, J.; Chen, J.; Jia, N. A binary system based DNA tetrahedron and fluorogenic RNA aptamers for highly specific and label-free mRNA imaging in living cells. Talanta 2024, 269, 125465. [Google Scholar]
- Jiang, Y.; Xu, X.; Fang, X.; Cai, S.; Wang, M.; Xing, C.; Lu, C.; Yang, H. Self-Assembled mRNA-Responsive DNA Nanosphere for Bioimaging and Cancer Therapy in Drug-Resistant Cells. Anal. Chem. 2020, 92, 11779–11785. [Google Scholar] [CrossRef]
- Li, X.; Zou, R.; Chen, F.; Chen, C.; Gong, H.; Cai, C. Stimulus-responsive strategy based on MnO2 nanosheet-modified mesoporous silica nanoprobes for accurate multiple mRNAs detection. Talanta 2023, 255, 124179. [Google Scholar]
- Wu, M.-J.; Tseng, W.-L. Rapid, facile, reagentless, and room-temperature conjugation of monolayer MoS2 nanosheets with dual-fluorophore-labeled flares as nanoprobes for ratiometric sensing of TK1 mRNA in living cells. J. Mater. Chem. B 2020, 8, 1692–1698. [Google Scholar]
- Ma, W.; Chen, B.; Jia, R.; Sun, H.; Huang, J.; Cheng, H.; Wang, H.; He, X.; Wang, K. In Situ Hand-in-Hand DNA Tile Assembly: A pH-Driven and Aptamer-Targeted DNA Nanostructure for TK1 mRNA Visualization and Synergetic Killing of Cancer Cells. Anal. Chem. 2021, 93, 10511–10518. [Google Scholar] [CrossRef]
- Gong, H.; Yao, S.; Zhao, X.; Chen, F.; Chen, C.; Cai, C. Construction of an autofluorescence interference-free phosphorescence biosensor for the specific detection of TK1 mRNA. Anal. Chim. Acta 2024, 1303, 342508. [Google Scholar] [CrossRef]
- Buckhout-White, S.; Spillmann, C.M.; Algar, W.R.; Khachatrian, A.; Melinger, J.S.; Goldman, E.R.; Ancona, M.G.; Medintz, I.L. Assembling programmable FRET-based photonic networks using designer DNA scaffolds. Nat. Commun. 2014, 5, 5615. [Google Scholar] [CrossRef]
- Klein, W.P.; Díaz, S.A.; Buckhout-White, S.; Melinger, J.S.; Cunningham, P.D.; Goldman, E.R.; Ancona, M.G.; Kuang, W.; Medintz, I.L. Resonance Energy Transfer: Utilizing HomoFRET to Extend DNA-Scaffolded Photonic Networks and Increase Light-Harvesting Capability. Adv. Opt. Mater. 2018, 6, 1700679. [Google Scholar] [CrossRef]
- Chen, C.; Midelet, C.; Bhuckory, S.; Hildebrandt, N.; Werts, M.H.V. Nanosurface Energy Transfer from Long-Lifetime Terbium Donors to Gold Nanoparticles. J. Phys. Chem. C 2018, 122, 17566–17574. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Wang, W.; Zou, Y.; Li, C.; Zou, H.; Huang, C.; Zhan, L. Stable Gold@Polydopamine@ssDNA Bioconjugates for Highly Efficient Detection of Tumor-Related mRNA in Living Cells. Molecules 2025, 30, 3551. https://doi.org/10.3390/molecules30173551
Hu S, Wang W, Zou Y, Li C, Zou H, Huang C, Zhan L. Stable Gold@Polydopamine@ssDNA Bioconjugates for Highly Efficient Detection of Tumor-Related mRNA in Living Cells. Molecules. 2025; 30(17):3551. https://doi.org/10.3390/molecules30173551
Chicago/Turabian StyleHu, Senhao, Wenjing Wang, Yu Zou, Chunmei Li, Hongyan Zou, Chengzhi Huang, and Lei Zhan. 2025. "Stable Gold@Polydopamine@ssDNA Bioconjugates for Highly Efficient Detection of Tumor-Related mRNA in Living Cells" Molecules 30, no. 17: 3551. https://doi.org/10.3390/molecules30173551
APA StyleHu, S., Wang, W., Zou, Y., Li, C., Zou, H., Huang, C., & Zhan, L. (2025). Stable Gold@Polydopamine@ssDNA Bioconjugates for Highly Efficient Detection of Tumor-Related mRNA in Living Cells. Molecules, 30(17), 3551. https://doi.org/10.3390/molecules30173551






