Development and Evaluation of Egg-Free Mayonnaise Stabilized with Aquafaba and Gum Tragacanth: Functional, Sensory, and Storage Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) in Aquafaba Extracts
2.2. Anti-Oxidant Analysis
2.3. Physicochemical Analysis of Plant-Based Mayonnaise
2.4. Stability in Plant-Based Mayonnaise
2.5. Mold Count
2.6. Viscosity
2.7. Textural Analysis
2.8. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis of Mayonnaise Samples
2.9. Sensory Evaluation of Plant-Based Mayonnaise
3. Materials and Methods
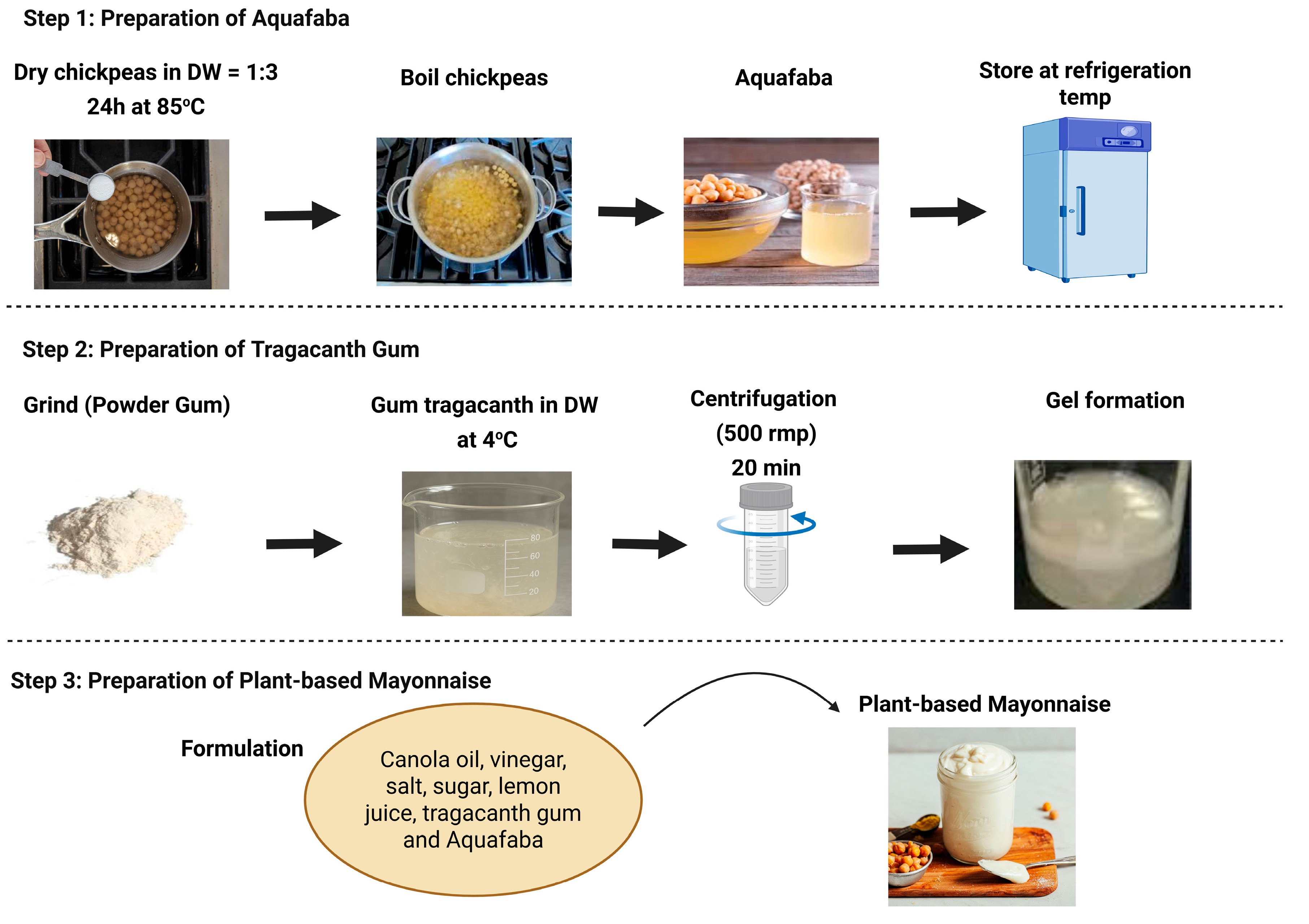
3.1. Raw Materials
3.2. Process of Aquafaba Production
3.3. Preparation of Gum Tragacanth Solution
3.4. Preparation of Plant-Based Mayonnaise
3.5. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) in Aquafaba Extracts
3.6. Anti-Oxidant Activity
3.7. Physicochemical Analysis
3.8. Creaming Index Analysis
3.9. Physical and Heat Stability (%)
3.10. Microbiological Analysis
3.11. Viscosity Analysis
3.12. Texture Analysis
3.13. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
3.14. Sensory Evaluation
3.15. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedramnia, A.; Elhamirad, A.H.; Habashi, V. Textural properties of low fat mayonnaise with whey protein concentrate and Tragacanth gum as egg and fat substitutes. Foods Raw Mater. 2021, 9, 19–23. [Google Scholar] [CrossRef]
- Balaghi, S.; Mohammadifar, M.A.; Zargaraan, A.; Gavlighi, H.A.; Mohammadi, M. Compositional analysis and rheological characterization of gum tragacanth exudates from six species of Iranian Astragalus. Food Hydrocoll. 2011, 25, 1775–1784. [Google Scholar] [CrossRef]
- Mirzanajafi-Zanjani, M.; Yousefi, M.; Ehsani, A. Challenges and approaches for production of a healthy and functional mayonnaise sauce. Food Sci. Nutr. 2019, 7, 2471–2484. [Google Scholar] [CrossRef]
- Dona, D.W.; Suphioglu, C. Egg Allergy: Diagnosis and Immunotherapy. Int. J. Mol. Sci. 2020, 21, 5010. [Google Scholar] [CrossRef]
- Mustafa, R.; He, Y.; Shim, Y.Y.; Reaney, M.J.T. Aquafaba, wastewater from chickpea canning, functions as an egg replacer in sponge cake. Int. J. Food Sci. Technol. 2018, 53, 2247–2255. [Google Scholar] [CrossRef]
- Serventi, L.; Wang, S.; Zhu, J.; Liu, S.; Fei, F. Cooking water of yellow soybeans as emulsifier in gluten-free crackers. Eur. Food Res. Technol. 2018, 244, 2141–2148. [Google Scholar] [CrossRef]
- Stantiall, S.E.; Dale, K.J.; Calizo, F.S.; Serventi, L. Application of pulses cooking water as functional ingredients: The foaming and gelling abilities. Eur. Food Res. Technol. 2017, 244, 97–104. [Google Scholar] [CrossRef]
- He, Y.; Meda, V.; Reaney, M.J.T.; Mustafa, R. Aquafaba, a new plant-based rheological additive for food applications. Trends Food Sci. Technol. 2021, 111, 27–42. [Google Scholar] [CrossRef]
- Menezes, R.C.F.d.; Gomes, Q.C.d.C.; Almeida, B.S.d.; Matos, M.F.R.d.; Pinto, L.C. Plant-based mayonnaise: Trending ingredients for innovative products. Int. J. Gastron. Food Sci. 2022, 30, 100599. [Google Scholar] [CrossRef]
- Amiri, M.S.; Joharchi, M.R.; TaghavizadehYazdi, M.E. Ethno-medicinal plants used to cure jaundice by traditional healers of Mashhad, Iran. Iran. J. Pharm. Res. 2014, 13, 157. [Google Scholar]
- Taghavizadeh Yazdi, M.E.; Nazarnezhad, S.; Mousavi, S.H.; Sadegh Amiri, M.; Darroudi, M.; Baino, F.; Kargozar, S. Gum Tragacanth (GT): A Versatile Biocompatible Material beyond Borders. Molecules 2021, 26, 1510. [Google Scholar] [CrossRef]
- Abdi, G.; Jain, M.; Patil, N.; Tariq, M.; Choudhary, S.; Kumar, P.; Raj, N.S.; Mohsen Ali, S.S.; Uthappa, U.T. Tragacanth gum-based hydrogels for drug delivery and tissue engineering applications. Front. Mater. 2024, 11, 1296399. [Google Scholar] [CrossRef]
- Pocan, P.; Ilhan, E.; Oztop, M.H. Characterization of Emulsion Stabilization Properties of Gum Tragacanth, Xanthan Gum and Sucrose Monopalmitate: A Comparative Study. J. Food Sci. 2019, 84, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Ham, S.H.; Hahn, J.; Choi, Y.J. Developing plant-based mayonnaise using pea protein-xanthan gum conjugates: A maillard reaction approach. Lwt 2023, 185, 115137. [Google Scholar] [CrossRef]
- Coelho, P.; Serrano, C.; Komora, N.; Raymundo, A. From a Coriander Mayonnaise to a Vegan Analogue: Assessing pH and Salt Influence in a Saccharomyces cerevisiae Yeast Protein Extract and Chlorella vulgaris Mixed System. Foods 2025, 14, 587. [Google Scholar] [CrossRef] [PubMed]
- Abedi-Firoozjah, R.; Azadinejad, H.; Hosseini, A.; Afrah, A.; Abdolmaleki, K. Potential of Fat Replacers To Develop Low/Or Reduced-Fat Mayonnaise and Salad Dressing Systems: An Update Review. Curr. Nutr. Food Sci. 2025, 21, 156–178. [Google Scholar] [CrossRef]
- Gavlighi, H.A.; Meyer, A.S.; Zaidel, D.N.A.; Mohammadifar, M.A.; Mikkelsen, J.D. Stabilization of emulsions by gum tragacanth (Astragalus spp.) correlates to the galacturonic acid content and methoxylation degree of the gum. Food Hydrocoll. 2013, 31, 5–14. [Google Scholar] [CrossRef]
- Nejatian, M.; Abbasi, S.; Azarikia, F. Gum Tragacanth: Structure, characteristics and applications in foods. Int. J. Biol. Macromol. 2020, 160, 846–860. [Google Scholar] [CrossRef]
- Shen, Y.; Babu, K.S.; Amamcharla, J.; Li, Y. Emulsifying properties of pea protein/guar gum conjugates and mayonnaise application. Int. J. Food Sci. Technol. 2022, 57, 3955–3966. [Google Scholar] [CrossRef]
- Odep, L.A.; Mahungu, S.M.; Omwamba, M.N. Physico-Chemical, and Sensory Properties of Mayonnaise Substitute Prepared from Chia Mucilage (Salvia hispanica L.) and Gum Arabic from Acacia senegal var. kerensis. Food Nutr. Sci. 2024, 15, 880–898. [Google Scholar] [CrossRef]
- Hashemi, M.; Aminlari, M.; Forouzan, M.; Moghimi, E.; Tavana, M.; Shekarforoush, S.; Mohammadifar, M. Production and Application of Lysozyme-Gum Arabic Conjugate in Mayonnaise as a Natural Preservative and Emulsifier. Pol. J. Food Nutr. Sci. 2018, 68, 33–43. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X.; Goff, H.D.; Cui, S.W. Application of yellow mustard gum in preparation of egg-free mayonnaise. Int. J. Food Sci. Technol. 2024, 59, 8972–8982. [Google Scholar] [CrossRef]
- Jeong, H.; Oh, I. Physicochemical and structural properties of vegan mayonnaise prepared with peanut sprout oil and aquafaba. Food Chem. X 2025, 27, 102463. [Google Scholar] [CrossRef]
- Yousefi, M.; Shadnoush, M.; Sohrabvandi, S.; Khorshidian, N.; Mortazavian, A.M. Encapsulation systems for delivery of flavonoids: A Review. Biointerface Res. Appl. Chem. 2021, 11, 13934–13951. [Google Scholar] [CrossRef]
- Sørensen, A.D.M.; Nielsen, N.S.; Decker, E.A.; Let, M.B.; Xu, X.; Jacobsen, C. The Efficacy of Compounds with Different Polarities as Antioxidants in Emulsions with Omega-3 Lipids. J. Am. Oil Chem. Soc. 2010, 88, 489–502. [Google Scholar] [CrossRef]
- Evanuarini, H.; Susilo, A. The Quality of Low Fat Mayonnaise Using Banana Peel Flour as Stabilizer. IOP Conf. Ser. Earth Environ. Sci. 2020, 478, 012091. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I. Advances in the Design and Production of Reduced-Fat and Reduced-Cholesterol Salad Dressing and Mayonnaise: A Review. Food Bioprocess Technol. 2013, 6, 648–670. [Google Scholar] [CrossRef]
- Ali, M.R.; El Said, R.M. Assessment of the potential of Arabic gum as an antimicrobial and antioxidant agent in developing vegan “egg-free” mayonnaise. J. Food Saf. 2020, 40, e12771. [Google Scholar] [CrossRef]
- Orgulloso-Bautista, S.; Ortega-Toro, R.; Garcia Zapateiro, L.A. Design and Application of Hydrocolloids from Butternut Squash (Cucurbita moschata) Epidermis as a Food Additive in Mayonnaise-type Sauces. ACS Omega 2021, 6, 5499–5508. [Google Scholar] [CrossRef]
- McClements, J.D. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Amin, M.H.H.; Elbeltagy, A.E.; Mustafa, M.; Khalil, A.H. Development of low fat mayonnaise containing different types and levels of hydrocolloid gum. J. Agroaliment. Process. Technol. 2014, 20, 54–63. [Google Scholar]
- Khalid, S.; Arshad, M.; Mahmood, S.; Siddique, F.; Roobab, U.; Ranjha, M.M.A.N.; Lorenzo, J.M. Extraction and Quantification of Moringa oleifera Leaf Powder Extracts by HPLC and FTIR. Food Anal. Methods 2023, 16, 787–797. [Google Scholar] [CrossRef]
- He, Y.; Purdy, S.K.; Tse, T.J.; Tar’an, B.; Meda, V.; Reaney, M.J.T.; Mustafa, R. Standardization of Aquafaba Production and Application in Vegan Mayonnaise Analogs. Foods 2021, 10, 1978. [Google Scholar] [CrossRef] [PubMed]
- Raikos, V.; Hayes, H.; Ni, H. Aquafaba from commercially canned chickpeas as potential egg replacer for the development of vegan mayonnaise: Recipe optimisation and storage stability. Int. J. Food Sci. Technol. 2019, 55, 1935–1942. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Raventos, R.M.L. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; The Association of Official Analytical Chemicals, U.V.: Rockville, MD, USA, 1990; Volume 1. [Google Scholar]
- Marshall, I.H.; Jones, R. International Conference on Composite Structures (10th) (ICCS/10) Held in Melbourne, Australia, 15–17 November 1999. In Composite Structures; Elsevier: Amsterdam, The Netherlands, 1999; Volume 47, pp. 1–4, (No. ISSN02638223). [Google Scholar]
- AOAC. Official Methods of Analysis; AOAC: Arlington, VA, USA, 2000. [Google Scholar]
- Shantha, N.C.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [CrossRef]
- Shamooshaki, M.G.; Mahounak, A.S.; Ghorbani, M.; Maghsouldloo, Y.; Ziaeifar, A.M. Effect of Milk and Xanthan as Egg Replacement on the Physical Properties of Mayonnaise. Int. Lett. Nat. Sci. 2015, 49, 24–34. [Google Scholar] [CrossRef][Green Version]
- Mun, S.; Kim, Y.L.; Kang, C.G.; Park, K.H.; Shim, J.Y.; Kim, Y.R. Development of reduced-fat mayonnaise using 4alphaGTase-modified rice starch and xanthan gum. Int. J. Biol. Macromol. 2009, 44, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, I.; Chhibber, S.; Capalash, N.; Sharma, P. Production of cellulase-free xylanase from Bacillus megaterium by solid state fermentation for biobleaching of pulp. Curr. Microbiol. 2006, 53, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Chippie, A.L.; Jamieson, P.R.; Golt, C.M.; Hsu, C.H.; Martin Lo, Y. Quantitative analysis of fat and moisture in mayonnaise using Fourier Transform Infrared Spectrometer. Int. J. Food Prop. 2002, 5, 655–665. [Google Scholar] [CrossRef]
- Herald, T.J.; Abugoush, M.; Aramouni, F. Physical and sensory properties of egg yolk and egg yolk substitutes in a model mayonnaise system. J. Texture Stud. 2009, 40, 692–709. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics, a Biometrical Approach, 2nd ed.; McGraw-Hill Kogakusha, Ltd.: Tokyo, Japan, 1981; 633p. [Google Scholar]










| Samples | ||||
|---|---|---|---|---|
| Ingredients (%) | Control (T0) | GT 30% (T1) | GT 50% (T2) | GT 100% (T3) |
| Canola oil | 80 | 80 | 75 | 70 |
| Egg | 15 | - | - | - |
| Vinegar | 4 | 3 | 3 | 3 |
| Salt | 0.5 | 0.5 | 0.5 | 0.5 |
| Sugar | 0.5 | 0.5 | 0.5 | 0.5 |
| Lemon Juice | 1 | 1 | 1 | 1 |
| Tragacanth Gum | - | 0.3 | 0.5 | 1 |
| Aquafaba | - | 15 | 20 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafique, B.; Murtaza, M.A.; Farid, M.S.; Ameer, K.; Hussain, M.I.; Sienkiewicz, M.; Lichota, A.; Łopusiewicz, Ł. Development and Evaluation of Egg-Free Mayonnaise Stabilized with Aquafaba and Gum Tragacanth: Functional, Sensory, and Storage Properties. Molecules 2025, 30, 3511. https://doi.org/10.3390/molecules30173511
Shafique B, Murtaza MA, Farid MS, Ameer K, Hussain MI, Sienkiewicz M, Lichota A, Łopusiewicz Ł. Development and Evaluation of Egg-Free Mayonnaise Stabilized with Aquafaba and Gum Tragacanth: Functional, Sensory, and Storage Properties. Molecules. 2025; 30(17):3511. https://doi.org/10.3390/molecules30173511
Chicago/Turabian StyleShafique, Bakhtawar, Mian Anjum Murtaza, Muhammad Salman Farid, Kashif Ameer, Muhammad Imran Hussain, Monika Sienkiewicz, Anna Lichota, and Łukasz Łopusiewicz. 2025. "Development and Evaluation of Egg-Free Mayonnaise Stabilized with Aquafaba and Gum Tragacanth: Functional, Sensory, and Storage Properties" Molecules 30, no. 17: 3511. https://doi.org/10.3390/molecules30173511
APA StyleShafique, B., Murtaza, M. A., Farid, M. S., Ameer, K., Hussain, M. I., Sienkiewicz, M., Lichota, A., & Łopusiewicz, Ł. (2025). Development and Evaluation of Egg-Free Mayonnaise Stabilized with Aquafaba and Gum Tragacanth: Functional, Sensory, and Storage Properties. Molecules, 30(17), 3511. https://doi.org/10.3390/molecules30173511








