Evaluation of Salvia yangii Extract as a Promising Protective Raw Material Applied Topically to the Skin
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Constituents of Extracts
2.2. Antioxidant Activity of Extract
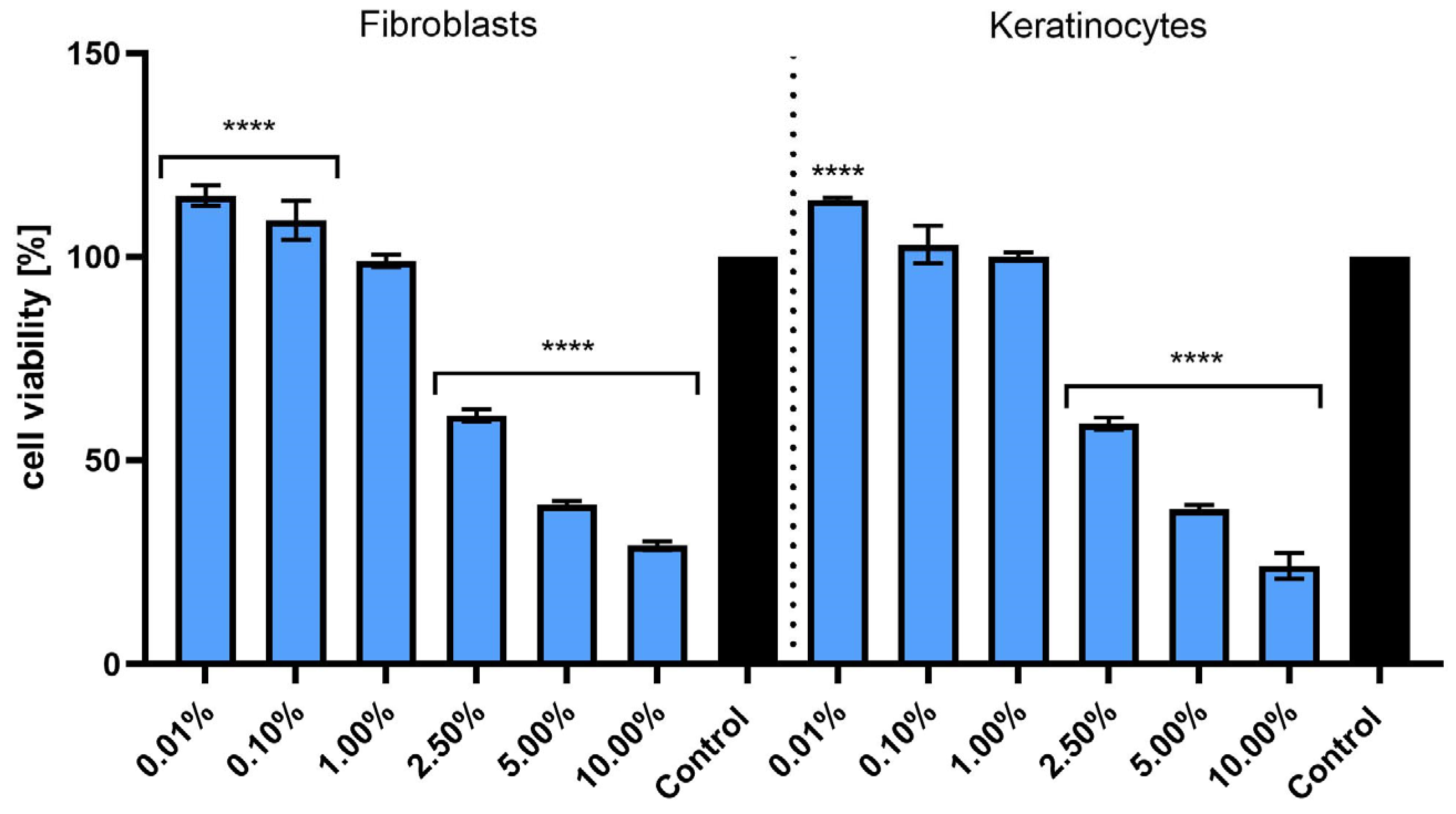
2.3. Effect of Extract on Skin Cell Viability
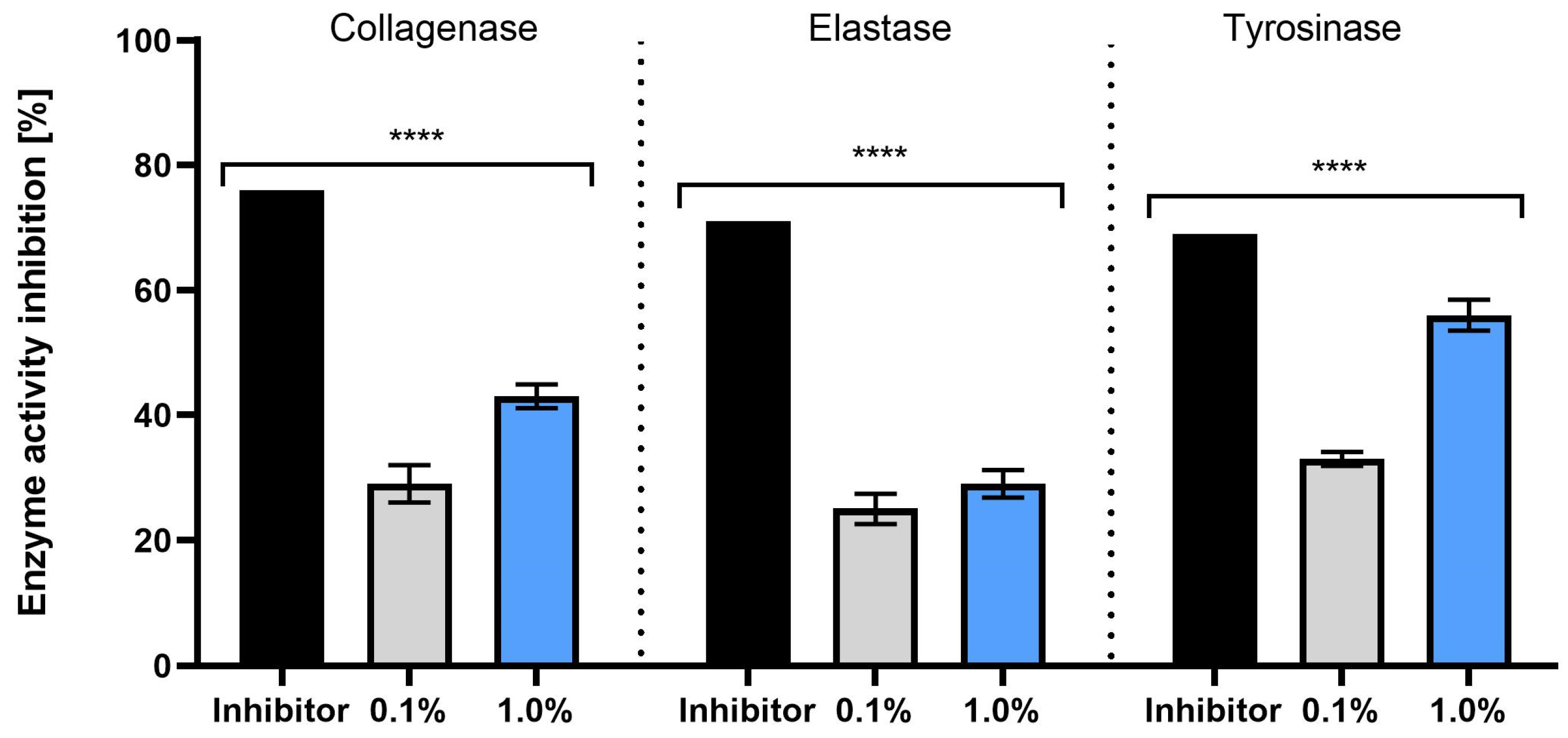
2.4. Anti-Aging Activity of Extract
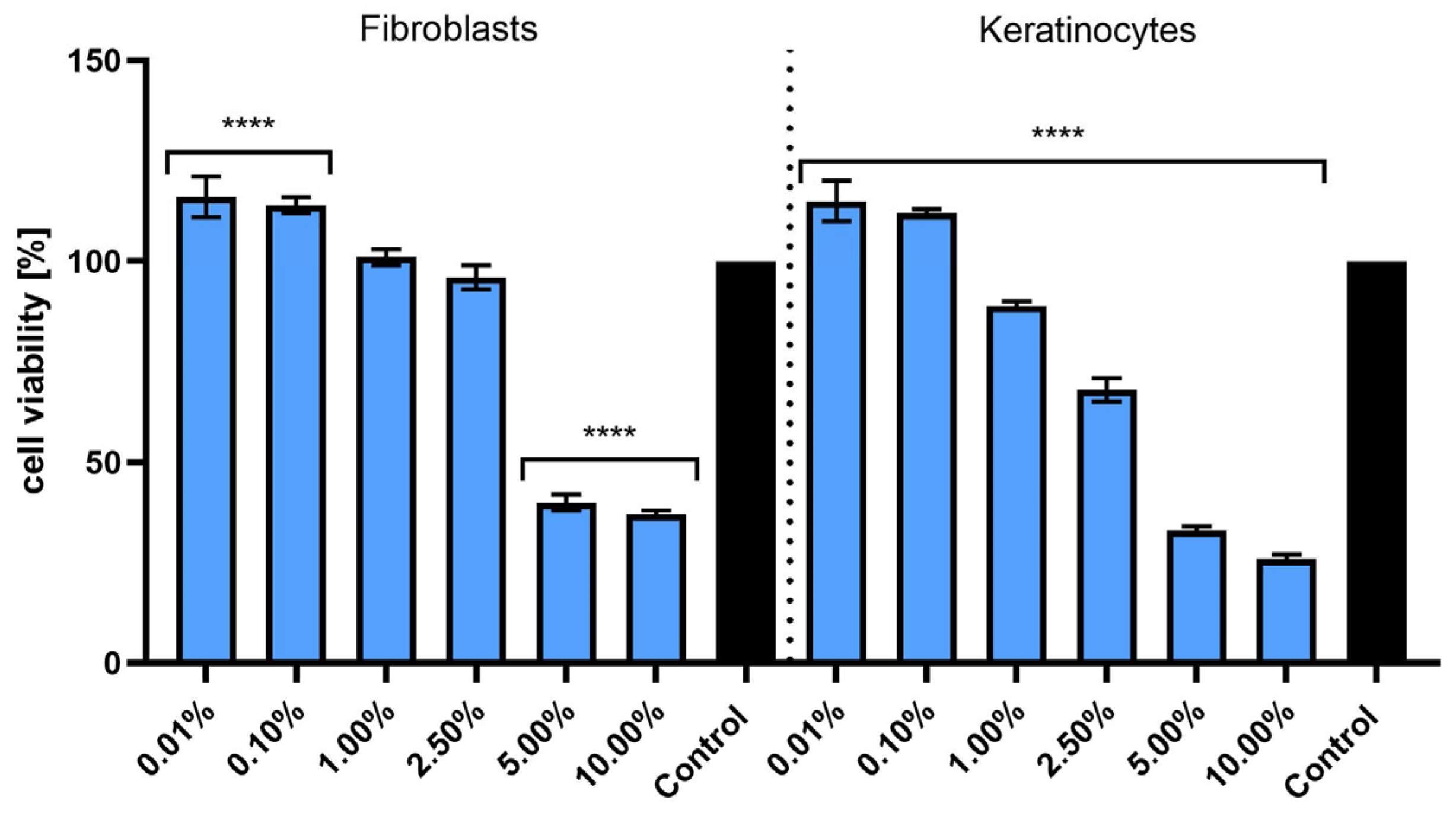
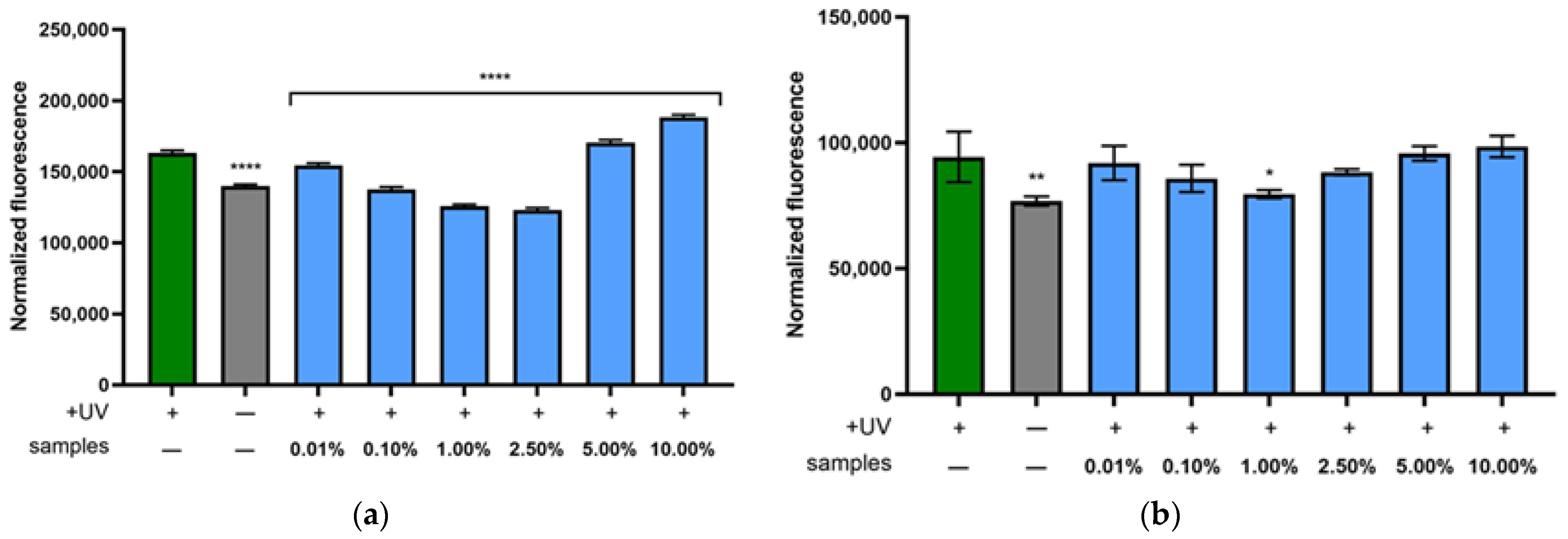
2.5. Assessment of Photoprotective Activity of Extract on Keratinocytes and Fibroblasts
2.6. Antimicrobial and Antibiofilm Activity of Extract
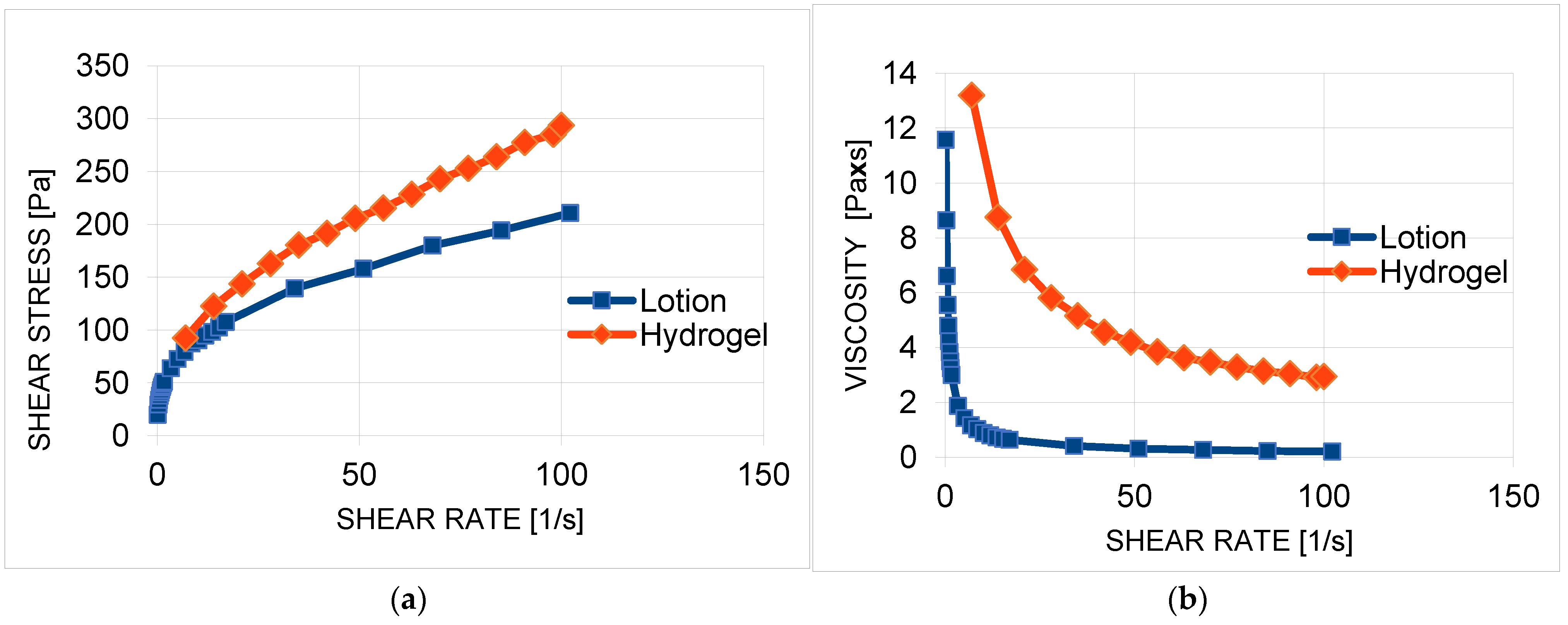
2.7. Rheology and Texture Analysis of Extract-Based Formulation
3. Materials and Methods
3.1. Plant Material and Extraction
3.2. Phytochemical Characteristics of Extract
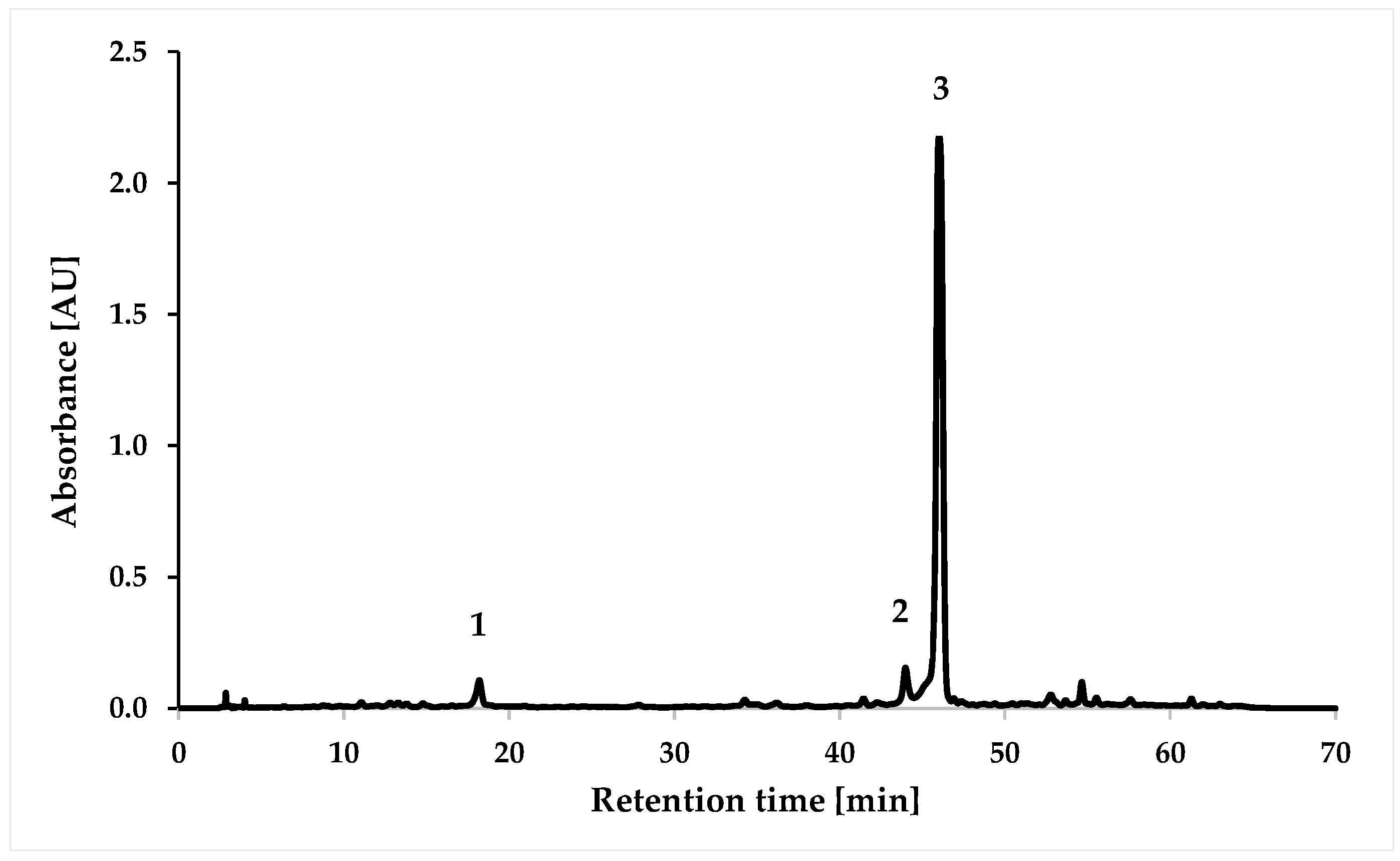
3.3. HPLC Analysis of Extract
3.4. Antioxidant Activity Evaluation of Extract
3.5. Cell Culture
3.6. Assessment of Cytotoxicity—Alamar Blue (AB) and Neutral Red (NR) Uptake Assays
3.7. Evaluation of Intracellular Reactive Oxygen Species (ROS) Levels in Skin Cells Without and After UVB Radiation Exposure
3.8. Determination of Anti-Collagenase and Anti-Elastase Activity of Extract
3.9. Determination of Anti-Tyrosinase Activity of Extract
3.10. Evaluation of the Antimicrobial Activity and Biofilm Formation Inhibition of Extract
3.11. Determination of Model Cosmetics Containing Extract
3.11.1. Preparation of Formulations
3.11.2. Rheological Properties of Extract-Based Formulation
3.11.3. Textural Properties of Extract-Based Formulation
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UAE | Ultrasound-assisted extraction |
| SFE | Supercritical fluid extraction |
| TP | Total polyphenol |
| GAE | Gallic acid equivalents |
| TF | Total flavonoid |
| CE | Catechin equivalents |
| TPA | Total phenolic acid |
| CAE | Caffeic acid equivalents |
| CT | Condensed tannin |
| DpE | Delphinidin equivalents |
| EHMC | Ethylhexyl methoxycinnamate |
| OCT | Octocrylene |
| EHS | Ethylhexyl salicylate |
| BMDBM | Avobenzone |
| BEMT | Bemotrizinol |
| FRAP | Ferric Reducing Antioxidant Power |
| TE | Trolox equivalent |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| TPTZ | 2,4,6-tripyridyl-s-triazine |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| MIC | Minimal Inhibitory Concentration |
| CLSI | Clinical and Laboratory Standards Institute |
| MBC | Minimum Bactericidal Concentration |
| MFC | Minimum Fungicidal Concentration |
| AB | Alamar Blue |
| NR | Neutral Red |
| ROS | Reactive Oxygen Species |
| CRT | Compression/Relaxation/Tension |
| ITPA | Instrumental Texture Profile Analysis |
| SPF | Sun Protection Factor |
| CF | Correction factor |
| EE | Erythemogenic effect |
References
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef] [PubMed]
- Cannavacciuolo, C.; Pagliari, S.; Celano, R.; Campone, L.; Rastrelli, L. Critical analysis of green extraction techniques used for botanicals: Trends, priorities, and optimization strategies-A review. TrAC Trends Anal. Chem. 2024, 173, 117627. [Google Scholar] [CrossRef]
- Ahmad, I.; Waheed, A.; Tahir, N.B.; Rais, A.K. Anti-inflammatory constituents from Perovskia atriplicifolia. Pharm. Biol. 2015, 53, 1628–1631. [Google Scholar] [CrossRef]
- Perveen, S.; Al-Taweel, A.M.; Fawzy, G.A.; Ibrahim, T.A.; Malik, A.; Khan, A. Cholinesterase Inhibitory Triterpenes from Perovskia atriplicifolia. Asian J. Chem. 2014, 26, 6163–6166. [Google Scholar] [CrossRef]
- Ślusarczyk, S.; Deniz, F.S.S.; Abel, R.; Pecio, Ł.; Pérez-Sánchez, H.; Cerón-Carrasco, J.P.; den-Haan, H.; Banerjee, P.; Preissner, R.; Krzyżak, E.; et al. Norditerpenoids with Selective Anti-Cholinesterase Activity from the Roots of Perovskia atriplicifolia Benth. Int. J. Mol. Sci. 2020, 21, 4475. [Google Scholar] [CrossRef]
- Gostin, I.N.; Popescu, I.E. Russian Sage Revealed: Exploring Biology, Cultivation, and Chemical Dimensions of Salvia yangii. Agronomy 2025, 15, 868. [Google Scholar] [CrossRef]
- Miroliaei, M.; Aminjafari, A.; Ślusarczyk, S.; Nawrot-Hadzik, I.; Rahimmalek, M.; Matkowski, A. Inhibition of glycation-induced cytotoxicity, protein glycation, and activity of proteolytic enzymes by extract from Perovskia atriplicifolia Roots. Pharmacogn. Mag. 2017, 13, 676–683. [Google Scholar] [CrossRef]
- Tarawneh, A.; León, F.; Pettaway, S.; Elokely, K.M.; Klein, M.L.; Lambert, J.; Mansoor, A.; Cutler, S.J. Flavonoids from Perovskia atriplicifolia and Their in Vitro Displacement of the Respective Radioligands for Human Opioid and Cannabinoid Receptors. J. Nat. Prod. 2015, 78, 1461–1465. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Yu, Y.-J.; Huang, C.-G.; Huang, X.-Z.; Hu, Q.-F.; Yang, G.-Y.; Wang, H.-B.; Zhang, X.-Y.; Li, G.-P. Icetexane Diterpenoids from Perovskia atriplicifolia. Planta Medica 2015, 81, 241–246. [Google Scholar] [CrossRef]
- Rabiee, M.; Manafi, N. Healing Effect of Perovskia Abrotanoides Karel and Expression of VEGF and TGF-Β Genes in Burn Injury of Rats. Int. J. Nutr. Sci. 2020, 5, 43–44. [Google Scholar]
- Bielecka, M.; Pencakowski, B.; Stafiniak, M.; Jakubowski, K.; Rahimmalek, M.; Gharibi, S.; Matkowski, A.; Ślusarczyk, S. Metabolomics and DNA-Based Authentication of Two Traditional Asian Medicinal and Aromatic Species of Salvia subg. Perovskia. Cells 2021, 10, 112. [Google Scholar] [CrossRef]
- Ślusarczyk, S.; Topolski, J.; Domaradzki, K.; Adams, M.; Hamburger, M.; Matkowski, A. Isolation and Fast Selective Determination of Nor-abietanoid Diterpenoids from Perovskia atriplicifolia Roots Using LC-ESI-MS/MS with Multiple Reaction Monitoring. Nat. Prod. Commun. 2015, 10, 1149–1152. [Google Scholar] [CrossRef]
- Senol, F.S.; Ślusarczyk, S.; Matkowski, A.; Pérez-Garrido, A.; Girón-Rodríguez, F.; Cerón-Carrasco, J.P.; Den-Haan, H.; Peña-García, J.; Pérez-Sánchez, H.; Domaradzki, K.; et al. Selective in vitro and in silico butyrylcholinesterase inhibitory activity of diterpenes and rosmarinic acid isolated from Perovskia atriplicifolia Benth. And Salvia glutinosa L. Phytochemistry 2017, 133, 33–44. [Google Scholar] [CrossRef]
- Perveen, S.; Khan, S.B.; Malik, A.; Tareen, R.B.; Nawaz, S.A.; Choudhary, M.I. Phenolic constituents from Perovskia atriplicifolia. Nat. Prod. Res. 2006, 20, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, Z.; Rahimmalek, M.; Sabzalian, M.R. Variations in Essential Oil Composition and Antioxidant Activity in Perovskia abrotanoides KAR. Collected from Different Regions in Iran. Chem. Biodivers. 2018, 15, e1700565. [Google Scholar] [CrossRef] [PubMed]
- Topal, M.; Gulcin, I. Evaluation of the in vitro antioxidant, antidiabetic and anticholinergic properties of rosmarinic acid from rosemary (Rosmarinus officinalis L.). Biocatal. Agric. Biotechnol. 2022, 43, 102417. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Aslam Gondal, T.; Imran, A.; Shahbaz, M.; Muhammad Amir, R.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G.; et al. Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]
- Spagnol, C.M.; Assis, R.P.; Brunetti, I.L.; Isaac, V.L.B.; Salgado, H.R.N.; Corrêa, M.A. In vitro methods to determine the antioxidant activity of caffeic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 358–366. [Google Scholar] [CrossRef]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef]
- Zuo, A.R.; Dong, H.H.; Yu, Y.Y.; Shu, Q.L.; Zheng, L.X.; Yu, X.Y.; Cao, S.W. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin. Med. 2018, 13, 51. [Google Scholar] [CrossRef]
- Okediran, A.P. Evaluation Of In Vitro Methods of Antioxidant Activity Determination Using Caffeic Acid. Ph.D. Thesis, University of Ghana, Accra, Ghana, 2015; pp. 1–82. [Google Scholar]
- Al-Ashaal, H.A.; El-Sheltawy, S.T. Antioxidant capacity of hesperidin from Citrus peel using electron spin resonance and cytotoxic activity against human carcinoma cell lines. Pharm. Biol. 2011, 49, 276–282. [Google Scholar] [CrossRef]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef]
- Farhan, M. The Promising Role of Polyphenols in Skin Disorders. Molecules 2024, 29, 865. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Jodynis-Liebert, J.; Kujawska, M. Biphasic Dose-Response Induced by Phytochemicals: Experimental Evidence. J. Clin. Med. 2020, 9, 718. [Google Scholar] [CrossRef] [PubMed]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic dietary phytochemicals. Neuromolecular Med. 2008, 10, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Skalicka-Woźniak, K.; Mihai, C.T.; Gradinaru, A.C.; Mandici, A.; Ciocarlan, N.; Miron, A.; Aprotosoaie, A.C. Chemical Profile and Bioactivity Evaluation of Salvia Species from Eastern Europe. Antioxidants 2023, 12, 1514. [Google Scholar] [CrossRef]
- Zhumaliyeva, G.; Zhussupova, A.; Zhusupova, G.E.; Błońska-Sikora, E.; Cerreto, A.; Omirbekova, N.; Zhunusbayeva, Z.; Gemejiyeva, N.; Ramazanova, M.; Wrzosek, M.; et al. Natural Compounds of Salvia L. Genus and Molecular Mechanism of Their Biological Activity. Biomedicines 2023, 11, 3151. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants: A comprehensive review. Arch. Toxicol. 2025, 99, 1893–1997. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-Oxidant Activity of Polyphenols and its Implication on Cancer Chemoprevention and Chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef]
- Chedea, V.S.; Tomoiagǎ, L.L.; Macovei, Ş.O.; Mǎgureanu, D.C.; Iliescu, M.L.; Bocsan, I.C.; Buzoianu, A.D.; Voşloban, C.M.; Pop, R.M. Antioxidant/pro-oxidant Actions of Polyphenols from grapevine and Wine By-Products-Base for Complementary Therapy in Ischemic Heart Diseases. Front. Cardiovasc. Med. 2021, 8, 750508. [Google Scholar] [CrossRef]
- Ertürk, E.; Onur, O.E.; Aydin, I.; Ozel, M.Z.; Firat, M.; Ari, F. Cytotoxic Potential of Rare Plant Salvia candidissima subsp. candidissima on Breast Cancer Cells. Braz. Arch. Biol. Technol. 2023, 66, e23220358. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.S.F.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.F.R.; Cardoso, S.M. The Health-Benefits and Phytochemical Profile of Salvia apiana and Salvia farinacea var. Victoria Blue Decoctions. Antioxidants 2019, 8, 241. [Google Scholar] [CrossRef]
- He, X.; Gao, X.; Guo, Y.; Xie, W. Research Progress on Bioactive Factors against Skin Aging. Int. J. Mol. Sci. 2024, 25, 3797. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Matwiejczuk, N.; Galicka, A.; Zaręba, I.; Brzóska, M.M. The Protective Effect of Rosmarinic Acid Against Unfavorable Influence of Methylparaben and Propylparaben on Collagen in Human Skin Fibroblasts. Nutrients 2020, 12, 1282. [Google Scholar] [CrossRef] [PubMed]
- Yücel, Ç.; Şeker Karatoprak, G.; Değim, İ.T. Anti-aging formulation of rosmarinic acid-loaded ethosomes and liposomes. J. Microencapsul. 2019, 36, 180–191. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef]
- Jesus, E.G.; Souza, F.F.; Andrade, J.V.; Andrade ESilva, M.L.; Cunha, W.R.; Ramos, R.C.; Campos, O.S.; Santos, J.A.N.; Santos, M.F.C. In silico and in vitro elastase inhibition assessment assays of rosmarinic acid natural product from Rosmarinus officinalis Linn. Nat. Prod. Res. 2024, 38, 879–884. [Google Scholar] [CrossRef]
- Sutkowska, J.; Hupert, N.; Gawron, K.; Strawa, J.W.; Tomczyk, M.; Forlino, A.; Galicka, A. The Stimulating Effect of Rosmarinic Acid and Extracts from Rosemary and Lemon Balm on Collagen Type I Biosynthesis in Osteogenesis Imperfecta Type I Skin Fibroblasts. Pharmaceutics 2021, 13, 938. [Google Scholar] [CrossRef]
- Galicka, A.; Sutkowska-Skolimowska, J. The Beneficial Effect of Rosmarinic Acid on Benzophenone-3-Induced Alterations in Human Skin Fibroblasts. Int. J. Mol. Sci. 2021, 22, 11451. [Google Scholar] [CrossRef]
- Kang, H.S.; Kim, H.R.; Byun, D.S.; Park, H.J.; Choi, J.S. Rosmarinic Acid as a Tyrosinase Inhibitors from Salvia miltiorrhiza. Nat. Prod. Sci. 2004, 10, 80–84. [Google Scholar]
- Tousif, M.I.; Nazir, M.; Riaz, N.; Saleem, M.; Tauseef, S.; Azam, S.M.; Arfan Yawer, M.; Zengin, G. Terpenoids as Human Neutrophil Elastase (HNE) Inhibitors: A Comprehensive Review of Natural Anti-inflammatory Isoprenoids. Chembiochem 2023, 24, e202300346. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.M.; Domínguez-Martín, E.M.; Nicolai, M.; Faustino, C.; Rodrigues, L.M.; Rijo, P. Screening the dermatological potential of plectranthus species components: Antioxidant and inhibitory capacities over elastase, collagenase and tyrosinase. J. Enzym. Inhib. Med. Chem. 2021, 36, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Sung, J.; Lee, H.; Kim, Y.; Jeong, H.S.; Lee, J. Protective activity of caffeic acid against UVB-induced photoaging in human fibroblasts via MAPKs/NF-κB inactivation. J. Food Biochem. 2019, 43, e12701. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Cho, S.H.; Park, D.; Jung, E. Anti-skin aging properties of protocatechuic acid in vitro and in vivo. J. Cosmet. Dermatol. 2020, 19, 977–984. [Google Scholar] [CrossRef]
- Li, H.-R.; Habasi, M.; Xie, L.-Z.; Aisa, H.A. Effect of Chlorogenic Acid on Melanogenesis of B16 Melanoma Cells. Molecules 2014, 19, 12940–12948. [Google Scholar] [CrossRef]
- Chen, Q.-X.; Song, K.-K.; Qiu, L.; Liu, X.-D.; Huang, H.; Guo, H.-Y. Inhibitory effects on mushroom tyrosinaseby p-alkoxybenzoic acids. Food Chem. 2005, 91, 269–274. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, P.; Masur, K.; Shome, D.; Kaushik, N.; Nguyen, L.N.; Kaushik, N.K.; Choi, E.H. Influence of Redox Stress on Crosstalk between Fibroblasts and Keratinocytes. Biology 2021, 10, 1338. [Google Scholar] [CrossRef]
- Barygina, V.; Becatti, M.; Prignano, F.; Lotti, T.; Taddei, N.; Fiorillo, C. Fibroblasts to Keratinocytes Redox Signaling: The Possible Role of ROS in Psoriatic Plaque Formation. Antioxidants 2019, 8, 566. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef]
- Ciobotaru, G.V.; Goje, I.-D.; Dehelean, C.A.; Danciu, C.; Magyari-Pavel, I.Z.; Moacă, E.-A.; Muntean, D.; Imbrea, I.M.; Sărățeanu, V.; Pop, G. Analysis of the Antioxidant and Antimicrobial Activity, Cytotoxic, and Anti-Migratory Properties of the Essential Oils Obtained from Cultivated Medicinal Lamiaceae Species. Plants 2025, 14, 846. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Macedo, P.I.d.S.; Pinto, C.A.S.d.O.; Hiraishi, C.F.; Marques, G.d.A.; Escudeiro, C.C.; Pessoa, F.V.L.S.; Gregorio, J.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. Enhancing Photoprotection and Mitigating Ex Vivo Stratum Corneum Oxidative Stress: A Multifunctional Strategy Combining Rosmarinic Acid with UVB Filters. Antioxidants 2025, 14, 274. [Google Scholar] [CrossRef] [PubMed]
- Cândido, T.M.; Ariede, M.B.; Pinto, C.A.S.d.O.; Lima, F.V.; Magalhães, W.V.; Pedro, N.M.E.; Padovani, G.; Sufi, B.d.S.; Rijo, P.; Velasco, M.V.R.; et al. Rosmarinic Acid Multifunctional Sunscreen: Comet Assay and In Vivo Establishment of Cutaneous Attributes. Cosmetics 2022, 9, 141. [Google Scholar] [CrossRef]
- de Oliveira Bispo, M.; Morocho-Jácome, A.L.; Escudeiro, C.C.; Martinez, R.M.; de Oliveira Pinto, C.A.S.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. Photoprotective Efficacy of the Association of Rosmarinic Acid 0.1% with Ethylhexyl Methoxycinnamate and Avobenzone. Cosmetics 2023, 10, 11. [Google Scholar] [CrossRef]
- Bittner Fialová, S.; Rendeková, K.; Mučaji, P.; Nagy, M.; Slobodníková, L. Antibacterial Activity of Medicinal Plants and Their Constituents in the Context of Skin and Wound Infections, Considering European Legislation and Folk Medicine—A Review. Int. J. Mol. Sci. 2021, 22, 10746. [Google Scholar] [CrossRef]
- Iancu, A.V.; Maftei, N.M.; Dumitru, C.; Baroiu, L.; Gurau, G.; Elisei, A.M.; Stefan, C.S.; Tatu, A.L.; Iancu, A.F.; Arbune, M. Prevalence of Multidrug Resistance Pathogens in Dermatology: A Retrospective Study in Romania, 2018–2022. Electron. J. Gen. Med. 2024, 21, em582. [Google Scholar] [CrossRef]
- Del Giudice, P. Skin Infections Caused by Staphylococcus aureus. Acta Derm. Venereol. 2020, 100, 208–215. [Google Scholar] [CrossRef]
- Pato, C.; Melo-Cristino, J.; Ramirez, M.; Friães, A. Streptococcus pyogenes Causing Skin and Soft Tissue Infections Are Enriched in the Recently Emerged emm89 Clade 3 and Are Not Associated With Abrogation of CovRS. Front. Microbiol. 2018, 9, 2372. [Google Scholar] [CrossRef]
- AlSheikh, H.M.A.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Tasleem Jan, A.; Haq, Q.M.R. Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, G.; Khan, G.Z.; Sivasamugham, L.A.; Wong, L.S.; Kidd, S.; Yap, C.K. Antimicrobial and anti-biofilm activities of plant extracts against Pseudomonas aeruginosa—A review. J. Exp. Biol. Agric. Sci. 2023, 11, 780–790. [Google Scholar]
- Quradha, M.M.; Duru, M.E.; Kucukaydin, S.; Tamfu, A.N.; Iqbal, M.; Bibi, H.; Khan, R.; Ceylan, O. Comparative assessment of phenolic composition profile and biological activities of green extract and conventional extracts of Salvia sclarea. Sci. Rep. 2024, 14, 1885. [Google Scholar] [CrossRef]
- Iobbi, V.; Parisi, V.; Bernabè, G.; De Tommasi, N.; Bisio, A.; Brun, P. Anti-Biofilm Activity of Carnosic Acid from Salvia rosmarinus against Methicillin-Resistant Staphylococcus aureus. Plants 2023, 12, 3679. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H.G. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T.; et al. Biofilms: Formation, drug resistance and alternatives to conventional approaches. AIMS Microbiol. 2022, 8, 239–277. [Google Scholar] [CrossRef]
- Sarojini, S.; Jayaram, S.; Kalathilparambil Santhosh, S.; Priyadarshini, P.; Pappuswamy, M.; Balasubramanian, B. Harnessing the Potential of Antibacterial and Antibiofilm Phytochemicals in the Combat Against Superbugs: A One Health Perspective. Antibiotics 2025, 14, 692. [Google Scholar] [CrossRef]
- Ivanov, M.; Kostić, M.; Stojković, D.; Soković, M. Rosmarinic acid common plant polyphenol. S. Afr. J. Bot. 2022, 146, 521–527. [Google Scholar] [CrossRef]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus aureus Clinical Strains. BioMed Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef]
- Luis, A.; Silva, F.; Sousa, S.; Duarte, A.P.; Domingues, F. Antistaphylococcal and bioflm inhibitory activities of gallic, cafeic, and chlorogenic acids. Biofouling 2014, 30, 69–79. [Google Scholar] [CrossRef]
- Vijayakumar, K.; Muhilvannan, S.; Vignesh, A. Hesperidin inhibits biofilm formation, virulence and staphyloxanthin synthesis in methicillin resistant Staphylococcus aureus by targeting Sar A and Crt M: An in vitro and in silico approach. World J. Microbiol. Biotechnol. 2022, 38, 20. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef]
- Michalak, M.; Błońska-Sikora, E.; Paradowska, K.; Zielińska, A. Pharmaceutical availability of hydrogels with extracts of Arnica montana, Aesculus hippocastanum and Ruscus aculeatus and their potential use as antioxidant polyphenol-rich material. Med. Stud. 2023, 39, 223–229. [Google Scholar] [CrossRef]
- Franceschini, M.; Pizzetti, F.; Rossi, F. On the Key Role of Polymeric Rheology Modifiers in Emulsion-Based Cosmetics. Cosmetics 2025, 12, 76. [Google Scholar] [CrossRef]
- Wróblewska, M.; Słyż, J.; Winnicka, K. Rheological and textural properties of hydrogels, containing sulfur as a model drug, made using different polymers types. Polimery 2019, 64, 208–215. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Maciążek-Jurczyk, M.; Pożycka, J.; Dolińska, B. Pre-Formulation Studies: Physicochemical Characteristics and In Vitro Release Kinetics of Insulin from Selected Hydrogels. Pharmaceutics 2021, 13, 1215. [Google Scholar] [CrossRef]
- Czarnecka-Operacz, M. Sucha skóra jako aktualny problem kliniczny. Int. J. Adv. Integr. Med. Sci. 2006, 23, 49–56. [Google Scholar]
- Kim, J.Y.; Song, J.Y.; Lee, E.J.; Park, S.K. Rheological properties and microstructures of Carbopol gel network system. Colloid Polym. Sci. 2003, 281, 614–623. [Google Scholar] [CrossRef]
- Huynh, A.; Garcia, A.G.; Young, L.K.; Szoboszlai, M.; Liberatore, M.W.; Baki, G. Measurements Meet Perceptions: Rheology–Texture–Sensory Relations When Using Green, Bio-Derived Emollients in Cosmetic Emulsions. Int. J. Cosmet. Sci. 2021, 43, 11–19. [Google Scholar] [CrossRef]
- Ali, A.; Skedung, L.; Burleigh, S.; Lavant, E.; Ringstad, L.; Anderson, C.D.; Wahlgren, M.; Engblom, J. Relationship between sensorial and physical characteristics of topical creams: A comparative study on effects of excipients. Int. J. Pharm. 2022, 613, 121370. [Google Scholar] [CrossRef]
- Michalak, M.; Błońska-Sikora, E.; Stryjecka, M.; Zagórska-Dziok, M.; Klimek-Szczykutowicz, M.; Szopa, A. Phytochemical Profile and Antioxidant and Protective Activities of Various Types of Extracts from Hyssopus officinalis L. and Grindelia robusta Nutt. Herb Grown in Poland. Curr. Top Med. Chem. 2024, 24, 2238–2253. [Google Scholar] [CrossRef]
- Kostrzewa, D.; Dobrzyńska-Inger, A.; Turczyn, A. Optimization of supercritical carbon dioxide extraction of sweet paprika (Capsicum annuum L.) using response surface methodology. Chem. Eng. Res. Des. 2020, 160, 39–51. [Google Scholar] [CrossRef]
- Michalak, M.; Zagórska-Dziok, M.; Żarnowiec, P.; Bocho-Janiszewska, A.; Stryjecka, M.; Kostrzewa, D.; Dobros, N.; Paradowska, K. Evaluation of Achillea millefolium var. Paprika Extract with Antioxidant, Antimicrobial, and Skin Protection Potential in Topical Application. Appl. Sci. 2025, 15, 4631. [Google Scholar] [CrossRef]
- Agbor, G.A.; Vinson, J.A.; Donnelly, P.E. Folin-Ciocalteau Reagent for Polyphenolic Assay. Int. J. Food Sci. Nutr. Diet. 2014, 3, 147–156. [Google Scholar] [CrossRef]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321. [Google Scholar] [CrossRef]
- Jain, R.; Rao, B.; Tare, A.B. Comparative analysis of the spectrophotometry based total phenolic acid estimation methods. J. Anal. Chem. 2017, 72, 972–976. [Google Scholar] [CrossRef]
- Tlili, H.; Hanen, N.; Ben Arfa, A.; Neffati, M.; Boubakri, A.; Buonocore, D.; Dossena, M.; Verri, M.; Doria, E. Biochemical profile and in vitro biological activities of extracts from seven folk medicinal plants growing wild in southern Tunisia. PLoS ONE 2019, 14, e0213049. [Google Scholar] [CrossRef]
- Dobros, N.; Zawada, K.; Paradowska, K. Phytochemical Profile and Antioxidant Activity of Lavandula angustifolia and Lavandula x intermedia Cultivars Extracted with Different Methods. Antioxidants 2022, 11, 711. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Ziemlewska, A.; Wójciak, M.; Sowa, I.; Wąsik-Szczepanek, E.; Nizioł-Łukaszewska, Z. Comparison of Cytotoxicity and Antioxidant, Antibacterial, and Anti-Inflammatory Activity of Aqueous and Ethanolic Extracts from Malus domestica, Prunus armeniaca, and Prunus cerasus Leaves. Molecules 2025, 30, 2085. [Google Scholar] [CrossRef]
- Krochmal-Marczak, B.; Zagórska-Janiszewska, M.; Michalak, M.; Kiełtyka-Dadasiewicz, A. Comparative assessment of phenolic content, cellular antioxidant, antityrosinase and protective activities on skin cells of extracts from three sweet potato (Ipomoea batatas (L.) Lam.) cultivars. J. King Saud Univ. Sci. 2021, 33, 6. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 9th ed.; CLSI document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and analyzing static biofilms. Curr. Protoc. Microbiol. 2005, 22, 1B.1.1–1B.1.18. [Google Scholar] [CrossRef]
- Terzić, J.; Stanković, M.; Stefanović, O. Extracts of Achillea millefolium L. inhibited biofilms and biofilm-related virulence factors of pathogenic bacteria isolated from wounds. Microb. Pathog. 2025, 199, 107219. [Google Scholar] [CrossRef] [PubMed]
- Goliszek-Chabros, M.; Xu, T.; Bocho-Janiszewska, A.; Podkościelna, B.; Sevastyanova, O. Lignin nanoparticles from softwood and hardwood as sustainable additives for broad-spectrum protection and enhanced sunscreen performance. Wood Sci. Technol. 2025, 59, 60. [Google Scholar] [CrossRef]

| Extract | TP | TF | TPA | CT |
|---|---|---|---|---|
| UAE | 64.46 ± 0.09 a | 17.39 ± 0.06 a | 4.92 ± 0.04 a | 4.12 ± 0.03 a |
| SFE | 4.29 ± 0.25 b | 1.43 ± 0.08 b | 0.96 ± 0.02 a | 0.74 ± 0.02 a |
| Number | Compound | Retention Time [min] | [mg/mL] ± SD |
|---|---|---|---|
| 1 | Caffeic acid | 17.8 | 0.04 ± 0.00 |
| 2 | Hesperidin | 43.9 | 0.16 ± 0.01 |
| 3 | Rosmarinic acid | 45.9 | 1.32 ± 0.04 |
| Test Microorganism | MIC | MBC/MFC |
|---|---|---|
| Candida albicans ATCC10231 | 2 | 16 |
| Streptococcus agalactiae PCM 2683 | 8 | 8 |
| Enterococcus faecalis PCM 2784 | 8 | 8 |
| Proteus mirabilis ATCC 29906 | 8 | 16 |
| Streptococcus mutans ATCC 25175 | 4 | 8 |
| Staphylococcus epidermidis ATCC 8853 | 8 | 8 |
| Streptococcus pyogenes ATCC 19615 | 8 | 16 |
| Escherichia coli UPEC PCM 176 | 8 | 8 |
| Enterococcus hirae ATCC 10541 | 8 | 16 |
| Bacillus subtilis PCM 486 | 2 | 16 |
| Staphylococcus aureus 6538P | 4 | 4 |
| Staphylococcus epidermidis PCM 2118 | 4 | 4 |
| Escherichia coli ATCC 8739 | 16 | 16 |
| Pseudomonas aeruginosa PAO1 | 2 | 8 |
| Ralstonia solanacearum Z1 | 4 | 4 |
| Shear Rate [s−1] | Hydrogel Viscosity [Paxs] | Lotion Viscosity [Paxs] | p |
|---|---|---|---|
| 30 | 5.16 ± 0.09 | 0.49 ± 0.06 | p < 0.05 |
| 50 | 3.86 ± 0.10 | 0.30 ± 0.05 | p < 0.05 |
| 100 | 2.55 ± 0.01 | 0.21 ± 0.01 | p < 0.05 |
| Parameter | Hydrogel | Lotion | p |
|---|---|---|---|
| Relaxation [%] | 79.90 ± 0.87 | 92.60 ± 13.15 | NS |
| Hardness 1 [N] | 0.11 ± 0.02 | 0.05 ± 0.00 | p < 0.05 |
| Hardness 2 [N] | 0.13 ± 0.01 | 0.05 ± 0.00 | p < 0.05 |
| Cohesiveness [-] | 1.45 ± 0.19 | 1.31 ± 0.06 | NS |
| Adhesiveness [mJ] | 0.40 ± 0.10 | 0.20 ± 0.00 | NS |
| Elasticity [-] | 1.32 ± 0.12 | 0.85 ± 0.00 | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalak, M.; Zagórska-Dziok, M.; Żarnowiec, P.; Ostróżka-Cieślik, A.; Bocho-Janiszewska, A.; Stryjecka, M.; Dobros, N.; Kostrzewa, D.; Paradowska, K. Evaluation of Salvia yangii Extract as a Promising Protective Raw Material Applied Topically to the Skin. Molecules 2025, 30, 3505. https://doi.org/10.3390/molecules30173505
Michalak M, Zagórska-Dziok M, Żarnowiec P, Ostróżka-Cieślik A, Bocho-Janiszewska A, Stryjecka M, Dobros N, Kostrzewa D, Paradowska K. Evaluation of Salvia yangii Extract as a Promising Protective Raw Material Applied Topically to the Skin. Molecules. 2025; 30(17):3505. https://doi.org/10.3390/molecules30173505
Chicago/Turabian StyleMichalak, Monika, Martyna Zagórska-Dziok, Paulina Żarnowiec, Aneta Ostróżka-Cieślik, Anita Bocho-Janiszewska, Małgorzata Stryjecka, Natalia Dobros, Dorota Kostrzewa, and Katarzyna Paradowska. 2025. "Evaluation of Salvia yangii Extract as a Promising Protective Raw Material Applied Topically to the Skin" Molecules 30, no. 17: 3505. https://doi.org/10.3390/molecules30173505
APA StyleMichalak, M., Zagórska-Dziok, M., Żarnowiec, P., Ostróżka-Cieślik, A., Bocho-Janiszewska, A., Stryjecka, M., Dobros, N., Kostrzewa, D., & Paradowska, K. (2025). Evaluation of Salvia yangii Extract as a Promising Protective Raw Material Applied Topically to the Skin. Molecules, 30(17), 3505. https://doi.org/10.3390/molecules30173505










