Quinazolines [a]-Annelated by Five-Membered Heterocycles: Synthesis and Biological Activity
Abstract
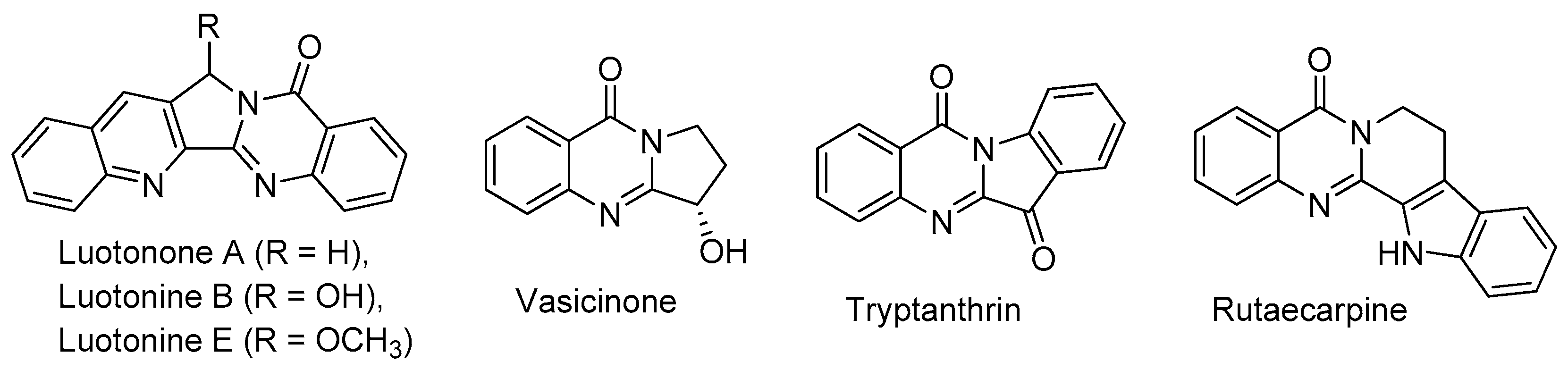
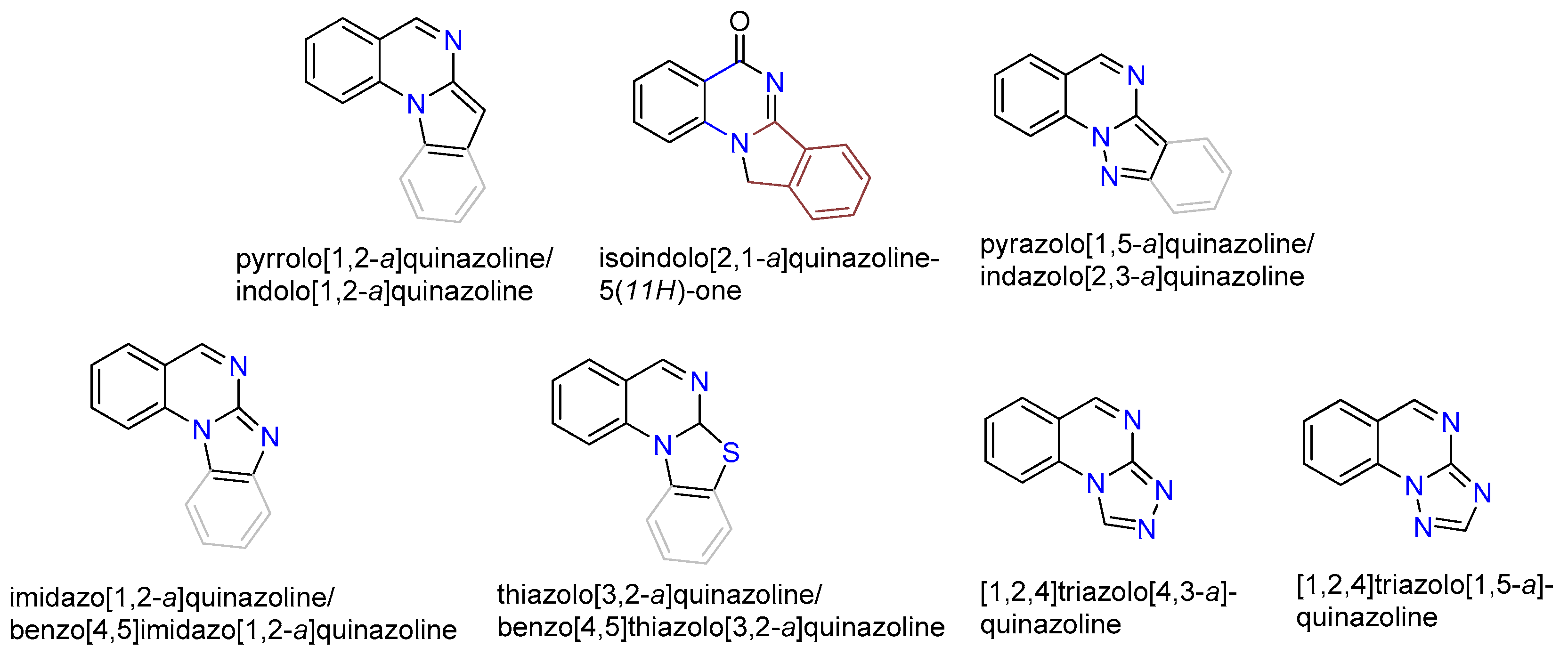
1. Introduction
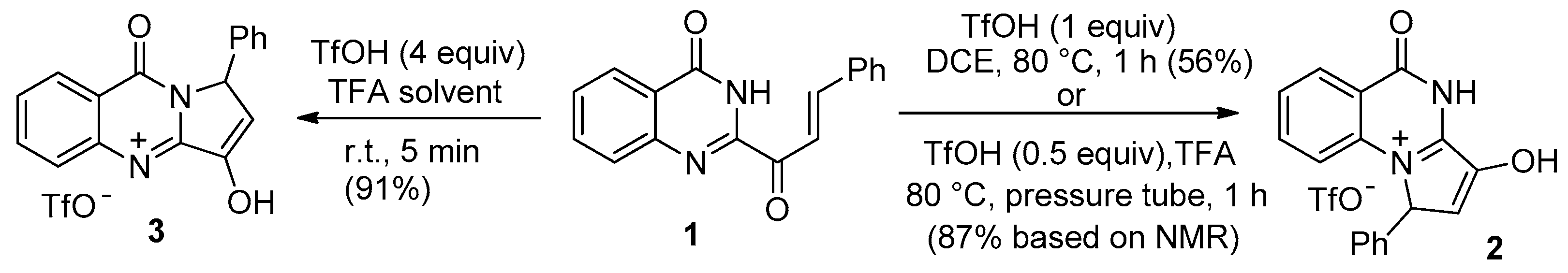
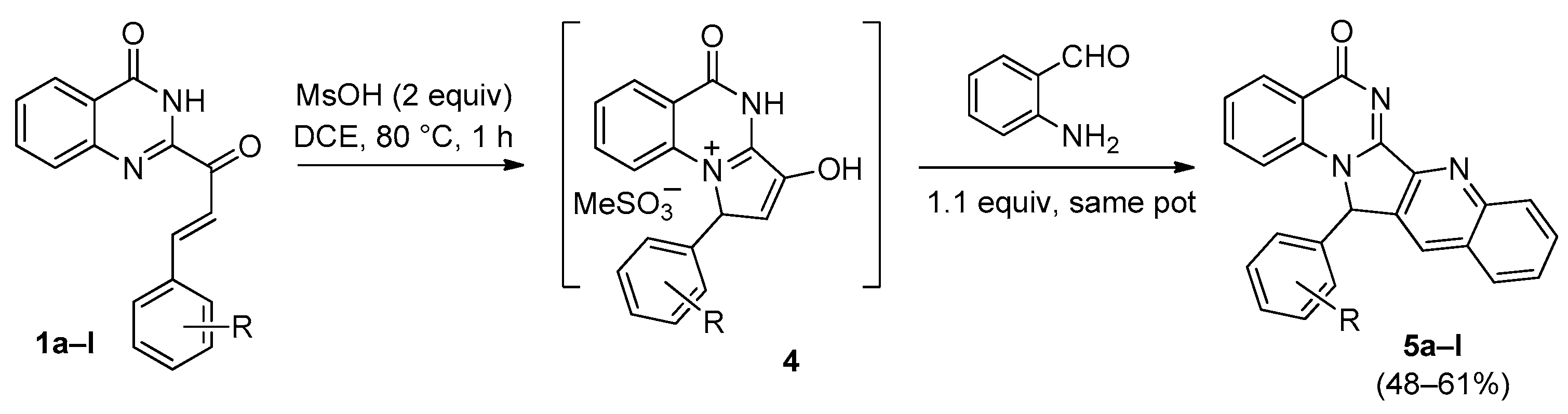
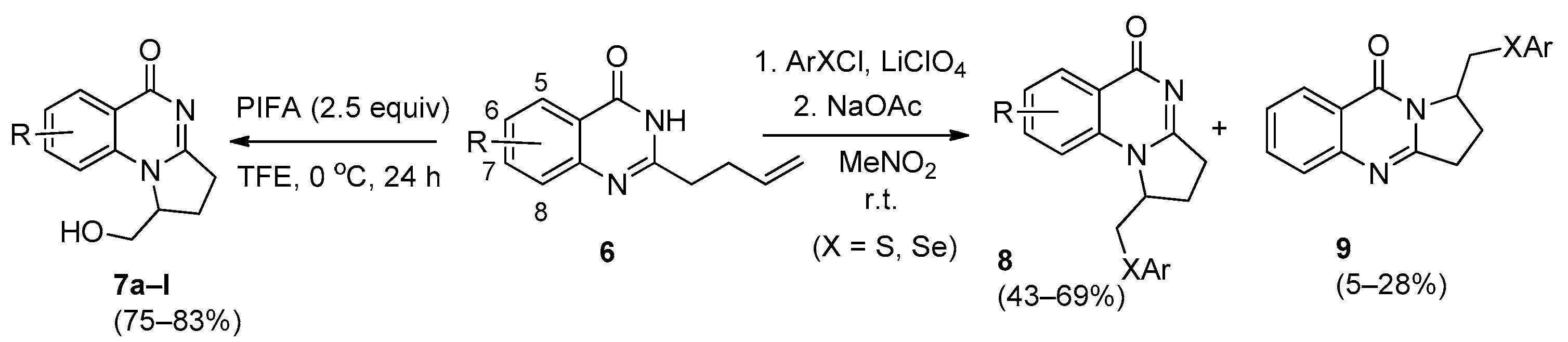
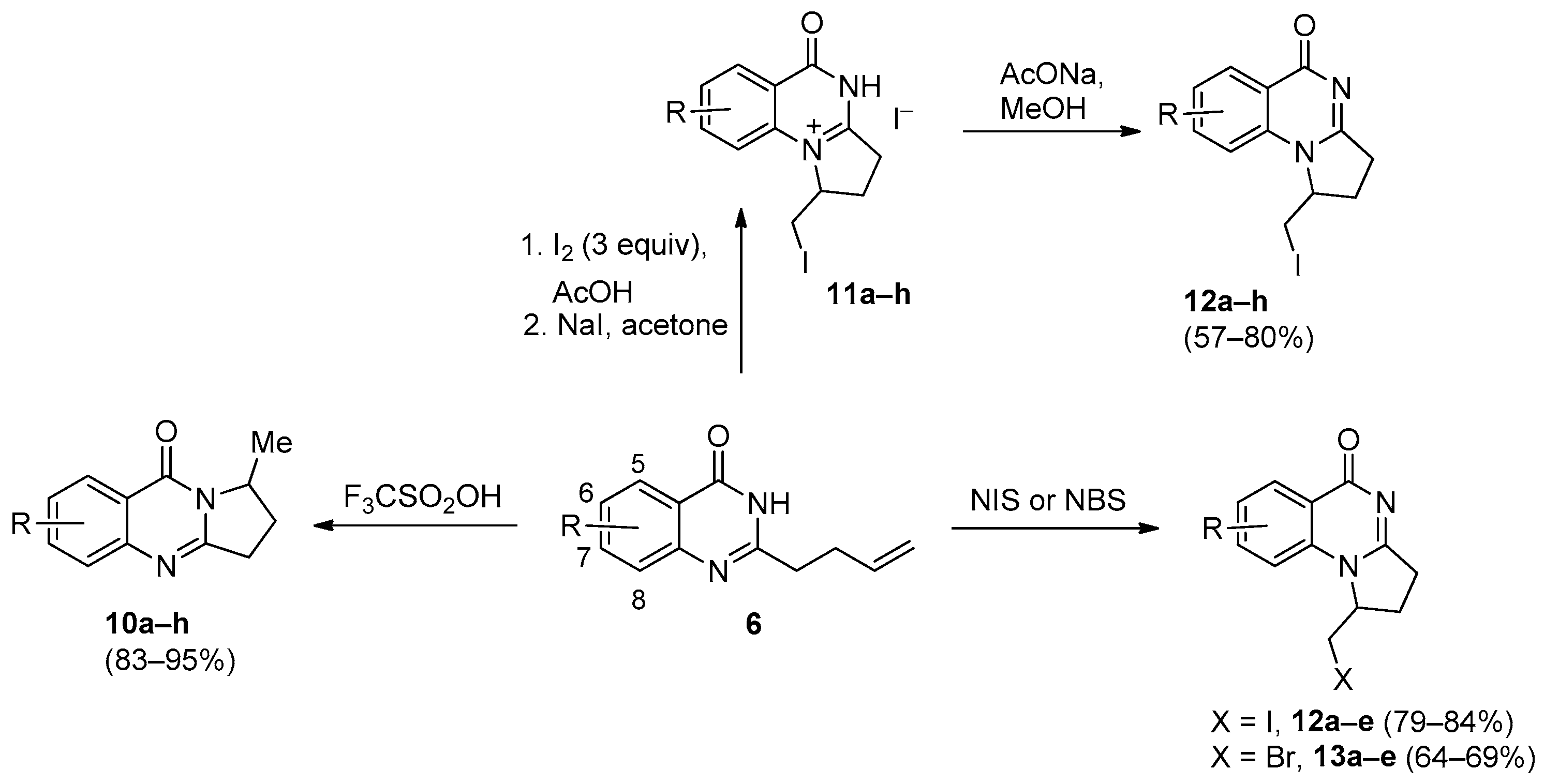
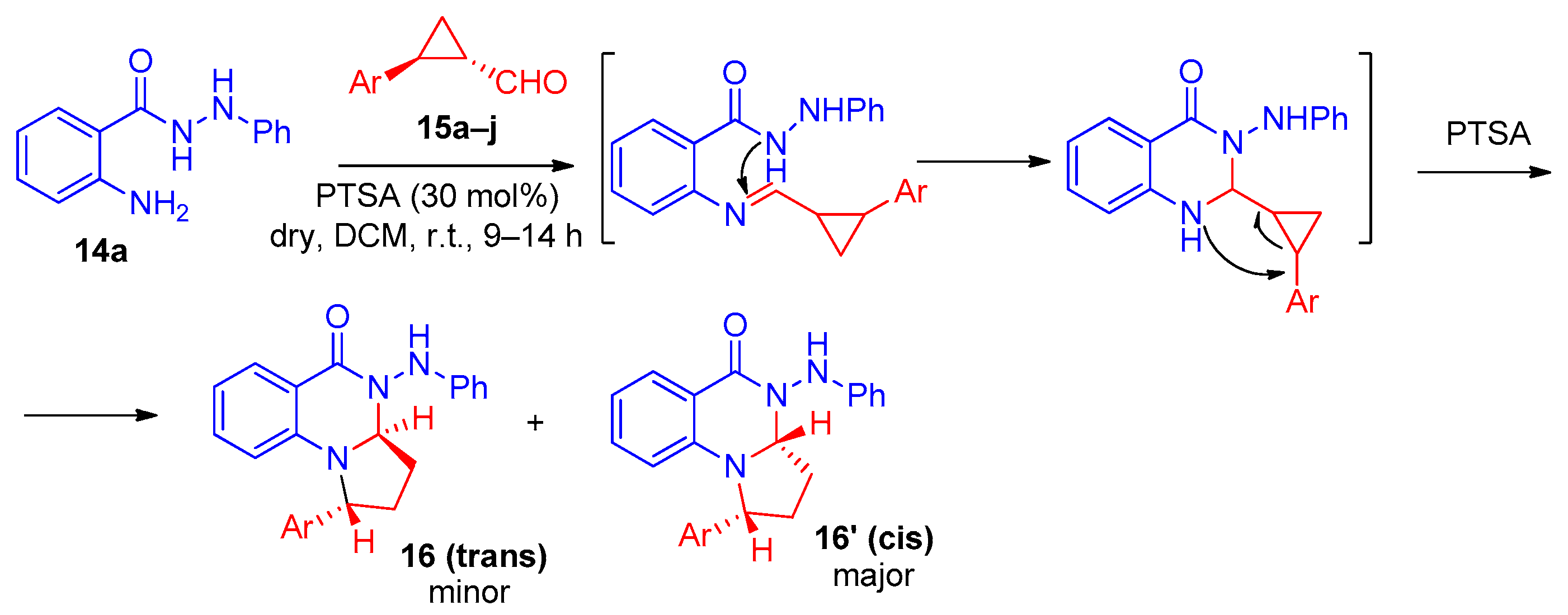
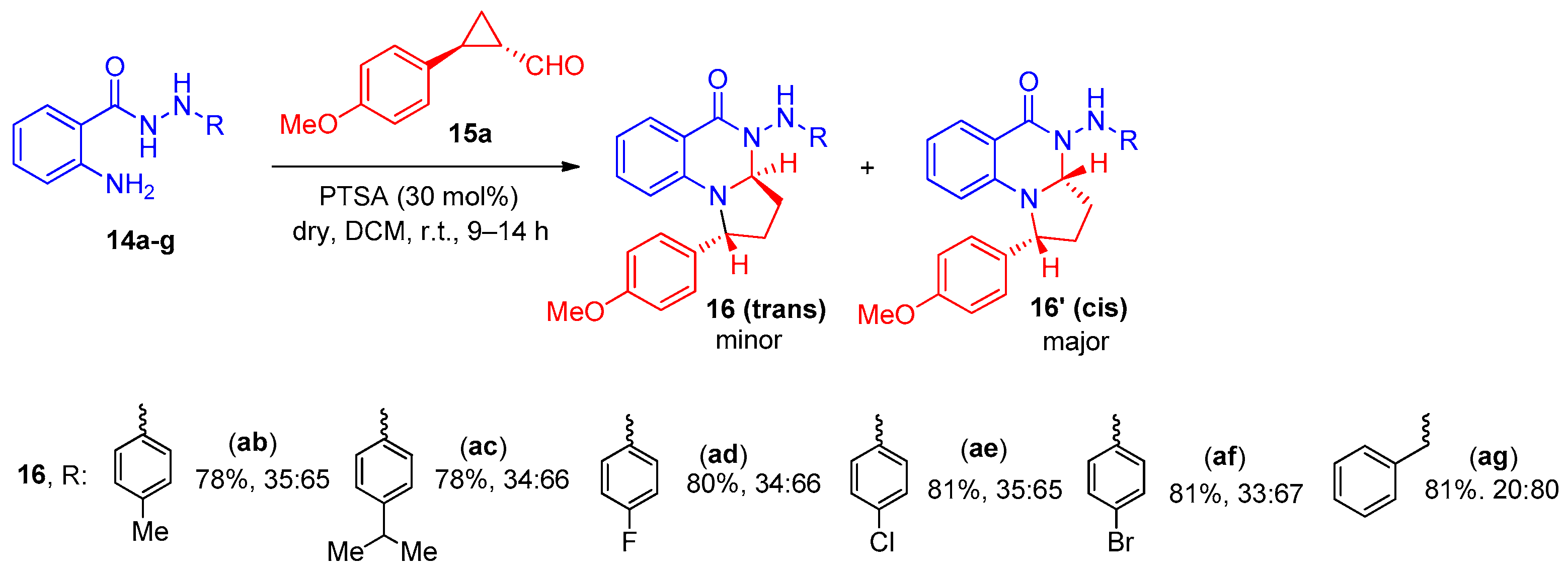
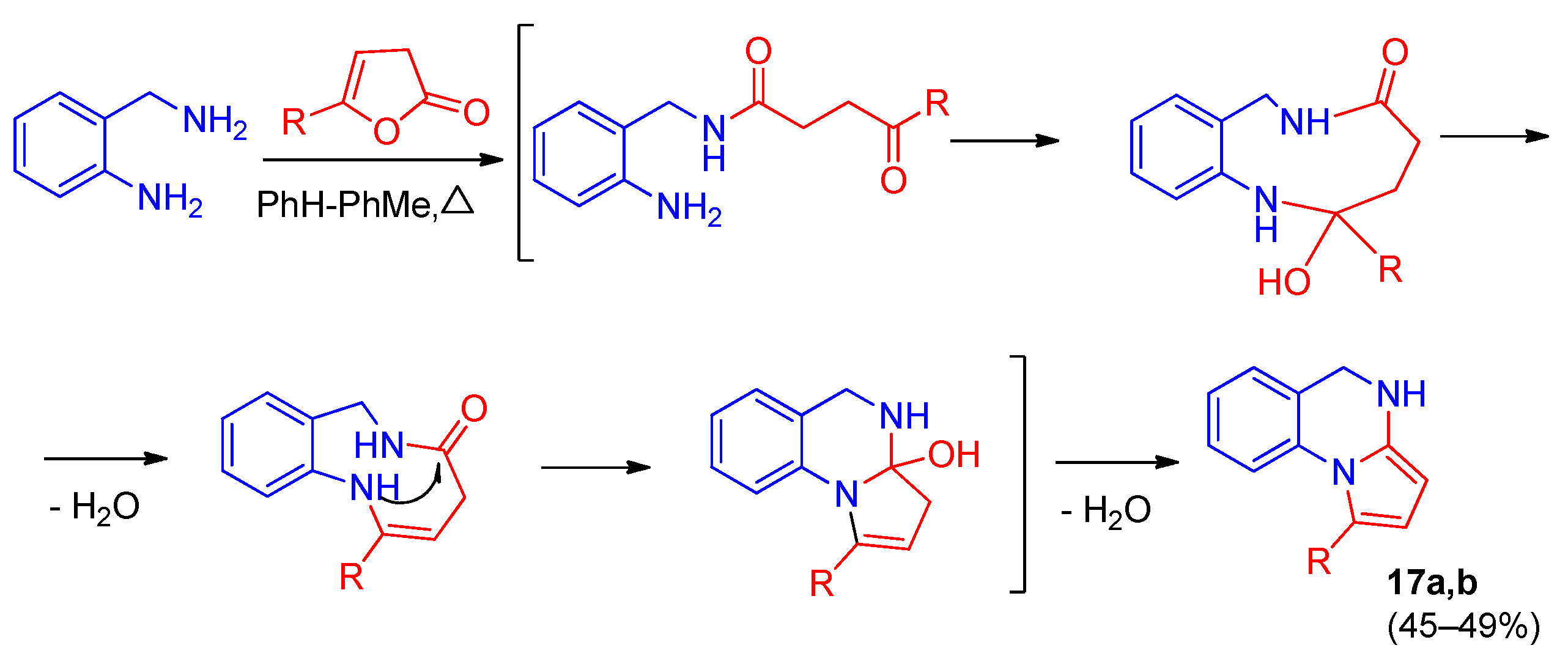
2. Pyrrolo[1,2-a]quinazolinones
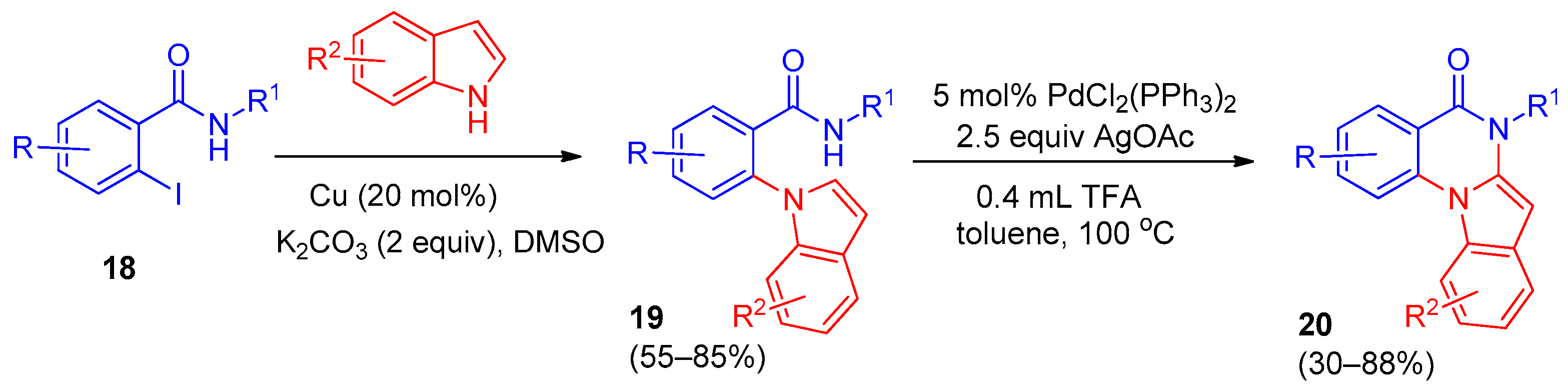
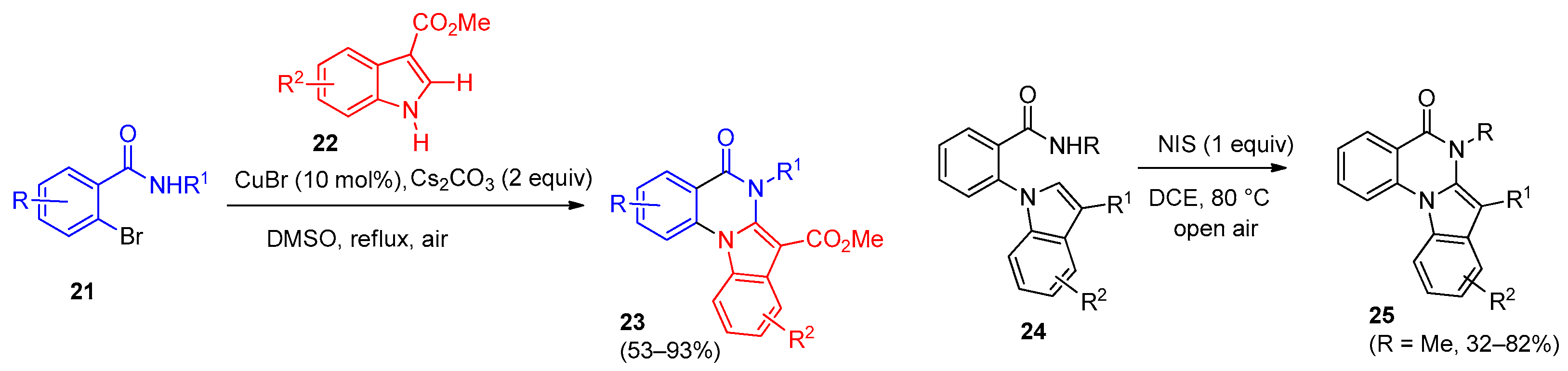
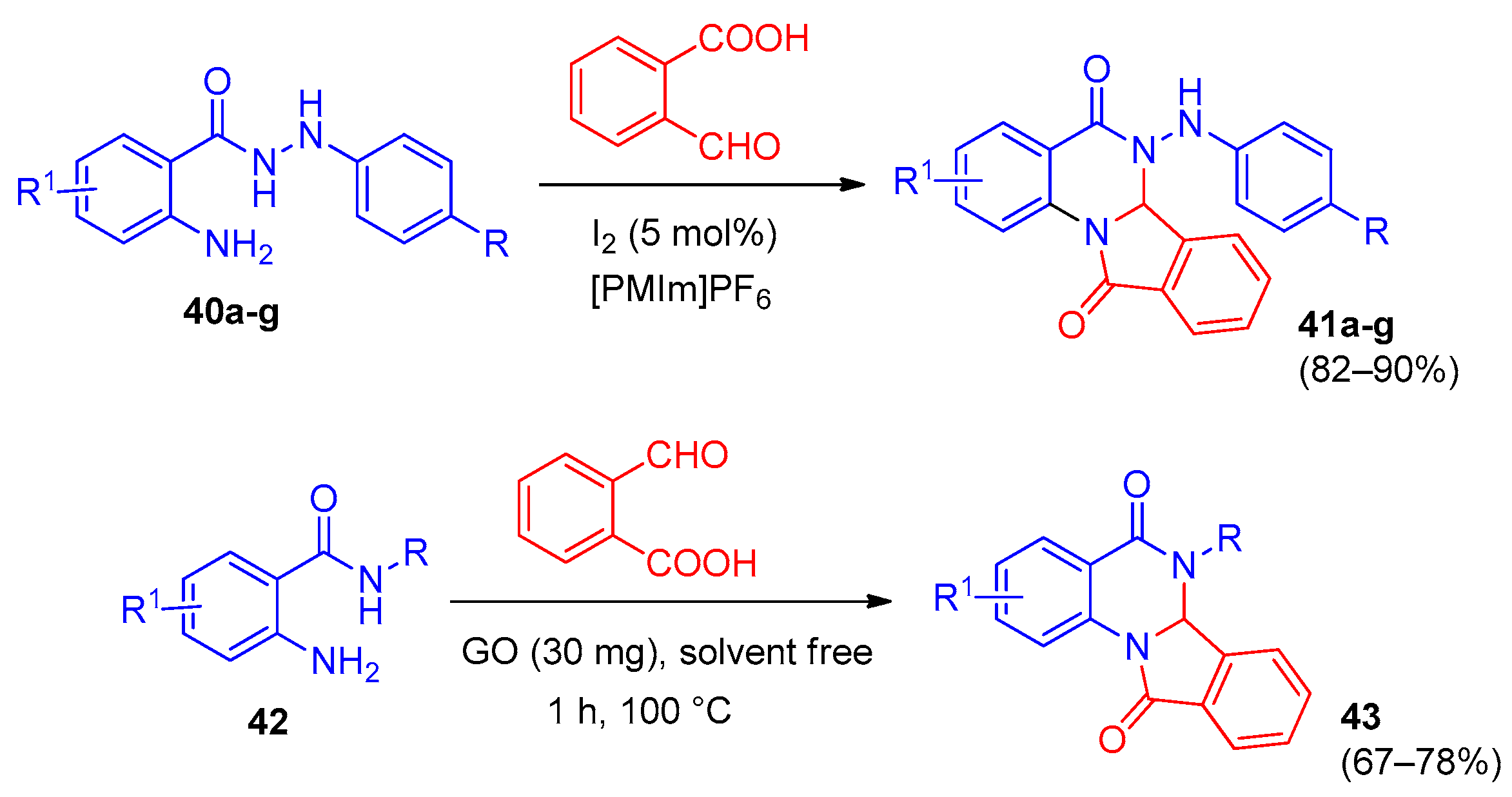
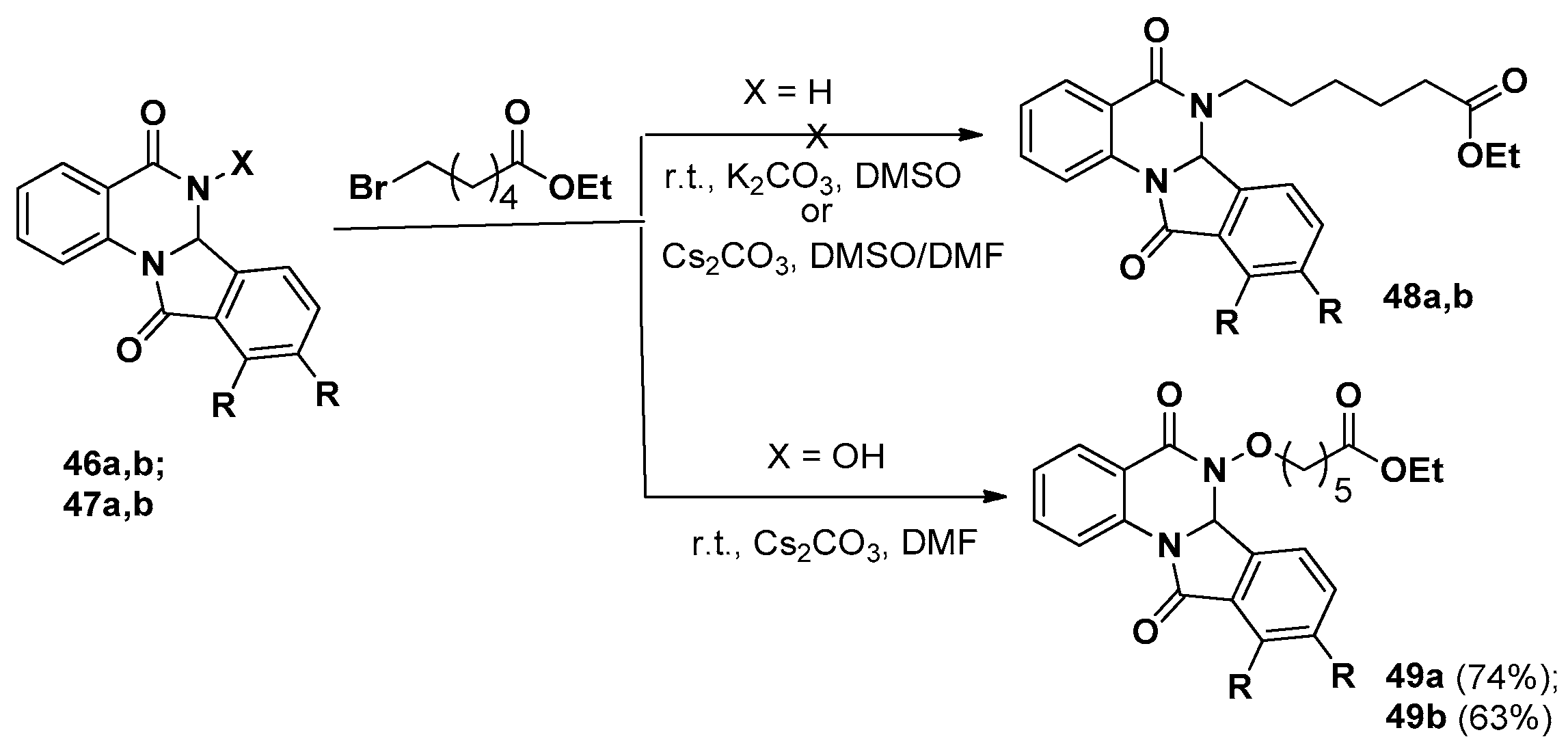
3. Indolo[1,2-a]quinazolinones and Isoindolo[2,1-a]quinazolinediones
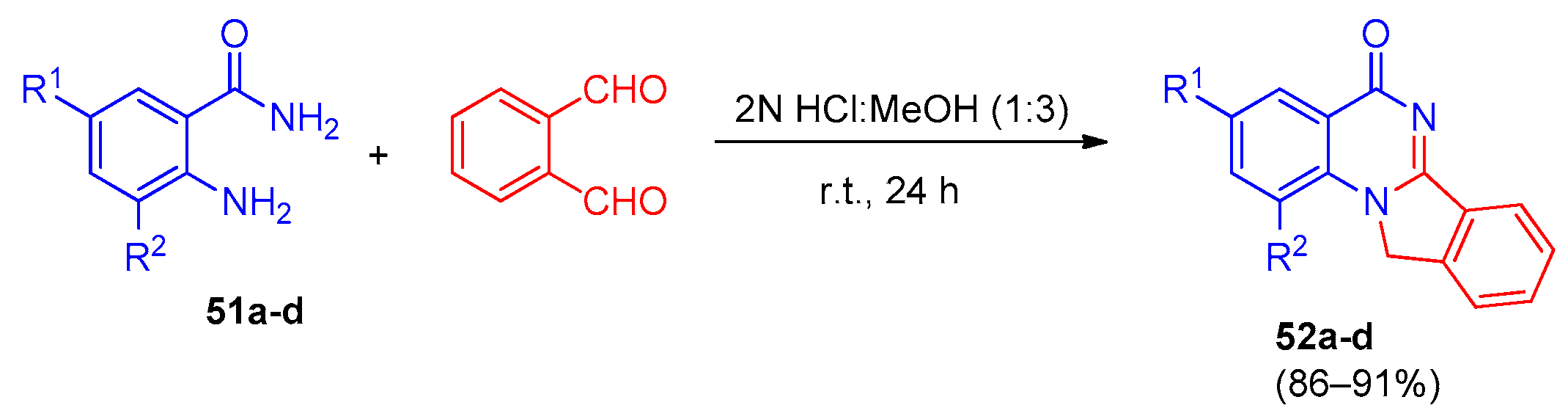
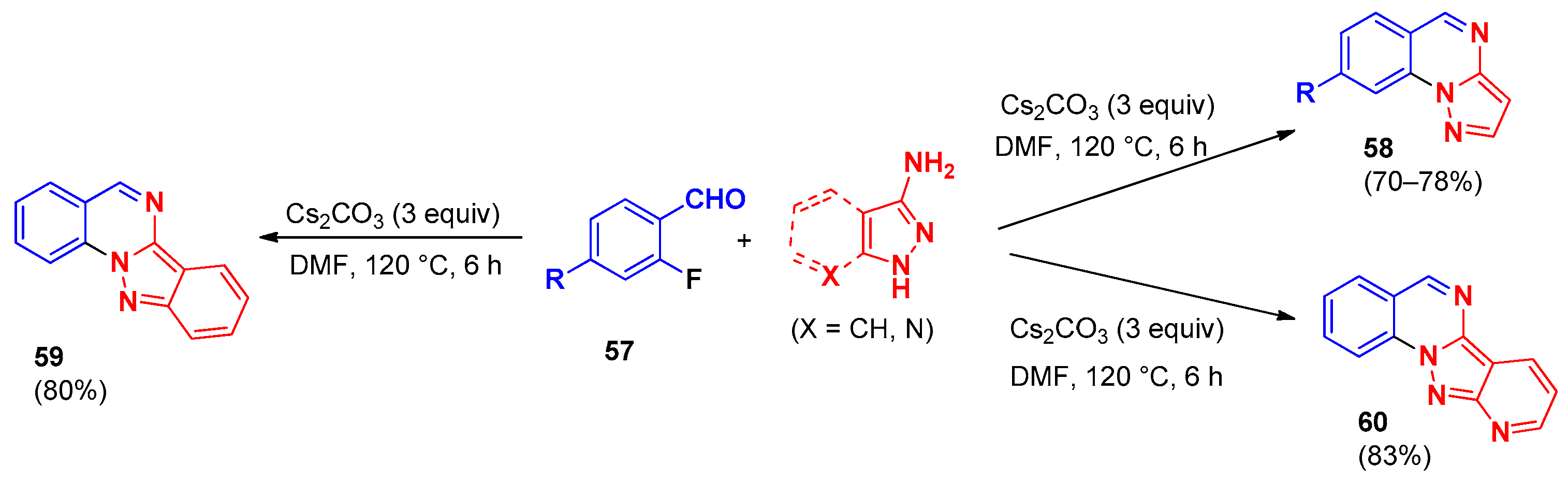
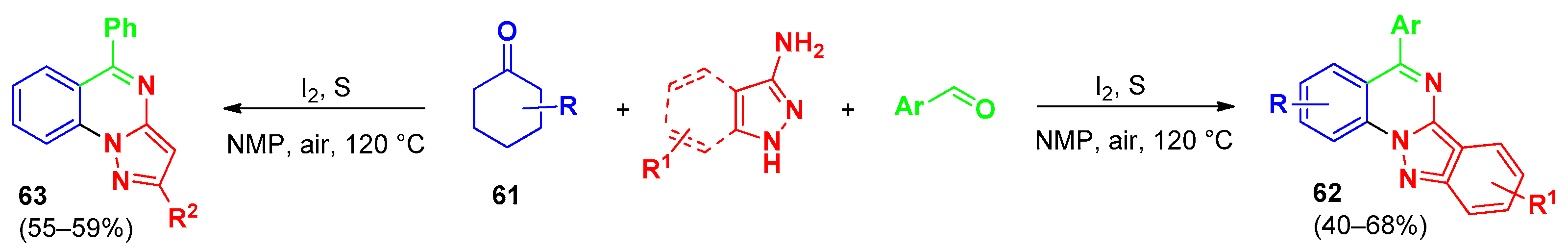
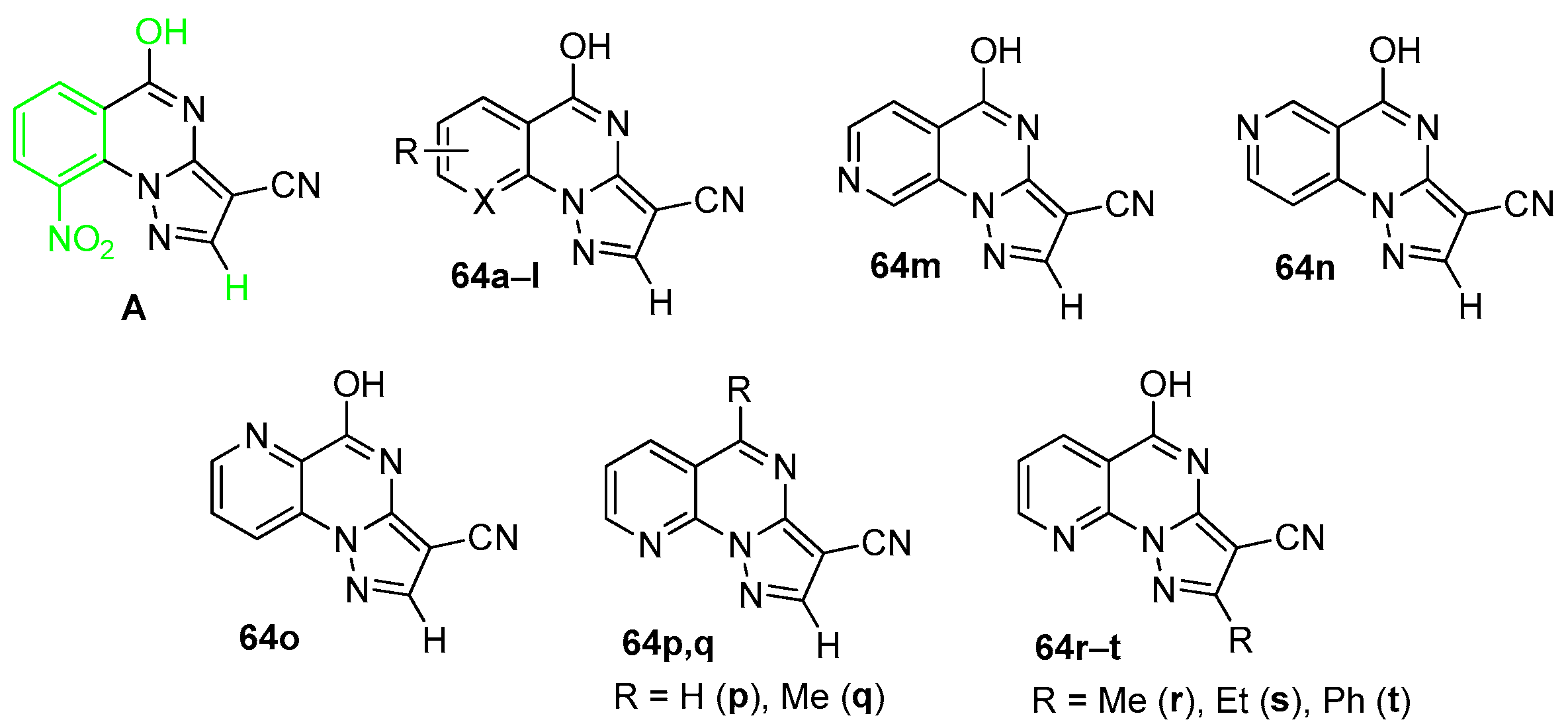
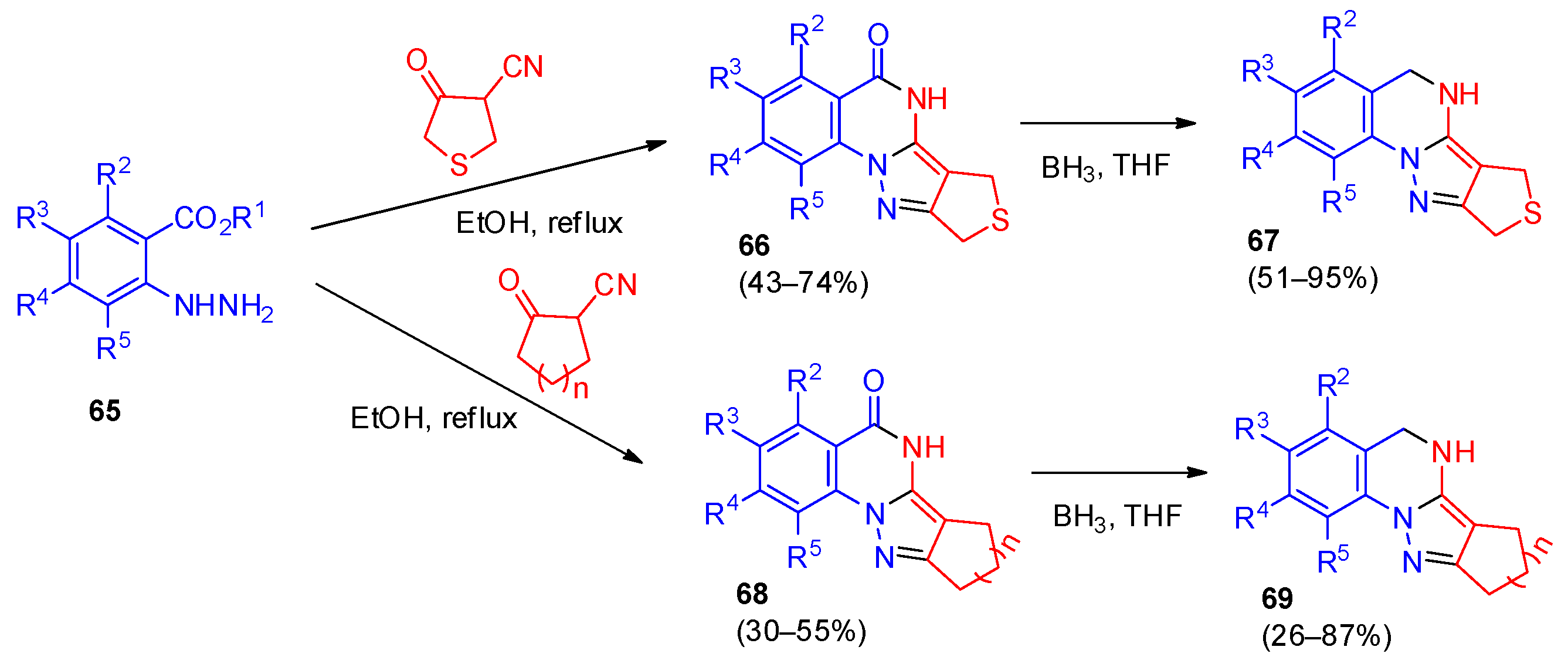
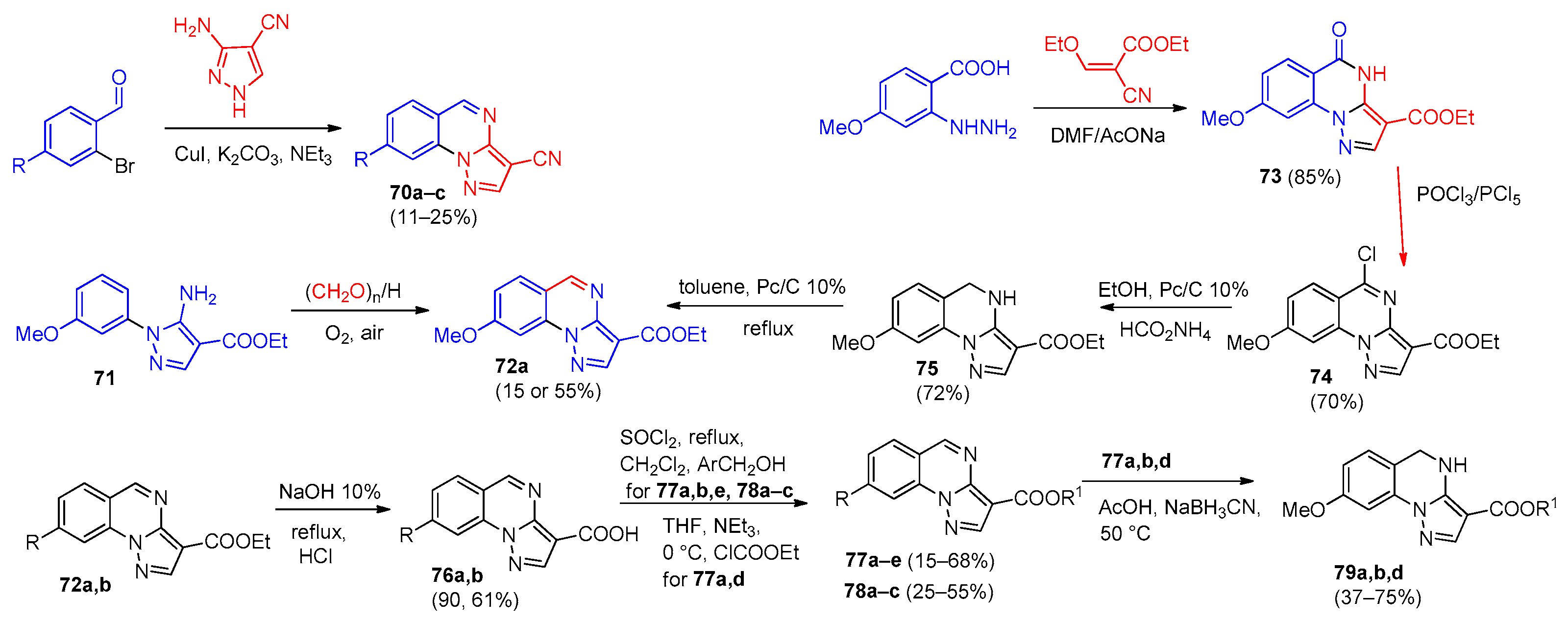
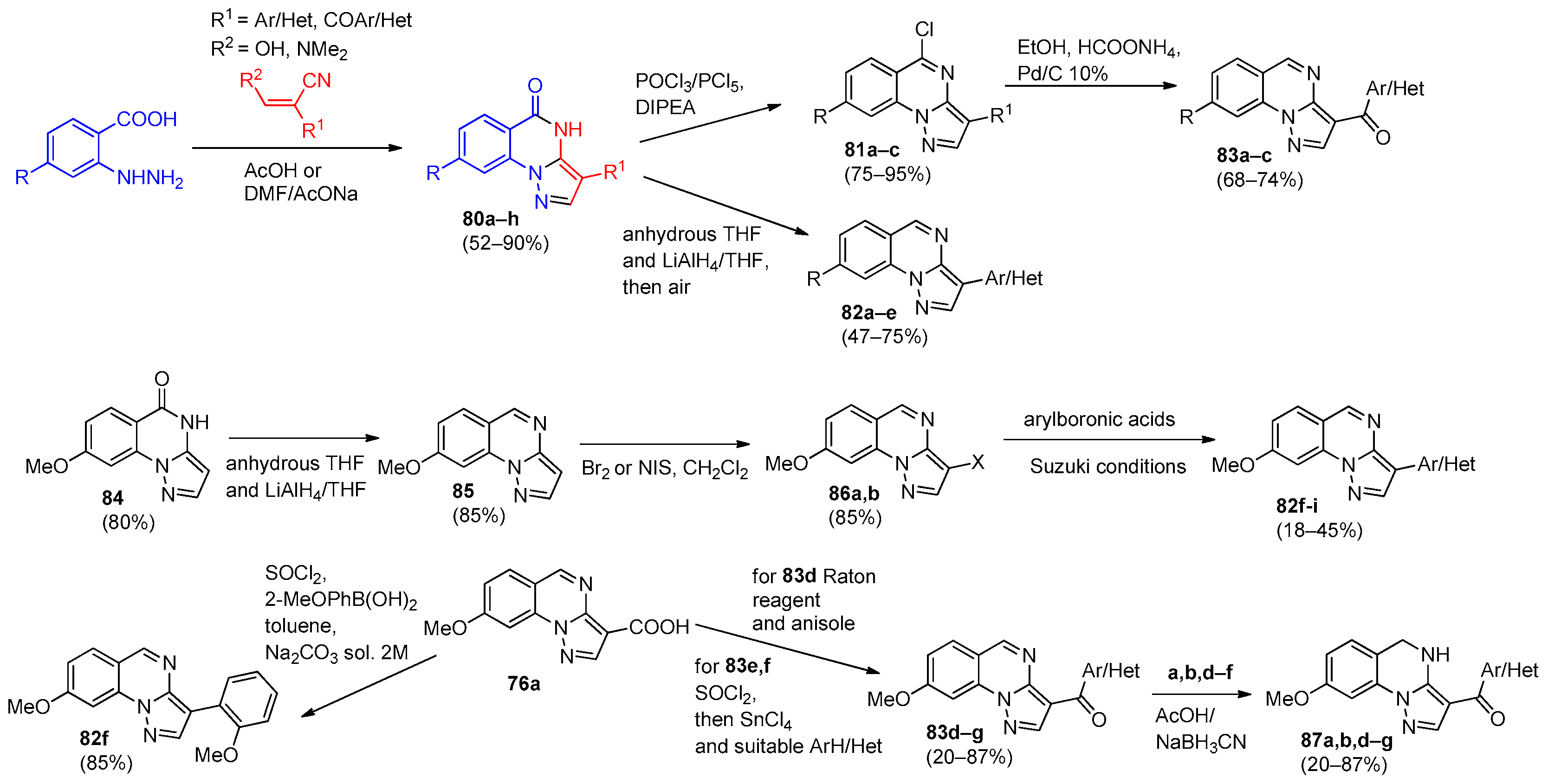
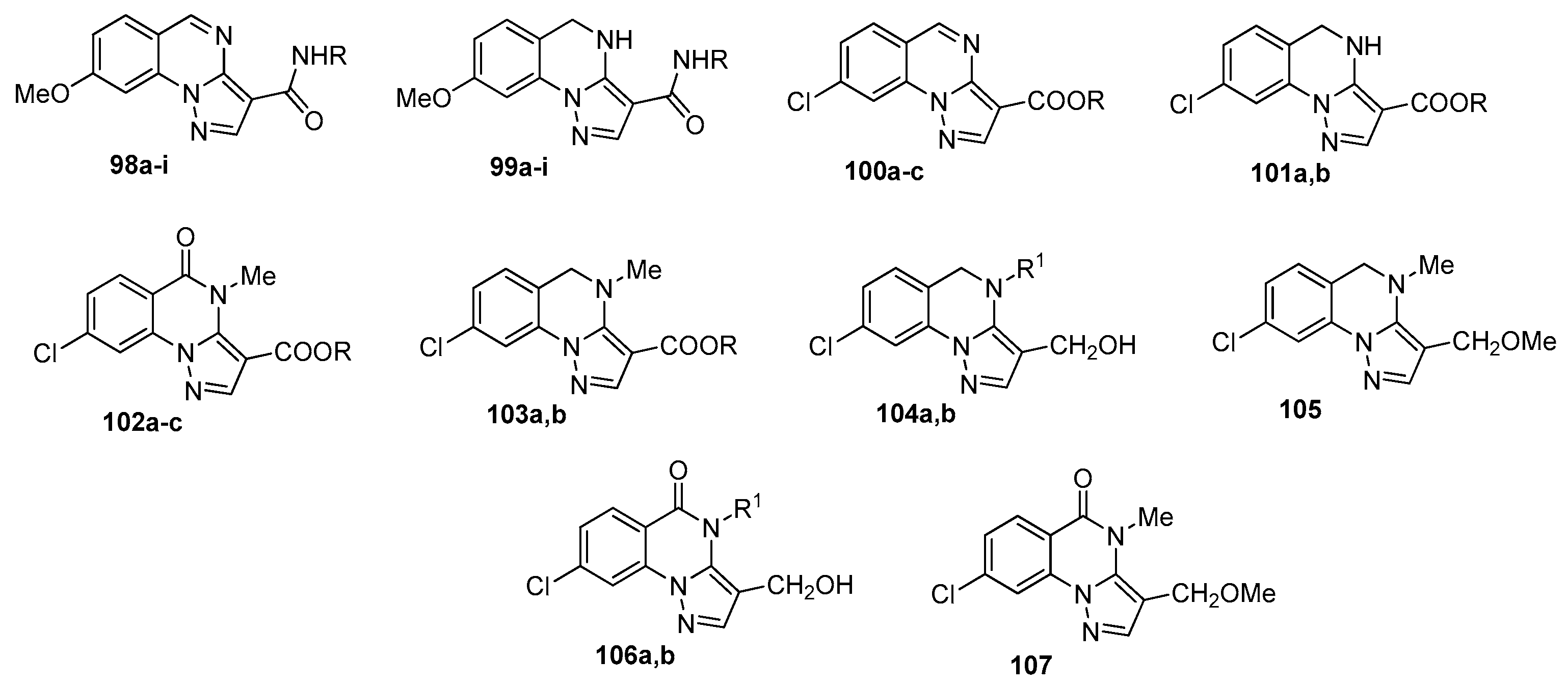
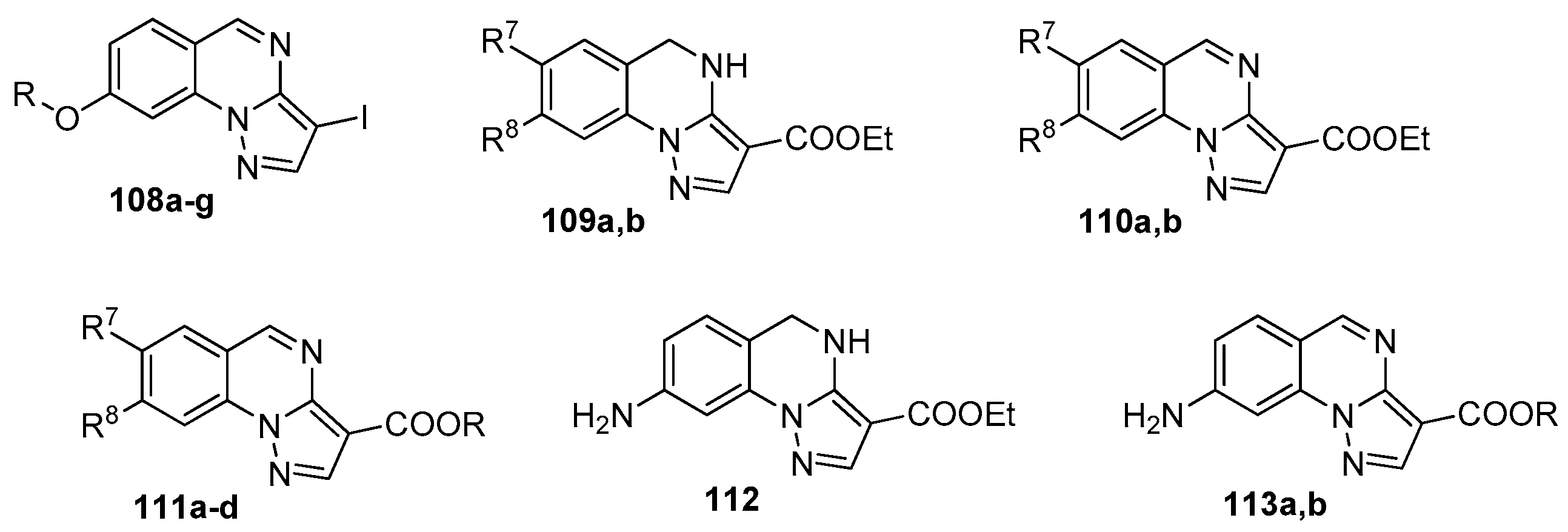
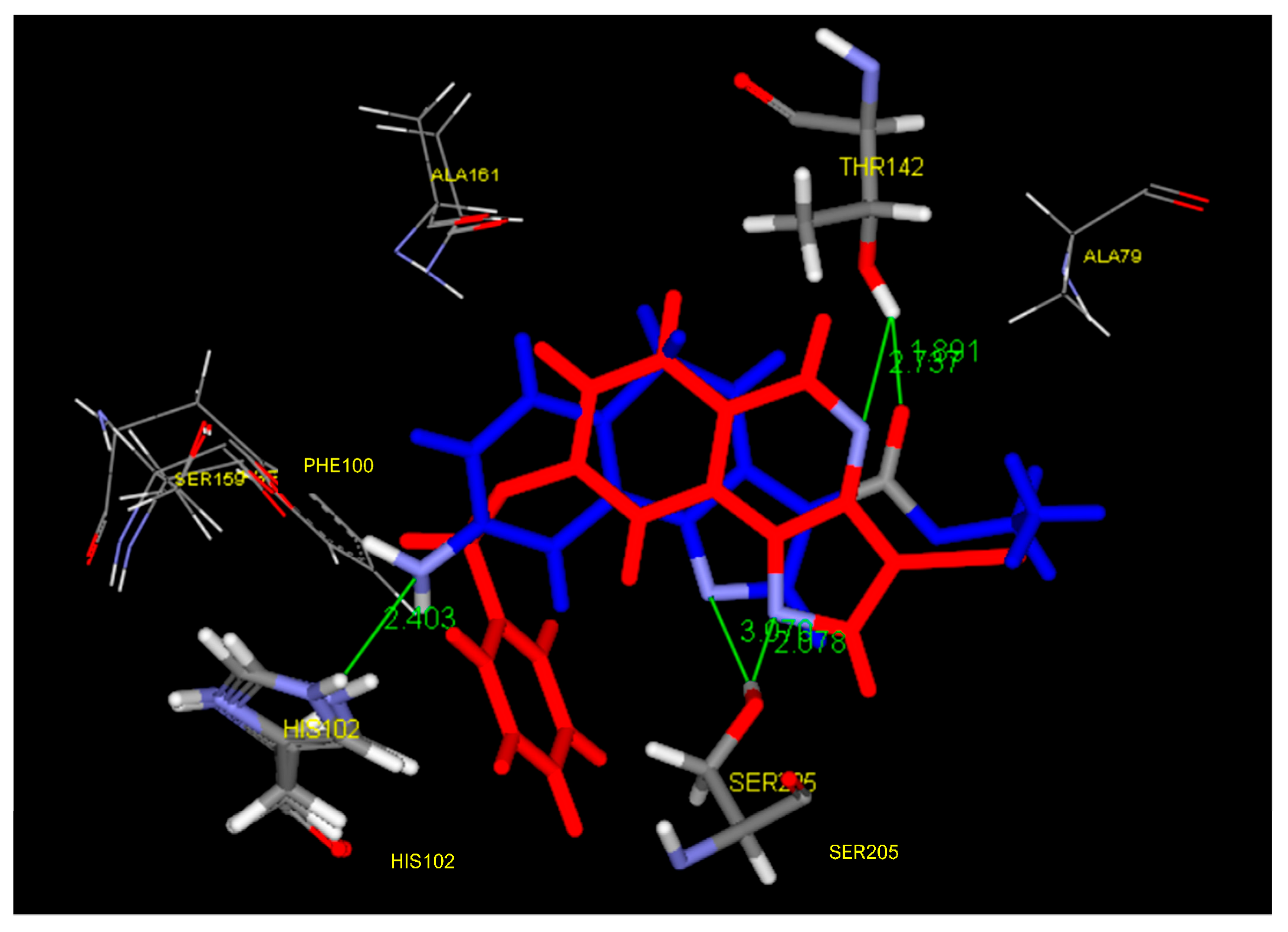
4. Pyrazolo[1,5-a]quinazolines and Indazolo[2,3-a]quinazolines
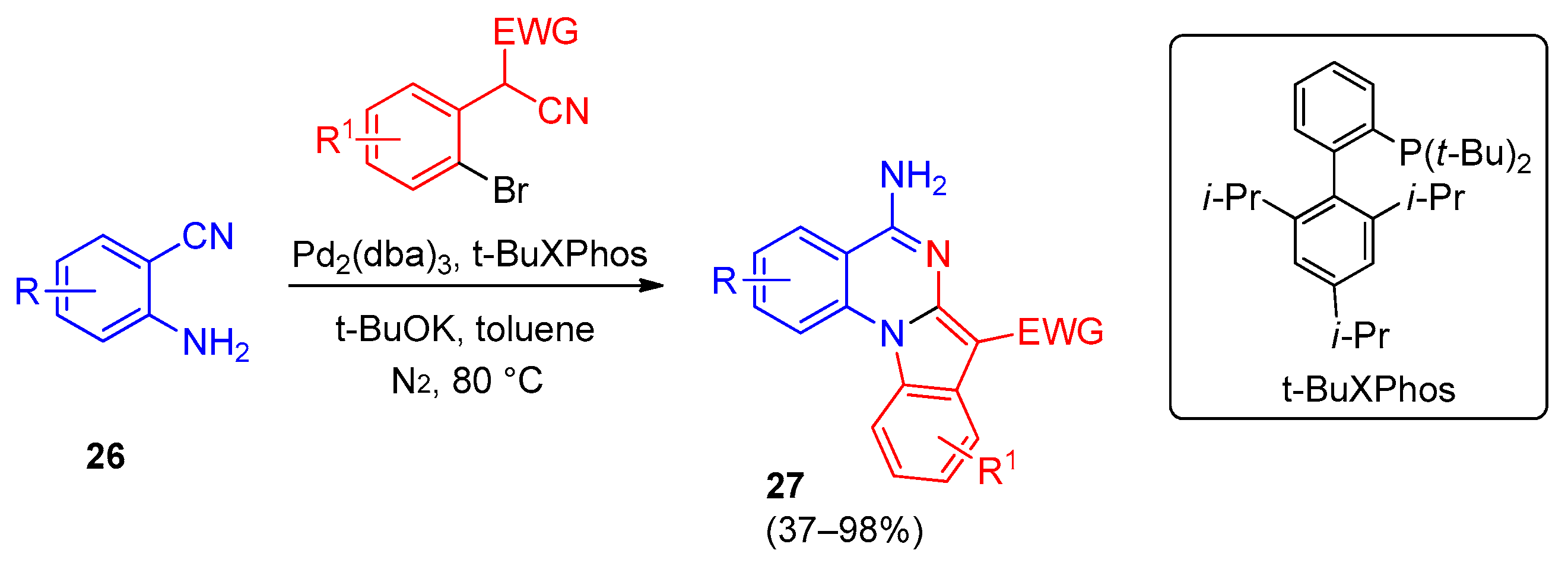
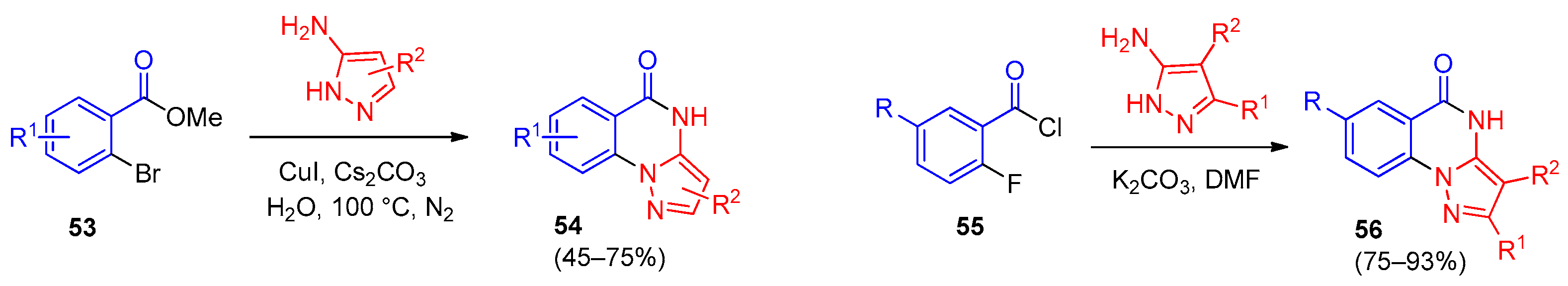
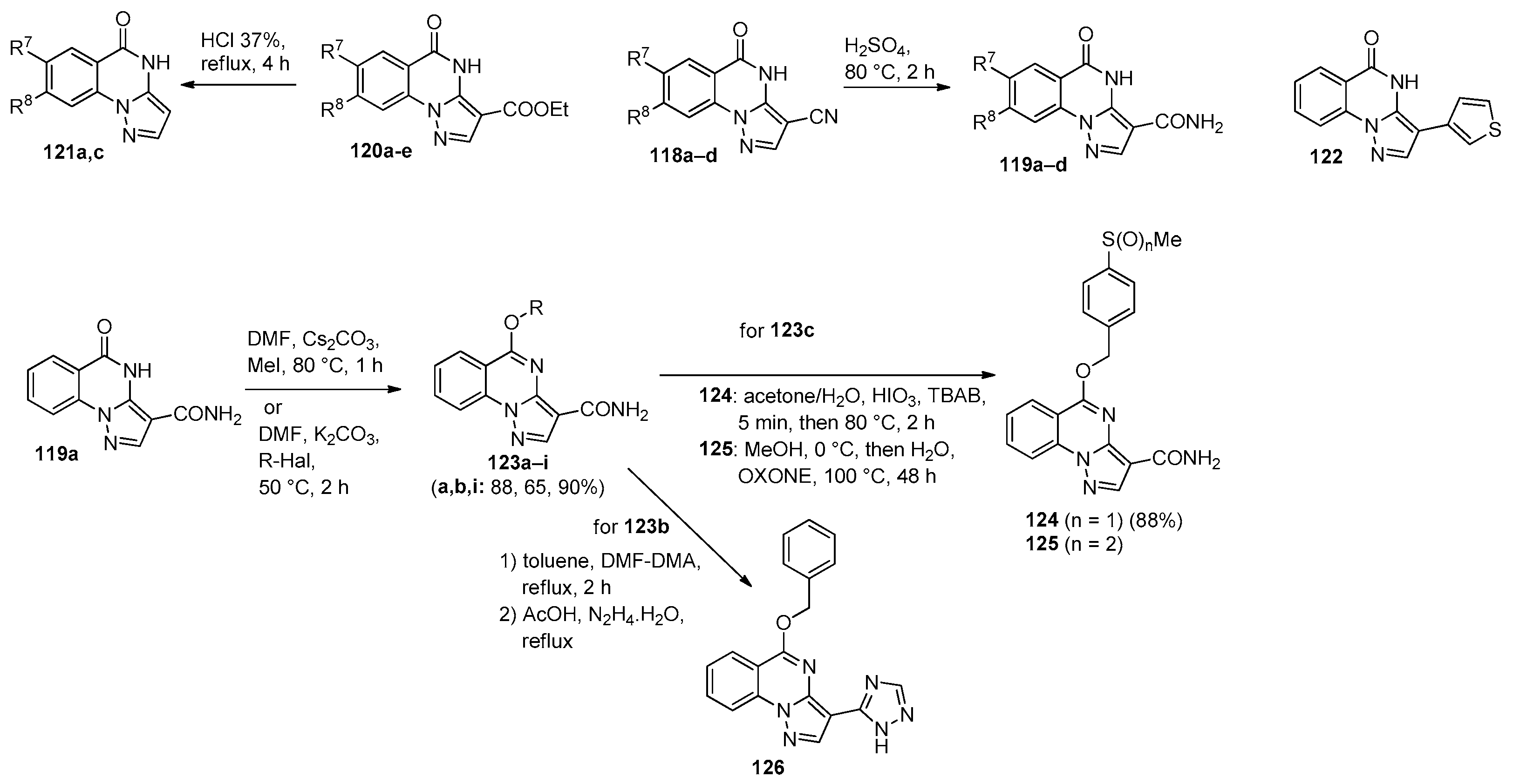
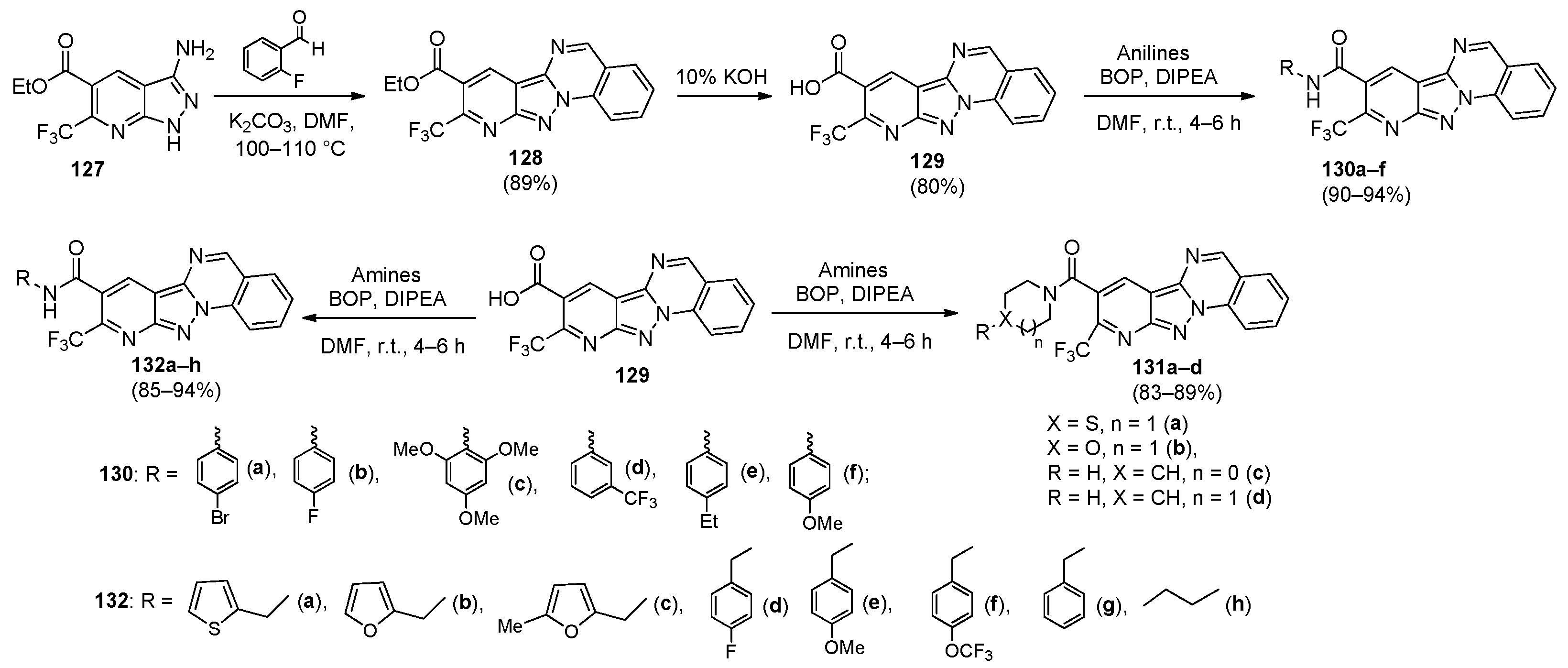
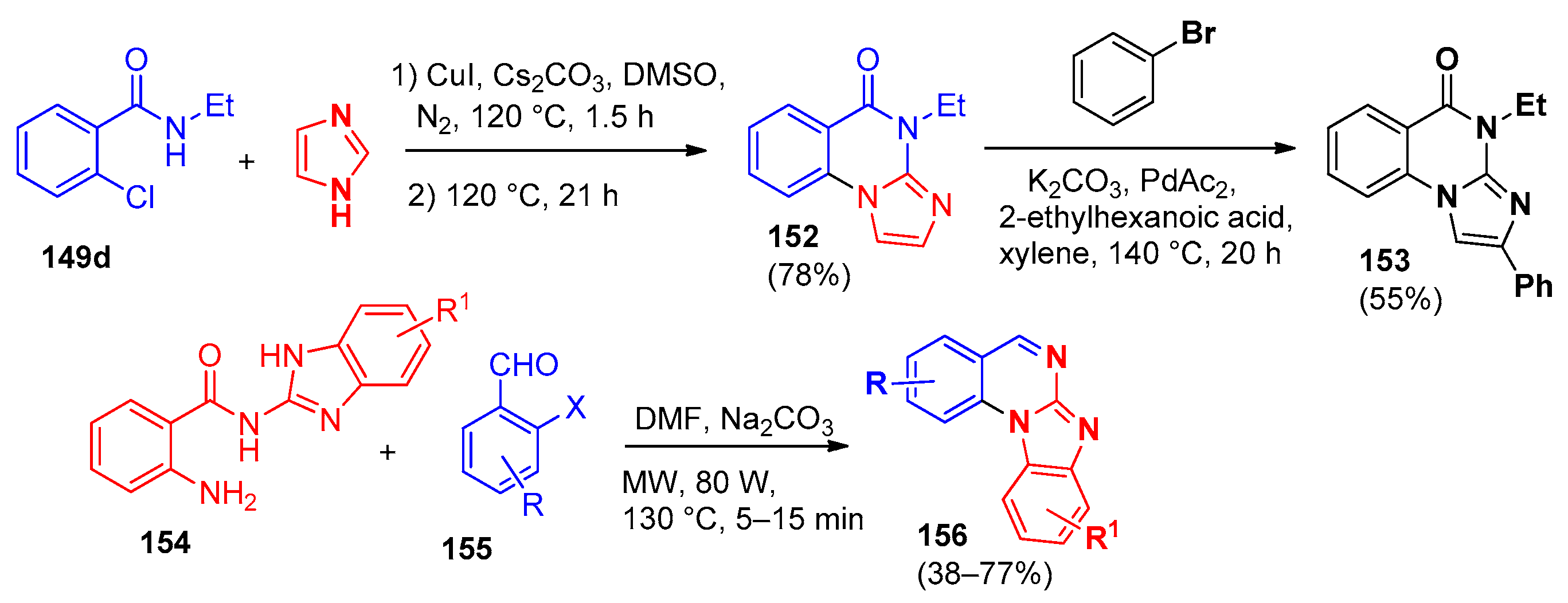
5. Imidazo[1,2-a]quinazolines, Benzimidazo[1,2-a]quinazolines
6. Triazolo[a]quinazolines
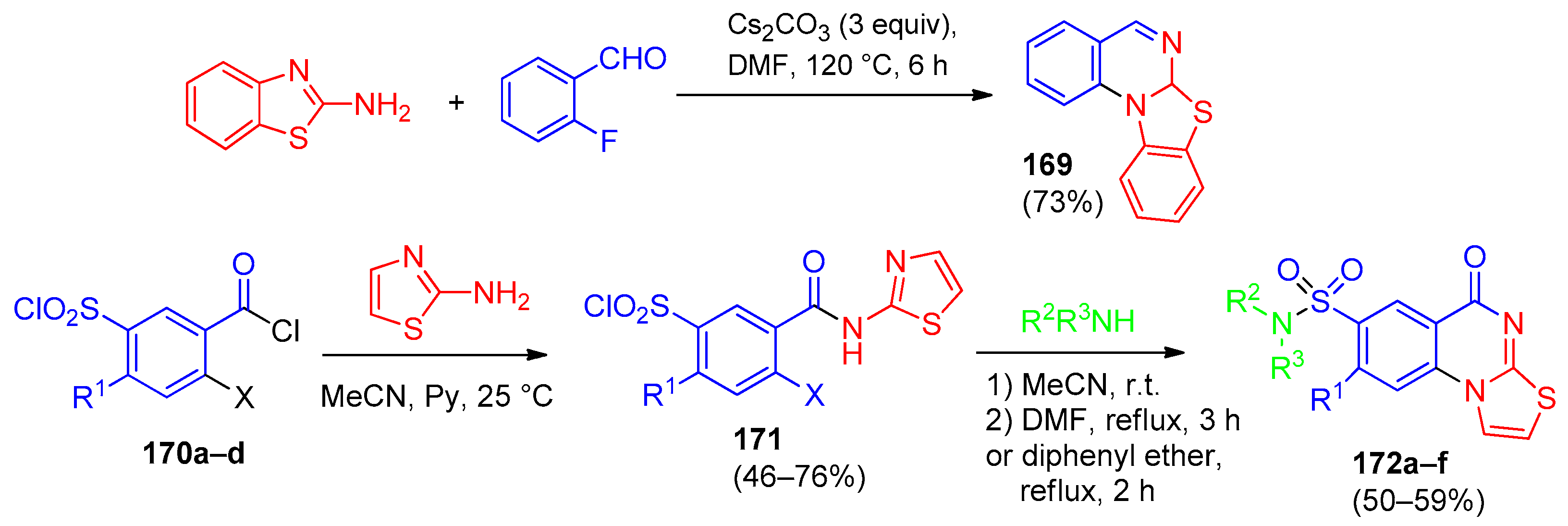
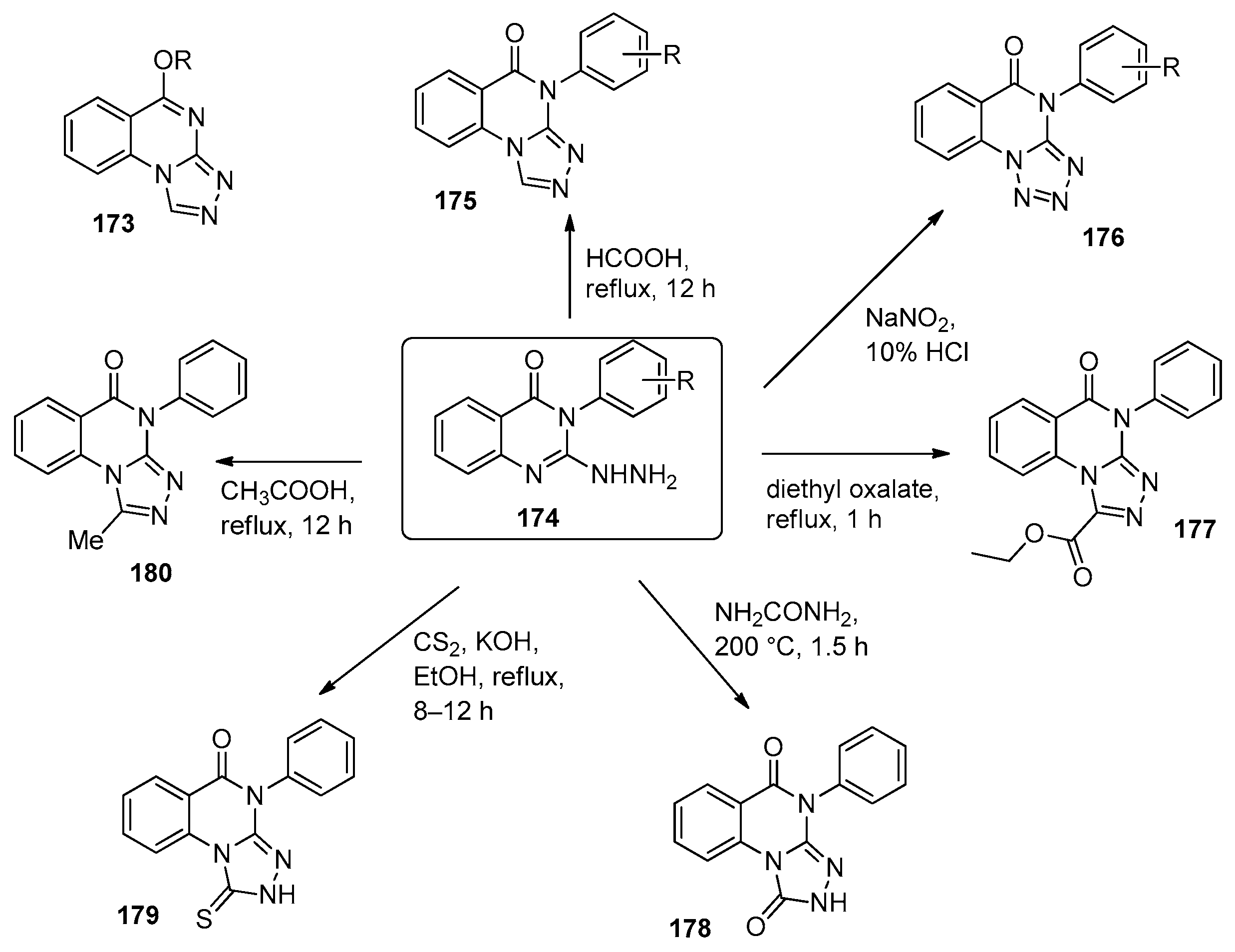
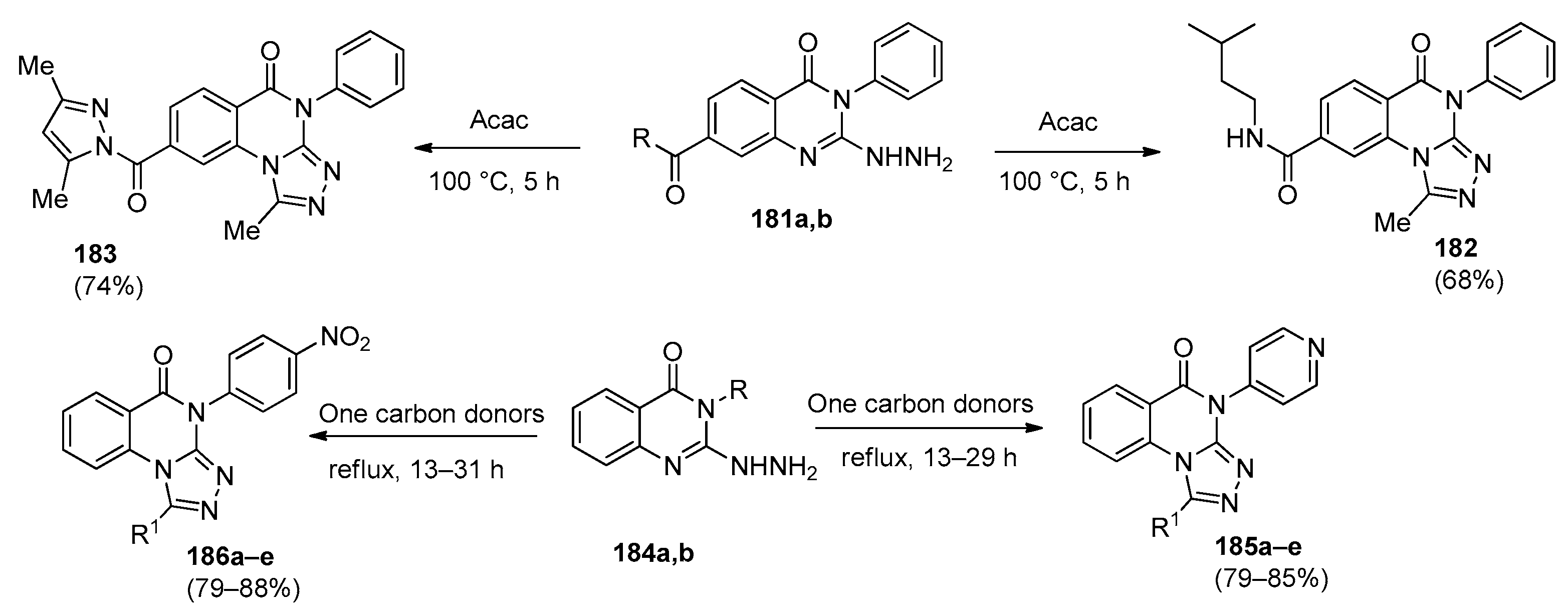
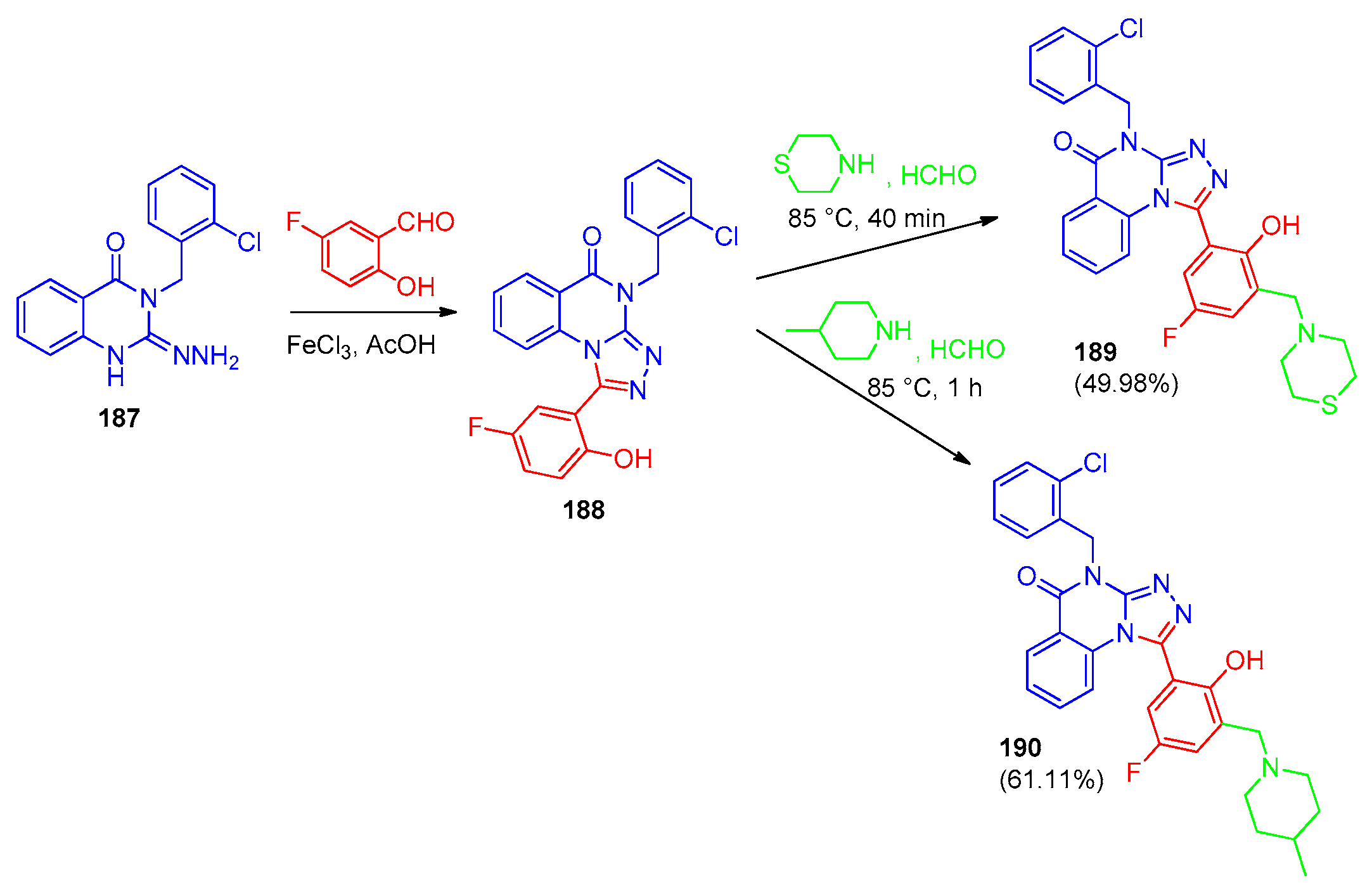
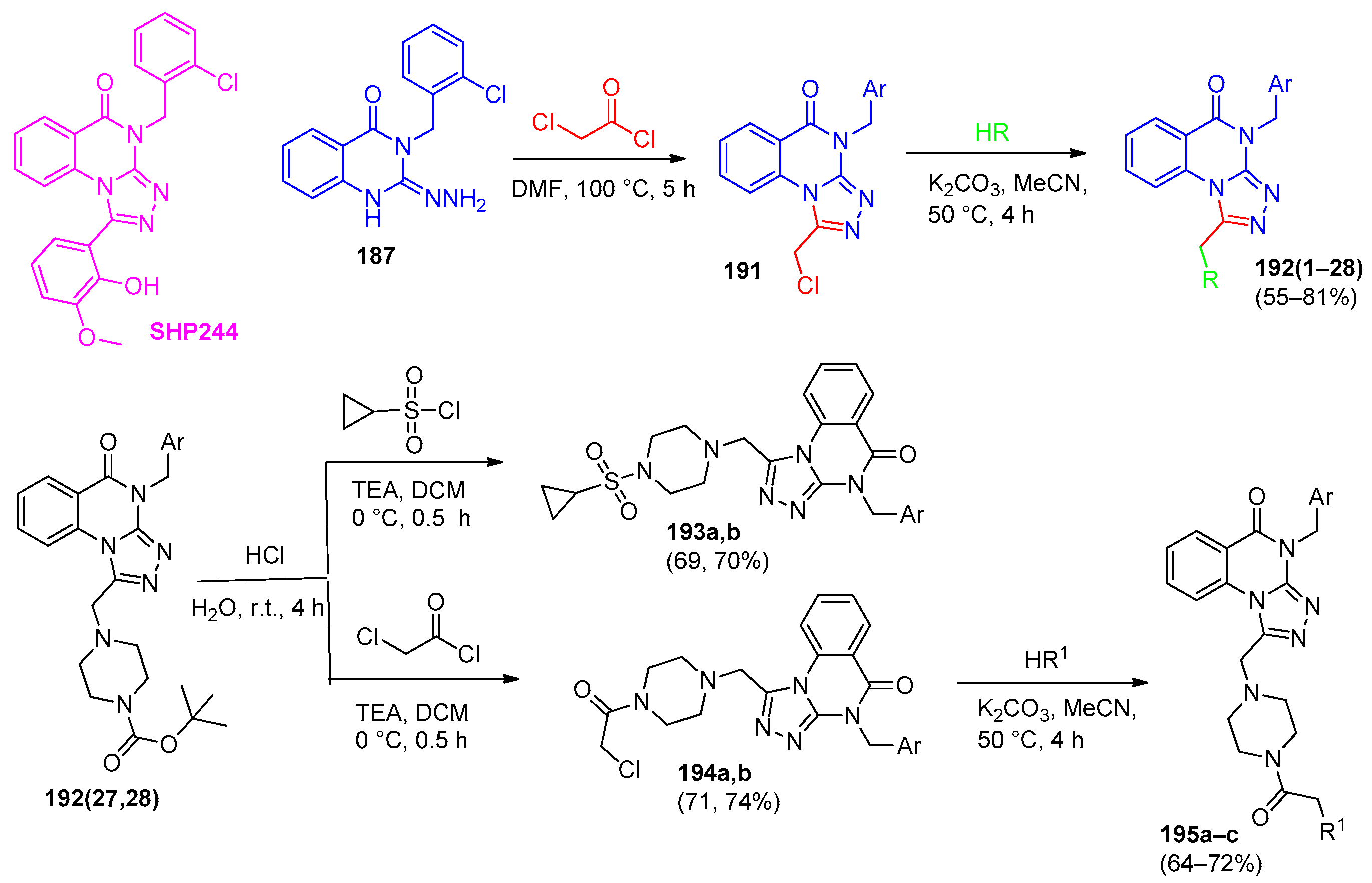
6.1. [1,2,4]-Triazolo[4,3-a]quinazolines
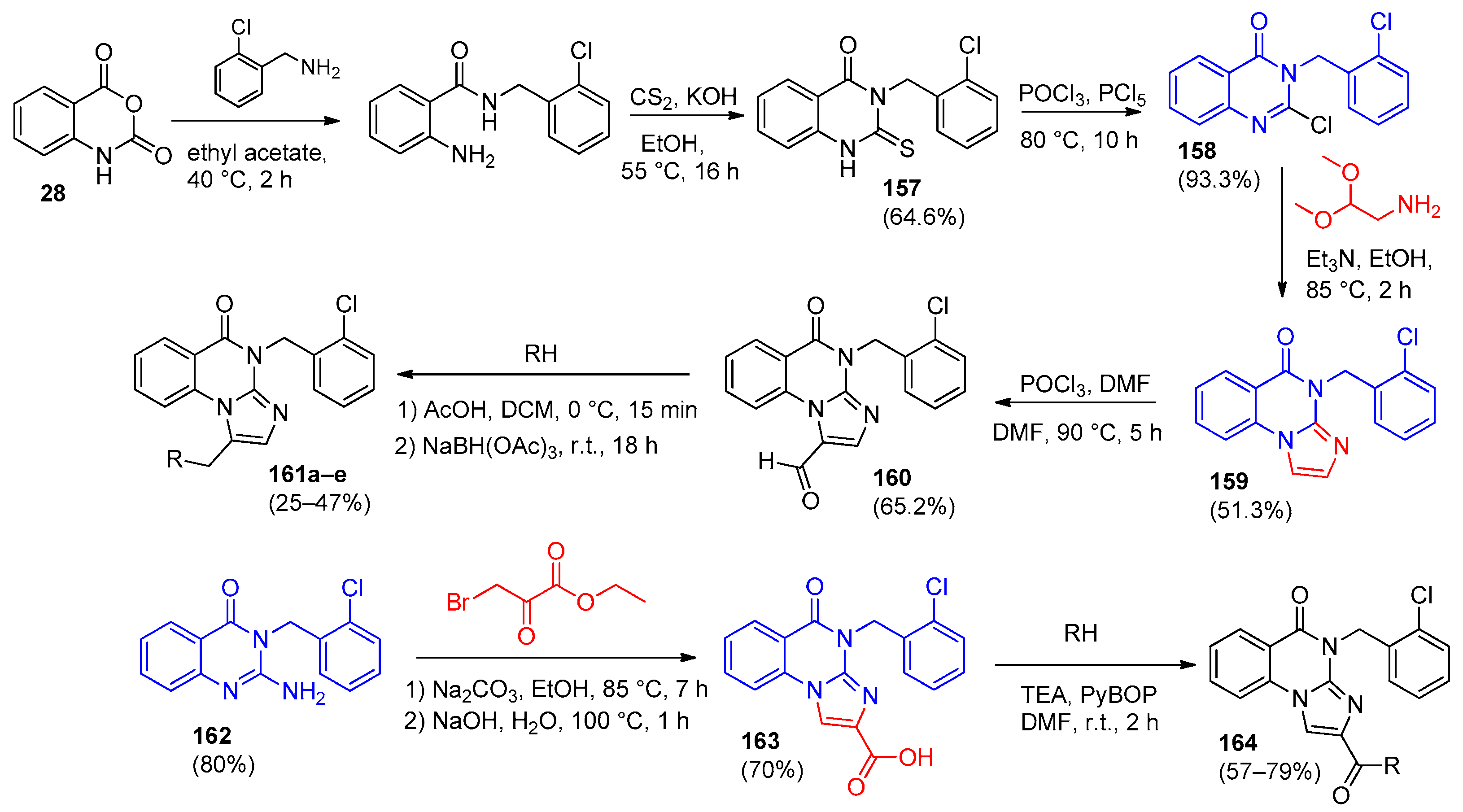
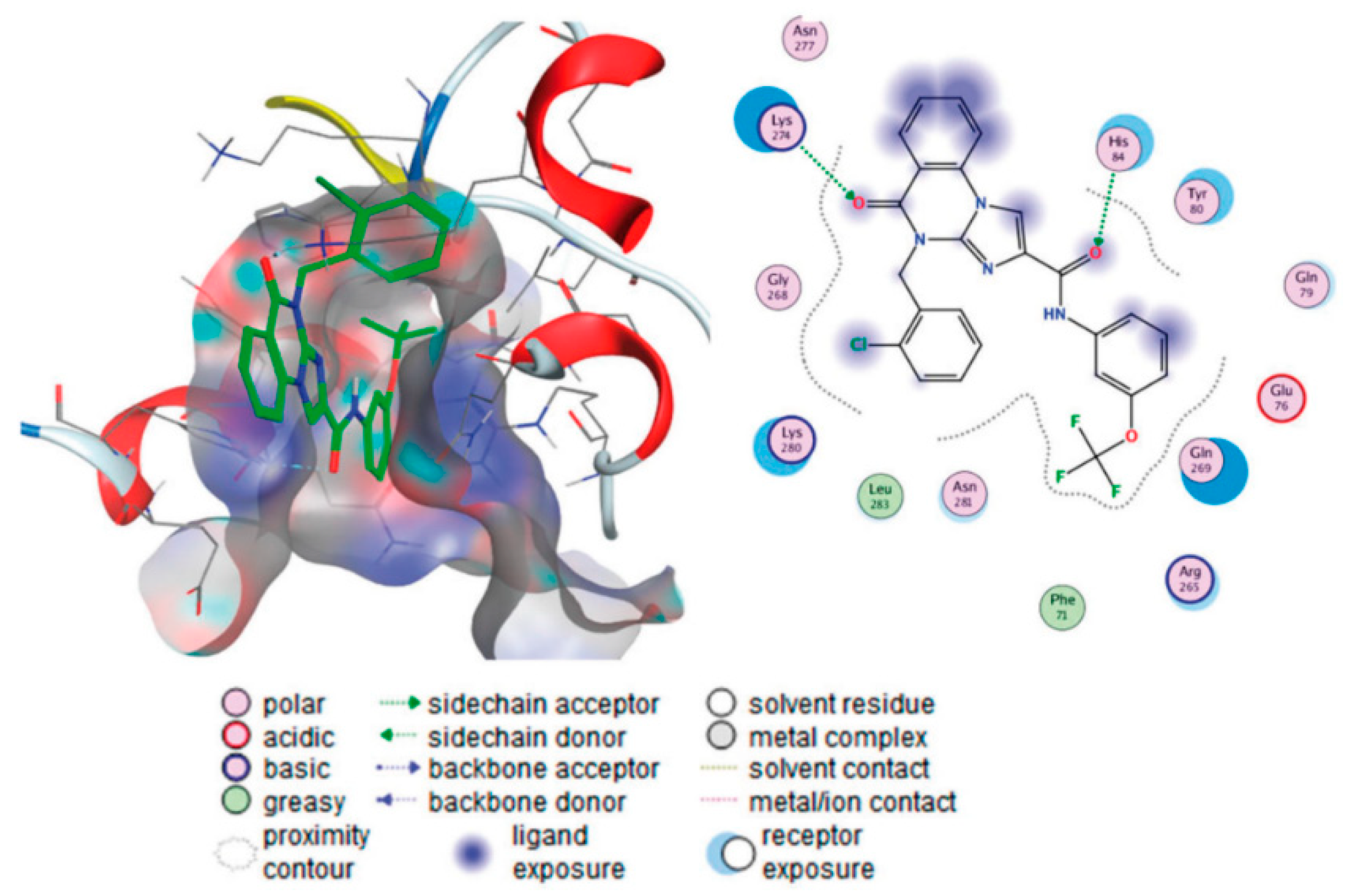
6.2. [1,2,4]-Triazolo[1,5-a]quinazolines
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TFA | Trifluoroacetic acid |
| CPT | Camptothecin |
| NIS | N-iodosuccinimide |
| NBS | N-bromosuccinimide |
| NOE | Nuclear Overhauser effect |
| DMSO | Dimethyl sulfoxide |
| TBAB | Tetrabutylammonium bromide |
| PEG | Polyethylene glycol |
| TFE | 2,2,2-Trifluoroethanol |
| LAG | Liquid-assisted grinding |
| HTS | High-throughput screening |
| DMF | N,N-Dimethylformamide |
| GABA | Gamma-aminobutyric acid |
| HCA | Hierarchical Cluster Analysis |
| ERK | Extracellular signal-regulated kinase |
| GBBR | Groebke–Blackburn–Bienaymé reaction |
| MES | Maximal electroshock |
| ATP | Adenosine triphosphate |
| SAR | Structure–activity relationship |
| DFT | Density Functional Theory |
| FMO | Frontier molecular orbital |
| TfOH | Trifluoromethanesulfonic acid |
| PTSA, TsOH | p-Toluenesulfonic acid |
| DCM | Dichloromethane |
| DCE | 1,2-Dichloroethane |
| NMR | Nuclear magnetic resonance |
| MsOH | Methanesulfonic acid |
| DMF-DtBA | Dimethylformamide-di-tert-butylacetate |
| PIFA | (Bis(trifluoroacetoxy)iodo)benzene |
| DABCO | 1,4-diazabicyclo[2.2.2]octane |
| BOP | Benzotriazol-1-yloxy-tris(dimethylamino)phosphonium hexafluorophosphate |
| DPPH | 1,1-diphenyl-2-picryl-hydrazyl |
| MOF | Metal–organic frameworks |
| NCI | National Cancer Institute |
| HDAC | Histone deacetylase |
| VEGFR | Vascular endothelial growth factor receptor |
| HREI-MS | High-resolution electron ionization mass spectrometry |
| TNF | Tumor Necrosis Factor |
| TLC | Thin-layer chromatography |
References
- Shagufta, I.A. An insight into the therapeutic potential of quinazoline derivatives as anticancer agents. Med. Chem. Commun. 2017, 8, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Halappanavar, V.; Teli, S.; Sannakki, H.B.; Teli, D. Quinazoline scaffold as a target for combating microbial resistance: Synthesis and antimicrobial profiling of quinazoline derivatives. Results Chem. 2025, 13, 101955. [Google Scholar] [CrossRef]
- Alagarsamy, V.; Chitra, K.; Saravanan, G.; Solomon, V.R.; Sulthana, M.T.; Narendhar, B. An overview of quinazolines: Pharmacological significance and recent developments. Eur. J. Med. Chem. 2018, 151, 628–685. [Google Scholar] [CrossRef]
- Shang, X.F.; Morris-Natschke, S.L.; Liu, Y.Q.; Guo, X.; Xu, X.S.; Goto, M.; Li, J.C.; Yang, G.Z.; Lee, K.H. Biologically active quinoline and quinazoline alkaloids Part II. Med. Res. Rev. 2018, 38, 1614–1660. [Google Scholar] [CrossRef] [PubMed]
- Gatadi, S.; Lakshmi, T.V.; Nandur, S. 4(3H)-Quinazolinone derivatives: Promising antibacterial drug leads. Eur. J. Med. Chem. 2019, 170, 157–172. [Google Scholar] [CrossRef]
- Bakheit, A.H.; Abuelizz, H.A.; Al-Salahi, R. Crystallographic analysis, Hirshfeld surface investigation, and DFT calculations of 2-phenoxy-triazoloquinazoline molecule: Implications for drug design. J. Mol. Struct. 2025, 1319, 139436. [Google Scholar] [CrossRef]
- Bakheit, A.H.; Abuelizz, H.A.; Al-Salahi, R. A DFT study and Hirshfeld surface analysis of the molecular structures, radical scavenging abilities and ADMET properties of 2-methylthio(methylsulfonyl)-[1,2,4]triazolo[1,5-a]quinazolines: Guidance for antioxidant drug design. Crystals 2023, 13, 1086. [Google Scholar] [CrossRef]
- Auti, P.S.; George, G.; Paul, A.T. Recent advances in the pharmacological diversification of quinazoline/quinazolinone hybrids. RSC Advances 2020, 10, 41353–41392. [Google Scholar] [CrossRef]
- Kushwaha, P.; Bhardwaj, A.; Rashi. One pot synthesis and mechanistic insights of quinazoline and related molecules. Tetrahedron 2025, 179, 134635. [Google Scholar] [CrossRef]
- Nosova, E.V.; Lipunova, G.N.; Permyakova, Y.V.; Charushin, V.N. Quinazolines annelated at the N(3)–C(4) bond: Synthesis and biological activity. Eur. J. Med. Chem. 2024, 271, 116411. [Google Scholar] [CrossRef]
- Abuelizz, H.A.; Al-Salahi, R. An overview of triazoloquinazolines: Pharmacological significance and recent developments. Bioorg. Chem. 2021, 115, 105263. [Google Scholar] [CrossRef]
- Jabeen, T.; Aslam, S.; Ahmad, M.; ul Haq, A.; Al-Hussain, S.A.; Zaki, M.E.A. Triazoloquinazoline: Synthetic strategies and medicinal importance. In Recent Advances on Quinazoline; IntechOpen: London, UK, 2023; pp. 1–27. [Google Scholar]
- Rafiq, A.; Aslam, S.; Ahmad, M.; Saif, M.J.; Al-Hussain, S.A.; Zaki, M.E.A. Recent approaches for the synthesis of imidazoquinazolines and benzimidazoquinazolines. In Recent Advances on Quinazoline; IntechOpen: London, UK, 2023; pp. 1–19. [Google Scholar]
- Garg, M.; Chauhan, M.; Singh, P.K.; Alex, J.M.; Kumar, R. Pyrazoloquinazolines: Synthetic strategies and bioactivities. Eur. J. Med. Chem. 2015, 97, 444–461. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, X.; Zhang, Y.; Tang, C.; Feng, B. Progress in synthesis and bioactivity evaluation of pyrazoloquinazolines. Lett. Drug Des. Discov. 2020, 17, 104–113. [Google Scholar] [CrossRef]
- Vaskevych, A.I.; Dekhtyar, M.; Vovk, M. Cyclizations of alkenyl(alkynyl)-functionalized quinazolinones and their heteroanalogues: A powerful strategy for the construction of polyheterocyclic structures. Chem. Rec. 2024, 24, e202300255. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, F.; Georgescu, F.; Georgescu, E.; Cairad, M.R. Chapter Three—Pyrroloquinolines, Imidazoquinolines, and Pyrroloquinazolines with a Bridgehead Nitrogen. Adv. Heterocycl. Chem. 2019, 129, 155–244. [Google Scholar]
- Rasapalli, S.; Sammeta, V.R.; Murphy, Z.F.; Huang, Y.; Boerth, J.A.; Golen, J.A.; Savinov, S.N. Synthesis of C-ring-substituted vasicinones and luotonins via regioselective aza-Nazarov cyclization of quinazolinonyl enones. Org. Lett. 2019, 21, 9824–9828. [Google Scholar] [CrossRef] [PubMed]
- Rasapalli, S.; Sammeta, V.R.; Murphy, Z.F.; Golen, J.A.; Agama, K.; Pommier, Y.; Savinov, S.N. Design and synthesis of C-aryl angular luotonins via a one-pot aza-Nazarov–Friedlander sequence and their topo-I inhibition studies along with C-aryl vasicinones and luotonins. Bioorg. Med. Chem. Lett. 2021, 41, 127998. [Google Scholar] [CrossRef]
- Vaskevych, A.I.; Savinchuk, N.O.; Vaskevych, R.I.; Rusanov, E.B.; Grygorenko, O.O.; Vovk, M.V. PIFA-Initiated oxidative cyclization of 2-(3-butenyl)quinazolin-4(3H)-ones—An efficient approach to 1-(Hydroxymethyl)-2,3-dihydropyrrolo[1,2-a]quinazolin-5(1H)-ones. Beilstein J. Org. Chem. 2021, 17, 2787–2794. [Google Scholar] [CrossRef]
- Vaskevych, A.I.; Savinchuk, N.O.; Vaskevych, R.I.; Rusanov, E.B.; Vovk, M.V. Chalcogenation/Pyrrolo(Pyrido)Annulation of 2-(3-butenyl)quinazolin-4(3H)-ones by Arylsulfenyl(Selenyl) Chlorides. Tetrahedron 2022, 111, 132722. [Google Scholar] [CrossRef]
- Vaskevych, R.I.; Savinchuk, N.O.; Vaskevych, A.I.; Rusanov, E.B.; Bylina, D.V.; Kyrylchuk, A.A.; Vovk, M.V. Proton- and halogen-induced cyclizations of 2-(3-butenyl) quinazolin-4(3H)-ones in the synthesis of pyrrolo[2,1-b]- and pyrrolo[1,2-a]quinazolinone derivatives. J. Heterocycl. Chem. 2023, 60, 431–448. [Google Scholar] [CrossRef]
- Singh, P.; Kaur, N.; Banerjee, P. Regioselective Bronsted Acid-Catalyzed Annulation of Cyclopropane Aldehydes with N’-Aryl Anthranil Hydrazides: Domino Construction of Tetrahydropyrrolo[1,2-a]quinazolin-5(1H)-ones. J. Org. Chem. 2020, 85, 3393–3406. [Google Scholar] [CrossRef] [PubMed]
- Grinev, V.S.; Amalchieva, O.A.; Egorova, A.Y. Features of Reaction of Substituted 4-Oxobutanoic Acids and 3H-Furan-2-ones with 1,3-binucleophiles. Russ. J. Org. Chem. 2017, 53, 1669–1674. [Google Scholar] [CrossRef]
- Ferraris, D.V. Evolution of Poly(ADP-ribose) Polymerase-1 (PARP-1) Inhibitors. From Concept to Clinic. J. Med. Chem. 2010, 53, 4561. [Google Scholar] [CrossRef]
- Kumar, K.S.; Kumar, P.M.; Kumar, K.A.; Sreenivasulu, M.; Jafar, A.A.; Rambabu, D.; Krishna, G.R.; Reddy, C.M.; Kapavarapu, R.; Shivakumar, K.; et al. A new three-component reaction: Green synthesis of novel isoindolo[2,1-a]quinazoline derivatives as potent inhibitors of TNF-α. Chem. Commun. 2011, 57, 5010–5012. [Google Scholar] [CrossRef]
- Kotipalli, T.; Kavala, V.; Janreddy, D.; Kuo, C.-W.; Kuo, T.-S.; Huang, H.-N.; He, C.-H.; Yao, C.-F. Syntheses of indolo[1,2-a]quinazolinone derivatives via palladium-catalyzed intramolecular C–H amidation. RSC Adv. 2014, 4, 2274–2283. [Google Scholar] [CrossRef]
- Abe, T.; Takahashi, Y.; Matsubara, Y.; Yamada, K. Ullmann N-Arylation/2-Amidation Cascade by Self-Relay Copper Catalysis: One-pot Synthesis of Indolo[1,2-a]quinazolinones. Org. Chem. Front. 2017, 4, 2124–2127. [Google Scholar] [CrossRef]
- Badigenchala, S.; Sekar, G. NIS-mediated cross-coupling of C(sp2)–H and N–H bonds: A transition metal-free approach towards indolo[1,2-a]quinazolinones. J. Org. Chem. 2017, 82, 7657–7665. [Google Scholar] [CrossRef]
- Jiang, M.; Xiang, H.; Zhu, F.; Xu, X.; Deng, L.; Yang, C. Efficient Pd-catalyzed domino synthesis of 1-phenyl-1H-indol-2-amine and 5-amino-indolo[1,2-a]quinazoline derivatives. Org. Biomol. Chem. 2015, 13, 10122. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Palnati, G.R.; Dodda, R.P.; Avula, S.R.; Swami, P. Studies on novel synthetic methodologies, part XII: An efficient one-pot access to 6,6a-dihydroisoindolo[2,1-a]quinazoline-5,11-diones and 5-phenylisoindolo[2,1-a]quinazolin-11(6aH)-ones. Synlett 2013, 24, 105–113. [Google Scholar] [CrossRef][Green Version]
- Reddy, G.R.; Reddy, T.R.; Chary, R.G.; Joseph, S.C.; Mukherjee, S.; Pal, M. β-Cyclodextrin-mediated MCR in water: Synthesis of dihydroisoindolo[2,1-a]quinazoline-5,11-dione derivatives under microwave irradiation. Tetrahedron Lett. 2013, 54, 6744–6746. [Google Scholar] [CrossRef]
- Esmaeili-Marandi, F.; Saeedi, M.; Yavari, I.; Mahdavi, M.; Shafiee, A. Synthesis of novel isoinodolo[2,1-a]quinazolinedione derivatives containing a 1,2,3-triazole ring system. Helv. Chim. Acta 2016, 99, 37–40. [Google Scholar] [CrossRef]
- Mahdavi, M.; Lotfi, V.; Saeedi, M.; Kianmehr, E.; Shafiee, A. Synthesis of novel fused quinazolinone derivatives. Mol. Divers. 2016, 20, 677–685. [Google Scholar] [CrossRef]
- Madhubabu, M.V.; Shankar, R.; Reddy, G.R.; Rao, T.S.; Rao, M.V.B.; Akula, R. Metal-catalyst-free green and efficient synthesis of five and six-membered fused N-heterocyclic quinazoline derivatives. Tetrahedron Lett. 2016, 57, 5033–5037. [Google Scholar] [CrossRef]
- Abbasian, S.; Kabirifard, H.; Mahdavi, M. The synthesis of 2,3-dihydroquinazoline-4(1H)-one and dihydroisoindolo[2,1-a]quinazoline-5,11-dione derivatives in the presence of imidazolium ionic liquid sulfonic acid functionalized SBA-15: A novel feature of SBA-15. Arkivoc 2018, 3, 302–314. [Google Scholar] [CrossRef]
- Rayatzadeh, A.; Haghipour, S. Efficient synthesis of 6,6a-dihydroisoindolo[2,1-a]quinazoline-5,11-dione derivatives catalyzed by functionalized nanoporous silica. Monatshefte Chem. 2021, 152, 103–107. [Google Scholar] [CrossRef]
- Devi, R.V.; Garande, A.M.; Bhate, P.M.; Sirisha, K.; Ganapathi, T. Synthesis of isoindolo[2,1-a]quinazoline, isoindolo[2,1-a]pyrrolo[2,1-c]quinoxalinone, and indolo[1,2-a]isoindolo[1,2-c]quinoxalinone derivatives in a deep eutectic solvent. Synlett 2016, 27, 2807–2810. [Google Scholar]
- Khachatryan, D.S.; Belus, S.K.; Misyurin, V.A.; Baryshnikova, M.A.; Kolotaev, A.V.; Matevosyan, K.R. Synthesis and properties of 1,2-dihydro-4(3H)-quinazolinones. Russ. Chem. Bull. 2017, 66, 1044–1058. [Google Scholar] [CrossRef]
- Lohar, T.; Mane, A.; Kamat, S.; Kumbhar, A.; Salunkhe, R. Trifluoroethanol and liquid-assisted grinding method: A green catalytic access for multicomponent synthesis. Res. Chem. Intermed. 2017, 44, 1919–1933. [Google Scholar] [CrossRef]
- Jin, R.-Z.; Zhang, W.-T.; Zhou, Y.-J.; Wang, X.-S. Iodine-catalyzed synthesis of H-phthalazino[1,2-b]quinazoline and isoindolo[2,1-a]quinazoline derivatives via chemoselective reaction of 2-aminobenzohydrazide and 2-formylbenzoic acid in ionic liquids. Tetrahedron Lett. 2016, 57, 2515–2519. [Google Scholar] [CrossRef]
- Bodhak, C.; Hazra, S.; Pramanik, A. Graphene oxide: An efficient carbocatalyst for the facile synthesis of isoindolo[2,1-a]quinazoline-5,11-diones via domino condensation under solvent-free conditions. ChemistrySelect 2018, 3, 7707–7712. [Google Scholar] [CrossRef]
- Guo, S.; Zhai, J.; Wang, F.; Fan, X. One-pot three-component selective synthesis of isoindolo[2,1-a]quinazoline derivatives via a palladium-catalyzed cascade cyclocondensation/cyclocarbonylation sequence. Org. Biomol. Chem. 2017, 15, 3674–3680. [Google Scholar] [CrossRef] [PubMed]
- Kolotaev, A.V.; Matevosyan, K.R.; Osipov, V.N.; Khachatryan, D.S. Synthesis of new quinazoline-containing hydroxamic acids as potential HDAC/VEGFR inhibitors. Unusual rearrangements with pyrrolidone ring opening and dehydration of 3-N-hydroxyquinazoline fragment containing tetracycles. Tetrahedron Lett. 2019, 60, 151315. [Google Scholar] [CrossRef]
- Mondal, M.A.; Mondal, S.; Khan, A.A. Mechanistic insight into the acid-catalyzed, one-pot synthesis of isoindole-fused quinazolin-4-ones. J. Chem. Sci. 2020, 132, 63–68. [Google Scholar] [CrossRef]
- Orvieto, F.; Branca, D.; Giomini, C. Identification of substituted pyrazolo[1,5-a]quinazolin-5(4H)-one as potent poly(ADP-ribose) polymerase-1 (PARP-1) inhibitors. Bioorg. Med. Chem. 2009, 19, 4196–4200. [Google Scholar] [CrossRef]
- Petrov, A.; Kasatochkin, A. Synthesis of 6,7,8,9-tetrahydropyrazolo[1,5-a]quinazolines containing a 5-phenylamine fragment. Russ. J. Org. Chem. 2013, 49, 1250–1252. [Google Scholar] [CrossRef]
- Ferraguti, F.; Shigemoto, R. Metabotropic Glutamate Receptors. Cell. Tissue Res. 2006, 326, 483–504. [Google Scholar] [CrossRef]
- Taliani, S.; Pugliesi, I.; Barresi, E. Phenylpyrazolo[1,5-a]quinazolin-5(4H)-one: A suitable scaffold for the development of noncamptothecin topoisomerase I (Top1) inhibitors. J. Med. Chem. 2013, 56, 7458–7462. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roopan, S.M.; Palaniraja, J. Synthesis of indazoloquinazoline system (microreview). Chem. Heterocycl. Comp. 2016, 52, 93–95. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, L.; Wang, Z.; Fan, X. Water-mediated selective synthesis of pyrazolo[1,5-a]quinazolin-5(4H)-ones and [1,2,4]triazolo[1,5-a]quinazolin-5(4H)-one via copper-catalyzed cascade reactions. Synth. Commun. 2015, 45, 2426–2435. [Google Scholar] [CrossRef]
- Gnanasekaran, K.K.; Muddala, N.P.; Bunce, R.A. Pyrazoloquinazolinones and pyrazolopyridopyrimidinones by a sequential N-acylation–SNAr reaction. Tetrahedron Lett. 2015, 56, 1367–1369. [Google Scholar] [CrossRef]
- Annareddygari, S.; Kasireddy, V.R.; Reddy, J. Transition-metal-free N-arylation: A general approach to aza-fused poly-heteroaromatics. J. Heterocycl. Chem. 2019, 56, 3267–3276. [Google Scholar] [CrossRef]
- Gao, Q.; Guo, Y.; Cao, P.; Fan, G.; Xu, Y. Regioselective synthesis of indazolo[2,3-a]quinazolines enabled by I2/S-facilitated annulation relay dehydrogenative aromatization of cyclohexanones. Chem. Commun. 2023, 59, 13835–13838. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Wang, T.-Q.; Li, H.; Zhang, G.; Wu, X.-A.; Yang, L.; Peng, Y.-L.; Zou, J.; Li, L.-L.; Xiang, R.; et al. Discovery of pyrazolo[1,5-a]pyrimidine-3-carbonitrile derivatives as a new class of histone lysine demethylase 4D (KDM4D) inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 3201–3204. [Google Scholar] [CrossRef]
- Kovács, D.; Molnár-Tóth, J.; Blaskó, G.; Fejes, I.; Nyerges, M. Synthesis of new pyrazolo[1,5-a]quinazoline derivatives. Synth. Commun. 2015, 45, 1675–1680. [Google Scholar] [CrossRef]
- Guerrini, G.; Ciciani, G.; Ciattini, S.; Crocetti, L.; Daniele, S.; Martini, C.; Melani, F.; Vergelli, C.; Giovannoni, M.P. Pyrazolo[1,5-a]quinazoline scaffold as 5-deaza analogue of pyrazolo[5,1-c][1,2,4]benzotriazine system: Synthesis of new derivatives, biological activity on GABAA receptor subtype and molecular dynamic study. J. Enzym. Inhib. Med. Chem. 2016, 31, 195–204. [Google Scholar] [CrossRef]
- Guerrini, G.; Ciciani, G.; Crocetti, L.; Daniele, S.; Ghelardini, C.; Giovannoni, M.P.; Iacovone, A.; Mannelli, L.D.C.; Martini, C.; Vergelli, C. Identification of a new pyrazolo[1,5-a]quinazoline ligand highly affine to γ-aminobutyric type A (GABAA) receptor subtype with anxiolytic-like and antihyperalgesic activity. J. Med. Chem. 2017, 60, 9691–9702. [Google Scholar] [CrossRef]
- Hassan, A.Y.; Saleh, N.M.; Kadh, M.S.; Abou-Amra, E.S. New fused pyrazolopyrimidine derivatives; heterocyclic styling, synthesis, molecular docking and anticancer evaluation. J. Heterocycl. Chem. 2020, 57, 2704–2721. [Google Scholar] [CrossRef]
- Guerrini, G.; Vergelli, C.; Cantini, N.; Giovannoni, M.P.; Daniele, S.; Mascia, M.P.; Martini, C. Synthesis of new GABAA receptor modulator with pyrazolo[1,5-a]quinazoline (PQ) scaffold. Int. J. Mol. Sci. 2019, 20, 1438. [Google Scholar] [CrossRef]
- Guerrini, G.; Crocetti, L.; Daniele, S.; Lacovone, A.; Cantini, N.; Martini, C.; Melani, F.; Vergelli, C.; Giovannoni, M.P. New 3,6-disubstituted pyrazolo[1,5-a]quinazolines as ligands to GABAA receptor subtype. J. Heterocycl. Chem. 2019, 56, 1571–1589. [Google Scholar] [CrossRef]
- Bruni, F.; Chimichi, S.; Cosimelli, B.; Costanzo, A.; Guerrini, G.; Selleri, S. A new entry to pyrazolo[1,5-a]pyrido[3,4-e]pyrimidine derivatives. Heterocycles 1990, 31, 1141. [Google Scholar] [CrossRef]
- Crocetti, L.; Guerrini, G.; Cantini, N.; Vergelli, C.; Melani, F.; Mascia, M.P.; Giovannoni, M.P. ‘Proximity frequencies’: A new parameter to evaluate the profile of GABAAR modulators. Bioorg. Med. Chem. Lett. 2021, 34, 127755. [Google Scholar] [CrossRef]
- Crocetti, L.; Guerrini, G.; Melani, F.; Vergelli, C.; Mascia, M.P.; Giovannoni, M.P. GABAA receptor modulators with a pyrazolo[1,5-a]quinazoline core: Synthesis, molecular modelling studies and electrophysiological assays. Int. J. Mol. Sci. 2022, 23, 13032. [Google Scholar] [CrossRef]
- Crocetti, L.; Guerrini, G.; Melani, F.; Mascia, M.P.; Giovannoni, M.P. 3,8-Disubstituted pyrazolo[1,5-a]quinazoline as GABAA receptor modulators: Synthesis, electrophysiological assays, and molecular modelling studies. Int. J. Mol. Sci. 2024, 25, 10840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, W.; Zhang, G.; Fang, Z.; Chen, X.; Li, L. Design, synthesis, and biological screening of a series of pyrazolo[1,5-a]quinazoline derivatives as SIRT6 activators. Eur. J. Pharm. Sci. 2023, 185, 106424. [Google Scholar] [CrossRef]
- Chen, X.; Sun, W.; Huang, S.; Zhang, H.; Lin, G.; Li, H.; Qiao, J.; Li, L.; Yang, S. Discovery of potent small-molecule sirt6 activators: Structure-activity relationship and anti-pancreatic ductal adenocarcinoma activity. J. Med. Chem. 2020, 63, 10474–10495. [Google Scholar] [CrossRef]
- Crocetti, L.; Khlebnikov, A.I.; Guerrini, G.; Schepetkin, I.A.; Melani, F.; Giovannoni, M.P.; Quinn, M.T. Anti-inflammatory activity of pyrazolo[1,5-a]quinazolines. Molecules 2024, 29, 2421. [Google Scholar] [CrossRef]
- Kumar, N.R.; Swaroop, D.K.; Punna, N.; Sirisha, K.; Ganapathi, T.; Kumar, C.G.; Narsaiah, B. Synthesis of Novel Pyrido[2′,3′:3,4]Pyrazolo[1,5-a]Quinazoline Derivatives, Their Biological Evaluation and Molecular Modelling Studies. ChemistrySelect 2018, 3, 7813–7821. [Google Scholar] [CrossRef]
- Jiang, X.; Wei, X.; Lin, F.; Zhang, Z.; Yao, G.; Yang, S.; Zhao, W.; Zhao, C.; Xu, H. Substrate controlled [5+1] annulation of 5-amino-1H-phenylpyrazoles with alkenes: Divergent synthesis of multi-substituted 4,5-dihydropyrazolo[1,5-a]quinazolines. Eur. J. Org. Chem. 2020, 3997–4003. [Google Scholar] [CrossRef]
- Yang, S.; Lai, Q.; Lai, F.; Jiang, X.; Zhao, C.; Xu, H. Design, Synthesis, and Insecticidal Activities of Novel 5-Substituted 4,5-Dihydropyrazolo[1,5-a]quinazoline Derivatives. Pest. Man. Sci. 2021, 77, 1013–1022. [Google Scholar] [CrossRef]
- Yang, S.; Li, B.; Tang, J.; Peng, H.; Pu, C.; Zhao, C.; Xu, H. Structural optimization based on 4,5-dihydropyrazolo[1,5-a]quinazoline scaffold for improved insecticidal activities. Pest. Biochem. Physiol. 2023, 195, 105533. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, C.-J.; Luo, X.-F.; Li, A.-P.; Zhang, S.-Y.; An, J.-X.; Zhang, Z.-J.; Ma, Y.; Zhang, B.-Q.; Liu, Y.-Q. Design, synthesis, and biological evaluation of novel berberine derivatives against phytopathogenic fungi. Pest. Manag. Sci. 2022, 78, 4361–4376. [Google Scholar] [CrossRef]
- Ho, S.L.; Dao, P.D.Q.; Cho, C.S. Microwave-Assisted Synthesis of Benzo[4,5]imidazo[1,2-a]pyrimidines from β-Bromo-α,β-unsaturated Aldehydes and 2-Aminobenzimidazoles. Synlett 2017, 28, 1811–1815. [Google Scholar] [CrossRef]
- Kim, D.Y.; Dao, P.D.Q.; Cho, C.S. Synthesis of Pyrimidine- and Quinazoline-Fused Benzimidazole-4,7-diones Using Combinatorial Cyclocondensation and Oxidation. ACS Omega 2018, 3, 17456–17465. [Google Scholar] [CrossRef]
- Dao, P.D.Q.; Cho, C.S.; Ho, S.L.; Sohn, H.-S. Microwave-assisted Copper Powder-Catalyzed Synthesis of Azole-Fused Pyrimidinones. Curr. Org. Chem. 2018, 22, 85–93. [Google Scholar]
- Ma, P.; Meng, F.; Wang, N.; Zhang, J.; Xie, J.; Dai, B. Heterogeneous Amorphous Cu–MOF-74 Catalyst for C–N Coupling Reaction. ChemistrySelect 2018, 3, 10694–10700. [Google Scholar] [CrossRef]
- Kumpulainen, E.T.T.; Hognasbacka, A. Modular Approach to Tricyclic Heterocycles through Copper Catalysis and Functionalization by Palladium-Catalyzed C–H Arylation. Eur. J. Org. Chem. 2017, 2017, 2610–2614. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, A.K.; Bahadur, V.; Len, C.; Richards, N.G.J.; Parmar, V.S.; Van der Eycken, E.V.; Singh, B.K. Microwave Assisted Metal Free, Base mediated C–N Bond Formation/Cleavage: Synthesis of Benzimidazo[1,2-a]quinazoline Derivatives. ACS Sust. Chem. Eng. 2016, 4, 2206–2210. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Z.; Deng, H.; Ren, Q.; Zhou, Z.; Zhao, C.; Chai, H. Synthesis and characterization of a new compound 4-(2-chlorobenzyl)imidazo[1,2-a]quinazolin-5(4H)-one: DFT study, crystal structure, MEP, and HOMO–LUMO values. J. Struct. Chem. 2021, 62, 1285–1292. [Google Scholar] [CrossRef]
- Ye, W.; Liu, Y.; Ren, Q.; Liao, T.; Chen, Y.; Chen, D.; Wang, S.; Yao, L.; Jia, Y.; Zhao, C.; et al. Design, synthesis and biological evaluation of novel triazoloquinazolinone and imidazoquinazolinone derivatives as allosteric inhibitors of SHP2 phosphatase. J. Enzyme Inhibit. Med. Chem. 2022, 37, 1495–1513. [Google Scholar] [CrossRef] [PubMed]
- Ghashghaei, O.; Caputo, S.; Sintes, M.; Revés, M.; Kielland, N.; Estarellas, C.; Luque, F.J.; Avińȯ, A.; Eritja, R.; Serna-Gallego, A.; et al. Multiple Multicomponent Reactions: Unexplored Substrates, Selective Processes, and Versatile Chemotypes in Biomedicine. Chem. Eur. J. 2018, 24, 14513–14521. [Google Scholar] [CrossRef]
- Shlenev, R.M.; Filimonov, S.I.; Tarasov, A.V.; Danilova, A.S.; Agat’ev, P.A. Synthesis of New Sulfonamide Derivatives of Thiazolo[3,2-a]quinazolin-5-one. Russ. J. Org. Chem. 2016, 52, 69–75. [Google Scholar] [CrossRef]
- Deng, X.Q.; Xiao, C.R.; Wei, C.X.; Quan, Z.S. Synthesis and anticonvulsant activity of substituted[1,2,4]triazolo[4,3-a]quinazolines. Chin. J. Org. Chem. 2011, 31, 2082–2087. [Google Scholar]
- Zhang, H.-J.; Jin, P.; Wang, S.-B.; Li, F.-N.; Guan, L.-P.; Quan, Z.-S. Synthesis and Anticonvulsant Activity Evaluation of 4-Phenyl[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one and Its Derivatives. Arch. Pharm. Chem. Life Sci. 2015, 348, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; Wang, S.-B.; Quan, Z.-S. Synthesis and antidepressant activities of 4-(substituted-phenyl)triazolo[1,5-a]quinazolin-5(4H)-ones and their derivatives. Mol. Divers. 2015, 19, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, V.; Raja, S.V.; Dhanabal, K. Synthesis and pharmacological evaluation of some 3-phenyl-2-substituted-3H-quinazolin-4-one as analgesic, anti-inflammatory agents. Bioorg. Med. Chem. 2007, 15, 235–241. [Google Scholar] [CrossRef]
- Cheng, G.; Mei, X.-B.; Yan, Y.-Y.; Chen, J.; Zhang, B.; Li, J.; Dong, X.-W.; Lin, N.-M.; Zhou, Y.-B. Identification of new NIK inhibitors by discriminatory analysis-based molecular docking and biological evaluation. Arch. Pharm. Chem. Life Sci. 2019, 352, e1800374. [Google Scholar] [CrossRef]
- Abuelizz, H.A.; Soliman, S.M.; Ghabbour, H.A.; Marzouk, M.; Abdellatif, M.M.; Al-Salahi, R. DFT calculation, Hirshfeld analysis and X-ray crystal structure of some synthesized N-alkylated (S-alkylated) [1,2,4]triazolo[1,5-a]quinazolines. Crystals 2021, 11, 1195. [Google Scholar] [CrossRef]
- Yu, S.; Danylchenko, O.G.; Drushlyak, S.S.; Kovalenko, S.M. Formation of 1-methyl[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-ones by reaction of 2-hydrazinoquinazolin-4(3H)-ones with acetylacetone. Heterocycl. Commun. 2015, 21, 195–197. [Google Scholar]
- Gobinath, M.; Subramanian, N.; Alagarsamy, V.; Nivedhitha, S.; Solomon, V.R. Synthesis of 1-Substituted-4-(Pyridin-4-yl) [1,2,4]Triazolo[4,3-a]Quinazolin-5(4H)-ones as a New Class of H1-Antihistaminic Agents. Trop. J. Pharm. Res. 2015, 14, 271–277. [Google Scholar] [CrossRef]
- Gobinath, M.; Subramanian, N.; Alagarsamy, V.; Nivedhitha, S.; Solomon, V.R. Design and Synthesis of 1-Substituted-4-(4-Nitrophenyl)-[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-ones as a New Class of Antihistaminic Agents. Russ. J. Bioorg. Chem. 2020, 46, 403–408. [Google Scholar] [CrossRef]
- Hu, W.; Liao, T.; Deng, L.; Sun, H.; Zhou, Z.; Chai, H.; Zhao, C. Synthesis, crystal structure, and density functional theory study of a new compound 4-(2-chlorobenzyl)-1-(5-fluoro-2-hydroxy-3-(thiomorpholinomethyl)phenyl)[1,2,4]triazolo[4,3-a]quinazolin-5(4H)-one. J. Heterocycl. Chem. 2021, 58, 2102–2108. [Google Scholar] [CrossRef]
- Zhou, Z.-X.; Wu, Q.-M.; Huang, Z.-Y.; Yu, D.-H.; Lu, H.-G. Synthesis, crystal structure, DFT, molecular docking and antitumor activity of 4-(2-chlorobenzyl)-1-(5-fluoro-2-hydroxy-3-((4-methylpiperidin-1-yl)methyl)phenyl)[1,2,4]-triazolo[4,3-a]quinazolin-5(4H)-one. Res. Chem. Intermed. 2021, 47, 3609–3627. [Google Scholar] [CrossRef]
- Luo, R.; Wang, Z.; Luo, D.; Qin, Y.; Zhao, C.; Yang, D.; Lu, T.; Zhou, Z.; Huang, Z. Design, synthesis, and biological evaluation of novel triazoloquinazolinone derivatives as SHP2 protein inhibitors. J. Enzym. Inhib. Med. Chem. 2021, 36, 2170–2182. [Google Scholar] [CrossRef]
- Pandey, S.K.; Yadava, U.; Upadhyay, A.; Sharma, M.L. Synthesis, biological evaluation and molecular docking studies of novel quinazolinones as antitubercular and antimicrobial agents. Bioorg. Chem. 2021, 108, 104611. [Google Scholar]
- Švajger, U.; Horvat, Ž.; Knez, D.; Rožman, P.; Turk, S.; Gobec, S. New antagonists of toll-like receptor 7 discovered through 3D ligand-based virtual screening. Med. Chem. Res. 2015, 24, 362–371. [Google Scholar] [CrossRef]
- Driowya, M.; Leclercq, J.; Verones, V.; Barczyk, A.; Lecoeur, M.; Renault, N.; Flouquet, N.; Ghinet, A.; Berthelot, P.; Lebegue, N. Synthesis of triazoloquinazolinone based compounds as tubulin polymerization inhibitors and vascular disrupting agents. Eur. J. Med. Chem. 2016, 115, 393–405. [Google Scholar] [CrossRef]
- Álvarez, C.N.; Park, J.-E.; Toti, K.S.; Xia, Y.; Krausz, K.W.; Rai, G.; Bang, J.K.; Gonzalez, F.J.; Jacobson, K.A.; Lee, K.S. Identification of a new heterocyclic scaffold for inhibitors of the polo-box domain of polo-like kinase 1. J. Med. Chem. 2020, 63, 14087–14117. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.Y.; Sarg, M.T.; Bayoumi, A.H.; El-Deebb, M.A. Synthesis and anticancer evaluation of some novel 5-amino[1,2,4]triazole derivatives. J. Heterocycl. Chem. 2018, 55, 1450–1478. [Google Scholar] [CrossRef]
- Abdelhamid, I.A.; Abdelmoniem, A.M.; Ghozlan, S.A.S.; Butenschön, H. Hydrazononitriles as precursors for 4-aminotriazoles and 3-aminoisoxazoles: One pot synthesis of triazolo[1,5-a]quinazoline derivatives. J. Heterocycl. Chem. 2016, 53, 1251–1258. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Marzouk, M.S. Synthesis of novel 2-phenoxy-benzo[g][1,2,4]triazolo[1,5-a]quinazoline and its derivatives starting with diphenyl-N-cyanoimidocarbonate. Russ. J. Gen. Chem. 2016, 86, 1741–1746. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Abuelizz, H.A.; Wadi, M.; El Dib, R.A.; Alotaibi, M.A.; Marzouk, M. Antimicrobial activity of synthesized 2-methylthiobenzo[g][1,2,4]-triazolo[1,5-a]quinazoline derivatives. Medicinal Chem. 2016, 12, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Al-Salahi, R.; El-Tahir, K.-E.; Alswaidan, I.; Lolak, N.; Hamidaddin, M.; Marzouk, M. Biological effects of a new set 1,2,4-triazolo[1,5-a]quinazolines on heart rate and blood pressure. Chem. Cent. J. 2014, 8, 3. [Google Scholar] [CrossRef]
- Bakheit, A.H.; Abuelizz, H.A.; Al-Salahi, R. Hirshfeld surface analysis and density functional theory calculations of 2-benzyloxy-1,2,4-triazolo[1,5-a]quinazolin-5(4H)-one: A comprehensive study on crystal structure, intermolecular interactions, and electronic properties. Crystals 2023, 13, 1410. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Geffken, D. Synthesis of novel 2-methylsulfanyl-4H-[1,2,4]triazolo[1,5-a]quinazolin-5-one and derivatives. Synth. Commun. 2011, 41, 3512–3523. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Al-Omar, M.A.; Alswaidan, I.; Marzouk, M.; El-Senousy, W.M.; Amr, A.E.-G.E. Antiviral activities of some synthesized methylsulfanyltriazoloquinazoline derivatives. Res. Chem. Intermed. 2015, 41, 151–161. [Google Scholar] [CrossRef]
- Al-Salahi, R.; Anouar, E.-H.; Marzouk, M.; Taie, H.A.A.; Abuelizz, H.A. Screening and evaluation of antioxidant activity of some 1,2,4-triazolo[1,5-a]quinazoline derivatives. Future Med. Chem. 2017, 10, 379–390. [Google Scholar] [CrossRef]
- Abuelizz, H.A.; Anouar, E.-H.; Ahmad, R.; Azman, N.I.I.N.; Marzouk, M.; Al-Salahi, R. Triazoloquinazolines as a new class of potent α-glucosidase inhibitors: In vitro evaluation and docking study. PLoS ONE 2019, 14, e0220379. [Google Scholar] [CrossRef]
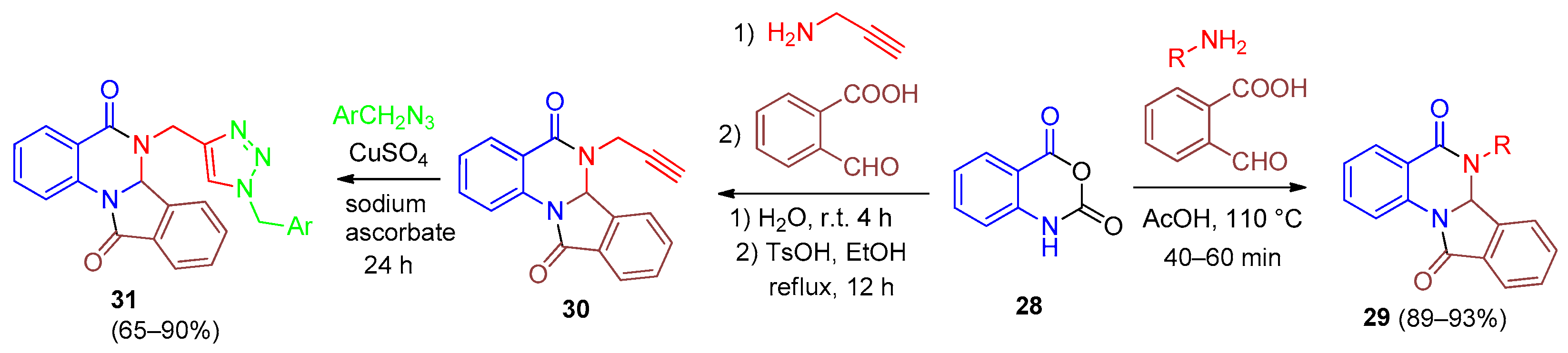
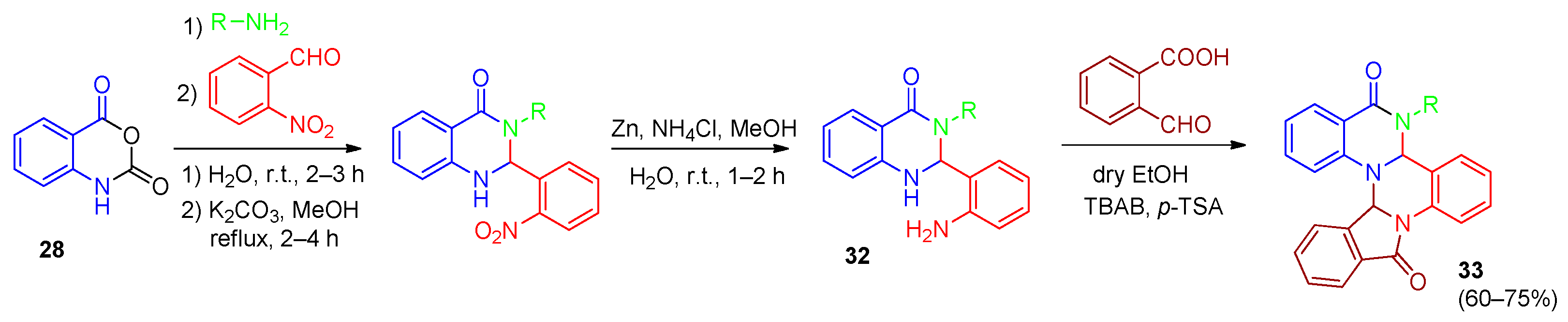
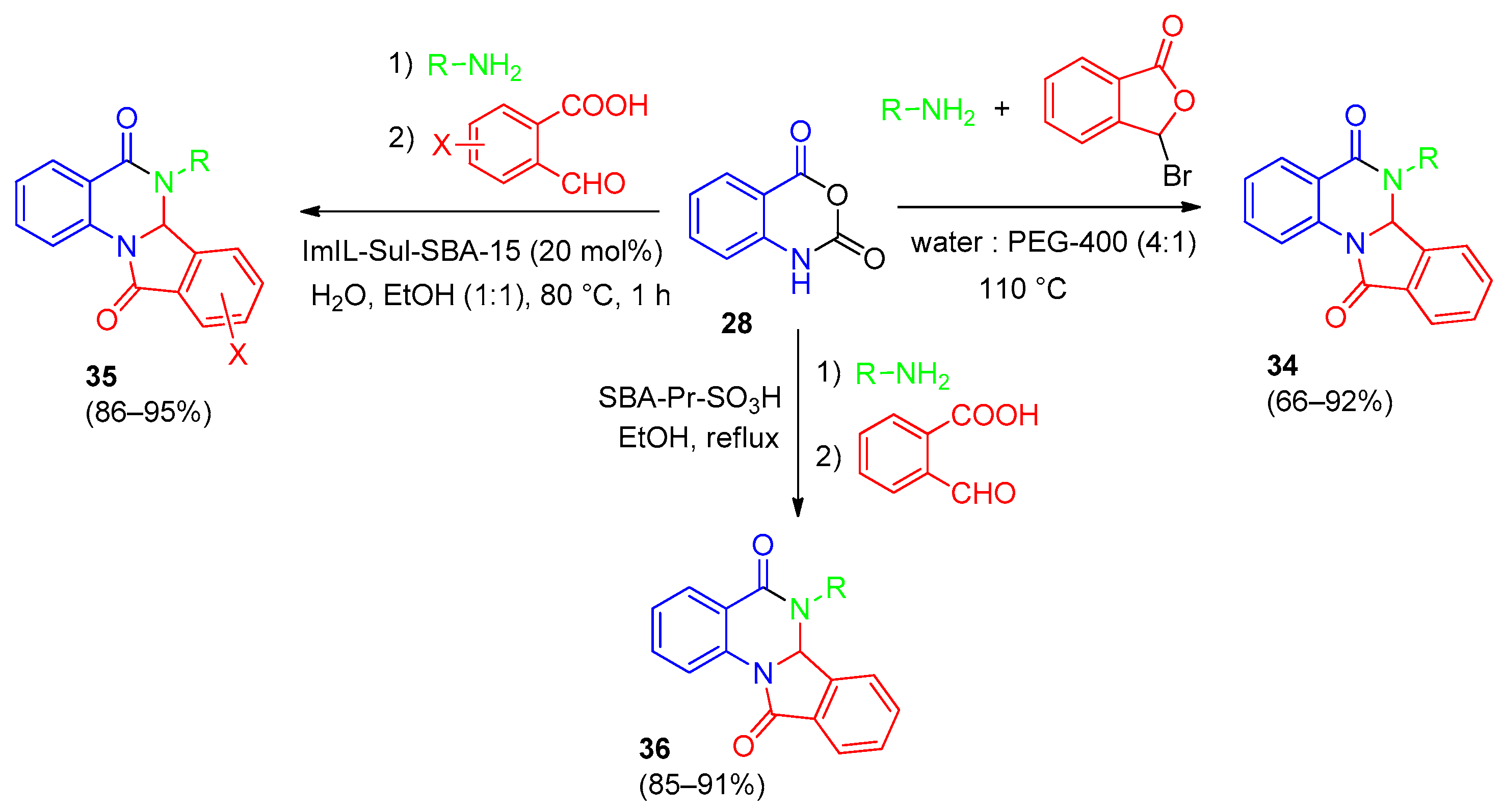
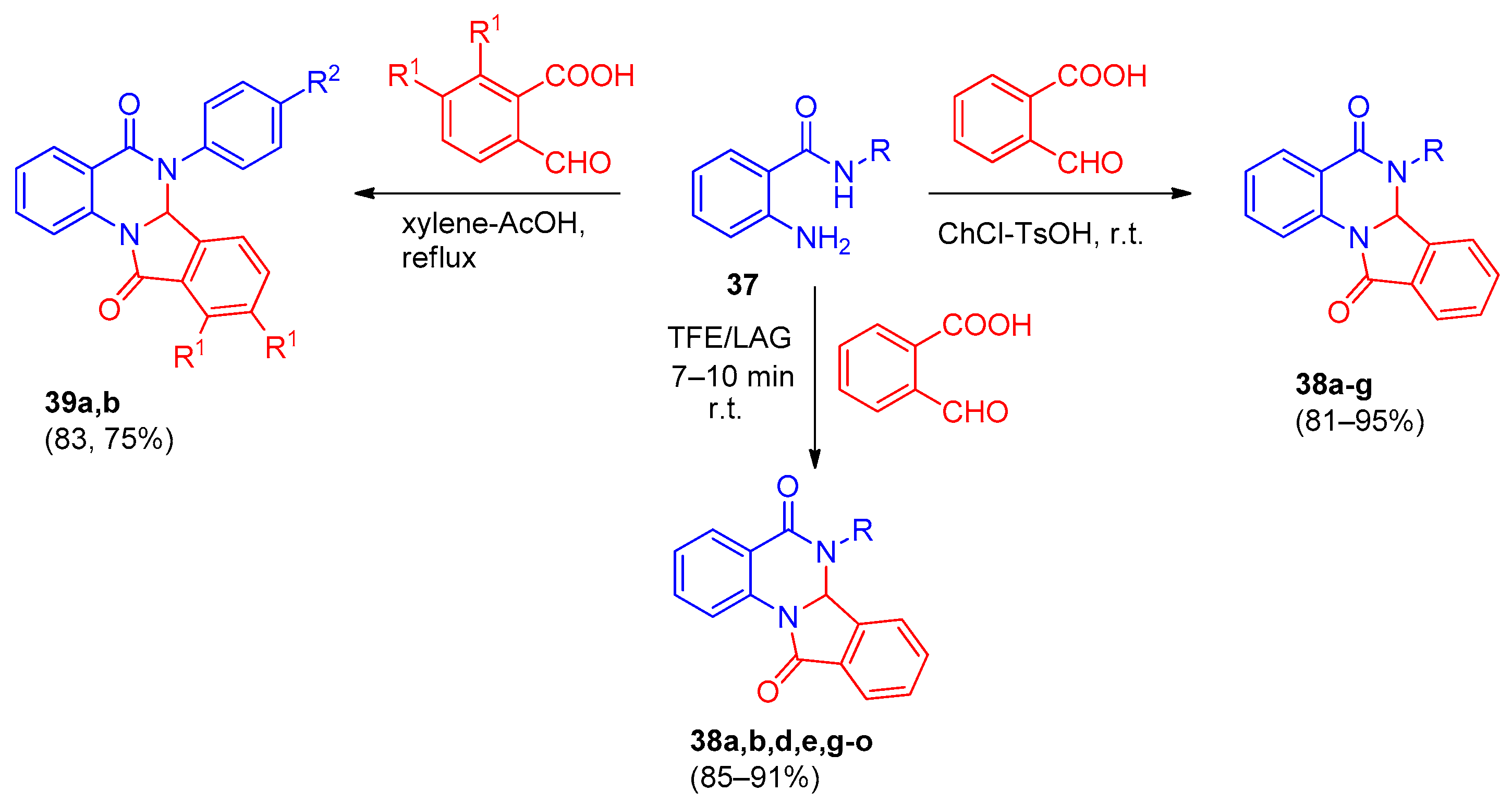


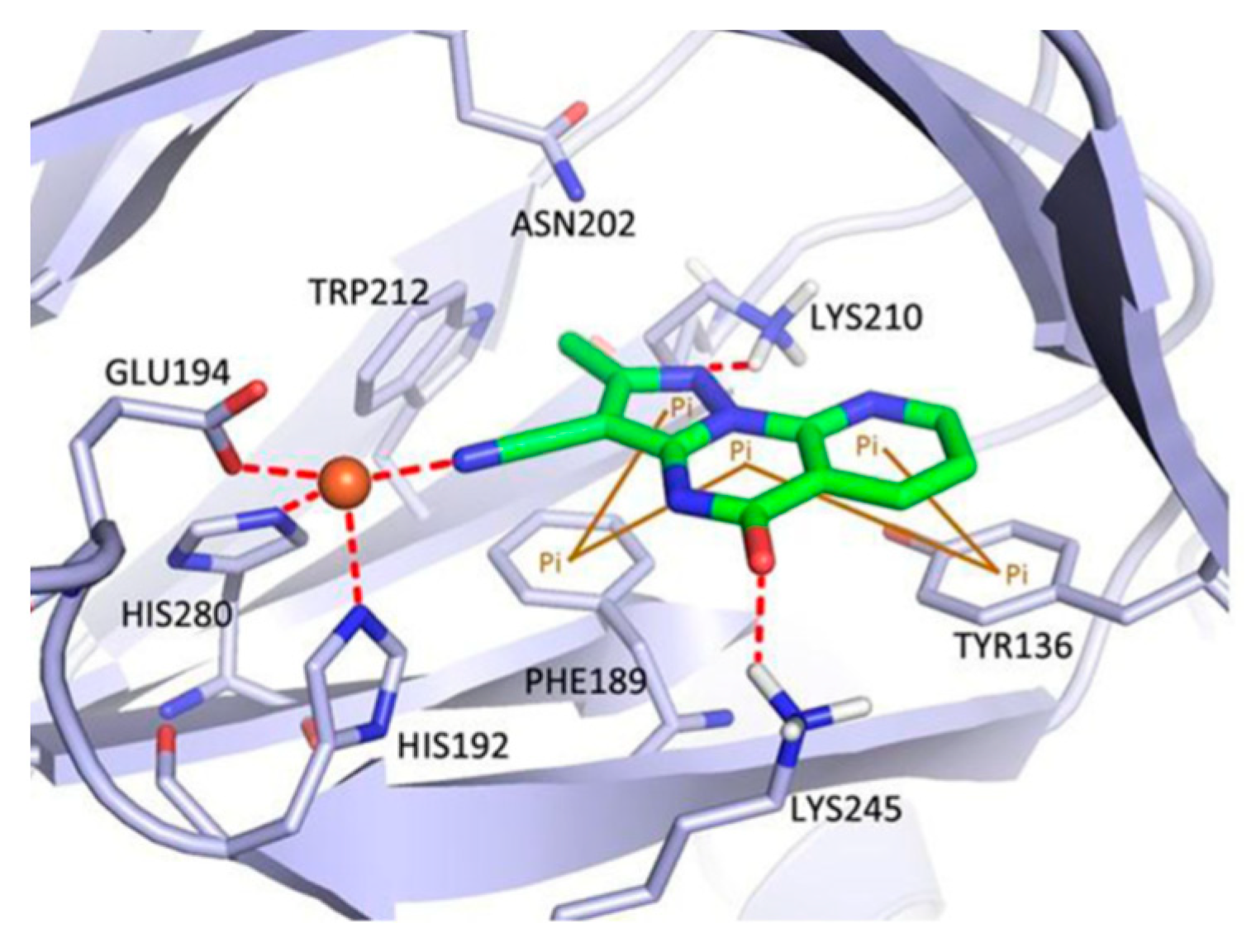


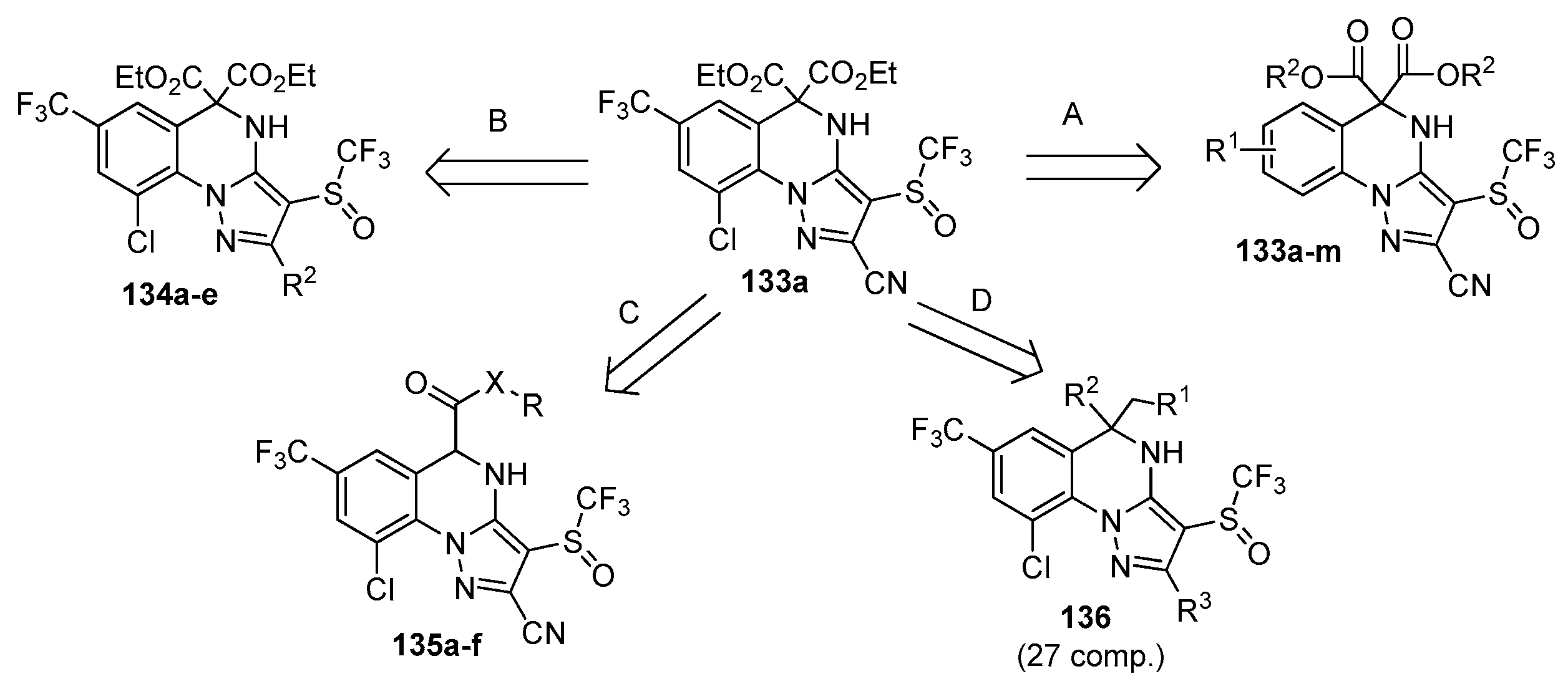
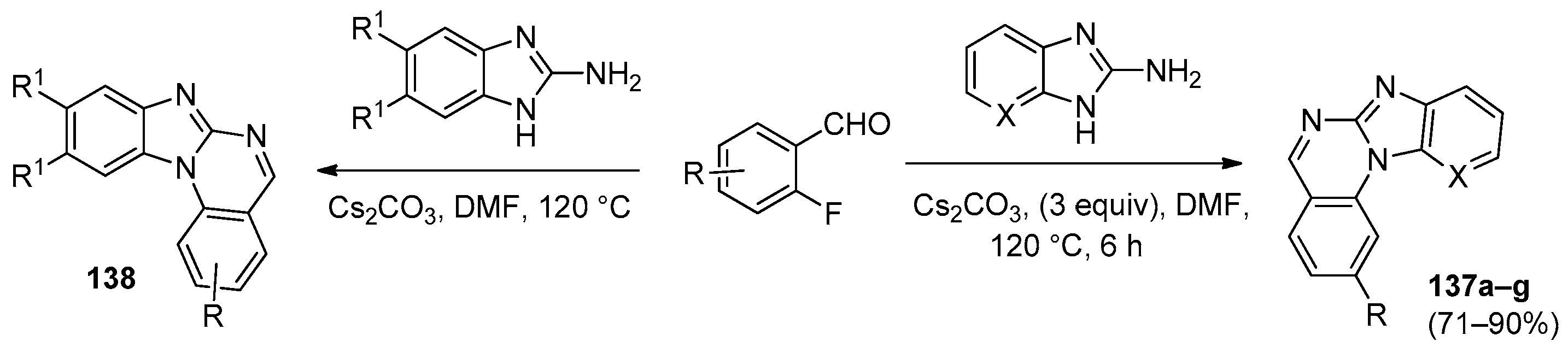
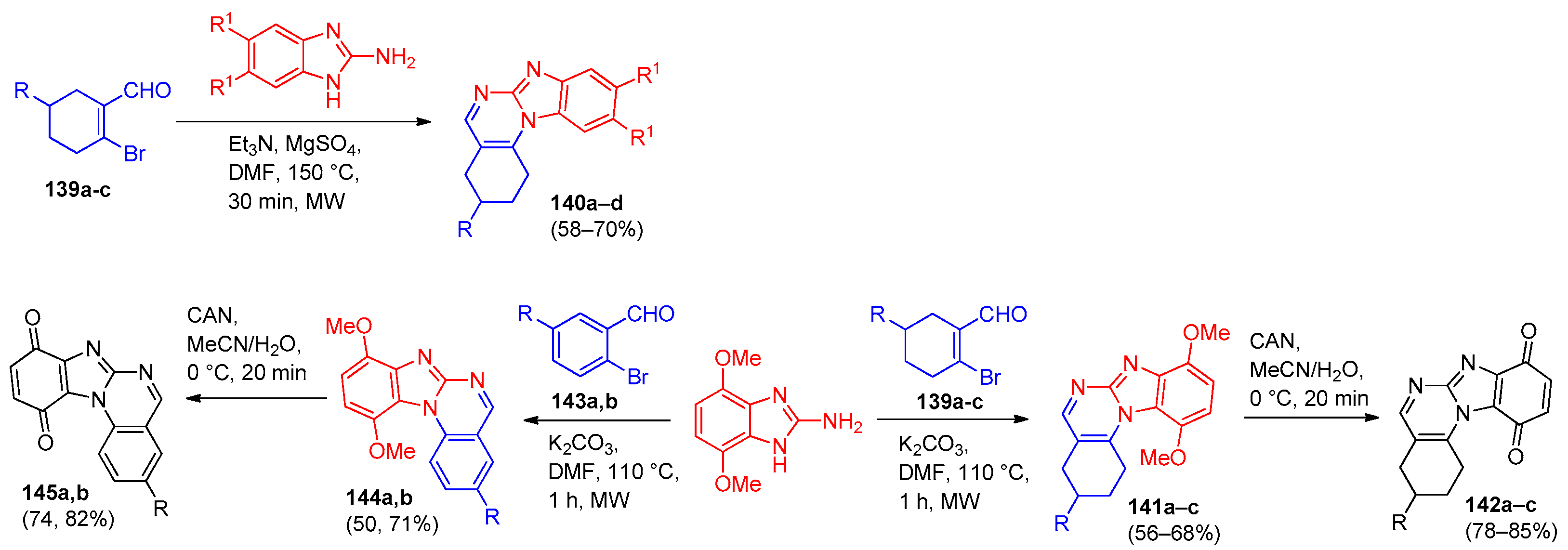
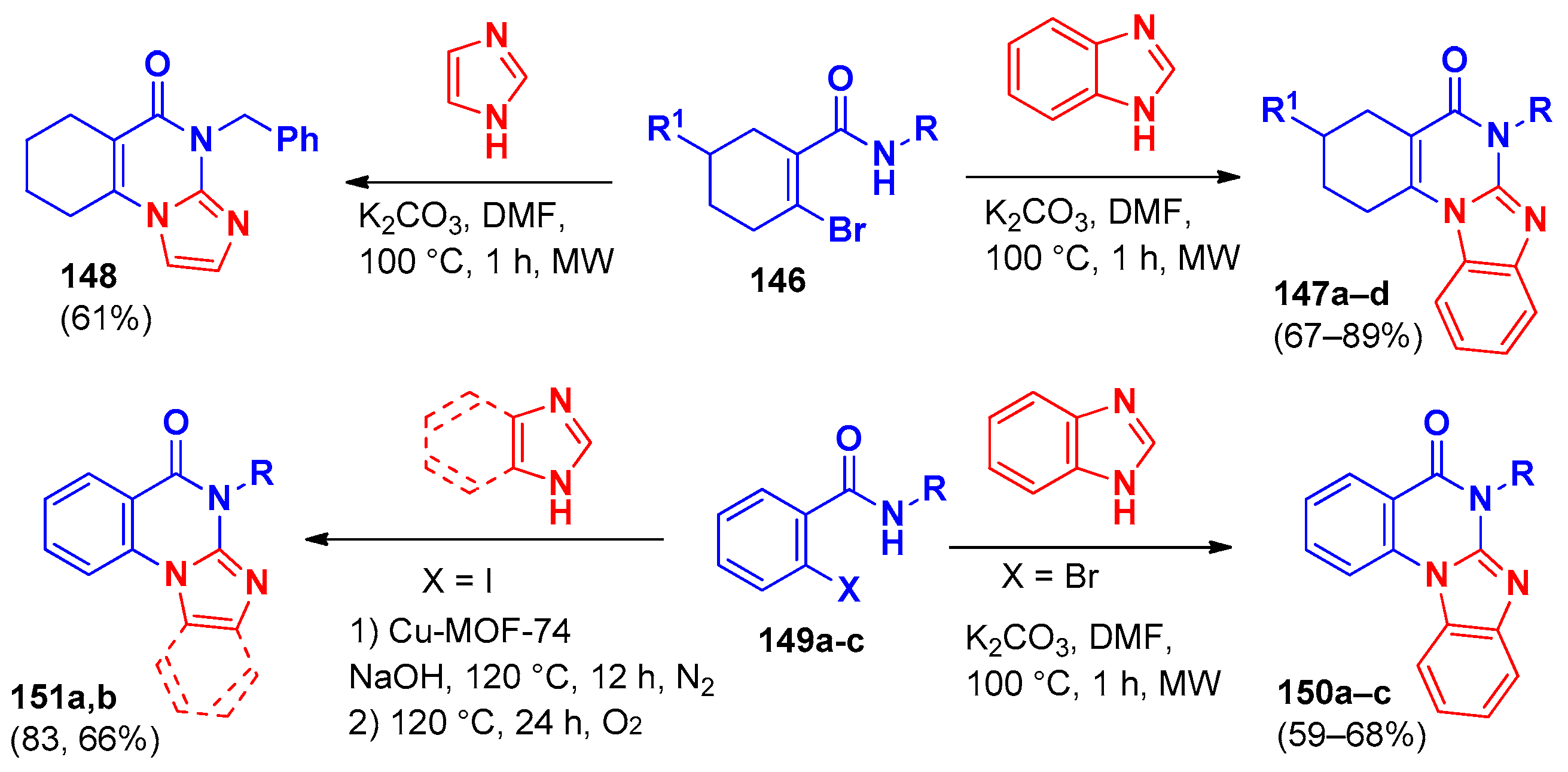

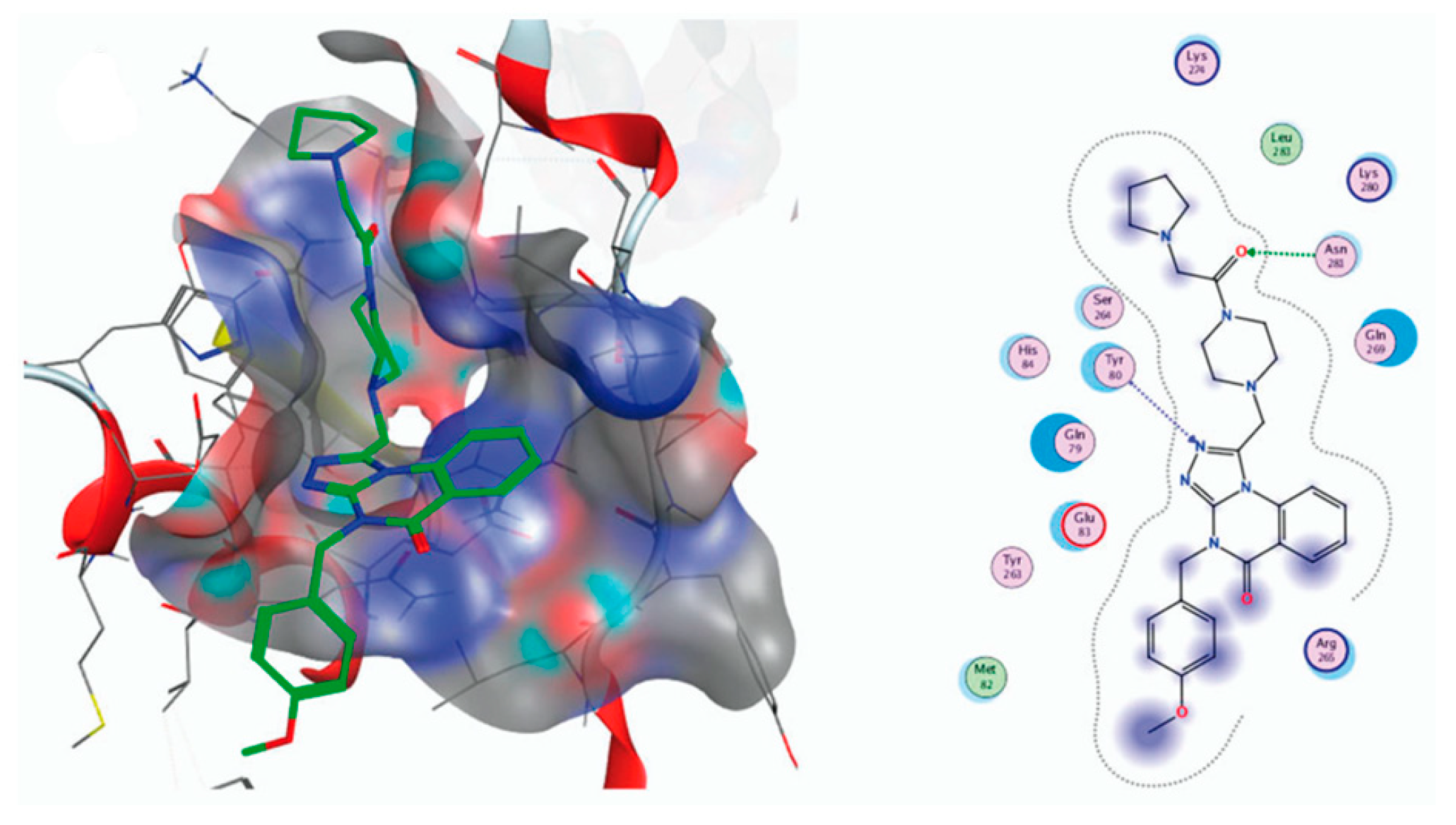
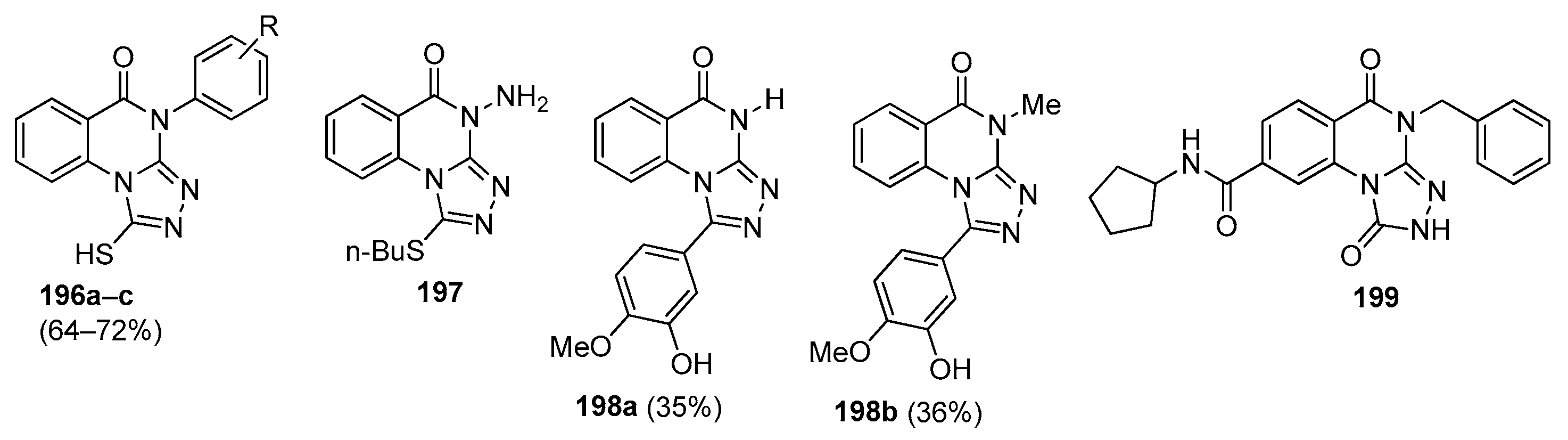
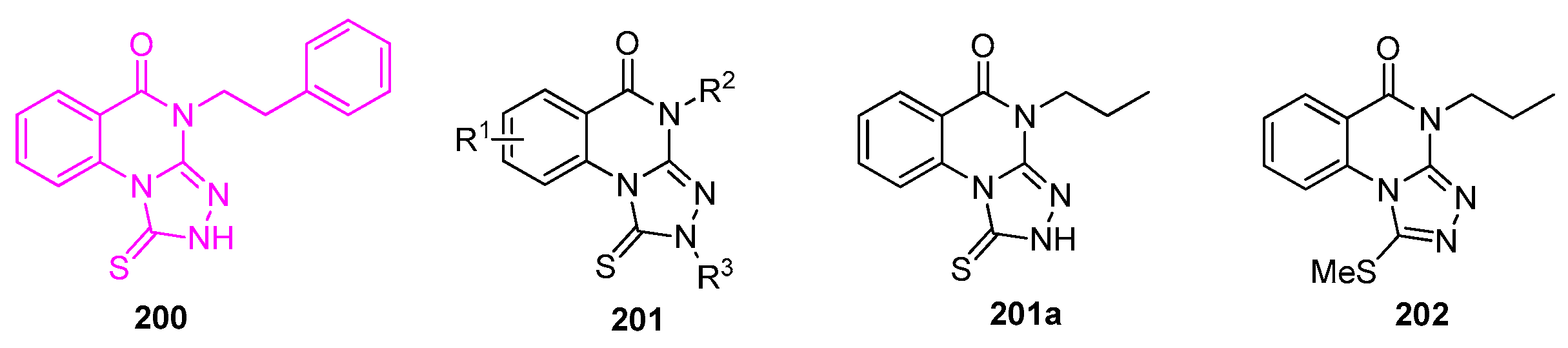
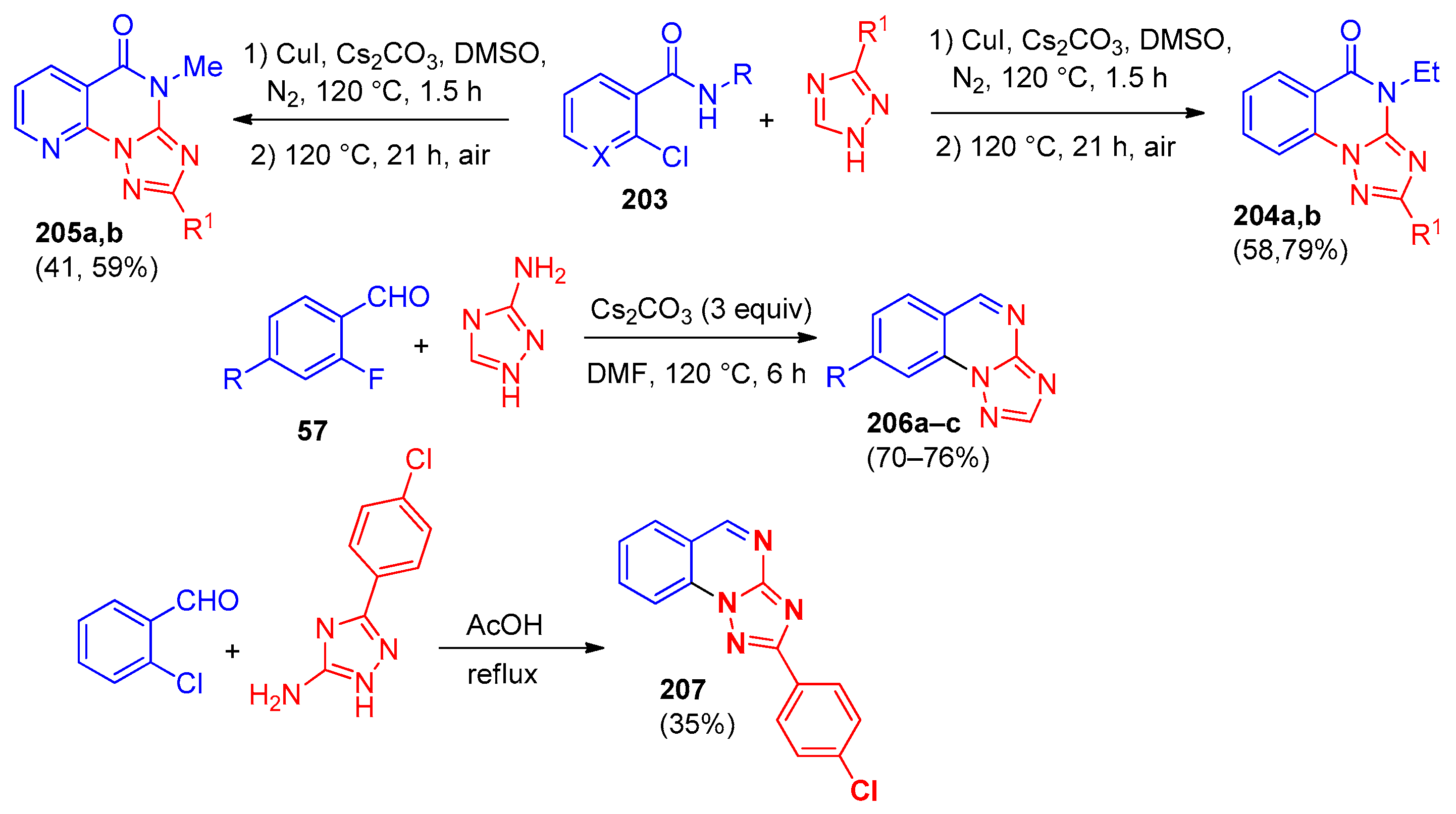
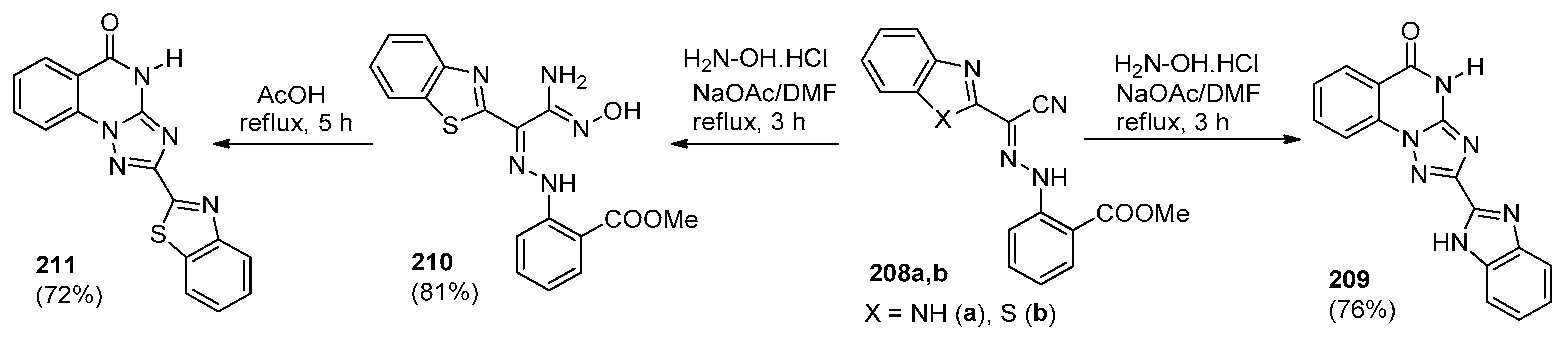
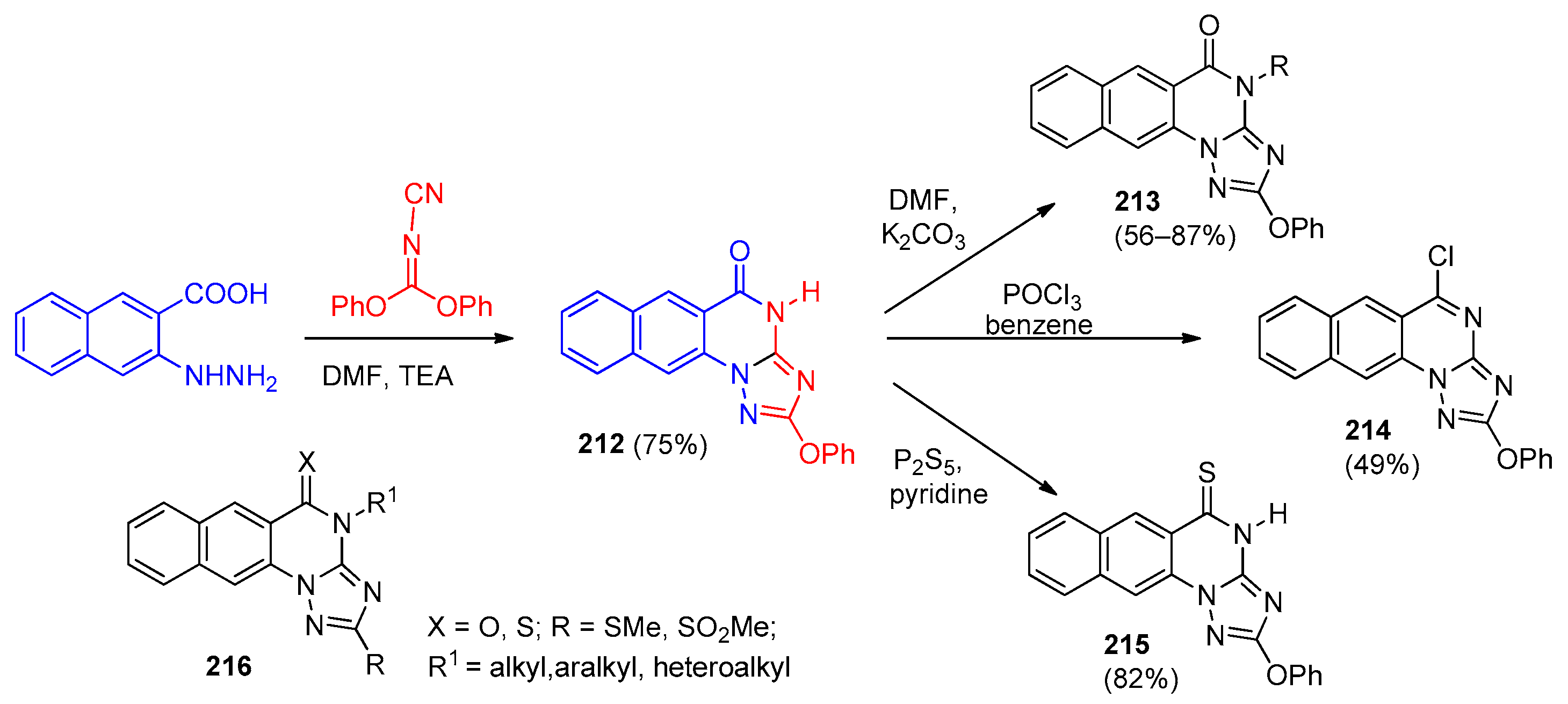
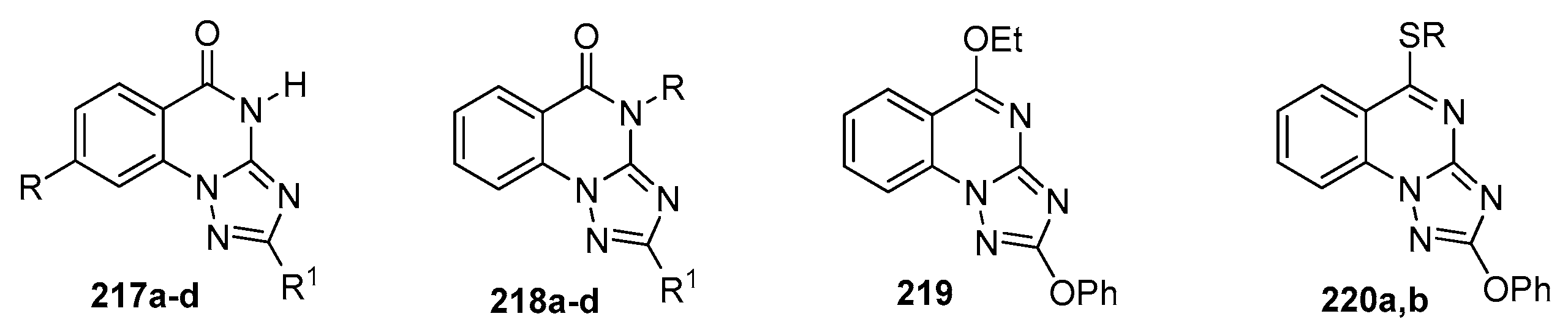
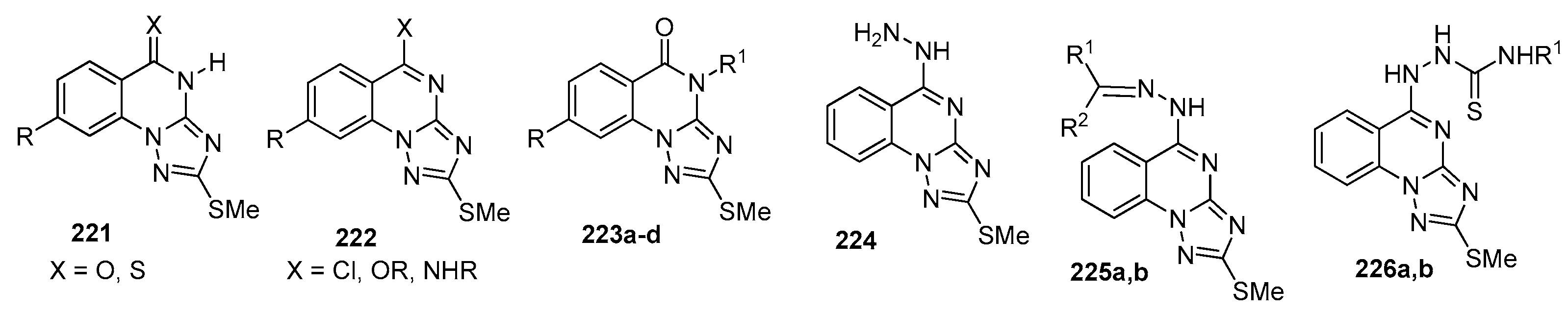

| Compound | Inhibition Rate at 10 μM | IC50 (μM) |
|---|---|---|
| A | 73.33 ± 19.14 | 3.14 ± 0.18 |
| 64p | 85.06 ± 3.54 | 4.03 ± 1.02 |
| 64r | 88.82 ± 9.58 | 0.41 ± 0.03 |
| 64s | 78.06 ± 6.27 | 1.85 ± 0.14 |
 | |||
|---|---|---|---|
| Compound | R | R1 | Ki (nM) |
| 72a | OMe | Et | 102.4 ± 10.4 |
| 72b | H | Et | 529.3 ± 58.8 |
| 77a | OMe | CH2Ph | 19.0 ± 1.9 |
| 77b | OMe | CH2-(2-OMe)Ph | 0.27 ± 0.04 |
| 77c | OMe | CH2-2-thienyl | 16.7 ± 0.9 |
| 77d | OMe | CH2-2-furyl | 15.2 ± 1.4 |
| 77e | OMe | CHMe2 | 97.4 ± 13.8 |
| 78a | H | CH2Ph | 25.9 ± 1.98 |
| 78b | H | CH2-(2-OMe)Ph | 3.16 ± 0.54 |
| 78c | H | CH2-2-thienyl | 13.2 ± 0.7 |
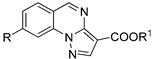
 | |||
|---|---|---|---|
| Compound | R | R1 | I, % |
| 89a | CN | H | 2 |
| 89b | COOEt | H | 32 |
| 90a | CN | CH2Ph | 5 |
| 90b | COOEt | CH3 | 19 |
| 91a | COOCH2Ph | CH3 | 14 |
| 91b | COOCH2(2-OCH3Ph) | CH3 | 18 |
| 91c | COOCH2-2-furyl | CH3 | 10 |
| 92a | COOCH2Ph | - | 14 |
| 92b | COOCH2(2-OCH3Ph) | - | 14 |
| 92c | COOCH2-2-furyl | - | 7 |
| 92d | COOEt | - | 1 |
| 93 | 1,2,4-oxadiazol-3-yl-5-methyl | CH2Ph | 10 |
| 94a | CONH2 | H | 5 |
| 94b | CONH2 | CH2Ph | 5 |
| 96a | 1,2,4-triazol-3-yl | CH3 | 9 |
| 96b | 1,2,4-triazol-3-yl | CH2Ph | 9 |
| 97 | I | CH2Ph | 43.7 |
| Compound | Staphylococcus Aureus MTCC96 | Bacillus Subtilis MTCC121 | Staphylococcus Aureus MLS162940 | Micrococcus Luteus 2470MTCC | Klebsiella Planticola MTCC530 |
|---|---|---|---|---|---|
| 130a | 3.9 | 3.9 | 7.8 | 3.9 | 3.9 |
| 130b | >125 | 15.6 | >125 | >125 | >125 |
| 130c | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 |
| 130f | 3.9 | 3.9 | 7.8 | 3.9 | >125 |
| 131a | 3.9 | 3.9 | 15.6 | 7.8 | 3.9 |
| 132c | 3.9 | 3.9 | 7.8 | >125 | >125 |
| 132d | >125 | >125 | >125 | >125 | 7.8 |
| 132e | 15.6 | 15.6 | >125 | >125 | >125 |
| 132f | 3.9 | 3.9 | 7.8 | 7.8 | 7.8 |
| 132h | 3.9 | 3.9 | 7.8 | >125 | 7.8 |
| Ciprofloxacin | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 |
 | |||||
|---|---|---|---|---|---|
| Compound | R | % Inhibition | Compound | R | % Inhibition |
| 161 | 4-Methylpiperazin-1-yl | 76.15 | 164 | (3-MeOC6H4)NH | 24.42 |
| 164 | 3-(OCF3)C6H4NH | 24.08 | 164 | 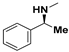 | 21.89 |
| 164 | 3-CF3C6H4NH | 27.93 | 164 | 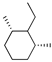 | 24.77 |
| SHP244 | 13.81 | Sorafenib | 14.79 | ||
| Compound | Substituents | Biological Activity | LC50 Value | Reference |
|---|---|---|---|---|
| 175 | R = 4-Cl | Anticonvulsant | 88.02 mg/kg | [85] |
| 175 | R = 4-Br | Anticonvulsant | 94.60 mg/kg | [85] |
| 176 | R = 4-Me | Antidepressant | Percentage decrease in immobility duration 82.69% at a dose of 50 mg/kg | [86] |
| 193 | Ar = 4-MeOC6H4 | Inhibitory activity against SHP2 protease | Percentage inhibition 28.20% at 100 μM | [81] |
| 201 | R1 = R3 = H, R2 = Pr | PBD of Plk1 Inhibitory | 1.03 μM | [88] |
| 231 | α-Glucosidase inhibitor | 12.70 μM | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipunova, G.N.; Nosova, E.V.; Charushin, V.N. Quinazolines [a]-Annelated by Five-Membered Heterocycles: Synthesis and Biological Activity. Molecules 2025, 30, 3506. https://doi.org/10.3390/molecules30173506
Lipunova GN, Nosova EV, Charushin VN. Quinazolines [a]-Annelated by Five-Membered Heterocycles: Synthesis and Biological Activity. Molecules. 2025; 30(17):3506. https://doi.org/10.3390/molecules30173506
Chicago/Turabian StyleLipunova, Galina N., Emiliya V. Nosova, and Valery N. Charushin. 2025. "Quinazolines [a]-Annelated by Five-Membered Heterocycles: Synthesis and Biological Activity" Molecules 30, no. 17: 3506. https://doi.org/10.3390/molecules30173506
APA StyleLipunova, G. N., Nosova, E. V., & Charushin, V. N. (2025). Quinazolines [a]-Annelated by Five-Membered Heterocycles: Synthesis and Biological Activity. Molecules, 30(17), 3506. https://doi.org/10.3390/molecules30173506







