Visible-Light-Controlled Thermal Energy Storage and Release: A Tetra-Ortho-Fluorinated Azobenzene-Doped Composite Phase Change Material
Abstract
1. Introduction
2. Results and Discussion
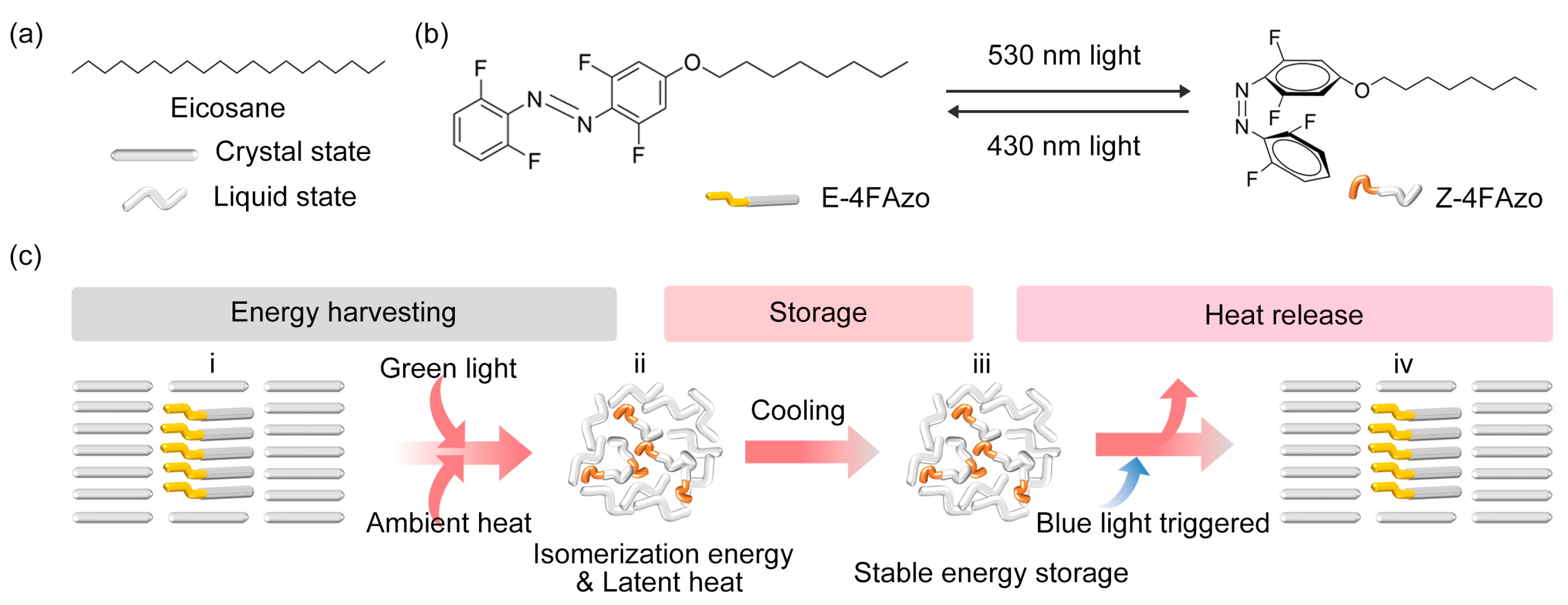
2.1. Molecule Design and Energy Havesting–Storage–Release Mechanism
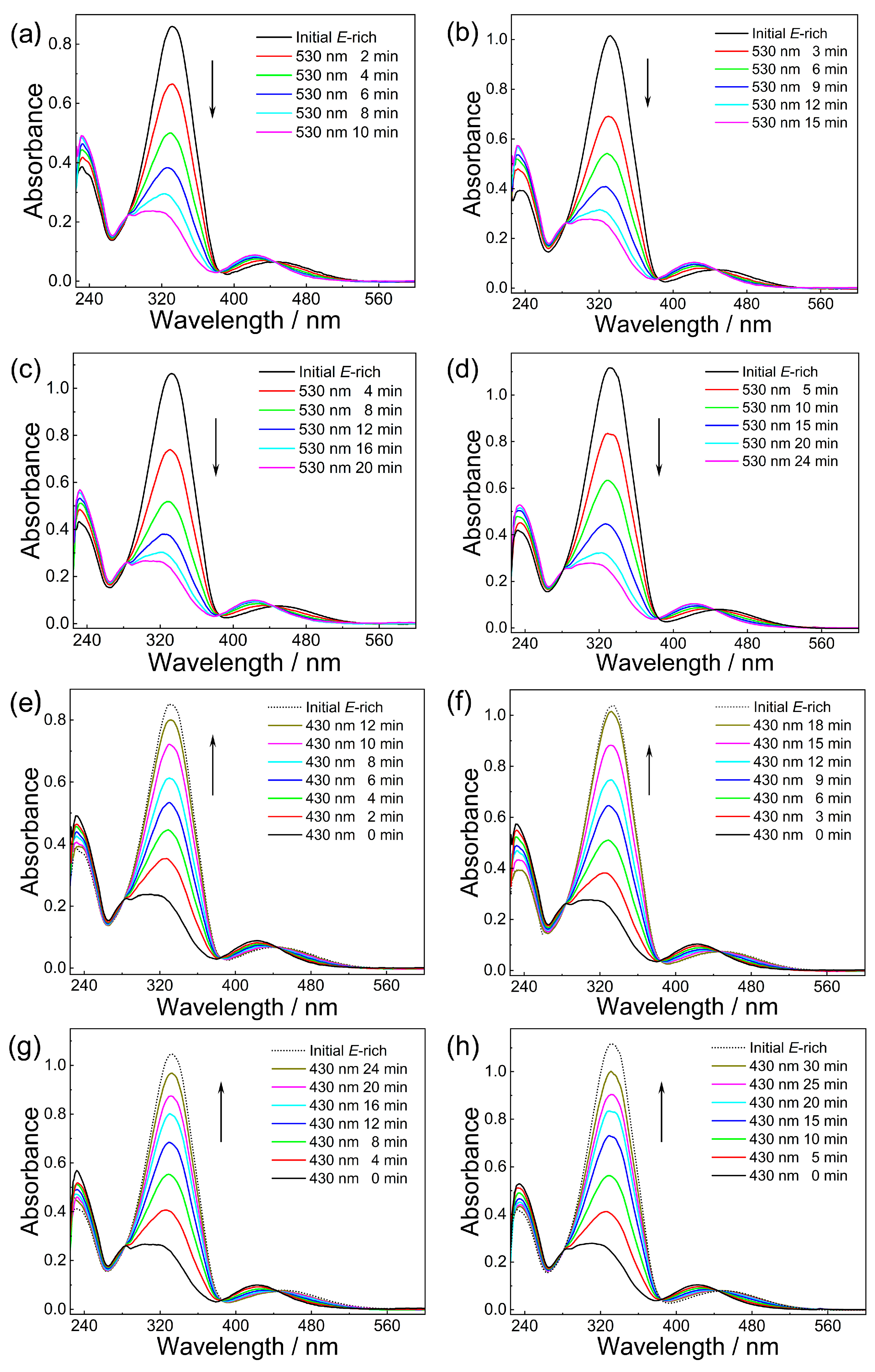
2.2. Photoisomerization Performance
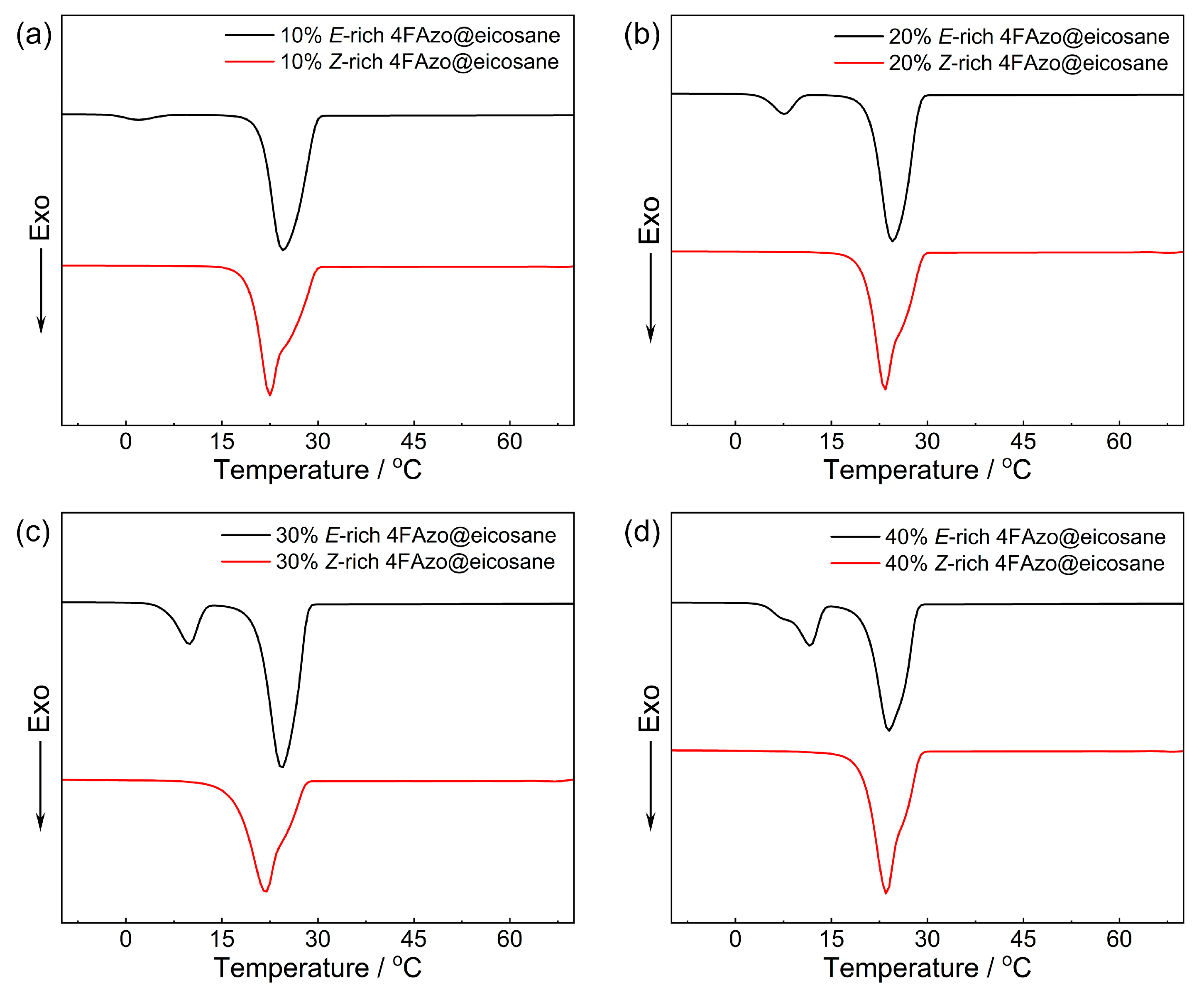
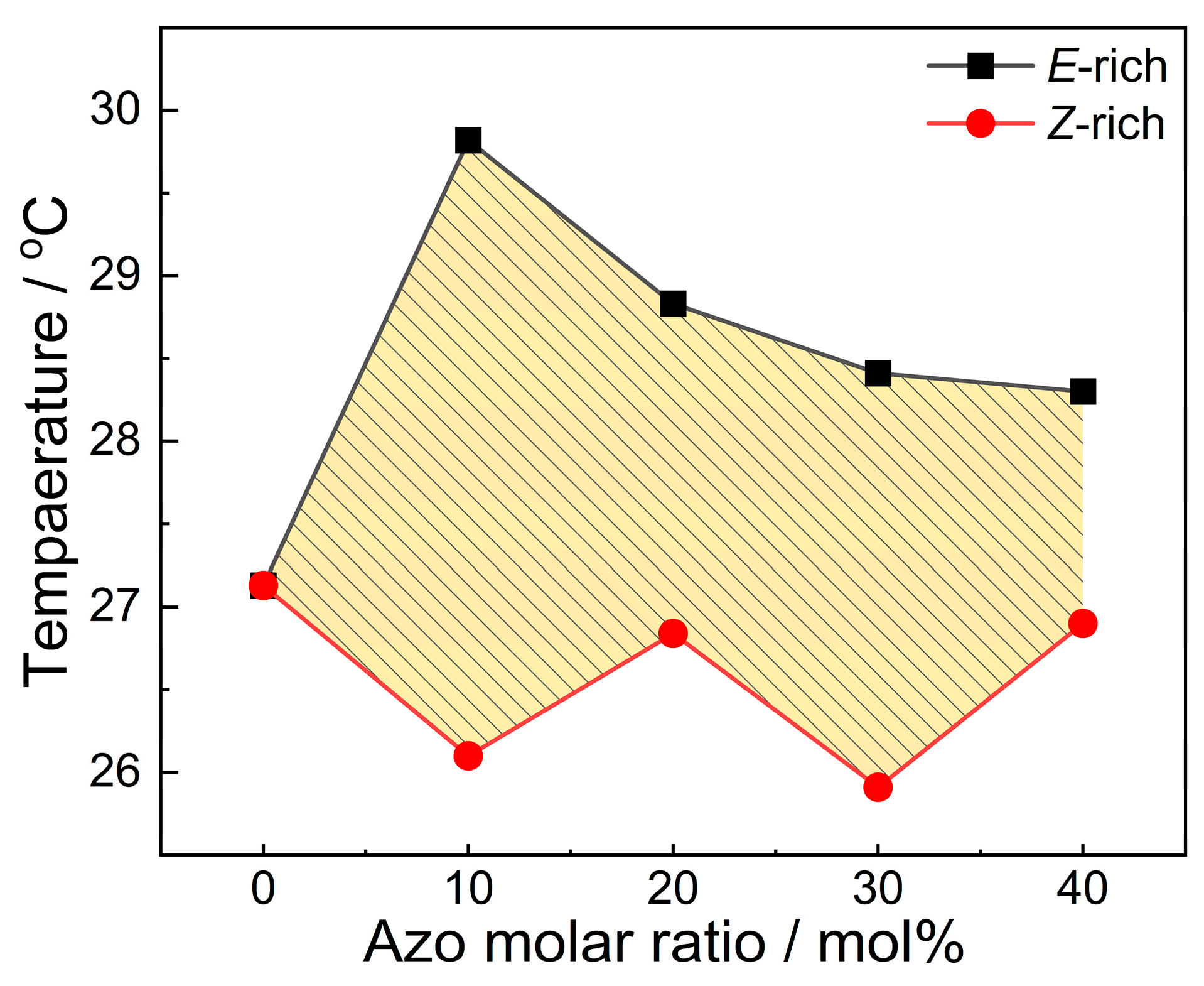
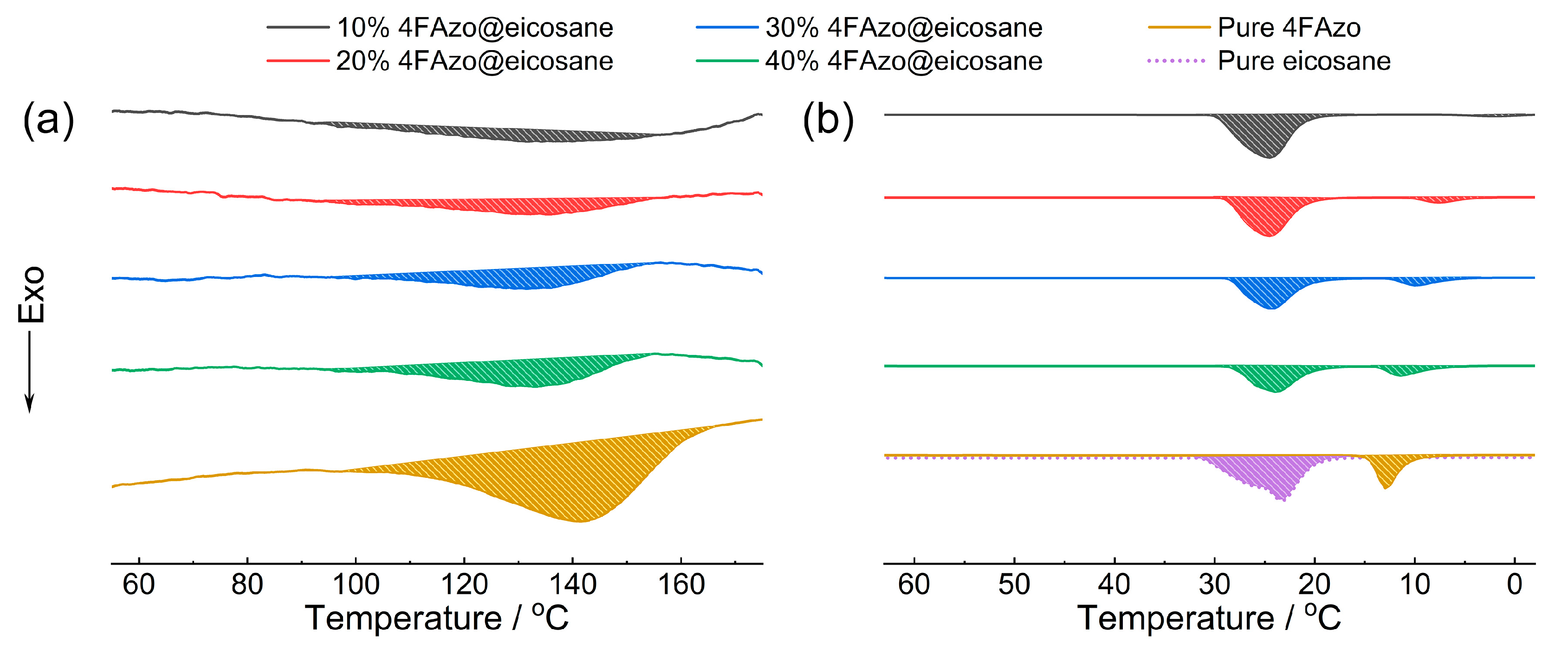
2.3. Phase Transition Property
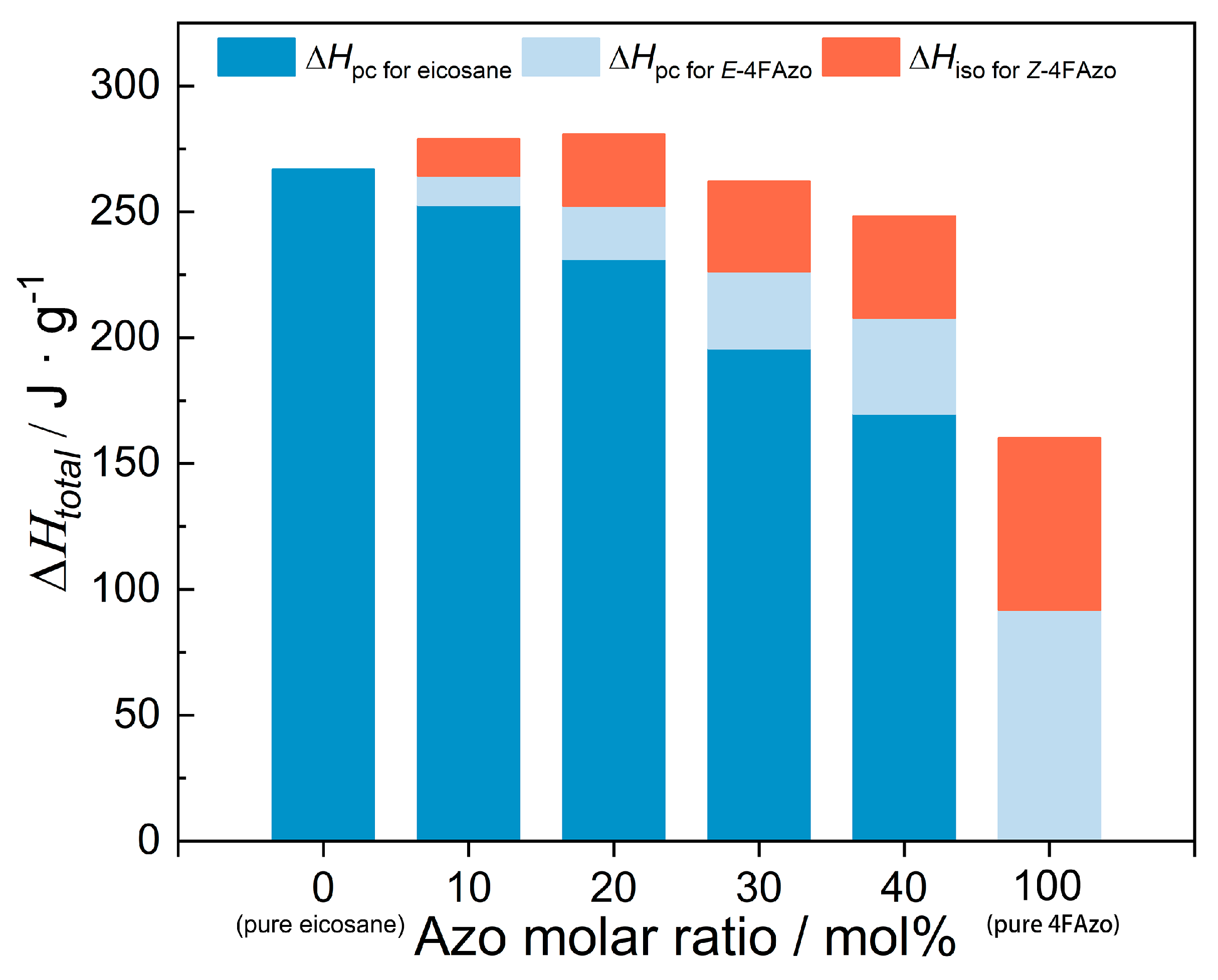
2.4. Energy Storage Density
- (1)
- Isomerization Enthalpy
- (2)
- Latent Heat of Phase Change
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Preparation of 4FAzo@eicosane Composite Materials
3.4. UV–Vis Absorption Spectroscopy
3.5. DSC Measurements
3.6. Statistical Analysis:
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OPCMs | Organic solid–liquid phase change materials |
| CPCMs | Composite phase change material |
| Azo | Azobenzene |
| 4FAzo | (E)-1-(2,6-difluoro-4-(octyloxy)phenyl)-2-(2,6-difluorophenyl)diazene |
| DSC | Differential scanning calorimetry |
References
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Bolivar Osorio, F.J.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Pereira da Cunha, J.; Eames, P. Thermal energy storage for low and medium temperature applications using phase change materials—A review. Appl. Energy 2016, 177, 227–238. [Google Scholar] [CrossRef]
- Jayathunga, D.S.; Karunathilake, H.P.; Narayana, M.; Witharana, S. Phase change material (PCM) candidates for latent heat thermal energy storage (LHTES) in concentrated solar power (CSP) based thermal applications—A review. Renew. Sustain. Energy Rev. 2024, 189, 113904. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, M.; Zhai, F.; Wei, H.; Liu, S.; Wang, P.; Liu, Z.; Ji, Z.; Wang, X. 3D printed modular Bouligand dissipative structures with adjustable mechanical properties for gradient energy absorbing. Mater. Futures 2024, 3, 025001. [Google Scholar] [CrossRef]
- Reddy, V.J.; Ghazali, M.F.; Kumarasamy, S. Advancements in phase change materials for energy-efficient building construction: A comprehensive review. J. Energy Storage 2024, 81, 110494. [Google Scholar] [CrossRef]
- Han, M.; Lu, L. Phase change materials integrated into building envelopes for thermal management: A review. J. Build. Eng. 2025, 103, 112063. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Luo, Z.; Wang, K.; Shah, S.P. A critical review on phase change materials (PCM) for sustainable and energy efficient building: Design, characteristic, performance and application. Energy Build. 2022, 260, 111923. [Google Scholar] [CrossRef]
- Karlessi, T.; Santamouris, M.; Apostolakis, K.; Synnefa, A.; Livada, I. Development and testing of thermochromic coatings for buildings and urban structures. Sol Energy 2009, 83, 538–551. [Google Scholar] [CrossRef]
- Lei, Q.; Wang, L.; Xie, H.; Yu, W. Active-passive dual-control smart window with thermochromic synergistic fluidic glass for building energy efficiency. Build. Environ. 2022, 222, 109407. [Google Scholar] [CrossRef]
- Mondal, S. Phase change materials for smart textiles—An overview. Appl. Therm. Eng. 2008, 28, 1536–1550. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Organic phase change materials and their textile applications: An overview. Thermochim. Acta 2012, 540, 7–60. [Google Scholar] [CrossRef]
- Zare, P.; Perera, N.; Lahr, J.; Hasan, R. Solid-liquid phase change materials for the battery thermal management systems in electric vehicles and hybrid electric vehicles—A systematic review. J. Energy Storage 2022, 52, 105026. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, B.; Zhang, T.; Jiang, Z.; Ding, J.; Ding, Y. A comprehensive review of composite phase change material based thermal management system for lithium-ion batteries. Renew. Sustain. Energy Rev. 2022, 167, 112667. [Google Scholar] [CrossRef]
- Han, G.G.D.; Li, H.S.; Grossman, J.C. Optically-controlled long-term storage and release of thermal energy in phase-change materials. Nat. Commun. 2017, 8, 1446. [Google Scholar] [CrossRef]
- Zhang, X.-x.; Fan, Y.-f.; Tao, X.-m.; Yick, K.-l. Crystallization and prevention of supercooling of microencapsulated n-alkanes. J. Colloid Interface Sci. 2005, 281, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Zahir, M.H.; Mohamed, S.A.; Saidur, R.; Al-Sulaiman, F.A. Supercooling of phase-change materials and the techniques used to mitigate the phenomenon. Appl. Energy 2019, 240, 793–817. [Google Scholar] [CrossRef]
- Fan, Y.F.; Zhang, X.X.; Wu, S.Z.; Wang, X.C. Thermal stability and permeability of microencapsulated n-octadecane and cyclohexane. Thermochim. Acta 2005, 429, 25–29. [Google Scholar] [CrossRef]
- Qiu, Q.; Shi, Y.; Han, G.G.D. Solar energy conversion and storage by photoswitchable organic materials in solution, liquid, solid, and changing phases. J. Mater. Chem. C 2021, 9, 11444–11463. [Google Scholar] [CrossRef]
- Le, M.; Han, G.G.D. Stimuli-responsive organic phase change materials: Molecular designs and applications in energy storage. Acc. Mater. Res. 2022, 3, 634–643. [Google Scholar] [CrossRef]
- Zhou, H.W.; Xue, C.G.; Weis, P.; Suzuki, Y.; Huang, S.L.; Koynov, K.; Auernhammer, G.K.; Berger, R.; Butt, H.J.; Wu, S. Photoswitching of glass transition temperatures of azobenzene-containing polymers induces reversible solid-to-liquid transitions. Nat. Chem. 2017, 9, 145–151. [Google Scholar] [CrossRef]
- Xu, W.-C.; Sun, S.; Wu, S. Photoinduced reversible solid-to-liquid transitions for photoswitchable materials. Angew. Chem. Int. Ed. 2019, 58, 9712–9740. [Google Scholar] [CrossRef]
- Goulet-Hanssens, A.; Eisenreich, F.; Hecht, S. Enlightening materials with photoswitches. Adv. Mater. 2020, 32, 1905966. [Google Scholar] [CrossRef]
- Wu, S.; Li, T.; Zhang, Z.-Y.; Li, T.; Wang, R. Photoswitchable phase change materials for unconventional thermal energy storage and upgrade. Matter 2021, 4, 3385–3399. [Google Scholar] [CrossRef]
- Huang, X.; Shangguan, Z.; Zhang, Z.-Y.; Yu, C.; He, Y.; Fang, D.; Sun, W.; Li, Y.-C.; Yuan, C.; Wu, S.; et al. Visible-light-induced reversible photochemical crystal–liquid transitions of azo-switches for smart and robust adhesives. Chem. Mater. 2022, 34, 2636–2644. [Google Scholar] [CrossRef]
- Dong, L.Q.; Feng, Y.Y.; Wang, L.; Feng, W. Azobenzene-based solar thermal fuels: Design, properties, and applications. Chem. Soc. Rev. 2018, 47, 7339–7369. [Google Scholar] [CrossRef] [PubMed]
- Gerkman, M.A.; Han, G.G.D. Toward controlled thermal energy storage and release in organic phase change materials. Joule 2020, 4, 1621–1625. [Google Scholar] [CrossRef]
- Liu, H.; Feng, Y.Y.; Feng, W. Alkyl-grafted azobenzene molecules for photo-induced heat storage and release via integration function of phase change and photoisomerization. Compos. Commun. 2020, 21, 100402. [Google Scholar] [CrossRef]
- Li, X.; Cho, S.; Wan, J.; Han, G.G.D. Photoswitches and photochemical reactions for optically controlled phase transition and energy storage. Chem 2023, 9, 2378–2389. [Google Scholar] [CrossRef]
- Han, G.G.D.; Deru, J.H.; Cho, E.N.; Grossman, J.C. Optically-regulated thermal energy storage in diverse organic phase-change materials. Chem. Commun. 2018, 54, 10722–10725. [Google Scholar] [CrossRef]
- Liu, H.; Tang, J.W.; Dong, L.Q.; Wang, H.; Xu, T.Y.; Gao, W.C.; Zhai, F.; Feng, Y.Y.; Feng, W. Optically triggered synchronous heat release of phase-change enthalpy and photo-thermal energy in phase-change materials at low temperatures. Adv. Funct. Mater. 2021, 31, 2008496. [Google Scholar] [CrossRef]
- Liang, R.; Yuan, B.; Zhang, F.; Feng, W. Azopyridine polymers in organic phase change materials for high energy density photothermal storage and controlled release. Angew. Chem. Int. Ed. 2025, 64, e202419165. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, J.; Luo, W.; Quan, X.; Li, H.; Huang, J.; Feng, W. High-energy and light-actuated phase change composite for solar energy storage and heat release. Surf. Interfaces 2021, 24, 101071. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, R.; Luo, W.; Hu, Y.; Wang, H.; Wang, C.; Li, X.; Huang, J. Photoguided AZO-phase change composite for high-energy solar storage and heat release at near ambient temperature. J. Energy Storage 2024, 101, 113974. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, L.; Tang, S.; Liu, X.; Han, Y.; Zhang, S.; Liu, K.; Feng, W. An innovative azobenzene-based photothermal fabric with excellent heat release performance for wearable thermal management device. Small 2024, 20, 2404310. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhang, Y.; Xia, J.; Qi, J.; Tang, F.; Zhai, F.; Dong, L. A tetra-ortho-chlorinated azobenzene molecule for visible-light photon energy conversion and storage. Molecules 2025, 30, 2333. [Google Scholar] [CrossRef]
- Bleger, D.; Schwarz, J.; Brouwer, A.M.; Hecht, S. o-Fluoroazobenzenes as readily synthesized photoswitches offering nearly quantitative two-way isomerization with visible light. J. Am. Chem. Soc. 2012, 134, 20597–20600. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; Tomberg, A.; Friščić, T.; Barrett, C.J. Shaping crystals with light: Crystal-to-crystal isomerization and photomechanical effect in fluorinated azobenzenes. J. Am. Chem. Soc. 2013, 135, 12556–12559. [Google Scholar] [CrossRef]
- Ge, F.; Yu, W.; Yin, Y.; Wang, C. Solar-driven thermochromic fabric based on photothermal conversion for light intensity monitoring. J. Mater. Chem. A 2021, 9, 20565–20575. [Google Scholar] [CrossRef]
- Kwaria, D.; McGehee, K.; Liu, S.; Kikkawa, Y.; Ito, S.; Norikane, Y. Visible-light-photomeltable azobenzenes as solar thermal fuels. ACS Appl. Opt. Mater. 2023, 1, 633–639. [Google Scholar] [CrossRef]
- Xu, X.; Wang, G. Molecular solar thermal systems towards phase change and visible light photon energy storage. Small 2022, 18, 2107473. [Google Scholar] [CrossRef]
- Zhao, F.; Grubert, L.; Hecht, S.; Bléger, D. Orthogonal switching in four-state azobenzene mixed-dimers. Chem. Commun. 2017, 53, 3323–3326. [Google Scholar] [CrossRef]
- Shi, Y.; Gerkman, M.A.; Qiu, Q.; Zhang, S.; Han, G.G.D. Sunlight-activated phase change materials for controlled heat storage and triggered release. J. Mater. Chem. A 2021, 9, 9798–9808. [Google Scholar] [CrossRef]
- Rezvanpour, M.; Hasanzadeh, M.; Azizi, D.; Rezvanpour, A.; Alizadeh, M. Synthesis and characterization of micro-nanoencapsulated n-eicosane with PMMA shell as novel phase change materials for thermal energy storage. Mater. Chem. Phys. 2018, 215, 299–304. [Google Scholar] [CrossRef]
- Alkan, C.; Sarı, A.; Karaipekli, A. Preparation, thermal properties and thermal reliability of microencapsulated n-eicosane as novel phase change material for thermal energy storage. Energy Convers. Manag. 2011, 52, 687–692. [Google Scholar] [CrossRef]
- Karaipekli, A.; Biçer, A.; Sarı, A.; Tyagi, V.V. Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Convers. Manag. 2017, 134, 373–381. [Google Scholar] [CrossRef]
- Knie, C.; Utecht, M.; Zhao, F.; Kulla, H.; Kovalenko, S.; Brouwer, A.M.; Saalfrank, P.; Hecht, S.; Bléger, D. ortho-Fluoroazobenzenes: Visible light switches with very long-lived Z isomers. Chem.—A Eur. J. 2014, 20, 16492–16501. [Google Scholar] [CrossRef]
- Konrad, D.B.; Savasci, G.; Allmendinger, L.; Trauner, D.; Ochsenfeld, C.; Ali, A.M. Computational design and synthesis of a deeply red-shifted and bistable azobenzene. J. Am. Chem. Soc. 2020, 142, 6538–6547. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Ashy; Krishna Km, A. Sunlight driven E–Z isomerization of liquid crystals based on hexahydroxytriphenylene nano-templates for enhanced solid-state solar thermal energy storage. J. Mater. Chem. A 2024, 12, 27373–27380. [Google Scholar] [CrossRef]
- Gerkman, M.A.; Gibson, R.S.L.; Calbo, J.; Shi, Y.R.; Fuchter, M.J.; Han, G.G.D. Arylazopyrazoles for long-term thermal energy storage and optically triggered heat release below 0 °C. J. Am. Chem. Soc. 2020, 142, 8688–8695. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; He, Y.; Wang, Z.; Xu, J.; Xie, M.; Tao, P.; Ji, D.; Moth-Poulsen, K.; Li, T. Photochemical phase transitions enable coharvesting of photon energy and ambient heat for energetic molecular solar thermal batteries that upgrade thermal energy. J. Am. Chem. Soc. 2020, 142, 12256–12264. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Feng, Y.Y.; Fang, W.Y.; Wang, H.; Ge, J.; Yang, X.Y.; Yu, H.T.; Qin, M.M.; Feng, W. Co-harvest phase-change enthalpy and isomerization energy for high-energy heat output by controlling crystallization of alkyl-grafted azobenzene molecules. Energy Environ. Mater. 2023, 7, e12607. [Google Scholar] [CrossRef]
- Yang, Q.B.; Ge, J.; Qin, M.M.; Wang, H.; Yang, X.Y.; Zhou, X.L.; Zhang, B.; Feng, Y.Y.; Feng, W. Controllable heat release of phase-change azobenzenes by optimizing molecular structures for low-temperature energy utilization. Sci. China-Mater. 2023, 66, 3609–3620. [Google Scholar] [CrossRef]
- Wang, L.; Yu, H.; Feng, W. Photothermal phase change energy storage materials: A groundbreaking new energy solution. Research 2024, 7, 0460. [Google Scholar] [CrossRef]
- Hu, J.; Huang, S.; Yu, M.; Yu, H. Flexible solar thermal fuel devices: Composites of fabric and a photoliquefiable azobenzene derivative. Adv. Energy Mater. 2019, 9, 1901363. [Google Scholar] [CrossRef]
- Tao, J.; Luan, J.; Liu, Y.; Qu, D.; Yan, Z.; Ke, X. Technology development and application prospects of organic-based phase change materials: An overview. Renew. Sustain. Energy Rev. 2022, 159, 112175. [Google Scholar] [CrossRef]
- Gao, F.; Wang, X.; Wu, D. Design and fabrication of bifunctional microcapsules for solar thermal energy storage and solar photocatalysis by encapsulating paraffin phase change material into cuprous oxide. Sol. Energy Mater. Sol. Cell 2017, 168, 146–164. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Yeol Yun, B.; Uk Kim, Y.; Wi, S.; Kim, S. Introduction of eicosane into biochar derived from softwood and wheat straw: Influence of porous structure and surface chemistry. Chem. Eng. J. 2021, 415, 128887. [Google Scholar] [CrossRef]
- Yadav, A.; Pandey, A.; Samykano, M.; Kalidasan, B.; Said, Z. A review of organic phase change materials and their adaptation for thermal energy storage. Int. Mater. Rev. 2024, 69, 380–446. [Google Scholar] [CrossRef]
- Dong, L.; Zhai, F.; Wang, H.; Peng, C.; Feng, Y.; Feng, W. An azobenzene-based photothermal energy storage system for co-harvesting photon energy and low-grade ambient heat via a photoinduced crystal-to-liquid transition. Energy Mater. 2022, 2, 200025. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Y.; Yu, H.; Dong, L.; Zhai, F.; Tang, J.; Ge, J.; Feng, W. Utilisation of photo-thermal energy and bond enthalpy based on optically triggered formation and dissociation of coordination bonds. Nano Energy 2021, 89, 106401. [Google Scholar] [CrossRef]
- Wang, Z.; Hölzel, H.; Moth-Poulsen, K. Status and challenges for molecular solar thermal energy storage system based devices. Chem. Soc. Rev. 2022, 51, 7313–7326. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, T.J.; Ferralis, N.; Kolpak, A.M.; Zheng, J.O.; Nocera, D.G.; Grossman, J.C. Templated assembly of photoswitches significantly increases the energy-storage capacity of solar thermal fuels. Nat. Chem. 2014, 6, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Feng, Y.Y.; Qin, C.Q.; Li, M.; Li, S.P.; Cao, C.; Long, P.; Liu, E.Z.; Hu, W.P.; Yoshino, K.; et al. High-energy, stable and recycled molecular solar thermal storage materials using AZO/graphene hybrids by optimizing hydrogen bonds. Nanoscale 2015, 7, 16214–16221. [Google Scholar] [CrossRef]
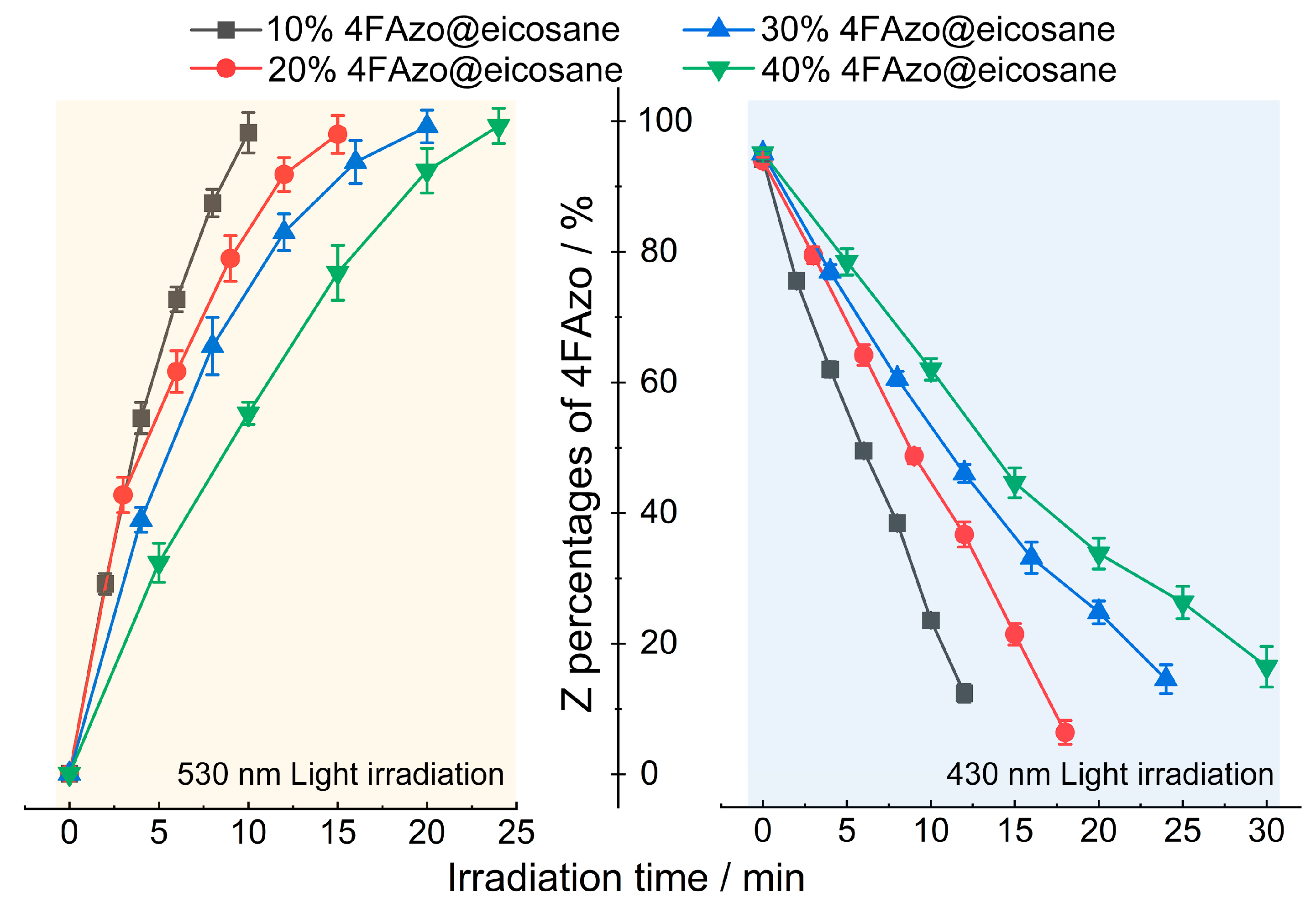
| 4FAzo Ratios (mol%) | 10 | 20 | 30 | 40 | ||||
|---|---|---|---|---|---|---|---|---|
| 530 nm | Irradiation Time (min) | Z Percent (%) | Irradiation Time (min) | Z Percent (%) | Irradiation Time (min) | Z Percent (%) | Irradiation Time (min) | Z Percent (%) |
| 2 | 29 | 3 | 43 | 4 | 39 | 5 | 32 | |
| 4 | 54 | 6 | 62 | 8 | 65 | 10 | 55 | |
| 6 | 73 | 9 | 79 | 12 | 83 | 15 | 77 | |
| 8 | 87 | 12 | 92 | 16 | 94 | 20 | 92 | |
| 10 | 98 | 15 | 97 | 20 | 99 | 24 | 99 | |
| 430 nm | 2 | 78 | 3 | 82 | 4 | 79 | 5 | 81 |
| 4 | 63 | 6 | 65 | 8 | 61 | 10 | 63 | |
| 6 | 49 | 9 | 49 | 12 | 45 | 15 | 44 | |
| 8 | 37 | 12 | 35 | 16 | 32 | 20 | 32 | |
| 10 | 21 | 15 | 19 | 20 | 22 | 25 | 24 | |
| 12 | 9 | 18 | 2 | 24 | 11 | 30 | 13 | |
| 4FAzo Ratio (mol%) | Before 530 nm Irradiation (E-rich) | After 530 nm Irradiation (Z-rich) | ΔTc (°C) | |||
|---|---|---|---|---|---|---|
| Tm (°C) | Tc (°C) | Tm (°C) | Tc (°C) | |||
| 0 (pure eicosane) | 32.86 | 27.13 | 32.86 | 27.13 | ||
| 10 | 30.51 | 5.02 | 29.82 | 31.01 | 26.10 | 3.72 |
| 20 | 31.01 | 10.33 | 28.83 | 30.56 | 26.84 | 1.99 |
| 30 | 31.00 | 11.90 | 28.41 | 32.52 | 25.91 | 2.50 |
| 40 | 31.07 | 13.73 | 28.30 | 30.52 | 26.90 | 1.40 |
| 100 (Pure 4FAzo) | 42.06 | 13.00 | none | none | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Qi, J.; Xia, J.; Zhai, F.; Dong, L. Visible-Light-Controlled Thermal Energy Storage and Release: A Tetra-Ortho-Fluorinated Azobenzene-Doped Composite Phase Change Material. Molecules 2025, 30, 3576. https://doi.org/10.3390/molecules30173576
Zhang Y, Qi J, Xia J, Zhai F, Dong L. Visible-Light-Controlled Thermal Energy Storage and Release: A Tetra-Ortho-Fluorinated Azobenzene-Doped Composite Phase Change Material. Molecules. 2025; 30(17):3576. https://doi.org/10.3390/molecules30173576
Chicago/Turabian StyleZhang, Yating, Jing Qi, Jun Xia, Fei Zhai, and Liqi Dong. 2025. "Visible-Light-Controlled Thermal Energy Storage and Release: A Tetra-Ortho-Fluorinated Azobenzene-Doped Composite Phase Change Material" Molecules 30, no. 17: 3576. https://doi.org/10.3390/molecules30173576
APA StyleZhang, Y., Qi, J., Xia, J., Zhai, F., & Dong, L. (2025). Visible-Light-Controlled Thermal Energy Storage and Release: A Tetra-Ortho-Fluorinated Azobenzene-Doped Composite Phase Change Material. Molecules, 30(17), 3576. https://doi.org/10.3390/molecules30173576







