An Integrative Approach to Hazardous Effects Caused by Pharmaceutical Contaminants on Aquatic Effluents
Abstract
1. Introduction
2. Presence of Pharmaceutical Substances in Aquatic Systems
2.1. Occurrence, Pollution Sources, and Existing Techniques for Pharmaceuticals Removal
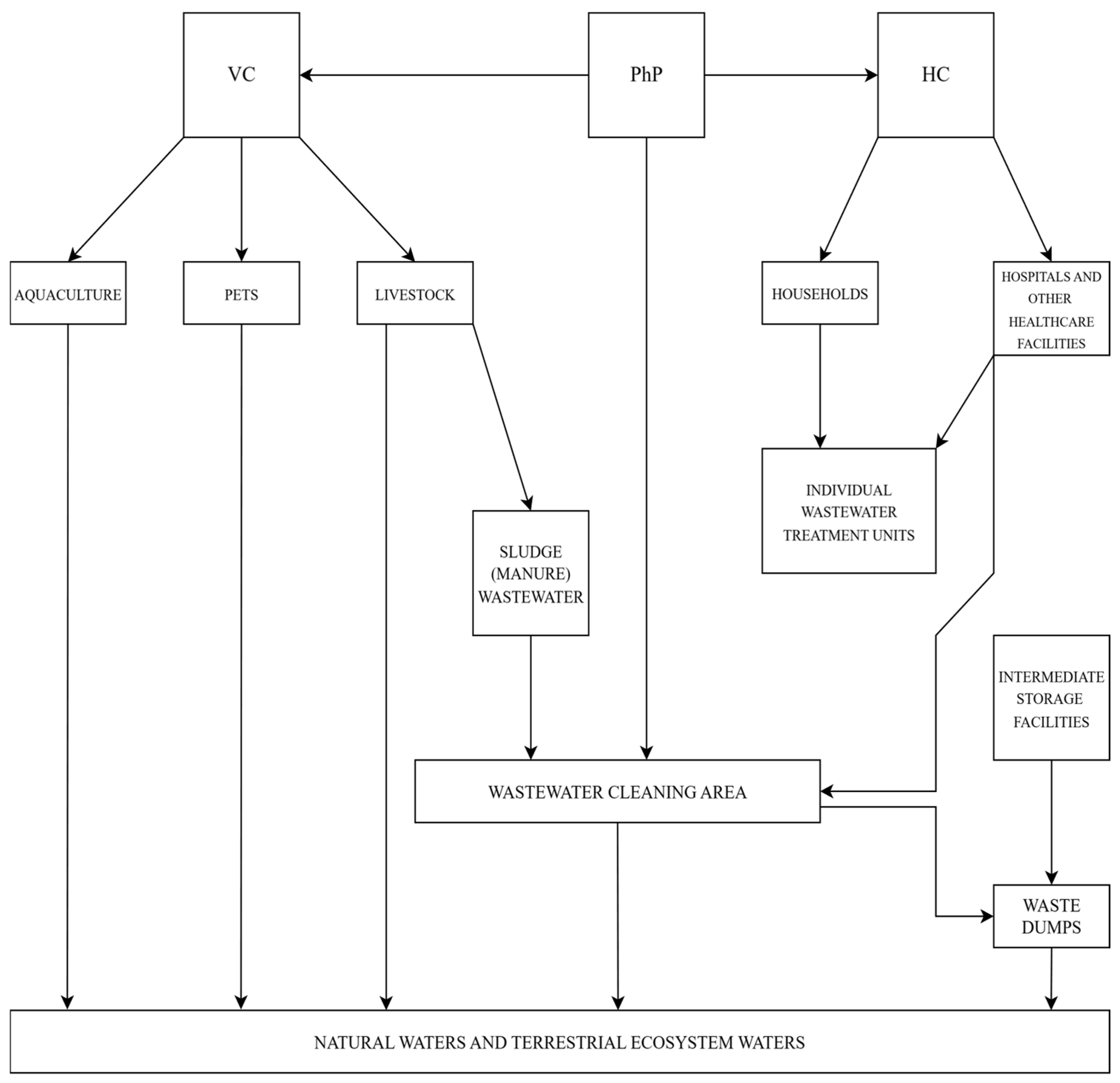
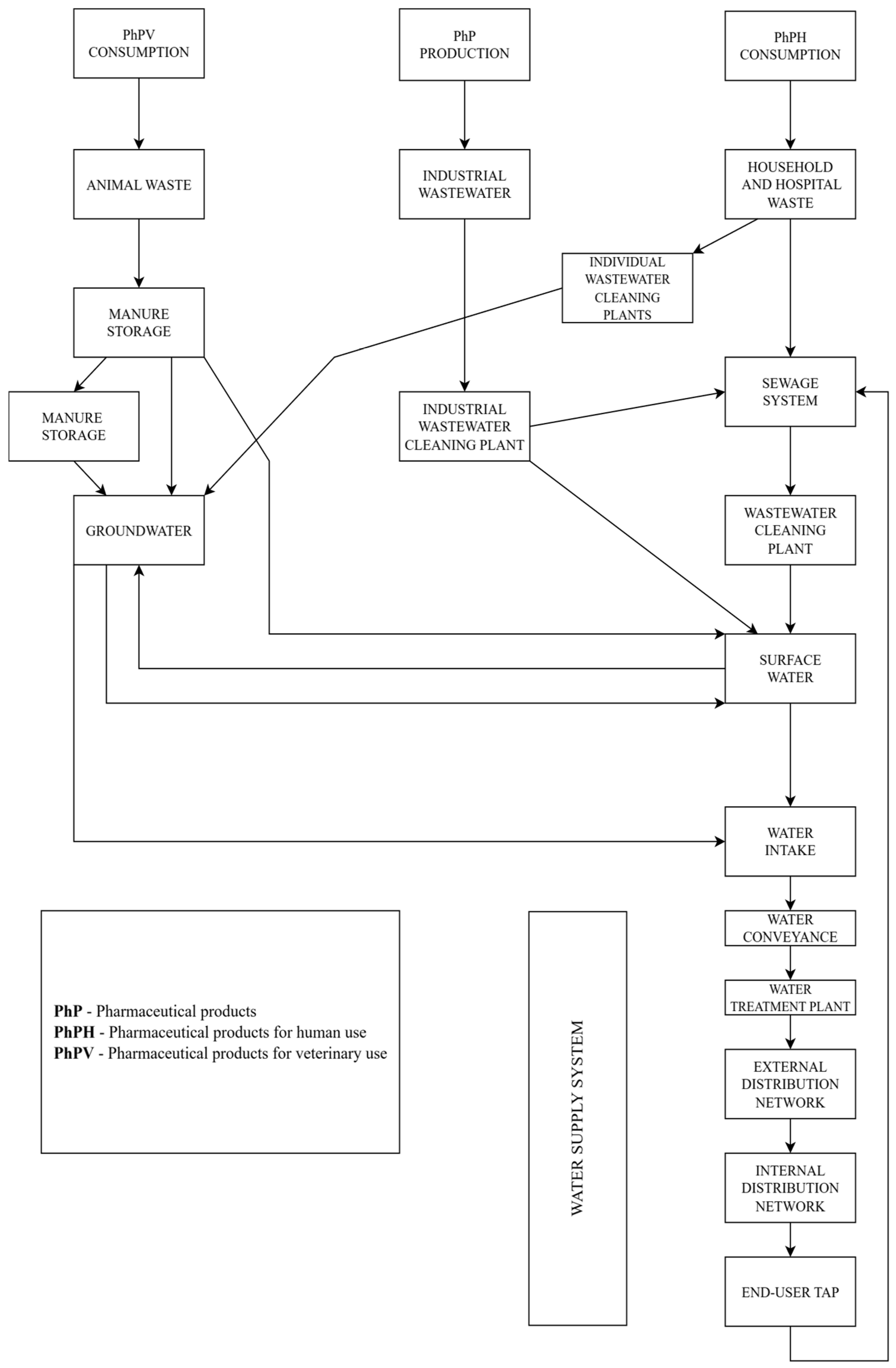
2.2. Trajectories of Pharmaceutical Substances from Production, Consumption and Evacuation in Environment Until Household Tap
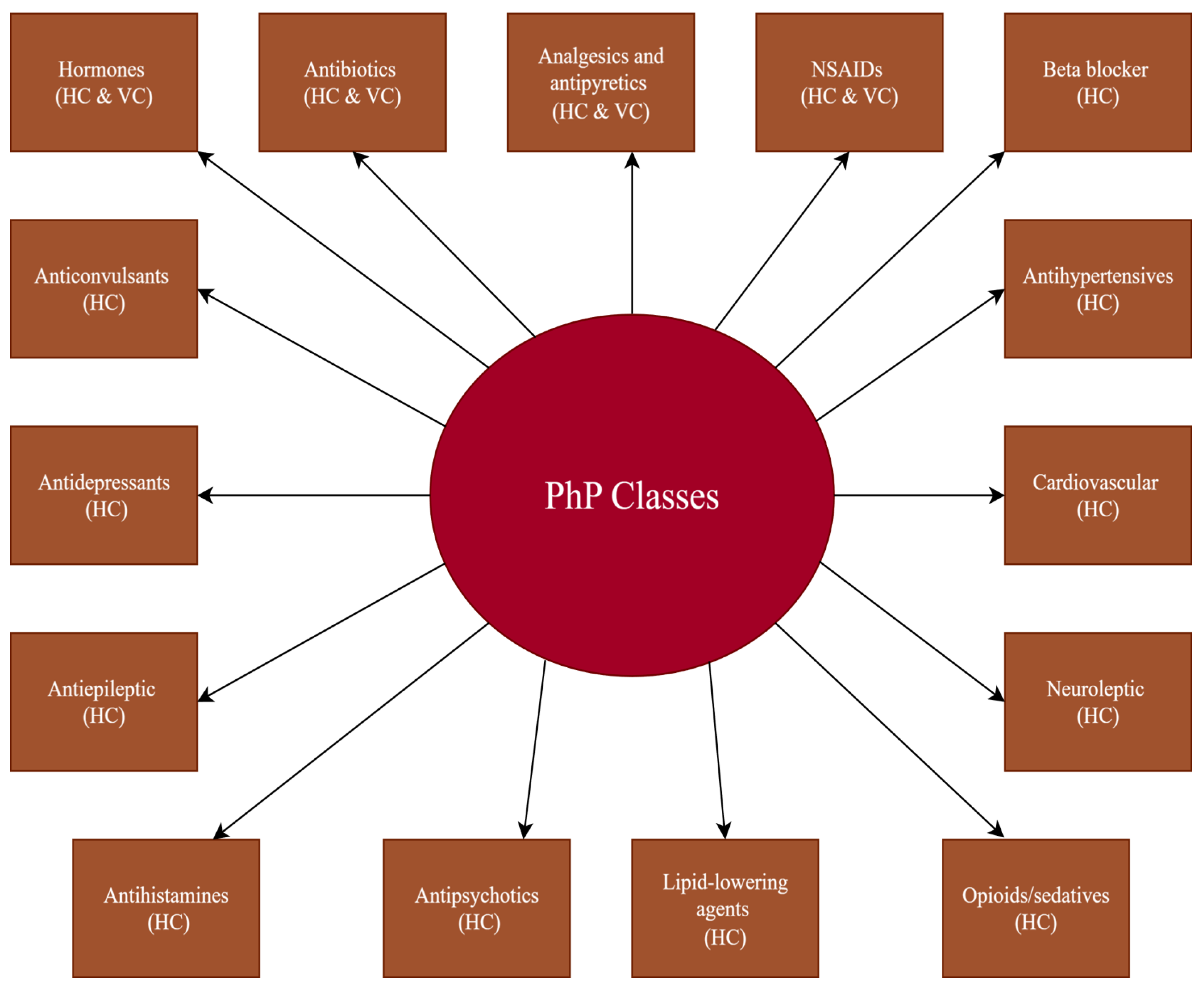
2.3. Production and Consumption of Pharmaceutical Products
2.4. Concentrations of Pharmaceutical Contaminants in Natural Water
2.5. Monitoring and Statistics of Water Contaminants—General Procedures
2.6. Impact of Pharmaceutical Wastes on Environment, Effects on Human Health, and Global Risks
3. Removal of Pharmaceutical Contaminants from Aqueous Effluents
3.1. Adequate Methods to Remove Pharmaceutical Pollutants
3.1.1. Advanced Oxidation Processes
| PhC/Class of PhPs | Cleaning/Treatment Method | Efficiency | Citation |
|---|---|---|---|
| NSAIDs | Membrane filtration and AOPs | 90–94% | [108] |
| NSAIDs | EAOPs | >90% | [110] |
| Ibuprofen | UV/Fe(III)/Oxone | ~100% | [113] |
| Ibuprofen | photo-Fenton | 96% | [114] |
| Ibuprofen | Fenton-based AOPs | 66% | [124] |
| Ibuprofen | Sonophotocatalysis/TiO2 or Fe3+ | ~100% | [126] |
| Acetaminophen | electro-Fenton & photo-electro-Fenton | 100% | [116] |
| Naproxen | electrochemical oxidation | ~100% | [118] |
| Naproxen | EAOPs | 91% | [125] |
| Ibuprofen | 3D electro-Fenton | 93.51% | [121] |
| Naproxen | 3D electro-Fenton | 98.14% | [121] |
| Diclofenac | electrocatalysis & photo-electrocatalysis | >90% | [122] |
| Diclofenac | Fenton-based AOPs | 86% | [124] |
| Diclofenac | AOPs (UV/TiO2/H2O2) | 100% | [125] |
3.1.2. Cleaning Technologies for Pharmaceutical Contaminants Removal from Aqueous Effluents
3.2. Studies Focused on One or Two Specific Pharmaceutical Contaminants
3.3. Research Devoted to the Removal of Pharmaceutical Contaminant Classes
3.4. Remediation Potential and Strategies
3.5. Technical and Economic Aspects
4. Conclusions, Proposals, and Recommendations
- It is necessary to draw up an inventory of the PhPs that are used for human and animal purposes which are contaminating water systems across different countries.
- We must include PhC monitoring in programs for water quality control from natural water sources, wastewater, and drinkable water based on research which must be performed a priori to monitor some PhCs. However, such research must not distract from the attention given to the control of existent parameters and indicators.
- It is very important to establish a methodology for sampling and determining PhCs from water; special attention should be paid to the standardization of the accepted limits for PhC concentrations in natural water, wastewater, and drinkable water.
- It is very important to perform appropriate research to establish the effects of the presence of PhP micropollutants in drinkable water and aquatic systems in general on humans and on flora and fauna, respectively.
- The initiation/continuation of research to establish appropriate rehabilitation solutions for existent wastewater cleaning and treatment plants for the removal of micropollutants (including PhCs) from water.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PhPs | pharmaceutical products |
| POPs | persistent organic pollutants |
| DDT | dichlorodiphenyltrichloroethane |
| PCBs | polychlorinated biphenyls |
| HCH | hexachlorocyclohexane |
| PhCs | pharmaceutical contaminants |
| CFC | chlorofluorocarbon |
| PAHs | polyaromatic hydrocarbons |
| OECD | Organization for Economic Co-operation and Development |
| HC | human consumption |
| VC | veterinary consumption |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| HDCs | human drug consumers |
| ADCs | animal drug consumers |
| DWC | drinking water consumer |
| PhPHs | pharmaceutical products for human use |
| PhPVs | pharmaceutical products for veterinary use |
| MSTs | multivariate statistical techniques |
| PCA | principal component analysis |
| MANOVA | multivariate analysis of variance |
| ANOVA | analysis of variance |
| AOPs | advanced oxidation processes |
| NTs | nanotubes |
| COD | chemical oxygen demand |
| PPCPs | pharmaceutical and personal care products |
| EAOPs | electrochemical advanced oxidation processes |
| CTAB | cethyltrimethyl ammonium bromide |
Appendix A
Appendix A.1
| PhPs/Class and Application/Usage | Concentration Level Detected, ng/L | Ecosystem | Country/Citation |
|---|---|---|---|
| Azithromycin/Antibiotic/HC | 2.6–166.5 | Surface | China/[2] |
| Ciprofloxacin/Antibiotic/HC | 1.5–2.4 | Surface | Sweden/[2] |
| Clarithromycin/Antibiotic/HC | 123 | Surface | China/[2] |
| Chloramphenicol/Antibiotic/HC | 0.0002–0.016 | Surface | Sweden/[2] |
| Erythromycin/Antibiotic/HC and VC | 80 | Surface | UK/[1] |
| 6.46 | Surface | Bangladesh/[2] | |
| 22.3 | Surface | China/[2] | |
| 2.2–54.8 | Underground | Taiwan/[2] | |
| Metronidazole/Antibiotic/HC | 1.41–156 | Surface | China/[2] |
| Norfloxacin/Antibiotic/HC | 4.5–83 | Surface | UK/[1] |
| 2.8–9.3 | Underground | Taiwan/[2] | |
| Roxithromycin/Antibiotic/HC | 0.6–88.4 | Surface | China/[2] |
| 0.0013 | Surface | Sweden/[2] | |
| Sulfamethazine/Antibiotic/HC and VC | 3.7 | Surface | China/[2] |
| 4.19 | Surface | Bangladesh/[2] | |
| 0.3–28.9 | Underground | Taiwan/[2] | |
| Sulfamethoxazole/Antibiotic/HC and VC | 0.55 | Surface | UK/[1] |
| 0.44–115.3 | Surface | China/[2] | |
| 11.35 | Surface | Bangladesh/[2] | |
| 0.1–1820 | Underground | Taiwan/[2] | |
| Tetracycline/Antibiotic/HC and VC | 1000 | Surface | UK/[1] |
| Trimethoprim/Antibiotic/HC | 17.20 | Surface | Bangladesh/[2] |
| 15.6 | Surface | China/[2] | |
| 0.33 | Surface | Sweden/[2] | |
| 0.0–0.68 | Surface | USA/[2] | |
| Aspirin/Analgesics and anti-inflammatories/HC and VC | 25–294 | Surface | Columbia/[2] |
| 0.0007 | Seawater | Sweden/[2] | |
| 2.5 | Underground | Serbia/[2] | |
| Acebutolol/Antihypertensive/HC | 0.7 | Underground | Taiwan/[2] |
| Atenolol/Antihypertensive/HC | 181 | Surface | China/[2] |
| 3.5–8.5 | Surface | Sweden/[2] | |
| 0–14.2 | Lake | USA/[2] | |
| 3.6 | Underground | Taiwan/[2] | |
| Metoprolol/Beta-blocker/HC | 0.46–17.3 | Surface | China/[2] |
| 0.04–21.2 | Estuary | China/[2] | |
| 3.5 | Underground | Serbia/[2] | |
| 0.04–21.2 | Underground | Taiwan/[2] | |
| Propranolol/Beta-blocker/HC | 29 | Surface | UK/[1] |
| 0.9 | Estuary | China/[2] | |
| 0.0006 | Surface | Sweden/[2] | |
| 4.5 | Underground | Serbia/[2] | |
| Furosemide/Antihypertensive/HC | 4.7–9.4 | Surface | Sweden/[2] |
| Verapamil/Antihypertensive/HC | 0.053 | Surface | Sweden/[2] |
| Carbamazepine/Anticonvulsants/HC | 8.80 | Surface | Bangladesh/[2] |
| 25.3, 21.3, 0.41–10.3 | Surface | China/[2] | |
| 37.4 | Estuary | China/[2] | |
| 0.38–0.51 | Surface | Sweden/[2] | |
| 3.4 | Underground | Serbia/[2] | |
| Diazepam/Antidepressants/HC | 24.3 | Surface | China/[2] |
| 6.1 | Estuary | China/[2] | |
| Gabapentin/Antiepileptic/HC | 4.5 | Surface | Sweden/[2] |
| Cimetidine/Antihistamine/HC | 0.36 | Surface | Sweden/[2] |
| Fluoxetine/Antidepressants/HC | 5 | Surface | UK/[1] |
| 0.4 | Surface | China/[2] | |
| 0.4–4.7 | Surface | Sweden/[2] | |
| Simvastatin/Lipid-lowering agent/HC | 2.3–16.7 | Estuary | China/[2] |
| Tramadol/Analgesic/HC | 3.01 | Surface | Sweden/[2] |
| Diclofenac/NSAIDs/HC and VC | 195 | Surface | UK/[1] |
| 20–64 | Surface | Austria/[1] | |
| 18–41 | Surface | France/[1] | |
| 150–1200 | Surface | Germany/[1] | |
| 1.5 | Surface | China/[2] | |
| Ibuprofen/NSAIDs/HC and VC | 826–5044 | Surface | UK/[1] |
| 23–120 | Surface | France/[1] | |
| 70–530 | Surface | Grece/[1] | |
| 0.86–115.8 | Surface | China/[2] | |
| 0.2 | Underground | Serbia/[2] | |
| 7–836.7 | Underground | Taiwan/[2] | |
| Ketoprofen/NSAIDs/HC and VC | 1.4–54.5 | Estuary | China/[2] |
| 75.3 | Surface | Colombia/[2] | |
| 10.3–89 | Seawater | Portugal/[2] | |
| Naproxen/NSAIDs/HC and VC | 3.5 | Surface | China/[2] |
| 260 | Surface | Colombia/[2] | |
| 1178 | Seawater | Portugal/[2] |
Appendix A.2
| Pharmaceutical Compound/Class | Impact on the Environment/References | Risk Assessment/References |
|---|---|---|
| Antibiotics (Inhibiting and toxic compounds) | Antibiotic resistance/[100,186,187] Haematological and hormonal imbalances in fish/[188,189,190,191] Growth of phytoplankton/[101] Great negative effects on marine amphipods & aquatic invertebrates/[101] | Antibiotic resistance/[100,186,187] Marine biodiversity/[69,103,108] Aquatic ecosystems/[43,103,104] Antimicrobial resistance/[105,106] Worldwide effects/[107] |
| Alcohols, acetone (High content of organic matter) | Oxygen depletion/[100,192,193] Eutrophication (algal bloom)/[194] | Marine biodiversity/[100] Mussels’ digestive glands/[104] Worldwide effects/[107] |
| Aromatic compounds, chlorinated hydrocarbons (Slowly biodegradable organic compounds) | Disruption of immune responses in aquatic fauna/[100,195,196] | Effects on aquatic and terrestrial wildlife/[105] Potential human exposure/[105] Worldwide effects/[107] |
| NSAIDs | Great negative effects on marine amphipods & aquatic invertebrates/[101] | Aquatic ecosystems/[103] Worldwide effects/[107] |
| Beta-blockers | Great negative effects on marine amphipods & aquatic invertebrates/[37] | Biodiversity/[37] Worldwide effects/[107] |
| Analgesics | Human health and environment/[43] | Ecological health risks/[43] Worldwide effects/[107] |
Appendix A.3
| Pharmaceutical Product/Class and Therapeutical Application | Chemical Formula and Molecular Mass (g/mol) | Structural Formula | References |
|---|---|---|---|
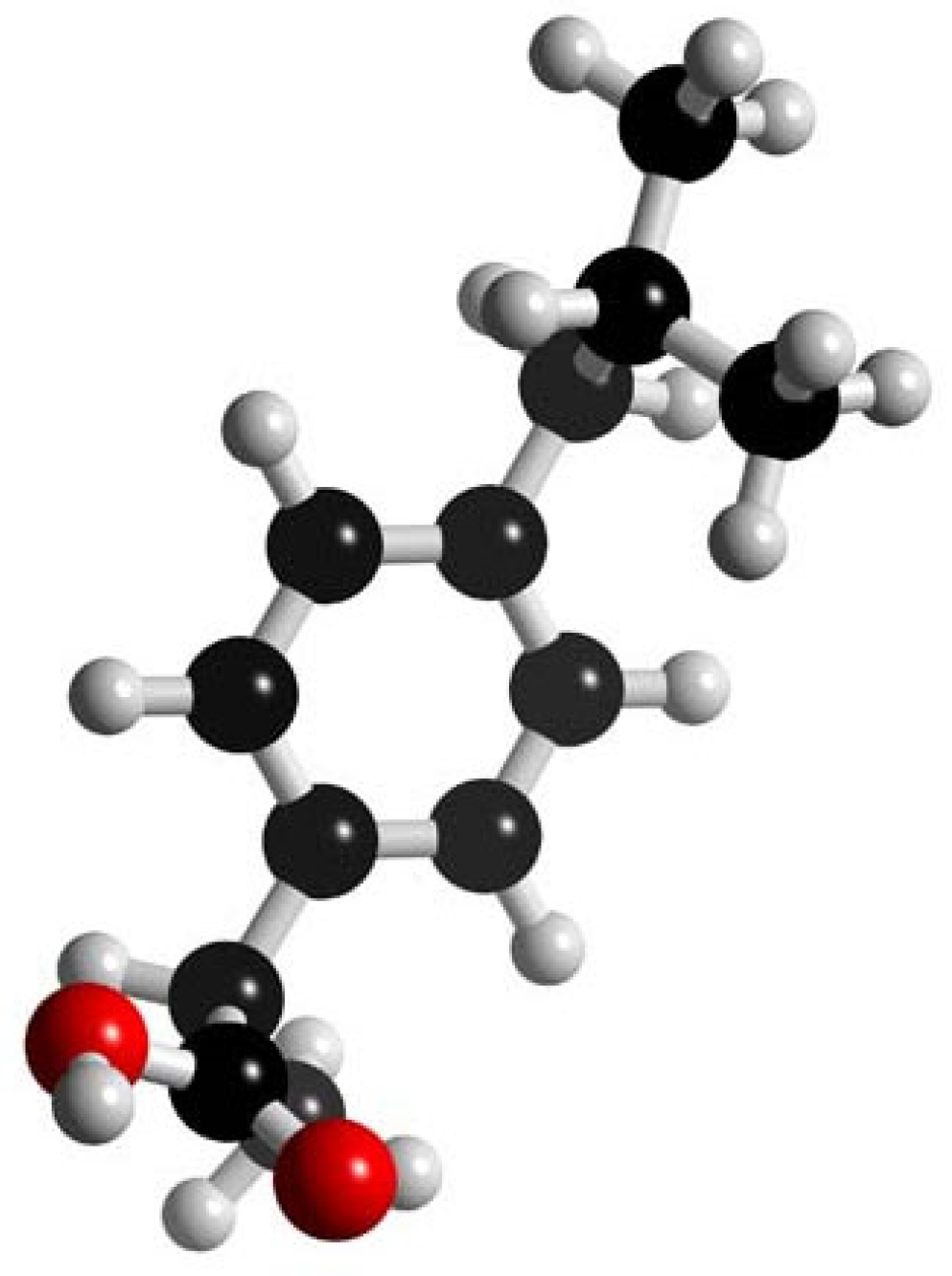
| Ibuprofen/ NSAIDs | C13H18O2 206.285 | 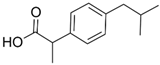 | [113,114,121,124,126,140,143,152,153,155,159,164,197] |
| Naproxen/ NSAIDs | C14H14O3 230.263 | 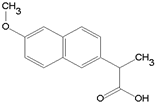 | [118,120,121,140,144,147,148,166] |
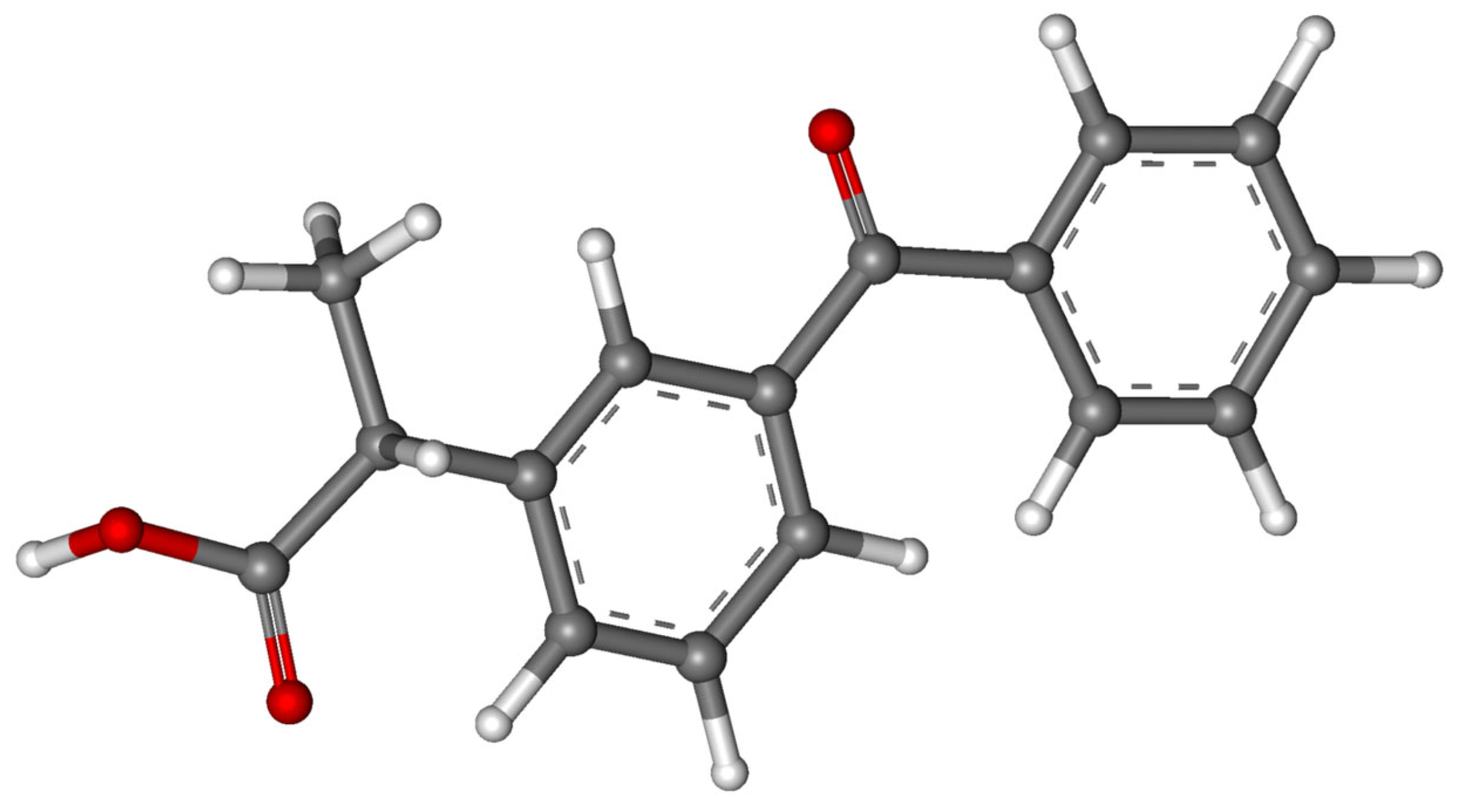
| Ketoprofen/ NSAIDs | C16H14O3 254.285 | 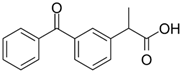 | [111,114,139,144,155,158,166] |
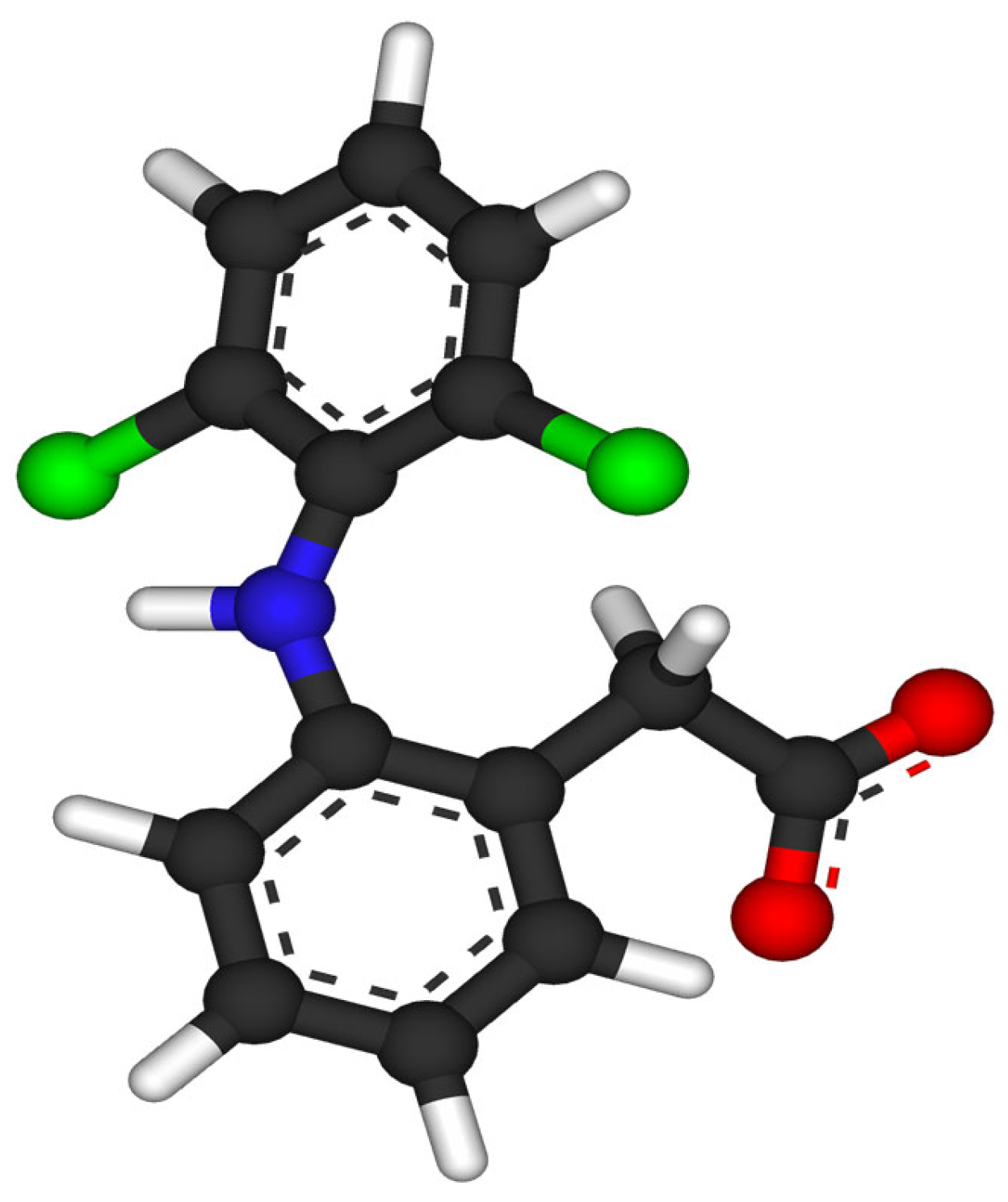
| Diclofenac/ NSAIDs | C14H11Cl2NO2 296.147 | 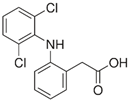 | [122,124,125,146,149,150,151,158,162,164] |
| Aspirin/ Analgesic and anti-inflammatory | C9H8O4 180.159 | 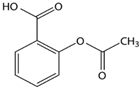 | [162] |
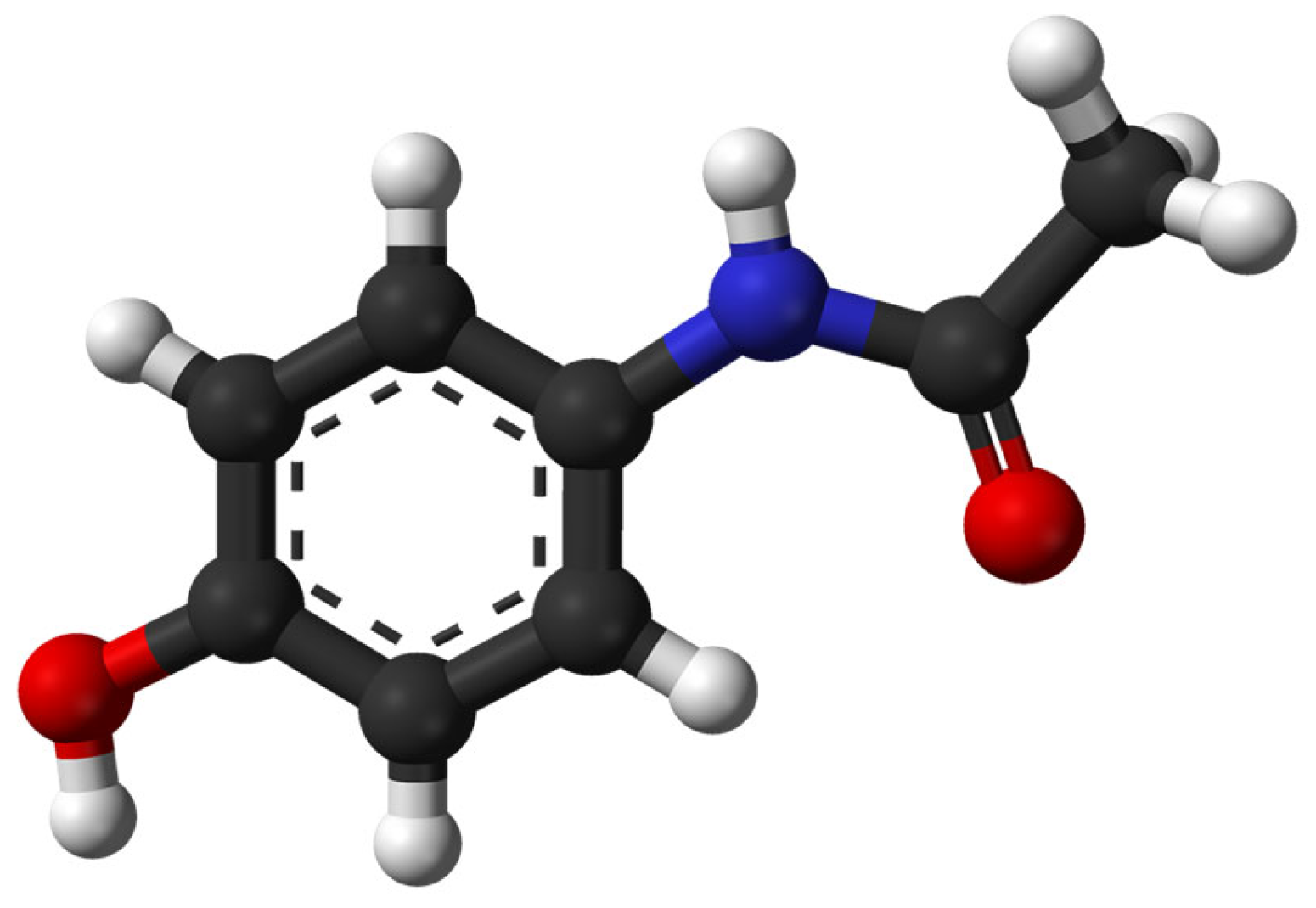
| Paracetamol (Acetaminophen)/ Analgesic and anti-inflammatory | C8H9NO2 151.063 | 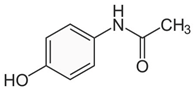 | [116] |
| Sulfamethoxazole/ Antibiotic | C10H11N3O3S 253.28 | 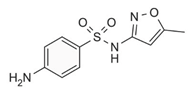 | [143,198] |
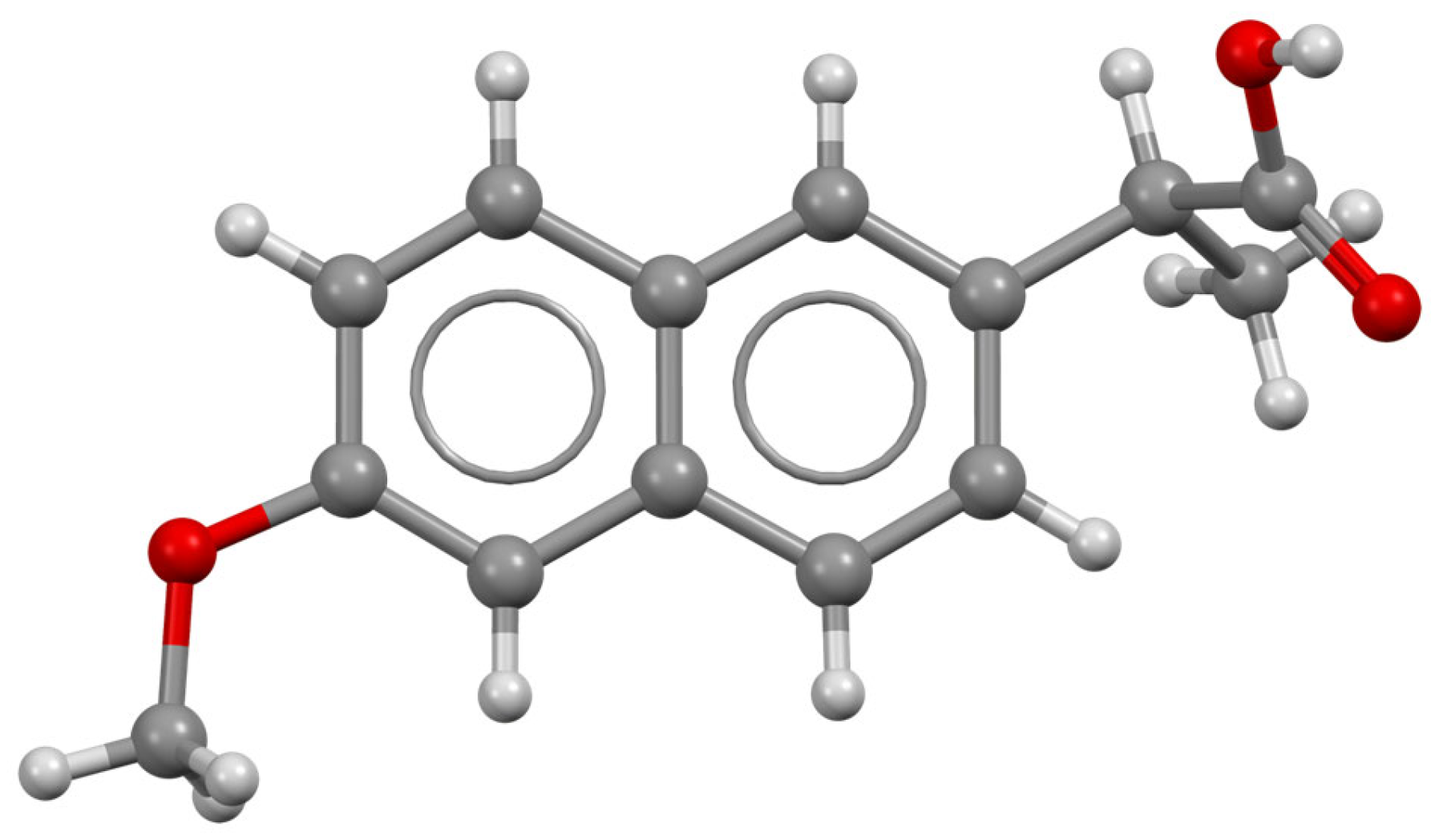
| Carbamazepine (Tegretol)/ Anticonvulsant and neuropathic pain | C15H12N2O 236.274 | 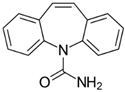 | [153,161] |
Appendix A.4
| Class and Therapeutical Application | PhPs | References |
|---|---|---|
| NSAIDs | Ibuprofen, Naproxen, Ketoprofen, Diclofenac, Acetaminophen (Paracta-mol), Acetylsalicylic acid (aspirin), Fenoprofen, Mefenamic acid, Metho-carbamol, Chlorzoxazone, Lidocaine hydrochloride, Indomethacin | [40,44,121,128,140,141,145,155,156,157,160,161,167,168,170,171,172,173,174,175,176] |
| Analgesic PhPs | Propyphenazone, Indomethacin, Codeine, Dexamethasone, Tramadol | [127,157,172,178] |
| Antibiotics | Amoxicillin, Cefaclor, Cefazolin, Ciprofloxacin, Sulfadimethoxine, Tetracycline, Trimethoprim, Cefotaxime, Chlortetracycline, Oxytetracycline, Levofloxacin, Enrofloxacin, Norfloxacin, Daptomycin, Erythromycin, Streptomycin, Cephalexin, Metronidazole, Sparfloxacin, Cefotaxime, Ceftriaxone, Chloramphenicol, Ampicillin, Enrofloxacin, Marbofloxacin, Penicillin G, Trimethoprim, Clarithromycin, Sulfamethoxazole | [40,44,141,170,178,199] |
| Hormones/ Endocrine disrupter | 17-alpha-ethinylestradiol, Bisphenol A, Nonylphenol | [40,156,178,200] |
| Proton pump inhibitors | Omeprazole, Ranitidine | [40] |
| Anticonvulsant PhPs/Neuropathic pain/ Antidepressant | CBZ-Diol, Carbamazepine, Gabapentin, Lamotrigine, Amitriptyline, Diazepam, Doxepin, Imipramine, Oxazepam, Amisulpride, Citalopram, Venlafaxine | [141,157,178,197] |
| Beta-blocker/ Antihypertensive | Acebutolol, Atenolol, Bezafibrate, Clofibric acid, Gemfibrozil, Losartan, Metoprolol, Propranolol, Hydrochlorothiazide | [141,161,178,201] |
| Antihistamine/ Antiallergic | Chlorpheniramine, Dexamethasone | [178] |
| Antifungal | Clotrimazole, Econazole | [40] |
Appendix A.5
| Pharmaceutical Compound/Class | Removal Methods/Citation |
|---|---|
| NSAIDs | AOPs/[108,110,130,132] |
| NSAIDs | EAOPs/[128] |
| NSAIDs | Activated carbon/[156] |
| Naproxen and Diclofenac | Advanced Membranes Technologies/[110,137] |
| Antibiotics | AOPs/[129,130,132,134] |
| Antibiotics | Advanced Membranes Technologies/[133] |
| Diazepam and Carbamazepine | AOPs/[132] |
| Ketoprofen | AOPs/[111] |
| Ketoprofen | Ultrafiltration and photocatalysis/[139] |
| Ibuprofen | UV/Fe(III)/Oxone process/[113] |
| Ibuprofen | Photo-Fenton process/[114] |
| Ibuprofen and naproxen | Zeolite-based filters/[140,141] |
| Naproxen and ketoprofen | Carbonaceous adsorbents/[144] |
| Acetaminophen | Electro-Fenton processes/[116] |
| NSAIDs | Electro-Fenton processes/[121] |
| Naproxen | Chlorination and biofilm processes/[147] |
| Naproxen | Photocatalysis process/[120] |
| Ibuprofen | Photocatalysis process/[114] |
| Diclofenac | Photo-electrocatalytic processes/[122] |
| Ibuprofen | Oxidation & sonophotocatalytic degradation/[126] |
| Analgesics | AOPs/[127] |
| Disinfectant | AOPs/[127] |
| Antibiotics | AOPs/[127] |
References
- WHO. Pharmaceuticals in Drinking Water; WHO Library: Paris, France, 2012; ISBN 978-924-150-2085. [Google Scholar]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Taylor, D.; Senac, T. Human Pharmaceutical Products in the Environment—The “Problem” in Perspective. Chemosphere 2014, 115, 95–99. [Google Scholar] [CrossRef]
- Daughton, C.G. Non-Regulated Water Contaminants: Emerging Research. Environ. Impact Assess. Rev. 2004, 24, 711–732. [Google Scholar] [CrossRef]
- Zwiener, C. Occurrence and Analysis of Pharmaceuticals and Their Transformation Products in Drinking Water Treatment. Anal. Bioanal. Chem. 2007, 387, 1159–1162. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical Residues in Environmental Waters and Wastewater: Current State of Knowledge and Future Research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. The Presence of Pharmaceuticals in the Environment Due to Human Use—Present Knowledge and Future Challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.A.; Prados-Joya, G.; Ocampo-Perez, R. Pharmaceuticals as Emerging Contaminants and Their Removal from Water: A Review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Nassri, I.; Khattabi Rifi, S.; Sayerh, F.; Souabi, S. Occurrence, pollution sources, and mitigation prospects of Antibiotics, anti-inflammatories, and endocrine disruptors in the aquatic environment. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100878. [Google Scholar] [CrossRef]
- Hignite, C.; Azaroff, D.L. Drugs and drug metabolites as environmental contaminants: Chlorophenoxyisobutyrate and salicyclic acid in sewage water effluent. Life Sci. 1977, 20, 337. [Google Scholar] [CrossRef]
- Velpandian, T.; Halder, N.; Nath, M.; Das, U.; Moksha, L.; Gowtham, L.; Batta, S.P. Un-segregated waste disposal: An alarming threat of antimicrobials in surface and ground water sources in Delhi. Environ. Sci. Pollut. Res. Int. 2018, 25, 29518–29528. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, P.; Li, J.; Cao, Y.; Zhang, J. Phosphatidylinositol 4-kinase β is required for the ciliogenesis of zebrafish otic vesicle. J. Genet. Genom. 2020, 47, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Kock-Schulmeyer, M.; Ginebreda, A.; Gonzalez, S.; Cortina, J.L.; De Alda, M.L.; Barcelo, D. Analysis of the occurrence and risk assessment of polar pesticides in the Llobregat River Basin (NE Spain). Chemosphere 2012, 86, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Zioris, I.; Danis, T.; Bikiaris, D.; Lambropoulou, D. Comprehensive investigation of a wide range of pharmaceuticals and personal care products in urban and hospital wastewaters in Greece. Sci Total Environ. 2019, 694, 133565. [Google Scholar] [CrossRef]
- Azzouz, A.; Colón, L.P.; Souhail, B.; Ballesteros, E. A multi-residue method for GC-MS determination of selected endocrine disrupting chemicals in fish and seafood from European and North African markets. Environ. Res. 2019, 178, 108727. [Google Scholar] [CrossRef]
- Akhter, P.; Nawaz, S.; Shafiq, I.; Nazir, A.; Shafique, S.; Jamil, F.; Park, Y.-K.; Hussain, M. Efficient visible light assisted photocatalysis using ZnO/TiO2 nanocomposites. Mol. Catal. 2023, 535, 112896. [Google Scholar] [CrossRef]
- Arun, A.K.; Rustveld, L.; Sunny, A. Association between Water Fluoride Levels and Low Birth Weight: National Health and Nutrition Examination Survey (NHANES) 2013–2016. Int. J. Environ. Res. Public Health 2022, 19, 8956. [Google Scholar] [CrossRef]
- Suebi, B.; Balakrishna, K.; Joshua, D.L.; Kanan, K. Mass Loading and Removal of Pharmaceuticals and Personal Care Products Including Psychoactives, Antihypertensives and Antibiotics in Two Sewage Treatment Plants in Southern India. Chemosphere 2017, 167, 429–437. [Google Scholar] [CrossRef]
- Duan, H.; Zhao, Y.; Koch, K.; Wells, G.F.; Zheng, M.; Yuan, Z.; Ye, L. Insights into Nitrous Oxide Mitigation Strategies in Wastewater Treatment and Challenges for Wider Implementation. Environ. Sci. Technol. 2021, 55, 7208–7224. [Google Scholar] [CrossRef]
- Fu, F.; Deng, S.; Wu, D.; Liu, W.; Bai, Z. Research on the spatiotemporal evolution of land use landscape pattern in a county area based on CA-Markov model. Sustain. Cities Soc. 2022, 80, 103760. [Google Scholar] [CrossRef]
- Hanna, D.E.L.; Tomscha, S.A.; Dallaire, C.O.; Bennett, E.M. A review of riverine ecosystem service quantification: Research gaps and recommendations. J. Appl. Ecol. 2018, 55, 1299–1311. [Google Scholar] [CrossRef]
- Xiang, Y.; Lam, P.J.; Lee, J.M. Diel redox cycle of manganese in the surface Arctic Ocean. Geophys. Res. Lett. 2021, 48, e2021GL094805. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, G.; Liu, J.; Yan, Z.; Ma, B.; Zhang, Z.; Chen, W. Occurrence, bioaccumulation, and trophic magnification of pharmaceutically active compounds in Taihu Lake, China. Chemosphere 2015, 138, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bao, T.; Wei, F.; Wang, S. Zeotype porous coordination networks as potential adsorbents for removing nonsteroidal anti-inflammatory drugs from water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128401. [Google Scholar] [CrossRef]
- Afsa, S.; Hamden, K.; Lara Martin, P.A.; Mansour, H.B. Occurrence of 40 pharmaceutically active compounds in hospital and urban wastewaters and their contribution to Mahdia coastal seawater contamination. Environ. Sci. Pollut. Res. 2020, 27, 1941–1955. [Google Scholar] [CrossRef]
- Ayadi, Y.; Mokadem, N.; Besser, H.; Redhaounia, B.; Khelifi, F.; Harabi, S.; Nasri, T.; Hamed, Y. Statistical and geochemical assessment of groundwater quality in Teboursouk area (Northwestern Tunisian Atlas). Environ. Earth Sci. 2018, 77, 349. [Google Scholar] [CrossRef]
- Ashfaq, M.; Khan, K.N.; Saif, M.; Rehman, U.; Mustafa, G.; Nazar, M.F.; Sun, Q.; Iqbal, J.; Mulla, S.I.; Yu, C. Ecological risk assessment of pharmaceuticals in the receiving environment of pharmaceutical wastewater in Pakistan. Ecotoxicol. Environ. Saf. 2017, 136, 31–39. [Google Scholar] [CrossRef]
- Azanu, D.; Adu-Poku, D.; Saah, S.A.; Appaw, W.O. Prevalence of Pharmaceuticals in Surface Water Samples in Ghana. J. Chem. 2021, 2021, 7829477. [Google Scholar] [CrossRef]
- Böger, B.; Surek, M.; de O Vilhena, R.; Fachi, M.M.; Junkert, A.M.; Santos, J.M.M.F.; Domingos, E.L.; de F Cobre, A.; Momade, D.R.; Pontarolo, R. Occurrence of antibiotics and antibiotic resistant bacteria in subtropical urban rivers in Brazil. J. Hazard. Mater. 2021, 402, 123448. [Google Scholar] [CrossRef]
- Branco, G.S.; Moreira, R.G.; Borella, M.I.; de Paiva Camargo, M.; Muñoz-Peñuela, M.; Dal’Olio Gomes, A.; Tolussi, C.E. Nonsteroidal anti-inflammatory drugs act as endocrine disruptors in Astyanax lacustris (Teleostei: Characidae) reproduction: An ex vivo approach. Aquat. Toxicol. 2021, 232, 105767. [Google Scholar] [CrossRef]
- Chafi, S.; Azzouz, A.; Ballesteros, E. Occurrence and distribution of endocrine disrupting chemicals and pharmaceuticals in the river bouregreg (Rabat, Morocco). Chemosphere 2022, 287, 132202. [Google Scholar] [CrossRef]
- Chaib, O.; Arhoune, B.; Achour, S.; Moreau-Guigon, E.; Alliot, F.; Chevreuil, M.; El Fakir, S.; El Arabi, I.; Oumokhtar, B. Occurrence and Seasonal Variation of Antibiotics in Fez-Morocco Surface Water. Am. J. Environ. Sci. 2019, 15, 127–136. [Google Scholar] [CrossRef]
- Jari, Y.; Roche, N.; Necibi, M.C.; El Hajjaji, S.; Dhiba, D.; Chehbouni, A. Emerging Pollutants in Moroccan Wastewater: Occurrence, Impact, and Removal Technologies. J. Chem. 2022, 2022, 9727857. [Google Scholar] [CrossRef]
- Hernández-Tenorio, R. Pharmaceutical active compounds at drugs manufacturing wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100870. [Google Scholar] [CrossRef]
- Guruge, K.S.; Goswami, P.; Tanoue, R.; Nomiyama, K.; Wijesekara, R.G.S.; Dharmaratne, T.S. First nationwide investigation and environmental risk assessment of 72 pharmaceuticals and personal care products from Sri Lankan surface waterways. Sci. Total Environ. 2019, 690, 683–695. [Google Scholar] [CrossRef]
- Kairigo, P.; Ngumba, E.; Sundberg, L.-R.; Gachanja, A.; Tuhkanen, T. Occurrence of antibiotics and risk of antibiotic resistance evolution in selected Kenyan wastewaters, surface waters and sediments. Sci. Total Environ. 2020, 720, 137580. [Google Scholar] [CrossRef]
- Munzhelele, E.P.; Mudzielwana, R.; Ayinde, W.B.; Gitari, W.M. Pharmaceutical contaminants in wastewater and receiving water bodies of South Africa: A review of sources, pathways, occurrence, effects, and geographical distribution. Water 2024, 16, 796. [Google Scholar] [CrossRef]
- Khumalo, S.M.; Makhathini, T.P.; Bwapwac, J.K.; Bakare, B.F.; Rathilal, S. The occurrence and fate of antibiotics and nonsteroidal anti-inflammatory drugs in water treatment processes: A review. J. Hazard. Mater. Adv. 2023, 10, 100330. [Google Scholar] [CrossRef]
- Huynh, N.C.; Nguyen, T.T.T.; Nguyen, D.T.C.; Tran, T.V. Occurrence, toxicity, impact and removal of selected non-steroidal anti-inflammatory drugs (NSAIDs): A review. Sci. Total Environ. 2023, 898, 165317. [Google Scholar] [CrossRef]
- Lozano, I.; Pérez-Guzmán, C.J.; Mora, A.; Mahlknecht, J.; López Aguilar, C.; Cervantes-Avilés, P. Pharmaceuticals and personal care products in water streams: Occurrence, detection, and removal by electrochemical advanced oxidation processes. Sci. Total Environ. 2022, 827, 154348. [Google Scholar] [CrossRef]
- Kumar, R.; Qureshi, M.; Vishwakarma, D.K.; Al-Ansari, N.; Kuriqi, A.; Elbeltagi, A.; Saraswat, A. A review on emerging water contaminants and the application of sustainable removal technologies. Case Stud. Chem. Environ. Eng. 2022, 6, 100219. [Google Scholar] [CrossRef]
- Koumaki, E.; Mamais, D.; Noutsopoulos, C. Environmental fate of non-steroidal anti-inflammatory drugs in river water/sediment systems. J. Hazard. Mater. 2017, 323, 233–241. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Cui, H.; Gao, S.; Ye, L.; Li, Z.; Nie, S.; Han, J.; Wang, A.; Liang, B. Environmental occurrence, risk, and removal strategies of pyrazolones: A critical review. J. Hazard. Mater. 2023, 460, 132471. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Jin, Y.; Chen, Y.; Li, Y.; Chen, J.; Zhuang, X.; Mo, P.; Liu, H.; Chen, P.; Lv, W.; et al. Leaf-like ionic covalent organic framework for the highly efficient and selective removal of non-steroidal anti-inflammatory drugs: Adsorption performance and mechanism insights. J. Colloid Interface Sci. 2023, 645, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Yuan, S.; Li, Z.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Degradation of the anti-inflammatory drug ibuprofen by electro-peroxone process. Water Res. 2014, 63, 81–93. [Google Scholar] [CrossRef]
- Ayati, A.; Tanhaei, B.; Beiki, H.; Krivoshapkin, P.; Krivoshapkina, E.; Tracey, C. Insight into the adsorptive removal of ibuprofen using porous carbonaceous materials: A review. Chemosphere 2023, 323, 138241. [Google Scholar] [CrossRef]
- Al-Khateeb, L.A.; Hakami, W.; Salam, M.A. Removal of non-steroidal anti-inflammatory drugs from water using high surface area nanographene: Kinetic and thermodynamic studies. J. Mol. Liq. 2017, 241, 733–741. [Google Scholar] [CrossRef]
- Phasuphan, W.; Praphairaksit, N.; Imyim, A. Removal of ibuprofen, diclofenac, and naproxen from water using chitosan-modified waste tire crumb rubber. J. Mol. Liq. 2019, 294, 111554. [Google Scholar] [CrossRef]
- Yu, T.-H.; Lin, A.Y.-C.; Lateef, S.K.; Lin, C.-F.; Yang, P.-Y. Removal of antibiotics and non-steroidal anti-inflammatory drugs by extended sludge age biological process. Chemosphere 2009, 77, 175–181. [Google Scholar] [CrossRef]
- Kim, M.; Njaramba, L.K.; Yoon, Y.; Jang, M.; Park, C.M. Thermally-activated gelatin–chitosan–MOF hybrid aerogels for efficient removal of ibuprofen and naproxen. Carbohydr. Polym. 2024, 324, 121436. [Google Scholar] [CrossRef]
- Tan, C.; Cui, X.; Sun, K.; Xiang, H.; Du, E.; Deng, L.; Gao, H. Kinetic mechanism of ozone activated peroxymonosulfate system for enhanced removal of anti-inflammatory drugs. Sci. Total Environ. 2020, 733, 139250. [Google Scholar] [CrossRef]
- OECD. Pharmaceutical Residues in Freshwater Hazards and Policy Responses; OECD: Paris, France, 2019. [Google Scholar]
- Weber, F.-A.; Beek, T.; Bergmann, A.; Carius, A.; Grüttner, G.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A.; Rose, J.; et al. Pharmaceuticals in the Environment—The Global Perspective. Occurrence, Effects, and Potential Cooperative Action Under SAICM; German Federal Environmental Agency: Dessau-Roßlau, Germany, 2014.
- BIO Intelligence Service. Study on the Environmental Risks of Medicinal Products; Final Report Prepared for Executive Agency for Health and Consumers; BIO Intelligence Service: Paris, France, 2013. [Google Scholar]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones and Other Organic Wastewater Contaminants in U.S. Streams, 1999–2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar]
- Beek, T.; Weber, F.-A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment—Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef]
- Wise, R. Antimicrobial resistance: Priorities for action. J. Antimicrob. Chemother. 2002, 49, 585–586. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Lindim, C.; van Gils, J.; Cousins, I.T.; Kühne, R.; Georgieva, D.; Kutsarova, S.; Mekenyan, O. Model-predicted occurrence of multiple pharmaceuticals in Swedish surface waters and their flushing to the Baltic Sea. Environ. Pollut. 2017, 223, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Bound, J.P.; Voulvoulis, N. Predicted and measured concentrations for selected pharmaceuticals in UK rivers: Implications for risk assessment. Water Res. 2006, 40, 2885–2892. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the Biological and Chemical Treatment Technologies for Emerging Contaminant Removal from Wastewater: A Critical Review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef]
- Joss, A.; Zabczynski, S.; Göbel, A.; Hoffmann, B.; Löffler, D.; McArdell, C.S.; Ternes, T.A.; Thomsen, A.; Siegrist, H. Biological Degradation of Pharmaceuticals in Municipal Wastewater Treatment: Proposing a Classification Scheme. Water Res. 2006, 40, 1686–1696. [Google Scholar] [CrossRef]
- United Nations. World Contraceptive Use; United Nations: New York, NY, USA, 2004. [Google Scholar]
- Meghea, I. Statistical Methods and Models for Pollutant Control in Municipal Surface Waters. Water 2023, 15, 4178. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Tian, W.; Li, C.; Yang, Y. Characterizing Long-Term Water Quality Variation with Multivariate Statistical Methods: A Case Study in the Two Tributaries of Yellow River, China. Environ. Eng. Manag. J. 2022, 21, 1117–1125. [Google Scholar] [CrossRef]
- Ismail, A.; Robescu, D. Application of Multivariate Statistical Techniques in Water Quality Assessment of Danube River, Romania. Environ. Eng. Manag. J. 2019, 18, 719–726. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, M.; Pandita, S.; Kumar, V.; Kour, J.; Sharma, N. Assessment of water quality using different pollution indices and multivariate statistical techniques. In Heavy Metals in the Environment. Impact, Assessment, and Remediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 165–178. [Google Scholar]
- Hue, N.H.; Thanh, N.H. Assessment of Surface Water Quality by Using Multivariate Statistical Analysis Techniques: A Case Study of Nhue River, Vietnam. Int. J. Environ. Sci. Dev. 2020, 11, 488–492. [Google Scholar] [CrossRef]
- da Silva, D.F.M.; Silva, L.M.L.; Gamier, J.; Araújo, D.F.; da Silva, L.A.; Mulholland, D.S. Linking Multivariate Statistical Methods and Water Quality Indices to Evaluate the Natural and Anthropogenic Geochemical Processes Controlling the Water Quality of a Tropical Watershed. Environ. Monit. Assess. 2023, 195, 1240. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.S.; Faroon, M.A.; Yousif, Y.T. The use of multivariate statistical techniques in the assessment of river water quality. Anbar J. Eng. Sci. 2020, 11, 102–112. [Google Scholar] [CrossRef]
- Abed, S.A.; Ewaid, S.H.; Al-Ansari, N. Evaluation of Water quality in the Tigris River within Baghdad, Iraq using Multivariate Statistical Techniques. IOP Conf. Ser. J. Phys. 2019, 1294, 072025. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, G.; Yu, R. Assessment of Surface Water Quality using Multivariate Statistical Techniques: A Case Study in China. Irrigat. Drain. Sys. Eng. 2018, 7, 1000214. [Google Scholar]
- Nguyen, H.Q.; Vuong, Q.P.; Pham-Thi, N.A.; Tran-Thi, T.T.; Ho, T.P.; Lam, S.H.T. Assessment of Surface Water Quality Using Multivariate Statistical Techniques: A Case Study of Saigon River. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, D.; Song, S.; Sun, J. Assessments of surface water quality through the use of multivariate statistical tech-niques: A case study for the watershed of the Yuqiao Reservoir, China. Front. Environ. Sci. 2023, 11, 1107591. [Google Scholar]
- Liu, J.; Zhang, D.; Tang, Q.; Xu, H.; Huang, S.; Shang, D.; Liu, R. Water quality assessment and source identification of the Shuangji River (China) using multivariate statistical methods. PLoS ONE 2021, 16, e0245525. [Google Scholar] [CrossRef]
- Özdemir, Ö. Application of Multivariate Statistical Methods for Water Quality Assessment of Karasu-Sarmisakli Creeks and Kizilirmak River in Kayseri, Turkey. Pol. J. Environ. Stud. 2016, 25, 1149–1160. [Google Scholar] [CrossRef]
- Ma, X.; Wang, L.; Yang, H.; Li, N.; Gong, C. Spatiotemporal Analysis of Water Quality Using Multivariate Statistical Tech-niques and the Water Quality Identification Index for the Qinhuai River Basin, East China. Water 2020, 12, 2764. [Google Scholar] [CrossRef]
- Gyimah, R.A.A.; Gyamfi, C.; Anornu, G.K.; Karikari, A.Y.; Tsyawo, F.W. Multivariate statistical analysis of water quality of the Densu River, Ghana. Int. J. River Basin Manag. 2020, 19, 189–199. [Google Scholar] [CrossRef]
- Dawood, A.S.; Jabbar, M.T.; Al-Temeemi, H.H.; Baer, E.M. Application of Water Quality Index and Multivariate Statistical Techniques to Assess and Predict of Groundwater Quality with Aid of Geographic Information System. J. Ecol. Eng. 2022, 23, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Koklu, R.; Sengorur, B.; Topal, B. Water Quality Assessment Using Multivariate Statistical Methods—A Case Study: Melen River System (Turkey). Water Resour. Manag. 2010, 24, 959–978. [Google Scholar] [CrossRef]
- Leventeli, Y.; Yalcin, F. Data analysis of heavy metal content in riverwater: Multivariate statistical analysis and inequality expressions. J. Inequal Appl. 2021, 2021, 14. [Google Scholar] [CrossRef]
- Gholikandi, G.B.; Orumieh, H.R.; Haddadi, S.; Mojir, N. Application of multivariate statistical techniques for surface water quality assessment: Case study of Karaj River, Iran. WIT Transactions on Ecology and the Environment. Water Resour. Manag. 2011, 145, 361–370. [Google Scholar]
- Mohammed, A.; Samara, F.; Alzaatreh, A.; Knuteson, S.L. Statistical Analysis for Water Quality Assessment: A Case Study of Al Wasit Nature Reserve. Water 2022, 14, 3121. [Google Scholar] [CrossRef]
- Bărbulescu, A.; Dumitriu, C.Ş. Assessing Water Quality by Statistical Methods. Water 2021, 13, 1026. [Google Scholar] [CrossRef]
- Schreiber, S.G.; Schreiber, S.; Tanna, R.N.; Roberts, D.R.; Arciszewski, T.J. Statistical tools for water quality assessment and monitoring in river ecosystems—A scoping review and recommendations for data analysis. Water Qual. Res. 2022, 57, 40. [Google Scholar] [CrossRef]
- Kovrov, O.; Kulikova, D.; Pavlychenko, A. Statistical analysis of Samara River pollution impact on the population morbidity rate in Western Donbas (Ukraine). In Proceedings of the IV International Conference “ESSAYS OF MINING SCIENCE AND PRACTICE” (RMGET-2022), Dnipro, Ukraine, 9–11 November 2022; Volume 1156, p. 012025. [Google Scholar]
- Antonopoulos, V.Z.; Papamichail, D.M.; Mitsiou, K.A. Statistical and trend analysis of water quality and quantity data for the Strymon River in Greece. Hydrol. Earth Syst. Sci. 2001, 5, 679–691. [Google Scholar] [CrossRef]
- Majerek, D.; Duda, S.; Babko, R.; Widomski, M.K. Statistical analysis of the water pollution indicators pertaining to treated municipal sewage introduced to the river. MATEC Web Conf. 2019, 252, 09009. [Google Scholar] [CrossRef]
- Stričević, L.; Pavlović, M.; Filipović, I.; Radivojević, A.; Bursać, N.M.; Gocić, M. Statistical analysis of water quality parameters in the basin of the Nišava River (Serbia) in the period 2009–2018. Geografie 2021, 126, 55–73. [Google Scholar] [CrossRef]
- Khouri, L.; Al-Muft, M.B. Assessment of surface water quality using statistical analysis methods: Orontes River (Case study). Baghdad Sci. J. 2022, 19, 981–989. [Google Scholar] [CrossRef]
- Bhat, S.A.; Meraj, G.; Yaseen, S.; Pandit, A.K. Statistical Assessment of Water Quality Parameters for Pollution Source Identification in Sukhnag Stream: An Inflow Stream of Lake Wular (Ramsar Site), Kashmir Himalaya. J. Ecosyst. 2014, 2014, 898054. [Google Scholar] [CrossRef]
- Le, R.K.; Rackauckas, C.V.; Ross, A.S.; Ulloa, N. Assessment of Statistical Methods for Water Quality Monitoring in Maryland’s Tidal Waterways REU Site: Interdisciplinary Program in High Performance Computing. 2012. Available online: www.umbc.edu/hpcreu (accessed on 6 July 2024).
- Andrejiová, M.; Kimáková, Z.; Knežo, D. Analysis of Water Pollution Indicators with the Use of Selected Statistical Methods. Acta Mech. Slovaca 2011, 15, 52–61. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Wen, J.; Wang, S.; Zhu, M. Monitoring and statistical methods of irrigation water consumption of Wanyao Irrigation Area. In Proceedings of the 2020 4th International Workshop on Renewable Energy and Development (IWRED 2020), Sanya, China, 24–26 April 2020; Volume 510, p. 032025. [Google Scholar]
- Fu, L.; Wang, Y.G. Statistical Tools for Analyzing Water Quality Data. In Water Quality Monitoring and Assessment; Voudouris, K., Voutsa, D., Eds.; IntechOpen: Rijeka, Croatia, 2012; pp. 143–168. Available online: https://www.intechopen.com/chapters/35050 (accessed on 5 May 2024).
- Helsel, D.R.; Hirsch, R.M. Statistical Methods in Water Resources. In Techniques of Water-Resources Investigations of the United States Geological Survey; Book 4, Hydrologic Analysis and Interpretation; USGS Science for a Changing World, Chapter A3; USGS: Reston, VA, USA, 2002. [Google Scholar]
- Bal, K.J. Statistical Analysis for the Quality of Water. Master’s Thesis, Institute of Applied Statistics, Johannes Kepler University, Linz, Austria, 2023. [Google Scholar]
- Hajigholizadeh, M. Water Quality Modelling Using Multivariate Statistical Analysis and Remote Sensing in South Florida. Ph.D. Thesis, Florida International University, Miami, FL, USA, 2016. [Google Scholar]
- Meghea, I.; Lesnic, M.; Manea-Saghin, A.-M. Innovative technological solutions for removal of pharmaceutical contaminants from water sources. Proc. Int. Struct. Eng. Constr. 2024, 11, AAW-01. [Google Scholar] [CrossRef]
- Kayode-Afolayan, S.D.; Ahuekwe, E.F.; Nwinyi, O.C. Impacts of pharmaceutical effluents on aquatic ecosystems. Sci. Afr. 2022, 17, e01288. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Narvaez V, J.F.; Jimenez C, C. Pharmaceutical products in the environment: Sources, effects and risks. Vitae 2012, 19, 93–108. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Barros, R. Pharmaceuticals in water: Risks to aquatic life and remediation strategies. Hydrobiology 2023, 2, 395–409. [Google Scholar] [CrossRef]
- Mezzelani, M.; Notarstefano, V.; Panni, M.; Giorgini, E.; Gorbi, S.; Regoli, F. Exposure to environmental pharmaceuticals afects the macromolecular composition of mussels digestive glands. Sci. Rep. 2024, 14, 9369. [Google Scholar] [CrossRef]
- Ashiwaju, B.I.; Uzougbo, C.G.; Orikpete, O.F. Environmental impact of pharmaceuticals: A comprehensive review. Matrix Sci. Pharma (MSP) 2023, 7, 85–94. [Google Scholar] [CrossRef]
- Manole, F.; Marian, P.; Mekeres, G.M.; Csep, A.N. A review of the effects of pharmaceutical waste on the environment and human health. Pharmacophore 2023, 14, 106–110. [Google Scholar] [CrossRef]
- Ortúzar, M.; Esterhuizen, M.; Olicón-Hernández, D.R.; González-López, J.; Aranda, E. Pharmaceutical pollution in aquatic environments: A concise review of environmental impacts and bioremediation systems. Front. Microbiol. 2022, 13, 869332. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.L.; Dias Costa, C.S.; da Silva, M.G.C.; Adeodato Vieira, M.G. Overview of non-steroidal anti-inflammatory drugs degradation by advanced oxidation processes. J. Clean. Prod. 2022, 346, 131226. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, H.; Zhang, Z.; Yang, W.; Sun, J.; Liu, Y.; Xia, M.; Jiang, Q. Construction of a photocatalytic-membrane separation coupled system via click chemistry-synthesized TiO2/biochar composites for ranitidine degradation in water: Mechanisms, pathways, and theoretical calculations. Sep. Purif. Technol. 2025, 371, 133327. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Mei, Q.; Qiu, Z.; Jiang, J.; Li, M.; Wang, Q.; He, M. Ozonolysis of ketoprofen in polluted water: Reaction pathways, kinetics, removal efficiency, and health effects. J. Environ. Sci. 2025, 147, 451–461. [Google Scholar] [CrossRef]
- Available online: https://en.wikipedia.org/wiki/Ketoprofen#/media/File:Ketoprofen_ball-and-stick.png (accessed on 14 August 2025).
- Rao, Y.F.; Xue, D.; Pan, H.; Feng, J.; Li, Y. Degradation of ibuprofen by a synergistic UV/Fe(III)/Oxone process. Chem. Eng. J. 2016, 283, 65–75. [Google Scholar] [CrossRef]
- Loaiza-Ambuludi, S.; Panizza, M.; Oturan, N.; Oturan, M.A. Removal of the anti-inflammatory drug ibuprofen from water using homogeneous photocatalysis. Catal. Today 2014, 224, 29–33. [Google Scholar] [CrossRef]
- Ibuprofen-3D-balls. Ibuprofen. Wikipedia. Available online: https://commons.wikimedia.org/wiki/File:Ibuprofen-3D-balls.png (accessed on 14 August 2025).
- de Luna, M.D.G.; Veciana, M.L.; Su, C.-C.; Lu, M.-C. Acetaminophen degradation by electro-Fenton and photoelectro-Fenton using a double cathode electrochemical cell. J. Hazard. Mater. 2012, 217–218, 200–207. [Google Scholar] [CrossRef]
- Available online: https://en.wikipedia.org/wiki/Paracetamol#/media/File:Paracetamol-from-xtal-3D-balls.png (accessed on 14 August 2025).
- Chin, C.-J.M.; Chen, T.-Y.; Lee, M.; Chang, C.-F.; Liu, Y.-T.; Kuo, Y.-T. Effective anodic oxidation of naproxen by platinum nanoparticles coated FTO glass. J. Hazard. Mater. 2014, 277, 110–119. [Google Scholar] [CrossRef]
- Available online: https://upload.wikimedia.org/wikipedia/commons/2/2b/%28S%29-naproxen-from-xtal-3D-bs-17.png (accessed on 14 August 2025).
- Coria, G.; Sirés, I.; Brillas, E.; Nava, J.L. Influence of the anode material on the degradation of naproxen by Fenton-based electrochemical processes. Chem. Eng. J. 2016, 304, 817–825. [Google Scholar] [CrossRef]
- Mohammadi, H.; Bina, B.; Ebrahimi, A. A novel three-dimensional electro-Fenton system and its application for degradation of anti-inflammatory pharmaceuticals: Modeling and degradation pathways. Process Saf. Environ. Prot. 2018, 117, 200–213. [Google Scholar] [CrossRef]
- Cerro-Lopez, M.; Castro-Pastrana, L.I.; Campos-Delgado, J.; Rubio-Rosas, E.; Bustos, E.; Martínez-Huitle, C.A. Mesostructured lead dioxide grown on titania nanotubes for diclofenac water removal through electrocatalytic and photoelectrocatalytic processes. Environ. Res. 2023, 231, 116094. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://upload.wikimedia.org/wikipedia/commons/1/1f/Diclofenac_3D_2ek.png (accessed on 14 August 2025).
- Rahman, K.O.; Hama Aziz, K.H. Utilizing scrap printed circuit boards to fabricate efficient Fenton-like catalysts for the removal of pharmaceutical diclofenac and ibuprofen from water. J. Environ. Chem. Eng. 2022, 10, 109015. [Google Scholar] [CrossRef]
- Tra, V.T.; Pham, V.T.; Tran, T.-D.; Tran, T.H.; Tran, T.K.; Nguyen, T.P.T.; Nguyen, V.T.; Dao, T.-V.-H.; Tran, P.-Y.-N.; Le, V.-G.; et al. Enhance diclofenac removal in wastewater by photocatalyst process combination with hydrogen peroxide. Case Stud. Chem. Environ. Eng. 2023, 8, 100506. [Google Scholar] [CrossRef]
- Madhavan, J.; Grieser, F.; Ashokkumar, M. Combined advanced oxidation processes for the synergistic degradation of ibuprofen in aqueous environments. J. Hazard. Mater. 2010, 178, 202–208. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Biruntha, M.; Govarthanan, M.; Karmegam, N. Removal of emerging micropollutants originating from pharmaceuticals and personal care products (PPCPs) in water and wastewater by advanced oxidation processes: A review. Environ. Technol. Innov. 2021, 23, 101757. [Google Scholar] [CrossRef]
- Feng, L.; van Hullebusch, E.D.; Rodrigo, M.A.; Esposito, G.; Oturan, M.A. Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chem. Eng. J. 2013, 228, 944–964. [Google Scholar] [CrossRef]
- Kim, S.; Eichhorn, P.; Jensen, J.N.; Weber, A.S.; Aga, D.S. Removal of antibiotics in wastewaters: Effect of hydraulic and solid retention times on the fate of tetracycline in the active sludge process. Environ. Sci. Technol. 2005, 39, 5816–5823. [Google Scholar] [CrossRef]
- Jones, O.A.; Lester, J.N.; Voulvoulis, N. Pharmaceuticals: A threat for drinking water? Trends Biotechnol. 2005, 23, 163–167. [Google Scholar] [CrossRef] [PubMed]
- DEFRA. Desk Based Review of Current Knowledge on Pharmaceuticals in Drinking Water and Estimation of Potential Levels; DWI 70/2/213; DEFRA: London, UK, 2007.
- Ternes, T.A.; Joss, A.; Siegrist, H. Peer reviewed: Scrutinizing pharmaceuticals and personal care products in wastewater treatment. Environ. Sci. Technol. 2004, 38, 392A–399A. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.M.; Canonica, S.; Park, G.-Y.; von Gunten, U. Oxidation of pharmaceuticals during ozonation and advanced oxidation processes. Environ. Sci. Technol. 2005, 37, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Rosman, N.; Salleh, W.N.W.; Mohamed, M.A.; Jaafar, J.; Ismail, A.F.; Harun, Z. Hybrid membrane filtration—Advanced oxidation processes for removal of pharmaceutical residues. J. Colloid Interface Sci. 2018, 532, 236–260. [Google Scholar] [CrossRef]
- Drewes, J.E.; Heberer, T.; Rauch-Williams, T.; Reddersen, K. Fate of pharmaceuticals during ground water recharge. Groundw. Monit. Remediat. 2003, 23, 64–72. [Google Scholar] [CrossRef]
- Radjenović, J.; Petrović, M.; Ventura, F.; Barceló, D. Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 2008, 42, 3601–3610. [Google Scholar] [CrossRef]
- Qurie, M.; Khamis, M.; Scrano, L.; Bufo, S.A.; Mecca, G.; Karaman, R. Removal of Two NSAIDs: Naproxen and Diclofenac and a Heavy Metal Cr (VI) by Advanced Membranes Technology. Case Stud. J. 2015, 4, 51–63. [Google Scholar]
- Meghea, I.; Lesnic, M.; Manea-Saghin, A.-M. Design solutions for rehabilitation of a water treatment plant. Proc. Int. Struct. Eng. Constr. 2024, 11, AAW-02. [Google Scholar] [CrossRef]
- Szymanski, K.; Mozia, S. Submerged photocatalytic membrane reactor utilizing ultrafiltration for ketoprofen removal from surface water. Chem. Eng. Process. Process Intensif. 2023, 183, 109251. [Google Scholar] [CrossRef]
- Smiljanić, D.; de Gennaro, B.; Daković, A.; Galzerano, B.; Germinario, C.; Izzo, F.; Rottinghaus, G.E.; Langella, A. Removal of non-steroidal anti-inflammatory drugs from water by zeolite-rich composites: The interference of inorganic anions on the ibuprofen and naproxen adsorption. J. Environ. Manag. 2021, 286, 112168. [Google Scholar] [CrossRef]
- Cuomo, M.; Konig, R.; Zanardini, E.; Di Guardo, A.; Bianchi, G.; Ortona, A.; Principi, P. Using zeolite filters to reduce activated carbon use in micropollutant removal from wastewater. J. Water Process Eng. 2023, 56, 104298. [Google Scholar] [CrossRef]
- Ishak, F.A.; Razak, A.S.A.; Krishnan, S.; Zularisama, A.W.; Nasrullah, M. Water hyacinth (Eichhornia crassipes) for organic contaminants removal in water—A review. J. Hazard. Mater. Adv. 2022, 7, 100092. [Google Scholar]
- Priyan V, V.; Narayanasamy, S. Effective removal of pharmaceutical contaminants ibuprofen and sulfamethoxazole from water by Corn starch nanoparticles: An ecotoxicological assessment. Environ. Toxicol. Pharmacol. 2022, 94, 103930. [Google Scholar] [CrossRef] [PubMed]
- Cuerda-Correa, E.M.; Domínguez-Vargas, J.R.; Olivares-Marín, F.J.; de Heredia, J.B. On the use of carbon blacks as potential low-cost adsorbents for the removal of non-steroidal anti-inflammatory drugs from river water. J. Hazard. Mater. 2010, 177, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Rozaini, M.N.H.; Semail, N.-F.; Zango, Z.U.; Lim, J.W.; Yahaya, N.; Setiabudi, H.D.; Tong, W.-Y.; Shamsuddin, R.; Chan, Y.J.; Khoo, K.S.; et al. Advanced adsorptions of non-steroidal anti-inflammatory drugs from environmental waters in improving offline and online preconcentration techniques: An analytical review. J. Taiwan Inst. Chem. Eng. 2023, 166, 105020. [Google Scholar] [CrossRef]
- Valadez-Renteria, E.; Perez-Gonzalez, R.; Gomez-Solis, C.; Diaz-Torres, L.A.; Encinas, A.; Oliva, J.; Rodriguez-Gonzalez, V. A novel and stretchable carbon-nanotube/Ni@TiO2: W photocatalytic composite for the complete removal of diclofenac drug from the drinking water. J. Environ. Sci. 2023, 126, 575–589. [Google Scholar] [CrossRef]
- Boyd, G.R.; Zhang, S.; Grimm, D.A. Naproxen removal from water bychlorination and biofilm processes. Water Res. 2005, 39, 668–676. [Google Scholar] [CrossRef]
- Sarici-Özdemir, Ç.; Önal, Y. Study to investigate the importance of mass transfer of naproxen sodium onto activated carbon. Chem. Eng. Process. 2010, 49, 1058–1065. [Google Scholar] [CrossRef]
- Martín, I.; Renones, P.; Rodríguez-Chueca, J.; de la Peña O’Shea, V.A.; Fresno, F.; García-Muñoz, P. Independent and simultaneous photocatalytic decontamination and disinfection of water over In2O3/TiO2 heterojunctions. Catal. Today 2024, 426, 114397. [Google Scholar] [CrossRef]
- Mustafa, F.S.; Hama Aziz, K.H. Heterogeneous catalytic activation of persulfate for the removal of rhodamine B and diclofenac pollutants from water using iron-impregnated biochar derived from the waste of black seed pomace. Process Saf. Environ. Prot. 2023, 170, 436–448. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. Positively charged covalent organic framework modified magnetic chitosan as a smart device for efficient diclofenac sodium removal from water. Chem. Eng. J. 2023, 452, 139557. [Google Scholar] [CrossRef]
- Joodaki, S.; Mollahosseini, A. Evaluation modified luffa with silver nanoparticles (LF/AgNPs) for removal of a nonsteroidal anti-inflammatory (IBUPROFEN) from aqueous media. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100823. [Google Scholar] [CrossRef]
- Ahmad, F.A. The use of agro-waste-based adsorbents as sustainable, renewable, and low-cost alternatives for the removal of ibuprofen and carbamazepine from water. Heliyon 2023, 9, e16449. [Google Scholar] [CrossRef] [PubMed]
- Amaral, E.T.; Castro Bender, L.B.Y.; Rizzetti, T.M.; de Cassia de Souza Schneider, R. Removal of organic contaminants in water bodies or wastewater by microalgae of the genus Chlorella: A review. Case Stud. Chem. Environ. Eng. 2023, 8, 100476. [Google Scholar] [CrossRef]
- Hashim, N.M.; Mohamad, M.; Kamal, N.N.S.N.M.; Aziz, M.Y.; Mohamad, S.; Yahaya, N.; Zain, N.N.M. Revolutionizing drug removal: Green magnetic nanoparticles functionalized with hydrophobic deep eutectic solvents for anti-inflammatory drug adsorption. J. Mol. Liq. 2023, 383, 122082. [Google Scholar] [CrossRef]
- Noutsopoulos, C.; Mamais, D.; Mpouras, T.; Kokkinidou, D.; Samaras, V.; Antoniou, K.; Gioldasi, M. The role of activated carbon and disinfection on the removal of endocrine disrupting chemicals and non-steroidal anti-inflammatory drugs from wastewater. Environ. Technol. 2014, 35, 698–708. [Google Scholar] [CrossRef]
- Jiménez-Bambague, E.M.; Villarreal-Arias, D.S.; Ramírez-Vanegas, O.D.; Gómez-Gómez, D.D.; Madera-Parra, C.A.; Peña-Salamanca, E.J.; Mota-Filho, C.R.; Machuca-Martínez, F. Removal of pharmaceutical compounds from real urban wastewater by a continuous bio-electrochemical process at pilot scale. J. Environ. Chem. Eng. 2023, 11, 110130. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Lo, S.-L.; Liou, Y.-H.; Hu, C.-Y. Removal of nonsteroidal anti-inflammatory drugs (NSAIDs) by electrocoagulation–flotation with a cationic surfactant. Sep. Purif. Technol. 2015, 152, 148–154. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ekpe, O.D.; Lee, H.-J.; Oh, J.-E. Perfluoroalkyl substances and pharmaceuticals removal in full-scale drinking water treatment plants. J. Hazard. Mater. 2020, 400, 123235. [Google Scholar] [CrossRef]
- Malesic-Eleftheriadou, N.; Evgenidou, E.; Lazaridou, M.; Bikiaris, D.N.; Yang, X.; Kyzas, G.Z.; Lambropoulou, D.A. Simultaneous removal of anti-inflammatory pharmaceutical compounds from an aqueous mixture with adsorption onto chitosan zwitterionic derivative. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126498. [Google Scholar] [CrossRef]
- Malesic-Eleftheriadou, N.; Trikkaliotis, D.G.; Evgenidou, E.; Kyzas, G.Z.; Bikiaris, D.N.; Lambropoulou, D.A. New biobased chitosan/polyvinyl alcohol/graphene oxide derivatives for the removal of pharmaceutical compounds from aqueous mixtures. J. Mol. Liq. 2023, 387, 122673. [Google Scholar] [CrossRef]
- Zaka, A.; Ibrahim, T.H.; Khamis, M. Removal of selected non-steroidal anti-inflammatory drugs from wastewater using reduced graphene oxide magnetite. Desalination Water Treat. 2021, 212, 401–414. [Google Scholar] [CrossRef]
- Selvakumar, R.; Guhananthan, A.; Palanisami, T. Recent advances in micropollutant removal and mitigation from water using three dimensional adsorbent materials. Curr. Opin. Environ. Sci. Health 2023, 34, 100475. [Google Scholar] [CrossRef]
- Koltsakidou, A.; Terzopoulou, Z.; Liakos, E.V.; Evgenidou, E.; Lambropoulou, D.A.; Bikiaris, D.N.; Kyzas, G.Z. Colloids and Surfaces A: Physicochemical and Engineering Aspects. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127382. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Zhang, Y.; Ma, J.; Huang, L.; Yu, S.; Chen, L.; Song, G.; Qiu, M.; Wang, X. Applications of water-stable metal-organic frameworks in the removal of water pollutants: A review. Environ. Pollut. 2021, 291, 118076. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.; Song, J.Y.; Jhung, S.H. Adsorptive removal of anti-inflammatory drugs from water using graphene oxide/metal-organic framework composites. Chem. Eng. J. 2018, 335, 74–81. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Zhang, Y.; Bian, Y.; Zhang, Y.-X.; Du, R.-Z.; Li, M.; Tan, Y.; Feng, X.-S. Non-steroidal anti-inflammatory drugs (NSAIDs) in the environment: Recent updates on the occurrence, fate, hazards and removal technologies. Sci. Total Environ. 2023, 904, 166897. [Google Scholar] [CrossRef]
- Mlunguza, N.Y.; Ncube, S.; Mahlambi, P.N.; Chimuka, L.; Madikizela, L.M. Adsorbents and removal strategies of non-steroidal anti-inflammatory drugs from contaminated water bodies. J. Environ. Chem. Eng. 2019, 7, 103142. [Google Scholar] [CrossRef]
- Barasarathi, J.; Abdullah, P.S.; Uche, E.C. Application of magnetic carbon nanocomposite from agro-waste for the removal of pollutants from water and wastewater. Chemosphere 2022, 305, 135384. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Soltys, L.; Macyk, W. Magnetic adsorbents for removal of pharmaceuticals: A review of adsorption properties. J. Mol. Liq. 2023, 384, 122174. [Google Scholar] [CrossRef]
- Tavares Costa, R.L.; do Nascimento, R.A.; Santos de Araújo, R.C.; Adeodato Vieira, M.G.; da Silva, M.G.C.; de Carvalho, S.M.L.; de Faria, L.J.G. Removal of non-steroidal anti-inflammatory drugs (NSAIDs) from water with activated carbons synthetized from waste murumuru (Astrocaryum murumuru Mart.): Characterization and adsorption studies. J. Mol. Liq. 2021, 343, 116980. [Google Scholar] [CrossRef]
- Gallardo-Altamirano, M.J.; Maza-Márquez, P.; Peña-Herrera, J.M.; Rodelas, B.; Osorio, F.; Pozo, P. Removal of anti-inflammatory/analgesic pharmaceuticals from urban wastewater in a pilot-scale A2O system: Linking performance and microbial population dynamics to operating variables. Sci. Total Environ. 2018, 643, 1481–1492. [Google Scholar] [CrossRef]
- Michelon, A.; Bortoluz, J.; Raota, C.S.; Giovanela, M. Agro-industrial residues as biosorbents for the removal of anti-inflammatories from aqueous matrices: An overview. Environ. Adv. 2022, 9, 100261. [Google Scholar] [CrossRef]
- Kaur, A.; Umar, A.; Kansal, S.K. Heterogeneous photocatalytic studies of analgesic and non-steroidal anti-inflammatory drugs. Appl. Catal. A Gen. 2016, 510, 134–155. [Google Scholar] [CrossRef]
- Valadez-Renteria, E.; Perez-Carrasco, C.; Medina-Velazquez, D.Y.; Rodriguez-Gonzalez, V.; Oliva, J. Efficient removal of the recalcitrant metamizole contaminant from drinking water by using a CaLaCoO9 perovskite supported on recycled polyethylene. J. Environ. Sci. 2024, 136, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Lakkaboyana, S.K.; Sharma, N.; Chakraborty, P.; Umesh, M.; Pasrija, R.; Thomas, J.; Kalebar, V.U.; Jayaraj, I.; Awasthi, M.K.; et al. A critical assessment of technical advances in pharmaceutical removal from wastewater—A critical review. Case Stud. Chem. Environ. Eng. 2023, 8, 100363. [Google Scholar] [CrossRef]
- Rana, S.; Kumar, A.; Dhiman, P.; Mola, G.T.; Sharma, G.; Lai, C.W. Recent advances in photocatalytic removal of sulfonamide pollutants from wastewater by semiconductor heterojunctions: A review. Mater. Today Chem. 2023, 30, 101603. [Google Scholar] [CrossRef]
- França, D.B.; Oliveira, L.S.; Nunes Filho, F.G.; Silva Filho, E.C.; Osajima, J.A.; Jaber, M.; Fonseca, M.G. The versatility of montmorillonite in water remediation using adsorption: Current studies and challenges in drug removal. J. Environ. Chem. Eng. 2022, 10, 107341. [Google Scholar] [CrossRef]
- Fakhri, M.S.B.; Barghi, N.G.; Mehdikhanmahaleh, M.M.; Zadeh, S.M.M.R.; Mousavi, T.; Rezaee, R.; Daghighi, M.; Abdollahi, M. Pharmaceutical wastewater toxicity: An ignored threat to the public health. Sustain. Environ. 2024, 10, 2322821. [Google Scholar] [CrossRef]
- Miettinen, M.; Khan, S.A. Pharmaceutical pollution: A weakly regulated global environmental risk. Rev. Eur. Comp. Int. Environ. Law 2022, 31, 75–88. [Google Scholar] [CrossRef]
- Hejna, M.; Kapuścińska, D.; Aksmann, A. Pharmaceuticals in the aquatic environment: A review on eco-toxicology and the remediation potential of algae. Int. J. Environ. Res. Public Health 2022, 19, 7717. [Google Scholar] [CrossRef]
- Sharma, J.; Joshi, M.; Bhatnagar, A.; Chaurasia, A.K.; Nigam, S. Pharmaceutical residues: One of the significant problems in achieving ‘clean water for all’ and its solution. Environ. Res. 2022, 215, 114219. [Google Scholar] [CrossRef]
- Caban, M.; Stepnowski, P. How to decrease pharmaceuticals in the environment? A review. Environ. Chem. Lett. 2021, 19, 3115–3138. [Google Scholar] [CrossRef]
- UN Environment. Global Chemicals Outlook II: From Legacies to Innovative Solutions; UN Environment Program; UN: Nairobi, Kenya, 2019; Available online: https://www.unep.org/resources/report/global-chemicals-outlook-ii-legacies-innovative-solutions (accessed on 14 August 2025).
- Natural Resource Management Ministerial Council; Environment Protection and Heritage Council; National Health and Medical Research Council. Australian Guidelines for Water Recycling: Managing Health and Environmental Risks (Phase 2), Augmentation of Drinking Water Supplies; Biotext Pty Ltd.: Canberra, Australia, 2008. [Google Scholar]
- Li, D.; Yang, M.; Hu, J.; Zhang, J.; Liu, R.; Gu, X.; Zhang, Y.; Wang, Z. Antibiotic-resistance profile in environmental bacteria isolated from penicillin production wastewater treatment plant and the receiving river. Environ. Microbiol. 2009, 11, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, X.; Feng, H.; Shi, H.; Sun, L.; Tao, W.; Xi, Q.; Wang, D. Simultaneous exposure to estrogen and androgen resulted in feminization and endocrine disruption. J. Endocrinol. 2016, 228, 205–218. [Google Scholar] [CrossRef]
- Fick, J.; Lindberg, R.H.; Parkkonen, J.; Arvidsson, B.; Tysklind, M.; Joakim Larsson, D.G. Therapeutic Levels of Levonorgestrel Detected in Blood Plasma of Fish: Results from Screening Rainbow Trout Exposed to Treated Sewage Effluents. Environ. Sci. Technol. 2010, 44, 2661–2666. [Google Scholar] [CrossRef]
- Thrupp, T.J.; Runnalls, T.J.; Scholze, M.; Kugathas, S.; Kortenkamp, A.; Sumpter, J.P. The consequences of exposure to mixtures of chemicals: Something from ‘nothing’ and ‘a lot from a little’ when fish are exposed to steroid hormones. Sci. Total Environ. 2018, 619–620, 1482–1492. [Google Scholar] [CrossRef]
- Davis, T.W.; Bullerjahn, G.S.; Tuttle, T.; McKay, R.M.; Watson, S.B. Effects of Increasing Nitrogen and Phosphorus Concentrations on Phytoplankton Community Growth and Toxicity During Planktothrix Blooms in Sandusky Bay, Lake Erie. Environ. Sci. Technol. 2015, 49, 7197–7207. [Google Scholar] [CrossRef]
- Ogueji, E.; Nwani, C.; Iheanacho, S.; Mbah, C.; Okeke, C.; Yaji, A. Acute toxicity effects of ibuprofen on behaviour and haematological parameters of African catfish Clarias gariepinus (Burchell, 1822). Afr. J. Aquat. Sci. 2018, 43, 293–303. [Google Scholar] [CrossRef]
- Makowska, N.; Zawierucha, K.; Nadobna, P.; Piątek-Bajan, K.; Krajewska, A.; Szwedyk, J.; Iwasieczko, P.; Mokracka, J.; Koczura, R. Occurrence of integrons and antibiotic resistance genes in cryoconite and ice of Svalbard, Greenland, and the Caucasus glaciers. Sci. Total Environ. 2020, 716, 137022. [Google Scholar] [CrossRef]
- Chislock, M.F.; Doster, E.; Zitomer, R.A.; Wilson, A.E. Eutrophication: Causes, Consequences, and Controls in Aquatic Ecosystems. Nat. Educ. Knowl. 2013, 4, 10. [Google Scholar]
- Zhang, Y.; Marrs, C.F.; Simon, C.; Xi, C. Wastewater treatment contributes to selective increase of antibiotic resistance among Acinetobacter spp. Sci. Total Environ. 2009, 407, 3702–3706. [Google Scholar] [CrossRef]
- Vasanthi, L.A.; Revathi, P.; Mini, J.; Munuswamy, N. Integrated use of histological and ultrastructural biomarkers in Mugil cephalus for assessing heavy metal pollution in Ennore estuary, Chennai. Chemosphere 2013, 91, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Mira, J.-D.; Valderrama, S.; Londoño, M.-J.; Giraldo-Pérez, E.; Betancur, E.; Osorio-Gómez, G. Preliminary design tools applied to a solar powered vessel design: A South American river analysis. In Proceedings of the 2020 Fifteenth International Conference on Ecological Vehicles and Renewable Energies (EVER), Monte-Carlo, Monaco, 10–12 September 2020. [Google Scholar]
- Achilleos, A.; Hapeshi, E.; Xekoukoulotakis, N.P.; Mantzavinos, D.; Fatta-Kassinos, D. UV-A and Solar Photodegradation of Ibuprofen and Carbamazepine Catalyzed by TiO2. Sep. Sci. Technol. 2010, 45, 1564–1570. [Google Scholar] [CrossRef]
- Ioannidou, E.; Frontistis, Z.; Antonopoulou, M.; Venieri, D.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Solar photocatalytic degradation of sulfamethoxazole over tungsten—Modified TiO2. J. Chem. Eng. 2017, 318, 143–152. [Google Scholar] [CrossRef]
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekoukoulotakis, N.P.; Venieri, D.; Mantzavinos, D. Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. J. Environ. Manag. 2012, 98, 168–174. [Google Scholar] [CrossRef]
- Frontistis, Z.; Daskalaki, V.M.; Katsaounis, A.; Poulios, I.; Mantzavinos, D. Electrochemical enhancement of solar photocatalysis: Degradation of endocrine disruptor bisphenol-A on Ti/TiO2 films. Water Res. 2011, 45, 2996–3004. [Google Scholar] [CrossRef]
- Ioannou, L.A.; Hapeshi, E.; Vasquez, M.I.; Mantzavinos, D.; Fatta-Kassinos, D. Solar/TiO2 photocatalytic decomposition of β-blockers atenolol and propranolol in water and wastewater. Sol. Energy 2011, 85, 1915–1926. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meghea, I.; Stefan, D.S.; Ioniţă, F.; Lesnic, M.; Manea-Saghin, A.-M. An Integrative Approach to Hazardous Effects Caused by Pharmaceutical Contaminants on Aquatic Effluents. Molecules 2025, 30, 3483. https://doi.org/10.3390/molecules30173483
Meghea I, Stefan DS, Ioniţă F, Lesnic M, Manea-Saghin A-M. An Integrative Approach to Hazardous Effects Caused by Pharmaceutical Contaminants on Aquatic Effluents. Molecules. 2025; 30(17):3483. https://doi.org/10.3390/molecules30173483
Chicago/Turabian StyleMeghea, Irina, Daniela Simina Stefan, Florina Ioniţă, Mihai Lesnic, and Ana-Maria Manea-Saghin. 2025. "An Integrative Approach to Hazardous Effects Caused by Pharmaceutical Contaminants on Aquatic Effluents" Molecules 30, no. 17: 3483. https://doi.org/10.3390/molecules30173483
APA StyleMeghea, I., Stefan, D. S., Ioniţă, F., Lesnic, M., & Manea-Saghin, A.-M. (2025). An Integrative Approach to Hazardous Effects Caused by Pharmaceutical Contaminants on Aquatic Effluents. Molecules, 30(17), 3483. https://doi.org/10.3390/molecules30173483










